ABSTRACT
Chronic kidney disease (CKD) is a major public health issue affecting an estimated 850 million people globally. The leading causes of CKD is diabetes and hypertension, which together account for >50% of patients with end-stage kidney disease. Progressive CKD leads to the requirement for kidney replacement therapy with transplantation or dialysis. In addition, CKD, is a risk factor for premature cardiovascular disease, particularly from structural heart disease and heart failure (HF). Until 2015, the mainstay of treatment to slow progression of both diabetic and many non-diabetic kidney diseases was blood pressure control and renin-angiotensin system inhibition; however, neither angiotensin-converting enzyme inhibitors (ACEIs) nor angiotensin receptor blockers (ARBs) reduced cardiovascular events and mortality in major trials in CKD. The emergence of cardiovascular and renal benefits observed with sodium-glucose cotransporter-2 inhibitors (SGLT2i) from clinical trials of their use as anti-hyperglycaemic agents has led to a revolution in cardiorenal protection for patients with diabetes. Subsequent clinical trials, notably DAPA-HF, EMPEROR, CREDENCE, DAPA-CKD and EMPA-KIDNEY have demonstrated their benefits in reducing risk of HF and progression to kidney failure in patients with HF and/or CKD. The cardiorenal benefits—on a relative scale—appear similar in patients with or without diabetes. Specialty societies’ guidelines are continually adapting as trial data emerges to support increasingly wide use of SGLT2i. This consensus paper from EURECA-m and ERBP highlights the latest evidence and summarizes the guidelines for use of SGLT2i for cardiorenal protection focusing on benefits observed relevant to people with CKD.
Keywords: cardiorenal syndrome, cardiovascular, chronic renal failure, diabetic kidney disease, heart failure
EPIDEMIOLOGY OF CKD
Approximately 850 million patients in the world have chronic kidney disease (CKD), with ∼4 million receiving kidney replacement therapy [1]. Diabetic kidney disease (DKD) is the foremost cause of CKD globally [2]. In 2015, there were ∼415 million people living with diabetes mellitus with the prevalence predicted to rise to 642 million by 2040, largely driven by an ageing population and lifestyle factors [2, 3]. DKD develops in ∼40% of patients with diabetes mellitus and the incidence is increasing with the growing prevalence of diabetes [2]. Until recently the mainstay of renal protection in patients with type 2 diabetes mellitus (T2DM) and proteinuric CKD has been the use of renin-angiotensin system inhibitors (RASi) based on the Reduction of Endpoints with the Angiotensin II Antagonist Losartan (RENAAL) [4] and the Irbesartan in Diabetic Nephropathy Trial (IDNT) [5] with both studies published >20 years ago. Similarly, RASi was the mainstay of treatment for hypertensive kidney disease, the second most common cause of CKD, based mainly of the results of the African American Study on Kidney Disease [6]. However, the residual renal risk remained very high [2, 7]. Furthermore, in all outcome trials in DKD, the use of RASi was not associated with reduction in cardiovascular events and mortality [8].
Cardiovascular events and mortality increase exponentially with decreasing glomerular filtration rate or increasing albuminuria independent of age, sex, and other risk factors [9, 10]. Furthermore, as kidney function decreases, there is a shift from predominantly atherosclerotic/thrombo-embolic/vasculo-occlusive cardiovascular diseases to a proportionally increasing incidence of heart failure (HF) and sudden cardiac death [11]. In a large United States study (1998–2006) of 15 762 patients aged >20 years, the 10-year cumulative cardiovascular mortality for patients without neither diabetes or CKD, with diabetes and no CKD, and with CKD and no diabetes was 3.4%, 6.7%, and 9.9% respectively [12]. However, for patients with both diabetes and CKD the 10-year cumulative mortality was a staggering 19.6% [12]. Until 2015, although several novel agents had emerged for managing hyperglycaemia in T2DM, limited progress had been made in reducing the incidence of kidney failure due to diabetes [13]. The emergence of sodium-glucose cotransporter-2 inhibitors (SGLT2i) as a beneficial evidence-based therapy for improving cardiovascular and renal outcomes potentially revolutionizes therapy in this group of patients. This position paper integrates the current evidence base for therapy with SGLT2i with professional societies guidelines for their use.
CARDIOVASCULAR AND RENAL BENEFITS OF SGLT2i IN THE ORIGINAL CARDIOVASCULAR OUTCOME TRIALS
The cardiovascular and kidney benefits of SGLT2i were initially observed in cardiovascular outcomes trials (CVOT) mandated by the US Food and Drug Administration to assess cardiovascular (CV) safety in T2DM. Three CVOT shared a common three-point major adverse cardiovascular events (MACE) primary outcome and additionally had secondary kidney outcomes. Table 1 summarizes the major CVOTs and as well the renal outcome trials with SGLT2i.
Table 1:
Main: clinical trials evaluating cardiorenal outcomes in patients treated with SGLT2i. CKD: chronic kidney disease; MACE: major adverse cardiovascular event (CV death, nonfatal MI, or nonfatal stroke); CV: cardiovascular; eGFR: estimated GFR; HF: heart failure; HHF: hospitalised heart failure; EF: ejection fraction; ESKD: end-stage kidney disease; T2DM Type Diabetes mellitus.
| Trials (ref) | Year of completion | SGLT2i | Patient population | Number of patients | Median follow up | Mean eGFR (ml/min/1.73 m2) | Μedian uACR mg/g (IQR) | CV outcome | SGLT2i vs. placebo group | HR (95%CI) | Renal outcome | SGLT2i vs. placebo group | HR (95%CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EMPA-REG OUTCOME [48] | 2015 | empagliflozin (10 or 25 mg) | T2DM and CVD | 7020 | 3.1 years(2.6 treatment) | 74 ± 21 | ΝΑ(28.7% microalbuminuria and 11% macroalbuminuria) | MACEHF or CVdeath (excluding fatal stroke)CV death | 10.5% vs. 12.1%5.7% vs. 8.5%3.7% vs. 5.9 | 0.86(0.74–0.99)0.66 (0.55–0.79)0.62(0.49–0.77) | (Post hoc)Incident or worsening nephropathy or CV deathincident orworsening nephropathydoubling of the serum creatinineinitiation of renal-replacement therapy | 16.2% vs. 23.6%12.7% vs 18.8%1.5% vs. 2.6%0.3% vs. 0.6% | 0.61 (0.55–0.69)0.61 (0.53–0.7)0.56 (0.39–0.79)0.45 (0.21–0.97) |
| CANVAS [17] | 2017 | canagliflozin(100 or 300 mg) | T2D andhigh CVD risk | 10 142 | 2.6 years | 76. 5 ± 20. 5 | 12.3 (6.65–42.1) | MACEHospitalization for HF | 26.9% vs. 31.5%5.5 vs. 8.68 per 1000 patient-years | 0.86(0.75–0.97)0.67 (0.52–0.87) | progression of albuminuriasustained 40% reduction in eGFR, need for renal-replacement therapy, or death from renal causes | 89.4 vs. 128.7per 1000 patient-years5.5 vs. 9.0 | 0.73 (0.67–0.79)0.60 (0.47–0.77) |
| DECLARE-TIMI58 [20] | 2018 | Dapagliflozin(10 mg) | T2DMand ≥ 1 CVDrisk factor | 17.160 | 4.2years | 85.2 ± 16 | NA | MACECV death or hospitalization for HF | 8.8 vs. 9.4%4.9% vs. 5.8% | 0.93 (0.84–1.03)0.83 (0.73–0.95) | ≥40% reduction in eGFR, ESKD≥90 days, (dialysis, sustained eGFR <15 ml/min/1.73 m2, orkidney transplantation), or renal/CV death | 4.3% vs. 5.6% | 0.76 (0.67–0.87) |
| CREDENCE [18] | 2019 | canagliflozin(100 mg) | T2DM and CKD | 4401 | 2.6 years | 56.2 ± 18.2 | 927 [463–1833] | MACEHHF or CVdeath | 9.9 vs. 12.2%8.1 vs. 11.5% | 0.80 (0.67–0.95)0.69 (0.57–0.83) | doubling ofserum creatinine, ESKD (dialysis, renal transplantation, or sustainedeGFR <15 ml/min/1.73 m2), or renal/CV death | 11.1% vs. 15.4% | 0.70 (0.59–0.82) |
| VERTIS CV [80] | 2019 | ertugliflozin(5 or 15 mg) | T2D and established CVD | 8246 | 3.5 years | 76.1 ± 20.9(75.7 ± 20.8 placebo group) | NA | hospitalization for heartfailureDeath from CV causes or hospitalizationfor HF | 11.9% vs. 11.9%8.1% vs. 9.1% | 0.97 (0.85–1.11)0.88 (0.75–1.03) | death from renal causes,renal-replacement therapy, or doubling of theserum creatinine | 3.2% vs. 3.9% | 0.81 (0.63–1.04) |
| EMPEROR reduced [50] | 2020 | empagliflozin(10 mg) | HF with reduced EF | 3730 | 16 months | 61.8 ± 21.7 (62.2 ± 21.5 placebo group) | NA | death fromCV causes or hospitalization for HFhospitalization for HF | 19.4% vs24.7%20.8% vs. 29.6% | 0.75 (0.65–0.86)0.70 (0.58–0.85) | dialysis or renal transplantation orsustained reduction in the eGFReGFR decline | 1.6% s 3.1%–0.93 vs. –4.21 ml perminute per 1.73 m2 | 0.50 (0.32–0.77)(95%CI –1.97–0.11) and(95% CI, −5.26 to −3.17) |
| EMPEROR –preserved [51] | 2021 | empagliflozin(10 mg) | HF with preserved EF | 5988 | 26.2 months | 60.6 ± 19.8 | NA | death fromCV causes or hospitalization for HFhospitalization for HF | 13.8% vs. 17.1%8.6% vs. 11.8% | 0.79 (0.69–0.90)0.73 (0.61–0.83) | Rate of declinein the eGFR | (−1.25 vs. −2.62 ml per minute per 1.73 m2 per year | |
| DAPA-CKD [35] | 2020 | dapagliflozin(10 mg) | CKD (T2D and non-diabetics) | 4304 | 2.4 years | 43.1 ± 12.4 | 965 (472–1903)934 (482–1868) for the placebo group | Death CV causes or hospitalization for HF | 4.6% vs. 6.4% | 0.71 (0.55–0.92) | decline in e-GFR of at least 50%, ESKD, or death from renal, or CV causessustained decline in the eGFR of at least 50%, ESKD, or death from renal causes | 9.2% vs. 14.5%6.6% vs. 11.3% | 0.61 (0.51 −0.72)0.56 (0.45–0.68) |
| EMPA-KIDNEY [58] | 2022 | Empagliflozin(10 mg) | CKD (T2D and non-diabetics) | 6609 | 2.0 years | 37.5 ± 14.8 | 412 (94–1190) | hospitalization for HF or cardiovascular death | 4.0% vs. 4.6% | 0.84 (0.67–1.07) | ESKD, a sustained decline in eGFR to < 10 mL/min/1.73m², renal death, or a sustained decline of ≥ 40% in eGFR from randomization) or (ii) Cardiovascular death | 13.1% vs. 16.9% | 0.72 (0.64–0.82) |
The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial studied empagliflozin versus placebo (81% subjects on RASi at baseline) in T2DM with established atherosclerotic cardiovascular disease (ASCVD) (coronary, peripheral vascular, or cerebral artery disease). During median follow up of 3.1 years, empagliflozin reduced risk of MACE and kidney disease outcomes [14]. A post hoc analysis of new onset or worsening nephropathy in 2250 patients with prevalent CKD at baseline demonstrated the benefit of empagliflozin on cardiovascular risk reduction by 29% [hazard ratio (HR), 0.71; 95% confidence interval (CI), 0.52–0.98] independently of estimated glomerular filtration rate (eGFR) category and urinary albumin-creatinine ratio (uACR) baseline status [14, 15].
The Canagliflozin Cardiovascular Assessment Study (CANVAS) programme assessed canagliflozin versus placebo in T2DM, with 66% of patients with established ASCVD. Canagliflozin reduced risk of primary MACE outcome, and also found similar reductions in kidney disease progression outcomes to EMPA-REG OUTCOME. Post hoc analyses of CANVAS reported consistency of effects on cardiovascular and renal outcomes at different albuminuric stages [16]. An increased risk of lower limb amputation risk led to regulatory warnings, but this observation has not been replicated in subsequent trials of canagliflozin [17, 18] or other SGLT2i [19].
The Dapagliflozin Effect on Cardio-vascular Events–Thrombolysis in Myocardial Infarction 58 trial (DECLARE-TIMI 58) included T2DM patients at high risk of cardiovascular disease, ∼60% of patients without previous ASCVD (59%). During median follow up of 4.2 years, compared to placebo, dapagliflozin did not reduce risk of MACE but did reduce risk of its co-primary outcome of CV death or hospitalization for HF (4.9 vs. 5.8%, HR 0.83; 95% CI, 0.73 to 0.95), as well as the composite kidney outcomes [20].
RENAL MECHANISMS OF ACTION OF SGLT2i
A key concept that may explain both acute and long-term SGLT2i protective effects on renal function is linked to the decreased reabsorption of glucose and sodium (Na) in the S1 and S2 portions of the proximal tubule where SGLT2i have their intended mechanism of action. Higher Na delivery to the macula densa stimulates adenosine release, inducing vasoconstriction of the afferent and vasodilatation of the efferent arterioles, reducing intraglomerular pressure and restoring tubuloglomerular feedback [21]. This effect leads to an initial dip in eGFR and reduced albuminuria but preserves renal function in the longer term [22]. A natriuretic effect associated to the inhibition of the Na/H+ exchanger (NHE3) has been also described [23]. The increase in diuresis and reduction in blood pressure (BP) confers extra beneficial long-term effects than usual diuretics as fluid redistribution between intracellular and extracellular compartments is achieved [24]. Furthermore, the reduction in BP does not increase heart rate, so a direct inhibition of sympathetic activity is suggested [25]. Decreases in the tubular transport workload reduce glucotoxicity and oxygen consumption, increasing cortical oxygen tension in this highly aerobic part which may explain the mechanism by which acute kidney injury (AKI) is reduced by these agents [26]. The increased glucose delivery to subsequent tubular segments, increases SGLT1 activity decreasing oxygen tension in the outer medulla and stimulating erythropoietin production [27]. Another beneficial effect of SGLT2i is the increased uricosuria, due to a decrease in urate absorption by competition of the excess glucose delivered with urate in the urate transporter [28]. A direct effect in podocytes with a potential of decreasing proteinuria has been also described [29].
Indirect effects are mainly metabolic, as SGLT2i induce weight loss, perirenal fat, and obesity-induced inflammation [30]. SGLT2i have been associated with increases in adiponectin and ketogenesis, and decreases in leptin release, mimicking a fasting-like state [31]. This will lead to an increase in sirtuin-1 and adenosine monophosphate-activated protein kinase (AMPK), which in turn decrease oxidative and endoplasmic reticulum stress and inflammation [32, 33]. Furthermore, a decrease in epithelial-mesenchymal transition has been demonstrated [34]. All those changes lead to a decrease in renal damage and fibrosis.
RENAL OUTCOME TRIALS- CREDENCE AND DAPA-CKD
Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) was the first trial evaluating the effect of an SGLT2i on major renal outcomes in CKD [18], randomizing 4401 patients with T2DM, eGFR 30 to <90 ml/min/1.73 m2 and uACR 300–5000 mg/g already on maximum tolerated dose of an ACEI or ARB to canagliflozin or placebo. The primary outcome was end-stage kidney disease (ESKD) defined as dialysis, transplantation, or sustained eGFR <15 ml/min/1.73 m2; doubling of serum creatinine; or death from renal or cardiovascular causes. Mean eGFR was 56.2 ml/min/1.73 m2; median uACR 927 mg/g; mean age of participants 63 years; and 33.9% of patients were women. The study was stopped early for efficacy (median follow up 2.62 years). The relative risk of the primary outcome was 30% lower with canagliflozin, with event rates of 43.2 and 61.2 per 1000 patient-years, respectively (HR 0.70; 95%CI 0.59–0.82). The risk of the renal-specific composite of ESKD, doubling of creatinine, or death from renal causes was lower by 34% (HR 0.66; 95%CI 0.53–0.81), and that of ESKD by 32% (HR 0.68; 95%CI 0.54–0.86) with canagliflozin. Furthermore, the geometric mean of uACR was 31% lower with canagliflozin (95%CI, 26 to 35%), while the eGFR slope was typical of an acute eGFR dip with canagliflozin during the first 3 weeks (−3.72 ± 0.25 vs. −0.55 ± 0.25 ml/min/1.73 m2), followed by slower eGFR decline thereafter (−1.85 ± 0.13 vs. −4.59 ± 0.14 ml/min/1.73 m2 for canagliflozin versus placebo) [18].
The Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with CKD (DAPA-CKD) evaluated the effect of dapagliflozin versus placebo in addition to a maximum tolerated dose of an ACEI or ARB in patients with eGFR ≥ 25 and ≤75 ml/min/1.73 m2, and uACR ≥ 200 and ≤5000 mg/g. The primary outcome was a composite of ≥50% sustained decline in eGFR, ESKD, or cardiovascular or renal death [35]. DAPA-CKD recruited 4304 participants with mean eGFR 43.1 ml/min/1.73 m2 and median uACR of 949 mg/g. Here, 2906 participants (67.5%) had T2DM, but in 396 of them CKD was ascribed to causes different than DKD [36]. DAPA-CKD was also stopped early (median of 2.4 years) due to overwhelming efficacy. The primary endpoint occurred in 9.2% versus 14.5% of the participants in the dapagliflozin and the placebo groups, respectively (HR 0.61; 95% CI 0.51–0.72). The renal component of the primary composite outcome of ≥50% sustained decline in eGFR, ESKD and renal death was reduced with dapagliflozin (HR 0.56; 95% CI, 0.45–0.68), including reductions in each component of this renal composite. Dapagliflozin reduced geometric mean uACR by 29.3% (95% CI −33.1 to −25.2; P < 0.0001) [37], a typical eGFR dip during the first 2 weeks was evident with dapagliflozin (−3.97 ± 0.15 vs. −0.82 ± 0.15 ml/min/1.73 m2), which was followed by a smaller annual eGFR loss thereafter (−1.67 ± 0.11 vs. −3.59 ± 0.11 ml/min/1.73 m2) [35].
SGLT2i IN NON-DIABETIC CKD
DAPA-CKD and the Multicentre International Randomized Parallel Group Double-blind Placebo-controlled Clinical Trial of EMPAgliflozin Once Daily to Assess Cardio-renal Outcomes in Patients With Chronic KIDNEY Disease trial (EMPA-KIDNEY) enrolled, among other non-diabetic diseases, patients with IgA nephropathy (IgAN) and focal segmental glomerulosclerosis (FSGS) with or without diabetes (Table 1) [38]. DAPA-CKD provided pre-specified analyses of 270 participants with IgAN (94% biopsy confirmed) and 104 with biopsy-confirmed FSGS [39, 40]. The size of DAPA-CKD compares favourably with phase 2 and 3 (Supplementary Table S1) and recent trials in IgAN, such as Therapeutic Evaluation of Steroids in IgA Nephropathy Global Study (TESTING) and Supportive Versus Immunosuppressive Therapy for the Treatment Of Progressive IgA Nephropathy (STOP-IgAN). IgAN patients in DAPA-CKD had lower baseline eGFR and similar proteinuria but were older and were more frequently diabetic than patients in TESTING and STOP-IgAN (Supplementary Table S2) [41–44].
In DAPA-CKD, mean eGFR slopes for IgAN patients on dapagliflozin and placebo were −3.5 and −4.7 ml/min/1.73 m2/year, respectively, and dapagliflozin reduced the uACR by 26% relative to placebo [39]. The primary outcome occurred in six (4%) participants on dapagliflozin and 20 (15%) on placebo (HR, 0.29; 95% CI, 0.12, 0.73). EMPA-KIDNEY has confirmed important kidney benefits in IgAN, adding a further 80 such outcomes. Once pooled, SGLT2i appeared to reduce kidney disease progression substantially compared to placebo (37/550 vs. 68/537; HR 0.49, 95% CI 0.32–0.74) [45]. Data are more limited in FSGS, with only 28 kidney disease progression outcomes when DAPA-CKD and EMPA-KIDNEY are combined [40, 45]. However, when considered as a group of diseases, pooled analysis of DAPA-CKD and EMPA-KIDNEY suggest SGLT2i reduce risk of kidney disease progression by about 40% compared to placebo (HR 0.60, 95% CI 0.46–0.78) [45]. It is now proposed that IgAN and podocytopathy trials should include SGLT2i as background therapy and assess GFR decline as primary outcome [46, 47]. Clinical guidelines for the treatment of these conditions should be updated to include combined RASi plus SGLT2i as the recommended new standard of care.
CARDIOVASCULAR BENEFITS OF SGLT2i IN HEART FAILURE
Following the observation of major clinical benefits of HF outcomes in the initial CVOTs [20, 48], recent trials of SGLT2i as an adjunctive therapy in HF have demonstrated significant benefits with these agents, both in people with and without diabetes. In DAPA-HF trial of 4744 people with HF with reduced ejection fraction (HFrEF) with and without diabetes, compared to placebo, dapagliflozin was associated with reduced incidence of the of primary outcome of worsening HF or cardiovascular death [49]. Similarly, in the EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction (EMPEROR Reduced) trial of 3730 people with HFrEF empagliflozin with or without diabetes, empagliflozin reduced risk of the primary composite outcome of cardiovascular death or hospitalization for worsening HF compared to placebo [50]. Moreover, in the EMPEROR with Preserved Ejection Fraction (EMPEROR-Preserved) trial, empagliflozin reduced the incidence of the composite of cardiovascular death or hospitalization for HF in patient with and without diabetes considered separately [51], and the drug now has a US licence for the treatment of HF regardless of ejection fraction—the first therapy to demonstrate clear efficacy in HF with preserved ejection fraction (HFpEF). The benefits of empagliflozin were consistent across the range of eGFR in HF patients in EMPEROR Reduced [52]. These data has been followed by the Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure (DELIVER) trial in 6263 patients with HF and a left ventricular ejection fraction >40%, where compared to placebo, dapagliflozin significantly reduced the incidence of a primary outcome (composite of worsening HF or cardiovascular death) (HR, 0.82; 95% CI, 0.73 to 0.92; P < 0.001) [53].
Given the high prevalence of HF in general and more specifically HFpEF in patients with CKD, these observations should herald a new era of SGLT2i for cardiorenal protection where people with CKD may stand to experience considerable benefit [54]. For example, it is estimated from event rates from the CKD trials, that each 1000 patient-years of treatment with an SGLT2i is predicted to prevent 11 first hospitalization for HF or cardiovascular deaths in patients with diabetes, and many more if they have pre-existing heart failure (Supplementary Fig. S1, see the online supplementary material for a colour version of this figure) [45].
HOW EMPA-KIDNEY AND ONGOING TRIALS ARE PUSHING THE EVIDENCE IN CKD
With the growing number of large placebo-controlled SGLT2i confirming their efficacy in increasingly broad populations [19], certainty about their safety is increasing and the opportunity for new placebo-controlled trials in CKD has diminished rapidly. CREDENCE and DAPA-CKD provide clear evidence of cardiorenal benefits among patients with T2DM and DKD [18, 35], and other trials suggest renal benefits extend to those with T2DM without albuminuria [55]. Nevertheless, it is important for the renal community to see more randomized data in patients without T2DM and with low eGFR.
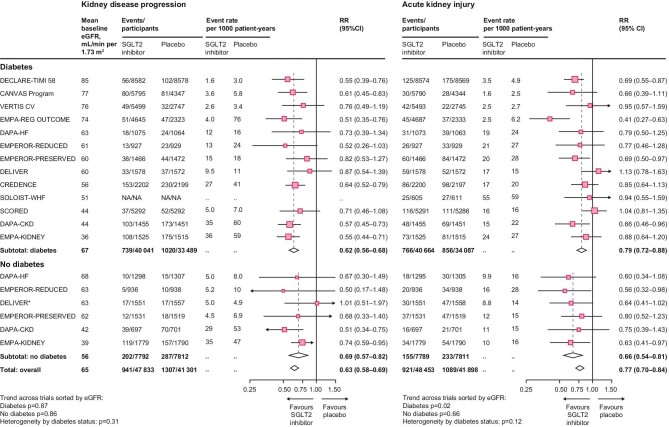
The EMPA-KIDNEY trial assessed the effect of 10 mg of empagliflozin versus placebo on a composite primary outcome of kidney disease progression or cardiovascular death in an even broader range of patients with CKD at risk of progression. In total, 6609 patients with eGFRs as low as 20 ml/min/1.73 m2 were screened, and by the time of randomization 254 had eGFRs 15–20 ml/min/1.73 m2 [45, 56]. The types of patient recruited into EMPA-KIDNEY who were under-represented in previously reported SGLT2i trials including a large proportion without diabetes (54%), with an eGFR <30 ml/min/1.73 m2 (35%), and with a uACR <300 mg/g at recruitment (48%). The trial also included 1669 people with glomerular disease, of which 817 (49%) had IgAN, plus 68 patients with type 1 diabetes [57]. Empagliflozin had beneficial effect on the primary renal outcome in EMPA-KIDNEY (HR 0.72, 95% CI 0.64–0.82) (Table 1) [58]. All the reported large SGLT2i trials in CKD excluded patients with polycystic kidney disease or a kidney transplant. Patients with extremely low eGFR and on dialysis are still being studied in the RENAL LifeCycle trial (NCT05374291). The cumulative of impact of SGLT2i on renal outcomes is highlighted in an updated meta-analysis with SGLT2i use having 37% lower risk of kidney disease progression (HR 0.63, 95% CI 0.58–0.69) (Fig. 1), with consistent effects in patients with and without diabetes, across primary kidney diagnoses and with different SGLT2 inhibitors [45].
Figure 1:
Effect of sodium-glucose cotransporter-2 inhibition on kidney disease outcomes from meta-analysis of large placebo-controlled randomized controlled trials. Figure adopted from [45]. eGFR: estimated glomerular filtration rate; SGLT2i: sodium-glucose cotransporter-2 inhibitor; RR: relative risk; NA: not available.
ROLE OF SGLT2i COMPARED TO/COMBINED WITH OTHER NEPHROPROTECTIVE THERAPY-NOW AND IN FUTURE
In parallel to the advent of SGLT2i as foundational therapy for CKD, contemporary data from the Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease (FIDELIO-DKD) and Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD) trials suggest the novel non-steroidal mineralocorticoid receptor antagonist (ns-MRA) finerenone on top of standard of care RAS inhibition can also significantly improve a range of both kidney and cardiovascular outcomes [59, 60]. FIDELIO-DKD (n = 5674) and FIGARO-DKD (n = 7352) trials determined the effect of finerenone on CKD progression and CV mortality and morbidity in patients with CKD and T2DM and a combined analysis termed FIDELIO-DKD and FIGARO-DKD Trial programme analYsis (FIDELITY) has been published [59–61]. Both trials randomized patients receiving a standard of care with the maximum tolerated dose of RASi.
In FIDELIO-DKD, finerenone in combination with maximal tolerated RASi significantly reduced the risk of the kidney and CV composite endpoints by 23% (0.77, 95%CI 0.67–0.88) and 14% (0.86, 95%CI 0.78–0.95), respectively [59]. In the FIDELITY analysis, just 6.7% of participants received an SGLT2i at baseline and 8.5% initiated one during the trial [62]. Baseline SGLT2i use did not affect risk reduction for the cardiovascular or kidney composites with finerenone. Indeed, numerically, the lowest risk corresponded to patients with combined SGLT2i and finerenone: for the cardiovascular composite, the HRs were 0.87 (95% CI 0.79–0.96) without SGLT2i and 0.67 (95% CI 0.42–1.07) with SGLT2i; for the kidney composite, the HRs were 0.80 (95% CI 0.69–0.92) without SGLT2i and 0.42 (95% CI 0.16–1.08) with SGLT2i [62].
Combination of ns-MRA and SGLT2i seems to be very attractive not only for efficacy but also as the potential risk of hyperkalaemia when adding MRA to ACEIs/ARBs might be counteracted by the potassium lowering effect of SGLT2i [63].
SAFETY PROFILE OF SGLT2i
The renal safety profile of SGLT2i has been quantified by meta-analysis [45, 64]. Data from 13 studies of 90 413 participants showed that beyond the known excess risk of mycotic genital infections (RR, 3.57; 95% CI, 3.14–4.06), there was no overall increased risk of other safety outcomes, including serious urinary tract infections, amputations, and fractures [45]. In patients with diabetes, the risk of ketoacidosis is approximately doubled, but the risk is higher for states of insulin deficiency and T1 diabetes mellitus and patients with T2DM in the trials remained at low absolute risk. In the trial populations studies to date, the absolute harms of SGLT2i in patients with and without diabetes are clearly lower than the substantial absolute benefits [45]. This is particularly the case in patients with CKD, and especially those without diabetes [19, 45]. Furthermore, meta-analysis of the large placebo-controlled SGLT2i trials show they reduce the risk of AKI (HR 0.77, 0.70–0.84) [45] and reduce risk of serious adverse events of hyperkalaemia by 16% (0.84, 95% CI 0.76–0.93) [19, 63]. Although there is a risk of ketoacidosis and hypoglycaemia with these agents, there were no differences in the incidence of these events between placebo and control groups [35, 45].
Reductions in serious hyperkalaemia were consistent across the trials and a range of subgroups, including baseline kidney function, history of HF, and use of RASi, diuretic, and MRA. Of note, SGLT2i did not increase the risk of hypokalaemia [HR, 1.04 (95% CI, 0.94–1.15)] [64]. In the HFrEF setting, beneficial effects of empagliflozin were unmodified in a secondary analysis of EMPEROR Reduced when those prescribed an MRA at baseline or not were compared, and allocation to empagliflozin was associated with less discontinuation of MRAs (perhaps due to less severe hyperkalaemia with empagliflozin [65]). In DAPA-HF moderate/severe hyperkalaemia (potassium >6.0 mmol/l) was less common among patients assigned to dapagliflozin, compared with placebo in patients taking an MRA at baseline [HR 0.50 (0.29–0.85); P = 0.01, P value for interaction 0.08] [66].
In the HFpEF setting, the benefit of empagliflozin to reduce the primary outcome was not significantly different between steroidal MRA nonusers and steroidal MRA users [67]. The effect of empagliflozin to reduce first and recurrent HF hospitalizations was more pronounced in MRA nonusers [HR: 0.60 (95% CI: 0.47–0.77) compared to MRA users HR: 0.90 (95% CI: 0.68–1.19); interaction P = 0.038]. Empagliflozin reduced hyperkalaemia or initiation of potassium binders, with no significant treatment-by-MRA subgroup interaction. Multiple mechanisms have been proposed to explain the mechanism by which SGLT2i reduce hyperkalaemia [63].
REVIEW OF GUIDELINES AND RECOMMENDATIONS ON THE USE SGLT2I BY DIFFERENT SPECIALTIES (SUPPLEMENTARY TABLE S3)
Diabetology guidelines
In 2018 the Canadian Diabetes Association [68] published its recommendations for antihyperglycemic medication selection and dosing in CKD. In this guideline, SGLT2i are used as ‘anti-hyperglycaemic drugs’ in people who cannot achieve glycaemic targets. The authors recommend using SGLT2i with proven renal benefits for reducing the risk of CKD progression in people with T2DM, cardiovascular disease and CKD who have eGFR >30 ml/min/1.73 m2 with a level for recommendation ‘2B for empagliflozin’ and ‘3C for canagliflozin’.
The most recent 2022 joint American Diabetes Association (ADA) European Association for Study of Diabetes (EASD) consensus statement regarding the choice of glucose-lowering therapy in patients with T2DM. The recommendation is that the therapy should be based on assessment of established ASCVD, CKD or HF with goals of glycaemic control and cardiorenal protection considered in partnership [69]. SGLT2i are indicated in people with T2DM independently of baseline HbA1c or individualized HbA1c target to reduce cardiac events, HF, cardiovascular death, and progression of CKD. In particular, SGLT2i were recommended for people with HF with moderately reduced ejection fraction or CKD (eGFR 30 to 60 ml/min/1.73 m2) or T2DM and HFrEF because they reduce cardiac events and cardiovascular death. As a distinct recommendation, for patients with high risk for amputation, SGLT2i should be prescribed after risks are explained and with comprehensive education on foot care. The 2023 updated ADA ADA guideline on Standards of Medical Care in Diabetes suggests that both cardiorenal risk and glycaemic control should be assessed in parallel with SGLT2i first line therapy for cardiorenal risk [70].
Cardiology guidelines
In 2019, the revised version of the European Society of Cardiology (ESC) guidelines on diabetes, pre-diabetes, and cardiovascular diseases first mentioned the use of SGLT2i as recommended for glucose-lowering treatment in patients with T2DM [71]. This guideline recognized the paradigm shift in glucose-lowering treatment demonstrated by several cardiovascular outcome trials that indicate cardiovascular benefits from the use of SGLT2i and GLP-1-receptor agonists (GLP-1RA) in patients with ASCVD or at very high/high cardiovascular risk.
SGLT2i are indicated as glucose-lowering agents in patients with cardiovascular disease or high cardiovascular risk to reduce cardiovascular events with class of recommendation ‘I’ and level of evidence ‘A’. Also, SGLT2i are recommended to lower the risk of HF hospitalization with class of recommendation ‘I’ and level of evidence ‘A’. Finally, empagliflozin is recommended in patients with T2DM and CVD to reduce the risk of death with a class I recommendation and level B of evidence.
The ESC guideline for acute and chronic HF [72] described a four-pillar approach in managing HFrEF patients irrespective of presence of T2DM. These four pillars are represented by four classes of medication that are used to reduce mortality: beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor-neprilysin inhibitor, MRA, and SGLT2i. The main SGLT2i are dapagliflozin and empagliflozin. They are recommended to reduce hospitalization for HF and to reduce death with class of recommendation ‘I’ and level of evidence ‘A’.
ERA AND KIDNEY DISEASE IMPROVING GLOBAL OUTCOMES (KDIGO) GUIDELINES
The first mention of SGLT2i was in the ERBP Clinical Practice Guideline on management of patients with diabetes and CKD stage 3b or higher (eGFR <45 ml/min), published in 2015 [73]. There, SGLT2i were used as anti-hyperglycaemic drugs. The recommendation of use for SGLT2i was for CKD stage 3B or less, but the authors mention a ‘limited experience’. On this topic, this guideline is outdated and undergoing revision.
In 2019 the EURECA-m and the DIABESITY working groups of the ERA-EDTA made a consensus statement recommending that patients with T2DM and CKD, that are not on HbA1c target on recommended Metformin therapy or for whom Metformin is not tolerated or is contraindicated, to use SGLT2i with evidence for cardio and renal protection [74]. Therefore, SGLT2i were historically recommended for patients with T2DM and CKD with eGFR >60 ml/min/1.73 m2 or with eGFR <60 ml/min/1.73 m2 but above the licensed range who have either micro- or macro-albuminuria who do not achieve glycaemic targets under Metformin or do not tolerate Metformin. While these guidelines emphasized the role of SGLT2i in glycaemic control, we have moved to an era of SGLT2i for kidney protection rather than glycaemic control in isolation, and, in reality, the effect of SGLT2i on glycaemia is limited when eGFR <45 ml/min/1.73 m2.
KDIGO published an updated guideline on Diabetes Management in CKD in 2022 in which it recommends a comprehensive treatment of patients with diabetes and CKD to reduce risks of kidney disease progression and CV disease [75]. SGLT2i are now considered a first line drug for patients with T2DM and CKD. The target population for SGLT2i were people with T2DM and CKD who have eGFR ≥20 ml/min/1.73 m2. The level of recommendation for the SGLT2 administration was ‘1A’.
UPDATED UNITED KINGDOM GUIDANCE SPECIFIC TO KIDNEY INDICATIONS OF SGLT2i
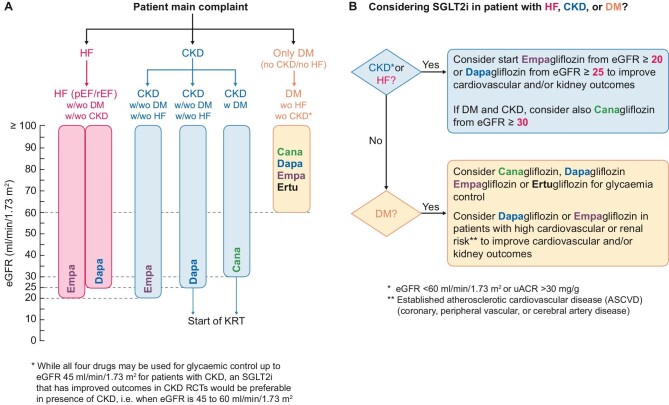
The guideline from the National Institute for Health and Care Excellence (NICE) focused on drug treatment of T2DM, specifically on ‘anti-hyperglycaemic drugs’ was published in February 2022 [76]. For SGLT2i, the target population are patients with T2DM and one other disease: HF, ASCVD, or people at risk for development cardiovascular disease. Also, SGLT2i are suggested as anti-hyperglycaemic drug if people have contraindication for Metformin in chronic HF, atherosclerotic cardiovascular disease, or in people at risk for development cardiovascular disease. Perhaps more importantly, in CKD, a NICE update now recommends use of dapagliflozin, in addition to RASi, in patients with T2DM, or patients with a uACR of 22.6 mg/mol or more (irrespective of diabetes) [77]. This is consistent with the UK Kidney Association recommendations, with the exception that there is an effort to simplify the summary of where there is clear evidence for use of an SGLT2i, suggesting any member of the SGLT2i class could be used in CKD. To facilitate implementation, the UK Kidney Association guideline also has a full lay summary and template patient information leaflets [78]. A pragmatic algorithm for using SGLT2i across the range of indications is proposed in Fig. 2.
Figure 2:
Proposed algorithm for selection of SGLT2i based on large clinical trial evidence/indication and threshold of eGFR for initiation based on patient's main complaint (A) and clinical indication (B). Note that current prescribing licences for agents may not apply in all regions. CKD: chronic kidney disease, defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 or urine albumin-creatinine ratio (uACR) >30 mg/g; HF: heart failure; pEF: preserved ejection fraction; rEF: reduced ejection fraction; DM: Type 2 diabetes mellitus: eGFR values expressed in ml/min/1.73 m2, w/: with; wo: without, RCT: randomized controlled trials; KRT: kidney replacement therapy, SGLT2i: sodium-glucose cotransporter-2 inhibitor; ASCVD: atherosclerotic cardiovascular disease.
CONCLUSIONS AND FUTURE GUIDELINES
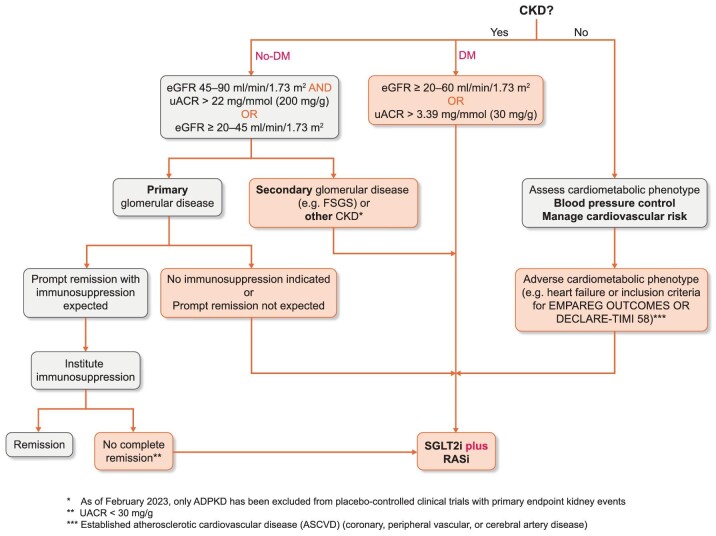
The emergence of SGLT2i represents a major leap forward in the evidence base for cardiorenal protection in CKD. DAPA-CKD has shown the renal benefits of SGLT2i, clearly proven in patients with T2DM with proteinuric CKD, appear to extend to patients with non-diabetic proteinuric CKD and EMPA-KIDNEY trial has provided more evidence on benefits in patients without diabetes, and included one-third of participants with an eGFR <30 ml/min/1.73 m2 and those without albuminuria. Nephrologists are required to assimilate existing evidence and guidelines and apply these to the broad spectrum of patients with CKD, including those with primary glomerular diseases. Recent proposals for integrating SGLT2i into the management of glomerular disease have been proposed [79] (Fig. 3). We propose that this broad pragmatic approach is appropriate, as SGLT2i appears to be safe in CKD, particularly in patients without diabetes and potentially highly effective. Further efforts are required by nephrologists and aligned professional organizations to ensure that these agents with evidence-based benefits for reducing rate of progression of CKD to kidney failure are prescribed to eligible patients to address the global burden of CKD and its cardiovascular complications.
Figure 3:
Broad schematic to support clinical use of SGLT2i in as foundational therapy for CKD considering need for specific treatment in both cardiometabolic and glomerular disease. CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate (eGFR); FSGS: focal segmental glomerulosclerosis; DM: type 2 diabetes mellitus; SGLT2i: sodium-glucose cotransporter-2 inhibitor; uACR: urine albumin-creatinine ratio; ASCVD: atherosclerotic cardiovascular disease; RASi renin-angiotensin system inhibition.
Supplementary Material
ACKNOWLEDGEMENTS
EURECA-m and ERBP are official bodies of the ERA.
Contributor Information
Patrick B Mark, School of Cardiovascular and Metabolic Health, University of Glasgow, Glasgow, UK.
Pantelis Sarafidis, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Robert Ekart, Faculty of Medicine, University of Maribor, Taborska 8, Maribor, Slovenia.
Charles J Ferro, Renal Unit, University Hospitals Birmingham and Institute of Cardiovascular Science, University of Birmingham, Birmingham, UK.
Olga Balafa, Department of Nephrology, University Hospital of Ioannina, Ioannina, Greece.
Beatriz Fernandez-Fernandez, Division of Nephrology and Hypertension, IIS-Fundación Jiménez Díaz-Universidad Autónoma Madrid. Spain, Spain.
William G Herrington, Medical Research Council Population Health Research Unit, Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Patrick Rossignol, Université de Lorraine, INSERM CIC-P 1433, CHRU de Nancy, INSERM U1116, F-CRIN INI-CRCT (Cardiovascular and Renal Clinical Trialists), Nancy, France; Service de Spécialités Médicales et de Néphrologie-Hémodialyse Centre Hospitalier Princesse Grace de Monaco, Monaco, Monaco.
Lucia Del Vecchio, Department of Nephrology and Dialysis, ASST Lecco, Lecco, Italy.
Jose M Valdivielso, Vascular and Renal Translational Research Group and UDETMA, IRBLleida, Lleida, Spain.
Francesca Mallamaci, CNR-IFC, Clinical Epidemiology and Physiopathology of Renal Diseases and Hypertension, Reggio Calabria, Italy.
Alberto Ortiz, Division of Nephrology and Hypertension, IIS-Fundación Jiménez Díaz-Universidad Autónoma Madrid. Spain, Spain.
Ionut Nistor, Faculty of Medicine, University of Medicine and Pharmacy ‘Grigore T. Popa’, Iași, Romania.
Mario Cozzolino, Renal Division, ASST Santi Paolo e Carlo, Department of Health Sciences, University of Milan, Milan, Italy.
FUNDING
A.O. and B.F.F.’s research is supported by FIS/Fondos FEDER (PI18/01366, PI19/00588, PI19/00815, PI21/00251, PI20/00744), ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064, ISCIII-RETIC REDinREN RD016/0009), Sociedad Española de Nefrología, Sociedad Madrileña de Nefrología (SOMANE), FRIAT, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM. Instituto de Salud Carlos III (ISCIII) RICORS programme to RICORS2040 (RD21/0005/0001) funded by European Union—NextGenerationEU, Mecanismo para la Recuperación y la Resiliencia (MRR) and SPACKDc PMP21/00109, FEDER funds.
CONFLICT OF INTEREST STATEMENT
P.B.M. reports lecture fees and travel to meetings support from Vifor, Astrazeneca, Pharmacomsos, Napp, Astellas, lecture fees from Novartis, Astellas, GSK, and grants from Boehringer Ingelheim outside the submitted work. A.O. has received grants from Sanofi and consultancy or speaker fees or travel support from Advicciene, Astellas, Astrazeneca, Amicus, Amgen, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex and Vifor Fresenius Medical Care Renal Pharma and is Director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra Astrazeneca-UAM of chronic kidney disease and electrolytes.
REFERENCES
- 1. Jager KJ, Kovesdy C, Langham R et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019;34:1803–5. 10.1093/ndt/gfz174. [DOI] [PubMed] [Google Scholar]
- 2. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017;12:2032–45. 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saeedi P, Petersohn I, Salpea P et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2019;157:107843. 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4. Brenner BM, Cooper ME, de Zeeuw D et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–9. 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 5. Lewis EJ, Hunsicker LG, Clarke WR et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–60. 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 6. Wright JTJr., Bakris G, Greene T et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421–31. 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 7. Ortiz A, Ferro CJ, Balafa O et al. Mineralocorticoid receptor antagonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. Nephrol Dial Transplant 2023;38:10–25. 10.1093/ndt/gfab167. [DOI] [PubMed] [Google Scholar]
- 8. Sarafidis PA, Stafylas PC, Kanaki AI et al. Effects of renin-angiotensin system blockers on renal outcomes and all-cause mortality in patients with diabetic nephropathy: an updated meta-analysis. Am J Hypertens 2008;21:922–9. 10.1038/ajh.2008.206. [DOI] [PubMed] [Google Scholar]
- 9. Go AS, Chertow GM, Fan D et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 10. Matsushita K, van der Velde M, Astor BC et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wanner C, Amann K, Shoji T. The heart and vascular system in dialysis. Lancet 2016;388:276–84. 10.1016/S0140-6736(16)30508-6. [DOI] [PubMed] [Google Scholar]
- 12. Afkarian M, Sachs MC, Kestenbaum B et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302–8. 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gregg EW, Li Y, Wang J et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med 2014;370:1514–23. 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 14. Wanner C, Inzucchi SE, Lachin JM et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–34. 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 15. Wanner C, Lachin JM, Inzucchi SE et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018;137:119–29. 10.1161/CIRCULATIONAHA.117.028268. [DOI] [PubMed] [Google Scholar]
- 16. Neuen BL, Ohkuma T, Neal B et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS Program. JASN 2019;30:2229–42. 10.1681/ASN.2019010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neal B, Perkovic V, Mahaffey KW et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 18. Perkovic V, Jardine MJ, Neal B et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 19. Staplin N, Roddick AJ, Emberson J et al. Net effects of sodium-glucose co-transporter-2 inhibition in different patient groups: a meta-analysis of large placebo-controlled randomized trials. EClinicalMedicine 2021;41:101163. 10.1016/j.eclinm.2021.101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiviott SD, Raz I, Bonaca MP et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 21. Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 2006;86:901–40. 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 22. Cherney DZ, Perkins BA, Soleymanlou N et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–97. 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 23. Onishi A, Fu Y, Patel R et al. A role for tubular Na(+)/H(+) exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol 2020;319:F712–28. 10.1152/ajprenal.00264.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jensen J, Omar M, Kistorp C et al. Effects of empagliflozin on estimated extracellular volume, estimated plasma volume, and measured glomerular filtration rate in patients with heart failure (Empire HF Renal): a prespecified substudy of a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2021;9:106–16. 10.1016/S2213-8587(20)30382-X. [DOI] [PubMed] [Google Scholar]
- 25. Scheen AJ. Effect of SGLT2 inhibitors on the sympathetic nervous system and blood pressure. Curr Cardiol Rep 2019;21:70. 10.1007/s11886-019-1165-1. [DOI] [PubMed] [Google Scholar]
- 26. O'Neill J, Fasching A, Pihl L et al. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol 2015;309:F227–34. 10.1152/ajprenal.00689.2014. [DOI] [PubMed] [Google Scholar]
- 27. Mazer CD, Hare GMT, Connelly PW et al. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation 2020;141:704–7. 10.1161/CIRCULATIONAHA.119.044235. [DOI] [PubMed] [Google Scholar]
- 28. Chino Y, Samukawa Y, Sakai S et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos 2014;35:391–404. 10.1002/bdd.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cassis P, Locatelli M, Cerullo D et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 2018;3:e98720. 10.1172/jci.insight.98720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Packer M. Mitigation of the adverse consequences of nutrient excess on the kidney: a unified hypothesis to explain the renoprotective effects of sodium-glucose cotransporter 2 inhibitors. Am J Nephrol 2020;51:289–93. 10.1159/000506534. [DOI] [PubMed] [Google Scholar]
- 31. Wu P, Wen W, Li J et al. Systematic review and meta-analysis of randomized controlled trials on the effect of SGLT2 inhibitor on blood leptin and adiponectin level in patients with type 2 diabetes. Horm Metab Res 2019;51:487–94. [DOI] [PubMed] [Google Scholar]
- 32. Umino H, Hasegawa K, Minakuchi H et al. High basolateral glucose increases sodium-glucose cotransporter 2 and reduces sirtuin-1 in renal tubules through glucose transporter-2 detection. Sci Rep 2018;8:6791. 10.1038/s41598-018-25054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inoue MK, Matsunaga Y, Nakatsu Y et al. Possible involvement of normalized Pin1 expression level and AMPK activation in the molecular mechanisms underlying renal protective effects of SGLT2 inhibitors in mice. Diabetol Metab Syndr 2019;11:57. 10.1186/s13098-019-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J, Liu H, Takagi S et al. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight 2020;5:e129034. 10.1172/jci.insight.129034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heerspink HJL, Stefansson BV, Correa-Rotter R et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 36. Wheeler DC, Stefansson BV, Batiushin M et al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant 2020;35:1700–11. 10.1093/ndt/gfaa234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jongs N, Greene T, Chertow GM et al. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021;9:755–66. 10.1016/S2213-8587(21)00243-6. [DOI] [PubMed] [Google Scholar]
- 38. Herrington WG, Preiss D, Haynes R et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018;11:749–61. 10.1093/ckj/sfy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wheeler DC, Toto RD, Stefansson BV et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int 2021;100:215–24. 10.1016/j.kint.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 40. Wheeler DC, Jongs N, Stefansson BV et al. Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: a prespecified analysis of the DAPA-CKD trial. Nephrol Dial Transplant 2022;37:1647–56. 10.1093/ndt/gfab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wong MG, Lv J, Hladunewich MA et al. The therapeutic evaluation of steroids in IgA Nephropathy Global (TESTING) Study: trial design and baseline characteristics. Am J Nephrol 2021;52:827–36. 10.1159/000519812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lv J, Zhang H, Wong MG et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING Randomized Clinical Trial. J Am Med Assoc 2017;318:432–42. 10.1001/jama.2017.9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fellstrom BC, Barratt J, Cook H et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet North Am Ed 2017;389:2117–27. 10.1016/S0140-6736(17)30550-0. [DOI] [PubMed] [Google Scholar]
- 44. Rauen T, Eitner F, Fitzner C et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 2015;373:2225–36. 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 45. Baigent C, Emberson J, Haynes R et al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet North Am Ed 2022;400:1788–801. 10.1016/S0140-6736(22)02074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anders HJ, Peired AJ, Romagnani P. SGLT2 inhibition requires reconsideration of fundamental paradigms in chronic kidney disease, ‘diabetic nephropathy’, IgA nephropathy and podocytopathies with FSGS lesions. Nephrol Dial Transplant 2022;37:1609–15. 10.1093/ndt/gfaa329. [DOI] [PubMed] [Google Scholar]
- 47. Barratt J, Floege J. SGLT-2 inhibition in IgA nephropathy: the new standard of care? Kidney Int 2021;100:24–26. 10.1016/j.kint.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 48. Zinman B, Wanner C, Lachin JM et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 49. McMurray JJV, Solomon SD, Inzucchi SE et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 50. Packer M, Anker SD, Butler J et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 51. Anker SD, Butler J, Filippatos G et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–61. 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 52. Zannad F, Ferreira JP, Pocock SJ et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function. Circulation 2021;143:310–21. 10.1161/CIRCULATIONAHA.120.051685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Solomon SD, McMurray JJV, Claggett B et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089–98. 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 54. Mark PB, Mangion K, Rankin AJ et al. Left ventricular dysfunction with preserved ejection fraction: the most common left ventricular disorder in chronic kidney disease patients. Clin Kidney J 2022;15:2186–99. 10.1093/ckj/sfac146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Neuen BL, Young T, Heerspink HJL et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7:845–54. 10.1016/S2213-8587(19)30256-6. [DOI] [PubMed] [Google Scholar]
- 56. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2022;388:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Group E-KC., EMPA-KIDNEY Collaborative Group. Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant 2022;37:1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Herrington WG, Staplin N, Wanner C et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bakris GL, Agarwal R, Anker SD et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–29. 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 60. Pitt B, Filippatos G, Agarwal R et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021;385:2252–63. 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 61. Agarwal R, Filippatos G, Pitt B et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022;43:474–84. 10.1093/eurheartj/ehab777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rossing P, Anker SD, Filippatos G et al. Finerenone in patients with chronic kidney disease and type 2 diabetes by sodium-glucose cotransporter 2 inhibitor treatment: the FIDELITY Analysis. Diabetes Care 2022;45:2991–8. 10.2337/dc22-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Neuen BL, Oshima M, Agarwal R et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation 2022;145:1460–70. 10.1161/CIRCULATIONAHA.121.057736. [DOI] [PubMed] [Google Scholar]
- 64. Toyama T, Neuen BL, Jun M et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab 2019;21:1237–50. 10.1111/dom.13648. [DOI] [PubMed] [Google Scholar]
- 65. Ferreira JP, Zannad F, Pocock SJ et al. Interplay of mineralocorticoid receptor antagonists and empagliflozin in heart failure: eMPEROR-reduced. J Am Coll Cardiol 2021;77:1397–407. 10.1016/j.jacc.2021.01.044. [DOI] [PubMed] [Google Scholar]
- 66. Shen L, Kristensen SL, Bengtsson O et al. Dapagliflozin in HFrEF patients treated with mineralocorticoid receptor antagonists: an analysis of DAPA-HF. JACC: Heart Failure 2021;9:254–64. 10.1016/j.jchf.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 67. Ferreira JP, Butler J, Zannad F et al. Mineralocorticoid receptor antagonists and empagliflozin in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2022;79:1129–37. 10.1016/j.jacc.2022.01.029. [DOI] [PubMed] [Google Scholar]
- 68. McFarlane P, Cherney D, Gilbert RE et al. Chronic kidney disease in diabetes. Can J Diabetes 2018;42:S201–9. 10.1016/j.jcjd.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 69. Davies MJ, Aroda VR, Collins BS et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022;45:2753–86. 10.2337/dci22-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. ElSayed NA, Aleppo G, Aroda VR et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2023. Diabetes Care 2023;46:S140–57. 10.2337/dc23-S009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cosentino F, Grant PJ, Aboyans V et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J 2019;41:255–323. [DOI] [PubMed] [Google Scholar]
- 72. McDonagh TA, Metra M, Adamo M et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 73. ERBP . Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min). Nephrol Dial Transplant 2015;30:ii1–142. [DOI] [PubMed] [Google Scholar]
- 74. Sarafidis P, Ferro CJ, Morales E et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol Dial Transplant 2019;34:208–30. 10.1093/ndt/gfy407. [DOI] [PubMed] [Google Scholar]
- 75. Rossing P, Caramori ML, Chan JCN et al. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2022;102:S1–S127. 10.1016/j.kint.2022.06.008. [DOI] [PubMed] [Google Scholar]
- 76. Moran GM, Bakhai C, Song SH et al. Type 2 diabetes: summary of updated NICE guidance. BMJ 2022;377:o775. 10.1136/bmj.o775. [DOI] [PubMed] [Google Scholar]
- 77. National Institute for Health and Care Excellence. Dapagliflozin for treating chronic kidney disease 2022.
- 78. UK Kidney Association. 2022; https://ukkidney.org/sites/renal.org/files/UKKA%20guideline_SGLT2i%20in%20adults%20with%20kidney%20disease%20v1%2018.10.21.pdf
- 79. McQuarrie EP, Gillis KA, Mark PB. Seven suggestions for successful SGLT2i use in glomerular disease - a standalone CKD therapy? Curr Opin Nephrol Hypertens 2022;31:272–7. 10.1097/MNH.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 80. Cannon CP, Pratley R, Dagogo-Jack S et al. Cardiovascular outcomes with ertugliflozin in Type 2 diabetes. N Engl J Med 2020;383:1425–35. 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.