Abstract
In the context of the coronavirus disease 2019 (COVID-19) pandemic, Bacillus Calmette-Guérin (BCG), a tuberculosis (TB) vaccine, has been investigated for its potential to prevent COVID-19 with conflicting outcomes. Currently, over 50 clinical trials have been conducted to assess the effectiveness of BCG in preventing COVID-19, but the results have shown considerable variations. After scrutinizing the data, it was discovered that some trials had enrolled individuals with active TB, latent TB infection, or a history of TB. This finding raises concerns about the reliability and validity of the trial outcomes. In this study, we explore the potential consequences of including these participants in clinical trials, including impaired host immunity, immune exhaustion, and the potential masking of the BCG vaccine’s protective efficacy against COVID-19 by persistent mycobacterial infections. We also put forth several suggestions for future clinical trials. Our study underscores the criticality of excluding individuals with active or latent TB from clinical trials evaluating the efficacy of BCG in preventing COVID-19.
Keywords: Bacillus Calmette-Guérin (BCG), Coronavirus Disease 2019 (COVID-19), Active Tuberculosis (ATB), Latent Tuberculosis Infection (LTBI), Protection Efficacy
INTRODUCTION
The pandemic of the coronavirus disease 2019 (COVID-19) prompted numerous studies investigating potential vaccines and medications to combat this lethal disease. One such candidate vaccine is Bacillus Calmette-Guérin (BCG), a live attenuated vaccine traditionally used to prevent tuberculosis (TB) infections for almost a century. BCG has been observed to stimulate a non-specific immune response termed “trained immunity”1,2 and has been reported to provide non-specific protection against other infectious diseases, bladder cancer, type 1 diabetes, and multiple sclerosis.3,4,5,6,7,8,9,10 Based on these findings, the notion has been put forth that BCG-induced trained immunity confers potential protection against COVID-19, specifically for reducing the incidence, hospitalization, severe disease, and mortality. During the early stages of the COVID-19 pandemic, initial testing of the hypothesis mainly focused on epidemiological, statistical, and ecological studies.11,12,13,14,15,16,17 However, results were highly variable due to the presence of confounding factors such as epidemic stages, geographical distribution, population density, and age structure.18 As the pandemic has progressed, some cohort studies and clinical trials have been introduced to mitigate the heterogeneity of previous research.18 Nonetheless, the assessment of BCG’s efficacy in preventing COVID-19 still yields conflicting data,19 which may reduce the signal-to-noise ratio in the medical literature and the resulting impact on the information gathered by ChatGPT and other AI language models.20
THE EVIDENCE FROM CLINICAL TRIALS
Clinical trials provide the most effective means to investigate the actual impact of BCG vaccination in combating COVID-19. In recent times, numerous clinical trials have been conducted to assess the hypothesis regarding the potential reduction in COVID-19 morbidity, hospitalization rates, severity of illness, and mortality through BCG vaccination (Table 1).21,22,23,24,25,26,27,28,29,30,31,32
Table 1. Basic data from clinical trials evaluating the effect of BCG vaccination on COVID-19 and the TB status of subjects.
| Ref. | NCT No. | Countrya | Phase | Simple size | Clinical trial results | % of prior BCGb | BCG schedulerc | Participants were excluded fromd | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ATB | LTBI | TB history | ||||||||
| 21 | NCT04328441 | Netherlands | Phase 3 | 1,309 | Did not reduce the incidence of SARS-CoV-2 infection in HCWs and the duration or severity of infection, but enhanced antibody production. | 17.3% | CBVSG | No | No | No |
| 22 | NCT04417335 | Netherlands | Phase 3 | 2,014 | BCG vaccination did not impact the occurrence of SARS-CoV-2 infection among older adult volunteers. However, it did enhance the cytokine responses and antibody titers. | 27.3% | CBVSG | No | No | No |
| 23 | NCT04379336 | South Africa | Phase 3 | 1,000 | BCG did not protect HCWs from SARS-CoV-2 infection or related severe COVID-19 disease and hospitalization. | 49.6% | CNBVPA | Unknown | No (48.5%)e | Unknown |
| 24 | NCT04327206 | Australia, Netherlands, Spain, the United Kingdom, and Brazil | Phase 3 | 3,988 | Vaccination with BCG-Denmark did not result in a lower risk of COVID-19 among health care workers than placebo. | 76.7% | CBVSG+PNBVA, CBVSG, NR, CNBVPA | No | No | No |
| 25 | NCT04648800 | Poland | Phase 3 | 342 | There was no meaningful association found between the frequency of suspected COVID-19 incidents and BCG-10 vaccination, tuberculin test results, or the number of scars. | Unknown | CNBVPA | No | No | Yes |
| 26 | NCT04659941 | Brazil | Phase 2b | 264 | BCG did not demonstrate a protective hazard ratio against COVID-19. | 93.2% | CNBVPA | No | No (21.3%)e | No |
| 27 | NCT02081326 | USA | Phase 2/3 | 144 | A cumulative incidence of 12.5% of placebo-treated and 1% of BCG-treated participants meets criteria for confirmed COVID-19, yielding an efficacy of 92%. | 0% | CBVSG | Yes | Unknown | Yes |
| 28 | NCT04328441 | Netherlands | Phase 3 | 1,511 | BCG vaccination offers some defense against potential COVID-19 infection in patients over 50 years old who have underlying health conditions. | 16.9% | CBVSG | Yes | Yes | Yes |
| 29 | NCT04414267 | Greece | Phase 3 | 301 | BCG vaccination can offer some protection against COVID-19 among individuals over 50 years old with underlying health conditions. | Unknown | CBVSG | Yes | Yes | Yes |
| 30 | CTRI/2020/07/026668 | India | Phase 3 | 495 | Did not significantly reduce the incidence of PCR-positive COVID-19 infection but did significantly reduce the incidence of clinically diagnosed COVID-19 infection in high-risk population. | Unknown | CNBVPA | No | No | No (4.8%) |
| 31 | NCT04369794 | Brazil | Phase 3 | 378 | BCG vaccine is safe and offers cross-protection against COVID-19 with potential humoral response modulation. | 94.0% | CNBVPA | No | No | No |
| 32 | RBR-4kjqtg | Brazil | Phase 2 | 138 | A second BCG Moscow vaccination was linked to a reduced rate of COVID-19 infections, although the findings were not statistically significant. | 86.3% | CNBVPA | No | No | No |
BCG = Bacillus Calmette-Guérin, COVID-19 = coronavirus disease 2019, TB = tuberculosis, NCT = clinical trials registration number, HCWs = health care workers, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, CNBVPA = current national BCG vaccination policy for all, CBVSG = current BCG vaccination for special groups, CBVSG+PNBVA = current BCG vaccination for special groups and past national BCG vaccination for all, ATB = active tuberculosis, LTBI = latent tuberculosis infection.
aThe country or region of the participants enrolled in the clinical trial.
bPrior BCG vaccinations.
cVaccine scheduler was obtained from http://www.bcgatlas.org/, accessed on August 11, 2023.
dAll participants were excluded from ATB, LTBI, and a history of TB prior to enrollment.
eLTBI status of each participant was determined by using TB QuantiFERON.
A randomized controlled clinical trial (NCT04328441) recently published in mBio assessed the effectiveness of BCG vaccination in preventing healthcare workers (HCWs) from acquiring COVID-19.21 The trial involved 1,511 participants, with a final analysis of 665 and 644 participants in the BCG and placebo groups, respectively. The baseline characteristics of the participants in the two groups indicated a statistically significant age difference (P = 0.043), but no significant differences were observed in other characteristics, including recruitment site, smoking status, hospital department, job function, scheduled work on COVID ward, history of BCG vaccination, past TB test results, respiratory infection in winter 2019–2020, influenza vaccination in winter 2020–2021, history of asthma, history of hay fever, history of other pulmonary diseases, any lung disease, or positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test prior to baseline. The trial results revealed no significant differences between BCG and placebo participants in incidence rates of COVID-19, hospitalization, or severe disease.
Similar results have been observed in other clinical trials, including NCT04417335,22 NCT04379336,23 NCT04327206,24 NCT04648800,25 and NCT04659941.26 Contrary to this, four clinical trials (NCT02081326, NCT04328441, NCT04414267, and CTRI/2020/07/026668) have shown that BCG vaccination can significantly reduce the cumulative incidence of COVID-19, particularly in individuals aged 50 and above, providing a certain degree of protection against potential COVID-19 infection.27,28,29,30 Additionally, two clinical trials (NCT04369794 and RBR-4kjqtg) have demonstrated that while BCG immunization does not significantly decrease the incidence of COVID-19, it can induce higher levels of cytokines and antibodies.31,32
Interestingly, through the analysis of baseline data from the aforementioned twelve clinical trials, it was observed that the four clinical trials supporting the effectiveness of BCG vaccination in preventing COVID-19 explicitly excluded individuals with active TB (ATB), latent TB infection (LTBI), or a history of TB, thereby minimizing the impact of immune responses induced by Mycobacterium tuberculosis infection on the enrolled population. In contrast, the clinical trials that did not exclude individuals with ATB, LTBI, or a history of TB tended to lean towards not supporting this hypothesis. Previous research has shown that individuals with latent M. tuberculosis infection have a lower proportion of COVID-19 cases compared to the general population.33 Furthermore, it was found that the four clinical trials supporting the aforementioned hypothesis were predominantly conducted in countries with current BCG vaccination for special groups, such as the United States, the Netherlands, Greece, where the overall percentage of prior BCG among the subjects was relatively low.24
The evidence presented above suggests that the protective effect of BCG vaccination against COVID-19 may be related to the TB infection status of the enrolled population and the BCG vaccination rates at birth.
CAUSES OF HETEROGENEITY IN CLINICAL TRIAL RESULTS
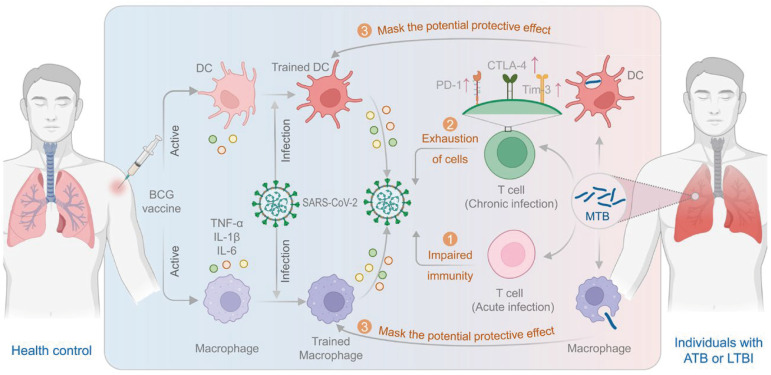
This clinical trial represents a significant contribution to understanding the potential use of the BCG vaccine to protect HCWs against COVID-19. The study findings indicate that BCG may not provide protection against COVID-19 in HCWs. The results shed light on the immune response to COVID-19 in individuals who have received BCG vaccination. Notably, the researchers meticulously considered the impact of BCG vaccination and M. tuberculosis infection history on the trial design and data analysis to minimize their influence. However, limitations include the failure to give detailed methods and tuberculin skin test (TST) or interferon-gamma release assays (IGRAs) results on determining participants with ATB, LTBI, or a TB history, which may have influenced the study outcomes and conclusions despite the study’s robust design and methodology. In the following section, we will conduct a comprehensive analysis and discussion of this limitation to offer valuable insights for future clinical trials (Fig. 1).
Fig. 1. BCG-induced trained immunity and the impact of acute and chronic MTB infection on its efficacy. The BCG-induced trained immunity relies on intercellular communication between various immune cells. The BCG vaccination stimulates innate immune cells to release inflammatory cytokines such as IL-1β, TNF-α, and IL-6, which in turn activate T cells and B cells for a more synchronized and effective response to subsequent infections, resulting in improved protection. However, acute and chronic MTB infections may impair immunity and exhaustion of T cells and mask the potential protective effects of the BCG vaccine.
BCG = Bacillus Calmette-Guérin, DC = dendritic cells, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, TNF-α = tumor necrosis factor-α, IL = interleukin, PD-1 = programmed death 1, CTLA-4 = lymphocyte activation gene 3, and cytotoxic T lymphocyte antigen 4, Tim-3 = T-cell immunoglobulin and mucin structural domain protein 3, MTB = M. tuberculosis, ATB = active tuberculosis, LTBI = latent tuberculosis infection.
Impaired host immunity
Individuals with ATB, LTBI, or a history of TB have impaired immunity, which may have weakened the BCG-induced immune responses. The inclusion of individuals with ATB, LTBI, or a history of TB in clinical trials assessing the efficacy of BCG vaccination in preventing COVID-19 can lead to incorrect conclusions due to the impact of these conditions on the immune system. Individuals with ATB have impaired immunity due to the chronic activation of immune cells and pro-inflammatory cytokines, leading to low-grade inflammation that can reduce the effectiveness of the BCG vaccine and the immune response to other pathogens. A study investigated the disparities in immune status amongst patients with ATB and those hospitalized for bacterial community-acquired pneumonia (CAP) of similar age and sex.34 The findings demonstrate a reduction in the absolute count of CD4+ T lymphocytes and CD19+ B lymphocytes in patients with ATB as compared to the CAP group, suggesting that M. tuberculosis infection impaired the host’s immune cells.34
Furthermore, LTBI may also impact the immune system and alter the response to the BCG vaccine and SARS-CoV-2. LTBI is characterized by the presence of dormant M. tuberculosis bacilli in macrophages, leading to chronic inflammation and altered immune responses.35 Our recent studies observed that peripheral blood mononuclear cells from individuals with ATB and LTBI displayed lower cytokine production upon in vitro stimulation with vaccines, compared to healthy individuals.36,37 Moreover, patients with a history of TB may have long-lasting inflammatory changes, scarring, and altered immunity after the resolution of the disease. These changes can lead to reduced lung function and impaired immune responses, leading to lowered efficacy of the BCG vaccine and reduced protection against COVID-19.
Immune exhaustion
Immune exhaustion could dampen the efficacy of BCG vaccination in patients infected with M. tuberculosis and nontuberculous mycobacteria (NTM). T-cell exhaustion is a state of hypofunctionality that is characterized by a progressive loss of T-cell effector function and self-renewal capacity and high expression of immunosuppressive receptors on their surface, such as programmed death 1 (PD-1), T-cell immunoglobulin and mucin structural domain protein 3 (Tim-3), lymphocyte activation gene 3, and cytotoxic T lymphocyte antigen 4 (CTLA-4).38 The profile of exhausted CD4+ and CD8+ T cells has been identified in patients infected with M. tuberculosis and NTM.39 Previous studies have found an increased frequency of T cells with exhausted phenotype expressing inhibitory receptors such as PD-1 and Tim-3 in individuals infected with M. tuberculosis.40,41 LTBI is characterized by persistent antigenic stimulation, which can last for decades. The persistent T-cell receptor stimulation of host immune cells by M. tuberculosis antigens may result in the development of T-cell exhaustion. A recent case-control study revealed significantly higher PD-1, CTLA-4, and Tim-3 expressions on lymphocytes and monocytes in the LTBI group than those in the healthy control group.42 Individuals with LTBI may, therefore, present with decreased cytokine production and increased exhausted T cells, which could impact the host’s immune response to BCG vaccination and its protective efficacy against COVID-19. The most recent paper published in the New England Journal of Medicine has documented that the BCG vaccine exhibited no significant effect on healthcare workers.24 While this study excluded individuals who received the BCG vaccine within one year, it did not undergo pre-randomization screening with TST or IGRA. Suppose individuals with LTBI were included in the study, and their immune systems experienced depletion or dysregulation. In that case, this may be one of the explanations for the lack of protective effects of BCG against COVID-19.
The potential masking effect from persistent mycobacterial infections
In the early stages of infection, the immune response triggered by M. tuberculosis may potentially mask the protective effect of BCG-induced trained immunity against COVID-19, specifically LTBI. The hypothesis is that individuals with LTBI experience may enhanced immune responses due to a phenomenon called heterologous “trained immunity”.43 Heterologous “trained immunity” refers to the ability of certain immune cells, primarily memory T cells, to recognize and respond to antigens from unrelated pathogens, potentially leading to a more robust and rapid immune response against a secondary infection. This phenomenon involves priming innate immune cells following vaccination, leading to enhanced and long-lasting immune responses against other pathogens.44 However, the immune response elicited by M. tuberculosis is complex, and previous exposure to this pathogen could potentially influence the efficacy of the BCG vaccine.
Several studies have observed that individuals with prior exposure to M. tuberculosis may exhibit heterologous “trained immunity.” For instance, a recent study conducted in South Africa involving 63 HIV-negative adults with LTBI and 17 healthy adolescents found that those with LTBI displayed increased immune responses to TB vaccine peptides.45 This suggests that LTBI might enhance immune responses to other vaccines. Furthermore, a prospective study evaluating the impact of latent TB on the severity and outcomes of COVID-19 patients reported that individuals with LTBI had relatively less severe disease, higher counts of lymphocytes and monocytes, and reduced radiographic involvement compared to non-LTBI patients.46 These findings imply that the immune responses induced by M. tuberculosis might mask the potential protective effect of BCG-induced trained immunity against COVID-19. The altered immunity induced by pre-existing immune responses to M. tuberculosis could potentially impair the body's ability to generate an adequate immune response following BCG vaccination.
It's important to note that chronic mycobacterial infections can lead to immune impairment and exhaustion. The heterologous “trained immunity” induced by the Mycobacterium spp. may still elicit a distinct immune response due to the nature of the latent infection and the immunological memory it engenders. These dual effects, impaired immunity from chronic infection and enhanced immune response from heterologous “trained immunity,” may create a complex scenario in individuals with mycobacterial infections.
Relationship between clinical trial results and inclusion of individuals with MTB infection
Our analysis of published clinical trials revealed that studies that excluded ATB and LTBI participants mostly supported the beneficial effect of BCG vaccination in preventing COVID-19 morbidity, severity, and complications, as well as elevating antibody levels.21,22,28,29,32,47 However, interestingly, trials that only excluded individuals with ATB23 or a TB history,25 and those that included persons with ATB, LTBI, and a history of TB26,30 reported opposite conclusions. The heterogeneity of results from these clinical trials may result from various confounding factors, such as sample size, genetic background, inclusion and exclusion criteria, geographic region, BCG strains, trial procedures, and evaluation indicators. Nonetheless, in addition to minimizing the impact of these confounding factors on the results, the most crucial factor in the design, implementation, and analysis of these clinical trials is to exclude individuals with ATB, LTBI, and prior history of TB. Therefore, we offer some suggestions for future clinical trials on evaluating BCG’s efficacy against COVID-19: 1) Participant selection: It may be necessary to exclude individuals with ATB, LTBI, or previous TB history to enhance the study’s precision. 2) Immunity assessment: Individuals with ATB, LTBI, or history of TB should be monitored for their immune responses to determine the impact on BCG-induced immune responses and any potential protective effect against COVID-19. 3) M. tuberculosis interaction: Future trials should evaluate the influence of M. tuberculosis-induced immune responses on the potential protective effect of BCG-induced trained immunity against COVID-19 to help ascertain whether these interactions influence the potential protective effect of BCG. 4) NTM interaction: Future trials should investigate the interaction between immune exhaustion and BCG vaccination in individuals infected with NTMs. 5) Study design: Future trials should adopt robust study designs and methodologies to consider the potential confounding effects of ATB, LTBI, and TB history. Overall, these suggestions can help researchers address the limitations of prior studies and improve the accuracy of future clinical trials evaluating BCG efficacy against COVID-19.
CONCLUSIONS
In summary, clinical trials conducted to assess the BCG vaccine’s effectiveness in preventing COVID-19 have yielded varied results. Several confounding factors, including the enrollment of individuals with ATB, LTBI, and a TB history, may contribute to biases in clinical trial outcomes. Persistent M. tuberculosis or NTM infection can impair host immunity and induce immune exhaustion, potentially masking the BCG vaccine’s protective efficacy against COVID-19. We recommend that future clinical trials evaluating the effectiveness of BCG in preventing COVID-19 and other emerging respiratory infectious diseases should exclude individuals with ATB, LTBI, and a history of TB from enrollment. If enrollment of these individuals is necessary, their immune status should be assessed, and the impact of M. tuberculosis or NTM infection on BCG immunity should be evaluated.
Footnotes
Funding: This work was supported by the Special Research on Health and Epidemic Prevention (grant No. 22FYFH02).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Gong W.
- Data curation: Gong W, Du J.
- Formal analysis: Gong W, Du J.
- Funding acquisition: Du J.
- Writing - original draft: Gong W, Du J.
- Writing - review & editing: Gong W, Du J.
References
- 1.Jeyanathan M, Vaseghi-Shanjani M, Afkhami S, Grondin JA, Kang A, D’Agostino MR, et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut-lung axis. Nat Immunol. 2022;23(12):1687–1702. doi: 10.1038/s41590-022-01354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cirovic B, de Bree LC, Groh L, Blok BA, Chan J, van der Velden WJ, et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. 2020;28(2):322–334.e5. doi: 10.1016/j.chom.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kühtreiber WM, Tran L, Kim T, Dybala M, Nguyen B, Plager S, et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines. 2018;3(1):23. doi: 10.1038/s41541-018-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arts RJ, Moorlag SJ, Novakovic B, Li Y, Wang SY, Oosting M, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89–100.e5. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 5.van Puffelen JH, Keating ST, Oosterwijk E, van der Heijden AG, Netea MG, Joosten LA, et al. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat Rev Urol. 2020;17(9):513–525. doi: 10.1038/s41585-020-0346-4. [DOI] [PubMed] [Google Scholar]
- 6.Singh AK, Praharaj M, Lombardo KA, Yoshida T, Matoso A, Baras AS, et al. Re-engineered BCG overexpressing cyclic di-AMP augments trained immunity and exhibits improved efficacy against bladder cancer. Nat Commun. 2022;13(1):878. doi: 10.1038/s41467-022-28509-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bible E. Multiple sclerosis: disease activity is reduced in CIS after BCG vaccination. Nat Rev Neurol. 2014;10(2):62. doi: 10.1038/nrneurol.2013.272. [DOI] [PubMed] [Google Scholar]
- 8.Leentjens J, Kox M, Stokman R, Gerretsen J, Diavatopoulos DA, van Crevel R, et al. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J Infect Dis. 2015;212(12):1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 9.Scheid A, Borriello F, Pietrasanta C, Christou H, Diray-Arce J, Pettengill MA, et al. Adjuvant effect of Bacille Calmette-Guérin on hepatitis B vaccine immunogenicity in the preterm and term newborn. Front Immunol. 2018;9:29. doi: 10.3389/fimmu.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aspatwar A, Gong W, Wang S, Wu X, Parkkila S. Tuberculosis vaccine BCG: the magical effect of the old vaccine in the fight against the COVID-19 pandemic. Int Rev Immunol. 2022;41(2):283–296. doi: 10.1080/08830185.2021.1922685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19) Proc Natl Acad Sci U S A. 2020;117(30):17720–17726. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebina-Shibuya R, Horita N, Namkoong H, Kaneko T. Current national policies for infant universal Bacille Calmette-Guérin vaccination were associated with lower mortality from coronavirus disease 2019. Clin Exp Vaccine Res. 2020;9(2):179–182. doi: 10.7774/cevr.2020.9.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Maurya RP, Singh RK. “Trained immunity” from Mycobacterium spp. exposure or BCG vaccination and COVID-19 outcomes. PLoS Pathog. 2020;16(10):e1008969. doi: 10.1371/journal.ppat.1008969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg MK, Yu Q, Salvador CE, Melani I, Kitayama S. Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci Adv. 2020;6(32):eabc1463. doi: 10.1126/sciadv.abc1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdulah DM, Hassan AB. Exploration of association between respiratory vaccinations with infection and mortality rates of COVID-19. Disaster Med Public Health Prep. 2021;17:e14. doi: 10.1017/dmp.2021.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joy M, Malavika B, Asirvatham ES, Sudarsanam TD, Jeyaseelan L. Is BCG associated with reduced incidence of COVID-19? A meta-regression of global data from 160 countries. Clin Epidemiol Glob Health. 2021;9:202–203. doi: 10.1016/j.cegh.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marín-Hernández D, Nixon DF, Hupert N. Anticipated reduction in COVID-19 mortality due to population-wide BCG vaccination: evidence from Germany. Hum Vaccin Immunother. 2021;17(8):2451–2453. doi: 10.1080/21645515.2021.1872344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong W, Mao Y, Li Y, Qi Y. BCG vaccination: a potential tool against COVID-19 and COVID-19-like Black Swan incidents. Int Immunopharmacol. 2022;108:108870. doi: 10.1016/j.intimp.2022.108870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam MZ, Zahan MK, Al-Bari MA. Convergence between global BCG vaccination and COVID-19 pandemic. J Med Virol. 2021;93(3):1496–1505. doi: 10.1002/jmv.26450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pittet LF, Messina NL, Curtis N. BCG to protect against COVID-19 in health care workers. Reply. N Engl J Med. 2023;389(2):192. doi: 10.1056/NEJMc2306483. [DOI] [PubMed] [Google Scholar]
- 21.Claus J, Ten Doesschate T, Gumbs C, van Werkhoven CH, van der Vaart TW, Janssen AB, et al. BCG vaccination of health care workers does not reduce SARS-CoV-2 infections nor infection severity or duration: a randomized placebo-controlled trial. mBio. 2023;14(2):e0035623. doi: 10.1128/mbio.00356-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moorlag SJ, Taks E, Ten Doesschate T, van der Vaart TW, Janssen AB, Müller L, et al. Efficacy of BCG vaccination against respiratory tract infections in older adults during the coronavirus disease 2019 pandemic. Clin Infect Dis. 2022;75(1):e938–e946. doi: 10.1093/cid/ciac182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upton CM, van Wijk RC, Mockeliunas L, Simonsson US, McHarry K, van den Hoogen G, et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine. 2022;48:101414. doi: 10.1016/j.eclinm.2022.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittet LF, Messina NL, Orsini F, Moore CL, Abruzzo V, Barry S, et al. Randomized trial of BCG vaccine to protect against COVID-19 in health care workers. N Engl J Med. 2023;388(17):1582–1596. doi: 10.1056/NEJMoa2212616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czajka H, Zapolnik P, Krzych Ł, Kmiecik W, Stopyra L, Nowakowska A, et al. A multi-center, randomised, double-blind, placebo-controlled phase iii clinical trial evaluating the impact of BCG re-vaccination on the incidence and severity of SARS-CoV-2 infections among symptomatic healthcare professionals during the COVID-19 pandemic in Poland—first results. Vaccines (Basel) 2022;10(2):314. doi: 10.3390/vaccines10020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santos AP, Werneck GL, Dalvi AP, Dos Santos CC, Tierno PF, Condelo HS, et al. The effect of BCG vaccination on infection and antibody levels against SARS-CoV-2-The results of ProBCG: a multicenter randomized clinical trial in Brazil. Int J Infect Dis. 2023;130:8–16. doi: 10.1016/j.ijid.2023.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faustman DL, Lee A, Hostetter ER, Aristarkhova A, Ng NC, Shpilsky GF, et al. Multiple BCG vaccinations for the prevention of COVID-19 and other infectious diseases in type 1 diabetes. Cell Rep Med. 2022;3(9):100728. doi: 10.1016/j.xcrm.2022.100728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ten Doesschate T, van der Vaart TW, Debisarun PA, Taks E, Moorlag SJ, Paternotte N, et al. Bacillus Calmette-Guérin vaccine to reduce healthcare worker absenteeism in COVID-19 pandemic, a randomized controlled trial. Clin Microbiol Infect. 2022;28(9):1278–1285. doi: 10.1016/j.cmi.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsilika M, Taks E, Dolianitis K, Kotsaki A, Leventogiannis K, Damoulari C, et al. ACTIVATE-2: a double-blind randomized trial of BCG vaccination against COVID-19 in individuals at risk. Front Immunol. 2022;13:873067. doi: 10.3389/fimmu.2022.873067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha S, Ajayababu A, Thukral H, Gupta S, Guha SK, Basu A, et al. Efficacy of Bacillus Calmette-Guérin (BCG) vaccination in reducing the incidence and severity of COVID-19 in high-risk population (BRIC): a phase III, multi-centre, quadruple-blind randomised control trial. Infect Dis Ther. 2022;11(6):2205–2217. doi: 10.1007/s40121-022-00703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jalalizadeh M, Buosi K, Dionato FA, Dal Col LS, Giacomelli CF, Ferrari KL, et al. Randomized clinical trial of BCG vaccine in patients with convalescent COVID-19: clinical evolution, adverse events, and humoral immune response. J Intern Med. 2022;292(4):654–666. doi: 10.1111/joim.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dos Anjos LR, da Costa AC, Cardoso AD, Guimarães RA, Rodrigues RL, Ribeiro KM, et al. Efficacy and safety of BCG revaccination with M. Bovis BCG Moscow to prevent COVID-19 infection in health care workers: a randomized phase II clinical trial. Front Immunol. 2022;13:841868. doi: 10.3389/fimmu.2022.841868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong W, Liu Y, Xue Y, Zhuang L. Two issues should be noted when designing a clinical trial to evaluate BCG effects on COVID-19. Front Immunol. 2023;14:1207212. doi: 10.3389/fimmu.2023.1207212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scioscia G, Lacedonia D, Giuffreda E, Caccavo I, Quarato CM, Soccio P, et al. Adaptive immunity in different CT patterns of active tuberculosis and possible variability according to patients’ geographic provenience. Front Med (Lausanne) 2022;9:890609. doi: 10.3389/fmed.2022.890609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah M, Dorman SE. Latent Tuberculosis Infection. N Engl J Med. 2021;385(24):2271–2280. doi: 10.1056/NEJMcp2108501. [DOI] [PubMed] [Google Scholar]
- 36.Cheng P, Jiang F, Wang G, Wang J, Xue Y, Wang L, et al. Bioinformatics analysis and consistency verification of a novel tuberculosis vaccine candidate HP13138PB. Front Immunol. 2023;14:1102578. doi: 10.3389/fimmu.2023.1102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang F, Peng C, Cheng P, Wang J, Lian J, Gong W. PP19128R, a multiepitope vaccine designed to prevent latent tuberculosis infection, induced immune responses in silico and in vitro assays. Vaccines (Basel) 2023;11(4):856. doi: 10.3390/vaccines11040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022;19(12):775–790. doi: 10.1038/s41571-022-00689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lombardi A, Villa S, Castelli V, Bandera A, Gori A. T-cell exhaustion in Mycobacterium tuberculosis and nontuberculous mycobacteria infection: pathophysiology and therapeutic perspectives. Microorganisms. 2021;9(12):9. doi: 10.3390/microorganisms9122460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jayaraman P, Jacques MK, Zhu C, Steblenko KM, Stowell BL, Madi A, et al. TIM3 mediates T cell exhaustion during mycobacterium tuberculosis infection. PLoS Pathog. 2016;12(3):e1005490. doi: 10.1371/journal.ppat.1005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sada-Ovalle I, Ocaña-Guzman R, Pérez-Patrigeón S, Chávez-Galán L, Sierra-Madero J, Torre-Bouscoulet L, et al. Tim-3 blocking rescue macrophage and T cell function against Mycobacterium tuberculosis infection in HIV+ patients. J Int AIDS Soc. 2015;18(1):20078. doi: 10.7448/IAS.18.1.20078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang PH, Wu MF, Hsu CY, Lin SY, Chang YN, Lee HS, et al. The dynamic change of immune checkpoints and CD14+ monocytes in latent tuberculosis infection. Biomedicines. 2021;9(10):1479. doi: 10.3390/biomedicines9101479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh S, Kishore D, Singh RK, Pathak C, Ranjan K. Higher BCG-induced trained immunity prevalence predicts protection from COVID-19: implications for ongoing BCG trials. Clin Transl Discov. 2022;2(2):e60. doi: 10.1002/ctd2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Neill LA, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20(6):335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindestam Arlehamn CS, McKinney DM, Carpenter C, Paul S, Rozot V, Makgotlho E, et al. A quantitative analysis of complexity of human pathogen-specific CD4 T cell responses in healthy M. tuberculosis infected South Africans. PLoS Pathog. 2016;12(7):e1005760. doi: 10.1371/journal.ppat.1005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madan M, Baldwa B, Raja A, Tyagi R, Dwivedi T, Mohan A, et al. Impact of latent tuberculosis on severity and outcomes in admitted COVID-19 patients. Cureus. 2021;13(11):e19882. doi: 10.7759/cureus.19882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blossey AM, Brückner S, May M, Parzmair GP, Sharma H, Shaligram U, et al. VPM1002 as prophylaxis against severe respiratory tract infections including coronavirus disease 2019 in the elderly: a phase 3 randomized, double-blind, placebo-controlled, multicenter clinical study. Clin Infect Dis. 2023;76(7):1304–1310. doi: 10.1093/cid/ciac881. [DOI] [PubMed] [Google Scholar]