Abstract
Herpes simplex virus type 1 (HSV-1) protein ICP27 interacts with the cellular export adaptor protein Aly/REF, which is part of the exon junction complex implicated in cellular mRNA export. We previously reported that Aly/REF was no longer associated with splicing factor SC35 sites during infection but instead colocalized with ICP27 in distinct structures. Here we show that these structures colocalize with ICP4 and are sites of HSV-1 transcription. ICP27 mutants with lesions in the region required for the interaction with Aly/REF failed to recruit Aly/REF to viral transcription sites; however, ICP27 export to the cytoplasm was unimpaired, indicating that the interaction of ICP27 with Aly/REF is not required for ICP27 shuttling. ICP27 has also been shown to interact with the cellular mRNA export receptor TAP/NXF1. We report that ICP27 interacts directly with TAP/NXF1 and does not require Aly/REF to bridge the interaction. The C terminus of ICP27 is required; however, the N-terminal leucine-rich region also contributes to the interaction of ICP27 with TAP/NXF1. In contrast to the results found for Aly/REF, mutants that failed to interact with TAP/NXF1 were not exported to the cytoplasm, and TAP/NXF1 was not recruited to sites of HSV-1 transcription. Therefore, the interaction of ICP27 with TAP/NXF1 occurs after ICP27 leaves viral transcription sites. We conclude that ICP27 and the viral RNAs to which it binds are exported via the TAP/NXF1 export receptor.
The herpes simplex virus type 1 (HSV-1) immediate-early protein ICP27 is essential for viral replication (56). ICP27 functions principally at the posttranscriptional level, affecting RNA processing and export (37, 58, 61). Early in infection, ICP27 associates with spliceosomal proteins (45, 59, 60) and mediates an inhibition of host cell splicing (3, 19, 34, 62). This process contributes to the shutoff of host protein synthesis because cellular pre-mRNAs are incompletely spliced and thus are retained in the nucleus in stalled spliceosomal complexes. ICP27 inhibits host cell splicing by recruiting a primarily cytoplasmic kinase, termed SR protein kinase 1, to the nucleus, where its interaction with ICP27 alters its ability to phosphorylate essential splicing factors, termed SR proteins (62). This process results in stalled splicing complex formation (3, 34, 62). In metazoans, the nuclear export of mRNAs has been linked to pre-mRNA splicing (36, 47, 55). The basis of this connection was revealed by the discovery of a protein complex that is deposited on pre-mRNAs undergoing splicing at a specific position upstream of exon junctions (30-32, 49). This exon junction complex (EJC) consists of at least six proteins, which have been shown to function in splicing, RNA export, cytoplasmic localization, mRNA surveillance, and translational efficiency (14, 28, 30, 73).
ICP27 interacts with spliceosomal components (3, 62), including the protein Aly/REF, which is part of the EJC (4, 29). Aly/REF has been shown to have a role in mRNA export because it remains bound to the spliced mRNA (49, 77). Antibodies to Aly/REF that block its interaction with RNA reduced mRNA export in Xenopus oocyte microinjection assays (54), and excess Aly/REF increased the rate and efficiency of mRNA export in vivo (54, 77). Aly/REF interacts directly with TAP/NXF1 (71), the nuclear export receptor for mRNAs in metazoans (2, 10, 25-27, 72) and the homologue of Mex67p, the mRNA export receptor in yeasts (22, 63, 70). ICP27 was found to colocalize with Aly/REF in HSV-1-infected cells (4); in addition, Aly/REF was redistributed from spliceosomal sites to structures that resemble HSV-1 replication compartments (4), where viral transcription and DNA replication occur (7, 35).
Here we show that these structures to which Aly/REF was redistributed colocalized with ICP4 and thus are sites of HSV-1 transcription. Further, ICP27 mutants that are unable to interact with Aly/REF were unable to recruit Aly/REF to centers of ICP4 staining; instead, Aly/REF remained associated with splicing factor SC35. However, a failure to interact with Aly/REF did not impair the export of ICP27 to the cytoplasm at late times after infection. Further, although it has been suggested that efficient shuttling of ICP27 requires RNA binding (67, 68), an ICP27 mutant that lacks the essential RGG box RNA binding domain and thus cannot bind RNA (40, 58) was efficiently exported to the cytoplasm, whereas an ICP27 mutant that has a mutation in a predicted KH domain and that is able to bind RNA was largely retained in the nucleus.
To further explore the export requirements for ICP27, we investigated its interaction with TAP/NXF1, the cellular mRNA export receptor. ICP27 was shown to interact with TAP/NXF1 both in vitro and in infected cells (4, 29); however, it was not shown whether ICP27 interacted directly with TAP/NXF1 or whether the interaction required Aly/REF as a bridging protein. Here we show that ICP27 interacts directly with TAP/NXF1 in vitro and also that an interaction with Aly/REF is not required for ICP27 to interact with TAP/NXF1 in vivo. The C terminus of ICP27 is required for the interaction, but the N-terminal leucine-rich region is also necessary for efficient binding to TAP/NXF1. Further, ICP27 mutants with C-terminal and N-terminal mutations were defective in export. Interestingly, although TAP/NXF1 was seen to colocalize with ICP27, there was no redistribution of TAP/NXF1 to compartments containing ICP4, as was seen with Aly/REF. Therefore, the association of ICP27 with TAP/NXF1 occurs after ICP27 has left sites of viral transcription. These data support the conclusion that ICP27 mediates viral RNA export through the TAP/NXF1 pathway.
MATERIALS AND METHODS
Cells, viruses, and recombinant plasmids.
Rabbit skin fibroblasts (RSFs) and HeLa cells were grown in minimal essential medium containing 10% fetal calf serum. HSV-1 wild-type strain KOS, ICP27 null mutant 27-LacZ, and temperature-sensitive mutant tsLG4 were previously described (64). ICP27 viral mutants dLeu, d1-2, d3-4, d4-5, d5-6, m15, m16, and n406R were generously provided by Steve Rice (33, 50, 52). ICP27 mutants were propagated on 2-2 cells as described previously (64). ICP27 mutant plasmids pCMV-ΔLRR, pCMV-D2ΔS5, pCMV-S5, pCMV-R1, pCMV-H17, and pCMV-ΔC were described previously (4, 21, 75). Plasmid pEGFP-Aly/REF was constructed by ligating Aly/REF cDNA (4) in frame into pEGFP-C3 (Clontech). pEGFP-TAP was kindly provided by E. Izaurralde (1). Full-length TAP cDNA was synthesized by PCR and cloned into pCS2-Flag to create pCS2-Flag-TAP (4) or into pGEX to create pGEX-GST-TAP. TAP truncations were created by cutting at appropriate restriction enzyme sites or by PCR amplification of partial TAP cDNAs. All constructs were verified by sequencing.
Immunofluorescence microscopy.
Cells were grown on coverslips, transfected, and infected as described in the figure legends. At various times, cells were fixed in 3.7% formaldehyde, and immunofluorescence staining was performed with anti-ICP27 monoclonal antibodies H1119 and H1113 (Goodwin Institute) or anti-ICP4 antibody H1114 (Goodwin Institute). Enhanced green fluorescent protein (EGFP)-tagged proteins EGFP-Aly/REF and EGFP-TAP were visualized directly by fluorescence microscopy. Cells were viewed by fluorescence microscopy at a magnification of ×100 with a Zeiss Axiovert S100 microscope. Images were pseudocolored and merged by using Adobe Photoshop.
UV irradiation, RNA-protein complex purification, and RNase protection assays.
RSFs were infected with HSV-1 KOS, tsLG4, and d4-5 at a multiplicity of infection of 10 at 39.5°C. At 6 h after infection, infected cell monolayers were UV irradiated as described previously (57, 58). Nuclear extracts were prepared, and ICP27-bound RNA and unbound RNA fractions were isolated as described previously (58). RNase protection assays and antisense RNA probes for ICP27, glycoprotein B (gB), and gD were described previously (58). For analysis of ICP27-RNA complexes by Western blot analysis, ICP27 and ICP27-RNA complexes were isolated by immunoprecipitation with anti-ICP27 antibody H1119. Protein-RNA complexes were fractionated directly on sodium dodecyl sulfate (SDS)-10% polyacrylamide gels without prior treatment with RNase. Blots were probed with anti-ICP27 antibody and analyzed by enhanced chemiluminescence as described previously (4).
Transfection, infection, and immunoprecipitation procedures.
Cells were transfected with pFlag-TAP in Lipofectamine (Invitrogen) and were infected 24 h later with HSV-1 KOS or with the viral mutants at a multiplicity of infection of 10. Infections with tsLG4 were performed at 39.5°C. Nuclear extracts were prepared as described previously (4, 58) with extraction buffer containing 400 mM NaCl (62). Immunoprecipitations were performed with anti-ICP27 monoclonal antibodies H1119 and H1113 as described previously (4, 62). Proteins were fractionated on SDS-polyacrylamide gels and transferred to nitrocellulose. Blots were probed with anti-ICP27 antibody or anti-Flag antibody and analyzed by enhanced chemiluminescence as described previously (4).
In vitro nuclear export assays.
Cells were infected with KOS or with the ICP27 viral mutants. Nuclear export assays were carried out at various times after infection. Cytoplasmic membranes were permeabilized with digitonin and then extensively washed with phosphate-buffered saline. Export was initiated by the addition of 50% rabbit reticulocyte lysate and an ATP regeneration system as described previously (4). Assays were stopped at various times by the addition of cold transport buffer (4), and exported proteins were washed away by repeated ice-cold washes. Proteins that were retained in the nucleus were harvested by direct lysis in 2× SDS-polyacrylamide gel electrophoresis loading buffer (59). Western blot analysis was performed as described previously (4). The antibodies that were used included anti-ICP27 antibodies H1119 and H1113 and anti-TAP/NXF1 (BD Biosciences), anti-CRM1 (BD Biosciences), and anti-Smad2/3 (BD Biosciences) antibodies. Anti-Aly/REF (28) and anti-hnRNP A1 (46) antibodies were kindly provided by Gideon Dreyfuss. In all experiments, three independent export assays were performed at each time point. The relative amount of ICP27 or cellular protein that was present in the nucleus was calculated by scanning X-ray films with Personal FX (Bio-Rad) for quantification with Quantity One (Bio-Rad).
In vitro binding assays.
Glutathione S-transferase (GST) binding assays were performed as described previously (62) in the presence of 2.5 U of RNase A and 200 U of RNase T1 (Ambion).
RESULTS
Aly/REF colocalizes with ICP27 and is relocalized to centers of ICP4 staining during HSV-1 infection.
The cellular mRNA export adaptor protein Aly/REF, which is part of the EJC, colocalizes with essential splicing factor SC35 in uninfected cells (4, 77) (Fig. 1a to c). In HSV-1-infected cells, Aly/REF was seen to colocalize with ICP27 early in infection (4) (Fig. 1g to i), and as infection progressed, Aly/REF was relocalized into structures that were distinct from SC35 speckles (Fig. 1d to f). These structures resembled HSV-1 replication compartments, where viral transcription and DNA replication occur (7, 35). To determine whether these structures were viral transcription sites, colocalization studies were performed with Aly/REF and ICP4, the HSV-1 transcriptional activator. It should be noted that in these and in all subsequent colocalization experiments, representative cells are shown, and in all instances, 75% or more of the cells displayed the colocalization pattern shown. Aly/REF was seen to colocalize in part with sites of ICP4 staining as early as 4 h after infection (Fig. 1v to x) and to be present almost entirely within centers of ICP4 staining by 8 h after infection (Fig. 1y to aa). Further, ICP4 was seen to localize in structures that were quite distinct from SC35 speckles (Fig. 1bb to dd). It has been reported that ICP27 interacts with ICP4 (8, 44); therefore, we examined the colocalization of ICP27 and ICP4. ICP27 was seen to colocalize in part with ICP4 at 4 h after infection; however, by 8 h after infection, while there was some nuclear colocalization of the two proteins, ICP27 was prominently cytoplasmic, in accordance with its shuttling activity at later times after infection (Fig. 1p to u). However, at early times, it was clear that ICP27 moved to ICP4 transcription sites.
FIG. 1.
ICP27 interacts with Aly/REF, which is relocalized to centers of ICP4 staining and away from SC35 splice sites during infection. RSFs were transfected with EGFP-Aly/REF and 20 h later were infected with wild-type HSV-1 KOS. At the indicated times, cells were fixed and then stained with antibodies directed to the indicated proteins. Immunofluorescence staining was performed with anti-SC35 hybridoma supernatant (11), anti-ICP27 antibody H1119, or anti-ICP4 antibody H1114. EGFP expression was detected by direct fluorescence. WT, wild type.
Because ICP27 colocalized with Aly/REF at early times after infection and because Aly/REF was relocalized to sites of ICP4 staining, where ICP27 can also be found at early times (Fig. 1), we examined whether ICP27 recruited Aly/REF to viral transcription sites. Cells were infected with ICP27 viral mutants that had small deletions within and around the region that was mapped previously as the ICP27-Aly/REF interaction region (4, 29). The positions of the mutations are depicted in Fig. 2. Specifically, in vitro binding assays and coimmunoprecipitation experiments indicated that the interaction region was located between residues 104 and 138 (4, 29). ICP27 deletion mutants d1-2, in which residues 12 to 63 were deleted, and d4-5, in which residues 139 to 153 were deleted (33), colocalized with Aly/REF at 4 h after infection, and Aly/REF was redistributed to sites of ICP4 staining and away from SC35 speckles by 8 h after infection (Fig. 3). In contrast, mutant d2-3, which lacks residues 64 to 108, and mutant d3-4, which lacks residues 109 to 138 (33), did not colocalize with Aly/REF. Further, Aly/REF was not redistributed to sites of ICP4 staining but instead remained localized to SC35 spliceosomal sites (Fig. 3). Both the d2-3 and the d3-4 deletions occur within the region of ICP27 that is required for the interaction with Aly/REF. The deletion in d3-4 spans the major nuclear localization signal sequence (NLS) of ICP27 (21, 38); therefore, ICP27 was inefficiently imported into the nucleus (Fig. 3). However, mutant d2-3 was strictly nuclear at 4 h after infection, yet there was pronounced cytoplasmic staining by 8 h. We conclude that ICP27 interacts with and recruits Aly/REF to ICP4-containing transcription foci and away from SC35 speckles. Further, an interaction with Aly/REF is not required for ICP27 export to the cytoplasm.
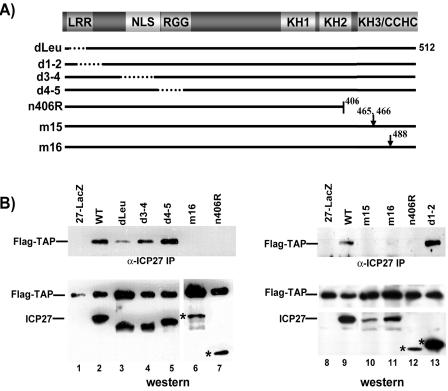
FIG. 2.
ICP27 deletion, truncation, and substitution mutants. (A) A schematic representation of the ICP27 512-amino-acid protein shows the leucine-rich region (LRR), the NLS, the RGG box RNA binding domain, three predicted KH motifs, and a zinc finger-like domain (CCHC). The positions of the mutations are shown. (B) The residues that are deleted or altered are indicated. The ability of the mutant viruses to replicate on noncomplementing cells is indicated relative to that of the wild-type virus, with ++++ indicating nearly wild-type levels and − indicating that these mutants require an ICP27-complementing cell line to replicate. tsLG4 fails to replicate at a nonpermissive temperature. Ref., reference.
FIG. 3.
ICP27 recruits Aly/REF to ICP4 transcription sites. RSFs were transfected with EGFP-Aly/REF and then infected with the indicated ICP27 viral mutants. At 4 and 8 h after infection, cells were fixed and stained with anti-ICP27, anti-ICP4, and anti-SC35 antibodies.
RNA binding by ICP27 is not required for ICP27 export to the cytoplasm.
Previous studies reported that viral late RNAs must be present for ICP27 to shuttle during infection, suggesting that ICP27 must bind to its RNA cargo to access the export receptor (29, 67-69). To determine whether RNA binding is required for the export of the ICP27 protein itself, we measured the efficiency of export in in vitro export assays (4) with ICP27 viral mutants that are or are not able to bind RNA. We first probed the abilities of two mutants with lesions in RNA binding domains to bind RNA. In a previous study, Soliman and Silverstein (68) showed that following UV cross-linking, ICP27 could be copurified with poly(A)+ RNA fractions from cells infected with the wild type but not from cells infected with a temperature-sensitive mutant with an amino acid substitution within a predicted KH RNA binding domain (69). Further, this mutant, termed tsLG4, was shown to be defective in shuttling (67). However, Soliman and Silverstein (68) did not examine directly the ability of mutant tsLG4 to bind RNA but rather examined its copurification with total poly(A)+ RNA. In their study, the recovery of wild-type ICP27 was close to the limit of detection; therefore, RNA binding by tsLG4 might have been missed.
To address whether tsLG4 can bind RNA, we performed in vivo UV cross-linking (4, 58) with cells infected with wild-type HSV-1 KOS and with mutants tsLG4 and d4-5. The RGG box RNA binding domain, which is required for ICP27 RNA binding in vivo (58) and in vitro (40), is deleted in d4-5 (33). ICP27-RNA complexes were immunopurified from nuclear extracts of infected cells that were UV irradiated at 6 h after infection. RNase protection assays performed with the ICP27-bound RNA fractions showed that mutant tsLG4 bound its own transcripts and those of the gD gene as efficiently as the wild type (Fig. 4A). Compared to the results obtained with the wild type, the recovery of gB RNA was significantly diminished in the tsLG4-bound RNA fractions; however, the expression of gB RNA was greatly reduced in tsLG4-infected cells, as shown for the unbound fraction (Fig. 4A). These results indicated that there was a transcriptional effect on this late viral RNA, in accordance with the results of Jean et al. (24). Thus, the reduced recovery of gB transcripts in the bound fractions was reflective of the reduced abundance of these transcripts. As expected, none of the three transcripts probed were recovered in the d4-5-bound RNA fractions, although all three were abundant in the unbound fraction (Fig. 4A). These results confirmed that the RGG box is required for RNA binding by ICP27.
FIG. 4.
In vivo RNA binding by wild-type (WT) ICP27 and ICP27 mutants at two RNA binding motifs. RSFs were infected with HSV-1 KOS, tsLG4, and d4-5 at 39.5°C for 6 h, at which time infected cell monolayers were UV irradiated as described previously (58). Nuclear extracts were prepared, and RNA bound to ICP27 was isolated by immunoprecipitation with anti-ICP27 antibody H1119. (A) ICP27-bound and unbound RNA fractions were hybridized to antisense RNA probes for ICP27, gB, and gD transcripts as described previously (58). (B) ICP27-bound RNA fractions were fractionated on SDS-polyacrylamide gels and transferred to nitrocellulose. The blots were probed with anti-ICP27 antibody.
The RNA sequence specificity for ICP27 binding has not been determined; however, a number of viral transcripts to which ICP27 binds were identified in a yeast three-hybrid screen (66). Further, a mutant with an amino acid substitution within the predicted KH3 domain (69) of ICP27 showed reduced binding in three-hybrid assays (66). The tsLG4 mutation also occurs within KH3 (65). Therefore, it is possible that tsLG4 binds some viral RNAs less efficiently.
To compare the abilities of tsLG4 ICP27 and wild-type ICP27 to bind RNA during viral infection, UV cross-linking was performed, and ICP27-RNA complexes were isolated by immunoprecipitation with an ICP27 monoclonal antibody (58). In this experiment, total RNA binding rather than binding to specific RNAs was measured. To accomplish this goal, ICP27-RNA complexes were fractionated by SDS-polyacrylamide gel electrophoresis and analyzed by immunoblotting. This procedure was used because UV cross-linking induces the covalent binding of proteins in direct contact with RNA, and bound RNA would reduce the mobility of ICP27 by increasing the apparent molecular mass by that of the RNA bound to each protein molecule. Consequently, in addition to a band at 63 kDa, the apparent molecular mass of ICP27, a smear of higher-molecular-mass species would be expected and would represent ICP27 bound to RNA. This result in fact was observed (Fig. 4B). Further, there was no apparent difference between the tsLG4 sample and the wild-type sample, indicating that tsLG4 ICP27 binds RNA as efficiently as wild-type ICP27. As predicted, slower-migrating species were absent in the d4-5 sample. We cannot exclude the possibilities that the predicted KH3 domain contributes to RNA binding specificity and therefore that tsLG4 binds some viral transcripts less efficiently. These possibilities are currently being explored. However, we can conclude that an inability to bind RNA likely is not the basis of an export defect for tsLG4.
To compare the efficiencies of ICP27 export in cells infected with the wild type and in mutant-infected cells, in vitro export assays were performed as described previously (4). In accordance with our previous results (4), at later times after infection, 80 to 90% of nuclear ICP27 was exported to the cytoplasm by 15 min after the start of the in vitro export assay (Fig. 5). Next, we analyzed viral mutants that were previously shown to be defective in export or to have mutations in regions required for the interaction with Aly/REF or for RNA binding. Mutant dLeu (33) has a deletion of residues 6 to 19 (Fig. 2), which includes a leucine-rich region that resembles a nuclear export signal sequence (NES) (58) of the type that interacts with the CRM1 export receptor (9). This region was shown previously to be required for the efficient export of ICP27 (33, 58, 69). We found that the level of expression of the dLeu protein was too low to be detected in this assay at early times after infection; therefore, infections with dLeu were performed for 8 to 16 h. Export was reduced compared to that seen with the wild type because about 60% of the protein remained nuclear (Fig. 5). Mutant d1-2 (Fig. 2), which has a deletion of a portion of the leucine-rich region (33), was exported less efficiently than the wild type at 4 and 6 h after infection, but by 8 h, only about 30% of the protein remained in the nucleus. Mutant d3-4 has a deletion within the Aly/REF interaction region, but it also spans the ICP27 NLS (Fig. 2). However, we measured the decrease in the amount of ICP27 that was present in the nucleus at the start of the assay; therefore, inefficient import of ICP27 did not affect the ability to measure export. Mutant d3-4 export was even more efficient than that of the wild type; therefore, an interaction with Aly/REF is not required for ICP27 cytoplasmic export. Similarly, mutant d4-5, which has a deletion of the RGG box RNA binding domain, was efficiently exported, confirming that ICP27 binding to RNA is not required for ICP27 export to the cytoplasm.
FIG. 5.
ICP27 export to the cytoplasm is impaired in ICP27 N-terminal and C-terminal mutant-infected cells. RSFs were infected with HSV-1 KOS and the indicated viral mutants. In vitro export assays were performed at the indicated times after infection. The assays were stopped, and nuclear proteins were harvested at the indicated times. Western blots were scanned and quantified as described previously (4). All export assays were performed three or more times, and representative data are shown.
In contrast, the export of ICP27 mutants with mutations in the C terminus was greatly impaired. Mutant n406R, which expresses an ICP27 protein that is truncated at amino acid 406 (53), and two substitution mutants, m15 and m16 (51), were largely retained in the nucleus, in accordance with a previous report by Mears and Rice, who used a heterokaryon assay (39). Further, tsLG4 ICP27 was inefficiently exported, and about 60% of the protein remained nuclear, similar to what was observed for dLeu ICP27 (Fig. 5). Although the export of the tsLG4 protein was impaired, this result was not due to an inability to bind RNA (Fig. 4). Further, mutant d4-5, which cannot bind RNA, showed efficient export (Fig. 5). Therefore, the regions involved in the export of ICP27 to the cytoplasm include the N-terminal leucine-rich region and the C-terminal region. Neither RNA binding nor an interaction with Aly/REF is required.
ICP27 interacts with TAP/NXF1 without Aly/REF bridging, and both the N terminus and the C terminus are involved in the interaction.
We showed previously that TAP/NXF1 coimmunoprecipitated and colocalized with ICP27 in HSV-1-infected cells and that a dominant-negative TAP/NXF1 mutant with a deletion of the C-terminal domain, required for its interaction with nucleoporins, retained ICP27 in the nucleus (4). These results showed that the export of ICP27 occurs through TAP/NXF1. However, it was not clear whether ICP27 bound directly to TAP/NXF1 or whether the interaction required bridging by Aly/REF, which has been shown to interact directly with TAP/NXF1 (71).
To address this question, in vitro binding assays were performed with GST-TAP and with wild-type and mutant versions of ICP27 that were translated in vitro. The input 35S-labeled proteins are shown in Fig. 6C. A schematic showing the deleted regions in the ICP27 mutants used is shown in Fig. 6A. Wild-type ICP27 and mutants that lacked the Aly/REF interaction region (DΔS, ΔNLS, and R1), the RGG box RNA binding domain (DΔS), or the predicted KH1 and KH2 domains (H17) bound to GST-TAP (Fig. 6B). An N-terminal deletion mutant that lacked the leucine-rich region (ΔLRR) showed greatly reduced binding relative to that of the wild type. No binding was detected with a truncation mutant (ΔC) that expressed amino acids 1 to 450 and thus lacked the C-terminal region containing the predicted KH3 domain and the zinc finger-like region (Fig. 6B). The C terminus of ICP27 was shown previously to be required for multimerization (75) and for interactions with SR proteins (62) and RNA polymerase II (76) and therefore appears to be important for protein-protein interactions. Interestingly, the N-terminal leucine-rich region also appeared to be involved in the interaction of ICP27 with TAP/NXF1, despite its resemblance to NESs that bind to CRM1. We reported previously that we were unable to detect any interaction between ICP27 and CRM1 (4). Thus, the N-terminal leucine-rich region contributes to ICP27 export through TAP/NXF1 rather than CRM1. Further, these data confirm that Aly/REF is not required to bridge the interaction between ICP27 and GST-TAP because we were unable to detect Aly/REF in in vitro binding assays with a specific monoclonal antibody and because ICP27 mutants that lacked the Aly/REF interaction region bound efficiently to GST-TAP.
FIG. 6.
ICP27 interacts with TAP/NXF1 in vitro, and Aly/REF is not required to bridge the interaction. (A) A schematic diagram of ICP27 shows the leucine-rich region (LRR), the NLS, the RGG box RNA binding domain, three predicted KH domains, and a zinc finger-like motif (CCHC). The positions of the deletion mutations that were used in the in vitro binding assays are shown. (B) GST binding assays were performed with GST-TAP and in vitro-translated wild-type (WT) ICP27 and ICP27 mutants ΔLRR, D2ΔS5, S5, R1, ΔNLS, H17, and ΔC. Aly/Ref was included as a positive control, and luciferase (Luc) was included as a negative control. The in vitro binding assays were performed in the presence of RNase to eliminate the possibility of RNA bridging. (C) Input 35S-labeled proteins. (D) GST binding assays were performed with in vitro-translated WT ICP27, GST-TAP, and TAP truncations. A schematic diagram of the TAP/NXF1 protein is shown.
To map the region of TAP/NXF1 that interacts with ICP27, we used GST-TAP truncations in the binding assays. A schematic of 619-amino-acid TAP is shown in Fig. 6. ICP27 bound to full-length GST-TAP and to GST-TAP-1-506, which lacks the C-terminal nucleoporin binding domain (1) (Fig. 6D). ICP27 also bound to GST-TAP-1-373 (data not shown), which lacks the region required for binding to its heterodimeric partner, p15 (17), but includes the Aly/REF interaction region. The Aly/REF interaction requires the entire N terminus of TAP/NXF1 from residues 1 to 373 (1, 71). Binding of ICP27 was not detected with GST-TAP-61-373, suggesting that the N terminus of TAP/NXF1 is required for binding to ICP27 (Fig. 6D). This finding was confirmed with GST-TAP-1-99, to which ICP27 bound efficiently (Fig. 6D). Thus, the N terminus of TAP/NXF1 from amino acids 1 to 99 is sufficient for the interaction with ICP27.
To confirm the region of ICP27 required for the interaction in vivo, coimmunoprecipitation experiments were performed with cells infected with wild-type HSV-1 and with ICP27 viral mutants. Flag-TAP coimmunoprecipitated with the wild type and with mutants d1-2, d3-4, and d4-5 (Fig. 7). A reduced amount of Flag-TAP was seen in the dLeu sample despite equivalent levels of expression of the dLeu protein and of the wild-type protein, confirming that the N-terminal leucine-rich region contributes to the interaction of ICP27 with TAP/NXF1. Flag-TAP did not coimmunoprecipitate with C terminus mutants n406R, m15, and m16 (Fig. 7). The expression of these mutant proteins was reduced compared to that of the wild-type protein and may have reflected decreased protein stability. Nonetheless, in multiple experiments, two of which are shown in Fig. 7, Flag-TAP did not coimmunoprecipitate with mutants n406R, m15, and m16.
FIG. 7.
Mapping the regions of ICP27 that are involved in the interaction with TAP/NXF1 in infected cells. (A) Diagram of the ICP27 coding region, along with the positions of the mutations in the ICP27 viral mutants. See the legends to Fig. 2 and 6 for definitions. (B) RSFs were transfected with Flag-TAP and then infected with HSV-1 KOS and the indicated ICP27 viral mutants. At 6 h after infection, nuclear extracts were prepared, and immunoprecipitation (IP) was performed with anti-ICP27 antibody. Immune complexes were fractionated and transferred to nitrocellulose. Blots shown in the upper panels were probed with anti-Flag antibody. The middle and lower panels show Western blots of nuclear extracts before immunoprecipitation. The blots in the middle panels were probed with anti-Flag antibody, and the blots in the lower panels were probed with anti-ICP27 antibody. Asterisks mark the positions of ICP27 mutant proteins. WT, wild type.
To further investigate the in vivo interaction between TAP/NXF1 and ICP27, we performed colocalization experiments. As reported previously, wild-type ICP27 colocalized with TAP/NXF1 at 4 h after infection, but by 8 h, there was prominent cytoplasmic staining, although some nuclear colocalization was observed (Fig. 8). These findings were also observed at 8 h after infection with d1-2 and d4-5, both of which coimmunoprecipitated with Flag-TAP (Fig. 7). Further, the overexpression of GFP-TAP facilitated the export of d1-2 ICP27, which was found to be less efficient than wild-type ICP27 export in in vitro export assays (Fig. 5). This finding can be seen for the d1-2-infected cell that expressed GFP-TAP compared to the one that did not (Fig. 8). We previously found that excess TAP also facilitated wild-type ICP27 export (4). In contrast, dLeu did not colocalize with GFP-TAP and remained strictly nuclear at 8 h after infection (Fig. 8). Similarly, n406R, m15, m16, and tsLG4 did not colocalize with GFP-TAP, and all remained confined to the nucleus at 8 h after infection. These results correlate with the results obtained for these mutants in in vitro export assays (Fig. 5) and confirm that C terminus mutants cannot interact with TAP/NXF1 and that an interaction with TAP/NXF1 is required for the export of ICP27 to the cytoplasm.
FIG. 8.
ICP27 mutants that do not interact with TAP/NXF1 are confined to the nucleus. RSFs were transfected with EGFP-TAP and then infected with HSV-1 KOS or the indicated viral mutants. At 4 and 8 h after infection, cells were fixed and stained with anti-ICP27 antibody. EGFP expression was detected by direct fluorescence. WT, wild type.
TAP/NXF1 is not recruited to ICP4 transcription sites.
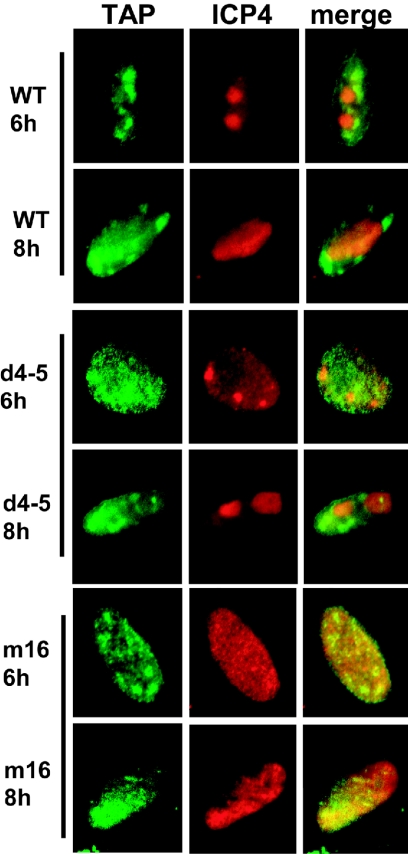
To determine whether TAP/NXF1 is recruited to sites of HSV-1 transcription like Aly/REF, colocalization studies were performed. TAP/NXF1 was not observed to colocalize with centers of ICP4 staining in wild-type HSV-1-infected cells at 6 or 8 h after infection (Fig. 9), and this was also the situation for d4-5, which also interacted with TAP/NXF1 (Fig. 7 and 8). In mutant m16-infected cells, ICP4 transcription sites were poorly formed, but it was still quite evident that TAP/NXF1 did not colocalize with ICP4 (Fig. 9). Therefore, unlike Aly/REF, TAP/NXF1 is not recruited to ICP4 transcription sites but instead must interact with ICP27 after it has left viral replication sites.
FIG. 9.
TAP/NXF1 does not move to centers of ICP4 staining. RSFs were transfected with EGFP-TAP and then infected with HSV-1 KOS or mutant d4-5 or m16. At 6 and 8 h after infection, cells were stained with anti-ICP4 antibody. WT, wild type.
HSV-1 infection does not affect the nucleocytoplasmic transport of several cellular shuttling proteins.
Recent studies showed that certain RNA viruses that replicate in the cytoplasm, including picornaviruses and vesicular stomatitis virus, disrupt normal nucleocytoplasmic trafficking (15, 16). This situation would benefit the cytoplasmic replication of these RNA viruses because the inhibition of cellular mRNA export would reduce competition for the translation machinery, and the prevention of import might contribute to the accumulation of nuclear factors in the cytoplasm, where they might benefit viral replication (reviewed in reference 15). This inhibition of transport occurs through targeting of components of the nuclear pore complex (NPC). HSV-1 infection inhibits host cell splicing, resulting in the retention of host pre-mRNAs in stalled splicing complexes (18). Further, ICP27 interacts with cellular export factors to facilitate viral RNA export. Therefore, we examined whether HSV-1 infection had an effect on the nucleocytoplasmic export of cellular proteins that are known to shuttle between the nucleus and the cytoplasm.
In vitro export assays were performed with HeLa cells that were mock infected or infected with HSV-1 KOS (Fig. 10). The export of ICP27 was slower than what we observed previously with RSFs (Fig. 4), a result which could reflect a slower progression of infection in HeLa cells than in RSFs. Still, at 8 h after infection, about 70% of ICP27 had exited the nucleus by 20 min after the start of the export assay (Fig. 10A). Importantly, TAP/NXF1 export was unaffected by HSV-1 infection (Fig. 10B). This was also the situation for Aly/REF (Fig. 10E), which has been shown to be largely nuclear, although it shuttles (71), suggesting that it dissociates from RNA when the RNPs reach the NPC or that it dissociates soon after and returns to the nucleus (30). Interestingly, CRM1, a member of the RAN-GTP-dependent exportin family, which mediates the export of proteins and some cellular RNAs (9, 12, 43), remained entirely nuclear throughout the assay in both uninfected and HSV-1-infected cells (Fig. 10C). The export of Smad2, which is a nucleoporin-dependent but CRM1-independent shuttling protein (74), also was not affected by infection (Fig. 10D), nor was hnRNP A1 (Fig. 10F), whose export is mediated by transportin through an M9 import-export signal (23, 41). Thus, HSV-1 infection does not disrupt the nucleocytoplasmic trafficking of export factors that shuttle through a number of different export pathways.
FIG. 10.
HSV-1 infection does not affect the export of cellular shuttling proteins. HeLa cells were either mock infected or infected with HSV-1 KOS. At the indicated times, in vitro export assays were performed. The amount of protein remaining in the nucleus at each time point after the start of the assay was quantified by Western blot analysis with anti-ICP27 (A), anti-TAP/NXF1 (B), anti-CRM1 (C), anti-Smad2 (D), anti-Aly/REF (E), and anti-hnRNP A1 (F) antibodies.
DISCUSSION
In this study, we used in vitro and in vivo approaches to elucidate the interaction of HSV-1 ICP27 with cellular RNA export factors and to define the consequences of these interactions on their localization and on ICP27 export. As reported previously (4, 29), ICP27 interacted with EJC export adaptor protein Aly/REF; further, we showed that ICP27 recruited Aly/REF to centers of ICP4 staining, where viral transcription occurs (Fig. 3). These findings are in accordance with the results of recent studies with in situ hybridization and confocal microscopy, which demonstrated an accumulation of EJC proteins, including Aly/REF, at sites of transcription in mammalian cell nuclei (6). It was shown that excess Aly/REF facilitated the cytoplasmic export of some HSV-1 late mRNAs (4, 29); however, an interaction with Aly/REF was not required for the export of ICP27 (Fig. 4). Because ICP27 binds viral RNAs independently of Aly/REF (4), Aly/REF may not be required for HSV-1 RNA export but may merely enhance efficiency. This situation was found for Aly/REF in Drosophila by small interfering RNA approaches (13). In contrast to the results obtained for Aly/REF, TAP/NXF1 was not recruited to transcription sites that were marked by ICP4 staining (Fig. 9). This result is in agreement with the results of Custodio et al. (6), who also found that there was not a concentration of TAP/NXF1 on nascent transcripts. Because ICP27 interacts with and colocalizes with TAP/NXF1 during infection (Fig. 7 and 8), these results suggest that TAP/NXF1 binds ICP27-RNA complexes after their release from transcription sites. Further, ALY/REF is not required to bridge the interaction of ICP27 with TAP/NXF1, although it may facilitate the interaction of ICP27-RNA complexes with TAP/NXF1.
TAP/NXF1 is to date the most important receptor involved in the export of mRNA from the nucleus to the cytoplasm (5, 20, 48). Here we confirmed our previous results (4) showing that ICP27 is exported to the cytoplasm via TAP/NXF1. In the previous study, we showed that a dominant-negative TAP/NXF1 mutant that was unable to interact with the NPC confined ICP27 to the nucleus (4). Here we found that ICP27 mutants that failed to coimmunoprecipitate or colocalize with TAP/NXF1 were restricted to the nucleus. Interestingly, two regions of ICP27 were involved in the interaction. The C terminus from residues 406 to 508 was required for the interaction, but the N-terminal leucine-rich region from amino acids 6 to 12 also contributed because in vitro and in vivo binding was reduced when this region was deleted (Fig. 6 and 7). This leucine-rich region resembles an NES of the type that interacts with the CRM1 export receptor (9, 12). Further, it was reported that ICP27 export was sensitive to leptomycin B, a CRM1-specific inhibitor (42, 68, 69). We (4) and others (29) were not able to confirm that finding, but instead it was found that ICP27 was efficiently exported in the presence of leptomycin B. Further, we could not detect an interaction between ICP27 and CRM1 in yeast two-hybrid assays, coimmunoprecipitation experiments, or colocalization studies with HSV-1-infected cells (4). However, mutation or deletion of the leucine-rich region did impair ICP27 export (33, 58, 69) (Fig. 5). The finding that this region contributes to the interaction with TAP/NXF1 thus clarifies the requirement for the N terminus for ICP27 export. Interestingly, TAP/NXF1 also has a leucine-rich region that lies within the region of TAP/NXF1 that interacts with ICP27 (Fig. 6) and also with Aly/REF (71). We conclude that an interaction with TAP/NXF1 is required for ICP27 to exit the nucleus. Contrary to previous reports, we found that the ability of ICP27 to bind RNA did not affect ICP27 export during infection (Fig. 4 and 5). Thus, ICP27 export requires an interaction with TAP/NXF1 but does not require an interaction with Aly/REF or RNA. The regions of ICP27 that are required for its facilitation of viral RNA export do include the RGG box RNA binding domain, however. Deletions within the RGG box, the N-terminal leucine-rich region, and the C-terminal region impair viral RNA export (S. Rojas, A. Sun, G. B. Devi-Rao, E. K. Wagner and R. M. Sandri-Goldin, unpublished data).
Finally, unlike the situation with RNA viruses (15, 16), HSV-1 infection does not have an impact on cellular nucleocytoplasmic trafficking. Shuttling of cellular proteins that use several different export pathways was not disrupted or modified. These data suggest that while ICP27 hijacks Aly/REF from sites of cellular splicing and uses TAP/NXF1 to reach the cytoplasm, cellular mRNA export can continue. Thus, the inhibition of splicing by ICP27 early in infection, which results in the nuclear retention of incompletely processed cellular pre-mRNAs, may be as important to shifting the balance to viral RNA export as the subsequent recruitment of Aly/REF.
Acknowledgments
We thank Steve Rice for the generous gift of ICP27 viral mutants and Gideon Dreyfuss for antibodies.
These studies were supported by U.S. Public Health Service grant AI21515 from NIAID. I.-H.B.C. was supported by a University of California biotechnology training grant. L.S. was supported for part of these studies by a UCI undergraduate minority access to research training grant.
REFERENCES
- 1.Bachi, A., I. C. Braun, J. P. Rodrigues, N. Pante, K. Ribbeck, C. von Kobbe, U. Kutay, M. Wilm, D. Gorlich, M. Carmo-Fonseca, and E. Izaurralde. 2000. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6:136-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun, I. C., A. Herold, M. Rode, E. Conti, and E. Izaurralde. 2001. Overexpression of TAP/p15 heterodimers bypasses nuclear retention and stimulates nuclear mRNA export. J. Biol. Chem. 276:20536-20543. [DOI] [PubMed] [Google Scholar]
- 3.Bryant, H. E., S. Wadd, A. I. Lamond, S. J. Silverstein, and J. B. Clements. 2001. Herpes simplex virus IE63 (ICP27) protein interacts with spliceosome-associated protein 145 and inhibits splicing prior to the first catalytic step. J. Virol. 75:4376-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, I. B., K. S. Sciabica, and R. M. Sandri-Goldin. 2002. ICP27 interacts with the export factor Aly/REF to direct herpes simplex virus 1 intronless RNAs to the TAP export pathway. J. Virol. 76:12877-12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen, B. R. 2003. Nuclear RNA export. J. Cell Sci. 116:587-597. [DOI] [PubMed] [Google Scholar]
- 6.Custodio, N., C. Carvalho, I. Condado, M. Antoniou, B. J. Blencowe, and M. Carmo-Fonseca. 2004. In vivo recruitment of exon junction complex proteins to transcription sites in mammalian cell nuclei. RNA 10:622-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bruyn Kops, A., and D. M. Knipe. 1988. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell 55:857-868. [DOI] [PubMed] [Google Scholar]
- 8.Everett, R. D., G. Sourvinos, C. Leiper, J. B. Clements, and A. Orr. 2004. Formation of nuclear foci of the herpes simplex virus type 1 regulatory protein ICP4 at early times of infection: localization, dynamics, recruitment of ICP27, and evidence for the de novo induction of ND10-like complexes. J. Virol. 78:1903-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 10.Fribourg, S., I. C. Braun, E. Izaurralde, and E. Conti. 2001. Structural basis for the recognition of a nucleoporin FG repat by the NTF2-like domain of the TAP/p15 mRNA nuclear export factor. Mol. Cell 8:645-656. [DOI] [PubMed] [Google Scholar]
- 11.Fu, X. D., and T. Maniatis. 1992. The 35-kDa mammalian splicing factor SC35 mediates specific interactions between U1 and U2 small nuclear ribonucleoprotein particles at the 3′ splice site. Proc. Natl. Acad. Sci. USA 89:1725-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda, M., S. Asano, T. Nakamura, M. Adachi, M. Yoshida, M. Yanagida, and E. Nishida. 1997. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390:308-311. [DOI] [PubMed] [Google Scholar]
- 13.Gatfield, D., and E. Izaurralde. 2002. REF/Aly and the additional exon junction complex proteins are dispensable for nuclear mRNA export. J. Cell Biol. 159:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatfield, D., H. LeHir, C. Schmitt, I. C. Braun, T. Kocher, M. Wilm, and E. Izaurralde. 2001. The DexH/D box protein HEL/UAP56 is essential for mRNA export in Drosophila. Curr. Biol. 11:1716-1721. [DOI] [PubMed] [Google Scholar]
- 15.Gustin, K. E. 2003. Inhibition of nucleo-cytoplasmic trafficking by RNA viruses: targeting the nuclear pore complex. Virus Res. 95:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustin, K. E., and P. Sarnow. 2002. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 76:8787-8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzik, B. W., L. Levesque, S. Prasad, Y.-C. Bor, B. E. Black, B. M. Paschal, D. Rekosh, and M.-L. Hammarskjold. 2001. NXT1 (p15) is a crucial cellular cofactor in TAP-dependent export of intron-containing RNA in mammalian cells. Mol. Cell. Biol. 21:2545-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardwicke, M. A., and R. M. Sandri-Goldin. 1994. The herpes simplex virus regulatory protein ICP27 can cause a decrease in cellular mRNA levels during infection. J. Virol. 68:4797-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy, W. R., and R. M. Sandri-Goldin. 1994. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J. Virol. 68:7790-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold, A., L. Teixeira, and E. Izaurralde. 2003. Genome-wide analysis of nuclear mRNA export pathways in Drosophila. EMBO J. 22:2472-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hibbard, M. K., and R. M. Sandri-Goldin. 1995. Arginine-rich regions succeeding the nuclear localization region of the herpes simplex virus type 1 regulatory protein ICP27 are required for efficient nuclear localization and late gene expression. J. Virol. 69:4656-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurt, E., K. Straber, A. Segref, S. Bailer, N. Schlaich, C. Presutti, D. Tollervey, and R. Jansen. 2000. Mex67p mediates nuclear export of a variety of RNA polymerase II transcripts. J. Biol. Chem. 275:8361-8368. [DOI] [PubMed] [Google Scholar]
- 23.Izaurralde, E., A. Jarmolowski, C. Beisel, I. W. Mattaj, and G. Dreyfuss. 1997. A role for the M9 transport signal of hnRNP A1 in mRNA nuclear export. J. Cell Biol. 137:27-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jean, S., K. M. LeVan, B. Song, M. Levine, and D. M. Knipe. 2001. Herpes simplex virus 1 ICP27 is required for transcription of two viral late (γ2) genes in infected cells. Virology 283:273-284. [DOI] [PubMed] [Google Scholar]
- 25.Kang, Y., and B. R. Cullen. 1999. The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences. Genes Dev. 13:1126-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katahira, J., K. Straber, A. Podtelejnikov, M. Mann, J. U. Jung, and E. Hurt. 1999. The Mex-67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J. 18:2593-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katahira, J., K. Straesser, T. Saiwaki, Y. Yoneda, and E. Hurt. 2002. Complex formation between Tap and p15 affects binding to FG-repeat nucleoporins and nucleocytoplasmic shuttling. J. Biol. Chem. 277:9242-9246. [DOI] [PubMed] [Google Scholar]
- 28.Kim, V. N., J. Yong, N. Kataoka, L. Abel, M. D. Diem, and G. Dreyfuss. 2001. The Y14 protein communicates to the cytoplasm the position of exon-exon junctions. EMBO J. 20:2062-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 20:5769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeHir, H., D. Gatfield, E. Izaurralde, and M. J. Moore. 2001. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 20:4987-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeHir, H., E. Izaurralde, L. E. Maquat, and M. J. Moore. 2000. The spliceosome deposits multiple proteins 20-24 nucleotides upstream of mRNA exon-exon junctions. EMBO J. 19:6860-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeHir, H., M. J. Moore, and L. E. Maquat. 2000. Pre-mRNA splicing alters mRNP composition: evidence for stable association of proteins at exon-exon junctions. Genes Dev. 14:1098-1108. [PMC free article] [PubMed] [Google Scholar]
- 33.Lengyel, J., C. Guy, V. Leong, S. Borge, and S. A. Rice. 2002. Mapping of functional regions in the amino-terminal portion of the herpes simplex virus ICP27 regulatory protein: importance of the leucine-rich nuclear export signal and RGG box RNA-binding domain. J. Virol. 76:11866-11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linberg, A., and J. P. Kreivi. 2002. Splicing inhibition at the level of spliceosome assembly in the presence of herpes simplex virus protein ICP27. Virology 294:189-198. [DOI] [PubMed] [Google Scholar]
- 35.Lukonis, C. J., and S. K. Weller. 1997. Formation of herpes simplex virus type 1 replication compartments by transfection: requirements and localization to nuclear domain 10. J. Virol. 71:2390-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo, M. J., and R. Reed. 1999. Splicing is required for rapid and efficient mRNA export in metazoans. Proc. Natl. Acad. Sci. USA 96:14937-14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLauchlan, J., A. Phelan, C. Loney, R. M. Sandri-Goldin, and J. B. Clements. 1992. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3′ processing. J. Virol. 66:6939-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mears, W. E., V. Lam, and S. A. Rice. 1995. Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J. Virol. 69:935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between the nucleus and cytoplasm. Virology 242:128-137. [DOI] [PubMed] [Google Scholar]
- 40.Mears, W. E., and S. A. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 70:7445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michael, W. M., M. Choi, and G. Dreyfuss. 1995. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell 83:415-422. [DOI] [PubMed] [Google Scholar]
- 42.Murata, T., F. Goshima, T. Koshizuka, H. Takakuwa, and Y. Nishiyama. 2001. A single amino acid substitution in the ICP27 protein of herpes simplex virus type 1 is responsible for its resistance to leptomycin B. J. Virol. 75:1039-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ossareh-Nazari, B., F. Bachelerie, and C. Dargemont. 1997. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278:141-144. [DOI] [PubMed] [Google Scholar]
- 44.Panagiotidis, C. A., E. K. Lium, and S. Silverstein. 1997. Physical and functional interactions between herpes simplex virus immediate-early proteins ICP4 and ICP27. J. Virol. 71:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phelan, A., M. Carmo-Fonseca, J. McLauchlan, A. I. Lamond, and J. B. Clements. 1993. A herpes simplex virus type 1 immediate-early gene product, IE63, regulates small nuclear ribonucleoprotein distribution. Proc. Natl. Acad. Sci. USA 90:9056-9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinol-Roma, S., and G. Dreyfuss. 1992. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature 355:730-732. [DOI] [PubMed] [Google Scholar]
- 47.Rafiq, M., C. K. Suen, N. Choudhury, C. L. Joannou, K. N. White, and R. W. Evans. 1997. Expression of recombinant human ceruloplasmin—an absolute requirement for splicing signals in the expression cassette. FEBS Lett. 407:132-136. [DOI] [PubMed] [Google Scholar]
- 48.Reed, R., and E. Hurt. 2002. A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell 108:523-531. [DOI] [PubMed] [Google Scholar]
- 49.Reichert, V. L., H. LeHir, M. S. Jurica, and M. J. Moore. 2002. 5′ exon interactions within the human spliceosome establish a framework for exon junction complex strucutre and assembly. Genes Dev. 16:2778-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 64:1704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rice, S. A., and V. Lam. 1994. Amino acid substitution mutations in the herpes simplex virus ICP27 protein define an essential gene regulation function. J. Virol. 68:823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rice, S. A., V. Lam, and D. M. Knipe. 1993. The acidic amino-terminal region of herpes simplex virus type 1 alpha protein ICP27 is required for an essential lytic function. J. Virol. 67:1778-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rice, S. A., L. Su, and D. M. Knipe. 1989. Herpes simplex virus alpha protein ICP27 possesses separable positive and negative regulatory activities. J. Virol. 63:3399-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodrigues, J. P., M. Rode, D. Gatfield, B. J. Blencowe, M. Carmo-Fonseca, and E. Izaurralde. 2001. REF proteins mediate the export of spliced and unspliced mRNAs from the nucleus. Proc. Natl. Acad. Sci. USA 98:1030-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryu, W. S., and J. E. Mertz. 1989. Simian virus 40 late transcripts lacking excisable intervening sequences are defective in both stability in the nucleus and transport to the cytoplasm. J. Virol. 63:4386-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sacks, W. R., C. C. Greene, D. P. Ashman, and P. A. Schaffer. 1985. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J. Virol. 55:796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandri-Goldin, R. M. 1998. Interactions between an HSV regulatory protein and cellular mRNA processing pathways. Methods 16:95-104. [DOI] [PubMed] [Google Scholar]
- 58.Sandri-Goldin, R. M. 1998. ICP27 mediates herpes simplex virus RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12:868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandri-Goldin, R. M., and M. K. Hibbard. 1996. The herpes simplex virus type 1 regulatory protein ICP27 coimmunoprecipitates with anti-Sm antiserum, and the C terminus appears to be required for this interaction. J. Virol. 70:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandri-Goldin, R. M., M. K. Hibbard, and M. A. Hardwicke. 1995. The C-terminal repressor region of herpes simplex virus type 1 ICP27 is required for the redistribution of small nuclear ribonucleoprotein particles and splicing factor SC35; however, these alterations are not sufficient to inhibit host cell splicing. J. Virol. 69:6063-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandri-Goldin, R. M., and G. E. Mendoza. 1992. A herpes virus regulatory protein appears to act posttranscriptionally by affecting mRNA processing. Genes Dev. 6:848-863. [DOI] [PubMed] [Google Scholar]
- 62.Sciabica, K. S., Q. J. Dai, and R. M. Sandri-Goldin. 2003. ICP27 interacts with SRPK1 to mediate HSV-1 inhibition of pre-mRNA splicing by altering SR protein phosphorylation. EMBO J. 22:1608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Segref, A., K. Sharma, V. Doye, A. Hellwig, J. Huber, R. Luhrmann, and E. Hurt. 1997. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J. 16:3256-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 186:74-86. [DOI] [PubMed] [Google Scholar]
- 65.Smith, I. L., R. E. Sekulovich, M. A. Hardwicke, and R. M. Sandri-Goldin. 1991. Mutations in the activation region of herpes simplex virus regulatory protein ICP27 can be trans dominant. J. Virol. 65:3656-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sokolowski, M., J. E. Scott, R. P. Heaney, A. H. Patel, and J. B. Clements. 2003. Identification of herpes simplex virus RNAs that interact specifically with regulatory protein ICP27 in vivo. J. Biol. Chem. 278:33540-33549. [DOI] [PubMed] [Google Scholar]
- 67.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soliman, T. M., and S. J. Silverstein. 2000. Herpesvirus mRNAs are sorted for export via CRM1-dependent and -independent pathways. J. Virol. 74:2814-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soliman, T. M., and S. J. Silverstein. 2000. Identification of an export control sequence and a requirement for the KH domains in ICP27 from herpes simplex virus type 1. J. Virol. 74:7600-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strasser, K., J. Bassler, and E. Hurt. 2000. Binding of the Mex67p/Mtr2p heterodimer to FXFG, GLFG and FG repeat nucleoporins is essential for nuclear mRNA export. J. Cell Biol. 150:695-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stutz, F., A. Bachi, T. Doerks, I. C. Braun, B. Seraphin, M. Wilm, P. Bork, and E. Izaurralde. 2000. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 6:638-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiegand, H. L., G. A. Coburn, Y. Zeng, Y. Kang, H. P. Bogerd, and B. R. Cullen. 2002. Formation of Tap/NXT1 heterodimers activates Tap-dependent nuclear mRNA export by enhancing recruitment to nuclear pore complexes. Mol. Cell. Biol. 22:245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiegand, H. L., S. Lu, and B. R. Cullen. 2003. Exon junction complexes the enhancing effect of splicing on mRNA expression. Proc. Natl. Acad. Sci. USA 100:11327-11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu, L., Y. Kang, S. Col, and J. Massague. 2002. Smad2 nucleocytoplasmic shuttling by nucleoporins CAN/Nup214 and Nup153 feeds TGFβ signalling complexes in the cytoplasm and in the nucleus. Mol. Cell 10:271-282. [DOI] [PubMed] [Google Scholar]
- 75.Zhi, Y., K. S. Sciabica, and R. M. Sandri-Goldin. 1999. Self interaction of the herpes simplex virus type 1 regulatory protein ICP27. Virology 257:341-351. [DOI] [PubMed] [Google Scholar]
- 76.Zhou, C., and D. M. Knipe. 2001. Association of herpes simplex virus type 1 ICP8 and ICP27 with cellular RNA polymerase II holoenzyme. J. Virol. 76:5893-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou, Z., M. J. Luo, K. Straesser, J. Katahira, E. Hurt, and R. Reed. 2001. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature 407:401-405. [DOI] [PubMed] [Google Scholar]