Abstract
Ammonia monooxygenase (AMO) from Nitrosomonas europaea catalyzes the oxidation of ammonia to hydroxylamine and has been shown to oxidize a variety of halogenated and nonhalogenated hydrocarbons. As part of a program focused upon extending these observations to natural systems, a study was conducted to examine the influence of soil upon the cooxidative abilities of N. europaea. Small quantities of Willamette silt loam (organic carbon content, 1.8%; cation-exchange capacity, 15 cmol/kg of soil) were suspended with N. europaea cells in a soil-slurry-type reaction mixture. The oxidations of ammonia and three different hydrocarbons (ethylene, chloroethane, and 1,1,1-trichloroethane) were compared to results for controls in which no soil was added. The soil significantly inhibited nitrite production from 10 mM ammonium by N. europaea. Inhibition resulted from a combination of ammonium adsorption onto soil colloids and the exchangeable acidity of the soil lowering the pH of the reaction mixture. These phenomena resulted in a substantial drop in the concentration of NH4+ in solution (10 to 4.5 mM) and, depending upon the pH, in a reduction in the amount of available NH3 to concentrations (8 to 80 μM) similar to the Ks value of AMO for NH3 (∼29 μM). At a fixed initial pH (7.8), the presence of soil also modified the rates of oxidation of ethylene and chloroethane and changed the concentrations at which their maximal rates of oxidation occurred. The modifying effects of soil on nitrite production and on the cooxidation of ethylene and chloroethane could be circumvented by raising the ammonium concentration in the reaction mixture from 10 to 50 mM. Soil had virtually no effect on the oxidation of 1,1,1-trichloroethane.
Ammonia-oxidizing bacteria initiate the process of nitrification in soils and other matrices. The chemolithotroph Nitrosomonas europaea is the most extensively studied of the ammonia-oxidizing bacteria and has provided much of our current knowledge of ammonia oxidation at the molecular and cellular levels (16). Ammonia is converted to nitrite by the sequential action of two enzymes: ammonia monooxygenase (AMO), which oxidizes ammonia to hydroxylamine in a reductant-dependent reaction, and hydroxylamine oxidoreductase, which oxidizes hydroxylamine to nitrite with the release of four electrons. Two electrons are returned to AMO to provide reductant for subsequent oxidations. The other two electrons can fulfill the remaining reductant needs of the cell.
Nitrification in soils has received considerable attention, though historically it has been studied in the context of nitrogen cycling in terrestrial ecosystems (5, 6, 33). A number of physical and chemical factors affecting nitrification rates in soils have been identified as a result. Nitrification typically requires O2 and is inhibited by soil conditions which limit O2 availability (e.g., water-saturated soils) (1). Ammonia oxidation is most rapid in neutral to alkaline soils (11, 12). Nitrification rates are inhibited under acidic conditions where the NH4+-NH3 equilibrium is driven toward NH4+ and the availability of NH3 becomes limiting. The substrate for AMO is NH3 and not NH4+ (35). There have been a number of studies investigating the effects of phenolics, terpenes, and other organic molecules derived from plants on nitrification activity in soils (4, 7, 25, 26, 32). In general, these studies were concerned with the process of nitrification in soils and not with specific nitrifying bacteria.
Through the action of AMO, N. europaea can oxidize a number of aliphatic and aromatic compounds in addition to NH3. Examples of cooxidants include alkanes (17, 20), alkenes (17, 21), aromatics (24), ethers (18), and thioethers (22). The oxidation of these alternative substrates does not provide any known energy benefit to the cell and is dependent on NH3 oxidation for a sustained supply of reductant. The substrate range of AMO also extends to several halogenated hydrocarbons such as trichloroethylene, methyl bromide, vinyl chloride and dibromochloropropane (2, 9, 19, 28–30). For many of these substrates, oxidation is followed by dehalogenation which decreases the recalcitrance of these compounds. Many of these halogenated compounds are soil contaminants resulting from industrial, agricultural, or military activities, and it is possible that nitrifiers, among other bacteria, may play some role in natural bioremediation processes.
In previous experiments, the degradation of cosubstrates by N. europaea was extensively studied in liquid culture, but the degree to which these results can be extrapolated to soil environments has not been addressed. Because some of the potential applications of biodegradation by ammonia-oxidizing bacteria would take place in soils, it is desirable to relate the oxidative activities seen in liquid cultures to situations where these reactions occur in the presence of soil. Toward this end, three representative cosubstrates were chosen for experiments designed to analyze the effect of soil on their oxidation. The three cosubstrates, ethylene, chloroethane, and 1,1,1-trichloroethane (TC), are well-characterized inhibitors of AMO activity in N. europaea (21, 23, 28, 30). TC is oxidized by AMO but yields products that inactivate ammonia oxidation, while ethylene and chloroethane are oxidized by AMO with little or no toxic effect on the cell in short time course experiments (23, 28).
MATERIALS AND METHODS
Growth and preparation of cells.
N. europaea ATCC 19718 cells were grown in shaken (150 rpm) batch cultures (1.5 liters) at 30°C in an unlit room in previously described media (10). The cells were harvested by centrifugation after 3 days of growth and suspended in buffer (50 mM NaH2PO4 and 2 mM MgCl2 [pH 7.8] at 1.0 ml of buffer/liter of culture). Cell suspensions were prepared daily, stored on ice, and used within 8 h. Storage on ice for periods up to 24 h caused little or no loss of activity.
Collection and preparation of soil.
Soil was collected from the North Willamette Research and Extension Center, Aurora, Oreg. The soil is classified as a Willamette silt loam (fine-silty, mixed, mesic, Pachic Ultic Haploxeroll) in the soil survey of Clackamas County, Oreg. Soil was sampled from depths of 0 to 20 cm. Particle size analysis showed the soil to be composed of 13.4, 53.5, and 33.1% (wt/wt) clay, silt, and sand size particles, respectively. Whole-soil samples were ground with a mortar and pestle to pass through a 0.25-mm-pore-size sieve, and total soil organic carbon was determined by direct combustion to CO2 in a DC-80 carbon analyzer (Dohrmann Inc., Santa Clara, Calif.) equipped with an infrared detector. The cation-exchange capacity (CEC) of the soil was determined by the ammonium acetate displacement method used by the soil testing laboratory of the Department of Crop and Soil Sciences at Oregon State University. The Willamette soil was found to contain about 28 μg of ammonium-N per gram of soil, as determined by the ammonia diffusion method described below (Table 1). The pHs of the soil slurries were determined by measuring the pHs of the supernatants after the soil was removed by centrifugation. Because the initial pH of the soil (2:1, water:soil [vol/wt]) ranged between 6.0 and 6.2, the soil was neutralized (pH 7.2) by the addition of 3 g of Ca(OH)2/kg of soil. Furthermore, the pHs of reaction mixtures containing 0.5 g of neutralized Willamette soil in 1 ml of buffer (50 mM NaH2PO4, 2 mM MgCl2 [pH 7.8]) were adjusted to 7.8 by the addition of NaOH and monitored for 30 min to ensure that the pH was stable prior to the addition of cells.
TABLE 1.
Ammonium binding to Willamette soila
| Amt of NH4+ (μmol):
|
% NH4+ in supernatant | Amt of NH4+ (μmol):
|
||
|---|---|---|---|---|
| Added to soil | Recovered in supernatant | Recovered in sediment | Recovered total | |
| 0 | 0.6 | 60 | 0.4 | 1.0 |
| 5 | 1.8 | 38 | 3.0 | 4.8 |
| 10 | 4.5 | 45 | 5.5 | 9.9 |
| 20 | 10.4 | 49 | 10.7 | 21.1 |
Soil (0.5 g) and ammonium sulfate solution in phosphate buffer (1 ml) were mixed for 90 min and then centrifuged. Ammonium was recovered from the supernatant and the sediment by the NH4+ diffusion assay following resuspension in a solution of K2CO3 and quantified by Nessler’s assay (8).
Substrate oxidations.
Reactions were carried out in 6.5-ml serum vials stoppered with gray butyl rubber stoppers (Baxter Scientific, McGraw Park, Ill.) or Teflon-lined gray butyl rubber stoppers (Alltech Associates Inc., Deerfield, Ill.) and capped with aluminum seals (VWR Scientific, Seattle, Wash.). The reaction mixtures were made up to a volume of 1 ml. Each reaction was buffered with 50 mM NaH2PO4–2 mM MgCl2 (pH 7.8). Reactions were run in the presence of 10 mM NH4+ unless indicated otherwise. All reactions were initiated by the addition of 20 μl of cell suspension (ca. 400 μg of protein). Each reaction was incubated in a 30°C water bath while being shaken at 150 cycles/min for 30 min unless indicated otherwise. Samples were removed with gastight syringes at the indicated times and analyzed as described below. In the cooxidation experiments, after the addition of ethylene, chloroethane, or TC to the sealed vials containing the reaction components, the cosubstrate was allowed to equilibrate for 30 min at 30°C while being shaken before the reaction was started by the addition of cells.
Analytical procedures.
The protein contents of the cell suspensions were estimated by the biuret assay (14) after the cells were solubilized in 3 N NaOH for 30 min at 65°C. Bovine serum albumin was used as a standard. An ammonia diffusion assay (8) was used to determine the ammonia (NH3 plus NH4+) concentration. In this assay, the solution to be tested is made basic with K2CO3, converting any NH4+ to NH3. The stoppered vial contains a wick in the headspace which is saturated with H2SO4. Ammonia diffuses into the headspace and is trapped on the wick. The wick is then removed and dipped into a solution containing Nessler’s reagent (8), and the resulting color development is measured spectrophotometrically at 490 nm. For nitrite analyses, liquid samples (5 μl; test performed in duplicate) were taken at specified time intervals and analyzed as described previously (15). For samples containing soil, the soil was removed by centrifugation in a microcentrifuge at 16,000 × g for 5 min prior to taking samples for nitrite analyses. Gas samples were analyzed by a gas chromatograph (model GC-8A; Shimadzu, Kyoto, Japan) equipped with a stainless-steel column packed with Porapak Q 80-100 mesh (Waters Associates Inc., Framingham, Mass.) and a flame ionization detector interfaced to an integrator (model C-R3A; Shimadzu). To determine the amount of ethylene oxide produced from ethylene, gas samples (500 μl) were injected into a Porapak Q 80-100 mesh stainless-steel column (1/8 by 48 in [0.3 by 121.9 cm]) at 130°C. To determine the amount of acetaldehyde formed from the oxidation of chloroethane, gas samples (1 ml) were injected into a Porapak Q 80-100 mesh column (1/8 by 48 in [0.3 by 121.9 cm]) at 90°C. To determine the amount of 2,2,2-trichloroethanol produced from TC, liquid samples (10 μl) were injected into a Porapak Q 80-100 mesh column (1/8 by 36 in [0.3 by 91.4 cm]) at 175°C. Within a given type of experiment, replicate analyses indicated standard errors of about 5% for the nitrite assays, about 6% for the ammonium diffusion assays, and about 8% for the gas chromatography experiments. Between experiment types, the variability was greater, as indicated below.
RESULTS
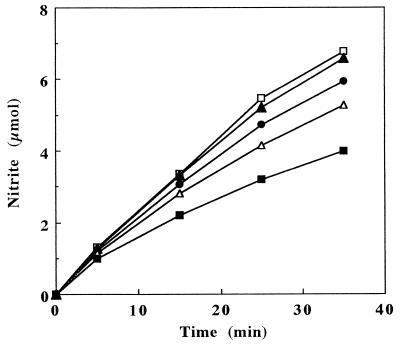
Ammonia oxidation by cell suspensions of N. europaea was affected by the presence of unsterilized soil. The amount of NO2− produced by cells was measured over time in the presence of varying amounts of Willamette silt loam soil ranging from 0 to 1.0 g per ml of reaction mixture. The addition of soil to cultures had a pronounced inhibitory effect on the oxidation of NH3 (Fig. 1). The degree of inhibition increased with increasing amounts of soil present. The addition of soil potentially could influence NO2− production in a number of ways, e.g., by altering the pH, by adsorbing ammonium (NH4+), or through endogenous ammonium- and nitrite-oxidizing activities. Therefore, experiments were done to identify and characterize the nature of the inhibition of NH3 oxidation by soil.
FIG. 1.
Time course of nitrite production by N. europaea in the presence of various amounts of soil added to 1 ml of reaction mixture. No soil, □; 0.1 g of soil, ▴; 0.25 g of soil, •; 0.5 g of soil, ▵; 1.0 g of soil, ▪.
When nonsterilized soil not augmented with N. europaea was tested for endogenous NH3-oxidizing activity, NO2− production was not detected after a 60-min incubation (data not shown). In addition, NH4+ added to soil samples was almost completely recoverable after a 90-min incubation (Table 1), suggesting that there was little if any endogenous NH4+ oxidation or assimilation activity in the soil under the conditions tested. Likewise, soil samples did not consume added NO2−, indicating that endogenous NO2−-oxidizing activity was also not significant in these experiments (data not shown). In the short-term assays used in this study, we obtained no evidence for any biological activity of the air-dried soil that compromised the measurements that we made. Therefore, nonsterilized soil was used in subsequent experiments, and NO2− production was used as a measure of NH3 oxidation. In a separate experiment, the addition of washed sand instead of Willamette soil was tested for its effect on nitrification. The sand did not bind NH4+ or alter the reaction pH, and no decrease in NO2− production was observed (data not shown).
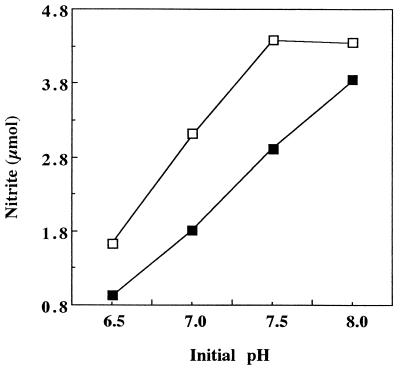
Because NH3 oxidation by N. europaea is sensitive to pH, the addition of soil could affect activity simply by lowering the pH of the reaction medium. Indeed, the addition of Willamette soil (pH 6.1 in a 1:2 [wt/vol] soil-deionized-water mixture) resulted in a soil-buffer suspension with a pH of 6.8 before ammonia oxidation was initiated. Furthermore, when soil neutralized with Ca(OH)2 (to pH 7.2) was used, the initial soil-buffer pH was 7.2. Nitrite production was determined in reactions with different starting pHs both in the presence and absence of 0.5 g of soil (Fig. 2). In the presence of soil, it can be seen that substantially less NO2− was produced as the starting pH was lowered from 8.0. The addition of soil reduced NO2− production by as little as 12% at pH 8.0 and by greater percentages (33 to 40%) at pH values lower than 8.0. Thus, changes in the initial pH of the reaction mixture (caused by the addition of unbuffered soil) had a significant effect on NO2− production. For this reason, in subsequent experiments the pHs of the reaction mixtures containing soil were adjusted to 7.8 prior to the start of the reaction.
FIG. 2.
Nitrite production versus starting pH. Samples either without soil (□) or with 0.5 g of soil (▪) were incubated for 30 min at 30°C in the presence of 10 mM NH4+.
The soil might also be expected to affect the buffering capacity of the reaction mixture. To determine if this was a significant factor, an experiment was done in which 1 ml of buffer, with and without the addition of 0.5 g of soil, was adjusted to pH 7.8. Cells were added, and the mixture was incubated at 30°C for 30 min. In the absence of soil, 5.5 mM NO2− was produced and the final pH was 7.21, while in the presence of soil, 4.6 mM NO2− was produced and the final pH was 7.33. Therefore, when the pHs of the assay buffers of the soil and nonsoil samples were both adjusted to an initial value of 7.8, the effects of any changes in buffering capacity on NO2− production were small.
Most soils have a capacity to bind cations from aqueous solutions. Therefore, we considered the possibility that part of the observed decrease in NO2− formation was a result of NH4+ bound to the soil surfaces and thereby lowering the free-NH4+ concentration governing the rate of ammonia oxidation. The amount of NH4+ which bound to 0.5 g of soil was determined for amounts ranging from 0 to 20 μmol of NH4+/ml. After a 1.5-h incubation in buffer containing NH4+, the soil was sedimented by centrifugation and the NH4+ concentrations in the supernatant and sediment were measured (Table 1). Our data clearly indicated that the concentration of ammonium in the solution phase was lower than anticipated if soil was not present. The sedimented soil retained a large proportion of the NH4+, ranging from 40 to 62% of the initial amount. For 10 μmol of NH4+, 55% of the added NH4+ was bound to the soil.
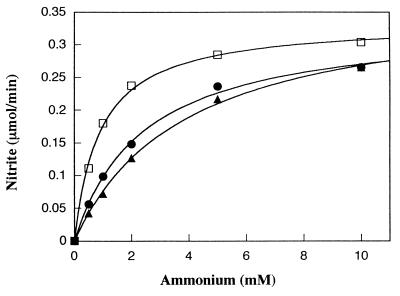
Given that the Willamette soil bound a large portion of the added NH4+, we wished to determine if increasing the NH4+ concentration in the reaction mixture could restore activity in the presence of soil. The rate of NO2− production as a function of the NH4+ concentration in the presence of various amounts of soil (Fig. 3) was measured. As before, for a given NH4+ concentration, increasing the amount of soil in the reaction decreased the rate of NO2− formation. However, the inhibition of activity by a given amount of soil could be overcome by the addition of more NH4+. More soil required more NH4+ to reach maximal activity. Plots of the data with the best least-squares fit to the equation v = [Vmax × S/(Ks + S)] (Fig. 3) showed that the net effect of the addition of soil was to raise the apparent Ks values for ammonium from 0.83 mM (in the absence of soil) to 1.9 and 3.6 mM in the presence of 0.25 and 0.5 g of soil/ml of reaction mixture, respectively. Given an apparent Ks of 3.6 mM, the lowering of the NH4+ concentration in solution from 10 to 4.5 mM upon the addition of 0.5 g of soil should result in a 21% decrease in activity ([1 − (0.73 Vmax/0.92 Vmax)] × 100). This predicted decrease in activity is compared to the 22% decrease for 0.5 g of soil at 30 min seen in Fig. 1 and the 14% decrease seen in Fig. 3 with 0.5 g of soil at 10 mM NH4+.
FIG. 3.
Nitrite production per minute versus NH4+ concentration in the presence of varying amounts of soil in assays ranging in length from 2 to 15 min. Amounts added were 0.0 g of soil (□), 0.25 g of soil (•), and 0.5 g of soil (▴). Lines were plotted with the best least-squares fits to the equation v = [Vmax × S/(KS + S)].
The availability of soil-bound ammonium for nitrifying bacteria can vary and is strongly influenced by the availability of competing cations such as potassium (27, 36). In our system, it should be noted that we routinely used a sodium phosphate buffer. However, the use of KH2PO4 buffer and KOH for pH adjustments instead of NaH2PO4 and NaOH made no difference with regard to the observed pH effects or the amount of nitrite produced in 30 min at the same pH.
Having characterized the effect of Willamette soil on nitrite production by cultures of N. europaea, we next wished to determine the effect of soil on the oxidation of the cosubstrates ethylene, chloroethane, and TC. We first considered the possibility that these compounds might bind to the soil, thereby changing the effective concentrations of the compounds, as we had observed with ammonium. However, there was no detectable change in the headspace concentrations of these compounds over 2 h after the addition of soil to the reaction mixture, which indicates that there was no substantial binding of these three compounds to the soil nor endogenous biological oxidation activity under the conditions tested. Our results do not rule out the possibility that these compounds may bind to the soil over longer time periods and especially under conditions where the soil structure remains intact. Ammonia is a required component in the reaction mixtures because the oxidation of ammonia provides the reductant for AMO-catalyzed transformation of these alternative substrates. The experiments were done in the presence of 10 mM NH4+.
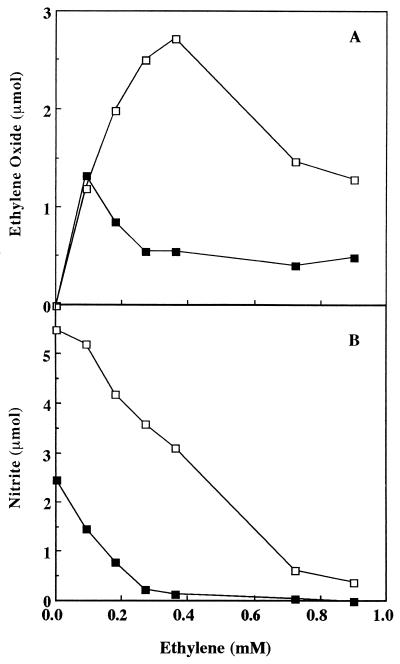
Ethylene is a competitive inhibitor of ammonia oxidation (with a Ki of about 660 μM) by AMO (23). Both the production of NO2− and the formation of ethylene oxide from ethylene were measured as functions of increasing ethylene concentration in the presence or absence of soil. As expected of a competitive inhibitor, NO2− production decreased as the cosubstrate concentration increased (Fig. 4B). The addition of soil increased the inhibition of NO2− production by ethylene. The point of 50% inhibition of NO2− formation was at about 0.41 mM ethylene in the absence of soil and about 0.12 mM ethylene in the presence of 0.5 g of soil. The addition of soil had two effects on the oxidation of ethylene. First, the maximal amount of ethylene oxide produced was about 50% less in the presence of soil (Fig. 4A). Second, the maximum activity we observed was shifted downward from a 0.36 mM concentration of added ethylene to about 0.09 mM. In the presence of soil, at the highest ethylene concentrations, about 0.5 μmol of ethylene oxide was produced, even though little NO2− was produced. Apparently, the oxidation of endogenous storage compounds provided the reductant for the AMO-catalyzed transformation of ethylene to ethylene oxide.
FIG. 4.
Ethylene oxide (A) and NO2− production (B) versus ethylene concentration in the presence (▪) or absence (□) of 0.5 g of soil with 10 mM NH4+. Samples were withdrawn from the headspace (for ethylene oxide determinations) or liquid (for nitrite determinations) after a 30-min incubation.
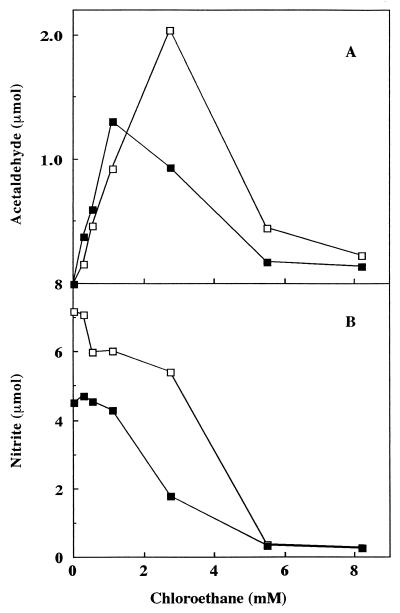
The second cosubstrate examined was chloroethane, which is oxidized to acetaldehyde and Cl− by AMO (28). Chloroethane is a noncompetitive inhibitor of NH3 oxidation (KiE and KiES of 1.4 mM) and does not inactivate AMO (23, 30). Nitrite production (from ammonia) and acetaldehyde production (from chloroethane) were monitored at various concentrations of chloroethane. Nitrite production steadily declined with increasing chloroethane concentration (Fig. 5B). The addition of soil lowered the 50% inhibition point in nitrite formation from about 3.8 mM chloroethane to about 2.4 mM. As expected, the amount of acetaldehyde produced first increased with increasing chloroethane concentration and then decreased as reductant became limiting (due to the inhibition of ammonia oxidation) (Fig. 5A). The addition of soil reduced the maximal activity by about 37% and shifted the peak of maximal activity from about 2.8 mM chloroethane to about 1.1 mM. A residual acetaldehyde production of about 0.3 μmol was observed at the higher chloroethane concentrations.
FIG. 5.
Acetaldehyde (A) and NO2− production (B) versus chloroethane concentration in the presence (▪) or absence (□) of 0.5 g of soil with 10 mM NH4+. Samples were withdrawn from the headspace (for acetaldehyde determinations) or liquid (for nitrite determinations) after a 30-min incubation.
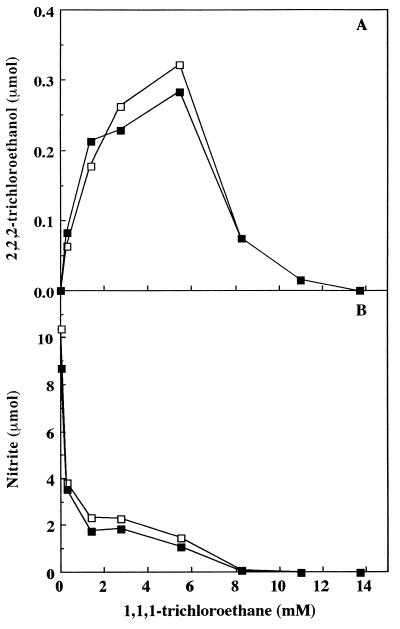
The third cosubstrate tested, TC, is a potent inactivator of NH3 oxidation (30). TC is oxidized to 2,2,2-trichloroethanol. Even low concentrations of TC (0.2 mM) drastically lowered the amount of NO2− produced (Fig. 6B). The inhibition of nitrite production in the presence of soil was only slightly greater than that observed in its absence and in the presence of TC. As with chloroethane and ethylene, the profile for 2,2,2-trichloroethanol production first increased with increasing TC concentrations and then decreased (Fig. 6A). Unlike with ethylene and chloroethane, however, the addition of soil appeared to have only a small effect on the oxidation of TC. The maximum for 2,2,2-trichloroethanol production was not shifted to a lower value in the presence of soil. The maximal amount of 2,2,2-trichloroethanol produced (0.33 μmol) was also much less than that observed for ethylene oxide (2.7 μmol) or acetaldehyde (2.1 μmol). Another distinction between the results with this compound and those with the other two cosubstrates was that no residual activity was observed at high concentrations of TC. Apparently this is a result of the ability of TC to inhibit AMO.
FIG. 6.
2,2,2-trichloroethanol (A) and NO2− production (B) versus TC concentration in the presence (▪) or absence (□) of 0.5 g of soil with 10 mM NH4+. Liquid samples were withdrawn for 2,2,2-trichloroethanol and nitrite determinations after a 30-min incubation.
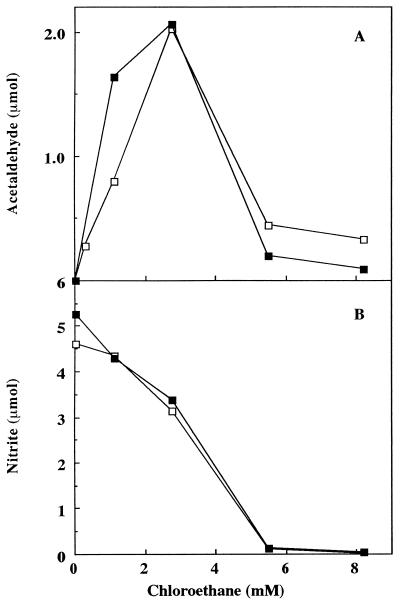
Higher concentrations of NH4+ added to the reaction mixture could compensate for the inhibitory effects of soil on NO2− production in the absence of a cosubstrate (Fig. 3). To determine if increased NH4+ concentrations could also compensate for the observed differences in cosubstrate oxidations, the experiment with chloroethane (Fig. 5) was repeated in the presence of 50 mM NH4+. Both the maximum activity and the chloroethane concentration at which this maximum occurred were now the same, regardless of the presence or absence of soil (Fig. 7). In fact, the curves were similar at all concentrations of chloroethane. The amounts of nitrite produced during the incubation were also similar.
FIG. 7.
Acetaldehyde (A) and NO2− production (B) versus chloroethane concentration in the presence (▪) or absence (□) of 0.5 g of soil with 50 mM NH4+. Samples were withdrawn from the headspace (for acetaldehyde determinations) or liquid (for nitrite determinations) after a 30-min incubation.
DISCUSSION
Our primary interest in these experiments was to extend the present knowledge of NH3 and cosubstrate oxidation by N. europaea in liquid cultures to situations where these reactions take place in the presence of soil. The results showed that while the oxidation of NH3 and cosubstrates by N. europaea occurred in the presence of soil, the soil does significantly modify the kinetics of these reactions. We have considered two primary mechanisms by which soil could influence the rate of NH3 oxidation: by changing the pH of the reaction mixture, which consequently influences the NH3-NH4+ equilibrium, and by binding NH4+ and thereby lowering the equilibrium concentration available for the cells. It is well recognized that ammonium binds to a majority of soils because of their CECs (27). Indeed, the CECs of many soils are routinely measured by determining the amount of ammonium that binds to the soil after suspension in an ammonium salt solution (31). Young (37) showed that both organic matter and clay content were influential in the binding of ammonium to Oregon soils. Despite the fact that the Willamette silt loam possesses a relatively low CEC (15 cmol/kg) and organic carbon content (1.8%), this soil lowered the amount of ammonium present in solution significantly, lowered the pH of the reaction mixture, and subsequently influenced the rate of ammonia oxidation. When the pH of the reaction mixture was lowered by the addition of soil with exchangeable acidity, the inhibition could be substantial (Fig. 2). At pH values of 7.5 or lower, the more exaggerated effect of soil could be attributed to a shift in the NH3-NH4+ equilibrium (pKa = 9.2) towards NH4+ and away from NH3, the substrate for AMO. Reduced AMO activity was observed when the NH3 concentration dropped into the range of the Ks value for NH3. For example, at pH 8.0, 4.5 mM NH4+ is in equilibrium with 250 μM NH3, while within the pH range 6.5 to 7.5, the NH3 concentration associated with 4.5 mM NH4+ ranges between 7.9 and 79 μM. These values are similar to the Ks of AMO for NH3 (∼29 μM) calculated from the apparent Ks for NH4+ (0.83 mM) at pH 7.8 (Fig. 3).
However, even when the pH of the reaction mixture was carefully controlled, upon addition of soil, inhibition of nitrite production was still observed (Fig. 1). In most of our experiments, the addition of 0.5 g of soil decreased the nitrite production 14 to 55%, with a median decrease near 27%. Considering the kinetic saturation profile and an apparent Ks of 0.83 mM NH4+, the decrease from 10 to 4.5 mM NH4+ which occurred upon addition of 0.5 g of soil should result in about a 9% decrease in nitrite production ([1 − (0.84 Vmax/0.92 Vmax)] × 100). However, the increase in apparent Ks to 3.6 mM which occurred upon addition of 0.5 g of soil predicts a 21% decrease in activity ([1 − (0.73 Vmax/0.92 Vmax)] × 100). Therefore, while a significant portion of the soil effect on nitrite production can be attributed to the loss of free NH4+ due to binding to soil, there appear to be other factors associated with the addition of soil which also contribute to the reduction of activity. For example, the presence of competitive inhibitors of nitrification in the Willamette soil would be consistent with the data presented in this study. In light of the literature concerning the presence of naturally occurring inhibitory compounds in soils, it might not be surprising if Willamette soil contained organic substances inhibitory to NO2− production. Although the earlier studies concerning phenolics and terpenoids in soils were somewhat contradictory concerning the effects of these substances on nitrification, recent studies with pure cultures showed that AMO in N. europaea is inhibited by aromatic compounds (24). Regardless of the source of the inhibition of nitrite production, the inhibition was almost completely compensated for by increasing the NH4+ concentration (Fig. 3).
As was the case with NH3 oxidation, the addition of soil had a significant impact on the oxidation of the cosubstrates ethylene and chloroethane. The effect of adding soil to these reactions is complex, since the NH4+ concentration in the reaction is altered, which in turn affects the competitive inhibition of AMO by the cosubstrate. The effect of soil on the substrate inhibition curves for the oxidations of ethylene and chloroethane were similar in that the peak of maximal activity shifted toward lower cosubstrate concentrations and the amplitude of the maximal activity was significantly lowered in the presence of soil. As with NO2− production, part of the decrease in substrate oxidation activity would be expected from the adsorption of NH4+ to the soil particles. Less free NH4+ would result in less reductant available for the oxidation of the cosubstrate, lowering the rate of product formation. Indeed, when the NH4+ concentration was increased to 50 mM, the results with soil were similar to those without soil. In the case of the competitive inhibitor ethylene, less NH4+ also means ethylene competes more effectively for the active site on AMO, resulting in even greater inhibition. This fact could explain why soil depressed ethylene oxidation to a greater extent than chloroethane. Rasche et al. (28) had previously shown that the concentration of chloroethane optimal for cooxidation dropped significantly when ammonium concentrations were lowered from 10 to 1 mM. The effect of soil on chloroethane oxidation was entirely consistent with a lowering of ammonium availability.
TC degradation was largely unaffected by the addition of soil. This result is understandable when one considers that TC is oxidized slowly relative to the other substrates considered in this study. TC drastically inhibited NH4+ oxidation, thereby lowering reductant levels, but sufficient reductant, either from endogenous respiration or NH3 oxidation, was available to drive TC oxidation. At 8 mM TC, NO2− was no longer produced and electrons were no longer available from NH3 oxidation. Yet TC oxidation continued at a reduced rate at this concentration, suggesting that endogenous sources of reductant could support some TC oxidation (Fig. 6). Similarly, ethylene and chloroethane oxidation also showed a basal rate at the higher substrate concentrations, which might also be accounted for by endogenous sources supplying some low level of reductant. Rates of NO2− production in the presence of TC were much lower than with ethylene and chloroethane, which is consistent with TC being a stronger inhibitor of AMO. The addition of soil resulted in a slight drop in NO2− production. However, the effect of soil on nitrite production might be expected to be less for TC than for ethylene and chloroethane, since the slow rate of TC turnover would require a lower reductant availability.
Sustaining the rate of cooxidation requires the achievement of a balance between the concentrations of the growth-supporting substrate and the cooxidant so that sufficient energy is generated for the maintenance of cell processes and turnover of the catalytic enzyme. Although several kinds of bacteria possess the ability to cooxidize chlorinated aliphatic hydrocarbons, their primary energy sources are volatile compounds (e.g., toluene, phenol, methane, and butane [3]) whose concentrations are difficult to control accurately under most environmental conditions. In this context, ammonium has some useful attributes, since it can be added and retained by soil in amounts that will be influenced by the magnitude of the CEC of the solid matrix. Furthermore, it is conceptually possible for the solution phase NH4+ concentration to be maintained from this solid phase adsorbed supply at a concentration sufficient to support growth and cooxidation.
Our results show that soil can have a profound impact on the oxidation of alternative substrates as well as on the oxidation of NH3 by N. europaea. With the Willamette silt loam used in this study, NH3 oxidation was influenced both by exchangeable acidity and by the adsorption of NH4+ to the soil. While our work focused on a single soil type, it does serve to highlight some of the soil factors that can influence both NH3 oxidation and cosubstrate oxidation. Although we used soil slurries in our experiments, the factors we identified are also likely to be important in structured soils at or below field moisture capacity. Furthermore, with other soils, or with intact soils with different moisture contents, other factors may be identified that are more or less important. For example, Stark and Firestone (34) described the importance of soil moisture to the availability of NH4+ for nitrification. Fuller and Scow (13) observed biocidal effects of chlorinated aliphatic hydrocarbons on cell growth of ammonia oxidizers in experiments that occurred over long time periods. The effects of soil would be particularly relevant to in situ bioremediation or bioaugmentation plans where rates of cosubstrate oxidation would be sensitive to the sequestering of NH4+ by the soil, with the result that rates of cosubstrate oxidation could be reductant limited.
ACKNOWLEDGMENT
This work was supported by the U.S. Environmental Protection Agency (grant R821405 to D.J.A. and P.J.B.).
Footnotes
Technical paper 11,230 of the Oregon Agricultural Experiment Station.
REFERENCES
- 1.Alexander M. Introduction to soil microbiology. J. New York, N.Y: Wiley & Sons; 1976. Nitrification; p. 467. [Google Scholar]
- 2.Arciero D, Vannelli T, Logan M, Hooper A B. Degradation of trichloroethylene by the ammonia-oxidizing bacterium Nitrosomonas europaea. Biochem Biophys Res Commun. 1989;159:640–643. doi: 10.1016/0006-291x(89)90042-9. [DOI] [PubMed] [Google Scholar]
- 3.Arp D J. Understanding the diversity of trichloroethene co-oxidations. Curr Biol. 1995;6:352–358. [Google Scholar]
- 4.Baldwin I T, Olson R K, Reiners W A. Protein binding phenolics and the inhibition of nitrification in subalpine balsam fir soils. Soil Biol Biochem. 1983;15:419–423. [Google Scholar]
- 5.Belser L W. Population ecology of nitrifying bacteria. Ann Rev Microbiol. 1979;33:309–333. doi: 10.1146/annurev.mi.33.100179.001521. [DOI] [PubMed] [Google Scholar]
- 6.Belser L W, Schmidt E L. Diversity in the ammonia-oxidizing nitrifier population of a soil. Appl Environ Microbiol. 1978;36:584–588. doi: 10.1128/aem.36.4.584-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bremner J M, McCarty G W. Effects of terpenoids on nitrification in soil. Soil Sci Soc Am J. 1988;52:1630–1633. [Google Scholar]
- 8.Burris R H. Nitrogen fixation—assay methods and techniques. Methods Enzymol. 1972;24B:415–431. doi: 10.1016/0076-6879(72)24088-5. [DOI] [PubMed] [Google Scholar]
- 9.Ely R L, Hyman M R, Arp D J, Guenther R B, Williamson K J. A cometabolic biodegradation model incorporating enzyme inhibition, inactivation, and recovery. II. Trichloroethylene degradation experiments. Biotechnol Bioeng. 1995;46:232–245. doi: 10.1002/bit.260460306. [DOI] [PubMed] [Google Scholar]
- 10.Ensign S A, Hyman M R, Arp D J. In vitro activation of ammonia monooxygenase from Nitrosomonas europaea by copper. J Bacteriol. 1993;175:1971–1980. doi: 10.1128/jb.175.7.1971-1980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Focht D D, Chang A C. Nitrification and denitrification processes related to waste water treatment. Adv Appl Microbiol. 1975;19:153–186. doi: 10.1016/s0065-2164(08)70428-3. [DOI] [PubMed] [Google Scholar]
- 12.Frijlink M J, Abee T, Laanbroek H J, deBoer W, Konings W N. The bioenergetics of ammonia and hydroxylamine oxidation in Nitrosomonas europaea at acid and alkaline pH. Arch Microbiol. 1992;157:194–199. [Google Scholar]
- 13.Fuller M E, Scow K M. Impact of trichloroethylene and toluene on nitrogen cycling in soil. Appl Environ Microbiol. 1997;63:4015–4019. doi: 10.1128/aem.63.10.4015-4019.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gornall A G, Bardawill C J, David M M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 15.Hageman R H, Hucklesby D P. Nitrate reductase in higher plants. Methods Enzymol. 1971;23:491–503. [Google Scholar]
- 16.Hooper A B. Ammonia oxidation and energy transduction in the nitrifying bacteria. In: Strohl W R, Tuovinen O H, editors. Microbial chemoautotrophy: Ohio State University 8th Bioscience Colloquium. Columbus, Ohio: Ohio State University Press; 1984. pp. 133–167. [Google Scholar]
- 17.Hyman M R, Murton I B, Arp D J. Interaction of ammonia monooxygenase from Nitrosomonas europaea with alkanes, alkenes, and alkynes. Appl Environ Microbiol. 1988;54:3187–3190. doi: 10.1128/aem.54.12.3187-3190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyman M R, Page C L, Arp D J. Oxidation of methyl fluoride and dimethyl ether by ammonia monooxygenase in Nitrosomonas europaea. Appl Environ Microbiol. 1994;60:3033–3035. doi: 10.1128/aem.60.8.3033-3035.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyman M R, Russell S A, Ely R L, Williamson K J, Arp D J. Inhibition, inactivation, and recovery of ammonia-oxidizing activity in cometabolism of trichloroethylene by Nitrosomonas europaea. Appl Environ Microbiol. 1995;61:1480–1487. doi: 10.1128/aem.61.4.1480-1487.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyman M R, Wood P M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983;212:31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyman M R, Wood P M. Ethylene oxidation by Nitrosomonas europaea. Arch Microbiol. 1984;137:155–158. [Google Scholar]
- 22.Juliette L Y, Hyman M R, Arp D J. Inhibition of ammonia oxidation in Nitrosomonas europaea by sulfur compounds—thioethers are oxidized to sulfoxides by ammonia monooxygenase. Appl Environ Microbiol. 1993;59:3718–3727. doi: 10.1128/aem.59.11.3718-3727.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keener W K, Arp D J. Kinetic studies of ammonia monooxygenase inhibition in Nitrosomonas europaea by hydrocarbons and halogenated hydrocarbons in an optimized whole-cell assay. Appl Environ Microbiol. 1993;59:2501–2510. doi: 10.1128/aem.59.8.2501-2510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keener W K, Arp D J. Transformations of aromatic compounds by Nitrosomonas europaea. Appl Environ Microbiol. 1994;60:1914–1920. doi: 10.1128/aem.60.6.1914-1920.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarty G W, Bremner J M. Effects of phenolic compounds on nitrification in soil. Soil Sci Soc Am J. 1986;50:920–923. [Google Scholar]
- 26.Moore D R E, Waid J S. The influence of living roots on nitrification. Soil Biol Biochem. 1971;3:69–83. [Google Scholar]
- 27.Nommik H, Vahtras K. Retention and fixation of ammonium and ammonia in soils. In: Stevenson F J, editor. Nitrogen in agricultural soil. Madison, Wis: American Society of Agronomy; 1982. pp. 123–171. [Google Scholar]
- 28.Rasche M E, Hicks R E, Hyman M R, Arp D J. Oxidation of monohalogenated ethanes and n-chlorinated alkanes by whole cells of Nitrosomonas europaea. J Bacteriol. 1990;172:5368–5373. doi: 10.1128/jb.172.9.5368-5373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasche M E, Hyman M R, Arp D J. Biodegradation of halogenated hydrocarbon fumigants by nitrifying bacteria. Appl Environ Microbiol. 1990;56:2568–2571. doi: 10.1128/aem.56.8.2568-2571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasche M E, Hyman M R, Arp D J. Factors limiting aliphatic chlorocarbon degradation by Nitrosomonas europaea—cometabolic inactivation of ammonia monooxygenase and substrate specificity. Appl Environ Microbiol. 1991;57:2986–2994. doi: 10.1128/aem.57.10.2986-2994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhoades J D. Cation exchange capacity. In: Page A L, editor. Methods of soil analysis. Madison, Wis: American Society of Agronomy; 1982. pp. 149–159. [Google Scholar]
- 32.Rice E L, Pancholy S K. Inhibition of nitrification by climax ecosystems. II. Additional evidence and possible role of tannins. Am J Bot. 1973;60:691–698. [Google Scholar]
- 33.Schmidt E L. Nitrification in soil. In: Stevenson F J, editor. Nitrogen in agricultural soils. Madison, Wis: American Society of Agronomy; 1982. pp. 253–288. [Google Scholar]
- 34.Stark J M, Firestone M K. Mechanisms for soil moisture effects on activity of nitrifying bacteria. Appl Environ Microbiol. 1995;61:218–221. doi: 10.1128/aem.61.1.218-221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki I, Dular U, Kwok S C. Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J Bacteriol. 1974;120:556–558. doi: 10.1128/jb.120.1.556-558.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welch L F, Scott A D. Nitrification of fixed ammonium in clay minerals as affected by added potassium. Soil Sci. 1960;90:79–85. [Google Scholar]
- 37.Young J L. Ammonia and ammonium reactions with some Pacific Northwest soils. Soil Sci Soc Am Proc. 1964;28:339–345. [Google Scholar]