Summary
Background
Malaria transmission-blocking vaccines target mosquito-stage parasites and will support elimination programs. Gamete vaccine Pfs230D1-EPA/Alhydrogel® (“Pfs230D1”) induced superior activity to zygote vaccine Pfs25-EPA/Alhydrogel® (“Pfs25”) in malaria-naïve US adults. Here we compare these vaccines in malaria-experienced Malians.
Methods
We conducted a pilot dose-escalation then double-blind, block-randomized, comparator-controlled main-phase trial (ClinicalTrials.gov, NCT02334462) in malaria-intense Bancoumana, Mali. 18–50-year-old healthy nonpregnant/nonbreastfeeding consenting adult residents were allocated 1:1:1:1 to receive four doses at months 0, 1, 4·5, 16·5 of either 47μg Pfs25, 40μg Pfs230D1 or comparator (TWINRIX®, Menactra)—all co-administered with normal saline for blinding—or 47μg Pfs25+40μg Pfs230D1 co-administered. We documented safety/tolerability (primary; as-treated populations) and immunogenicity (secondary; as-treated populations: ELISA, standard-membrane-feeding assay, and mosquito direct-skin-feed assay).
Findings
Vaccinations were well-tolerated in dose-escalation and main phases. Most vaccinees became seropositive after two Pfs230D1 or three Pfs25 doses; peak titres increased with each dose thereafter [Pfs230D1 GM(95%CI): 77·8(56·9-106·3), 146·4(108·3-198·0), 410·2(301·6-558·0); Pfs25: 177·7(130·3-242·4), 315·7(209·9-474·6)]. Functional activity [mean peak transmission-reducing activity(95%CI)] appeared for Pfs230D1 and Pfs25+Pfs230D1 post-dose 3 [74·5%(66·6-82·5) and 68·6%(57·3-79·8), respectively] and post-dose 4 [88·9%(81·7-96·2) and 85·0%(78·4-91·5), respectively] but not for Pfs25 [58·2%(49·1-67·3) and 58·2(48·5-67·9) post-dose 3 and 4, respectively]. Pfs230D1 transmission-reducing activity [73·7%(64·1-83·3)] persisted 10 weeks post-dose 4. Transmission-reducing activity of 80% was estimated at Pfs25 ELISA Units=1659 and Pfs230D1 ELISA Units=218 (Pfs230D1 alone) or 223 (Pfs230D1 + Pfs25 arm).
After 3850 direct-skin-feed assays, 35 participants [12 Pfs25; 8 Pfs230D1; 5 Pfs25+Pfs230D1; 10 comparator] had transmitted parasites at least once. Proportion positive assays in vaccine arms [Pfs25, 33/982 (3·4%); Pfs230D1, 22/954 (2·3%); combination, 11/940 (1·2%)] did not differ (95%CI = −0·013-0·014; −0·005-0·027; −0·024-0·002, respectively) from comparator [22/974 (2·3%)], nor did Pfs230D1 and combination arms differ (95%CI = −0·024-0·001).
Interpretation
Pfs230D1 but not Pfs25 vaccine induces durable serum functional activity in Malian adults. Direct-skin-feed assays detect parasite transmission to mosquitoes but increased event rates are needed to assess vaccine effectiveness.
Introduction
Malaria is a global scourge with a marked increase in cases and deaths since 2019, mostly due to Plasmodium falciparum (Pf).2 Existing tools are insufficient to achieve global eradication or even elimination in many countries where malaria remains entrenched. New interventions are urgently needed.
In 2021, the World Health Organization recommended3 wider deployment of the first approved anti-parasite vaccine RTS,S/AS01 (Mosquirix®, GSK Vaccines) which reduces clinical malaria and malaria deaths in under-fives. Candidates R21/Matrix M™4 and PfSPZ Vaccine5 have shown efficacy in field trials, and like RTS,S/AS01, target pre-erythrocytic parasites (sporozoite and liver stages).
Malaria transmission-blocking vaccines (TBV) target gametes or zygotes in the mosquito to block transmission6,7 and can be used for elimination. TBV have no activity against pre-erythrocytic or blood-stage parasites.6,7 Leading TBV candidate antigens include gamete surface proteins Pfs230 and Pfs48/45 and zygote/ookinete surface protein Pfs25.8,9 We previously reported that Pichia-expressed Pfs25 conjugated to carrier-protein ExoProtein A (EPA) and formulated in Alhydrogel® (Pfs25H-EPA/Alhydrogel®) was well-tolerated and induced functional antisera in US10 and Malian volunteers1 that reduced Pf transmission to mosquitoes in a laboratory assay. However, Pfs25 titres decayed rapidly and functional activity measured 2 weeks post-dose 41,10 was lost by 8 weeks.1 We subsequently reported Pichia-expressed Pfs230 domain 1 (Pfs230D1) vaccine (Pfs230D1-EPA/Alhydrogel®) induced serum functional activity superior to Pfs25-EPA/Alhydrogel® after two vaccine doses in malaria-naïve US volunteers.11
Vaccine responses can be impaired in malaria-exposed populations.1 Here, we compared Pfs230D1-EPA/Alhydrogel® and Pfs25-EPA/Alhydrogel®, alone or in combination, in a malaria-experienced target population in Mali.
Methods
Study design
The study was conducted Mar 2015 to Sep 2017 at John LaMontagne Malaria Research Center in Bancoumana, Mali, ~60 km southwest of Bamako with highly seasonal (Jun-Dec) hyperendemic malaria transmission. Cohort enrollment was staggered for safety; Pfs25+Pfs230D1 combination subjects enrolled after single-antigen (Pfs25 or Pfs230D1) vaccine immunizations were reviewed for safety [Appendix, pages 6-7; Figure S1, Appendix, page 40] Main cohort participants followed 0-1-4·5-16·5-month dosing schedules (rather than planned 0-1-6-18-month) to complete vaccination before malaria transmission seasons; follow up ended ~6 months post-dose 4.
Healthy 18-50-year-old study village residents (men or non-pregnant, non-breastfeeding women; residency established in village census) were eligible to enroll after informed consent, if available for trial duration and (for main cohort) willing to undergo direct-skin-feed (DSF) mosquito assays. Women-of-child-bearing-potential used reliable contraception during vaccinations. Individuals were excluded for abnormal laboratories (including HIV, hepatitis B/C tests), previous malaria vaccine, or recent vaccines, blood products, or immunosuppressive drugs Full inclusion/exclusion criteria are in Appendix, pages 9-11.
The trial adhered to Good Clinical Practice and institutional procedures and guidelines. Participating villages provided community permission; participants provided informed consent.12 The study was approved in Mali (Faculte de Medecine de Pharmacie et d’OdontoStomatologie ethics committee and Mali national regulatory authority) and U.S.A. (NIAID institutional review board), registered at ClinicalTrials.gov (NCT02334462), and conducted under FDA IND#16251. Safety was monitored by an independent Data and Safety Monitoring Board (DSMB) and local medical monitor.
Randomization and masking
Subjects were block-randomized at enrollment to receive: 1) Pfs25-EPA/Alhydrogel® [pilot 16μg Pfs25; main 47μg Pfs25+saline]; 2) Pfs230D1-EPA/Alhydrogel® [pilot 15μg Pfs230D1; main 40μg Pfs230D1+saline]; 3) combination [pilot 16μg Pfs25+15μg Pfs230D1; main 47μg Pfs25+40μg Pfs230D1]; or 4) comparator (TWINRIX for vaccinations #1, 2, 3; Menactra for #4) +/−saline as needed for blinding. The study investigators, vaccinators, laboratory personnel, and participants were blinded to treatment group assignment. Unblinded site pharmacist received randomization codes via secure email. Product syringes were masked by opaque tape and labeled only with study identification number. Group assignments were unmasked at final study visit: ~6 months post-dose 2 in pilot-safety cohort; ~6 months post-dose 4 in main cohort. After unblinding, investigational vaccine arms were offered TWINRIX and Menactra.
Vaccines
Pfs25-EPA/Alhydrogel® and Pfs230D1-EPA/Alhydrogel® vaccine consist of Pichia pastoris-expressed recombinant Pfs2513 (UniProt 25 kDa ookinete surface antigen) and Pfs230D1 (UniProt gametocyte surface protein P230),14 respectively (manufactured at the Walter Reed Army Institute of Research Pilot Bioproduction Facility [WRAIR PBF], Silver Spring, Maryland) conjugated to E. coli-expressed recombinant Pseudomonas aeruginosa ExoProtein A15 (EPA; WRAIR PBF) and adjuvanted with Alhydrogel® (Brenntag, Denmark). Additional vaccine information is in Appendix, pages 7-9.
Procedures
Per study design (Figure S1, Appendix, page 40), pilot-safety cohorts (n=25) received two doses at months 0 and 1 of either 0·2mL (Pfs25, 16μg) and/or 0·3mL (Pfs230D1, 15μg), or comparator vaccine TWINRIX (1mL recombinant hepatitis A and B vaccine; GlaxoSmithKline) with (n=5) or without (n=5) 1mL saline (0·9% sodium chloride injection, USP, Hospira). Main cohort (n=200) participants received 0·6 mL (Pfs25, 47μg) and/or 0·8 mL (Pfs230D1, 40μg) or comparator at months 0, 1, 4·5, 16·5; single-antigen (Pfs25 or Pfs230D1 or comparator) was co-administered with 1mL saline to maintain blind; comparator arm received TWINRIX months 0, 1, 4·5 in year 1, and Menactra (0·5mL meningococcal polysaccharide vaccine; Sanofi Pasteur Inc., Swiftwater, Pennsylvania) at month 16·5. Local pediatricians not involved in follow-up or adverse event (AE) assessment completed deltoid injections in alternating (pilot-safety cohort only) or both arms (co-administration; alternating with successive doses; both cohorts). Participants were considered enrolled upon first vaccination.
Participants were monitored 30 minutes post-vaccination for AEs, then on days 1, 3, 7, 14, 28, then monthly until unblinding. Medical personnel were always available for unscheduled visits. Solicited AEs were recorded 14 days after each vaccination (Table S1, Appendix, page 41). Unsolicited AEs, including symptomatic malaria, serious AEs (SAE), and new onset of chronic illness (NOCIs) were recorded throughout the study. SAEs include death, life-threatening event, inpatient hospitalization, persistent or significant incapacity, congenital anomaly or birth defect, or medically important event. Complete-blood-count with differential, creatinine, alanine-aminotransferase, and urinalysis were assessed before and 3 and 14 days after each vaccination. AE grading used US FDA guidelines16 adapted to local laboratory reference ranges (Tables S2, S3; Appendix, pages 42-43).
Blood smears (BS) were prepared before and at least monthly after vaccination, twice weekly with DSF visits following 3rd/4th vaccinations, and when clinically indicated. Symptomatic malaria was defined as asexual parasitemia with axillary temperature ≥37·5°C, clinical signs/symptoms of malaria, or both, and was treated with artemether/lumefantrine; asymptomatic parasitemia was not treated per Malian Government guidelines. BS were examined under standard procedures by trained technicians with skills regularly documented using blinded BS sets.
Immunogenicity samples were collected at various timepoints (Table S4, Appendix, page 44-45). Antibodies to Pfs25, Pfs230D1 and EPA were measured by ELISA on vaccination day, 14 days post-vaccination, then periodically post-3rd/4th vaccination, using previously published methods.1,10,11 For descriptive analyses of raw data, seropositive ELISA values were defined as greater than the mean limit-of-detection, based on the standard curve for each plate averaged across all ELISA plates. Supplementary modeling of ELISA data used plate-specific limit-of-detection.
Serum functional activity was measured in standard membrane feeding assay (SMFA) in which mosquitoes feed on cultured gametocytes in the presence of test (immune) or control (naïve) sera, using previously published methods.10 At least 20 mosquitoes were dissected ~1 week after feeding to count infected mosquitoes and parasites (oocysts) per infected mosquito. Transmission-reducing-activity (TRA) was defined as ((mean-oocyst-count in control sera – mean-oocyst count in test sera)/mean-oocyst-count in control sera) x100. Transmission-blocking-activity (TBA) was defined as ((mean-infection-prevalence in control sera– mean-infection-prevalence in test sera)/ mean-infection-prevalence in control sera) x100.
To assess vaccine effectiveness, DSF were conducted twice-weekly for six weeks on all main cohort participants (regardless of BS results) starting 7 days after 3rd/4th vaccinations for a maximum of 12 DSF each year. In brief (see details in Appendix, pages 16-18), two mesh-covered feeding pints, each containing up to 30 (first year) or 15 (second year) pre-starved lab-adapted female A. coluzzi were placed on participants’ calf/forearm by trained entomology staff to blood-feed 15-20 mins. Afterward, participants were offered topical antihistamine and/or topical antipruritic and followed for AEs. Only fed mosquitoes were transported to Bamako, stored in the secure insectary, and dissected a week later to count oocysts. Midguts of a subset of infected mosquitoes were PCR-tested for parasite speciation (further details in Appendix, pages 19-21).
Experimental hut (EH) studies (details in Appendix, pages 18-19) were explored to measure transmission and vaccine efficacy, whereby BS-positive individuals slept alone in huts modified to limit mosquito ingress/egress overnight; bloodied mosquitoes captured in morning underwent forensic testing of human blood source.
We assessed schistosomiasis and helminth/protozoan infections using previously published methods,17 at baseline in main cohort (see details in Appendix, page 19).
Primary and Secondary Outcomes
The primary outcome (as-treated population) in the pilot-safety and main cohorts was safety, tolerability, and reactogenicity of repeated immunization with increasing doses of vaccines, based on occurrence/severity of local AEs, systemic AEs, and laboratory abnormalities after each vaccination. Unsolicited AEs (including symptomatic malaria), SAEs, UPs, and NOCIs were reported throughout the study. AEs related to DSF were recorded for 7 days post-feed.
The secondary outcome (as-treated population) in main cohort was immunogenicity, measured starting 2 weeks post-vaccination as seroreactivity by IgG ELISA, antibody functionality by SMFA (2 weeks post-dose 3, and 2 and (for Pfs230D1 and comparator arms) 10 weeks post-dose 4), and vaccine effectiveness against parasite transmission to mosquitoes by DSF performed across two malaria seasons. Additional analyses to assess seropositive rates, antibody durability, and ELISA relationships to SMFA and DSF were conducted on an exploratory basis, and comparability between Malian and US vaccinee responses (post-hoc analysis). Similar analyses (ELISA and SMFA) were completed in the pilot-safety cohort as exploratory objectives. Additional exploratory analyses were completed and are reported in the Appendix, pages 12-13, 18-23.
Statistical analysis
Primary safety endpoint was analysed by as-treated and intention-to-treat (ITT), which differed by a single participant (Figure 1); thus only as-treated analysis is reported. Secondary immunogenicity endpoints (ELISA; SMFA; DSF) were analysed by as-treated. The two pilot comparator arms were combined for analyses.
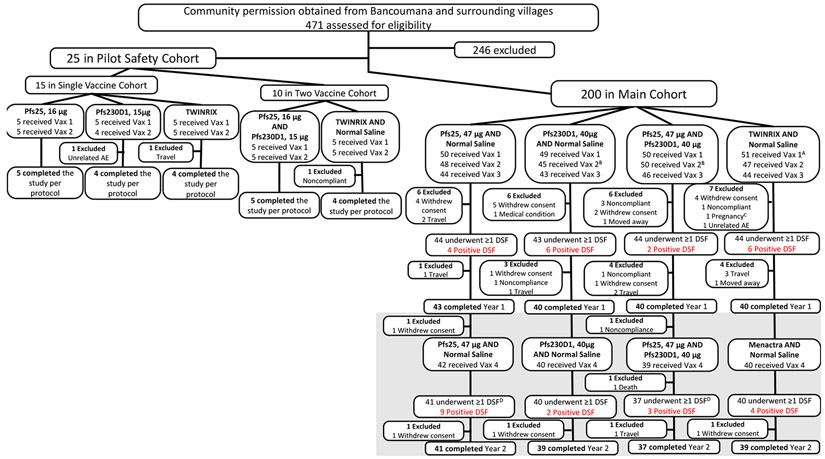
Figure 1. Trial Profile.
Trial included Pilot Safety Cohort then Main Cohort. Subjects in Pilot Safety Cohort were enrolled (April 2015) in a double-blind, comparator-controlled pilot study to receive single vaccinations (16μg Pfs25, 15μg Pfs230D1, TWINRIX) on days 0 and 28, or to receive co-administered (two syringes, separate arms) vaccinations (16μg Pfs25 + 15μg Pfs230D1, TWINRIX + normal saline) on the same schedule; pilot safety cohort participants were followed for 6 months post-dose 2 for safety and immunogenicity. For Pilot Safety Cohort, “completed study per protocol” was defined as completed through study day 196 (~6 months post vaccination #2). For analysis purposes, subjects in the TWINRIX and TWINRIX + normal saline arms (n=10) were combined as a single comparator arm. Subjects were then enrolled into the main double-blind, comparator-controlled study (n=200) and divided into 4 arms: 47μg Pfs25 + normal saline; 40μg Pfs230D1 + normal saline; 47μg Pfs25 + 40μg Pfs230D1; and comparator (TWINRIX, Menactra + normal saline). Main cohort participants were initially scheduled to receive vaccinations on a 0, 1, 6, 18-month schedule, but ultimately received these on a 0, 1, 4·5, 16·5-month schedule in order to complete dosing before the peak malaria transmission season (dose 3 = 15 Sep to 16 Oct 2015; dose 4 = 15 Sep to 17 Oct 2016). For the Main Cohort, completed Year 1= completed through study day 510 (~11 months post dose 3; end of Year 1); completed Year 2 = completed study day 730 (~6 months post dose 4). Year 2 is indicated in grey. DSF Year 1 was defined as completing at least 1 DSF from study day 175 (7 days post dose 3) to study day 213 (45 days post dose 3); maximum of 12 DSFs were completed in Year 1 (2015). DSF Year 2 was defined as completing at least 1 DSF from study day 547 (7 days post dose 4) to study day 585 (45 days post dose 4); maximum of 12 DSFs were completed in Year 2 (2016). Pfs25 = Pfs25-EPA/Alhydrogel®; Pfs230D1 = Pfs230D1-EPA/Alhydrogel®; μg = micrograms; Vax = vaccine; DSF = direct skin feeds. AOne subject randomized to 40μg Pfs230D1 + normal saline was erroneously administered comparator for vaccination #1, then continued to receive comparator throughout study (subject and clinical team remained blinded); for analysis considered comparator subject (for as-treated analysis) and Pfs230D1 subject (for ITT). BOne Pfs230D1-randomized subject was administered 47μg Pfs25 + 40μg Pfs230D1 for vaccination #2; considered Pfs230D1 subject for both as-treated and ITT analysis. COne subject (TWINRIX + normal saline) became pregnant just prior to vaccination #2 and was intentionally unblinded early for counseling of risk given vaccine received. DTwo subjects (one Pfs25; one Pfs25+Pfs230D1) did not complete a single DSF in Year 2 but completed the study.
Analysis populations:
Pilot Safety Cohort primary (safety, as-treated): Pfs25, 16μg (N=5), Pfs230D1, 15μg (N=5), TWINRIX (N=5), Pfs25, 16μg and Pfs230D1, 15μg (N=5), TWINRIX and Normal Saline (N=5)
Pilot Safety Cohort secondary (ELISA, SMFA; 2 weeks post dose 2, as-treated): Pfs25, 16μg (N=5), Pfs230D1, 15μg (N=4), TWINRIX (N=5), Pfs25, 16μg and Pfs230D1, 15μg (N=5), TWINRIX and Normal Saline (N=5)
Year 1
Main Cohort primary (safety, as-treated): Pfs25, 47μg and Normal Saline (N=50), Pfs230D1, 40μg and Normal Saline (N=49), Pfs25, 47μg and Pfs230D1, 40μg (N=50), TWINRIX and Normal Saline (N=51)
Main Cohort secondary (ELISA, SMFA; 2 weeks post dose 3, as-treated): Pfs25, 47μg and Normal Saline (N=44), Pfs230D1, 40μg and Normal Saline (N=39), Pfs25, 47μg and Pfs230D1, 40μg (N=46), TWINRIX and Normal Saline (N=44)
Main Cohort secondary (DSF, as-treated): Pfs25, 47μg and Normal Saline (N=44), Pfs230D1, 40μg and Normal Saline (N=43), Pfs25, 47μg and Pfs230D1, 40μg (N=44), TWINRIX and Normal Saline (N=44)
Year 2
Main Cohort primary (safety, as-treated): Pfs25, 47μg and Normal Saline (N=42), Pfs230D1, 40μg and Normal Saline (N=40), Pfs25, 47μg and Pfs230D1, 40μg (N=39), TWINRIX and Normal Saline (N=40)
Main Cohort secondary (ELISA, SMFA; 2 weeks post dose 4, as-treated): Pfs25, 47μg and Normal Saline (N=42), Pfs230D1, 40μg and Normal Saline (N=39), Pfs25, 47μg and Pfs230D1, 40μg (N=37), TWINRIX and Normal Saline (N=40)
Main Cohort secondary (DSF, as-treated): Pfs25, 47μg and Normal Saline (N=41), Pfs230D1, 40μg and Normal Saline (N=40), Pfs25, 47μg and Pfs230D1, 40μg (N=37), TWINRIX and Normal Saline (N=40)
Safety analyses included all participants who received at least one vaccine dose and examined AEs as proportions of unique subjects (Fisher’s exact and Chi-square tests) and overall counts (Wilcoxon-Mann-Whitney tests), including by grade and relationship to vaccination.
Descriptive statistics for proportions were assessed by modified Wald test with 95% confidence intervals. Categorical data were analysed by two-tailed Fisher’s Exact or Chi-square tests.
We compared ELISA levels between groups by Wilcoxon-Mann-Whitney test at specific time points. We compared seroconversion rates using conditional exact test for given time points (R package “exact2x2”). As supplementary analysis, two Bayesian proportional odds models were fit for the number of doses required to elicit an immune response. Antibody decay profiles were also modeled with a hierarchical Bayesian model. Modeling details are provided in Appendix, pages 21-23.
Group SMFA measures were compared using Kruskal-Wallis with Dunn’s correction for multiple comparisons, while individual comparison between Pfs230D1-alone vs. comparator 10 weeks post-dose 4 were analysed using Wilcoxon-Mann-Whitney. The association of SMFA with longitudinal ELISA values was assessed using GEE and estimates for ELISA values that would achieve 80% TRA by linear regression. We assessed DSF oocyst counts in zero-inflated negative binomial random effect models, with the traditional log-link and normally distributed random effects (this model has a parameter to account for excess 0 counts and the negative binomial accounts for overdispersion better than Poisson models).
Role of the funding source
Intramural Research Program of NIAID, NIH funded the study. NIAID scientists but not NIAID officials were involved in study design, study management, data collection, analysis, interpretation, report writing, and decision to submit.
Results
Between 19 Mar and 2 Jun 2015, 471 individuals were screened. 25 were randomized to pilot-safety cohort groups of 5 to receive a two-dose series of: Pfs25-EPA/Alhydrogel® (16μg), Pfs230D1-EPA/Alhydrogel® (15μg) or comparator, followed by Pfs25-EPA/Alhydrogel® (16μg) + Pfs230D1-EPA/Alhydrogel® (15 μg) or comparator + saline. First vaccinations occurred Apr 2015, last vaccinations May 2015, and scheduled unblinding Nov 2015 (Figures 1, S1, Appendix, page 40). Nine of 10 individuals who received comparator and 13/15 who received experimental vaccines continued to pilot-safety study end.
For main cohort (Figures 1, S1, Appendix, page 40), 200 participants enrolled May-Jun 2015 to receive four-dose series of 47μg Pfs25-EPA/Alhydrogel® + saline (n=50; Pfs25); or 40μg Pfs230D1-EPA/Alhydrogel® + saline (n=49; Pfs230D1); or 47μg Pfs25-EPA/Alhydrogel® + 40μg Pfs230D1-EPA/Alhydrogel® (n=50; Pfs25+Pfs230D1); or comparator (TWINRIX, Menactra) + saline (n=51; comparator). A Pfs230D1-randomised subject erroneously received comparator at first vaccination; this subject was not unblinded and continued receiving comparator. Vaccine doses (Figures 1, S1, Appendix page 40) in 2015 were given 11 May-2 Jun, 9-25 Jun, 15 Sep-16 Oct, and booster dose in 2016 from 15 Sep-17 Oct. DSFs were conducted post-dose 3 (22 Sep-2 Dec 2015) and post-dose 4 (22 Sep-30 Nov 2016). All randomised participants received at least one vaccination and were eligible for safety analyses (as-treated). Drop out was similar across arms, with 78% (range: 74-82%) completing the two-year study including DSF (Figure 1).
Main cohort study arms were similar with majority male participants (140/200; 70%, 95%CI: 63·3-76·0%) drawn equally from sites (Bancoumana: 103/200; 51·5%, 95%CI: 44·6-58·3%), and similar baseline parasitemia and gametocytemia rates (Table 1).
Table 1: Baseline characteristics of participants.
Age presented is age at enrollment (day of vaccine #1). AOne subject randomized to Pfs230D1, 40μg + normal saline was erroneously administered comparator for vaccination #1; reviewed by study team, statistician, Sponsor, and DSMB and recommended the subject continue to receive comparator for the rest of the study (subject and clinical team remained blinded); for analysis considered comparator subject (for as-treated analysis) and Pfs230D1 subject (for ITT). BUrine and stool samples were obtained from subjects during screening. Urine schistosomiasis screening was completed on site and positive subjects were treated with praziquantel; all enrolled subjects underwent urinary schistosomiasis testing. Urinary S. haematobium was further quantified for density of infection (slightly infected = 1-49 eggs/10mL of urine; moderately infected = 50-100 eggs/10mL; heavily infected = >100 eggs/10mL of urine) – all reported infections were slightly infected except 1 subject with heavily infected sample (main cohort TWINRIX/Menactra + normal saline subject). Stool PCR was completed retrospectively at NIH and did not impact clinical care; testing was completed for the following pathogens and grouped accordingly: Helminth: Ascaris lumbricoides (nematode/roundworm), Trichuris trichiura (nematode/roundworm), Strongyloides stercoralis (nematode/roundworm), Necator americanus (hookworm), Ancylostoma duodenale (hookworm), Protozoa: Giardia lamblia, Cryptospordium parvum/hominis, Entamoeba histolytica. Stool (no result) = either stool not collected, assay not completed, or NIC. S. haematobium = Schistosoma haematobium. Calculated % for stool results are based off assayed results (Pilot safety cohort: Pfs25, n=4; Pfs230, n=4; Pfs25 + Pfs230, n=5; TWINRIX +/− NS, n=10; main cohort: Pfs25 + NS, n=31; Pfs230D1 + NS, n=27; Pfs25 + Pfs230, n=33; TWINRIX, Menactra + NS, n=26). CParasitemia defined as blood smear >0 asexual Plasmodium falciparum on day of vaccine 1 (study day 0) or on day of vaccine 4 (main cohort only; study day 540). Gametocytemia is defined as blood smear >0 gametocytes (sexual Plasmodium falciparum) seen by at least one reader on day of vaccine 1 (study day 0) or on day of vaccine 4 (main cohort only; study day 540). DFor prior to dose four, n/arm (main cohort only) shifted to Pfs25 + NS, n=42; Pfs230D1 + NS, n=40; Pfs25 + Pfs230, n=39; TWINRIX, Menactra + NS, n=40. Pfs25 = Pfs25-EPA/Alhydrogel®; Pfs230 = Pfs230D1-EPA/Alhydrogel®; μg = micrograms; SD = standard deviation. Min = minimum. Max = maximum. N/A = not applicable.
| Pilot Safety Cohort | Main Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Pfs25, 16 μg (N=5) |
Pfs230D 1, 15 μg (N=5) |
Pfs25, 16 μg + Pfs230D 1, 15 μg (N=5) |
TWINRI X +/− NS (N=10) |
Pfs25, 47 μg + NS (N=50) |
Pfs230D 1, 40 μg + NS (N=49)A |
Pfs25, 47 μg + Pfs230D 1, 40 μg (N=50) |
TWINRIX/Mena ctra + NS (N=51)A |
|
| Gender | ||||||||
| Male | 4 (80%) | 4 (80%) | 4 (80%) | 8 (80%) | 35 (70%) | 33 (67%) | 36 (72%) | 36 (71%) |
| Age | ||||||||
| Mean ± SD | 35·6 ± 12·5 | 38·6 ± 8·9 | 34·6 ± 10·3 | 31·5 ± 10·2 | 38 ± 9·3 | 38·3 ± 8·8 | 36·6 ± 9·5 | 37·8 ± 10·4 |
| Min, Max | 19, 50 | 29, 48 | 19, 46 | 19, 47 | 18, 50 | 19, 50 | 19, 50 | 18, 50 |
| Village | ||||||||
| Bancoumana | 5 (100%) | 5 (100%) | 5 (100%) | 10 (100%) | 29 (58%) | 24 (49%) | 28 (56%) | 22 (43%) |
| Koursale | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (10%) | 6 (12%) | 7 (14 %) | 7 (14%) |
| Kolle | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (12%) | 6 (12%) | 3 (6%) | 3 (6%) |
| Samako | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (10%) | 4 (8%) | 6 (12%) | 8 (16%) |
| Nankilabougou | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Missira | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Djiguidala | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 1 (2%) | 1 (2%) | 2 (4%) |
| Tema | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 2 (4%) | 1 (2%) | 3 (6%) |
| Djoliba | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (4%) | 6 (12%) | 2 (4%) | 6 (12%) |
| Co-infectionsB | ||||||||
| Urinary S. haematobium | 0 (0%) | 0 (0%) | 0 (0%) | 1 (10%) | 3 (6%) | 2 (4%) | 4 (8%) | 5 (10%) |
| Stool helminth | 1 (25%) | 0 (0%) | 1 (20%) | 2 (20%) | 6 (19%) | 4 (15%) | 7 (21%) | 4 (15%) |
| Stool protozoa | 2 (50%) | 2 (50%) | 0 (0%) | 5 (50%) | 14(45%) | 15 (56%) | 13 (39%) | 6 (23%) |
| Stool (no result) | 1 (20%) | 1 (20%) | 0 (0%) | 0 (0%) | 19 (38%) | 22 (45%) | 17 (34%) | 25 (49%) |
| ParasitemiaC | ||||||||
| Prior to dose 1 | 1 (20%) | 1 (20%) | 1 (20%) | 0 (0%) | 4 (8%) | 6 (12%) | 6 (12%) | 9 (18%) |
| Prior to dose 4D | N/A | N/A | N/A | N/A | 4 (10%) | 7 (18%) | 0 (0%) | 6 (15%) |
| GametocytemiaC | ||||||||
| Prior to dose 1 | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (4%) | 1 (2%) | 4 (8%) |
| Prior to dose 4D | N/A | N/A | N/A | N/A | 5 (12%) | 8 (20%) | 2 (5%) | 1 (3%) |
Vaccinations in the low dose, pilot-safety arms were relatively well-tolerated (summary in Appendix, pages 23-25; Tables S5-S7, pages 46–50).
Vaccinations in high-dose arms (47μg Pfs25 and/or 40μg Pfs230D1) were also well-tolerated (Table 2; Appendix, pages 25-30; Tables S8-S10, pages 51-56). Most commonly reported AEs were Grade 1/2 and most related AEs were injection site reactogenicity, reported more frequently for Pfs25 and Pfs230D1 arms than comparator (Table S8, Appendix, pages 51-52). Reactogenicity was more common in Pfs25 and Pfs230D1 limbs (and in comparator limbs post-dose 2 and 4) than saline limbs. Local reactogenicity in Pfs25+Pfs230D1 arm was attributed equally to either Pfs25 or Pfs230D1 limbs.
Table 2. Safety summary of main cohort.
Reporting periods for adverse events (AEs) were protocol specific. Unsolicited AEs, serious AEs (SAEs), unanticipated problems (UPs), and (new onset chronic illness (NOCIs) were recorded through the end of the study (study day 730, ~6 months post vaccination #4). Vaccinations were administered on a 0, 1, 4·5. 16·5 month schedule from May to October 2015 (for dose 1, 2, 3) and September to October 2016 (for dose 4). The following reporting periods were defined as follows: during entire study period (for Vax 1 = ~1 month, Vax 2 = ~3·5 months, Vax 3 = ~12 months, Vax 4 = ~6 months); local reactogenicity was assessed until 7 days post vaccination; solicited reactogenicity was assessed until 14 days post vaccination; laboratory AEs were assessed until 14 days post vaccination + visit window timeframe (+3 days). Local injection site reactogenicity included: pain/tenderness, erythema/redness, swelling, induration, and pruritus. Systemic solicited reactogenicity included: fever, headache, nausea, malaise, myalgia, arthralgia, and urticaria. Scheduled labs (complete blood cell count with differential, alanine transaminase, creatinine) were completed 3 and 14 days post vaccination. Given all subjects received two vaccinations (co-administration), if local reactogenicity reported and attributed to both upper arms, two individual AEs are reported in one subject. Symptomatic malaria was reported as an AE (defined as Plasmodium asexual parasitaemia accompanied by an axillary temperature of at least 37·5 °C and/or clinical signs and symptoms compatible with malaria) and collected throughout the study duration. Follow-up concluded in March to April 2017. All AEs were coded using MedDRA and preferred terms provided. X (XX%) X = number of unique subjects experiencing AEs (percentage of subjects with AEs) absolute number of AEs. Vax = Vaccination. AE = adverse events; SAE = serious adverse events. μg = micrograms. A For fair comparison between study year 1 (2015) and year 2 (2016), symptomatic malaria cases reported were assessed for a 6-month period post dose 3 and dose 4. Significant differences from the control are noted with an *.
| Pfs25, 47 μg + NS | Pfs230, 40 μg + NS | Pfs25, 47 μg + Pfs230, 40 μg |
TWINRIX/Menactra + NS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vax 1 (N=50 ) |
Vax 2 (N=4 8) |
Vax 3 (N=4 4) |
Vax 4 (N=4 2) |
Vax 1 (N=4 9) |
Vax 2 N=4 5) |
Vax 3 (N=4 3) |
Vax 4 (N=40 ) |
Vax 1 (N= 50) |
Vax 2 (N= 50) |
Vax 3 (N= 46) |
Vax 4 (N=3 9) |
Vax 1 (N=51 ) |
Vax 2 (N=4 7) |
Vax 3 (N=44 ) |
Vax 4 (N=4 0) |
|
| Reported during entire study period | ||||||||||||||||
| Total AE | 37* (74%) 70 | 45 (93·8%) 133 | 41 (93·2%) 249 | 40 (95·2%) 179 | 35 (71·4%) 67 | 40 (88·9%) 105 | 39 (90·7%) 189 | 39 (97·5%) 152 | 36(72%)82 | 43(86%)126 | 44 (95·7%) 282 | 37 (94·9%) 178 | 27 (52·9%) 48 | 42 (89·4%) 103 | 43 (97·7%) 225 | 40 (100%) 149 |
| Grade 1 | 33* (66%) 49 | 38 (79·2%) 67 | 39 (88·6%) 85 | 20 (47·6%) 26 | 28* (57.1%) 46 | 36 (80%) 57 | 29 (67·4%) 68 | 20 (50%) 33 | 32* (64%) 60 | 38 (76%) 88 | 38 (82·6%) 103 | 21 (53·8%) 36 | 18 (35·3%) 29 | 36 (76·6%) 62 | 32 (72·7%) 75 | 18 (45%) 24 |
| Grade 2 | 16 (32%) 21 | 34 (70·8%) 65 | 40 (90·9%) 156 | 39 (92·9%) 151 | 17 (34·7%) 21 | 29 (64·4%) 46 | 32* (74·4%) 115 | 37 (92·5%) 117 | 17 (34%) 21 | 26 (52%) 37 | 42 (91·3%) 176 | 34 (87·2%) 139 | 16 (31·4%) 18 | 26 (55·3%) 38 | 43 (97·7%) 145 | 40 (100%) 124 |
| Grade 3 | 0 (0%) 0 | 2 (4·2%) 2 | 8 (18·2%) 8 | 2 (4·8%) 2 | 0 (0%) 0 | 2 (4·4%) 2 | 4 (9·3%) 5 | 2 (5%) 2 | 1 (2%) 1 | 1 (2%) 1 | 3 (6·5%) 3 | 2 (5·1%) 2 | 0 (0%) 0 | 2 (4·3%) 3 | 4 (9·1%) 5 | 1 (2·5%) 1 |
| Grade 4 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (2·3%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (2%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 |
| Grade 5 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (2·6%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 |
| Related AE | 31* (62%) 44 | 35* (72·9%) 52 | 20* (45·5%) 28 | 21 (50%) 25 | 23 (46·9%) 33 | 28* (62·2%) 31 | 18* (41·9%) 23 | 17 (42·5%) 20 | 29* (58%) 53 | 26 (52%) 48 | 23* (50%) 45 | 23* (59%) 37 | 16 (31·4%) 19 | 17 (36·2%) 23 | 10 (22·7%) 12 | 12 (30%) 17 |
| SAE | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (2·2%) 1 | 1 (2·3%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (2·6%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 |
| Malaria AEA | 2 (4%) 2 | 13 (27·1%) 13 | 29 (65·9%) 37 | 25 (59·5%) 32 | 2 (4·1%) 2 | 12 (26·7%) 12 | 24 (55·8%) 29 | 20 (50%) 25 | 0 (0%) 0 | 12 (24%) 13 | 29 (63%) 40 | 22 (56·4%) 26 | 2 (3·9%) 2 | 16 (34%) 17 | 31 (70·5%) 44 | 22 (55%) 26 |
| Reported within 7 days of vaccination | ||||||||||||||||
| Local Reactogenicity | 26* (52%) 32 | 31* (64·6%) 42 | 16* (36·4%) 23 | 20 (47·6%) 23 | 21* (42·9%) 24 | 22 (48·9%) 22 | 17* (39·5%) 21 | 16 (40%) 16 | 26* (52%) 48 | 23 (46%) 43 | 22*(47·8%) 40 | 17 (43·6%) 28 | 6 (11·8%) 9 | 14 (29·8%) 16 | 4 (9·1%) 4 | 10 (25%) 15 |
| Reported within 14 days of vaccination | ||||||||||||||||
| Solicited Reactogenicity | 3 (6%) 3 | 3 (6·3%) 3 | 4 (9·1%) 4 | 3 (7·1%) 4 | 5 (10·2%) 5 | 3 (6·7%) 3 | 1 (2·3%) 1 | 5 (12·5%) 5 | 2 (4%) 2 | 3 (6%) 3 | 2 (4·3%) 3 | 8* (20·5%) 8 | 5 (9·8%) 5 | 4 (8·5%) 4 | 2 (4·5%) 3 | 1 (2·5%) 1 |
| Laboratory AE | 8 (16%) 8 | 8 (16·7%) 9 | 4 (9·1%) 4 | 0 (0%) 0 | 5 (10·2%) 5 | 8 (17·8%) 10 | 2 (4·7%) 2 | 5 (12·5%) 5 | 4 (8%) 4 | 5 (10%) 5 | 2 (4·3%) 2 | 5 (12·8%) 6 | 7 (13·7%) 8 | 6 (12·8%) 7 | 6 (13·6%) 7 | 4 (10%) 4 |
Solicited AEs were few (most commonly headache) and were only significantly more frequent (p=0·039) in Pfs25+Pfs230D1 arm post-dose 4 (Table S9, Appendix, pages 53-54). Laboratory abnormalities were similar across arms and Grade 1/2 except two unrelated Grade 4 creatine elevations (Pfs230D1; TWINRIX) (Table S10, Appendix, pages 55-56). Three unrelated SAEs (2 Pfs230D1: snake bite, peritonsillar abscess; 1 Pfs25+Pfs230D1: cerebrovascular accident/death) are further detailed in the Appendix, page 29. There were no Grade 3, 4, or 5 related AEs.
Malaria endpoints (symptomatic malaria, severity of malaria cases, parasitaemia, gametocytaemia) were assessed throughout the study period. Comparison of malaria AE (frequency, duration, severity), parasitaemia, and gametocytaemia showed no significant difference between vaccine versus comparator arms (Table 2). Further details are available in the Appendix (pages 29-30).
Pilot cohort antibody titres against Pfs25 and Pfs230D1 are shown in Figure S2 (Appendix, page 57).
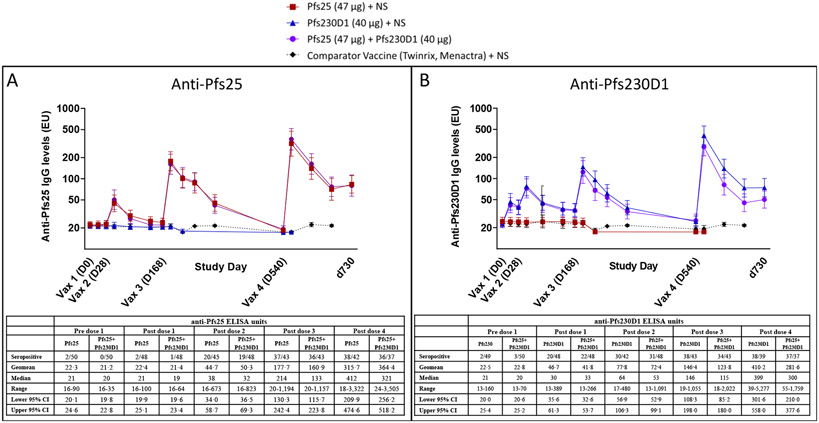
In main cohort, significant Pfs25 antibody responses were observed 2 weeks after second Pfs25 vaccination [p<0·0001 for Pfs25-alone (95%CI: 34·0-58·7) and Pfs25+Pfs230D1 (95%CI: 36·5-69·3) vs. comparator (95%CI: 19·4-22·3); Figure 2A]. In contrast, significant antibody levels were detected after first Pfs230D1 vaccination [p=0·0001 for Pfs230D1-alone (95%CI: 35·6-61·3) vs. comparator (20·8-25·2); p=0·0024 for Pfs25+Pfs230D1 (95%CI: 32·6-53·7) vs comparator; Figure 2B]. Peak titres increased after each subsequent dose [Pfs230D1 GM (95%CI): 77·8 (56·9-106·3), 146·4 (108·3-198·0), 410·2 (301·6-558·0); Pfs25: 177·7 (130·3-242·4), 315·7 (209·9-474·6)].
Figure 2. Antibody titres for single and combination immunogen arms by ELISA.
Anti-Main cohort participants received vaccinations on a 0, 1, 4·5, 16·5-month schedule from May to Oct 2015 (for dose 1, 2, 3) and Sep to Oct 2016 (for dose 4), and samples were collected at post-vaccination timepoints (schedule in Table S4B, Appendix, page 45) to assess anti-Pfs25 (Figure 2A) and anti-Pfs230D1 (Figure 2B) antibody titres by ELISA. Values are presented as ELISA EU. Geometric means are presented with error bars indicating 95% confidence interval. Differences in antibody titres induced by vaccines versus comparator were analyzed by Mann-Whitney test. For anti-Pfs25 titres, significant differences were observed 2 weeks after vaccinations 2, 3, and 4 (p<0·0001 for each Pfs25-containing group versus comparator). For anti-Pfs230D1 titres, significant differences were observed 2 weeks post-vaccination 1 (p=0·0001 for Pfs230D1 alone; p=0·0024 for Pfs25+Pfs230D1), and 2 weeks after each subsequent vaccination (p<0·0001 for each Pfs230D1-containing group). d730 = study day 730, ~6 months post dose 4 (end of study); comparator antibody titres to Pfs25 and Pfs230D1 were not completed for d730. NS = normal saline. Associated tables with anti-Pfs25 (Figure 2A) and anti-Pfs230D1 (Figure 2B) ELISA data at peak timepoints (2 weeks post vaccination) are provided below each associated figure. Seropositive is defined as EU > mean + 3SD of plate level of detection (Pfs25=55 EU; Pfs230D1=43 EU). Post vaccination receipt sample missingness (due to missed visit, off study post vaccination) ranged from 0-3 participants per each time point and was evenly disbursed between arms (Pfs25: pre dose 1 = 0, post dose 1 = 2 off study; post dose 2 = 3 missed visits; post dose 3 = 1 off study; post dose 4 = 0; Pfs230D1: pre dose 1 = 0, post dose 1 = 1 off study; post dose 2 = 3 missed visits; post dose 3 = 0; post dose 4 = 1 missed visit; Pfs25+Pfs230D1: pre dose 1 = 0, post dose 1 = 1 off study, 1 missed visit; post dose 2 = 2 off study; post dose 3 = 1 off study, 2 missed visits; post dose 4 = 1 off study, 1 missed visit) . Pfs25 = 47 μg of Pfs25-EPA/Alhydrogel® + normal saline; Pfs230D1 = 40 μg of Pfs230D1-EPA/Alhydrogel® + normal saline; Pfs25+Pfs230D1 = 47 μg of Pfs25-EPA/Alhydrogel® + 40 μg of Pfs230D1-EPA/Alhydrogel®; comparator = Twinrix (dose 1-3) or Menactra (dose 4) + normal saline.
We also examined the number of doses needed to elicit an immune response, with seropositivity defined as limit-of-detection (LoD, determined by standard curve on each plate) averaged across all plates. Pfs230D1-alone responders were more frequent after 1 or 2 doses [20/48 (42%) and 30/42 (71%), respectively] than Pfs25-alone responders [2/48 (4%) and 20/45 (44%), Fisher exact p<0·0001 and p=0·017, respectively). Most vaccinees became seropositive after two Pfs230D1 or three Pfs25 doses; proportion of responders after 3 and 4 doses was high and similar between groups (Figures S3, S4, Appendix, pages 58-59). Pfs230D1-seropositive individuals were significantly more frequent in Pfs230D1-alone versus combination arm at later time points post-dose 4 [at 10, 19, 27 weeks post-dose 4 (Fisher exact, p=0·031; 0·0039; 0·060; respectively); Figure S4, Appendix, page 59], suggesting impaired antibody durability after co-administration.
Significant anti-Pfs25 antibody levels remained detectable in both Pfs25-containing arms at 19 weeks post-dose 3 [p<0·0001 for Pfs25-alone (95%CI: 62·9-84·6) and Pfs25+Pfs230D1 (95%CI: 60·9-79·2) vs. comparator (95%CI: 55·0-55·0); Figure 2A], and at 19 weeks post-dose 4 [p<0·0001 for Pfs25-alone (95%CI: 76·6-123·4) and Pfs25+Pfs230D1 (95%CI: 76·4-122·7) vs. comparator (95% CI: 55·0-55·0); Figure 2A]. Similarly, both Pfs230-containing arms showed significant antibody levels at 19 weeks post-dose 3 [p<0·0001 for Pfs230D1-alone (95%CI: 48·0-63·4) and Pfs25+Pfs230D1 (95%CI: 47·3-61·7) vs. comparator (95%CI: 43·0-43·0); Figure 2B], and at 19 weeks post-dose 4 [p<0·0001 for Pfs230D1-alone (95%CI: 64·9-105·9) and Pfs25+Pfs230D1 (95%CI: 53·4-76·7) vs. comparator (95%CI: 43·0-43·0)]; Figure 2B).
At the time of the fourth vaccination dose, anti-Pfs25 antibody levels were not significantly different between Pfs25-containing arms and comparator, whereas anti-Pfs230D1 levels remained significantly higher in the combination arm (but not Pfs230D1-alone arm) versus comparator (p=0·017 for combination arm; p=0·15 for single Pfs230D1 antigen; Figure 2B).
In exploratory analysis, we compared ELISA responses in this Mali population to a US safety cohort (N=5/arm) who received two vaccine doses under this protocol as previously reported.11 Pfs25 antibody levels were 2-fold higher in US than Mali subjects in response to high dose (47 μg) Pfs25 at 2 weeks (day 42, p=0·0086) and 8 weeks (day 84, p=0·031) post-dose 2 (Figure S5A, Appendix page 60). In contrast, Pfs25 antibody levels induced by the low dose (16 μg) Pfs25 single antigen may be higher in Mali than US (p=0·056). Pfs230D1 antibody levels did not significantly differ between US and Mali populations (Figure S5B, Appendix page 60).
Responses to EPA appeared after dose 1 in all vaccine groups and geometric mean titres increased with successive doses (Appendix, pages 31-32; Figure S6, page 61). Post-vaccination EPA titres were consistently higher in the combination arm versus single antigen arms.
Stool co-infections reduced Pfs230D1 titres post-dose 2, but not post-dose 3; details of these results are in the Appendix, page 33.
Proportions of responders at peak titres (2 weeks post-vaccine) did not significantly differ in supplementary modeling analyses (Figure S7, Appendix page 62). Supplementary Bayesian models for immune responses suggest that the combined administration of Pfs230D1 and Pfs25 did not affect the number of doses needed to elicit a Pfs230D1 immune response when compared to administering Pfs230D1-alone (COR, 1·08; 95% CI, 0·52, 2·21) or Pfs25-alone (COR, 0·86; 95% CI, 0·4, 1·82). Posterior log geometric mean (GM) titres were estimated for Pfs230D1 and Pfs25 (Figure S8, Appendix, page 63): while log GM titres only slightly exceed LoD after the second Pfs25 dose, they already exceed assay LoD after first Pfs230D1 dose.
Supplementary Bayesian antibody decay models suggested Pfs230D1 antibodies were more durable than Pfs25 antibodies (Figure S7-S11, Appendix, page 62-66): we estimated that after 16 weeks Pfs230D1 GM titres are 30% (95% CI: 26%, 34%) of peak versus 22% (95% CI: 19%, 26%) for Pfs25. Durability of the response was not greater in the combination arm versus single antigen vaccination arms: estimated Pfs230D1 titres were slightly higher in Pfs230D1-alone versus combination arm following doses 3 and 4 while Pfs25 titres did not differ between single and combination arms (Figure S11, Appendix page 66).
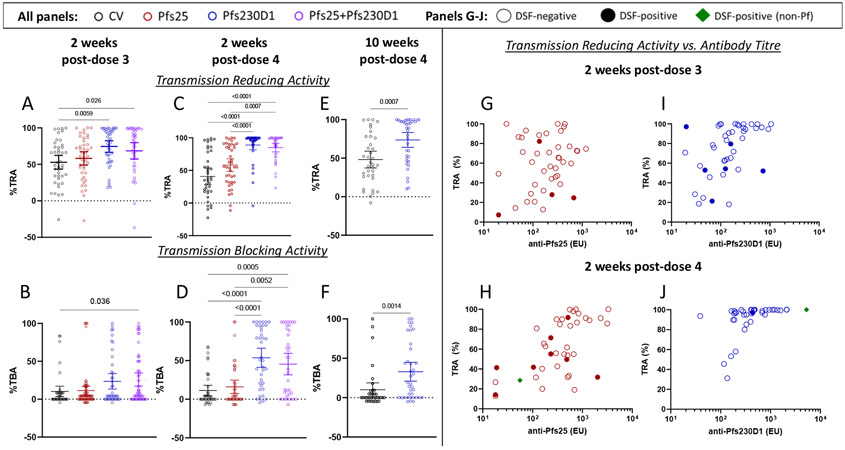
Serum functional activity was assessed by SMFA in each arm using all available sera collected 2 weeks post-dose 3 and 4; and for all available sera in Pfs230D1 monovaccination and comparator vaccine groups at 10 weeks post-dose 4. Significant transmission-reducing activity appeared for Pfs230D1 and Pfs25+Pfs230D1 post-dose 3 [mean(95% CI): 74·5%(66·6-82·5) and 68·6%(57·3-79·8), respectively] and post-dose 4 [88·9%(81·7-96·2) and 85·0%(78·4-91·5), respectively] but not for Pfs25 [58·2%(49·1-67·3) and 58·2(48·5-67·9) post-dose 3 and 4, respectively; Figures 3A-D]. Pfs230D1 transmission-reducing-activity [73·7% (64·1-83·3%)] persisted 10 weeks post-dose-4 (Figures 3E-F).
Figure 3. Pfs230D1 shows superior transmission reducing activity (TRA), transmission-blocking activity (TBA) and durability.
TBV functional activity was assessed by standard membrane feeding assay. Transmission reducing activity (TRA; Figures 3A, 3C, 3E) was defined as ((mean oocyst count in control sera – mean oocyst count in test sera)/mean oocyst count in control sera) x 100. Transmission blocking activity (TBA; Figures 3B, 3D, 3F) was defined as ((mean infection prevalence in assay control – mean prevalence in the test sample)/mean prevalence in the assay control) x 100. For 2 weeks post-each vaccination (post dose 3, Figures 3A, 3B; post dose 4: Figures 3C, 3D), differences between groups were analysed by Kruskal-Wallis test with Dunn’s correction for multiple comparisons; at 10 weeks post-dose 4 (Figures 3E, 3F), differences between Pfs230D1 group and comparator was analyzed by Mann-Whitney test. Significant p-values are presented. Transmission reducing plotted as a function of ELISA titre for Pfs25 and Pfs230D1 single antigen arms at 2 weeks post-dose 3 (Figures 3G, 3I), and 2 weeks post-dose 4 (Figure 3H, 3J). Results for the Pfs25+Pfs230D1 combination arm are presented in Figure S12, Appendix, page 67. Results for transmission reducing activity at 10 weeks post dose 4 for Pfs230D1 single antigen arm can be found in Figure S13, Appendix, page 68. Empty circles represent participants with negative DSF results; closed circles indicate participants with positive DSF results; green diamonds indicate DSFs that were positive for a non-falciparum Plasmodium species detected by PCR analysis of a single midgut selected from the feed. Dotted horizontal lines represent no difference from assay control (non-immune sera).
To relate titres and functional activity (TRA), linear regressions were fitted regressing TRA onto the log10 ELISA titres at 2 weeks post-dose 3 (Figures 3G, 3I) and 2 weeks post-dose 4 (Figures 3H, 3J) for single antigens; the effect was significant in each model. To achieve a TRA of 80%, it was estimated that an ELISA value of 1659 would be required for Pfs25 vaccine, compared to an ELISA value of 218 for the Pfs230D1 vaccine (Figures 3G-J). For the Pfs25+Pfs230D1 combination vaccine, TRA of 80% required a similar Pfs230D1 ELISA value of 223 (Figure S12, Appendix page 67). The relationship for the Pfs230D1-alone group appeared similar 10 weeks post-vaccination 4 (Figure S13, Appendix, page 68).
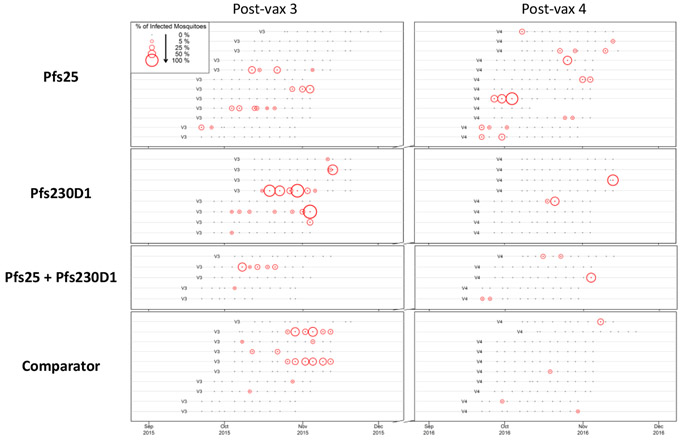
Direct-skin-feed assays were conducted twice-weekly for 6 weeks post-doses 3 and 4, using 60 (Year 1) or 30 (Year 2) mosquitoes per feed [evident as two distributions of mosquito numbers dissected among the negative DSFs (Figure S14A, Appendix, page 69)]. After 3850 direct-skin-feed assays, 35 participants [8 Pfs230D1; 12 Pfs25; 5 Pfs25+Pfs230D1; 10 comparator] had transmitted parasites at least once (Figure 4, Table S11, Appendix, page 70). As expected,1 a small fraction of DSFs were positive, typically with a minority of mosquitoes infected (Figures 4, S14B, Appendix, page 69).
Figure 4. DSF assay results for the 35 participants who transmitted parasites in at least one DSF assay.
Of 175 individuals who underwent at least one DSF assay, 35 transmitted parasites on at least one occasion. One of these 35 DSF+ participants yielded positive transmission events in both years, 17 in Year 1 only, and 17 in Year 2 only. Each subject is depicted by timelines over two seasons that indicate DSF timepoints and their outcomes, stratified by trial arm. Feeds used 60 mosquitoes in Year 1 and 30 in Year 2. Dissections were performed 7 days after feed to assess transmission, with each surviving mosquito surveyed for the presence of oocysts with feeding and survival rates per arm available in Table S11, Appendix, page 70. The dots and colored circles in the figure represent DSF assays, with small black dots denoting negative DSF where no dissected mosquitoes had oocysts. Red circles are positive DSF transmission events, with the circle size proportional to the percentage of dissected mosquitoes that contained oocysts.
Proportion positive assays in vaccine arms [Pfs25, 33/982 (3·4%); Pfs230D1, 22/954 (2·3%); combination, 11/940 (1·2%)] (Figure 4, Table S11, Appendix, page 70) did not differ (respectively, 95%CI=−0·013-0·014; −0·005-0·027; −0·024-0·002) from comparator (22/974 (2·3%)), nor did Pfs230D1 and combination arms differ (95%CI=−0·024-0·001). The low rate of DSF positivity provided limited power to detect differences.
While a minority (35 of the 175 subjects who underwent at least one DSF assay) had a positive DSF during follow up, DSF-positive subjects were as likely to transmit parasites in 2 or more assays (12/18 DSF-positive individuals in season 1 and 9/18 in season 2) as to transmit during a single assay during the same season (Figures 4, S15, Appendix, pages 71-75; Table S11, Appendix, page 70). Multiple positive feeds occurred consecutively in several individuals. Conversely, only 1 subject had positive DSF in both seasons, which is no greater than predicted by random chance (Enrichment analysis predicted 2·1 individuals with positive DSF both seasons; p=0·36).
We assessed DSF oocyst counts in zero-inflated negative binomial random effect models. No significant associations were found in these models that explored peak dose-4 ELISA titre relationships to DSF outcomes.
Forensic typing of blood-fed mosquitoes collected from EH suggested a high rate of contamination by mosquitoes [66/189 (35%)] that had fed outside of the hut (Appendix, page 32-33), limiting usefulness of the approach to assess vaccine effectiveness.
Discussion
Gains in malaria control have plateaued since 2015 and reversed in high-burden regions,2 mandating new control tools. Here, we report two leading TBV candidates to be safe, alone or in combination, in rural Malian adults. Seroconversion occurred after fewer Pfs230D1 than Pfs25 doses, and Pfs230D1 antibodies persisted longer. Pfs230D1 serum functional activity appeared after dose 3 and persisted at least 10 weeks after dose 4; Pfs25 did not induce significant functional activity and did not enhance Pfs230D1 activity in a combination vaccine. These data firmly establish Pfs230D1superiority over Pfs25 in the target population.
We earlier reported that Pfs230D1 induces functional activity after 2 doses in malaria-naïve US subjects,11 whereas Pfs25 in Alhydrogel required 4 doses to achieve significant activity in most US10 or Mali vaccinees.1 Here, Pfs230D1 but not Pfs25 conferred statistically significant functional activity after both primary vaccine series and booster dose that persisted at least 10 weeks post-dose 4. Using human monoclonal antibodies generated from this trial, we previously observed that Pfs230D1 functional antibodies bind native antigen on the gamete surface, fix complement and lyse the parasite; non-functional Pfs230D1 antibodies often fail to bind native antigen likely because epitopes are masked by downstream Pfs230 domains.1,10
As in US adults,11 the Pfs230D1+Pfs25 combination in Malian adults did not enhance immunogenicity over single antigens nor improve serum functional activity achieved with Pfs230D1 alone. Peak antibody levels against Pfs230D1 (but not Pfs25) were lower with the combination, although differences were not statistically significant. At later post-dose 4 timepoints, the proportion of Pfs230D1-seropositive individuals was significantly greater in Pfs230D1-alone versus combination arm. Future studies may examine the combination at different doses or using different adjuvants.
Pfs230 but not Pfs25 is expressed during gametocyte development in humans, and exposed populations acquire Pfs230 antibodies. Naturally acquired transmission-reducing activity has been related to Pfs230 antibodies,18 and Pfs230 vaccines hypothetically might benefit from antibody boosting during Pf infections. Conversely, pre-existing antibody responses can impair vaccine responses, as suggested for the malaria circumsporozoite protein repeat-region.19 Here, Pfs230D1 responses did not significantly differ between Mali and US populations, whereas Pfs25 responses post-dose 1 and 2 were significantly lower in Mali, similar to our previous study.1 Pfs230D1 responses in the small number of subjects with pre-existing antibody were not lower than for other subjects (Figure S16, Appendix, page 76). Naturally acquired transmission-reducing activity may have hindered our ability to statistically confirm the Pfs25 functional activity that appeared to increase after 4th vaccine dose (Figure 3).
As TBV clinical development advances, efficacy trials will require large cluster-randomized designs to measure herd immunity.19 We are exploring DSF as an interim endpoint, whereby colony-raised mosquitoes are fed directly on trial participants.20 Here, we established safety and tolerability of twice-weekly DSF, which yielded measurable endpoints (infected mosquitoes) but with too few unique DSF-positive individuals to confirm vaccine effectiveness. DSFs with 30 mosquitoes detected fewer transmission events versus DSFs with 60 mosquitoes. Conversely, transmission events were often observed in consecutive DSF, suggesting oversampling with our twice-weekly schedule. In future, we plan to reduce DSF sampling frequency and extend the period of DSF using 60 mosquitoes per feed in order to achieve sufficient endpoint events in unique individuals and confirm vaccine effectiveness in-vivo.
Overall, our results are promising. Pfs230D1 requires fewer doses than Pfs25 for serum functional activity, Pfs230D1 antibodies persist longer, and Pfs230D1 serum activity persists at least 10 weeks post-dose 4 unlike Pfs25 activity that disappeared within eight weeks in prior testing.10 In preclinical studies, more potent adjuvants such as liposomal formulations21 improved durability of Pfs25 and Pfs230D1 vaccines.22-24 We are now evaluating Pfs230D1-EPA and Pfs25-EPA with such adjuvants in humans.
Our study had limitations. Dropout rates were similar in study arms over the 2-year trial, but selection bias may occur due to missing outcome data. Our direct-skin-feed assays that measure the rate at which participants transmit naturally-circulating parasites to mosquitoes detected transmission events in a low proportion of participants. Future trials should perform direct-skin-feed assays less frequently over a more extended time period to increase the proportion of participants with detectable transmission. Trials in younger age groups known to transmit parasites more frequently25 may increase power to measure vaccine effectiveness.
In conclusion, Pfs25 and Pfs230D1 conjugate vaccines adjuvanted with Alhydrogel® are safe, well-tolerated and immunogenic in Malian adults. Pfs230D1 is superior to Pfs25 based on functional immunogenicity by SMFA and combining Pfs230D1 with Pfs25 does not improve serum functional activity. DSF is a valuable in-vivo measurement of parasite transmission but must be further optimized to enhance statistical power for vaccine trial endpoints.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed, the Cochrane Library, and other relevant source data sources on February 27, 2023, for English-language articles on randomised controlled trials of malaria vaccines in adults published between January 1, 1980, and Feb 27, 2023. We searched using the following terms (“malaria vaccines” [MeSH Terms] OR “malaria”[All Fields] AND “vaccines”[All Fields]) OR “malaria vaccines”[All Fields] OR (“malaria”[All Fields] AND “vaccine”[All Fields]) OR “malaria vaccine”[All Fields]) AND (Pfs25 [All Fields] AND Pfs230 [All Fields]))). For the Cochrane Library and other data sources, we used the key search terms “Pfs25”, “Pfs230”, “malaria vaccines”, AND “clinical trials”. Although transmission blocking vaccine studies have been previously conducted in malaria endemic regions, no trial of a Pfs230 vaccine in malaria-experienced populations has been published. Another leading transmission-blocking vaccine target is gamete surface protein Pfs48/45, a long-established prime candidate that induces antibodies that prevent parasite development in the mosquito vector. Pfs48/45 first entered clinical trials early 2021 in the Netherlands in the form of subunit R0.6C, after overcoming nearly 20 years of challenges to manufacturing, particularly yielding sufficient quantities of a properly folded, functional activity-inducing protein.
We previously reported that Pfs25 or Pfs230 domain 1 (D1) vaccines (prepared as Pichia-expressed recombinant proteins conjugated to carrier-protein ExoProtein A (EPA) and formulated in the alum-based adjuvant Alhydrogel®: Pfs25H-EPA/Alhydrogel® and Pfs230D1-EPA/Alhydrogel®) were well-tolerated and induced functional antisera in U.S. volunteers. Pfs25H-EPA/Alhydrogel® also induced functional antisera in malaria-experienced Malian volunteers, that reduced Pf transmission to mosquitoes in a laboratory assay, but Pfs25 titers decayed rapidly after peak, and functional activity seen at peak titres in U.S. vaccinees was lost by 8 weeks post-vaccination.1 In U.S. volunteers, Pfs230D1 vaccine induced serum functional activity superior to Pfs25 after two doses, and the combination of Pfs230D1+Pfs25 did not increase serum activity.
Added value of this study
Here, we compared Pfs230D1 and Pfs25 vaccines, alone or in combination, as 4-dose regimens in a malaria-experienced target population in Mali. Both vaccines were safe, well-tolerated and immunogenic in Malian adults. As seen in the U.S. trial, Pfs230D1 was superior to Pfs25 for functional immunogenicity by SMFA; combining Pfs230 with Pfs25 did not improve serum functional activity.
Implications of all the available evidence
Pfs230D1-EPA is now the leading malaria transmission-blocking vaccine candidate. Both Pfs230D1-EPA and Pfs25-EPA candidates are safe and can be assessed with more potent adjuvants to enhance functional antibody responses. The direct skin feeding assay measures wild-type parasite transmission to mosquitoes but will require increased event rates to establish vaccine effectiveness in future field trials.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Funding
Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflicts of interest are associated with this manuscript.
Contributor Information
Issaka Sagara, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Sara A. Healy, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Mahamadoun H. Assadou, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Mamady Kone, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Bruce J. Swihart, Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Jennifer L. Kwan, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Jonathan Fintzi, Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Kourane Sissoko, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Bourama Kamate, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Yacouba Samake, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Merepen A. Guindo, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
M’Bouye Doucoure, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Karamoko Niaré, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Amagana Dolo, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Balla Diarra, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Kelly M. Rausch, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
David L. Narum, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
David S. Jones, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Nicholas J. MacDonald, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Daming Zhu, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
J. Patrick Gorres, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Alemush Imeru, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Rathy Mohan, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Ismaila Thera, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Irfan Zaidi, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Fernando Salazar-Miralles, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Junhui Duan, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Jillian Neal, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Robert D. Morrison, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA
Olga Muratova, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Daman Sylla, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Elise M. O’Connell, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Yimin Wu, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Jen. C.C. Hume, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Mamadou B. Coulibaly, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Charles F. Anderson, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Sekou F. Traore, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Ogobara K. Doumbo, Malaria Research and Training Center, Mali- National Institute of Allergy and Infectious Diseases International Center for Excellence in Research, University of Sciences, Techniques and Technologies of Bamako, Mali.
Patrick E. Duffy, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Data Sharing
All data associated with this study are present in the paper or Supplementary Materials and are available from the authors upon reasonable request. Individual level deidentified participant data will be made available with publication and upon execution of inter-institutional human data sharing agreement. Data can include all those described in the manuscript.
References
- 1.Sagara I, Healy SA, Assadou MH, et al. Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: a randomised, double-blind, comparator-controlled, dose-escalation study in healthy Malian adults. Lancet Infect Dis 2018; 18(9): 969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. World Malaria Report 2021. Geneva, Switzerland: World Health Organization, 2021. [Google Scholar]
- 3.WHO. WHO recommends groundbreaking malaria vaccine for children at risk. Geneva: World Health Organization; 2021. [Google Scholar]
- 4.Datoo MS, Natama HM, Some A, et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years' follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect Dis 2022; 22(12): 1728–36. [DOI] [PubMed] [Google Scholar]
- 5.Sissoko MS, Healy SA, Katile A, et al. Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: a randomised, controlled phase 1 trial. Lancet Infect Dis 2022; 22(3): 377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter R, Chen DH. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature 1976; 263(5572): 57–60. [DOI] [PubMed] [Google Scholar]
- 7.Gwadz RW. Successful immunization against the sexual stages of Plasmodium gallinaceum. Science 1976; 193(4258): 1150–1. [DOI] [PubMed] [Google Scholar]
- 8.Carter R, Kaushal DC. Characterization of antigens on mosquito midgut stages of Plasmodium gallinaceum. III. Changes in zygote surface proteins during transformation to mature ookinete. Molecular and biochemical parasitology 1984; 13(2): 235–41. [DOI] [PubMed] [Google Scholar]
- 9.Grotendorst CA, Kumar N, Carter R, Kaushal DC. A surface protein expressed during the transformation of zygotes of Plasmodium gallinaceum is a target of transmission-blocking antibodies. Infection and immunity 1984; 45(3): 775–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talaat KR, Ellis RD, Hurd J, et al. Safety and Immunogenicity of Pfs25-EPA/Alhydrogel(R), a Transmission Blocking Vaccine against Plasmodium falciparum: An Open Label Study in Malaria Naive Adults. PioS one 2016; 11(10): e0163144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healy SA, Anderson C, Swihart BJ, et al. Pfs230 yields higher malaria transmission-blocking vaccine activity than Pfs25 in humans but not mice. J Clin Invest 2021; 131(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diallo DA, Doumbo OK, Plowe CV, Wellems TE, Emanuel EJ, Hurst SA. Community permission for medical research in developing countries. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2005; 41(2): 255–9. [DOI] [PubMed] [Google Scholar]
- 13.Shimp RL Jr., Rowe C, Reiter K, et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 2013; 31(28): 2954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald NJ, Nguyen V, Shimp R, et al. Structural and Immunological Characterization of Recombinant 6-Cysteine Domains of the Plasmodium falciparum Sexual Stage Protein Pfs230. J Biol Chem 2016; 291(38): 19913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkhardt M, Reiter K, Nguyen V, et al. Assessment of the impact of manufacturing changes on the physicochemical properties of the recombinant vaccine carrier ExoProtein A. Vaccine 2019; 37(38): 5762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidance for industry: Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. U.S. Department of Health and Human Services, Food and Drug Administration Center for Biologics Evaluation and Research; September 2007. [Google Scholar]
- 17.Easton AV, Oliveira RG, O'Connell EM, et al. Multi-parallel qPCR provides increased sensitivity and diagnostic breadth for gastrointestinal parasites of humans: field-based inferences on the impact of mass deworming. Parasit Vectors 2016; 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter R, Graves PM, Quakyi IA, Good MF. Restricted or absent immune responses in human populations to Plasmodium falciparum gamete antigens that are targets of malaria transmission-blocking antibodies. The Journal of experimental medicine 1989; 169(1): 135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White MT, Verity R, Churcher TS, Ghani AC. Vaccine approaches to malaria control and elimination: Insights from mathematical models. Vaccine 2015; 33(52): 7544–50. [DOI] [PubMed] [Google Scholar]
- 20.Brickley EB, Coulibaly M, Gabriel EE, et al. Utilizing direct skin feeding assays for development of vaccines that interrupt malaria transmission: A systematic review of methods and case study. Vaccine 2016; 34(48): 5863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck Z, Matyas GR, Jalah R, Rao M, Polonis VR, Alving CR. Differential immune responses to HIV-1 envelope protein induced by liposomal adjuvant formulations containing monophosphoryl lipid A with or without QS21. Vaccine 2015; 33(42): 5578–87. [DOI] [PubMed] [Google Scholar]
- 22.Radtke AJ, Anderson CF, Riteau N, et al. Adjuvant and carrier protein-dependent T-cell priming promotes a robust antibody response against the Plasmodium falciparum Pfs25 vaccine candidate. Sci Rep 2017; 7: 40312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scaria PV, Anderson C, Muratova O, et al. Malaria transmission-blocking conjugate vaccine in ALFQ adjuvant induces durable functional immune responses in rhesus macaques. NPJ Vaccines 2021; 6(1): 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scaria PV, Chen BB, Rowe CG, et al. Comparison of carrier proteins to conjugate malaria transmission blocking vaccine antigens, Pfs25 and Pfs230. Vaccine 2020; 38(34): 5480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goncalves BP, Kapulu MC, Sawa P, et al. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun 2017; 8(1): 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or Supplementary Materials and are available from the authors upon reasonable request. Individual level deidentified participant data will be made available with publication and upon execution of inter-institutional human data sharing agreement. Data can include all those described in the manuscript.