Abstract
Objective:
We examined the long-term effects of premenopausal bilateral oophorectomy (PBO) with or without concurrent or preceding hysterectomy on physical and cognitive function and on odds of chronic conditions.
Methods:
We enrolled 274 women with PBO with or without concurrent or preceding hysterectomy and 240 referents aged 55 and older who were residents of Olmsted County, MN as of the PBO or index date. Chronic conditions were assessed via medical record abstraction. Cognitive diagnoses were based on neurocognitive testing. A physical function assessment included measures of strength and mobility. Multivariable regression models compared characteristics for women with PBO <46 years, PBO 46–49 years, and referent women with adjustments for age and other confounders.
Results:
The clinical visits (median age=67) were a median of 22 years after the PBO or index date. Of 274 women with PBO, 161 (59%) were <46 years at PBO and 113 (41%) were 46–49 years. Compared to referents, women with a history of PBO <46 years had increased odds of arthritis (odds ratio [OR], 1.64; 95% confidence interval [CI], 1.06–2.55), asthma (OR, 1.74; 95% CI, 1.03–2.93), obstructive sleep apnea (OR, 2.00; 95% CI, 1.23–3.26), and bone fractures (OR, 2.86; 95% CI, 1.17–6.98), and walked a shorter mean distance on a 6-minute walk test (b, −18.43; P, 0.034). Compared to referents, women with a history of PBO at age 46–49 years had increased odds of arthritis (OR, 1.92; 95% CI, 1.16–3.18) and obstructive sleep apnea (OR, 2.21; 95% CI, 1.33–3.66). There were no significant differences in cognitive status in women with PBO compared to referents.
Conclusions:
Women with a history of PBO with or without concurrent or preceding hysterectomy, especially at age <46 years, have more chronic conditions in late mid-life compared to referents.
Keywords: Premenopausal bilateral oophorectomy, Chronic conditions, Physical function, Aging
Introduction
Hysterectomy is the second most frequently performed surgical operation for women after cesarean section. Historically, an estimated 23% of women aged 40–44 years and 45% of women aged 45–49 years have undergone premenopausal bilateral oophorectomy (PBO) at the time of hysterectomy for the prevention of subsequent ovarian cancer.1,2 PBO was often performed in women at average risk of ovarian cancer. However, there is increasing concern that PBO may have harmful long-term effects that may negate the benefit conferred by protection from ovarian cancer,3 particularly among women with an average baseline risk of ovarian cancer, who are the majority of women undergoing this surgery. As a result of this new evidence, the rates of PBO have started to decline, at least in some geographical areas.4
In addition to their reproductive function, the ovaries are important endocrine organs that secrete hormones both before (primarily estrogen, progesterone, and testosterone) and after menopause (primarily testosterone, and androstenedione). Disruption of the hypothalamic-pituitary-ovarian axis due to removal of the ovaries is associated with an abrupt increase in gonadotropins (luteinizing hormone and follicle-stimulating hormone). Thus, ovaries have many important non-reproductive actions mediated via receptors spread throughout most tissues and organs of the body including the brain, muscle, bone, blood vessels, heart, and the gastrointestinal tract.5 As a result, removal of the ovaries, especially before natural menopause, can contribute to significant endocrine disruption, affecting multiple organs and systems throughout the body. Indeed, some studies suggest associations between PBO and increased risk of cardiovascular disease,6–9 cognitive impairment or dementia,10,11 and multimorbidity, a clinical marker of aging, compared to women with ovarian conservation.12 Notably, most studies found that the risk of these outcomes was greatest for women who underwent PBO before the age of 46 years.3 Use of estrogen and/or progesterone therapy among women who underwent PBO may attenuate some of these associations if used up to the median age of natural menopause.
Studies of PBO with or without hysterectomy to date have primarily utilized passive collection of outcomes through medical record abstraction, diagnostic codes, or hospital records. As a result, it is not clear whether specific domains of physical function or other aging-related measures are more affected than others. The current study was conducted to measure outcomes requiring in-person assessment such as physical function estimates, frailty, and functional scales. We hypothesized that the significant endocrine disruption caused by premenopausal PBO with or without hysterectomy would contribute to accelerated aging, as measured by a greater decline in physical and cognitive function, and that these declines would be most pronounced among women who underwent PBO at younger ages (<46 years). To test this hypothesis, we recruited women with a history of PBO with or without concurrent or preceding hysterectomy and age-matched referent women who were previously passively followed through medical record abstraction in the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2). Referent women in the MOA-2 study were randomly selected from the general population and the majority of women had not undergone any gynecologic surgery. This selection of referent women allowed us to address the broad epidemiological question of the effects of PBO with or without hysterectomy on long-term outcomes. In-person clinical visits collected objective information on physical and cognitive function and body composition at a median age of 67 years of age.
METHODS
Study Sample
We utilized the existing infrastructure of the Rochester Epidemiology Project (REP) medical records-linkage system and the established Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2). The MOA-2 study passively identified all women who underwent PBO with or without concurrent or preceding hysterectomy (defined as complete removal of both ovaries or as a second unilateral oophorectomy) for a non-malignant indication between 1988 and 2007 and age-matched referent women who had not undergone PBO before the index date. All women gave consent to use their medical records for research. The methods used to identify women who underwent PBO with or without hysterectomy have been described previously.13 Details about the Olmsted County population have been reported elsewhere.14–17 Briefly, the electronic indexes of the REP were searched for surgical procedure codes for unilateral or bilateral oophorectomy assigned from January 1, 1988 through December 31, 2007. Women were excluded if they underwent PBO with or without hysterectomy: 1) for ovarian cancer (primary or metastatic); 2) for the treatment of another estrogen-sensitive malignancy (usually breast cancer); or 2) for a high risk of ovarian cancer as judged by the gynecologist or confirmed by genetic testing. The study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
As of March 1, 2018, the start of the current in-person study, 1,522 of the 1,661 (91.6%) women who underwent PBO with or without hysterectomy and 1,471 of 1,580 (93.1%) referent women were alive. Women were eligible for the current study if they were: 1) identified and passively followed in MOA-2; 2) aged 55 years and older at enrollment; 3) more than six months post chemotherapy or major surgery requiring general anesthesia; and 4) willing and able to sign the informed consent. Women were ineligible if they: 1) were not able to read or speak English; 2) were receiving hospice care; 3) did not have a residence within approximately 200 miles of Rochester, MN due to the need for in-person clinic visits; 4) were found to have a malignancy on the pathology report after PBO; or 5) did not have a clinical visit documented in the REP in the past 5 years.
We created one randomized list that included all women who underwent PBO with or without hysterectomy and referent women, and we started recruitment from the top. Thus, recruitment of women into the current study was not age-matched. We contacted 916 women with a history of PBO with or without hysterectomy and 896 referent women for recruitment (Fig 1). We enrolled 274 women (29.9% of those contacted) with a history of PBO and 240 referent women (26.8% of those contacted) in the present study (Figure 1).
FIG. 1.
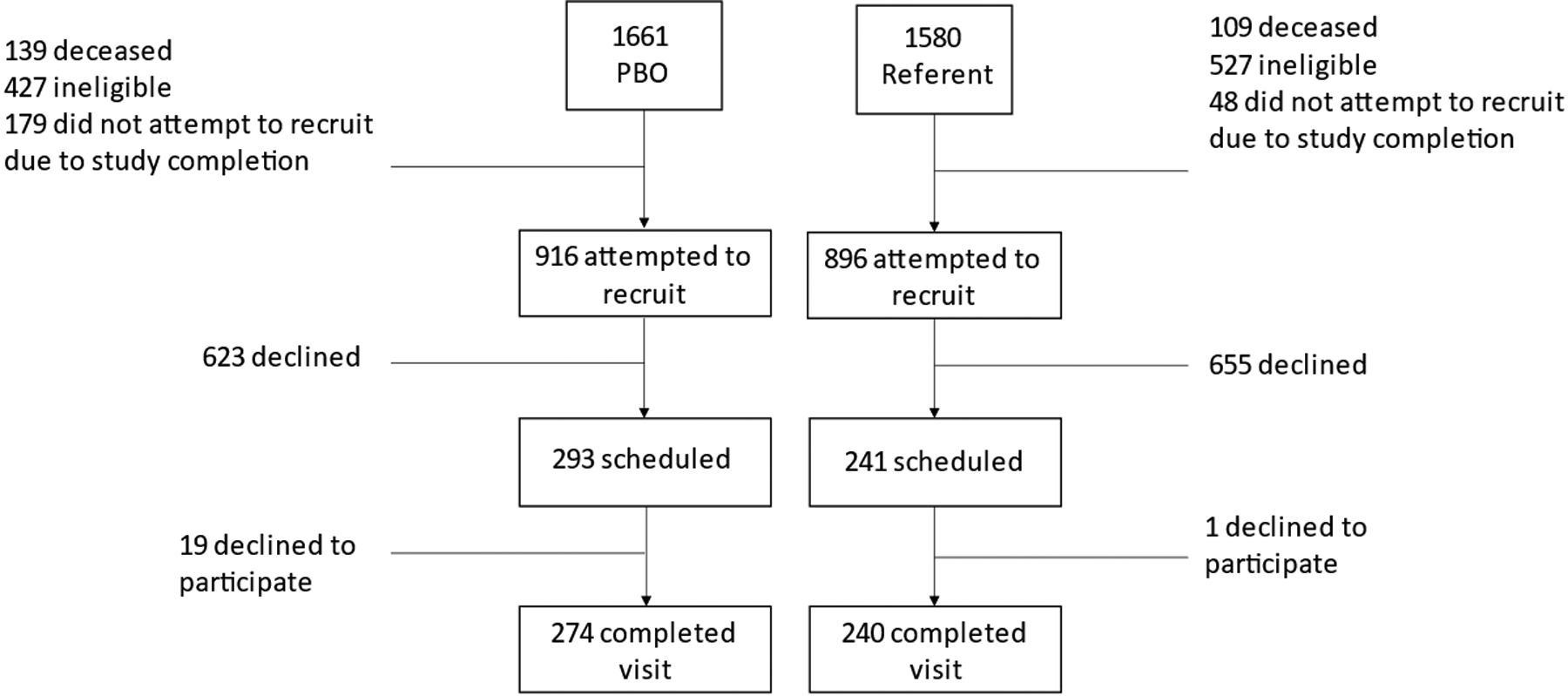
CONSORT diagram. PBO, premenopausal bilateral oophorectomy with or without concurrent or preceding hysterectomy.
To examine a possible participation bias, chi-square tests were used to compare eligible women who did and did not enroll for the frequencies of eighteen Department of Health and Human Services (DHHS) chronic conditions present in the medical record within five years of the study initiation. The enrolled women with PBO were less likely to have a DHHS code for hypertension (39% vs 52%, P = 0.002), cancer (10% vs 16%, P = 0.04), or chronic kidney disease (10% vs 15%, P = 0.048) compared to women with PBO who declined to enroll (Supplemental Digital Content 1). Among the referent women, those who enrolled were less likely to have DHHS codes for chronic obstructive pulmonary disease (COPD; 2.6% vs 6.3%, P = 0.048) or coronary artery disease (2.6% vs 6.7%, P = 0.03) compared to referent women who did not enroll (Supplemental Digital Content 2).
This study is a subsample of the original MOA-2 study. The sample size was designed to measure differences in physical function scales, neuroimaging outcomes and cognitive scales (to be reported elsewhere). The sample size was also determined by the cost of the imaging tests. Although the current study is smaller than several of the previous passive data collection studies, it is large when considering the in-person physical function assessments
Data Collection
At the time of the PBO with or without concurrent or preceding hysterectomy or index date, the following variables were passively collected from the medical records: ovarian indication for PBO and subsequent pathology, other gynecological surgeries (e.g., hysterectomy), education, and smoking status. Income was assessed via census data. The in-person study visit included self-reported demographics (age, race, marital status, education), a medical history, smoking status, limited physical examination, blood collection, multiple questionnaires, a DEXA scan (dual energy x-ray absorptiometry), neurocognitive testing, and a physical function assessment. The medical history included reproductive history, assessment of symptoms or chronic medical conditions, and use of medications, including estrogen therapy. Medical records were also abstracted to confirm medical diagnoses and medication use.
The physical examination included the assessment of height and weight to calculate body mass index (BMI); measurement of abdominal girth, hip circumference, and waist-hip ratio; blood pressure; and pulse rate. DEXA scans (Lunar IDEXA, GE Healthcare Lunar, Madison, WI) were utilized to measure spine bone mineral density (BMD), the ratio of trunk to hip body fat (android/gynoid fat ratio), appendicular lean mass (ALM), and percent fat mass.18
Physical function was assessed as the total meters walked in 6 minutes.19 Maximal muscle strength of the upper and lower extremities was assessed using the Keiser A420 research grade exercise system (Keiser Inc., Fresno, CA). We determined the One-Repetition Maximum (1RM) for the leg press as a measure of lower extremity strength, and the 1RM for the chest press as a measure of upper body strength.20 Grip strength was assessed in the dominant hand and measured with a Jamar electronic dynamometer (NK Biotechnical Corp., Minneapolis, MN). The Short Physical Performance Battery (SPPB), a validated measure of functional performance comprising measures of standing balance, gait speed, and repeated chair rise time, was also conducted.21
The neurocognitive testing included 11 tests covering four cognitive domains. Tests of memory included the Auditory Verbal Learning Test and Wechsler Memory Scale-Revised Logical Memory and Visual Reproduction.22,23 An index of working memory was obtained from the Letter-Number Sequencing and Spatial Span subtests of the Wechsler Memory Scale, 3rd Edition.24 Tests of language included the Boston Naming Test and Category Fluency.25,26 Tests of attention/executive function included the Trail Making Test and the Wechsler Memory Scale-Revised Digit Symbol subtest.27,28 Tests of visuospatial function included the Wechsler Memory Scale-Revised Picture Completion and Block Design subtests.28 In addition, the modified Mini-Mental State Exam was administered. The study neuropsychologist utilized the individual test age-adjusted scaled scores for each participant to determine the cognitive classification based on published criteria for MCI and dementia.29,30
Statistical analyses
Demographic characteristics were summarized using medians and interquartile ranges or frequencies and percentages. The associations between PBO with or without concurrent or preceding hysterectomy and outcomes were examined using three comparisons: all women with PBO versus referent women (n=274 vs n=240); women with Early PBO (<46 years) versus referent women (n=161 vs n=240); and women with Late PBO (aged 46–49) versus referent women (n=113 vs n=240). Logistic regression was used to compare binary or categorical variables, and linear regression was used to compare continuous variables. Multivariable linear and logistic regression models were used to compare characteristics or comorbidities adjusting for age. Additionally, models examining systolic or diastolic blood pressure were adjusted for the use of antihypertensive medications. Sensitivity analyses were performed to assess the effect of estrogen therapy among women who underwent PBO with or without hysterectomy and the impact of adjusting for BMI. In addition, we repeated the analyses stratified by ovarian indication for the PBO to assess whether the association between PBO and any of the outcomes (chronic conditions, physical function, body composition measures) differed by ovarian indication. Statistical testing was conducted at the conventional two-sided alpha=0.05 and results were not adjusted for multiple testing. Analyses were conducted in SAS (version 9.4; Cary, NC).
RESULTS
Participant characteristics
Of the 274 women with a history of PBO, 161 (58.8%) underwent the PBO procedure before the age of 46 years, 113 (41.2%) underwent the procedure between the ages of 46 and 49 years, 16 (5.8%) had a hysterectomy prior to the PBO, 9 (3.3%) did not have a hysterectomy concurrent with or prior to the PBO, and 249 (90.9%) had a hysterectomy concurrently with the PBO. There was no ovarian indication for 52.8% of women with Early PBO with or without hysterectomy (aged <46 years) and for 76.1% of women with Late PBO with or without hysterectomy (aged 46–49 years). Women who underwent PBO had lower income at the time of the PBO and were more like to be currently taking an antihypertensive and to be obese compared to referent women at the time of the in-person clinic visit (Table 1). As expected, women who underwent Early PBO were younger at the study visit compared to women who underwent Late PBO (Table 1).
TABLE 1.
Characteristics of women by age at premenopausal bilateral oophorectomy (PBO) with or without concurrent or preceding hysterectomy or referents at the time of the in-person visit, as median (IQR) or n(%), adjusted for age
| Referents | Early PBO (<46 years) | Late PBO (46–49 years) | PBO vs. Ref | Early PBO vs. Ref | Late PBO vs. Ref | |
|---|---|---|---|---|---|---|
| Characteristics | (N = 240) | (N = 161) | (N = 113) | P | P | P |
| Current visit age, y | 67.2 (63.6–−71.0) | 64.6 (61.2–−69.0) | 68.3 (64.8–−72.8) | 0.099 | <0.0001 | 0.013 |
| Racea | 0.264 | 0.281 | 0.528 | |||
| White | 233 (97.1%) | 159 (98.8%) | 111 (98.2%) | |||
| Black | 1 (0.4%) | - | 1 (0.9%) | |||
| Asian | 5 (2.1%) | 1 (0.6%) | - | |||
| Other | 1 (0.4%) | 1 (0.6%) | 1 (0.9%) | |||
| Education, y | 0.676 | 0.438 | 0.855 | |||
| High school or less | 32 (13.3%) | 30 (18.6%) | 17 (15.1%) | |||
| Some college | 91 (37.9%) | 64 (39.8%) | 44 (38.9%) | |||
| 4-year college degree | 70 (29.2%) | 41 (25.5%) | 22 (19.5%) | |||
| Graduate or professional degree | 47 (19.6%) | 26 (16.1%) | 30 (26.6%) | |||
| Current marital status | 0.286 | 0.468 | 0.287 | |||
| Never married | 12 (5.0%) | 6 (3.7%) | 8 (6.9%) | |||
| Separated/divorced | 41 (17.1%) | 30 (18.3%) | 10 (8.6%) | |||
| Widowed | 18 (7.5%) | 8 (4.9%) | 10 (8.6%) | |||
| Married | 165 (68.8%) | 117 (71.3%) | 83 (71.6%) | |||
| Living as married | 4 (1.7%) | 2 (1.2%) | 4 (3.4%) | |||
| Income quartile | 0.053 | 0.033 | 0.383 | |||
| <$42,000 | 39 (16.3%) | 29 (18.0%) | 20 (17.7%) | |||
| $42,000–56,999 | 58 (24.2%) | 49 (30.4%) | 31 (27.4%) | |||
| $57,000–71,999 | 67 (27.9%) | 52 (32.2%) | 31 (27.4%) | |||
| $72,000+ | 76 (31.7%) | 31 (19.3%) | 31 (27.4%) | |||
| Systolic BP (mmHg)b | 134.0 (122.5–149.0) | 128.7 (118.0–138.0) | 135.6 (122.0–149.0) | 0.017 | 0.005 | 0.599 |
| Diastolic BP (mmHg)b | 77.0 (71.0–83.0) | 76.8 (72.0–83.0) | 78.9 (74.0–84.0) | 0.400 | 0.740 | 0.016 |
| Current antihypertensive medication | 126 (52.5%) | 94 (58.4%) | 77 (68.1%) | 0.009 | 0.075 | 0.018 |
| BMI (kg/m2) | 27.8 (24.2–32.3) | 29.2 (24.2–33.6) | 30.8 (26.6–34.8) | 0.085 | 0.719 | 0.006 |
| Obese | 82 (34.2%) | 65 (40.4%) | 73 (48.7%) | 0.026 | 0.206 | 0.022 |
| Abdominal girth (cm) | 96.7 (87.0–108.5) | 98.3 (85.4–109.0) | 101.5 (91.0–110.0) | 0.150 | 0.752 | 0.027 |
| Hip measurement (cm) | 107.3 (99.0–116.4) | 109.8 (99.5–117.1) | 112.4 (103.6–119.5) | 0.309 | 0.928 | 0.041 |
| Waist/hip ratio | 0.9 (0.8–0.9) | 0.9 (0.8–0.9) | 0.9 (0.9–1.0) | 0.229 | 0.446 | 0.277 |
| Smoking status | 0.632 | 0.539 | 0.906 | |||
| Never | 146 (60.8%) | 93 (57.8%) | 68 (60.2%) | |||
| Former or current | 94 (39.2%) | 68 (42.2%) | 45 (39.8%) | |||
| Estrogen use at 50th birth date | 33 (13.8%) | 110 (68.3%) | 88 (77.9%) | <0.0001 | <0.0001 | <0.0001 |
| Menopause or PBO age, y | 50 (48–52) | 42 (40–44) | 47 (46–48) | NA | NA | NA |
| Any endometriosis during pathology | NA | 51 (31.7%) | 22 (22.1%) | NA | NA | NA |
| Hysterectomy status | NA | NA | NA | |||
| None | 218 (90.8%) | 2 (1.2%) | 7 (6.2%) | |||
| Before index date | 22 (9.2%) | 12 (7.5%) | 4 (3.5%) | |||
| Concurrent | NA | 147 (91.3%) | 102 (90.3%) | |||
| Ovarian indication | NA | NA | NA | |||
| None | NA | 85 (52.8%) | 86 (76.1%) | |||
| Benign | NA | 76 (47.2%) | 27 (23.9%) |
BMI, body mass index; BP, blood pressure; IQR, interquartile range; NA, not applicable; PBO, premenopausal bilateral oophorectomy.
Models compare white vs. non-white.
These models were adjusted for age and anti-hypertensive medications.
Associations between PBO with or without hysterectomy and chronic medical conditions
Compared to referent women, women with a history of PBO with or without hysterectomy had greater odds of arthritis (OR, 1.74; 95% CI, 1.19–2.53), COPD (OR, 2.69; 95% CI, 1.03–7.05), and obstructive sleep apnea (OR, 2.06; 95% CI, 1.36–3.11) (Figure 2 and Table 2). In additional analyses, associations were stratified by age at PBO. Compared to referent women, women with a history of Early PBO, had increased odds of arthritis (OR, 1.64; 95% CI, 1.06–2.55), asthma (OR, 1.74; 95% CI, 1.03–2.93), obstructive sleep apnea (OR, 2.00; 95% CI, 1.23–3.26), and bone fractures (OR, 2.86; 95% CI, 1.17–6.98). Women with a history of Late PBO had increased odds of arthritis (OR, 1.92; 95% CI, 1.16–3.18) and obstructive sleep apnea (OR, 2.21; 95% CI, 1.33–3.66) compared to referents. There were no associations between PBO with or without hysterectomy and MCI ordementia.
FIG. 2.
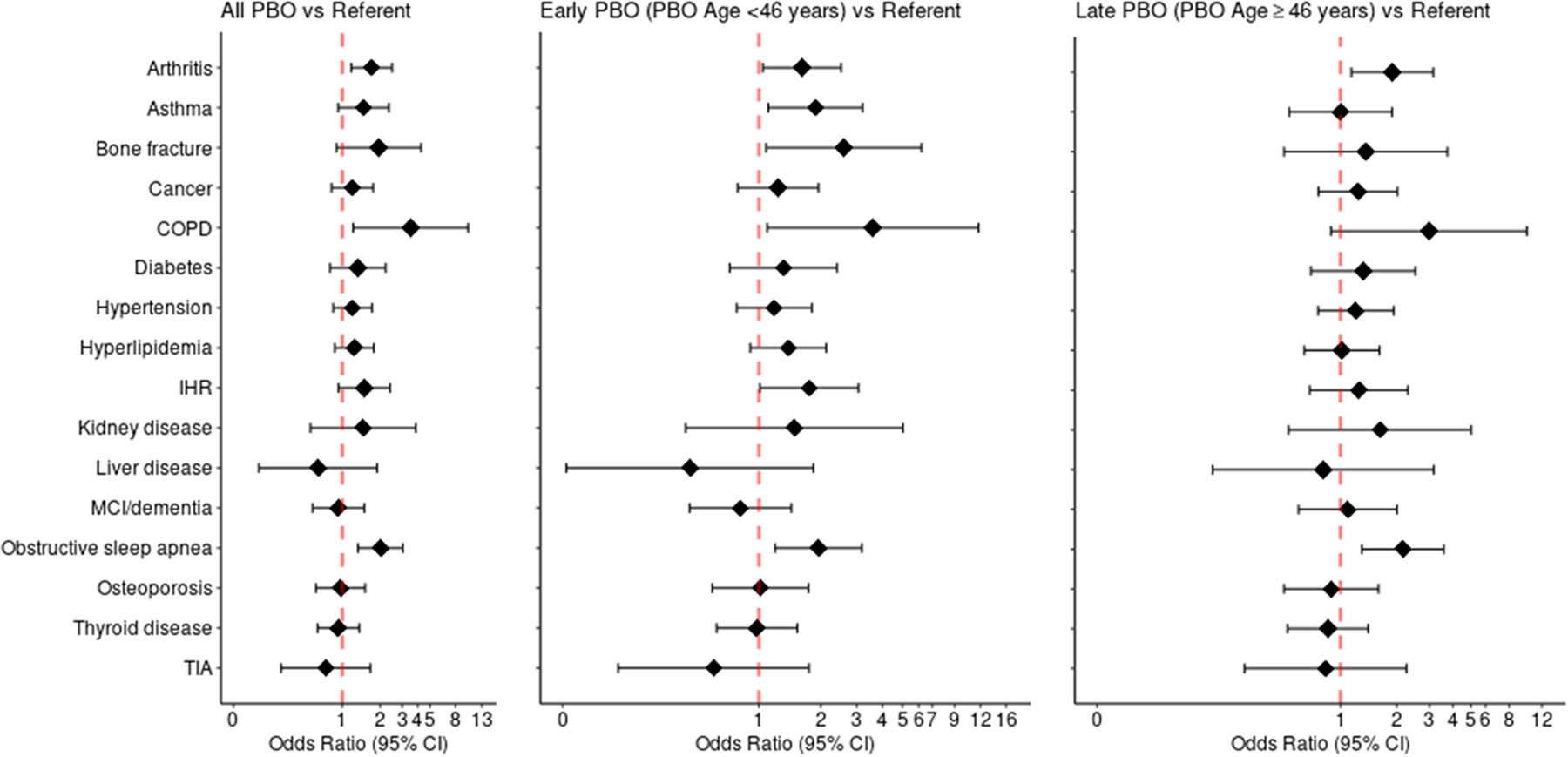
Odds ratios of chronic conditions associated with premenopausal bilateral oophorectomy (PBO) with or without concurrent or preceding hysterectomy, early PBO, and late PBO, adjusted for age. CI, confidence interval; COPD, chronic obstructive pulmonary disease; IHR, irregular heart rhythm; MCI, mild cognitive impairment; PBO, premenopausal bilateral oophorectomy; TIA, transient ischemic attack.
TABLE 2.
Associations of premenopausal bilateral oophorectomy (PBO), with or without concurrent or preceding hysterectomy, with odds of chronic conditions overall and by age at PBO
| All PBO vs. referents | Early PBO (<46 years) vs. referents | Late PBO (46–49 years) vs. referents | ||||
|---|---|---|---|---|---|---|
| Chronic condition | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Arthritis | 1.74 (1.19–2.53) | 0.004 | 1.64 (1.06–2.55) | 0.026 | 1.92 (1.16–3.18) | 0.011 |
| Asthma | 1.37 (0.87–2.18) | 0.176 | 1.74 (1.03–2.93) | 0.037 | 0.94 (0.50–1.76) | 0.848 |
| Bone fracture | 2.17 (0.97–4.83) | 0.059 | 2.86 (1.17–6.98) | 0.021 | 1.52 (0.54–4.25) | 0.426 |
| Cancer | 1.19 (0.81–1.74) | 0.371 | 1.21 (0.77–1.91) | 0.402 | 1.25 (0.76–2.03) | 0.378 |
| COPD | 2.69 (1.03–7.05) | 0.044 | 2.86 (0.96–8.59) | 0.060 | 2.25 (0.58–6.97) | 0.161 |
| Diabetes | 1.32 (0.79–2.20) | 0.287 | 1.31 (0.72–2.38) | 0.378 | 1.31 (0.69–2.49) | 0.412 |
| Hypertension | 1.11 (0.78–1.58) | 0.577 | 1.08 (0.71–1.64) | 0.725 | 1.12 (0.70–1.78) | 0.634 |
| Hyperlipidemia | 1.18 (0.83–1.69) | 0.359 | 1.32 (0.86–2.02) | 0.200 | 0.96 (0.61–1.53) | 0.878 |
| Irregular heart rhythm | 1.542 (0.89–2.27) | 0.146 | 1.68 (0.97–2.91) | 0.064 | 1.18 (0.64–2.15) | 0.594 |
| Kidney disease | 1.47 (0.56–3.89) | 0.435 | 1.48 (0.44–5.00) | 0.526 | 1.64 (0.53–5.09) | 0.388 |
| Liver disease | 0.71 (0.23–2.17) | 0.546 | 0.51 (0.13–2.08) | 0.347 | 0.89 (0.22–3.55) | 0.868 |
| MCI/dementia | 0.90 (0.56–1.45) | 0.665 | 0.79 (0.77–1.91) | 0.406 | 1.06 (0.58–1.94) | 0.850 |
| Obstructive sleep apnea | 2.06 (1.36–3.11) | <0.001 | 2.00 (1.23–3.26) | 0.005 | 2.21 (1.33–3.66) | 0.002 |
| Osteoporosis | 1.00 (0.63–1.57) | 0.997 | 1.06 (0.62–1.82) | 0.836 | 0.92 (0.51–1.64) | 0.768 |
| Thyroid disease | 0.96 (0.65–1.41) | 0.834 | 1.02 (0.65–1.60) | 0.942 | 0.88 (0.53–1.45) | 0.607 |
| TIA | 0.78 (0.34–1.78) | 0.553 | 0.64 (0.22–1.88) | 0.417 | 0.87 (0.32–2.39) | 0.787 |
All models adjusted for age.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; MCI, mild cognitive impairment; OR, odds ratio; PBO, premenopausal bilateral oophorectomy; TIA, transient ischemic attack.
Association between PBO with or without hysterectomy and physical function or body composition
Women who underwent Early PBO with or without hysterectomy (b = −18.43, P = 0.034), but not Late PBO with or without hysterectomy (b = −8.65, P = 0.336), walked a shorter distance in the 6-minute walk test (Table 3). However, there were no associations between PBO and total SPPB score or measures of muscle strength including 1RM chest and leg press and grip strength. Overall, women who underwent PBO had a higher upper to lower body fat ratio compared to referent women and this finding was driven by women who underwent Late PBO. In addition, women who underwent Late PBO, but not Early PBO, had higher mean percent fat mass, android/gynoid fat ratio, ALM, and spine BMD.
TABLE 3.
Associations of premenopausal bilateral oophorectomy (PBO), with or without concurrent or preceding hysterectomy, with measures of physical function and body composition overall and by age at PBO
| All PBO vs. referents | Early PBO (<46 years) vs. referents | Late PBO (46–49 years) vs. referents | ||||
|---|---|---|---|---|---|---|
| Physical function and body composition | Parameter estimate (SE) | P | Parameter estimate (SE) | P | Parameter estimate (SE) | P |
| 6-minute walk, meters | −14.05 (7.15) | 0.049 | −18.43 (8.69) | 0.034 | −8.65 (8.98) | 0.336 |
| 1RM chest, newtons | −4.29 (6.29) | 0.495 | −5.02 (7.29) | 0.492 | −3.00 (8.03) | 0.709 |
| 1RM legs, newtons | 10.41 (24.46) | 0.671 | −0.09 (28.94) | 0.997 | 16.68 (30.90) | 0.600 |
| Total SPPB score | 0.02 (0.11) | 0.880 | −0.05 (0.14) | 0.729 | 0.12 (0.14) | 0.405 |
| Grip strength, kg | −0.61 (0.44) | 0.168 | −0.62 (0.50) | 0.219 | −0.50 (0.58) | 0.393 |
| % Fat mass | 1.25 (0.65) | 0.054 | 0.43 (0.78) | 0.587 | 2.34 (0.83) | 0.005 |
| Android/gynoid fat ratioa | 0.033 (0.015) | 0.030 | 0.020 (0.018) | 0.269 | 0.047 (0.019) | 0.015 |
| ALM (g/m2) | 0.11 (0.10) | 0.271 | −0.03 (0.12) | 0.802 | 0.303 (0.13) | 0.018 |
| Spine BMD | 0.023 (0.01) | 0.117 | 0.006 (0.02) | 0.740 | 0.045 (0.02) | 0.016 |
All models adjusted for age.
1RM, One-Repetition Maximum; ALM, appendicular lean mass; BMD, bone mineral density; PBO, premenopausal bilateral oophorectomy; SE, standard error; SPPB, Short Physical Performance Battery.
Android/gynoid fat ratio is indicative of trunk to hip fat ratio.
Sensitivity analyses
We additionally assessed the effects of the use of estrogen therapy up to the age of 50 years among women with PBO with or without hysterectomy. Of the 274 women who underwent PBO, 76 (27.7%) did not use estrogen therapy up to age 50 years, including 51 of 161 (31.7%) who underwent PBO <46 years and 25 of 113 (22.1%) who underwent PBO between the ages of 46–49 years. We excluded these 76 women and repeated the analyses. Although some of the associations were slightly attenuated, partially due to the smaller sample size, the results generally remained similar to those from the primary analyses (Supplemental Digital Content 3 and Supplemental Digital Content 4). In additional sensitivity analyses, we adjusted for BMI in all models, but the results did not change. Lastly, we examined whether the association between PBO with or without hysterectomy and any of the outcomes (chronic conditions, physical function, body composition measures) differed by ovarian indications. We found no statistically significant differences (all p-values>0.05), indicating that ovarian indication did not affect the observed associations (data not shown).
DISCUSSION
In this community-based study, we comprehensively assessed the relationship between PBO with or without concurrent or preceding hysterectomy and chronic conditions, cognitive status, and physical function among women at a median age of 67 years. Women who underwent PBO with or without hysterectomy had increased odds of arthritis, COPD, and obstructive sleep apnea compared to women who did not. When stratified by age at PBO, women who underwent Early PBO had increased odds of arthritis, asthma, bone fractures, and obstructive sleep apnea and walked fewer meters on average in a 6-minute walk test. These results further emphasize that PBO with or without hysterectomy performed for a benign gynecologic condition or for ovarian cancer prevention, especially before the age of 46 years, is associated with increased odds of multiple chronic conditions.
Previous studies, including the larger passively followed MOA-2 study from which the women were recruited, have reported associations between PBO and an increased risk of cardiovascular disease,6–9 stroke,8 arthritis,12 asthma,12 COPD31 and the accelerated accumulation of multiple chronic conditions and a multimorbidity score.12 Compared to the larger MOA-2 study, results were similar in this subset of women recruited for an in-person visit, including the greater odds of several of these conditions for women who underwent Early PBO. Although there was a trend, we did not find significant associations between PBO with or without hysterectomy and odds of hypertension, hyperlipidemia, or diabetes, likely due to the smaller sample size. These results suggest that the participants in the present study are representative of the larger passively-followed MOA-2 cohort.12,13
Although we did not find an association between PBO with or without hysterectomy and osteoporosis, Early PBO was associated with increased odds of bone fractures. A potential explanation for the lack of association with osteoporosis is the high percent of women with PBO who took estrogen therapy up to the age of 50 years. Bone density is not the only determinant of bone fragility, and fractures may occur even in women with normal bone density if the bone quality is compromised.
PBO has been associated with an increased risk of cognitive impairment and dementia.10,11,32–36 In the current study, we did not find an association between PBO with or without hysterectomy and a diagnosis of MCI or dementia. One potential explanation is the younger age of our cohort compared to previous studies because the prevalence of cognitive impairment greatly increases with age. Recent studies of younger women have suggested that PBO may be associated with subjective cognitive decline37 or with lower performance in specific cognitive domains including scene memory, working memory, or attention/executive function.11,38 Future research in this cohort will assess the association between PBO with or without hysterectomy and specific cognitive domains or subjective cognitive complaints.
Previous studies examining the relationship between PBO and physical function yielded conflicting results. Two studies reported that women who underwent PBO, mainly before the age of 45 years, self-reported more physical function limitations, had greater declines in gait speed, and an increased risk of disability.39,40 However, self-reported PBO was not associated with a greater risk of pre-frailty or frailty over 18 years of follow-up in the Study of Osteoporotic Fractures.41 Our results are more consistent with the first two studies because women who underwent PBO with or without hysterectomy before the age of 46 years walked shorter distances in the 6-minute walk test.
Both BMI and fat mass have been shown to increase after PBO, and lean mass to decrease, with an earlier age of PBO associated with a greater change.42,43 In the present study, we found higher fat mass among women who underwent Late PBO with or without hysterectomy, but not Early PBO with or without hysterectomy, compared to referent women. A possible explanation is the higher body weight among the women with Late PBO compared to Early PBO.
The series of events linking PBO to somatic aging and to the development of chronic conditions remains unknown. However, it has been hypothesized that the premature or early loss of ovarian hormones may affect aging processes at the cellular, tissue, organ, or system level (e.g., inflammation, accumulation of senescent cells, protein aggregation, epigenetic alterations, or mitochondrial dysfunction).12
A strength of the study includes the comprehensive in-person assessments on women with a medical-record documented history of PBO with or without hysterectomy. In addition, the women in the original MOA-2 study were a representative sample from a geographically-defined population, thus reducing possible selection biases. We compared women who underwent PBO with or without concurrent or preceding hysterectomy to referent women from the general population. Therefore, the majority of our referent women had not undergone any gynecologic surgery as of the index date. Limitations should also be considered. First, the study design was cross-sectional so causality cannot be inferred. Longitudinal studies of PBO and cognitive and functional decline are needed. Second, the overall participation rate was low and there was some evidence of participation bias, with healthier women enrolled more frequently into the PBO and referent groups. This bias would lead to conservative estimates. Third, the study included women residing in Olmsted County, Minnesota, and most were White. Results may not be generalizable to other populations with different socioeconomic or racial and ethnic characteristics. Fourth, we had limited power to examine some of the associations and to adjust for some potential additional confounders. The number of comparisons could also have led to some type 1 errors. Fifth, it is possible that the underlying indication for the PBO with or without hysterectomy could contribute to adverse outcomes instead of the PBO itself. We note that none of the women recruited had PBO due to a malignancy. Moreover, we conducted additional analyses to assess whether the association between PBO and any of the outcomes (chronic conditions, physical function, body composition measures) differed by ovarian indications (none vs benign) and found it did not. However, it is still possible that other factors related to the choice to undergo surgery could have contributed to the results. Sixth, most women who underwent PBO with or without hysterectomy took estrogen therapy up to the age of 50 years. Although the numbers were too small to stratify analyses by estrogen therapy, the exclusion of the 76 women without estrogen therapy up to age 50 did not substantially change the results. It is possible that some of the associations reported in other studies, but not observed in this study, were due to a lower percentage of use of estrogen therapy. Last, it is possible that some women sought care outside of the REP medical care providers and thus escaped the passive follow-up. Women were not eligible for the in-clinic visit if they did not have a residence within approximately 200 miles of Rochester, MN. Moreover, all women had medical records in the REP within 5 years of the clinical visit. The REP currently covers 27 counties in Southeastern Minnesota and West Central Wisconsin.
CONCLUSION
Women with a history of PBO with or without concurrent or preceding hysterectomy have more chronic medical conditions and subtle physical function changes in late mid-life compared to referent women without PBO. Notably, most of the findings were stronger among women who underwent Early PBO with or without hysterectomy. These results, highlighting potential negative long-term effects of PBO, are important for women with benign or no ovarian indications at average genetic risk of ovarian cancer to weigh in their consideration of a PBO with or without hysterectomy. Longitudinal studies with extended follow-up are needed to assess whether additional differences in cognitive and physical function emerge at older ages. In addition, patient registries are needed to study the trajectories of clinical course after PBO because it is possible that chronic conditions develop at different times with variable effects of estrogen therapy, aging, and other factors.
Supplementary Material
Sources of funding:
Funding for this study was provided by grants from the NIH (U54 AG044170, RF1 AG055151). This study used the resources of the Rochester Epidemiology Project (REP) medical records-linkage system, which is supported by the National Institute on Aging (R33 AG058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. However, the content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or the Mayo Clinic. Dr. Rocca was partly funded by the Ralph S. and Beverley E. Caulkins Professorship of Neurodegenerative Diseases Research of the Mayo Clinic.
Financial disclosure/conflicts of interest:
Dr. Mielke has served on scientific advisory boards and/or has consulted for Biogen, LabCorp, Lilly, Merck, PeerView Institute, Roche, Siemens Healthineers, and Sunbird Bio unrelated to the current manuscript. Dr. Kapoor has no conflicts of interest directly related to the subject of this manuscript. However, over the past 36 months she has been a consultant for Astellas and Mithra Pharmaceuticals, Scynexis and Womaness; received grant support form Mithra Pharmaceuticals; received payment for development of educational content from Med Learning Group and Academy of Continued Healthcare Learning; and received honoraria for CME activity from PriMed and OBG Management. Dr. Fields serves on the SWAN-Aging Study Observational Study Monitoring Board. Dr. Morrow was on the board for American Society of Biomechanics, which is unrelated to this work. Dr. Kantarci has served on data safety monitoring boards and/or was a consultant for Pfizer, Takeda, and Biogen. She received research support from Eli Lilly. The other authors declare no competing interests.
Footnotes
Presented at a national meeting: Results from this study were presented as a poster at the Organization for the Study of Sex Differences (OSSD) annual meeting May 7–11, 2023.
REFERENCES
- 1.Asante A, Whiteman MK, Kulkarni A, Cox S, Marchbanks PA, Jamieson DJ. Elective oophorectomy in the United States: trends and in-hospital complications, 1998–2006. Obstet Gynecol 2010;116(5):1088–1095. doi: 10.1097/AOG.0b013e3181f5ec9d [DOI] [PubMed] [Google Scholar]
- 2.Chan JK, Urban R, Capra AM, et al. Ovarian cancer rates after hysterectomy with and without salpingo-oophorectomy. Obstet Gynecol 2014;123(1):65–72. doi: 10.1097/AOG.0000000000000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocca WA, Mielke MM, Gazzuola Rocca L, Stewart EA. Premature or early bilateral oophorectomy: a 2021 update. Climacteric 2021;24(5):466–473. doi: 10.1080/13697137.2021.1893686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol 2022;139(5):724–734. doi: 10.1097/AOG.0000000000004728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wend K, Wend P, Krum SA. Tissue-specific effects of loss of estrogen during menopause and aging. Front Endocrinol (Lausanne) 2012;3:19. doi: 10.3389/fendo.2012.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard BV, Kuller L, Langer R, et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative Observational Study. Circulation 2005;111(12):1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD [DOI] [PubMed] [Google Scholar]
- 7.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009;16(1):15–23. doi: 10.1097/gme.0b013e31818888f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol 2009;113(5):1027–1037. doi: 10.1097/AOG.0b013e3181a11c64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mytton J, Evison F, Chilton PJ, Lilford RJ. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ 2017;356:j372. doi: 10.1136/bmj.j372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocca WA, Grossardt BR, Shuster LT, Stewart EA. Hysterectomy, oophorectomy, estrogen, and the risk of dementia. Neurodegener Dis 2012;10(1–4):175–178. doi: 10.1159/000334764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocca WA, Lohse CM, Smith CY, Fields JA, Machulda MM, Mielke MM. Association of premenopausal bilateral oophorectomy with cognitive performance and risk of mild cognitive impairment. JAMA Netw Open 2021;4(11):e2131448. doi: 10.1001/jamanetworkopen.2021.31448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocca WA, Gazzuola-Rocca L, Smith CY, et al. Accelerated accumulation of multimorbidity after bilateral oophorectomy: a population-based cohort study. Mayo Clin Proc 2016;91(11):1577–1589. doi: 10.1016/j.mayocp.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocca WA, Gazzuola Rocca L, Smith CY, et al. Cohort profile: the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2) in Olmsted County, Minnesota (USA). BMJ Open 2017;7(11):e018861. doi: 10.1136/bmjopen-2017-018861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St. Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St. Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41(6):1614–1624. doi: 10.1093/ije/dys195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocca WA, Yawn BP, St. Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebrasseur NK, Achenbach SJ, Melton LJ 3rd, Amin S, Khosla S. Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res 2012;27(10):2159–2169. doi: 10.1002/jbmr.1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy CC, Novotny PJ, LeBrasseur NK, Wise RA, Sciurba FC, Benzo RP. Frailty and clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2019;16(2):217–224. doi: 10.1513/AnnalsATS.201803-175OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeBrasseur NK, Bhasin S, Miciek R, Storer TW. Tests of muscle strength and physical function: reliability and discrimination of performance in younger and older men and older men with mobility limitations. J Am Geriatr Soc 2008;56(11):2118–2123. doi: 10.1111/j.1532-5415.2008.01953.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 22.Rey A L’examen Clinique en Psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 23.Wechsler D Manual for the Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation: Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 24.Wechsler D WMS-III: Wechsler Memory Scale Administration and Scoring Manual. 3rd ed. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 25.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 26.Lucas JA, Ivnik RJ, Smith GE, et al. Mayo’s Older Americans Normative Studies: category fluency norms. J Clin Exp Neuropsychol 1998;20(2):194–200. doi: 10.1076/jcen.20.2.194.1173 [DOI] [PubMed] [Google Scholar]
- 27.Reitan R Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–276. doi: [Google Scholar]
- 28.Wechsler D Wechsler Adult Intelligence Scale-Revised [Manual]. San Antonio, TX: Psychological Corporation: Harcourt Brace Jovanovich; 1981. [Google Scholar]
- 29.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256(3):183–194. doi: doi: 10.1111/j.1365-2796.2004.01388 [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th ed. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 31.Nguyen TT, Smith CY, Gazzuola Rocca L, Rocca WA, Vassallo R, Dulohery Scrodin MM. A population-based cohort study on the risk of obstructive lung disease after bilateral oophorectomy. NPJ Prim Care Respir Med 2022;32(1):52. doi: 10.1038/s41533-022-00317-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007;69(11):1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6 [DOI] [PubMed] [Google Scholar]
- 33.Phung TK, Waltoft BL, Laursen TM, et al. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord 2010;30(1):43–50. doi: 10.1159/000314681 [DOI] [PubMed] [Google Scholar]
- 34.Bove R, Secor E, Chibnik LB, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 2014;82(3):222–229. doi: 10.1212/WNL.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uldbjerg CS, Wilson LF, Koch T, et al. Oophorectomy and rate of dementia: a prospective cohort study. Menopause 2022;29(5):514–522. doi: 10.1097/GME.0000000000001943 [DOI] [PubMed] [Google Scholar]
- 36.Blumel JE, Arteaga E, Vallejo MS, et al. Association of bilateral oophorectomy and menopause hormone therapy with mild cognitive impairment: the REDLINC X study. Climacteric 2022;25(2):195–202. doi: 10.1080/13697137.2021.1951203 [DOI] [PubMed] [Google Scholar]
- 37.Reuben R, Karkaby L, McNamee C, Phillips NA, Einstein G. Menopause and cognitive complaints: are ovarian hormones linked with subjective cognitive decline? Climacteric 2021;24(4):321–332. doi: 10.1080/13697137.2021.1892627 [DOI] [PubMed] [Google Scholar]
- 38.Gervais NJ, Gravelsins L, Brown A, et al. Scene memory and hippocampal volume in middle-aged women with early hormone loss. Neurobiol Aging 2022;117:97–106. doi: 10.1016/j.neurobiolaging.2022.05.003 [DOI] [PubMed] [Google Scholar]
- 39.Wilson LF, Pandeya N, Byles J, Mishra GD. Hysterectomy and perceived physical function in middle-aged Australian women: a 20-year population-based prospective cohort study. Qual Life Res 2018;27(6):1501–1511. doi: 10.1007/s11136-018-1812-9 [DOI] [PubMed] [Google Scholar]
- 40.Canonico M, Artaud F, Tzourio C, Elbaz A. Association of reproductive history with motor function and disability in aging women. J Am Geriatr Soc 2020;68(3):585–594. doi: 10.1111/jgs.16257 [DOI] [PubMed] [Google Scholar]
- 41.Huang G, Coviello A, LaValley MP, et al. Surgical menopause and frailty risk in community-dwelling older women: study of osteoporotic fractures. J Am Geriatr Soc 2018;66(11):2172–2177. doi: 10.1111/jgs.15505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarthy AM, Menke A, Visvanathan K. Association of bilateral oophorectomy and body fatness in a representative sample of US women. Gynecol Oncol 2013;129(3):559–564. doi: 10.1016/j.ygyno.2013.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karia PS, Joshu CE, Visvanathan K. Association of oophorectomy and fat and lean body mass: evidence from a population-based sample of U.S. women. Cancer Epidemiol Biomarkers Prev 2021;30(7):1424–1432. doi: 10.1158/1055-9965.EPI-20-1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


