Abstract
Large-scale genome-wide association studies (GWASs) on bipolar disorder (BD) have implicated the involvement of the fatty acid desaturase (FADS) locus. These enzymes (FADS1 and FADS2) are involved in the metabolism of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are thought to potentially benefit patients with mood disorders. To model reductions in the activity of FADS1/2 affected by the susceptibility alleles, we generated mutant mice heterozygously lacking both Fads1/2 genes. We measured wheel-running activity over six months and observed bipolar swings in activity, including hyperactivity and hypoactivity. The hyperactivity episodes, in which activity was far above the norm, usually lasted half a day; mice manifested significantly shorter immobility times on the behavioral despair test performed during these episodes. The hypoactivity episodes, which lasted for several weeks, were accompanied by abnormal circadian rhythms and a marked decrease in wheel running, a spontaneous behavior associated with motivation and reward systems. We comprehensively examined lipid composition in the brain and found that levels of certain lipids were significantly altered between wild-type and the heterozygous mutant mice, but no changes were consistent with both sexes and either DHA or EPA was not altered. However, supplementation with DHA or a mixture of DHA and EPA prevented these episodic behavioral changes. Here we propose that heterozygous Fads1/2 knockout mice are a model of BD with robust constitutive, face, and predictive validity, as administration of the mood stabilizer lithium was also effective. This GWAS-based model helps to clarify how lipids and their metabolisms are involved in the pathogenesis and treatment of BD.
Subject terms: Bipolar disorder, Neuroscience
Introduction
Bipolar disorder (BD) is a chronic mental illness characterized by recurrent manic and depressive episodes interspersed with an absence of symptoms (a euthymic state). Large-scale genome-wide association studies (GWASs) have identified dozens of loci associated with BD [1–3]. Among them, the FADS1/2 region was first highlighted in Japanese population [1] and replicated in the large European population [2, 3], being the only locus with a genome-wide significant difference in multiple populations.
The FADS1 and FADS2 genes on a tight linkage disequilibrium (LD) block are located head-to-head in the GWAS-identified region (Supplementary Fig. 1a). These genes encode fatty acid desaturases, rate-limiting enzymes involved in the biosynthesis of ω3 (n-3) and ω6 (n-6) long-chain polyunsaturated fatty acids (PUFAs) (Fig. 1a). Linoleic acid (LA; 18:2n-6), which is abundant in the oils of grains such as corn, is converted to arachidonic acid (AA; 20:4n-6) by a two-step desaturating reaction catalyzed by FADS2 and FADS1. α-Linolenic acid (ALA; 18:3n-3), which is enriched in some seed oils such as linseed oil, is converted to eicosapentaenoic acid (EPA; 20:5n-3) by FADS2 and FADS1 and then to docosahexaenoic acid (DHA; 22:6n-3) by further unsaturation catalyzed by FADS2.
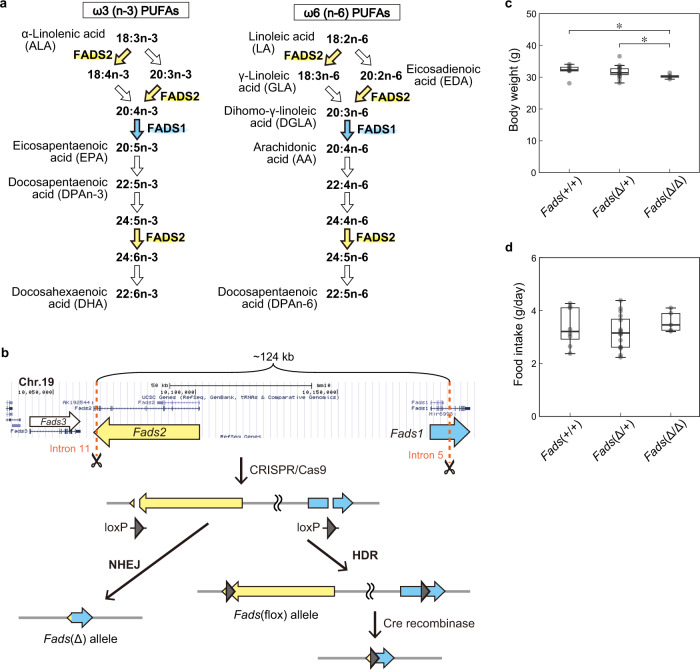
Fig. 1. Generation of mutant mice.
a The PUFA biosynthesis pathways. The desaturation reactions of ω3 and ω6 PUFAs are catalyzed by FADS1 (cyan arrows) or FADS2 (yellow arrows). b A strategy for simultaneous generation of alleles using the CRISPR/Cas9 system. A CRISPR/Cas9 cocktail containing two gRNAs that targeted the Fads1/2 genes and two single-stranded DNA donor templates with the loxP sequence (Supplementary Table 3) was microinjected into mouse fertilized eggs (C57BL/6JJcl strain). Nonhomologous end joining (NHEJ) resulted in the deletion of ~124 kb (Fads(Δ)) and homology-directed repair (HDR) generated the floxed allele. c Body weight of heterozygous and homozygous Fads1/2 deficient mice. Fads(Δ/Δ) mice were significantly leaner than the Fads(Δ/+) and WT (Fads(+/+)) mice (*P < 0.05, d = 0.80 and 1.24 [large effect size (ES)], respectively, t-test with Bonferroni correction). Males, 18–33 weeks old. The box length and a horizontal bar show the interquartile range (IQR) and median, respectively. The length of the whiskers is defined as 1.5 times the upper and lower limits of the IQR. d Food intake. Daily food intake did not differ by genotype.
Even before this FADS1/2 genomic region attracted attention as a locus of BD susceptibility shared across populations, it had seized the spotlight due to the significant changes in haplotype diversity (Supplementary Fig. 1b) since humans commenced crop agriculture [4–6]. The increased intake of grain oils has resulted in an increase in the proportion of people carrying a haplotype associated with higher FADS1/2 activity (Haplotype D) [4]. This haplotype has a protective effect against BD [1–3]. GWASs of blood lipid composition have also demonstrated a pivotal role of the FADS1/2 locus in the plasma levels of ω3 and ω6 PUFAs [7]. Consistent with these results, expression quantitative trait loci (eQTL) analysis suggested that the other major haplotype (Haplotype A) conferring susceptibility to BD was associated with decreased expression of FADS1/2 and likely with lower enzyme activity [8]. Although the odds ratio for the susceptible alleles is not high (at most 1.18) [1–3], these evolutional and functional underpinnings of the locus collectively suggest that an animal mimicking a decreased, but not completely nullified, activity of both FADS1/2 can be a valid model and contribute to a better understanding of the pathogenesis of BD. In the present study, to generate such a model, we deleted the region containing the mouse Fads1 and Fads2 genes in a heterozygous manner (referred to as Fads(Δ/+) mice).
Single knockout (KO) mice deficient in either Fads1 or Fads2 have been investigated prior to our study; [9–15] however, if these mice are crossed, double KO mice cannot be generated because these genes are located only approximately 100 kb apart. Additionally, since studies using single KO mice were conducted from the perspective of nutrition, they mainly analyzed homozygous KO mice fed semipurified diets [9–15]. Data on behavioral phenotypes were not reported so far.
The inverse relationship between seafood consumption and the prevalence of depression and BD highlights a possible nutritional or pharmacological effect of ω3 PUFAs, such as DHA and EPA [16]. Several previous studies reported the therapeutic efficacy of ω3 PUFAs for depressive episodes in BD [17, 18], but the results from randomized clinical trials were debatable [19]. Data on plasma PUFA levels in patients are also inconsistent; however, recent studies with the largest sample size to date have reported low levels of EPA and DHA and high levels of AA in patients with BD [20]. In addition, studies of lipids in postmortem brains are limited, with small sample sizes and inconsistent results [21–23]. Moreover, food and medication can be major confounding factors in human studies, requiring analysis using animal models that provide greater experimental control.
In this study, we established Fads(Δ/+) mice as a preclinical model of the GWAS-identified risk factor in BD; these mice exhibited both mania- and depression-like episodic behavioral changes. To detect these infrequent episodic behavioral changes, we monitored the wheel-running activity of Fads(Δ/+) mice continuously for six months. Unlike other locomotor activities, wheel running in mice is a strongly goal-directed behavior having a significant reward value [24, 25]; thus, a reduction in wheel running is associated with “markedly diminished pleasure (anhedonia)”, a core symptom of a depressive episode [26]. In addition, we provided proof of concept that long-term supplementation with DHA improved the behavioral abnormalities in the model mice.
Methods
Generation of Fads1/2 mutant mice
All animal procedures were approved by the Wako Animal Experiment Committee of RIKEN (H27-2-233, H29-2-230, W2019-2-040, W2021-2-042). We developed Fads(Δ/+) and Fads(flox/+) mice by the CRISPR/Cas9 system, which have been deposited in RIKEN BioResource Center (RBRC11813 and RBRC11814). A detailed description of the procedure and animal husbandry is provided in Supplementary Methods.
Determination of the lipid composition
Lipidomics analysis of brain samples was performed using liquid chromatography (LC)–tandem mass spectrometry (LC-MS/MS). All the lipidomics data were provided in Supplementary Table 1. Total fatty acid levels in plasma samples were determined by gas chromatography–mass spectrometry (GC-MS) analysis. For complete details, see Supplementary Methods.
Behavioral testing
Recording and analyses of wheel-running activity were performed as previously described [27, 28]. Hyperactivity bouts and hypoactivity episodes were defined operationally. Tail suspension test was performed during hyperactivity bouts. IntelliCage analysis and open-field, splash, accelerating rotarod, sucrose preference tests were conducted in non-episodic periods. Detailed procedures are provided in Supplementary Methods.
Lithium treatment and PUFA supplementation
We prepared a lithium-containing normal chow and PUFA-supplemented AIN93G diets and fed them to mice. The composition of the diets is provided in Supplementary Table 2. Since hypoactivity episode frequency was age-dependent (Supplementary Fig. 2), the effect of lithium was examined by a two-group, two-period crossover design using a cohort obtained by a single in-vitro fertilization.
Statistics
U-test, t-test, Fisher’s exact probability test, or analysis of variance (ANOVA) was used. The Benjamin-Hochberg false discovery rate (FDR) or Bonferroni correction was applied to correct multiple comparisons. Statistical analyses were performed using Excel (Microsoft) or R (R Development Core Team). For all analyses, P < 0.05 was considered statistically significant. Substantive significance (effect size) was calculated using Cohen’s d for t-test, r for U-test, η2 for ANOVA, and φ for Fisher’s exact test. d > 0.01, 0.2, 0.5, and 0.8 were considered as very small, small, medium, and large effect sizes, respectively. r > 0.1, 0.3, 0,5; η2 > 0.01, 0.06, 0.14; and φ > 0.1, 0.3, 0.5 were considered as small, medium, and large effect sizes, respectively.
Results
Generation of Fads(Δ/+) and Fads(flox/+) mice by CRISPR/Cas9-mediated genome editing in zygotes
To model the susceptibility haplotype to BD, we generated two kinds of mutant mice by means of the CRISPR/Cas9 system: Fads(Δ/+) mice carrying a 124-kb genomic fragment deletion and Fads(flox/+) mice in which the same deletion occurs in the presence of Cre recombinase (Fig. 1b). F0 mice carrying either the Fads(Δ) or Fads(flox) allele were crossed with wild-type (WT) mice to obtain F1 mice, and we confirmed germline transmission of the genome-edited alleles. We selected strains of Fads(Δ/+) or Fads(flox/+) mice that did not harbor damaging mutations due to possible off-target effects of Cas9 or de novo mutagenesis through whole-exome sequencing and subsequent genotyping.
Both male and female Fads(Δ/+) mice were fed a normal chow diet (CRF-1 diet, Jackson Laboratory Japan), which contained fish meat components, and were fertile. Fads(Δ/Δ) mice were obtained by intercrossing the heterozygous mice. These Fads(Δ/Δ) mice weighed significantly less than WT and Fads(Δ/+) mice, even though they ate the similar amount of the normal chow diet (Fig. 1c, d). We did not use Fads(Δ/Δ) mice in this study because they completely lost FADS1/2 and therefore did not model the results of the GWAS, namely, reduced FADS1/2 activity.
We performed histological staining and found no apparent difference in gross brain structure between Fads(Δ/+) and WT mice (Supplementary Fig. 3). We conducted open-field, rotarod, sucrose preference, and splash tests and revealed that Fads(Δ/+) mice had a normal sensorimotor function and emotional response in a euthymic state (Supplementary Fig. 4). Additionally, we measured the plasma levels of seven inflammatory markers and detected no changes in Fads(Δ/+) mice (Supplementary Fig. 5).
GWAS analysis on blood fatty acids revealed that the PUFA composition is affected by the genotype (or haplotype) of FADS1/2 [7]. We measured plasma levels of total fatty acids in Fads(Δ/+) and WT mice by GC-MS. Intermediate metabolites in the ω6 PUFA pathway, 18:3n-6 (γ-linolenic acid, GLA) and 20:2n-6 (eicosadienoic acid, EDA), were significantly decreased and increased, respectively, but no significant change in DHA, EPA, or AA was detected (Fig. 2a). The changes in the plasma fatty acid levels in Fads(Δ/+) mice were similar to those observed in BD patients carrying the susceptibility allele (Saito et al., submitted).
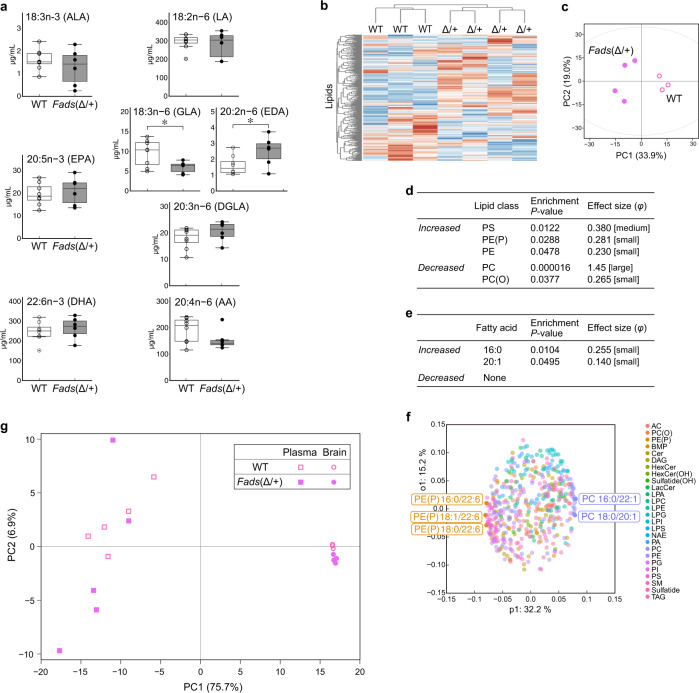
Fig. 2. Lipid analysis of male Fads(Δ/+) mice fed a normal chow diet.
a Plasma fatty acid levels in mice fed a normal chow diet. The intermediate metabolites GLA and EDA significantly differed between WT (n = 8) and Fads(Δ/+) (n = 6) mice (*P < 0.05, d = 0.658 [medium ES] and 0.272 [small ES], respectively, t-test with Bonferroni correction). Note that the values on the vertical axis are very different in each graph. For the boxplot description, see the legend of Fig. 1c. b Dendrogram of unsupervised clustering analysis of 464 brain lipids in mice fed a normal chow diet. c PCA plot of mice based on brain lipids. This is a part of Fig. 4b. PC1 and PC2 were calculated using all brain lipidomics data from males in this study (Fig. 4b and Supplementary Fig. 9). d, e Lipid class (d) and fatty acid (e) enrichment analysis of differentially changed brain lipids between Fads(Δ/+) and WT mice. Enrichment P-values are given by Fisher’s exact test. The abbreviations for the lipid classes are listed in Supplementary Table 4. f OPLS-DA loading plot of the 464 brain lipids from Fads(Δ/+) and WT mice. The top 1% of lipid molecules are highlighted. The comparisons between genotypes for these individual lipids are shown in Supplementary Fig. 7. g PCA plot of brain and plasma samples based on 282 lipids that were detected all the brain and plasma samples.
Lipid profiles in the brain and plasma are altered in Fads(Δ/+) mice fed a normal chow diet
To date, data on the brain lipid compositions are lacking in studies of single KO mice of Fads1 and Fads2, especially mice fed a normal chow diet that contained DHA and EPA [9–15], as well as in studies of human patients. We thus used a targeted lipidomics approach and measured the levels of 464 lipids (26 different lipid classes, 29 types of fatty acids) by LC-MS/MS in brain samples from male Fads(Δ/+) and WT mice. Unsupervised hierarchical clustering and principal component analysis (PCA) based on the 464 lipids categorized Fads(Δ/+) and WT mice into two distinctive groups (Fig. 2b, c). To identify the lipids, lipid classes, or fatty acids that contributed to the overall difference in brain lipid composition, we looked for lipids that were altered in the mutant mice. Of the lipids measured, 70 were significantly altered with large effect sizes (P < 0.05 and d > 0.8). Lipid class enrichment analysis showed that five classes including phosphatidylcholine (PC) and phosphatidylserine (PS) significantly changed by genotype (Fig. 2d). Fatty acid enrichment analysis revealed a significant change in 16:0 and 20:1 PUFAs but not ω3 or ω6 PUFAs (Fig. 2e). We also examined the brain samples from female mice (Supplementary Fig. 6). Although female Fads(Δ/+) mice had a distinct brain lipid composition as compared to WT mice, the lipid classes and fatty acids that were significantly altered differed from those in males. We performed an orthogonal partial least squares discriminant analysis (OPLS-DA) to uncover individual lipid molecules contributing to the lipidomic difference between Fads(Δ/+) and WT mice. There were no identical molecules in the lipids identified in the comparison between males and those identified in females (Fig. 2f and Supplementary Figs. 6e, 7).
Little DHA is synthesized locally in the brain even in WT mice, and DHA in the brain mainly is derived from the blood [29]. Thus, we examined the plasma of male Fads(Δ/+) and WT mice in detail by LC-MS/MS and compared them with changes in brain lipids (Fig. 2g and Supplementary Fig. 8). We found that blood and brain have different lipid changes in response to reduced FADS1/2 activity, and the composition of brain lipids varied less by genotype and also less between individuals than that in the blood (Fig. 2g), probably due to robust homeostatic mechanisms in the brain [29, 30].
Spontaneous behavioral changes, hyperactivity bouts and hypoactivity episodes in Fads(Δ/+) mice
BD is characterized by recurrent manic and depressive episodes [26]. To detect these infrequent episodic behavioral changes, the wheel-running activity of Fads(Δ/+) mice of both sexes fed a normal chow diet was recorded for six months.
Male Fads(Δ/+) mice showed a marked episodic increase in wheel-running activity (Fig. 3a, b). This episodic high activity hereafter referred to as a hyperactivity bout (HAB), typically lasted approximately half a day (~6 h to 1 day). We operationally defined HABs as behavioral changes that lasted more than 6 h with sufficiently heightened activity to be considered a statistical outlier (see Supplementary Methods for details). Male Fads(Δ/+) mice exhibited HABs at a significantly higher frequency (~2.4 episodes in 6 months) than that of WT mice (Fig. 3c). Several female Fads(Δ/+) mice also exhibited HABs, but less frequently than male Fads(Δ/+) mice (Fig. 3c). HABs were often observed even during the light period when nocturnal mice should have been resting or sleeping (Fig. 3a).
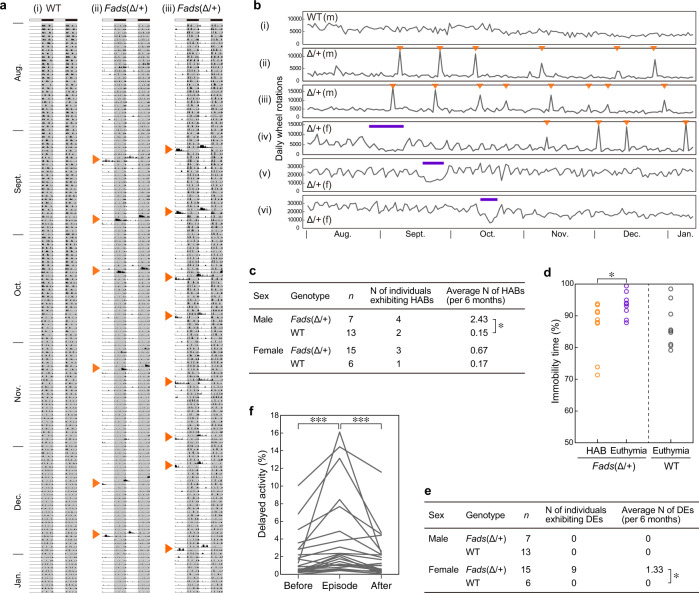
Fig. 3. Spontaneous behavioral changes in Fads(Δ/+) mice fed a normal chow diet.
a Representative double-plotted actograms of wheel-running activity in WT (i) and Fads(Δ/+) mice (ii, iii). Arrowheads depict hyperactivity bouts (HABs). b Total daily wheel-running activity for six months. Arrowheads depict HABs, and thick lines indicate depressive-like episodes. Individuals (i)–(iii) are identical to those (i)–(iii) in panel a, respectively. m, male; f, female. c Frequency of HABs. Male Fads(Δ/+) mice exhibited HABs significantly more often than WT mice (*P < 0.05, r = 0.503 [large ES], U-test). d Immobility time in the tail suspension test during HAB or the euthymic state. The immobility time of Fads(Δ/+) mice was significantly shorter during HABs (*P < 0.05, d = 1.03 [large ES], t-test). Data for euthymic WT mice are shown as a reference. There is a significant difference in immobility time between euthymic Fads(Δ/+) mice and euthymic WT mice. e Frequency of depression-like episodes. Female Fads(Δ/+) mice exhibited depression-like episodes (DEs) significantly more often than WT mice (*P < 0.05, r = 0.516 [large ES]). We have observed four female individuals that exhibited both DE and HAB; one of them is the individual (iv) in panel (b). The four animals were among 47 females that were fed a normal chow diet and examined for wheel-running behavior for more than four months. f Comparison of delayed activity, an indicator of abnormal circadian rhythms, during and two weeks before and after a depression-like episode. Fads(Δ/+) mice showed significantly higher delayed activity during episodes (***P < 0.001, r = 0.91 and 0.80 [large ES], paired U-test).
It is difficult to verify whether the HABs in Fads(Δ/+) mice correspond to manic episodes. The DSM-5 criteria for a manic episode are inapplicable to mice because most of the criteria are related to subjective experiences [26]. In addition, it was impossible to predict when HABs developed, and they lasted only approximately half a day. Given the limitations of this investigation, we performed a tail suspension test during HABs, which required no prior training and only 6 min of testing time. The tail suspension test is a behavioral despair experiment that has been used in antidepressant screening (antidepressant administration shortens immobility time) and that has sometimes been used to evaluate mouse phenotype in models for depression (in general, immobility time is increased in depression models) [31]. During HABs, Fads(Δ/+) mice had shorter immobility times than concurrently tested littermate Fads(Δ/+) mice during non-episodic periods (Fig. 3d).
Female Fads(Δ/+) mice also exhibited several weeks of hypoactivity, and some mice showing these episodes showed HABs as well (Fig. 3b). These hypoactivity episodes were also operationally defined according to our previous study [27]. Female Fads(Δ/+) mice showed a significantly higher frequency of these hypoactivity episodes (~1.3 episodes in 6 months) than WT mice (Fig. 3e). As an indicator of circadian rhythm disturbances associated with the hypoactivity episodes, we used the “delayed activity index” [27], which reflects the extent to which the mice continued to run on a wheel even in the light period (i.e., morning). This index was more marked during hypoactivity episodes than during the two-week euthymic state surrounding the episodes (Fig. 3f). The frequency of hypoactivity episodes with circadian rhythm disturbances was comparable to that of depression-like episodes in mutant Polg1 Tg mice, a mouse model of recurrent depression [27]. No such depression-like episodes were observed in male mice.
Long-term DHA supplementation prevents depression-like episodes in Fads(Δ/+) mice
We investigated whether PUFA supplementation (EPA, DHA, or EPA + DHA) was effective for treating Fads(Δ/+) mice. We thus prepared semipurified diets supplemented with EPA and/or DHA to AIN93G that did not contain fish powder (Supplementary Table 2). Mice were raised until 25 weeks of age on a normal chow diet, and their wheel-running activity was measured at the time that they were started on the AIN93G-based diets for six months.
HABs were not observed in Fads(Δ/+) mice of either sex fed the AIN93G control diet or the PUFA-supplemented diets. This obviously indicates that diet has a significant effect on the behavioral changes. However, depression-like episodes were observed in female Fads(Δ/+) mice fed AIN93G; the frequency of the hypoactivity episodes was similar to that in normal chow-fed mice (~1.0 vs. ~1.3 episodes in 6 months). The occurrence of depression-like episodes was significantly reduced in Fads(Δ/+) mice fed a DHA-supplemented diet compared to that in mice fed the AIN93G control diet (Fig. 4a). Supplementation with EPA + DHA that mimicked the composition of Lotriga (Takeda Pharmaceutical) also tended to reduce the frequency of these depression-like episodes. No apparent effects of the diet supplemented with EPA alone were observed.
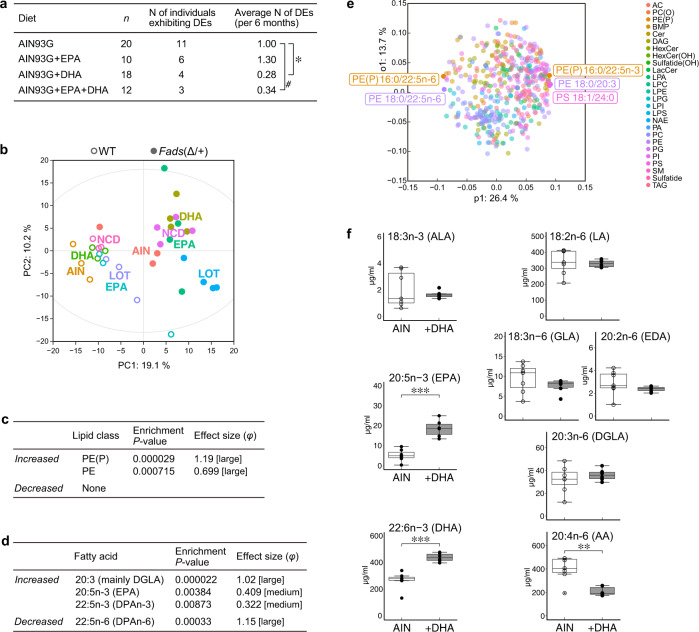
Fig. 4. Effect of dietary DHA supplementation on the lipid compositions and behavior of Fads(Δ/+) mice.
a Frequency of depression-like episodes in female Fads(Δ/+) mice fed the AIN93G diet (control) and AIN93G supplemented with DHA and/or EPA. DHA supplementation significantly reduced the frequency of DEs (*P < 0.05, #P < 0.1, r = 0.331 and 0.315 [medium ES], U-test with Bonferroni correction following Kruskal-Wallis test). b PCA plot of Fads(Δ/+) and WT mice fed the five different diets based on brain lipidomics data (Supplementary Table 1a). A two-way ANOVA revealed a significant and large effect of genotype (P < 0.001, η2 = 0.15) but not diet on the Euclidean distance between the lipid compositions of the samples measured. c, d Lipid class (c) and fatty acid (d) enrichment analyses of differentially changed brain lipids between Fads(Δ/+) mice fed AIN93G and those supplemented with DHA. The abbreviations for the lipid classes are listed in Supplementary Table 4. e OPLS-DA loading plot of the 464 brain lipids from Fads(Δ/+) mice fed AIN93G and from those supplemented with DHA. The top 1% of lipid molecules are highlighted. The comparisons of these individual lipids between diet groups are shown in Supplementary Fig. 10. f Plasma fatty acid levels in Fads(Δ/+) mice fed the AIN93G diet (AIN, n = 7) and those of mice supplemented with DHA (n = 6). In Fads(Δ/+) mice supplemented with DHA, DHA itself and its precursor metabolite, EPA, were significantly increased (***P < 0.001, d = 1.884 and 1.419 [large ES], t-test), and the major ω6 metabolite AA was reduced (**P < 0.05, d = 1.437 [large ES]). For the boxplot description, see the legend of Fig. 1c.
Additionally, we examined the brain lipid composition in Fads(Δ/+) and WT mice fed 5 different diets (normal chow, AIN93G, AIN93G + DHA, AIN93G + EPA, and AIN93G + EPA + DHA) for 2 months. The PCA and unsupervised hierarchical clustering indicated that the effect of the Fads1/2 genotype was significantly greater than the effect of the diet on the brain lipid composition (Fig. 4b and Supplementary Fig. 9). We focused on the DHA-supplemented diet, which notably prevented the depression-like episodes (Fig. 4a), and further analyzed its effect on brain lipid composition. In total, 49 lipids were altered with statistical and substantive significance in Fads(Δ/+) mice fed a DHA-supplemented diet compared to those of the mutant mice fed the control diet. Lipid class enrichment analysis showed that PE(P) and PE were significantly altered by diet (Fig. 4c). Fatty acid enrichment and OPLS-DA analyses revealed a significant increase in several ω3 fatty acids, such as DGLA and EPA, but in DHA (Fig. 4d, e and Supplementary Fig.10). Plasma levels of total fatty acids in these mice were also measured. DHA and EPA were increased, and AA was decreased significantly in the blood of DHA-supplemented mutant mice (Fig. 4f), indicating that lipid metabolism and homeostasis are different in the brain than the periphery.
Lithium has a prophylactic effect on depression-like episodes in Fads(Δ/+) mice
To evaluate the predictive validity of these mutant mice as a BD model, we tested the effects of administering lithium, a mood-stabilizing treatment for manic and depressive episodes [32]. Since it is difficult to maintain the therapeutic plasma level of lithium in male mice for months, likely due to less robustness of lithium clearance, only female mice were used in this study. To examine the effect of lithium on depression-like episodes exhibited by female Fads(Δ/+) mice, we administered a lithium-containing CRF-1 chow in a two-period crossover design, with each period lasting for 12 weeks (Fig. 5). After 12 weeks of baseline measurement, the mice were randomly divided into two groups (A and B). Lithium significantly decreased the frequency of depression-like episodes in these mice. In addition, we observed more frequent episodes after terminating the treatment (Fig. 5), which suggests that lithium withdrawal triggered new episodes. This finding is similar to observations in BD patients and another mouse model of mood disorders [27].
Fig. 5. Prophylactic effect of lithium in Fads(Δ/+) mice.
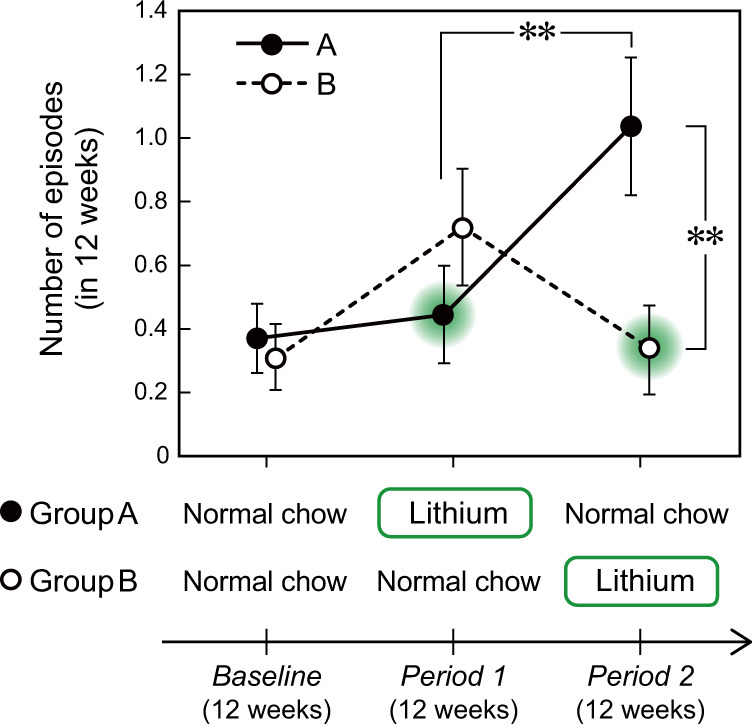
Effect of lithium treatment on the frequency of depression-like episodes. There was a significant effect of lithium treatment (P < 0.001, η2 = 0.061 [medium ES], two-way repeated-measures ANOVA). The episodes were significantly more frequent after treatment was terminated (Group A in Period 2) than during treatment (Group A in Period 1) (**P < 0.01, r = 0.583 [large ES], paired U-test). The episode frequency after treatment was terminated was also higher than that of another treated group (comparing Group A and Group B in Period 2) (**P < 0.01, r = 0.373 [medium ES], U-test). Data are expressed as the means ± s.e.m. Group A, n = 27; Group B, n = 25.
Brain-specific deletion of Fads1/2 had no significant impact on behavioral phenotypes
In the brain, Fads1 is expressed in both neurons and glia, while Fads2 is expressed mainly in glial cells and subsets of neurons according to the Single Cell Portal [33]. Since these enzymes are also widely expressed in peripheral, non-neural tissues, we next examined whether the HABs and depression-like episodes in Fads(Δ/+) mice were caused by abnormal PUFA metabolism in the brain or by peripheral metabolic disorders. We crossed Fads(flox/+) mice with Nestin-Cre (NC) mice (a brain-specific Cre driver) and obtained brain-specific conditional knockout (cKO) mice of the genotype Fads(flox/+);NC/+, in which Fads1/2 genes were heterozygously deleted in ~80% of cells in the brain (Supplementary Fig. 11). These mice and control Fads(+/+);NC/+ mice were fed a normal chow diet, and their wheel-running activity was measured for six months. Neither male nor female cKO mice exhibited HABs or depressive-like episodic behavioral changes, in contrast to Fads(Δ/+) mice (Supplementary Fig. 12). No sleep-wake rhythm abnormalities were detected either (Supplementary Fig. 13). To detect non-episodic behavioral phenotypes, we performed a battery of behavioral tests assessing place learning ability, impulsivity, attention control, etc. using the IntelliCage [34–36]. We compared Fads cKO mice of both sexes fed a normal chow diet (under group-feeding conditions) compared with controls and observed that none of the tested behaviors displayed any differences (Supplementary Fig. 14). Moreover, there were no significant changes in the plasma fatty acid levels of Fads cKO mice compared with those of the controls (Supplementary Fig. 15). These results suggest that the behavioral abnormalities in Fads(Δ/+) mice (i.e., the HABs and depression-like episodes) were due not to reduced FADS1/2 activity in the neurons or astrocytes but to reduced FADS1/2 activity in the periphery or microglia and endothelial cells in the brain where NC did not work.
Discussion
In this study, we focused on the FADS1/2 gene region, which was identified in large-scale GWASs of BD in multiple populations [1–3]. We generated heterozygous KO mice (Fads(Δ/+)) to clarify the functional relevancy of the genes and the susceptibility alleles to BD. Using behavioral and lipidomics approaches, we confirmed that they have construct, face, and predictive validity [37] as an animal model of BD. Its high construct validity is conferred by heterozygous deletion of the Fads1/2 gene, which mimics the reduced FADS1/2 enzyme activity observed in the BD risk haplotype. Previously proposed mouse models of mania [38, 39], such as Clock mutant mice and methamphetamine-treated mice, had a certain level of construct validity, but most of them lacked episodic phenotypes. In contrast, Fads(Δ/+) mice exhibited episodic behavioral changes, depression-like episodes and HABs (Fig. 3a, b). However, it is fundamentally difficult to evaluate HABs in mice using the DSM-5 diagnostic criteria for manic episodes because the primary criterion is an abnormally and persistently elevated mood, which is a subjective perception or experience. Other diagnostic criteria, such as inflated self-esteem and flight of ideas, are also difficult to evaluate in mouse models. However, the HABs of Fads(Δ/+) mice are considered to meet the following DSM-5 diagnostic criteria for manic episodes: a decreased need for sleep and an increase in goal-directed activity. This is because the mutant mice exhibited sustained wheel-running activity even in the light phase during days of HABs (Fig. 3a); wheel running is a goal-directed behavior in rodents and is closely linked to the reward system [24, 25, 27]. In addition, these mice exhibited decreases in immobility time in the tail suspension test during HABs (Fig. 3d), which supports the idea that the HAB is a mania-like episode. Fads(Δ/+) mice also spontaneously showed hypoactive episodes that lasted for two weeks or more (Fig. 3b). This behavioral phenotype was accompanied by abnormal circadian rhythms (Fig. 3f) and is very similar to the hypoactive episodes exhibited by mutant Polg1 Tg mice, which satisfied the DSM-5 criteria for depressive episodes [27, 28]. To the best of our knowledge, these findings indicate that the Fads(Δ/+) mouse model has the most clinically relevant face validity for BD to date.
Regarding the predictive validity of this model, we demonstrated that DHA supplementation (and also DHA + EPA supplementation) was effective in reducing the frequency of depression-like episodes (Fig. 4a). Comprehensive lipid analysis showed that DHA supplement slightly altered the brain lipid composition of Fads(Δ/+) mice, but did not alter the level of DHA, nor did it alter the lipid composition to resemble that of WT mice (Fig. 4b and Supplementary Fig. 16). This result may reflect strong homeostasis to maintain constant PUFA levels, especially DHA in the brain. Nevertheless, DHA supplement exerted the prophylactic effect (Fig. 4a), possibly because it facilitated the homeostatic responses to lower FADS1/2 activities in Fads(Δ/+) mice. Although seemingly contrary to the results of the meta-analysis of clinical studies that EPA supplement, rather than DHA, is more effective in treating BD [17, 18, 40], clinical studies include the problem of not being able to control diet and DHA supplement could be particularly effective in BD patient with FADS1/2 risk allele. Lastly, we emphasize that lithium treatment also had a prophylactic effect in these mice (Fig. 5), which further supports the model’s predictive validity.
One of the limitations of the model is sex differences in the behavioral phenotypes. Male mice did not experience depression-like episodes, and female mice exhibited less frequent HABs than males (Fig. 3c, e). This appears to be inconsistent with the lack of substantial sex difference in the prevalence of BD [41]. A recent paper, however, reports that depressive episodes are more frequent in female patients with BD than in males, which would be consistent with the fact that BD-II is more prevalent in females [42]. Thus, we would need to investigate the sex ratio of BD patients who have the susceptibility allele, as well as their detailed symptoms, in addition to studying the mechanism underlying the sex differences in Fads(Δ/+) mice. Another limitation is that we have not been able to evaluate the efficacy of lithium on male Fads(Δ/+) mice or the effects of other therapeutic drugs because of the lack of a chronic administration regimen over a period of several months. Because of the infrequency of episodic behavioral changes exhibited by this model (Figs. 3–5), there is an urgent need to establish methods for the long-term administration of various psychotropic drugs to mice.
Curiously and importantly, brain-specific cKO mice lacked apparent behavioral phenotypes (Supplementary Figs. 12–14). It suggests that the reduced activity of FADS1/2 enzymes in peripheral tissues primarily leads the BD-like episodic behavioral change in Fads(Δ/+) mice. The contribution of microglia and endothelial cells in the brain, in which Nestin-Cre does not work in cKO mice, cannot be ruled out, but this is unlikely because the expression of Fads1/2 is very low. In the systemic KO mice, plasma inflammatory markers were unaltered (Supplementary Fig. 5), but blood lipid composition was altered to a greater extent than in the brain (Fig. 2g). Blood levels of fatty acids were completely unchanged in cKO mice (Supplementary Fig. 15). Even in animal studies, it will be necessary to study BD not only as a disease of the brain but also as a disease of the whole body. Fads(Δ/+) mice, which satisfy all three validities, will particularly help us to understand the pathogenesis of BD and develop therapeutic interventions in patients carrying the FADS1/2 susceptibility allele (approximately half of all patients with BD).
Supplementary information
Acknowledgements
The authors are grateful to Prof. Kiyoto Kasai (The University of Tokyo), HY’s supervisor, for his dedicated support. We thank Fukiko Isono, Naoko Kume, Miki Aizawa, and Mika Morino for technical assistance. This research was supported by AMED under Grant Numbers JP16dm0107098, JP20dm017097, JP22wm0425008, and JP22dm0207074.
Author contributions
T Kat and T Kas conceptualized and designed the studies and jointly supervised this work. TY, NI, T Kat, and T Kas acquired funding. HY and T Kas performed most experiments, data analysis, and wrote the manuscript. HY, H-CL-O, MI, and TS conducted lipid analysis under the supervision of TY and NI. TN provided technical supports for behavioral analyses. AT provided technical supports for statistical analyses. HY and T Kas wrote the first draft of the manuscript and all authors have read and approved the manuscript.
Competing interests
NI has received research support or speakers’ honoraria from Jansen, Takeda, Sumitomo Dainippon Pharma, and Otsuka. T Kat has received a research grant and personal fees from Sumitomo Dainippon Pharma outside of this work. The other authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tadafumi Kato, Email: tadafumi.kato@juntendo.ac.jp.
Takaoki Kasahara, Email: takaoki.kasahara@uni-oldenburg.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-023-01988-2.
References
- 1.Ikeda M, Takahashi A, Kamatani Y, Okahisa Y, Kunugi H, Mori N, et al. A genome-wide association study identifies two novel susceptibility loci and trans population polygenicity associated with bipolar disorder. Mol Psychiatry. 2018;23:639–47. doi: 10.1038/mp.2016.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51:793–803. doi: 10.1038/s41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29. doi: 10.1038/s41588-021-00857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ameur A, Enroth S, Johansson A, Zaboli G, Igl W, Johansson ACV, et al. Genetic adaptation of fatty-acid metabolism: a human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am J Hum Genet. 2012;90:809–20. doi: 10.1016/j.ajhg.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieson S, Mathieson I. FADS1 and the timing of human adaptation to agriculture. Mol Biol Evol. 2018;35:2957–70. doi: 10.1093/molbev/msy180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathias RA, Fu W, Akey JM, Ainsworth HC, Torgerson DG, Ruczinski I, et al. Adaptive evolution of the FADS gene cluster within Africa. PLoS One. 2012;7:e44926. doi: 10.1371/journal.pone.0044926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI study. PLoS Genet. 2009;5:e1000338. doi: 10.1371/journal.pgen.1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Z, Zhang R, Jiang F, Zhang H, Zhao A, Xu B, et al. FADS1-FADS2 genetic polymorphisms are associated with fatty acid metabolism through changes in DNA methylation and gene expression. Clin Epigenet. 2018;10:113. doi: 10.1186/s13148-018-0545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Y-Y, Monk JM, Hou TY, Callway E, Vincent L, Weeks B, et al. Characterization of an arachidonic acid-deficient (Fads1 knockout) mouse model. J Lipid Res. 2012;53:1287–95. doi: 10.1194/jlr.M024216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroud CK, Nara TY, Roqueta-Rivera M, Radlowski EC, Lawrence P, Zhang Y, et al. Disruption of FADS2 gene in mice impairs male reproduction and causes dermal and intestinal ulceration. J Lipid Res. 2009;50:1870–80. doi: 10.1194/jlr.M900039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi Y, Lee-Okada H-C, Nakamura E, Tada N, Yokomizo T, Fujiwara Y, et al. Ablation of fatty acid desaturase 2 (FADS2) exacerbates hepatic triacylglycerol and cholesterol accumulation in polyunsaturated fatty acid-depleted mice. FEBS Lett. 2021;595:1920–32. doi: 10.1002/1873-3468.14134. [DOI] [PubMed] [Google Scholar]
- 12.Powell DR, Gay JP, Smith M, Wilganowski N, Harris A, Holland A, et al. Fatty acid desaturase 1 knockout mice are lean with improved glycemic control and decreased development of atheromatous plaque. Diabetes Metab Syndr Obes. 2016;9:185–99. doi: 10.2147/DMSO.S106653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monk JM, Liddle DM, Cohen DJA, Tsang DH, Hillyer LM, Abdelmagid SA, et al. The delta 6 desaturase knock out mouse reveals that immunomodulatory effects of essential n-6 and n-3 polyunsaturated fatty acids are both independent of and dependent upon conversion. J Nutr Biochem. 2016;32:29–38. doi: 10.1016/j.jnutbio.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Stoffel W, Holz B, Jenke B, Binczek E, Günter RH, Kiss C, et al. Δ6-desaturase (FADS2) deficiency unveils the role of ω3- and ω6-polyunsaturated fatty acids. EMBO J. 2008;27:2281–92. doi: 10.1038/emboj.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ri K, Lee-Okada H-C, Yokomizo T. Omega-6 highly unsaturated fatty acids in Leydig cells facilitate male sex hormone production. Commun Biol. 2022;5:1001. doi: 10.1038/s42003-022-03972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–7. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 17.Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–12. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 18.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomized double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 19.Bozzatello P, Rocca P, Mantelli E, Bellino S. Polyunsaturated fatty acids: what is their role in treatment of psychiatric disorders? Int J Mol Sci. 2019;20:5257. doi: 10.3390/ijms20215257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koga N, Ogura J, Yoshida F, Hattori K, Hori H, Aizawa E, et al. Altered polyunsaturated fatty acid levels in relation to proinflammatory cytokines, fatty acid desaturase genotype, and diet in bipolar disorder. Transl Psychiatry. 2019;9:208. doi: 10.1038/s41398-019-0536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNamara RK, Jandacek R, Rider T, Tso P, Stanford KE, Hahn C-G, et al. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–99. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamazaki K, Choi KH, Kim H-Y. Phospholipid profile in the postmortem hippocampus of patients with schizophrenia and bipolar disorder: no changes in docosahexaenoic acid species. J Psychiatr Res. 2010;44:688–93. doi: 10.1016/j.jpsychires.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh S, Dyer RA, Beasley CL. Evidence for altered cell membrane lipid composition in postmortem prefrontal white matter in bipolar disorder and schizophrenia. J Psychiatr Res. 2017;95:135–42. doi: 10.1016/j.jpsychires.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Vargas-Pérez H, Mena-Segovia J, Giordano M, Díaz JL. Induction of c-fos in nucleus accumbens in naive male Balb/c mice after wheel running. Neurosci Lett. 2003;352:81–4. doi: 10.1016/j.neulet.2003.08.073. [DOI] [PubMed] [Google Scholar]
- 25.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). 5th ed. Arlington, TX: American Psychiatric Association Publishing; 2013.
- 27.Kasahara T, Takata A, Kato TM, Kubota-Sakashita M, Sawada T, Kakita A, et al. Depression-like episodes in mice harboring mtDNA deletions in paraventricular thalamus. Mol Psychiatry. 2016;21:39–48. doi: 10.1038/mp.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T, et al. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol Psychiatry. 2006;11:577–93. doi: 10.1038/sj.mp.4001824. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from α-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007;48:1150–8. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Rapoport SI, Igarashi M, Gao F. Quantitative contributions of diet and liver synthesis to docosahexaenoic acid homeostasis. Prostaglandins Leukot Ess Fat Acids. 2010;82:273–6. doi: 10.1016/j.plefa.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Kato T. Current understanding of bipolar disorder: toward integration of biological basis and treatment strategies. Psychiatry Clin Neurosci. 2019;73:526–40. doi: 10.1111/pcn.12852. [DOI] [PubMed] [Google Scholar]
- 33.Single Cell Portal. https://singlecell.broadinstitute.org/single_cell. Accessed November 13, 2022.
- 34.Nakamura T, Nakajima K, Kobayashi Y, Itohara S, Kasahara T, Tsuboi T, et al. Functional and behavioral effects of de novo mutations in calcium-related genes in patients with bipolar disorder. Hum Mol Genet. 2021;30:1851–62. doi: 10.1093/hmg/ddab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krackow S, Vannoni E, Codita A, Mohammed AH, Cirulli F, Branchi I, et al. Consistent behavioral phenotype differences between inbred mouse strains in the IntelliCage. Genes Brain Behav. 2010;9:722–31. doi: 10.1111/j.1601-183X.2010.00606.x. [DOI] [PubMed] [Google Scholar]
- 36.Masuda A, Kobayashi Y, Itohara S. Automated, long-term behavioral assay for cognitive functions in multiple genetic models of Alzheimer’s disease, using IntelliCage. J Vis Exp. 2018;138:58009. doi: 10.3791/58009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chadman KK, Yang M, Crawley JN. Criteria for validating mouse models of psychiatric diseases. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1–11. doi: 10.1002/ajmg.b.30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Logan RW, McClung CA. Animal models of bipolar mania: the past, present and future. Neuroscience. 2016;321:163–88. doi: 10.1016/j.neuroscience.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beyer DKE, Freund N. Animal models for bipolar disorder: from bedside to the cage. Int J Bipolar Disord. 2017;5:35. doi: 10.1186/s40345-017-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciappolino V, Delvecchio G, Agostoni C, Mazzocchi A, Altamura AC, Brambilla P. The role of n-3 polyunsaturated fatty acids (n-3PUFAs) in affective disorders. J Affect Disord. 2017;224:32–47. doi: 10.1016/j.jad.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 41.Diflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22:437–52. doi: 10.3109/09540261.2010.514601. [DOI] [PubMed] [Google Scholar]
- 42.Dell’Osso B, Cafaro R, Ketter TA. Has Bipolar Disorder become a predominantly female gender related condition? Analysis of recently published large sample studies. Int J Bipolar Disord. 2021;9:3. doi: 10.1186/s40345-020-00207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.