Abstract
Concurrent cocaine and alcohol use is among the most frequent drug combination, and among the most dangerous in terms of deleterious outcomes. Cocaine increases extracellular monoamines by blocking dopamine (DA), norepinephrine (NE) and serotonin (5-HT) transporters (DAT, NET and SERT, respectively). Likewise, ethanol also increases extracellular monoamines, however evidence suggests that ethanol does so independently of DAT, NET and SERT. Organic cation transporter 3 (OCT3) is an emergent key player in the regulation of monoamine signaling. Using a battery of in vitro, in vivo electrochemical, and behavioral approaches, as well as wild-type and constitutive OCT3 knockout mice, we show that ethanol’s actions to inhibit monoamine uptake are dependent on OCT3. These findings provide a novel mechanistic basis whereby ethanol enhances the neurochemical and behavioral effects of cocaine and encourage further research into OCT3 as a target for therapeutic intervention in the treatment of ethanol and ethanol/cocaine use disorders.
Subject terms: Neuroscience, Physiology
Introduction
Alcohol use disorder (AUD) is the most common substance use disorder (SUD) in the US [1]. Problematic alcohol use contributes significantly to disease burden worldwide and is associated with various adverse economic and social outcomes [2]. Even more concerning, alcohol is often used with other substances [3–10], frequently with cocaine [11–16]. This drug pairing, which often causes emergency hospitalization, is likely more toxic than each substance alone [15, 16]. There is currently no treatment for co-abuse of, and overdose with, alcohol and cocaine, further highlighting the hazards of their concurrent use and underscoring the need to understand its mechanistic basis. Furthering this understanding is necessary to discover new targets for therapeutic intervention in treating AUD, including co-abuse of alcohol and cocaine.
The popularity of co-abuse of alcohol and cocaine could result from an increased “high” compared with taking either drug alone, as well as from a prolongation of cocaine-induced euphoria [16–18], possibly because increases in dopamine (DA), norepinephrine (NE), and serotonin (5-HT) may be greatest when cocaine and alcohol are taken together. This is supported by several lines of evidence. For example, coadministration of ethanol with cocaine accelerates cocaine absorption, increasing the concentration of cocaine in brain extracellular fluid [19]. Ethanol also enhances cocaine-induced increases of extracellular DA in the nucleus accumbens [20], a critical brain area in the reward pathway [21]. Finally, preclinical self-administration and conditioned place preference (CPP) results, and clinical findings in humans, suggest that the rewarding properties of ethanol combined with cocaine are greater than those of each drug alone [22]. Similar findings are reported across species, including planarians [23], rodents [24, 25], non-human primates [26, 27], and humans [17, 28].
Cocaine and ethanol each increase extracellular levels of DA, NE, and 5-HT, neurotransmitters linked to drug reward. Cocaine does this by blocking the high-affinity transporters for these neurotransmitters: the DA transporter (DAT), NE transporter (NET), and 5-HT transporter (SERT), respectively [29]. While alcohol has a broad and complex range of actions [30], which includes increasing extracellular levels of monoamines [31], how it does this remains unclear.
Alcohol inhibits monoamine uptake (e.g., [32–36]), but we and others provide evidence that ethanol lacks activity at DAT [33], NET [34], and SERT [36]. Instead, organic cation transporter 3 (OCT3) could potentially contribute to ethanol’s monoamine uptake inhibition. OCT3 is a low-affinity transporter capable of transporting DA, 5-HT, and NE at high capacity (also known as an “uptake-2” transporter) [37, 38]. OCT3, located on neurons and glia in reward-related brain regions [39–44], may be an important regulator of monoamines [39–41, 43, 45–54]. Supporting a role for OCT3 in effects of ethanol, we found that ethanol-induced inhibition of 5-HT clearance from hippocampus was greater in SERT knockout (KO, -/-) mice than in wild-type (+/+) mice [36], corresponding with greater OCT3 expression in SERT-/- than SERT+/+ mice [54, 55]. We also found that histamine (a substrate for OCT3 [48]) is cleared faster from hippocampal extracellular fluid in SERT-/- mice than SERT+/+ mice [54]. Together, these findings suggest that activity of ethanol at OCT3 may account for greater inhibition of 5-HT clearance in SERT-/- mice than in SERT+/+ mice [36]. More support for ethanol’s action at OCT3 comes from findings that ethanol inhibits uptake of the prototypical cation, tritiated 1-methyl-4-phenylpyridinium ([3H]MPP+), into human epithelial colorectal adenocarcinoma (Caco-2) cells, which are rich in OCT3 [56].
Literature evidence supports the premise that ethanol inhibits DA, NE, and 5-HT clearance from extracellular fluid but does so in a manner independent of high-affinity, “uptake-1” transporters, DAT, NET, and SERT [33, 34, 36]. This raises the possibility that ethanol’s actions on monoamine uptake are mediated, at least in part, via OCT3, and provides a potential mechanism by which ethanol (via inhibition of OCT3) enhances cocaine-induced increases in extracellular monoamines, as well as cocaine’s rewarding properties. Here we focus on DA because of its important role in reward pathways [57] and provide novel evidence that ethanol acts in an OCT3-dependent manner to inhibit DA clearance and augment the rewarding properties of cocaine.
Materials and methods
Naïve adult male and female OCT3+/+ and OCT3-/- mice bred on a C57BL/6 background were used for in vivo chronoamperometry, conditioned place preference (CPP) and locomotor sensitization studies. Male OCT3+/+, OCT3-/-, SERT+/+ and SERT-/- mice were used for loss of righting reflex (LORR) studies. All mice were from our in-house colonies. For details, see Supplementary Information (SI).
To assess binding of ethanol to the orthosteric site of DAT, NET and/or SERT we measured its ability to displace binding of [3H]WIN35,428, [3H]nisoxetine and [3H]citalopram, respectively, from striatal (DAT) or hippocampal (NET, SERT) homogenates, compared with high-affinity ligands for DAT, NET and SERT (GBR12909, reboxetine and citalopram, respectively) using established methods [58–60] (and SI).
To gain direct insight into ethanol’s interference with OCT3-mediated uptake we measured inhibition of [3H]MPP+ (Perkin Elmer, Boston, MA, USA) uptake into human embryonic kidney (HEK293) cells stably expressing the human (h) isoform of OCT3 (hOCT3), or mock transfected HEK293 cells, according to Janowsky et al. [61]. Additionally, we used HEK293 cells stably expressing the yellow fluorescent protein (YFP) tagged (to the N-terminus) isoforms of either hOCT3, hDAT, hNET or hSERT, and measured the ability of ethanol, cocaine (DAT, NET, SERT blocker) or corticosterone (OCT3 blocker) to inhibit uptake of the fluorescent substrates 4-(4-diemethylamino-styryl)-N-methylpyridinium (ASP+ for OCT3) or 4-(4-(dimethylamino)phenyl)-1-methylpyridinium, (APP+ for DAT, NET and SERT) according to established methods [44, 62, 63] (see SI).
High-speed chronoamperometry was used in vivo to assess the ability of ethanol to inhibit DA clearance, and to enhance the ability of cocaine to do so, in dorsal striatum according to established methods where a Nafion-coated carbon fiber electrode is coupled to a glass multibarreled micropipette containing DA and drugs of interest [50, 64, 65, 66] (see SI). Barrels were filled with either dopamine (200 μM), ethanol (100 mM), cocaine (400 μM), ethanol + cocaine, or vehicle (phosphate-buffered saline, (PBS)). Pressure-ejection of ~20 nL of 200 µM dopamine, 200 µm away from the recording electrode, results in signal amplitudes of ~0.5–1.0 µM [65], consistent with those elicited by stimulated release of DA [67]. Thus, the concentration of neurotransmitter and drug reaching the recording electrode is ~200–400 fold less than the barrel concentration. Consequently, ethanol’s concentration at the recording site is ~0.5–10 mM, consistent with extracellular brain concentrations after systemic administration of 1 g/kg of ethanol [68, 69]. Cocaine was locally applied to dorsal striatum to yield concentrations at the recording site of 1–10 μM, consistent with extracellular brain concentrations after systemic administration of behaviorally relevant doses of cocaine [70, 71]. Importantly, these concentrations of cocaine robustly inhibit DA clearance, whereas lower concentrations are known to increase DA clearance by trafficking DAT to the plasma membrane [72].
Rewarding effects of cocaine, ethanol, and their combination were measured by CPP using established methods [73, 74]. In the same procedure, sensitization of cocaine-induced locomotion (in the presence and absence of ethanol) was measured. Loss of righting reflex (LORR) was assessed as described previously [36]. See SI for details.
Competition binding and uptake of [3H]MPP+ into hOCT3 HEK293 cells were analyzed using non-linear regression, and means were compared by ANOVA. Uptake of APP+ and ASP+ in cells, chronoamperometry and behavioral data were analyzed by ANOVA and post-hoc analyses described in the figure legends. Assumptions underlying the statistical tests were met. Results are expressed as mean and standard error (SEM) or 95% confidence interval (CI). Tests were performed with GraphPad Prism (GraphPad, La Jolla, CA, USA), with statistical significance defined as p < 0.05. See SI for details.
Results
Ethanol does not displace radioligand binding to DAT, NET, and SERT in mouse brain tissue
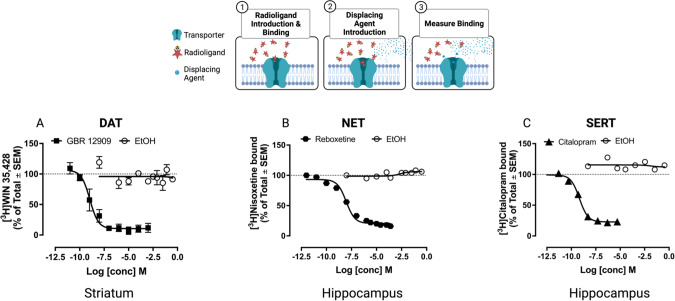
Ki values for displacement of [3H]WIN35,428, [3H]nisoxetine, and [3H]citalopram binding to DAT, NET, and SERT by GBR12909, reboxetine, and citalopram, respectively, in striatal (for DAT) or hippocampal (for NET and SERT) homogenates were in the low nanomolar range, consistent with previous reports [58–60] (Fig. 1, Table 1). In contrast, ethanol (up to 1 M) did not displace [3H]WIN35,428, [3H]nisoxetine and [3H]citalopram, evidencing that ethanol does not interact with binding sites of well-established ligands at DAT, NET and SERT (Fig. 1, Table 1).
Fig. 1. Ethanol fails to displace radioligand binding to DAT (A), NET (B) and SERT (C).
In contrast, known inhibitors of these transporters displaced binding with Ki values in the nM range, as expected (see Table 1). Data shown are mean and SEM, N = 3 mice per assay. Where the error bar is not visible, it is obscured by the symbol. Note: Radioligand binding to DAT was performed on striatal homogenates using 2.7 nM [3H]WIN 35,428, unlabeled GBR 12909 at 11 concentrations from 0.01 nM to 1 mM and ethanol from 3.4 nM to 1.0 M. Radioligand binding to NET was performed on hippocampal homogenates using 1.7 nM [3H]nisoxetine, unlabeled reboxetine at 11 concentrations from 100 pM to 250 µM, and ethanol from 3.4 nM to 1.0 M. Radioligand binding to SERT was performed on hippocampal homogenates using 1.5 nM [3H]citalopram, unlabeled citalopram at 7 concentrations ranging from 5 pM to 5 µM and ethanol from 3.4 nM to 1.0 M. Cartoon created with Biorender.com.
Table 1.
Ki values for displacement of radioligands for DAT, NET and SERT by known inhibitors in mouse brain homogenates.
| Transporter | Hot ligand | nM | Kd | Cold ligand | Ki (nM) |
|---|---|---|---|---|---|
| DAT | [3H]WIN35,428 | 2.7 | 7.8 | GBR12909 | 1.00 (0.01 to 79) |
| NET | [3H]Nisoxetine | 1.7 | 2.5 | Reboxetine | 1.26 (0.10 to 32) |
| SERT | [3H]Citalopram | 1.5 | 1.5 | Citalopram | 0.32 (0.20 to 0.40) |
Data are shown as mean (95% CI).
Ethanol inhibits [3H]MPP+ uptake in hOCT3 HEK293 cells at concentrations relevant in vivo
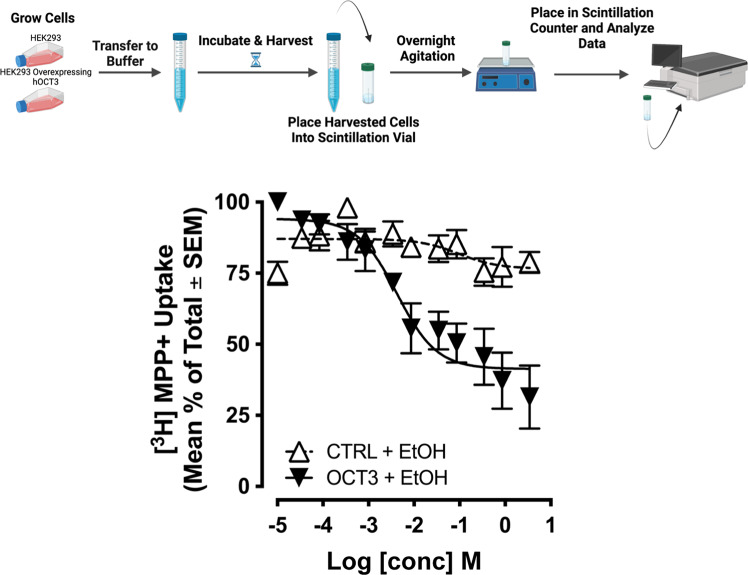
To directly assess putative activity of ethanol at OCT3, we used HEK293 cells stably expressing hOCT3 [75], examined ethanol’s inhibition of [3H]MPP+ uptake into these cells, and obtained a Ki = 4.2 ± 0.2 mM (Fig. 2). Ethanol inhibited uptake into hOCT3 transfected cells to a greater extent than into mock-transfected control cells (interaction F(11, 88) = 10.66, p < 0.0001; ethanol concentration F(11,88) = 17.3, p < 0.001; cell type F(1,8) = 10.18, p = 0.013) (Fig. 2). Because (i) systemic administration of 1 mg/kg of ethanol (i.p.) results in dialysate ethanol levels of approximately 6 mM in the nucleus accumbens of rats [68, 69], and (ii) human subjects may achieve similar [76] or considerably higher brain ethanol levels, with an emphasis on regular ethanol consumers [77], these results are consistent with ethanol inhibiting activity of OCT3 at behaviorally/physiologically relevant concentrations.
Fig. 2. Ethanol inhibits [3H]MPP+ uptake in hOCT3 HEK293 cells to a greater extent than in mock-transfected cells.
Assays were performed in triplicate. N = 5 replicate assays. As ethanol concentrations increased, inhibition of uptake was greater in hOCT3 expressing cells than in mock-transfected cells, p < 0.05. Filled symbols represent significantly different from mock-transfected cells. Data are shown as mean and SEM. Cartoon created using Biorender.com.
Ethanol concentration-dependently inhibits uptake of fluorescent substrate via hOCT3 at concentrations that do not interfere with hDAT, hNET and hSERT-mediated uptake in vitro
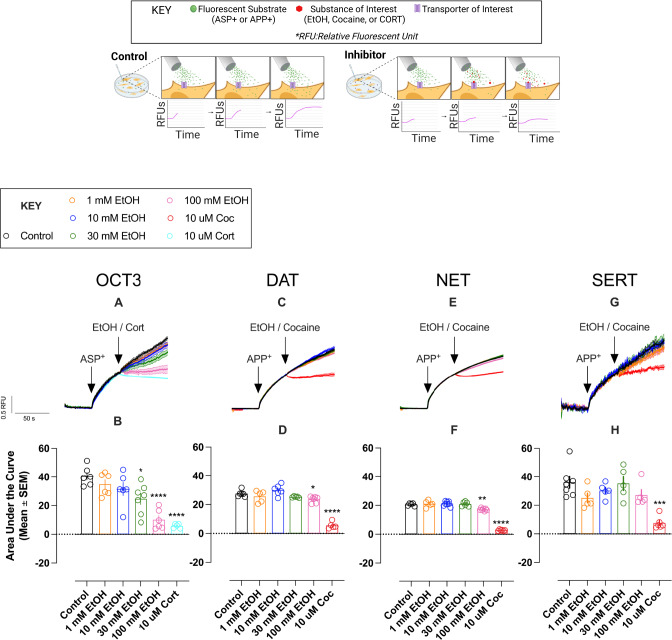
To further probe activity of ethanol at OCT3 versus “uptake-1” [37, 78] monoamine transporters, we measured its ability to inhibit uptake of fluorescent substrates in HEK293 cells expressing either the YFP tagged human isoforms of OCT3, DAT, NET or SERT. Uptake of the fluorescent compounds APP+ and ASP+ afford the temporal resolution to monitor instantaneous effects of test drugs on transporter activity, providing a direct readout of substrate transport that is mediated by electrochemical gradient-driven OCT3 and the secondary-active “uptake-1” transporters. Cells were superfused with fluorescent substrate (10 µM of APP+ for SERT, DAT and NET; 3 µM of ASP+ for OCT3) for 40 s to establish the initial uptake rate before cells were exposed to ethanol or well-established inhibitors of these transporters. As in previous studies, we used ASP+ to measure OCT3-mediated transport fluxes [44, 63]. Owing to the antagonistic properties of ASP+ at SERT [79], APP+ was used for SLC6 transporters, as it reliably labels DAT- and NET-expressing cells and behaves as a substrate of SERT [79, 80]. Ethanol concentration-dependently inhibited substrate uptake only in hOCT3 HEK293 cells (F(5, 22.62) = 16.99, p < 0.0001; Fig. 3A, B). Both the selective OCT3 blocker corticosterone (10 µM; t(5.16) = 12.37, CI95: 24.81, 44.72, p < 0.0001) and ethanol (100 mM; t(9.99) = 7.78, CI95: 18.28, 42.55, p < 0.0001), fully blocked uptake of ASP+, while 30 mM ethanol inhibited uptake by ~50% (t(10.29) = 3.31, CI95: 0.99, 30.74, p = 0.04). In contrast, ethanol did not impact fluorescent substrate uptake in hSERT HEK293 cells (Fig. 3G, H), and only modestly inhibited uptake into hDAT (F(5, 16.58) = 53.95, p < 0.0001; Fig. 3C, D) and hNET (F(5, 15.52) = 162.40, p < 0.0001; Fig. 3E, F) HEK293 cells at the highest concentration tested (100 mM). As expected, the DAT, NET, SERT blocker cocaine (10 µM) blocked APP+ uptake into cells expressing these transporters (Fig. 3C–H). We have previously shown that cocaine does not block fluorescent substrate uptake in hOCT3 HEK293 cells [44], which further supports the interpretation that the combination affects transporters of multiple families, i.e., SLC22 (OCT3 [37]) and SLC6 (DAT, NET, and SERT [81]).
Fig. 3. Real time assessment of substrate uptake reveals ethanol inhibits uptake at physiologically relevant concentrations in OCT3-expressing cells, but not DAT, NET or SERT expressing cells.
A EtOH inhibited accumulation of ASP+ (3 µM) via OCT3 in a concentration-dependent manner. Addition of the OCT3-inhibitor corticosterone (cort, 10 µM) is shown as control. B Total A.U.C. (T80-150 s) from data shown in A. C, E Ethanol was without effect on DAT- or NET-mediated uptake at physiologically relevant concentrations and was without effect on SERT-mediated uptake (G). Addition of cocaine (10 µM) inhibited intracellular accumulation of APP+ (10 µM) via DAT, NET, and SERT (C, E, G). D, F, H Total A.U.C. (T80-150 s) from data shown in C, E, G. Data are shown as mean and SEM of 5-8 individual recordings per condition. Data shown in B, D, F, & H were analyzed using Brown-Forsythe ANOVA test, Dunnett’s T3 multiple comparisons test versus control. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Cartoon created with Biorender.com.
Ethanol inhibits DA clearance in dorsal striatum, and enhances cocaine-induced inhibition of DA clearance, in an OCT3-dependent manner
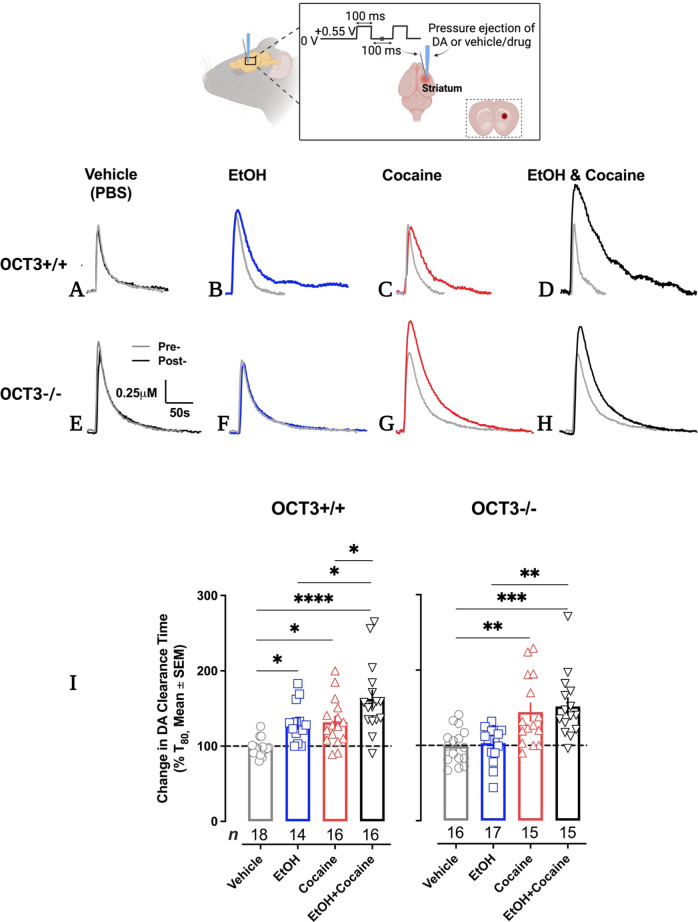
Important for the translational relevance of our in vitro data, which strongly point to OCT3 as a mechanism by which ethanol inhibits monoamine uptake, we used high-speed chronoamperometry to investigate effects of ethanol on OCT3-mediated DA clearance in vivo. We measured clearance of exogenously applied DA (to achieve signal amplitudes of ~0.5–1.0 μM) to dorsal striatum, a brain region implicated in the locomotor stimulant and rewarding effects of cocaine, in OCT3+/+ and OCT3-/- mice and tested the effect of ethanol (0.5–10 mM) and cocaine (1–10 µM), also locally applied to dorsal striatum, to inhibit DA clearance (see methods and SI methods for details). There were no statistically significant main or interaction effects of sex for any of the dependent variables, so data from males and females were pooled. Dependent variables were peak amplitude of the DA signal, time to clear between 20% and 60% of the peak signal (T20-60, the pseudo linear portion of the descending limb of the DA signal), and the time to clear 80% of peak signal (T80). The most marked effects were on T80. There were significant differences in DA clearance time (T80) among groups administered ethanol, cocaine, or ethanol and cocaine for OCT3+/+ (F(3, 60) = 12.36, p < 0.0001 (Fig. 4)) and OCT3-/- (F (3, 59) = 9.52, p < 0.0001 (Fig. 4)) mice. Intrastriatally applied ethanol increased T80 DA clearance time (i.e., inhibited DA clearance) in OCT3+/+ animals (p = 0.01), an effect that was lost in OCT3-/- mice (p = 0.98), consistent with OCT3 being a key player in the action of ethanol to inhibit DA uptake. As expected, a maximally effective pmol amount of cocaine locally applied to dorsal striatum inhibited DA clearance in both OCT3+/+ (p = 0.01) and OCT3-/- (p = 0.003) mice (Fig. 4). Examining different pmol amounts of cocaine confirmed that there were no significant differences in the maximal effect, or EC50 value, of cocaine to inhibit DA clearance between OCT3+/+ and OCT3-/- mice (see SI Fig. 1). Consistent with ethanol and cocaine acting at different sites, ethanol enhanced cocaine’s inhibition of DA clearance in OCT3+/+ mice (p = 0.03), but not in OCT3-/- animals (p = 0.93) (Fig. 4). Drug effects on T20-60 clearance time followed similar patterns (SI Table 1). Drugs generally did not have significant effects on signal amplitude. Although cocaine trended to increase signal amplitude in OCT3-/- mice, the only significant effect on amplitude was in OCT3+/+ mice following ethanol and cocaine, which increased DA signal amplitude (SI Table 2).
Fig. 4. Ethanol inhibits DA clearance in dorsal striatum, and enhances cocaine-induced inhibition of DA clearance, in an OCT3-dependent manner.
Representative traces of oxidation currents (converted to a micromolar value using a calibration factor determined in vitro prior to using electrodes in vivo) showing that in OCT3+/+ mice, vehicle had no effect on DA clearance time (T80) (A), whereas ethanol (~0.5-10 mM, see methods) (B) and cocaine (1–10 µM, see methods) (C) inhibited DA clearance. Coadministration of ethanol and cocaine inhibited DA clearance to a markedly greater extent than either drug alone (D). As expected, in OCT3-/- mice, vehicle had no effect on DA clearance (E), and cocaine inhibited DA clearance (G). However, unlike in OCT3+/+ mice, ethanol did not inhibit DA clearance (F), nor did it enhance the ability of cocaine to inhibit DA clearance (H). Summary data are shown in (I). Data are shown as mean and SEM change in T80 value 2 minutes following administration of drug or vehicle, n = 14–18 per condition. Data were analyzed using thee-factor ANOVA and followed up with separate 1-way ANOVAs per genotype, with Tukey’s test for post-hoc multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Cartoon created with Biorender.com.
There were significant differences between genotypes for baseline (i.e., pre-drug) T80 values. DA clearance time was significantly longer in OCT3-/- mice (T80 49 ± 3 s, n = 63) than their OCT3+/+ counterparts (T80 37 ± 2 s, n = 64) (T80 t125 = 2.72, p = 0.01). Likewise T20-60 values were longer in OCT3-/- mice than OCT3+/+ mice (see SI). Baseline signal amplitude was not significantly different between genotypes, which was expected given we controlled the amount of DA pressure-ejected to yield similar signal amplitudes between genotypes. Neither the volume or pmol amount of DA delivered differed as a function of genotype (see SI).
Reinforcing and locomotor sensitizing effects of cocaine are enhanced by OCT3-dependent actions of ethanol
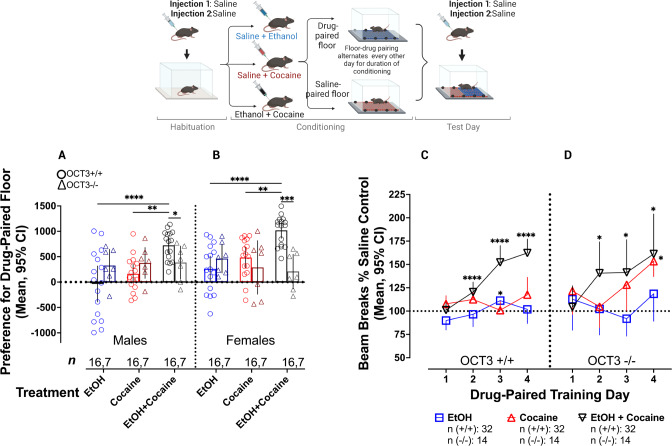
To determine whether the ability of ethanol to augment cocaine-induced inhibition of DA clearance mapped onto behavioral outcomes in an OCT3-dependent manner, we assessed CPP and sensitization to the locomotor stimulant effects of cocaine in the presence and absence of ethanol. The CPP protocol is often used to model abuse-related drug effects, and is demonstrated by an animal’s preference for an environment that has been previously paired with a drug of interest in comparison to an environment paired with vehicle [82, 83]. Based on pilot studies (data not shown), we selected a dose of cocaine (3.2 mg/kg) and ethanol (1 g/kg) that generally failed to produce CPP, and in cases where CPP developed (e.g., female OCT3+/+ mice given cocaine), it was only modest (see SI methods). Three-way ANOVA was used to assess CPP in OCT3+/+ and OCT3-/- animals receiving ethanol, cocaine, or ethanol+cocaine (see Fig. 5).
Fig. 5. Ethanol enhances the rewarding and locomotor sensitizing effects of cocaine in an OCT3-dependent manner.
A, B Ethanol (1 g/kg) enhanced CPP for cocaine (3.2 mg/kg) in male and female OCT3+/+ mice. This effect was lost in OCT3-/- mice. C In OCT3+/+ mice, the combination of cocaine and ethanol resulted in sensitization to locomotor stimulant effects of this drug pairing, whereas, at the doses used, neither drug alone produced sensitization. D OCT3-/- mice developed sensitization to cocaine, but coadministration with ethanol did not significantly enhance this effect at drug paired training days 3 and 4. N = 7-16/condition. Error bars represent 95% CI. Post-hoc comparisons for CPP were conducted with Tukey’s post-hoc, whereas Dunnet’s post-hoc test versus day 1 was used for sensitization analysis. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Cartoon created with Biorender.com.
We found significant interactions between treatment and genotype (F(2,126) = 10.68, p < 0.0001) as well as genotype and sex (F(1,126) = 5.02, p = 0.03). There was also a main effect for treatment (F (2, 126) = 6.74, p = 0.002). Consistent with abundant literature [84–89], cocaine elicited significant CPP in female OCT3+/+ animals (p = 0.002) (Fig. 5B). Also in line with previous literature [90–93], in wild-type mice co-administration of cocaine and ethanol led to a significant increase in preference for the drug-paired floor compared to either drug administered alone for both males (ethanol vs. ethanol+cocaine: p < 0.0001; cocaine vs. ethanol+cocaine: p = 0.001, Fig. 5A) and females (ethanol vs. ethanol+cocaine: p < 0.0001; cocaine vs. ethanol+cocaine: p = 0.002, Fig. 5B). Moreover, OCT3 +/+ male and female mice displayed a greater preference for the ethanol+cocaine-paired floor compared to male (treatment*genotype: F(2,63) = 3.54, p = 0.035, Fig. 5A) and female (treatment*genotype: F(2,63) = 7.83, p = 0.001, Fig. 5B) OCT3-/- mice. There was no significant effect of treatment on drug-paired floor preference for male or female OCT3-/- mice (Fig. 5A, B). Although the sample size for OCT3-/- mice was approximately half that for OCT3+/+ mice, this smaller size still afforded adequate power (i.e., 0.97, calculated with G*Power) to detect CPP differences like those observed in OCT3+/+ mice (i.e., Cohen’s d = 1.5-1.6 using Student’s t-test to compare the combination of ethanol and cocaine with either drug alone). Because the sample size in OCT3-/- mice afforded more than 80% power to detect CPP differences like those observed in OCT3+/+ mice, we truncated our CPP studies in OCT3-/- mice for ethical reasons.
Sensitization describes the phenomenon of enhanced locomotor-stimulating effects that are observed in response to repeated drug administration, thought to reflect brain changes leading to drug addiction [88]. Importantly, it appears sensitization is involved only in certain phases of drug addiction, and is thought to be an important initial step in the drug addiction process [94]. Sensitization to the locomotor stimulant effects of cocaine was first assessed using a four-way repeated measures ANOVA with Greenhaus-Geisser correction, with sex, genotype, treatment, and training day as independent variables. There were no interaction or main effects of sex on sensitization, so male and female data were pooled for further analysis. There was a significant three-way interaction of drug-paired training day, treatment, and genotype (F (5.282, 348.59) = 4.02, p = 0.001). To examine this three-way interaction in more detail, the interaction between drug-paired training day and genotype was assessed separately for each treatment condition. There was no effect of training day or genotype on the effect of ethanol to impact locomotor activity (Fig. 5C, D). There was no ethanol-induced sensitization. For the cocaine-treated group, there were significant main effects of training day (F(2.63, 115.8) = 3.82, p = 0.02) and genotype (F(1, 44) = 8.84, p = 0.01). Interestingly, OCT3-/- (first vs. 4th session: Mean Difference = -31.91, p = 0.04, Fig. 5D), but not OCT3+/+ (first vs. 4th session: Mean Difference = -9.84, p = 0.75, Fig. 5C) mice developed sensitization to the locomotor stimulant effects of cocaine alone, suggesting that OCT3-/- mice are more sensitive to this effect of cocaine than their wild-type counterparts. For the groups administered ethanol + cocaine, we observed a significant main effect of training day (F (2.67, 117.6) = 22.57, p < 0.0001), but no effect of genotype. OCT3+/+ mice administered both ethanol and cocaine developed significant sensitization to the locomotor stimulant effect of this drug combination (first vs. 4th session: Mean Difference = −61.19, p < 0.0001, Fig. 5C). Moreover, the locomotor increase from repeated coadministration of ethanol and cocaine was substantially greater compared to OCT3+/+ mice that received either ethanol (first vs. 4th session: Mean Difference = −12.01, p = 0.42) or cocaine (first vs. 4th session: Mean Difference = -9.84, p = 0.69) alone, (Fig. 5C), consistent with this drug combination amplifying the effect of either drug alone. OCT3-/- mice also developed sensitization to the locomotor stimulant effects of ethanol+cocaine (first vs. 4th session: Mean Difference = -56.92, p = 0.02, Fig. 5D). Importantly, consistent with activity of ethanol at OCT3, when given in combination with cocaine there was no further significant enhancement of sensitization to the locomotor stimulant effect of cocaine in OCT3-/- mice at days 3 and 4, though it appears the combination of drugs may have accelerated sensitization to cocaine, being evident at day 2 in OCT3-/- mice, whereas sensitization to cocaine alone was not apparent until day 3. This could indicate actions of ethanol elsewhere, but does not preclude a major role for OCT3 in the actions of ethanol.
Lack of OCT3 Significantly Reduces Sensitivity to the Sedative/Hypnotic Effects of Ethanol
Greater sensitivity to the sedative/hypnotic effects of ethanol is associated with a higher incidence of alcohol use disorder in humans [95]. We therefore investigated the ability of ethanol (3.2 g/kg) to induce loss of righting reflex (LORR), a commonly used indicator of sedative/hypnotic effects in rodents, in wild-type (OCT3+/+ and SERT+/+), OCT3-/- and SERT-/- mice. We included SERT-/- mice as a comparator because we have previously shown that they have higher expression of OCT3 than SERT+/+ mice, which is associated with longer LORR [36, 96], though the mechanism for this effect remains unknown. Replicating our previous findings [36, 96], SERT-/- mice regained their righting reflex at a rate 14.34 times slower than SERT+/+ controls (χ2(1) = 9.99, p = 0.003, HR = 14.34, CI95: 2.75, 74.78) indicating that these animals are significantly more sensitive to the sedative/hypnotic effects of alcohol (Fig. 6). Consistent with increased expression of OCT3 playing a mechanistic role in this finding, OCT3-/- mice regained their righting reflex at a rate approximately 4.65 times faster than OCT3+/+ controls (χ2(1)=6.64, p = 0.01, HR = 4.65, CI95: 1.45, 14.96), indicating that mice lacking OCT3 are significantly less sensitive to the sedative/hypnotic effects of ethanol, and that the sedative effects of ethanol appear to be dependent on degree of OCT3 expression. There was no effect of genotype on LORR latency (χ2(3) = 0.87, p = 0.83; data not shown).
Fig. 6. Sedative/hypnotic effects of ethanol are dampened in OCT3-/- mice and enhanced in SERT-/- mice.
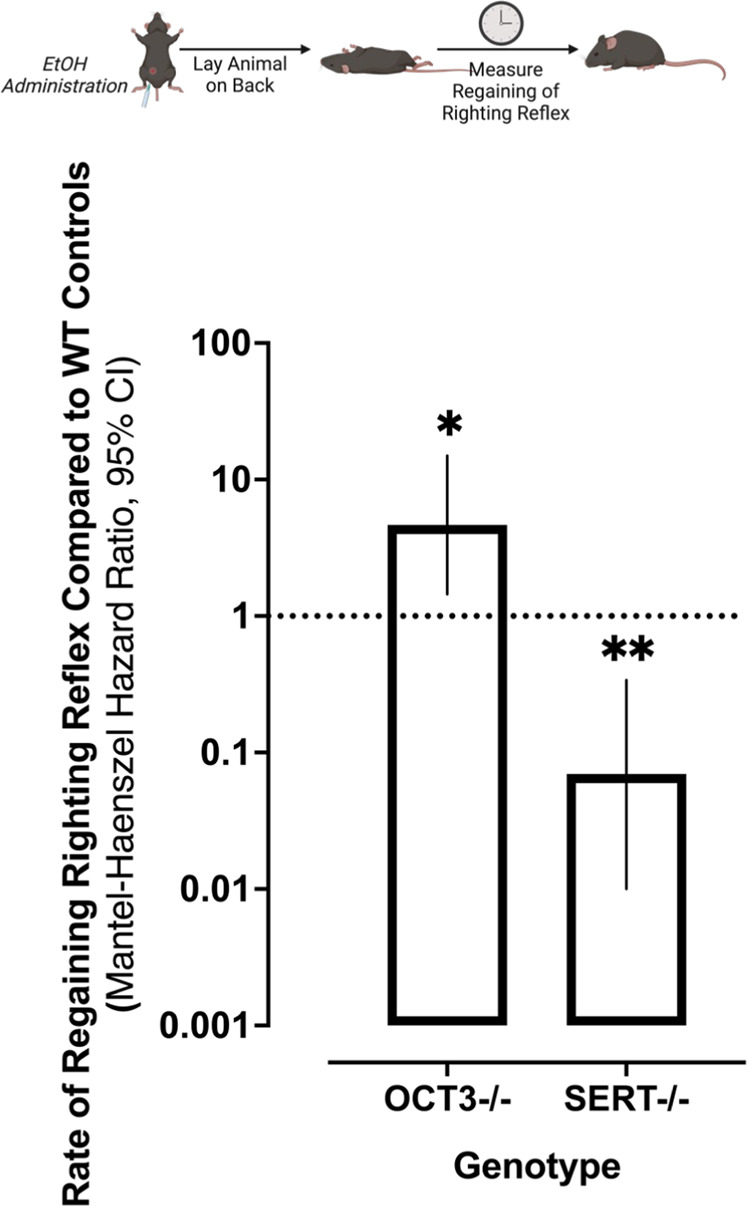
Replicating our previously published findings [36, 98] SERT-/- mice, which have greater expression of OCT3 than wild-type mice, administered ethanol (3.2 g/kg) regained their righting reflex at a significantly slower rate than their wild-type controls. In contrast, OCT3-/- mice regained their righting reflex significantly faster than wild-type controls. Data were analyzed via survival analysis, using the Mantel-Cox log-rank test and the Mentel-Haenszel hazard ratio followed by Holmes-Šidák correction for multiple comparisons. *p < 0.05, **p < 0.01 compared to within-genotype control mice; error bars represent 95% CI, n = 6 – 9. Cartoon created with Biorender.com.
Discussion
Here we report the novel finding that ethanol acts at OCT3 to inhibit monoamine uptake. We base this on our findings that (1) ethanol inhibits fluorescent and radiolabeled substrate uptake in hOCT3 HEK293 cells at physiologically relevant concentrations; (2) in wild-type, but not in OCT3-/- mice, ethanol (a) inhibits DA clearance in striatum in vivo; (b) enhances the ability of cocaine to inhibit DA clearance in striatum in vivo; (c) augments CPP for cocaine; and (d) augments sensitization to the locomotor stimulant effects of cocaine; (3) sedative/hypnotic effects of ethanol are less in OCT3-/- mice than wild-type mice; and (4) ethanol does not displace binding of well-established ligands that bind to the S1 site of DAT, NET or SERT nor does it interfere with uptake of substrates via each transporter at concentrations lower than 100 mM. Together with our published findings that cocaine does not interact with OCT3 at physiologically relevant concentrations (e.g., concentrations that produce maximal behavioral effects) [44] these findings provide a novel mechanistic basis for the increased and prolonged subjective euphoria experienced by individuals who use cocaine and alcohol concurrently. By acting at different sites to inhibit monoamine uptake (i.e., ethanol at OCT3, cocaine at DAT, NET, and SERT), the ensuing increase in monoamines, and therefore conceivably the “high”, is greater when ethanol and cocaine are taken concurrently than when they are taken separately.
We focus our discussion on DA, since it is considered the primary player in rewarding effects of ethanol, however, since OCT3 also transports other cations with varying efficiencies [38, 97], our discussion applies to other monoamines as well. Among its many actions, ethanol increases extracellular DA, assessed predominanty using microdialysis, but the mechanisms by which it does are not clear. Often the increase in extracellular DA has been attributed to ethanol increasing DA release. However, this does not take into account that changes in microdialysate DA reflect a number of processes occurring in tandem, including DA release, uptake and metabolism. Electrophysiological studies show that ethanol increases firing of DA neurons [98–101], and there is some evidence that ethanol influences DA metabolism [102], whereas studies of the effect of ethanol on DA uptake have been mixed. This is likely due to different preparations and approaches used; however, using fast-scan cyclic voltammetry to measure DA clearance in striatum in vivo, Wightman’s group found that ethanol slowed DA clearance [32]. Subsequent studies from the Jones’ group, using striatal slices prepared from DAT-/- mice, showed this effect of ethanol to be DAT-independent [33]. These findings, together with those showing that ethanol potentiates cocaine-induced increases in extracellular DA [20] suggest that ethanol acts at a site(s) other than DAT to exert this effect. Our findings provide evidence that ethanol acts via OCT3 to inhibit DA uptake.
Because DAT, NET and SERT take up substrates other than their native neurotransmitters [37], we first investigated if ethanol had activity at these high-affinity “uptake-1” transporters to inhibit monoamine clearance. Evidence has been reported both for [32, 35, 103] and against [33, 34, 36] activity of ethanol at these transporters, with different observations likely influenced by use of different expression systems [104] and different concentrations of ethanol. We found that ethanol, at concentrations as high as 1 M, did not compete against, [3H]WIN35,428 binding to DAT in striatal homogenates, or against [3H]nisoxetine and [3H]citalopram binding to NET and SERT, respectively, in hippocampal homogenates. In contrast, high affinity ligands for these transporters (GBR12909, reboxetine and citalopram,) displaced [3H]WIN35,428, [3H]nisoxetine and [3H]citalopram to DAT, NET and SERT, respectively, with Ki values in the nM range. However, the pharmacology of monoamine transporters is complex and modulators may bind to ortho- and allosteric sites [105]. Ethanol interacts with a binding site that is located on the extracellular loop 2 of the DAT [106]. Hence, ligand-displacement assays carried out at equilibrium may not allow for identification of modulatory properties, or lack thereof. Consequently, we also performed functional assays to determine the effect of ethanol on DAT, NET, SERT and OCT3 with high temporal resolution. Ethanol at concentrations as high as 100 mM did not inhibit uptake of fluorescent substrate into hSERT HEK293 cells, and at this high concentration, only modestly inhibited uptake into hDAT and hNET HEK293 cells. In contrast, cocaine (10 µM) fully blocked substrate uptake in these cell lines. Combined with existing literature [33, 34, 36], our data support findings that ethanol does not perturb function of DAT, NET or SERT, with regards to inwardly-directed transport fluxes.
Rapidly growing evidence supports an important role for OCT3 in monoamine neurotransmission [39–41, 43, 45–54]. Because ethanol concentration-dependently inhibits uptake of [3H]MPP+ into Caco-2 cells, which are rich in OCT3 [56], we hypothesized that ethanol acts to inhibit monoamine uptake via actions at OCT3. Consistent with our hypothesis, we found that ethanol inhibited uptake of [3H]MPP+ into hOCT3 HEK293 cells with a Ki of 4.2 ± 0.2 mM. Considering that brain concentrations of ethanol reported for rodents and human subjects are in the low mM range [68, 76], this observation suggests that the inhibitory properties of ethanol at OCT3 contribute to elevations in extracellular monoamines observed following ethanol exposure. Consistent with this, ethanol inhibited fluorescent substrate uptake into hOCT3 HEK293 cells. These novel findings further support the conclusion that ethanol acts to inhibit monoamine uptake via its action at OCT3. Based on the prolonged exposure times (minutes) to ethanol during binding experiments and uptake utilizing [3H]MPP+ as a substrate, it remains possible that the antagonistic activity of ethanol at OCT3 involves other effects, such as disruptions of membrane composition and intracellular signaling cascades affecting OCT3 more than members of the SLC6 family. However, the temporal resolution of the fluorescent tracer-based uptake experiments allowed us to uncover an instantaneous effect of ethanol on OCT3-mediated transport processes. This observation strongly supports the interpretation that ethanol directly and instantaneously perturbs the function of OCT3.
To interrogate the translational relevance of these in vitro findings we turned to constitutive OCT3-/- mice. Consistent with earlier reports [32], ethanol inhibited DA clearance in striatum of wild-type (OCT3+/+) mice. This effect was lost in OCT3-/- mice, consistent with a crucial role for OCT3 in the actions of ethanol to inhibit DA clearance. We [44], and others [39] have previously shown that cocaine does not have activity at OCT3 at concentrations lower than 100 µM, and our data show that ethanol does not interfere with the function of DAT, NET or SERT. Consistent with these drugs acting at different sites, ethanol enhanced the ability of a maximally effective concentration of cocaine to inhibit DA clearance in striatum of OCT3+/+ mice, but not in OCT3-/- mice. The genotype-dependency of ethanol’s effects on DA clearance make non-specific effects of ethanol, such as fluidization of the cell membrane [107], unlikely. Related to our findings, the OCT3 blocker corticosterone has been shown to enhance cocaine-induced DA signaling and behaviors via nongenomic glucocorticoid actions, suggesting involvement of an OCT3-dependent mechanism [49, 52]. Taken together, these results suggest that cocaine-induced increases in DA signaling can be potentiated by concurrent blockade of OCT3. These findings are in line with our previous studies showing that the OCT blocker, decynium-22, enhances the antidepressant-like effects of SERT and NET blockers most likely via its action at OCT3 [50, 108].
Although preclinical investigations using self-administration and CPP across species [23, 25–28, 92], as well as clinical findings in humans [16–18], suggest that rewarding properties of the combination of ethanol and cocaine are greater than those of each drug alone, the mechanism(s) underlying these observations remain unknown. Consistent with these reports, ethanol potentiated CPP for cocaine in OCT3+/+ mice. This effect was more pronounced in females than males, consistent with findings that women are more sensitive to the effects of psychostimulants and progress from initial use to addiction faster than men [109, 110]. Importantly, in agreement with our in vivo neurochemical data, ethanol did not potentiate CPP for cocaine in OCT3-/- mice, revealing OCT3 as an important mechanism in this action of ethanol. Though no significant sex differences were observed in sensitization to the locomotor effects of ethanol, cocaine or their combination, doses of ethanol and cocaine that did not elicit sensitization when given alone, did when given together in OCT3+/+ mice. Interestingly, sensitization to the locomotor stimulant effect of cocaine did emerge in OCT3-/- mice, suggesting potential compensation in these mice as a result of constitutive ablation of OCT3. Importantly however, when cocaine was given together with ethanol in OCT3-/- mice, sensitization was not enhanced, again pointing to a crucial role for OCT3 in this effect of ethanol. In line with our observations, previous reports have shown that ethanol, at a dose that does not cause CPP per se, enhances the conditioning properties of the DAT, NET and SERT-targeting cathinone mephedrone [111] and enhances its effects on extracellular monoamines [112]. Related to potential compensation in constitutive OCT3 KO mice, available data suggest that there is no overt compensation that would account for the present findings [41, 44, 113] (see SI).
To examine the generality of our findings, we assessed the sedative/hypnotic effects of ethanol in wild-type, OCT3-/- and SERT-/- mice. We, and others, have shown OCT3 expression to be greater in SERT-/- mice than in wild-type mice [54, 55], and that ethanol’s inhibition of 5-HT clearance in hippocampus of SERT-/- mice is more robust than in SERT+/+ mice [36], consistent with a role of OCT3. Likewise, we found that the sedative/hypnotic effects of ethanol were more pronounced in SERT-/- mice [36, 96]. We replicated this finding, and extended it by showing that ethanol-induced LORR is less in OCT3-/- mice, consistent with OCT3 contributing to ethanol-induced LORR. Although we did not measure blood alcohol concentration in OCT3+/+ and OCT3-/- mice, differences in ethanol metabolism between the two genotypes are unlikely [36, 96] (see SI). Taken together with data presented here, it appears that LORR is related to level of OCT3 expression.
Collectively, our data provide compelling evidence for OCT3 as a novel and previously unsuspected player in the actions of ethanol to inhibit monoamine uptake. Here we identified OCT3 as an important mediator of the acute effects of ethanol to inhibit DA uptake and produce rewarding effects. These novel findings raise several avenues for exciting future studies, including investigations of (and SI for details) (1) the effect of chronic cocaine and ethanol use in combination or alone, (2) potential actions of cocaethylene, a psychoactive substance formed in the liver when cocaine and alcohol coexist in blood [114], at OCT3, as well as acetaldehyde, the main metabolite of ethanol, which is readily self-administered in rodents and could play a role in the rewarding properties of ethanol [115], (3) self-administration of ethanol, cocaine and their combination, since behavioral and neurochemical effects of most drugs of abuse, including ethanol and cocaine, differ depending on whether they are administered contingently vs. non-contingently (e.g., [116–118]), (4) how ethanol interacts with OCT3 to inhibit substrate uptake. These efforts will build on the recently published structural basis for OCT3 inhibition [119].
In sum, these data raise the provocative idea that it may be possible to pharmacologically prevent ethanol’s actions at OCT3, and/or to enhance OCT3 transporter function, as a therapeutic approach to treating alcohol dependence, and alcohol and cocaine co-abuse [120], and open an avenue rich for future research.
Supplementary information
Acknowledgements
This work was supported by R21DA046044, U18DA052527 and R01 MH093320 to LCD, W81XWH-12-1-0506 to GGG, and by grant P34670-B (to HHS) from the Austrian Science Fund/FWF. FPM received support from the Austrian Academy of Sciences (DOC and MAX KADE Fellowships, respectively).
Author contributions
NJC, FPM, WAO, MV, KMC, MAB, REH, DS, MH, and GGG performed experiments; NJC, FPM, KMC, GGG, WK, and LCD analyzed data and prepared figures; LCD contributed original concept; DG, WK, HHS contributed to experimental design and editing of the manuscript; NJC, FPM, KMC, GGG, LCD wrote the manuscript, GGG, HHS, LCD provided funding.
Data availability
Data supporting these findings are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: N. J. Clauss, F. P. Mayer.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-023-02064-5.
References
- 1.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and dsm-iv alcohol use disorder in the united states, 2001-2002 to 2012-2013: Results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry. 2017;74:911–23. doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of dsm-5 alcohol use disorder: Results from the national epidemiologic survey on alcohol and related conditions iii. JAMA Psychiatry. 2015;72:757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staines GL, Magura S, Foote J, Deluca A, Kosanke N. Polysubstance use among alcoholics. J Addict Dis. 2001;20:53–69. doi: 10.1300/J069v20n04_06. [DOI] [PubMed] [Google Scholar]
- 4.Jones AW, Kugelberg FC, Holmgren A, Ahlner J. Drug poisoning deaths in sweden show a predominance of ethanol in mono-intoxications, adverse drug-alcohol interactions and poly-drug use. Forensic Sci Int. 2011;206:43–51. doi: 10.1016/j.forsciint.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Koski A, Ojanpera I, Vuori E. Interaction of alcohol and drugs in fatal poisonings. Hum Exp Toxicol. 2003;22:281–7. doi: 10.1191/0960327103ht324oa. [DOI] [PubMed] [Google Scholar]
- 6.van Amsterdam J, Brunt TM, Pierce M, van den Brink W. Hard boiled: Alcohol use as a risk factor for MDMA-induced hyperthermia: A systematic review. Neurotox Res. 2021;39:2120–33. doi: 10.1007/s12640-021-00416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thai D, Dyer JE, Benowitz NL, Haller CA. Gamma-hydroxybutyrate and ethanol effects and interactions in humans. J Clin Psychopharmacol. 2006;26:524–9. doi: 10.1097/01.jcp.0000237944.57893.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liechti ME, Kunz I, Greminger P, Speich R, Kupferschmidt H. Clinical features of gamma-hydroxybutyrate and gamma-butyrolactone toxicity and concomitant drug and alcohol use. Drug Alcohol Depend. 2006;81:323–6. doi: 10.1016/j.drugalcdep.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Connor JP, Gullo MJ, White A, Kelly AB. Polysubstance use: Diagnostic challenges, patterns of use and health. Curr Opin Psychiatry. 2014;27:269–75. doi: 10.1097/YCO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 10.Barrett SP, Gross SR, Garand I, Pihl RO. Patterns of simultaneous polysubstance use in Canadian rave attendees. Subst Use Misuse. 2005;40:1525–37. doi: 10.1081/JA-200066866. [DOI] [PubMed] [Google Scholar]
- 11.Grant BF, Harford TC. Concurrent and simultaneous use of alcohol with cocaine: Results of national survey. Drug Alcohol Depend. 1990;25:97–104. doi: 10.1016/0376-8716(90)90147-7. [DOI] [PubMed] [Google Scholar]
- 12.Caetano R, Weisner C. The association between dsm-iii-r alcohol dependence, psychological distress and drug use. Addiction. 1995;90:351–9. doi: 10.1111/j.1360-0443.1995.tb03783.x. [DOI] [PubMed] [Google Scholar]
- 13.Martin CS, Clifford PR, Maisto SA, Earleywine M, Kirisci L, Longabaugh R. Polydrug use in an inpatient treatment sample of problem drinkers. Alcohol Clin Exp Res. 1996;20:413–7. doi: 10.1111/j.1530-0277.1996.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Williamson V, Setlow B, Cottler LB, Knackstedt LA. The importance of considering polysubstance use: Lessons from cocaine research. Drug Alcohol Depend. 2018;192:16–28. doi: 10.1016/j.drugalcdep.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanek VW, Dickey-White HI, Signs SA, Schechter MD, Buss T, Kulics AT. Concurrent use of cocaine and alcohol by patients treated in the emergency department. Ann Emerg Med. 1996;28:508–14. doi: 10.1016/S0196-0644(96)70114-2. [DOI] [PubMed] [Google Scholar]
- 16.McCance-Katz EF, Kosten TR, Jatlow P. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone—a multiple-dose study. Biol Psychiatry. 1998;44:250–9. doi: 10.1016/S0006-3223(97)00426-5. [DOI] [PubMed] [Google Scholar]
- 17.Farre M, de la Torre R, Llorente M, Lamas X, Ugena B, Segura J, et al. Alcohol and cocaine interactions in humans. J Pharm Exp Ther. 1993;266:1364–73. [PubMed] [Google Scholar]
- 18.Higgins ST, Roll JM, Bickel WK. Alcohol pretreatment increases preference for cocaine over monetary reinforcement. Psychopharmacology. 1996;123:1–8. doi: 10.1007/BF02246274. [DOI] [PubMed] [Google Scholar]
- 19.Hedaya MA, Pan WJ. Effect of alcohol coadministration on the plasma and brain concentrations of cocaine in rats. Drug Metab Dispos. 1997;25:647–50. [PubMed] [Google Scholar]
- 20.Lindholm S, Rosin A, Dahlin I, Georgieva J, Franck J. Ethanol administration potentiates cocaine-induced dopamine levels in the rat nucleus accumbens. Brain Res. 2001;915:176–84. doi: 10.1016/S0006-8993(01)02847-5. [DOI] [PubMed] [Google Scholar]
- 21.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–25. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennings EJ, Leccese AP, Wolff FA. Effects of concurrent use of alcohol and cocaine. Addiction. 2002;97:773–83. doi: 10.1046/j.1360-0443.2002.00158.x. [DOI] [PubMed] [Google Scholar]
- 23.Tallarida CS, Bires K, Avershal J, Tallarida RJ, Seo S, Rawls SM. Ethanol and cocaine: Environmental place conditioning, stereotypy, and synergism in planarians. Alcohol. 2014;48:579–86. doi: 10.1016/j.alcohol.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busse GD, Riley AL. Cocaine, but not alcohol, reinstates cocaine-induced place preferences. Pharm Biochem Behav. 2004;78:827–33. doi: 10.1016/j.pbb.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Ding ZM, Oster SM, Hauser SR, Toalston JE, Bell RL, McBride WJ, et al. Synergistic self-administration of ethanol and cocaine directly into the posterior ventral tegmental area: Involvement of serotonin-3 receptors. J Pharm Exp Ther. 2012;340:202–9. doi: 10.1124/jpet.111.187245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aspen JM, Winger G. Ethanol effects on self-administration of alfentanil, cocaine, and nomifensine in rhesus monkeys. Psychopharmacology. 1997;130:222–7. doi: 10.1007/s002130050232. [DOI] [PubMed] [Google Scholar]
- 27.John WS, Nader MA. Effects of ethanol on cocaine self-administration in monkeys responding under a second-order schedule of reinforcement. Drug Alcohol Depend. 2017;170:112–9. doi: 10.1016/j.drugalcdep.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira RB, Andrade PB, Valentao P. A comprehensive view of the neurotoxicity mechanisms of cocaine and ethanol. Neurotox Res. 2015;28:253–67. doi: 10.1007/s12640-015-9536-x. [DOI] [PubMed] [Google Scholar]
- 29.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: Structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 30.Ron D, Barak S. Molecular mechanisms underlying alcohol-drinking behaviours. Nat Rev Neurosci. 2016;17:576–91. doi: 10.1038/nrn.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand I, Fliegel S, Spanagel R, Noori HR. Global ethanol-induced enhancements of monoaminergic neurotransmission: A meta-analysis study. Alcohol Clin Exp Res. 2013;37:2048–57. doi: 10.1111/acer.12207. [DOI] [PubMed] [Google Scholar]
- 32.Robinson DL, Volz TJ, Schenk JO, Wightman RM. Acute ethanol decreases dopamine transporter velocity in rat striatum: In vivo and in vitro electrochemical measurements. Alcohol Clin Exp Res. 2005;29:746–55. doi: 10.1097/01.ALC.0000164362.21484.14. [DOI] [PubMed] [Google Scholar]
- 33.Mathews TA, John CE, Lapa GB, Budygin EA, Jones SR. No role of the dopamine transporter in acute ethanol effects on striatal dopamine dynamics. Synapse. 2006;60:288–94. doi: 10.1002/syn.20301. [DOI] [PubMed] [Google Scholar]
- 34.Lin AM, Bickford PC, Palmer MR, Cline EJ, Gerhardt GA. Effects of ethanol and nomifensine on NE clearance in the cerebellum of young and aged Fischer 344 rats. Brain Res. 1997;756:287–92. doi: 10.1016/S0006-8993(97)00229-1. [DOI] [PubMed] [Google Scholar]
- 35.Lin AM, Bickford PC, Palmer MR, Gerhardt GA. Ethanol inhibits the uptake of exogenous norepinephrine from the extracellular space of the rat cerebellum. Neurosci Lett. 1993;164:71–75. doi: 10.1016/0304-3940(93)90860-N. [DOI] [PubMed] [Google Scholar]
- 36.Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, et al. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–8. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daws LC. Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressant efficacy. Pharm Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koepsell H. General overview of organic cation transporters in brain. Handb Exp Pharm. 2021;266:1–39. doi: 10.1007/164_2021_449. [DOI] [PubMed] [Google Scholar]
- 39.Amphoux A, Vialou V, Drescher E, Bruss M, Mannoury La Cour C, Rochat C, et al. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–52. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, et al. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci USA. 2009;106:8043–8. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vialou V, Balasse L, Callebert J, Launay JM, Giros B, Gautron S. Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J Neurochem. 2008;106:1471–82. doi: 10.1111/j.1471-4159.2008.05506.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, et al. Identity of the organic cation transporter oct3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem. 1998;273:32776–86. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- 43.Schomig E, Lazar A, Grundemann D. Extraneuronal monoamine transporter and organic cation transporters 1 and 2: A review of transport efficiency. Handb Exp Pharm. 2006;175:151–80. doi: 10.1007/3-540-29784-7_8. [DOI] [PubMed] [Google Scholar]
- 44.Mayer FP, Schmid D, Owens WA, Gould GG, Apuschkin M, Kudlacek O, et al. An unsuspected role for organic cation transporter 3 in the actions of amphetamine. Neuropsychopharmacology. 2018;43:2408–17. doi: 10.1038/s41386-018-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baganz N, Horton R, Martin K, Holmes A, Daws LC. Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: Organic cation transporter 3, the smoking gun. J Neurosci. 2010;30:15185–95. doi: 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng N, Telefont M, Kelly KJ, Orchinik M, Forster GL, Renner KJ, et al. Local perfusion of corticosterone in the rat medial hypothalamus potentiates d-fenfluramine-induced elevations of extracellular 5-ht concentrations. Horm Behav. 2009;56:149–57. doi: 10.1016/j.yhbeh.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol. 2009;512:529–55. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- 48.Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: Potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci. 2006;26:8758–66. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, et al. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci. 2013;33:11800–10. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horton RE, Apple DM, Owens WA, Baganz NL, Cano S, Mitchell NC, et al. Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: Uncovering novel targets to treat depression. J Neurosci. 2013;33:10534–43. doi: 10.1523/JNEUROSCI.5687-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitaichi K, Fukuda M, Nakayama H, Aoyama N, Ito Y, Fujimoto Y, et al. Behavioral changes following antisense oligonucleotide-induced reduction of organic cation transporter-3 in mice. Neurosci Lett. 2005;382:195–200. doi: 10.1016/j.neulet.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 52.McReynolds JR, Taylor A, Vranjkovic O, Ambrosius T, Derricks O, Nino B, et al. Corticosterone potentiation of cocaine-induced reinstatement of conditioned place preference in mice is mediated by blockade of the organic cation transporter 3. Neuropsychopharmacology. 2017;42:757–65. doi: 10.1038/npp.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solanki RR, Scholl JL, Watt MJ, Renner KJ, Forster GL. Amphetamine withdrawal differentially increases the expression of organic cation transporter 3 and serotonin transporter in limbic brain regions. J Exp Neurosci. 2016;10:93–100. doi: 10.4137/JEN.S40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, et al. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci USA. 2008;105:18976–81. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmitt A, Mossner R, Gossmann A, Fischer IG, Gorboulev V, Murphy DL, et al. Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res. 2003;71:701–9. doi: 10.1002/jnr.10521. [DOI] [PubMed] [Google Scholar]
- 56.Monteiro R, Calhau C, Martel F, Guedes de Pinho P, Azevedo I. Intestinal uptake of mpp+ is differently affected by red and white wine. Life Sci. 2005;76:2483–96. doi: 10.1016/j.lfs.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Volkow ND, Morales M. The brain on drugs: From reward to addiction. Cell. 2015;162:712–25. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 58.Fraser-Spears R, Krause-Heuer AM, Basiouny M, Mayer FP, Manishimwe R, Wyatt NA, et al. Comparative analysis of novel decynium-22 analogs to inhibit transport by the low-affinity, high-capacity monoamine transporters, organic cation transporters 2 and 3, and plasma membrane monoamine transporter. Eur J Pharm. 2019;842:351–64. doi: 10.1016/j.ejphar.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell NC, Gould GG, Koek W, Daws LC. Ontogeny of SERT expression and antidepressant-like response to escitalopram in wild-type and SERT mutant mice. J Pharm Exp Ther. 2016;358:271–81. doi: 10.1124/jpet.116.233338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell NC, Gould GG, Smolik CM, Koek W, Daws LC. Antidepressant-like drug effects in juvenile and adolescent mice in the tail suspension test: Relationship with hippocampal serotonin and norepinephrine transporter expression and function. Front Pharm. 2013;4:131. doi: 10.3389/fphar.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janowsky A, Neve K, Eshleman AJ Uptake and release of neurotransmitters. Curr Protoc Neurosci. 2001;2:7.9.1–7.9.22. [DOI] [PubMed]
- 62.Mayer FP, Luf A, Nagy C, Holy M, Schmid R, Freissmuth M, et al. Application of a combined approach to identify new psychoactive street drugs and decipher their mechanisms at monoamine transporters. Curr Top Behav Neurosci. 2017;32:333–50. doi: 10.1007/7854_2016_63. [DOI] [PubMed] [Google Scholar]
- 63.Mayer FP, Schmid D, Holy M, Daws LC, Sitte HH. “Polytox” synthetic cathinone abuse: A potential role for organic cation transporter 3 in combined cathinone-induced efflux. Neurochem Int. 2019;123:7–12. doi: 10.1016/j.neuint.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, et al. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 2007;5:e274. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daws LC, Owens WA, Toney GM Using high-speed chronoamperometry to measure biogenic amine release and uptake in vivo. In: Bönisch H, Sitte HH (eds). Neurotransmitter transporters, vol. 118. Springer New York: New York, NY, 2016, pp 53-81.
- 66.Daws LC, Toney GM High-speed chronoamperometry to study kinetics and mechanisms for serotonin clearance in vivo. In: Michael AC, Borland LM (eds). Electrochemical methods for neuroscience. CRC Press/Taylor & Francis: Boca Raton (FL), 2007. [PubMed]
- 67.Holleran KM, Rose JH, Fordahl SC, Benton KC, Rohr KE, Gasser PJ, et al. Organic cation transporter 3 and the dopamine transporter differentially regulate catecholamine uptake in the basolateral amygdala and nucleus accumbens. Eur J Neurosci. 2020;52:4546–62. doi: 10.1111/ejn.14927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson DL, Lara JA, Brunner LJ, Gonzales RA. Quantification of ethanol concentrations in the extracellular fluid of the rat brain: In vivo calibration of microdialysis probes. J Neurochem. 2000;75:1685–93. doi: 10.1046/j.1471-4159.2000.0751685.x. [DOI] [PubMed] [Google Scholar]
- 69.Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res. 1998;22:367–74. doi: 10.1111/j.1530-0277.1998.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 70.Fuh MR, Tai YL, Pan WH. Determination of free-form of cocaine in rat brain by liquid chromatography-electrospray mass spectrometry with in vivo microdialysis. J Chromatogr B Biomed Sci Appl. 2001;752:107–14. doi: 10.1016/S0378-4347(00)00531-4. [DOI] [PubMed] [Google Scholar]
- 71.Nicolaysen LC, Pan HT, Justice JB., Jr Extracellular cocaine and dopamine concentrations are linearly related in rat striatum. Brain Res. 1988;456:317–23. doi: 10.1016/0006-8993(88)90234-X. [DOI] [PubMed] [Google Scholar]
- 72.Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, et al. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290:1545–50. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- 73.Clauss NJ, Koek W, Daws LC. Role of organic cation transporter 3 and plasma membrane monoamine transporter in the rewarding properties and locomotor sensitizing effects of amphetamine in male andfemale mice. Int J Mol Sci. 2021;22:13420. doi: 10.3390/ijms222413420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koek W. Morphine-induced conditioned place preference and effects of morphine pre-exposure in adolescent and adult male c57bl/6j mice. Psychopharmacology. 2016;233:2015–24. doi: 10.1007/s00213-014-3695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1:349–51. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- 76.Thierauf-Emberger A, Echle J, Dacko M, Lange T. Comparison of ethanol concentrations in the human brain determined by magnetic resonance spectroscopy and serum ethanol concentrations. Int J Leg Med. 2020;134:1713–8. doi: 10.1007/s00414-020-02325-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–49. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharm Ther. 2001;91:35–62. doi: 10.1016/S0163-7258(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 79.Solis E, Jr, Zdravkovic I, Tomlinson ID, Noskov SY, Rosenthal SJ, De Felice LJ. 4-(4-(dimethylamino)phenyl)-1-methylpyridinium (app+) is a fluorescent substrate for the human serotonin transporter. J Biol Chem. 2012;287:8852–63. doi: 10.1074/jbc.M111.267757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karpowicz RJ, Jr, Dunn M, Sulzer D, Sames D. App+, a fluorescent analogue of the neurotoxin mpp+, is a marker of catecholamine neurons in brain tissue, but not a fluorescent false neurotransmitter. ACS Chem Neurosci. 2013;4:858–69. doi: 10.1021/cn400038u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, et al. Slc6 neurotransmitter transporters: Structure, function, and regulation. Pharm Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 82.Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 83.Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology. 1992;107:385–93. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- 84.Beatty WW, Holzer GA. Sex differences in stereotyped behavior in the rat. Pharm Biochem Behav. 1978;9:777–83. doi: 10.1016/0091-3057(78)90356-8. [DOI] [PubMed] [Google Scholar]
- 85.Brass CA, Glick SD. Sex differences in drug-induced rotation in two strains of rats. Brain Res. 1981;223:229–34. doi: 10.1016/0006-8993(81)90830-1. [DOI] [PubMed] [Google Scholar]
- 86.Siuciak JA, McCarthy SA, Chapin DS, Reed TM, Vorhees CV, Repaske DR. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-1b (pde1b) enzyme. Neuropharmacology. 2007;53:113–24. doi: 10.1016/j.neuropharm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 87.van den Buuse M, Halley P, Hill R, Labots M, Martin S. Altered n-methyl-d-aspartate receptor function in reelin heterozygous mice: Male-female differences and comparison with dopaminergic activity. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:237–46. doi: 10.1016/j.pnpbp.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 89.Becker JB, Chartoff E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology. 2019;44:166–83. doi: 10.1038/s41386-018-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Althobaiti YS, Sari Y. Alcohol interactions with psychostimulants: An overview of animal and human studies. J Addict Res Ther. 2016;7:281. doi: 10.4172/2155-6105.1000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Busse GD, Lawrence ET, Riley AL. The modulation of cocaine-induced conditioned place preferences by alcohol: Effects of cocaine dose. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:149–55. doi: 10.1016/j.pnpbp.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 92.Lewis MJ, June HL. Synergistic effects of ethanol and cocaine on brain stimulation reward. J Exp Anal Behav. 1994;61:223–9. doi: 10.1901/jeab.1994.61-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Busse GD, Lawrence ET, Riley AL. The effects of alcohol preexposure on cocaine, alcohol and cocaine/alcohol place conditioning. Pharm Biochem Behav. 2005;81:459–65. doi: 10.1016/j.pbb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 94.Vanderschuren LJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2010;3:179–95. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- 95.Parker CC, Lusk R, Saba LM. Alcohol sensitivity as an endophenotype of alcohol use disorder: Exploring its translational utility between rodents and humans. Brain Sci. 2020;10:725. doi: 10.3390/brainsci10100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, et al. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–65. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- 97.Bonisch H. Substrates and inhibitors of organic cation transporters (OCTS) and plasma membrane monoamine transporter (PMAT) and therapeutic implications. Handb Exp Pharm. 2021;266:119–67. doi: 10.1007/164_2021_516. [DOI] [PubMed] [Google Scholar]
- 98.Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–52. doi: 10.1111/j.1530-0277.1999.tb04082.x. [DOI] [PubMed] [Google Scholar]
- 99.Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–3. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- 100.Mereu G, Fadda F, Gessa GL. Ethanol stimulates the firing rate of nigral dopaminergic neurons in unanesthetized rats. Brain Res. 1984;292:63–69. doi: 10.1016/0006-8993(84)90890-4. [DOI] [PubMed] [Google Scholar]
- 101.Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves n-methyl-d-aspartate receptors. J Pharm Exp Ther. 2004;311:282–9. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- 102.Saeed Dar M, Wooles WR. The effect of acute ethanol on dopamine metabolism and other neurotransmitters in the hypothalamus and the corpus striatum of mice. J Neural Transm. 1984;60:283-94. [DOI] [PubMed]
- 103.Mayfield RD, Maiya R, Keller D, Zahniser NR. Ethanol potentiates the function of the human dopamine transporter expressed in xenopus oocytes. J Neurochem. 2001;79:1070–9. doi: 10.1046/j.1471-4159.2001.00656.x. [DOI] [PubMed] [Google Scholar]
- 104.Ho M, Segre M. Individual and combined effects of ethanol and cocaine on the human dopamine transporter in neuronal cell lines. Neurosci Lett. 2001;299:229–33. doi: 10.1016/S0304-3940(01)01526-9. [DOI] [PubMed] [Google Scholar]
- 105.Niello M, Gradisch R, Loland CJ, Stockner T, Sitte HH. Allosteric modulation of neurotransmitter transporters as a therapeutic strategy. Trends Pharm Sci. 2020;41:446–63. doi: 10.1016/j.tips.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maiya R, Buck KJ, Harris RA, Mayfield RD. Ethanol-sensitive sites on the human dopamine transporter. J Biol Chem. 2002;277:30724–9. doi: 10.1074/jbc.M204914200. [DOI] [PubMed] [Google Scholar]
- 107.Alexi T, Azmitia EC. Ethanol stimulates [3h]5-ht high-affinity uptake by rat forebrain synaptosomes: Role of 5-ht receptors and voltage channel blockers. Brain Res. 1991;544:243–7. doi: 10.1016/0006-8993(91)90060-9. [DOI] [PubMed] [Google Scholar]
- 108.Bowman MA, Mitchell NC, Owens WA, Horton RE, Koek W, Daws LC, et al. Effect of concurrent organic cation transporter blockade on norepinephrine clearance inhibiting- and antidepressant-like actions of desipramine and venlafaxine. Eur J Pharm. 2020;883:173285. doi: 10.1016/j.ejphar.2020.173285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: Evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- 111.Ciudad-Roberts A, Camarasa J, Ciudad CJ, Pubill D, Escubedo E. Alcohol enhances the psychostimulant and conditioning effects of mephedrone in adolescent mice; postulation of unique roles of d3 receptors and bdnf in place preference acquisition. Br J Pharm. 2015;172:4970–84. doi: 10.1111/bph.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lopez-Arnau R, Buenrostro-Jauregui M, Camarasa J, Pubill D, Escubedo E. Effect of the combination of mephedrone plus ethanol on serotonin and dopamine release in the nucleus accumbens and medial prefrontal cortex of awake rats. Naunyn Schmiedebergs Arch Pharm. 2018;391:247–54. doi: 10.1007/s00210-018-1464-x. [DOI] [PubMed] [Google Scholar]
- 113.Duan H, Wang J. Impaired monoamine and organic cation uptake in choroid plexus in mice with targeted disruption of the plasma membrane monoamine transporter (slc29a4) gene. J Biol Chem. 2013;288:3535–44. doi: 10.1074/jbc.M112.436972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perez-Reyes M, Jeffcoat AR. Ethanol/cocaine interaction: Cocaine and cocaethylene plasma concentrations and their relationship to subjective and cardiovascular effects. Life Sci. 1992;51:553–63. doi: 10.1016/0024-3205(92)90224-D. [DOI] [PubMed] [Google Scholar]
- 115.Melis M, Diana M, Enrico P, Marinelli M, Brodie MS. Ethanol and acetaldehyde action on central dopamine systems: Mechanisms, modulation, and relationship to stress. Alcohol. 2009;43:531–9. doi: 10.1016/j.alcohol.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: Differences in the lethal effects of the drug. Psychopharmacology. 1995;117:262–6. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- 117.Moolten M, Kornetsky C. Oral self-administration of ethanol and not experimenter-administered ethanol facilitates rewarding electrical brain stimulation. Alcohol. 1990;7:221–5. doi: 10.1016/0741-8329(90)90008-Z. [DOI] [PubMed] [Google Scholar]
- 118.Porrino LJ, Esposito RU, Seeger TF, Crane AM, Pert A, Sokoloff L. Metabolic mapping of the brain during rewarding self-stimulation. Science. 1984;224:306–9. doi: 10.1126/science.6710145. [DOI] [PubMed] [Google Scholar]
- 119.Khanppnavar B, Maier J, Herborg F, Gradisch R, Lazzarin E, Luethi D, et al. Structural basis of organic cation transporter-3 inhibition. Nat Commun. 2022;13:6714. doi: 10.1038/s41467-022-34284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin L, Yee SW, Kim RB, Giacomini KM. Slc transporters as therapeutic targets: Emerging opportunities. Nat Rev Drug Discov. 2015;14:543–60. doi: 10.1038/nrd4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting these findings are available from the corresponding author upon reasonable request.