Abstract
Cross-sectional neuroimaging studies show that bipolar disorder is associated with structural brain abnormalities, predominantly observed in prefrontal and temporal cortex, cingulate gyrus, and subcortical regions. However, longitudinal studies are needed to elucidate whether these abnormalities presage disease onset or are consequences of disease processes, and to identify potential contributing factors. Here, we narratively review and summarize longitudinal structural magnetic resonance imaging studies that relate imaging outcomes to manic episodes. First, we conclude that longitudinal brain imaging studies suggest an association of bipolar disorder with aberrant brain changes, including both deviant decreases and increases in morphometric measures. Second, we conclude that manic episodes have been related to accelerated cortical volume and thickness decreases, with the most consistent findings occurring in prefrontal brain areas. Importantly, evidence also suggests that in contrast to healthy controls, who in general show age-related cortical decline, brain metrics remain stable or increase during euthymic periods in bipolar disorder patients, potentially reflecting structural recovering mechanisms. The findings stress the importance of preventing manic episodes. We further propose a model of prefrontal cortical trajectories in relation to the occurrence of manic episodes. Finally, we discuss potential mechanisms at play, remaining limitations, and future directions.
Subject terms: Prognostic markers, Neuroscience, Bipolar disorder
Introduction
Cross-sectional neuroimaging studies show that bipolar disorder is associated with structural brain abnormalities, predominantly observed in prefrontal and temporal cortex, cingulate gyrus, and subcortical regions [1–4], and less consistently in insula and visual cortex [1–8]. Large-scale studies from the ENIGMA (Enhancing Neuro Imaging Genetics through Meta Analysis) bipolar disorder working group found the most pronounced cortical alterations in pars opercularis and rostral middle frontal and fusiform cortex [2]. Subcortical abnormalities have been observed in amygdala, hippocampus, and thalamus in bipolar disorder patients. Finally, enlarged ventricles have been observed in bipolar disorder [1].
The causes of these findings remain unknown and the question whether the observed brain aberrancies presage disease onset or are consequences of disease processes cannot be resolved due to inherent limitations of cross-sectional study designs. Some observations suggest that brain abnormalities might be related to worsening along the course of illness and decline in general functioning, at least in some patients with bipolar disorder [9–13]. Such progressive deterioration of illness has—along with hypothesized neuroanatomical changes over time—been denoted ‘neuroprogression’ [13] and has also been described in bipolar disorder [12–14]. It is still disputed, however, whether bipolar disorder entails such neuroprogression [15], mainly because longitudinal studies investigating brain changes over time are scarce.
Recently, however, a few longitudinal studies of brain morphology that enable conclusions about possible brain changes in bipolar disorder have been performed. These do in fact indicate that aberrant brain changes occur. Single-center studies [16–20], multi-center studies [21], and recent reviews [13, 14, 22] have observed structural changes mainly in the prefrontal and temporal cortices. Notably, however, the largest longitudinal imaging study in bipolar disorder to date—a multi-center effort conducted by the ENIGMA bipolar disorder working group—found no decrease in cortical measures over time, but in fact slower thinning of specific cortical measures in some brain areas than controls [21]. However, bipolar disorder patients did show an accelerated enlargement of ventricles compared with healthy controls [21]. While the causes of abnormal brain changes remain undetermined, medication use [23, 24], genetic factors [17], and the occurrence of mood episodes [16–21] have been hypothesized as probable contributing factors.
Not only is the occurrence of manic episodes the hallmark of bipolar disorder, but the number of manic episodes has also been associated with worsening illness severity over time [9–13]. The aim of this narrative review was therefore to advance our understanding of the consequences manic episodes might have on neuroanatomical structures. We review and summarize longitudinal structural magnetic resonance imaging studies that relate imaging outcomes to manic episodes. We first provide a brief overview of longitudinal case-control studies in bipolar disorder, then discuss factors that might contribute to structural brain changes, and subsequently, we provide a more detailed outline of studies reporting mania-related structural changes. Based on the reviewed studies, we finally propose a model describing the pathogenesis of mania. Finally, we discuss limitations and suggestions for future research.
Selection of studies
Since 2011 and up to December 10th 2022, we regularly searched PubMed (NLM) using the following keywords: longitudinal, MRI, structural, neuroimaging, magnetic resonance imaging, mania, manic episodes, mood episodes, bipolar disorder, brain morphology, brain changes, cortical thickness, cortical volume, cortical surface area, subcortical volume, gray matter. Two of the authors (C.A. and L.K.) checked all hits for relevance. We did not rate risk of bias or quality of evidence as this was narrative review. No statistical analyses were performed. We considered only original studies in English and selected 36 studies relevant for structural brain changes in bipolar disorder. Table 1 lists the 7 studies on longitudinal structural brain changes in relation to mania, which was the main focus of this review.
Table 1.
Overview of the main studies reviewed that related structural longitudinal brain imaging outcomes to manic episodes.
| Reference | Diagnosis and sample characteristics | Sample size | Follow-up time | Method; MRI metric | Main result | Brain area affected |
|---|---|---|---|---|---|---|
| Moorhead et al., 2007 | Bipolar type 1 (male and female adults). | 20 | 4 years (two timepoints) | VBM/TBM (region of interest); gray matter density | Number of manic/hypomanic episodes during follow-up correlated with gray matter loss in cerebellar and temporal lobe. | Temporal lobe (including hippocampus and fusiform) |
| Gogtay, 2007 | Heterogenous clinical picture with/without first manic episode, healthy controls (male children) | 8/9, 18 | 4–8 years (scanned every two years, incl. scans pre/post first manic episode) | Surface-based; gray matter density | Results suggestive for faster gray matter decreases in children who experienced first manic episode, but not in children that did not experience a manic episode. | Bilateral anterior (and sub genual) cingulate cortex |
| Abé et al., 2015 | Bipolar type 1 with/without manic episodes (male and female adults). | 18/21 | 6 years (two timepoints) | Surface based (region of interest): cortical thickness, surface area, volume | Patients who experienced mania decreased in cortical volume, whereas those without manic episodes did not. Patients who had no manic episodes displayed increases in cortical thickness and decreases in surface area. | Bilateral dlPFC, inferior frontal cortex |
| Zak et al., 2019 | Bipolar type 2 with few/many hypomanic episodes between time points, healthy controls (male and female adults). | 12/16, 35 | 2.4 years (two timepoints) | Surface based (vertex-wise, region of interest, and whole brain) | Patients with few (0–3) hypomanic episodes had greater temporal cortical thinning than patients with more (>3) hypomanic episodes. | left/right temporal cortex |
| Abé et al., 2020 | Bipolar type 1 with/without manic episodes between timepoints, bipolar type 2 with/without hypomanic episodes, healthy controls (male and female adults). | 35/21, 8/15, 46 | 6 years (two timepoints) | Surface based (vertex-wise, whole brain): cortical thickness | Bipolar disorder type 1 patients, who experienced mania, showed greater cortical thinning than controls and patients without manic episodes. The same pattern was observed in bipolar disorder type 2 with respect to hypomania. Cortical change rates did not differ between patients without manic episodes and controls, who displayed normal age-related thinning. | left inferior frontal cortex |
| Abé et al., 2021 | Bipolar type 1, type 2, NOS (male and female adults). | 230 | 0.5–9 years (two timepoints) | Surface based (region of interest, whole brain): cortical thickness, surface area; subcortical volumes | Negative correlation between yearly thickness change rates and the number of manic, hypomanic, and/or mixed episodes between time points. No correlation between the number of mood episodes and surface area or subcortical volumes. Follow-up tests: Patient who experienced manic, hypomanic, and/or mixed episodes showed cortical thinning, while those without showed no changes or increases in cortical thickness. Results remained when excluding patient included in Abé et al. 2015 and 2020, and when controlling for various potential confounders, incl. depressive episodes. | Frontal: left frontal pole, bilateral inferior frontal, right caudal anterior cingulate, left dm/dlPFC. Other brain areas: left lingual, right paracentral, left isthmus, left transverse temporal. |
| Cahn et al., 2021 (review) | Bipolar type 1 following first manic episode (male and female adults and children). | Sample sizes ranged from 8–41 patients and 17–70 controls | >1 year | Various methods; gray matter volume (cortical or subcortical), cortical thickness, or surface area. | Various findings. Most consistently reported finding was a faster gray matter volume decreases in patients that experienced a first manic episode compared with controls in anterior cingulate cortex. | Anterior cingulate cortex |
Sample characteristics, investigated MRI metrics, and main results are summarized.
dm dorsomedial, dlPFC dorsolateral prefrontal cortex, MRI magnetic resonance imaging, NOS not otherwise specified, TBM tensor-based morphometry, VBM voxel-based morphometry.
Brain morphometric measures
Studies of bipolar disorder have used several brain morphometric measures. The most common are subcortical and cortical volumes. Cortical volume is a function of cortical surface area and cortical thickness, which are two genetically and phenotypically distinct measures [25, 26]. These can be assessed separately by using surface-based measures—provided by, e.g., FreeSurfer [27–30] - to obtain more detailed anatomical information. Cortical surface area is largely determined by the number of cortical columns. Cortical thickness is related to the size, number, and density of cells and dendrites in a cortical column [31]. Cortical thickness therefore serves as a proxy marker of the integrity of the cerebral cortex [1–4, 17, 31]. Here, we considered studies that measured volumes of subcortical and cortical structures, as well as density, thickness, and surface area of cortical structures derived from tensor/voxel-based morphometry or surface-based methods.
Summary of longitudinal brain changes in bipolar disorder
Even though limited in numbers, studies using a longitudinal case-control design to assess brain changes along the course of bipolar disorder suggest that noticeable structural brain changes in bipolar disorder do occur over time. Several studies report abnormal changes in the prefrontal and temporal cortices as well as in subcortical structures, in particular amygdala [13, 14, 16–19, 21, 22]. For example, in a 6-year follow-up study using vertex-analyses [17], bipolar disorder patients showed an increase of cortical thickness in visual/somatosensory brain areas, whereas healthy controls showed an expected age-related thinning. Differences were observed in the bilateral medial occipital cortex (including pericalcarine and cuneus cortex), bilateral central sulcus, posterior cingulate cortex, and left anterior insula. However, bipolar patients showed a faster cortical thinning than controls in the middle temporal cortex.
More recently, a large multi-center EMIGMA study, including 307 bipolar disorder patients and 925 controls from 14 international sites, investigated yearly change rates of subcortical volumes, regional cortical thickness, and surface area [21]. The thickness change rates of the right fusiform gyrus and right parahippocampal regions differed significantly between bipolar patients and controls: The thickness decreased over time in healthy controls, whereas patients showed less or no decline. No significant difference in surface area or subcortical volumes were noted. The study’s most significant and robust finding was faster ventricular enlargement in bipolar patients than in controls. Interestingly, ventricular change rates correlated negatively with subcortical volume change rates, indicating that bipolar patients with greater ventricle enlargement also had greater subcortical decline over time. While the study populations differed across individual centers (e.g., sample size varied, some were medical trial populations, some patients were followed after their first manic episode), this multicenter study covered a wide range of follow-up periods and leave-one-site-out analyses validated that the reported results were robust. This is the largest longitudinal brain imaging study in bipolar disorder yet performed, and therefore the most relevant for the present review.
In summary, longitudinal brain imaging studies suggest that bipolar disorder in general is associated with deviant brain changes. However, both aberrant decreases and increases in morphometric measures have been reported and it is uncertain if bipolar disorder per se adversely affects the brain.
Factors contributing to brain changes
The mechanisms underlying observed brain changes in bipolar disorder remain to be elucidated. We outline below some of the most relevant factors that have been suggested to be of importance.
Pharmacological treatments
Pharmacological treatments of bipolar disorder have diverse effects on brain structure [32]. Lithium—the prototypical mood stabilizer—has been ascribed neurotrophic and neuroprotective properties [24, 33–36]. Lithium use has been associated with increased thickness of the prefrontal and medial occipital cortex [17, 37, 38]. However, such effects need to be interpreted with caution, since lithium itself has an effect on the MR signal which may affect the measured outcomes [39]. Many patients with bipolar disorder are treated with antipsychotic drugs during acute manic episodes, and some patients continue with the antipsychotic medication during the maintenance phase [40]. Gray matter decrease has been associated with antipsychotic drug use [41] - including newer second-generation drugs albeit to a lesser degree [42]. However, findings are mixed with some reviews [43, 44] suggesting that the exact effect of antipsychotic medication on gray matter volumes is still unclear. These inconsistencies are partially due to results being derived from cross-sectional studies, which do not allow conclusions on brain changes over time [45].
Genetic factors
Bipolar disorder is a highly heritable disorder. Interestingly, cross-sectional studies have found structural differences similar to those in bipolar disorder in unaffected relatives to patients with bipolar disorder [46–49]. Moreover, higher polygenic liability for bipolar disorder and schizophrenia, as indexed by polygenic risk scores, have been associated with thinner ventromedial prefrontal cortex [50]. With respect to specific genetic variants, a recent systematic review of genetic neuroimaging findings from cross-sectional studies concluded that the most consistent finding is the influence of the CACNA1C rs1006737 polymorphism on cortical thickness [51], albeit that most studies remain to be replicated.
A longitudinal study found a positive correlation between polygenic risk for bipolar disorder and thickness of medial occipital cortex and central sulcus [17]. This may indicate that genetic factors modulate the risk of disorder-related brain changes over time. However, a longitudinal twin-study reported that the liability to bipolar disorder was not associated with structural brain changes over time [52], whereas a more recent narrative review suggests that heritable changes are primarily confined to white matter [53]. Taken together, longitudinal imaging-genetics studies in bipolar disorder are scarce and show mixed results. Some findings suggest genetic contributions to brain gray matter structure, and possibly a genetic modulation of longitudinal changes.
Comorbid conditions
Comorbid medical conditions such as substance use disorders, cardiovascular diseases, or obesity may confound observed effects of manic episodes on brain changes. The life-time prevalence of substance use—known to be associated with brain abnormalities [54] and longitudinal brain changes [55, 56] - is high in bipolar disorder, especially during manic episodes. Substance abuse can also trigger manic episodes and affect outcomes and recurrence rates [57, 58]. The risk of adverse cardiovascular events (e.g., stroke) that can adversely affect brain structure [59–61] is higher in bipolar disorder than in the general population, also at younger ages [62]. Bipolar disorder patients are furthermore at increased risk for being overweight or obese, and have elevated risk for metabolic syndrome [63, 64]. In fact, recent studies suggest that comorbid obesity could explain why neurostructural alterations are more pronounced in some individuals with bipolar disorder [65]. Obesity is also a risk factor for accelerated brain ageing in first-episode psychosis patients [66].
Differentiating between bipolar disorder and schizophrenia sometimes poses challenges. Even though these conditions are mutually exclusive according to diagnostic systems, patients might in fact have a lifetime history of both disorders. Psychotic symptoms as seen in schizophrenia are also most commonly observed during manic episodes and, consistent with overlapping symptoms [67], bipolar disorder and schizophrenia share around half of their genetic liability [68, 69]. Schizophrenia is known to be associated with progressive gray matter loss confined to fronto-temporal cortical regions [70]. A greater diagnostic challenge presents the dissociation between bipolar disorder (with psychotic symptoms) and schizoaffective disorder bipolar type (featuring manic episodes) [71]. A recent study aimed at discriminating between schizophrenia, schizoaffective disorder, and psychotic bipolar disorder applying a multimodal brain imaging approach and revealing activity of the salience network as discriminative along the psychosis spectrum [72]. Comparing gray matter volume among these three disorders, schizoaffective disorder displayed on the one hand similar but less extensive gray matter reductions than schizophrenia but on the other hand more gray matter reductions compared to bipolar disorder [73].
These are just to name a few comorbid conditions that are important to consider when investigating longitudinal brain changes in bipolar disorder.
Accelerated aging
A recent systematic review and meta-analysis found accelerated brain aging in bipolar disorder that was more pronounced in older patients, suggesting the possibility of a cumulative effect of the disease burden [74]. This may also explain the clinical association between bipolar disorder and behavioral variant of frontotemporal dementia [75, 76], as well as the suggested protective effect of lithium against dementia [77]. A recent investigation into the neurobiology underlying age-related brain changes suggest that cortical thinning is associated with inter-regional expression of genes specific to CA1 pyramidal cells, astrocytes, and microglia during both development and aging [78]. In line with this, a study of 1547 bipolar disorder patients showed that cortical thinning is associated with gene expression profile of CA1 pyramidal cells and microglia, and with genes involved in axon guidance during neurodevelopment, synaptic activity, and neuroplasticity [79].
Manic episodes and neuroinflammatory processes
While depressive episodes [19] may also affect brain structure, several lines of evidence suggest that manic episodes are detrimental for the integrity of cortical structure. Mechanisms underlying pathological gray matter loss may include increased neurodegeneration, neurotoxic susceptibility, neuronal apoptosis, and altered neuroplasticity caused by neuroinflammatory processes and/or oxidative stress during mood episodes [14, 16, 80]. Mania has been related to biochemical changes that may have adverse effects on brain structure and function [81]. For example, stress-induced elevation of cortisol and adrenocorticotrophic hormone has been suggested to play a role during manic episodes [82]. One study found higher cerebrospinal fluid levels of neurofilament light chain—indicative of axonal damage—in bipolar disorder compared with healthy controls [83], and a recent longitudinal study showed increased concentrations of an oxidative stress marker in cerebrospinal fluid following mania [84]. With respect to neuroinflammation, higher cerebrospinal fluid concentrations of interleukin 8 [85] and interleukin-1ß [86] was found in bipolar disorder patients compared with controls. Manic episodes have also been associated with immunological activation of microglia [87], with perisynaptic release of proinflammatory cytokines such as interleukin-1ß, interleukin-2, and tumor necrosis factor [11, 88]. Abnormal synaptic plasticity involving altered secretion of neurotrophic factors could be at play during mania, as a recent study found lower levels of secretogranin II—a neuroprotective compound that reflects secretion of neurotrophins—in patients with bipolar disorder type 1 (but not type 2) compared with controls [89].
Brain changes associated with manic episodes
Early cross-sectional studies suggested that the number of manic episodes correlated negatively with gray matter volume in dorsolateral prefrontal cortex (dlPFC) [90] and inferior frontal cortex [91]. However, it was not possible to determine if these correlations reflect temporal relationships between brain changes and manic episodes, or whether premorbid trait abnormalities increased the risk for manic episodes. Longitudinal studies can address this question. Below we summarize the findings from longitudinal studies that related structural brain imaging outcomes to manic episodes. In Table 1, we present the characteristics of the studies discussed.
In 2007, Moorhead and coworkers [18] performed the first structural longitudinal brain imaging studies in bipolar disorder type 1 using voxel-based morphometry to measure gray matter density. This 4-year follow-up study of 20 patients reported that the number of manic/hypomanic episodes correlated with gray matter loss. Although the study was small and limited the investigation to temporal cortex where case-control differences had been observed, it was the first indication that manic episodes may be related to gray matter changes.
A decade later, Abé and colleagues examined 13 bipolar I disorder patients who had experienced manic episodes during a mean follow-up time of six years along with 18 patients who had not [16]. The study used a region of interest approach and investigated cortical volume, thickness, and surface area. Patients who had experienced manic episodes showed faster volume decrease in the dlPFC (comprising the superior frontal, caudal middle frontal, and rostral middle frontal cortex) and the inferior frontal cortex (including pars opercularis, pars triangularis and pars orbitalis). Intriguingly, patients who did not experience manic episodes showed no volume changes, but increases in thickness and decreases in surface area were indicated. The mania-related changes in dlPFC volume were driven by changes in rostral middle frontal cortex volume. Sensitivity analyses showed that the results remained when patients with more than one manic episode were excluded, suggesting that even a single manic episode may adversely affect cortical volume. However, no healthy controls were included in this study, which precluded conclusions about how the changes related to normal ageing.
In 2020, the study by Abé and coworkers [16] was extended to include both bipolar type 1 (including some patients from the previous study), bipolar type 2, and healthy controls [17]. This study used a validated longitudinal image processing pipeline and more advanced data analyses, e.g., linear mixed effects modeling, as well as a more fine-grained vertex-wise anatomical analysis. After the follow-up period of 6 years, similar effects of manic, hypomanic and/or mixed episodes on cortical thickness were observed in left inferior frontal cortex. The study also indicated that the mania-related changes reflected accelerated decline compared with controls. The findings remained when the previously investigated patients were excluded [16], and similar results were obtained after adjusting for depressive episodes between timepoints. Finally, change rates in inferior frontal cortex correlated positively with change rates in middle temporal cortex indicating a potential mania-related decline in other brain areas.
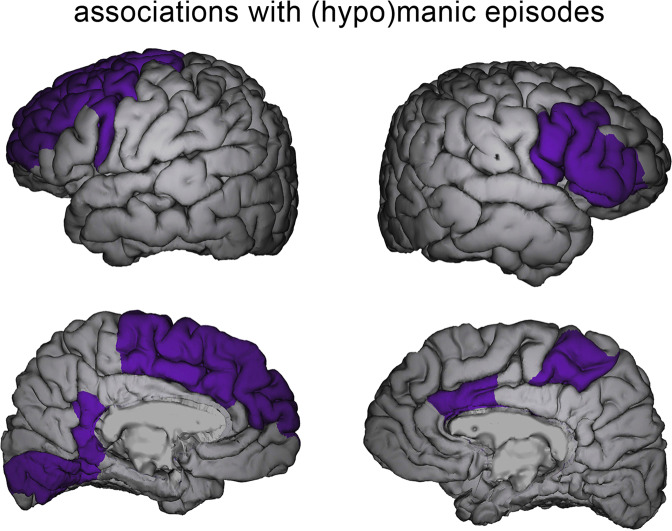
In 2022, the largest longitudinal neuroimaging study in bipolar disorder to date was published [21]. This international multi-center study, conducted within the ENIGMA consortium, investigated yearly change rates of 11 subcortical regions and 64 cortical brain regions covering the whole brain. Changes in subcortical volume, as well as cortical thickness and surface area were quantified. The study found negative correlations between the number of manic episodes between imaging time points and cortical thickness change in frontal pole and the lingual gyrus of the medial occipital cortex. When considering the number of all manic, hypomanic, and/or mixed mood episodes, negative correlations were observed with the medial occipital cortex and in widespread manner over frontal cortex—including dlPFC and inferior frontal cortex—and anterior cingulate cortex (Fig. 1). The results remained after adjusting for the number of depressive episodes between timepoints, after excluding the cohort previously reported by Abé and colleagues [17], as well as after excluding first-episode mania cohorts. Further analyses suggested that more frequent manic episodes were associated with faster cortical thinning, and that patients with no manic episodes showed no change or increased cortical thickness depending on brain region.
Fig. 1. Associations between longitudinal cortical thickness changes and (hypo)manic episodes.
Figure obtained from Abé et al. [21] showing anatomical locations of brain regions in which negative correlations between thickness change rates and the number of (hypo)manic episodes between imaging timepoints were observed in the ENIGMA-BD study [21]. The brain areas identified in the ENIGMA-BD study largely cover regions identified in this review (see Table 1 for details).
Since most studies reviewed above included patients that had had bipolar disorder for many years, they had received treatment and sometimes experienced several manic episodes at the first scanning. To disentangle effects of mood episodes per se from those of illness duration and medication, it is valuable to investigate patients in proximity to the onset of their first manic episode. In 2007, Gogtay and colleagues conducted a pre-post onset longitudinal imaging study in pediatric bipolar disorder [92]. The authors suggested that, after onset of bipolar I disorder, i.e., after the first manic episode, children may show faster gray matter decreases bilaterally in the anterior (and sub-genual) cingulate cortex than those that did not develop bipolar disorder type 1. Note that the investigated samples were small (n = 8–9 per group) and the results were only suggestive and not statistically significant in direct group comparisons. Also, the children were ‘multi-dimensionally impaired’, i.e., they presented with a heterogenous clinical picture, comprising emotion dysregulation, attention deficit hyperactivity disorder, developmental disorders, and psychosis. Most recently, van Rheenen et al. revealed that first episode of mania in adolescent and young adults (age 15–25) did not impact cortical thickness or gyrification but increased surface area was found in inferior and middle prefrontal and occipitoparietal cortices [93].
A recent review on longitudinal gray matter changes following the first mania in bipolar disorder type 1 included 15 studies (the study by Gogtay et al. was not included) and concluded that the most replicated finding was a decrease in anterior cingulate cortical volume following first mania [22]. Although conclusions on regional specificity may be difficult given the inconsistency of findings and methodology applied in the individual studies, this report suggests changes in prefrontal brain structural following the first manic episode in bipolar disorder.
Hypomania denotes a milder form of mania [94]. Patients with bipolar disorder type 2 experience recurrent depressive and hypomanic episodes, but, by definition, no manic episodes. In the study by Abé and colleagues, the pattern of mania-related inferior frontal cortical thinning in bipolar disorder type 2 was also observed for hypomanic episodes in bipolar type 2 patients [17]. In contrast, however, Zak and coworkers reported that bipolar disorder type 2 patients with few (0–3) hypomanic episodes between baseline and follow-up scan had greater temporal cortical thinning than patients with many (>3) hypomanic episodes [19]. However, no correlations between the actual number of mood episodes were observed and the dichotomization of continuous variables performed in this study hamper the interpretation [95].
Taken together, the most consistent finding is that manic episodes relate to brain changes in prefrontal brain areas. Changes in temporal regions have been reported as well, but less consistently.
Prefrontal cortical integrity trajectory in relation to the occurrence of manic episodes
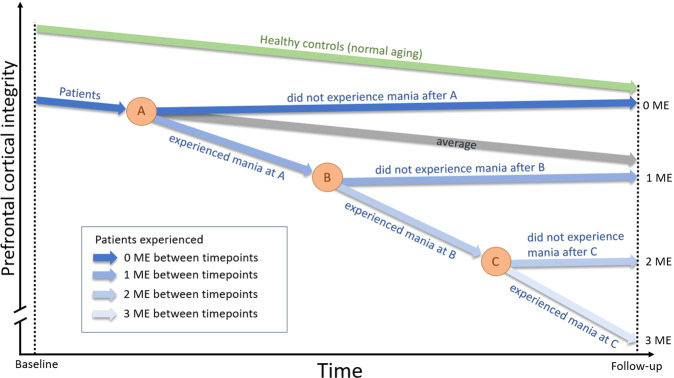
Based on the studies reviewed, a picture emerges where periods of inter-episodic euthymia are associated with no structural change or even an increase in gray matter, whereas manic episodes are associated with gray matter decrease predominantly in the prefrontal cortex. Hence, longitudinal studies suggest that the structural prefrontal cortical integrity is unaffected or improved in the absence of manic episodes: On average, patients follow a similar trajectory as healthy individuals and the slope is similar to that of normal age-related cortical decline. It is in relation to manic episodes that the prefrontal cortical integrity deteriorates. Figure 2 illustrates proposed trajectories of how prefrontal cortical integrity may change over time in relation to the occurrence of manic episodes.
Fig. 2. Cortical integrity trajectory model.
This simplified scheme illustrates structural gray matter changes in relation to the occurrence of manic episodes in bipolar disorder type 1. This model is based on results of the reviewed longitudinal studies depicting changes in prefrontal cortical integrity, the area most consistently reported, as representative example. Structural integrity is defined by the various structural brain imaging outcomes investigated in these studies (e.g., cortical thickness). Three events in time (A, B, C) between baseline and follow-up scans are indicated, at which a manic episode may or may not have occurred. Healthy control trajectory is shown in green, patient trajectories in blue. Patient average trajectory (gray) is based on general longitudinal findings showing no case-control differences with respect to change rates. Different shades of blue show example trajectories for patients who experienced none, one, two, or three manic episodes (ME) between imaging time points. Arbitrary units are used to express approximate trends. Additional notes: The illustration assumes that patients and controls are age matched at baseline. Although age-related slopes are not necessarily linear and may depend on age and follow-up period, linear slopes were illustrated for simplicity. Further, the average trajectory (gray arrow; same slope as controls) could potentially result from counterbalancing sub-group trajectories, where one group declines faster than controls and one show increased gray matter. This could potentially be the case if some patients experienced mania shortly before baseline imaging time point.
However, cross-sectional studies report lower frontal cortical volume and thickness in bipolar disorder [2–4]. These aberrancies may be consequences of manic episodes accumulated before first imaging timepoint. They may also reflect static/premorbid conditions associated with the disorder that are unrelated to previous manic episodes. Therefore, and regardless of the origin of such baseline abnormalities, the proposed trajectory model shows lower cortical integrity in bipolar disorder at baseline (Fig. 2).
Available data suggest that when bipolar patients experience manic episodes, structural brain metrics decrease faster not only in comparison with healthy controls but also compared with patients that remain well (Fig. 2, line labeled ‘experienced mania at A’). Since the degree of decline seems to correlate with the number of manic episodes between imaging timepoints [21], each additional manic episode presumably add to the abnormal gray matter decline, and the change rate might even accelerate (Fig. 2, line labeled ‘experienced mania at B/C’). Importantly, however, we also propose that patients who remain well may show structural recovery if manic episodes can be prevented (related or unrelated to treatment). This proposition rests on some studies reporting that structural brain measures do not change or increase in patients that did not experience manic episodes between timepoints (Fig. 2, lines labeled ‘did not experience mania after A/B/C’). It should be noted that size increases of cortical structures do not necessarily reflect beneficial effects. They may also be explained by e.g., neuroinflammatory processes, previously suggested to occur in bipolar disorder [96]. However, as stated above, the neurobiological mechanisms behind the observed structural changes remain to be clarified.
Limitations of previous studies and suggestions for future research
Even though longitudinal studies can distinguish static abnormalities from dynamic changes, causal links to associated factors cannot be established. It thus remains unclear whether structural changes cause manic episodes or vice versa. It is also unknown if mania induces brain changes directly, e.g., through biochemical mechanisms as discussed above, or whether a third unrelated factor causes both structural decline and mania. To answer such questions and to validate the proposed model, we need larger datasets designed to specifically study brain trajectories with a fine-grained time resolution. Of great interest would be studies covering the period around the first manic episode, preferably in a pre- and post-first mania design similar to that used in [92].
Although this review focuses on the association of structural brain changes and mania, bipolar patients also experience episodes of depression, often severe and long lasting, which might also be associated with brain changes. In addition, medication effects also need to be disentangled from mania-related effects. For example, although challenged and still inconclusive [43, 44], antipsychotic drug use has been associated with gray matter decrease [41]. Moreover, lithium use has been associated with gray matter volume increases [33, 36, 37] and has been attributed neuroprotective effects [34, 35], which might explain the observation that some patients who remain well show no cortical decline or even structural recovery. Since medication effects may be confounded by indication, these are better addressed in interventional experimental study designs, such as randomized controlled trials. A recent study examining brain structure networks investigated individuals with a manic episode over the course of treatment with quetiapine or lithium: Unmedicated manic patients featured global and nodal network deviances compared with controls, which, however, normalized with treatment [97].
Another important factor to consider is that bipolar disorder is highly heritable. Thus, genetic effects may play a role in structural brain trajectories over time. For example, Abé and colleagues found relationships between brain changes and polygenic risk scores for bipolar disorder and schizophrenia that warrant further investigation [17]. Finally, comorbidity with other medical conditions may be crucial to consider as they may affect brain trajectories over time.
Apart from disentangling mania effects from other factors contributing to gray matter changes, it is important to investigate if mania-related brain changes translate to changes in symptomatology, as well as social and cognitive functioning. Meta-analyses suggest that executive functions are impaired in bipolar patients [98], but it is less clear how such cognitive measures change over time and how they relate to structural brain changes. There are longitudinal studies indicating that the number of manic episodes relates to changes in cognitive function [99], but the findings in other studies are mixed [100].
Although this review focuses on structural brain imaging findings, other imaging modalities like functional imaging (fMRI) and diffusion tensor imaging (DTI) also have the potential to advance our understanding of mania-related brain alterations in BD [101–104]. To date, however, longitudinal studies of mania-related changes are scarce. Hence, future longitudinal studies using fMRI and/or DTI are warranted to further our understanding of how manic episodes cause longitudinal functional and white matter integrity changes, respectively.
Conclusion
In this narrative review, we summarized results from longitudinal studies in bipolar disorder that relate structural brain imaging outcomes to manic episodes. The results suggest that mania is associated with brain changes, i.e., decreases in gray matter metrics, most consistently reported in the prefrontal cortex. Evidence also suggests increases in brain metrics if manic episodes do not occur. The latter potentially reflects structural improvement mechanisms and emphasizes the importance of preventing manic episodes. Finally, to pinpoint targets for improving outcomes, future studies should attempt to disentangle the effects of mania from other factors associated with brain changes.
Acknowledgements
ML is funded by the Swedish Medical Research Council (2022–01643), the Swedish Brain foundation (FO2020–0261), the Wenner-Gren foundation (SSv2019–0008), and by the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG- 965444).
Author contributions
The study was conceived by CA and ML. CA and ALK conducted the literature search. CA, BL, PP, and ML drafted the manuscript. BL and ALK provided critical input for the interpretation. All authors critically revised and approved the final version of the manuscript.
Funding
Open access funding provided by University of Gothenburg.
Competing interests
ML reports that he has received lecture honoraria from Lundbeck pharmaceutical. CA is an employee of Quantify Research. BL, ALK and PP report no potential conflict of interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hibar DP, Westlye LT, Van Erp TGM, Rasmussen J, Leonardo CD, Faskowitz J, et al. Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry. 2016;21:1710–6. doi: 10.1038/mp.2015.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23:932–42. doi: 10.1038/mp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abé C, Ekman CJ, Sellgren C, Petrovic P, Ingvar M, Landén M. Cortical thickness, volume and surface area in patients with bipolar disorder types I and II. J Psychiatry Neurosci. 2016;41:240–50. doi: 10.1503/jpn.150093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanford LC, Nazarov A, Hall GB, Sassi RB. Cortical thickness in bipolar disorder: a systematic review. Bipolar Disord. 2016;18:4–18. doi: 10.1111/bdi.12362. [DOI] [PubMed] [Google Scholar]
- 5.Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- 6.Selvaraj S, Arnone D, Job D, Stanfield A, Farrow TF, Nugent AC, et al. Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord. 2012;14:135–45. doi: 10.1111/j.1399-5618.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- 7.Savitz JB, Price JL, Drevets WC. Neuropathological and neuromorphometric abnormalities in bipolar disorder: View from the medial prefrontal cortical network. Neurosci Biobehav Rev. 2014;42:132–47. doi: 10.1016/j.neubiorev.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Maller JJ, Thaveenthiran P, Thomson RH, McQueen S, Fitzgerald PB. Volumetric, cortical thickness and white matter integrity alterations in bipolar disorder type I and II. J Affect Disord. 2014;169:118–27. doi: 10.1016/j.jad.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Passos IC, Mwangi B, Vieta E, Berk M, Kapczinski F. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134:91–103. doi: 10.1111/acps.12581. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso T, Bauer IE, Meyer TD, Kapczinski F, Soares JC. Neuroprogression and cognitive functioning in bipolar disorder: a systematic review. Curr Psychiatry Rep. 2015;17:75. doi: 10.1007/s11920-015-0605-x. [DOI] [PubMed] [Google Scholar]
- 11.Barbosa IG, Bauer ME, Machado-Vieira R, Teixeira AL. Cytokines in bipolar disorder: paving the way for neuroprogression. Neural Plast. 2014;2014:1–9. doi: 10.1155/2014/360481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider MR, DelBello MP, McNamara RK, Strakowski SM, Adler CM. Neuroprogression in bipolar disorder. Bipolar Disord. 2012;14:356–74. doi: 10.1111/j.1399-5618.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- 13.Serafini G, Pardini M, Monacelli F, Orso B, Girtler N, Brugnolo A, et al. Neuroprogression as an illness trajectory in bipolar disorder: a selective review of the current literature. Brain Sci. 2021;11:276. doi: 10.3390/brainsci11020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim CS, Baldessarini RJ, Vieta E, Yucel M, Bora E, Sim K. Longitudinal neuroimaging and neuropsychological changes in bipolar disorder patients: review of the evidence. Neurosci Biobehav Rev. 2013;37:418–35. doi: 10.1016/j.neubiorev.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Martino DJ, Samamé C, Marengo E, Igoa A, Strejilevich SA. A critical overview of the clinical evidence supporting the concept of neuroprogression in bipolar disorder. Psychiatry Res. 2016;235:1–6. doi: 10.1016/j.psychres.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Abé C, Ekman C-J, Sellgren C, Petrovic P, Ingvar M, Landén M. Manic episodes are related to changes in frontal cortex: a longitudinal neuroimaging study of bipolar disorder 1. Brain. 2015;138:3440–8. doi: 10.1093/brain/awv266. [DOI] [PubMed] [Google Scholar]
- 17.Abé C, Liberg B, Song J, Bergen SE, Petrovic P, Ekman CJ, et al. Longitudinal cortical thickness changes in bipolar disorder and the relationship to genetic risk, mania, and lithium use. Biol Psychiatry. 2020;87:271–81. doi: 10.1016/j.biopsych.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Moorhead TWJ, McKirdy J, Sussmann JED, Hall J, Lawrie SM, Johnstone EC, et al. Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry. 2007;62:894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Zak N, Bøen E, Boye B, Andreassen OA, Doan NT, Malt UF, et al. Mood episodes are associated with increased cortical thinning: A longitudinal study of bipolar disorder type II. Bipolar Disord. 2019;21:525–38. doi: 10.1111/bdi.12771. [DOI] [PubMed] [Google Scholar]
- 20.Kozicky J-M, McGirr A, Bond DJ, Gonzalez M, Silveira LE, Keramatian K, et al. Neuroprogression and episode recurrence in bipolar I disorder: A study of gray matter volume changes in first-episode mania and association with clinical outcome. Bipolar Disord. 2016;18:511–9. doi: 10.1111/bdi.12437. [DOI] [PubMed] [Google Scholar]
- 21.Abé C, Ching CRK, Liberg B, Lebedev AV, Agartz I, Akudjedu TN, et al. Longitudinal structural brain changes in bipolar disorder: a multicenter neuroimaging study of 1232 individuals by the ENIGMA bipolar disorder working group. Biol Psychiatry. 2022;91:582–92. doi: 10.1016/j.biopsych.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Cahn AJ, Keramatian K, Frysch C, Yatham LN, Chakrabarty T. Longitudinal grey matter changes following first episode mania in bipolar I disorder: a systematic review. J Affect Disord. 2021;291:198–208. doi: 10.1016/j.jad.2021.04.051. [DOI] [PubMed] [Google Scholar]
- 23.Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes. Arch Gen Psychiatry. 2011;68:128. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald C. Brain structural effects of psychopharmacological treatment in bipolar disorder. Curr Neuropharmacol. 2015;13:445–57. doi: 10.2174/1570159X13666150403231654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–35. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–46. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 28.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rakic P. Specification of cerebral cortical areas. Science. 1979;1988:170–6. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 31.Abé C, Rolstad S, Petrovic P, Ekman CJ, Sparding T, Ingvar M, et al. Bipolar disorder type I and II show distinct relationships between cortical thickness and executive function. Acta Psychiatr Scand. 2018;138:325–35. doi: 10.1111/acps.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anand A, Nakamura K, Spielberg JM, Cha J, Karne H, Hu B. Integrative analysis of lithium treatment associated effects on brain structure and peripheral gene expression reveals novel molecular insights into mechanism of action. Transl Psychiatry. 2020;10:103. doi: 10.1038/s41398-020-0784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajek T, Bauer M, Simhandl C, Rybakowski J, O’Donovan C, Pfennig A, et al. Neuroprotective effect of lithium on hippocampal volumes in bipolar disorder independent of long-term treatment response. Psychol Med. 2014;44:507–17. doi: 10.1017/S0033291713001165. [DOI] [PubMed] [Google Scholar]
- 34.Berk M, Dandash O, Daglas R, Cotton SM, Allott K, Fornito A, et al. Neuroprotection after a first episode of mania: a randomized controlled maintenance trial comparing the effects of lithium and quetiapine on grey and white matter volume. Transl Psychiatry. 2017;7:e1011–e1011. doi: 10.1038/tp.2016.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dwivedi T, Zhang H. Lithium-induced neuroprotection is associated with epigenetic modification of specific BDNF gene promoter and altered expression of apoptotic-regulatory proteins. Front Neurosci. 2015;8:457. doi: 10.3389/fnins.2014.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun YR, Herrmann N, Scott CJM, Black SE, Khan MM, Lanctôt KL. Global grey matter volume in adult bipolar patients with and without lithium treatment: a meta-analysis. J Affect Disord. 2018;225:599–606. doi: 10.1016/j.jad.2017.08.078. [DOI] [PubMed] [Google Scholar]
- 37.Monkul ES, Matsuo K, Nicoletti MA, Dierschke N, Hatch JP, Dalwani M, et al. Prefrontal gray matter increases in healthy individuals after lithium treatment: a voxel-based morphometry study. Neurosci Lett. 2007;429:7–11. doi: 10.1016/j.neulet.2007.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyoo IK, Dager SR, Kim JE, Yoon SJ, Friedman SD, Dunner DL, et al. Lithium-induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: a longitudinal brain imaging study. Neuropsychopharmacology. 2010;35:1743–50. doi: 10.1038/npp.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cousins DA, Aribisala B, Nicol Ferrier I, Blamire AM. Lithium, gray matter, and magnetic resonance imaging signal. Biol Psychiatry. 2013;73:652–7. doi: 10.1016/j.biopsych.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 40.Tohen M, Zhang F, Taylor CC, Burns P, Zarate C, Sanger T, et al. A meta-analysis of the use of typical antipsychotic agents in bipolar disorder. J Affect Disord. 2001;65:85–93. doi: 10.1016/S0165-0327(00)00162-2. [DOI] [PubMed] [Google Scholar]
- 41.Vita A, de Peri L, Deste G, Barlati S, Sacchetti E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry. 2015;78:403–12. doi: 10.1016/j.biopsych.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Lieberman JA. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 43.Lawrie SM. Are structural brain changes in schizophrenia related to antipsychotic medication? A narrative review of the evidence from a clinical perspective. Ther Adv Psychopharmacol. 2018;8:319–26. doi: 10.1177/2045125318782306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cattarinussi G, Delvecchio G, Prunas C, Brambilla P. Effects of pharmacological treatments on neuroimaging findings in first episode affective psychosis: a review of longitudinal studies. J Affect Disord. 2020;276:1046–51. doi: 10.1016/j.jad.2020.07.118. [DOI] [PubMed] [Google Scholar]
- 45.Abé C, Liberg B, Petrovic P, Ingvar M, Landén M. Reply to: tripping over the same stone. Biol Psychiatry. 2020;88:e13. doi: 10.1016/j.biopsych.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 46.Mikolas P, Bröckel K, Vogelbacher C, Müller DK, Marxen M, Berndt C, et al. Individuals at increased risk for development of bipolar disorder display structural alterations similar to people with manifest disease. Transl Psychiatry. 2021;11:485. doi: 10.1038/s41398-021-01598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fusar-Poli P, Howes O, Bechdolf A, Borgwardt S. Mapping vulnerability to bipolar disorder: a systematic review and meta-analysis of neuroimaging studies. J Psychiatry Neurosci. 2012;37:170–84. doi: 10.1503/jpn.110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarıçiçek A, Yalın N, Hıdıroğlu C, Çavuşoğlu B, Taş C, Ceylan D, et al. Neuroanatomical correlates of genetic risk for bipolar disorder: A voxel-based morphometry study in bipolar type I patients and healthy first degree relatives. J Affect Disord. 2015;186:110–8. doi: 10.1016/j.jad.2015.06.055. [DOI] [PubMed] [Google Scholar]
- 49.Roberts G, Lenroot R, Frankland A, Yeung PK, Gale N, Wright A, et al. Abnormalities in left inferior frontal gyral thickness and parahippocampal gyral volume in young people at high genetic risk for bipolar disorder. Psychol Med. 2016;46:2083–96. doi: 10.1017/S0033291716000507. [DOI] [PubMed] [Google Scholar]
- 50.Abé C, Petrovic P, Ossler W, Thompson WH, Liberg B, Song J, et al. Genetic risk for bipolar disorder and schizophrenia predicts structure and function of the ventromedial prefrontal cortex. J Psychiatry Neurosci. 2021;46:E441–E450. doi: 10.1503/jpn.200165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janiri D, Kotzalidis GD, di Luzio M, Giuseppin G, Simonetti A, Janiri L, et al. Genetic neuroimaging of bipolar disorder: a systematic 2017-20 update. Psychiatr Genet. 2021;31:50–64. doi: 10.1097/YPG.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 52.Bootsman F, Brouwer RM, Schnack HG, Kemner SM, Hillegers MHJ, Sarkisyan G, et al. A study of genetic and environmental contributions to structural brain changes over time in twins concordant and discordant for bipolar disorder. J Psychiatr Res. 2016;79:116–24. doi: 10.1016/j.jpsychires.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Delvecchio G, Pigoni A, Bauer IE, Soares JC, Brambilla P. Disease-discordant twin structural MRI studies on affective disorders. Neurosci Biobehav Rev. 2020;108:459–71. doi: 10.1016/j.neubiorev.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 54.Pando-Naude V, Toxto S, Fernandez-Lozano S, Parsons CE, Alcauter S, Garza-Villarreal EA. Gray and white matter morphology in substance use disorders: a neuroimaging systematic review and meta-analysis. Transl Psychiatry. 2021;11:29. doi: 10.1038/s41398-020-01128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.el Marroun H, Klapwijk ET, Koevoets M, Brouwer RM, Peters S, Van’t Ent D, et al. Alcohol use and brain morphology in adolescence: a longitudinal study in three different cohorts. Eur J Neurosci. 2021;54:6012–26. doi: 10.1111/ejn.15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parvaz MA, Rabin RA, Adams F, Goldstein RZ. Structural and functional brain recovery in individuals with substance use disorders during abstinence: a review of longitudinal neuroimaging studies. Drug Alcohol Depend. 2022;232:109319. doi: 10.1016/j.drugalcdep.2022.109319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tolliver BK, Anton RF. Assessment and treatment of mood disorders in the context of substance abuse. Dialogues Clin Neurosci. 2015;17:181–90. doi: 10.31887/DCNS.2015.17.2/btolliver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levin FR, Hennessy G. Bipolar disorder and substance abuse. Biol Psychiatry. 2004;56:738–48. doi: 10.1016/j.biopsych.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Song R, Xu H, Dintica CS, Pan K-Y, Qi X, Buchman AS, et al. Associations Between cardiovascular risk, structural brain changes, and cognitive decline. J Am Coll Cardiol. 2020;75:2525–34. doi: 10.1016/j.jacc.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marebwa BK, Adams RJ, Magwood GS, Basilakos A, Mueller M, Rorden C, et al. Cardiovascular risk factors and brain health: impact on long‐range cortical connections and cognitive performance. J Am Heart Assoc. 2018;7:e010054. doi: 10.1161/JAHA.118.010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, et al. Silent brain infarction and risk of future stroke. Stroke. 2016;47:719–25. doi: 10.1161/STROKEAHA.115.011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossom RC, Hooker SA, O’Connor PJ, Crain AL, Sperl‐Hillen JM. Cardiovascular risk for patients with and without schizophrenia, schizoaffective disorder, or bipolar disorder. J Am Heart Assoc. 2022;11:e021444. doi: 10.1161/JAHA.121.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vancampfort D, Vansteelandt K, Correll CU, Mitchell AJ, de Herdt A, Sienaert P, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013;170:265–74. doi: 10.1176/appi.ajp.2012.12050620. [DOI] [PubMed] [Google Scholar]
- 64.Vancampfort D, Stubbs B, Mitchell AJ, de Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14:339–47. doi: 10.1002/wps.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McWhinney SR, Abé C, Alda M, Benedetti F, Bøen E, del Mar Bonnin C, et al. Association between body mass index and subcortical brain volumes in bipolar disorders–ENIGMA study in 2735 individuals. Mol Psychiatry. 2021;26:6806–19. doi: 10.1038/s41380-021-01098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McWhinney S, Kolenic M, Franke K, Fialova M, Knytl P, Matejka M, et al. Obesity as a risk factor for accelerated brain ageing in first-episode psychosis—a longitudinal study. Schizophr Bull. 2021;47:1772–81. doi: 10.1093/schbul/sbab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pearlson GD. Etiologic, phenomenologic, and endophenotypic overlap of schizophrenia and bipolar disorder. Annu Rev Clin Psychol. 2015;11:251–81. doi: 10.1146/annurev-clinpsy-032814-112915. [DOI] [PubMed] [Google Scholar]
- 68.Lee S, Ripke S, Neale B, Faraone S, Purcell S, Perlis R, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liberg B, Rahm C, Panayiotou A, Pantelis C. Brain change trajectories that differentiate the major psychoses. Eur J Clin Invest. 2016;46:658–74. doi: 10.1111/eci.12641. [DOI] [PubMed] [Google Scholar]
- 71.Pagel T, Baldessarini RJ, Franklin J, Baethge C. Heterogeneity of schizoaffective disorder compared with schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2013;128:238–50. [DOI] [PubMed]
- 72.Liang C, Pearlson G, Bustillo J, Kochunov P, Turner JA, Wen X, et al. Psychotic symptom, mood, and cognition-associated multimodal MRI reveal shared links to the salience network within the psychosis spectrum disorders. Schizophr Bull. 2023;49:172–84. doi: 10.1093/schbul/sbac158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ivleva EI, Bidesi AS, Thomas BP, Meda SA, Francis A, Moates AF, et al. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res Neuroimaging. 2012;204:13–24. doi: 10.1016/j.pscychresns.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ballester PL, Romano MT, Azevedo Cardoso T, Hassel S, Strother SC, Kennedy SH, et al. Brain age in mood and psychotic disorders: a systematic review and meta‐analysis. Acta Psychiatr Scand. 2022;145:42–55. doi: 10.1111/acps.13371. [DOI] [PubMed] [Google Scholar]
- 75.Mendez MF, Parand L, Akhlaghipour G. Bipolar disorder among patients diagnosed with frontotemporal dementia. J Neuropsychiatry Clin Neurosci. 2020;32:376–84. doi: 10.1176/appi.neuropsych.20010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roman Meller M, Patel S, Duarte D, Kapczinski F, Azevedo, Cardoso T. Bipolar disorder and frontotemporal dementia: a systematic review. Acta Psychiatr Scand. 2021;144:433–47. doi: 10.1111/acps.13362. [DOI] [PubMed] [Google Scholar]
- 77.Kessing LV, Søndergård L, Forman JL, Andersen PK. Lithium treatment and risk of dementia. Arch Gen Psychiatry. 2008;65:1331. doi: 10.1001/archpsyc.65.11.1331. [DOI] [PubMed] [Google Scholar]
- 78.Vidal-Pineiro D, Parker N, Shin J, French L, Grydeland H, Jackowski AP, et al. Cellular correlates of cortical thinning throughout the lifespan. Sci Rep. 2020;10:21803. doi: 10.1038/s41598-020-78471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel Y, Parker N, Shin J, Howard D, French L, Thomopoulos SI, et al. Virtual histology of cortical thickness and shared neurobiology in 6 psychiatric disorders. JAMA Psychiatry. 2021;78:47. doi: 10.1001/jamapsychiatry.2020.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders. Nat Rev Dis Prim. 2018;4:18008. doi: 10.1038/nrdp.2018.8. [DOI] [PubMed] [Google Scholar]
- 81.Magioncalda P, Martino M. A unified model of the pathophysiology of bipolar disorder. Mol Psychiatry. 2022;27:202–11. doi: 10.1038/s41380-021-01091-4. [DOI] [PubMed] [Google Scholar]
- 82.Schmider J, Lammers C-H, Gotthardt U, Dettling M, Holsboer F, Heuser IJE. Combined dexamethasone/corticotropin-releasing hormone test in acute and remitted manic patients, in acute depression, and in normal controls: I. Biol Psychiatry. 1995;38:797–802. doi: 10.1016/0006-3223(95)00064-X. [DOI] [PubMed] [Google Scholar]
- 83.Jakobsson J, Bjerke M, Ekman CJ, Sellgren C, Johansson AG, Zetterberg H, et al. Elevated concentrations of neurofilament light chain in the cerebrospinal fluid of bipolar disorder patients. Neuropsychopharmacology. 2014;39:2349–56. doi: 10.1038/npp.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knorr U, Simonsen AH, Roos P, Weimann A, Henriksen T, Christensen E-M, et al. Cerebrospinal fluid oxidative stress metabolites in patients with bipolar disorder and healthy controls: a longitudinal case-control study. Transl Psychiatry. 2019;9:325. doi: 10.1038/s41398-019-0664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Isgren A, Jakobsson J, Pålsson E, Ekman CJ, Johansson AGM, Sellgren C, et al. Increased cerebrospinal fluid interleukin-8 in bipolar disorder patients associated with lithium and antipsychotic treatment. Brain Behav Immun. 2015;43:198–204. doi: 10.1016/j.bbi.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Söderlund J, Olsson S, Samuelsson M, Walther-Jallow L, Johansson C, Erhardt S, et al. Elevation of cerebrospinal fluid interleukin-1β in bipolar disorder. J Psychiatry Neurosci. 2011;36:114–8. doi: 10.1503/jpn.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ohgidani M, Kato TA, Haraguchi Y, Matsushima T, Mizoguchi Y, Murakawa-Hirachi T, et al. Microglial CD206 gene has potential as a state marker of bipolar disorder. Front Immunol. 2017;7:676. doi: 10.3389/fimmu.2016.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munkholm K, Vinberg M, Vedel Kessing L. Cytokines in bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2013;144:16–27. doi: 10.1016/j.jad.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 89.Jakobsson J, Stridsberg M, Zetterberg H, Blennow K, Ekman C-J, Johansson A, et al. Decreased cerebrospinal fluid secretogranin II concentrations in severe forms of bipolar disorder. J Psychiatry Neurosci. 2013;38:E21–E26. doi: 10.1503/jpn.120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ekman CJ, Lind J, Rydén E, Ingvar M, Landén M. Manic episodes are associated with grey matter volume reduction - a voxel-based morphometry brain analysis. Acta Psychiatr Scand. 2010;122:507–15. doi: 10.1111/j.1600-0447.2010.01586.x. [DOI] [PubMed] [Google Scholar]
- 91.Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–51. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 92.Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, et al. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48:852–62. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 93.van Rheenen TE, Cotton SM, Dandash O, Cooper RE, Ringin E, Daglas-Georgiou R, et al. Increased cortical surface area but not altered cortical thickness or gyrification in bipolar disorder following stabilisation from a first episode of mania. Prog Neuropsychopharmacol Biol Psychiatry. 2023;122:110687. doi: 10.1016/j.pnpbp.2022.110687. [DOI] [PubMed] [Google Scholar]
- 94.World Health Organization. International statistical classification of diseases and related health problems. 11th ed. World Health Organization; 2019.
- 95.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muneer A. Bipolar disorder: role of inflammation and the development of disease biomarkers. Psychiatry Investig. 2016;13:18. doi: 10.4306/pi.2016.13.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lei D, Li W, Tallman MJ, Strakowski SM, DelBello MP, Rodrigo Patino L, et al. Changes in the structural brain connectome over the course of a nonrandomized clinical trial for acute mania. Neuropsychopharmacology. 2022;47:1961–8. doi: 10.1038/s41386-022-01328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cotrena C, Damiani Branco L, Ponsoni A, Samamé C, Milman Shansis F, Paz, et al. Executive functions and memory in bipolar disorders I and II: new insights from meta‐analytic results. Acta Psychiatr Scand. 2020;141:110–30. doi: 10.1111/acps.13121. [DOI] [PubMed] [Google Scholar]
- 99.Sánchez-Morla EM, López-Villarreal A, Jiménez-López E, Aparicio AI, Martínez-Vizcaíno V, Roberto R-J, et al. Impact of number of episodes on neurocognitive trajectory in bipolar disorder patients: a 5-year follow-up study. Psychol Med. 2019;49:1299–307. doi: 10.1017/S0033291718001885. [DOI] [PubMed] [Google Scholar]
- 100.Sparding T, Joas E, Clements C, Sellgren CM, Pålsson E, Landén M. Long-term trajectory of cognitive performance in people with bipolar disorder and controls: 6-year longitudinal study. BJPsych Open. 2021;7:e115. doi: 10.1192/bjo.2021.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schumer MC, Chase HW, Rozovsky R, Eickhoff SB, Phillips ML. Prefrontal, parietal, and limbic condition-dependent differences in bipolar disorder: a large-scale meta-analysis of functional neuroimaging studies. Mol Psychiatry. 2023;13:2023. doi: 10.1038/s41380-023-01974-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cotovio G, Oliveira-Maia AJ. Functional neuroanatomy of mania. Transl Psychiatry. 2022;12:29. doi: 10.1038/s41398-022-01786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brady RO, Margolis A, Masters GA, Keshavan M, Öngür D. Bipolar mood state reflected in cortico-amygdala resting state connectivity: a cohort and longitudinal study. J Affect Disord. 2017;217:205–9. doi: 10.1016/j.jad.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rey G, Bolton TAW, Gaviria J, Piguet C, Preti MG, Favre S, et al. Dynamics of amygdala connectivity in bipolar disorders: a longitudinal study across mood states. Neuropsychopharmacology. 2021;46:1693–701. doi: 10.1038/s41386-021-01038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]