Abstract
Background
Over the past few decades, neuroimaging research in Bipolar Disorder (BD) has identified neural differences underlying cognitive and emotional processing. However, substantial clinical and methodological heterogeneity present across neuroimaging experiments potentially hinders the identification of consistent neural biomarkers of BD. This meta-analysis aims to comprehensively reassess brain activation and connectivity in BD in order to identify replicable differences that converge across and within resting-state, cognitive, and emotional neuroimaging experiments.
Methods
Neuroimaging experiments (using fMRI, PET, or arterial spin labeling) reporting whole-brain results in adults with BD and controls published from December 1999—June 18, 2019 were identified via PubMed search. Coordinates showing significant activation and/or connectivity differences between BD participants and controls during resting-state, emotional, or cognitive tasks were extracted. Four parallel, independent meta-analyses were calculated using the revised activation likelihood estimation algorithm: all experiment types, all resting-state experiments, all cognitive experiments, and all emotional experiments. To confirm reliability of identified clusters, two different meta-analytic significance tests were employed.
Results
205 published studies yielding 506 individual neuroimaging experiments (150 resting-state, 134 cognitive, 222 emotional) comprising 5745 BD and 8023 control participants were included. Five regions survived both significance tests. Individuals with BD showed functional differences in the right posterior cingulate cortex during resting-state experiments, the left amygdala during emotional experiments, including those using a mixed (positive/negative) valence manipulation, and the left superior and right inferior parietal lobules during cognitive experiments, while hyperactivating the left medial orbitofrontal cortex during cognitive experiments. Across all experiments, there was convergence in the right caudate extending to the ventral striatum, surviving only one significance test.
Conclusions
Our findings indicate reproducible localization of prefrontal, parietal, and limbic differences distinguishing BD from control participants that are condition-dependent, despite heterogeneity, and point towards a framework for identifying reproducible differences in BD that may guide diagnosis and treatment.
Subject terms: Biomarkers, Neuroscience
Introduction
Bipolar Disorder (BD) is a common, debilitating psychiatric disorder resulting in disease burden worldwide [1]. The past several decades of neuroimaging research have investigated the neural substrates of mechanisms underlying differences in cognitive and emotional processing that are characteristic of BD [2, 3] which has enabled the conceptualization of neural models [2, 4] that are critical to understanding it. The study of neural differences in BD associated with both brain activation (i.e., regional BOLD signaling) and functional connectivity (i.e., the correlation between different brain regions that can elucidate the nature of neural network dynamics) [5] enables the identification of biomarkers that improve diagnostic precision, facilitate early identification, and inform targets for treatment developments [6]. However, the presence of clinical heterogeneity [7, 8] (e.g., differences in healthcare systems [9, 10], diagnostic subtypes [11, 12], mood state [13, 14], treatment response [7, 15], comorbidity [7], chronicity, severity [16]), methodological differences (imaging modality, paradigm), and analytical flexibility [17, 18], as well as the impact of physiological noise sources [19–21] and variability of neural responses to cognitive manipulations [22–25] may all hinder the identification of consistent neural biomarkers of Bipolar-related illness [7, 12, 26, 27].
While high-powered structural studies [28] and qualitative reviews [2, 4] are informative to the development of theoretical models of BD, coordinate-based meta-analysis techniques, such as activation likelihood estimation (ALE) [29, 30], can test meta-analytic hypotheses at the level of the whole brain in a spatially unbiased fashion [31], taking into account hundreds to thousands of participants and disparities in experimental design decisions [29]. Moreover, depending on how the hypothesis is constructed, successful refutation of the null hypothesis can provide preliminary evidence for potential reproducible differences distinguishing individuals with BD from control participants [32]. However, the extent to which this is possible depends on the quality and number of studies included.
It is widely known that functional neuroimaging studies are hampered by great heterogeneity and low power due to small sample sizes, leading to the use of lower, often uncorrected, thresholds to obtain positive results, and thus a substantial risk of frequent false positive findings [33]. It is thus important to acknowledge that the neuroimaging literature on BD is likely to include numerous under-powered studies using phenotypically heterogenous samples and disproportionately characterized by positive exploratory findings rather than evaluation of the magnitude of a priori hypothesized effects [4]. Nevertheless, meta-analyses are needed to reconcile the literature’s pitfalls and provide a framework to test whether the findings of small, heterogenous studies can be reproducible across different studies [31]. Meta-analyses can be used to synthesize results of individual studies in spite of heterogeneity, thereby allowing readers to draw wider conclusions about the state of the literature at large (including whether any reported effects are reproducible). They also highlight irregularities and issues present in the field which, importantly, provides transparency that can guide future study designs and encourage replications [31]. Given the rapid rate at which neuroimaging studies of BD are being conducted and published, meta-analyses are useful in that they comprehensively, quantitatively summarize and integrate disparate findings, building cumulative knowledge and guiding future work [33]. ALE is also statistically conservative [34], using cluster-level family-wise error correction which leads to a low likelihood of false positive convergence, especially if a significant region includes contributing foci from several studies rather than a disproportionate contribution from a single study [31, 35]. Individual studies reporting results at uncorrected thresholds may be used with ALE, given that uncorrected thresholds can provide a favorable balance between false positives and false negatives [36].
Previous coordinate-based meta-analyses have found correlates of BD across emotion-processing experiments distinguishing BD from both non-clinical [37] and clinical controls, such as unipolar depression [38] and schizophrenia [39], across resting-state experiments [40, 41], and across both cognitive and emotional experiments [42]. However, these meta-analyses had a narrower focus and were limited by the available data which often had smaller sample sizes. Additionally, they did not incorporate techniques that have been used more recently in psychiatric neuroimaging research (e.g., Amplitude of Low Frequency Fluctuations (ALFF) [43, 44], Independent Component Analysis (ICA) [45], Regional Homogeneity (ReHo) [46], degree centrality (DC) [47], functional connectivity strength (FCS) [47]). Furthermore, despite there being extensive neurocognitive differences in BD [48–51], there are no ALE meta-analyses of BD solely examining cognitive experiments.
Notwithstanding these gaps and advancements, no meta-analyses have examined the effect of condition (i.e., changes in neural activity and connectivity in response to changing task requirements and/or the level of arousal) via testing for a potential invariant condition-independent, or condition-dependent (i.e., clusters that converge across experiments or paradigms of one type, but not across experiments of a different type) functional marker of BD. Given the extensive evidence showing functional and structural differences in limbic regions, particularly the amygdala [4], across different mood states and neuroimaging modalities (e.g., structural magnetic resonance imaging (MRI), diffusion tensor imaging, resting-state, emotional and cognitive paradigms) [4], there are empirical grounds for suggesting the existence of a condition-independent marker of BD. Such a marker could manifest across a variety of cognitive (e.g., working memory), and emotional paradigms, and contribute to the differences in these processes distinguishing BD [51–57]. Alternatively, functional neural differences in BD may be more selective, with distinct markers being observed across different paradigm types.
Thus, the objective of this investigation was to comprehensively reassess brain activation and functional connectivity in BD in order to identify a reproducible, condition-independent neural correlate of BD that converges across resting-state, cognitive, and emotional experiments combined. To our knowledge, this study is the largest, high-powered, most comprehensive meta-analysis of BD functional neuroimaging experiments to date, which is necessary to justify whether the current state of the literature allows for identification of replicable differences in BD despite significant heterogeneity and the mixed reliability of task-based functional MRI (fMRI) for brain biomarker discovery [58, 59]. An omnibus meta-analysis across all experiment types tested the hypothesis that there would be a condition-independent neural marker differentiating BD from controls that can be seen regardless of task type, akin to a simple localized deficit which is generalizable across paradigms and modalities [28]. Parallel independent, individual meta-analyses of resting-state, cognitive, and emotional processing experiments each tested for condition-dependent neural signatures of BD. Meta-analysis across resting-state experiments tested for a potential core functional difference [2] that is reliable [43, 60] and relatively unconfounded by task effects compared to task-based fMRI [61]. Across cognitive tasks using non-affective stimuli, we hypothesized that there would be a functional difference related to cognition given that individuals with BD have impaired executive functioning, sustained attention, working and verbal memory [3, 48, 50, 51, 57], and this cognitive signature would significantly differ from both the null distribution as well as resting-state and emotional experiments. We further tested whether any significant cognitive differences were hyper/hypoactive in participants with BD. Furthermore, we hypothesized that there would be an emotion-related difference specific to emotion processing-related paradigms, given behavioral differences associated with mood lability and emotion dysregulation in BD [2, 52, 53]. We also tested whether this signature was specific to valence via post-hoc subgroup meta-analyses of experiments associated with negative, positive and mixed (negative and positive) valence, and whether any significant emotion-processing differences were hyper/hypoactive in participants with BD. Finally, we examined the contribution of clinical confounds and nesting (i.e., a single study contributing more than one experiment or contrast to a cluster) using two different meta-analytic significance tests to confirm the reliability of meta-analytic findings.
Methods
Search and selection
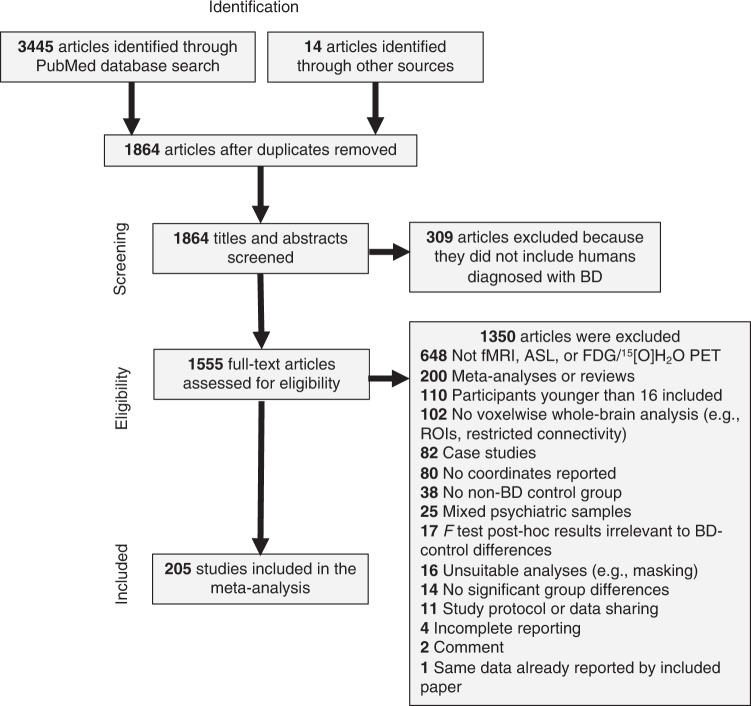
Figure 1 depicts the study selection process and reasons for exclusion. Details on eligibility criteria and literature search terms can be found in the Supplementary Methods. In brief, resting-state and task-based (cognitive and emotional) functional neuroimaging experiments using fMRI, positron emission tomography (PET), or arterial spin labeling (ASL) published online from December 1, 1999 through July 18, 2019 were identified from a systematic PubMed search. To be eligible for inclusion, experiments had to report voxelwise whole-brain results via standard whole-brain analyses, seed-to-voxel functional connectivity (including psychophysiological interactions (PPIs), granger causality mapping (GCM), and beta-series correlation for task-based experiments), ICA, ReHo, ALFF/fractional ALFF (fALFF), voxel-mirrored homotopic connectivity (VMHC), FCS, DC, eigenvector centrality mapping (ECM), in standard stereotaxic space (Montreal Neuroimaging Institute (MNI) or Talairach) that statistically compared adults (≥16 years old) diagnosed with BD to an adult non-BD control group (non-clinical and/or clinical). The minimum age was 16 and the mean age was over 18. While the majority of included studies measured adults aged 18 or older, a minority of studies included participants aged 16. These studies were included so as to increase the number of relevant studies in the meta-analysis and be as inclusive as possible. Pediatric (<16 years of age) and at-risk cases of BD were excluded to mitigate variability in neural activations that might be secondary to developing sex hormone effects [62–66].
Fig. 1. Flowchart of study selection.
ASL Arterial Spin Labeling, BD Bipolar Disorder, FDG Fluorodeoxyglucose, fMRI Functional Magnetic Resonance Imaging, PET Positron Emission Tomography, ROI Region Of Interest.
Cognitive experiments were operationalized as tasks using a cognitive paradigm with non-affective stimuli, and contrasts comparing a cognitive challenge to either a less-challenging control (e.g., 3-back vs. 1-back) or a baseline condition (e.g., 0-back, rest) were both included.
Emotional experiments were operationalized as tasks presenting an emotional visual, auditory, or sensory (e.g., pain, odor) stimulus or invoking an emotion (e.g., sad mood induction). Contrasts comparing an emotional condition to either a non-emotional/neutral condition, resting/baseline condition, or other emotional condition were all included. Compound emotional/cognitive tasks, operationalized as cognitive paradigms with an emotional manipulation (e.g., go/no-go with emotional distractors), were also included. Emotional tasks were then further separated into valence classes for post-hoc meta-analyses: Negative valence was operationalized as stimuli representing or invoking fear, sadness, anger, disgust, pain, loss, or punishment; positive valence was operationalized as stimuli representing or invoking happiness, pleasure, or rewards; mixed valence was operationalized as positive and negative valence stimuli/conditions collapsed (e.g., all emotional faces vs. baseline); neutral was defined as a non-emotional condition or stimulus (e.g., blank face, shape).
Data extraction and experimental design
Information was extracted from each experiment on (a) sample size, (b) imaging technique (fMRI, PET, or ASL), (c) task type (resting-state, cognitive, or emotional), (d) directionality (i.e., group differences reported by t-tests, or nondirectional group effects and group-by-condition interactions reported by F statistics), (e) peak MNI or Talairach coordinates, (f) level of arousal (emotional conditions were arousing; cognitive and non-emotional/neutral conditions were non-arousing), and (g) valence. Additional experiment characteristics such as BD mood state (hypo/manic or mixed (collapsed due to low power i.e., there were fewer than 17 mixed state experiments [35]), depressed, euthymic/remitted, or combined/not reported), current presence and/or history of psychosis, medication status, BD diagnostic subtype (I, II, Not Otherwise Specified), control group type (non-clinical and/or clinical), and BD participants’ age and gender were also extracted. Further details on data extraction are provided in the Supplementary Methods.
Activation likelihood estimation
Meta-analyses were performed using the revised ALE algorithm [30] (detailed description in the Supplementary Methods). All ALE results are reported at p < .05 family-wise error (FWE) cluster-level corrected (cluster-forming threshold at voxel-level p < .001) in MNI space, consistent with previous ALE meta-analyses. The SPM Anatomy Toolbox [67] was used to obtain MNI coordinates and z statistics. In addition to the location of significant clusters, the ALE software indicates the experiments which contributed to a given cluster. This contribution information was used for post-hoc analyses (see ‘assessments of robustness and post-hoc analyses’ section).
Planned meta-analyses
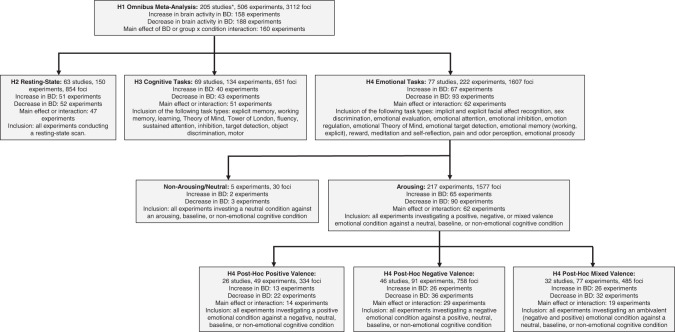
In total, 4 primary independent meta-analyses were performed: omnibus, resting-state, cognition, and emotion (Fig. 2; Supplementary Tables 1–4). Meta-analyses were calculated across reported patterns of BD hyper/hypoactivation (see previous ALE meta-analyses [68–70]), which accommodates flexibility in how research groups calculated group differences, i.e., some task-based experiments contrast a task with either a less-difficult control condition (e.g., 3-back >1-back) or a less-tightly controlled comparison condition (e.g., crosshair) on the subject-level, and group-level comparisons are subsequently calculated; whereas other experiments report group-by-condition interactions calculated on the second-level. In this way, the direction of the BD vs. control difference (i.e., hyper/hypoactivation) can depend on the type of control condition, and given that control conditions vary widely across experiments, different calculation approaches may influence the direction of group differences. Thus, this pooled analysis allowing for the discovery of converging activation differentiating BD from controls provides the most comprehensive summary of neuroimaging findings in BD. Additionally, meta-analyses were pooled across mood states and control groups due to asymmetric power i.e., the vast majority of experiments either examined euthymia, depression, or combined mood states, with a small number of hypo/manic and mixed state experiments (these latter two states were often collapsed within a sample), and most experiments compared participants with BD to non-clinical controls (74%). Although there were experiments with clinical controls (26%), half of those comparisons used F statistics generated across non-clinical, clinical, and BD groups, while the other half compared participants with BD directly to clinical controls.
Fig. 2. Description of meta-analyses.
BD Bipolar Disorder, H Hypothesis. *One study contributed both resting-state and cognitive experiments; two studies contributed both cognitive and emotional experiments. The number of foci for each meta-analysis reflects the total number of foci when all individual (nested) experiments were included.
The omnibus meta-analysis, collapsed across all experiments, tested for a condition-independent difference in BD i.e., a concordant signature that could be seen across all task types. To confirm that the omnibus meta-analysis represented a distinct, independent hypothesis from the other three, we added a secondary stipulation for this hypothesis, namely that any regions identified must not show a significant bias for one paradigm type over another i.e. that all paradigm types (emotion, cognition, rest) should contribute to the cluster in an approximately similar fashion. To test this hypothesis, we conducted Bayesian χ2 tests assuming a Poisson sampling plan, which can provide evidence for and against the null hypothesis of no difference between contributing paradigm types [71]. Exploratory meta-analyses of task-based activation experiments are reported in the Supplementary Results and Supplementary Table 6.
The resting-state meta-analysis tested for convergence across resting-state experiments followed by separate post-hoc contrasts against cognitive and emotional experiments testing whether resting-state signatures were significantly different from task-based experiments to provide further evidence for the specificity of group differences for cognitive and emotional manipulations. Post-hoc meta-analyses tested whether there were dissociations in these signatures across mood states. Exploratory meta-analyses of seed-to-voxel resting-state functional connectivity experiments are reported in the Supplementary Results and Supplementary Table 6. We did not perform planned tests of additional meta-analytic connectivity differences specific to other resting-state methods if there were fewer than 17 studies [35]. We did not test for directionality effects in BD due to the variety of different seeds and methods being used.
The cognition meta-analysis tested for a neural signature associated with cognitive task differences in BD that would significantly converge across cognitive experiments and further tested for their specificity to cognitive manipulations only via separate post-hoc contrasts against resting-state and emotional experiments. Post-hoc meta-analyses tested whether these signatures were hyper/hypoactive in participants with BD compared to controls, and whether there were dissociations in these signatures across mood states. Exploratory meta-analyses of working memory paradigms are reported in the Supplementary Results and Supplementary Table 6. We were did not perform planned meta-analyses of additional cognitive domains if there were fewer than 17 studies [35]).
The emotion meta-analysis, collapsed across all emotional tasks, tested for an emotion-specific neural signature of BD, followed by post-hoc subgroup meta-analyses of valence (negative, positive, mixed) that examined the locus of the difference (Supplementary Table 5), and whether any significant effects were significantly different from cognitive and resting-state experiments via separate post-hoc contrasts to further establish that emotional task signatures are unique to emotional manipulations. Additional post-hoc meta-analyses tested for emotional task signatures that were hyper/hypoactive in participants with BD compared to controls as well as whether there were dissociations in these clusters across mood states. Exploratory meta-analyses of emotional reactivity and emotion regulation paradigms are reported in the Supplementary Results and Supplementary Table 6.
Assessments of robustness and post-hoc analyses
Converging clusters were evaluated for reliability using two different meta-analytic methods. The first, primary method pooled together all coordinates from all contrasts (if more than one was included) from a given study into one experiment [70], such that each study only contributed one experimental contrast to the ALE, thereby enabling the modeling of random effects [72]. To assess the robustness of meta-analytic findings yielded by this initial inference with a different set of assumptions, we also conducted a two-part robustness test. The first step treated each individual contrast as a separate experiment to examine the contribution of different experiment characteristics (e.g., studies that included more than one mood state contrast); this initial approach intentionally allowed for within-study clustering, or nesting, of effects. The second step examined the impact of nesting on meta-analytic findings yielded by the first step: for studies contributing more than one contrast to a significant cluster (i.e., nested studies), we re-ran the ALE keeping the least contributing experiment to the cluster (i.e., having the lowest ALE contribution score; detailed description in the Supplementary Methods) and removing the other experiments belonging to the same study, both the ones that more strongly contributed to the cluster and the ones that did not contribute, thus keeping only one contrast per nested study. This analysis was performed in order to reduce within-study bias that nesting can introduce, which is not accounted for by the ALE algorithm. Clusters that reached significance both when coordinates were initially pooled and then after nested experiments were removed were considered to be significant effects. For each meta-analysis, we focus on findings that survived both the pooled test and the subsequent robustness assessment.
We also examined the contribution of overlapping study samples (i.e., multiple studies whose samples came from the same research group/laboratory) post-hoc for findings passing both significance tests; this information can be found in the Supplementary Results.
Details of the experiments that contributed to each cluster from the pooled, nested, post-hoc, and exploratory meta-analyses are provided in Supplementary Tables 7–32.
Post-hoc analyses of the experiment contribution information were conducted to assess the impact of directionality, mood state, BD diagnostic subtype, medication status, psychosis, age, gender, and control group type so as to examine whether certain experiment types were over/underrepresented in the findings; these analyses were performed to evaluate whether these clinical heterogeneity factors confounded the significant findings of interest [12, 73, 74]. These analyses were conducted using the contribution information from the nested analysis approach that allows for the examination of different experiment characteristics. We conducted two-tailed Fisher’s Exact Tests of independence in SPSS Version 27, in which one variable designated whether a given experiment was contributing or not contributing to a significant cluster and the other represented the observed frequencies for each sub-category of the above factors. Results were FDR-corrected using the Benjamini-Hochberg procedure [75], although uncorrected p values are reported alongside FDR-corrected q values. Results of all post-hoc analyses are reported in the Supplementary Results, while a summary is provided in the main text.
In addition, we sought to use the contribution information to provide an estimate of the underlying effect size. Effect size estimation in fMRI can be difficult as the statistic representing a cluster’s peak provides a biased (inflated) estimate of effect size [76]. Instead, we used the (observed) proportion of experiments contributing versus not contributing to a cluster for the pooled method, and, using a Fisher’s Exact Test, compared it to a null distribution in which the (expected) rate of contribution was equivalent to the expected false positive rate given an effect size of zero (i.e. alpha). We used p < 0.01 uncorrected as an estimate of alpha, given that we included coordinate maps obtained using uncorrected thresholds. The resulting p value from the Fisher’s Exact test was converted into a χ2 statistic via an inverse χ2 distribution, which was converted into a Pearson’s r statistic [77] and then an effect size estimate (Cohen’s d [78]).
Results
205 published studies with 506 individual neuroimaging experiments (yielding 3112 foci total) published from 1999–2019 met the criteria for inclusion in this meta-analysis. Demographic, clinical, and methodological details and citations of included papers are provided in Supplementary Table 33. The total sample covering 13768 participants comprised 5745 individuals with BD, 5919 non-clinical controls, and 2104 clinical controls (Tables 1 and 2).
Table 1.
Significant clusters and contrasts from a priori ALE meta-analyses.
| Task type or contrast | N foci | N BD | N controls | Peak coordinates (MNI) | Location | Cluster Size (k voxels) | Peak Intensity (Z) |
|---|---|---|---|---|---|---|---|
| All task types | 2910 | 5745 | 8023 |
10, 10, −8 14, 12, 14 12, 14, 10 10, 12, −2 |
Anterior Caudate ext. to Ventral Striatuma | 157 |
4.44 3.94 3.86 3.42 |
| Resting-state | 786 | 2218 | 3093 | 6, −52, 32 | Posterior Cingulate Cortexb | 144 | 4.47 |
| Cognitive tasks | 619 | 1848 | 3034 |
38, −44, 46 36, −56, 48 −20, −70, 52 −2, 52, −14 −10, 8, −2 −16, 6, −18 −2, 12, −4 |
Inferior Parietal Lobuleb Superior Parietal Lobuleb Medial Orbitofrontal Cortexa Anterior Caudate ext. to Ventral Striatum, Subgenual Anterior Cingulate Cortexa |
154 121 115 144 |
5.21 4.97 5.87 5.27 4.53 4.21 3.94 |
| Emotional tasks | 1505 | 1762 | 2028 |
−26, −6, −20 −20, −14, −14 |
Amygdala ext. to Hippocampusb | 128 |
4.25 3.47 |
| Resting-state > cognitive | 1405 | 4066 | 6127 | 4, −50, 28 | Posterior Cingulate Cortexc | 143 | 3.43 |
| Cognitive > resting-state | 1405 | 4066 | 6127 |
42, −46, 50 38, −54, 52 −24, −70, 46 2, 50, −12 −6, 10, −2 −18, 4, −16 |
Inferior Parietal Lobulec Superior Parietal Lobulec Medial Orbitofrontal Cortex Ventral Striatum ext. to Subgenual Anterior Cingulate Cortex Posterior Central Orbitofrontal Cortex |
119 83 82 92 10 |
2.91 2.18 2.51 2.64 2.92 1.94 |
| Resting-state > emotional | 2291 | 3980 | 5121 | 2, −54, 36 | Posterior Cingulate Cortexc | 93 | 3.29 |
| Emotional > resting-state | 2291 | 3980 | 5121 | −20, −6, −18 | Amygdalac | 108 | 3.34 |
| Cognitive > emotional | 2124 | 3610 | 5062 |
40, −46, 44 −18, −70, 50 0, 54, −10 −14, 8, −14 |
Inferior Parietal Lobulec Superior Parietal Lobulec Medial Orbitofrontal Cortex Subgenual Anterior Cingulate Cortex |
101 93 9 12 |
2.32 2.57 1.86 2.02 |
| Emotional > cognitive | 2124 | 3610 | 5062 |
−26, −6, −20 −20, −12, −14 |
Amygdala ext. to Hippocampusc | 128 |
4.25 3.47 |
To avoid double counting, sample sizes (N) correspond to the total number of study participants, not the number of individual experiment participants.
BD Bipolar Disorder, C Controls, ext. Extended, MNI Montreal Neuroimaging Institute.
aSeen when experiments were pooled.
bSeen both when nested experiments were removed and when experiments were pooled.
cClusters passing both significance tests that were also observed in contrasts.
Table 2.
Significant clusters and contrasts from post-hoc ALE meta-analyses.
| Task type, contrast, or interaction | N foci | N BD | N controls | Peak coordinates (MNI) | Location | Cluster size (k voxels) | Peak intensity (Z) |
|---|---|---|---|---|---|---|---|
| Cognitive tasks (BD > C) | 118 | 660 | 781 |
−4, 52, −16 −4, 36, −14 0, 38, 4 −10, 46, −6 |
Medial Orbitofrontal Cortexb Ventral Anterior Cingulate Cortexa,c |
156 220 |
5.56 4.6 4.17 3.42 |
| Cognitive tasks (C > BD) | 250 | 816 | 963 | −32, −2, 52 | Premotor/Supplementary Motor Cortexa,c | 121 | 5.47 |
| Emotional tasks (BD > C) | 451 | 621 | 824 | – | No Convergence | – | – |
| Emotional tasks (C > BD) | 690 | 709 | 896 | 32, 30, −14 | Ventrolateral Prefrontal Cortexa,c | 125 | 5.46 |
| Positive valence | 334 | 623 | 739 | – | No Convergence | – | – |
| Negative valence | 706 | 1127 | 1220 | – | No Convergence | – | – |
| Mixed valence | 436 | 723 | 988 |
−28, −8, −24 −30, −2, −28 −22, −12, −14 |
Amygdala ext. to Hippocampusb | 196 |
3.77 3.66 3.38 |
| Euthymic BD & Resting-state | 230 | 526 | 561 | – | No Convergence | – | – |
| Depressed BD & Resting-state | 372 | 1107 | 1694 |
6, −52, 32 −6, −54, 30 |
Posterior Cingulate Cortexb | 157 |
4.62 3.51 |
| Hypo/manic BD & Resting-state | 28 | 52 | 103 | – | No Convergence | – | – |
|
Euthymic BD & Cognitive tasks |
288 | 738 | 1170 | −20, −70, 52 | Superior Parietal Lobuleb | 121 | 6.21 |
| Depressed BD & Cognitive tasks | 220 | 329 | 481 | – | No Convergence | – | – |
| Hypo/manic BD & Cognitive tasks | 27 | 163 | 198 | −32, −2, 58 | Premotor/Supplementary Motor Cortexa,c | 118 | 5.62 |
| Euthymic BD & Emotional tasks | 663 | 828 | 945 |
10, 10, −6 10, 16, 10 |
Anterior Caudate ext. to Ventral Striatuma | 131 |
4.5 3.65 |
| Depressed BD & Emotional tasks | 546 | 321 | 504 | – | No Convergence | – | – |
| Hypo/manic BD & Emotional tasks | 110 | 123 | 135 | – | No Convergence | – | – |
To avoid double counting, sample sizes (N) correspond to the total number of study participants, not the number of individual experiment participants. No Convergence indicates a null meta-analytic result i.e., no significant clusters were identified.
BD Bipolar Disorder, C Controls, ext. Extended, MNI Montreal Neuroimaging Institute.
aSeen when experiments were pooled.
bSeen both when nested experiments were removed and when experiments were pooled.
cNovel clusters which emerged in the post-hoc meta-analyses and were not observed in the a priori meta-analyses.
Meta-analyses across all experiments
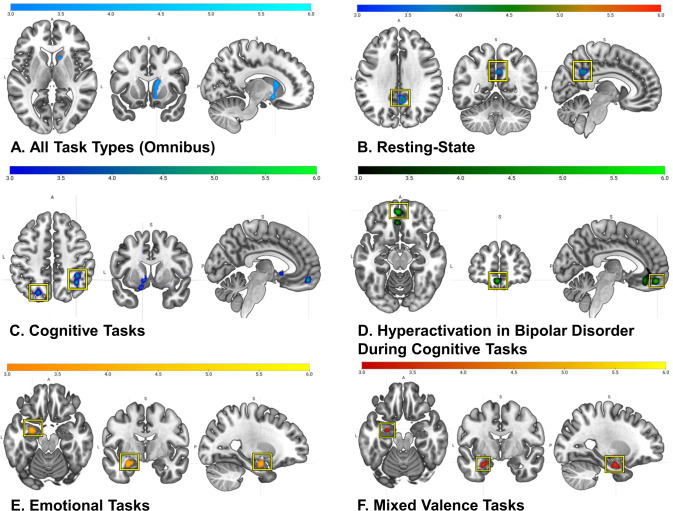
There were no significant findings from the omnibus meta-analysis that survived both the initial pooled test and the subsequent nested robustness assessment. Across all experiments (number of included experiments (NIE) = 205 in the pooled meta-analysis; NIE with nesting = 506), there was convergence in the right anterior caudate extending to the ventral striatum that survived the pooled test, but not the nested test, yielding an estimated effect size d = 0.52 (Fig. 3A). Of the 28 experiments that contributed to the striatal cluster in the pooled analysis, 25% were resting-state experiments (primarily employing seed-to-voxel functional connectivity) and 75% were task-based (primarily activation), of which 38% were cognitive paradigms and 62% were emotional. To confirm the independence of these observations across paradigm types, a Bayesian χ2 test revealed evidence in favor of the null hypothesis of no differences between contributing paradigms in the striatum (Bayes Factor (BF) = 0.18 with an inverse of 5.61, evidence for the null hypothesis).
Fig. 3. Overview of all significant clusters (pFWE < .05) from the a priori ALE meta-analyses.
Meta-analyses with clusters highlighted in yellow boxes indicate that these clusters survived both significance tests, whereas non-highlighted clusters only survived one significance test (i.e., the pooled analysis). Activations displayed in the sagittal slices reflect the position in the y/z axis.
Meta-analyses across resting-state experiments
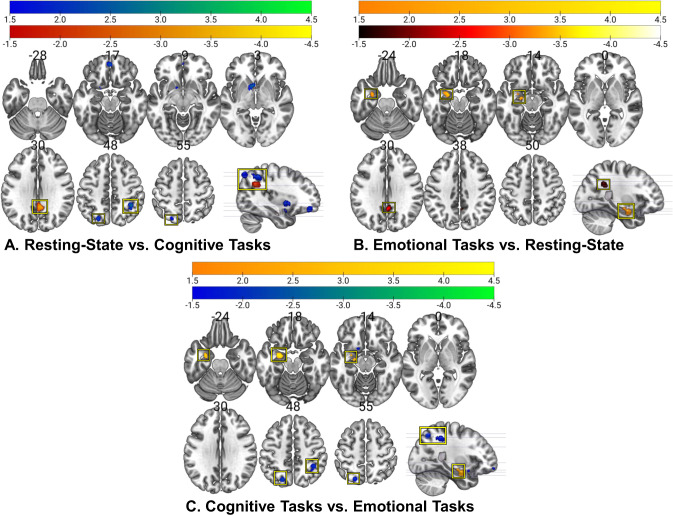
Across resting-state experiments (pooled NIE = 63; nested NIE = 150), there was significant convergence in the right ventral posterior cingulate cortex (PCC), which survived both significance tests and yielded an estimated effect size d = 0.53 (Fig. 3B). The PCC significantly differed from both cognitive experiments (Fig. 4A) and emotional experiments (Fig. 4B), demonstrating that connectivity in this region distinguishing BD from controls is unique to resting-state experiments.
Fig. 4. Contrast maps of activation distinguishing BD from controls (pFWE < .05) for specific task types.
Activations displayed in the sagittal slices reflect the position in the y/z axis. A Resting-state > cognitive clusters are shown in orange (slice 30) and red (sagittal slice); cognitive > resting-state clusters are shown in blue. B Resting-state > emotional clusters are shown in red (slice 30) and burgundy (sagittal slice); emotional > resting-state clusters are shown in yellow. C Emotional > cognitive clusters are shown in yellow; cognitive > emotional clusters are shown in blue.
Post-hoc tests evaluating the effect of mood state on resting-state differences revealed preliminary evidence for dissociations across mood states: the meta-analysis of resting-state experiments in depressed BD participants (pooled NIE = 29; nested NIE = 62) revealed significant convergence in the right ventral PCC that survived both significance tests (Supplementary Fig. 1); but meta-analyses of resting-state experiments in euthymic (pooled NIE = 18; nested NIE = 45) and hypo/manic BD participants (pooled NIE = 3; nested NIE = 4) did not reveal significant results.
Meta-analyses across cognitive experiments
Meta-analysis across cognitive experiments (pooled NIE = 69; nested NIE = 134) yielded four significant clusters of convergence: the right inferior parietal lobule (IPL) extending to the right angular gyrus (estimated effect size d = 0.41) and the left superior parietal lobule (SPL) (estimated effect size d = 0.41), both of which survived both significance tests, the left medial orbitofrontal cortex (mOFC), which survived the pooled test, but not the nested test (estimated effect size d = 0.41) and the left anterior caudate extending to the ventral striatum and subgenual anterior cingulate cortex, which was observed in the pooled analysis only (estimated effect size d = 0.54) (Fig. 3C). All four clusters significantly differed from resting-state experiments (Fig. 4A) and from emotional experiments (Fig. 4C), indicating that these signatures are specific to cognitive experiments.
A post-hoc test examining BD hyperactivation experiments alone (pooled NIE = 27; nested NIE = 40) revealed significant convergence in the left mOFC, which survived both significance tests (estimated effect size d = 0.65), as well as in one new cluster (i.e., clusters not initially observed in the a priori cognitive tasks meta-analysis): the left ventral anterior cingulate cortex, observed only when experiments were pooled (d = 0.84) (Fig. 3D). The meta-analysis of BD hypoactivation experiments alone (pooled NIE = 29; nested NIE = 43) revealed significant convergence in a new cluster, the left premotor/supplementary motor cortex, which survived the pooled test, but not the nested test (estimated effect size d = 0.43) (Supplementary Fig. 2).
Post-hoc tests evaluating the effect of mood state on cognitive task activations revealed preliminary evidence for dissociations across mood states, such that the post-hoc meta-analysis across cognitive tasks in euthymic BD participants (pooled NIE = 31; nested NIE = 55) revealed significant convergence in the left SPL, which survived both significance tests (Supplementary Fig. 3). The meta-analysis of cognitive tasks in depressed BD participants (pooled NIE = 16; nested NIE = 41) did not reveal significant results. Despite being underpowered to test the interaction between hypo/manic BD participants and cognitive tasks (pooled NIE = 7; nested NIE = 12), there was still significant convergence, albeit in a new cluster: the left premotor/supplementary motor cortex, which survived the pooled test only, as there was no nesting observed (Supplementary Fig. 4).
Meta-analyses across emotional experiments
Meta-analysis of emotional experiments (pooled NIE = 77; nested NIE = 222), pooled across valence, showed significant convergence in the left amygdala extending to the left hippocampus, which survived both significance tests (estimated effect size d = 0.75) (Fig. 3E). The amygdala significantly differed from resting-state experiments (Fig. 4B) and from cognitive experiments (Fig. 4C) demonstrating its specificity to emotional processing in BD.
There were no significant findings from the meta-analyses of BD hyper- and hypoactivation emotional experiments alone that survived both significance tests. Meta-analysis of BD hypoactivation experiments alone (pooled NIE = 36; nested NIE = 93) revealed significant convergence in one new cluster not previously observed in the emotional tasks meta-analysis: the right ventrolateral prefrontal cortex (VLPFC), which survived the pooled test, but not the nested test (estimated effect size d = 0.85) (Supplementary Fig. 5). Meta-analysis of BD hyperactivation experiments alone (pooled NIE = 32; nested NIE = 67) did not reveal significant results.
The post-hoc meta-analysis across mixed valence experiments (pooled NIE = 32; nested NIE = 76) showed significant convergence in the left basolateral amygdala extending to the left hippocampus (Fig. 3F); this finding survived both significance tests (estimated effect size d = 1.00). Meta-analyses across negative valence experiments (pooled NIE = 46; nested NIE = 91) and positive valence experiments (pooled NIE = 26; nested NIE = 49) did not reveal significant results.
The meta-analysis across emotional tasks in euthymic BD participants (pooled NIE = 39, nested NIE = 101) revealed significant convergence in the right anterior caudate extending to the ventral striatum, surviving the pooled test (Supplementary Fig. 6). Post-hoc meta-analyses across emotional tasks in depressed (pooled NIE = 14; nested NIE = 44) and hypo/manic BD participants (pooled NIE = 8; NIE = 23) did not reveal significant results.
Summary of post-hoc fisher’s exact tests of independence
None of the post-hoc Fisher’s Exact Tests of potential confounds were significant at FDR-corrected thresholds for any of the primary or post-hoc meta-analytic findings passing both significance tests. Further details are provided in the Supplemental Results.
Discussion
The present study yielded several major findings that survived both significance tests: (1) focused meta-analyses of resting-state and cognitive tasks revealed differences distinguishing BD from controls in connectivity of the right PCC specific to resting-state experiments and in activation of the right IPL and left SPL specific to cognitive experiments; (2) there was hyperactivation in BD of the left mOFC across cognitive tasks; and (3) meta-analysis of emotional tasks revealed differences in activation of the left amygdala specific to emotional experiments, including mixed valence manipulations. The omnibus meta-analysis across all experiments revealed differences in activation of the right striatum, surviving one significance test. The absence of findings surviving both significance tests from the omnibus meta-analysis likely reflects the fact that neural differences in BD are context-specific rather than generalizable across different tasks, as we had sufficient power to detect the latter if present. These findings thus collectively suggest that the extant literature provides support for reproducible localization of context-dependent differences in BD despite significant heterogeneity.
Resting-state experiments revealed differences in connectivity of the PCC, a core default mode network (DMN) node implicated in mood disorders [79, 80], consistent with prior evidence [81, 82]. Altered functional coupling and engagement of the PCC in BD may reflect rumination and/or attention dysregulation at rest and potentially during task performance [83–86]. Across cognitive experiments, there were differences in activation of the IPL and SPL, hubs of the frontoparietal network [87, 88]; contributing experiments covered executive control domains such as response inhibition, working memory and sustained/selective attention, suggesting a possible locus of sustained attentional deficits specific to BD [49, 89–91]. Additionally, the mOFC was hyperactivated in BD primarily during working memory and sustained attention tasks. OFC hyperactivation in non-emotional contexts has been proposed to reflect emotional processing interference due to a heightened salience to emotional perception in BD [2] and has also been found across psychiatric disorders during working memory tasks [92], indicating this finding may be transdiagnostic. Differences in amygdala activation observed across all mood states during emotional and mixed valence experiments is consistent with previous BD consensus models showing state- and direction-independent, emotionally-sensitive amygdala dysfunction [4].
While there was indeed convergence across all experiment types in the right striatum (consistent with previous models and reviews [2, 4, 93]) which survived the pooled test, this finding did not survive the nested test, thus we cannot draw definitive, confident conclusions about whether convergence in the striatum is indicative of an invariant, condition-independent difference in BD because this finding was biased by nesting and thus overly dependent on several experiments. Nonetheless, the null Bayesian χ2 result shows that this region’s activation appears largely independent from the aforementioned condition-dependent differences. The omnibus results are also encouraging in regard to clinical relevance, as they raise the possibility that therapeutics targeting this region (e.g., neuromodulatory interventions such as transcranial magnetic stimulation) may prove widely useful in treating and potentially preventing the onset and occurrence of different presentations and subtypes of BD.
The main strength of this meta-analysis is the inclusion of a large number of experiments, demonstrating one of the largest efforts to increase power to detect underlying associations with BD and allowing us to perform numerous well-powered subgroup meta-analyses that highlighted effects specific to resting-state, emotional, and cognitive processing. However, a consequence of this strength is the extensive clinical and methodological heterogeneity that comes with including such a diverse set of experiments, as well as substantial nesting that introduces within-study bias. In response to this limitation, we developed a robustness assessment that screened for significant effects yielded by our primary inference. There were also methodological biases: resting-state experiments differed from the other conditions in that a wide mixture of methods was used, primarily employing functional connectivity (i.e., seed-to-voxel, ALFF, fALFF, ICA, ReHo, DC, FCS, ECM, VMHC), whereas cognitive and emotional conditions primarily employed GLM-based analyses of task manipulations. Another important limitation of this study was that we did not preregister the meta-analysis before conducting it (see ‘protocol and registration’ section in the Supplementary Methods).
An additional consequence of heterogeneity in included studies meant that several ad-hoc methodological and eligibility criteria needed to be specified. In light of these limitations, we made efforts to be transparent and exhaustive in our reporting of the literature search, eligibility criteria, and methods for quantitative synthesis so as to enable replication of our work, all of which may aid in the formulation of comprehensive meta-analytic methods for future studies. Furthermore, in an effort to be inclusive and comprehensive, this meta-analysis included multiple publications that came from the same laboratory, with a strong accompanying risk of the same patients being included in separate studies (see Supplementary Results). Ideally, many different research groups should contribute a significant finding, and this was generally the case, although some laboratories did contribute two or more studies. In the case of the mOFC hyperactivation finding (BD > non-clinical controls), one laboratory contributed the large majority of studies (see Supplementary Results). Intriguingly, a similar finding has been observed in a transdiagnostic meta-analysis [92], and, of the various possible reasons for this laboratory’s over-representation in identifying this effect, it may be 1) that many research groups are not routinely considering hyperactivity of the DMN in their hypothesis generation, and/or 2) that the methods employed by this laboratory are particularly sensitive to this effect. Overall, our findings may provide a basis for future replication attempts by independent laboratories, and/or more ambitious demonstrations (i.e. employing more divergent techniques) of the same phenomenon. Finally, we did not run a priori illness-specificity meta-analyses examining the effect of control group type (non-clinical vs. clinical) due to power asymmetry in included experiments (and were underpowered to conduct separate meta-analyses directly comparing participants with BD to those with unipolar depression and schizophrenia). Post-hoc analyses did not reveal significant moderation effects of mood state, diagnostic subtype, medication, psychosis, age, gender, or control group type on the primary findings, however, raising the likelihood that these neural differences are reliable across different presentations of BD and relatively robust to mood state-related effects. However, the aforementioned power asymmetry across mood states, i.e., nearly 70% of experiments sampled euthymic or depressed participants with BD, may potentially be introducing a mood state-related bias. Post-hoc mood state by task type dissociations revealing differences in SPL activation converging in euthymic BD participants during cognitive tasks and in PCC connectivity in depressed BD participants during resting-state experiments may be reflective of a potential bias. This can be mitigated in future meta-analyses by conducting more and larger studies of hypo/mania and mixed states, especially because these mood states uniquely characterize BD [94]. Thus, identifying robust mania-related markers will greatly inform our ability to elucidate a neural marker of objective risk for BD. Furthermore, there was a relatively small number of experiments using reward processing paradigms, and thus it was not possible to perform a separate analysis of these data. Future studies should determine whether these functional differences are pathognomonic of BD or illness-severity effects (hence more studies with clinical controls are needed), and whether these effects hold in larger studies of hypo/manic and mixed samples. A next stage will be to determine how the neural differences identified in the present adult BD meta-analysis compare with child onset BD, and in at-risk child and adolescent groups. This may inform early risk identification. Another future direction will be to examine effects of mood state, treatments and illness duration.
In the present work, we included a novel estimate of the underlying effect size, based on a χ2 distribution. This estimate suggested that effect sizes tended to be medium to large. This effect size estimate does not include an estimate of the effect of heterogeneity, which is likely to be substantial in this sample due to the great variety of experimental approaches which were adopted. Further work may clarify whether there is indeed a medium-large underlying effect size in the regions identified, or whether there are even larger effect sizes which are obscured by the heterogeneity of the included studies.
Conclusions
The present study aimed to evaluate whether identification of a concordant, reproducible functional difference distinguishing BD from controls was tractable given substantial heterogeneity across experiments and found reliable, stable neural signatures that were condition-dependent. This study is the most comprehensive meta-analysis of BD functional neuroimaging studies to date. Our findings highlight core regions involved in BD that are not only context-specific, but also observed across mood states, given that our analyses were pooled across euthymic, depressed, hypo/manic and mixed mood state participants. We acknowledge, however, that these regions might more likely represent trait-like markers due to an overrepresentation of euthymic/remitted participants, which is a limitation of this meta-analytic dataset. To move towards the goal of being able to identify robust, group-level trait- and/or state-like markers of BD in future work, including future meta-analyses, better power-matched subgroup comparisons and larger subgroup analyses of different mood states are needed. These findings nevertheless build on existing neural models of BD (e.g., the OFC, amygdala, striatum, and VLPFC are components of previous models of BD [2, 4, 5]) and may help feed back onto studies that will develop new models, as our resting-state and cognition meta-analyses revealed additional condition-specific regions (e.g., the PCC, SPL, and IPL) that evolve and expand the consensus, complexities, and context of the neural circuitry implicated in BD, which can guide future hypothesis-testing [95]. Our present findings highlighting associations with BD of the amygdala and key prefrontal and parietal cortical regions in large-scale networks (e.g., the central executive network) also support other models of BD which emphasize that different mood episodes in BD might result from a progression from prefrontal cortical-subcortical differences to differences in functional coupling among largescale neural networks [96–98].
Future research should compare task and resting-state differences in the PCC and OFC, determine the neurodevelopmental trajectories of these regions in at-risk populations, and investigate whether BD medications (e.g., lithium) modulate these regions in non-clinical controls [99], which can elucidate potentially stabilizing or even normalizing [100] mechanisms of pharmacological treatments on functional differences in BD. Future studies should further examine these regions when shaping neural models of BD, and aim to identify mood state-related neural differences in BD, given that there are not enough existing studies of hypo/mania, mixed states, and clinical comparisons. Nevertheless, we hope the findings of this meta-analysis show promise for the ability of functional neuroimaging to identify group-level differences distinguishing BD that can be leveraged to advance therapeutics in the coming years.
Supplementary information
Acknowledgements
Maya Schumer gratefully acknowledges the National Science Foundation Graduate Research Fellowship Program for their support during the time this meta-analysis was conducted. We also thank the authors who sent us additional data to supplement their published manuscripts. All files used for analyses, associated references for all included papers, and all meta-analytic maps and contrasts have been made available to download and visualize from the Archive of Neuroimaging Meta-Analyses (ANIMA) database (https://anima.fz-juelich.de/studies/Schumer_BipolarDisorderMetaAnalysis_2023). Supplementary information is available at Molecular Psychiatry’s website.
Author contributions
All authors have full access to all the data in the study. Study concept and design: Schumer, Chase, Eickhoff. Acquisition, analysis, or interpretation of data: Schumer, Chase, Rosovsky, Eickhoff, Phillips. Statistical analysis: Schumer, Eickhoff, Chase. Drafting of the manuscript: Schumer. Critical revision of the manuscript for important intellectual content: Schumer, Chase, Eickhoff, Phillips. Study supervision: Chase, Eickhoff, Phillips.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-023-01974-8.
References
- 1.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171:829–43. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellani M, Biagianti B, Zovetti N, Rossetti MG, Bressi C, Perlini C, et al. The effects of cognitive remediation on cognitive abilities and real-world functioning among people with bipolar disorder: A systematic review: Special Section on “Translational and Neuroscience Studies in Affective Disorders”. Section Editor, Maria Nobile MD, PhD. This Section of JAD focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders. The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders. J Affect Disord. 2019;257:691–7. doi: 10.1016/j.jad.2019.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–25. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chase HW, Phillips ML. Elucidating neural network functional connectivity abnormalities in bipolar disorder: toward a harmonized methodological approach. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:288–98. doi: 10.1016/j.bpsc.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertocci MA, Chase HW, Graur S, Stiffler R, Edmiston EK, Coffman BA, et al. The impact of targeted cathodal transcranial direct current stimulation on reward circuitry and affect in Bipolar Disorder. Mol Psychiatry. 2021;26:4137–45. doi: 10.1038/s41380-019-0567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alda M. The phenotypic spectra of bipolar disorder. Eur Neuropsychopharmacol. 2004;14:S94–9. doi: 10.1016/j.euroneuro.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Angst J, Gerber-Werder R, Zuberbühler HU, Gamma A. Is bipolar I disorder heterogeneous? Eur Arch Psychiatry Clin Neurosci. 2004;254:82–91. doi: 10.1007/s00406-004-0501-6. [DOI] [PubMed] [Google Scholar]
- 9.Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113–21. doi: 10.2105/AJPH.2005.077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams JS, Walker RJ, Egede LE. Achieving equity in an evolving healthcare system: opportunities and challenges. Am J Med Sci. 2016;351:33–43. doi: 10.1016/j.amjms.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charney AW, Ruderfer DM, Stahl EA, Moran JL, Chambert K, Belliveau RA, et al. Evidence for genetic heterogeneity between clinical subtypes of bipolar disorder. Transl Psychiatry. 2017;7:e993–e993. doi: 10.1038/tp.2016.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hozer F, Houenou J. Can neuroimaging disentangle bipolar disorder? J Affect Disord. 2016;195:199–214. doi: 10.1016/j.jad.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 13.Dilsaver SC, Chen YR, Shoaib AM, Swann AC. Phenomenology of mania: evidence for distinct depressed, dysphoric, and euphoric presentations. Am J Psychiatry. 1999;156:426–30. doi: 10.1176/ajp.156.3.426. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg JF, Garno JL, Portera L, Leon AC, Kocsis JH. Qualitative differences in manic symptoms during mixed versus pure mania. Compr Psychiatry. 2000;41:237–41. doi: 10.1053/comp.2000.7427. [DOI] [PubMed] [Google Scholar]
- 15.Swann AC, Lafer B, Perugi G, Frye MA, Bauer M, Bahk WM, et al. Bipolar mixed states: an international society for bipolar disorders task force report of symptom structure, course of illness, and diagnosis. Am J Psychiatry. 2013;170:31–42. doi: 10.1176/appi.ajp.2012.12030301. [DOI] [PubMed] [Google Scholar]
- 16.Solé B, Bonnin CM, Jiménez E, Torrent C, Torres I, Varo C, et al. Heterogeneity of functional outcomes in patients with bipolar disorder: a cluster-analytic approach. Acta Psychiatr Scand. 2018;137:516–27. doi: 10.1111/acps.12871. [DOI] [PubMed] [Google Scholar]
- 17.Botvinik-Nezer R, Holzmeister F, Camerer CF, Dreber A, Huber J, Johannesson M, et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020;582:84–8. doi: 10.1038/s41586-020-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botvinik-Nezer R, Iwanir R, Holzmeister F, Huber J, Johannesson M, Kirchler M, et al. fMRI data of mixed gambles from the Neuroimaging Analysis Replication and Prediction Study. Sci Data. 2019;6:106. doi: 10.1038/s41597-019-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodurka J, Ye F, Petridou N, Murphy K, Bandettini PA. Mapping the MRI voxel volume in which thermal noise matches physiological noise—Implications for fMRI. NeuroImage. 2007;34:542–9. doi: 10.1016/j.neuroimage.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birn RM, Murphy K, Handwerker DA, Bandettini PA. fMRI in the presence of task-correlated breathing variations. Neuroimage. 2009;47:1092–104. doi: 10.1016/j.neuroimage.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy K, Birn RM, Bandettini PA. Resting-state FMRI confounds and cleanup. Neuroimage. 2013;80:349–59. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kherif F, Josse G, Seghier ML, Price CJ. The main sources of intersubject variability in neuronal activation for reading aloud. J Cogn Neurosci. 2009;21:654–68. doi: 10.1162/jocn.2009.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrett DD, Kovacevic N, McIntosh AR, Grady CL. Blood oxygen level-dependent signal variability is more than just noise. J Neurosci. 2010;30:4914–21. doi: 10.1523/JNEUROSCI.5166-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrett DD, Samanez-Larkin GR, MacDonald SWS, Lindenberger U, McIntosh AR, Grady CL. Moment-to-moment brain signal variability: a next frontier in human brain mapping? Neurosci Biobehav Rev. 2013;37:610–24. doi: 10.1016/j.neubiorev.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The modulation of BOLD variability between cognitive states varies by age and processing speed. Cereb Cortex. 2013;23:684–93. doi: 10.1093/cercor/bhs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ching CRK, Hibar DP, Gurholt TP, Nunes A, Thomopoulos SI, Abé C, et al. What we learn about bipolar disorder from large-scale neuroimaging: findings and future directions from the ENIGMA Bipolar Disorder Working Group. Human Brain Mapping. 2020. https://onlinelibrary.wiley.com/doi/abs/10.1002/hbm.25098. [DOI] [PMC free article] [PubMed]
- 27.Gonzalez R, Gonzalez SD, McCarthy MJ. Using chronobiological phenotypes to address heterogeneity in bipolar disorder. Mol Neuropsychiatry. 2019;5:72–84. doi: 10.1159/000506636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA bipolar disorder working group. Mol Psychiatry. 2018;23:932–42. doi: 10.1038/mp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:2349–61. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, et al. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. 2018;84:151–61. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–67. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acar F, Seurinck R, Eickhoff SB, Moerkerke B. Assessing robustness against potential publication bias in Activation Likelihood Estimation (ALE) meta-analyses for fMRI. PLOS One. 2018;13:e0208177. doi: 10.1371/journal.pone.0208177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham J, Salimi-Khorshidi G, Hagan C, Walsh N, Goodyer I, Lennox B, et al. Meta-analytic evidence for neuroimaging models of depression: State or trait? J Affect Disord. 2013;151:423–31. doi: 10.1016/j.jad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Eickhoff SB, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, et al. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage. 2016;137:70–85. doi: 10.1016/j.neuroimage.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thirion B, Pinel P, Mériaux S, Roche A, Dehaene S, Poline JB. Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. NeuroImage. 2007;35:105–20. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 37.Houenou J, Frommberger J, Carde S, Glasbrenner M, Diener C, Leboyer M, et al. Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. J Affect Disord. 2011;132:344–55. doi: 10.1016/j.jad.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Delvecchio G, Fossati P, Boyer P, Brambilla P, Falkai P, Gruber O, et al. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012;22:100–13. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Delvecchio G, Sugranyes G, Frangou S. Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychological Med. 2013;43:553–69. doi: 10.1017/S0033291712001432. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Gao Y, Tang S, Lu L, Zhang L, Bu X, et al. Large-scale network dysfunction in the acute state compared to the remitted state of bipolar disorder: a meta-analysis of resting-state functional connectivity. EBioMedicine. 2020;54:102742. doi: 10.1016/j.ebiom.2020.102742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong J, Wang J, Chen P, Qi Z, Luo Z, Wang J, et al. Large-scale network abnormality in bipolar disorder: a multimodal meta-analysis of resting-state functional and structural magnetic resonance imaging studies. J Affect Disord. 2021;292:9–20. doi: 10.1016/j.jad.2021.05.052. [DOI] [PubMed] [Google Scholar]
- 42.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 43.Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. The oscillating brain: complex and reliable. NeuroImage. 2010;49:1432–45. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu-Feng Z, Yong H, Chao-Zhe Z, Qing-Jiu C, Man-Qiu S, Meng L, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 47.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keener MT, Phillips ML. Neuroimaging in bipolar disorder: a critical review of current findings. Curr Psychiatry Rep. 2007;9:512–20. doi: 10.1007/s11920-007-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. J Affect Disord. 2002;72:209–26. doi: 10.1016/S0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 50.Solé B, Jiménez E, Torrent C, Reinares M, del Bonnin CM, et al. Cognitive impairment in bipolar disorder: treatment and prevention strategies. Int J Neuropsychopharmacol. 2017;20:670–80. doi: 10.1093/ijnp/pyx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93:105–15. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 52.Miola A, Cattarinussi G, Antiga G, Caiolo S, Solmi M, Sambataro F. Difficulties in emotion regulation in bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2022;302:352–60. doi: 10.1016/j.jad.2022.01.102. [DOI] [PubMed] [Google Scholar]
- 53.Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Res. 2011;188:303–9. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 54.Bora E. Neurocognitive features in clinical subgroups of bipolar disorder: a meta-analysis. J Affect Disord. 2018;229:125–34. doi: 10.1016/j.jad.2017.12.057. [DOI] [PubMed] [Google Scholar]
- 55.Depp CA, Mausbach BT, Harmell AL, Savla GN, Bowie CR, Harvey PD, et al. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord. 2012;14:217–26. doi: 10.1111/j.1399-5618.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soraggi-Frez C, Santos FH, Albuquerque PB, Malloy-Diniz LF. Disentangling working memory functioning in mood states of bipolar disorder: a systematic review. Front Psychol. 2017;8. https://www.frontiersin.org/article/10.3389/fpsyg.2017.00574. [DOI] [PMC free article] [PubMed]
- 57.Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: profile and effects of clinical state. Neuropsychology. 2009;23:551–62. doi: 10.1037/a0016277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elliott ML, Knodt AR, Ireland D, Morris ML, Poulton R, Ramrakha S, et al. What Is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychol Sci. 2020;31:792–806. doi: 10.1177/0956797620916786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kragel P, Han X, Kraynak TE, Gianaros P, Wager T. fMRI can be highly reliable, but it depends on what you measure. 2020. 10.31234/osf.io/9eaxk. [DOI] [PMC free article] [PubMed]
- 60.Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–29. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fox M, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ladouceur CD, Peper JS, Crone EA, Dahl RE. White matter development in adolescence: the influence of puberty and implications for affective disorders. Dev Cogn Neurosci. 2012;2:36–54. doi: 10.1016/j.dcn.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dai J, Scherf KS. Puberty and functional brain development in humans: convergence in findings? Dev Cogn Neurosci. 2019;39:100690. doi: 10.1016/j.dcn.2019.100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goddings AL, Beltz A, Peper JS, Crone EA, Braams BR. Understanding the role of puberty in structural and functional development of the adolescent brain. J Res Adolescence. 2019;29:32–53. doi: 10.1111/jora.12408. [DOI] [PubMed] [Google Scholar]
- 65.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–74. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 66.Peper JS, Dahl RE. The teenage brain: surging hormones—brain-behavior interactions during puberty. Curr Dir Psychol Sci. 2013;22:134–9. doi: 10.1177/0963721412473755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 68.McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017;174:676–85. doi: 10.1176/appi.ajp.2017.16040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McTeague LM, Rosenberg BM, Lopez JW, Carreon DM, Huemer J, Jiang Y, et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiatry. 2020;177:411–21. doi: 10.1176/appi.ajp.2019.18111271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Müller VI, Cieslik EC, Serbanescu I, Laird AR, Fox PT, Eickhoff SB. Altered brain activity in unipolar depression revisited: meta-analyses of neuroimaging studies. JAMA Psychiatry. 2017;74:47–55. doi: 10.1001/jamapsychiatry.2016.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jamil T, Ly A, Morey RD, Love J, Marsman M, Wagenmakers EJ. Default “Gunel and Dickey” Bayes factors for contingency tables. Behav Res. 2017;49:638–52. doi: 10.3758/s13428-016-0739-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turkeltaub P, Eickhoff S, Laird A, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bora E, Yücel M, Pantelis C. Neurocognitive markers of psychosis in bipolar disorder: a meta-analytic study. J Affect Disord. 2010;127:1–9. doi: 10.1016/j.jad.2010.02.117. [DOI] [PubMed] [Google Scholar]
- 74.Potash JB. Carving chaos: genetics and the classification of mood and psychotic syndromes. Harv Rev Psychiatry. 2006;14:47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- 75.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. doi: 10.1016/S0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 76.Vul E, Pashler H. Voodoo and circularity errors. NeuroImage. 2012;62:945–8. doi: 10.1016/j.neuroimage.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 77.Rosenberg MS. A generalized formula for converting chi-square tests to effect sizes for meta-analysis. PLOS One. 2010;5:e10059. doi: 10.1371/journal.pone.0010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Routledge; 1988. 567 p.
- 79.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–11. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zovetti N, Rossetti MG, Perlini C, Maggioni E, Bontempi P, Bellani M, et al. Default mode network activity in bipolar disorder. Epidemiol Psychiatr Sci. 2020;29. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7503172/. [DOI] [PMC free article] [PubMed]
- 82.Syan SK, Smith M, Frey BN, Remtulla R, Kapczinski F, Hall GBC, et al. Resting-state functional connectivity in individuals with bipolar disorder during clinical remission: a systematic review. J Psychiatry Neurosci. 2018;43:298–316. doi: 10.1503/jpn.170175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malhi GS, Lagopoulos J, Owen AM, Ivanovski B, Shnier R, Sachdev P. Reduced activation to implicit affect induction in euthymic bipolar patients: An fMRI study. J Affect Disord. 2007;97:109–22. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 84.Wessa M, Houenou J, Paillère-Martinot ML, Berthoz S, Artiges E, Leboyer M, et al. Fronto-striatal overactivation in euthymic bipolar patients during an emotional Go/NoGo task. AJP. 2007;164:638–46. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- 85.Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychological Med. 2004;34:795–802. doi: 10.1017/S0033291704002557. [DOI] [PubMed] [Google Scholar]
- 86.Malhi GS, Lagopoulos J, Sachdev P, Mitchell PB, Ivanovski B, Parker GB. Cognitive generation of affect in hypomania: an fMRI study. Bipolar Disord. 2004;6:271–85. doi: 10.1111/j.1399-5618.2004.00123.x. [DOI] [PubMed] [Google Scholar]
- 87.Uddin LQ, Yeo BTT, Spreng RN. Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 2019;32:926–42. doi: 10.1007/s10548-019-00744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Witt ST, van Ettinger-Veenstra H, Salo T, Riedel MC, Laird AR. What executive function network is that? An image-based meta-analysis of network labels. Brain Topogr. 2021. 10.1007/s10548-021-00847-z. [DOI] [PubMed]
- 89.Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry. 2002;180:313–9. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- 90.Harmer CJ, Clark L, Grayson L, Goodwin GM. Sustained attention deficit in bipolar disorder is not a working memory impairment in disguise. Neuropsychologia. 2002;40:1586–90. doi: 10.1016/S0028-3932(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 91.Clark L, Kempton MJ, Scarnà A, Grasby PM, Goodwin GM. Sustained attention-deficit confirmed in euthymic bipolar disorder but not in first-degree relatives of bipolar patients or euthymic unipolar depression. Biol Psychiatry. 2005;57:183–7. doi: 10.1016/j.biopsych.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 92.Farruggia MC, Laird AR, Mattfeld AT. Common default mode network dysfunction across psychopathologies: a neuroimaging meta-analysis of the n-back working memory paradigm. 2020. https://europepmc.org/article/ppr/ppr111170.
- 93.Marchand WR, Yurgelun‐Todd D. Striatal structure and function in mood disorders: a comprehensive review. Bipolar Disord. 2010;12:764–85. doi: 10.1111/j.1399-5618.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- 94.Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington, VA: American Psychiatric Association; 2013.
- 95.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–78. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 96.Perry A, Roberts G, Mitchell PB, Breakspear M. Connectomics of bipolar disorder: a critical review, and evidence for dynamic instabilities within interoceptive networks. Mol Psychiatry. 2019;24:1296–318. doi: 10.1038/s41380-018-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bi B, Che D, Bai Y. Neural network of bipolar disorder: Toward integration of neuroimaging and neurocircuit-based treatment strategies. Transl Psychiatry. 2022;12:1–12. doi: 10.1038/s41398-022-01917-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Magioncalda P, Martino M. A unified model of the pathophysiology of bipolar disorder. Mol Psychiatry. 2022;27:202–11. doi: 10.1038/s41380-021-01091-4. [DOI] [PubMed] [Google Scholar]
- 99.Volman I, Pringle A, Verhagen L, Browning M, Cowen PJ, Harmer CJ. Lithium modulates striatal reward anticipation and prediction error coding in healthy volunteers. Neuropsychopharmacology. 2021;46:386–93. doi: 10.1038/s41386-020-00895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.