Abstract
Bipolar disorders (BD) represent a severe leading disabling mental condition worldwide characterized by episodic and often progressive mood fluctuations with manic and depressive stages. The biological mechanisms underlying the pathophysiology of BD remain incompletely understood, but it seems that there is a complex picture of genetic and environmental factors implicated. Nowadays, gut microbiota is in the spotlight of new research related to this kind of psychiatric disorder, as it can be consistently related to several pathophysiological events observed in BD. In the context of the so-called microbiota–gut–brain (MGB) axis, it is shown to have a strong influence on host neuromodulation and endocrine functions (i.e., controlling the synthesis of neurotransmitters like serotonin or mediating the activation of the hypothalamic–pituitary–adrenal axis), as well as in modulation of host immune responses, critically regulating intestinal, systemic and brain inflammation (neuroinflammation). The present review aims to elucidate pathophysiological mechanisms derived from the MGB axis disruption and possible therapeutic approaches mainly focusing on gut microbiota in the complex network of BD. Understanding the mechanisms of gut microbiota and its bidirectional communication with the immune and other systems can shed light on the discovery of new therapies for improving the clinical management of these patients. Besides, the effect of psychiatric drugs on gut microbiota currently used in BD patients, together with new therapeutical approaches targeting this ecosystem (dietary patterns, probiotics, prebiotics, and other novelties) will also be contemplated.
Subject terms: Bipolar disorder, Physiology
Introduction
What are bipolar disorders?
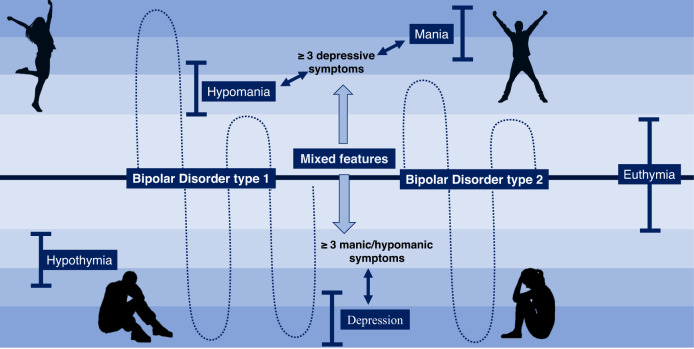
Bipolar disorders (BD) are a group of complex, severe, episodic and often progressive mood disorders considered as one of the leading causes of disability in the world. This cyclic disorder is distinguished by mood fluctuations, combining manic (bipolar mania), hypomanic and depressive phases (bipolar depression) [1]. Although a trimodal age‐at‐onset distribution has been proposed, this disease has typically its onset between adolescence and early adulthood [2]. According to Diagnostic and Statistical Manual of Mental Disorders V (DSM-V) [3] and International Statistical Classification of Diseases and Related Health Problems 11th (ICD-11) [4], BD can be classified into two main categories considering clinical features: BD type 1, when patients experience at least one manic episode and depressive episodes regularly, and BD type 2 when they undergo at least one depressive episode and at least one hypomanic episode and have no history of manic episodes [5]. Likewise, nowadays current clinical guidelines recognize that patients may exhibit episodes with “mixed features”. This term can be used for manic, hypomanic or depressive episodes in bipolar spectrum (type I and II) and major depressive disorders (MDD), characterize by the co-occurrence of three or more manic/hypomanic symptoms in a depressive episode or three or more depressive symptoms in a manic/hypomanic episode [6]. Recent evidence suggest that type 1 and type 2 BD should be considered as different entities. According to some studies, type 1 BD is associated with a more pronounced clinical presentation in both mania and depression [7], whereas type 2 BD cases are characterized by more marked and longer depressions with some hypomania and mixed features but not mania and rarely psychosis [8, 9]. They also had higher socioeconomic and functional status together with high levels of long-term morbidity and suicidal risk, hence denoting that both types are distinct, but not necessarily more or less severe than the others [8]. There is a third type of BD, cataloged as “substance/medication-induced bipolar and related disorder”, when those mood fluctuations are due to substance abuse or therapeutic drugs [1]. Schematically, these fluctuations can be visually understood through Fig. 1.
Fig. 1. Characteristic fluctuations in two main types of bipolar disorder (BD).
In BD type 1, patients experience at least one manic episode and various depressive episodes, whereas in BD type 2 they undergo at least one depressive episode and at least one hypomanic episode and have no history of manic episodes. A patient with type I or type II BD with “mixed features” consist of the co-occurrence either of hypomanic/manic episodes with 3 or more depressive symptoms or depressive episodes with 3 or more hypomanic/manic symptoms.
Epidemiologically, systematic review and meta-analysis found that BD type 1 has a pooled lifetime prevalence of 1.06% and 1.57% for BD type 2 [10], but numbers keep rising. On the other hand, in the last years, large sample studies have been analyzed reporting higher frequency in female patients in both types of BD [11, 12]. These studies argue that there must be sex differences as women have reported to present rapid cycling more often than men, besides the onset in female patients occurs with depressive phases and present more mixed manic presentations Moreover, BD can be accompanied by psychiatric comorbidities such as anxiety or substance abuse and impulse control disorders [13]. Not only BD may have devastating impact on patients but also an important economic burden for healthcare systems, with a range of direct costs per patient and per year oscillating from $881 to $27,617 (plus $1568–$116,062 indirect costs) [14]. On the other hand, this disease also involves high social stigma; one in four patients with BD reports high internalized stigma [15]. In relation to this fact, suicide affects 23–26% of BD patients, which represents about 3.4–14% of all suicide deaths [16], and sex-specific prevalence in this sense has also been observed, being relative suicide attempts higher in bipolar women, although less violent than men [11].
Nowadays, due to the complexity of this psychiatric disorder, there is neither molecular biomarker nor biological sign used for the diagnosis of BD in the clinical routine, so this entity remains a descriptive syndrome whose diagnosis is eminently clinical, based on the already mentioned clinical manuals such as the DSM-V and the ICD [17]. The pathophysiological bases of BD are not fully understood and deepen on the biological mechanisms underlying this intricate malady is an imperative need, as this could open potential translational applications.
What is the pathophysiological basis of bipolar disorders?
To date, the etiopathogenesis of this disorder remains uncompleted and unclear, being plausible the hypothesis about the conjunction of both genetic, environmental and psychosocial factors. The heritability of BD seems to obey a complex non‐Mendelian inheritance [18] and some risk variants have been identified in previous works [19]. Thus, more than genes of major effect, evidence suggest that there are multiple susceptibility loci, each one with small effect. Furthermore, these loci seem to overlap with other psychiatric disorders, specially schizophrenia (SZ) [20]. Regarding environmental factors, compelling evidence support that they may influence the development of many psychiatric disorders, including BD. There is circumstantial evidence that certain environmental factors in pregnancy or in adulthood like viral infections could be associated with the clinical course of BD, although more evidence in this sense is warranted [21]. In the interface between genetics and environmental factors, epigenetics can be considered as the major biological mechanism explaining the etiopathogenesis of many psychiatric disorders [22, 23]. There are different epigenetic mechanisms involved in the regulation of DNA expression, including DNA methylation, histone methylation/acetylation and other modifications, along with non-coding RNAs such as micro RNAs (miRNAs) or long non-coding RNAs (lncRNAs) [24]. In the event of BD, obtained results are more limited than in other psychiatric disorders, although some authors argue that because of the nature of BD and its therapy, the field of epigenetics can be critical for understanding the clinical course of this complex disorder, aiding to define the role of environmental factors, and the possible identification of promising state and traits biomarkers [25].
From a molecular level, alterations in calcium signaling appear to be clearly associated with the development of BD [20, 26]. The intracellular calcium is essential for the modulation of several intracellular signaling cascades and neurotransmitter release [27]. Indeed, neurotransmitter dysregulation strongly underlies the pathophysiology of BD. Several neurotransmitters must be mentioned here, including monoamines (serotonin, dopamine and norepinephrine), glutamate, acetylcholine and gamma-aminobutyric acid (GABA). Importantly, the levels of these neurotransmitters can be different according to the phase of BD (Fig. 2) and can partially explain the cyclic fluctuations observed in these patients [28–35]. The aberrant neurotransmission is associated with several structural and functional changes in the brain and other encephalic structures, as it has been observed that patients with BD display abnormalities in neural circuitries supporting emotion processing, emotion regulation and reward processing [36]. These changes can also be different according to the history of these patients. For instance, image studies have found significant differences in brain activation of psychotic versus non-psychotic BD patients, showing that the former appears to be related to altered activation in left-sided regions whereas the latter exhibited right-sided functional alterations [37]. The brain of patients with BD are also characterized by impaired neurogenesis and neuroplasticity [38, 39]. In this sense, peripheral variations in some critical molecular markers involved in these processes such as brain-derived neurotrophic factor (BDNF) can be applied to understand the significant changes occurred in the brain, showing promising translational applications [40]. Alterations in different neuropeptides such as neuropeptide Y (NPY) and somatostatin have also been observed in patients with BD [41].
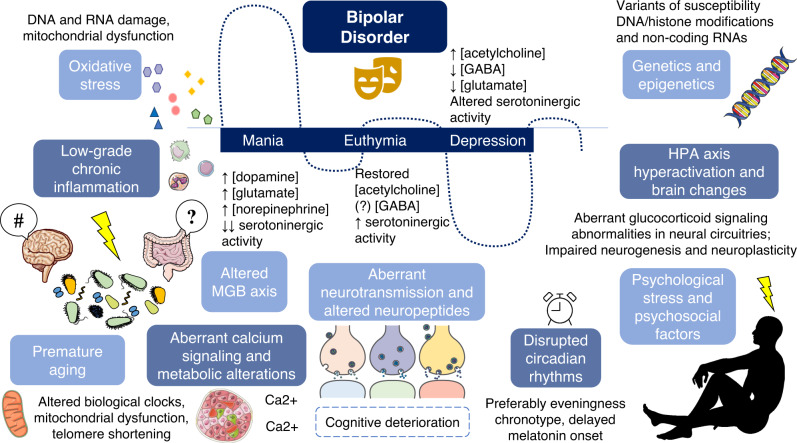
Fig. 2. Network of a wide variety of factors involved in the etiopathogenesis of bipolar affective disorder (BD).
Main factors described are differences in genetics (especially risk variants), epigenetics (DNA and histone modifications, non-coding RNAs), metabolic alterations; aberrant calcium signaling; alterations in circadian rhythms; oxidative stress, characteristic low-grade chronic inflammation, HPA axis hyperactivation, related to psychological stress and different psychosocial factors, accompanied with multiple changes in different neural circuitries, aberrant neurotransmission and altered neuropeptides. Peripheral blood concentrations of neurotransmitters are altered in BD, where in each phase there seems to be a different pattern of activity for every neurotransmissor. These differences are relevant with respect to healthy controls. Many of these events, together with additional mechanisms like altered biological clocks, mitochondrial dysfunction or telomere shortening can be considered as signs of premature aging. Beyond, all these factors influences and are directly influence by the dysregulation of the MGB axis which acts as a central element of psychiatric disorders. HPA hypothalamic–pituitary–adrenal, GABA gamma-aminobutyric acid.
In addition, the role of oxidative stress markers in BD has been explored, as it seems that numerous lines of investigation of BD pathophysiology converge on oxidative stress and aberrations in oxidative energy generation. Although findings are not always steady, an increase in lipid peroxidation and in nitric oxide levels have been reported in BD patients, as well as increased damage in deoxyribonucleotide acid (DNA) and ribonucleic acid (RNA), compared with healthy controls [42]. Similarly, patients with BD exhibit telomere shortening which in turn can be associated with oxidative damage and other pathogenic mechanisms involved in BD [31]. Oxidative stress is also closely linked to mitochondrial dysfunction and despite findings are not consistent, it seems that deleted mitochondrial DNA is a manifestation in postmortem brains of patients with BD, which can be equally related to calcium dysregulation [43]. Even alterations in circadian rhythms seem to be implicated in BD [44], and it is thought that this fact potentiate episodes of mania and depression [45]. The dysregulation of sleep/wake cycle mediated by a delayed melatonin hormone onset reveals in most cases an eveningness chronotype [46] in these patients.
Similarly, hyperactivation of the hypothalamic–pituitary–adrenal (HPA) axis and aberrant glucocorticoid signaling is critically associated with this disorder. Although there is disagreement in affirming it is an etiological factor, what is undeniable is that this is a contributor to the BD clinical presentation and increases the risk of cognitive deterioration [47]. The hyperactivation of the HPA axis is closely related to the response of an individual to different psychosocial stressors. Among some of the most relevant psychosocial factors potentially related to BD, it must be highlighted cognitive dysfunction, altered domains of social cognition (theory of mind, emotion comprehension, empathy), autobiographical memory, temperament and personality factors (i.e. ruminative tendencies and neuroticism), which may drive to several difficulties in familial and social relationships [48]. Furthermore, there is a significant low-grade chronic inflammation reported in patients with BD, with an aberrant immune activation observed in the gut and systemically, which influences HPA axis, gut microbiota, metabolic functions, and the brain, driving to a phenomenon of neuroinflammation [49]. Likewise, dysregulation in the brain and systemic metabolism together with an altered hormonal profile represents another major feature of BD [50, 51].
As shown, the many factors involved in the etiopathogenesis of BD cannot be understood separately, but collectively exert a synergic effect, interacting bidirectionally. Beyond, many of the observed changes occurred in patients with BD can be considered as signs of premature aging (telomere length, oxidative stress, inflammation, disruptions on biological clocks, epigenetic aging and mitochondrial dysfunction), as prior works hypothesize that accelerated aging process is potentially implicated in the development and course of BD [52]. Likewise, another key branch to understand BD pathogenesis is the disruption of the so-called microbiota–gut–brain (MGB) axis [53–55]. Hence, the MGB axis can be considered a part of a great picture that influences and in turn is influenced by the different mechanisms involved in BD pathobiology. Because of that, there is a growing claim to study the gut microbiota and MGB axis as a potential biomarker for BD patients, offering promising clinical and translational opportunities [56]. Overall, a global picture of the plenty biological mechanisms involved in the etiopathogenesis of BD is summarized in Fig. 2.
How is operating the microbiota–gut–brain axis in bipolar disorders?
Gut microbiota typifies a complex ecosystem consisting of trillions of microbes that inhabit the human intestine and which maintain a symbiotic relationship with their host [57]. This ecosystem represents about 2% of our body weight (approximately 1.5 kg) [58]. On the other hand, the available studies are also focused on gut microbiome, which refers not only to the different microbial populations that inhabit in the gut, but also their genome and end-products [59]. Bacteria are the most important component in gut microbiota, but also viruses—especially phages—Fungi, Amoebozoa or Archaea can be found. Among bacteria, Firmicutes, Bacteroides, Proteobacteria and Actinobacteria phyla represent over 90% of the gut microbiota community [60]. The shaping of gut microbiota starts at birth. The composition of gut microbiota differs according to delivery mode, so vaginal birth is preferable to cesarean section, which is linked with gut microbiota dysbiosis and even with subsequent repercussions in immunological and metabolic status [61, 62]. Successively, the gut microbiota is highly sensitive to environmental signals, receiving, integrating and responding to the information not only from the different organs of the body, but also from external influences like diet, physical activity, psychological and physical stress, sleep restrictions, socioeconomic status, drugs, antibiotics, exposure to pets, noise, and temperature [63]. In this sense, gut microbiota signifies a field of growing interest in the understanding of several processes in the organism regulating a plethora of metabolic routes, having a very close interplay with the immune system [60]. Because of the many functions that the gut microbiota fulfills, compelling evidence suggest that gut microbiota dysregulation could be directly associated with low-grade inflammation and a wide range of pathological conditions including obesity and metabolic disorders like type 2 diabetes [64, 65], inflammatory bowel disease (IBD) [66], gastrointestinal cancer [67] and psychiatric disorders, including BD [68].
There are several ways by which MGB axis works. A complex interplay between the gut microbiota, the parasympathetic nervous system—with a prominent role of vagus nerve-immune system and the different cells located in the gut is continuously occurring [69]. These interactions can occur by direct contact or indirectly through the secretion of a plenty of specific products and metabolites which influence the different parts of the MGB axis and the whole organism [70, 71]. The mucus layer of the gut is the scenario where most of the host-microbiota interactions take place. In these interactions, there is a basal and physiological inflammatory environment induced by gut microbiota that allows the regulation of bacterial populations, preventing its spread [71]. In this context, enterocytes communicate through innate immune receptors, chemokines and cytokines. For instance, Toll-like receptors (TLR) enable host innate immune system to identify pathogen-associated molecular patterns (PAMP) of microbes, such as lipopolysaccharide (LPS), lipoproteins or flagellin [72]. The brain receives and integrates signals from the gut directly from the afferent fibers of the vagus nerve [73] or due to the aforementioned products secreted by the different cells to the systemic circulation. In turn, the brain exerts a direct influence on the MGB axis through the efferent fibers of the vague nerve, or indirectly (i.e., by the activation of the HPA axis), denoting the bidirectional interplay occurred in this axis [74].
In this attractive and promising background, the aim of the present work is to study the relationships between BD and gut microbiota in the context of pathophysiology, and how the MGB axis interacts with the multiple systems and mechanisms behind this psychiatric condition. Equally, we will also collect the most relevant insights on the impact of BD pharmacotherapy on the gut microbiota, reviewing promising translational approaches targeting the MGB axis as well.
Microbial ecosystem in patients with bipolar disorders
Gut microbiota composition of BD patients is different from healthy individuals, supporting its possible involvement in the pathogenesis of these complex entities [75]. BD, as many other psychiatric conditions, is related to a reduced microbial diversity and different relative abundance of bacterial phyla compared to controls [68]. Also, alterations in specific microbial populations are reported in these patients. For instance, Coello et al. [75] found that Flavonifractor, a genus linked to oxidative stress inducement, was associated with an odds ratio of 2.9 for having BD, but smoking could act as a potential confounder and more research is needed to confirm these results. Another study has also stated the existence of a potential causality between Betaproteobacteria and BD, associated with alterations in mucosal permeability and intestinal inflammation [76]. Oppositely, Aizawa et al. [77] failed to find any significant difference in the levels of Lactobacillus and Bifidobacterium between BD and healthy subjects; although their count appears to be directly correlated with sleep and serum cortisol levels. Besides, Lu et al. [78] reported that levels of Faecalibacterium prausnitzii, Bacteroides–Prevotella group, Atopobium Cluster, Enterobacter spp. and Clostridium Cluster IV were higher in BD patients than healthy subjects along with a reduced Bifidobacteria to Enterobacteriaceae ratio, having these changes a possible impact on brain function in these patients.
Interestingly, the differences in gut microbiota composition could partially explain clinical manifestations (types and phases) of BD. Notwithstanding there are no studies evaluating the gut microbiota composition in maniac episodes, it seems that the use of probiotics could prevent rehospitalization in patients who had suffered a recent episode of acute mania, thereby suggesting the involvement of gut microbiota in the development of maniac episodes [79]. In contrast, prior works have found that bacterial diversity differs in euthymic versus depressive phases in BD subjects, having this fact an epigenetic impact on the circadian clock gene ARNTL [80]. Despite the sample size being low (N = 32) and these results may be attributed to differences in their dietary patterns, the authors observed that these microbial changes appear to contribute to the pathogenesis of BD. Depressive stages in BD patients manifested in form of melancholia are associated with higher IgA responses to Citrobacter koseri than in healthy or non-melancholic depressed individuals [56]. Similarly, Painold et al. [81] found an inverse correlation between microbial alpha-diversity and illness duration in BD patients with a depressive episode, with increased levels of Coriobacteria and Actinobacteria together with decreased Ruminococcaceae and Faecalibacterium. Despite there is a lack of studies yet, we consider that future works could be directed to study the gut microbiota in episodes with “mixed features”, also focusing on the study of hypomanic/manic phases in order to establish and understand differences occurred in each phase (Fig. 3).
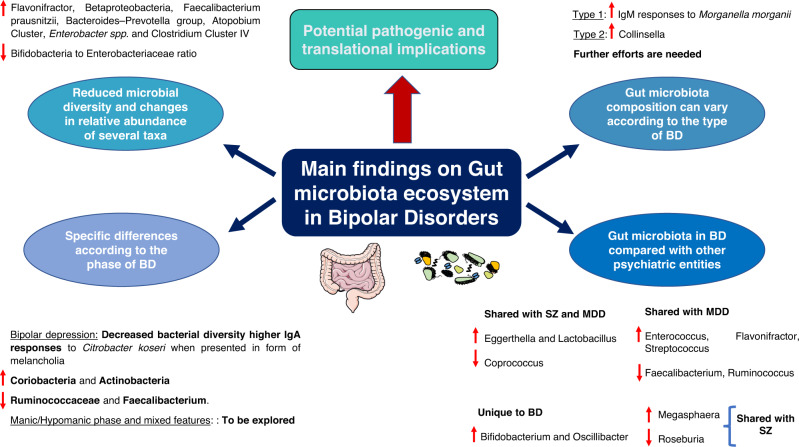
Fig. 3. Main findings on gut microbiota ecosystem in bipolar disorders.
As shown, BD patients are characterized by presenting a reduced microbial diversity and changes in relative abundance of several taxa, which may be involved in the pathogenesis of this complex disorder. Besides, specific differences according to the type and clinical manifestation, although further efforts are needed in hypomanic/manic phases, mixed features and also in the comparison between type 1 and 2 BD. Finally, compelling evidence is also supporting the notion that the gut microbiota can present some similarities and disparities across psychiatric disorders, opening the use of gut microbiota as potential biomarkers.
On the other hand, there are also some studies evaluating the gut microbiota composition in relation to the type of BD. Interestingly, there are works focused on comparing gut microbiota composition in patients with MDD, type I and type II BD. Notwithstanding there are some similarities in the MGB axis disruption in these three groups in comparison to healthy subjects, it seems that these aberrations are greater in type I BD patients and melancholia [56]. Indeed, individuals with type I BD showed higher IgM responses to Morganella morganii than patients with MDD and type II BD. Similarly, McIntyre et al. [82] reported that patients with type II BD exhibit a greater abundance of Collinsella in comparison to those with type I, although they noticed about the small sample size and insufficient control for some potential moderating factors like medication. More studies could be aimed to find if there is a differential gut microbiota profile in patients with type 1 versus type 2 BD (Fig. 3).
Also, some researchers have found in gut microbiota a promising point to compare and distinguish between BD and other psychiatric disorders. For instance, it seems that Prevotella 2 and Ruminococcaceae UCG-002 are more prevalent in patients with MDD than BD patients [83]. Likewise, compared with healthy individuals, MDD is associated with alterations in Bacteroidaceae family, whereas disturbances in Lachnospiraceae, Prevotellaceae, and Ruminococcaceae families are related to BD [84]. Furthermore, the abundance of Fusobacteriaceae, Escherichia blattae DSM 4481 and Klebsiella oxytoca were significantly augmented, whereas the Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 was significantly reduced in BD group compared with MDD group [85]. Besides, a very recent systematic review compared the gut microbiota in BD, MDD and SZ [86]. Interestingly, they observe that an increase in Eggerthella and Lactobacillus, together with a decrease in Coprococcus was common for the three conditions in comparison to controls. Likewise, MDD and SZ shared higher levels of Escherichia/Shigella and Veillonella whereas increased Megasphaera and lower Roseburia are common for BD and SZ. MDD and BD had more commonalities, including higher Enterococcus, Flavonifractor, and Streptococcus, and lower Faecalibacterium and Ruminococcus. They reported that each mental disorder could be potentially characterized by a different microbial profile: MDD was often characterized by higher Alistipes and Parabacteroides and lower Prevotella; BD by higher Bifidobacterium and Oscillibacter; and SZ by higher Prevotella and lower Bacteroides, Haemophilus, and Streptococcus [86]. Other studies have also found that BD and SZ are characterized by higher serum antibody levels to fungal pathogens Saccharomyces cerevisiae and Candida albicans and how these changes could be related to cognitive performance or the onset of psychotic symptoms [87].
Collectively, as shown in Fig. 3, these studies support the notion that there is an altered gut microbiota profile in patients with BD when compared to healthy subjects, and that changes observed in gut microbiota can correlate with the clinical manifestations, including both types and phases. Besides, by the analysis of gut microbiota in different psychiatric disorders the discovery of potential biomarkers that aid in the clinical diagnosis, prognosis or therapy prediction can also be opened. Due to the promising implications related to this field, we encourage further and more precise works to evaluate and study the gut microbiota in BD and other mental disorders.
Microbiota–gut–brain axis in the context of bipolar disorder pathophysiology
To simplify, the pathophysiological mechanisms by which the MGB axis seems to influence the development of BD are (1) through modulating the enteric and central nervous system (CNS), with a focus on its immunomodulatory actions while influencing the intestinal, systemic and brain inflammation; and (2) because of the production of microbial metabolites with pleiotropic actions. In the following section, we will focus on the pathophysiological role of gut microbiota in BD patients.
Gut and immune dysfunction in bipolar disorder
Intestinal epithelium disruption
The intestinal barrier constitutes the interface between the gut lumen and blood torrent, being crucial for the preservation of homeostasis. The gut harbors its own immune system, which is called intestine and gut-associated lymphoid tissue (GALT), key for whole body immune function. In physiological conditions, GALT should allow a tolerance of commensal bacteria and dietary antigens and act as a primary line of defense against luminal antigens and harmful substances in the host [88].
Intestinal barrier has varying degrees of permeability throughout the gastrointestinal tract. Zonulin modulates intercellular tight junctions and increases intestinal permeability in jejunum and ileum [89]. The increment of serum zonulin levels may be related to higher susceptibility for depression induced by stimuli [90], and zonulin and claudin-5 have seen increased in patients with BD [91]. Evidence has even found that levels of claudin-5 are associated with an earlier onset of BD, while reduced levels of this protein is associated with an extended duration of BD [92]. Prior studies suggest that there is an association between stress from early adverse life events and the development of irritable bowel syndrome (IBS), a functional disorder associated with a higher intestinal permeability [93, 94]. Increases in intestinal permeability have also been associated with autoimmunity diseases, inflammatory bowel disease (IBD) or celiac disease [95, 96]. Similarly, emerging evidence indicates that patients with mental disorders seem to present alterations of the gut microbiota and increased intestinal permeability [97, 98]. Specifically, IBS [99], celiac disease [100] and IBD [101, 102] have been associated with an increment in the risk of developing BD. In fact, both incidence and prevalence of psychiatric disorders (including BD) are higher in IBD patients [103].
Bacterial translocation
Gut microbiota can influence enterocyte junctions and therefore intestinal permeability [104]. When symbiotic relationship between gut microbiota and host is interrupted, dysbiosis leads to dysfunctions in mucosal barrier function, translocation of commensal microbes and chronic proinflammatory states [105, 106]. This disruption may have consequences CNS and immune response, potentially resulting in systemic disease [59, 107]. But, what is more; rising evidence supports that MGB axis establishes a bidirectional connection, so that central injury not only disrupts CNS, but also causes a gastrointestinal injury [108]. Furthermore, it has been well described that depression and other psychiatric disorders in which stress plays a key role, show alterations in gut microbiota, what consequently underpins translocation of bacterial products and triggers HPA axis activation [109]. Indeed, in BD bacterial translocation signs can be found. For instance, anti-Saccharomyces cerevisiae antibodies are higher in BD patients than in healthy controls, although these antibodies did not show to have an association with symptom severity or pharmacological treatment [110]. Candida albicans exposure has been associated with BD, especially in males and patients with somatic conditions [111].
An analyzable marker of bacterial translocation is the soluble cluster of differentiation (CD)-14; its levels are higher in BD patients compared with healthy individuals [112]. An association between soluble CD14 and anti-tissue transglutaminase IgG has also been described [112]. BD patients have increased levels of IgG antibodies to gliadin, especially during mania, compared to controls [113]. Further to this, the persistence of elevated antibodies to gliadin in patients with mania showed an association with rehospitalization rates in a 6-month follow-up [113]. Hence, bacterial translocation might be related to the clinical switch observed in these patients, although further studies are required in this field.
Moreover, microbial components can affect the intestinal barrier through diverse mechanisms. For instance, LPS is an endotoxin located in the outer membranes of numerous Gram-negative bacteria. This normally does not penetrate paracellular junctions because of its size, so that systems such as lipid rafts or clathrin-dependent mechanisms are necessary for LPS to penetrate into the cell [114]. Independently, when intestinal barrier is compromised, there is an increase in endotoxin in the blood torrent [114]. When LPS penetrates, the endotoxemia stimulates innate immune response, triggering systemic inflammation and aggravating neuroinflammation [115]. In this way, LPS levels can be used as a marker of bacterial translocation, presumably indicating that there is a weakened intestinal barrier, which is related to chronic systemic inflammation and insulin resistance [116, 117].
On the contrary, there are certain microbial products that modulate positively intestinal barrier function, besides, stimulating regulatory T CD4 cells (Tregs) and preserving intestinal epithelial lymphocytes [118]. These metabolites are short-chain fatty acids (SCFAs) among others and seem to be altered in BD as will be later discussed.
Intestinal inflammation and consequent systemic inflammation
Cytokines and chemokines are crucial for intercellular communication and contribute to maintain intestinal homeostasis through inflammatory mechanisms, but when these are constantly elevated, epithelial barrier integrity results compromised [119]. Proinflammatory cytokines such as Tumor Necrosis Factor (TNF)-α, interleukin (IL)-1β or IL-18 induce the endocytosis of epithelial apical junctional proteins [120], promoting an increment in intestinal permeability [121]. IL-1β is a key player in inducing intestinal inflammation; it increases epithelial tight junctions permeability, disrupting this barrier thorough the canonical NF-κB pathway [122]. Also, IL-18 can damage intestinal barrier, as it has been involved in tight junction modulation, mucosal apoptosis [123] and in inhibiting goblet cell maturation [124].
Altered levels in some of these cytokines have been observed in patients with BD, although more studies are needed in order to establish this relationship with intestinal permeability. Meanwhile, studies with varying results can be found in literature. Concretely, in a study with rapid cycling BD patients, IL-6 and IL-18 have been suggested as markers of manic episodes, due to significant levels in manic/hypomanic stages; however, IL-1 β was almost undetectable in plasma samples [125]. In another study, TNF-α, IL-6 and IL-18 were measured in serum from BD patients in manic, depressive and mixed state. The results concluded that TNF-α and IL-6 serum levels were significantly higher in manic, depressive and mixed-state patients compared with controls, but IL-18 was significantly higher only in depressive states. Nevertheless, it was alleged that confounding factors cannot confirm precisely the roles of these cytokines in the psychopathology of BD [126].
In general terms, systematic reviews of limited evidence affirm that it seems that TNF superfamily and proinflammatory cytokines contribute to the neuroprogression of the disease [127]. Moreover, what is known so far is that the abnormal immune response influence all stages of the disease, and potentially explains the elevated rates of comorbid inflammatory diseases found in these patients [128]. In fact, alterations can also be found in euthymia, when patients show to have greater levels of IL-8 in cerebrospinal fluid and monocyte chemoattractant proteins [129]. In patients with BD, stress-related neuroendocrine responses seem to be diminished while there is an increase in immune activation, related to incapability in reducing NF-κB and MAPK signaling [130].
Furthermore, during manic and depressive states, an elevated inflammatory signaling can be found; phospholipase A2 activity increases -liberating more arachidonic acid-, as well as levels of C-reactive protein, compliment and proinflammatory cytokines, compared to healthy individuals [131]. These alterations are attenuated in euthymia and with pharmacological treatment [131]. In relation to this, IL-6 in cerebrospinal fluid is significantly higher in patients with suicide attempts than in healthy controls, especially in violent attempts [132]. IL-13 levels have also been seen higher in euthymia and in mania [133]. Other findings also indicate that IL-6 and CRP levels are significantly higher in unipolar mania than in BD [134]. All these studies denote that BD is a group of complex mood disorders in which not only MGB axis is the central element. Evidence in recent years is demonstrating that elevated levels of peripheral proinflammatory signals are observed during all phases of BD, and that patients with autoimmune diseases present an increased risk of BD diagnosis.
Both innate and adaptive immunity play a prominent role in pathophysiology. Concretely, T helper-1 cells (Th1), which are IL-2, IL-6 and TNF-α producers, are hyperactivated in patients with BD; and also, a hyperactivation of Th2 has been associated with BD although more studies are required to elucidate clear links [135]. Also, some studies have exposed differences with MDD regarding immune cell populations. For instance, circulating levels of several subtypes of CD4+ Th cells, are higher in BD than in MDD including Th1, Th2, Th17 and Tregs [136]. However, there are contradictory results in other studies, as Treg populations are observed to decrease, but Th1 expansion is maintained as well as CD8+ cytotoxic T cell expansion [137]. All in all, intestinal integrity disruption and local and systemic inflammation go together with gut dysbiosis. The ecosystem harbored inside the intestine results damaged and, consequently, this increases inflammation (by bacterial components as described) and neuroinflammation, by neuroactive compounds (even from microbial metabolism) and other neuromodulatory mechanisms, as explained below.
Neuroinflammation and hypothalamic–pituitary–adrenal axis
Neuroinflammation consists of an inflammatory process within the brain or spinal cord, mediated by cytokines, chemokines, free radicals and other active ligands. Cytokines involvement in neurodegeneration has been discussed although it is not widely explained. A link between the elevated cytokines levels in CNS and in peripheral blood has been observed in postmortem studies of BD patients [138].
Low-grade systemic inflammation driven by several mediators previously debated, such as LPS, bacterial translocation and the increased proinflammatory cytokines and Th1 response, are all contributing to an immunological response in the brain [139]. A blood–brain barrier (BBB) dysfunction associated with BD is observed, also linked to gut microbiota, substance abuse and insulin resistance, all altering neuronal plasticity [140, 141]. BBB functions as a selective barrier that regulates the transport of molecules between blood and CNS, keeping necessities of nutrients and neurotransmitters in the brain. The dysregulation at this level enhances the activation of glial cells. Neuroinflammatory mediators are produced by these glial cells (microglia and astrocytes), besides endothelial and peripheral immune cells, all located in CNS [142]. Microglial hyperactivation induces damage to oligodendrocytes, affecting negatively myelination and neural circuits [143].
These disturbances would explain the findings in neuroimaging studies about white matter abnormalities in BD [144]. The inflammatory environment created by the release of cytokines by microglia in stressful situations seems to have a repercussion in behavioral phenotype in stress models, which is linked to neuropsychiatric disorders [145]. What is known so far is that cytokines-mediated neuroinflammation leads to dysregulations in the HPA axis, where the central regulator is cortisol releasing factor (CRF). Its hyperactivation produces an excess of cortisol, inducing depression-like behavior. This mechanism has been deeply studied in other psychiatric disorders like MDD [146]. As previously commented, this is consistent with the fact that so many patients with inflammatory-based diseases develop anxiety, MDD or BD [135]. Recent studies have found in BD a certain correlation between HPA axis dysfunction and the increased risk of relapses and cognitive impairments [47]. There is a growing body of evidence that suggest that dysfunctional microglia, oxidative damage, and mitochondrial dysfunction can play compelling roles in BD etiopathogenesis. Thus, an overactive neuroinflammatory response of astrocytes and microglia can contribute to BD development by alterations in immune response that leads to an increase in not only BBB integrity but also in intestinal permeability [147]. In fact, zonulin and claudin-5 have been proposed as biomarkers to detect BBB disruption [140] now that a diminished BBB integrity has been observed in patients with intestinal inflammation [148].
Although glucose is the principal energy fuel of the brain in normal conditions, lactate can be an alternative source during hypoglycemia and because of mitochondrial shifts in redox state or ischemia. Compared to healthy controls, higher levels of lactate from glycolysis in BD have been reported, which can relate to metabolic dysfunctions and a decrease in oxidative phosphorylation [149].
Abnormalities in mitochondria signaling lead to the production of free radicals like reactive oxygen species (ROS), which causes DNA damage, affecting neural plasticity and behavior [150]. ROS are produced in the brain mostly by microglia [32], and these products appear to be raised in patients with BD, which leads to oxidative and nitrosative damage. Indeed, dopamine, which is higher during mania, increases ROS, and it seems that, during mania and depression, there is a compensatory increase in antioxidant marker levels [151]. Furthermore, oxidative stress is associated with lower brain-derived neurotrophic factor (BDNF) levels [152]. This factor is crucial for the development, differentiation, plasticity, and survival of neurons, and it is decreased during mania and depression, but recovers at normal level in euthymic patients [32].
Sleep disruption may also aggravate this immune activation and neuroinflammation. Recent discoveries in animal models have demonstrated that some components from circadian system are mediators of microglial activation and neuroinflammation [153]. Mechanisms studied allege that immune activity and brain function are subjected to circadian clock machinery [154]. Moreover, sleep hygiene depends on the dynamics of the longest nerve in the autonomic nervous system, the vagus nerve—which controls the viscera—and also cerebral blood flow [155]. Chronic sleep disturbances have also been reported in BD. It is known that dysregulations in the circadian system can weaken cell antioxidant mechanisms, favoring an increased oxidative stress and lipid peroxidation, as there is a maintained damage from oxidative and nitrosative stress and higher levels of proinflammatory cytokines [156]. A consequence of this disruption resides in the abnormality of neurotransmitter release, related to HPA axis [157]. At the same time, stressful life events in patients with BD have been reported to be higher than in healthy people. These events affect vulnerability, onset and recurrence of BD, and increase cortisol levels [158], then following the process herein described in inverse direction. Figure 4 intends to summarize all these ideas.
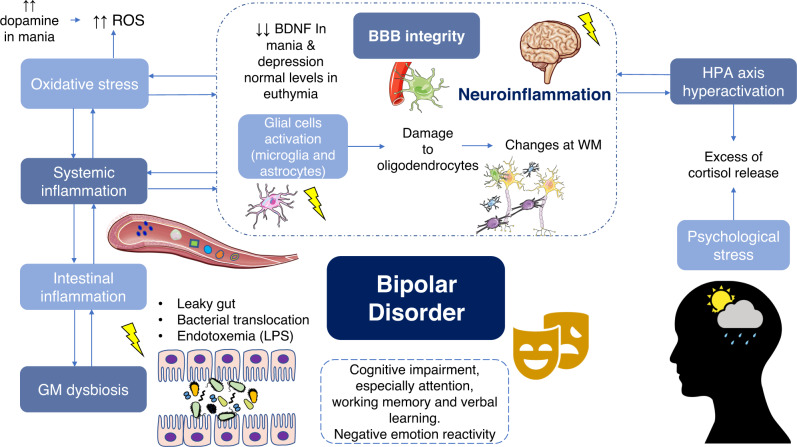
Fig. 4. Factors involved in neuroinflammation in the context of bipolar disorder physiopathology.
Leaky gut promotes bacterial translocation and consequent endotoxemia, what is a major contributor of inflammation, locally (intestinal) and systemically. Oxidative stress is also represented, and it seems to be even increased in certain phases; particularly, in mania, an increase in dopamine causes higher ROS production. Besides HPA axis hyperactivation is related to the aberrant control release in addition to psychological stress. Chronic inflammation causes impairment at BBB integrity, and then glial activation (microglia and astrocytes) is triggered. These mechanisms cause damage to oligodendrocytes, which causes imbalance at myelinization and therefore changes at WM. This cascade of events causes the so-called neuroinflammation, which promotes clinical manifestations of BD like cognitive impairment and negative emotion reactivity. ROS reactive oxygen species, BDNF brain-derived neurotrophic factor, BBB blood–brain barrier, WM white matter, HPA hypothalamic–pituitary–adrenal, GM gut microbiota, LPS lipopolysaccharide.
Particularly, numerous BD patients manifest HPA axis hyperactivity in whole circadian rhythm, especially during mania [47]. Childhood trauma has been demonstrated to have a crucial role in HPA axis alterations in BD patients [159]. These patients showed to have increased levels of cortisol as well as lower glucocorticoid receptor’s function and alterations in glucocorticoid signaling. The hyperactivity of HPA axis is not the etiological factor that triggers BD, but undoubtable, it contributes to modify clinical presentations of BD and influence the genetic and environmental basic interaction in BD etiopathogenesis [47]. However, in patients with BD, neuropsychological performance is closely related to cortisol [160], and higher levels of this hormone have been related to poorer performance in some cognitive tasks [161, 162], especially working memory [163]. Moreover, abnormalities in this axis and in cortisol levels can be related to higher cardiovascular risk [164, 165], which may explain the increase in the mortality rate and in the cardiovascular risk factors prevalence observed in affective disorders, including BD [166].
Gut microbiota has bidirectional communication with HPA axis: certain microbial metabolites can attenuate it whereas translocated microbial antigens and exerted cytokines or prostaglandins productions activate it [95]. Moreover, dysbiosis status is associated with a constant increase in corticosterone synthesis in ileum, and, consequently, to glucocorticoid and insulin resistance, hyperglycemia and dyslipidemia [167]. This hypercortisolism is also associated with reduction in the immune response [97]. Then, HPA axis has an important impact on gut microbiota composition and neuroenteroendocrine functions, increasing gastrointestinal permeability, and contributing to the chronic low-grade inflammation [168]. Gut microbiota is not only a key player in regulating intestinal permeability, inflammation and neuroinflammation, but also in modulating behavior acting as an endocrine organ and modulating neural pathways through MGB axis as commented below.
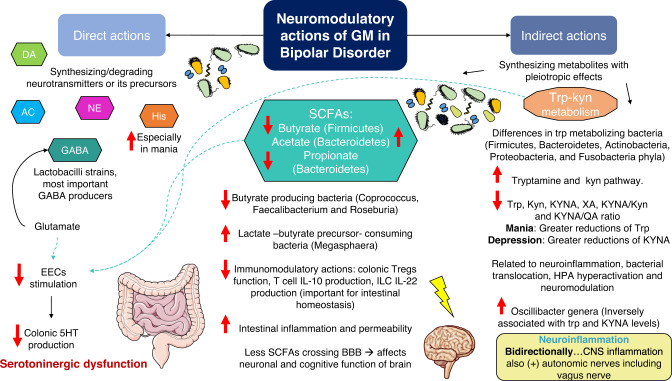
Neuromodulatory actions of gut microbiota in bipolar disorder
Gut microbiota can have important neuromodulatory activities either by direct actions (synthesizing or degrading neurotransmitters) or indirectly through the production of different microbial metabolites, which exert a plethora of systemic actions [169]. Besides, enteric and CNS can also influence neuromodulation actions of gut microbiota. In this section we will summarize the neuromodulatory implications of gut microbiota in patients with BD, focusing on its direct actions on neurotransmission and the main microbial metabolites (SCFAs, tryptophan metabolites).
Direct actions of gut microbiota on neurotransmission in bipolar disorder
As previously described, neurotransmitter dysregulation is a major pathophysiological signature of BD. Especially, GABA, glutamate, serotonin, dopamine, norepinephrine and acetylcholine appears to be importantly dysregulated in these patients [31, 170]. Gut microbiota is a central modulator of several neurotransmitters, being able to synthesize them and their precursors as well as promoting their degradation or metabolization to other products [171]. There are some species of bacteria grown in culture that can produce serotonin, although it seems that the most prominent role of gut microbiota in serotonin synthesis is achieved through the stimulation of enteroendocrine cells (EECs), especially by spore-forming bacteria [172]. The interplay between gut microbiota and EECs mostly occurs through different microbial metabolites, with a central role of SCFAs and tryptophan, as it will be later discussed.
Glutamate and GABA can be obtained from dietary sources or produced by the gut microbiota. Several bacteria are able of producing glutamate including Coryneform and lactic acid bacteria (LAB). Interestingly, glutamate also exert important modulatory effects on EECs, sending sensory signals to the vagus nerve [173] GABA is biosynthesized from glutamate due to the action of the enzyme glutamate decarboxylase, which is found in eukaryotic cells and in a broad spectrum of bacterial species [174]. However, LAB and more specifically certain Lactobacilli strains are the most important GABA producers in the gut [175]. In this sense, one study explored the possible role of Lactobacillus and Bifidobacterium in patients with BD. Interestingly, they show that notwithstanding the counts of Bifidobacterium or Lactobacillus were not significantly varied in patients with BD, it seems to exist a negative correlation between Lactobacillus and Bifidobacterium counts with sleep and cortisol levels respectively, being both important mechanisms in BD pathophysiology [77].
A broader number of bacteria have been reported to be able to produce other monoamines like dopamine and norepinephrine whereas only Lactobacillus plantarumseems to take part in the synthesis of acetylcholine [176, 177]. Despite the role of gut microbiota in the modulation of dopamine and norepinephrine remains to be fully understood, germ-free (GF) animal models have shown that there is a noteworthy alteration of these neurotransmitters in the gut as well as in the brain, which could have important consequences in the behavior [178].
Last but not least, nearly 117 types of bacteria have been identified as major histamine producers [179]. Apart from its well-known immunomodulatory role, histamine can act as a neurotransmitter in the brain, acting as a critical modulator of other neurotransmitters, and influencing arousal, motivation, and energy balance [180]. Neurons located in the posterior hypothalamus projects to all the major regions of the CNS, also participating in the regulation of sleep-wakefulness [181]. Likewise, the central histamine system in the brain is mediated via G-protein-coupled H1-H4 receptors and also appears to be involved in additional functions like arousal, control of pituitary hormone secretion, suppression of eating and cognitive functions [182]. Currently, there are some hypotheses supporting that patients with BD may have an altered histaminergic system, with upregulated histamine levels in manic phases and downregulated in depression, being their fluctuations closely related to other pathophysiological mechanisms [183]. An upregulated histamine production in the brain seems to drive to sleep-wake alterations in vivo, being associated with behavioral and metabolic disorders similar to those caused by voluntary sleep restriction in humans [184]. Conversely, animal models have also established that low levels of histamine-induced depression-like behavior, decreased locomotor activity in the home cage, and impaired aversive memory, leading to a decrease in wakefulness as well [185]. Hence, it is likely that the different alterations reported in the gut microbiota of patients with BD can be partially involved in the differential production of histamine and in turn this may be related to the described effects. Similarly, histamine production from gut microbiota appears to provide anti-inflammatory effects by suppressing TNF-α [186]. Thus, compelling evidence suggest the relevance of gut microbiota as pivotal player of brain function, influencing in the levels of several neurotransmitters and neuromodulators [187].
Short-chain fatty acids
SCFAs are end-products of saccharolytic fermentation of indigestible carbohydrates from our diet [188]. Also, proteins and peptides can be metabolized in the cecum and colon [189], as protein fermentation lead to branched SCFAs production [188]. The concentration of SCFAs is higher in cecum and proximal colon, and their levels decrease toward the distal colon [189]. The most important SCFAs are butyrate, propionate, and acetate, accounting approximately for 80% of the total, although the production of others like formate and valerate should also be highlighted [188–190]. Acetate and propionate are mainly produced by members of Bacteroidetes whereas butyrate is mainly synthesized by Firmicutes. Furthermore, butyrate can also be synthesized by the gut microbiota from acetate and lactate, although the last is not considered a SCFAs [191]. Notwithstanding mostly SCFAs uptake, signaling and functions occurring in the gut, associative studies show that SCFAs have an important role in human metabolism, the immune system and the entire organism, being able to cross the BBB [192, 193]. Critically, SCFAs are master epigenetic regulators, acting as inhibitors of the enzyme histone deacetylase (HDAC), which is involved in the histone modification [194, 195]. Thus, SCFAs are pleiotropic components produced by the gut microbiota involved in multiple processes of health and disease.
In this great context, butyrate is the most widely studied SCFA. Patients with BD show reductions in butyrate-producing bacteria which, being a central feature of MGB axis disruption [68]. In this sense, lower levels of Coprococcus, Faecalibacterium and Roseburia have been reported, while there seems to be an increase in bacteria populations that consume lactate, such as Megasphaera [86]. A recent systematic review has shown that despite the need for further studies, changes in butyrate-producer bacteria like Faecalibacterium may characterize BD in both a trait and state-dependent fashion [196]. In the gut, colonocytes consume butyrate locally, and this SCFA is crucial for maintaining the intestinal barrier [197]. SCFAs also stimulate enteroendocrine cells (EECs), promoting serotonin production in the colon [198]. This augmented production of serotonin leads to enhanced levels of serotonin in systemic circulation and in the brain, also stimulating vagal pathways and similar events occur with GABA, glucagon-like peptide 1 (GLP1) and peptide YY (PYY) [190].
Besides, immune cells have a high expression of SCFAs receptors, and it seems that SCFAs can regulate the function of colonic Treg cells [189, 199]. Despite butyrate and propionate seem to have the greatest immunomodulatory properties, all SCFAs can regulate the immune response [200]. Mostly, SCFAs exert anti-inflammatory properties. They induce the production of IL-10, closely related to intestinal homeostasis [201], and the production of IL-22 by innate lymphoid cells (ILC) requires the presence of gut microbiota IL-22 is not only crucial in intestinal homeostasis and mucosal barrier function, but also in host defense against infections [202]. Interestingly, most of the pharmacological treatment used in BD showed to increase IL-22, so the efficacy of these drugs may also be in relation with changes in this immune altered response [203]. Thus, reduced levels of butyrate and other SCFAs in patients with BD can have important consequences in the intestinal inflammation and permeability.
Moreover, SCFAs also have direct effects on the brain. For instance, SCFAs are associated with improved neuronal and cognitive function, BBB integrity and decreased neuroinflammation in the brain, representing a critical mechanism of interplay between gut microbiota and glial cells [190, 204]. Hence, despite more investigation is needed, it is conceivable that SCFAs can play a key role in the neural alterations observed in psychiatric disorders. The role of SCFAs in BD symptomatology has not been studied yet. However, a recent work analyzed SCFAs in fecal samples from 125 patients with psychiatric disorders (including 23 with BD). Interestingly, they found that self-reported depressive symptoms were positively associated with fecal acetate concentrations and negatively associated with butyrate and propionate levels [205]. Furthermore, there are in vivo studies showing that SCFAs can alleviate repeated psychosocial stress alterations, exerting antidepressant and anxiolytic effects [206]. Moreover, the therapeutic use of butyrate has been demonstrated to revert manic-like behavior in rats and regulates the antioxidant enzyme activities, protecting the brain against oxidative damage [207] therefore supporting the important role that SCFAs may play in the brain alterations in patients with BD.
Overall, there are plenty of potential effects of SCFAs in patients with BD, although more studies are required to unleash the microbial populations involved in this dysregulation. Furthermore, studying local and systemic levels of SCFAs would be of great aid to understand the pathophysiological basis of MGB axis disruption in BD, especially focusing on its different phases and types.
Tryptophan-kynurenine metabolism
Tryptophan is an essential amino acid with multiple metabolic fates and crucial in cell danger response, being again gut microbiota a modulator of its metabolic pathways [208]. Indeed, differences in tryptophan metabolizing bacterial pathways can be found in patients with neurological diseases [209] and psychiatric disorders including BD [210].
Gut microbiota can play a pivotal role in the metabolism of tryptophan. Five bacterial phyla including Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Fusobacteria can participate in tryptophan metabolism, being those belonging to the Clostridium, Burkholderia, Streptomyces, Pseudomonas or Bacillus genera the most important modulators [209]. First, via hydroxylated pathway tryptophan is the precursor for serotonin or melatonin [176]. Thus, a large amount of serotonin is produced by enterochromaffin cells of the gastrointestinal tract [58, 208] and it is also present in enteric nerves [208]. Although tryptophan can cross the BBB, serotonin produced in the gut cannot [211]. However, serotonin can affect both vagus nerve and BBB permeability [69], and it modulates intestinal inflammation [212] and numerous physiological processes, including gastrointestinal secretion and peristalsis, vasoconstriction, behavior, and other neurological functions [177]. Besides, it can indirectly affect central serotoninergic pathways by modulating tryptophan and tryptamine availability. Thus, gut microbiota can play a central role in the serotoninergic dysfunction observed in patients with psychiatric disorders, although further studies are required.
The role of tryptamine in the brain is not well understood, although it is hypothesized that they may cross the BBB and exert important neurotransmitter or neuromodulatory actions [213]. Besides, increased tryptamine production can be associated with decreased circulating tryptophan and serotonin synthesis in the brain and could represent one mechanism by which tryptamine influence behavior [214]. Furthermore, tryptophan can be metabolized by gut microbiota to form indole and their derivates including indole-3-aldehyde, indole-3-acetic-acid and indole-3-propionic acid [176]. The role of tryptamine, indole and their derivate has been suggested in different psychiatric disorders, although little is known about their possible implication in BD [215].
On the other hand, tryptophan can be metabolized via the indoleamine 2,3-dioxygenase (IDO) [58, 176] or through the enzyme tryptophan-2,3-dioxygenase (TDO) to form N-formylkynurenine (Kyn) [216]. This is known as the Kyn pathway, and accounts for ~95% of dietary tryptophan degradation [217]. The indoles and their derivates can also be transformed into Kyn [58, 218]. Many components of the Kyn pathway are neuroactive, and influence neuroplasticity and/or exert neurotoxic effects; and this pathway is modulated by, and in turn modulates many other systems that are commonly disrupted in psychiatric disorders, including immune, endocrine, metabolic, and hormonal systems [219]. In a simple manner, Kyn can be metabolized either to kynurenic acid (KYNA), with neuroprotective properties, or to the neurotoxic components quinolinic acid (QA) and 3-hydroxykynurenine (3-HK) [208]. KYNA acts as an NMDA receptor antagonist or through the inhibition of the α7 nicotinic acetylcholine (α7nACh) receptors whereas QA acts as an agonist toward the NMDA receptors, thus promoting excitotoxic neuronal damage [220]. Regarding 3-HK, this molecule seems to exert a dual role in the CNS. On the one hand, it appears to induce oxidative damage and cell death, and high levels of 3-HK are associated with several psychiatric disorders. In contrast, some experimental studies have provided evidence of antioxidant and scavenging properties inherent to this component [221]. Howsoever, the synthesis of 3-HK from Kyn by the flavoprotein kynurenine-3-monoxygenase (KMO) is another critical point in the Kyn pathway, determining the balance between the neurotoxic component 3-HK and the neuroprotective KYNA [216]. Besides, it seems that Kyn and its downstream products exert pivotal immunomodulatory actions in the brain [222]. Thus, alterations in the Kyn pathway, with an augmentation of neurotoxic components like QA at the expense of neuroprotective derivates like KYNA, seem to have a central role in neuroinflammation in many neuropsychiatric disorders [223]. Likewise, systemic inflammation and glucocorticoids can also cross the BBB and influence the formation of QA in the brain by the microglia and infiltrated macrophages, enhancing the neuroinflammatory cascade [224]. Moreover, Kyn pathway may critically influence glutamate neurotransmission [225]. Thus, the gut microbiota can be a major modulator of Kyn pathway in different mental disorders, impairing tryptophan metabolism and influencing the brain and systemic inflammation [226]. Indeed, Kyn pathway seems to be abnormally activated in BD patients [227, 228]. Significant changes in different molecules of the Kyn pathway in BD patients have been described. For instance, meta-analysis on the peripheral blood levels of these components demonstrates that individuals with BD present lower peripheral blood levels of tryptophan, Kyn, KYNA, xanthurenic acid (a component derived from 3-HK), KYNA/Kyn and KYNA/QA ratio [229]. Besides, it seems that individuals with manic episode showed the greatest reductions in tryptophan levels whereas KYNA levels were more notably reduced among individuals in the depressive phase [229]. Other meta-analyses, however, despite showing that patients with BD present lower peripheral levels of tryptophan, Kyn or KYNA, did not find any significant differences between manic and depressed phases [230]. Intriguingly, it seems that this shift in the tryptophan metabolism from serotonin to the kyn pathway is associated with BD, MDD and SZ, but only in mood disorders (BD and MDD) there was a preferential metabolism of Kyn to the potentially neurotoxic QA [231], demonstrating the biological differences in this pathway between various psychiatric disorders. Interestingly, Kyn/tryptophan relation, which appears to represent IDO activity, is also elevated in BD patients [232]. Importantly, the increased IDO activity is a crucial mechanism related to the inflammation-induced depressive-like behavior by endotoxemia [233], thus showing the relevance that gut dysbiosis and bacterial translocation may have on BD patients. Van den Ameele et al. [234] observed that there was a strong relation between TNF-α and Kyn, Kyn/tryptophan, 3-HK and QA in the manic subgroup whereas in the depressed subgroup the KYNA/3-HK decreased and there was a strong association between interferon-y and Kyn pathway activation. Besides, both depressive and manic subgroups were characterized by presenting low levels of KYNA in comparison to healthy controls, which seems to support the relevance of the Kyn pathway in the context of neuroinflammation in BD and how it may be differentially modulated. Beyond, it seems that there is an inverse relationship between Oscillibacter (a genera increased in patients with BD) and the levels of tryptophan and KYNA, thereby supporting the relevance of gut microbiota in the altered Kyn pathway and neuroinflammation [235]. These findings could have important therapeutical implication for BD patients; as some antidepressant like ketamine can influence the Kyn pathway [227].
Taken together, this evidence suggests that gut microbiota plays a key role in BD. Gut microbiota is responsible for the disruption of intestinal permeability, which results in an upregulation of inflammatory cytokines. The gastrointestinal tract and brain maintain a crosstalk connection in which gut microbiota and inflammatory response are crucial, and this immune disruption is inextricably related to a neuroinflammatory response mediated by abnormalities in microglia and mitochondrial functions, as well as an increase in oxidative damage. CNS injury is a cause of inflammation, but this inflammation activates also autonomic nerves, including vagus nerve, feeding back this detrimental inflammation system. Aberrant neuroendocrine and immune responses seem to be critical in the precipitation and the feedback of neuroinflammatory-related disorders, such as BD. Neuromodulatory actions herein expressed in the section “Neuromodulatory actions of gut microbiota in bipolar disorder” are summarized in Fig. 5.
Fig. 5. Summary of main neuromodulatory actions of gut microbiota (GM) in bipolar disorder (BD).
There are direct (synthesis or degradation of neurotransmitters and their precursors) and indirect (synthesis of microbial metabolites with systemic pleiotropic effects) actions. In the indirect actions the main metabolites, SCFAs and tryptophan (trp) derived metabolites, are represented. Red arrows represent the result of pathological condition of BD, meaning the increased or decreased level in certain metabolic routes. Decreased levels of butyrate and propionate besides increased levels of acetate have been related to higher depressive symptoms. Tryptamine levels are risen whereas tryptophan levels are decreased in BD. Kynurenine pathway is also altered in BD and it seems to be related to neuroinflammation, bacterial translocation, HPA dysfunction, neuromodulation. As shown, a peripheral decrease in many metabolites of the Kyn pathway is shown, with specific differences in manic versus depressive stages and inversely correlated with certain gut microbiota populations (Oscillibacter genera). Glutamate concentration, abnormal SCFAs production and decreased trp, all confluence in less enteric serotonin (5HT) production due to decreased stimulation of enteroendocrine cells (EECs). All these mechanisms disruption contribute to bidirectional intestinal inflammation-neuroinflammation. Central nervous system (CNS) also sends signals activating vagus nerve. Studying local and systemic levels of SCFAs in patients with BD can be of aid to better understand microbiota–gut–brain (MGB) axis disruption in this malady. More studies focusing on its different phases and types are needed. This scenario is just the beginning of new therapeutical approaches. GM gut microbiota, DA dopamine, AC acetylcholine, NE norepinephrine, his histamine, GABA gamma-aminobutyric acid, EECs enteroendocrine cells, 5HT serotonin, SCFAs short-chain fatty acids, trp tryptophan, kyn kynurenine, KYNA Kynurenic acid, XA Xanthurenic acid, QA Quinolic acid, Tregs T regulator cells, ILC innate lymphoid cell, BBB blood–brain barrier, CNS central nervous system; (+): activates.
Medical management of patients with bipolar disorders and the impact of pharmacological treatment on gut microbiota
Currently, the medical treatment of BD is eminently pharmacological, and it is based on antipsychotics, antidepressants, and other molecules such as lithium [18]. More detailly, mania should be treated first-line with lithium, divalproex, or an atypical antipsychotic medication; mixed episodes with first-line divalproex or an atypical antipsychotic; Bipolar depression treatment has limited evidence, although lots of drugs have been proposed as treatment options with similar efficacy but varying tolerability, such as lamotrigine, fluoxetine or antipsychotics as quetiapine or olanzapine, among others [236–238]. Despite the relevance of these clinical approaches, non-adherence rates under antipsychotics or mood stabilizers at long-term treatment in BD are high, with an estimation of about 40–50% [239]. In the non-adherence phenomenon, patient-related factors such as stigma or the knowledge about their illness, seem to be more influential than demographic or illness related variables [239]. Because of that, all patients should be offered individual, or group psychoeducation and a proper therapeutic drug monitoring could aid to inquire the effect of the treatment selected for each patient [238]. Similarly, electroconvulsive therapy (ECT) has proven to be a useful and safe option in severe and drug-resistant phases of BD episodes [240].
There is preliminary evidence that non-pharmacological therapies used in mental disorders like ECT are able to induce changes in gut microbiota [241, 242]. However, it has not been evaluated in patients with BD yet, and future studies can be focused on exploring the effects of this approach in this psychiatric condition. Conversely, compelling evidence points gut microbiota as a potential marker of pharmacological therapy in patients with BD [243]. Because of that, the field of “pharmacomicrobiomics” is gaining more attention in recent years, as it considers that both genetic and gut microbiota can influence the interindividual heterogeneity in drug responses [244]. Moreover, in a scoping review, the use of psychotropics for neurocognitive disorders, including BD, SZ, MDD or obsessive-compulsive disorder, could exert important antimicrobial effects on gut microbiota [210]. Thus, pharmacological treatment can have an important impact on gut microbiota, and in turn, gut microbiota can induce pharmacodynamical alterations by transforming drugs or by modifying host metabolism and immune system [244, 245]. Hence the bidirectional interaction between pharmacology and gut microbiota could be used as an important biomarker for patients with BD, allowing the identification of novel therapeutic targets or agents with better tolerability.
Antipsychotics and gut microbiota
As aforementioned, antipsychotics are critical therapeutic agents for the clinical management of BD. Despite their benefits, weight gain, diabetes, metabolic syndrome, and cardiovascular disease are common side effects of this type of drugs, and insulin resistance that may be induced because of their use has been related to changes in DNA methylation, particularly with hypomethylation in fatty acyl CoA reductase 2 [246]. To this fact it must be added lipid and glucose dysregulation are common in BD patients, even before the diagnosis and treatment [247] and atypical antipsychotics can worsen these alterations. Previous works have demonstrated that atypical antipsychotics drive to notable alterations in gut microbiota populations, increasing the Firmicutes: Bacteroidetes ratio, fermentative metabolism as well as monosaccharide absorption or adipocyte fatty acid storage [248]. Thus, gut dysbiosis may be, at least partly, responsible for the metabolic dysfunctions induced by antipsychotics. Similarly, children and adolescents under chronic risperidone treatment showed a higher weight gain and, concurrently, changes in gut microbiota consisting in an altered ratio of Bacteroidetes: Firmicutes and an upregulation of metabolic pathways linked with weight gain, including alterations in butanoate, propanoate, fatty acid and tryptophan pathways [249]. In rats, olanzapine modifies gut microbiota to an obesogenic bacterial profile (similarly to the alterations observed in humans), so that gut microbiota is sufficient and essential to induce this weight gain [250]. Moreover, olanzapine not only acts synergistically with a high-fat diet, but it also proved to have antimicrobial activity in vitro against resident enteric bacterial strains [250]. Conversely, in rats, the coadministration of olanzapine and a purified prebiotic Bimuno™ galacto-oligosaccharides powder increased fecal Bifidobacterium and attenuated weight gain induced by olanzapine [251]. Also, the coadministration of olanzapine and an antibiotic combination consisting of neomycin, metronidazole and polymyxin B attenuated weight gain and metabolic dysfunction markers in rats [252]. Collectively, these studies appear to indicate that gut microbiota can be used as a promising therapeutic target to limit the development of different metabolic and cardiovascular risk factors observed in BD patients.
On the other hand, 4 weeks of treatment of quetiapine (300 mg/day) in patients with bipolar depression led to increased levels of Eubacterium rectale, Bifidobacteria, and Bifidobacteria to Enterobacteriacea ratio, having this fact a potential impact on brain function [78]. Another study conducted by Hu et al. [253] reported changes in 30 microbial markers after quetiapine treatment, and 10 of them presented an area under the curve (AUC) of 0.93 between responder and no responder patients, denoting a potential application of gut microbiota as a predictive biomarker.
Women under atypical antipsychotic treatment showed to have a decreased species diversity in their gut microbiota compared to healthy controls, while men did not show to have this difference [254]. Among these changes, a significant decrease in Akkermansia muciniphila was reported. This is a crucial Gram-negative bacterium capable of improving enterocyte layer integrity [255], whose levels decrease under high-fat diets and ageing [256]. The abundance of this strain has been inversely associated with inflammation markers, obesity, and metabolic disruption, including insulin resistance, cardiovascular risk parameters and adiposity [256], supporting again the possible role of gut microbiota in the metabolic adverse effects of antipsychotics. This information could be crucial, as BD is associated with a two-fold risk of suffering from cardiovascular disease [257] and we can find that changes in gut microbiota induced by certain drugs may be partly involved in the increased risk.
On the other hand, Cussoto et al. [258] found that aripiprazole, another atypical antipsychotic, was associated with a significant increase in the bacterial richness and diversity in mice. More detailly, aripiprazole induced an increase in Firmicutes phyla, Peptostreptococcaceae, Clostridiaceae and Ruminococcaceae family and in multiple minor genera such as Clostridium sensu stricto 1, Ruminiclostridium 5, Intestinibacter, Eubacterium coprostanoligens, Peptoclostridium, Eubacterium oxidoreducens, Christensenellaceae uncultured and Clostridia Family XIII, with a decrease in the relative abundance of Ruminococcus 1. Likewise, aripiprazole drive to an increase in the production of the SCFAs acetate and isovalerate. The role of isovalerate in psychiatric disorders is however uncertain, and there are some studies evidencing that an increase of this component is directly correlated with depression and cortisol levels, being able to interfere with synaptic neurotransmitter release after crossing the BBB [259]. Thus, further studies are needed to unravel the effects of antipsychotics on gut microbiota and how this may impact the response and side effects reported by the patients receiving this therapeutic regimen.
Antidepressants and gut microbiota
Nowadays, neither monotherapy nor the use of antidepressants in rapid cycling BD is recommended, but antidepressants can be used as adjunctive treatment in BD as a second-line treatment. Thus, in BD type 1, serotonin reuptake inhibitors (SSRIs) and bupropion associated with lithium, divalproex or an atypical antipsychotic are reasonable options, while serotonin‐ norepinephrine reuptake inhibitors (SNRIs) and monoamine oxidase inhibitors (MAOIs) have a have higher propensity to induce manic switch and to produce mood destabilization. In BD type 2 sertraline and venlafaxine are preferred [13].
Moreover, the use of antidepressants has been contestable in BD because of the risk of manic switch or rapid cycling. In addition to this risk, it is important to highlight that antidepressants such as mirtazapine (especially associated with trazodone), fluoxetine, and nortriptyline have been associated with Clostridium difficile infection in depressed patients [260]. Moreover, some antidepressants seem to exert noteworthy antimicrobial effects, having been proposed that their effectiveness can be, at least partially, consequence of the effects on gut microbiota [261]. In turn, antimicrobials can also have antidepressant effects. However, further studies are needed to establish if the antimicrobial effects of antidepressants are associated with beneficial or detrimental mechanisms of these drugs, aiding to explain the therapeutic success or antidepressant resistance. For instance, SSRIs showed to have antimicrobial effects, mostly against Gram-positive microorganisms, acting as efflux pump inhibitors and having a synergistic activity when combined with some antibiotics. Sertraline, as a SSRIs, has an intrinsic antibacterial and antifungal activity in vitro, and it is capable of increasing antibacterial activities of antibiotics and making susceptible some previously resistant strains [262]. In murine models, fluoxetine, escitalopram, venlafaxine, duloxetine and desipramine showed to alter gut microbiota composition, mainly reducing Ruminococcus, Adlercreutzia and an unclassified Alphaproteobacteria [263]. Interestingly, Ruminococcus flavefaciens showed to alter the expression of genes linked with mitochondrial and neuronal processes in the medial prefrontal cortices, and the decrease of its levels could be associated with a relief in depressive-like behavior [263]. Thus, there is a promising line of research to explore the pharmacomicrobiomics of antidepressants in patients with BD and its clinical implications.
Lithium and gut microbiota
Lithium is the mainstay of the treatment since it was discovered in 1949 for prophylaxis of BD [264]. This drug is used as an attenuator of calcium dysregulation, a critical mechanism involved in BD etiopathogenesis [20]. Although little is known about the effects of lithium on gut microbiota, it seems to favorably increase microbial species richness and diversity in vivo [258]. More precisely, at the phylum level, lithium appears to increase Actinobacteria and reduce Bacteroidetes, increasing the family of Peptostreptococcaceae, Clostridiaceae and Ruminococcaceae. Lithium also led to an increase in the relative abundance of Clostridium sensu stricto 1, Ruminiclostridium 5, Intestinibacter, Eubacterium coprostanoligens, Peptoclostridium, Eubacterium oxidoreducens, Christensenellaceae uncultured and Clostridia Family XIII and a decrease in the relative abundance of Bacteroides and Ruminococcus 1 [258]. Likewise, a very recent study showed that lithium carbonate was able to lighten colon inflammation by inducing changes in gut microbiota and increasing its diversity, especially expanding Akkermansia municiphila [243]. In this study, lithium could also activate anti-inflammatory Treg cell activity in lamina propria via metabolite-sensing G-protein coupled receptors 43 (GPR43), which mediates anti-inflammatory effects of the SCFA [243]. In relation to this fact, the immunomodulatory role of lithium in BD has been formerly discussed. Thus, chronic lithium therapy in euthymic BD patients can normalize immune parameters, leading to a decrease in cytokine-producing peripheral blood lymphocytes [265].
Despite the possible favorable effects of lithium on gut microbiota composition, the use of this drug has been associated with numerous withdrawals in patients with BD, including nausea, diarrhea, weight gain, fine tremor, hypothyroidism, electrocardiogram anomalies, alterations in renal function and even cognitive side effects [266]. This could be explained by two main alternatives: (1) these adverse outcomes can be independent of gut microbiota or (2) the use of lithium may have some unexplored effects on gut microbiota that can explain the occurrence of some adverse outcomes that could be used for therapy monitoring in patients receiving this medication.
Anticonvulsants and gut microbiota
Anticonvulsants such as valproic acid, lamotrigine or carbamazepine are eligible therapeutical options in BD as mood stabilizers [267]. The effects of valproic acid from a biological perspective are multiple. On the one hand, some studies have described that valproic acid protects BBB function and integrity in a similar way to lithium [140]. On the other hand, valproic acid is detrimentally associated with metabolic anomalies, including higher levels of insulin and triglyceride, weight gain and increased body mass index [268], similar to antipsychotics. Considering its effects on gut microbiota, the study conducted by Cusotto et al. [258] also demonstrated that valproic acid raised bacterial diversity and richness in mice. This drug leads to an increase in Actinobacteria and Firmicutes phyla along with a decrease in Bacteroidetes. Likewise, valproic acid increase Peptostreptococcaceae, Clostridiaceae and Ruminococcaceae family, decreased the relative abundance of S24-7 uncultbact and enhance the relative abundance of Ruminococcaceae uncultured, Clostridium sensu stricto 1, Ruminiclostridium 5, Intestinibacter, Eubacterium coprostanoligens, Peptoclostridium, Eubacterium oxidoreducens, Christensenellaceae uncultured and Clostridia Family XIII. Also, according to this study, valproic acid administration induced a significant decrease in the levels of propionate and butyrate while augmenting the levels of isovalerate. Oppositely, in a murine model of valproic acid-induced autism, valproic acid administered to pregnant females significantly decreased the fecal microbiome diversity in pups, altering the composition of gut microbiota to resemble those derived from patients with autism spectrum disorder [269]. Similarly, the use of valproic acid at high doses used by patients can have noteworthy effects on the gut microbiota, altering the biosynthesis of fatty acid by different microorganisms [270]. Hence, more evidence is required to understand the favorable or detrimental effects of the use of valproic acid in the gut microbiota and its impact on BD.
On the other hand, lamotrigine does not have the metabolic adverse effects of, but it showed to have a temperature-dependent activity inhibiting ribosome biogenesis in Escherichia coli and Salmonella enterica [271], also displaying antimicrobial activity towards Gram-positive, such as Bacillus subtilis and Staphylococcus aureus [56]. Although there are few studies exploring the effects of carbamazepine on gut microbiota composition, carbamazepine also appears to exert notable antimicrobial effects, particularly on Gram-negative bacteria, inducing cytotoxicity in colonocytes [272]. This cytotoxic effect is shared with lamotrigine and appears to be contrary to the cytoprotective effects of certain microbial metabolites.
Nowadays, the implications of anticonvulsive therapy in gut microbiota remain unclear, but it seems to alter bacterial composition and metabolism. Future research might clarify the repercussion of these medications and if this interaction is meaningful or if it has any relation with its efficacy or adverse effects. The main findings collected about the impact of pharmacological treatment, its uses, side effects and consequences in gut microbiota are summarized in Table 1.
Table 1.
Pharmacological treatment in bipolar disorder and its impact on gut microbiota.
| Drug | Therapeutic effect | Clinical evidence | Side effects | Main findings on gut microbiota | References |
|---|---|---|---|---|---|
| Risperidone |
Antipsychotic Anti-manic |
Risperidone is one of the first-line treatment during acute mania. It could be also an option in maintenance treatment | Metabolic disruption (moderate risk): weight gain, diabetes, metabolic syndrome, insulin resistance and cardiovascular disease |
Decreased Akkermansia muciniphila Decreased gut microbiota diversity in women Altered Firmicutes/Bacteroidetes ratio Modifications in butyrate, propionate and tryptophan pathways |
[13, 246, 249, 251, 252, 254] |
| Olanzapine |
Antipsychotic Anti-manic |
Olanzapine is one of the second-line treatment during acute mania, but it is also effective in maintaining and preventing also depressive episodes in BD-1 | Metabolic disruption (high risk): weight gain, diabetes, metabolic syndrome, insulin resistance and cardiovascular disease. These side effects position olanzapine as a second-line treatment |
Decreased Akkermansia muciniphila Decreased gut microbiota diversity in women Increased Firmicutes/Bacteroidetes ratio In rats, olanzapine modifies gut microbiota to an obesogenic bacterial profile It also proved to have antimicrobial activity in vitro against resident enteric bacterial strains |
[13, 246, 250, 254] |
| Quetiapine |
Antipsychotic Antidepressant in BD |
First-line treatment for both maintenance and acute depression in BD-1 and 2 | Metabolic disruption (moderate risk): weight gain, diabetes, metabolic syndrome, insulin resistance and cardiovascular disease |
Increased Firmicutes/Bacteroidetes ratio Decreased Akkermansia muciniphila Decreased gut microbiota diversity in women |
[246, 248, 254] |
| Aripiprazole |
Antipsychotic Anti-manic |
First-line treatment in acute mania and maintenance in BD-1. First-line treatment in agitation | Metabolic disruption (very low risk) |
Decreased Akkermansia muciniphila Decreased gut microbiota diversity in women Increase bacterial richness and diversity (increase in Firmicutes phyla, Peptostreptococcaceae, Clostridiaceae and Ruminococcaceae family and in minor genera like Clostridium sensu stricto 1, Ruminiclostridium 5, Intestinibacter, Eubacterium coprostanoligens, Peptoclostridium, Eubacterium oxidoreducens, Christensenellaceae uncultured and Clostridia Family XIII, with a decrease in the relative abundance of Ruminococcus 1) Increase acetate and isovalerate production |
[246, 254, 258] |
| SSRIs | Antidepressant Antimicrobial | As a second-line treatment, SSRI can be used as an adjunctive treatment for acute depression in BD-1. Specifically, sertraline can be used as a second-line treatment for acute depression in BD-2 |
Antidepressant therapy may have a potential risk of inducing modifications or resistances in gut microbiota There is a risk of manic switch or rapid cycling, so they should not be used in monotherapy |
Antimicrobial and antifungal properties have been described. Some antidepressants showed to reduce Ruminococcus, Adlercreutzia and an unclassified Alphaproteobacteria. Interestingly, lower levels of Ruminococcus flavefaciens can relieve depressive-like behavior The effectiveness of antidepressants could be related to their antimicrobial effects |
[13, 260, 261, 263, 366] |
| Lithium | Anti-inflammatory. Mood stabilizer | Mainstay of the prophylaxis in BD (first-line treatment for maintenance in BD-1 and -2). It can also be used in bipolar depression (first-line treatment in BD-1 and second-line in BD-2). Commonly used in combination with other drugs | Nausea, diarrhea, Weight gain, fine tremor, hypothyroidism, electrocardiogram anomalies, alterations in renal function and cognitive side effects |
Increase bacterial richness and diversity in mice (enhance Actinobacteria growth and reduce Bacteroidetes, increasing the family of Peptostreptococcaceae, Clostridiaceae and Ruminococcaceae and the relative abundance of Clostridium sensu stricto 1, Ruminiclostridium 5, Intestinibacter, Eubacterium coprostanoligens, Peptoclostridium, Eubacterium oxidoreducens, Christensenellaceae uncultured and Clostridia Family XIII decreasing the relative abundance of Bacteroides and Ruminococcus 1 Expands Akkermansia municiphila Modulates expression of a receptor that mediates anti-inflammatory effects of the SCFA |
[13, 243, 258, 265, 266] |
| Valproic acid |
Mood stabilizer Anticonvulsant |
First-line maintenance treatment in BD-1. Second-line treatment in BD-1 depression | Metabolic anomalies, including higher levels of insulin and triglyceride, weight gain and increased body mass index |
Increase bacterial diversity and richness in mice (increase Actinobacteria and Firmicutes phyla along with a decrease in Bacteroidetes; Promote the growth of Peptostreptococcaceae, Clostridiaceae and Ruminococcaceae family, decrease the relative abundance of S24-7 uncultbact and enhance the relative abundance of Ruminococcaceae uncultured, Clostridium sensu stricto 1, Ruminiclostridium 5, Intestinibacter, Eubacterium coprostanoligens, Peptoclostridium, Eubacterium oxidoreducens, Christensenellaceae uncultured and Clostridia Family XIII Valproic acid can alter the biosynthesis of the fatty acid productions in some gut microbiota species |
[13, 258, 268, 270] |
| Lamotrigine and carbamazepine |
Mood stabilizer Anticonvulsant |
It can be used during bipolar depression (first-line treatment in BD-1 and second-line in BD-2) and as maintenance treatment (first-line treatment in BD-1 and 2) |
Lamotrigine and carbamazepine induce cytotoxicity in colonocytes and present antimicrobial effects: Lamotrigine showed to have a temperature-dependent activity inhibiting ribosome biogenesis in Escherichia coli and Salmonella enterica, exerting antimicrobial effects against Gram-positive Bacillus subtilis and Staphylococcus aureus Carbamazepine has antimicrobial effects on Gram-negative bacteria |
[13, 271, 272] |
Translational approaches modulating gut microbiota
Up to this point, it has been observed an important link between gut microbiota with several pathogenic mechanisms involved in BD. Likewise, the study of gut microbiota after treatment offers promising applications for therapy monitory or as biomarkers. On the other hand, a growing number of studies point MGB axis as a potential target of a broad spectrum of psychiatric disorders [63, 69, 187, 273]. In this section, we will collect the most relevant and updated knowledge related to the targeting of gut microbiota in BD patients. Nevertheless, it is important to notice that up to date there are no relevant conclusions drawn yet, as this type of therapy entails numerous challenges, such as the fact that gut microbiota is a dynamic structure continuously interacting with the inner and outer environment. Beyond, nowadays it has not been possible to identify a unique signature of a “healthy gut microbiota”, and the interindividual variation of this microbial ecosystem makes it hard to find adequate formulas or therapeutic approaches, critically determining the response of each patient to these interventions [274, 275].
Dietary interventions
Diet is considered the greatest shaper of gut microbiota, as different dietary sources are needed for the biosynthesis of SCFAs and other microbial metabolites, determining the differential growth of certain bacterial populations [60, 276]. Besides, dietary patterns, food and nutrients exert pleiotropic effects in the entire organism, including in the MGB axis; being the gut microbiota a critical mediator of their benefits [277]. Thus, the modulatory effect of diet on gut microbiota can have in turn a direct influence on the immune system, the brain and the rest of the tissues in the body, regulating multiple biological processes epigenetically [23]. Because of that, dietary interventions have demonstrated their usefulness in multiple diseases characterized by an altered gut microbiota like mental disorders [278–280].
One of the most widely studied dietary interventions is Mediterranean Diet (MedDiet), characterized by a combination of high complex carbohydrates rich in fiber (vegetables, fruits, cereals and legumes), polyunsaturated fatty acids like omega-3 polyunsaturated fatty acids (PUFAs) or monounsaturated fatty acids (MUFA) with antiatherogenic and anti-inflammatory properties (found in olive oil, fish, seafood and nuts), and bioactive compounds found in plants with antioxidative properties such as flavonoids, phytosterols, terpenes and polyphenols. All these nutrients have beneficial effects on gut microbiota composition and function, contrary to westernized diets, abundant in refined carbohydrates (sugar or flour), low-quality fats (i.e., trans fatty acids), salt, additives and other detrimental components [71, 281]. In this sense, Łojko et al. [282] evaluated the dietary pattern followed by 113 euthymic BD patients in comparison with 160 healthy control subjects. Interestingly, they found that BD patients had lower Mediterranean Diet adherence than controls, showing unhealthy dietary patterns (western-type, pro-healthy carbohydrates, unhealthy snacks, and meats and potatoes). Likewise, they observed 70% of patients with BD had body mass index (BMI) >25 kg/m2 and they tend to present increased values of insulin resistance, with higher levels of fasting triglycerides, glucose index and waist circumference than the healthy subjects. Despite, they did not find any association between diet quality and the clinical course of BD, there is some preliminary findings supporting the role of diet in multiple biological mechanisms involved in BD, possibly influencing the clinical course and therapy success [279, 283]. The effects of diet on gut microbiota can be critically involved in this fact, representing a potential adjuvant therapy for BD and other mental disorders [284]. In this sense, a parallel randomized controlled trial also demonstrated that an isocaloric MedDiet in obese and overweight patients led to an increase in SCFA levels, Faecalibacterium strains (linked to butyrate metabolism) and in gene bacterial gene richness, diminishing Ruminococcus gnavus (with potentially proinflammatory properties) and decreasing systemic inflammation [285]. Other reported changes from MedDiet in gut microbiota include augmentation of Bacteroides, Lactobacilli, Bifidobacteria, Oscillospira, Roseburia, Clostridium cluster XIVa along with a reduction in Firmicutes and Proteobacteria phyla [286].
Of the many nutrients found in MedDiet, omega-3 polyunsaturated fatty acid (PUFAs) is perhaps the most widely studied, exerting the greatest benefits as an adjuvant for mental disorders [287]. In this line, a very recent systematic review [288] collecting results from 33 observational trials and 27 interventional studies established that dietary intake or supplementation of unsaturated fatty acids, mainly omega-3 PUFA seems to be associated with improved BD symptoms, together with seafood, folic acid and zinc. Nevertheless, omega-3 PUFA showed to improve depressive manifestations in BD, but not manic symptoms [289, 290] and seems to attenuate variability in mood, energy, irritability, and pain [291]. Intriguingly, some of the benefits of Omega-3 PUFA could be attributed to its modulatory role on gut microbiota. More detailly, omega-3 PUFA seems to modify its diversity by increasing the abundance of Bifidobacteria and Akkermansia as well as lowering Enterobacteria abundance [292]. Other reported changes include increase of Lactobacillus and Butyrivibrio, together with restoration of Firmicutes/Bacteroidetes ratio [293]. Moreover, they seem to enhance the mucosal barrier function and mitigate the inflammatory response linked with metabolic endotoxemia [292]. Conversely, some studies have found that nitrated dry cured meat has been associated with mania in humans, while alimentation with nitrated-added cured meat products leads to hyperactivity behavior (similar to mania) in rats [294]. These results were accompanied by increases in Lachnospiraceae and Erysipelotrichales abundance, two families linked to potential disruptions in fat and energy metabolism.
Overall, all these studies support that there is a possible, but still inconclusive role of diet in patients with BD, and these effects are partly mediated by gut microbiota. Further studies should deepen on the promising role of nutrition in the clinical course, monitoring or as an adjunctive therapy for these patients, especially for those with metabolic disturbances, overweight and unhealthy dietary patterns. Likewise, the recommendation and elimination of certain nutrients or foods, and nutritional education for these patients will surely bring notable benefits for patients with BD, being an additional part of the complex picture of this psychiatric malady.
Prebiotics, probiotics and postbiotics
Probiotics are defined as: “live microorganisms, preferentially of human origin, that upon ingestion in specific and sufficient numbers confer unspecified health benefits to the host” [295]. For its part, prebiotics are “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora that confers benefits upon host wellbeing and health” [296]. Dietary fibers are the principal prebiotics, and some examples of prebiotics include resistant starch, non-starch polysaccharides, inulin, and oligosaccharides such as fructooligosaccharides, galacto-oligosaccharides, and xylooligosaccharides [297]. These ingredients can resist hydrolysis in the human small intestine so they are fermented by colonic bacteria resulting in metabolites such as SCFA, whose importance has been previously discussed [297]. Pro- and prebiotics have numerous health benefits. Related to their favorable effect on gut microbiota, both seem to reduce inflammation, decrease some risk factors for cardiovascular disease, enhance satiety and promote weight loss and improve the bioavailability of minerals [298, 299]. Simultaneously, current evidence suggests that pro- and prebiotics seem to have anxiolytic and antidepressant effects in healthy population and in patients with mental disorders, improving psychological function via several mechanisms [300–302]. In this line, the term psychobiotic has been proposed as “a live organism that, when ingested in adequate amounts, produces a health benefit in patients suffering from psychiatric illness” [303].
The effects of probiotics in BD are not steady. Some researchers have not found significant differences in the severity of mania and depression in BD type 1 patients after probiotics supplementation [304]. In other studies, the probiotic supplementation of Lactobacillus GG and Bifidobacterium lactis strains showed to lessen the risk of rehospitalization in patients who were recently discharged from the hospital due to a mania episode. Interestingly, the benefits and efficacy of probiotic supplementation were significantly highly observed in patients with increased levels of systemic inflammation [79]. In a recent interventional study, 80 first-episode drug-naive patients with BD who received psychotropic therapy supplemented with either probiotic or placebo were followed up for 3 months for observing clinical symptom improvements and changes in oxidative stress markers [305]. After follow-up, decreased serum levels of lysophosphatidylcholines (LPCs) and increased serum levels of other six oxidative stress markers (creatine, inosine, hypoxanthine, choline, uric acid, allantoic acid) were observed in both placebo and probiotic groups. However, the adjuvant use of probiotics shows a positive correlation between changes in LPC (18:0) and Young Mania Rating Scale (YMRS scale), and in addition, the mania symptom greatly ameliorated in patients who received probiotic supplements as compared with the placebo. Moreover, dietary fiber, as natural prebiotic, combined with probiotics may be a useful therapeutic weapon to deal with the side effects of atypical antipsychotics [306]. In another intervention study, Reininghaus et al. [307] proved the benefits of probiotic supplementation on cognitive function in 20 euthymic individuals with BD over a time of 3 months. Interestingly, they found that this supplementation brought significant improvements to attention and psychomotor processing speed after 1 and 3 months of treatment while executive function seemed to improve 3 months after supplementation.
Overall, despite more studies are needed before drawing any conclusions, there are plenty of preliminary and promising evidence of the potential of probiotics (alone or in combination with prebiotics) for improving the clinical management of BD patients, as multiple studies have proven their benefits in mood, depressive symptoms and other mental health parameters [284, 308]. Because of the growing attention that is receiving, we also propose to drive further studies on the field of postbiotics, which can be defined as “a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” [309]. In this line, SCFAs represent the postbiotic most widely studied, particularly due to their epigenetic activity modulating histone acetylation and, consequently, gene expression. In bovine mammary epithelial cells, sodium propionate and sodium butyrate showed to reduce the activity of certain histone deacetylases as well as increase the acetylation of particular histones [310]. Thus, after the administration of LPS in bovine mammary epithelial cell, sodium butyrate showed to inactivate NF-κB signaling and, consequently, diminishing inflammatory response and apoptosis [311]. Notwithstanding the fact that more evidence is needed, this protective effect may represent a promising therapeutical alternative to modulate gut microbiota in BD. In rats, sodium butyrate showed to have an antimanic effect by attenuating the hyperactivity and the neurotrophic factors and metabolic disruption induced by ouabain, similar to mood stabilizers [207, 312, 313]. Because of the multiple mechanisms that postbiotic exert in the MGB axis [314], we expect that exploring the therapeutic use of postbiotics can represent an attractive line of research for future works.
Other therapeutic approaches
Apart from diet, probiotics and prebiotics, there are interesting lines of research that could be used as valuable alternatives to consider for therapeutic studies.
Antimicrobials
As aforementioned, not only certain drugs used in BD exerted antimicrobial activity, but also antimicrobials could have noteworthy effects on mental functioning, because of their direct effect on gut microbiota. For instance, it has been suggested that tetracycline antibiotics might represent an attractive therapeutic alternative for different psychiatric disorders [315]. Minocycline is a tetracycline antibiotic that showed to increase neuronal survival, as well as being able to reduce proinflammatory cytokines production and modulating glutamate and monoaminergic pathways, which explains its anti-inflammatory and neuroprotective activity [316]. Aspirin and minocycline combination showed to be effective in BD depression, and minocycline was especially effective at higher baseline levels of IL-6, suggesting that both options could be evaluated as an adjunctive therapy [317]. Likewise, the use of minocycline can be particularly effective for the therapy of bipolar depression in patients with high glutathione (GSH) levels [318]. Similar roles have been given to another tetracycline, doxycycline, which appears to be a promising adjunctive therapy with lithium for treating BD, according to preclinical models [319]. Other studies and systematic reviews, however, did not find any benefits from using minocycline as adjunctive therapy in patients with BD [320, 321], and contrary to some proposals, doxycycline exposure in the adolescence did not report any causal relationship between this tetracycline and BD development [322]. Regarding the effects of minocycline treatment on gut microbiota, it seems that this therapy decreases the Firmicutes/Bacteroidetes ratio [323]. Likewise, changes in other genera have been reported under minocycline treatment, such as decreases in Allobaculum, Bifidobacterium, Turicibacter and Clostridium or increases in Akkermansia muciniphilla, Lachnospiraceae and Ruminococcaceae incertae sedis [324]. Regarding the effects of doxycycline on gut microbiota, previous studies have noticed an effect of this antibiotic on a short-term reduction of LAB like Bifidobacterium or Lactobacillus [325, 326]. On the other hand, there are some antibiotics that may be associated with the development of mania, what experts have designed as “antibiomania” [327]. According to a systematic review of 47 cases of antibiomania, 12 different antibacterial agents were implicated, with antitubercular agents, macrolides and quinolones being the most common causative groups [328]. Despite the pathophysiological basis of antibiomania remains to be fully unraveled, the suspected drug should be immediately discontinued and manic symptoms according to the clinical guidelines. Hence, despite some preliminary evidence is available supporting the use of antimicrobials in the clinical management of BD, further studies are warranted to evaluate the effects of antimicrobials in the gut microbiota and its association with clinical outcomes in these patients.
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) is another promising translational approach targeting gut microbiota. In a simple manner, FMT consists of transferring stool from a healthy donor into the colon of a patient with an established pathology with the aim to restore the normal microbiota and cure or at least ameliorate the disease [329]. Currently, FMT can be considered to treat recurrent Clostridium difficile infection, although there are multiple lines of research opened in intestinal and extraintestinal pathologies [330]. In the literature, there is a case report of a woman diagnosed with BD who went through FMT from her healthy husband at least nine times. Afterward, she had neither manic nor depressive symptoms in the following sixth months, also showing an important weight loss of nearly 33 kg [331]. However, despite these promising results, further studies are needed to evaluate FMT in patients with psychiatric disorders, as this field entails several challenges regarding donors and recipients [55]. Currently, there is a clinical trial (NCT03279224) conducted to assess the feasibility, efficacy, safety, and tolerability of FMT on patients with BD depression. The results of this trial are not available yet (on November 4, 2022), but in this workshop participants are randomized to receive either screened and processed donor stool (allogenic FMT) or their own stool (autologous FMT) via colonoscopy and monitored for 24 weeks post intervention. Then, depressive and manic symptoms, treatment acceptability, gastrointestinal and other side effects are assessed at baseline (prior to randomization) and weekly, whereas stool samples to evaluate microbiome composition are obtained at baseline and 3 and 6 months [332].
Immune-based approaches
Immune-based therapeutic strategies have been suggested for some patients with BD and MDD, although due to the biological and clinical heterogeneity of these complex disorders, this type of therapy could be considered as a part of personalized approach, beneficiating approximately one in three patients, according to previous studies [333]. Thus, some immunomodulatory drugs such as COX inhibitors exert not only anti-inflammatory outcomes but also antidepressant effects [334]. In this sense celecoxib showed to be a safe and a highly effective therapeutical option in resistant bipolar depression, reducing anxiety and accelerating treatment response [335], in a similar way to aspirin [336]. It should be highlighted that, although short-term administration of celecoxib does not seem to alter the bacterial abundance, relative changes in some bacterial populations have been described although changes in butyrate production are not observed after its administration [337]. TNF-α inhibitors have also shown antidepressant properties [338], so they have also been proposed as a potential treatment in BD with contradictory and unsatisfactory results [339]. For instance, in a study with BD patients, the administration of the TNF-α inhibitor infliximab did not report either clinical improvement after its use or significant differences in gut microbiota composition [340]. Despite not exerting a direct anti-inflammatory but an antioxidant effect, N-acetylcysteine (NAC) has also proven potential benefits for patients with BD, especially as a coadjutant for ameliorating depressive symptoms [333, 341]. Likewise, according to previous studies, the use of NAC alleviates gut dysbiosis and glucose disturbances in mice fed with high-fat diet [342], denoting the potential benefits that this drug may have on gut microbiota and BD.
Other lifestyle interventions
Active lifestyle approaches with regular physical activity and sleep hygiene as well as psychosocial interventions are prominent coadjutants to pharmacotherapy [343]. Regarding physical activity levels, patients with BD appear to be less active and more sedentary than the general population [344]. Exercise also displays pleiotropic effects in the organism, including favorable effects on gut microbiota, including the augmentation of the number of beneficial bacteria, microbial diversity while improving the functioning of the whole MGB axis [345]. Because of that, compelling evidence is supporting the role of exercise in patients with BD, improving health measures including depressive symptoms, functioning and quality of life [346]. However, there is still a lack of studies that do not permit to establish a cause-effect relationship between mood and physical exercise and further research is needed to determine the recommended intensity, duration and frequency of exercise programs [347]. An interesting approach could be to study changes in gut microbiota composition before and after following an adapted physical activity training program and possible implications in patients with BD, aiding to understand the effects derived from this intervention.
On the other hand, irregular circadian patterns can promote mania and depression episodes, and potential interventions to ameliorate this disruption have been explored [46]. Aberrant light cycles can modify gut microbiota composition, altering especially relative abundance of Lactobacillus and Bacteroidetes [348]. In this way, novel approaches have been proposed, such as light and social rhythm therapies, which aim to regulate everyday activities, to enhance social relationships and, consequently, reduce the effect of circadian patterns disturbances [46]. These approaches showed to modify gut microbiota in a beneficial way [349] and it seems to be helpful in BD [350], but the capacity of ameliorate mood symptoms and preventing episodes is yet uncertain [351]. Similarly, midday bright light has been proposed, which has already shown efficacy in increasing remission rates in BD [352]. In relation to this fact, not only circadian but also seasonal variations appear to have a major effect on BD patients. Indeed, it seems that there is a peak in hypomanic/manic symptoms in the spring and early summer months versus a peak in depressive symptoms in the late fall and early winter; and these observations may correspond to seasonal changes in solar insolation, alterations in melatonin production and seasonal influence on the hypothalamic–pituitary–thyroid (HPT) axis, especially at locations of increasing distance from the equator [353]. Gut microbiota is equally affected by seasonal variations. For instance, increased levels of Actinobacteria and Firmicutes to Bacteroidetes ratio can be observed in summer [354], partially explained by dietary fluctuations across seasons [355]. Thus, it would be interesting to research if there is a specific relationship between seasonal variations in gut microbiota with the onset and/or switch of different episodes in patients with BD, with a special focus on dietary variations in these patients.
Some studies have reported associations between the menstrual cycle and BD, particularly in a subgroup of women with enhanced hormonal sensitivity, who seem to experience menstrual cycle effects on depressive, hypomanic, and manic episodes. These phase-episode effects appear to be heterogeneous and may have implications for treatment [356]. Although some studies have failed to find these associations [357, 358], a recent narrative review on this topic [359] supports that, despite there are little works in the field (only 22), certain women with BD may have their mood shifts affected by menstrual cycle events, with different patterns according to the type of BD. The co-occurrence of BD with menstrual cycle-related disorders such as premenstrual dysphoric disorder (PMDD) appears to have a noteworthy impact on these patients, leading to greater chronobiological disruptions across the follicular and luteal phases of the menstrual cycle [360]. In this sense, prior works have related menstrual cycles variations with gut microbiota, and nowadays it is widely accepted that there are certain microorganisms directly implicated in the metabolism and effects mediated by estrogens and sex hormones, conforming what is designed as the estrobolome [361]. There are no studies evaluating the role of the estrobolome in women with BD yet, and how variations in these microorganisms can be important in this group. However, deepening on this field could be specially beneficial for these patients, aiding to develop specific interventions to ameliorate the effects of menstrual cycle in mental disorders [362].
Notwithstanding current therapies reduce rates of relapse in BD patients, these effects might not be translated into an increased quality of life for the patient, also neglecting the impact of BD on suicidal thoughts, besides actions and functional outcomes [363]. In this sense, it is important to find personalized therapies using multidisciplinary approaches that fit patients’ needs [364, 365]. For this reason, understanding the role of gut microbiota in BD pathology is extremely important, as this may aid to identify novel therapeutic targets for maximizing the clinical management of these patients. The main findings about translational approaches reviewed in this section are summarized in Table 2.
Table 2.
Translational approaches modulating gut microbiota in patients with BD.
| Adjuvant approach | Therapeutic effect | Preliminary evidence in BD | Main findings on gut microbiota | References |
|---|---|---|---|---|
| Dietary interventions (Mediterranean diet) | Pleiotropic actions |
Patients with BD frequently present lower Mediterranean Diet adherence and evidence of malnutrition (insulin resistance, higher levels of fasting triglycerides, glucose index and waist circumference) Diet modulates multiple biological mechanisms involved in BD, possibly influencing the clinical course and therapy success Mediterranean diet appears to decrease systemic inflammation Diet should be considered as an important coadjutant to pharmacotherapy |
Increased SCFA and Faecalibacterium Increased gene bacterial gene richness Decreased Ruminococcus strains |
[275, 279, 282–285, 288] |
| Omega-3 PUFA | Anti-inflammatory; antidepressant; pleiotropic actions | Improve depressive manifestations in BD, but not manic symptoms and seems to attenuate variability in mood, energy, irritability, and pain |
Omega-3 PUFAs modify gut microbiota diversity by increasing the abundance of Bifidobacteria and Akkermansia as well as lowering Enterobacteria abundance Increase of Lactobacillus and Butyrivibrio, together with restoration of Firmicutes/Bacteroidetes ratio |
[290–292] |
| Probiotics/psychobiotics and prebiotics | Health benefits; anti-inflammatory; decrease some risk factors for cardiovascular disease, enhance satiety and promote weight loss | Pro and prebiotics seem to have anxiolytic and antidepressant effect. Its usefulness in BD are promising, but still controversial | Probiotics are live microorganisms (generally Lactobacillus and Bifidobacterium strains) which can interact with gut microbiota, whereas prebiotics induce changes in the composition and/or activity in the gut microbiota, resulting in critical metabolites and products | [79, 295–298, 300, 301, 304, 306] |
| Postbiotics (SCFA) | Anti-inflammatory and immunomodulatory |
SCFAs diminish inflammatory response and apoptosis In rats, sodium butyrate showed to have an antimanic effect SCFA seems to be associated with BD symptomatology |
SCFA could have an epigenetic activity and they may modulate histone acetylation and NF-κB signaling | [205, 207, 311–313] |
| Antimicrobials (tetracyclines, i.e., minocycline and doxycycline) | Antibiotic and antidepressant effect |
Anti-inflammatory and neuroprotective activity. Tetracycline antibiotics might have a lithium-like effect and an antidepressants activity Minocycline may be useful for patients with high GSH levels Aspirin and minocycline combination shown to be effective in BD depression |
Minocycline modulates glutamate and monoaminergic pathways. It also Reduces gut microbiota diversity and reduces the Firmicutes/Bacteroidetes ratio Decreases Allobaculum, Bifidobacterium, Turicibacter and Clostridium genera. In humans, it lowers Lactobacillus salivarius, Bifidobacterium adolescentis and Bifidobacterium breve strains Increases Akkermansia, Lachnospiraceae and Ruminococcaceae incertae sedis Doxycycline drives to a short-term reduction of lactic acid bacteria like Bifidobacterium or Lactobacillus |
[316–319, 323–325] |
| Fecal microbiota transplantation (FMT) | Gut microbiota restoration | A woman diagnosed with BD went through FMT from her healthy husband at least nine times. Afterward, she had neither manic nor depressive symptoms in the following sixth months, also presenting an important weight loss of nearly 33 kg | Possible beneficial changes of gut microbiota according to the healthy donor | [329, 331] |
| Immune-based approaches | Anti-inflammatory; antidepressant effects |
Celecoxib reduces anxiety and accelerates treatment response in BD TNF-α inhibitors have also shown antidepressant properties NAC can be beneficial for alleviating depressive symptoms in patients with BD |
Celecoxib does not seem to alter bacterial abundance, but relative changes in some bacterial populations have been described and it reduces butyrate production The administration of infliximab did not show significant differences in gut microbiota composition NAC alleviates gut dysbiosis and alterations in glucose metabolism in mice |
[333, 334, 336–338, 340–342] |
| Physical activity | Pleiotropic effects |
Patients with BD are less active and more sedentary than general population Physical activity can be a promising coadjutant to pharmacotherapy in BD patients |
Physical activity widely increases bacterial diversity and beneficial bacteria | [344–347] |
| Light therapy and social rhythm therapy | Mood stabilizer; improves quality of life | Increases remission rates in BD. Clinical benefits have been reported, but the capacity of ameliorate mood symptoms and preventing episodes is yet uncertain |
Beneficial changes in gut microbiota have been reported. Increases in Akkermansia muciniphila, Bifidobacterium sp., and Faecalibacterium sp Decreases in the Firmicutes:Bacteroides ratio |
[350–352] |
Conclusions
The dialog between MGB axis and immune system represents a crucial biological mechanism in BD, being tightly linked to other pathophysiological events. There seems to be an interplay within aberrant neurotransmission, chronic low-grade inflammation and host metabolic state that would explain characteristic mood fluctuations in this complex and incapacitating disorder. In our search for evidence about BD-gut microbiota, we realized that there seem to be more studies related to depressive symptoms and changes in the dynamism of the gut microbiota (different concentrations of SCFAs or relative bacterial abundances). Hence, it would be equally important to deepen investigations related to other clinical phases like hypomania/mania and mixed features, also differencing between type I and type II BD. Pharmacological treatment of BD, which is of great importance in the clinical management of BD, has also critical effects on gut microbiota, opening the opportunity to use this knowledge as potential biomarkers. Finally, the very probable involvement of gut microbiota in the pathogenesis of this psychiatric disorder opens promising approaches to modulate the gut microbiota and the MGB axis, including dietary interventions, the use of pre-, pro- and postbiotics and other therapeutic approaches like FMT and other lifestyle interventions. Overall, the MGB axis represents an additional but a critical point of study in the complex field of BD and deepening on this exciting line of research could allow the development of promising translational approaches together with better monitoring of BD individuals, potentially improving the clinical management and quality of life of these patients.
Acknowledgements
This study was partially supported by grants from the Fondo de Investigación de la Seguridad Social, Instituto de Salud Carlos III (PI21/01252), Spain, Programa de Actividades de I+D de la Comunidad de Madrid en Biomedicina (P2022/BMD-7321), and HALEKULANI S.L. and MJR.
Author contributions
MAO: conceptualization, investigation, formal analysis, visualization, writing—original draft. MAÁ-M: conceptualization, writing—review and editing. CG-M: conceptualization, investigation, formal analysis, visualization, writing—original draft. OF-M: conceptualization, investigation, formal analysis, visualization, writing—original draft. JM: conceptualization, writing—review and editing. LM-R: conceptualization, writing—review and editing. RR-J: conceptualization, writing—review and editing. MÁ-M: conceptualization, supervision, funding acquisition, writing—review and editing. GL: conceptualization, supervision, funding acquisition, writing—review and editing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Melchor Álvarez-Mon, Guillermo Lahera.
References
- 1.Jain A, Mitra P. Bipolar affective disorder. Treasure Island (FL): StatPearls Publishing; 2022.
- 2.Bolton S, Warner J, Harriss E, Geddes J, Saunders KEA. Bipolar disorder: trimodal age‐at‐onset distribution. Bipolar Disord. 2021;23:341–56. doi: 10.1111/BDI.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Association: Arlington, VA, USA; 2013.
- 4.World Health Organization. ICD-11: International Classification of Diseases (11th Revision). 2019.
- 5.Charney AW, Ruderfer DM, Stahl EA, Moran JL, Chambert K, Belliveau RA, et al. Evidence for genetic heterogeneity between clinical subtypes of bipolar disorder. Transl Psychiatry. 2017;7. 10.1038/TP.2016.242. [DOI] [PMC free article] [PubMed]
- 6.Muneer A. Mixed states in bipolar disorder: etiology, pathogenesis and treatment. Chonnam Med. J. 2017;53:1. doi: 10.4068/CMJ.2017.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzman-Parra J, Streit F, Forstner AJ, Strohmaier J, González MJ, Gil Flores S, et al. Clinical and genetic differences between bipolar disorder type 1 and 2 in multiplex families. Transl Psychiatry. 2021;11. 10.1038/S41398-020-01146-0. [DOI] [PMC free article] [PubMed]
- 8.Tondo L, Miola A, Pinna M, Contu M, Baldessarini RJ. Differences between bipolar disorder types 1 and 2 support the DSM two-syndrome concept. Int J Bipolar Disord. 2022;10. 10.1186/S40345-022-00268-2. [DOI] [PMC free article] [PubMed]
- 9.Faurholt-Jepsen M, Frost M, Busk J, Christensen EM, Bardram JE, Vinberg M, et al. Differences in mood instability in patients with bipolar disorder type I and II: a smartphone-based study. Int J Bipolar Disord. 2019;7. 10.1186/S40345-019-0141-4. [DOI] [PMC free article] [PubMed]
- 10.Clemente AS, Diniz BS, Nicolato R, Kapczinski FP, Soares JC, Firmo JO, et al. Bipolar disorder prevalence: a systematic review and meta-analysis of the literature. Braz J Psychiatry. 2015;37:155–61. doi: 10.1590/1516-4446-2012-1693. [DOI] [PubMed] [Google Scholar]
- 11.Dell’Osso B, Cafaro R, Ketter TA. Has bipolar disorder become a predominantly female gender related condition? Analysis of recently published large sample studies. Int J Bipolar Disord. 2021;9:3. doi: 10.1186/S40345-020-00207-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naguy A. Bipolar in women: any gender-based difference. Indian J Psychol Med. 2017;39:381–2. doi: 10.4103/0253-7176.207345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97–170. doi: 10.1111/BDI.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraiss JT, Wijnen B, Kupka RW, Bohlmeijer ET, Lokkerbol J. Economic evaluations of non-pharmacological interventions and cost-of-illness studies in bipolar disorder: a systematic review. J Affect Disord. 2020;276:388–401. doi: 10.1016/J.JAD.2020.06.064. [DOI] [PubMed] [Google Scholar]
- 15.Shumet S, W/michele B, Angaw D, Ergete T, Alemnew N. Magnitude of internalised stigma and associated factors among people with bipolar disorder at Amanuel Mental Specialized Hospital, Addis Ababa, Ethiopia: a cross-sectional study. BMJ Open. 2021;11. 10.1136/BMJOPEN-2020-044824. [DOI] [PMC free article] [PubMed]
- 16.Schaffer A, Isometsä ET, Tondo L, Moreno DH, Sinyor M, Lars Vedel K, et al. Epidemiology, neurobiology and pharmacological interventions related to suicide deaths and suicide attempts in bipolar disorder: Part I of a Report of the International Society for Bipolar Disorders Task Force on Suicide in Bipolar Disorder. Aust N Z J Psychiatry. 2015;49:785–802. doi: 10.1177/0004867415594427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angst J, Ajdacic-Gross V, Rössler W. Bipolar disorders in ICD-11: current status and strengths. Int J Bipolar Disord. 2020;8. 10.1186/S40345-019-0165-9. [DOI] [PMC free article] [PubMed]
- 18.Harrison PJ, Cipriani A, Harmer CJ, Nobre AC, Saunders K, Goodwin GM, et al. Innovative approaches to bipolar disorder and its treatment. Ann N Y Acad Sci. 2016;1366:76–89. doi: 10.1111/NYAS.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51:793–803. doi: 10.1038/S41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci. 2018;41:18–30. doi: 10.1016/J.TINS.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldinger F, Schulze TG. Environmental factors, life events, and trauma in the course of bipolar disorder. Psychiatry Clin Neurosci. 2017;71:6. doi: 10.1111/PCN.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahgoub M, Monteggia LM. Epigenetics and psychiatry. Neurotherapeutics. 2013;10:734. doi: 10.1007/S13311-013-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortega MA, Fraile-Martínez Ó, García-Montero C, Alvarez-Mon MA, Lahera G, Monserrat J, et al. Nutrition, epigenetics, and major depressive disorder: understanding the connection. Front Nutr. 2022;9. 10.3389/FNUT.2022.867150. [DOI] [PMC free article] [PubMed]
- 24.Herceg Z. Epigenetic mechanisms as an interface between the environment and genome. Adv Exp Med Biol. 2016;903:3–15. doi: 10.1007/978-1-4899-7678-9_1. [DOI] [PubMed] [Google Scholar]
- 25.Legrand A, Iftimovici A, Khayachi A, Chaumette B. Epigenetics in bipolar disorder: a critical review of the literature. Psychiatr Genet. 2021;31:1–12. doi: 10.1097/YPG.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 26.Nanou E, Catterall WA. Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron. 2018;98:466–81. doi: 10.1016/J.NEURON.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Won E, Kim YK. An oldie but goodie: lithium in the treatment of bipolar disorder through neuroprotective and neurotrophic mechanisms. Int J Mol Sci. 2017;18. 10.3390/IJMS18122679. [DOI] [PMC free article] [PubMed]
- 28.Yksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:794. doi: 10.1016/J.BIOPSYCH.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolaus S, Müller HW, Hautzel H. Different patterns of dopaminergic and serotonergic dysfunction in manic, depressive and euthymic phases of bipolar disorder. Nuklearmedizin. 2017;56:191–200. doi: 10.3413/NUKMED-0893-17-04. [DOI] [PubMed] [Google Scholar]
- 30.Van Enkhuizen J, Janowsky DS, Olivier B, Minassian A, Perry W, Young JW, et al. The catecholaminergic-cholinergic balance hypothesis of bipolar disorder revisited. Eur J Pharmacol. 2015;753:114–26. doi: 10.1016/J.EJPHAR.2014.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J, Badner JA, Hattori E, Potash JB, Willour VL, McMahon FJ, et al. Neurotransmission and bipolar disorder: a systematic family-based association study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1270–7. doi: 10.1002/AJMG.B.30769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigitova E, Fišar Z, Hroudová J, Cikánková T, Raboch J. Biological hypotheses and biomarkers of bipolar disorder. Psychiatry Clin Neurosci. 2017;71:77–103. doi: 10.1111/PCN.12476. [DOI] [PubMed] [Google Scholar]
- 33.Brady RO, Mccarthy JM, Prescot AP, Jensen JE, Cooper AJ, Cohen BM, et al. Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar Disord. 2013;15:434–9. doi: 10.1111/BDI.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KS, Park YM, Lee SH. Serotonergic dysfunction in patients with bipolar disorder assessed by the loudness dependence of the auditory evoked potential. Psychiatry Investig. 2012;9:298–306. doi: 10.4306/PI.2012.9.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hannestad JO, Cosgrove KP, Dellagioia NF, Perkins E, Bois F, Bhagwagar Z, et al. Changes in the cholinergic system between bipolar depression and euthymia as measured with [123I]5IA single photon emission computed tomography. Biol Psychiatry. 2013;74:768–76. doi: 10.1016/J.BIOPSYCH.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and roadmap for future research. Am J Psychiatry. 2014;171:829. doi: 10.1176/APPI.AJP.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sepede G, Chiacchiaretta P, Gambi F, Di Iorio G, De Berardis D, Ferretti A, et al. Bipolar disorder with and without a history of psychotic features: FMRI correlates of sustained attention. Prog Neuropsychopharmacol Biol Psychiatry. 2020;98:109817. doi: 10.1016/J.PNPBP.2019.109817. [DOI] [PubMed] [Google Scholar]
- 38.Gandhi AB, Kaleem I, Alexander J, Hisbulla M, Kannichamy V, Antony I, et al. Neuroplasticity improves bipolar disorder: a review. Cureus. 2020;12. 10.7759/CUREUS.11241. [DOI] [PMC free article] [PubMed]
- 39.Kucharska-Mazur J, Jabłoński M, Misiak B, Frydecka D, Rybakowski J, Ratajczak MZ, et al. Adult stem cells in psychiatric disorders – new discoveries in peripheral blood. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:23–27. doi: 10.1016/J.PNPBP.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Fernandes BS, Molendijk ML, Köhler CA, Soares JC, Leite CMGS, Machado-Vieira R, et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Med. 2015;13:1–22. doi: 10.1186/S12916-015-0529-7/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brockway DF, Crowley NA. Turning the ′tides on neuropsychiatric diseases: the role of peptides in the prefrontal cortex. Front Behav Neurosci. 2020;14:182. doi: 10.3389/FNBEH.2020.588400/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218:61–68. doi: 10.1016/J.PSYCHRES.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Kato T. Neurobiological basis of bipolar disorder: mitochondrial dysfunction hypothesis and beyond. Schizophr Res. 2017;187:62–66. doi: 10.1016/J.SCHRES.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 44.Viswanath B, Jose SP, Squassina A, Thirthalli J, Purushottam M, Mukherjee O, et al. Cellular models to study bipolar disorder: a systematic review. J Affect Disord. 2015;184:36–50. doi: 10.1016/J.JAD.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 45.Takaesu Y. Circadian rhythm in bipolar disorder: a review of the literature. Psychiatry Clin Neurosci. 2018;72:673–82. doi: 10.1111/PCN.12688. [DOI] [PubMed] [Google Scholar]
- 46.Gold AK, Kinrys G. Treating circadian rhythm disruption in bipolar disorder. Curr Psychiatry Rep. 2019;21:14. doi: 10.1007/S11920-019-1001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belvederi Murri M, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, et al. The HPA axis in bipolar disorder: systematic review and meta-analysis. Psychoneuroendocrinology. 2016;63:327–42. doi: 10.1016/J.PSYNEUEN.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 48.McKinnon MC, Cusi AM, MacQueen GM. Psychological factors that may confer risk for bipolar disorder. Cogn Neuropsychiatry. 2013;18:115–28. doi: 10.1080/13546805.2012.702505. [DOI] [PubMed] [Google Scholar]
- 49.Rosenblat JD, McIntyre RS. Bipolar disorder and immune dysfunction: epidemiological findings, proposed pathophysiology and clinical implications. Brain Sci. 2017;7:144. doi: 10.3390/BRAINSCI7110144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Port JD, Unal SS, Mrazek DA, Marcus SM. Metabolic alterations in medication-free patients with bipolar disorder: a 3T CSF-corrected magnetic resonance spectroscopic imaging study. Psychiatry Res. 2008;162:113–21. doi: 10.1016/J.PSCYCHRESNS.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Rosso G, Cattaneo A, Zanardini R, Gennarelli M, Maina G, Bocchio-Chiavetto L. Glucose metabolism alterations in patients with bipolar disorder. J Affect Disord. 2015;184:293–8. doi: 10.1016/J.JAD.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Fries GR, Zamzow MJ, Andrews T, Pink O, Scaini G, Quevedo J. Accelerated aging in bipolar disorder: a comprehensive review of molecular findings and their clinical implications. Neurosci Biobehav Rev. 2020;112:107–16. doi: 10.1016/J.NEUBIOREV.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 53.Hofer U. Gut–brain axis in ageing. Nat Rev Microbiol. 2022;20:446. doi: 10.1038/s41579-022-00762-5. [DOI] [PubMed] [Google Scholar]
- 54.Appleton J. The gut-brain axis: influence of microbiota on mood and mental health. Integr Med A Clin J. 2018;17:28. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang P, Kong L, Huang H, Pan Y, Zhang D, Jiang J, et al. Gut microbiota – a potential contributor in the pathogenesis of bipolar disorder. Front Neurosci. 2022;16:830748. doi: 10.3389/FNINS.2022.830748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucidi L, Pettorruso M, Vellante F, Di Carlo F, Ceci F, Santovito MC, et al. Gut microbiota and bipolar disorder: an overview on a novel biomarker for diagnosis and treatment. Int J Mol Sci. 2021;22:3723. doi: 10.3390/IJMS22073723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naviaux RK. Metabolic features of the cell danger response. Mitochondrion. 2014;16:7–17. doi: 10.1016/J.MITO.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Levy M, Blacher E, Elinav E. Microbiome, metabolites and host immunity. Curr Opin Microbiol. 2017;35:8–15. doi: 10.1016/j.mib.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Bibbò S, Ianiro G, Giorgio V, Scaldaferri F, Masucci L, Gasbarrini A, et al. The role of diet on gut microbiota composition. Eur Rev Med Pharmacol Sci. 2016;22:4742–9. [PubMed] [Google Scholar]
- 61.Korpela K. Impact of delivery mode on infant gut microbiota. Ann Nutr Metab. 2021;11–9. 10.1159/000518498. [DOI] [PubMed]
- 62.Zhang C, Li L, Jin B, Xu X, Zuo X, Li Y, et al. The effects of delivery mode on the gut microbiota and health: state of art. Front Microbiol. 2021;12:724449. doi: 10.3389/FMICB.2021.724449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chakrabarti A, Geurts L, Hoyles L, Iozzo P, Kraneveld AD, La Fata G, et al. The microbiota–gut–brain axis: pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell Mol Life Sci. 2022;79:1–15. doi: 10.1007/S00018-021-04060-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol. 2020;11. 10.3389/FIMMU.2020.571731. [DOI] [PMC free article] [PubMed]
- 65.Ortega MA, Fraile-Martínez O, Naya I, García-Honduvilla N, Álvarez-Mon M, Buján J, et al. Type 2 diabetes mellitus associated with obesity (Diabesity). The central role of gut microbiota and its translational applications. Nutrients. 2020;12. 10.3390/nu12092749. [DOI] [PMC free article] [PubMed]
- 66.Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2017;11:1–10. doi: 10.1007/S12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 67.Meng C, Bai C, Brown TD, Hood LE, Tian Q. Human gut microbiota and gastrointestinal cancer. Genomics Proteomics Bioinformatics. 2018;16:33–49. doi: 10.1016/J.GPB.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nikolova VL, Hall MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry. 2021;78:1343–54. doi: 10.1001/JAMAPSYCHIATRY.2021.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174:651–60. doi: 10.1002/AJMG.B.32567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ortega MA, Alvarez-Mon MA, García-Montero C, Fraile-Martinez O, Guijarro LG, Lahera G, et al. Gut microbiota metabolites in major depressive disorder-deep insights into their pathophysiological role and potential translational applications. Metabolites. 2022;12. 10.3390/METABO12010050. [DOI] [PMC free article] [PubMed]
- 71.García-Montero C, Fraile-Martínez O, Gómez-Lahoz AM, Pekarek L, Castellanos AJ, Noguerales-Fraguas F, et al. Nutritional components in western diet versus Mediterranean diet at the gut microbiota-immune system interplay. implications for health and disease. Nutrients. 2021;13:1–53. doi: 10.3390/nu13020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lim KH, Staudt LM. Toll-like receptor signaling. Cold Spring Harb Perspect Biol. 2013;5. 10.1101/CSHPERSPECT.A011247. [DOI] [PMC free article] [PubMed]
- 73.Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. doi: 10.3389/FPSYT.2018.00044/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2020;19:241–55. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 75.Coello K, Hansen TH, Sørensen N, Munkholm K, Kessing LV, Pedersen O, et al. Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain Behav Immun. 2019;75:112–8. doi: 10.1016/J.BBI.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 76.Ni J-J, Xu Q, Yan S-S, Han B-X, Zhang H, Wei X-T, et al. Gut microbiota and psychiatric disorders: a two-sample mendelian randomization study. Front Microbiol. 2022;12:737197. doi: 10.3389/FMICB.2021.737197/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, et al. Bifidobacterium and Lactobacillus counts in the gut microbiota of patients with bipolar disorder and healthy controls. Front Psychiatry. 2019;9:730. doi: 10.3389/FPSYT.2018.00730/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu Q, Lai J, Lu H, Ng C, Huang T, Zhang H, et al. Gut microbiota in bipolar depression and its relationship to brain function: an advanced exploration. Front Psychiatry. 2019;10:784. doi: 10.3389/FPSYT.2019.00784/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dickerson F, Adamos M, Katsafanas E, Khushalani S, Origoni A, Savage C, et al. Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: a randomized controlled trial. Bipolar Disord. 2018;20:614–21. doi: 10.1111/BDI.12652. [DOI] [PubMed] [Google Scholar]
- 80.Bengesser SA, Mörkl S, Painold A, Dalkner N, Birner A, Fellendorf FT, et al. Epigenetics of the molecular clock and bacterial diversity in bipolar disorder. Psychoneuroendocrinology. 2019;101:160–6. doi: 10.1016/J.PSYNEUEN.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 81.Painold A, Mörkl S, Kashofer K, Halwachs B, Dalkner N, Bengesser S, et al. A step ahead: exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019;21:40–49. doi: 10.1111/BDI.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McIntyre RS, Subramaniapillai M, Shekotikhina M, Carmona NE, Lee Y, Mansur RB, et al. Characterizing the gut microbiota in adults with bipolar disorder: a pilot study. Nutr Neurosci. 2021;24:173–80. doi: 10.1080/1028415X.2019.1612555. [DOI] [PubMed] [Google Scholar]
- 83.Rhee SJ, Kim H, Lee Y, Lee HJ, Park CHK, Yang J, et al. Comparison of serum microbiome composition in bipolar and major depressive disorders. J Psychiatr Res. 2020;123:31–38. doi: 10.1016/J.JPSYCHIRES.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 84.Zheng P, Yang J, Li Y, Wu J, Liang W, Yin B, et al. Gut microbial signatures can discriminate unipolar from bipolar depression. Adv Sci (Weinh) 2020;7:1902862. doi: 10.1002/advs.201902862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rong H, Xie XH, Zhao J, Lai WT, Wang MB, Xu D, et al. Similarly in depression, nuances of gut microbiota: evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J Psychiatr Res. 2019;113:90–99. doi: 10.1016/J.JPSYCHIRES.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 86.McGuinness AJ, Davis JA, Dawson SL, Loughman A, Collier F, O’Hely M, et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol Psychiatry. 2022;2022:1–16. doi: 10.1038/s41380-022-01456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nguyen TT, Kosciolek T, Eyler LT, Knight R, Jeste DV. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J Psychiatr Res. 2018;99:50–61. doi: 10.1016/J.JPSYCHIRES.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schoultz I, Keita ÅV. The intestinal barrier and current techniques for the assessment of gut permeability. Cells. 2020;9:1909. doi: 10.3390/CELLS9081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4:e1251384. doi: 10.1080/21688370.2016.1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maget A, Dalkner N, Hamm C, Bengesser SA, Fellendorf FT, Platzer M, et al. Sex differences in zonulin in affective disorders and associations with current mood symptoms. J Affect Disord. 2021;294:441–6. doi: 10.1016/J.JAD.2021.07.021. [DOI] [PubMed] [Google Scholar]
- 91.Kılıç F, Işık Ü, Demirdaş A, Doğuç DK, Bozkurt M. Serum zonulin and claudin-5 levels in patients with bipolar disorder. J Affect Disord. 2020;266:37–42. doi: 10.1016/J.JAD.2020.01.117. [DOI] [PubMed] [Google Scholar]
- 92.Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10:373. doi: 10.1038/S41398-020-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–90. doi: 10.1016/J.CGH.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park SH, Videlock EJ, Shih W, Presson AP, Mayer EA, Chang L. Adverse childhood experiences are associated with irritable bowel syndrome and gastrointestinal symptom severity. Neurogastroenterol Motil. 2016;28:1252–60. doi: 10.1111/NMO.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Misiak B, Łoniewski I, Marlicz W, Frydecka D, Szulc A, Rudzki L, et al. The HPA axis dysregulation in severe mental illness: can we shift the blame to gut microbiota? Prog Neuropsychopharmacology Biol Psychiatry. 2020;102. 10.1016/J.PNPBP.2020.109951. [DOI] [PubMed]
- 96.Kinashi Y, Hase K. Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Front Immunol. 2021;12. 10.3389/FIMMU.2021.673708. [DOI] [PMC free article] [PubMed]
- 97.Stevens BR, Goel R, Seungbum K, Richards EM, Holbert RC, Pepine CJ, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67:1555–7. doi: 10.1136/GUTJNL-2017-314759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ishida I, Ogura J, Aizawa E, Ota M, Hidese S, Yomogida Y, et al. Gut permeability and its clinical relevance in schizophrenia. Neuropsychopharmacol Rep. 2022. 10.1002/NPR2.12227. [DOI] [PMC free article] [PubMed]
- 99.Liu CJ, Hu LY, Yeh CM, Hu YW, Chen PM, Chen TJ, et al. Irritable brain caused by irritable bowel? A nationwide analysis for irritable bowel syndrome and risk of bipolar disorder. PLoS One. 2015;10. 10.1371/JOURNAL.PONE.0118209. [DOI] [PMC free article] [PubMed]
- 100.Giovanni Carta M, Conti A, Lecca F, Sancassiani F, Cossu G, Carruxi R, et al. The burden of depressive and bipolar disorders in celiac disease. Clin Pract Epidemiol Ment Heal. 2015;11:180–5. doi: 10.2174/1745017901511010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord. 2010;12:638–46. doi: 10.1111/J.1399-5618.2010.00853.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kao LT, Lin HC, Lee HC. Inflammatory bowel disease and bipolar disorder: a population-based cross-sectional study. J Affect Disord. 2019;247:120–4. doi: 10.1016/J.JAD.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 103.Bernstein CN, Hitchon CA, Walld R, Bolton JM, Sareen J, Walker JR, et al. Increased burden of psychiatric disorders in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:360–8. doi: 10.1093/IBD/IZY235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jakobsson HE, Rodríguez-Piñeiro AM, Schütte A, Ermund A, Boysen P, et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16:164. doi: 10.15252/EMBR.201439263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li XJ, You XY, Wang CY, Li XL, Sheng YY, Zhuang PW, et al. Bidirectional brain‐gut‐microbiota axis in increased intestinal permeability induced by central nervous system injury. CNS Neurosci Ther. 2020;26:783–90. doi: 10.1111/CNS.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hayes CL, Dong J, Galipeau HJ, Jury J, McCarville J, Huang X, et al. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci Rep. 2018;8:14184. doi: 10.1038/S41598-018-32366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blacher E, Levy M, Tatirovsky E, Elinav E. Microbiome-modulated metabolites at the interface of host immunity. J Immunol. 2017;198:572–80. doi: 10.4049/JIMMUNOL.1601247. [DOI] [PubMed] [Google Scholar]
- 108.Olsen AB, Hetz RA, Xue H, Aroom KR, Bhattarai D, Johnson E, et al. Effects of traumatic brain injury on intestinal contractility. Neurogastroenterol Motil. 2013;25:593. doi: 10.1111/NMO.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94. doi: 10.1016/J.BBI.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 110.Severance EG, Gressitt KL, Yang S, Stallings CR, Origoni AE, Vaughan C, et al. Seroreactive marker for inflammatory bowel disease and associations with antibodies to dietary proteins in bipolar disorder. Bipolar Disord. 2014;16:230–40. doi: 10.1111/BDI.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, et al. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophr. 2016;2:16018. doi: 10.1038/NPJSCHZ.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. 2013;148:130–7. doi: 10.1016/J.SCHRES.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Markers of gluten sensitivity in acute mania: a longitudinal study. Psychiatry Res. 2012;196:68–71. doi: 10.1016/J.PSYCHRES.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 114.Hollander D, Kaunitz JD. The “leaky gut”: tight junctions but loose associations? Dig Dis Sci. 2020;65:1277–87. doi: 10.1007/S10620-019-05777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sundman MH, Chen NK, Subbian V, Chou YH. The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun. 2017;66:31–44. doi: 10.1016/J.BBI.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 116.Stehle JR, Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol A Biol Sci Med Sci. 2012;67:1212–8. doi: 10.1093/GERONA/GLS178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saad MJA, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology. 2016;31:283–93. doi: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- 118.Scott SA, Fu J, Chang PV. Microbial tryptophan metabolites regulate gut barrier function via the Aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2020;117:19376–87. doi: 10.1073/PNAS.2000047117/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andrews C, McLean MH, Durum SK. Cytokine tuning of intestinal epithelial function. Front Immunol. 2018;9. 10.3389/FIMMU.2018.01270. [DOI] [PMC free article] [PubMed]
- 120.Jin Y, Blikslager AT. The regulation of intestinal mucosal barrier by myosin light chain kinase/Rho kinases. Int J Mol Sci. 2020;21:3550. doi: 10.3390/IJMS21103550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Al-Sadi R, Guo S, Ye D, Rawat M, Ma TY. TNF-α modulation of intestinal tight junction permeability is mediated by NIK/IKK-α axis activation of the canonical NF-ΚB pathway. Am J Pathol. 2016;186:1151–65. doi: 10.1016/J.AJPATH.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaminsky LW, Al-Sadi R, Ma TY. IL-1β and the intestinal epithelial tight junction barrier. Front Immunol. 2021;12. 10.3389/FIMMU.2021.767456. [DOI] [PMC free article] [PubMed]
- 123.Li X, Akhtar S, Choudhry MA. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochim Biophys Acta. 2012;1822:196–203. doi: 10.1016/J.BBADIS.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nowarski R, Jackson R, Gagliani N, De Zoete MR, Palm NW, Bailis W, et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell. 2015;163:1444–56. doi: 10.1016/J.CELL.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Munkholm K, Weikop P, Kessing LV, Vinberg M. Elevated levels of IL-6 and IL-18 in manic and hypomanic states in rapid cycling bipolar disorder patients. Brain Behav Immun. 2015;43:205–13. doi: 10.1016/J.BBI.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 126.Luo Y, He H, Zhang M, Huang X, Fan N. Altered serum levels of TNF-α, IL-6 and IL-18 in manic, depressive, mixed state of bipolar disorder patients. Psychiatry Res. 2016;244:19–23. doi: 10.1016/J.PSYCHRES.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 127.Castaño-Ramírez OM, Sepúlveda-Arias JC, Duica K, Díaz Zuluaga AM, Vargas C, López-Jaramillo C. Inflammatory markers in the staging of bipolar disorder: a systematic review of the literature. Rev Colomb Psiquiatr. 2018;47:119–28. doi: 10.1016/J.RCP.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 128.Jones GH, Vecera CM, Pinjari OF, Machado-Vieira R. Inflammatory signaling mechanisms in bipolar disorder. J Biomed Sci. 2021;28:45. doi: 10.1186/S12929-021-00742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Isgren A, Sellgren C, Ekman CJ, Holmén-Larsson J, Blennow K, Zetterberg H, et al. Markers of neuroinflammation and neuronal injury in bipolar disorder: relation to prospective clinical outcomes. Brain Behav Immun. 2017;65:195–201. doi: 10.1016/J.BBI.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 130.Wieck A, Grassi-Oliveira R, do Prado CH, Rizzo LB, de Oliveira AS, Kommers-Molina J, et al. Differential neuroendocrine and immune responses to acute psychosocial stress in women with type 1 bipolar disorder. Brain Behav Immun. 2013;34:47–55. doi: 10.1016/J.BBI.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 131.McNamara RK, Lotrich FE. Elevated immune-inflammatory signaling in mood disorders: a new therapeutic target? Expert review of neurotherapeutics. Expert Rev Neurother. 2012;12:1143–61. doi: 10.1586/ERN.12.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–92. doi: 10.1016/J.BIOPSYCH.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 133.Barbosa IG, Morato IB, De Miranda AS, Bauer ME, Soares JC, Teixeira AL. A preliminary report of increased plasma levels of il-33 in bipolar disorder: further evidence of pro-inflammatory status. J Affect Disord. 2014;157:41–44. doi: 10.1016/J.JAD.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 134.Gorgulu Y, Uluturk MK, Palabiyik O. Comparison of serum BDNF, IL-1β, IL-6, TNF-α, CRP and leucocyte levels in unipolar mania and bipolar disorder. Acta Neuropsychiatr. 2021;33:317–22. doi: 10.1017/NEU.2021.25. [DOI] [PubMed] [Google Scholar]
- 135.Benedetti F, Aggio V, Pratesi ML, Greco G, Furlan R. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71. doi: 10.3389/FPSYT.2020.00071/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Becking K, Haarman BCM, Grosse L, Nolen WA, Claes S, Arolt V, et al. The circulating levels of CD4+ t helper cells are higher in bipolar disorder as compared to major depressive disorder. J Neuroimmunol. 2018;319:28–36. doi: 10.1016/J.JNEUROIM.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 137.Do Prado CH, Rizzo LB, Wieck A, Lopes RP, Teixeira AL, Grassi-Oliveira R, et al. Reduced regulatory T cells are associated with higher levels of Th1/TH17 cytokines and activated MAPK in type 1 bipolar disorder. Psychoneuroendocrinology. 2013;38:667–76. doi: 10.1016/J.PSYNEUEN.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 138.Barbosa IG, Bauer ME, MacHado-Vieira R, Teixeira AL. Cytokines in bipolar disorder: paving the way for neuroprogression. Neural Plast. 2014;2014:360481. doi: 10.1155/2014/360481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoogland ICM, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12. 10.1186/S12974-015-0332-6/TABLES/5. [DOI] [PMC free article] [PubMed]
- 140.Zhao NO, Topolski N, Tusconi M, Salarda EM, Busby CW, Lima CNNC, et al. Blood-brain barrier dysfunction in bipolar disorder: molecular mechanisms and clinical implications. Brain Behav Immun Health. 2022;21:100441. doi: 10.1016/J.BBIH.2022.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Calkin C, McClelland C, Cairns K, Kamintsky L, Friedman A. Insulin resistance and blood-brain barrier dysfunction underlie neuroprogression in bipolar disorder. Front Psychiatry. 2021;12:636174. doi: 10.3389/FPSYT.2021.636174/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139:136–53. doi: 10.1111/JNC.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Patel JP, Frey BN. Disruption in the blood-brain barrier: the missing link between brain and body inflammation in bipolar disorder. Neural Plast. 2015;2015:708306. doi: 10.1155/2015/708306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barysheva M, Jahanshad N, Foland-Ross L, Altshuler LL, Thompson PM. White matter microstructural abnormalities in bipolar disorder: a whole brain diffusion tensor imaging study. Neuroimage Clin. 2013;2:558–68. doi: 10.1016/J.NICL.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rea K, Dinan TG, Cryan JF. The microbiome: a key regulator of stress and neuroinflammation. Neurobiol Stress. 2016;4:23–33. doi: 10.1016/J.YNSTR.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ahmad MH, Rizvi MA, Fatima M, Chandra Mondal A. Pathophysiological implications of neuroinflammation mediated HPA axis dysregulation in the prognosis of cancer and depression. Mol Cell Endocrinol. 2021;520:111093. doi: 10.1016/J.MCE.2020.111093. [DOI] [PubMed] [Google Scholar]
- 147.Yang L, Zhou Y, Jia H, Qi Y, Tu S, Shao A. Affective immunology: the crosstalk between microglia and astrocytes plays key role? Front Immunol. 2020;11. 10.3389/FIMMU.2020.01818. [DOI] [PMC free article] [PubMed]
- 148.Carloni S, Bertocchi A, Mancinelli S, Bellini M, Erreni M, Borreca A, et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science. 2021;374:439–48. doi: 10.1126/SCIENCE.ABC6108. [DOI] [PubMed] [Google Scholar]
- 149.Dogan AE, Yuksel C, Du F, Chouinard VA, Öngür D. Brain lactate and PH in schizophrenia and bipolar disorder: a systematic review of findings from magnetic resonance studies. Neuropsychopharmacology. 2018;43:1681–90. doi: 10.1038/S41386-018-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cyrino LAR, Delwing-de Lima D, Ullmann OM, Maia TP. Concepts of neuroinflammation and their relationship with impaired mitochondrial functions in bipolar disorder. Front Behav Neurosci. 2021;15. 10.3389/FNBEH.2021.609487. [DOI] [PMC free article] [PubMed]
- 151.Madireddy S, Madireddy S. Therapeutic interventions to mitigate mitochondrial dysfunction and oxidative stress-induced damage in patients with bipolar disorder. Int J Mol Sci. 2022;23:1844. doi: 10.3390/IJMS23031844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kato T. Current understanding of bipolar disorder: toward integration of biological basis and treatment strategies. Psychiatry Clin Neurosci. 2019;73:526–40. doi: 10.1111/PCN.12852. [DOI] [PubMed] [Google Scholar]
- 153.Griffin P, Dimitry JM, Sheehan PW, Lananna BV, Guo C, Robinette ML, et al. Circadian clock protein Rev-Erbα regulates neuroinflammation. Proc Natl Acad Sci USA. 2019;116:5102–7. doi: 10.1073/PNAS.1812405116/SUPPL_FILE/PNAS.1812405116.SM02.MP4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang XL, Li L. Circadian clock regulates inflammation and the development of neurodegeneration. Front Cell Infect Microbiol. 2021;11:696554. doi: 10.3389/FCIMB.2021.696554/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zielinski MR, Gibbons AJ. Neuroinflammation, sleep, and circadian rhythms. Front Cell Infect Microbiol. 2022;12:853096. doi: 10.3389/FCIMB.2022.853096/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Morris G, Stubbs B, Köhler CA, Walder K, Slyepchenko A, Berk M, et al. The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med Rev. 2018;41:255–65. doi: 10.1016/J.SMRV.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 157.Steardo LJ, De Filippis R, Carbone EA, Segura-Garcia C, Verkhratsky A, De Fazio P. Sleep disturbance in bipolar disorder: neuroglia and circadian rhythms. Front Psychiatry. 2019;10:501. doi: 10.3389/FPSYT.2019.00501/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sato A, Hashimoto T, Kimura A, Niitsu T, Iyo M. Psychological distress symptoms associated with life events in patients with bipolar disorder: a cross-sectional study. Front Psychiatry. 2018;9:200. doi: 10.3389/FPSYT.2018.00200/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aas M, Ueland T, Inova A, Melle I, Andreassen OA, Steen NE. Childhood trauma is nominally associated with elevated cortisol metabolism in severe mental disorder. Front Psychiatry. 2020;11. 10.3389/FPSYT.2020.00391. [DOI] [PMC free article] [PubMed]
- 160.Tournikioti K, Dikeos D, Alevizaki M, Michopoulos I, Ferentinos P, Porichi E, et al. Hypothalamus-pituitary-adrenal (HPA) axis parameters and neurocognitive evaluation in patients with bipolar disorder. Psychiatrike. 2018;29:199–208. doi: 10.22365/JPSYCH.2018.293.199. [DOI] [PubMed] [Google Scholar]
- 161.Tournikioti K, Alevizaki M, Michopoulos I, Mantzou A, Soldatos CR, Douzenis A, et al. Differential association of cortisol with visual memory/learning and executive function in bipolar disorder. Psychiatry Res. 2022;307. 10.1016/J.PSYCHRES.2021.114301. [DOI] [PubMed]
- 162.Lee HH, Chang CH, Wang LJ, Wu CC, Chen HL, Lu T, et al. The correlation between longitudinal changes in hypothalamic-pituitary-adrenal (HPA)-axis activity and changes in neurocognitive function in mixed-state bipolar II disorder. Neuropsychiatr Dis Treat. 2018;14:2703–13. doi: 10.2147/NDT.S173616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aas M, Pizzagalli DA, Laskemoen JF, Reponen EJ, Ueland T, Melle I, et al. Elevated hair cortisol is associated with childhood maltreatment and cognitive impairment in schizophrenia and in bipolar disorders. Schizophr Res. 2019;213:65–71. doi: 10.1016/J.SCHRES.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 164.Iob E, Steptoe A. Cardiovascular disease and hair cortisol: a novel biomarker of chronic stress. Curr Cardiol Rep. 2019;21:116. doi: 10.1007/S11886-019-1208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Crawford AA, Soderberg S, Kirschbaum C, Murphy L, Eliasson M, Ebrahim S, et al. Morning plasma cortisol as a cardiovascular risk factor: findings from prospective cohort and Mendelian randomization studies. Eur J Endocrinol. 2019;181:429–38. doi: 10.1530/EJE-19-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maripuu M, Wikgren M, Karling P, Adolfsson R, Norrback KF. Relative hypocortisolism is associated with obesity and the metabolic syndrome in recurrent affective disorders. J Affect Disord. 2016;204:187–96. doi: 10.1016/J.JAD.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 167.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812–27. doi: 10.1016/J.CELL.2013.04.020/ATTACHMENT/C4986E52-C823-458C-A35F-FE2F700249D1/MMC3.XLS. [DOI] [PubMed] [Google Scholar]
- 168.Farzi A, Fröhlich EE, Holzer P. Gut microbiota and the neuroendocrine system. Neurotherapeutics. 2018;15:5–22. doi: 10.1007/S13311-017-0600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Krautkramer KA, Fan J, Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2020;19:77–94. doi: 10.1038/s41579-020-0438-4. [DOI] [PubMed] [Google Scholar]
- 170.Manji HK, Quiroz JA, Payne JL, Singh J, Lopes BP, Viegas JS, et al. The underlying neurobiology of bipolar disorder. World Psychiatry. 2003;2:136–46. [PMC free article] [PubMed] [Google Scholar]
- 171.Chen Y, Xu J, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. 2021;13:2099. doi: 10.3390/NU13062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–76. doi: 10.1016/J.CELL.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, et al. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361:eaat5236. doi: 10.1126/SCIENCE.AAT5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mazzoli R, Pessione E. The neuro-endocrinological role of microbial glutamate and GABA signaling. Front Microbiol. 2016;7. 10.3389/FMICB.2016.01934. [DOI] [PMC free article] [PubMed]
- 175.Li H, Cao Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amin Acids. 2010;39:1107–16. doi: 10.1007/S00726-010-0582-7. [DOI] [PubMed] [Google Scholar]
- 176.Gao K, Mu CL, Farzi A, Zhu WY. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr. 2020;11:709–23. doi: 10.1093/ADVANCES/NMZ127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128. doi: 10.1016/J.BRAINRES.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–95. doi: 10.1152/AJPGI.00341.2012. [DOI] [PubMed] [Google Scholar]
- 179.Mou Z, Yang Y, Hall AB, Jiang X. The taxonomic distribution of histamine-secreting bacteria in the human gut microbiome. BMC Genomics. 2021;22:695. doi: 10.1186/S12864-021-08004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Passani MB, Panula P, Lin JS. Histamine in the brain. Front Syst Neurosci. 2014;8. 10.3389/FNSYS.2014.00064/BIBTEX. [DOI] [PMC free article] [PubMed]
- 181.Thakkar MM. Histamine in the regulation of wakefulness. Sleep Med Rev. 2011;15:65–74. doi: 10.1016/J.SMRV.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nuutinen S, Panula P. Histamine in neurotransmission and brain diseases. Adv Exp Med Biol. 2010;709:95–107. doi: 10.1007/978-1-4419-8056-4_10. [DOI] [PubMed] [Google Scholar]
- 183.Riveros ME, Retamal MA. Are polyunsaturated fatty acids implicated in histaminergic dysregulation in bipolar disorder?: an hypothesis. Front Physiol. 2018;9:693. doi: 10.3389/FPHYS.2018.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gondard E, Anaclet C, Akaoka H, Guo RX, Zhang M, Buda C, et al. Enhanced histaminergic neurotransmission and sleep-wake alterations, a study in histamine H3-receptor knock-out mice. Neuropsychopharmacology. 2013;38:1015–31. doi: 10.1038/NPP.2012.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yamada Y, Yoshikawa T, Naganuma F, Kikkawa T, Osumi N, Yanai K. Chronic brain histamine depletion in adult mice induced depression-like behaviours and impaired sleep-wake cycle. Neuropharmacology. 2020;175. 10.1016/J.NEUROPHARM.2020.108179. [DOI] [PubMed]
- 186.Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, et al. Histamine derived from probiotic lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One. 2012;7:e31951. doi: 10.1371/JOURNAL.PONE.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–48. doi: 10.1038/MP.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–45. doi: 10.1016/J.CELL.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 190.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 2020;11:25. doi: 10.3389/FENDO.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Venegas DP, De La Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/FIMMU.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21. 10.3390/IJMS21176356. [DOI] [PMC free article] [PubMed]
- 193.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5. 10.1038/NCOMMS4611. [DOI] [PMC free article] [PubMed]
- 194.Liu H, Wang J, He T, Becker S, Zhang G, Li D, et al. Butyrate: a double-edged sword for health. Adv Nutr. 2018;9:21–29. doi: 10.1093/ADVANCES/NMX009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mihaylova MM, Stratton MS. Chapter 23 – short chain fatty acids as epigenetic and metabolic regulators of neurocognitive health and disease. Nutr Epigenomics. 2019;14:381–97. doi: 10.1016/B978-0-12-816843-1.00023-0. [DOI] [Google Scholar]
- 196.Sublette ME, Cheung S, Lieberman E, Hu S, Mann JJ, Uhlemann AC, et al. Bipolar disorder and the gut microbiome: a systematic review. Bipolar Disord. 2021;23:544–64. doi: 10.1111/BDI.13049. [DOI] [PubMed] [Google Scholar]
- 197.Knudsen KEB, Lærke HN, Hedemann MS, Nielsen TS, Ingerslev AK, Nielsen DSG, et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients. 2018;10:1499. doi: 10.3390/NU10101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–403. doi: 10.1096/FJ.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. doi: 10.1126/SCIENCE.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Iraporda C, Errea A, Romanin DE, Cayet D, Pereyra E, Pignataro O, et al. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology. 2015;220:1161–9. doi: 10.1016/J.IMBIO.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 201.Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, et al. Microbiota-derived short-chain fatty acids promote Th1 Cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. doi: 10.1038/S41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Keir ME, Yi T, Lu TT, Ghilardi N. The role of IL-22 in intestinal health and disease. J Exp Med. 2020;217:e20192195. doi: 10.1084/JEM.20192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Himmerich H, Bartsch S, Hamer H, Mergl R, Schönherr J, Petersein C, et al. Impact of mood stabilizers and antiepileptic drugs on cytokine production in-vitro. J Psychiatr Res. 2013;47:1751–9. doi: 10.1016/J.JPSYCHIRES.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 204.Erny D, Hrabě de Angelis AL, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. 2017;150:7–15. doi: 10.1111/IMM.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Müller B, Rasmusson AJ, Just D, Jayarathna S, Moazzami A, Novicic ZK, et al. Fecal short-chain fatty acid ratios as related to gastrointestinal and depressive symptoms in young adults. Psychosom Med. 2021;83:693–9. doi: 10.1097/PSY.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, et al. Short‐chain fatty acids: microbial metabolites that alleviate stress‐induced brain–gut axis alterations. J Physiol. 2018;596:4923–44. doi: 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Valvassori SS, Dal-Pont GC, Steckert AV, Varela RB, Lopes-Borges J, Mariot E, et al. Sodium butyrate has an antimanic effect and protects the brain against oxidative stress in an animal model of mania induced by ouabain. Psychiatry Res. 2016;235:154–9. doi: 10.1016/J.PSYCHRES.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 208.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/J.BBR.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 209.Kaur H, Bose C, Mande SS. Tryptophan metabolism by gut microbiome and gut-brain-axis: an in silico analysis. Front Neurosci. 2019;13:1365. doi: 10.3389/FNINS.2019.01365/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Halverson T, Alagiakrishnan K. Gut microbes in neurocognitive and mental health disorders. Ann Med. 2020;52:423–43. doi: 10.1080/07853890.2020.1808239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–24. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 212.De Vadder F, Grasset E, Holm LM, Karsenty G, Macpherson AJ, Olofsson LE, et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci USA. 2018;115:6458–63. doi: 10.1073/PNAS.1720017115/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jones RSG. Tryptamine: a neuromodulator or neurotransmitter in mammalian brain. Prog Neurobiol. 1982;19:117–39. doi: 10.1016/0301-0082(82)90023-5. [DOI] [PubMed] [Google Scholar]
- 214.Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495–503. doi: 10.1016/J.CHOM.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jaglin M, Rhimi M, Philippe C, Pons N, Bruneau A, Goustard B, et al. Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats. Front Neurosci. 2018;12:216. doi: 10.3389/FNINS.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Breda C, Sathyasaikumar KV, Idrissi SS, Notarangelo FM, Estranero JG, Moore GGL, et al. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc Natl Acad Sci USA. 2016;113:5435–40. doi: 10.1073/PNAS.1604453113/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Badawy AAB. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:1178646917691938. doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. [DOI] [PMC free article] [PubMed]
- 219.Savitz J. The Kynurenine pathway: a finger in every pie. Mol Psychiatry. 2020;25:131–47. doi: 10.1038/S41380-019-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Comai S, Bertazzo A, Brughera M, Crotti S. Tryptophan in health and disease. Adv Clin Chem. 2020;95:165–218. doi: 10.1016/BS.ACC.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 221.Colín-González AL, Maldonado PD, Santamaría A. 3-Hydroxykynurenine: an intriguing molecule exerting dual actions in the central nervous system. Neurotoxicology. 2013;34:189–204. doi: 10.1016/J.NEURO.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 222.Bo L, Guojun T, Li G. An expanded neuroimmunomodulation axis: SCD83-indoleamine 2,3-dioxygenase—kynurenine pathway and updates of kynurenine pathway in neurologic diseases. Front Immunol. 2018;9:1. doi: 10.3389/FIMMU.2018.01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mithaiwala MN, Santana-Coelho D, Porter GA, O’connor JC. Neuroinflammation and the kynurenine pathway in CNS disease: molecular mechanisms and therapeutic implications. Cells. 2021;10:1548. doi: 10.3390/CELLS10061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Brown SJ, Huang XF, Newell KA. The kynurenine pathway in major depression: what we know and where to next. Neurosci Biobehav Rev. 2021;127:917–27. doi: 10.1016/J.NEUBIOREV.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 225.Schwarcz R. Kynurenines and glutamate: multiple links and therapeutic implications. Adv Pharmacol. 2016;76:13–37. doi: 10.1016/BS.APHA.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Więdłocha M, Marcinowicz P, Janoska-Jaździk M, Szulc A. Gut microbiota, kynurenine pathway and mental disorders – review. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110145. doi: 10.1016/J.PNPBP.2020.110145. [DOI] [PubMed] [Google Scholar]
- 227.Kadriu B, Farmer CA, Yuan P, Park LT, Deng Z, De, et al. The kynurenine pathway and bipolar disorder: intersection of the monoaminergic and glutamatergic systems and immune response. Mol Psychiatry. 2021;26:4085–95. doi: 10.1038/S41380-019-0589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Trepci A, Sellgren CM, Pålsson E, Brundin L, Khanlarkhani N, Schwieler L, et al. Central levels of tryptophan metabolites in subjects with bipolar disorder. Eur Neuropsychopharmacol. 2021;43:52–62. doi: 10.1016/J.EURONEURO.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 229.Bartoli F, Misiak B, Callovini T, Cavaleri D, Cioni RM, Crocamo C, et al. The kynurenine pathway in bipolar disorder: a meta-analysis on the peripheral blood levels of tryptophan and related metabolites. Mol Psychiatry. 2021;26:3419–29. doi: 10.1038/S41380-020-00913-1. [DOI] [PubMed] [Google Scholar]
- 230.Hebbrecht K, Skorobogatov K, Giltay EJ, Coppens V, De Picker L, Morrens M. Tryptophan catabolites in bipolar disorder: a meta-analysis. Front Immunol. 2021;12. 10.3389/FIMMU.2021.667179. [DOI] [PMC free article] [PubMed]
- 231.Marx W, McGuinness AJ, Rocks T, Ruusunen A, Cleminson J, Walker AJ, et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies. Mol Psychiatry. 2021;26:4158–78. doi: 10.1038/S41380-020-00951-9. [DOI] [PubMed] [Google Scholar]
- 232.Fellendorf FT, Gostner JM, Lenger M, Platzer M, Birner A, Maget A, et al. Tryptophan metabolism in bipolar disorder in a longitudinal setting. Antioxidants (Basel, Switz) 1795;2021:10. doi: 10.3390/ANTIOX10111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511. doi: 10.1038/SJ.MP.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.van den Ameele S, van Nuijs ALN, Lai FY, Schuermans J, Verkerk R, van Diermen L, et al. A mood state-specific interaction between kynurenine metabolism and inflammation is present in bipolar disorder. Bipolar Disord. 2020;22:59–69. doi: 10.1111/BDI.12814. [DOI] [PubMed] [Google Scholar]
- 235.Lai WT, Deng WF, Xu SX, Zhao J, Xu D, Liu YH, et al. Shotgun metagenomics reveals both taxonomic and tryptophan pathway differences of gut microbiota in major depressive disorder patients. Psychol Med. 2021;51:90–101. doi: 10.1017/S0033291719003027. [DOI] [PubMed] [Google Scholar]
- 236.Kishi T, Ikuta T, Matsuda Y, Sakuma K, Okuya M, Mishima K, et al. Mood stabilizers and/or antipsychotics for bipolar disorder in the maintenance phase: a systematic review and network meta-analysis of randomized controlled trials. Mol Psychiatry. 2021;26:4146–57. doi: 10.1038/S41380-020-00946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bahji A, Ermacora D, Stephenson C, Hawken ER, Vazquez G. Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: a systematic review and network meta-analysis. J Affect Disord. 2020;269:154–84. doi: 10.1016/J.JAD.2020.03.030. [DOI] [PubMed] [Google Scholar]
- 238.Connolly KR, Thase ME. The clinical management of bipolar disorder: a review of evidence-based guidelines. Prim Care Companion CNS Disord. 2011;13:6. doi: 10.4088/PCC.10R01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chakrabarti S. Treatment-adherence in bipolar disorder: a patient-centred approach. World J Psychiatry. 2016;6:399–409. doi: 10.5498/WJP.V6.I4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Perugi G, Medda P, Toni C, Mariani M, Socci C, Mauri M. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. 2017;15:359–71. doi: 10.2174/1570159X14666161017233642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kanayama M, Hayashida M, Hashioka S, Miyaoka T, Inagaki M. Decreased clostridium abundance after electroconvulsive therapy in the gut microbiota of a patient with schizophrenia. Case Rep Psychiatry. 2019;2019. 10.1155/2019/4576842. [DOI] [PMC free article] [PubMed]
- 242.Young J, Kritzer M, Mischel N, Taekman J, Weiner R. The microbiome-immune-brain axis and cognitive side effects of electroconvulsive therapy. Biol Psychiatry. 2020;87:S450–S451. doi: 10.1016/j.biopsych.2020.02.1147. [DOI] [Google Scholar]
- 243.Huang S, Hu S, Liu S, Tang B, Liu Y, Tang L, et al. Lithium carbonate alleviates colon inflammation through modulating gut microbiota and Treg cells in a GPR43-dependent manner. Pharmacol Res. 2022;175:105992. doi: 10.1016/J.PHRS.2021.105992. [DOI] [PubMed] [Google Scholar]
- 244.Doestzada M, Vila AV, Zhernakova A, Koonen DPY, Weersma RK, Touw DJ, et al. Pharmacomicrobiomics: a novel route towards personalized medicine. Protein Cell. 2018;9:432–45. doi: 10.1007/S13238-018-0547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pascale A, Marchesi N, Govoni S, Barbieri A. Targeting the microbiota in pharmacology of psychiatric disorders. Pharmacol Res. 2020;157:104856. doi: 10.1016/J.PHRS.2020.104856. [DOI] [PubMed] [Google Scholar]
- 246.Burghardt KJ, Goodrich JM, Dolinoy DC, Ellingrod VL. Gene-specific DNA methylation may mediate atypical antipsychotic-induced insulin resistance. Bipolar Disord. 2016;18:423–32. doi: 10.1111/BDI.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jones BDM, Farooqui S, Kloiber S, Husain MO, Mulsant BH, Husain MI. Targeting metabolic dysfunction for the treatment of mood disorders: review of the evidence. Life (Basel, Switz) 2021;11:819. doi: 10.3390/LIFE11080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Skonieczna-Żydecka K, Łoniewski I, Misera A, Stachowska E, Maciejewska D, Marlicz W, et al. Second-generation antipsychotics and metabolism alterations: a systematic review of the role of the gut microbiome. Psychopharmacology (Berl) 2019;236:1491–512. doi: 10.1007/S00213-018-5102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, et al. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry. 2015;5:e652. doi: 10.1038/TP.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, et al. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One. 2014;9:e115225. doi: 10.1371/JOURNAL.PONE.0115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kao ACC, Spitzer S, Anthony DC, Lennox B, Burnet PWJ. Prebiotic attenuation of olanzapine-induced weight gain in rats: analysis of central and peripheral biomarkers and gut microbiota. Transl Psychiatry. 2018;8:66. doi: 10.1038/S41398-018-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Davey KJ, Cotter PD, O’Sullivan O, Crispie F, Dinan TG, Cryan JF, et al. Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry. 2013;3:e309. doi: 10.1038/TP.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hu S, Li A, Huang T, Lai J, Li J, Sublette ME, et al. Gut microbiota changes in patients with bipolar depression. Adv Sci. 2019;6:1900752. doi: 10.1002/ADVS.201900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Flowers SA, Evans SJ, Ward KM, McInnis MG, Ellingrod VL. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37:261–7. doi: 10.1002/PHAR.1890. [DOI] [PubMed] [Google Scholar]
- 255.Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81:3655–62. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. doi: 10.1038/SREP16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder. Ann Clin Psychiatry. 2011;23:40. [PMC free article] [PubMed] [Google Scholar]
- 258.Cussotto S, Strain CR, Fouhy F, Strain RG, Peterson VL, Clarke G, et al. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology (Berl) 2019;236:1671–85. doi: 10.1007/S00213-018-5006-5/TABLES/2. [DOI] [PubMed] [Google Scholar]
- 259.Szczesniak O, Hestad KA, Hanssen JF, Rudi K. Isovaleric acid in stool correlates with human depression. Nutr Neurosci. 2016;19:279–83. doi: 10.1179/1476830515Y.0000000007. [DOI] [PubMed] [Google Scholar]
- 260.Rogers MAM, Greene MT, Young VB, Saint S, Langa KM, Kao JY, et al. Depression, antidepressant medications, and risk of clostridium difficile infection. BMC Med. 2013;11:121. doi: 10.1186/1741-7015-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Macedo D, Filho AJMC, Soares de Sousa CN, Quevedo J, Barichello T, Júnior HVN, et al. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord. 2017;208:22–32. doi: 10.1016/J.JAD.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 262.Ayaz M, Subhan F, Ahmed J, Khan A, Ullah F, Ullah I, et al. Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. J Biol Res. 2015;22:4. doi: 10.1186/S40709-015-0028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lukić I, Getselter D, Ziv O, Oron O, Reuveni E, Koren O, et al. Antidepressants affect gut microbiota and ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl Psychiatry. 2019;9:133. doi: 10.1038/S41398-019-0466-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Licht RW. Lithium: still a major option in the management of bipolar disorder. CNS Neurosci Ther. 2012;18:226. doi: 10.1111/J.1755-5949.2011.00260.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Boufidou F, Nikolaou C, Alevizos B, Liappas IA, Christodoulou GN. Cytokine production in bipolar affective disorder patients under lithium treatment. J Affect Disord. 2004;82:309–13. doi: 10.1016/J.JAD.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 266.Volkmann C, Bschor T, Köhler S. Lithium treatment over the lifespan in bipolar disorders. Front Psychiatry. 2020;11:377. doi: 10.3389/FPSYT.2020.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Grunze HCR. Anticonvulsants in bipolar disorder. J Ment Health. 2010;19:127–41. 10.3109/09638230903469186. [DOI] [PubMed]
- 268.Chang HH, Yang YK, Gean PW, Huang HC, Chen PS, Lu RB. The role of valproate in metabolic disturbances in bipolar disorder patients. J Affect Disord. 2010;124:319–23. doi: 10.1016/J.JAD.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 269.Liu F, Horton-Sparks K, Hull V, Li RW, Martínez-Cerdeño V. The valproic acid rat model of autism presents with gut bacterial dysbiosis similar to that in human autism. Mol Autism. 2018;9. 10.1186/S13229-018-0251-3. [DOI] [PMC free article] [PubMed]
- 270.Poolchanuan P, Unagul P, Thongnest S, Wiyakrutta S, Ngamrojanavanich N, Mahidol C, et al. An anticonvulsive drug, valproic acid (valproate), has effects on the biosynthesis of fatty acids and polyketides in microorganisms. Sci Rep. 2020;10:9300. doi: 10.1038/S41598-020-66251-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Stokes JM, Selin C, Cardona ST, Brown ED. Chemical inhibition of bacterial ribosome biogenesis shows efficacy in a worm infection model. Antimicrob Agents Chemother. 2015;59:2918–20. doi: 10.1128/AAC.04690-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ilhan ZE, Brochard V, Lapaque N, Auvin S, Lepage P. Exposure to anti-seizure medications impact growth of gut bacterial species and subsequent host response. Neurobiol Dis. 2022;167:105664. doi: 10.1016/J.NBD.2022.105664. [DOI] [PubMed] [Google Scholar]
- 273.Cryan JF, O’riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 274.Kerimi A, Kraut NU, da Encarnacao JA, Williamson G. The gut microbiome drives inter- and intra-individual differences in metabolism of bioactive small molecules. Sci Rep. 2020;10:1–12. doi: 10.1038/s41598-020-76558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Healey GR, Murphy R, Brough L, Butts CA, Coad J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr Rev. 2017;75:1059–80. doi: 10.1093/NUTRIT/NUX062. [DOI] [PubMed] [Google Scholar]
- 276.Williamson G. The role of polyphenols in modern nutrition. Nutr Bull. 2017;42:226–35. doi: 10.1111/NBU.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ortega MA, Fraile-Martínez Ó, García-Montero C, Alvarez-Mon MA, Lahera G, Monserrat J, et al. Biological role of nutrients, food and dietary patterns in the prevention and clinical management of major depressive disorder. Nutrients. 2022;14:3099. doi: 10.3390/NU14153099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Firth J, Marx W, Dash S, Carney R, Teasdale SB, Solmi M, et al. The effects of dietary improvement on symptoms of depression and anxiety: a meta-analysis of randomized controlled trials. Psychosom Med. 2019;81:265–80. doi: 10.1097/PSY.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Łojko D, Stelmach M, Suwalska A. Is diet important in bipolar disorder? Psychiatr Pol. 2018;52:783–95. doi: 10.12740/PP/ONLINEFIRST/78703. [DOI] [PubMed] [Google Scholar]
- 280.Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the “SMILES” Trial) BMC Med. 2017;15:1–13. doi: 10.1186/S12916-017-0791-Y/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128:229–38. doi: 10.1016/J.AMJMED.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Łojko D, Stelmach-Mardas M, Suwalska A. Diet quality and eating patterns in euthymic bipolar patients. Eur Rev Med Pharmacol Sci. 2019;23:1221–38. doi: 10.26355/EURREV_201902_17016. [DOI] [PubMed] [Google Scholar]
- 283.Lopresti AL, Jacka FN. Diet and bipolar disorder: a review of its relationship and potential therapeutic mechanisms of action. J Altern Complement Med. 2015;21:733–9. doi: 10.1089/ACM.2015.0125. [DOI] [PubMed] [Google Scholar]
- 284.Gondalia S, Parkinson L, Stough C, Scholey A. Gut microbiota and bipolar disorder: a review of mechanisms and potential targets for adjunctive therapy. Psychopharmacology (Berl) 2019;236:1433–43. doi: 10.1007/S00213-019-05248-6. [DOI] [PubMed] [Google Scholar]
- 285.Meslier V, Laiola M, Roager HM, De Filippis F, Roume H, Quinquis B, et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 2020;69:1258–68. doi: 10.1136/GUTJNL-2019-320438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A, et al. Influence of Mediterranean diet on human gut microbiota. Nutrients. 2021;13:1–12. doi: 10.3390/NU13010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Alvarez-Mon MA, Ortega MA, García-Montero C, Fraile-Martinez O, Monserrat J, Lahera G, et al. Exploring the role of nutraceuticals in major depressive disorder (MDD): rationale, state of the art and future prospects. Pharmaceuticals (Basel). 2021;14. 10.3390/PH14080821. [DOI] [PMC free article] [PubMed]
- 288.Gabriel FC, Oliveira M, Martella BDM, Berk M, Brietzke E, Jacka FN, et al. Nutrition and bipolar disorder: a systematic review. Nutr Neurosci. 2022. 10.1080/1028415X.2022.2077031. [DOI] [PubMed]
- 289.Montgomery P, Richardson AJ. Omega-3 fatty acids for bipolar disorder. Cochrane Database Syst Rev. 2008. 10.1002/14651858.CD005169.PUB2. [DOI] [PubMed]
- 290.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73:81–86. doi: 10.4088/JCP.10R06710. [DOI] [PubMed] [Google Scholar]
- 291.Saunders EFH, Mukherjee D, Myers T, Wasserman E, Hameed A, Bassappa Krishnamurthy V, et al. Adjunctive dietary intervention for bipolar disorder: a randomized, controlled, parallel-group, modified double-blinded trial of a high n-3 plus low n-6 diet. Bipolar Disord. 2022;24:171–84. doi: 10.1111/BDI.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Fu Y, Wang Y, Gao H, Li D, Jiang R, Ge L, et al. Associations among dietary Omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediators Inflamm. 2021;2021:8879227. doi: 10.1155/2021/8879227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Costantini L, Molinari R, Farinon B, Merendino N. Impact of Omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18. 10.3390/IJMS18122645. [DOI] [PMC free article] [PubMed]
- 294.Khambadkone SG, Cordner ZA, Dickerson F, Severance EG, Prandovszky E, Pletnikov M, et al. Nitrated meat products are associated with mania in humans and altered behavior and brain gene expression in rats. Mol Psychiatry. 2020;25:560–71. doi: 10.1038/S41380-018-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Caselli M, Cassol F, Calò G, Holton J, Zuliani G, Gasbarrini A. Actual concept of “probiotics”: is it more functional to science or business. World J Gastroenterol. 2013;19:1527–40. doi: 10.3748/WJG.V19.I10.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Gibson GR, Probert HM, Loo J, Van, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–75. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 297.Tsai YL, Lin TL, Chang CJ, Wu TR, Lai WF, Lu CC, et al. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci. 2019;26:3. doi: 10.1186/S12929-018-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–35. doi: 10.3390/NU5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, et al. Health benefits of probiotics: a review. ISRN Nutr. 2013;2013:1–7. doi: 10.5402/2013/481651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13–23. doi: 10.1016/J.NEUBIOREV.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8:483. doi: 10.3390/NU8080483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ansari F, Pourjafar H, Tabrizi A, Homayouni A. The effects of probiotics and prebiotics on mental disorders: a review on depression, anxiety, Alzheimer, and autism spectrum disorders. Curr Pharm Biotechnol. 2020;21:555–65. doi: 10.2174/1389201021666200107113812. [DOI] [PubMed] [Google Scholar]
- 303.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–6. doi: 10.1016/J.BIOPSYCH.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 304.Shahrbabaki ME, Sabouri S, Sabahi A, Barfeh D, Divsalar P, Esmailzadeh M, et al. The efficacy of probiotics for treatment of bipolar disorder-type 1: a randomized, double-blind, placebo controlled trial. Iran J Psychiatry. 2020;15:10–16. doi: 10.18502/ijps.v15i1.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zeng C, Qiu Y, Li S, Teng Z, Xiang H, Chen J, et al. Effect of probiotic supplements on oxidative stress biomarkers in first-episode bipolar disorder patients: a randomized, placebo-controlled trial. Front Pharmacol. 2022;13. 10.3389/FPHAR.2022.829815. [DOI] [PMC free article] [PubMed]
- 306.Liu C, Kang D, Xiao J, Huang Y, Peng X, Wang W, et al. Dietary fiber and probiotics for the treatment of atypical antipsychotic-induced metabolic side effects: study protocol for a randomized, double-blind, placebo-controlled trial. Trials. 2021;22:159. doi: 10.1186/S13063-021-05123-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Reininghaus EZ, Wetzlmair LC, Fellendorf FT, Platzer M, Queissner R, Birner A, et al. The impact of probiotic supplements on cognitive parameters in euthymic individuals with bipolar disorder: a pilot study. Neuropsychobiology. 2020;79:63–70. doi: 10.1159/000492537. [DOI] [PubMed] [Google Scholar]
- 308.Rios AC, Maurya PK, Pedrini M, Zeni-Graiff M, Asevedo E, Mansur RB, et al. Microbiota abnormalities and the therapeutic potential of probiotics in the treatment of mood disorders. Rev Neurosci. 2017;28:739–49. doi: 10.1515/REVNEURO-2017-0001. [DOI] [PubMed] [Google Scholar]
- 309.Vinderola G, Sanders ME, Salminen S. The concept of postbiotics. Foods (Basel, Switzerland). 2022;11. 10.3390/FOODS11081077. [DOI] [PMC free article] [PubMed]
- 310.Silva LG, Ferguson BS, Avila AS, Faciola AP. Sodium propionate and sodium butyrate effects on histone deacetylase (HDAC) activity, histone acetylation, and inflammatory gene expression in bovine mammary epithelial cells. J Anim Sci. 2018;96:5244–52. doi: 10.1093/JAS/SKY373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sun X, Luo S, Jiang C, Tang Y, Cao Z, Jia H, et al. Sodium butyrate reduces bovine mammary epithelial cell inflammatory responses induced by exogenous lipopolysaccharide, by inactivating NF-ΚB signaling. J Dairy Sci. 2020;103:8388–97. doi: 10.3168/JDS.2020-18189. [DOI] [PubMed] [Google Scholar]
- 312.Varela RB, Valvassori SS, Lopes-Borges J, Mariot E, Dal-Pont GC, Amboni RT, et al. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J Psychiatr Res. 2015;61:114–21. doi: 10.1016/J.JPSYCHIRES.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 313.Lopes-Borges J, Valvassori SS, Varela RB, Tonin PT, Vieira JS, Gonçalves CL, et al. Histone deacetylase inhibitors reverse manic-like behaviors and protect the rat brain from energetic metabolic alterations induced by ouabain. Pharmacol Biochem Behav. 2015;128:89–95. doi: 10.1016/J.PBB.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 314.Pandey M, Bhati A, Priya K, Sharma KK, Singhal B. Precision postbiotics and mental health: the management of post-COVID-19 complications. Probiotics Antimicrob Proteins. 2022;14:426. doi: 10.1007/S12602-021-09875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chaves Filho AJM, Mottin M, Soares MVR, Jucá PM, Andrade CH, Macedo DS. Tetracyclines, a promise for neuropsychiatric disorders: from adjunctive therapy to the discovery of new targets for rational drug design in psychiatry. Behav Pharmacol. 2021;123–41. 10.1097/FBP.0000000000000585. [DOI] [PubMed]
- 316.Soczynska JK, Mansur RB, Brietzke E, Swardfager W, Kennedy SH, Woldeyohannes HO, et al. Novel therapeutic targets in depression: minocycline as a candidate treatment. Behav Brain Res. 2012;235:302–17. doi: 10.1016/J.BBR.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 317.Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M, et al. Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2×2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry. 2018;8:27. doi: 10.1038/S41398-017-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Murrough JW, Huryk KM, Mao X, Iacoviello B, Collins K, Nierenberg AA, et al. A pilot study of minocycline for the treatment of bipolar depression: effects on cortical glutathione and oxidative stress in vivo. J Affect Disord. 2018;230:56–64. doi: 10.1016/J.JAD.2017.12.067. [DOI] [PubMed] [Google Scholar]
- 319.Chaves Filho AJM, Cunha NL, Rodrigues P, de A, de Souza AG, Soares MVR, et al. Doxycycline reverses cognitive impairment, neuroinflammation and oxidative imbalance induced by D-amphetamine mania model in mice: a promising drug repurposing for bipolar disorder treatment? Eur Neuropsychopharmacol. 2021;42:57–74. doi: 10.1016/J.EURONEURO.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 320.Husain MI, Chaudhry IB, Khoso AB, Husain MO, Hodsoll J, Ansari MA, et al. Minocycline and celecoxib as adjunctive treatments for bipolar depression: a multicentre, factorial design randomised controlled trial. Lancet Psychiatry. 2020;7:515–27. doi: 10.1016/S2215-0366(20)30138-3. [DOI] [PubMed] [Google Scholar]
- 321.Zheng W, Zhu XM, Zhang QE, Cheng G, Cai DB, He J, et al. Adjunctive minocycline for major mental disorders: a systematic review. J Psychopharmacol. 2019;33:1215–26. doi: 10.1177/0269881119858286. [DOI] [PubMed] [Google Scholar]
- 322.Upmark F, Sjöqvist H, Hayes JF, Dalman C, Karlsson H. Doxycycline exposure during adolescence and future risk of non-affective psychosis and bipolar disorder: a total population cohort study. Transl Psychiatry. 2021;11:1–8. doi: 10.1038/s41398-021-01574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Schmidt EKA, Raposo PJF, Torres-Espin A, Fenrich KK, Fouad K. Beyond the lesion site: minocycline augments inflammation and anxiety-like behavior following SCI in rats through action on the gut microbiota. J Neuroinflammation. 2021;18:144. doi: 10.1186/S12974-021-02123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21:797–805. doi: 10.1038/MP.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Robles-Vera I, de la Visitación N, Toral M, Sánchez M, Romero M, Gómez-Guzmán M, et al. Changes in gut microbiota induced by doxycycline influence in vascular function and development of hypertension in Doca-Salt Rats. Nutrients. 2021;13. 10.3390/NU13092971/S1. [DOI] [PMC free article] [PubMed]
- 326.Elvers KT, Wilson VJ, Hammond A, Duncan L, Huntley AL, Hay AD, et al. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: a systematic review. BMJ Open. 2020;10:e035677. doi: 10.1136/BMJOPEN-2019-035677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22:71–81. doi: 10.1097/00004714-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 328.Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149–56. doi: 10.1016/J.JAD.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 329.Vindigni SM, Surawicz CM. Fecal microbiota transplantation. Gastroenterol Clin North Am. 2017;46:171–85. doi: 10.1016/J.GTC.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 330.Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Ther Adv Gastroenterol. 2016;9:229–39. doi: 10.1177/1756283X15607414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hinton R. A case report looking at the effects of faecal microbiota transplantation in a patient with bipolar disorder. Aust N Z J Psychiatry. 2020;54:649–50. doi: 10.1177/0004867420912834. [DOI] [PubMed] [Google Scholar]
- 332.Cooke NCA, Bala A, Allard JP, Hota S, Poutanen S, Taylor VH. The safety and efficacy of fecal microbiota transplantation in a population with bipolar disorder during depressive episodes: study protocol for a pilot randomized controlled trial. Pilot Feasibility Stud. 2021;7. 10.1186/S40814-021-00882-4. [DOI] [PMC free article] [PubMed]
- 333.Colpo GD, Leboyer M, Dantzer R, Trivedi MH, Teixeira AL. Immune-based strategies for mood disorders: facts and challenges. Expert Rev Neurother. 2018;18:139–52. doi: 10.1080/14737175.2018.1407242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Köhler O, E Benros M, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91. doi: 10.1001/JAMAPSYCHIATRY.2014.1611. [DOI] [PubMed] [Google Scholar]
- 335.Halaris A, Cantos A, Johnson K, Hakimi M, Sinacore J. Modulation of the inflammatory response benefits treatment-resistant bipolar depression: a randomized clinical trial. J Affect Disord. 2020;261:145–52. doi: 10.1016/J.JAD.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 336.Rapoport SI. Aspirin and celecoxib may help to rectify a neurotransmission imbalance in bipolar disorder. Med Hypotheses. 2021;149:110536. doi: 10.1016/J.MEHY.2021.110536. [DOI] [PubMed] [Google Scholar]
- 337.Hernandez-Sanabria E, Heiremans E, Calatayud Arroyo M, Props R, Leclercq L, Snoeys J, et al. Short-term supplementation of celecoxib-shifted butyrate production on a simulated model of the gut microbial ecosystem and ameliorated in vitro inflammation. NPJ Biofilms Microbiomes. 2020;6:9. doi: 10.1038/S41522-020-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23:335–43. doi: 10.1038/MP.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Uzzan S, Azab AN. Anti-TNF-α compounds as a treatment for depression. Molecules. 2021;26:2368. doi: 10.3390/MOLECULES26082368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Bharwani A, Szamosi JC, Taylor VH, Lee Y, Bala A, Mansur R, et al. Changes in the gut microbiome associated with infliximab in patients with bipolar disorder. Brain Behav. 2021;11:e2259. doi: 10.1002/BRB3.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder—a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–75. doi: 10.1016/J.BIOPSYCH.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 342.Zheng J, Yuan X, Zhang C, Jia P, Jiao S, Zhao X, et al. N-acetylcysteine alleviates gut dysbiosis and glucose metabolic disorder in high-fat diet-fed mice. J Diabetes. 2019;11:32–45. doi: 10.1111/1753-0407.12795. [DOI] [PubMed] [Google Scholar]
- 343.Marzani G, Neff AP. Bipolar disorders: evaluation and treatment. Am Fam Physician. 2021;103:227–39. [PubMed] [Google Scholar]
- 344.Janney CA, Fagiolini A, Swartz HA, Jakicic JM, Holleman RG, Richardson CR. Are adults with bipolar disorder active? Objectively measured physical activity and sedentary behavior using accelerometry. J Affect Disord. 2014;152–154:498–504. doi: 10.1016/J.JAD.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Monda V, Villano I, Messina A, Valenzano A, Esposito T, Moscatelli F, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. 2017;2017. 10.1155/2017/3831972. [DOI] [PMC free article] [PubMed]
- 346.Melo MCA, Daher EDF, Albuquerque SGC, De Bruin VMS. Exercise in bipolar patients: a systematic review. J Affect Disord. 2016;198:32–38. doi: 10.1016/J.JAD.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 347.Thomson D, Turner A, Lauder S, Gigler ME, Berk L, Singh AB, et al. A brief review of exercise, bipolar disorder, and mechanistic pathways. Front Psychol. 2015;6. 10.3389/FPSYG.2015.00147. [DOI] [PMC free article] [PubMed]
- 348.Cui M, Xiao H, Luo D, Zhang X, Zhao S, Zheng Q, et al. Circadian rhythm shapes the gut microbiota affecting host radiosensitivity. Int J Mol Sci. 2016;17:1786. doi: 10.3390/IJMS17111786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Liebert A, Bicknell B, Johnstone DM, Gordon LC, Kiat H, Hamblin MR. “Photobiomics”: can light, including photobiomodulation, alter the microbiome? Photobiomodul Photomed Laser Surg. 2019;37:681–93. doi: 10.1089/PHOTOB.2019.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Steardo L, Luciano M, Sampogna G, Zinno F, Saviano P, Staltari F, et al. Efficacy of the Interpersonal and Social Rhythm Therapy (IPSRT) in patients with bipolar disorder: results from a real-world, controlled trial. Ann Gen Psychiatry. 2020;19:15. doi: 10.1186/S12991-020-00266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Haynes PL, Gengler D, Kelly M. Social rhythm therapies for mood disorders: an update. Curr Psychiatry Rep. 2016;18:75. doi: 10.1007/S11920-016-0712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Sit DK, McGowan J, Wiltrout C, Diler RS, Dills J, Luther J, et al. Adjunctive bright light therapy for bipolar depression: a randomized double-blind placebo-controlled trial. Am J Psychiatry. 2018;175:131–9. doi: 10.1176/APPI.AJP.2017.16101200. [DOI] [PubMed] [Google Scholar]
- 353.Rosenthal SJ, Josephs T, Kovtun O, McCarty R. Seasonal effects on bipolar disorder: a closer look. Neurosci Biobehav Rev. 2020;115:199–219. doi: 10.1016/J.NEUBIOREV.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 354.Koliada A, Moseiko V, Romanenko M, Piven L, Lushchak O, Kryzhanovska N, et al. Seasonal variation in gut microbiota composition: cross-sectional evidence from Ukrainian population. BMC Microbiol. 2020;20:1–9. doi: 10.1186/S12866-020-01786-8/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Davenport ER, Mizrahi-Man O, Michelini K, Barreiro LB, Ober C, Gilad Y. Seasonal variation in human gut microbiome composition. PLoS One. 2014;9. 10.1371/JOURNAL.PONE.0090731. [DOI] [PMC free article] [PubMed]
- 356.Teatero ML, Mazmanian D, Sharma V. Effects of the menstrual cycle on bipolar disorder. Bipolar Disord. 2014;16:22–36. doi: 10.1111/BDI.12138. [DOI] [PubMed] [Google Scholar]
- 357.Shivakumar G, Bernstein IH, Suppes T, Keck PE, McElroy SL, Altshuler LL, et al. Are bipolar mood symptoms affected by the phase of the menstrual cycle? J Womens Health (Larchmt) 2008;17:473–8. doi: 10.1089/JWH.2007.0466. [DOI] [PubMed] [Google Scholar]
- 358.Sit D, Seltman H, Wisner KL. Menstrual effects on mood symptoms in treated women with bipolar disorder. Bipolar Disord. 2011;13:310–7. doi: 10.1111/J.1399-5618.2011.00921.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Aragno E, Fagiolini A, Cuomo A, Paschetta E, Maina G, Rosso G. Impact of menstrual cycle events on bipolar disorder course: a narrative review of current evidence. Arch Womens Ment Health. 2022;25:257–66. doi: 10.1007/S00737-022-01217-9. [DOI] [PubMed] [Google Scholar]
- 360.El Dahr Y, de Azevedo Cardoso T, Syan SK, Caropreso L, Minuzzi L, Smith M, et al. Investigating biological rhythms disruptions across the menstrual cycle in women with comorbid bipolar disorder and premenstrual dysphoric disorder. Arch Womens Ment Health. 2022;25:345–53. doi: 10.1007/S00737-022-01220-0. [DOI] [PubMed] [Google Scholar]
- 361.Ervin SM, Li H, Lim L, Roberts LR, Liang X, Mani S, et al. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem. 2019;294:18586–99. doi: 10.1074/JBC.RA119.010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.García-Montero C, Ortega MA, Alvarez-Mon MA, Fraile-Martinez O, Romero-Bazán A, Lahera G, et al. The problem of malnutrition associated with major depressive disorder from a sex-gender perspective. Nutrients. 2022;14. 10.3390/NU14051107. [DOI] [PMC free article] [PubMed]
- 363.Miklowitz DJ, Johnson SL. The psychopathology and treatment of bipolar disorder. Annu Rev Clin Psychol. 2006;2:199–235. doi: 10.1146/ANNUREV.CLINPSY.2.022305.095332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Maassen EF, Regeer BJ, Regeer EJ, Bunders JFG, Kupka RW. The challenges of living with bipolar disorder: a qualitative study of the implications for health care and research. Int J Bipolar Disord. 2018;6. 10.1186/S40345-018-0131-Y. [DOI] [PMC free article] [PubMed]
- 365.Nestsiarovich A, Hurwitz NG, Nelson SJ, Crisanti AS, Kerner B, Kuntz MJ, et al. Systemic challenges in bipolar disorder management: a patient-centered approach. Bipolar Disord. 2017;19:676–88. doi: 10.1111/BDI.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Munoz-Bellido JL, Munoz-Criado S, Garcìa-Rodrìguez JA. Antimicrobial activity of psychotropic drugs: selective serotonin reuptake inhibitors. Int J Antimicrob Agents. 2000;14:177–80. doi: 10.1016/S0924-8579(99)00154-5. [DOI] [PubMed] [Google Scholar]