Abstract
Interleukin-15 (IL-15) is essential for the development, maturation, and function of NK and NKT cells, which are critical components of the innate immune defense against viral infections. We recently showed that mice lacking IL-15 and/or NK/NKT cells are significantly more susceptible to intravaginal (IVAG) herpes simplex virus type 2 (HSV-2) infection than control mice. For this study, we examined whether IL-15 has any direct antiviral activity, independent of NK/NKT cells, in innate protection against HSV-2 infection. A sensitive enzyme-linked immunosorbent assay for murine IL-15 was developed and used to show that IVAG HSV-2 infection induces IL-15 in vaginal washes. Using immunohistochemistry, we detected IL-15-positive cells in the submucosa and vaginal epithelium following IVAG HSV-2 infection. Local, but not systemic, delivery of murine recombinant IL-15 (mrIL-15) to the genital mucosae of IL-15−/− and RAG-2−/− γc−/− mice, which both lack NK and NKT cells, resulted in significant reductions in HSV-2 titers in genital washes and 60% survival following IVAG HSV-2 challenge. Furthermore, we showed that IL-15 is important for CpG oligodeoxynucleotide (ODN)-induced innate protection against genital HSV-2 infection. While 100% of CpG ODN-treated RAG2−/− γc−/− mice, which are capable of producing IL-15 but lack NK/NKT cells, survived an IVAG HSV-2 challenge, only 60% of CpG ODN-treated IL-15−/− mice survived, and all of these mice had similar vaginal viral titers to those in control mice by day 3 postchallenge. Lastly, a treatment of RAW264.7 cells with mrIL-15 induced the production of tumor necrosis factor alpha and beta interferon (IFN-β), but not IFN-α, and significantly protected them against HSV-2 infection in vitro. The results of these studies indicate that IL-15 can act independently of NK/NKT cells in mediating the innate defense against viral infection.
Innate immunity plays a crucial role in the defense against viral infections (6, 7). This is especially true for mucosal surfaces, where the vast majority of pathogens initiate infection (18). Indeed, as we and others (3, 21, 22, 32, 33) have shown, the activation of innate immunity following local delivery of Toll-like receptor (TLR) ligands, such as unmethylated CpG-containing DNA, to the vaginal mucosa can protect female mice against genital infection with herpes simplex virus type 2 (HSV-2). Numerous factors are involved in the innate immune defense, but NK and NKT cells and interleukin-15 (IL-15) are of particular interest since they can rapidly respond to viral pathogens (8, 11, 12, 19, 20, 29, 35). We recently demonstrated that IL-15 and NK and NKT cells are critical for innate protection against intravaginal (IVAG) HSV-2 infections (4). However, the mechanisms of regulation and the delivery of antiviral effects are not completely understood. Both NK and NKT cells have the ability to kill target cells without prior sensitization and/or to rapidly release gamma interferon (IFN-γ), a cytokine which is critical for the activation of antigen-presenting cells (APCs) (5, 6). The development, maturation, and activation of NK/NKT cells are regulated by IL-15. Mice lacking either IL-15 or IL-15 receptor α (IL-15Rα) are completely devoid of NK cells and have a severe deficiency in NKT cells (9, 24, 31).
IL-15 is a cytokine that was discovered in 1994 and that exhibits IL-2-like activity (10). Although IL-15 has no sequence homology with IL-2 at the amino acid level, the two resemble each other in their tertiary structures, and both belong to the four-alpha-helix-bundle cytokine family. Furthermore, IL-15 and IL-2 share the IL-2R beta and gamma chains for signal transduction, but analogous to IL-2R, IL-15 binds with a high affinity to the alpha chain of IL-15R (29, 30). Differences in the distributions of the IL-2R alpha and IL-15R alpha chains allows these cytokines to perform their distinct actions. IL-15 plays a very important role in innate immunity, as shown by various studies (19, 27, 29). It has been found that the IL-15R alpha chains are present on APCs such as dendritic cells and macrophages (14, 16, 29). As a result, IL-15 seems to be important for the functioning of APCs, particularly in the release of IL-12, IFN-γ, and nitric oxide (NO). IL-15 also strongly increases the cytotoxic activity and IFN-γ production of NK/NKT cells (13, 19, 34). The overexpression of IL-15 in mice (IL-15Tg mice) causes a significant increase in the number and activity of NKT cells (15, 16). Recently, we showed that NK/NKT cells are the early source of IFN-γ in the genital mucosa after IVAG HSV-2 infection (4). Furthermore, we demonstrated that IL-15−/− mice, which lack NK and NKT cells but possess B and T cells, and RAG-2−/− γc−/− mice, which lack all lymphoid cells, including NK and NKT cells, but have IL-15, are 100 times more susceptible to IVAG HSV-2 infection than their C57BL/6 congenic controls.
Although the effects of IL-15 on cells of the immune system have been relatively well studied, the role of IL-15 in antiviral defense is much less documented. A few reports have shown that IL-15 has antiviral activity. It has been suggested that the antiviral activity of IL-15 is primarily mediated via the activation of NK cells (2, 13, 17, 19). These reports have also shown that different viruses up-regulate NK cytotoxic activity via IL-15 induction (13). Gosselin et al. (19) reported that recombinant IL-15 (rIL-15) significantly reduced infections of peripheral blood mononuclear cells (PBMCs) by HSV-1 and human herpesvirus 6. In an in vivo study, a protective role for IL-15 against systemic HSV-2 was reported (36). The current understanding is that the antiviral activities of IL-15 are mediated via the activation of NK and NKT cells. However, little is known about the antiviral activity of IL-15 in the absence of NK/NKT cells.
The purpose of the present study was to determine whether IL-15 has any direct antiviral activity, independent of NK/NKT cells, in innate protection against HSV-2 infection in vivo and in vitro. Our results show that the delivery of murine rIL-15 (mrIL-15) to mice that lack IL-15 and NK and NKT cells can provide protection against genital HSV-2 infection in vivo and can protect RAW264.7 cells in vitro. IL-15 induced the production of tumor necrosis factor alpha (TNF-α), but not IFN-α, in RAW264.7 cells, as detected by enzyme-linked immunosorbent assay (ELISA). Supernatants from treated RAW264.7 cells showed significant IFN-α/β activity, as redetected by a standard vesicular stomatitis virus (VSV) plaque reduction assay. Furthermore, we show that IL-15 is important for CpG DNA-induced mucosal innate protection against IVAG HSV-2 infection.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice of 8 to 12 weeks of age were purchased from Charles River Laboratory (Quebec, Canada). Female IL-15−/− mouse breeding pairs were obtained from Immunex (Immunex, Seattle, Wash.) and were then bred in the barrier facilities at McMaster University, Hamilton, Ontario, Canada. RAG2−/− γc−/− mice were purchased from Taconic (Germantown, N.Y.). All mice followed a 12-h day and 12-h night schedule and were maintained under standard temperature-controlled conditions.
Viruses, cells, and reagents.
HSV-2 strain 333 was grown and titrated as previously described (28). VSV and interferon regulatory factor 3−/− (IRF3−/−) murine embryonic fibroblast (MEF) cells were kindly provided by Karen Mossman, McMaster University. Synthetic CpG phosphorothioate oligodeoxynucleotides (ODN) (1826) were provided by McMaster University's Molecular Biology Institute. Progesterone (Depo-Provera) was purchased from Upjohn (Don Mills, Ontario, Canada). mrIL-15 was obtained from RDI (Flanders, N.J.).
Genital HSV-2 inoculation and vaginal virus titration.
Mice were injected subcutaneously with 2 mg of progesterone/mouse. Five days later, the mice were anesthetized and infected IVAG with 10 μl of HSV-2 while being maintained under anesthetic. The mice were washed IVAG daily by pipetting 30 μl of PBS into and out of the vagina six to eight times (performed twice). Viral titers in IVAG washes were determined by plaque assays on monolayers of Vero cells as previously described (28). Genital pathology was monitored daily after infection. Pathology was scored on a five-point scale as follows: 0, no infection; 1, slight redness of external vagina; 2, swelling and redness of external vagina; 3, severe swelling of external vagina and hair loss in the surrounding area; 4, ulceration of vaginal tissue, redness, and swelling; 5, continued ulceration, redness, swelling, and sometimes paralysis in back legs—mice were sacrificed at this point.
Treatment of mice and RAW264.7 cells with mrIL-15 or CpG ODN.
IL-15−/−, RAG2−/− γc−/−, and C57BL/6 mice were subcutaneously injected with 2 mg of progesterone/mouse. Four days later, the mice were anesthetized and treated vaginally with 500 ng of mrIL-15, and the treatment was performed again on the following day. They were then infected with lethal doses of HSV-2, which differed for the three strains because of their various susceptibilities. C57BL/6 mice were infected with a lethal dose of 1 × 104 PFU of HSV-2, while IL-15−/− and RAG2−/− γc−/− mice were infected with a dose of 5 × 102 PFU. A second set of IL-15−/− and RAG2−/− γc−/− mice were treated with 500 ng of mrIL-15 intraperitoneally on days −3, −2, −1, 0, and 1 postinfection. Vaginal washes from all groups were collected, and pathologies were recorded as described above. RAW264.7 cells were grown to subconfluence and then treated with mrIL-15 (500 ng/ml) or CpG (10 μg/ml). At 24 h posttreatment, the cells were infected with HSV-2 at a multiplicity of infection (MOI) of 0.1, and at 20 h postinfection, the cells and the supernatant were removed from the flask and frozen at −70°C.
ELISA for murine IL-15.
Maxisorp 96-well ELISA plates from Invitrogen (Burlington, Ontario, Canada) were coated with 100 μl of rabbit anti-mouse IL-15 (RDI)/well at a concentration of 2.0 μg/ml and incubated overnight at 4°C. The plates were then blocked with 2% bovine serum albumin (BSA) and incubated at room temperature for 2 h. The sample dilutions were prepared in 0.1% BSA and then transferred to ELISA plates, and the plates were incubated overnight at 4°C. Following several washes with phosphate-buffered saline (PBS)-Tween 20, 100 μl of goat anti-mouse IL-15 (R&D Systems, Minneapolis, Minn.), at a concentration of 1 μg/ml, was added to the plate and incubated for 1 h in the dark at room temperature (RT). One hundred microliters of biotin-labeled donkey anti-goat antibody (RDI), at a concentration of 0.1 μg/ml, was then added to the plate and incubated at RT for 1 h, after which an Extravidin peroxidase conjugate from Sigma (Oakville, Ontario, Canada) was added to each well and incubated for 45 min at RT. Next, TMB microwell peroxidase (KPC, Gaithersburg, Md.) was added to each well and allowed to incubate for 30 min at RT. Finally, 100 μl of the stop solution, 1 M H2SO4, was added, and the plates were then read with a Sapphire ELISA plate reader at an optical density of 405 nm.
ELISA for IFN-α and TNF-α.
RAW264.7 cells were treated with either mrIL-15, CpG ODN, or poly(I · C) (positive control) or were left untreated. IFN-α and TNF-α ELISAs were conducted by the use of Quantikine murine kits from R&D Systems according to the manufacturer's instructions.
Bioassay for IFN-α/β activity.
To detect the presence of any IFN-α/β with biological activity in the supernatant of IL-15- or CpG ODN-treated RAW264.7 cells, we used a standard VSV plaque reduction assay. RAW 264.7 cells were treated with either mrIL-15 (500 ng/ml), CpG (10 μg/ml), or poly(I · C) (10 μg/ml; positive control) or were left untreated. The supernatants were serially diluted and transferred onto IRF3−/− cells plated in 12-well plates. The IRF3−/− cells were incubated with the supernatants at 37°C for 24 h. The supernatants were then removed, and the cells were infected with 200 μl of pretitrated VSV for 1 h. The plates were then overlaid with methylcellulose, incubated for 24 h, and stained for plaque counts.
Immunohistochemistry for IL-15.
Vaginal tissues from progesterone-treated B6 and IL-15−/− mice and CpG ODN-treated or IVAG HSV-2-infected B6 mice were dissected, and fresh frozen vaginal tissue slides were prepared. After several steps of acetone washing and air drying, the slides were washed three times in 0.1% BSA. Sections were placed in 3% H2O2 for 5 min and then washed three times again in 0.1% BSA. The primary antibody, goat anti-mouse IL-15, was placed on each slide (diluted 1:100) and allowed to incubate for 2 h in a humidifier. After incubation, the slides were washed three times with 0.1% BSA and then blocked with 1% BSA for 20 min. The sections were incubated with the secondary antibody, a donkey anti-goat antibody (1:250 dilution), for 2 h in the humidifier. They were then treated with horseradish peroxidase from DAKO Diagnostics Canada Inc. (Mississauga, Ontario, Canada) for 1 h. Next, they were washed in AEC (3-amino-9-ethylcarbazole) buffer for 3 min, after which a chromogen was added. The sections were incubated in the dark for 15 to 20 min, or until color development. After washes with normal H2O for 1 min, the sections were counterstained with hematoxylin from Biomeda Corp. (Foster City, Calif.) for 4 to 5 min. The slides were mounted by the use of crystal mount reagent (Sigma).
Statistical analysis.
Statistical differences in the viral titers were determined by analysis of variance followed by Tukey's test. The statistical significances of the survival rates were determined by either the χ2 test or the log-rank test. A P value of <0.05 was considered statistically significant. An unpaired t test was used to find the significant differences in IL-15 production.
RESULTS
Genital mucosa produces IL-15 in response to IVAG HSV-2 infection.
Currently there is no commercial source for a murine IL-15-specific ELISA. In order to have a better understanding of the role of IL-15 in the defense against mucosal viral infections, we first developed a sensitive ELISA to measure murine IL-15 by using the reagents and method described above. The detection limit was 100 pg/ml based on the standard curve obtained with mrIL-15 (data not shown). Samples from IL-15−/− mice were unreactive, showing the specificity of the reaction.
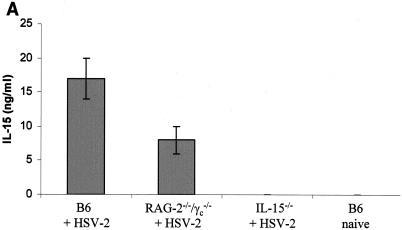
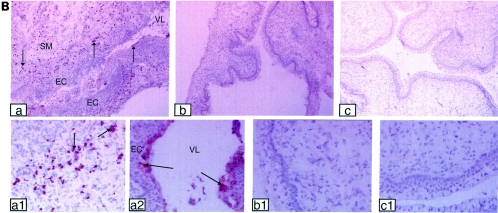
We previously showed that mice lacking IL-15 are very susceptible to IVAG HSV-2 infection (4). To determine whether IL-15 was produced in response to genital viral infection, we measured the levels of IL-15 in vaginal washes following IVAG HSV-2 infection. C57BL/6 and alymphoid RAG2−/− γc−/− mice had significant levels of IL-15 in vaginal washes at 24 h postinfection, whereas no IL-15 was detected in vaginal washes from uninfected B6 or IVAG HSV-2-infected IL-15−/− mice (Fig. 1A). To determine if the IL-15 found in the vaginal washes following IVAG HSV-2 infection was produced by vaginal mucosal cells, we localized the IL-15-producing cells in the vaginal mucosa by immunohistochemistry. Vaginal tissues from B6 and IL-15−/− mice were dissected at 24 and 48 h post-IVAG HSV-2 infection and then processed for the detection of IL-15-positive cells. Significant numbers of cells in the submucosa were positive for IL-15 in HSV-2-infected B6 mice (Fig. 1B, panels a and a1). Some vaginal epithelial cells were also positive for IL-15 (Fig. 1B, panel a2). Vaginal sections from uninfected B6 mice (Fig. 1B, panels b and b1) were negative for IL-15 protein, and as expected, no IL-15-positive cells were found in IVAG HSV-2-infected IL-15−/− mice (Fig. 1B, panels c and c1).
FIG. 1.
IVAG HSV-2 infection induces IL-15 production in the genital mucosa. Six- to 8-week-old B6, RAG2−/− γc−/−, and IL-15−/− mice were treated with progesterone and then infected with IVAG HSV-2 5 days later. (A) Vaginal washes were collected for IL-15 measurements by IL-15 ELISA. Significantly higher levels of IL-15 were found in washes from B6 mice than in those from control uninfected mice (P < 0.0001) (Fig. 1A). RAG-2−/− γc−/− also showed some level of IL-15 secretion postinfection compared to uninfected mice (P < 0.001), but the level was significantly lower (P < 0.01) than that for B6 infected mice. B6 naive or IVAG HSV-2-infected IL-15−/− mice did not have any IL-15 in their vaginal washes. (B) Photomicrographs of vaginal tract cross sections stained for IL-15. IL-15-positive cells were found in abundance in the subepithelial cells (a and a1). Epithelial cells were also positive for IL-15 (a and a2). No IL-15-positive cells were found in B6 controls (b and b1) or IVAG HSV-2-infected IL-15−/− mice (c and c1). SM, submucosa; EC, epithelial cells; VL, vaginal lumen. Magnifications, ×18 (a, b, and c) and ×360 (a1, a2, b1, and c1).
Mucosal delivery of mrIL-15 provides some protection against HSV-2 for IL-15−/− and RAG2−/− γc−/− mice.
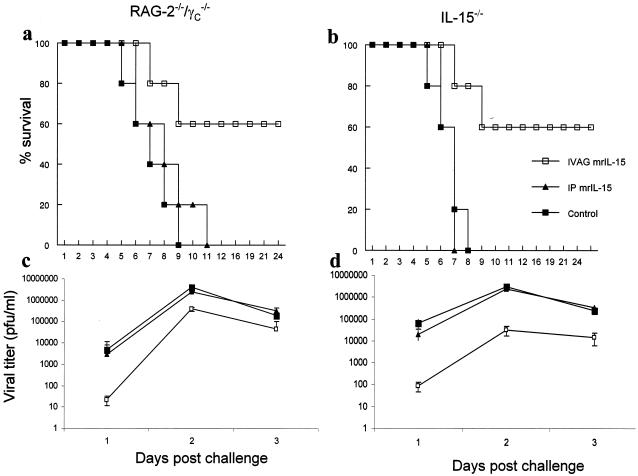
We next examined the effect that mrIL-15 had on protection against IVAG HSV-2. IL-15−/−, RAG2−/− γc−/−, and B6 mice were treated vaginally with mrIL-15 and then infected with lethal doses of HSV-2. Both RAG2−/− γc−/− and IL-15−/− mice showed a survival rate of 60% following local vaginal delivery of mrIL-15 (Fig. 2a and b). In contrast, RAG2−/− γc−/− and IL-15−/− mice that were not treated with mrIL-15 succumbed to the genital HSV-2 challenge in 8 or 9 days. Control B6 mice did not have an increase in survival rate or a change in viral titer compared to mice that were not treated with mrIL-15 (data not shown). We also examined the effect of the route of mrIL-15 delivery on IVAG HSV-2 infection. The results in Fig. 2 show that intraperitoneal (i.p.) delivery of mrIL-15 to RAG2−/− γc−/− or IL-15−/− mice did not protect them from a subsequent IVAG HSV-2 challenge. Viral titers of vaginal washes also demonstrated that both RAG2−/− γc−/− and IL-15−/− mice treated vaginally with mrIL-15 had lower titers than the untreated groups and the mice that received mrIL-15 i.p. (Fig. 2c and d). These results demonstrated that the local delivery of mrIL-15 directly to the genital mucosa was able to assist in protection against viral infection in the genital tract.
FIG. 2.
Vaginal, but not systemic, delivery of mrIL-15 increases resistance to IVAG HSV-2 infection in RAG2−/− γc−/− and IL-15−/− mice. mrIL-15 (500 ng/mouse) or PBS was administered IVAG or i.p. to RAG2−/− γc−/− and IL-15−/− mice (n = 10/group) 24 h prior to an IVAG HSV-2 challenge (5 × 102 PFU/mouse). Challenged mice were monitored daily for genital pathology, survival, and vaginal virus titers. (a and b) IVAG treated mice showed 60% survival, in contrast to those treated i.p. or with PBS (▴ and ▪, respectively). (c and d) Vaginal HSV-2 titers were examined on days 1 to 3 post-viral challenge. RAG2−/− γc−/− and IL-15−/− mice treated vagianlly with mrIL-15 showed significantly lower viral titers on days 1 (P < 0.001), 2, and 3 (P < 0.01) postchallenge than those for i.p. treated (▴) or PBS-treated control (▪) mice. There were no significant differences in vaginal wash HSV-2 titers between mice that received mrIL-15 i.p. and control mice (▴ and ▪, respectively).
IL-15 is important for CpG ODN-induced mucosal innate protection against IVAG HSV-2 infection.
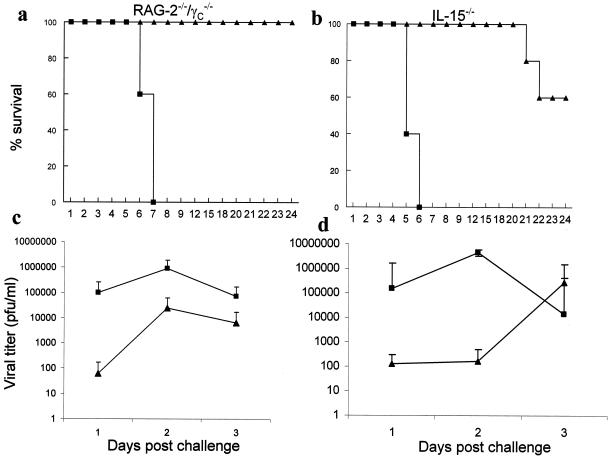
We and others previously showed that the local delivery of CpG ODN to the vaginal mucosa induced potent innate protection against a subsequent IVAG HSV-2 challenge in B6 mice (3, 21, 22, 32, 33). To examine if the CpG-induced innate protection was mediated through IL-15 and/or NK/NKT cells, we delivered CpG ODN locally to IL-15−/− mice (negative for NK/NKT cells and unable to produce IL-15) and RAG2−/− γc−/− mice (negative for NK/NKT cells, but able to produce IL-15) prior to an IVAG HSV-2 challenge. While 100% of CpG-treated RAG2−/− γc−/− mice survived the IVAG HSV-2 challenge, IL-15−/− mice showed 60% (P < 0.01) survival after IVAG CpG treatment (Fig. 3a and b). Furthermore, while CpG ODN-treated RAG2−/− γc−/− mice showed significantly lower viral titers in vaginal washes during the first 3 days after IVAG HSV-2 challenge (Fig. 3c), CpG-treated IL-15−/− mice had low viral titers on days 1 and 2, but their viral titers on day 3 postinfection were similar to those of the control group (Fig. 3d).
FIG. 3.
IL-15 contributes to CpG ODN-induced innate protection against IVAG HSV-2. CpG ODN or control ODN (100 μg) was delivered vaginally 24 h prior to IVAG HSV-2 challenge. Challenged mice were monitored daily for genital pathology, survival, and vaginal virus titers. (a and b) CpG ODN-treated RAG2−/− γc−/− mice (n = 20) (▴ in panel a) were 100% protected and IL-15−/− mice (n = 10) (▴ in panel b) were 60% protected against a lethal dose of HSV-2, in contrast to control ODN-treated mice (▪). (c and d) Vaginal HSV-2 titers were examined on days 1 to 3 post-viral challenge (n = 10/group). CpG ODN-treated RAG2−/− γc−/− mice (▴ in panel c) had significantly (P < 0.0001 for day 1, P < 0.001 for days 2 and 3) lower (up to 3 log) viral titers than did control ODN-treated mice (▪ in panel c). CpG-treated IL-15−/− mice (▴ in panel d) had significantly lower viral titers only on days 1 and 2 (P < 0.0001), but not on day 3, postchallenge than did control ODN-treated mice (▪ in panel d).
mrIL-15 provides protection to RAW264.7 cells in vitro.
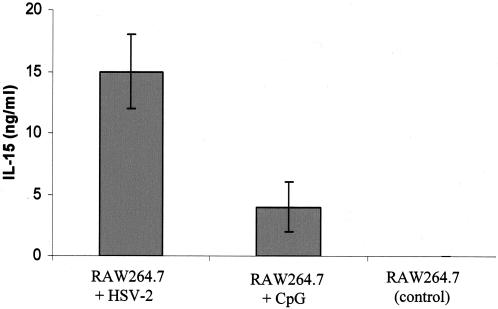
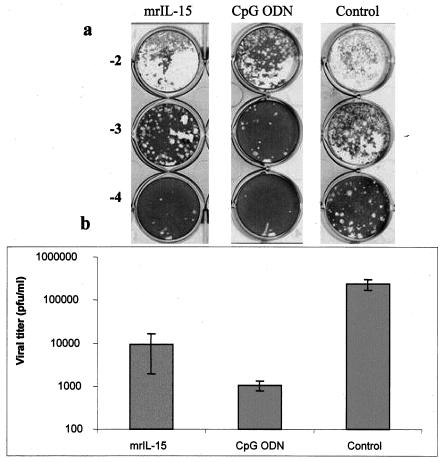
It has been reported that infections of human PBMCs with different viruses induce IL-15 production (13). Also, previous in vitro experiments have shown that rIL-15 significantly reduces infections of PBMC by HSV-1 and human herpesvirus 6 via the activation of NK cells (19). Here our results demonstrate that both HSV-2 infection and CpG ODN treatment of a murine macrophage cell line, RAW264.7, induced the production of IL-15 (Fig. 4). To confirm the direct antiviral effect of IL-15, we treated RAW264.7 cells with either CpG ODN, mrIL-15, or PBS and then infected them with HSV-2. Figure 5 shows that a treatment with CpG or mrIL-15 provided protection against HSV-2 infection in vitro. Both CpG ODN and mrIL-15-treated cells had significantly lower HSV-2 titers than the control group (Fig. 5b).
FIG. 4.
Both CpG ODN treatment and HSV-2 infection induce IL-15 production in RAW264.7 cells. RAW264.7 cells were either infected with HSV-2 (MOI of 0.1), treated with CpG (10 μg/ml), or left untreated. Twenty-four hours later, the supernatants were collected and stored at −70°C. The levels of IL-15 in the supernatants were detected by the murine IL-15 ELISA. HSV-2 infection induced significant levels of IL-15 compared to control cells (P < 0.001) and CpG ODN-treated cells (P < 0.01). CpG ODN also induced significant amounts of IL-15 production compared to samples from control cells (P < 0.001).
FIG. 5.
Treatment of RAW264.7 cells with mrIL-15 or CpG ODN significantly reduces replication of HSV-2. RAW264.7 cells were treated with either mrIL-15 (500 ng/ml) or CpG (10 μg/ml) for 24 h and then infected with HSV-2 (MOI of 0.1). At 20 h postinfection, the cells and supernatant were collected for each group and stored at −70°C for HSV-2 titration. The supernatants were titrated on Vero cell monolayers after three freeze-thaw steps (a). (b) RAW264.7 cells treated with CpG ODN had significantly lower HSV-2 titers than did control cells (P < 0.001). Cells treated with mrIL-15 also had significantly lower HSV-2 titers than did control cells (P < 0.01).
IL-15 induces production of IFN-β and TNF-α, but not IFN-α, in RAW264.7 cells.
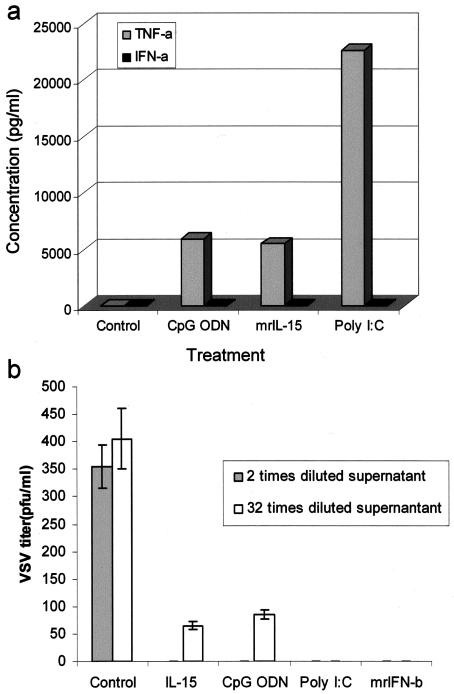
Currently, the mechanism by which IL-15 provides protection against HSV-2 is not completely understood. Our results show that upon treatment with mrIL-15 or CpG ODN, RAW264.7 cells produce TNF-α but no IFN-α (Fig. 6a). Our attempts to develop an ELISA for murine IFN-β failed with the currently available antibodies against murine IFN-β. To test the presence of IFN-β, we performed a standard VSV plaque reduction assay with MEF cells. The treatment of MEFs with supernatants from both mrIL-15- and CpG ODN-treated cells significantly reduced the formation of VSV plaques compared to supernatants from untreated cells (Fig. 6b).
FIG. 6.
Treatment of RAW264.7 cells with mrIL-15 or CpG ODN induces the production of TNF-α and IFN-β, but not IFN-α. RAW264.7 cells were treated with either mrIL-15 (500 ng/ml), CpG (10 μg/ml), or poly(I · C) (10 μg/ml; positive control). The supernatants were removed and used to measure the levels of IFN-α and TNF-α by ELISAs. All three treatments caused the production of TNF-α, but no IFN-α was detected in these supernatants (a). Since there is currently no ELISA available for murine IFN-β, biologically active IFN-α/β in the supernatants from treated cells was detected by a VSV plaque reduction assay. IRF-3−/− MEF cells were incubated with different dilutions of the mrIL-15 or CpG ODN-treated RAW264.7 supernatants. Supernatants from untreated cells were used as a control. Supernatants from RAW264.7 cells treated with poly(I · C) and different concentrations of mrIFN-β (from 1,000 to 31.5 U/ml) were used as positive controls. Supernatants from IL-15- and CpG ODN-treated cells showed significant VSV plaque reduction, even when diluted 32 times, compared to supernatants from untreated cells (b).
DISCUSSION
Previously, we demonstrated that IL-15 and NK/NKT cells are critical for innate protection against IVAG HSV-2 infection (4). For the present study, we investigated whether IL-15 has any direct antiviral activity, independent of NK/NKT cells, in the innate defense against HSV-2 infection. Three lines of evidence provided in this paper support a direct role of IL-15 in antiviral activity against genital HSV-2 infection. Firstly, the delivery of mrIL-15 to the genital mucosa, but not systemically, in knockout mice deficient in NK/NKT cells significantly reduced vaginal virus titers and provided some protection against IVAG HSV-2 challenge. Secondly, the treatment of RAW264.7 cells with mrIL-15 significantly reduced HSV-2 replication in vitro. Lastly, our results show that IL-15 is important for CpG ODN-induced innate antiviral protection against HSV-2 both in vivo and in vitro. Thus, RAG2−/− γc−/− mice, which are alymphoid and lack NK and NKT cells but can produce IL-15, were protected against IVAG HSV-2 infection after the local delivery of CpG to the vaginal tract, whereas only 60% of CpG-treated IL-15−/− mice survived IVAG HSV-2 infection, and all had similar vaginal virus titers by 3 days after infection. Furthermore, the treatment of RAW264.7 cells with CpG ODN led to the production of IL-15 and protection against viral infection in vitro. Thus, IL-15 has antiviral activity in the absence of NK/NKT cells.
To understand the role of IL-15 in defense against mucosal viral infection, we first needed to establish the presence and concentration of IL-15 produced mucosally at the site of initial infection, the first line of innate defense. Since there is no commercial ELISA kit for murine IL-15 and we found that the mouse cytolytic T lymphocyte cell line (CTLL-2) bioassay for IL-15 could not be used to measure IL-15 in vaginal washes following IVAG HSV-2 infection (data not shown), we developed a sensitive and specific ELISA for murine IL-15 by using a combination of antibodies that permitted us to assess IL-15 production in vivo and in vitro. This assay proved to be sensitive, with a detection limit of 100 pg/ml, and specific, since controls, including vaginal washes from IL-15−/− mice, were consistently negative. Using this murine IL-15 ELISA, we clearly demonstrated that IL-15 was produced in vivo in the genital tract shortly after IVAG HSV-2 infection. Interestingly, high levels of IL-15 were found in vaginal washes at 24 h post-IVAG HSV-2 infection for C57BL/6 and RAG-2−/− γc−/− mice, but not for uninfected B6 or IVAG HSV-2-infected IL-15−/− mice. It appears that IL-15 production in the genital tract peaks prior to an early peak in NK/NKT cell-derived IFN-γ 48 h after infection. The production of IL-15 in the genital tract was confirmed by immunohistochemistry. Significant numbers of cells in the vaginal submucosa and some vaginal epithelial cells were positive for IL-15 production following HSV-2 infection. Although we have not yet analyzed the phenotypes of the IL-15-positive cells, several of the IL-15-positive cells in the submucosa had a dendritic cell morphology. Moreover, it is clear from our results that vaginal epithelial cells also produced IL-15 in response to HSV-2 infection.
We recently reported that IL-15 and NK/NKT cells play a crucial role in the early innate defense against IVAG HSV-2 (4). It is well known that IL-15 is essential for NK and NKT cell development, maturation, and activation (9, 10, 24, 29-31). IL-15 was also shown to be secreted in response to various intracellular infectious agents and important for proper NK cell activity (8, 31). Furthermore, IL-15 plays an important role in the in vitro survival of NK cells by preventing or delaying apoptosis. Previous reports suggested that the antiviral activity of IL-15 is mediated through the activation of NK cells. We were interested in determining whether IL-15 could mediate antiviral activity independent of NK and/or NKT cells. Therefore, mrIL-15 was delivered directly to the vaginal mucosa or i.p. to RAG-2−/− γc−/−, IL-15−/−, and B6 mice prior to an IVAG challenge with lethal doses of HSV-2. Interestingly, 60% of both RAG-2−/− γc−/− and IL-15−/− mice treated IVAG with mrIL-15 were protected, whereas none of the mice left untreated or given mrIL-15 i.p. were protected. Furthermore, mice given mrIL-15 IVAG had significantly lower levels of virus in their vaginal washes for the first few days after the IVAG challenge. Thus, the direct delivery of mrIL-15 to mice that lacked NK and NKT cells provided a significant level of protection against IVAG viral challenge. The ability of mrIL-15 to provide protection to IL-15−/− mice suggests that IL-15 may work by some other mechanism than those mediated through NK/NKT cells. The treatment of normal B6 mice with mrIL-15 did not provide extra protection against IVAG HSV-2 infection (data not shown). Considering the fact that B6 mice are at least 100 times more resistant to IVAG HSV-2 than IL-15−/− and RAG-2−/− γc−/− mice, we speculate that there is a threshold for the antiviral activity of IL-15. The levels of IL-15 in genital washes from RAG-2−/− γc−/− mice were significantly lower than those for B6 mice, which may explain the moderate protection we observed in these mice after IVAG delivery of mrIL-15.
Recently, we and others reported that the local delivery of the TLR9 ligand CpG ODN induces an innate antiviral state that protects female mice from subsequent IVAG HSV-2 challenge (3, 21, 22, 32, 33). The direct delivery of CpG ODN to the vaginal mucosae of RAG-2−/− γc−/− female mice, which lack NK and NKT cells but can produce IL-15, completely protected them against IVAG HSV-2 challenge. This protection was accompanied by significant reductions in vaginal virus titers for the first 3 days after infection. In contrast, the local delivery of CpG ODN to the vaginal mucosae of IL-15−/− mice, which also lack NK and NKT cells but cannot produce IL-15, only reduced vaginal virus titers for 2 days after infection, and after about 20 days, 40% of the IL-15−/− mice had succumbed to IVAG infection. Thus, despite the lack of NK and NKT cells in both of these knockout strains, in the complete absence of IL-15 CpG ODN was unable to provide complete protection against genital infection. This indicates that IL-15 plays a critical role in the CpG ODN-induced innate antiviral response against IVAG HSV-2.
Similar to in vivo protection against IVAG viral challenge, the treatment of RAW264.7 macrophages in vitro with mrIL-15 or CpG ODN induced significant protection against HSV-2 infection. Interestingly, the treatment of RAW264.7 cells with CpG ODN induced significant levels of IL-15. Thus, these results reinforce the idea that IL-15, independent of NK/NKT cells, induces an innate antiviral response and that CpG-induced protection is associated with IL-15 production.
Our results suggest that IFN-β, and possibly TNF-α, but not IFN-α, plays an important role in IL-15-induced innate antiviral protection. We could not detect any IFN-α in the supernatants of treated cells. However, the bioassay for IFN-α/β (VSV plaque reduction) confirmed the presence of IFN-α/β in the supernatants from mrIL-15- and CpG ODN-treated cells. This strongly suggested the presence of IFN-β in the supernatants. Moreover, we used IRF-3−/− MEFs, which cannot produce IFN-α/β but can respond to it. Although the susceptibility of VSV to IFN-α/β is very well accepted, one might argue that some of the antiviral activity is mediated through TNF-α. A recent report by Adams et. al (1) showed that TNF-α alone has no antiviral activity against HSV-2. It has also been reported that IL-15 upregulates inducible NO synthase (iNOS) expression and NO production by RAW264.7 cells (25). A similar result has been reported for epithelial cells (37). Based on our results, it is likely that the NK/NKT-independent in vivo antiviral activity of IL-15 is mediated via IFN-β and/or TNF-α. We are currently developing a murine IFN-β-specific ELISA. This will enable us to compare the levels of IFN-β in vaginal washes from IL-15−/− and B6 mice after IVAG HSV-2 infection.
Similar to the induction of IL-15 in the vaginal washes of female mice after IVAG HSV-2 infection, the infection of RAW264.7 cells with HSV-2 in vitro induced high levels of IL-15. Indeed, higher levels of IL-15 were induced in these cells after HSV-2 infection than after CpG ODN treatment. These findings may be explained by recent results showing that the recognition of HSV-2 infection by plasmacytoid dendritic cells is mediated by TLR9 (26). The HSV-2 genome contains a high frequency of unmethylated CpG motifs that are active in vivo (23, 38). Indeed, the virus may take advantage of this pathway to induce local inflammation to attract increased numbers of target cells to sites of infection.
Overall, our results indicate that IL-15 plays an important role in innate protection against HSV-2. Cells from the genital mucosa and RAW264.7 cells produce significant amounts of IL-15 in response to HSV-2 infection. Interestingly, CpG ODN-induced innate protection against IVAG HSV-2 was partially mediated through IL-15. Furthermore, we have shown that IL-15 can act independently of NK/NKT cells via the induction of IFN-β and/or TNF-α in order to elicit an antiviral response. The results from our animal model, comprised of mice experimentally infected with HSV-2, clearly show the efficacy of IL-15 and the potential for selected clinical applications for this cytokine. Boosting of the innate immune response by IL-15 during primary viral infections of the genital tract, including HSV or human immunodeficiency virus type 1 infections, may therefore prove valuable clinically to help reduce viral spread and associated complications.
Acknowledgments
We thank Immunex Inc. for providing breeding pairs of IL-15−/− mice. We thank Karen Mossman for providing IRF-3−/− MEFs and VSV. We also thank Philip M. Deacon, Randy Elkasabgy, Jennifer Newton, and Amy Patrick for technical assistance.
This work was supported by grants from the Institute of Infection and Immunity of the Canadian Institutes of Health Research (CIHR) and the Canadian Network on Vaccines & Immunotherapeutics (CANVAC).
REFERENCES
- 1.Adams, O., K. Besken, C. Oberdorfer, C. R. MacKenzie, D. Russing, and W. Daubener. 2004. Inhibition of human herpes simplex virus type 2 by interferon gamma and tumor necrosis factor alpha is mediated by indoleamine 2,3-dioxygenase. Microbes Infect. 6:806-812. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, A., E. Sharif-Askari, L. Fawaz, and J. Menezes. 2000. Innate immune response of the human host to exposure with herpes simplex virus type 1: in vitro control of the virus infection by enhanced natural killer activity via interleukin-15 induction. J. Virol. 74:7196-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashkar, A. A., S. Bauer, W. J. Mitchell, J. Vieira, and K. L. Rosenthal. 2003. Local delivery of CpG oligodeoxynucleotides induces rapid changes in the genital mucosa and inhibits replication, but not entry, of herpes simplex virus type 2. J. Virol. 77:8948-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashkar, A. A., and K. L. Rosenthal. 2003. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77:10168-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biron, C. A. 1999. Initial and innate responses to viral infections—pattern setting in immunity or disease. Curr. Opin. Microbiol. 2:374-381. [DOI] [PubMed] [Google Scholar]
- 6.Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. [DOI] [PubMed] [Google Scholar]
- 7.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 8.Carson, W. E., and M. A. Caligiuri. 1996. Interleukin 15: a potential player during the innate immune response to infection. Exp. Parasitol. 84:291-294. [DOI] [PubMed] [Google Scholar]
- 9.Carson, W. E., T. A. Fehniger, S. Haldar, K. Eckhert, M. J. Lindemann, C. F. Lai, C. M. Croce, H. Baumann, and M. A. Caligiuri. 1997. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J. Clin. Investig. 99:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson, W. E., J. G. Giri, M. J. Lindemann, M. L. Linett, M. Ahdieh, R. Paxton, D. Anderson, J. Eisenmann, K. Grabstein, and M. A. Caligiuri. 1994. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 180:1395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duerst, R. J., and L. A. Morrison. 2003. Innate immunity to herpes simplex virus type 2. Viral Immunol. 16:475-490. [DOI] [PubMed] [Google Scholar]
- 12.Ellis, A. E. 2001. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 25:827-839. [DOI] [PubMed] [Google Scholar]
- 13.Fawaz, L. M., E. Sharif-Askari, and J. Menezes. 1999. Up-regulation of NK cytotoxic activity via IL-15 induction by different viruses: a comparative study. J. Immunol. 163:4473-4480. [PubMed] [Google Scholar]
- 14.Fehniger, T. A., and M. A. Caligiuri. 2001. Interleukin 15: biology and relevance to human disease. Blood 97:14-32. [DOI] [PubMed] [Google Scholar]
- 15.Fehniger, T. A., K. Suzuki, A. Ponnappan, J. B. VanDeusen, M. A. Cooper, S. M. Florea, A. G. Freud, M. L. Robinson, J. Durbin, and M. A. Caligiuri. 2001. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J. Exp. Med. 193:219-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehniger, T. A., K. Suzuki, J. B. VanDeusen, M. A. Cooper, A. G. Freud, and M. A. Caligiuri. 2001. Fatal leukemia in interleukin-15 transgenic mice. Blood Cells Mol. Dis. 27:223-230. [DOI] [PubMed] [Google Scholar]
- 17.Flamand, L., I. Stefanescu, and J. Menezes. 1996. Human herpesvirus-6 enhances natural killer cell cytotoxicity via IL-15. J. Clin. Investig. 97:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freihorst, J., and P. L. Ogra. 2001. Mucosal immunity and viral infections. Ann. Med. 33:172-177. [DOI] [PubMed] [Google Scholar]
- 19.Gosselin, J., A. TomoIu, R. C. Gallo, and L. Flamand. 1999. Interleukin-15 as an activator of natural killer cell-mediated antiviral response. Blood 94:4210-4219. [PubMed] [Google Scholar]
- 20.Grubor-Bauk, B., A. Simmons, G. Mayrhofer, and P. G. Speck. 2003. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J. Immunol. 170:1430-1434. [DOI] [PubMed] [Google Scholar]
- 21.Harandi, A. M., K. Eriksson, and J. Holmgren. 2003. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J. Virol. 77:953-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbst, M. M., and R. B. Pyles. 2003. Immunostimulatory CpG treatment for genital HSV-2 infections. J. Antimicrob. Chemother. 52:887-889. [DOI] [PubMed] [Google Scholar]
- 23.Karlin, S., W. Doerfler, and L. R. Cardon. 1994. Why is CpG suppressed in the genomes of virtually all small eukaryotic viruses but not in those of large eukaryotic viruses? J. Virol. 68:2889-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy, M. K., M. Glaccum, S. N. Brown, E. A. Butz, J. L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C. R. Willis, K. Brasel, P. J. Morrissey, K. Stocking, J. C. Schuh, S. Joyce, and J. J. Peschon. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191:771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, G., Q. Zhai, D. Schaffner, C. Bradburne, A. Wu, A. Hayford, S. Popov, E. Grene, C. Bailey, and K. Alibek. 2004. IL-15 induces IFN-beta and iNOS gene expression, and antiviral activity of murine macrophage RAW 264.7 cells. Immunol. Lett. 91:171-178. [DOI] [PubMed] [Google Scholar]
- 26.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastroianni, C. M., G. d'Ettorre, G. Forcina, M. Lichtner, F. Mengoni, C. D'Agostino, A. Corpolongo, A. P. Massetti, and V. Vullo. 2000. Interleukin-15 enhances neutrophil functional activity in patients with human immunodeficiency virus infection. Blood 96:1979-1984. [PubMed] [Google Scholar]
- 28.McDermott, M. R., J. R. Smiley, P. Leslie, J. Brais, H. E. Rudzroga, and J. Bienenstock. 1984. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J. Virol. 51:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohteki, T. 2002. Critical role for IL-15 in innate immunity. Curr. Mol. Med. 2:371-380. [DOI] [PubMed] [Google Scholar]
- 30.Ohteki, T., K. Suzue, C. Maki, T. Ota, and S. Koyasu. 2001. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat. Immunol. 2:1138-1143. [DOI] [PubMed] [Google Scholar]
- 31.Puzanov, I. J., M. Bennett, and V. Kumar. 1996. IL-15 can substitute for the marrow microenvironment in the differentiation of natural killer cells. J. Immunol. 157:4282-4285. [PubMed] [Google Scholar]
- 32.Pyles, R. B., D. Higgins, C. Chalk, A. Zalar, J. Eiden, C. Brown, G. Van Nest, and L. R. Stanberry. 2002. Use of immunostimulatory sequence-containing oligonucleotides as topical therapy for genital herpes simplex virus type 2 infection. J. Virol. 76:11387-11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sajic, D., A. A. Ashkar, A. J. Patrick, M. J. McCluskie, H. L. Davis, K. L. Levine, R. Holl, and K. L. Rosenthal. 2003. Parameters of CpG oligodeoxynucleotide-induced protection against intravaginal HSV-2 challenge. J. Med. Virol. 71:561-568. [DOI] [PubMed] [Google Scholar]
- 34.Sharif-Askari, E., L. M. Fawaz, P. Tran, A. Ahmad, and J. Menezes. 2001. Interleukin 15-mediated induction of cytotoxic effector cells capable of eliminating Epstein-Barr virus-transformed/immortalized lymphocytes in culture. J. Natl. Cancer Inst. 93:1724-1732. [DOI] [PubMed] [Google Scholar]
- 35.Singh, I. P., and S. Baron. 2000. Innate defences against viraemia. Rev. Med. Virol. 10:395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsunobuchi, H., H. Nishimura, F. Goshima, T. Daikoku, H. Suzuki, I. Nakashima, Y. Nishiyama, and Y. Yoshikai. 2000. A protective role of interleukin-15 in a mouse model for systemic infection with herpes simplex virus. Virology 275:57-66. [DOI] [PubMed] [Google Scholar]
- 37.Yanagita, M., Y. Shimabukuro, T. Nozaki, N. Yoshimura, J. Watanabe, H. Koide, M. Terakura, T. Saho, M. Takedachi, M. H. Jang, H. Kiyono, and S. Murakami. 2002. IL-15 up-regulates iNOS expression and NO production by gingival epithelial cells. Biochem. Biophys. Res. Commun. 297:329-334. [DOI] [PubMed] [Google Scholar]
- 38.Zheng, M., D. M. Klinman, M. Gierynska, and B. T. Rouse. 2002. DNA containing CpG motifs induces angiogenesis. Proc. Natl. Acad. Sci. USA 99:8944-8949. [DOI] [PMC free article] [PubMed] [Google Scholar]