Abstract
Background:
Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) is a clinicopathological construct proposed to facilitate studying TDP-43 pathology in older individuals.
Objective:
Our aim was to describe clinical and cognitive characteristics of LATE-NC without Alzheimer’s disease neuropathologic change (ADNC) and Lewy body (LB) and to compare this with ADNC and primary age related tauopathy (PART).
Methods:
In 364 autopsies of the oldest old of The 90+ Study, we identified those with LATE-NC without ADNC and LB. Control groups were participants with ADNC and PART.
Results:
Of 31% of participants who had LATE-NC, only 5 (1.4%) had LATE-NC without ADNC and LB, all of whom had tau. These participants had a gradual and progressive cognitive decline. Four (80%) had dementia at death, a rate that was higher than ADNC (50%) and PART (21.7%). Mean duration of cognitive impairment was twice as long in LATE-NC without ADNC and LB (6.2 years) compared to ADNC (2.9 years) and PART (3 years). LATE-NC without ADNC and LB group had a higher prevalence of syncope, depression, and extrapyramidal signs than the ADNC and PART groups.
Conclusions:
Despite the high prevalence of LATE-NC, LATE-NC without ADNC and LB was rare in this large oldest-old cohort, highlighting the very high prevalence of multiple pathologic changes in the oldest old. Slowly progressive cognitive decline, ubiquitous memory impairment, history of syncope and depression, and extrapyramidal signs were prominent features among our LATE-NC without ADNC and LB group.
Keywords: Alzheimer’s disease, case studies, dementia, oldest old, TDP-43 protein
INTRODUCTION
Pathological phosphorylation and cytoplasmic aggregation of TAR DNA-binding protein 43 kDa (TDP-43) is a common finding in frontotemporal lobar degeneration (FTLD-TDP) and in amyotrophic lateral sclerosis (ALS). Brain regional TDP-43 inclusions have recently been recognized as an important contributor to cognitive impairment in the oldest-old and a new disease entity limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) has been defined [1]. As described in thirteen community-based cohorts, LATE is present in 20–39% of individuals past the age of 80, illustrating its prevalence among the oldest-old [2]. LATE-NC often presents with other neuropathologic changes including those characteristic of Alzheimer’s disease (ADNC), hippocampal sclerosis (HS), Lewy bodies (LB), and primary-age related tauopathy (PART) [2–4]. This makes it hard to discern its pathognomonic and clinical characteristics.
Previous studies have reported gradual and insidious decline in episodic and working memory in individuals with LATE-NC [5–8]. However, these studies examine LATE-NC in individuals who were also harboring other neuropathologic changes. While statistical techniques can be used to infer the unique contribution of a given neuropathologic change, little is known about the clinical and cognitive impact of LATE-NC in those without coexisting diseases.
We aim to characterize clinical and cognitive features of LATE-NC without ADNC and LB in the oldest old, an age group where this neuropathologic change is most prevalent. Also, to better characterize clinical and cognitive features of LATE-NC we compared LATE-NC without ADNC and LB with ADNC and PART.
METHODS
Participants
Cases and comparison groups were selected from 364 consecutive autopsies of the oldest-old participants of The 90+ Study. The 90+ Study participants are evaluated every 6 months until death. Each evaluation includes neurological examination, neuropsychological testing, update of self-reported medical history and medication use. Trained examiners (physicians or nurse practitioners) perform neurological examination that includes the Clinical Dementia Rating scale [9] and Functional Activities Questionnaire [10]. Trained neuropsychological testers administer a neuropsychological test battery [11, 12], that includes the Mini-Mental State Examination (MMSE) [13], Modified MMSE (3MS) [14], Animal and Letter Fluency, Trail Making Test PartsA and B, Digit Span Forward and Backwards [15], California Verbal Learning Test-Short Form (CVLT-SF) [16], and Boston Naming Test (BNT) [17]. Postmortem neuropathological evaluation is performed on all participants who sign an autopsy consent.
The 90+ Study was approved by the Institutional Review Board of the University of California Irvine. All participants or their surrogates provided written informed consent.
Cognitive status determination
Cognitive status: normal cognition, cognitive impairment no dementia (CIND) [18], or the Diagnostic and Statistical Manual of Mental Disorders-IB (DSM-IV) [19] dementia was assigned at every visit by trained neurological examiners. At postmortem consensus case conference, cognitive status, date of onset of cognitive impairment, and clinical diagnosis were assigned by geriatric neurologists, neuropsychologists, and other members of the team after consideration of all available information that included neurological examination, neuropsychological testing, medical records, self-and informant-reported medical history, and medications. Cognitive status was assigned without knowledge of neuropathological evaluation. Clinical diagnoses of AD were assigned according to the National Institute of Neurological Disorders and Stroke, and the Alzheimer’s Disease and Related Disorders Association (NINDS/ADRDA) criteria [20].
Neuropathological evaluations
Before dissection, the whole brain was weighed, and one hemisphere was selected based on the clinician’s assessment of any asymmetry in clinical features. Then neuropathological evaluation was completed according to standard National Institute on Aging–Alzheimer’s Association (NIA-AA) guidelines [21] and was assessed without knowledge of cognitive status. The following pathologic changes were considered: 1) LATE-NC- defined as present when phosphorylated TDP-43 inclusions were found in the amygdala, hippocampus, and/or prefrontal cortex; 2) ADNC- defined as present when low, intermediate, or high in severity following NIA-AA criteria; 3) PART-defined as present when phosphorylated tau inclusions were found in the limbic system or cerebral cortex (Braak neurofibrillary tangle stage >2) but no amyloid-β plaques were identified (A score = 0 and C score = 0) [22]; 4) LB- defined as present when LBs were found in the limbic system or cerebral cortex (Braak stage ≥5) [23]; 5) arteriolosclerosis- defined as present when moderate or severe following National Alzheimer’s Coordinating Center (NACC) version (V10) criteria [24]; 6) atherosclerosis- defined as present when moderate or severe following NACC V10 criteria; 7) microvascular lesions (MVL) - defined as present when ≥2 MVLs were found in the cerebral cortex and/or deep gray matter [25]; 8) aging-related tau astrogliopathy (ARTAG)- defined as present when any ARTAG was found in the sections of cerebral cortex, hippocampus, or brainstem [26]; 9) cerebral amyloid angiopathy- defined as present when moderate or severe following NACC V10 criteria.
Statistical analysis
Analysis aimed to compare the clinical and cognitive features of the LATE-NC without ADNC and LB versus ADNC and PART groups. Statistical analyses were performed using GraphPad Prism. Due to the small sample size of the LATE-NC without ADNC and LB group (n = 5), a Fischer’s exact test was performed, and no adjustments were made for multiple comparisons.
Case and comparison groups determination
The LATE-NC without ADNC and LB cases and both comparison groups of ADNC and PART were selected from among a total of 364 participants of The 90+ Study who had brain autopsies (Fig. 1). 1) The LATE-NC without ADNC and LB cases had TDP-43 inclusions in the amygdala, hippocampus, or neocortex but no low, moderate, or severe ADNC using the NIA-AA criteria [21], LB in limbic region or neocortex, or ≥2 cortical MVLs. 2) The ADNC group had severe ADNC, but no TDP-43 in the amygdala, hippocampus, or neocortex, no LB in limbic regions or neocortex, and less than 2 MVLs. 3) The PART group had limbic or cortical phosphorylated tau inclusions (Braak stage >2) but no amyloid (A score = 0 and C score = 0), TDP-43 in the amygdala, hippocampus, or neocortex, or LB in limbic regions or neocortex, and less than 2 MVLs. Among the total of 364 autopsies, 5 met the criteria for LATE-NC without ADNC and LB, 46 for ADNC, and 23 for PART. In The 90+ Study cohort, no participant was found to have FTLD-TDP neuropathologic change.
Fig. 1.
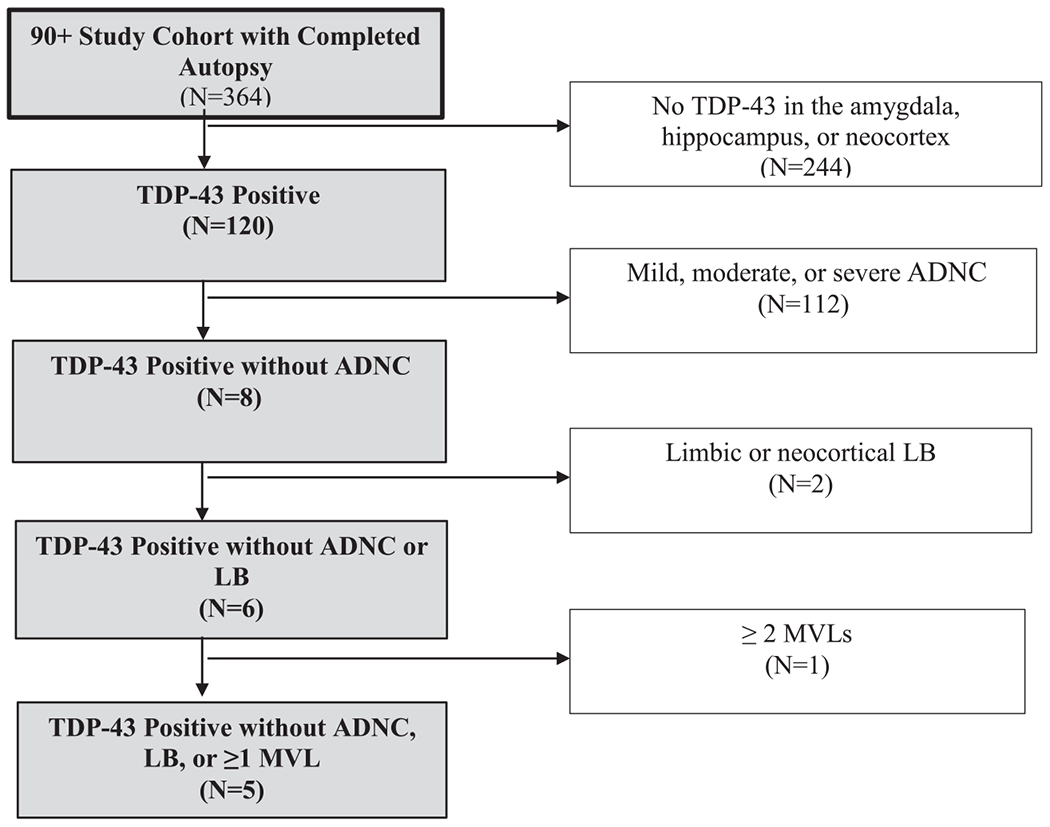
Flow chart for selection of LATE-NC without ADNC and LB cases. Starting from a group of 364 participants from The 90+ Study cohort with completed autopsy and after exclusion of participants without hippocampal or neocortical TDP-43 inclusions, a subgroup of 120 individuals were identified as TDP-43 positive. After further exclusion of dementia-related neuropathologies, including ADNC, limbic or neocortical LB, and ≥1 cortical MVL, the final group consisted of 5 LATE-NC without ADNC and LB cases.
Due to the ubiquitous presence of pathologic tau in the limbic structures in this cohort, all LATE-NC without ADNC and LB cases also fulfilled criteria for PART. Moreover, due to high prevalence of vascular pathologies, their presence was not an exclusion criterion for LATE-NC without ADNC and LB group membership. Table 1 summarizes presence and severity of cerebral arteriolosclerosis, atherosclerosis, and cerebral amyloid angiopathy, in cases with LATE-NC without ADNC and LB. In addition, Table 1 summarizes the demographics, medical history, and presence of extrapyramidal signs in cases with LATE-NC without ADNC and LB. Of note, while not an exclusion criterion for the LATE-NC without ADNC and LB cases, none of the LATE-NC without ADNC and LB cases had HS.
Table 1.
Demographics, clinical findings, and neuropathology in the LATE-NC without ADNC and LB cases
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Age at death, y | 95 | 105 | 100 | 98 | 101 |
| Sex | M | F | F | M | F |
| Education, y | College | College | College | College | College |
| Race | white | white | white | Asian | white |
| Clinical Findings | |||||
| Relevant medical history (Age, y) | TIA (82), stroke (88), syncope (90), COPD (91) | colon cancer (80), staphylococcal meningitis (82), osteoporosis (84), syncope (94), depression (95) | depression (95), syncope (98) | syncope (96), depression (96) | hypothyroidism (57), osteoarthritis (88), anxiety (91), depression (92), syncope (93) |
| Cardiovascular Disease, y | MI (88) | HTN (92), postural hypotension (95), MI (101), AF (105) | HTN (85), VHD (98), AF (98) | MI (90), HTN (91), Mixed HLD (91), CABG (91), chronic hypotension (93), arrythmia (96) | HTN (np), arrhythmia (91), AF (99), CHF (99) |
| Extra-pyramidal signs | Yes | Yes | Yes | Yes | No |
| Postmortem Case Conference Diagnosis | Possible AD | Possible AD | Probable AD | Possible AD | Probable AD |
| Neuropathology | |||||
| Atherosclerosis | No | No | Severe | No | No |
| Arteriolosclerosis | Moderate | Moderate | Moderate | Moderate | Moderate |
| Cerebral amyloid angiopathy | No | No | No | Moderate | No |
| Lewy bodies | No | No | Yes (only OB)* | Yes (only OB)* | No |
| Aging-related tau astrogliopathy | Yes, (MTL)* (HPC)* (SP)* (SUB)* (GM)*(WM)* (PV)* | No | Yes (HPC)* (SP)* | Yes (SMTG)* (WM)* | No |
Every LATE-NC without ADNC and LB case had TDP-43 stage II (hippocampus) and no HS. Medical history abbreviations: TIA, transient ischemic attack; MI, myocardial infarction; HTN, hypertension; VHD, valvular heart disease; AF, atrial fibrillation; HDL, hyperlipidemia; CHF, congestive heart failure; CABG, coronary artery bypass graft surgery. Region abbreviations: OB, olfactory bulb; MTL, medial temporal lobe; HPC, hippocampus; SMTG, superior & middle frontal gyrus. Location abbreviations: WM, white matter; SP, subpial; SUB, subependymal; GM, gray matter; PV, perivascular. Possible AD, clinical evidence to support diagnosis of AD with absence of neuroimaging scans. Probable AD, clinical evidence to support the diagnosis of AD with presence of neuroimaging scans.
RESULTS
Case 1
College educated white man who died at age 95 due to embolic stroke. Relevant history included TIA (82 years), stroke (88 years), myocardial infarction (88 years), syncope (90 years), and COPD (91 years). On neurological examination, he had bradykinesia and parkinsonian gait (Table 1). He was first diagnosed with CIND at age 90, and with dementia at age 93 years. He had impairments in the following cognitive domains: memory (90 years), executive function (91 years), visuospatial function (93 years), and orientation (94 years) (Table 2).
Table 2.
Age of impairment across cognitive domains among the LATE-NC without ADNC and LB cases
| Impaired Cognitive Domains | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Memory | |||||
| Onset: | 90 | 104 | 91 | 93 | 93 |
| Dementia DX: | 93 | - | 96 | 94 | 93 |
|
| |||||
| Orientation | |||||
| Onset: | 94 | - | 96 | 97 | 98 |
| Dementia DX: | 93 | 96 | 94 | 93 | |
|
| |||||
| Language | |||||
| Onset: | - | - | 99 | - | - |
| Dementia DX: | 96 | ||||
|
| |||||
| Executive Function | - | ||||
| Onset: | 91 | 104 | 96 | 101 | |
| Dementia DX: | 93 | - | 96 | 93 | |
|
| |||||
| Visuo-spatial | |||||
| Onset: | 93 | - | - | 94 | - |
| Dementia DX: | 93 | 94 | |||
|
| |||||
| Calculations | |||||
| Onset: | - | - | 97 | - | - |
| Dementia DX: | 96 | ||||
|
| |||||
| Constructions | |||||
| Onset: | - | - | 99 | - | - |
| Dementia DX: | 96 | ||||
Dx, diagnosis. Table 2 depicts the age at which the individual domain has an onset of impairment stratified by LATE-NC without ADNC and LB cases. Domains were considered separately and were derived based on neuropsychological assessment, neurological examination, and behavioral observations made at study visits. Domains that have a ‘-’ indicates no impairment was recorded for that specific domain. The cognitive diagnosis at death is described in the second column along with the age at which an individual began their onset of cognitive impairment, as defined by CIND or dementia, and the age at which they received a study diagnosis of dementia.
Case 2 had CIND, no dementia.
Case 2
College educated white woman who died at age 105 of unknown cause. Relevant history included colon cancer (80 years), staphylococcal meningitis (82 years), osteoporosis (84 years), hypertension (92 years), recurrent syncope of possible vasovagal cause (94 years), depression (95 years), episodic postural hypotension (95 years), myocardial infarction (101 years), and atrial fibrillation (105 years). On neurological examination, positive findings included bradykinesia, fluctuating vertical gaze limitation and parkinsonian gait (Table 1). She was first diagnosed with CIND at 104 years. She had impairment in the domains of memory and executive function (104 years) (Table 2).
Case 3
College educated white woman who died at age 100 of unknown cause. Relevant history included hypertension (85 years), depression (95 years), recurrent syncope (98 years), valvular heart disease (98 years), and atrial fibrillation (98 years). On neurological examination, she had bradykinesia and parkinsonian gait (Table 1). She was first diagnosed with CIND at 91 years and transitioned to dementia at the age of 96. She had impairment in memory (91 years), orientation and executive function (96 years), calculations (97 years), language, and constructional praxis (99 years) (Table 2).
Case 4
College educated Asian man who died at age 98 of congestive heart failure. Relevant history included myocardial infarction (90 years), hypertension (91 years), mixed hyperlipidemia (91 years), coronary bypass graft surgery (91 years), chronic hypotension (93 years), arrhythmia (96 years), syncope (96 years), and depression (96 years). On neurological examination, he had masked facies, bradykinesia, right upper limb rigidity, and parkinsonian gait (Table 1). He was diagnosed with CIND at age 93 and transitioned to dementia at age 94. Impaired cognitive domains included memory (93 years), visuospatial function (94 years), and orientation (97 years) (Table 2).
Case 5
College educated white woman who lived to be 101 years and died of congestive heart failure. Relevant history included hypothyroidism (57 years), hypertension (age not reported), osteoarthritis (88 years), arrhythmia (91 years), anxiety (91 years), depression (92 years), syncope (93 years), episodic orthostatic hypotension (95 years), atrial fibrillation (98 years), and congestive heart failure (99 years). Her neurological examination was unremarkable (Table 1). She was diagnosed with CIND at 93 years old and transitioned to dementia at 98 years. Impaired cognitive domains included memory (93 years), orientation (98 years), and executive functioning (101 years) (Table 2).
Photomicrographs of TDP-43 inclusions in the hippocampus are available for all five cases (Fig. 2).
Fig. 2.
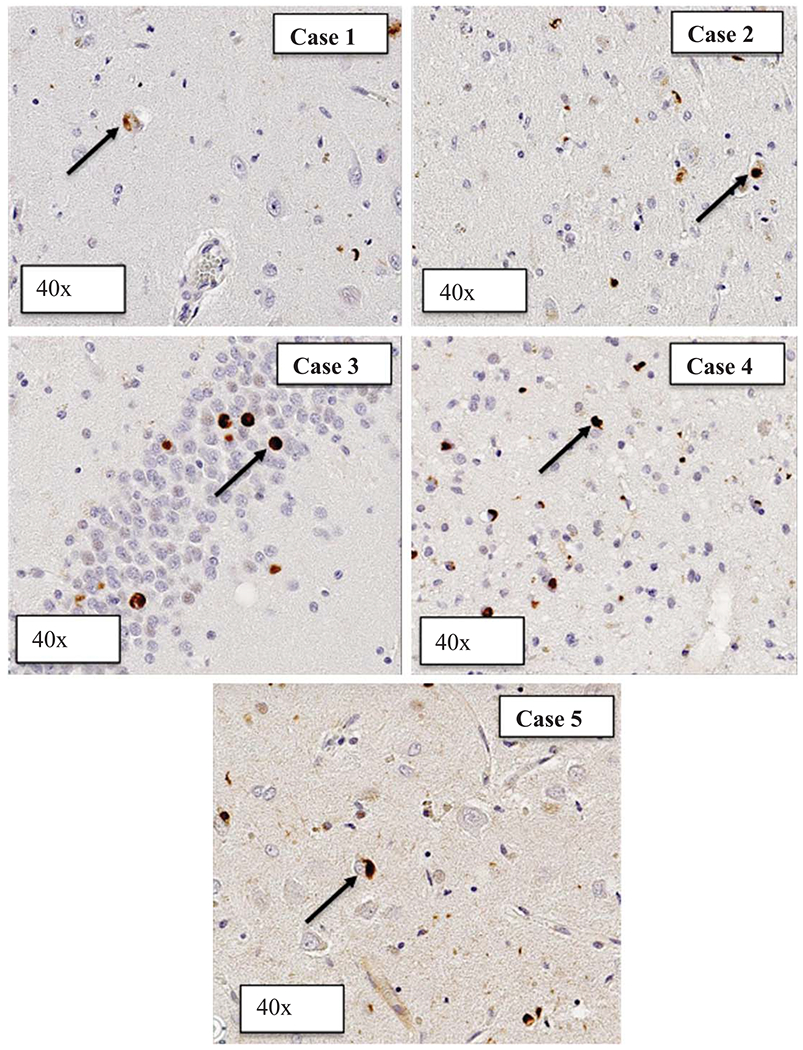
Phosphorylated TDP-43 inclusions in the hippocampi of LATE-NC without ADNC and LB cases. Photomicrographs of phosphorylated TDP-43 inclusions in the hippocampus in each of the 5 LATE-NC without ADNC and LB cases. The black arrow is identifying the p-TDP-43 cytoplasmic inclusions.
Cognition in LATE-NC without ADNC and LB compared to ADNC and PART
Dementia in last visit before death was more prevalent in LATE-NC without ADNC and LB (80%) compared to ADNC (43%, p = 0.05) and PART (22%, p = 0.02) (Table 3). Case conference diagnosis of AD was present in 100% of LATE-NC without ADNC and LB cases and in 44% of ADNC (p = 0.02) and 36% of PART cases (p = 0.01).
Table 3.
Demographics, clinical findings, cognition and neuropathology in LATE-NC without ADNC and LB versus ADNC and PART groups
| LATE-NC | ADNC | PART | PART Braak Stage ≥4 | |
|---|---|---|---|---|
| (n = 5) | (n = 46) | (n = 23) | (n =18) | |
| Age at Death (y) | ||||
| Mean (SD) | 100 (3.64) | 98.3 (3.24) | 96.1 (2.98) | 95.8 (2.97) |
| [Min, Max] | [95.7, 105] | [92.1, 109] | [91.7, 101] | [91.7, 100.6] |
| Women, n (%) | 3 (60) | 34 (73.9) | 18 (73.8) | 14 (77.8) |
| College education, n (%) | 5 (100) | 35 (76.1) | 18 (79.3) | 16 (88.9) |
| APOE4 Carrier, n (%) | 0 (0) | 13 (28.2) | 3 (13.0) | 0 (0%) |
| Clinical Findings | ||||
| Syncope, n (%) | 5 (100)*** | 9 (19.6)*** | 3 (13)*** | 2 (11.1)*** |
| Depression, n (%) | 4 (80)* | 8 (17.4)** | 4 (17.4)* | 4 (22.2)* |
| Cardiovascular Disease, n (%) | 5 (100) | 24 (52.2) | 14 (60.9) | 14 (77.8) |
| Osteoarthritis, n (%) | 1 (20) | 21 (45.6) | 17 (74) | 14 (77.8) |
| Extra-pyramidal signs, n (%) | 4 (80)** | 6 (13)** | 1 (4.4)** | 1 (5.6)** |
| Case Conference Diagnosis of AD, n (%) | 5 (100)* | 20 (44.4)* | 8 (36.4)* | 3 (16.7)** |
| Cognitive Measures | ||||
| Duration of Cognitive Impairment (y), n (%) | 6.2 | 3 | 2.9 | 3.2 |
| Dementia diagnosis at last visit before death, n (%) | 4 (80) | 23 (50.0) | 5 (21.7) | 1 (5.6)** |
| Memory domain impairment, n (%) | 5 (100) | 21 (45.7) | 10 (43.5) | 9 (50) |
| Executive function domain impairment, n (%) | 4 (80)* | 19 (41.3) | 6 (26.1)* | 6 (33.3) |
| Visuospatial domain impairment, n (%) | 2 (40) | 10 (21.7) | 2 (8.7) | 2 (11.1) |
| Orientation domain impairment, n (%) | 3 (60) | 9 (19.6) | 5 (8.7) | 5 (27.8) |
| Neuropathology | ||||
| Atherosclerosis, n (%) | 1 (20) | 9 (20) | 6 (27.3) | 4 (22.2) |
| Arteriolosclerosis, n (%) | 5 (100) | 27 (60) | 14 (63.6) | 12 (66.7) |
Comparison of social and physical characteristics among those with LATE-NC without ADNC and LB, ADNC, and PART. Syncope, cardiovascular disease, memory domain impairment, and cerebral arteriolosclerosis were found in all LATE-NC without ADNC and LB participants, who also demonstrated a higher prevalence of depression and dementia diagnosis at last visit before death compared to those with ADNC and PART. All participants in the ADNC group have high severity level of ADNC.
p < 0.05,
p < 0.01,
p < 0.001.
Impaired memory was present in all LATE-NC without ADNC and LB participants compared to 46% in ADNC (p = 0.05) and 44% in PART (p = 0.04) groups. Executive function impairment was observed in 80% of LATE-NC without ADNC and LB participants in comparison to 41.3% in ADNC (p = 0.16) and 26.1% in PART (p = 0.04). Duration of cognitive impairment was longest for LATE-NC without ADNC and LB group (6 years) compared to 3 years for ADNC and PART.
Health history in LATE-NC without ADNC and LB compared to ADNC and PART
LATE-NC without ADNC and LB group was older at death (100 years) compared to ADNC (98 years) and PART (96 years). There was slightly lower proportion of women in LATE-NC without ADNC and LB group (60%) compared to ADNC (74%, p = 0.61) and PART (74%, p = 0.57). The proportion of college educated participants was higher in LATE-NC without ADNC and LB (100%) then in ADNC (76%) (p = 0.55) and PART (79%) (p = 0.57) groups.
The LATE-NC without ADNC and LB group had more individuals with syncope, depression, and extrapyramidal signs compared to ADNC (p = 0.0009, p = 0.008, p = 0.0038 respectively) and PART groups (p = 0.0006, p = 0.008, p = 0.001 respectively) (Table 3). In contrast, osteoarthritis was significantly less frequent in the LATE-NC without ADNC and LB group compared to those in PART group (p = 0.03).
DISCUSSION
LATE-NC is a common neuropathological feature in the oldest old and is an important contributor to dementia in this age group. Similarity in clinical presentation [1, 3] and common coexistence of LATE-NC and ADNC [2, 22] have hampered efforts to elucidate the unique impact of LATE-NC. In this study, we characterize the clinical and cognitive manifestations of LATE-NC by studying participants who had LATE-NC pathology in the absence of comorbid ADNC or LB. We contrasted the cognitive and clinical characteristics of this group against participants from the same cohort with ADNC and PART. We found that those with LATE-NC without ADNC and LB, had a high prevalence of dementia (80%) and memory impairment (100%) at the time of death and had frequent history of depression (80%) and syncope (100%), and examination finding of extrapyramidal signs (80%). Additionally, we found those with LATE-NC without ADNC and LB had a longer duration of cognitive impairment. These findings provide possible future research directions to effectively distinguish LATE-NC from ADNC and other degenerative pathologies.
An important characteristic of LATE-NC neuropathology is that its ‘presence without ADNC and Lewy Bodies is rare. We identified only 5 cases (1.4%) with LATE-NC without ADNC and LB among 364 completed autopsies of The 90+ Study. In comparison, LATE with co-occurring neuropathologic changes is found in 36% of The 90+ Study cohort and is similarly reported in other old age cohorts [1, 2, 21, 23]. Interestingly, the LATE-NC without ADNC and LB group had no individuals with an APOE4 allele. Four cases had 3/3 and one case had 2/3 APOE allele genotype. Despite its relatively rare occurrence, study of LATE-NC without ADNC and LB is valuable to identify the unique impact of LATE-NC without the influence of ADNC.
To better characterize the clinical features of LATE-NC without ADNC and LB, two comparison groups were selected: 1) ADNC, as this neuropathologic feature closely mimics the clinical presentation of LATE-NC and 2) PART, a prevalent neuropathology found in the oldest-old. Individuals with LATE-NC without ADNC and LB had the longest mean duration of cognitive impairment of 6.2 years compared to 3 years in ADNC and 2.9 years in PART. This finding is consistent with previous literature which indicates LATE-NC is associated with prolonged cognitive decline in comparison to ADNC [26, 27]. In addition, those in the LATE-NC without ADNC and LB group died at an older age (100 years) in comparison to the ADNC (98.3 years) and PART groups (96.1 years). A previous study supports our results where individuals with LATE-NC died at an older age than those with ADNC [28].
Further, 80% of the LATE-NC without ADNC and LB cases had dementia at death, in comparison to the 42.6 % of the ADNC and 21.7% of the PART groups (Table 2). Previous studies have described the notable contribution of LATE-NC to cognitive impairment [4]. Our results suggest that in those with relatively minimal co-pathology, LATE-NC remains a strong contributor to dementia, potentially stronger than ADNC. Further research is warranted to support this observation, due to small sample size of our cohort. One caveat of the above conclusion is that the LATE-NC without ADNC and LB group has two pathologies: LATE and PART. Therefore, it could be argued that the cognitive signature of the LATE-NC without ADNC and LB group was due to the co-morbidity of two degenerative pathologies, TDP-43, and tau pathology in limbic structures. In the literature, the presence of PART alone does not appear to have a significant impact on cognition but the presence of two degenerative pathologies, such as PART and LATE, may have a greater cognitive impact than the effect of one pathology [28].
Memory impairment was present in every case with LATE-NC without ADNC and LB, whereas in ADNC and PART groups more than half of individuals died without memory impairment. Our results are congruent with the previous studies that have reported decline in memory in relation with TDP-43 [5, 27, 29]. Our results add to this body of literature by providing evidence that LATE-NC alone is sufficient to cause significant memory deficits in the oldest old. Further, impairments in executive functioning and orientation appeared in 80% of LATE-NC without ADNC and LB cases whereas less than 50% of ADNC and PART cases demonstrated these impairments. We have previously reported impaired orientation in relation to LATE-NC in a study that included all participants of this cohort [30]. A relation between impaired executive function and LATE-NC, however, has not been reported and will require further studies. One potential reason for the observed relation is the co-occurrence of cerebral arteriolosclerosis in our LATE-NC without ADNC and LB group since vascular-based injury is known to be associated with executive function impairment [31]. Future studies are needed to elucidate the relation of pathologic TDP-43 and impairment of executive function.
Depression and syncope were noticeably more frequent in those with LATE-NC without ADNC and LB than the other two groups (Table 2). However, the sample size warrants further research. The prevalence of depression and syncope in ADNC and PART were similar to that found in the healthy elderly population [31, 32]. Previous work has indicated neuropsychiatric symptoms, including depressed mood, can be prominent within a LATE-NC sample, with mixed neuropathologic features [32], making it a potentially clinically relevant feature of LATE-NC without ADNC and LB. Syncope, among other causes, can be related to cardiovascular disease or cerebral arteriolosclerosis [24], both of which were present in every case of LATE-NC without ADNC and LB. However, since both cardiovascular disease (including myocardial infarction, atrial fibrillation, hypertension, and valvular heart disease), and cerebral arteriolosclerosis were also prevalent in ADNC and PART, there may be other factors at play that merit further investigation.
From a clinical standpoint, extrapyramidal signs were a prominent feature in those with LATE-NC without ADNC and LB but rarely observed in the ADNC and PART groups. It is important to note that Lewy body co-morbidity was unlikely to have contributed to these extrapyramidal signs as the Lewy bodies present in two individuals with LATE-NC without ADNC and LB were limited to the olfactory bulb. A recent study corroborates our findings upon examination of FTLD-TDP substantia nigra tissue, where those with movement disorder features had a higher burden of TDP-43 than those without, suggesting there may be a link between TDP-43 pathology and extrapyramidal signs [33]. To our knowledge this is the first study to report extrapyramidal features in LATE-NC cases. As the sample size of LATE-NC without ADNC and LB is small, further research should be conducted to examine if extrapyramidal features may be an informative clinical measure to identify LATE-NC antemortem, as this is routinely measured upon neurological examination.
Among the LATE-NC without ADNC and LB, no cases had HS. A potential explanation for this finding is that none of the LATE-NC without ADNC and LB group had evidence of LATE-NC in the neocortex (stage 3) and in all participants LATE-NC was limited to hippocampus (stage 2). It has previously been shown that as LATE becomes more advanced; this increases the likelihood of comorbid HS [1]. This finding is also observed in our cohort, where individuals with LATE-NC stage 3, had a higher prevalence of HS (54.6%) in comparison to those with LATE stage 2 (16.4%). Since the LATE-NC without ADNC and LB cases in our study have only intermediate LATE-NC this may explain why there is no co-occurring HS in these participants. In the ADNC group, there are two cases (4.4%) with HS and in the PART group, there are two cases (9.5%) with HS as well. It is also important to mention, that there is no participant in The 90+ Study cohort with the presence of pathological FTLD-TDP.
We acknowledge several limitations. Low number of cases with LATE-NC without ADNC and LB was a major limitation of our study. Given the high frequency of multiple neuropathologic features in the oldest old cohorts, future studies can solve this problem by combining data from different cohorts or leveraging existing multi-center databases. Another limitation of our study is that the LATE-NC without ADNC and LB group has two pathologies: LATE and PART, and this group was compared to groups that contain only one pathology per group. The reason for presence of PART in LATE-NC without ADNC and LB cases is that every participant in The 90+ Study cohort (n = 386) has medial temporal tau pathology and those who do not have amyloid are given a label of PART. Previous studies suggest presence of PART alone does not have a significant impact on cognition but the synergy of two degenerative pathologies, i.e., PART and LATE might lead to a cognitive impact beyond the effect of each pathology alone [28]. Every LATE-NC without ADNC and LB case has Braak Stage IV, a more advanced spread of tau and this may contribute to the cognitive measures we examine. To see the impact of advanced PART alone, we performed additional analyses on PART with a Braak Stage IV or greater (n = 18) and compared this to the original PART group (N = 23) that includes lower Braak stage 3 and higher. Results from this analysis demonstrate that individuals in the PART Braak IV/V group did not differ in cognitive measures from the PART group that included lower Braak Stages. This finding suggests that more advanced Braak stage alone does not impact cognitive measures. However, we still acknowledge the potential synergistic effect of two degenerative pathologies (i.e., LATE-NC and PART) in the LATE-NC without ADNC and LB group.
Also, all cases with LATE-NC without ADNC and LB had cerebral arteriolosclerosis and as discussed above, some of the observed cognitive impairment of the LATE-NC group (i.e., executive function impairment) might be attributable to cerebral arteriolosclerosis. Additionally, as our sample is exclusively composed of individuals who were older than 90 years old at death, it is plausible that survival bias might have played a role in the findings of this study. It is noteworthy, however, that given that LATE-NC is a pathology with high prevalence in this age group, an oldest old cohort is an ideal cohort to study LATE-NC. Lastly, our cohort was predominantly white and all but one participant of the LATE-NC without ADNC and LB group were from this ethnicity. Future multi-ethnic studies of LATE-NC with appropriate representation from all races are required to elucidate the impact of LATE-NC in other ethnic groups.
LATE-NC most frequently occurs together with other neuropathologic features, and LATE-NC without ADNC and LB was rare in this large oldest old cohort. We found slowly progressive cognitive decline, ubiquitous presence of memory impairment, history of syncope and depression, and presence of extrapyramidal signs were prominent features among our LATE-NC without ADNC and LB group. Given the low number of LATE-NC without ADNC and LB, further research is needed to replicate these findings and tests their utility in antemortem diagnosis of LATE pathology.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was funded by the National Institute of Health (NIH) National Institute on Aging (NIA) grant # R01AG062706, grant # R01AG021055, and grant # P30AG066519. APOE genotyping from the National Centralized Repository for Alzheimer’s Disease and Related Dementias (NCRAD), which receives government support by NIA U24AG021886, was used in this study.
Footnotes
CONFLICT OF INTEREST
S. Ahmad Sajjadi is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review. All other authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from The 90+ Study. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- [1].Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K (2019) Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 142, 1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nelson PT, Brayne C, Flanagan ME, Abner EL, Agrawal S, Attems J, Castellani RJ, Corrada MM, Cykowski MD, Di J, Dickson DW, Dugger BN, Ervin JF, Fleming J, Graff-Radford J, Grinberg LT, Hokkanen SR, Hunter S, Kapasi A, Kawas CH, Keage HA, Keene CD, Kero M, Knopman DS, Kouri N, Kovacs GG, Labuzan SA, Larson EB, Latimer CS, Leite RE, Matchett BJ, Matthews FE, Merrick R, Montine TJ, Murray ME, Myllykangas L, Nag S, Nelson RS, Neltner JH, Nguyen AT, Petersen RC, Polvikoski T, Reichard RR, Rodriguez RD, Suemoto CK, Wang SJ, Wharton SB, White L, Schneider JA (2022) Frequency of LATE neuropathologic change across the spectrum of Alzheimer’s disease neuropathology: Combined data from 13 community-based or population-based autopsy cohorts. Acta Neuropathol 144, 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Besser LM, Teylan MA, Nelson PT (2020) Limbic predominant age-related TDP-43 encephalopathy (LATE): Clinical and neuropathological associations. J Neuropathol Exp Neurol 79, 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buciuc M, Tosakulwong N, Machulda MM, Whitwell JL, Weigand SD, Murray ME, Reichard RR, Parisi JE, Dickson DW, Boeve BF, Knopman DS, Petersen RC, Josephs KA (2021) Tar DNA-binding protein 43 is associated with rate of memory, functional and global cognitive decline in the decade prior to death. J Alzheimers Dis 80, 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA (2018) TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun 6, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nag S, Yu L, Wilson RS, Chen EY, Bennett DA, Schneider JA (2017) TDP-43 pathology, and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology 88, 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wilson RS, Wang T, Yu L, Bennett DA, Boyle PA (2020) Normative cognitive decline in old age. Ann Neurol 87, 816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- [10].Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S (1982) Measurement of functional activities in older adults in the community. J Gerontol 37, 323–329. [DOI] [PubMed] [Google Scholar]
- [11].Melikyan ZA, Corrada MM, Dick MB, Whittle C, Paganini-Hill A, Kawas CH (2019) Neuropsychological test norms in cognitively intact oldest-old. J Int Neuropsychol Soc 25, 530–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Whittle C, Corrada MM, Dick M, Ziegler R, Kahle-Wrobleski K, Paganini-Hill A, Kawas C (2007) Neuropsychological data in nondemented oldest old: The 90+ Study. J Clin Exp Neuropsychol 29, 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [14].Teng EL, Chui HC (1987) The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 48, 314–318. [PubMed] [Google Scholar]
- [15].Wechsler D (1997) WAIS-III administration and scoring manual. Psychological Corporation, San Antonio, TX. [Google Scholar]
- [16].Delis D, Kaplan E, Kramer JH (2001) The Delis–Kaplan Executive Function System (DK-EFS). Psychological Corporation, San Antonio, TX. [Google Scholar]
- [17].Kaplan E, Goodglass H, Weintraub S (1978) The Boston Naming Test. Kaplan & Goodglass, Boston. [Google Scholar]
- [18].Graham JE, Rockwood K, Beattie BL, Eastwood R, Gauthier S, Tuokko H, McDowell I (1997) Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 349, 1793–1796. [DOI] [PubMed] [Google Scholar]
- [19].American Psychiatric Association (1994) DSM-IV. Diagnostic and statistical manual of mental disorders (4th ed.) American Psychiatric Association, Washington, DC. [Google Scholar]
- [20].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- [21].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 123, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Josephs KA, Murray ME, Tosakulwong N, Whitwell JL, Knopman DS, Machulda MM, Weigand SD, Boeve BF, Kantarci K, Petrucelli L, Lowe VJ, Jack CR, Petersen RC, Parisi JE, Dickson DW (2017) Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: A clinico-imaging-pathological study of primary age-related tauopathy (PART). Acta Neuropathol 133, 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Besser LM, Kukull W, Teylan M, Bigio EH, Cairns NJ, Kofler JK, Montine TJ, Schneider JA, Nelson PT (2018) The Revised National Alzheimer’s Coordinating Center’s Neuropathology Form-available data and new analyses. J Neuropathol Exp Neurol 77, 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Agrawal S, Yu L, Kapasi A, James BD, Arfanakis K, Barnes LL, Bennett DA, Nag S, Schneider JA (2021) Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change and microvascular pathologies in community-dwelling older persons. Brain Pathol 31, e12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kovacs FI, Grinberg LT, Alafuzoff I, Attems J, Budka H, Cairns NJ, Crary JF, Duyckaerts C, Ghetti B, Halliday GM, Ironside JW, Love S, Mackenzie IR, Munoz DG, Murray ME, Nelson PT, Takahashi H, Trojanowski JQ, Beach TG (2016) Aging-related tau astrogliopathy (ARTAG): Harmonized evaluation strategy. Acta Neuropathol 131, 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Josephs KA, Dickson DW, Tosakulwong N, Weigand SD, Murray ME, Petrucelli L, Liesinger AM, Senjem ML, Spychalla AJ, Knopman DS, Parisi JE, Petersen RC, Jack CR, Whitwell JL (2017) Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer’s disease: A longitudinal retrospective study. Lancet Neurol 16, 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Butler Pagnotti RM, Pudumjee SB, Cross CL, Miller JB (2023) Cognitive and clinical characteristics of patients with limbic-predominant age-related TDP-43 encephalopathy. Neurology 100, e2027–e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Walker JM, Richardson TE, Farrell K, Iida MA, Foong C, Shang P, Attems J, Ayalon G, Beach TG, Bigio EH, Budson A, Cairns NJ, Corrada M, Cortes E, Dickson DW, Fischer P, Flanagan ME, Franklin E, Gearing M, Glass J, Hansen LA, Haroutunian V, Hof PR, Honig L, Kawas C, Keene CD, Kofler J, Kovacs GG, Lee EB, Lutz MI, Mao Q, Masliah E, McKee AC, McMillan CT, Mesulam MM, Murray M, Nelson PT, Perrin R, Pham T, Poon W, Purohit DP, Rissman RA, Sakai K, Sano M, Schneider JA, Stein TD, Teich AF, Trojanowski JQ, Troncoso JC, Vonsattel J, Weintraub S, Wolk DA, Wolder TL, Yamada M, Yu L, White CL, Crary JF (2021) Early selective vulnerability of the CA2 hippocampal subfield in primary age-related tauopathy. J Neuropathol Exp Neurol 80, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sajjadi SA, Bukhari S, Scambray K, Yan R, Kawas C, Montine TJ, Corrada MM (2023) Impact and risk factors of limbic predominant age-related TDP-43 encephalopathy neuropathologic change in an oldest-old cohort. Neurology 100, e203–e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Han JW, Maillard P, Harvey D, Fletcher E, Martinez O, Johnson DK, Olichney JM, Farias ST, Villeneuve S, Jagust W, Mungas D, DeCarli C (2020) Association of vascular brain injury, neurodegeneration, amyloid, and cognitive trajectory. Neurology 95, e2622–e2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen LY, Shen WK, Mahoney DW, Jacobsen SJ, Rodeheffer RJ (2006) Prevalence of syncope in a population aged more than 45 years. Am J Med 119, 1088. [DOI] [PubMed] [Google Scholar]
- [33].Fiondella L, Gami-Patel P, Blok CA, Rozemuller AJM, Hoozemans JM, Pijnenburg AL, Scarioni M, Dijkstra AA (2023) Movement disorders are linked to TDP-43 burden in the substantia nigra of FTLD-TDP brain donors. Acta Neuropathol Commun 11, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available on request from The 90+ Study. The data are not publicly available due to privacy or ethical restrictions.


