Abstract
Objective:
Regionalized sepsis care could improve sepsis outcomes by facilitating the interhospital transfer of patients to higher-capability hospitals. There are no measures of sepsis capability to guide the identification of such hospitals, although hospital case volume of sepsis has been utilized as a proxy. We evaluated the performance of a novel hospital sepsis-related capability (SRC) index as compared with sepsis case volume.
Design:
Principal Component Analysis (PCA) and Retrospective cohort study.
Setting:
182 New York (derivation) and 274 Florida and Massachusetts (validation) non-federal hospitals, 2018.
Patients:
89,069 and 139,977 adult (≥ 18 years) sepsis patients directly admitted into the derivation and validation cohort hospitals, respectively.
Interventions:
None
Measurements and Main Results:
We derived SRC scores by PCA of six hospital resource use characteristics (bed capacity, annual volumes of sepsis, major diagnostic procedures, renal replacement therapy, mechanical ventilation, and major therapeutic procedures) and classified hospitals into capability score tertiles: high, intermediate, and low. High capability hospitals were mostly urban teaching hospitals. Compared to sepsis volume, the SRC score explained more variation in hospital-level sepsis mortality in the derivation (unadjusted coefficient of determination [R2]: 0.25 vs. 0.12, P < .001 for both) and validation (0.18 vs. 0.05, P < .001 for both) cohorts; and demonstrated stronger correlation with outward transfer rates for sepsis in the derivation (Spearman’s coefficient [r]: 0.60 vs. 0.50) and validation (0.51 vs. 0.45) cohorts. Compared to low-capability hospitals, sepsis patients directly admitted into high-capability hospitals had a greater number of acute organ dysfunctions, a higher proportion of surgical hospitalizations, and higher adjusted mortality (Odds Ratio [OR], 1.55; 95% Confidence Interval, 1.25–1.92). In stratified analysis, worse mortality associated with higher hospital capability was only evident among patients with 3 or more organ dysfunctions (OR, 1.88 [1.50–2.34]).
Conclusions:
The SRC score has face validity for capability-based groupings of hospitals. Sepsis care may already be de facto regionalized at high-capability hospitals. Low-capability hospitals may have become more adept at treating less complicated sepsis.
Keywords: Sepsis, septic shock, Mortality, Regionalization, Healthcare Disparities, Interhospital Transfer, Principal Component Analysis, Hospital Bed Size, Low-volume Hospitals
INTRODUCTION
Sepsis is a multi-system inflammatory syndrome associated with life-threatening organ dysfunction resulting from a dysregulated host response to infection.1 Multiple studies have observed better outcomes for patients with sepsis treated at high-volume centers,2–5 and consequently there is growing interest in the possibilities for regionalized sepsis care. 6–8 Similar efforts for trauma,9–11 stroke,12 and acute myocardial infarction13,14 based on specific hospital capabilities have changed care and improved outcomes for these conditions. However, there is no currently available measure of hospital capability to care for sepsis patients, and sepsis case volume alone may be insufficient to characterize hospitals’ sepsis-specific capabilities.6,15,16 Consequently, while the transfer networks for many other acute, time-sensitive conditions are explicit, the transfer networks for sepsis, a more heterogeneous syndrome, are more implicit and based on informal transfer practices.17,18
Hospital capabilities represent a mechanistic link between case volume and outcomes and often are the specific reason leading to interhospital transfer in clinical practice when such capabilities may not be locally available.19,20 A few studies have previously characterized hospital capability. One study utilized condition-specific transfer frequency as a quantitative measure of hospital capability and regionalized care.21 Another study comparing outcomes of transferred and non-transferred sepsis patients empirically estimated hospital sepsis capability using a combination of hospital Intensive Care Unit (ICU) presence and annual emergency department case volume.22 However, prior work has lacked a broader approach to capability. As a multisystem illness, a hospital’s sepsis capability will also include the availability of resources to adequately diagnose and treat complicated sepsis. Such resources, including interventional radiology and surgical services for example, are lacking in many small, rural and critical access hospitals.23,24
Therefore, to better characterize a hospital’s capability to treat sepsis, we developed the Sepsis-Related Capability (SRC) score — an index empirically constructed from specific hospital-level characteristics using principal component analysis. We hypothesized that the hospital SRC score is a better system predictor of mortality and will explain more of the variation in hospital-level sepsis mortality than sepsis volume. Because interhospital transfers are reasonably expected to flow from lower to higher capability hospitals,21 we also hypothesized that the SRC score will be inversely proportional to hospitals’ outward transfer rates for sepsis. Finally, we hypothesized that sepsis patients treated at higher capability hospitals will have lower in-hospital mortality compared to low capability hospitals. System prediction models, in contrast to clinical prediction models intended for bedside use, are intended to characterize populations for research, benchmarking, and other administrative purposes.25 Thus, a validated SRC score can be deployed across hospital populations for research to better understand sepsis transfer networks, facilitate comparative effectiveness studies of interhospital transfer and other hospital-level interventions related to sepsis, and to model regionalized sepsis care.
MATERIALS AND METHODS
We performed a cross-sectional study with Principal Component Analysis (PCA) of resource characteristics at non-federal hospitals in New York, Massachusetts, and Florida, to develop and validate the SRC score. To characterize the association of the SRC score with sepsis outcomes, we then performed a retrospective cohort study of sepsis hospitalizations. For all analyses, we utilized the 2018 State Inpatient (SID) and Emergency Department Databases (SEDD). The SID and SEDD are a family of databases developed as part of the Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality.26 They contain the universe of inpatient and Emergency Department (ED) discharge abstracts from non-Federal hospitals in participating states. We obtained general hospital characteristics including urban versus rural location, ownership, teaching status, bed capacity, and Critical Access Hospital designation from the 2018 American Hospital Association annual survey database. The Human Research Protection Office at Washington University in St. Louis deemed this study exempt due to its use of de-identified administrative data (#202106079).
Hospital Resource-Use Characteristics
To explore potential resource-use characteristics, we performed a literature review and mapped hospital characteristics and capabilities that relate to sepsis care delivery, may necessitate interhospital transfer, or may explain sepsis volume-outcome effects (Figure 1). From this conceptual map, we classified candidate characteristics into 3 capability constructs: (i) bed capacity (total beds) and clinical volume; (ii) diagnostic; and (iii) therapeutic constructs. For clinical volume, we calculated hospital-specific case volumes of sepsis, ED visits, and ICU admissions. To calculate sepsis case volume, we defined sepsis as sepsis diagnosis complicated by organ dysfunction and identified patients with a principal (DX1) or other (DX2–25) International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnostic code for infection and an additional ICD-10 code for acute organ dysfunction, or patients assigned ICD-10 principal or other diagnosis codes for severe sepsis without septic shock [R6520] and severe sepsis with septic shock [R6521]1,27 (Supplemental Table 1). ED visit volume was estimated from the SEDD while ICU admission volume was estimated using ICU revenue codes.
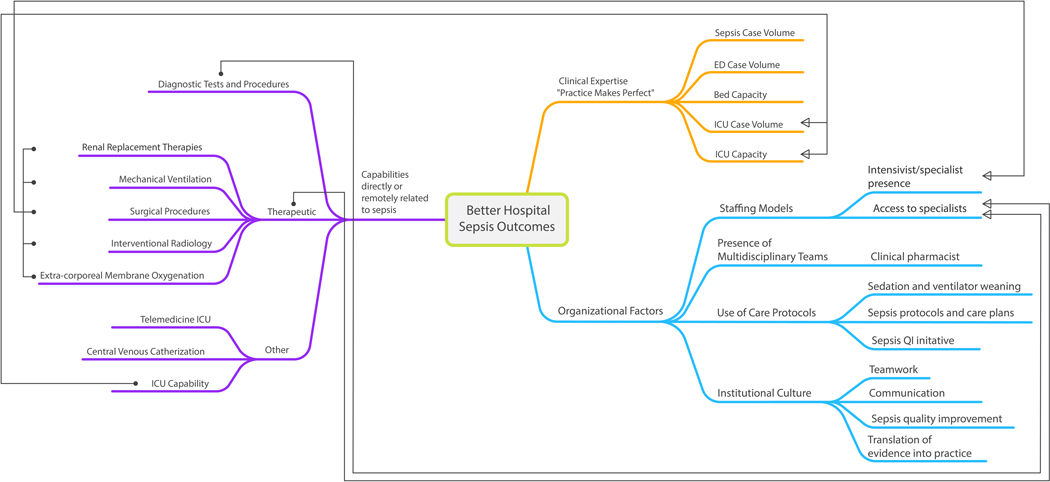
Figure 1:
Conceptual map of Sepsis-Related Hospital Characteristics
Conceptual map depicts three broad themes which then devolve into terminal branches that represent hospital characteristics and capabilities that relate to sepsis care delivery, potentially explain sepsis volume-outcome effects, or necessitate interhospital transfer. The directed arrows link a few related characteristics across broad themes. For example, ‘ICU Capability’ is linked by a directed arrow to ‘ICU Case Volume’ and ‘ICU Capacity’ as Clinical Expertise (or Practice Makes Perfect) constructs. Likewise, diagnostic, and therapeutic capabilities are linked to ‘Access to specialists’ as an organizational factor, e.g. hospitals that perform large numbers of diagnostic imaging procedures or surgical procedures are those that are more likely to have round-the-clock access to radiologists (for interpretation) or surgeons. Literature search and review performed by UO (first author), SB, and EL (see acknowledgement section). Conceptual mapping performed by UO.
ICU: Intensive Care Unit; ED: Emergency Department; QI: Quality Improvement.
We then measured these characteristics in a derivation cohort of 182 non-federal hospitals in New York and a validation cohort of 274 hospitals in Massachusetts and Florida. Because the HCUP lacks hospital-specific variables indicative of diagnostic and therapeutic capabilities, we constructed these characteristics using patient-level data associated with each hospital in the 2018 SID. For diagnostic resources, we captured radiological diagnostic resources using revenue codes for Magnetic Resonance Imaging (MRI), computerized tomography, and ultrasound.28 We also captured hospitals’ overall volumes of major diagnostic procedures using the HCUP’s refined procedure classes for ICD-10-PCS. For therapeutic resources, we utilized ICD-10 procedure codes for renal replacement therapies (5A1D70Z), mechanical ventilation (5A1935Z, 5A1945Z, 5A1955Z), non-intraoperative, veno-venous or veno-arterial extracorporeal membrane oxygenation (5A1522G, 5A1522H), percutaneous central venous catheters (Subclavian, 05H533Z, 05H633Z; Internal Jugular, 05HM33Z, 05HN33Z; Femoral, 06HM33Z, 06HN33Z), and any of two commonly performed interventional radiology procedures —percutaneous nephrostomy (0T9030Z, 0T903ZZ, 0T9130Z, 0T903ZZ) and cholecystostomy (0F943ZZ, 0F9430Z).29–35 Additionally, we identified major therapeutic procedures using the HCUP’s refined procedure classes for ICD-10-PCS.
Patient Study Population and Exclusions
The study population consisted of 229,046 adult (≥ 18 years) sepsis patient encounters in 2018. We excluded patients hospitalized at a non-acute care facility (rehabilitation, psychiatry, and drug and alcohol dependency center) or a long-term acute care facility 36 and “Transfer-In” patients whose admission source was designated as another acute care hospital, using previously described methods. 37
Patient Characteristics
Patient characteristics included demographic information (age, sex, race), sepsis type (community-acquired vs. nosocomial), surgical hospitalization, the Elixhauser co-morbidity index, 38 number of organ dysfunctions present on admission, primary insurance payer (Medicare, Medicaid, private), median household income of patient’s zip code (proxy for patient’s income level) and transfer status (non-transferred vs. transfer-out). Community-acquired sepsis was defined as a principal diagnosis (DX1) of sepsis, or any higher order diagnoses (DX2-DX25) that was designated as present on admission. We defined hospital mortality as a disposition of death in the hospital or transfer to hospice either in a medical facility or at home.
Statistical Analysis
Principal Component Analysis and Hospital Sepsis-Related Capability Score Estimation
Hospital resource use characteristics were summarized using medians and interquartile ranges. Principal Component Analysis (PCA) is a statistical process that converts the observations of correlated features into a set of linearly uncorrelated features (principal components). It simplifies the complexity in high-dimensional data while retaining trends and patterns by transforming the data into fewer dimensions, which act as summaries of data features.39,40 Details of the hospital resource-use characteristics and how the PCA was applied to derive the Sepsis-Related Capability Score are outlined in the Supplemental Methods. Briefly, PCA involved orthogonal transformations of selected hospital resource characteristics, yielding a corresponding number of principal components. Based on pre-defined criteria, we selected the fewest number of principal components that explained the largest amount of variance in the original data. We then calculated capability scores for each hospital by summing the values of each of the selected principal component items weighted by their respective loadings. We classified hospitals into 3 capability categories — high, intermediate, and low capability — based on tertiles of capability scores. We performed comparisons of hospital characteristics across hospital capability categories using the Cochran-Armitage test.
Sepsis-Related Capability Score Performance in the Derivation and Validation Cohort Patients
Descriptive statistics for continuous and categorical variables were presented as means with standard deviations and frequencies with percentages respectively. Standardized differences were used to compare patient characteristics across hospital capability categories.41 We deliberately separated patient populations from derivation (NY) and validation (MA, FL) hospitals to enable “state-level” contextual interpretation of our findings, especially as it relates to generalizability.
We calculated hospital transfer proportions for sepsis by dividing the number of sepsis patients transferred out of each hospital by the hospital’s total sepsis cases. (i) We evaluated the correlation between hospitals’ capability scores (as continuous variables) and outward transfer proportions for sepsis. (Hypothesis 1). (ii) We then fitted separate bivariate linear regression models for the SRC score and for sepsis case volume, with hospital sepsis mortality proportions as dependent variable, and evaluated each model’s coefficient of determination (R2) as a measure of the proportion of variance in hospital-level sepsis mortality explained by sepsis volume or the capability score (Hypothesis 2). For the correlation and linear regression analyses, we excluded hospitals with fewer than 20 patients to improve the reliability of the estimates. Next, we investigated whether the SRC in comparison to sepsis case volume (as hospital characteristics), would improve a clinical mortality prediction model that was based purely on patient characteristics. (iii) We then evaluated the association of hospital capability category (low, intermediate, and high) and patient-level sepsis mortality (Hypothesis 3). For all multivariate patient-level mortality models, we used generalized linear models with binomial distribution and exchangeable correlation structure for the hospital identification variable to account for within-hospital correlation. Patient-level variables included patient’s sex, age, sepsis type, surgical hospitalization, co-morbidity index, the number of organ dysfunctions, and payer type.
The discriminative abilities of the prediction models were compared using concordance statistic (C-statistic). Unadjusted and least squares adjusted means with standardized errors and adjusted Odds Ratios (OR) with 95 % Confidence Intervals (CI) were reported for in-hospital mortality. All hypothesis tests were 2-sided with a significance alpha level of 0.05. All analyses were performed with SAS Enterprise Guide Version 7.15 (SAS Institute Inc. Cary, NC) and R version 4.2.1.
Sensitivity and Secondary Analyses
Because lower capability hospitals are more likely to transfer their sepsis patients, who are sicker and more likely to die than their non-transferred counterparts,17 we performed a sensitivity analysis to ensure that our mortality estimates were not biased based on the potential for interhospital transfers to create a residual cohort of healthier patients at low capability hospitals by attributing any mortality of patients that were transferred out to the sending hospital. To ensure that our findings were not biased by inclusion of patients who may have been primarily admitted for reasons other than sepsis, we performed sensitivity analysis restricted to sepsis hospitalizations with principal diagnosis (DX1) of infection, severe sepsis without septic shock, or severe sepsis with septic shock, that most likely represented patients admitted with sepsis. Finally, to determine whether previously described relationships between sepsis case volume and mortality existed in our dataset, we estimated another generalized linear mortality model with sepsis volume as a continuous variable.
RESULTS
We presented results from both derivation (NY) and validation (MA, FL) hospitals so that relevant comparisons could be made. After exclusions, we identified 89,069 unique sepsis patients at 182 NY hospitals in 2018 among whom 1,654 (1.9%) were transferred out. Among 139,977 patients at 274 hospitals in Massachusetts and Florida in the same year, 3,504 (2.5%) were transferred out (Supplemental Figure 1). Hospital mortality rates were 23.7 % (n = 21,109) and 23.2% (n = 32,406) in the derivation and validation cohorts respectively.
Sepsis Capability Scores and Hospital Capability Categories
The frequency distribution of capability scores derived from PCA among the derivation and validation hospitals are illustrated in Supplemental Figure 2. The overall mean (range) capability scores were 0 (−3, +2), with higher scores denoting higher hospital capability. Among derivation hospitals that belonged to the highest tertile of capability score (high capability hospitals), 90% were teaching and all were in urban areas. In contrast, among low capability hospitals, 20% were teaching hospitals and approximately 60% were urban (Table 1). All 13 critical access hospitals were classified as low capability. High and intermediate capability hospitals, in contrast to low capability hospitals had annual sepsis volumes greater than the overall median values. The SRC scores derived from the validation cohort hospitals had similar distributions (Supplemental Figure 2) and yielded similar characteristics for high, intermediate, and low capability hospitals (Supplemental Table 2). The distribution of the original resource characteristics across hospital capability categories are tabulated for the derivation and validation cohort hospitals in Supplemental Tables 3 and 4 respectively.
Table 1:
Derivation Hospital Characteristics by Sepsis Capability Categories
| Hospital Capability Categories | ||||
|---|---|---|---|---|
| Characteristics | Total | High | Intermediate | Low |
| Hospitals, n (%) | 182 | 61 (33.5) | 60 (33.0) | 61 (33.5) |
| Ownership (profit status) | ||||
| Government, non-federal | 26 (14.3) | 9 (14.8) | 12 (20.0) | 5 (8.2) |
| Non-government, non-profit | 156 (85.7) | 52 (85.2) | 48 (80.0) | 56 (91.8) |
| Teaching Status a | ||||
| Teaching | 101 (55.5) | 55 (90.2) | 35 (58.3) | 11 (18.0) |
| Non-Teaching | 81 (44.5) | 6 (9.8) | 25 (41.7) | 50 (82.0) |
| Location a | ||||
| Rural | 32 (17.6) | 0 (0) | 6 (10.0) | 26 (42.6) |
| Urban | 150 (82.4) | 61 (100) | 54 (90.0) | 35 (57.4) |
| Critical Access Hospital n (%) a | 13 (7.1) | 0 (0) | 0 (0) | 13 (21.3) |
| Sepsis Volume, median (IQR) b | 432 (179,819) | 1103 (774,1578) | 440 (342,579) | 57 (11,183) |
P < .001 by Cochran-Armitage test
Comparison testing not performed as sepsis volume was an integral variable for capability score derivation.
IQR: Interquartile range.
Patient Characteristics
About two-thirds (67.6%) of people with sepsis who were admitted to derivation cohort hospitals were in high-capability hospitals, 47.8% were female, and 77.8% had community-acquired sepsis (Table 2).The most prevalent acute organ dysfunctions were related to the cardiovascular (61.9%), renal (58.8%), and respiratory (42.4%) systems. Approximately 40% had a single organ dysfunction. A majority (70.6%) had Medicare as their primary payer, and 19.7% belonged to the lowest quartile of median zip code income. Compared to low-capability hospitals, high-capability hospitals had a higher proportion of Black and a lower proportion of White sepsis patients and a lower proportion of patients with community-acquired sepsis. Patients admitted to high-capability hospitals also had higher co-morbidity burden and were more likely to have three or more organ dysfunctions. The validation cohort patients had similar characteristics as summarized in Supplemental Table 5.
Table 2:
Derivation Cohort Patient Characteristics by Hospital Sepsis Capability Categories
| Sepsis Capability Categories (n of hospitals) | |||||
|---|---|---|---|---|---|
| Characteristics | Total182 | High 61 (33.5) | Intermediate 60 (33.0) | Low 61 (33.5) | Standardized Differences |
| Sepsis cases, n (%) | 89,069 (100) | 60,212 (67.6) | 23,474 (26.4) | 5383 (6.0) | |
| Age, years, mean (SD) | 69.2 (15.1) | 68.7 (15.3) | 70.2 (14.5) | 69.7 (14.7) | 0.10 |
| Female (%) a | 47.8 | 47.4 | 48.5 | 48.3 | 0.02 |
| Race (%) | 0.77 | ||||
| White | 61.8 | 57.3 | 67.2 | 88.9 | |
| Black | 14.5 | 15.1 | 15.23 | 4.6 | |
| Hispanic | 10.3 | 12.0 | 7.6 | 2.7 | |
| Other | 13.4 | 15.6 | 10.0 | 3.8 | |
| Surgical Interventions (%) | 30.5 | 32.6 | 27.6 | 18.7 | 0.32 |
| Severe Sepsis code R6520 (%) | 55.3 | 56.4 | 53.6 | 49.7 | 0.14 |
| Community Sepsis (%) | 77.8 | 74.3 | 83.5 | 91.6 | 0.47 |
| Elixhauser Index, mean (SD) | 6.29 (2.77) | 6.33 (2.78) | 6.29 (2.76) | 5.83 (2.76) | 0.18 |
| Organ Dysfunction (%) | |||||
| Cardiovascular | 61.9 | 62.9 | 60.4 | 57.2 | 0.12 |
| Respiratory | 42.4 | 43.0 | 41.3 | 39.5 | 0.07 |
| Renal | 58.8 | 59.3 | 58.9 | 52.7 | 0.13 |
| Neurologic | 15.6 | 15.7 | 15.1 | 16.4 | 0.04 |
| Hematologic | 14.5 | 15.0 | 14.2 | 10.1 | 0.15 |
| Hepatic | 5.6 | 6.0 | 5.1 | 3.0 | 0.15 |
| Number of Organ Dysfunction (%) | 0.23 | ||||
| Any 1 | 40.1 | 39.0 | 41.4 | 46.8 | |
| Any 2 | 32.1 | 32.0 | 32.0 | 33.3 | |
| Any 3 | 18.8 | 19.3 | 18.7 | 14.9 | |
| Any 4+ | 9.0 | 9.7 | 8.0 | 5.1 | |
| Primary Payer (%) | 0.19 | ||||
| Medicare | 70.6 | 69.0 | 73.4 | 76.3 | |
| Medicaid | 13.7 | 14.3 | 12.9 | 10.1 | |
| Private insurance | 14.1 | 15.2 | 11.8 | 11.3 | |
| Other | 1.7 | 1.5 | 1.9 | 2.3 | |
| Median Income in Zip Code (%) | 0.59 | ||||
| Quartile 1 | 19.7 | 19.5 | 20.1 | 19.9 | |
| Quartile 2 | 21.1 | 18.0 | 22.8 | 49.1 | |
| Quartile 3 | 23.9 | 24.9 | 22.3 | 19.4 | |
| Quartile 4 | 34.7 | 37.2 | 33.8 | 10.3 | |
| Unknown | 0.7 | 0.5 | 1.0 | 1.3 | |
| Transferred-Out, n (%) | 1654 (1.9) | 796 (1.3) | 548 (2.3) | 310 (5.6) | 0.24 |
SD = Standard Deviation
Capability Scores in Relation to Outward Sepsis Transfer Proportions
The overall interhospital transfer rate (range: high to low capability) was 1.9% (1.3–5.6) in the derivation cohort and 2.5% (1.8–5.7) in the validation cohort (Table 2, Supplemental Table 5). Higher SRC scores were inversely and more strongly correlated with hospitals’ outward sepsis transfer proportions than higher sepsis volume. (Spearman’s Coefficient [r], derivation: − 0.60 vs. − 0.50; validation: − 0.51 vs. − 0.45, P< .001 for all tests).
Capability Scores in System and Clinical Prediction Models
The SRC score explained more variation in hospital-level sepsis mortality proportions than sepsis volume (Coefficient of Determination [R2], derivation: 0.25 vs. 0.12; validation: 0.18 vs. 0.05, P < .001 for all coefficients). A patient-level clinical mortality prediction model based on patient characteristics (Derivation and Validation AUC: 0.75 and 0.76, respectively) was not impacted by the addition of sepsis volume or the SRC scores (Supplemental Table 6).
Hospital Capability Categories in Relation to Patient-Level Sepsis Mortality
The overall (high, intermediate, and low capability) hospital mortality rates were 23.7% (24.6, 23.3, and 15.5%) and 23.2% (24.4, 21.9, and 18.9%) in the derivation and validation cohorts, respectively. Higher capability hospitals, compared to low capability hospitals, had higher adjusted mortality in the derivation (OR high vs. low: 1.55; 95% CI: 1.25–1.92); and validation cohorts (OR 1.30 [1.15–1.48]; Figure 2; Table 3).
Figure 2:
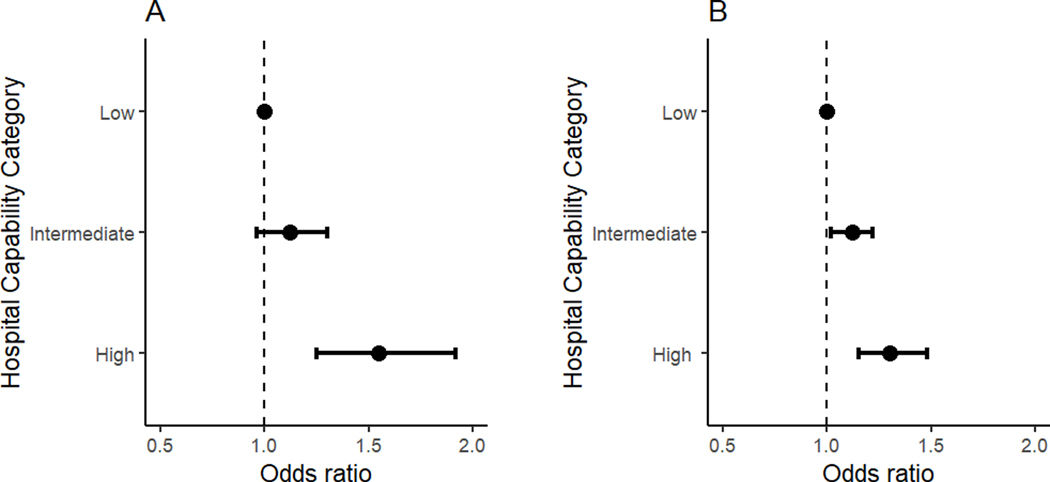
In-hospital Mortality Odds for Sepsis Patients. Odds Ratios and 95% Confidence Intervals showing differences between high and low capability hospitals for (A) the derivation cohort, and (B) the validation cohort.
Table 3:
Mortality Outcomes by Sepsis Capability Categories
| Derivation Cohort | Validation | |||||
|---|---|---|---|---|---|---|
| Capability Category | Unadjusted a | Adjusted a | Adjusted b | Unadjusted a | Adjusted a | Adjusted b |
| Low | 16.2 (13.9 – 18.8) | 16.4 (13.8 – 19.3) | reference | 19.2 (17.4 – 21.2) | 17.8 (16.2 – 19.5) | reference |
| Intermediate | 22.9 (21.0 – 24.9) | 21.3 (19.5 – 23.3) | 1.12 (0.96 – 1.30) | 22.3 (21.0 – 23.6) | 20.2 (19.1 – 21.3) | 1.12 (1.02 – 1.22) |
| High | 25.2 (23.8 – 26.6) | 23.2 (21.7 – 24.9) | 1.55 (1.25 – 1.92) | 24.6 (23.6 – 25.5) | 22.0 (21.1 – 23.0) | 1.30 (1.15 – 1.48) |
Least squares regression mean, % (95% Confidence Intervals)
Odds Ratios (95% Confidence Interval)
Hospital Capability and Patient-Level Sepsis Mortality by Organ Dysfunction
There was a significant interaction between capability category and the number of organ dysfunctions (P =.02). Among patients with one organ dysfunction, there was no significant difference in adjusted mortality at high vs. low capability hospitals in the derivation (OR 1.23 [0.92–1.65]) and validation (OR 1.17 [0.99–1.38]) cohorts. However, among patients with 3 or more organ dysfunctions, there was higher mortality at high compared to low capability hospitals in the derivation (OR 1.88 [1.50–2.34]) and validation (OR 1.35 [1.16–1.56]) cohorts (Figure 3 and Table 4).
Figure 3:
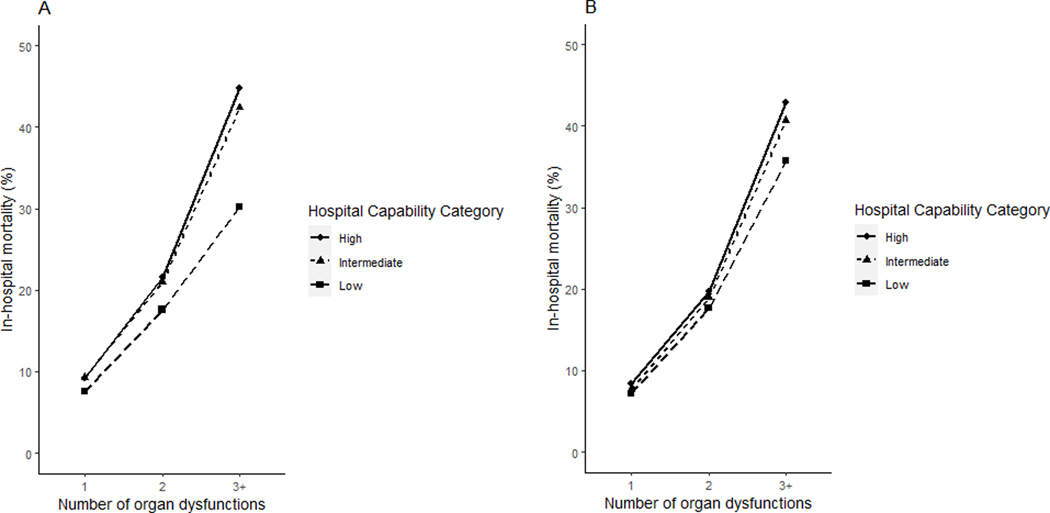
Adjusted Hospital Mortality, Hospital Capability Category, and Number of Organ Dysfunctions
Table 4:
Hospital Capability Categories and Adjusted Mortality by Organ Dysfunction
| Derivation | Validation | |||||
|---|---|---|---|---|---|---|
| Capability Category | Unadjusted a | Adjusted a | Adjusted b | Unadjusted a | Adjusted a | Adjusted b |
| 1 organ dysfunction | ||||||
| Low | 8.9 (6.9 – 11.4) | 7.6 (5.9 – 9.8) | reference | 9.7 (8.4 – 11.1) | 7.2 (6.3 – 8.3) | reference |
| Intermediate | 11.3 (10.0 – 12.8) | 9.3 (8.2 – 10.5) | 0.99 (0.84 – 1.16) | 10.2 (9.4 – 11.1) | 7.7 (7.2 – 7.7) | 1.10 (0.98 – 1.22) |
| High | 11.4 (10.4 – 12.4) | 9.2 (8.3 – 10.2) | 1.23 (0.92 – 1.65) | 11.3 (10.6 – 12.0) | 8.4 (7.8 – 9.0) | 1.17 (0.99 – 1.38) |
| 2 organ dysfunctions | ||||||
| Low | 18.2 (15.2 – 21.6) | 17.6 (14.6 – 21.1) | reference | 19.5 (17.3 – 21.9) | 17.7 (15.7 – 19.9) | reference |
| Intermediate | 22.4 (20.4 – 24.4) | 21.0 (19.2 – 23.0) | 1.03 (0.89 – 1.20) | 21.2 (19.7 – 22.7) | 18.9 (17.5 – 20.3) | 1.06 (0.95 – 1.18) |
| High | 23.3 (21.7 – 25.0) | 21.6 (20.0 – 23.3) | 1.29 (1.01 – 1.64) | 21.9 (20.9 – 23.0) | 19.7 (18.7 – 20.7) | 1.14 (0.98 – 1.33) |
| 3+ dysfunctions | ||||||
| Low | 28.8 (24.8 – 33.2) | 30.2 (26.1 – 34.7) | reference | 36.4 (33.5 – 39.4) | 35.8 (32.7 – 39.1) | reference |
| Intermediate | 41.5 (38.6 – 44.5) | 42.4 (39.6 – 45.4) | 1.10 (0.96 – 1.27) | 40.8 (39.0 – 42.6) | 40.7 (38.7 – 42.7) | 1.09 (0.99 – 1.21) |
| High | 45.4 (43.5 – 47.4) | 44.8 (43.0 – 46.8) | 1.88 (1.50 – 2.34) | 43.5 (42.3 – 44.8) | 42.9 (41.5 – 44.3) | 1.35 (1.16 – 1.56) |
Least squares regression mean, % (95% Confidence Intervals)
Odds Ratios (95% Confidence Interval)
Sensitivity and Secondary Analyses
In our sensitivity analysis attributing the mortality of transferred-out sepsis patients to the sending hospital, we found similar associations between hospital capability categories and sepsis mortality (Supplemental Table 7), as did sensitivity analysis restricted to hospitalizations with principal diagnosis of infection, severe sepsis, or septic shock (Supplemental Figure 8). In secondary analyses examining the relationship between sepsis volume and mortality, we observed higher but statistically insignificant odds of mortality associated with higher sepsis volume (OR + 0.8% per 100% increase in sepsis volume, 95% CI: − 0.8% to + 2.5% )
DISCUSSION
US hospitals differ in their capabilities for sepsis care. Informal interhospital transfer networks exist whereby patients are moved to hospitals best suited to care for them. A clearer understanding of hospital capability is necessary to better evaluate interhospital transfer networks and their impact on patient outcomes. By applying PCA to a set of hospital resource use characteristics we constructed a hospital sepsis capability index that classified hospitals into high, intermediate, and low capability categories. The capability-based index was inversely correlated with outward transfer rates for sepsis (face validity), explained more than twice the variance in hospital-level sepsis mortality than sepsis case volume (criterion and construct validity), and was not associated with sepsis clinical mortality prediction (discriminant validity). Interhospital transfer rates were low in our study cohorts and sicker patients were concentrated at high capability hospitals. High capability hospitals had higher overall adjusted sepsis mortality compared to low capability hospitals, mostly driven by mortality among patients with multi-organ dysfunction.
Case volume has been utilized as a surrogate measure of hospital’s capability to treat sepsis and as a categorical comparative measure in effectiveness studies of interhospital transfers. (4,5,17,42,43) Although higher capability hospitals had higher sepsis case volumes than lower capability hospitals, our study suggests that the capability score has better construct validity than sepsis volume and may be a more objective classifier. The face validity is further supported by the ability of the index to differentiate teaching hospitals that are more likely than non-teaching hospitals to have organizational characteristics related to better sepsis outcomes.7 From a conceptual point of view, when clinicians at smaller hospitals consider hospital characteristics in deciding to transfer patients with sepsis to larger hospitals, they think not in terms of case volume but in terms of expertise and capabilities (e.g., diagnostic, and therapeutic resources) that are not locally available.20 From a research perspective, any studies to characterize the potential impact of regionalized sepsis care by way of comparisons of outcomes of transferred and non-transferred patients must account for the capabilities of the sending and receiving hospitals. A valid measure of a hospital’s capability to treat sepsis can therefore facilitate clinical decision making, transfer network analysis, and comparative effective research relating to sepsis interhospital transfers.
The alignment of the derivative components of the SRC score with the clinical manifestations and management strategies of sepsis potentially explains why it accounts for more variation in hospital sepsis mortality than sepsis volume alone. Sepsis is associated with life-threatening organ dysfunction and management strategies that impact outcomes often include organ support by way of respiratory, renal, and circulatory support therapies, as well as sepsis source control by way of major surgery and other specialized diagnostic and therapeutic procedures. These sepsis-related capabilities were considered in our conceptual model and captured by the principal component variables that ultimately derived the score.
The concentration of sicker patients with higher co-morbidity indices and more organ dysfunctions at high capability hospitals, the low interhospital transfer rates in our study cohort, and the negative correlation between transfer rates and higher hospital capability scores, suggests that sepsis care may already be regionalized to an extent. In a regionalized system of care where sepsis patients are formally transferred from lesser to more capable hospitals, the correlation between transfer rates and hospital capability could be stronger. The observed moderate correlation may suggest residual inconsistencies in clinical practice relating to sepsis interhospital transfers. For example, there may be ongoing transfers between hospitals of similar capability categories. A previous study of sepsis interhospital transfer demonstrated a mismatch between subcategories of sepsis patients being transferred and apparent benefits of interhospital transfer for such patients.17 Additional studies are needed to better understand patterns of sepsis interhospital transfers. Such studies may also shed light on sepsis transfer characteristics into urban low capability hospitals considering that disparities in access to care often lead to rural residents bypassing rural hospitals in favor of more urban ones.44,45 Because rural hospital bypass has been associated with worse outcomes among sepsis patients,43 rural bypass or transfers into such low capability urban hospitals may not be ideal.
Our findings differ fundamentally from prior studies that have demonstrated better mortality outcomes for patients with sepsis treated at high-volume hospitals.4,5 In one of those studies,4 the apparent benefits of high case volume were limited to patients with 3 or less organ dysfunctions, with no difference in mortality among sepsis patients with 4 or more organ dysfunctions. However, our study found a clear association between higher hospital capability and worse sepsis outcomes, especially among the subgroup of patients with 3 or more organ dysfunctions. Although residual confounding may have contributed to these contrasting findings, it is also possible that de facto regionalization of sepsis care, whereby sicker patients with sepsis are concentrated at high capability hospitals may already be happening, possibly through selective referral to high capability hospitals by emergency medical services, patient choice (e.g., proximity to, or preference for a high capability hospital), or direct ED to ED transfers. Nearly two-thirds of our study cohort were directly admitted to high capability hospitals. Our finding of similar mortality at high and low capability hospitals among patients with less than 3 organ dysfunctions also suggests that low capability hospitals may be getting more adept at treating non-complicated sepsis, which may reflect the success of the surviving sepsis campaign and other public health initiatives targeted at sepsis awareness and performance improvement.46,47 Our findings suggest that regionalized sepsis care in the participating states may already be occurring, and that while increasingly centralized care cannot be supported, tools like the capability index we have developed might find utility in characterizing the efficiency of sepsis transfer networks more broadly.
Our study has strengths and limitations. The strength lies in the novelty of the sepsis capability construct and its conceptual and derivative methodology, the large sample size of hospitals and sepsis patients, and sensitivity analyses. Further, the utilization of data across 3 US states improves the generalizability of our findings. There are also several limitations. First, the billing information and procedure codes that we utilized to identify diagnoses and procedures may not always be coded correctly. Second, our study was retrospective in nature and the data source lacked variables relating to illness severity other than organ dysfunction. Third, although our study yields insights on general hospital characteristics associated with better sepsis capability, it sheds no light on evidence-based sepsis care processes.7 Fourth, our results may not fully generalize to states with lower population densities, where rural populations may be more geographically separated from urban areas. Finally, the SRC score was derived from hospital diagnostic and therapeutic resource-use characteristics that were conceptually derived with sepsis in mind but are not exclusively applicable to sepsis patients. As such, the score may lack specificity for sepsis and may instead describe a hospital’s overall capability to treat critical illness.
CONCLUSIONS
Capability based scores constructed from 6 hospital resource characteristics account for more variation in sepsis mortality than sepsis volume. The SRC score has face validity for categorizing hospitals of different sepsis capabilities and construct validity as a system predictor of hospital sepsis mortality and may find utility for improving system-based approaches to sepsis care. Further studies are needed to explore the relationship between the SRC score and evidence-based processes of sepsis care and to describe current sepsis interhospital transfer patterns in the context of hospital capabilities.
Supplementary Material
KEY POINTS.
Question:
How well does a novel capability index perform as a measure of hospital capability to treat sepsis compared with sepsis case volume and what is the association of hospital capability with sepsis mortality?
Findings:
Compared to sepsis case volume, the Sepsis-Related Capability (SRC) score, an index derived from 6 hospital characteristics explained more variation in hospital-level sepsis mortality and more strongly correlated with hospital outward sepsis transfer rates. Higher capability was associated with higher sepsis mortality among patients with multi-organ dysfunction.
Meaning:
The SRC score has face validity for capability-based groupings and comparisons of hospitals.
ACKNOWLEDGEMENTS
We thank Matthew R. Keller, MS of the Institute for Informatics at Washington University School of Medicine for technical assistance with advanced data programming, and Tierney Lanter, Public Health Research Coordinator, Cardiovascular Division at Washington University School of Medicine for formatting of analyzed data. We also thank Suresh Basnet, MD of the University of Maryland Medical Center, and Emile Ludeman, MSLIS, research librarian at the Health Sciences and Human Services Library of the University of Maryland for assistance with literature search and review.
Financial Support:
This project was supported by the National Center for Advancing Translational Sciences of the National Institutes of health under Award Number KL2 TR002346 (PI: Dominic N. Reeds, MD; Project Title: Washington University Institute of Clinical and Translational Sciences). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Dr Kollef is supported by the Barnes-Jewish Hospital Foundation
Dr. Joynt Maddox receives research support from the National Heart, Lung, and Blood Institute (R01HL143421 and R01HL164561), National Institute of Nursing Research (U01NR020555) and National Institute on Aging (R01AG060935, R01AG063759, and R21AG065526), and from Humana. She also serves on the Health Policy Advisory Council for the Centene Corporation (St. Louis, MO).
Copyright Form Disclosure: Drs. Ofoma and Maddox’s institutions received funding from the National Center for Advancing Translational Sciences. Dr. Ofoma’s institution received funding from the National Institute on Aging. Drs. Ofoma and Maddox received support for article research from the National Institutes of Health (NIH). Dr. Mohr’s institution received funding from Endpoint Health, Inc. Dr. Maddox’s institution received funding from the NIH; She received funding from Humana and Centene.
Footnotes
Conflict of Interest:
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama. Feb 23 2016;315(8):801–10. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peelen L, de Keizer NF, Peek N, Scheffer GJ, van der Voort PH, de Jonge E. The influence of volume and intensive care unit organization on hospital mortality in patients admitted with severe sepsis: a retrospective multicentre cohort study. Critical care. 2007;11(2):R40. doi: 10.1186/cc5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinikainen M, Karlsson S, Varpula T, et al. Are small hospitals with small intensive care units able to treat patients with severe sepsis? Intensive care medicine. Apr 2010;36(4):673–9. doi: 10.1007/s00134-009-1688-9 [DOI] [PubMed] [Google Scholar]
- 4.Gaieski DF, Edwards JM, Kallan MJ, Mikkelsen ME, Goyal M, Carr BG. The Relationship between Hospital Volume and Mortality in Severe Sepsis. Am J Respir Crit Care Med. Sep 15 2014;190(6):665–74. doi: 10.1164/rccm.201402-0289OC [DOI] [PubMed] [Google Scholar]
- 5.Walkey AJ, Wiener RS. Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med. Mar 1 2014;189(5):548–55. doi: 10.1164/rccm.201311-1967OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn JM. Volume, outcome, and the organization of intensive care. Critical care. 2007;11(3):129. doi: 10.1186/cc5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fawzy A, Walkey AJ. Association Between Hospital Case Volume of Sepsis, Adherence to Evidence-Based Processes of Care and Patient Outcomes. Critical care medicine. Mar 27 2017;doi: 10.1097/CCM.0000000000002409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walton NT, Mohr NM. Concept review of regionalized systems of acute care: Is regionalization the next frontier in sepsis care? J Am Coll Emerg Physicians Open. Feb 2022;3(1):e12631. doi: 10.1002/emp2.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma-center care on mortality. The New England journal of medicine. Jan 26 2006;354(4):366–78. doi: 10.1056/NEJMsa052049 [DOI] [PubMed] [Google Scholar]
- 10.DuBose JJ, Browder T, Inaba K, Teixeira PG, Chan LS, Demetriades D. Effect of trauma center designation on outcome in patients with severe traumatic brain injury. Archives of surgery. Dec 2008;143(12):1213–7; discussion 1217. doi: 10.1001/archsurg.143.12.1213 [DOI] [PubMed] [Google Scholar]
- 11.Demetriades D, Martin M, Salim A, Rhee P, Brown C, Chan L. The effect of trauma center designation and trauma volume on outcome in specific severe injuries. Annals of surgery. Oct 2005;242(4):512–7; discussion 517–9. doi: 10.1097/01.sla.0000184169.73614.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. Jama. Jan 26 2011;305(4):373–80. doi: 10.1001/jama.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs AK. Regionalized care for patients with ST-elevation myocardial infarction: it’s closer than you think. Circulation. Mar 7 2006;113(9):1159–61. doi: 10.1161/CIRCULATIONAHA.105.610345 [DOI] [PubMed] [Google Scholar]
- 14.Granger CB, Bates ER, Jollis JG, et al. Improving Care of STEMI in the United States 2008 to 2012. Journal of the American Heart Association. Jan 8 2019;8(1):e008096. doi: 10.1161/JAHA.118.008096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodek PM. Volume-outcome relationships in critical care. Understanding the mechanism. Am J Respir Crit Care Med. Sep 15 2014;190(6):601–3. doi: 10.1164/rccm.201401-0132ED [DOI] [PubMed] [Google Scholar]
- 16.Nguyen YL, Wallace DJ, Yordanov Y, et al. The Volume-Outcome Relationship in Critical Care: A Systematic Review and Meta-analysis. Chest. Jul 2015;148(1):79–92. doi: 10.1378/chest.14-2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ofoma UR, Dahdah J, Kethireddy S, Maeng D, Walkey AJ. Case Volume-Outcomes Associations Among Patients With Severe Sepsis Who Underwent Interhospital Transfer. Critical care medicine. Apr 2017;45(4):615–622. doi: 10.1097/CCM.0000000000002254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. Journal of critical care. Apr 2013;28(2):202–8. doi: 10.1016/j.jcrc.2012.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Critical care medicine. Aug 2012;40(8):2470–8. doi: 10.1097/CCM.0b013e318254516f [DOI] [PubMed] [Google Scholar]
- 20.Mohr NM, Wong TS, Faine B, Schlichting A, Noack J, Ahmed A. Discordance Between Patient and Clinician Experiences and Priorities in Rural Interhospital Transfer: A Mixed Methods Study. J Rural Health. Winter 2016;32(1):25–34. doi: 10.1111/jrh.12125 [DOI] [PubMed] [Google Scholar]
- 21.Franca UL, McManus ML. Transfer Frequency as a Measure of Hospital Capability and Regionalization. Health services research. Dec 2017;52(6):2237–2255. doi: 10.1111/1475-6773.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohr NM, Harland KK, Shane DM, Ahmed A, Fuller BM, Torner JC. Inter-hospital transfer is associated with increased mortality and costs in severe sepsis and septic shock: An instrumental variables approach. Journal of critical care. Dec 2016;36:187–194. doi: 10.1016/j.jcrc.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayirli TC, Warinner CB. Hospital Characteristics Associated with the Availability of Interventional Radiology Facilities and Services. Radiology. Dec 6 2022:221189. doi: 10.1148/radiol.221189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. The New England journal of medicine. Apr 11 2002;346(15):1128–37. doi: 10.1056/NEJMsa012337 [DOI] [PubMed] [Google Scholar]
- 25.Leisman DE, Harhay MO, Lederer DJ, et al. Development and Reporting of Prediction Models: Guidance for Authors From Editors of Respiratory, Sleep, and Critical Care Journals. Critical care medicine. May 2020;48(5):623–633. doi: 10.1097/CCM.0000000000004246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Databases HCUP. Healthcare Cost and Utilization Project (HCUP). June 2016. Agency for Healthcare Research and Quality, Rockville, MD. Accessed February 13, 2017, 2017. https://hcup-us.ahrq.gov/sidoverview.jsp [Google Scholar]
- 27.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. The New England journal of medicine. Apr 17 2003;348(16):1546–54. doi: 10.1056/NEJMoa022139 [DOI] [PubMed] [Google Scholar]
- 28.Adam EJ, Page JE. Intra-abdominal sepsis: the role of radiology. Baillieres Clin Gastroenterol. Sep 1991;5(3 Pt 1):587–609. doi: 10.1016/0950-3528(91)90044-2 [DOI] [PubMed] [Google Scholar]
- 29.Peerapornratana S, Manrique-Caballero CL, Gomez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. Nov 2019;96(5):1083–1099. doi: 10.1016/j.kint.2019.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sevransky JE, Levy MM, Marini JJ. Mechanical ventilation in sepsis-induced acute lung injury/acute respiratory distress syndrome: an evidence-based review. Critical care medicine. Nov 2004;32(11 Suppl):S548–53. doi: 10.1097/01.ccm.0000145947.19077.25 [DOI] [PubMed] [Google Scholar]
- 31.Zampieri FG, Mazza B. Mechanical Ventilation in Sepsis: A Reappraisal. Shock. Jan 2017;47(1S Suppl 1):41–46. doi: 10.1097/SHK.0000000000000702 [DOI] [PubMed] [Google Scholar]
- 32.Falk L, Hultman J, Broman LM. Extracorporeal Membrane Oxygenation for Septic Shock. Critical care medicine. Aug 2019;47(8):1097–1105. doi: 10.1097/CCM.0000000000003819 [DOI] [PubMed] [Google Scholar]
- 33.Park TK, Yang JH, Jeon K, et al. Extracorporeal membrane oxygenation for refractory septic shock in adults. Eur J Cardiothorac Surg. Feb 2015;47(2):e68–74. doi: 10.1093/ejcts/ezu462 [DOI] [PubMed] [Google Scholar]
- 34.Ilko SA, Vakkalanka JP, Ahmed A, Harland KK, Mohr NM. Central Venous Access Capability and Critical Care Telemedicine Decreases Inter-Hospital Transfer Among Severe Sepsis Patients: A Mixed Methods Design. Critical care medicine. May 2019;47(5):659–667. doi: 10.1097/CCM.0000000000003686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arabi M, Gemmete JJ, Arabi Y. The role of interventional radiology in the management of hemodynamically compromised patients. Intensive care medicine. Aug 2018;44(8):1334–1338. doi: 10.1007/s00134-018-5236-3 [DOI] [PubMed] [Google Scholar]
- 36.Liu K, Baseggio C, Wissoker D, Maxwell S, Haley J, Long S. Long-term care hospitals under Medicare: facility-level characteristics. Health Care Financ Rev. Winter 2001;23(2):1–18. [PMC free article] [PubMed] [Google Scholar]
- 37.Nadig NR, Goodwin AJ, Simpson AN, Simpson KN, Richards J, Ford DW. Patient and Hospital Characteristics Associated with Inter-hospital Transfer for Adults with Ventilator Dependent Respiratory Failure. Annals of the American Thoracic Society. Feb 15 2017;doi: 10.1513/AnnalsATS.201611-918OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical care. Jan 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 39.Ringnér M. What is principal component analysis? Nat Biotechnol. Mar 2008;26(3):303–4. doi: 10.1038/nbt0308-303 [DOI] [PubMed] [Google Scholar]
- 40.Lever J, Krzywinski M, Altman N. Principal component analysis. Nature Methods. 2017/07/01 2017;14(7):641–642. doi: 10.1038/nmeth.4346 [DOI] [Google Scholar]
- 41.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation. 2009/05/14 2009;38(6):1228–1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 42.Arulraja MD, Swanson MB, Mohr NM. Double inter-hospital transfer in Sepsis patients presenting to the ED does not worsen mortality compared to single inter-hospital transfer. Journal of critical care. Apr 2020;56:49–57. doi: 10.1016/j.jcrc.2019.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohr NM, Harland KK, Shane DM, et al. Rural Patients With Severe Sepsis or Septic Shock Who Bypass Rural Hospitals Have Increased Mortality: An Instrumental Variables Approach. Critical care medicine. Jan 2017;45(1):85–93. doi: 10.1097/CCM.0000000000002026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iglehart JK. The Challenging Quest to Improve Rural Health Care. The New England journal of medicine. Feb 1 2018;378(5):473–479. doi: 10.1056/NEJMhpr1707176 [DOI] [PubMed] [Google Scholar]
- 45.Malone T, Holmes M. Patterns of Hospital Bypass and Inpatient Care-Seeking by Rural Residents. NC Rural Health Research Program. 2019; [Google Scholar]
- 46.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive care medicine. Nov 2021;47(11):1181–1247. doi: 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dellinger RP, Rhodes A, Evans L, et al. Surviving Sepsis Campaign. Crit Care Med. 2023;51(4):431–444. doi: 10.1097/ccm.0000000000005804 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.