Abstract
The Coronavirus Disease 2019 (COVID-19) pandemic was caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which enters host cells through interactions of its spike protein to Angiotensin-Converting Enzyme 2 (ACE2). ACE2 is a peptidase that cleaves Angiotensin II, a critical pathological mediator. This study investigated if the spike protein binding to ACE2 compromises its peptidase activity. Spike/ACE2 Binding Assays suggested that spike proteins of SARS-CoV-2, SARS-CoV and MERS-CoV, but not HKU1, bind to ACE2. S1 and receptor-binding domain (RBD), but not S2, extracellular domain (ECD) or CendR domain, bind to ACE2. While glycosylated spike proteins prepared in HEK293 cells bind to ACE2, non-glycosylated proteins produced in E. coli do not. Cysteine residues of the spike protein expressed in HEK293 cells are fully oxidized, while those of the protein expressed in E. coli are reduced. The deglycosylation of HEK cell-produced protein attenuates the ACE2 binding, while the oxidation of the E. coli protein does not promote the binding. The S1 protein of SARS-CoV-2 enhances the ACE2 peptidase activity, while SARS-CoV, MERS-CoV or HKU1 does not. The ACE2 activity is enhanced by RBD, but not ECD or CendR. In contrast to distinct ACE2 binding capacities of proteins expressed in HEK293 cells and in E. coli, spike proteins expressed in both systems enhance the ACE2 activity. Thus, the spike protein of SARS-CoV-2, but not other coronaviruses, enhances the ACE2 peptidase activity through its RBD in a glycosylation-independent manner.
Keywords: ACE2, coronavirus, COVID-19, peptidase, SARS-CoV-2, spike protein
Graphical Abstract
Introduction
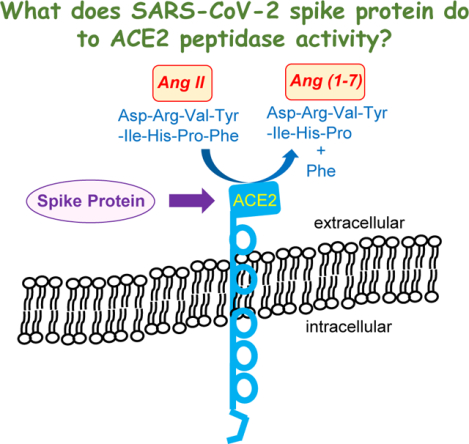
The Coronavirus Disease 2019 (COVID-19) pandemic was caused by a positive sense single stranded RNA virus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1,2]. This virus has similarities to Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) that caused the SARS outbreak between 2002 and 2004 [3]. Both SARS-CoV and SARS-CoV-2 use the viral fusion protein, spike protein, to enter the host cells by binding to their host cell receptor, Angiotensin-Converting Enzyme 2 (ACE2) [4–6]. ACE2 is a peptidase that cleaves Angiotensin II (Ang II) into Ang (1–7), thus regulating the levels of Ang II, a critical mediator of pathogenesis, especially in the cardiovascular system [7].
The S1 subunit of the spike protein contains the Receptor Binding Domain (RBD) that interacts with ACE2 [5,6]. In addition to ACE2 binding to the spike protein RBD of the intact virus to facilitate the viral entry, the S1 subunit of the spike protein can be cleaved off from the virus by proteases such as Furin and may affect various organs [8,9]. Widely used mRNA COVID-19 vaccines encode for the full-length spike protein S1+S2 [10] and may produce circulating S1 protein [11,12].
The binding of the SARS-CoV-2 spike protein to ACE2 downregulates ACE2 protein expression [13], resulting in the reduction of available ACE2 to degrade Ang II and thus the accumulation of this pathologic mediator, which may cause cardiovascular diseases associated with COVID-19 [14]. As the S1 protein binds to ACE2, it may interfere with the enzymatic activity of ACE2, thereby enhancing the Ang II-mediated pathology, which is worrisome. However, previous studies showed that the S1 protein does not inhibit, but rather slightly enhances, the peptidase activity of ACE2 [15]. Thus, S1 is capable of binding to ACE2 as well as enhancing the ACE2 enzymatic activity.
Currently, the relationships between the ability of RBD to bind to ACE2 and the enhancing action of S1 protein on the ACE2 peptidase activity have not been well defined. Therefore, the present study further investigates these events in order to provide mechanistic insights into the action of the SARS-CoV-2 spike protein.
Materials and Methods
Recombinant proteins
Recombinant SARS-CoV-2 Spike S1 (Cat# 40591-V08H), SARS-CoV-2 Spike RBD (Cat# 40592-V08H), SARS-CoV-2 Spike S1 Delta (Cat# 40591-V08H23), SARS-CoV-2 Spike S1 Omicron (Cat# 40591-V08H41), Middle East respiratory syndrome coronavirus (MERS-CoV) Spike S1 (Cat# 40069-V08H), HCoV-HKU1 (HKU1) Spike S1 (Cat# 40021-V08H), HIV-1 gp120 subtype A (Cat# 40403-V08H), HIV-1 gp120 subtype B (Cat# 40404-V08H), and human ACE2 (Cat# 10108-H08H) produced in HEK293 cells were purchased from Sino Biological Inc. (Wayne, PA, USA). Recombinant SARS-CoV Spike S1 (Cat# 40150-V08B1) produced in Baculovirusinsect cells was also purchased from Sino Biological. Recombinant SARS-CoV-2 Spike S1 (Cat# 230–01101) and SARS-CoV-2 Spike RBD (Cat# 230–01102) produced in E. coli were purchased from RayBiotech Life, Inc. (Peachtree Corners, GA, USA). Recombinant SARS-CoV-2 Spike N-terminal Extracellular Domain (ECD; Cat# 230–30191) and SARS-CoV-2 Spike CendR Domain (Cat# 230–30179) produced in HEK293 cells were also purchased from RayBiotech. Recombinant SARS-CoV-2 Spike CendR Domain expressed in E. coli were produced by Bio Basic Inc. (Markham, ON, Canada).
Spike Protein Binding Assays
RayBio COVID-19 Spike-ACE2 Binding Assay Kit I (Cat# CoV-SACE2), RayBio COVID-19 Spike-ACE2 Binding Assay Kit II (Cat# CoV-ACE2S2) and RayBio COVID-19 Spike-AXL Binding Assay Kit (Cat# CoV-AXLS1) were purchased from RayBiotech. SARS-CoV-2 S1 Protein-ACE2 Binding Inhibitor Screening Kit (Cat# K2050) was purchased from BioVision Inc. (Milpitas, CA, USA). Assays were performed in accordance with the manufactures’ instructions and the horseradish peroxidase (HRP) activity was detected at the absorbance of 450 nm. 10 nM of recombinant proteins were added.
Peptide-N-Glycosidase F (PNGase F) Treatment
Deglycosylation of the HEK293 cell-produced RBD protein was performed using Rapid PNGase F (non-reducing format) (Cat# P0711S) from New England Biolabs, Inc. (Ipswich, MA, USA). The sample proteins were incubated with PNGase F Buffer at 75°C for 5 min, then with Rapid PNGase F at 50°C for 10 min. The resultant proteins were subjected to SDS-PAGE or used in Spike-ACE2 binding assays.
SulfoBiotics Protein Redox State Monitoring
Protein thiol redox states were monitored using the -SulfoBiotics- Protein Redox State Monitoring Kit (Catalog # SB11; Dojindo Molecular Technologies). RBD proteins were labeled with the Protein-SHifter in accordance with the manufacturer’s instructions. Thereafter, proteins were subjected to SDS-PAGE and stained with Coomassie blue.
5,5’-dithiobis(2-nitrobenzoic acid) (DTNB) Assay
Reduced thiol contents in S1 proteins were measured using DTNB at an absorbance of 412 nm as previously described [16].
ACE2 Activity Assay
The cleavage of the Mca/Dnp fluorescence resonance energy transfer (FRET) peptide to the fluorescent MCA (7-Methoxycoumarin-4-acetic acid) peptide catalyzed by ACE2 was monitored using SensoLyte 390 ACE2 Activity Assay Kit (AnaSpec Inc., Fremont, CA, USA) using recombinant human ACE2 (0.002 μM) (Sino Biological). Results were confirmed using the ACE2 Activity Assay Kit (Cat# K897) from BioVision.
Furin Activity Assay
The protease activity of Furin was monitored by measuring the production of Rhodamine 110 fluorophore upon the Furin-dependent cleavage using SensoLyte Rh110 Furin Activity Assay Kit (AnaSpec).
Glycealdehyde-3-phosphate dehydrogenase (GAPDH) Activity Assay
The activity of GAPDH was determined by monitoring the conversion of Glycealdehyde-3-phosphase to 1,3-bisphosphoglycerate colorimetrically using the GAPDH Activity Assay Kit (Cat# MAK277, Sigma-Aldrich, St. Louis, MO, USA).
Dipeptidyl peptidase-4 (DPP4) Activity Assay
The peptidase activity of DPP4 was measured by monitoring the production of a fluorescent product, 7-amino-4-methyl coumarin using the DPP4 Activity Assay Kit (Cat# MAK088, Sigma-Aldrich).
Statistical analysis
Means and standard errors of mean (SEM) were computed. Two groups were compared by a two-tailed Student’s t test, and differences between more than two groups were determined by the analysis of variance (ANOVA). P < 0.05 was defined to be statistically significant.
Results
The use of Spike protein/ACE2 Binding Assays to study the biology of spike protein
The Spike protein/ACE2 Binding Assay Kits have been used to screen for inhibitors for the purpose of developing therapeutic agents to treat COVID-19. In this study, these assay kits were used to understand the mechanisms of spike protein biology. The RayBio COVID-19 Spike-ACE2 Binding Assay Kit I has the RBD protein coated at the bottom of the microplates to which ACE2 proteins are added. After washing unbound components, the antibody against ACE2 is added and the RBD/ACE2 binding is detected using the secondary antibody covalently bound to HRP (Fig. 1A). Using this assay kit, we found that the addition of the recombinant SARS-CoV-2 spike protein S1 subunit protein as well as the SARS-CoV-2 RBD-only containing protein competed with the RBD/ACE2 interactions, suggesting that the exogenously added S1 and RBD proteins competed for the ACE2 (Fig. 1A). Similar to SARS-CoV-2 proteins, SARS-CoV S1 as well as RBD from the earlier SARS outbreak reduced the signal, confirming that SARS-CoV spike also binds to ACE2 (Fig. 1A). Interestingly, we found that the S1 protein of MERS-CoV, whose known human target protein for infection is not ACE2, but DPP4 [17], also reduced the signal, indicating that MERS-CoV S1 can also interact with ACE2. By contrast, neither the S1 protein of coronavirus HKU1 that often causes common cold nor the viral fusion protein of HIV, gp120, bind to ACE2 (Fig. 1A).
Figure 1: The use of Spike/ACE2 Binding Assays to study the effects of spike proteins.
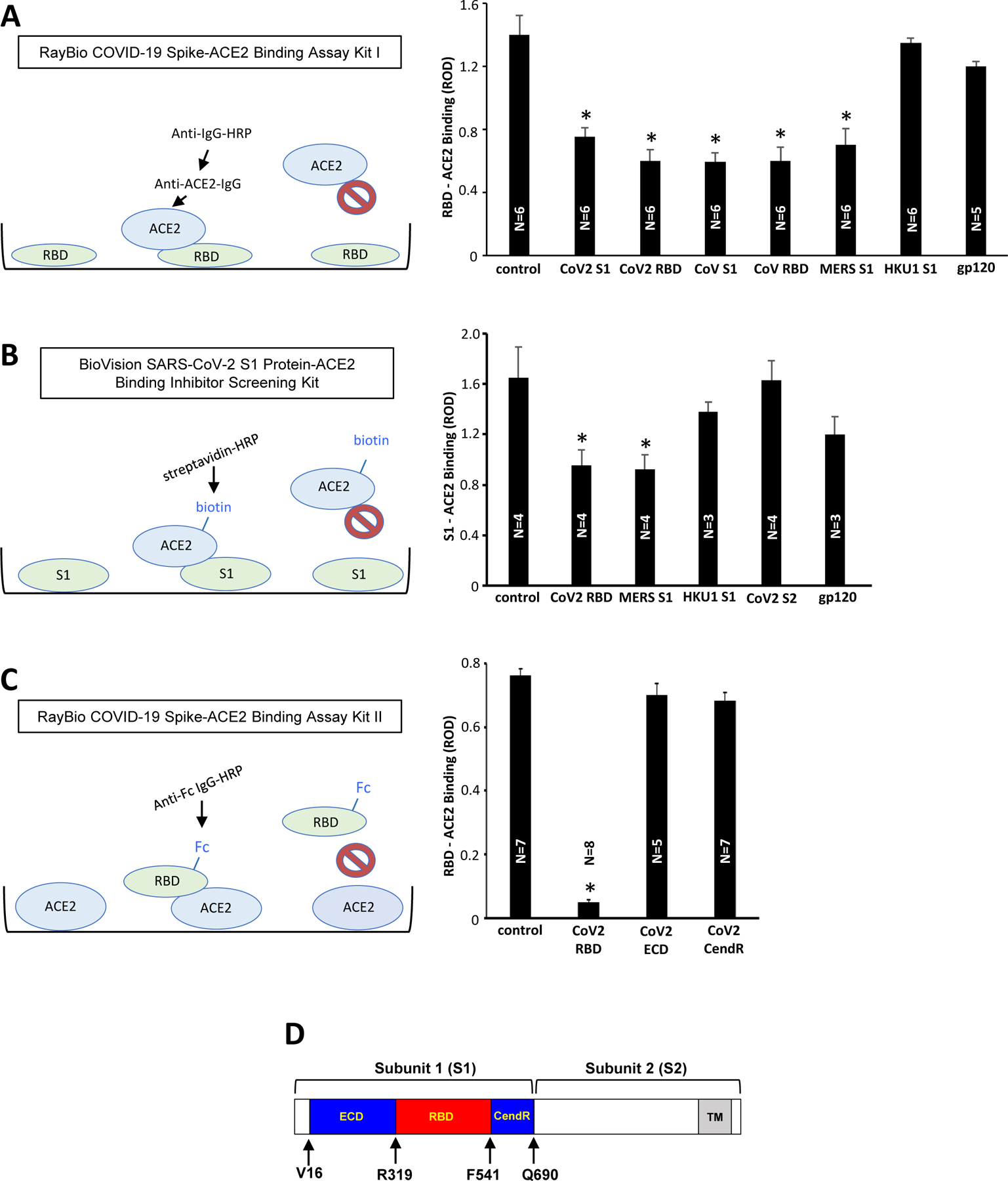
(A) The RayBio COVID-19 Spike-ACE2 Binding Assay Kit I has the SARS-CoV-2 RBD protein bound to the bottom of the microplate wells. The ACE2 protein is then added, washed, and bound ACE2 to RBD is detected using anti-ACE2-IgG and HRP-linked secondary antibody. The bar graph shows that the inclusion of SARS-CoV-2 spike protein S1 subunit (CoV2 S1), SARS-CoV-2 spike protein RBD-only containing protein (CoV2 RBD), SARS-CoV spike protein S1 subunit (CoV S1), SARS-CoV spike protein RBD-only containing protein (CoV RBD), and MERS-CoV spike protein S1 subunit (MERS S1) inhibited the binding of ACE2 to RBD in the kit. On the other hand, the HKU1 spike protein S1 subunit (HKU1 S1) and HIV-1 subunit B gp120 had no effects. (B) The BioVision SARS-CoV-2 S1 Protein-ACE2 Binding Inhibitor Screening Kit has the SARS-CoV-2 S1 protein bound to the bottom of the microplate wells. The biotin-linked ACE2 protein is then added, washed, and bound biotin-ACE2 to S1 is detected using streptavidin-HRP. The bar graph shows that the results of the above RayBio kit experiments were repeatable with the BioVision kit. (C) The RayBio COVID-19 Spike-ACE2 Binding Assay Kit II has the ACE2 protein bound to the bottom of the microplate wells. Fc-tagged RBD protein is then added, washed, and bound Fc-RBD to ACE2 is detected using HRP-linked anti-Fc-IgG. The bar graph shows that, while the inclusion of the SARS-CoV-2 spike protein RBD-only containing protein inhibited the binding of Fc-RBD to ACE2 in the assay kit, the ECD- or CendR domain-only containing proteins had no effects. The symbol * denotes significant difference from untreated control at P<0.05. (D) The structure of the SARS-CoV-2 spike protein. The spike protein consists of Subunit 1 (S1) and Subunit 2 (S2). The S1 subunit contains the RBD that binds to ACE2 of the host cell membrane. The S2 subunit is responsible for fusion. In this study, we used recombinant full-length S1 (Val16-Gln690), the RBD only-containing protein (Arg319-Phe541), ECD (Val16-Phe318), and CendR domain (Asn542-Arg685) (GenBank Accession Number: QHD43416.1). ROD: relative optical density. The symbol * denotes significant difference from untreated control at P<0.05.
To confirm these findings, we also used the BioVision SARS-CoV-2 S1 Protein-ACE2 Binding Inhibitor Screening Kit, which uses S1 protein on the bottom of the microplates and biotin-linked ACE2 (Fig. 1B). Similar to Fig. 1A, SARS-CoV-2 RBD and MERS-CoV S1 protein significantly inhibited the S1/ACE2 binding of the assay, while HKU1 S1, SARS-CoV-2 S2, and HIV-1 gp120 had no significant effects (Fig. 1B).
SARS-CoV-2 RBD also inhibited the binding of ACE2 at the bottom of the microplate with Fc-tagged RBD in the RayBio COVID-19 Spike-ACE2 Binding Assay Kit II (Fig. 1C). We further identified that only RBD, but not ECD or CendR domains of SARS-CoV-2 spike protein S1 subunit (see Fig. 1D), binds to ACE2 (Fig. 1C).
Both the BioVision Kit (Fig. 2A) and the RayBio Spike-ACE2 Binding Assay Kit II (Fig. 2B) showed that SARS-CoV-2 spike protein S1 subunit of the original Wuhan virus [1], the Delta variant, and the Omicron variant all competed with spike protein binding to ACE2 in the kits.
Figure 2: Effects of SARS-CoV-2 variants on Spike/ACE2 binding.
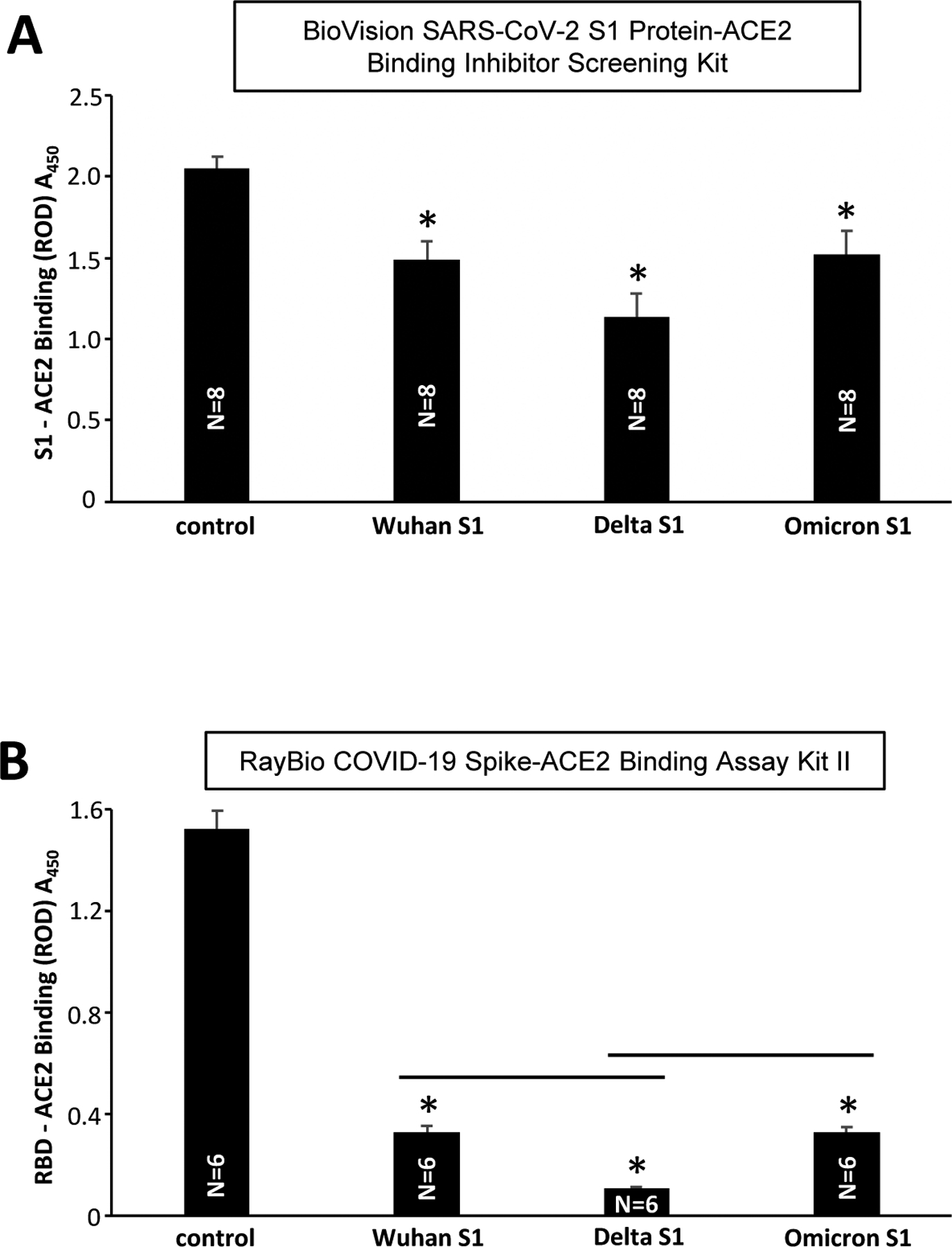
(A) The BioVision SARS-CoV-2 S1 Protein-ACE2 Binding Inhibitor Screening Kit and (B) RayBio COVID-19 Spike-ACE2 Binding Assay Kit II were used to determine the effects of the SARS-CoV-2 spike protein S1 subunit of the original Wuhan sequence (Wu et al., 2020), the S1 subunit of the SARS-CoV-2 spike protein Delta variant, and the S1 subunit of the SARS-CoV-2 spike protein Omicron variant. The symbol * denotes significant difference from untreated control at P<0.05. The lines above bars indicates significant difference from each other at P<0.05.
Effects of SARS-CoV-2 spike proteins produced in mammalian HEK293 cells and in E. coli
We obtained two commercially available preparations of SARS-CoV-2 spike protein S1 subunit and RBD-only containing proteins: (i) from Sino Biological that was produced in HEK293 cells and (ii) from RayBiotech that was produced in E. coli. All the proteins used in Figs. 1 and 2 were produced in HEK293 cells. However, interestingly, experiments using three different Spike/ACE2 Binding Assay Kits showed that, while HEK293 cell-produced S1 and RBD proteins are effective in competing with the Spike/ACE2 binding in the assay kits, E. coli-produced spike proteins had no effects (Fig. 3).
Figure 3: Effects of HEK293 cell- and E. coli-expressed S1 and RBD on Spike/ACE2 binding.
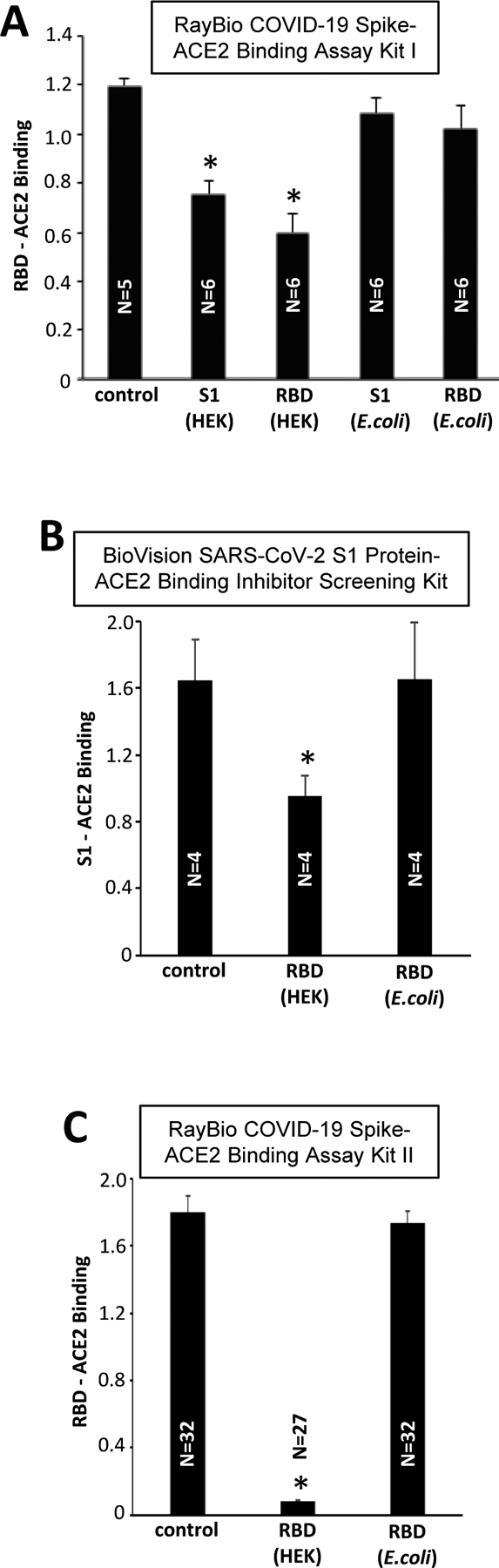
The recombinant SARS-CoV-2 S1 and RBD proteins produced in HEK293 cells and E. coli were added to RBD/ACE2 Binding Assays. All the binding assay kits showed that HEK293 cell-expressed spike proteins effectively competed with the spike protein binding to ACE2 in the assay kits. By contrast, the E. coli produced proteins had no effects. * denotes significant difference from untreated control at P<0.05.
Gel electrophoresis experiments showed that the E. coli-derived RBD-only containing protein migrated in SDS-PAGE at ~25 kD (Fig. 4A). By contrast, the RBD protein that was expressed in HEK293 cells migrated at molecular weight higher than 25 kD (at around 33 kD). The treatment with PNGase F resulted in this HEK293-expressed RBD protein migrating at ~25 kD (Fig. 4A). Thus, the RBD protein expressed in HEK293 cells is glycosylated, while the protein expressed in E. coli is not.
Figure 4: Mechanisms of differential actions of HEK293 cell- and E. coli-produced spike proteins.
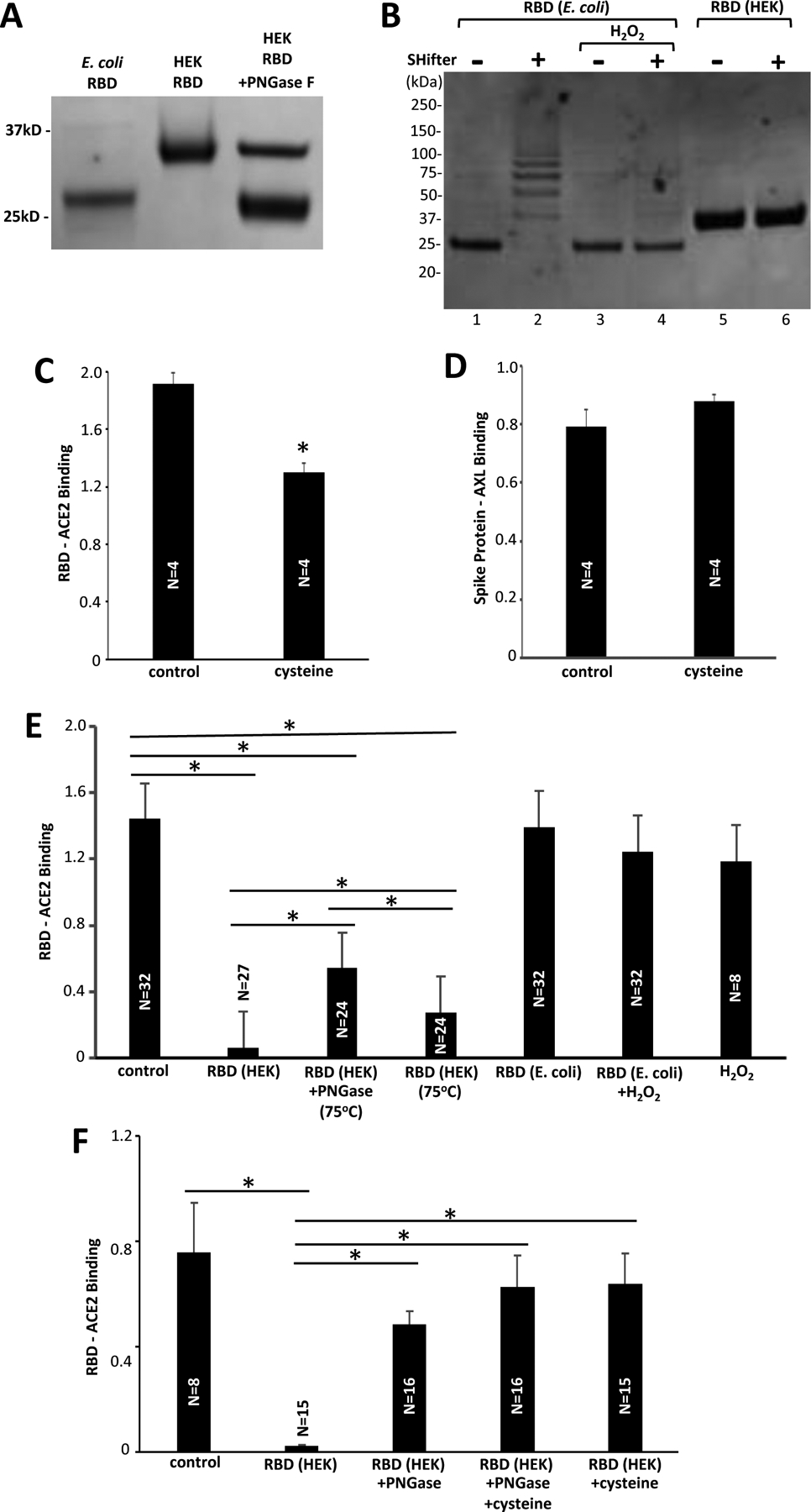
(A) The RBD protein expressed in HEK293 cells, but not that expressed in E. coli, is glycosylated. The SARS-CoV-2 RBD-only proteins were subjected to SDS-PAGE. While the RBD protein produced in E. coli migrates at expected 25 kD, the HEK293 cell-produced RBD migrates at a higher molecular weight. The PNGase F treatment caused the HEK293 cell-expressed RBD to migrate at ~25 kD. (B) The RBD protein expressed in E. coli is reduced, but that expressed in HEK293 cells is oxidized. The SulfoBiotics Protein Redox State Monitoring assay puts a 15-kD SHifter to reduce cysteine residues resulting in electrophoretic mobility shifts. Using this assay, the RBD protein that was produced in E. coli was found to be largely reduced as the 25-kD protein (Lane 1) became shifted in the presence of the SHifter (Lane 2). On the other hand, the glycosylated RBD protein produced in HEK293 cells was found to be fully oxidized (Lane 6). H2O2 treatment fully oxidized the E. coli-produced RBD protein (Lane 4). (C) The Fc-tagged RBD protein of RayBio COVID-19 Spike-ACE2 Binding Assay Kit II was pre-treated with cysteine. * denotes significant difference from untreated control at P<0.05. (D) The S1 protein of RayBio COVID-19 Spike-AXL Binding Assay Kit was pre-treated with cysteine. (E) The SARS-CoV-2 RBD protein expressed in HEK293 cells, RBD protein expressed in HEK293 cells treated with PNGase F at 75°C, RBD protein expressed in HEK293 cells heated at 75°C, RBD protein expressed in E. coli, RBD protein expressed in E. coli treated with H2O2, and H2O2 were added to the RayBio COVID-19 Spike-ACE2 Binding Assay Kit II. (F) Reduced cysteine was also added to some of experiments shown in Panel E. Lines above bars with * indicate significant difference from each other at P<0.05.
In addition to glycosylation states that are different between these two spike protein preparations, redox states are also different. The SulfoBiotics Protein Redox State Monitoring system [18] revealed that the RBD protein produced in E. coli is reduced, while the protein prepared in HEK293 cells is not. In this system, the 15-kD “SHifters” attached to reduced cysteine residues cause an upward shift of migrating protein bands in SDS-PAGE. Lane 1 of Fig. 4B is the E. coli-derived RBD protein without Shifters that migrates at ~25 kD. With the SHifters, proteins migrate at multiple molecular weights higher than 25 kD, depending on how many cysteine residues are reduced (Lane 2). The treatment of the E. coli-generated RBD protein with hydrogen peroxide (H2O2) caused the protein to migrate only at 25 kD even with the Shifters (Lane 4). By contrast, SHifters did not influence the migration of the HEK cell-generated RBD protein (Lanes 5 vs. 6). These results were confirmed using the DTNB assay which showed that the S1 protein preparation produced in E. coli exhibited 515 times higher reduced thiol levels than the S1 protein produced in HEK293 cells. These results indicated that the E. coli-produced RBD protein is nonglycosylated and its cysteine residues are highly reduced, while the HEK293 cell-produced RBD protein is glycosylated and its cysteine residues are oxidized.
The inability of the reduced RBD-only containing protein produced in E. coli to bind to ACE2 could be linked to the reported ability of reductants to interfere with RBD/ACE2 binding [19–21]. Indeed, we observed that the pretreatment of Fc-tagged RBD in the assay kit with reduced cysteine significantly suppressed Fc-RBD/ACE2 binding (Fig. 4C), while the pretreatment of S1 protein with reduced cysteine did not affect the binding of the spike protein (ECD) with another human host target of the SARS-CoV-2 spike protein, AXL (Fig. 4D).
To determine the role of glycosylation in the binding of the spike protein to ACE2, we tested the effects of the RBD protein produced in HEK293 cells with or without treatment with PNGase F to eliminate glycosylation (Fig. 4A). As shown in Fig. 4E, the treatment of the RBD protein with PNGase F significantly decreased its capacity to bind to ACE2 (2nd bar vs. 3rd bar). We further tested whether the oxidation of the E. coli-derived spike protein with H2O2 makes the E. coli protein interfere with the RBD/ACE2 binding. We found that H2O2 did not cause the E. coliderived RBD protein to bind to ACE2 (Fig. 4E, 5th bar vs. 6th bar). The addition of reduced cysteine to the reaction mixture in ACE2-coated wells with PNGase-treated RBD protein before the addition of Fc-RBD did not significantly alter the effects of RBD/ACE2 binding (Fig. 4F, 3rd bar vs. 4th bar), while effects of glycosylated RBD were affected by cysteine (Fig. 4F, 2nd bar vs. 5th bar).
Figure 5: Effects of spike proteins on ACE2 enzymatic activity.
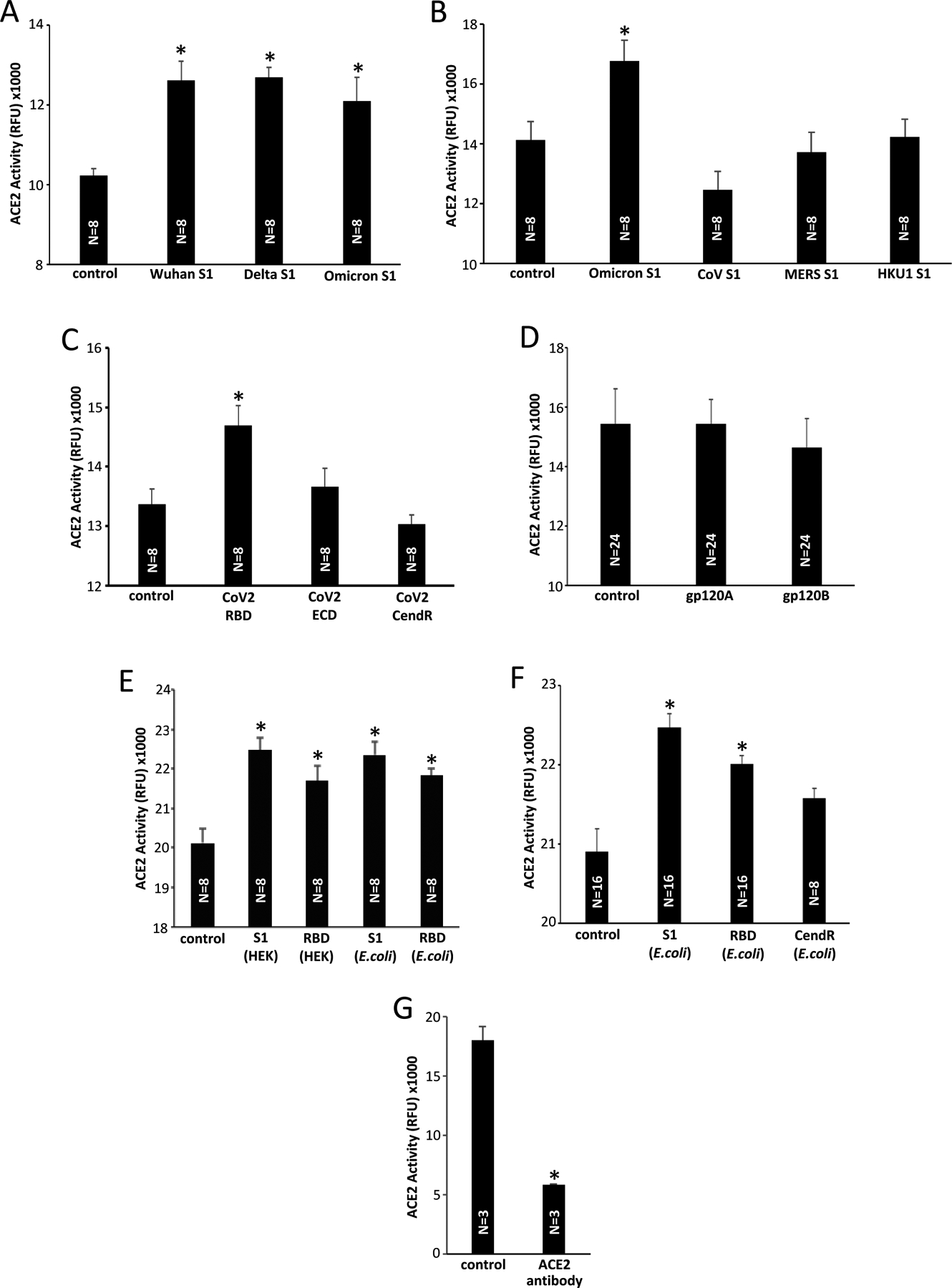
(A) Various variants of SARS-CoV-2 S1 protein (40 nM) slightly but significantly enhance the ACE2 activity. (B) The S1 proteins of SARS-CoV, MERS-CoV, and HKU1 (40 nM) do not enhance the ACE2 activity. (C) Only the SARS-CoV-2 RBD protein, but not the ECD- or CendR-only protein, enhance the ACE2 activity. (D) Neither gp120 of HIV-1 subtype A (gp120A) nor subtype B (gp120B) enhance the ACE2 activity. (E) The ACE2 activity was monitored using the ACE2 Activity Assay in the presence of recombinant S1 and RBD proteins. The HEK293 cell- and E. coli-expressed proteins were equally effective in enhancing the ACE2 activity. (F) The CendR domain protein produced in E. coli does not enhance the ACE2 activity. (G) The ACE2 antibody (mouse monoclonal IgG2A; Cat# MAB9331, R&D Systems Inc., Minneapolis, MN, USA) inhibited the ACE2 activity. RFU: relative fluorescence unit. The symbol * denotes significant difference from the untreated control values at P<0.05.
Effects of SARS-CoV-2 spike proteins on the peptidase activity of human ACE2
It has been reported that ACE2 enzymatic activity is enhanced by the spike protein through the binding via RBD [15]. To further investigate this event, we used the BioVision ACE2 Activity Assay and ANA SPEC SensoLyte 390 ACE2 Activity Assay kits that utilize artificial substrates for ACE2 to form fluorescence products in order to assay the peptidase activity of recombinant human ACE2.
Using these ACE2 activity assays, we also observed that the SARS-CoV-2 S1 proteins expressed in HEK293 cells caused a slight but consistent and statistically significant enhancement of ACE2 activity. Fig. 5A shows that the S1 protein of the original Wuhan virus, Delta variant, and Omicron variant all resulted in statistically significant enhancement of the ACE2 activity, albeit with small effects. These events were repeatedly observed using the ANA SPEC Kit and the BioVision Kit. This enhancement effect of the S1 protein was found to be unique to SARS-CoV-2, as the S1 proteins of neither SARS-CoV, MERS-CoV nor HKU1 enhanced the ACE2 activity (Fig. 5B). While the SARS-CoV-2 spike RBD-only containing protein (R319-F541) enhanced the ACE2 activity, ECD (V16-F318) and CendR Domain (N542-Q690) did not cause this enhancement (Fig. 5C). Neither the gp120 proteins of HIV-1 subtype A or subtype B caused the enhancement of the ACE2 activity (Fig. 5D).
Interestingly, this enhancement of ACE2 activity was also caused by the S1 and RBD proteins produced in E. coli which do not have the ability to tightly bind to ACE2 as shown in Fig. 3. Fig. 5E shows that both the HEK293 cell- and E. coli-produced S1 proteins equally enhance the ACE2 activity, and both HEK293 cell- and the E. coli-produced RBD proteins equally enhance the ACE2 activity. Like the HEK293 cell-produced CendR protein (Fig. 5C), the CendR domain-only protein expressed in E. coli did not significantly enhance the ACE2 activity (Fig. 5F). Thus, unlike ACE2 binding, the ability of the spike protein to enhance the ACE2 activity does not appear to be glycosylation-dependent, but still regulated by the RBD. The tight binding to ACE2 alone is not a factor for the enhancement either because the ACE2 antibody did not enhance, but rather inhibited the ACE2 peptidase activity (Fig. 5G).
In addition to the effects of SARS-CoV-2 S1 proteins on the ACE2 binding and the ACE2 peptidase activity, we found that the activities of other enzymes are also affected by the S1 protein. Fig. 6A shows that the S1 protein (produced in HEK293 cells) dramatically enhanced the Furin activity. The activity of glycolytic enzyme GAPDH was also enhanced by the S1 protein (Fig. 6B). Enzymatic activity of another peptidase, DPP4 was not affected by the SARS-CoV-2 S1 protein (Fig. 6C).
Figure 6: Effects of SAR-CoV-2 spike protein S1 on other enzymes.
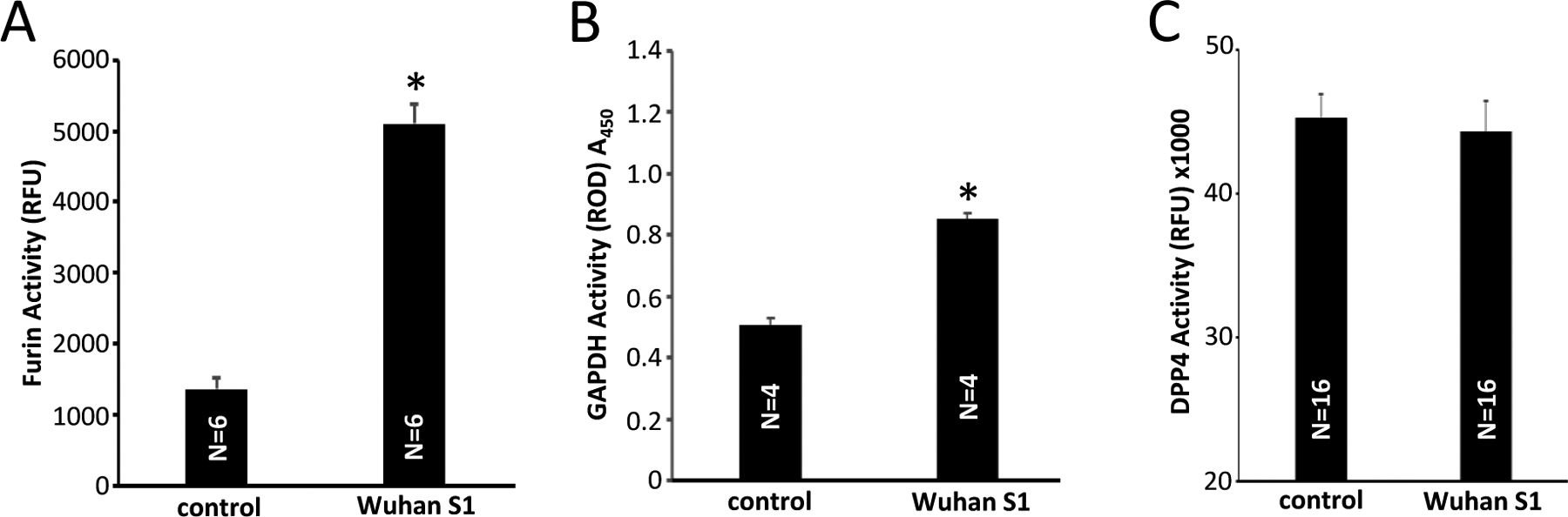
(A) Effects of the HEK293 cell-produced Wuhan SARS-CoV-2 spike protein S1 on the furin enzymatic activity using the Rh110 Furin Activity Assay. (B) The GAPDH activity was monitored at 450 nm in the presence of Wuhan SARS-CoV-2 spike protein S1 protein produced in HEK293 cells. (C) The Wuhan SARS-CoV-2 spike protein S1 does not influence the DPP4 activity. RFU: relative fluorescence unit. ROD: relative optical density. The symbol * denotes significant difference from the untreated control values at P<0.05.
Discussion
The spike protein plays a crucial role in the infection of human host cells with SARS-CoV-2 [5,6]. In addition, it appears that the shedding of the S1 subunit occurs, resulting in circulating S1 proteins and ACE2-dependent complications [8,9]. A number of people have been administered with COVID-19 vaccines that also produce the SARS-CoV-2 spike protein [10]. While the end of the COVID-19 pandemic has officially been declared by the World Health Organization, people still get infected with SARS-CoV-2, receive COVID-19 vaccines, and are coping with long COVID conditions as well as possible vaccine injuries [22]. Thus, understanding the molecular mechanisms of the actions of the SARS-CoV-2 spike protein remains highly important.
A number of assay kits are available to detect the binding between the SARS-CoV-2 spike protein and its host cell receptor ACE2, and allow for the screening of molecules that interfere with the RBD/ACE2 interactions. This aids the search for potential therapeutic agents to reduce a SARS-CoV-2 infection. We demonstrated that some of these assay kits can also be used to investigate the molecular mechanisms of spike protein/ACE2 interactions by adding recombinant spike proteins to the assays when the assay does not use the spike protein antibody for detection. For example, three kits used in this study utilize the ACE2 antibody, Fc tag antibody, and biotin-streptavidin reaction. As expected, our experiments showed that the S1 and RBD proteins of SARS-CoV-2 and SARS-CoV interfered with Spike/ACE2 binding in these assays. The S1 protein of MERS-CoV was also found to interfere with SARS-CoV-2 spike protein RBD binding to ACE2. On the other hand, the S1 protein of the HKU1 coronavirus and the viral fusion proteins of HIV-1 did not influence the RBD/ACE2 binding, serving as negative controls. Mechanistically, using truncated SARS-CoV-2 spike protein S1 subunit domains, only RBD (Arg319-Phe541), but not ECD (Val16-Phe318) or CendR (Asn542-Arg685), interfered with binding in the assays. The S1 proteins of different SARS-CoV-2 variants including the original Wuhan virus, Delta variant, and Omicron variant all interfered with the binding in the assays, with perhaps the Delta variant being most effective.
While all the recombinant proteins described above were produced in human HEK293 cells, the use of the E. coli-produced spike protein showed interesting results. The S1 and RBD proteins expressed in HEK293 effectively competed in the binding assays; however, the proteins produced in E. coli had no ability to do so, indicating that these E. coli-generated proteins do not tightly bind to ACE2. This is consistent with E. coli-produced recombinant proteins being non-glycosylated, and glycosylation playing a critical role in the binding of RBD to ACE2 in the SARS-CoV2 infection [23]. In addition to the glycosylation status, the SulfoBiotics Protein Redox State Monitoring system and DTNB assay revealed that redox states are different between the preparations of recombinant spike proteins produced in HEK293 cells and in E. coli. It has been reported that reductants interfere with the RBD/ACE2 binding [19–21]. Reduced cysteine also inhibited the RBD/ACE binding in our system, but not the ECD/AXL binding. Our experiments showed that the elimination of glycosylation from the HEK293 cell-produced spike protein attenuated the ability to bind to ACE2; however, the oxidation by H2O2 did not restore the ability of the E. coli-produced spike protein to bind to ACE2.
Despite a clear difference between the ability of the HEK293-produced and E. coli-produced spike proteins to bind to ACE2, these two proteins were equally effective in altering the ACE2 peptidase activity. Lu & Sun [15] previously reported that ACE2 enzymatic activity is enhanced by the spike protein. We also observed that S1 and RBD proteins of SARS-CoV-2 enhanced the ACE2 activity. Importantly, our results showed that this effect on ACE2 is specific to SARS-CoV-2 and is not exhibited by the SARS-CoV, MERS-CoV, or HKU S1 proteins. Mechanistically, only RBD, but not ECD or CendR, enhanced ACE2 activity, suggesting that the RBD is responsible for this mechanism. The present study generated an intriguing question about how the spike protein produced in E. coli without the ability to bind to ACE2 is still capable of affecting the ACE2 enzymatic activity. While further work is needed to completely understand the biology of the SARS-CoV-2 spike protein, our results suggest that the RBD-interacting sites for tight binding might be responsible for the viral infection, and the promotion of the peptidase activity might occur at distinct low affinity regions in the ACE2 structure in a manner independent of glycosylation status on the spike protein.
Our studies showed that ACE2 is not the only enzyme whose activity is enhanced by the SARS-CoV-2 spike protein. While the effects of the spike protein on ACE2 are quite small, the S1 protein more dramatically enhanced Furin and GAPDH activities. The full length S1+S2 spike protein of SARS-CoV-2 can be cleaved by Furin to produce S1 subunit. Thus, our results, which showed that the S1 protein increased the enzymatic activity of Furin, suggest the possibility of the S1 protein amplifying its production through a positive feedback mechanism. As Furin can occur in the extracellular space [24], circulating S1 may be produced from SARS-CoV-2 as well as from COVID-19 vaccines, causing complications. Furthermore, the influence of the spike protein on cytosolic enzymes regulating cellular metabolism such as GAPDH may have important implications on what COVID-19 vaccines may exert to our cells when they produce intracellular spike protein. These results may have important clinical implications, not only for SARS-CoV-2 infection, but also for adverse effects of COVID-19 vaccines that occur in some people as these vaccines encode for the spike protein [10,25–27].
In summary, the present study provided new information about the actions of SARS-CoV-2. The world is still affected by this virus as well as by the vaccines against it, both of which can generate spike proteins intracellularly and extracellularly. We need to understand biological actions of the spike protein on ACE2 as well as on other human host proteins. The accumulation of such knowledge should ultimately contribute to the development of therapeutic strategies to cope with current and possible future coronavirus infection and associated complications, long- or post-COVID syndromes as well as adverse events elicited by COVID-19 vaccines.
Highlights.
While glycosylated spike proteins prepared in HEK293 cells bind to ACE2, non-glycosylated proteins produced in E. coli do not.
Spike proteins expressed in both HEK293 cells and E. coli enhance the ACE2 activity.
Thus, the spike protein of SARS-CoV-2 enhances the ACE2 peptidase activity through its RBD in a glycosylation-independent manner.
Funding:
This research was funded by the National Institutes of Health (NIH), grant numbers R21AG073919 (to Y.J.S. and S.G.G.), R03AG071596 (to Y.J.S.), R01GM124020 (to T.I.B.), and R01CA252969 (to T.I.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ, A new coronavirus associated with human respiratory disease in China, Nature 579 (2020) 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet 395 (2020) 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E, COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect 26 (2020) 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M, Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus, Nature 426 (2003) 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q, Structural basis for the recognition of SARS-CoV-2 by full-length human ACE, Science 367 (2020) 1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L, Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine, Cell. Mol. Immunol 17 (2020) 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C, Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets, Nat. Rev. Cardiol 11 (2014) 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Suzuki YJ, The viral protein fragment theory of COVID-19 pathogenesis, Med. Hypotheses 144 (2020) 110267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ogata AF, Maley AM, Wu C, Gilboa T, Norman M, Lazarovits R, Mao CP, Newton G, Chang M, Nguyen K, Kamkaew M, Zhu Q, Gibson TE, Ryan ET, Charles RC, Marasco WA, Walt DR, Ultra-sensitive serial profiling of SARS-CoV-2 antigens and antibodies in plasma to understand disease progression in COVID-19 patients with severe disease, Clin. Chem 66 (2020) 1562–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Suzuki YJ, Gychka SG, SARS-CoV-2 spike protein elicits cell signaling in human host cells: Implications for possible consequences of COVID-19 vaccines, Vaccines 9 (2021) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ogata AF, Cheng CA, Desjardins M, Senussi Y, Sherman AC, Powell M, Novack L, Von S, Li X, Baden LR, Walt DR, Circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients, Clin. Infect. Dis 74 (2022) 715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yonker LM, Swank Z, Bartsch YC, Burns MD, Kane A, Boribong BP, Davis JP, Loiselle M, Novak T, Senussi Y, Cheng CA, Burgess E, Edlow AG, Chou J, Dionne A, Balaguru D, Lahoud-Rahme M, Arditi M, Julg B, Randolph AG, Alter G, Fasano A, Walt DR, Circulating spike protein detected in post-COVID-19 mRNA vaccine myocarditis, Circulation 147 (2023) 867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, Zhang Y, Yin Q, Cho Y, Andrade L, Shadel GS, Hepokoski M, Lei T, Wang H, Zhang J, Yuan JX, Malhotra A, Manor U, Wang S, Yuan ZY, Shyy JY, SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2, Circ. Res 128 (2021) 1323–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Seeherman S, Suzuki YJ, Viral infection and cardiovascular disease: Implications for the molecular basis of COVID-19 pathogenesis, Int. J. Mol. Sci 22 (2021) 1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lu J, Sun PD, High affinity binding of SARS-CoV-2 spike protein enhances ACE2 carboxypeptidase activity, J. Biol. Chem 295 (2020) 18579–18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Suzuki Y, Lyall V, Biber TU, Ford GD GD, A modified technique for the measurement of sulfhydryl groups oxidized by reactive oxygen intermediates, Free Radic. Biol. Med 9 (1990) 479484. [DOI] [PubMed] [Google Scholar]
- [17].Raj VS, Mou H, Smits SL, Dekkers DH, Müller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL, Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC, Nature 495 (2013) 251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Suzuki YJ, Marcocci L, Shimomura T, Tatenaka Y, Ohuchi Y, Brelidze TI, Protein redox state monitoring studies of thiol reactivity, Antioxidants 8 (2019)143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hati S, Bhattacharyya S, Impact of thiol-disulfide balance on the binding of Covid-19 spike protein with angiotensin-converting enzyme 2 receptor, ACS Omega. 5 (2020) 16292–16298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grishin AM, Dolgova NV, Landreth S, Fisette O, Pickering IJ, George GN, Falzarano D, Cygler M, Disulfide bonds play a critical role in the structure and function of the receptor-binding domain of the SARS-CoV-2 spike antigen, J. Mol. Biol 434 (2022) 167357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Murae M, Shimizu Y, Yamamoto Y, Kobayashi A, Houri M, Inoue T, Irie T, Gemba R, Kondo Y, Nakano Y, Miyazaki S, Yamada D, Saitoh A, Ishii I, Onodera T, Takahashi Y, Wakita T, Fukasawa M, Noguchi K, The function of SARS-CoV-2 spike protein is impaired by disulfide-bond disruption with mutation at cysteine-488 and by thiol-reactive N-acetyl-cysteine and glutathione, Biochem. Biophys. Res. Commun 597 (2022) 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].The Lancet. The COVID-19 pandemic in 2023: far from over, Lancet 401 (2023) 79. [DOI] [PubMed] [Google Scholar]
- [23].Gong Y, Qin S, Dai L, Tian Z, The glycosylation in SARS-CoV-2 and its receptor ACE2, Signal. Transduct. Target. Ther 6 (2021) 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Braun E, Sauter DD, Furin-mediated protein processing in infectious diseases and cancer, Clin. Transl. Immunology 8 (2019) e1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Suresh SJ, Suzuki YJ, SARS-CoV-2 spike protein and lung vascular cells, J. Respir 1 (2021) 40–48. [Google Scholar]
- [26].Trougakos IP, Terpos E, Alexopoulos H, Politou M, Paraskevis D, Scorilas A, Kastritis E, Andreakos E, Dimopoulos MA, Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis, Trends Mol. Med 28 (2022) 542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Parry PI, Lefringhausen A, Turni C, Neil CJ, Cosford R, Hudson NJ, Gillespie J, ‘Spikeopathy’: COVID-19 spike protein is pathogenic, from both virus and vaccine mRNA, Biomedicines 11 (2023) 2287. [DOI] [PMC free article] [PubMed] [Google Scholar]


