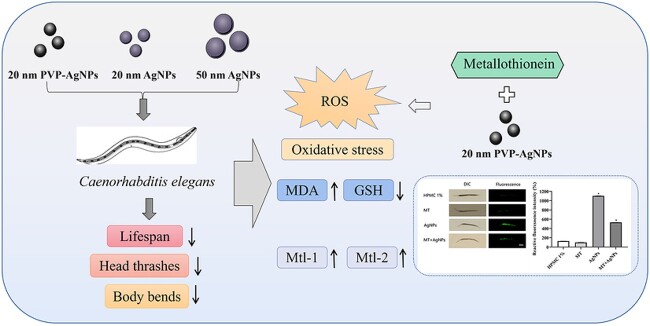
Abstract
Silver nanoparticles (AgNPs) are widely used in many commercial and medical products. Serious concerns are paid on their adverse potentials to the environment and human health. In this study, toxic effects and oxidative stress induced by AgNPs with different sizes and coatings (20 nm AgNPs, 20 nm polyvinylpyrrolidone (PVP) -AgNPs and 50 nm AgNPs) in Caenorhabditis elegans (C. elegans) were investigated. The toxic effects including the shortened lifespan and decreased frequency of head thrashes and body bends of C. elegans were induced in a dose-dependent manner by AgNPs. The reactive oxygen species (ROS) production and the oxidative stress-related indicators including malondialdehyde (MDA) and glutathione (GSH) in nematodes were changed after exposure to three kinds of AgNPs. These effects were the most obvious in a 20 nm PVP-AgNPs exposure group. AgNPs could also induce the expression of genes related to oxidative stress in nematodes. In addition, the up-regulation of mtl-1 and mtl-2 in nematodes might reduce the oxidative damage caused by AgNPs, by using transgenic strains CF2222 and CL2120 nematodes. Metallothionein (MT), an antioxidant, could relieve the oxidative damage caused by AgNPs. These results suggested that 20 nm PVP-AgNPs with a smaller particle size and better dispersion have stronger toxic effects and the oxidative damage to nematodes. Mtl-1 and mtl-2 might be involved in alleviating the oxidative damage caused by AgNPs. Our findings provide clues for the safety evaluation and mechanism information of metal nanoparticles.
Keywords: silver nanoparticles, caenorhabditis elegans, nanotoxicity, oxidative stress, metallothionein
Graphical Abstract
Graphical Abstract.
Introduction
Silver nanoparticles (AgNPs) can be used in nanomedicine, biosensing and biomedical engineering due to their unique antibacterial properties.1 According to a list of nanotechnology consumer products created by the Woodrow Wilson International Center for Scholars and the Emerging Nanotechnology Program, AgNPs-containing products account for more than 30% of total consumer nanoproducts and 75% of nanomedicine products.2 Their synthesis or use, whether industrial or domestic, leads to the release of AgNPs that eventually enter the environment. Although there is no exact information about the detailed concentration of AgNPs in the environment, the concentration of AgNPs in the environment of some countries and regions was predicted. The concentration of AgNPs in sewage treatment plant sludge is about 1–2 mg/kg and maybe as high as 10 mg/kg. The concentration of AgNPs in surface water is much lower and to be about 1 ng/L.3 AgNPs in the environment can be enriched into organisms by the biological amplification during migration and transformation. AgNPs can be absorbed through the respiratory and digestive systems as well as through the skin.4,5 What’s more, studies have shown that all of the above exposures can make AgNPs enter the lungs, liver, kidneys, spleen, heart and brain through the blood circulation.4,6,7 According to a recent opinion from the European Commission’s Scientific Committee on Consumer Safety, “due to some significant data gaps,” no conclusions can still be drawn on AgNP safety.8
As a model organism, Caenorhabditis elegans (C. elegans) is increasingly used in the toxicity evaluation of various exogenous compounds. In particular, the safety evaluation of man-made nanomaterials provides a basis for the formulation of safety standards.9 The genes in C. elegans have 40%–60% homology with human genes.10 Compared with the other mammals such as rats and mice, C. elegans has the characteristics of low costs, easy operations and short life cycles, which provides the possibility for various toxicological experiments using C. elegans.11C. elegans feeds on bacteria, usually live or dead Escherichia coli strain OP50 (E. coli OP50). In C. elegans, some useful sublethal endpoints, including development, reproduction, intestinal reactive oxygen species (ROS) production, and motor behavior, can be used to assess the toxic effects of exogenous compounds or stress.12 Studies by Braeckman et al. have shown that nematodes have highly complex but specialized ROS and redox control systems.13 Therefore, C. elegans is an excellent model organism to study the mechanisms of ROS production and oxidative stress.
The shape, size and surface morphology of AgNPs are important factors in determining their properties.14 Spherical AgNPs exhibit the stronger antibacterial activity than rod-shaped and wire-shaped AgNPs with similar diameters.15 Smaller nanoparticles had a more pronounced effect on the increased apoptosis induction and also increased reactive oxygen species production compared with larger nanoparticles.16 As naked AgNPs aggregate easily in suspension, they are synthesized with surface coatings to enhance a colloidal stability and extend a shelf life. Polyvinylpyrrolidone (PVP) is one of the most commonly used AgNPs stabilizers. Very tight binding of PVP to the metal surface makes AgNPs a highly stability in various solvents.17 The study by He et al. evaluated the anti-SARS-CoV-2 virus killing effect of AgNPs with three different surface modification types. AgNPs coated with PVP or branched polyethylenemine exhibited a stronger virucidal effect than AgNPs coated with citrate.18
At present, there is no unified conclusion about the toxicity mechanisms of AgNPs. One of the most important toxicity mechanisms of AgNPs is the generation of free radicals on the particle surface, which may affect biomolecules and cellular structures and cause oxidative stress. The antibacterial activity of AgNPs was also thought to be due to the increased ROS concentration. ROS leads to bacterial death by causing intracellular oxidation, changes in the membrane potential, and release of cellular contents.19 The decrease in cell viability exposure to AgNPs was preceded by increases in ROS levels and DNA repair foci.20,21 However, studies have shown that oxidative stress is not the only mechanism of AgNPs toxicity or antibacterial effects.22 Therefore, the research on the oxidative damage of AgNPs and its mechanism needs to be further confirmed.
Metallothionein (MT) is a low molecular weight protein that is ubiquitous in living organisms. It is a short cysteine-rich peptide with a high affinity for various heavy metals. Its sulfhydryl group can strongly chelate toxic metals to achieve detoxification.23 In this study, we used exogenous MT, a genetically engineered antioxidant produced by E. coli fermentation technology, to co-expose with 20 nm PVP-AgNPs for analyzing the effect of MT on AgNPs-induced ROS in C. elegans. In addition, the transgenic strains, the red fluorescent protein (RFP)-tagged mtl-1 nematodes (CF2222 nematode strain) and the green fluorescent protein (GFP)-tagged mtl-2 nematodes (CL2120 nematode strain) were also used. The effect of AgNPs on mtl-1 and mtl-2 gene expression was analyzed by observing the fluorescence intensity in nematodes.
In this study, the C. elegans was exposed to three types of AgNPs with different sizes and coatings, including 20 nm AgNPs, 20 nm PVP-AgNPs and 50 nm AgNPs. The effects of AgNPs on the nematode lifespan, head thrashes, body bends, ROS production and oxidative stress-related indicators malonaldehyde (MDA) and glutathione (GSH) were evaluated comparatively. Furthermore, the expression of oxidative stress-related genes in nematodes was detected by the quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR). The effects of MT induced by AgNPs were investigated by using transgenic nematode strains CF2222 and CL2120. The results provide clues for the safety evaluation and mechanistic information of AgNPs.
Materials and methods
AgNPs and their characterization
The 20 nm AgNPs and 50 nm AgNPs were obtained from Shanghai Yunfu Nano Technology Co., Ltd (Shanghai, China). The 20 nm PVP-AgNPs were obtained from Shanghai Huzheng Nano Technology Co., Ltd (Shanghai, China). The 20 nm PVP-AgNPs contain 25% silver content and 75% PVP surface coating. The particle size and shape of AgNPs were detected by transmission electron microscopy (TEM, JEM-2100, JEOL Corporation, Japan). Nano Measurer 1.2.5 software was used to analyze 500 nanoparticles taken by TEM to obtain the particle size distribution of AgNPs. The hydrated particle size and Zeta potential of AgNPs were detected by Malvern particle size analyzer (Nano ZS90, Malvern Instrument, UK).
C. elegans strains and culture
The Wild-type N2 C. elegans, CF2222 nematode strain (mtl-1::RFP) and CL2120 nematode strain (mtl-2::GFP) maintained in our laboratory were obtained from the Caenorhabditis Genetics Center (CGC, University of Minnesota, USA). We prepared age-synchronized L1-larval population by lysing hermaphroditic adults with a bleaching mixture solution (830 μL H2O, 50 μL NaOH, 120 μL NaClO). Nematodes were maintained on normal nematode growth medium (NGM) plates, and E. coli OP50 was used as food source. Nematodes were maintained in the 20 °C biochemical incubator (SHP150 biochemical incubator, Jinghong Test Equipment Co., Ltd, Shanghai, China). The approval of the Research Ethics Committee is not required to achieve the objectives of this study, as the experiment is carried out with the unregulated invertebrate species. This study does not involve ethical approval and informed consent.
C. elegans exposed to AgNPs
The solvent control group (1% HPMC) and the AgNPs exposure group (1, 10, 100 mg/L) were set up, respectively. Hydroxypropyl methylcellulose (HPMC) is usually used as an auxiliary material in the application of AgNPs. It is a dispersant and has the particle size and pH stability. Since metal nanoparticles are not soluble in aqueous medium, we used 1% HPMC as a dispersant and maintained the test suspensions stable throughout the experimental period. The three AgNPs powders were weighed, dispersed in 1% HPMC solution and diluted into 1, 10 and 100 mg/L AgNPs suspensions, respectively. Prior to exposure, the AgNPs suspensions were dispersed in an ultrasonic bath for 20 min, vortexed for 2 min. 200 μL of AgNPs (1, 10 and 100 mg/L) suspension was added to each NGM plate (35 mm plate) containing L1 larval nematodes. The plates were naturally dried and incubated in a biochemical incubator at 20 °C. The nematodes were synchronized to obtain the L1 larvae and then exposed to AgNPs for 48 h. L1-stage larvae could develop into the adult stage after approximately 46 h, so the toxic effects of AgNPs on the entire growth and development cycle of nematodes could be assessed after the prolonged exposure for 48 h to the L1 stage.
C. elegans lifespan assay
At the end of exposure, the nematodes (30 per dose group) were transferred onto new NGM plates coated with E. coli OP50. 2 mL of 0.1 mg/mL pentafluorouracil was added to each 500 mL of NGM medium to inhibit oviposition without affecting nematode lifespan. Three parallel groups were set up and the number of nematode survivors, deaths and losses were observed and recorded daily using a body vision microscope (Olympus SZ61, Olympus, Japan) until all nematodes were dead. The nematodes that did not respond to a gentle tapping with the picker were counted as dead.
C. elegans locomotion behavior assay
After the exposure, the nematodes were picked and placed in a new NGM plate without E. coli OP50 at 20 °C for 1 min recovery. The head thrashes and body bends were counted for 20 s under a stereomicroscope (Olympus, SZ61), respectively. A head thrash refers to a change in the direction of the nematode’s head thrashes, this change must be turned in the direction of its body. A body bend refers to the assumption that the direction along the nematode pharyngeal pump is the X axis, and the body changes along the corresponding Y axis when the nematode crawls. There are 3 parallel groups for each dose group, and 30 counts are selected for each group.
The detection of ROS, MDA and GSH in C. elegans
After the exposure, the nematodes were washed three times with M9 buffer and transferred to Eppendorf tubes. A commercial ROS assay kit (S0033, Beyotime Biotechnology Ltd, Shanghai, China) was used according to the instructions. The samples were incubated with 2 μL of the probe DCFH-DA and placed in a biochemical incubator at 25 °C for 3 h. The nematodes were transferred to an agarose gel pad, 20 μL of 0.5 mM sodium azide was dropped, covered with a coverslip, and the nematodes were observed and imaged on a fluorescence microscope (Ti-E Nikon inverted fluorescence microscope, Nikon, Japan). Fluorescence intensity was analyzed using Image J software. Each group was examined 30 nematodes, and the tests were performed three times.
The oxidative stress-related indicators including malondialdehyde (MDA) and glutathione (GSH) in nematodes were measured. The washed nematodes were shaken and grounded with tissue grinders (Tissuelyser-24, Jingxin Technology Co., Ltd, Shanghai, China) to prepare a 10% tissue homogenate. The specific steps were performed strictly following the MDA and GSH detection kit instructions in order. The entire process was operated on ice. A microplate reader (EPOCH, BIOTEK Corporation, USA) was used to determine the optical density (OD). The tests were performed three times.
The expression of genes related to oxidative stress in C. elegans
The amount of mRNA expression of nematode oxidative stress-related genes was analyzed by the quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR). The total RNA of nematodes was extracted with TRIzol Total RNA Extraction Kit (Sigma-Aldrich Trading Co., Ltd, USA). The OD at 260 nm and 280 nm were measured with a micro-ultraviolet spectrophotometer (Nanodrop 1000 Spectrophotometer, Thermo Scientific Company, USA) to ensure that the purity of the extracted RNA was 1.8–2.0. The cDNA was synthesized using the Mastercycler gradient PCR instrument (Eppendorf Company, USA). The StepOne fluorescent quantitative PCR instrument (ABI Company, USA) was used for a real-time fluorescent quantitative PCR detection.
The actin gene (Act-1) was used as an internal reference gene. The genes that needed to design primers were searched for on the Wormbase homepage. The Blast tool was used to select the sequence fragments with the highest homology to the mouse in the gene cDNA sequence. The primer sequences were designed using the software Primer Premier 6.0 software and Warmbase. The designed primers for oxidative stress-related genes were listed in Table 1.
Table 1.
Primers for oxidative stress related genes.
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| sod-1 | TCAGGTCTCCAACGCGATTT | ACCGGGAGTAAGTCCCTTGA |
| sod-2 | GAGGCGGTCTCCAAAGGAAA | CGCTCTTAATTGCGGTGAGC |
| sod-3 | CTCCAAGCACACTCTCCCAG | TCCCTTTCGAAACAGCCTCG |
| sod-4 | GACGCGGTACTTCAGACCAA | CTGGAGGAAGGGATGCTGTC |
| sod-5 | CCGATAAGGTGGTCAGCCTC | CAAAGACTCCTCGGCCTTGT |
| ctl-1 | GTGTCGTTCATGCCAAGGGAG | TGGATTGCGTCACGAATGAAG |
| ctl-2 | TCCCAGATGGGTACCGTCAT | GGTCCGAAGAGGCAAGTTGA |
| ctl-3 | ATGCCAATGCTTCCCCACAT | GCAGGTGGGGTTCCTGATTT |
| clk-1 | AACCCGTGGAGCACATACTG | AGCCAACTGTCCAGCGTAAA |
| gas-1 | TCACTCGCATCCAGAACCAC | TGGAAGATCCCAAGCGACAC |
| isp-1 | GAGTGTGCACCCATCTTGGA | GACGATGGTGGCATCCTTGA |
| mev-1 mtl-1 mtl-2 |
TCGATCGTCACCAAGTCCGA TGTGAGGAGGCCAGTGAGAA TTGTTCCTGCAACACCGGAA |
ATTCCTCCGACGAGAAGGGT TTGATGGGTCTTGTCTCCGC CTGAGCACATTCGCAGTTGG |
| act-1 | ATGTGTGACGACGAGGTT | GAAGCACTTGCGGTGAAC |
The detection of MT-related genes and antioxidative effect of MT
To determine the effects of AgNPs on mtl-1 and mtl-2 in nematodes, the Wild-type N2 C. elegans was exposed to 1, 10, and 100 mg/L of 20 nm PVP-AgNPs for 48 h, and MT-related genes including mtl-1 and mtl-2 in nematodes were detected by qRT-PCR. In addition, the fluorescence intensity in the transgenic nematodes was measured by an inverted fluorescence microscope after the CF2222 and CL2120 nematodes were exposed to 1, 10, and 100 mg/L of 20 nm PVP-AgNPs for 48 h.
To examine the antioxidant effect of MT (an antioxidant provided by Suzhou Hvha Medical Technology Development Co., Ltd China. The molecular weight is approximately 7 kDa containing 20 cysteines per molecule, purity ≥98% by SDS-PAGE) on the oxidative stress induced by AgNPs, Wild-type N2 C. elegans was exposed to 1% HPMC, 5 mg/L of MT, 100 mg/L of 20 nm PVP-AgNPs, and co-exposure to MT (5 mg/L) and 20 nm PVP-AgNPs (100 mg/L). After 48 h exposure, ROS levels in nematodes were detected by an inverted fluorescence microscope.
Statistical analysis
Each experiment was conducted at least three times. SPSS Statistics 22.0 software for Windows (SPSS Inc., Chicago, Illinois, USA) was used for a statistical analysis. Multiple groups were compared by homogeneity of variance tests and the one-way analysis of variances (One-Way ANOVA). For a comparison between the two groups, if the squareness is homogeneous, the LSD-t test method is used. If the variance is heterogeneous, the Games-Howell test method is used. The experimental data are all expressed as mean ± standard deviation (SD), and differences were considered statistically significant when the P value was <0.05. GraphPad Prism version 8.0.1 for Windows (GraphPad Software Inc., San Diego, California, USA) was used to make bar graphs.
Results
Characterization of AgNPs
The results in Table 2 showed the particle size, hydrated particle size and Zeta potential of the three AgNPs of 20 nm PVP-AgNPs, 20 nm AgNPs and 50 nm AgNPs. The hydrated particle size of 50 nm AgNPs was the largest, followed by 20 nm AgNPs, and the hydrated particle size of 20 nm PVP-AgNPs was the smallest. The absolute value of Zeta potential of 50 nm AgNPs was the smallest, followed by 20 nm AgNPs, and the absolute value of Zeta potential of 20 nm PVP-AgNPs was the largest. Combined with the results of the particle size analysis, the results of transmission electron microscopy in Fig. 1 showed that 20 nm PVP-AgNPs had less agglomeration and good dispersion. 20 nm AgNPs and 50 nm AgNPs had more agglomeration. The three AgNPs showed to be spherical. The three AgNPs were well dispersed under 1% HPMC solvent. After standing for 72 h, three kinds of AgNPs dispersions remained stable without significant precipitation. There were almost no silver ions released from 20 nm AgNPs and 50 nm AgNPs. Very few silver ions (about 6‰) were detected in the 20 nm PVP-AgNPs dispersion.
Table 2.
Characterization of three kinds of AgNPs (n = 3, mean ± SD).
| Kinds of AgNPs | particle size (nm) | Hydrated particle size (nm) | Zeta potential (mV) |
|---|---|---|---|
| 20 nm PVP-AgNPs | 22.08 ± 4.74 | 64.27 ± 21.42 | −14.21 ± 1.31 |
| 20 nm AgNPs | 20.14 ± 3.67 | 542.73 ± 64.83 | −9.51 ± 0.2 |
| 50 nm AgNPs | 49.84 ± 8.02 | 912.34 ± 76.25 | −8.93 ± 1.84 |
Note: 500 AgNPs were analyzed for obtaining size by Nano-Measurer1.2.5 software.
Fig. 1.
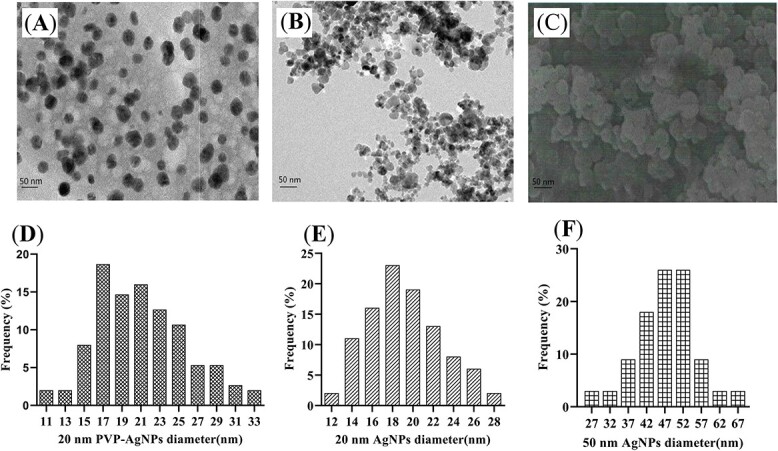
TEM of three AgNPs. A) 20 nm PVP-AgNPs; B) 20 nm AgNPs; C) 50 nm AgNPs. Particle size distributions of the three AgNPs were analyzed using Nano measurer 1.2.5 software for 500 nanoparticles taken by TEM, D) 20 nm PVP-AgNPs; E) 20 nm AgNPs; F) 50 nm AgNPs. AgNPs, silver nanoparticles; PVP, polyvinylpyrrolidone.
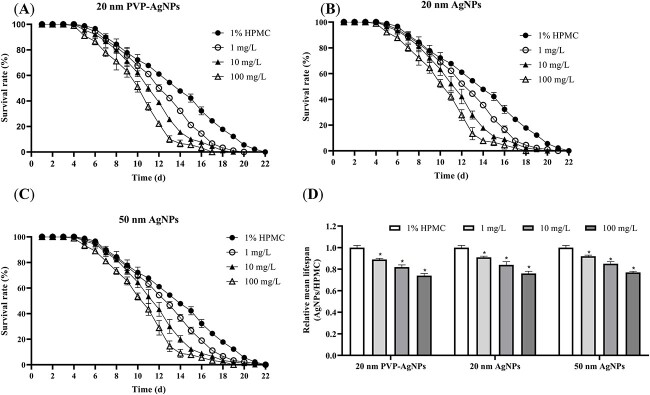
Effects of AgNPs on lifespan
Based on the results of nematode survival rates (Fig. 2), the maximum nematode lifespan in the control group was 22 days. From day 5 of exposure to AgNPs, a small number of nematodes died in each dose group. The relative mean lifespan of nematodes in each dose group of the three AgNPs showed a decreasing trend with an increasing dose. The smallest nematode relative mean lifespan was in the 20 nm PVP-AgNPs exposure group at 100 mg/L and the largest was in the 50 nm AgNPs exposure group at 1 mg/L. There was no significant difference in the mean lifespan of nematodes from the three AgNPs at the same exposure concentration.
Fig. 2.
Effects of 20 nm PVP-AgNPs (A), 20 nm AgNPs (B) and 50 nm AgNPs (C) on the survival rate of C. elegans after 48 h exposure; D) The relative average lifespan of C. elegans exposed to three AgNPs for 48 h. Experimental data were represented by mean ± SD in three parallel experiments. Compared with the control group, *P < 0.05. AgNPs, silver nanoparticles; PVP, polyvinylpyrrolidone; HPMC, hydroxypropyl methyl cellulose.
Effects of AgNPs on locomotion behavior
As shown in Fig. 3, compared with the control group, the head thrashes and body bends of the nematodes in the three AgNPs dose groups decreased with the increase of the dose. 20 nm PVP-AgNPs had the greatest effect on nematode head thrashes and body bends, followed by 20 nm AgNPs, and 50 nm AgNPs had the least effect on nematode head thrashes and body bends. The above results show that the 20 nm PVP-AgNPs are the most effective among the three AgNPs on the locomotor behavior of C. elegans.
Fig. 3.
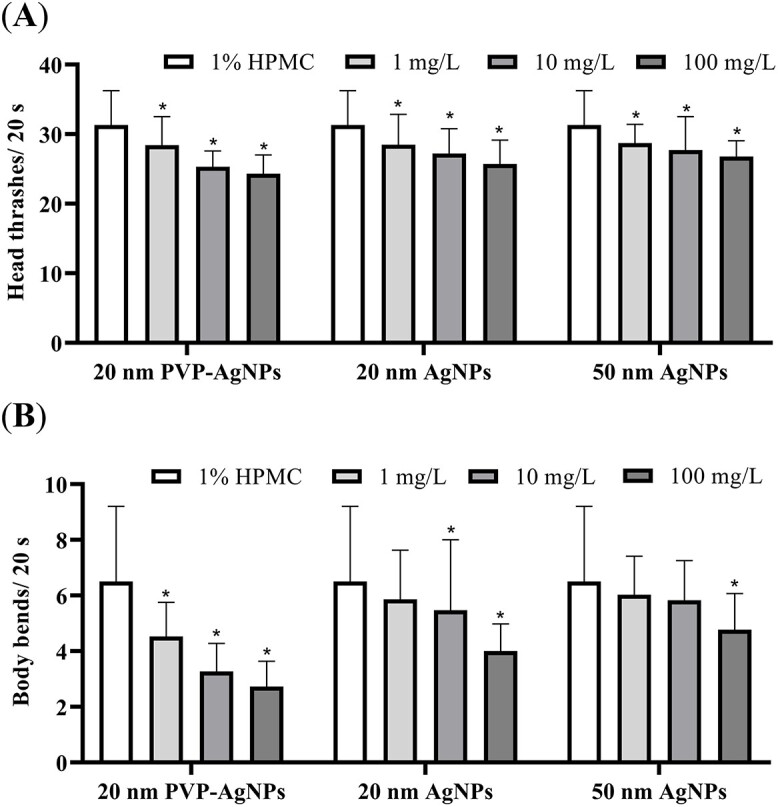
Effects of three AgNPs on the head thrashes (A) and body bends (B) in C. elegans after 48 h exposure. Experimental data were represented by mean ± SD in three parallel experiments. Compared with the control group, *P < 0.05. AgNPs, silver nanoparticles; PVP, polyvinylpyrrolidone; HPMC, hydroxypropyl methyl cellulose.
Effects of AgNPs on ROS, MDA, GSH contents
According to the ROS staining of nematodes and the fluorescence photography (Fig. 4A–D), the results showed that, compared with the control group, the fluorescence intensity of the three AgNPs dose groups in the nematodes increased with the increase of the dose, indicating that the ROS in the nematodes increased. Among them, the 100 mg/L group of 20 nm PVP-AgNPs had the strongest fluorescence intensity, and the 1 mg/L group of 50 nm AgNPs had the weakest fluorescence intensity. 20 nm PVP-AgNPs led to the highest level of oxidative stress in nematodes, resulting in the most ROS production and the strongest fluorescence intensity in nematodes.
Fig. 4.
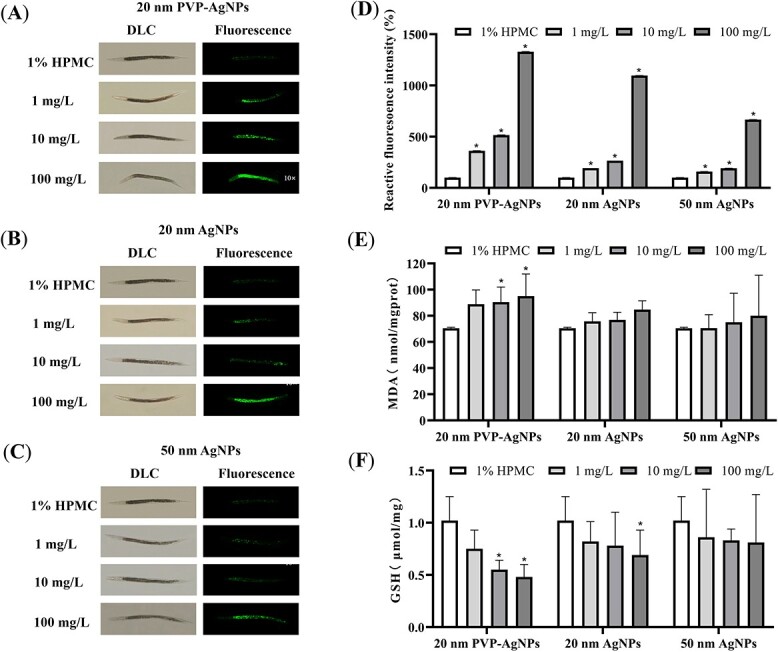
Effects of 20 nm PVP-AgNPs (A), 20 nm AgNPs (B) and 50 nm AgNPs (C) on ROS in C. elegans after 48 h exposure (10×). Effects of three AgNPs on the relative fluorescence intensity of ROS (D) in C. elegans after 48 h exposure. Effects of three AgNPs on MDA (E) and GSH (F) contents in C. elegans after 48 h exposure. Experimental data were represented by mean ± SD in three parallel experiments. Compared with the control group, *P < 0.05. AgNPs, silver nanoparticles; PVP, polyvinylpyrrolidone; HPMC, hydroxypropyl methyl cellulose.
By detecting the contents of MDA and GSH in the nematodes, as shown in Fig. 4E and F, compared with the control group, the contents of MDA in the nematodes in the three AgNPs dose groups increased with the increase of the exposure dose, and the contents of GSH decreased with the increase of the dose. The increase in MDA contents was due to oxidative stress in the nematodes, resulting in an increase in reactive oxygen species and lipid metabolites in the body. The decrease in GSH contents was due to excessive free radicals in nematodes, which exceeded the body’s compensatory ability, resulting in an increased consumption. In the results, only the 10 and 100 mg/L dose groups of 20 nm PVP-AgNPs and the 100 mg/L dose group of 20 nm AgNPs significantly changed the contents of MDA and GSH in nematodes, resulting in higher levels of oxidative stress in nematodes.
Effects of AgNPs on the expression of oxidative stress related genes
After exposure to 20 nm PVP-AgNPs for 48 h, the expression levels of oxidative stress-related genes in the nematodes were detected by qRT-PCR. As shown in Fig. 5, compared to the control group, the mRNA expression levels of ctl-1 were up-regulated in the 1, 10 and 100 mg/L dose groups. The mRNA expression levels of ctl-2, ctl-3, isp-1, clk-1, mev-1 and sod-5 were up-regulated in the 10, 100 mg/L dose group. The mRNA expression levels of sod-4 were up-regulated in the 10 mg/L dose group. The mRNA expression level of sod-1 was down-regulated in the 10 mg/L dose group. The mRNA expression levels of sod-2 and sod-3 were down-regulated in the 1 and 10 mg/L dose groups. The mRNA expression levels of gas-1 were down-regulated in the 1, 10 and 100 mg/L dose groups. The above differences were statistically significant. These results indicate that the AgNPs exposure affects the expression levels of oxidative stress-related genes in the nematodes.
Fig. 5.
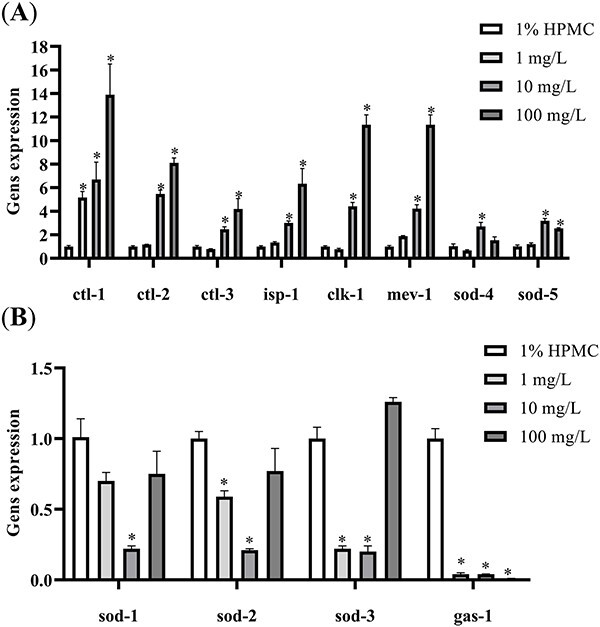
The influence of the expression of mRNA of oxidative stress related genes induced by 20 nm PVP-AgNPs in C. elegans. A) ctl-1, ctl2, ctl-3, isp-1, clk-1, mev-1, sod-4, sod-5. B) sod-1, sod-2, sod-3, gas-1. Experimental data were represented by mean ± SD in three parallel experiments. Compared with the control group, *P < 0.05. AgNPs, silver nanoparticles; PVP, polyvinylpyrrolidone; HPMC, hydroxypropyl methyl cellulose.
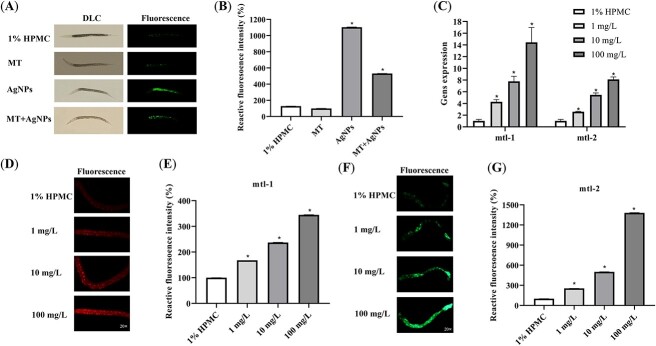
The effects of MT on oxidative stress induced by AgNPs
After exposure to 20 nm PVP-AgNPs for 48 h, the expression levels of mtl-1 and mtl-2 in the nematodes were detected by qRT-PCR. The mRNA expression levels of mtl-1 and mtl-2 in nematodes in each dose group of AgNPs showed a significant increase with the increase of dose (Fig. 6C). The CF2222 and CL2120 nematode strains were photographed by an inverted fluorescence microscope (Fig. 6D–G). Compared with the control group, the fluorescence intensity of nematodes in each dose group of AgNPs increased with the dose significantly, indicating that the expression levels of mtl-1 and mtl-2 in the nematodes were elevated by AgNPs exposure.
Fig. 6.
Effects of 20 nm PVP-AgNPs and MT on ROS (A) and the relative fluorescence intensity of ROS (B) in C. elegans after 48 h exposure (10×). The influence of the expression of mRNA of mtl-1 and mtl-2 induced by 20 nm PVP-AgNPs in C. elegans (C). Effects of 20 nm PVP-AgNPs in the expression of fluorescence of mtl-1 (D, E) in CF2222 nematode strain (mtl-1::RFP) after 48 h exposure (20×). Effects of 20 nm PVP-AgNPs in the expression of fluorescence of mtl-2 (F, G) in CL2120 nematode strain (mtl-2::GFP) after 48 h exposure (20×). Experimental data were represented by mean ± SD in three parallel experiments. Compared with the control group, *P < 0.05. AgNPs, silver nanoparticles; PVP, polyvinylpyrrolidone; HPMC, hydroxypropyl methyl cellulose.
As shown in Fig. 6A and B, the levels of ROS in nematodes in the 20 nm PVP-AgNPs group were significantly increased, and the levels of ROS in the nematodes in the MT + 20 nm PVP-AgNPs group were lower than that in the 20 nm PVP-AgNPs group, indicating that MT may be involved in alleviating the oxidative stress induced by AgNPs in nematodes.
Discussion
With the widespread use of AgNPs in commercial and industrial applications, the potential for human exposure to AgNPs in the environment is also increasing. Various factors such as sizes, surface coatings, charge and agglomeration are known to affect the toxicity of AgNPs.24C. elegans is the first animal to have its genome sequenced with 12 of the 17 known human signaling pathways.25 Due to its high sensitivity to environmental toxicants, C. elegans is also a suitable model for evaluating the safety and toxicity of NPs.9 In this study, we investigated comparatively the toxic effects and oxidative stress induced by three kinds of AgNPs with different sizes and coatings using C. elegans. Three AgNPs had different effects on the lifespan, head thrashes and body bends in C. elegans. The toxic effects of the three kinds of AgNPs on nematodes could be ranked as 20 nm PVP-AgNPs >20 nm AgNPs >50 nm AgNPs. Based on the characterization of AgNPs, the hydrated particle size of 20 nm PVP-AgNPs was the smallest and the absolute value of Zeta potential was the largest, which meant that PVP-coated AgNPs were more stable and less aggregated than the uncoated AgNPs. Our previous studies have found that 20 nm PVP-AgNPs were more cytotoxic than 20 nm AgNPs and were more likely to cause chromosomal aberrations in cells and animals.21 At the same dose, 20 nm PVP-AgNPs had higher oxidative stress and subacute toxicity than 20 nm AgNPs in mice.7 Therefore, the particle size and surface chemistry, as well as ion release, should be considered at least in part as potential factors in assessing the safety of metal nanomaterials.
In this study, AgNPs were involved in oxidative stress and ROS production in nematodes. 20 nm PVP-AgNPs caused the highest levels of oxidative stress in nematodes, resulting in the highest levels of ROS production in nematodes. The additional ROS production process by AgNPs may be a respiratory burst caused by NADPH oxidase activity, which is biologically related to the elimination of pathogens and foreign particles.26 AgNPs can deplete antioxidants such as GSH and inhibit antioxidant enzymes by reacting with the key thiol groups of proteins. The study by Piao et al. also showed that AgNPs can induce ROS production in human liver cells and depletion of intracellular GSH and lead to damage to cellular components.27 GSH is the main antioxidant in cells, which protects the body from damage caused by oxidative stress by scavenging free radicals and hydrogen peroxide. In this study, the decrease of GSH contents may be due to the excessive free radicals in nematodes, which exceed the body’s compensatory ability, resulting in the increased consumption and decreased GSH contents. MDA is the most important end product of lipid peroxidation. The increase in MDA contents is due to an increase in lipid metabolites in the body due to oxidative stress in nematodes. The study by Hassanen et al. also demonstrated that after rats were exposed to different concentrations of chitosan-coated AgNPs for 14 days, a dose of 50 mg/kg resulted in a significant increase in MDA levels and a significant decrease in GSH contents in different organs (liver, kidneys, and spleen).28 Many physiological and cellular events are caused by oxidative stress, including inflammation, DNA damage, and apoptosis. Current research on oxidative stress-related signaling pathways caused by AgNPs has identified endogenous ROS generators, including mitochondrial respiration, inflammatory response, microsomes and peroxisomes. The ROS generated interfere with or induce activation of the mitogen-activated protein kinase (MAPK) pathway.29 The oxidative stress-related signaling pathway induced by AgNPs is also associated with the activation of NF-E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1).30 A further study is needed to elucidate the oxidative stress-related signaling pathways induced by different kinds of AgNPs.
In order to explore the mechanisms of oxidative stress damage caused by AgNPs, we further studied the effects of AgNPs on the expression of oxidative stress-related genes in nematodes. When the body’s antioxidant capacity is exceeded, the antioxidant genes sod-1, sod-2, and sod-3 are down-regulated. The nematode genome contains a tandem array of three catalase (ctl) genes, which have a high sequence similarity. Among them, ctl-1 is expressed in the cytoplasm, and ctl-2 is a peroxisome ctl, accounting for 80% of the total cell ctl activity.13 Ctl-1, ctl-2, ctl-3 have catalase activity in vitro, so they may act as antioxidant enzymes in vivo to protect cells from ROS.31 The isp-1 gene encodes iron-sulfur protein (ISP), a subunit of mitochondrial complex III in the mitochondrial membrane.32 Clk-1 encodes coenzyme Q7/catalase 5 (COQ7/CAT5) and plays an important role in the normal growth, development, behavior and aging in nematodes.33 Mev-1 is an integral membrane protein that encodes the nematode succinate dehydrogenase cytochrome b subunit and is required for oxidative phosphorylation. Mev-1 is homologous to human succinate dehydrogenase cytochrome b isoform 1.34 In this study, the mRNA expression levels of ctl-1, ctl-2, ctl-3, isp-1, clk-1 and mev-1 were up-regulated. These results indicated that the activity of antioxidant enzymes and the synthesis of ISP in mitochondria increased after AgNPs exposure, in response to oxidative damage caused by AgNPs. The gas-1 gene is a mitochondrial complex encoding a 49 kDa subunit. It is an essential gene in the process of oxidative phosphorylation. It is expressed in the neuromuscular system and is involved in the regulation of the reproductive system and normal lifespan.35,36 The gas-1 mRNA expression levels were significantly down-regulated, which was consistent with the longevity results of nematodes and the results of head thrashes and body bends. The results suggested that AgNPs could damage the nematode lifespan and motor neurons.
MT is a highly conserved family of small, cysteine-rich metal-binding proteins. Compared to the traditional antioxidants, biological functions of MT include an effective regulation of metal ion metabolism, strong antioxidant capacity, scavenging of oxygen free radicals, and binding of non-essential metals for a removal. They transiently bind monovalent and divalent essential trace metals such as zinc (Zn), copper (Cu) and manganese (Mn), as well as non-essential metals such as cadmium (Cd) and mercury (Hg). Mammals have four distinct MT isoforms, of which mtl-1 and mtl-2 are the most sensitive to heavy metals. Unlike mammals, C. elegans has only two identified MT isoforms, mtl-1 and mtl-2. Mtl-1 and mtl-2 encode cysteine-rich metal-binding proteins that play important roles in metal detoxification, homeostasis, and stress adaptation. In the present study, exposure to AgNPs resulted in up-regulation of mRNA expression levels of mtl-1 and mtl-2 and increased the synthesis of MT, which may have been involved in attenuating oxidative damage to nematodes by AgNPs. Meanwhile, intervention using MT on the 100 mg/L 20 nm PVP-AgNPs exposed group found that the MT treatment could reduce ROS levels in nematodes. This effect may be related to MT’s ability to inhibit excessive free radicals in the body and improve the ability to scavenge free radicals. The study by Meyer et al. also found that the mtl-2 deletion nematodes (Strain JF23) were more susceptible to AgNPs-induced growth inhibition than the Wild-type.37 However, at present, there are few studies on exogenous MT, and its comparison with traditional antioxidants also needs further research.
Conclusion
In conclusion, this study was designed to investigate the toxic effects and oxidative damage comparatively in C. elegans by exposing to three types of AgNPs with different sizes and coatings. We found that the exposure AgNPs induced toxic effects and oxidative stress in nematodes in a dose-dependent manner. The particle sizes and surface coatings could significantly affect the toxicity of AgNPs. The 20 nm PVP-AgNPs with the smaller particle size and better dispersion had the greatest damage to the C. elegans lifespan, head thrashes and body bends. AgNPs increased the production of ROS and MDA and decreased the contents of GSH in nematodes. These effects were the most obvious in the 20 nm PVP-AgNPs exposure group. AgNPs could also induce the expression of genes related to oxidative stress in nematodes. Furthermore, mtl-1 and mtl-2 might be involved in the oxidative damage caused by AgNPs in nematodes. Metallothionein, an antioxidant, could relieve the oxidative damage caused by AgNPs at least partially. Our findings provide clues for the safety evaluation and mechanism information of metal nanoparticles.
Author contribution
Shuyan Niu (Conceptualization, Software, Validation, Data Curation, Writing—original draft preparation), Junjun Wang (Conceptualization, Investigation, Formal Analysis, Methodology), Xiaoru Chang (Writing—review & editing), Mengting Shang (Writing—review & editing), Menghao Guo (Writing—review & editing), Zuoyi Sun (Methodology, Resources), Yunjing Li (Methodology, Resources), and Yuying Xue (Conceptualization, Supervision, Project Administration, Funding Acquisition, Writing—review & editing)
Funding
This work was supported by the National Natural Science Foundation of China (NSFC Grant 82273674), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_0165).
Conflict of interest statement. The authors declare that there are no conflicts of interest.
Data availability
Data available on request from the authors.
Contributor Information
Shuyan Niu, Key Laboratory of Environmental Medicine and Engineering, Ministry of Education, School of Public Health, Southeast University, 87 Dingjiaqiao, Nanjing 210009, China.
Junjun Wang, Key Laboratory of Environmental Medicine and Engineering, Ministry of Education, School of Public Health, Southeast University, 87 Dingjiaqiao, Nanjing 210009, China.
Xiaoru Chang, Key Laboratory of Environmental Medicine and Engineering, Ministry of Education, School of Public Health, Southeast University, 87 Dingjiaqiao, Nanjing 210009, China.
Mengting Shang, Key Laboratory of Environmental Medicine and Engineering, Ministry of Education, School of Public Health, Southeast University, 87 Dingjiaqiao, Nanjing 210009, China.
Menghao Guo, Key Laboratory of Environmental Medicine and Engineering, Ministry of Education, School of Public Health, Southeast University, 87 Dingjiaqiao, Nanjing 210009, China.
Zuoyi Sun, Key Laboratory of Environmental Medicine and Engineering, Ministry of Education, School of Public Health, Southeast University, 87 Dingjiaqiao, Nanjing 210009, China.
Yunjing Li, Key Laboratory of Environmental Medicine and Engineering, Ministry of Education, School of Public Health, Southeast University, 87 Dingjiaqiao, Nanjing 210009, China.
Yuying Xue, Key Laboratory of Environmental Medicine and Engineering, Ministry of Education, School of Public Health, Southeast University, 87 Dingjiaqiao, Nanjing 210009, China.
References
- 1. Qamer S, Romli MH, Che-Hamzah F, Misni N, Joseph N, al-Haj NA, Amin-Nordin S. Systematic review on biosynthesis of silver nanoparticles and antibacterial activities: application and theoretical perspectives. Molecules. 2021:26(16):5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MJ, Rejeski D, Hull MS. Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 2015:6:1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gottschalk F, Sonderer T, Scholz RW, Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO(2), ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol. 2009:43(24):9216–9222. [DOI] [PubMed] [Google Scholar]
- 4. Bergin IL, Wilding LA, Morishita M, Walacavage K, Ault AP, Axson JL, Stark DI, Hashway SA, Capracotta SS, Leroueil PR, et al. Effects of particle size and coating on toxicologic parameters, fecal elimination kinetics and tissue distribution of acutely ingested silver nanoparticles in a mouse model. Nanotoxicology. 2016:10(3):352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang W, Wang L, Mettenbrink EM, DeAngelis PL, Wilhelm S. Nanoparticle toxicology. Annu Rev Pharmacol Toxicol. 2021:61(1):269–289. [DOI] [PubMed] [Google Scholar]
- 6. Jo MS, Kim JK, Kim Y, Kim HP, Kim HS, Ahn K, Lee JH, Faustman EM, Gulumian M, Kelman B, et al. Mode of silver clearance following 28-day inhalation exposure to silver nanoparticles determined from lung burden assessment including post-exposure observation periods. Arch Toxicol. 2020:94(3):773–784. [DOI] [PubMed] [Google Scholar]
- 7. Gan J, Sun J, Chang X, Li W, Li J, Niu S, Kong L, Zhang T, Wu T, Tang M, et al. Biodistribution and organ oxidative damage following 28 days oral administration of nanosilver with/without coating in mice. J Appl Toxicol. 2020:40(6):815–831. [DOI] [PubMed] [Google Scholar]
- 8. Pavičić I, Milić M, Pongrac IM, Brkić Ahmed L, Matijević Glavan T, Ilić K, Zapletal E, Ćurlin M, Mitrečić D, Vinković Vrček I. Neurotoxicity of silver nanoparticles stabilized with different coating agents: in vitro response of neuronal precursor cells. Food Chem Toxicol. 2020:136:110935. [DOI] [PubMed] [Google Scholar]
- 9. Wu T, Xu H, Liang X, Tang M. Caenorhabditis elegans as a complete model organism for biosafety assessments of nanoparticles. Chemosphere. 2019:221:708–726. [DOI] [PubMed] [Google Scholar]
- 10. Yanase S, Ishii N. Hyperoxia exposure induced hormesis decreases mitochondrial superoxide radical levels via Ins/IGF-1 signaling pathway in a long-lived age-1 mutant of Caenorhabditis elegans. J Radiat Res. 2008:49(3):211–218. [DOI] [PubMed] [Google Scholar]
- 11. Sinis SI, Gourgoulianis KI, Hatzoglou C, Zarogiannis SG. Mechanisms of engineered nanoparticle induced neurotoxicity in Caenorhabditis elegans. Environ Toxicol Pharmacol. 2019:67:29–34. [DOI] [PubMed] [Google Scholar]
- 12. Hall J, Haas KL, Freedman JH. Role of MTL-1, MTL-2, and CDR-1 in mediating cadmium sensitivity in Caenorhabditis elegans. Toxicol Sci. 2012:128(2):418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braeckman BP, Smolders A, Back P, De Henau S. In vivo detection of reactive oxygen species and redox status in Caenorhabditis elegans. Antioxid Redox Signal. 2016:25(10):577–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Talarska P, Boruczkowski M, Zurawski J. Current knowledge of silver and gold nanoparticles in laboratory research-application, toxicity, cellular uptake. Nanomaterials (Basel). 2021:11(9):2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raza MA, Kanwal Z, Rauf A, Sabri AN, Riaz S, Naseem S. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials (Basel). 2016:6(4):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhornik A, Baranova L, Volotovski I, Chizhik S, Drozd E, Sudas M, Buu Ngo Q, Chau Nguyen H, Huynh TH, Hien Dao T. Interaction of nanosilver particles with human lymphocyte cells. Adv Nat Sci Nanosci Nanotechnol. 2015:6(2):025003. [Google Scholar]
- 17. Nallanthighal S, Tierney L, Cady NC, Murray TM, Chittur SV, Reliene R. Surface coatings alter transcriptional responses to silver nanoparticles following oral exposure. NanoImpact. 2020:17:100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He Q, Lu J, Liu N, Lu W, Li Y, Shang C, Li X, Hu L, Jiang G. Antiviral properties of silver nanoparticles against SARS-CoV-2: effects of surface coating and particle size. Nanomaterials (Basel). 2022:12(6):990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Zhu S, Liu L, Li L. Flexible antibacterial film based on conjugated polyelectrolyte/silver nanocomposites. ACS Appl Mater Interfaces. 2017:9(10):9051–9058. [DOI] [PubMed] [Google Scholar]
- 20. Glinski A, Lima de Souza T, Zablocki da Luz J, Bezerra Junior AG, Camargo de Oliveira C, de Oliveira Ribeiro CA, Filipak Neto F. Toxicological effects of silver nanoparticles and cadmium chloride in macrophage cell line (RAW 264.7): An in vitro approach. J Trace Elem Med Biol. 2021:68:126854. [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Li T, Su X, Li J, Li W, Gan J, Wu T, Kong L, Zhang T, Tang M, et al. Genotoxic effects of silver nanoparticles with/without coating in human liver HepG2 cells and in mice. J Appl Toxicol. 2019:39(6):908–918. [DOI] [PubMed] [Google Scholar]
- 22. Radhakrishnan VS, Dwivedi SP, Siddiqui MH, Prasad T. In vitro studies on oxidative stress-independent, Ag nanoparticles-induced cell toxicity of Candida albicans, an opportunistic pathogen. Int J Nanomedicine. 2018:13(T-NANO 2014 Abstracts):91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopez-Alonso M, Benedito JL, Garcia-Vaquero M, Hernandez J, Miranda M. The involvement of metallothionein in hepatic and renal Cd, Cu and Zn accumulation in pigs. Livest Sci. 2012:150(1-3):152–158. [Google Scholar]
- 24. Moon J, Kwak JI, An YJ. The effects of silver nanomaterial shape and size on toxicity to Caenorhabditis elegans in soil media. Chemosphere. 2019:215:50–56. [DOI] [PubMed] [Google Scholar]
- 25. Hunt PR. The C. elegans model in toxicity testing. J Appl Toxicol. 2017:37(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pal R, Chakraborty B, Nath A, Singh LM, Ali M, Rahman DS, Ghosh SK, Basu A, Bhattacharya S, Baral R, et al. Noble metal nanoparticle-induced oxidative stress modulates tumor associated macrophages (TAMs) from an M2 to M1 phenotype: An in vitro approach. Int Immunopharmacol. 2016:38:332–341. [DOI] [PubMed] [Google Scholar]
- 27. Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, Choi J, Hyun JW. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett. 2011:201(1):92–100. [DOI] [PubMed] [Google Scholar]
- 28. Hassanen EI, Khalaf AA, Tohamy AF, Mohammed ER, Farroh KY. Toxicopathological and immunological studies on different concentrations of chitosan-coated silver nanoparticles in rats. Int J Nanomedicine. 2019:14:4723–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suthar JK, Vaidya A, Ravindran S. Toxic implications of silver nanoparticles on the central nervous system: a systematic literature review. J Appl Toxicol. 2023:43(1):4–21. [DOI] [PubMed] [Google Scholar]
- 30. Kang SJ, Ryoo IG, Lee YJ, Kwak MK. Role of the Nrf2-heme oxygenase-1 pathway in silver nanoparticle-mediated cytotoxicity. Toxicol Appl Pharmacol. 2012:258(1):89–98. [DOI] [PubMed] [Google Scholar]
- 31. Petriv OI, Rachubinski RA. Lack of peroxisomal catalase causes a progeric phenotype in Caenorhabditis elegans. J Biol Chem. 2004:279(19):19996–20001. [DOI] [PubMed] [Google Scholar]
- 32. Zhang J, Xue X, Yang Y, Ma W, Han Y, Qin X. Multiple biological defects caused by calycosin‐7‐O‐β‐d‐glucoside in the nematode Caenorhabditis elegans are associated with the activation of oxidative damage. J Appl Toxicol. 2018:38(6):801–809. [DOI] [PubMed] [Google Scholar]
- 33. Branicky R, Nguyen PA, Hekimi S. Uncoupling the pleiotropic phenotypes of clk-1 with tRNA missense suppressors in Caenorhabditis elegans. Mol Cell Biol. 2006:26(10):3976–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dancy BM, Sedensky MM, Morgan PG. Mitochondrial bioenergetics and disease in Caenorhabditis elegans. Front Biosci (Landmark Ed). 2015:20(2):198–228. [DOI] [PubMed] [Google Scholar]
- 35. Falk MJ, Kayser EB, Morgan PG, Sedensky MM. Mitochondrial complex I function modulates volatile anesthetic sensitivity in C. elegans. Curr Biol. 2006:16(16):1641–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kayser EB, Morgan PG, Hoppel CL, Sedensky MM. Mitochondrial expression and function of GAS-1 in Caenorhabditis elegans. J Biol Chem. 2001:276(23):20551–20558. [DOI] [PubMed] [Google Scholar]
- 37. Meyer JN, Lord CA, Yang XY, Turner EA, Badireddy AR, Marinakos SM, Chilkoti A, Wiesner MR, Auffan M. Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans. Aquat Toxicol. 2010:100(2):140–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.