Abstract
Borna disease virus (BDV) is a nonsegmented, negative-stranded RNA virus that causes neurological diseases in a variety of warm-blooded animal species. Recently, we showed that the nucleoside analog 1-β-d-arabinofuranosylcytosine (Ara-C) was a potent inhibitor of BDV. This finding was surprising for an RNA virus, since Ara-C is a DNA polymerase inhibitor. Thus, we sought to better define the mechanism of action of Ara-C on BDV. Here, we show that (i) this effect is specific for an arabinoside ring carrying a cytosine base, (ii) it requires phosphorylation of the nucleotide, and (iii) it can be reversed by an excess of cytidine. Using the recently described minigenome assay for BDV, we provide evidence suggesting that Ara-C may act as a competitive inhibitor of the BDV replication complex.
Borna disease virus (BDV) is a nonsegmented, negative-stranded (NNS) RNA virus belonging to the family Bornaviridae in the order Mononegavirales (7, 12). BDV is noncytolytic and highly neuronotropic (16). It replicates and transcribes its genome in the nucleus of the cell, a property unique among animal Mononegavirales (9).
BDV persists in the central nervous systems of infected animals, causing a broad range of neurological symptoms (13, 22, 27). The infection was initially described in horses and sheep as a nonpurulent, often fatal meningoencephalomyelitis (13, 22, 27). Infection can also become chronic, leading to persistent behavioral abnormalities (22, 27). Although most cases described have occurred in Central Europe, BDV seems to have a worldwide distribution (22). Recent data indicate that the natural host range of BDV is much broader than previously thought (22, 31). There is clear evidence that BDV can infect humans, although controversy still remains about the epidemiology and clinical consequences of human BDV infection (19, 26).
The importance of BDV in veterinary medicine and its possible implication as a human pathogen has stimulated many groups to search for a treatment for BDV infections. Amantadine was reported to have an effect against BDV (4), but other studies failed to confirm this result (8, 17, 32). Differences in susceptibility to amantadine may be related to the viral strain used, and thus the efficacy of amantadine awaits confirmation from more-comprehensive studies (5). The broad-spectrum antiviral ribonucleotide analog ribavirin proved to have an effect on BDV in vitro, decreasing both viral particle production and viral RNA levels (20, 23). However, the activity of ribavirin was modest, and the clinical benefits found in an in vivo trial turned out to be due to an indirect effect of ribavirin on the proliferation of microglial cells (30). Recently, we reported that the nucleoside analog 1-β-d-arabinofuranosylcytosine (Ara-C) possesses potent activity against BDV both in vitro and in vivo (1).
Our findings demonstrating an effect of Ara-C against BDV were unexpected, because Ara-C is a well-known specific inhibitor of viral and cellular DNA polymerases (14). It was surprising to find that it could inhibit BDV, a negative-stranded RNA virus that synthesizes only RNA. In addition, the effect against BDV could not be attributed to any known effect of Ara-C on the host cell (1). Therefore, we sought to elucidate the mechanism of action of Ara-C on BDV.
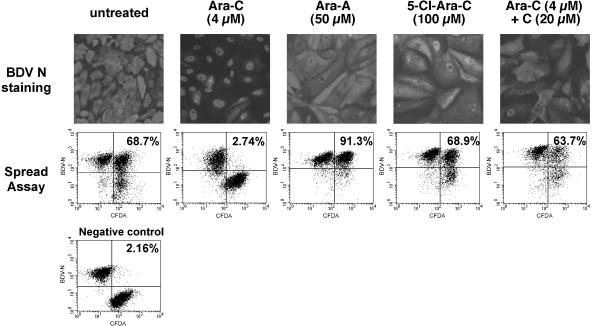
In order to test the inhibitory actions of various compounds, we used two previously described methods, allowing the assessment of BDV inhibition under different experimental conditions. The first method consists of analysis of the subcellular localization of BDV nucleoprotein (N) by immunofluorescence (Fig. 1). In persistently infected Vero cells (Vero-BV cells), BDV N is present both in the nucleus and in the cytoplasm. We showed previously that inhibition of BDV by Ara-C is accompanied by a nuclear relocalization of BDV N (1). This shift in the subcellular localization of BDV N correlates well with inhibition of BDV, and we therefore used this qualitative approach as a first criterion to determine the antiviral activities of the different compounds. The second method (1) is based on the analysis of virus spread between infected and noninfected Vero cells by using flow cytometry (fluorescence-activated cell sorting [FACS]). BDV is tightly cell associated (15), and efficient transmission requires close cell-to-cell contact. Virus titers in the supernatants of persistently infected cells are extremely low. Thus, the cell spread assay appears more relevant to the biology of BDV, and we have shown that it is better suited for quantitating viral inhibition, allowing a better comparison of BDV inhibition following different treatments (Fig. 1; Table 1). For both types of experiments, treatment was carried out daily at the indicated doses for a period of 5 days.
FIG. 1.
Representative examples of the inhibitory effects of compounds used in this study. (Top panels) Analysis by immunofluorescence of the subcellular localization of the BDV nucleocapsid protein (N) following treatment with different compounds. Vero-BV cells were treated for 5 days with the different compounds and stained with a rabbit anti-N polyclonal antibody, followed by a fluorescein isothiocyanate-conjugated anti-rabbit antibody. Of all compounds tested (only examples are shown here), only Ara-C resulted in nuclear retention of BDV N protein. Magnification, ×120 (original magnification, ×200). (Bottom panels) FACS analysis of BDV cell-to-cell spread. Confluent Vero cells were labeled with 5- (and 6-)carboxyfluorescein diacetate (CFDA) and were subsequently cocultivated for 5 days at a ratio of 1:1 with unlabeled Vero-BV cells. Cocultivation took place under daily treatment with 4 μM Ara-C or with various drugs (e.g., 50 μM Ara-A). Thereafter, cells were analyzed by flow cytometry. The percentage of viral dissemination during the cocultivation period was calculated as indicated in Table 1 and is shown in each case. Note that inhibition of BDV cell-to-cell spread is specific to Ara-C. The negative control consisted of a 1:1 mixture of CFDA-labeled Vero cells with Vero-BV cells, which were fixed directly after mixing.
TABLE 1.
Results of FACS analysis of BDV cell-to-cell spread assays following treatment with different compounds
| Treatment(concn [μM]) | % of viral disseminationa |
|---|---|
| None | 100 |
| Ara-C (4) | 7.4 ± 1.6 |
| Ara-A (100) | 142.9 ± 10.9 |
| 5-Cl-Ara-C (100) | 97.0 ± 4.1 |
| Ara-U (100) | 127.1 ± 5.8 |
| Ara-H (100) | 150.2 ± 16.6 |
| 5′-Deoxy-Ara-C (100) | 105.7 ± 6.0 |
| Ara-C (4) + C (20) | 72.3 ± 12.1 |
| Ara-C (4) + dC (20) | 97.1 ± 2.6 |
Calculated by using the following formula: (number of double-positive cells/total number of cells positive for carboxyfluorescein diacetate) × 100. To allow comparison between experiments, percentages of viral dissemination were normalized to the value obtained for the untreated cell control in each experiment. Each value given is the mean for three independent experiments ± the standard error of the mean.
We first tested if other nucleoside analogs with an arabinose sugar ring, like Ara-C, were able to inhibit BDV. We used 9-β-d-arabinofuranosyladenine (Ara-A), 1-β-d-arabinofuranosyluracil (Ara-U), and 9-β-d-arabinofuranosylhypoxanthine (Ara-H). Hypoxanthine is an intermediate compound in the synthesis of purinic bases. We also used 5-chloro-1-β-d-arabinofuranosylcytosine (5-Cl-Ara-C), a modified Ara-C carrying an atom of chlorine at position 5 of the cytosine base. Immunofluorescence analysis revealed that none of these compounds, even at high concentrations, was able to induce nuclear retention of BDV N comparable to that seen after treatment with Ara-C (Fig. 1 and data not shown). With Ara-A and 5-Cl-Ara-C, two compounds known to inhibit cellular DNA polymerases, cytotoxicity was clearly visible and could be appreciated by changes in cell morphology and cell density. This indicates that the lack of effect against BDV is not due to poor cellular uptake or metabolism, or to intracellular degradation. Nevertheless, we cannot totally exclude the possibility that differences in the pharmacokinetics of the different compounds may partly explain the results. However, this pharmacokinetic difference is likely to be minor, since all compounds were used over a large range of concentrations, exceeding 20- to 40-fold the optimal concentration of Ara-C against BDV, and we never noted any inhibition of BDV, except by Ara-C. In agreement with data from immunofluorescence studies, FACS analysis of virus spread also revealed that, of all these compounds, only Ara-C was able to inhibit BDV spread (Fig. 1 and Table 1; also data not shown). Interestingly, we observed that treatment with Ara-A, Ara-H, or Ara-U even increased viral spread relative to that among untreated cells. Since these compounds are cytostatic, this result could be related to the fact that BDV replication has been shown to be enhanced after cell growth arrest (24). Taken together, these data show that the antiviral effect is specific for an arabinose sugar ring carrying the nitrogen base cytosine.
To exert their antiviral effects, most nucleoside analogs must be phosphorylated. Thus, we investigated whether this requirement also applied to the inhibition of BDV by Ara-C. For this purpose, we synthesized a modified Ara-C, 5′-deoxy-1-β-d-arabinofuranosylcytosine (5′-deoxy-Ara-C) (18), that cannot be phosphorylated because of the absence of a 5′ hydroxyl group. Treatment of cells with 5′-deoxy-Ara-C had no effect on the localization of BDV N (data not shown) and did not inhibit viral spread (Table 1). These results demonstrate that Ara-C must be phosphorylated in order to inhibit BDV.
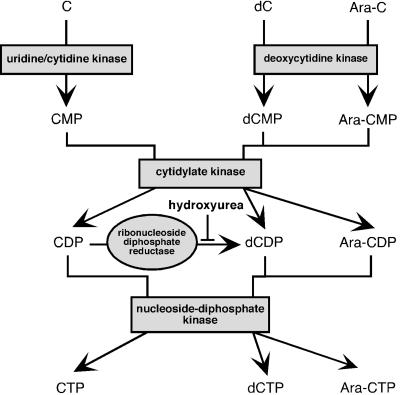
We then wondered if the antiviral effect of Ara-C could be reversed by adding cytidine (C). In cells treated with 4 μM Ara-C together with an excess of C (20 μM), BDV N was detected by immunofluorescence in both the nucleus and the cytoplasm (Fig. 1). In addition, FACS analysis showed a partial recovery of virus spread (Fig. 1 and Table 1). The cytotoxic effect of Ara-C was still visible when cytidine was added simultaneously, indicating that Ara-C could still be phosphorylated into Ara-CTP. These data suggest that cytidine is able to specifically reverse the antiviral effect of Ara-C and that the reversion involves competition between Ara-CTP and CTP, possibly at the level of the viral RNA polymerase. Since the cytotoxic effects of Ara-C were still clearly apparent, it is unlikely that the partial reversion mediated by simultaneous addition of C is a consequence of reversion of the genotoxic effects of Ara-C mediated by C. In addition, to make sure that the reversion seen when C was added was not a consequence of the transformation of CDP into dCDP by ribonucleotide diphosphate reductase (Fig. 2), we blocked this enzyme with hydroxyurea, a specific inhibitor (3). However, reversion of the antiviral effect of Ara-C still occurred when cytidine was added together with hydroxyurea (data not shown), confirming that reversion of the anti-BDV effect of Ara-C is indeed due to cytidine. Interestingly, reversion of both the antiviral and the cytotoxic effect of Ara-C occurred when cells were treated simultaneously with Ara-C (4 μM) and 2′-deoxycytidine (dC) (20 μM). This suggests that, in the case of dC, the reversion of the antiviral effect occurs at the initial step of Ara-C phosphorylation, probably by competition between Ara-C and dC for deoxycytidine kinase (Fig. 2), the enzyme catalyzing the rate-limiting step in the phosphorylation of Ara-C (3).
FIG. 2.
Metabolic phosphorylation pathways of cytidine and its derivatives. The mode of action of hydroxyurea is shown.
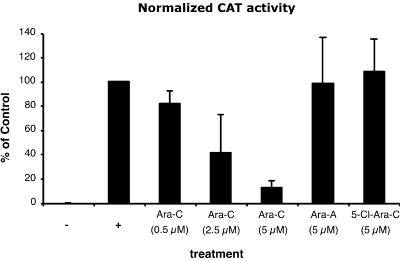
The results described above strongly suggest that the inhibitory effect of Ara-C on BDV is due to inhibition of the viral replication complex. The recent description of a functional assay for BDV polymerase complex, based on a chloramphenicol acetyltransferase (CAT)-expressing BDV RNA analog (or minigenome), has opened the way for assessing the effect of Ara-C on the BDV polymerase complex (25, 29). The BDV polymerase complex was reconstituted as described previously (29), by transfecting BSR-T7 cells with optimal quantities of plasmids expressing BDV proteins (L, N, and P) together with the BDV minigenome construct. Furthermore, we cotransfected a plasmid encoding Renilla luciferase under the control of a polymerase II promoter to normalize the CAT signal for transfection efficiency. Cells either were left untreated (positive control) or were treated with increasing doses of Ara-C or other nucleoside analogs, starting at 6 h before transfection. Three days after transfection, the cells were lysed and analyzed for CAT protein levels and luciferase activity by an enzyme-linked immunosorbent assay (ELISA) and luciferase-mediated light emission, respectively. CAT values were then normalized for transfection efficacy (Fig. 3). When cells were treated with Ara-C, we observed a dose-dependent reduction of CAT levels, dropping to <10% of those with the untreated control for a 5 μM concentration of Ara-C. In contrast, treatment with Ara-A or 5-Cl-Ara-C had no effect on the minigenome-driven expression of CAT protein. Thus, these results provide evidence suggesting that Ara-C inhibits BDV polymerase activity directly. Nevertheless, we cannot exclude the possibility that the expression of some yet unknown cellular factor(s) necessary for BDV replication may be modified by Ara-C and may contribute to the phenomenon observed. However, the fact that BDV is the only RNA virus that has been shown to exhibit sensitivity to Ara-C suggests that the effect is rather specific and is not due to a general deregulation of gene expression (1). Given our limited knowledge about the structure and function of the BDV polymerase complex, additional work will be needed to definitely prove that Ara-C indeed has a direct effect on this complex.
FIG. 3.
Dose-dependent, inhibitory effect of Ara-C in the BDV minireplicon assay. Analysis of reporter gene expression by a CAT ELISA. BSR-T7 cells (treated or not with the different compounds) were transfected with the minigenome construct together with optimal quantities of the expression plasmids for the BDV L, N, and P proteins. In the negative control (−), the plasmid encoding BDV L was replaced by the same amount of a plasmid encoding green fluorescent protein. The positive control (+) consisted of untreated cells and was set at 100% in each experiment. For an internal control for transfection efficiency, a plasmid encoding Renilla luciferase under the control of a polymerase II promoter was cotransfected. Seventy-two hours after transfection, the cells were lysed and analyzed for CAT protein and luciferase levels. CAT values were normalized for transfection efficiency and are expressed for each sample as the percentage of the value with the untreated control. Data shown are means (± standard deviations) for three independent experiments.
The results of this study, which was designed to elucidate the mechanism of the action of the nucleoside analog Ara-C on BDV, can be summarized as follows. (i) The effect against BDV is specific for an arabinoside carrying the nitrogen base cytosine and requires phosphorylation of the nucleoside. (ii) The inhibition of BDV by Ara-C can be reversed by treatment with an excess of cytidine. (iii) Using the recently developed BDV minigenome assay, we provide evidence suggesting a direct effect of Ara-C on the BDV polymerase complex. Taken together, these results show that Ara-C likely acts as a competitive inhibitor of the BDV polymerase complex. To our knowledge, this is the first indication that Ara-C, a well-known inhibitor of DNA polymerases, may inhibit an RNA-dependent RNA polymerase.
This possible sensitivity of the BDV polymerase complex to Ara-C raises interesting questions about the substrate selectivity of the BDV polymerase (L) and led us to suggest possible explanations, which are set forth below. Sequence comparisons between various RNA polymerases, including those of Mononegavirales (L family), have been reported. Four conserved motifs, designated A, B, C, and D, have been described and are also conserved within all RNA-dependent polymerases of eukaryotes. These motifs define a “polymerase module,” and two of them are also present in monomeric DNA-dependent polymerases (11, 28). The crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus has revealed that motif A is implicated in the binding of the incoming nucleoside triphosphate (NTP) (6). This motif A contains an invariant aspartate residue, present in all RNA polymerases, as well as an invariant lysine residue found in motif A of the polymerases of all NNS RNA viruses. Site-directed mutagenesis studies performed on human immunodeficiency virus type 1 reverse transcriptase have shown that mutation of the invariant aspartate residue completely destroys polymerase activity (21). For BDV L, this aspartate residue is also conserved. However, in BDV L, a serine residue is found in place of the invariant lysine residue present for all other negative-stranded RNA viruses (10). Thus, it is possible that this change might modify the selectivity of BDV L to nucleoside analogs and in particular to Ara-C. This provides a working hypothesis to explain why BDV is the only NNS RNA virus shown to date to be sensitive to Ara-C. Now that it is becoming possible to test the effect of mutations of the BDV polymerase complex by using the minigenome assay, it will be of interest in the future to assess the effects of mutations in BDV L, in particular in domain A, implicated in NTP selection and binding, on its sensitivity to Ara-C.
Given the importance of BDV in veterinary medicine and its possible involvement in the etiology of human behavioral diseases, the development of effective therapy against this infectious agent is needed. Despite its efficacy in vitro and in vivo, the toxicity of Ara-C poses an obstacle to its therapeutic use. Searching for a less toxic antiviral agent, we recently showed that the nucleoside analog 2′-fluoro-2′-deoxycytidine (2′-FdC), structurally close to Ara-C, is an attractive candidate (2). A better understanding of the mechanism of action of these nucleoside analogs on the BDV replication complex, such as that shown in this study, will contribute to the development of new therapeutic molecules aimed at the efficient control of BDV infections.
Acknowledgments
We thank Michel Brahic for continuous support and helpful discussions. We also thank Roland Liblau, Abdelhadi Saoudi, and Céline Monnet-Mars for critical reading of the manuscript.
This work was supported by the INSERM Avenir program and by grants from the Institut Pasteur and the CNRS. R.V. is a recipient of a doctoral fellowship from the Ministère de l'Education Nationale et de l'Enseignement Supérieur. J.J.B. is a recipient of a Marie Curie fellowship from the European Community program “Improving Human Research Potential and the Socio-economic Knowledge Base” (contract HPMF-CT-2000-01088).
REFERENCES
- 1.Bajramovic, J. J., S. Syan, M. Brahic, J. C. De La Torre, and D. Gonzalez-Dunia. 2002. 1-β-d-Arabinofuranosylcytosine inhibits Borna disease virus replication and spread. J. Virol. 76:6268-6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajramovic, J. J., R. Volmer, S. Syan, S. Pochet, and D. Gonzalez-Dunia. 2004. 2′-Fluoro-2′-deoxycytidine inhibits Borna disease virus replication and spread. Antimicrob. Agents Chemother. 48:1422-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhalla, K., P. Swerdlow, and S. Grant. 1991. Effects of thymidine and hydroxyurea on the metabolism and cytotoxicity of 1-β-d arabinofuranosylcytosine in highly resistant human leukemia cells. Blood 78:2937-2944. [PubMed] [Google Scholar]
- 4.Bode, L., D. E. Dietrich, R. Stoyloff, H. M. Emrich, and H. Ludwig. 1997. Amantadine and human Borna disease virus in vitro and in vivo in an infected patient with bipolar depression. Lancet 349:178-179. [DOI] [PubMed] [Google Scholar]
- 5.Bode, L., and H. Ludwig. 2003. Borna disease virus infection, a human mental-health risk. Clin. Microbiol. Rev. 16:534-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bressanelli, S., L. Tomei, A. Roussel, I. Incitti, R. L. Vitale, M. Mathieu, R. De Francesco, and F. A. Rey. 1999. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Proc. Natl. Acad. Sci. USA 96:13034-13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briese, T., W. I. Lipkin, and J. C. de la Torre. 1995. Molecular biology of Borna disease virus. Curr. Top. Microbiol. Immunol. 190:1-16. [DOI] [PubMed] [Google Scholar]
- 8.Cubitt, B., and J. C. de la Torre. 1997. Amantadine does not have antiviral activity against Borna disease virus. Arch. Virol. 142:2035-2042. [DOI] [PubMed] [Google Scholar]
- 9.Cubitt, B., and J. C. de la Torre. 1994. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J. Virol. 68:1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cubitt, B., C. Oldstone, and J. C. de la Torre. 1994. Sequence and genome organization of Borna disease virus. J. Virol. 68:1382-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delarue, M., O. Poch, N. Tordo, D. Moras, and P. Argos. 1990. An attempt to unify the structure of polymerases. Protein Eng. 3:461-467. [DOI] [PubMed] [Google Scholar]
- 12.de la Torre, J. C., K. Carbone, P. Staeheli, L. Stitz, J. A. Richt, K. Ikuta, B. Dietzschold, H. Ludwig, L. Bode, and W. I. Lipkin. 2000. Family Bornaviridae, p. 531-538. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wicker (ed.), Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. [Online.] Academic Press, San Diego, Calif. doi:10.1006/bkvt.2000.0052.
- 13.Dürrwald, R., and H. Ludwig. 1997. Borna disease virus (BDV), a (zoonotic?) worldwide pathogen. A review of the history of the disease and the virus infection with comprehensive bibliography. Zentbl. Veterinarmed. B 44:147-184. [DOI] [PubMed] [Google Scholar]
- 14.Furth, J. J., and S. S. Cohen. 1968. Inhibition of mammalian DNA polymerase by the 5′-triphosphate of 1-β-d-arabinofuranosylcytosine and the 5′-triphosphate of 9-β-d-arabinofuranoxyladenine. Cancer Res. 28:2061-2067. [PubMed] [Google Scholar]
- 15.Gosztonyi, G., B. Dietzschold, M. Kao, C. E. Rupprecht, H. Ludwig, and H. Koprowski. 1993. Rabies and Borna disease. A comparative pathogenetic study of two neurovirulent agents. Lab. Investig. 68:285-295. [PubMed] [Google Scholar]
- 16.Gosztonyi, G., and H. Ludwig. 1995. Borna disease—neuropathology and pathogenesis. Curr. Top. Microbiol. Immunol. 190:39-73. [PubMed] [Google Scholar]
- 17.Hallensleben, W., M. Zocher, and P. Staeheli. 1997. Borna disease virus is not sensitive to amantadine. Arch. Virol. 142:2043-2048. [DOI] [PubMed] [Google Scholar]
- 18.Hrebabecky, H., J. Brokes, and J. Beranek. 1980. Reaction of nucleosides with thionyl chloride; preparation of the deoxy derivatives of cytidine and adenosine. Collect. Czech. Chem. Commun. 45:599-605. [Google Scholar]
- 19.Ikuta, K., M. S. Ibrahim, T. Kobayashi, and K. Tomonaga. 2002. Borna disease virus and infection in humans. Front. Biosci. 7:470-495. [DOI] [PubMed] [Google Scholar]
- 20.Jordan, I., T. Briese, D. R. Averett, and W. I. Lipkin. 1999. Inhibition of Borna disease virus replication by ribavirin. J. Virol. 73:7903-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larder, B. A., D. J. Purifoy, K. L. Powell, and G. Darby. 1987. Site-specific mutagenesis of AIDS virus reverse transcriptase. Nature 327:716-717. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig, H., and L. Bode. 2000. Borna disease virus: new aspects on infection, disease, diagnosis and epidemiology. Rev. Sci. Tech. 19:259-288. [DOI] [PubMed] [Google Scholar]
- 23.Mizutani, T., H. Inagaki, K. Araki, H. Kariwa, J. Arikawa, and I. Takashima. 1998. Inhibition of Borna disease virus replication by ribavirin in persistently infected cells. Arch. Virol. 143:2039-2044. [DOI] [PubMed] [Google Scholar]
- 24.Mizutani, T., H. Inagaki, D. Hayasaka, H. Kariwa, and I. Takashima. 1999. Enhancement of Borna disease virus transcription in persistently infected cells by serum starvation. J. Vet. Med. Sci. 61:831-834. [DOI] [PubMed] [Google Scholar]
- 25.Perez, M., A. Sanchez, B. Cubitt, D. Rosario, and J. C. de la Torre. 2003. A reverse genetics system for Borna disease virus. J. Gen. Virol. 84:3099-3104. [DOI] [PubMed] [Google Scholar]
- 26.Planz, O., K. Bechter, and M. Schwemmle. 2002. Human Borna disease virus infection, p. 179-226. In K. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington, D.C.
- 27.Pletnikov, M., D. Gonzalez-Dunia, and L. Stitz. 2002. Experimental infection: pathogenesis of neurobehavioral disease, p. 125-178. In K. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington, D.C.
- 28.Poch, O., I. Sauvaget, M. Delarue, and N. Tordo. 1989. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 8:3867-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider, U., M. Naegele, P. Staeheli, and M. Schwemmle. 2003. Active Borna disease virus polymerase complex requires a distinct nucleoprotein-to-phosphoprotein ratio but no viral X protein. J. Virol. 77:11781-11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solbrig, M. V., R. Schlaberg, T. Briese, N. Horscroft, and W. I. Lipkin. 2002. Neuroprotection and reduced proliferation of microglia in ribavirin-treated bornavirus-infected rats. Antimicrob. Agents Chemother. 46:2287-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 32.Stitz, L., O. Planz, and T. Bilzer. 1998. Lack of antiviral effect of amantadine in Borna disease virus infection. Med. Microbiol. Immunol. 186:195-200. [DOI] [PubMed] [Google Scholar]