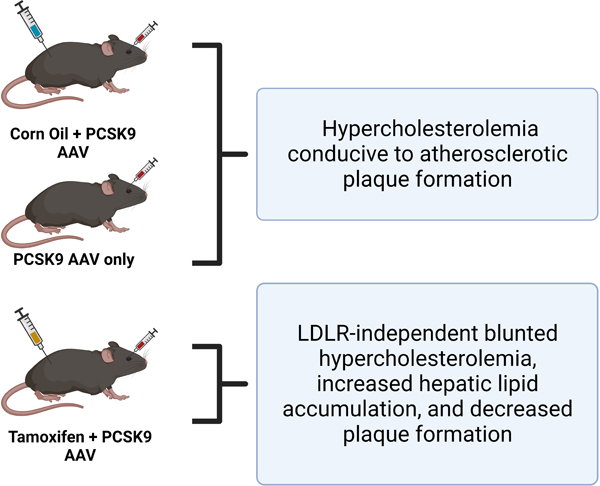
Abstract
Background:
In recent years fate-mapping lineage studies in mouse models have led to major advances in vascular biology by allowing investigators to track specific cell populations in vivo. One of the most frequently employed lineage tracing approaches involves tamoxifen-inducible CreERT-LoxP systems. However, tamoxifen treatment can also promote effects independent of Cre recombinase activation, many of which have not been fully explored.
Methods:
To elucidate off-target effects of tamoxifen, male and female mice were either unmanipulated or injected with tamoxifen or corn oil. All mice received PCSK9-AAV injections and a modified western diet to induce hypercholesterolemia. After 2 weeks, serum cholesterol and liver morphology were assessed. To determine the duration of any tamoxifen effects in long-term atherosclerosis experiments, mice received either 12 days of tamoxifen at baseline or 12 days plus two sets of 5-day tamoxifen boosters; all mice received PCSK9-AAV injections and a modified western diet to induce hypercholesterolemia. After 24 weeks, serum cholesterol and aortic sinus plaque burden were measured.
Results:
After 2 weeks of atherogenic treatment, mice injected with tamoxifen demonstrated significantly reduced serum cholesterol levels compared to uninjected- or corn oil-treated mice. However, there were no differences in PCSK9-mediated knockdown of LDL receptors between the groups. Additionally, tamoxifen treated mice exhibited significantly increased hepatic lipid accumulation compared to the other groups. Finally, the effects of tamoxifen remained for at least 8 weeks after completion of injections, with mice demonstrating persistent decreased serum cholesterol and impaired atherosclerotic plaque formation.
Conclusions:
In this study we establish that tamoxifen administration results in decreased serum cholesterol, decreased plaque formation, and increased hepatic lipid accumulation. These alterations represent significant confounding variables in atherosclerosis research, and we urge future investigators to take these findings into consideration when planning and executing their own atherosclerosis experiments.
Graphical Abstract
INTRODUCTION
In recent years fate-mapping lineage studies in mouse models have led to major advances in vascular biology by allowing investigators to track specific cell populations in vivo even when canonical markers are no longer expressed by the cell population of interest. For instance, lineage tracing of mature vascular smooth muscle cells (SMCs) has established major contributions of these cells to atherosclerotic plaques and neointima formation. However, many of these contributing cells lose expression of markers of contractile SMCs during the process of dedifferentiation; therefore studies that rely solely on expression of conventional markers to determine cell identity can underestimate their contribution.1
One of the most frequently employed lineage tracing approaches involves tamoxifen-inducible CreERT-LoxP systems. Using this approach, researchers exert both spatial and temporal control over the evaluation of genes of interest and/or permanent reporter knock-in through tamoxifen-mediated Cre recombinase activation and subsequent DNA manipulation.2 However, tamoxifen treatment can also promote effects independent of Cre recombinase activation, many of which have not been fully explored. In this manuscript we demonstrate that the timing of tamoxifen treatment can have major confounding effects in atherosclerosis research independent of Cre activation.
METHODS
The authors declare that all supporting data are available within the article.Reagents can be found in the Major Resources Table (Table 1).
Table 1:
Major Resources Table
| REAGENTS or RESOURCES | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies: Western Blotting | ||
| Rabbit polyclonal LDLR antibody | BioVision | 3839; lot 7B04L38390 |
| β-actin | Sigma | |
| IRDye® 800CW Goat anti-Rabbit IgG Secondary Antibody | Licor | 926–32211 |
| IRDye® 680RD Goat anti-Mouse IgG Secondary Antibody | Licor | 926–68070 |
| Bacterial and virus strains | ||
| AAV8-D377Y-mPCSK9 | Vector Biolabs | Addgene plasmid: 58376 |
| Chemicals, peptides, and recombinant proteins | ||
| Tamoxifen | Sigma | T5648–5G |
| Experimental models: Organisms/strains | ||
| Mouse: Gli1-CreER/Rosa -YFP | Multiple publications | N/A |
| Mouse: C57BL/6J | JAX | 000664 |
| Other | ||
| Atherogenic diet | Research Diets | D05060402 |
Animals
Mice used in this project were approved by our Institution IACUC, protocol #00066 Weiser-Evans. Animal numbers were approved by the University of Colorado Anschutz Medical Campus Animal Institute Committee. Two mouse strains were used for the experiments described here:
Gli1-CreER/Rosa-YFP (AdvSca1-SM lineage tracing mouse)
C57BL/6J (Jax 000664)
Age matched males and females were used for all experiments. For comparison of tamoxifen, corn oil, or no intervention, C57BL/6J mice were 8 weeks old at the start of the experiment; long-term atherosclerosis experiments utilized AdvSca1-SM lineage tracing mice (C57BL/6J background) between 6 and 8 weeks of age. Experimental mice were housed in the AALAC accredited RC-2 vivarium at the University of Colorado Anschutz Medical Campus. The vivarium is maintained at standard sub-thermoneutral temperatures (22–26°C) and with a 12-hour light-dark cycle. All animals had ad libitum access to both food and water. Atherogenic mice were fed Research Diets D05060402 (42.8% fat, 1.5% cholesterol, and 0.5% cholic acid). Animal health was monitored daily by veterinary staff. Cages were changed every 2 weeks and contained enrichment materials, including nesting material and/or cage furniture. Animals on long-term atherogenic diet received front and rear nail trims as needed to minimize scratch trauma resulting from diet-induced dermatitis.
Mouse Procedures
For the tamoxifen/corn oil experiments, mice received either 150 μl tamoxifen solution at a dose of 1.5 mg/day diluted in corn oil (“TMX”), 150 μl corn oil alone (“Corn Oil”), or no injections (“Control, No TMX”) at the start of the experiments. Tamoxifen solution was prepared in batches by resuspension in corn oil followed by 0.2 μm syringe filtration; aliquots were stored at −20°C. Animals received a total of 12 days of intraperitoneal (IP) tamoxifen or corn oil injections, followed by 1 day of rest. Mice then received a retroorbital injection of AAV bearing a mutant gain of function PCSK9 (Vector Biolabs, AAV8-D377Y-mPCSK9, Addgene 58376). When coupled with a high fat diet, injection of 1 × 1011 gene copies (gc) per mouse of AAV-m-PCSK9 has been shown to induce hypercholesterolemia and atherosclerotic lesion formation in mice without ApoE−/− or LDLR−/− mutations.3,4 The AAV stock was diluted in sterile saline such that each mouse received 1 × 1011 gc delivered in 200 μl of solution through a 28G syringe. The retroorbital injections were conducted under anesthesia per institutional protocol (induction at 3–5% isofluorane, and maintenance on the nose cone at 1.5–3% isofluorane). Following injection of the virus, animals were treated with Proparacaine HCl 0.5% ophthalmic eye drops (Bausch & Lomb 24208073006). Animals were observed for full recovery from the procedure, then placed in a new group housing cage and given atherogenic diet (2 weeks for tamoxifen/corn oil experiments, 24 weeks for long-term atherosclerosis experiments).
For long-term atherosclerosis experiments, AdvSca1-SM lineage mice received 12 days of tamoxifen for YFP reporter knock-in (standard protocol; “Baseline TMX”). A subset of mice (“Repeated TMX”) also received additional tamoxifen treatments at 8 and 16 weeks; these booster injections lasted 5 days as opposed to the initial 12 days. All mice received a retroorbital injection of AAV-m-PCSK9 to degrade LDL receptors at baseline (t=0, following tamoxifen dosing), 2 weeks, and 16 weeks. All animals for these experiments were fed atherogenic diet.
Mouse Tissues
Mice were euthanized and harvested according to institutional protocol. Specifically, mice were exposed to an overdose of isofluorane using the drop technique and left in the chamber until cessation of respiration. At that point, mice were removed from the chamber and cervical dislocation was performed as a secondary method of euthanasia (bilateral thoracotomy and exsanguination were also performed later during the harvest). Using a dissecting microscope, the abdominal cavity was opened, the diaphragm cut, and the ribcage removed. Blood was collected via cardiac puncture using a 25G syringe, then stored on ice for up to 1 hour during the harvest. Following exsanguination, mice were perfused with 10 ml of PBS/heparin (Sigma H3393, diluted to 0.08 KU/ml in PBS).
For the tamoxifen/corn oil experiments, two pieces of liver were collected after PBS/heparin but prior to fixation with PFA. These were snap frozen and stored at −80°C for future RNA or protein analysis. Following liver collection, mice were perfused with 10 ml of 4% PFA in PBS. The heart and an additional piece of liver were removed and placed in Eppendorf tubes containing 4% PFA. These samples were placed at 4°C overnight to ensure adequate fixation. After 24 hours, tissues were removed from the PFA, rinsed with PBS, then transferred to 30% sucrose/PBS. The sucrose/PBS incubation was for a minimum of 24 hours at 4°C to ensure adequate cryoprotection. Tissues were then embedded in Tissue-Tek O.C.T. Compound (Sakura) and frozen per manufacturer recommendations. Tissue blocks were stored at −80°C until sectioning into 6 μm thick serial sections. Aortic sinuses and livers were H&E stained for plaque measurements and changes in hepatic morphology, respectively.
Plaque quantification
Imaging of H&E slides was performed using a Keyence BZ-X710 microscope and BZ-X Viewer image acquisition software. Plaques were quantified using manual tracing in ImageJ following the guidelines provided by the 2017 AHA Statement.5 Each slide contained 3 sections of the aortic sinus taken at approximately 100 μm intervals. Only sections with all 3 valve leaflets represented were collected and analyzed. For quantification, measurements from each section were averaged. The mean area or percent coverage of 3 sections per mouse were used to compare plaque burden between the Baseline TMX and Repeated TMX groups.
Mouse Serum Analysis
For serum collection, tubes of blood were removed from the ice and left at room temperature for 30 minutes to clot, then the clots were dislodged using p200 pipette tips. Blood samples were spun down in a tabletop centrifuge for 10 minutes at 4°C and 10,000 G. Serum was removed and transferred to a new Eppendorf for storage at −80°C until analysis. Serum was analyzed with Wako Diagnostics kits for Cholesterol E (999–02601) using manufacturer recommendations. Samples were diluted 1:10 in sterile water for analysis. All tests were completed in triplicate.
Protein Harvest and Western Blot
Snap frozen livers were stored at −80°C following harvest, then thawed on ice for preparation of protein lysates. To prepare lysates, livers were ground using a ceramic mortar and pestle (pre-cooled in liquid nitrogen) then resuspended in 500 μl RIPA Lysis Buffer with PIC (protease inhibitor cocktail). Samples were sonicated twice for 10 seconds and samples spun down for 10 minutes at 14,000 G and 4°C. Supernatant was removed and stored at −80°C until use.
For western blots, protein lysates were diluted 1:10 in additional RIPA plus PIC. Bradford assays were performed to determine protein concentration. Samples were prepared at a target concentration of 0.5 μg/μl (20 μg prepared in 40 μl) under reducing conditions with DTT, then heated for 12 minutes at 95°C to denature the proteins. Samples were loaded into Novex™ WedgeWell™ 4 to 12% Tris-Glycine Mini Protein Gels and run at 75–110V. Proteins were transferred to PVDF membranes using a Trans-Blot® SD Semi-Dry Transfer Cell, run at 15V for 45 minutes. To confirm protein transfer, membranes were stained with Ponceau S. For analysis, membranes were blocked in 5% BSA in TBST for 1 hour. Membranes were cut at ~100 kDa with LDLR staining on the top portion and β-actin as a loading control on the bottom portion. For LDLR blotting, a rabbit polyclonal antibody (BioVision 3839–100, Lot: 7B04L38390) was diluted 1:1,000 in BSA/TBST; β-actin was used at a dilution of 1:60,000. Membranes were incubated on a rocker at 4°C overnight, rinsed 3 × 5 minutes in TBST, then incubated for 1 hour at room temperature on a rocker with the secondary antibodies in BSA/TBST (anti-mouse or anti-rabbit IRDye® 800CW or 680RD). Membranes were rinsed 3 × 5 minutes in TBST, 3 × 3 minutes in TBS, then imaged using a Licor Odyssey CLx. Densitometry analysis was conducted using Image Studio Ver. 5.2.
Statistics
All experimental data were evaluated for normality through comparison of median and means, standard deviations, and through formal normality tests (Shapiro-Wilk). The appropriate statistical analysis was used, dependent on the outcome of the normality analyses. For comparison of serum cholesterol in the tamoxifen/corn oil experiment, data passed tests for normality and equal variance and ANOVA with multiple comparisons was used. For comparison of serum cholesterol and plaque coverage by percent lesion area in long-term experiments, data passed tests for normality and equal variance and Student’s t-test was used. For plaque coverage by total lesion area in long-term experiments, data did not pass normality and equal variance and Mann Whitney was used. α level was set to 0.05 and statistical tests were completed using GraphPad Prism 9. Bar graph data was plotted as mean plus standard deviation, with all individual values displayed.
RESULTS
Our group and others routinely use tamoxifen-inducible fate mapping systems in combination with PCSK9 adeno-associated virus (AAV) and Western diet to elucidate the contribution of resident vascular cells to atherosclerosis progression. The current experiments were modeled off the groundbreaking work by the Bentzon Lab using a gain-of-function PCSK9 AAV to increase hepatic lysosomal degradation of low-density lipoprotein (LDL) receptors.3,4 However, in our atherosclerosis studies we observed that serum cholesterol from our animals was lower than reported in the literature (data not shown), with the only major difference being the genetic modifications to these animals through intraperitoneal injections of tamoxifen for 12 days for YFP reporter knock-in.
To test whether the decreased hypercholesterolemia was due to a tamoxifen effect, we conducted an experiment using male and female C57BL/6 mice and either performed no intervention (Control), injected them with corn oil control (Corn Oil), or injected tamoxifen suspended in corn oil (TMX) for 12 consecutive days (Figure 1A). One day later all animals received retroorbital PCSK9 AAV injections and were fed a modified Western diet for 2 weeks; an additional control group of mice that did not receive PCSK9, corn oil, or Western diet was used for baseline, physiological serum cholesterol levels. Following 2 weeks of Western diet, tamoxifen-treated mice had significantly lower serum cholesterol compared to the atherogenic Control and Corn Oil groups, but did exhibit higher cholesterol levels compared to non-atherogenic mice (Figure 1B). We initially hypothesized that tamoxifen might be interfering with PCSK9 AAV-mediated LDLR degradation, but all 3 PCSK9-treated atherogenic groups showed highly efficient depletion of the LDL receptor compared to animals that did not receive PCSK9 (Figure 1C; representative Western of a subset of all mice per condition). Finally, major changes in hepatic lipid accumulation were detected, with larger and more numerous lipid droplets observed in tamoxifen-treated mice compared to mice that received corn oil injections or no intervention (Figure Panel 1D). Our data provide clear evidence to support an off-target effect of tamoxifen on both serum lipids and hepatic lipid processing.
Figure 1: Off-target effects of tamoxifen decrease serum cholesterol and increase hepatic lipid accumulation.
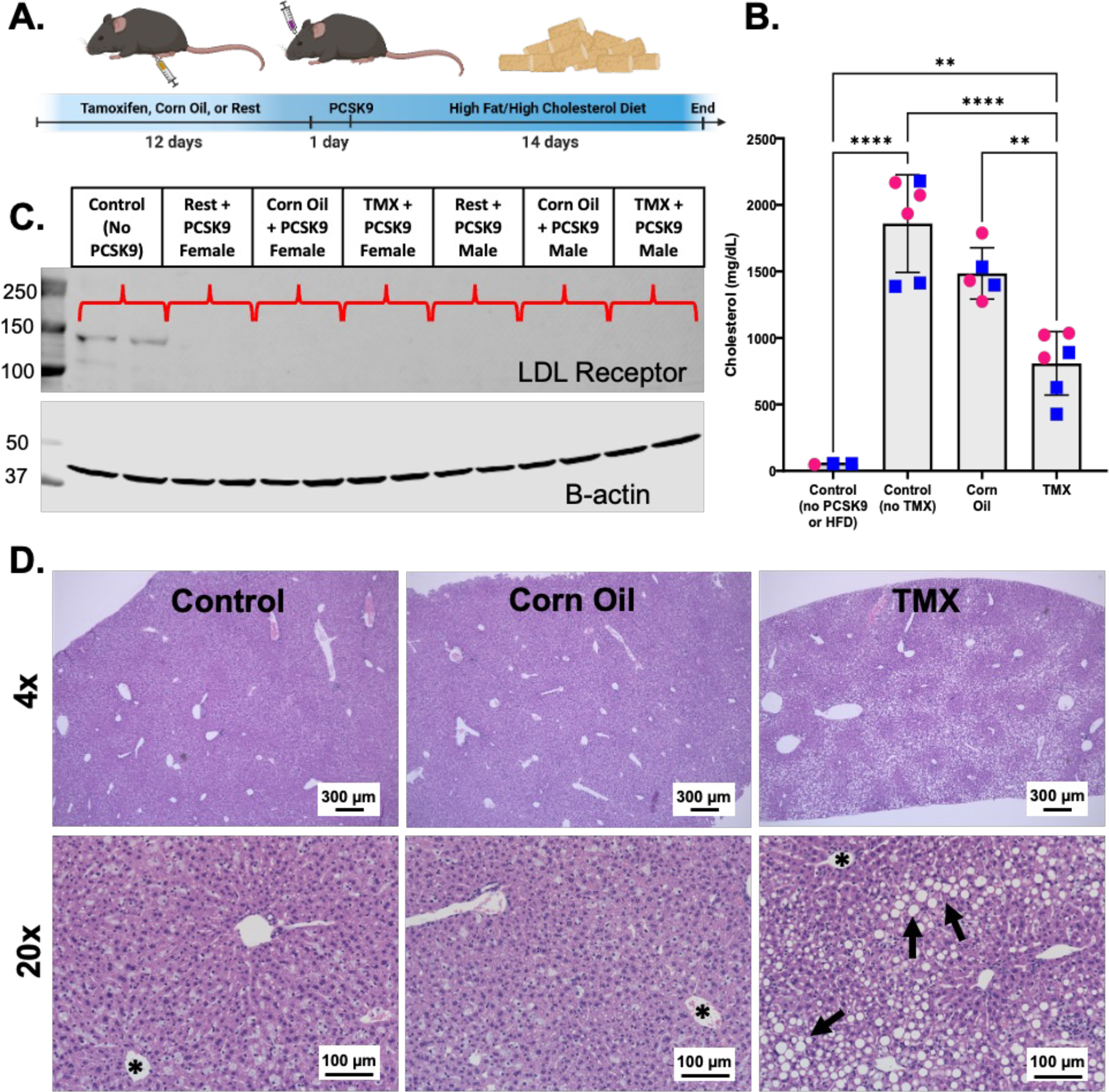
A. Model of experimental approach. 8-week-old C57BL/6 mice were either untreated (control), injected with corn oil (IP), or injected with 1.5 mg tamoxifen (TMX) dissolved in corn oil for 12 consecutive days. All mice received retro-orbital injections of 1 × 1011 gc per mouse AAV8-D377Y-mPCSK9 dissolved in sterile saline, then were fed a modified Western diet (42.8% fat, 1.5% cholesterol, and 0.5% cholic acid) for 14 days. B. Serum cholesterol levels of mice treated with TMX (n = 6; 3 male and 3 female), Corn Oil (n = 5; 2 male and 3 female), or untreated (Control no TMX)(n = 6; 3 male and 3 female) prior to PCSK9 AAV injection and 14 days of Western diet; control non-atherogenic mice did not receive PCSK9, corn oil/tamoxifen, or Western diet (Control no PCSK9/HFD). Data are shown as mean +/− SD. Males are marked as blue squares; females as pink circles. ANOVA with multiple comparisons to control non-atherogenic group **p<0.01; ****p<0.0001 C. Representative Western blot for LDL receptor in livers from one male and one female mouse from all 3 treatment groups compared to control mice without PCSK9 injections. D. 4x and 20x H&E images of livers from Control, Corn Oil, and TMX mice after 14 days of atherogenic treatment. Asterisks = hepatic central vein; arrows = representative lipid droplets. Scale bar for 4x images = 300 µm; scale bar for 20x images = 100 μm.
In addition to the 2-week timepoint, we also established that the observed tamoxifen effect persists for a minimum of 8 weeks. For long-term atherosclerosis studies using our current mouse model, a subset of mice received several rounds of tamoxifen injections applied throughout the experiment to efficiently label our cells of interest. For these experiments, mice received 12 days of tamoxifen at the beginning of the study (Baseline TMX), then 5 additional days of tamoxifen injections at 8 and 16 weeks (Repeated TMX)(Figure 2A). We found that after a total of 24 weeks of atherogenic conditions (PCSK9 AAV plus Western diet), the Repeated TMX mice exhibited significantly lower serum cholesterol and reduced aortic root plaque burden compared to the Baseline TMX group, which only received the initial 12 days of tamoxifen (Figure 2B–D). These findings demonstrate a long-term effect of tamoxifen, with altered lipid profiles and diminished plaque formation a full 8 weeks after cessation of tamoxifen treatment.
Figure 2: Off -target effects of tamoxifen impede atherosclerotic plaque formation.
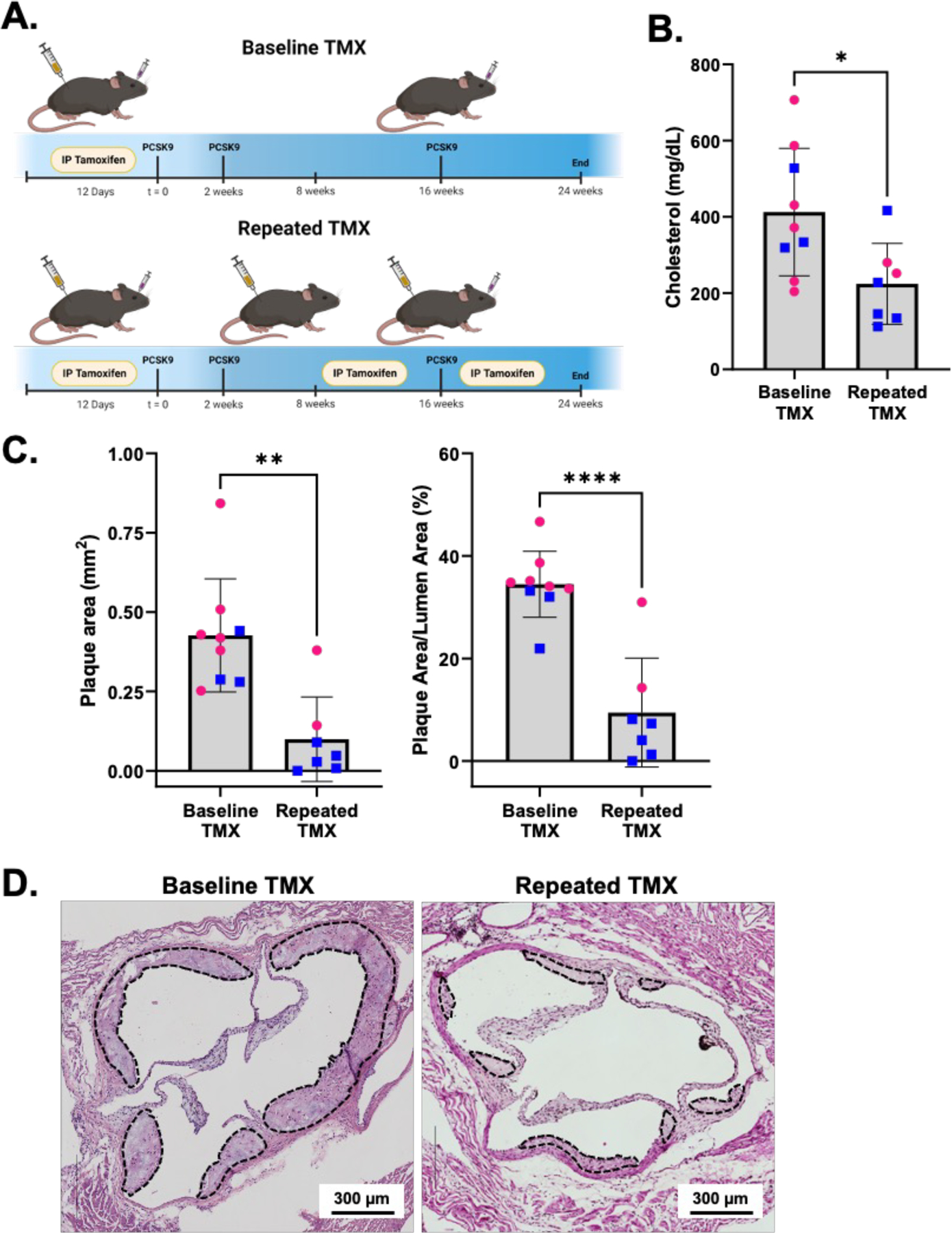
A. Model of experimental approach. Baseline TMX mice (n = 9; 3 male, 6 female) received 12 days of tamoxifen at t = 0, while Repeated TMX mice (n = 7; 5 male, 2 female) received 12 days of tamoxifen injections at baseline plus 5 additional days each at 8 and 16 weeks. Both groups of tamoxifen mice received PCSK9 AAV injections immediately following baseline tamoxifen, then were fed 24 weeks of Western diet. B. Serum cholesterol of mice after 24 weeks of atherogenic treatment. Data are shown as mean +/− SD. Males are marked as blue squares, females as pink circles. Student’s t test shows significant differences between the Baseline TMX and Repeated TMX groups (p=0.0099). C. Quantification of total plaque area (Left) and percent plaque areas (Right) from mice shown in panel B. Mann Whitney test test shows significant differences between total plaque area in Baseline and Repeated TMX groups (p<0.0012). Student’s t test shows significant differences between percent occlusion (plaque area/lumen area x 100) in Baseline and Repeated TMX groups (p<0.0001). Data are shown as mean +/− SD. Males are marked as blue squares, females as pink circles. D. Representative H&E images of aortic root plaques (dashed lines) from Baseline TMX (top) and Repeat TMX mice (bottom). Scale bars = 300 µm.
DISCUSSION
The findings from this study are consistent with clinical literature regarding women receiving tamoxifen treatment for breast cancer, where both altered hepatic lipid deposition and reduced serum cholesterol have been described.6–10 However, there have been fewer reports of tamoxifen effects in mouse studies despite the increased use of tamoxifen-inducible mouse models. One important study by Reckless et al. demonstrated that continuous tamoxifen administration through chow decreased serum cholesterol and inhibited plaque formation in ApoE null mice.11 In addition, the Bentzon group, in a follow up study to their initial study describing the use of PCSK9 to induce hyperlipidemia, noted a significant reduction in total cholesterol levels when introducing tamoxifen into the study protocol.12 To our knowledge, however, we are the first group to investigate the effects of timed tamoxifen administration on mouse serum lipids and atherosclerotic plaque development.
In this study we established that tamoxifen administration results in decreased serum cholesterol, decreased plaque formation, and increased hepatic lipid accumulation. We initially hypothesized that the decreased serum cholesterol was a result of some impairment of the PCSK9 AAV-mediated LDLR degradation. Although tamoxifen is generally considered safe, it has been reported to induce some hepatotoxicity, as indicated by increased ALT and AST after tamoxifen administration.13,14 Given that AAV8 demonstrates high liver tropism, we and others hypothesized that the hepatotoxic effects of tamoxifen might interfere with the activity of the PCSK9 AAV.15–18 However, in this study we demonstrated that the tamoxifen effects are independent of PCSK9-mediated knockdown of the LDLRs, suggesting that some other mechanism must be responsible for these observations. Some explanations for these phenomena include enhanced LDLR-independent lipid/cholesterol uptake, enhanced reverse cholesterol transport, increased hepatic fatty acid synthesis, or a VLDL secretion defect.9,19–21 Future studies will be needed to further define the mechanisms controlling tamoxifen-induced alterations to serum lipids and hepatic lipid accumulation.
In addition to overall evaluation of the effects of tamoxifen, it will be important to establish any sex-specific effects of the treatment. Tamoxifen is an estrogen receptor ligand and has been shown to interact with many biological systems in both males and females. Various studies have also reported differential effects of tamoxifen treatment prior to animal injury models in males compared to females.22–25 While the current study was not powered to evaluate sex differences in response to tamoxifen, this will be an important consideration for future analyses.
Collectively, these findings highlight a major cautionary tale when using tamoxifen-inducible mouse models in the setting of atherosclerosis. While we have not yet elucidated the mechanism driving these changes, in this study we establish that tamoxifen can have major influences on atherosclerosis studies by increasing hepatic lipid retention and decreasing serum cholesterol, leading to sub-optimal induction of atherosclerosis in murine models. This study also demonstrates that the effects of tamoxifen remain a full 8 weeks after cessation of injections, suggesting that a 2-week washout period, as proposed by some other groups, may be insufficient for many studies. In conclusion, we urge future investigators to take these findings into consideration when planning and executing their own atherosclerosis experiments.
Supplementary Material
HIGHLIGHTS.
Tamoxifen treatment prior to initiation of PCSK9-AAV plus western diet atherosclerosis studies significantly reduces plasma cholesterol and increases hepatic lipid retention compared to un-injected or corn oil-injected controls.
Tamoxifen-dependent alterations to serum cholesterol and hepatic lipid accumulation are independent of PCSK9-mediated low density lipoprotein receptor knockdown.
Tamoxifen treatment reduces serum cholesterol levels and impedes atherosclerotic plaque formation a full 8 weeks after cessation of treatment.
These findings highlight important concerns and considerations for researchers using tamoxifen-inducible mouse models for atherosclerosis research.
ACKNOWLEDGEMENTS
We would like to thank E. Erin Smith of the University of Colorado Cancer Center Pathology Shared Resource for her assistance with histological staining. This resource is supported in part by the Cancer Center Support Grant (no. P30CA046934). BioRender was used to create experimental approach schematics and graphical abstract.
SOURCES OF FUNDING
This work was supported by grants R01 HL 121877 (Weiser-Evans, Majesky) and R01 HL151331 (Weiser-Evans), and F31 HL160149–01 (Dubner) from the National Heart, Lung, and Blood Institute as well as TL1TR002533 (Dubner) from the National Center for Advancing Translational Sciences.
Footnotes
The authors have declared that no conflict of interest exists.
DISCLOSURES
The data that support the findings of this study are available from the corresponding author upon reasonable request. The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Shankman LS, Gomez D, Cherepanova OA, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 2015;21:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metzger D, Chambon P. Site-and Time-Specific Gene Targeting in the Mouse. METHODS 2001;24:71–80. [DOI] [PubMed] [Google Scholar]
- 3.Bjørklund MM, Hollensen AK, Hagensen MK, et al. Induction of atherosclerosis in mice and hamsters without germline genetic engineering. Circ Res 2014;114:1684–1689. [DOI] [PubMed] [Google Scholar]
- 4.Goettsch C, Hutcheson JD, Hagita S, et al. A single injection of gain-of-function mutant PCSK9 adeno-associated virus vector induces cardiovascular calcification in mice with no genetic modification. Atherosclerosis 2016;251:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daugherty A, Tall AR, Daemen MJAP, et al. Recommendation on Design, Execution, and Reporting of Animal Atherosclerosis Studies: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol 2017;37:e131–e157. [DOI] [PubMed] [Google Scholar]
- 6.Dnistrian AM, Schwartz MK, Greenberg EJ, Smith CA, Schwartz DC. Effect of tamoxifen on serum cholesterol and lipoproteins during chemohormonal therapy. Clin Chim Acta 1993;223:43–52. [DOI] [PubMed] [Google Scholar]
- 7.Joensuu H, Holli K, Oksanen H, Valavaara R. Serum lipid levels during and after adjuvant toremifene or tamoxifen therapy for breast cancer. Breast Cancer Res Treat 2000;63:225–234. [DOI] [PubMed] [Google Scholar]
- 8.Yoo JJ, Lim YS, Kim MS, et al. Risk of fatty liver after long-term use of tamoxifen in patients with breast cancer. PLoS One 2020;15. [DOI] [PMC free article] [PubMed]
- 9.Birzniece V, Barrett PHR, Ho KKY. Tamoxifen reduces hepatic VLDL production and GH secretion in women: a possible mechanism for steatosis development. Eur J Endocrinol 2017;177:137–143. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa Y, Murata Y, Nishioka A, Inomata T, Yoshida S. Tamoxifen-induced fatty liver in patients with breast cancer. Lancet 1998;351(9104):725. [DOI] [PubMed] [Google Scholar]
- 11.Reckless J, Metcalfe JC, Grainger DJ. Tamoxifen Decreases Cholesterol Sevenfold and Abolishes Lipid Lesion Development in Apolipoprotein E Knockout Mice. Circulation 1997;95:1542–1548. [DOI] [PubMed] [Google Scholar]
- 12.Bjørklund MM, Bernal JA, Bentzon JF. Atherosclerosis Induced by Adeno-Associated Virus Encoding Gain-of-Function PCSK9. Methods Mol Biol 2022;2419:461–473. [DOI] [PubMed] [Google Scholar]
- 13.Gao FF, Lv JW, Wang Y, et al. Tamoxifen induces hepatotoxicity and changes to hepatocyte morphology at the early stage of endocrinotherapy in mice. Biomed Reports 2016;4:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jena SK, Suresh S, Sangamwar AT. Modulation of tamoxifen-induced hepatotoxicity by tamoxifen–phospholipid complex. J Pharm Pharmacol 2015;67:1198–1206. [DOI] [PubMed] [Google Scholar]
- 15.Sands MS. AAV-Mediated Liver-Directed Gene Therapy. In: Methods in Molecular Biology Vol 807. ; 2011:141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA 2002;99:11854–11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenberghe LH, Xiao R, Lock M, Lin J, Korn M, Wilson JM. Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum Gene Ther 2010;21:1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsen K, Lund MB, Shim J, et al. Diverse cellular architecture of atherosclerotic plaque derives from clonal expansion of a few medial SMCs. JCI Insight 2017;2. [DOI] [PMC free article] [PubMed]
- 19.Cole LK, Jacobs RL, Vance DE. Tamoxifen induces triacylglycerol accumulation in the mouse liver by activation of fatty acid synthesis. Hepatology 2010;52:1258–1265. [DOI] [PubMed] [Google Scholar]
- 20.Le TT, Urasaki Y, Pizzorno G. Uridine prevents tamoxifen-induced liver lipid droplet accumulation. BMC Pharmacol Toxicol 2014;15:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Medina P, Payré BL, Bernad J, et al. Tamoxifen Is a Potent Inhibitor of Cholesterol Esterification and Prevents the Formation of Foam Cells. J Pharmacol Exp Ther 2004;308:1165–1173. [DOI] [PubMed] [Google Scholar]
- 22.Blum KM, Roby LC, Zbinden JC, et al. Sex and Tamoxifen confound murine experimental studies in cardiovascular tissue engineering. Sci Rep 2021;11:8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammad S, Othman A, Meyer C, et al. Confounding influence of tamoxifen in mouse models of Cre recombinase-induced gene activity or modulation. Arch Toxicol 2018;92:2549–2561. [DOI] [PubMed] [Google Scholar]
- 24.Falke LL, Broekhuizen R, Huitema A, Maarseveen E, Nguyen TQ, Goldschmeding R. Tamoxifen for induction of Cre-recombination may confound fibrosis studies in female mice. J Cell Commun Signal 2017;11:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Best KT, Studentsova V, Ackerman JE, et al. Effects of tamoxifen on tendon homeostasis and healing: Considerations for the use of tamoxifen-inducible mouse models. J Orthop Res 2021;39:1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


