Abstract
Objectives
To externally validate a published model predicting failure within 2 years after salvage focal ablation in men with localised radiorecurrent prostate cancer using a prospective, UK multicentre dataset.
Patients and methods
Patients with biopsy-confirmed ≤T3bN0M0 cancer after previous external beam radiotherapy or brachytherapy were included from the FORECAST trial (NCT01883128; 2014–2018; 6 centres), and from the HEAT and ICE UK-based registries (2006–2022; 9 centres). Eligible patients underwent either salvage focal HIFU or cryotherapy, with the choice based predominantly on anatomical factors. Per the original multivariable Cox regression model, the predicted outcome was a composite failure outcome. Model performance was assessed at 2 years post-salvage with discrimination (C-index), calibration (calibration curve and slope), and decision curve analysis. For the latter, two clinically-reasonable risk threshold ranges of 0.14–0.52 and 0.26–0.36 were considered, corresponding to previously-published pooled 2-year recurrence-free survival rates for salvage local treatments.
Results
168 patients were included, of whom 84/168 (50%) experienced the primary outcome in all follow-up, and 72/168 (43%) within 2 years. C-index was 0.65 (95%CI 0.58–0.71). On graphical inspection, there was close agreement between predicted and observed failure. Calibration slope was 1.01. In decision curve analysis, there was incremental net benefit versus a treat all strategy at risk thresholds ≥0.23. Net benefit was therefore higher across the majority of the 0.14–0.52 risk threshold range, and all of the 0.26–0.36 range.
Conclusion
In external validation using prospective, multicentre data, this model demonstrated modest discrimination but good calibration and clinical utility for predicting failure of salvage focal ablation within 2 years. This model could be reasonably used to improve selection of appropriate treatment candidates for salvage focal ablation, and its use should be considered when discussing salvage options with patients. Further validation in larger, international cohorts with longer follow-up is recommended.
Keywords: ablation, cryotherapy, failure, focal therapy, high-intensity focused ultrasound, prediction model, prostate cancer, radiotherapy, recurrence, salvage
INTRODUCTION
Radiotherapy is a common and effective prostate cancer treatment for many patients, with over 12,000 UK men undergoing external-beam radiotherapy each year [1]. However, approximately 10% with intermediate- or high-risk disease will develop recurrence localised to the prostate over long-term follow-up, an event independently predictive of metastasis and cancer-specific death [2]. Patients with localised radiorecurrence are typically offered surveillance or non-curative androgen deprivation therapy (ADT). Whole-gland salvage treatments are offered by some centres to highly-selected patients, but confer high rates of toxicity. Salvage radical prostatectomy, for example, leads to erectile dysfunction in nearly all, urinary incontinence in 80%, and rectal injury in 5–10% [3,4]. An emerging alternative is salvage focal ablation. Encompassing treatments like high-intensity focussed ultrasound (HIFU) and cryotherapy, this targets the recurrent lesion(s) alone. Preliminary data suggest this provides good early disease control with reduced toxicity [5,6].
Optimal patient selection for focal ablation, however, remains unknown. Certainly, the risk of treatment failure should be central to these decisions. In 2018, Peters et al. developed and internally validated a model for predicting failure after salvage focal HIFU [7]. To our knowledge, this is the only published model predicting failure after salvage focal ablation. This study aims to externally validate this model using prospective, UK multicentre data from a cohort study and 2 registries.
PATIENTS AND METHODS
Validation cohort
Patients were enrolled either within the FORECAST trial (NCT01883128), or the HIFU Evaluation and Assessment of Treatment (HEAT) and International Cryotherapy Evaluation (ICE) UK national registries [6,8,9]. All underwent salvage focal HIFU or cryotherapy after previous external beam radiotherapy and/or low/high dose rate brachytherapy with or without (neo)adjuvant ADT. For this analysis, only patients with ≤T3bN0M0 radiorecurrent disease were included, matching the inclusion criteria of the original model [7].
FORECAST
Between 2014–2018, 181 patients were prospectively enrolled to 6 UK centres who had biochemical failure defined by rising prostate-specific antigen (PSA) levels post-radiotherapy [6]. Those taking ADT within 6 months of enrolment, with a PSA doubling time of ≤3 months, with a total PSA of ≥20 ng/mL, unable to have an MRI, or with previous salvage treatment were ineligible.
Following 18F-Choline PET/CT and 99mTc MDP bone scan, patients underwent prostate multiparametric MRI (mpMRI) followed by transperineal mpMRI-targeted and template mapping biopsies [10]. Eligible patients were offered either salvage HIFU or cryotherapy, according to disease location, alongside other options like salvage prostatectomy or observation, as defined by a multi-disciplinary meeting. Cryotherapy was used for anterior tumours, larger tumours with an anterior-posterior distance of >3.5cm, and prostates with calcifications or previous brachytherapy seeds. All other patients with peripheral zone or posterior tumours underwent HIFU.
In the 93 patients who underwent focal ablation, PSA measurements were taken post-operatively at 1, 3, 6, 9, and 12 months, then every 6 months. A prostate mpMRI was also routinely performed at 12 months. Any further imaging or biopsy were ordered based on clinical judgement and were not protocol-mandated.
HEAT and ICE registries
Between 2006–2022, 292 patients with radiorecurrence undergoing salvage HIFU or cryotherapy from 9 UK centres were prospectively enrolled into HEAT and ICE [8,9]. Radiorecurrence was based on a rising PSA meeting Phoenix criteria, which triggered restaging investigations, comprising bone scan, CT, Choline PET/CT, or PSMA PET/CT dependent on local practice. Patients then underwent mpMRI with systematic and mpMRI-targeted biopsy.
Salvage focal ablation was offered to patients with non-metastatic disease requiring maximum 75% ablation of the gland. The decision of which energy to use was made locally, however generally HIFU was used for posterior disease, and cryotherapy for anterior or T3b disease. Post-operatively, subsequent PSAs, imaging, and biopsy were ordered based on local practice and protocols.
Outcome
The primary outcome was treatment failure as defined by the original model [7]. This was a composite of any of the following: biochemical failure (PSA value ≥2ng/mL above nadir), localised/distant disease on imaging (prostate mpMRI, PET/CT, bone scan), positive repeat biopsy, initiation of systemic treatment (ADT, chemotherapy), or cancer-specific death. Patients who did not fail were censored at the date of their latest appointment or investigation. Neither clinicians nor the study team were blinded to collection of predictor or outcome data.
Statistical analysis
The model by Peters et al. comprises a score developed from a multivariable Cox regression model [7]. Model variables measured at the time of radiorecurrence diagnosis, and their individual coefficients, were: Gleason score (Gleason 7: −0.083; Gleason 8–10: 0.48), radiological T-stage (T3: 0.314), PSA (ng/mL; 0.042), prostate volume (mL; 0.007), and disease-free survival interval (months; −0.007). Disease-free survival interval was the duration between finishing primary treatment and the mpMRI assessing for radiorecurrence. If mpMRI date was unavailable, biopsy date was used. As this variable was measured in months, if relevant dates were only available in year format rather than year and month, then this datapoint was considered missing so as to avoid inappropriately biasing this variable. For all variables, missing data were considered missing at random, and derived using multiple imputation by chain equations with 20 iterations and 1000 resamples using the mice R package. Model variables were used for imputation in addition to the binary failure outcome and the Nelson-Aalen estimate of the cumulative hazard function [11]. Missing data were imputed for PSA for 7 patients (4%), Gleason score for 18 (11%), prostate volume for 46 (27%), and disease-free survival interval for 61 (36%).
To calculate the risk score, variable coefficients were multiplied by 10 then multiplied by their respective value, and then summated. An additional 10 points were then added to obtain positive sum scores. For example, for a patient with radiorecurrent Gleason 8, T3a cancer with PSA 5 ng/mL, a prostate volume of 40mL and 72 month disease-free survival interval, the risk score would be calculated as follows:
(0.48 × 10) [Gleason 8] + (0.314 × 10) [T3 stage] + (5 × 0.042 × 10) [PSA 5ng/mL] + (40 × 0.007 × 10) [volume 40mL] + (72 x −0.007 × 10) [disease-free survival interval 72 months] + 10
= 4.8 + 3.14 + 2.1 + 2.8 – 5.04 + 10
= 17.8 points
Final risk scores were entered into Cox regression to predict failure. Model performance was assessed at 2 years post-ablation, with performance measures estimated in each imputed dataset and then summarised using Rubin’s rules [12]. Discrimination was evaluated using concordance index (C-index). Calibration was assessed graphically through plotting predicted versus observed failure, and through calculation of the calibration slope.
Decision curve analysis was also performed to determine clinical utility [13]. Here, net benefit (y-axis) is plotted against risk threshold (x-axis). Risk threshold refers to clinician preferences in regard to offering salvage focal ablation, taking account of its benefits versus harms. Lower risk thresholds reflect clinicians who are more concerned with missing the benefits of salvage focal ablation, that is the opportunity to treat any recurrence successfully. This therefore represents a low threshold for offering treatment. Higher risk thresholds reflect clinicians who are more concerned regarding the harms of salvage focal ablation, that is fewer, better selected patients should be treated in order to minimise any harms; thus these clinicians have a high threshold for offering treatment. A given risk threshold is defined as the minimum probability of failure at which salvage focal ablation would be warranted. Model net benefit, which takes into account both discrimination and calibration, is a combination of model-predicted false positives subtracted from true positives weighted against a given risk threshold.
In decision curve analysis, model-based decision making on whether to offer salvage focal ablation is compared against strategies of treating all patients, and treating no patients. The model with the highest net benefit across a clinically-reasonable range of risk thresholds has the greatest clinical utility and can be recommended for use. As the reference strategy in this scenario is treating all patients, net benefit can also be expressed in terms of true negatives, equivalent to the number of salvage focal ablation procedures that can be avoided. These patients who avoid a procedure reflect a high predicted risk of failure that may instead warrant whole-gland or multi-modal treatment strategies.
To determine a clinically-reasonable range of risk thresholds, we considered a 2021 systematic review that calculated pooled 2-year recurrence-free survival rates for 6 salvage local treatments post-radiotherapy. The lowest rate was reported for focal and whole-gland HIFU (54%; 95%CI 48–60%), and the highest rate for low dose-rate brachytherapy (81%; 74–86%) [4]. Taking the lowest and highest bounds of these 2 95%CIs, a 48–86% 2-year recurrence-free survival rate is equivalent to a 14–52% 2-year recurrence rate. This risk threshold range of 0.14–0.52 was the first clinically-reasonable range of risk thresholds considered. We also considered a second range as determined by the 2-year recurrence-free survival rate of salvage radical prostatectomy (69%; 95%CI 64–74%), a risk threshold range of 0.26–0.36.
Next, patients were categorised into 3 risk groups described by the original study, which were created based on 4-year failure-free survival proportions [7]. These were group 1 (score ≤7; best prognosis), group 2 (scores >7 and ≤15), and group 3 (scores >15; worst prognosis). Failure-free survival distributions were plotted using Kaplan-Meier curves and compared using log-rank tests adjusted for multiple comparisons using the Benjamini-Hochberg method [14].
Validation was performed using all patients who underwent salvage focal ablation. As subgroup analyses, discrimination and calibration were then estimated separately by ablation energy and data source.
Analyses were performed using R v.4.2.2. Statistical significance was set at p<0.05.
Sample size
The minimum sample size to provide precise estimation of the calibration slope was calculated as per Riley et al. (Appendix 1) [15]. As reported by the original study, for a C-index of 0.64 from internal validation and an estimated survival probability of 0.54 at 2 years, our cohort sample size of 164 would provide a 95% confidence interval (CI) of 0.77–1.23 for a calibration slope of 1 [7]. This assumes no censoring prior to the 2 year timepoint. Censoring prior to 2 years was not reported by the original study, however in our cohort this was 35%. Therefore, assuming a 35% censor rate prior to 2 years, a sample size of 164 would give 95%CI 0.05–1.95. For a target 95%CI of 0.9–1.1, as recommended by Riley et al., a minimum sample size of 8000 and 12500 would be required assuming no censoring and assuming a 35% censor rate by 2 years, respectively.
RESULTS
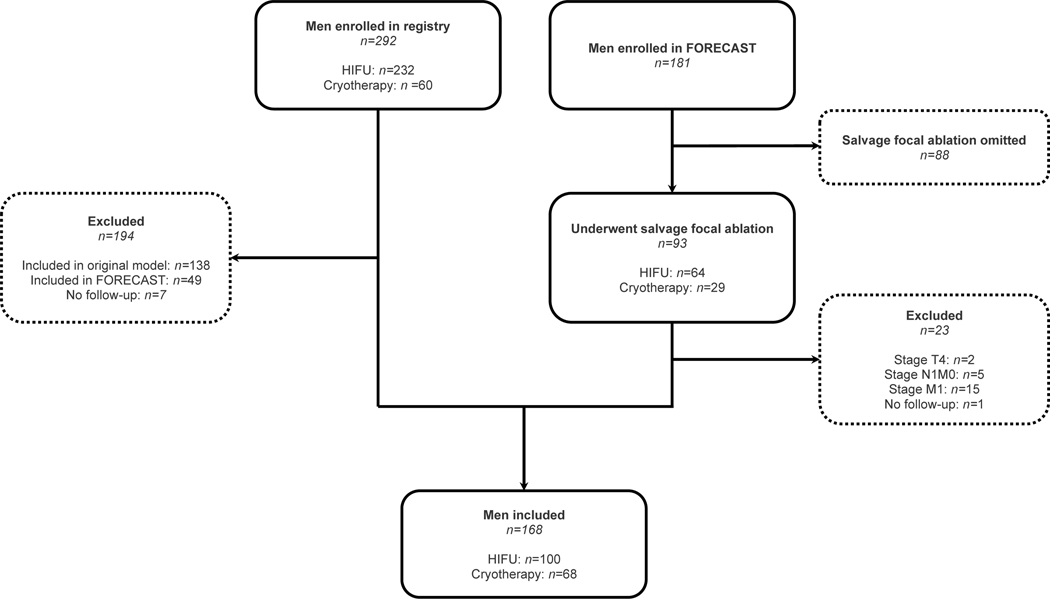
From 493 patients considered, 168 were eligible (Fig. 1). 100/168 (60%) underwent salvage focal HIFU, and 68/168 (40%) underwent salvage focal cryotherapy. Table 1 displays patient clinico-pathological data split by ablation energy, with Table S1 displaying combined data. Table S2 displays these data versus the original development cohort of 150 patients [7]. Ceharacteristics appeared generally comparable, though this external validation cohort comprised a greater proportion of brachytherapy patients (difference 17% [20% vs 3%], Fisher’s exact test p<0.0001). Furthermore, the original development cohort comprised purely HIFU-treated patients.
Fig. 1:
Flow chart detailing the exclusion and inclusion process of men in this study.
Table 1:
Patient characteristics, both at diagnosis and at time of enrolment in FORECAST or in the HEAT and ICE registries, split by ablation energy. Abbreviations: EBRT, external beam radiotherapy; MCCL, maximum cancer core length.
| Characteristic | HIFU, n = 1001 | Cryotherapy, n = 681 | p 2 |
|---|---|---|---|
| At diagnosis | |||
| Age | 62.0 (58.0, 66.0) | 64.0 (60.8, 69.0) | 0.01 |
| Unknown | 20 | 12 | |
| PSA (ng/mL) | 13.0 (7.7, 23.2) | 10.9 (7.8, 22.2) | 0.8 |
| Unknown | 20 | 20 | |
| Gleason score | 0.8 | ||
| ≤6 | 29 (36%) | 21 (41%) | |
| 7 | 40 (49%) | 22 (43%) | |
| ≥8 | 12 (15%) | 8 (16%) | |
| Unknown | 19 | 17 | |
| At trial or registry enrolment | |||
| EBRT use | 88 (88%) | 52 (78%) | 0.07 |
| Unknown | 0 | 1 | |
| Brachytherapy use | 13 (13%) | 21 (31%) | 0.004 |
| Unknown | 0 | 1 | |
| Neoadjuvant/adjuvant hormone use | 69 (80%) | 15 (83%) | 0.8 |
| Unknown | 14 | 50 | |
| Age | 69.0 (66.2, 73.0) | 72.0 (68.0, 76.0) | 0.02 |
| Unknown | 6 | 6 | |
| Disease-free survival interval (months) | 86 (61, 116) | 87 (53, 118) | 0.8 |
| Unknown | 40 | 21 | |
| PSA (ng/mL) | 4.7 (2.5, 8.0) | 5.1 (3.0, 7.2) | 0.9 |
| Unknown | 3 | 4 | |
| Prostate volume (mL) | 22.0 (19.0, 30.0) | 24.0 (20.0, 34.4) | 0.1 |
| Unknown | 19 | 27 | |
| MRI stage | 0.8 | ||
| T1/2 | 74 (79%) | 45 (82%) | |
| T3 | 1 (1.1%) | 0 (0%) | |
| T3a | 11 (12%) | 4 (7.3%) | |
| T3b | 8 (8.5%) | 6 (11%) | |
| Unknown | 6 | 13 | |
| Total biopsy cores | 29 (22, 40) | 23 (15, 36) | 0.1 |
| Unknown | 3 | 7 | |
| Positive biopsy cores | 5 (3, 9) | 6 (3, 8) | 0.6 |
| Unknown | 4 | 7 | |
| Grade Group | 0.03 | ||
| 1 | 3 (3.3%) | 3 (4.9%) | |
| 2 | 26 (29%) | 15 (25%) | |
| 3 | 37 (41%) | 19 (31%) | |
| 4 | 16 (18%) | 6 (9.8%) | |
| 5 | 9 (9.9%) | 18 (30%) | |
| Unknown or irradiation effect | 9 | 7 | |
| MCCL (mm) | 7 (4, 10) | 8 (5, 10) | 0.2 |
| Unknown | 11 | 8 |
Median (IQR); n (%).
Wilcoxon rank sum test; Pearson’s Chi-squared test; Fisher’s exact test.
In this external validation cohort, 84/168 patients (50%) experienced the primary failure outcome in all follow-up (HIFU n=50, cryotherapy n=34), Within 2 years, 72/168 patients (43%) experienced this within 2 years (HIFU n=43, cryotherapy n=29). Specifically, 34/72 (47%) experienced biochemical failure, 31/72 (43%) had positive re-imaging, 4/72 (6%) had a positive re-biopsy, and 3/72 (4%) started systemic treatment. In all cases, ADT was started prior to any other systemic treatment. No patient experienced cancer-specific mortality within 2 years, though in all follow-up this affected 2 patients at times of 8.65 and 10.62 years post-salvage focal ablation. Fig. 2 displays Kaplan-Meier curves illustrating failure-free survival distribution for all included patients and stratified by energy. Median time-to-failure was 0.99 years (interquartile range (IQR) 0.71–1.35), and median follow-up for censored patients was 1.09 years (IQR 0.68–2.21). Table S3 displays the number of patients reaching each individual outcome of the composite failure outcome in all follow-up, with Fig.S1 displaying corresponding Kaplan-Meier curves.
Fig. 2:
Kaplan-Meier curves plotting failure-free survival distributions for all patients undergoing salvage focal ablation (A), and stratified by focal ablation energy (B).
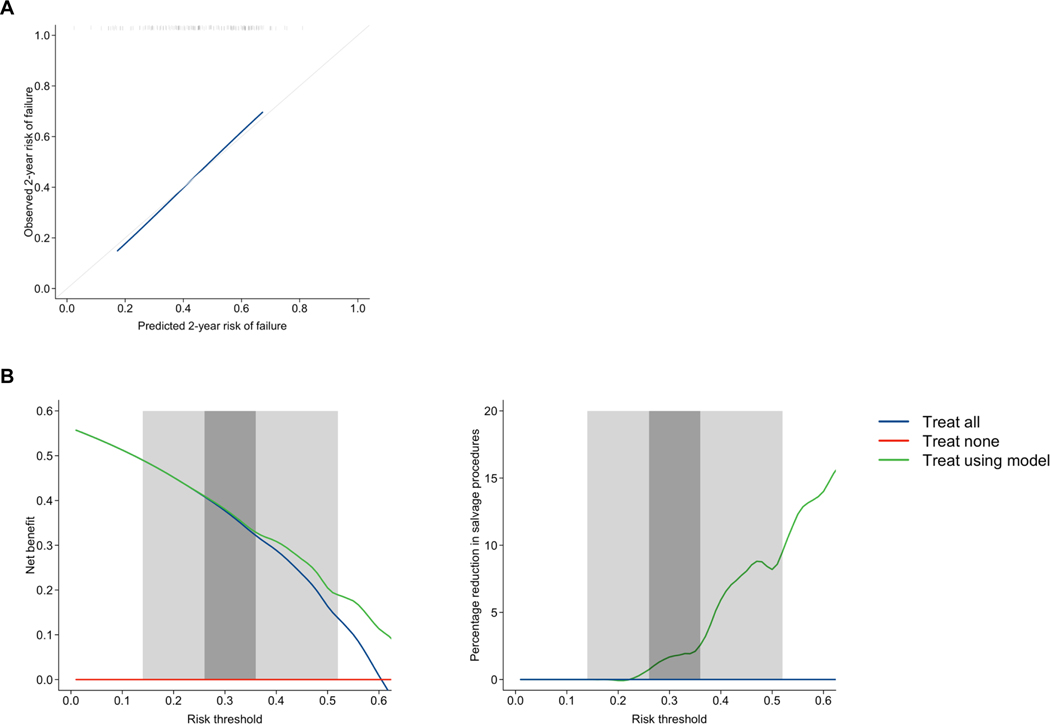
Model discrimination was modest (C-index 0.65, 95%CI 0.58–0.71). Calibration was good, however, with close agreement between predicted and observed failure on inspection of the calibration curve (Fig. 3). Furthermore, calibration slope was 1.01.
Fig. 3:
Calibration curve (A) and decision curve analysis (B) for model predictions of composite failure at 2 years post-salvage focal ablation for all included men undergoing salvage focal ablation. Calibration slope was 1.01. Decision curve analysis compares decision making to offer salvage focal ablation between model-based decision making and strategies of treating all patients and treating no patients. Plots demonstrating net benefit and percentage reduction in salvage focal ablation procedures are shown. Two clinically-reasonable ranges of risk threshold are highlighted: (i) 0.14–0.52 (light grey); and (ii) 0.26–0.36 (dark grey), based on previously-published pooled 2-year recurrence rates probabilities [4].
In decision curve analysis, there was incremental net benefit using model-based decision-making compared to a treat all strategy at risk thresholds ≥0.23 (Fig. 3) [13]. For risk thresholds <0.23, using the model to select patients for treatment had no benefit compared to offering treatment to all patients. Therefore, considering the risk threshold range of 0.14–0.52, model-based decision making represents the optimal strategy for the majority of these risk thresholds. In addition, a proportionally greater net benefit was observed at higher risk thresholds within this range, suggesting that proportionally greater model benefit lies with clinicians who prefer to be more selective of patients. In this range, using the model to select treatment candidates versus treating all patients would lead to a 0–9.5% reduction in salvage focal ablation procedures. Considering the second risk threshold range of 0.26–0.36, model-based decision making was the optimal strategy for all risk thresholds. However, on inspection, net benefit was only very marginally greater throughout this range. In this range, there would be a 0.7–2.6% reduction in salvage focal ablation procedures performed.
Fig. 4 displays Kaplan-Meier curves for each of the 3 risk score groups as detailed in the original development study [7]. In adjusted pairwise log-rank tests, there was a significant difference in failure-free survival distributions between groups 1 and 2 (p<0.0001), groups 1 and 3 (p<0.0001), and groups 2 and 3 (p=0.032), reflecting the good calibration of the model in this cohort.
Fig. 4:
Kaplan-Meier curves curve plotting time-to-failure stratified by risk groups. This is plotted for all included men undergoing salvage focal ablation. Log-rank test demonstrated a significant difference in survival distributions between groups (p<0.0001).
C-index across the 4 subgroup analyses ranged from 0.60–0.68 (HIFU only: 0.64, 95%CI 0.55–0.72; cryotherapy only: 0.68, 95%CI 0.57–0.78; FORECAST only: 0.60, 95%CI 0.51–0.79; HEAT/ICE registry only: 0.67, 95%CI 0.58–0.77). There remained generally good agreement between predicted and observed failure in the cryotherapy-only subgroup (calibration slope 1.10), the FORECAST-only subgroup (calibration slope 0.88), and HEAT/ICE registry-only subgroup (calibration slope 1.10; Fig.S2). However, for HIFU patients, the model slightly overestimated failure at lower predictions, and underestimated it at higher predictions (calibration slope 0.98).
DISCUSSION
Summary of results
In this external validation, the 43% 2-year and 50% all follow-up failure rate demonstrated emphasises the need for an effective risk model to predict which patients are likely to fail treatment, and thus for whom offering salvage focal ablation may not be warranted. The multivariable risk model here demonstrated comparably modest discrimination to internal validation (0.65 versus 0.64, respectively), but with good calibration [7]. Furthermore, compared to a treat all strategy, there was incremental net benefit for clinicians across the majority of risk thresholds in the range 0.14–0.52, corresponding to previously published pooled 2-year recurrence-free survival rates of local salvage treatments [4]. Importantly, there was also incremental net benefit for all risk thresholds in the range 0.26–0.36, which corresponds to the pooled 2-year recurrence-free survival rate of salvage radical prostatectomy from the same analysis. Certainly, therefore, this model does offer some utility to clinicians who desire to be more selective of treatment candidates for salvage HIFU and cryotherapy.
A nomogram to facilitate clinical use is detailed in Fig. 5, using the original model’s coefficients. Calculation of a patient’s risk score should be considered when discussing salvage options, and the risk groups detailed could be of use. Patients with higher risk predictions, for example risk group 3, can be more appropriately counselled and may benefit from alternative discussion of whole-gland treatments or multi-modal therapy. In contrast, patients with lower predictions, for example risk group 1, can be reassured regarding the early efficacy of salvage focal treatment. These risk groups could also be used to guide intensity of follow-up post-operatively.
Fig. 5:
Method of risk score calculation, presented with a nomogram presenting probability of failure-free survival by 2 years corresponding to the range of possible risk scores.
It should be noted that HIFU and cryotherapy are deemed to be complementary rather than competing treatments, with selection of either therapy based predominantly on anatomical factors. In subgroup analysis, the calibration in HIFU-treated patients was notably worse on inspection of the calibration curve compared to cryotherapy-treated patients, and this should be considered with clinical use of the model. This may be due to a higher proportion of the external validation HIFU-treated cohort having undergone previous brachytherapy compared to the development cohort (difference 11% [13% vs 3%], Fisher’s exact test p<0.001). The presence of brachytherapy seeds, typically, is a relative indication to treat with cryotherapy over HIFU [6]. Nonetheless, despite being developed in an exclusively HIFU-treated cohort, it is encouraging to see good calibration in cryotherapy-treated patients in this validation.
The primary composite failure outcome is designed to reflect potential disease progression as reached via different scenarios [7]. Consequently, the next steps for patients experiencing this outcome are not uniform. Nonetheless, unless already performed, this may include any combination of local and/or whole-body re-imaging, re-biopsy, ongoing observation, further local salvage treatment, and commencement of ADT. Importantly, this model and its predicted outcome do not seek to recommend specific next steps; instead, for a patient that has met the composite failure outcome, this implies that further investigation and potentially treatment is indicated as decided by their clinician.
Context
Improving the management of radiorecurrence is an important but under-studied research need. Considering that in the UK alone, over 12,000 patients undergo external beam radiotherapy for prostate cancer each year, approximately 20% will develop biochemical failure [1,16]. Furthermore, within five years of biochemical failure, 50% will develop distant metastases, and 20% will die from their cancer [16]. Overall, recurrence confined to the prostate affects 10% of patients with intermediate- and high-risk disease, and is independently predictive of metastasis and cancer-specific death [2]. It follows that preventing or delaying metastases and subsequent death in these patients through effective treatment of localised disease is therefore crucial.
At the point of biochemical failure, watchful waiting or non-curative ADT is typically offered. However, the latter carries bothersome side effects like hot flushes and reduced libido, plus significant metabolic toxicity [17]. Furthermore, castrate-resistant disease develops after 2–3 years, requiring expensive 2nd and 3rd line systemic agents [18]. As an alternative, our group has previously shown that, based on transperineal template mapping biopsies, as many as three-quarters of patients with localised radiorecurrent disease may be anatomically suitable for salvage focal ablation [19]. In support of the salvage focal approach, FORECAST demonstrated that focal ablation provides good early cancer control with 66% progression-free survival and preserved urinary continence in 84% at 2 years follow-up [6]. This is also supported by a 2020 systematic review, concluding a 48–72% 3-year disease-free survival rate [5].
At present, few centres perform salvage therapy post-radiotherapy, and even fewer offer salvage focal therapy. Wider application of salvage focal treatments will require optimisation of 2 key areas: (i) accurate diagnosis and localisation of radiorecurrent disease; and (ii) patient selection for salvage treatment. FORECAST addressed the former, showing that radiorecurrent cancer is prevalent in those with a rising PSA (80%), and that mpMRI followed by both systematic and targeted biopsies is important for detecting this [6]. The model by Peters et al. and the present external validation, addresses the second area, showing that short-term oncological outcome post-ablation can be predicted with reasonable performance, and that higher-risk versus lower-risk treatment candidates can be distinguished [7].
To our knowledge, this model is the only published tool predicting failure after salvage focal ablation. There exists one other published model by Wiligenburg et al. in the setting of salvage focal brachytherapy, but this has not been externally validated [20].
Future directions
After FORECAST, further prospective, ideally randomised, studies with longer-term follow-up are required addressing both the diagnosis of radiorecurrent disease, and treatment using salvage focal ablation. These will drive development of an optimised diagnostic and therapeutic paradigm.
It will be useful to evaluate how novel radiological parameters may improve models. Between the models by Peters et al. and Wiligenburg et al., radiological variables considered were prostate volume, lesion volume, and stage [7,20]. However, these do not necessarily quantify the likelihood of radiorecurrent disease. The recently-published Prostate Magnetic Resonance Imaging for Local Recurrence Reporting (PI-RR) guidelines provide a 5-point assessment system for mpMRI post-radiotherapy [21]. Although not yet prospectively validated, incorporating this score into models may prove beneficial. For example, higher PI-RADS score in the primary setting is associated with biochemical failure and metastases by 7 years post-radiotherapy [22]. Other radiomic parameters may also be important; our group previously evaluated pre-operative mpMRI pharmacokinetic quantitative variables [23]. After adjustment for 7 factors, median interstitial space volume (Ve) independently predicted failure after salvage focal HIFU. Furthermore, PSMA PET/CT is increasingly used in this population to identify any extra-prostatic disease; maximum standardised uptake value (SUV max) values of any visualised intra-prostatic lesion could also be considered.
Last, on the subject of PSMA PET/CT, data from novel imaging techniques could improve diagnostics and modelling. PSMA PET/CT is increasingly replacing other forms of cross-sectional imaging, including 18F-Choline PET/CT and bone scan, the standard at the time of the FORECAST trial [6]. PSMA PET/CT may improve patient selection for salvage focal ablation through greater ability firstly to rule out distant disease, and secondly to rule in local recurrence in conjunction with mpMRI [24,25]. When using both 68Ga-PSMA-11 PET/CT and mpMRI, one study found a positive predictive value of 98% with targeted biopsy [26]. PET/MR and whole body MR imaging are also emerging tools that warrant further study [27,28].
Limitations
Strengths of this study include use of prospective, multicentre UK-wide data with few exclusion criteria. Most importantly, despite using 3 sources, our validation cohort is ultimately small, reflecting the paucity of patients undergoing salvage focal therapy. A larger sample size that could predict calibration slope with ideal precision is likely unobtainable given the relatively few centres globally that perform salvage focal ablation. Our cohort is in fact larger than the majority of published cohorts in both salvage radical prostatectomy and salvage focal therapy [4,5,29]. Second, follow-up was limited to 2 years, whereas the model by Peters et al. was developed with follow-up to 4 years. International collaboration with colleagues from non-UK centres to facilitate further validation and refinement of this model is welcomed, particularly with larger datasets and longer-term follow-up.
Third, registry data are limited by less structured follow-up versus protocol-driven follow-up in FORECAST, though this is arguably more representative of clinical practice.
Last, missing data was another limitation, particularly involving disease-free survival interval (35%) and prostate volume (28%). Missing disease-free survival interval data mainly stemmed from incomplete reporting of when primary treatment was completed. For these patients, the year of primary treatment completion was often available. However, as disease-free survival interval is a variable measured in months, it was decided to omit calculation of this variable if a specific month was not available. Multiple imputation was instead used to impute these data; an approach that is effective in yielding unbiased results even with large proportions of missingness [30]. We argue this is preferable to the alternative strategy of implementing a rule such as assuming the month of finishing treatment is the mid-point of that year.
In conclusion, for patients who have previously undergone prostate radiotherapy, this external validation demonstrates that a previously-published risk model can, with reasonable performance, predict if a patient will fail salvage focal ablation by 2 years. Its use should be considered to facilitate appropriate patient selection for salvage focal ablation. Additional external validation in large, non-UK cohorts with longer-term follow-up is needed to further evaluate model performance.
Supplementary Material
Funding:
The FORECAST study was funded by the Pelican Cancer Foundation, the US National Institutes of Health, and the UK Medical Research Council (NCT01883128). The HEAT registry is supported by an unrestricted grant from Sonablate. The funders played no direct role in the study.
Taimur T. Shah certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Alexander Light receives funding from the UK National Institute of Health Research (NIHR) and Imperial Health Charity. Hashim U. Ahmed receives core funding from the UK NIHR Imperial Biomedical Research Centre, the Wellcome Trust, the UK NIHR, the UK Medical Research Council, Cancer Research UK, Prostate Cancer UK, The Urology Foundation, the BMA Foundation, Imperial Healthcare Charity, Sonablate, Trod Medical, and Sophiris Biocorp; has received a travel allowance from Sonablate; was a paid consultant for Sophiris Biocorp and Sonablate; and is a proctor for Rezūm treatment and cryotherapy for Boston Scientific. Mark Emberton receives funding from NIHR-i4i, the UK Medical Research Council, Cancer Research UK, the Jon Moulton Charitable Foundation, Sonacblate, Trod Medical, the Cancer Vaccine Institute, and Sophiris Biocorp; and is a consultant and/or trainer and proctor for Sonablate, Angiodynamics, and Exact Imaging. Caroline Moore is funded by the NIHR Research Professorship, and receives funding from the European Association of Urology Research Foundation, the UK Medical Research Council, Cancer Research UK, Prostate Cancer UK, Movember, and the Cancer Vaccine Institute; and has received advisory board fees from Genomic Health. Shonit Punwani receives sessional funding from UCLH Biomedical Research Centre and funding from Prostate Cancer UK, the UK Medical Research Council, and Cancer Research UK.
Footnotes
Conflicts of interest:
The remaining authors have nothing to disclose.
Ethics considerations: UK ethics committee approval was received for the FORECAST trial under reference 13/LO/1401.
REFERENCES
- [1].Results of the NPCA Prospective Audit in England and Wales for men diagnosed from 1 National Prostate Cancer Audit 2022. [Google Scholar]
- [2].Ma TM, Chu FI, Sandler H, Feng FY, Efstathiou JA, Jones CU, et al. Local Failure Events in Prostate Cancer Treated with Radiotherapy: A Pooled Analysis of 18 Randomized Trials from the Meta-analysis of Randomized Trials in Cancer of the Prostate Consortium (LEVIATHAN). Eur Urol 2022;82:487–98. 10.1016/J.EURURO.2022.07.011. [DOI] [PubMed] [Google Scholar]
- [3].Golbari NM, Katz AE. Salvage Therapy Options for Local Prostate Cancer Recurrence After Primary Radiotherapy: a Literature Review. Curr Urol Rep 2017;18. 10.1007/S11934-017-0709-4. [DOI] [PubMed] [Google Scholar]
- [4].Valle LF, Lehrer EJ, Markovic D, Elashoff D, Levin-Epstein R, Karnes RJ, et al. A Systematic Review and Meta-analysis of Local Salvage Therapies After Radiotherapy for Prostate Cancer (MASTER). Eur Urol 2021;80:280–92. 10.1016/J.EURURO.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Khoo CC, Miah S, Connor MJ, Tam J, Winkler M, Ahmed HU, et al. A systematic review of salvage focal therapies for localised non-metastatic radiorecurrent prostate cancer. Transl Androl Urol 2020;9:1535–45. 10.21037/TAU.2019.08.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shah TT, Kanthabalan A, Otieno M, Pavlou M, Omar R, Adeleke S, et al. Magnetic Resonance Imaging and Targeted Biopsies Compared to Transperineal Mapping Biopsies Before Focal Ablation in Localised and Metastatic Recurrent Prostate Cancer After Radiotherapy. Eur Urol 2022;81:598–605. 10.1016/j.eururo.2022.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Peters M, Kanthabalan A, Shah TT, McCartan N, Moore CM, Arya M, et al. Development and internal validation of prediction models for biochemical failure and composite failure after focal salvage high intensity focused ultrasound for local radiorecurrent prostate cancer: Presentation of risk scores for individual patient prognoses. Urol Oncol 2018;36:13.e1–13.e10. 10.1016/J.UROLONC.2017.08.022. [DOI] [PubMed] [Google Scholar]
- [8].Shah TT, Peters M, Eldred-Evans D, Miah S, Yap T, Faure-Walker NA, et al. Early-Medium-Term Outcomes of Primary Focal Cryotherapy to Treat Nonmetastatic Clinically Significant Prostate Cancer from a Prospective Multicentre Registry. Eur Urol 2019;76:98–105. 10.1016/J.EURURO.2018.12.030. [DOI] [PubMed] [Google Scholar]
- [9].Reddy D, Peters M, Shah TT, van Son M, Tanaka MB, Huber PM, et al. Cancer Control Outcomes Following Focal Therapy Using High-intensity Focused Ultrasound in 1379 Men with Nonmetastatic Prostate Cancer: A Multi-institute 15-year Experience. Eur Urol 2022;81:407–13. 10.1016/J.EURURO.2022.01.005. [DOI] [PubMed] [Google Scholar]
- [10].Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815–22. 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- [11].Nelson W. Hazard Plotting for Incomplete Failure Data. Https://DoiOrg/101080/00224065196911980344 2018;1:27–52. 10.1080/00224065.1969.11980344. [DOI]
- [12].Rubin DB, editor. Multiple Imputation for Nonresponse in Surveys 1987. 10.1002/9780470316696. [DOI] [Google Scholar]
- [13].Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565–74. 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research 2001;125:279–84. 10.1016/S0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- [15].Riley RD, Collins GS, Ensor J, Archer L, Booth S, Mozumder SI, et al. Minimum sample size calculations for external validation of a clinical prediction model with a time-to-event outcome. Stat Med 2022;41:1280–95. 10.1002/SIM.9275. [DOI] [PubMed] [Google Scholar]
- [16].Zumsteg ZS, Spratt DE, Romesser PB, Pei X, Zhang Z, Polkinghorn W, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol 2015;67:1009–16. 10.1016/J.EURURO.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mitsuzuka K, Arai Y. Metabolic changes in patients with prostate cancer during androgen deprivation therapy. Int J Urol 2018;25:45–53. 10.1111/IJU.13473. [DOI] [PubMed] [Google Scholar]
- [18].Sternberg CN, Fizazi K, Saad F, Shore ND, De Giorgi U, Penson DF, et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2020;382:2197–206. 10.1056/NEJMOA2003892. [DOI] [PubMed] [Google Scholar]
- [19].Kanthabalan A, Arya M, Freeman A, Mitra AV., Payne H, Peters M, et al. Intraprostatic Cancer Recurrence following Radical Radiotherapy on Transperineal Template Mapping Biopsy: Implications for Focal Ablative Salvage Therapy. J Urol 2020;204:950–5. 10.1097/JU.0000000000001201. [DOI] [PubMed] [Google Scholar]
- [20].Willigenburg T, van Son MJ, van de Pol SMG, Eppinga WSC, Lagendijk JJW, de Boer HCJ, et al. Development and internal validation of multivariable prediction models for biochemical failure after MRI-guided focal salvage high-dose-rate brachytherapy for radiorecurrent prostate cancer. Clin Transl Radiat Oncol 2021;30:7. 10.1016/J.CTRO.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Panebianco V, Villeirs G, Weinreb JC, Turkbey BI, Margolis DJ, Richenberg J, et al. Prostate Magnetic Resonance Imaging for Local Recurrence Reporting (PI-RR): International Consensus -based Guidelines on Multiparametric Magnetic Resonance Imaging for Prostate Cancer Recurrence after Radiation Therapy and Radical Prostatectomy. Eur Urol Oncol 2021;4:868–76. 10.1016/J.EUO.2021.01.003. [DOI] [PubMed] [Google Scholar]
- [22].Turchan WT, Kauffmann G, Patel P, Oto A, Liauw SL. PI-RADS score is associated with biochemical control and distant metastasis in men with intermediate-risk and high-risk prostate cancer treated with radiation therapy. Urol Oncol 2020;38:600.e1–600.e8. 10.1016/J.UROLONC.2019.12.015. [DOI] [PubMed] [Google Scholar]
- [23].Rakauskas A, Shah TT, Peters M, Randeva JS, Hosking-Jervis F, Schmainda MJ, et al. Can quantitative analysis of multi-parametric MRI independently predict failure of focal salvage HIFU therapy in men with radio-recurrent prostate cancer? Urol Oncol 2021;39:830.e1–830.e8. 10.1016/J.UROLONC.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur Urol 2020;77:403–17. 10.1016/J.EURURO.2019.01.049. [DOI] [PubMed] [Google Scholar]
- [25].Light A, Ahmed HU, Shah TT. The unclear role of PET-CT in localized radiorecurrent prostate cancer. Nat Rev Urol 2022;19:573–4. 10.1038/S41585-022-00635-9. [DOI] [PubMed] [Google Scholar]
- [26].Rasing M, van Son M, Moerland M, de Keizer B, Wessels F, Jonges T, et al. Value of Targeted Biopsies and Combined PSMA PET/CT and mp-MRI Imaging in Locally Recurrent Prostate Cancer after Primary Radiotherapy. Cancers (Basel) 2022;14. 10.3390/CANCERS14030781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jentjens S, Mai C, Ahmadi Bidakhvidi N, De Coster L, Mertens N, Koole M, et al. Prospective comparison of simultaneous [68Ga]Ga-PSMA-11 PET/MR versus PET/CT in patients with biochemically recurrent prostate cancer. Eur Radiol 2022;32:901–11. 10.1007/S00330-021-08140-0. [DOI] [PubMed] [Google Scholar]
- [28].Adeleke S, Latifoltojar A, Sidhu H, Galazi M, Shah TT, Clemente J, et al. Localising occult prostate cancer metastasis with advanced imaging techniques (LOCATE trial): a prospective cohort, observational diagnostic accuracy trial investigating whole-body magnetic resonance imaging in radio-recurrent prostate cancer. BMC Med Imaging 2019;19. 10.1186/S12880-019-0380-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Grubmüller B, Jahrreiss V, Brönimann S, Quhal F, Mori K, Heidenreich A, et al. Salvage Radical Prostatectomy for Radio-Recurrent Prostate Cancer: An Updated Systematic Review of Oncologic, Histopathologic and Functional Outcomes and Predictors of Good Response. Curr Oncol 2021;28:2881–92. 10.3390/CURRONCOL28040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Madley-Dowd P, Hughes R, Tilling K, Heron J. The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol 2019;110:63–73. 10.1016/J.JCLINEPI.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.