Abstract
Intestinal parasitic infections such as amoebiasis, ascariasis, hookworm infection, and trichuriasis are the most common infections among non-human primates (NHPs). There are always the possibilities of transmission these parasites between humans and NHPs. Multiple groups of rhesus macaques (Macaca mulatta) live in the urban area of Kathmandu Valley near human settlements, however the gastrointestinal (GI) parasitic infections in those macaques are understudied. This study aimed to explore the GI parasites in free-ranging macaques from Pashupatinath, Swayambhunath, Tripureshwor, Nilbarahi temples and a group of captive rhesus macaques in the Central Zoo, Kathmandu. Fecal samples were collected from the macaques between October 2021 to September 2022 and assessed for parasites by the both wet mount method and concentration technique. There is high prevalence of GI parasite infection; out of 121 fecal samples examined, 87.6% of samples were positive. Six species of protozoans and eight species of helminths were identified from the fecal samples including the first report of Iodamoeba butschlii in monkeys of Nepal. Among the protozoan parasites, Entamoeba coli (54.71%) showed the highest prevalence followed by Balantioides coli (44.33%), E. histolytica (19.81%), and Iodamoeba butschlii (10%). Among the helminths, Trichuris spp. (31.13%) and Strongyloides spp. (31.13%) showed the highest prevalence followed by Hookworm (24.52%), and Strongyle spp. (23.58%). The likelihood ratio test suggested that the prevalence differed significantly with the seasons for Iodamoeba butschlii, Giardia spp., Strongyles spp., Hookworm, and Trichostrongylus spp. The prevalence of E. histolytica, E. coli, Iodamoeba. butschlii, Trichuris spp., Trichostrongylus spp., and Unknown spp.1 differed with sampling localities. The high prevalence of GI parasites found in the macaques living in the densely urbanized Kathmandu presents a potential threat to humans and warrants further study as well as increased education of the public and management of the human-macaque interface in the urban landscape of the Valley.
Keywords: Helminths, Rhesus macaque, Temple and shrines, Gastro-intestinal parasites, Zoonosis, Public health
Graphical abstract
Highlights
-
•
87.6% of fecal samples of rhesus macaques (Macaca mulatta) were positive for GI parasite species.
-
•
The prevalence of GI parasites is significantly different with the seasons.
-
•
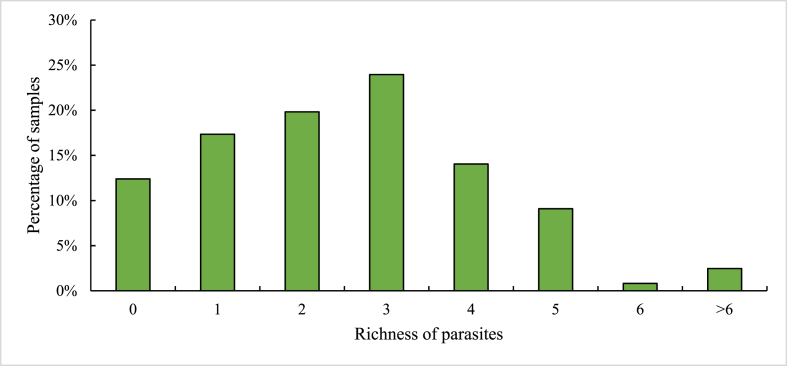
2.47% of the samples had a parasite species richness more than six.
-
•
The first report on presence of Iodamoeba butschlii in non-human primates of Nepal.
1. Introduction
Gastrointestinal protozoan and helminth parasites (GI parasites hereafter), including Amoeba, Ascaris, Hookworm, and Trichuris are common in both humans and non-human primates worldwide (Mogaji et al., 2020). In both urban and rural areas of Indian Subcontinent, humans coexist with non-human primates [NHPs hereafter], particularly rhesus macaques (Macaca mulatta) (Arunachalam et al., 2015; Cawthon, 2005; Mariadoss et al., 2019). In and around temples, monkeys not only received from human food but also exchange parasites with them. In rapidly urbanizing areas, the health of rhesus macaques is deteriorating because of their dependence on contaminated food, contaminated water, and habitat loss (Jha et al., 2011). Due to the close phylogenetic relatedness between humans and macaques, there is a considerable evidence for parasitic exchange between humans and macaques (Chapman et al., 2005; Dogel, 1964; Pedersen and Davies, 2009). Many parasites are known to be transmissible between NHPs and humans (Brown, 2004; Huffman et al., 2013; M. Li et al., 2015).
A number of studies have examined GI parasites of NHPs in captive settings (Khatun et al., 2014; M. Li et al., 2015; Tabasshum et al., 2018), the wild (Adrus et al., 2019; Chalise et al., 2013; Gillespie et al., 2010; Munene et al., 1998), and in urban areas (Jha et al., 2011; Sapkota et al., 2020; Schurer et al., 2019). Evidence suggests that many newly emerging parasitic diseases in humans have been acquired from NHPs and there is a significant risk of human pathogen transmission to free-ranging NHPs (Jones-Engel et al., 2006a, Jones-Engel et al., 2006b). Although there has been an increase in human-NHP interaction and conflict throughout Asia (Chalise, 2006; Khatun et al., 2014; Tabasshum et al., 2018), few studies have addressed GI parasite infection of NHPs living in the urban areas of Nepal.
In Nepal, rhesus macaques are abundant, roam freely, and often live in temples, stupas, and other public areas. Chalise (2006) estimated about 1000 rhesus macaques live in the Kathmandu Valley's temple areas, including Pashupatinath, Swayambhunath, Tripureshwor Mahadev Temple, and others. These temples are visited everyday by a large number of devotees who provide supplemental food to the macaques, and dispose of waste that results in the contamination of water sources. Visitors provide food materials to primates in these temples (Jones-Engel et al., 2006a, Jones-Engel et al., 2006b) so, there is an increased risk of parasite transmission between the macaques and the visitors as a result of direct physical contact or indirect contact through contaminated food, water, or soil (Hsu et al., 2009). Given the potential zoonotic risk, the purpose of this study was to examine the prevalence and intensity of GI parasites in the urban macaques of the Kathmandu Valley and site-specific variation.
2. Methods
2.1. Study area
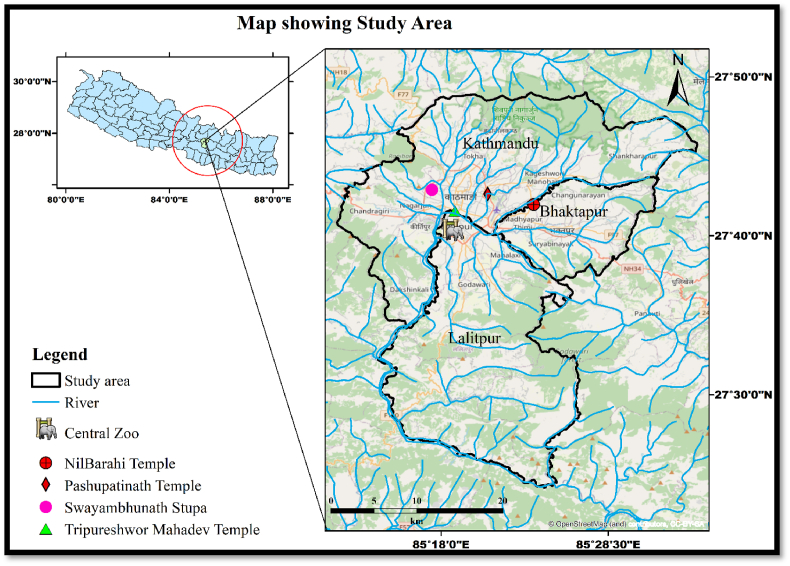
This study was carried out in temples and shrines of the Kathmandu Valley with resident populations of rhesus macaques. Kathmandu Valley encompasses an area of 600 sq. km and sits at an elevation of about 1425 m above sea level (Mahapatra et al., 2019). The area has four main temples/shrines with residential populations of rhesus macaques namely: Pashupatinath, Swayambhunath, Tripureshwor Mahadev, and Nilbarahi temples. All of these temples are located close to or surrounded by the densely populated city and heavily polluted Bagmati, Hanumante, Manohara, and Bishnumati rivers (Fig. 1). Thousands of visitors and pilgrims visit these temple sites daily. Except for Tripureshwor Mahadev temple, the other temple sites have small forest patches. The rhesus macaques feed on offerings provided in the temple or food snatched from visitors. Some of the macaques even raid crops from nearby farmland or human settlements.
Fig. 1.
Map showing the four fecal collection sites of the urban rhesus macaques in the Kathmandu Valley.
2.2. Sample collection
A total of 121 fecal samples were collected non-invasively sampling from the rhesus macaques at all four temple sites from October 2021 to September 2022, and additional six samples were collected from six rhesus macaques at the central zoo for comparison. The sampling period spanned all seasons: winter, spring, summer and rainy season. Fecal samples were collected from the rhesus macaques without causing any harm or disturbance to the animals or their habitat. Additionally, we acquired an official permit (reference number 274/080/081) from the Department of Forest and Soil Conservation, Government of Nepal to ensure that our research followed the ethical and legal regulations of the government.
The collection of feces (observed fresh drops) was carried out opportunistically during the early morning hours. To minimize the risk of repeats in sample collection from the same animal, we closely followed the macaques during the collection process, but individuals could not be recognized and each sample was treated as a sample from one distinguished individual. Thus, individual characteristics like age and sex could not be accounted for in subsequent analyses (although only adults individuals were sampled). There are also no repeat samples from the same individual, so each sample is considered by itself. Rigorous precautions were taken to maintain sample integrity, including the use of separate spatulas to prevent contamination, as well as the utilization of masks and gloves to ensure the safety of both the researchers and the samples.
Approximately 10 g of the fecal sample was placed in a clean, sterile bottle containing 2.5% potassium dichromate solution. This solution helps preserve the sample as it stops helminth eggs and larva from developing further and helps in maintaining their morphology. Each sample was carefully labeled at the time of collection.
2.3. Laboratory methods
2.3.1. Direct wet mount methods
2.3.1.1. Saline wet mount method
A drop of saline was placed on a clean, grease-free slide and a small amount of stool sample was spread over it. The examination was first done under low power (10 × ) with a compound light microscope and then under high power (40 × ).
2.3.1.2. Iodine wet mount method
About two gm of the fecal sample was emulsified in a drop of Lugol's Iodine solution on a clean glass slide and then covered with a clean cover-slip. The smear was examined under an electric microscope at 10 × and 40 × (Soulsby, 1982). This technique is generally used for the recovery of oocysts and motile trophozoites of protozoan parasites such as Eimeria spp. and Giardia spp. respectively.
2.3.2. Concentration techniques
Eggs, cysts, and trophozoites are often in such low number in feces that they are difficult to detect in direct smears or mounts. Therefore, the concentration procedures were performed which include floatation and sedimentation techniques (Soulsby, 1982; Zajac et al., 2021).
2.3.2.1. Floatation technique
This technique ensures the eggs float in the floatation liquid, which helps to identify the nematode and cestode eggs present in the macaque's feces. Approximately two grams of fecal sample was placed in a beaker and 28 ml of water was added. The sample was lightly mixed with the help of a rod and the solution was filtered by cotton gauge. The filtrate solution was poured into a 15 ml centrifuge tube, and centrifuged at 1000 rpm for 5 min. The tube's water was replaced with a super saturated ZnSO4 solution and again centrifuged. After being centrifuged, a higher saturated ZnSO4 solution was added to develop a convex meniscus at the top of the tube and one drop of Methylene blue was also added for staining purposes. A cover slip was placed for 5 min. It was then removed from the tube, placed on a glass slide, and examined at 10 × and 40 × . Photographs of the parasite eggs and cysts were taken and identified based on shape, shell, and size.
2.3.2.2. Sedimentation technique
The saturated ZnSO4 solution was carefully removed from the centrifuge tube after examination of the floatation portion and the sediment content was poured into a watch glass and stirred gently to mix it. One drop of the mixture was taken to prepare a second slide. The specimen was stained with Iodine wet mount's solution and examined at 10 × and 40 × . This technique is primarily used to identify eggs of internal parasites that do not float well due to high specific gravity, or the presence of an operculum (eggs of flukes and false tapeworms) such as eggs of trematodes. Following this technique, two slides were prepared from one sample (one from floatation and one from sedimentation) as Soulsby (1982).
2.4. Laboratory analysis and identification
The wet mount method and concentration (sedimentation and floatation) technique were used for fecal sample processing following Soulsby (1982) and Zajac et al. (2021). In this study, we followed Chatterjee (1976) for identification of parasites and helminthiasis. The study limited its investigation to Entamoeba histolytica and Entamoeba coli. To differentiate between trophozoites and cysts of these common intestinal Entamoeba species, we employed specific criteria, including parasite size, nuclei characteristics, and motility, as described by Fotedar et al. (2007), Li et al. (2015), Soulsby (1982), and Zajac et al. (2021). Additionally, we have updated the taxonomy nomenclature from Balantidium coli to Balantioides coli, in accordance with the revisions proposed by Li et al. (2020) and Ponce-Gordo and García-Rodríguez (2021). The study did not include an assessment of parasite egg density per gram of feces (OPG). This limitation is duly acknowledged.
2.5. Data analysis
The parasitic prevalence was expressed in percentage of the samples infected by the specific parasite and the intensity of the infection was the mean number of parasite cyst, oocyst, trophozoite eggs, or larvae per infected sample (Turgeon et al., 2018). The richness of parasites in each sample was expressed as the number of parasite species detected in the sample. For statistical analysis, the likelihood ratio test was used. In all cases, 95% confidence interval (CI) and P < 0.05 were considered for a statistically significant difference.
To assess the multivariate relationships among parasitic prevalence in response to the seasons and locations, we performed a Permutational Multivariate Analysis of Variance (PerMANOVA) at 999 permutations. In this analysis, parasitic prevalence served as the multivariate response variable, while seasons and locations acted as categorical explanatory variables.
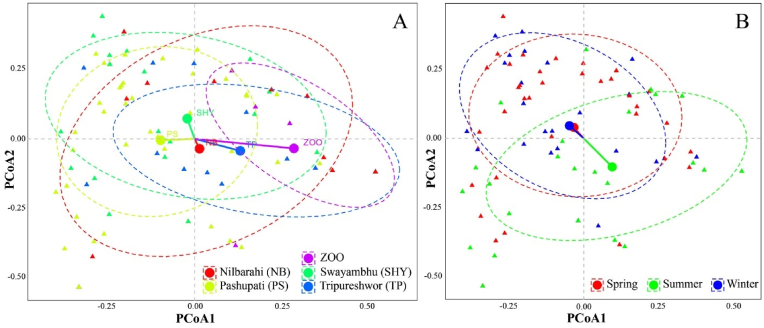
The distance between points in the sampled data was calculated using the Jaccard dissimilarity index because the multivariate response variables used in the analysis were present-or-absent (binary) variables. Additionally, pairwise Adonis tests were run using the “pairwiseAdonis” function in the “devtools” package (Martinez Arbizu, 2020) to determine whether there were significant differences in the multivariate response variable across different locations or seasons. To visualize the multivariate relationships and patterns revealed by PerMANOVA, we performed Principal Coordinate Analysis (PCoA) on the parasitic prevalence data. The PCoA scatterplots were used for the graphical representation of parasitic prevalence patterns across different locations and seasons, in which triangles represented coordinates of the individual data points and the big circles represented the centroid for each factor (location and season). The PCoA scatterplots were prepared using ggplot2 package in R version 4.2.1.
3. Results
3.1. Prevalence of intestinal parasites
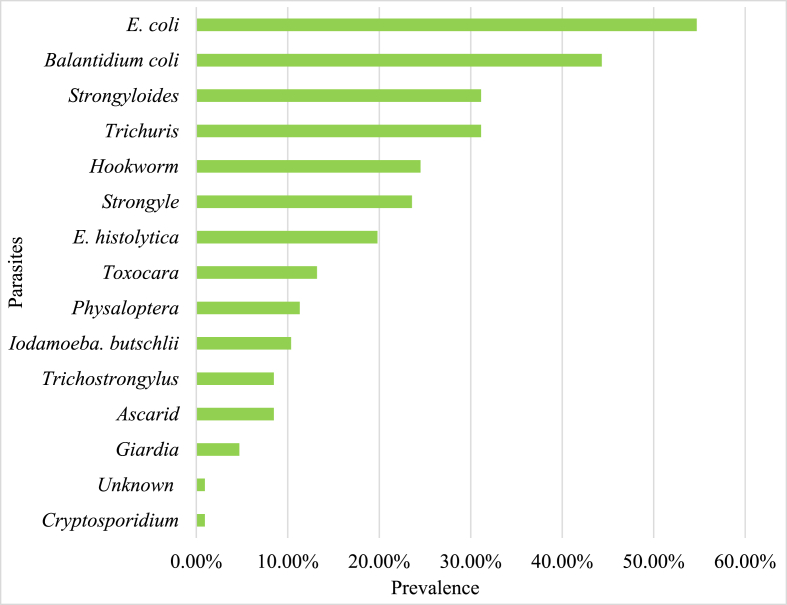
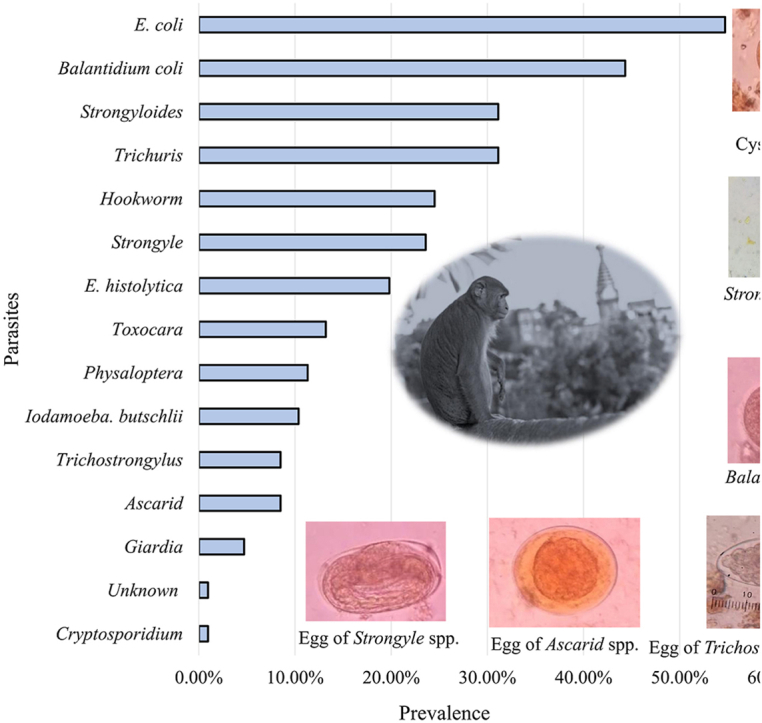
Among 121 fecal samples collected and examined, 106 (87.6%) were infected with one or more intestinal parasites. A total of 14 different species of intestinal parasites, including 5 protozoans, 1 coccidian, and 8 helminths, were identified. The most prevalent protozoan parasites were Entamoeba coli (54.71%) and Balantioides coli (44.33%).
The most prevalent helminth parasites included Trichuris spp. (31.13%) and Strongyloides spp. (31.13%) (Fig. 2, Fig. 3). A fecal smear test provided the first evidence of the presence of Iodamoeba butschlii in NHPs of Nepal (identified in samples from the Pashupatinath Temple, Swayambhunath Stupa and Tripureshwor Mahadev Temple with a 10% prevalence).
Fig. 2.
Prevalence of gastro-intestinal parasites in the rhesus macaques of Kathmandu Valley.
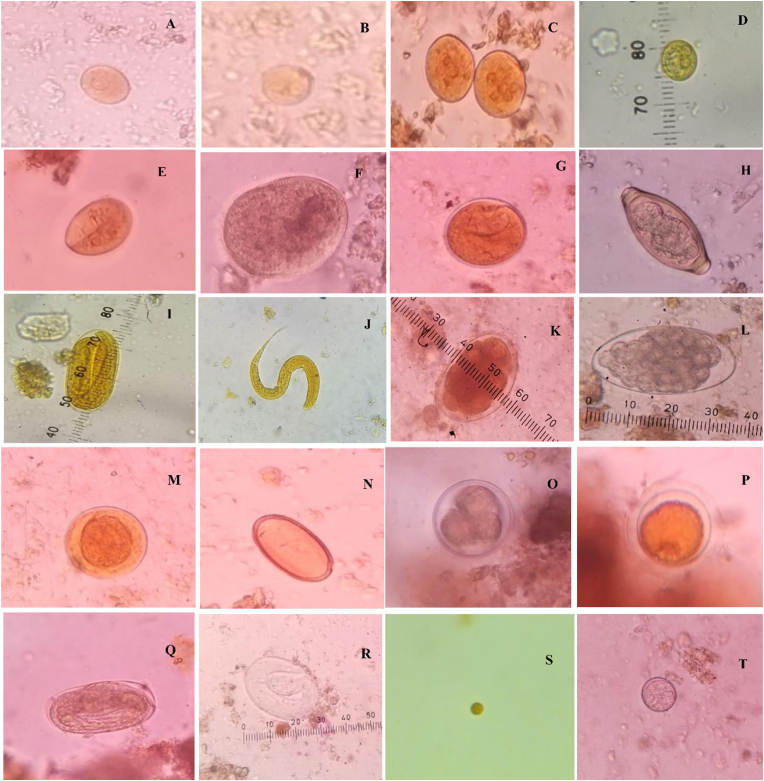
Fig. 3.
Photomicrographs of various GI parasites of the rhesus macaques at 400×: Trophozoite of E. histolytica (A), Cyst of E. histolytica (B), Cyst of E. coli (C), Cyst of Iodomoeba butschlii (D), Cyst of Giardia spp. (E), Trophozoite of Balantioides coli (F), Cyst of Balantioides coli (G), Egg of Trichuris spp. (H), Egg of Strongyloides spp. (I), Larva of Strongyloides spp. (J), Egg of Hookworm (K), Egg of Trichostrongylus spp. (L), Egg of Ascarid spp. (M), Egg of Physaloptera spp. (N), Egg of Toxocara spp.(O), Egg of Toxocara spp. (P), Egg of Strongyle spp. (Q), Egg of Strongyle spp.(R), Oocyst of Cryptosporidium spp. (S), Unknown spp. 1 (T).
3.2. Location-wise richness of GI parasites
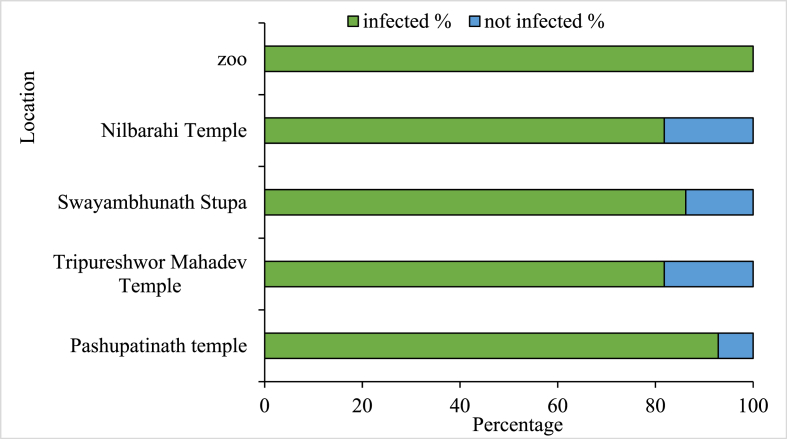
All the sampled locations had infection rates higher than 80%. Pashupatinath Temple (92.85%, n = 42) had the highest among the five sampling locations, followed by Swayambhunath Stupa (86.20%, n = 29), Tripureshwor Mahadev Temple (81.81%, n = 18), Nilbarahi Temple (81.81%, n = 18), and the Central Zoo (100%, n = 6) (Fig. 4).
Fig. 4.
Location-wise richness of parasitic infection in rhesus macaques of the Kathmandu Valley.
3.3. Species richness of GI parasite infection
The mean richness of parasites was 2.56 ± 0.25 (SD) species. A total of 87.56% of the samples had a parasite species richness of more than one, and 2.47% of the samples had a parasite species richness more than six. The most common species richness consisted of three parasite species, found in 23.96% of the samples, and 17.35% of the samples had only a single parasite species (Fig. 5).
Fig. 5.
Species Richness of GI parasite infection in rhesus macaques of the Kathmandu Valley.
3.4. Likelihood ratio test between season and the presence of parasites
The likelihood ratio test between seasons and the presence of parasites suggested that the prevalence of Iodamoeba butschlii, Giardia spp., Strongyloides spp., Hookworm, and Trichostrongylus spp. showed significant variation with seasons. Similarly, the presence of E. histolytica, E. coli, Iodamoeba butschlii, Trichuris spp., Trichostrongylus spp. and Unknown spp. 1 parasite showed the variation with location (Table 1).
Table 1.
Test of significance of difference in prevalence of GI parasites between the seasons and the sampling sites.
| Name of parasite | Prevalence of parasites by season |
Prevalence of parasites by site |
||
|---|---|---|---|---|
| χ2 | p value | χ2 | p value | |
| E. histolytica | 4.881 | 0.087 | 24.225 | 0.000 |
| E. coli | 1.288 | 0.525 | 11.300 | 0.023 |
| Iodamoeba butschlii | 10.351 | 0.006 | 13.242 | 0.010 |
| Giardia | 6.685 | 0.035 | 0.601 | 0.963 |
| Balantioides coli | 2.019 | 0.364 | 3.416 | 0.491 |
| Trichuris | 5.783 | 0.055 | 25.104 | 0.000 |
| Strongyloides | 1.776 | 0.411 | 1.600 | 0.809 |
| Strongyle | 14.023 | 0.001 | 8.126 | 0.087 |
| Ascaris | 1.773 | 0.412 | 3.059 | 0.548 |
| Toxocara | 4.388 | 0.111 | 5.054 | 0.282 |
| Physaloptera | 1.950 | 0.377 | 3.563 | 0.468 |
| Hookworm | 7.518 | 0.023 | 3.662 | 0.454 |
| Trichostrongylus | 7.812 | 0.020 | 10.347 | 0.035 |
| Cryptosporidium | 2.231 | 0.328 | 3.447 | 0.486 |
| Unknown sp 1 | 3.750 | 0.153 | 12.739 | 0.013 |
3.5. Parasitic prevalence in response to location and season
The PerMANOVA revealed a significant effect of location on parasite prevalence (F = 2.343, p < 0.001), indicating that the prevalence of parasites varied significantly among different locations. Pairwise Adonis tests indicated that parasitic prevalence at Swayambhu, Zoo, Tripureshwor and Nilbaharahi differed significantly with Pashupati (p < 0.01, p. adjusted <0.05, Fig. 6a). Further, the analysis revealed a significant influence of seasonality on the parasitic prevalence (F = 2.2197, p < 0.05). winter vs. summer (p < 0.05, p. adjusted <0.05) and spring vs. summer (p < 0.05, p. adjusted <0.05) were found to differ significantly in parasitic prevalence (Fig. 6b). These results suggest that locality and seasonality play a noticeable role in shaping the prevalence of parasitic infections in rhesus macaques.
Fig. 6.
The prevalence of parasites is examined in relation to a) location and b) season. Multicolor triangles and circles in the plots represent individual data points (triangles) and centroid of each specific grouping factor (circle).
4. Discussion
This study examined the prevalence and intensity of gastrointestinal (GI) parasites based on single sample per individual in urban rhesus macaques of the Kathmandu Valley. A total of 87.6% of the samples tested positive for one or more types of parasites. This finding was higher than previous studies in macaques of Nepal (Jha et al., 2011; Paudel, 2020; Pokhrel & Maharjan, 2014) but lower than the findings from Sapkota et al. (2020) who found 100% prevalence. A total of 12.3% of the monkeys in this study were free from GI parasites which could either due to a very low parasitic burden such that the parasitic output was too low to detect or they were in fact parasite free. This study revealed the presence of Iodamoeba butschlii in the NHPs of Nepal for the first time although it has been reported in NHPs in other countries (Levecke et al., 2007; Cordón et al., 2008; Kouassi et al., 2015). It also has been reported in domesticated livestock (Adhikari et al., 2021) and humans (Pandey et al., 2002; Moffat, 2003) in Nepal. The prevalence of this parasite is consistent with reports in pet macaques in Indonesia where a 21% prevalence was noted (Jones-Engel et al., 2004) and also in Chimpanzees of Cantanhez National Park (Sá et al., 2013). The prevalence in the current study, however, was lower than has been reported for M. fascicularis in a study by Zanzani et al. (2016) with 42.96% positive cases. The parasite may be transmitted to rhesus macaques from infected humans or livestock, especially from swine farming around the Kathmandu area. This zoonotic parasite can seriously damage the macaques' gastrointestinal tracts, resulting in symptoms including diarrhea and rectal prolapse (Burrows, 1972; Kuhn et al., 1997; Toft, 1986). As such, greater attention should be focused on monitoring parasite status and developing proactive approaches to risk mitigation in Kathmandu (Roberts et al., 2018; Roberts et al., 2020; Monecke et al., 2022; Napit et al., 2023).
Among the helminth parasites, Trichuris spp. and Strongyloides spp. showed the highest prevalence compared to the other parasites. Previous studies of NHPs in Nepal have reported similar prevalence rates: 51.61% Strongyloides spp. (Paudel, 2020) and 23.56% Trichuris spp. (Adhikari and Dhakal, 2018). The results however, differ from a number of other studies (Jha et al., 2011; Pokhrel & Maharjan, 2014; Sapkota, 2020). Climate change is altering the intestinal microbiome of wildlife, and these modifications may intensify the adverse effects of climate change (Risely et al., 2023). It is suggested that Oesophagostomum spp., Ascaris spp., and Trichuris spp. exists in a warm moist environment within temperate and tropical climates, with low light and wet soil, the high prevalence of Trichuris spp. in this study may indicate a potential change in climatic conditions (Schmidt and Roberts, 1977) of the Kathmandu Valley. If climate change leads to a warmer and/or moister climate, we could expect to see higher prevalence of these parasites. While lacking the longitudinal data to draw conclusions in the present study, but refer to the future study potential and how this is both timely and a pressing issue.
The rate of prevalence of Ascaris spp. was 8.49%, which is consisted with other studies from Nepal (11.82%) (Adhikari and Dhakal, 2018), (10.48%) (Paudel, 2020), (10.58%) (Pokhrel & Maharjan, 2014) and contrary with findings by Sapkota (2020) (21.4%). The prevalence of hookworm species was 24.52%. When compared to the reports by (Hilser, 2011; Pokhrel & Maharjan, 2014), the outcome was found to be much higher. Different parasite species may exist and thrive as a result of variation in environmental, genetics, gender and behavioral variables (Balasubramaniam et al., 2018). Mutani et al. (2003) who studied Cercopithecus aethiops sabaeus, documented the prevalence of Physaloptera spp. to be 58.5%. However, in the current study, the prevalence was much lower at 11.32%. Similarly, the rate of prevalence of Toxocara spp. was 13.20%, which was higher than the findings of Jha et al. (2011) and Paudel (2020). Toxocara typically infects members of the Canidae and Felidae families. The presence of this parasite in the macaques at the temple sites suggested that parasites are being exchanged between the macaques and canines (stray dogs) in study area where they share food and shelter. Obanda et al. (2019) suggested that a diverse array of gastrointestinal helminths flourishes within the interface zone that is frequented by wild ungulates, livestock, and non-human primates. Notably, many of these helminths exhibit a high degree of cross-species sharing among the host populations. Furthermore, Sirima et al., 2021 reported the prevalence of soil-transmitted helminth infections from wild non-human primate populations across various African nations.
Entamoeba coli was predominate among the protozoans with a prevalence of 54.71% which was lower than findings from China (89.96%) (Zhang et al., 2019), Nepal (66.7%) (Sapkota, 2020), but higher than those reported from other parts of Nepal (13.97% – 32%) (Adhikari and Dhakal, 2018; Bhattarai et al., 2019; Jha et al., 2011; Pokhrel & Maharjan, 2014), and from India (10% – 23.07%) (Jaiswal et al., 2014; Parmar et al., 2012). The prevalence rate of Cryptosporidium spp. was 0.94%, which is lower than the findings of Bhattarai et al. (2019) and (Sapkota, 2020) in different parks of Nepal. The prevalence of Giardia, however, was similar to that reported in a study by Sapkota (2020). Moreover, the prevalence of Balantioides coli is greater than that reported by Adhikari and Dhakal (2018); Bhattarai et al. (2019); Jha et al. (2011); and Pokhrel & Maharjan (2014) and but lower than the findings of Sapkota (2020). About one-fourth of the samples had a parasite richness of three species indicating the macaques' guts are highly infected. Similar co-infections were reported in macaques from temples in Lalitpur District, Nepal (Sapkota, 2020). All sampling locations in this study had very high rates of infection (>80%). The sacred but heavily polluted Bagmati River passes by the Pashupatinath Temple and Tripureshwor mahadev area, the Bishnumati River is near to the Swayambhunath Stupa and the Manahara River runs close to the Nilbarahi Temple. These rivers serve as public drainage systems of the Kathmandu Valley where macaques were often seen bathing, drinking or collecting food (Baral, 2014; Green, 2003). This in turn may have a significant impact on risk of infection.
In the current study, samples collected at the Central Zoo in Kathmandu, showed a 100% prevalence for Entamoeba coli, Hookworm, Balantioides coli, Entamoeba histolytica, Strongyloides spp., Ascaris spp. and unidentified parasites. These findings differed from the research with eight species of primates at the Rangpur Recreational Garden and Zoo of Bangladesh which found infection with only Trichuris spp. and Balantioides coli. (Khatun et al., 2014) and at the Nandan Van Zoo in Raipur where only Toxocara spp. was recorded in the captive rhesus macaques (Thawait et al., 2014). Li et al. (2015) emphasized that direct or indirect contact with contaminated food, water, or hands might elevate the risk of parasite transmission from primates to visitors or zoo keepers. Cibot et al. (2015) documented the circulation of three distinct Oesophagostomum species in both human and non-human primate populations within the Sebitoli region of Uganda. Thus, the parasites found in this study are among those associated with potential zoonotic risk to human health too and therefore appropriate animal care and husbandry protocols in zoo settings are essential to prevent the transmission of parasites.
5. Conclusion
Protozoan and helminthic gastrointestinal parasites are prevalent in the rhesus macaques of the Kathmandu Valley with multiple infections and high GI parasitic load. We confirmed the presence of Iodamoeba butschlii in NHPs of Nepal. Entamoeba coli was the most frequently present GI parasite, whereas Balantioides coli, Trichuris spp., Strongyloides spp. and Hookworm were among the more common ones. Iodamoeba butschlii, Giardia, Strongyloides, Hookworm, and Trichostrongylus prevalence varied significantly with season. Similarly, the presence of E. histolytica, E. coli, Iodamoeba butschlii, Trichuris, and Trichostrongylus showeda variation among study sites. Given the potential zoonotic health risks of these parasites, appropriate steps should be taken to mitigate pathogen transmission from macaques to humans and vice versa and to improve the habitat quality of rhesus macaques in the temples and shrines of the Kathmandu Valley.
Declaration of competing interest
The authors declare there is no conflict interest that could have appeared to influence the work reported in this paper.
Acknowledgements
We are thankful to Prof. Dr. Mahendra Maharjan (T.U) and Asst. Prof. Dr. Tirtha Raj Ghimire (T.U) for their support in identification of gastro-intestinal parasite species, Mr. Shailendra Sharma for support in data analysis. RCK's effort was supported in part by the National Institutes of Health (NIH) Office of Research Infrastructure Programs (ORIP) under award number P51OD010425 to the Washington National Primate Research Center, USA. The research paper is a part of MSc thesis of the first author AS. The MSc thesis research was supported by University Grants Commission Nepal (Grant no: MRS – 77/78 – S&T-42, Grant type: A).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2023.10.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adhikari P.P., Dhakal P. Prevalence of gastro-intestinal parasites of rhesus macaque (Macaca mulatta zimmermann, 1780) and hanuman langur (Semnopithecus entellus Dufresne, 1797) in Devghat, Chitwan, Nepal. Journal of Institute of Science and Technology. 2018;22(2):12–18. doi: 10.3126/jist.v22i2.19590. [DOI] [Google Scholar]
- Adhikari R.B., Adhikari Dhakal M., Thapa S., Ghimire T.R. Gastrointestinal parasites of indigenous pigs (Sus domesticus) in south‐central Nepal. Veterinary Medicine and Science. 2021;7(5):1820–1830. doi: 10.1002/vms3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrus M., Zainudin R., Ahamad M., Jayasilan M.-A., Abdullah M.T. Gastrointestinal parasites of zoonotic importance observed in the wild, urban, and captive populations of non-human primates in Malaysia. J. Med. Primatol. 2019;48(1):22–31. doi: 10.1111/jmp.12389. [DOI] [PubMed] [Google Scholar]
- Arunachalam K., Senthilvel K., Anbarasi P. Vol. 6. 2015. https://www.cabdirect.org/cabdirect/abstract/20153292760 (Endo Parasitic Infections in Free Living Rhesus Macaque Macaca mulatta of Namakkal, Tamil Nadu, India). [Google Scholar]
- Balasubramaniam K.N., Beisner B.A., Berman C.M., De Marco A., Duboscq J., Koirala S., Majolo B., MacIntosh A.J., McFarland R., Molesti S., Ogawa H. The influence of phylogeny, social style, and sociodemographic factors on macaque social network structure. Am. J. Primatol. 2018;80(1) doi: 10.1002/ajp.22727. [DOI] [PubMed] [Google Scholar]
- Baral K. Norwegian University of Life Sciences; 2014. Conservation and Threat to Selected Monkey Species in Nepal Compared to Selected Species in Tanzania [Master's Thesis.https://static02.nmbu.no/mina/studier/moppgaver/2014-Baral.pdf Ås] [Google Scholar]
- Bhattarai B.P., Adhikari J.N., Dhakal D.N. Impact of prevalence of gastrointestinal parasites in rhesus macaque (Macaca mulatta Zimmermann, 1780) in Chitwan-Annapurna landscape, Nepal. International Journal of Zoology Studies. 2019;4(2):34–42. doi: 10.3126/njz.v6iS1.50527. [DOI] [Google Scholar]
- Brown C. Emerging zoonoses and pathogens of public health significance–an overview. Revue Scientifique et Technique-Office International Des Epizooties. 2004;23(2):435–442. doi: 10.20506/rst.23.2.1495. [DOI] [PubMed] [Google Scholar]
- Burrows R.B. Pathology of Simian Primates. Karger Publishers; 1972. Protozoa of the intestinal tract; pp. 2–28. [DOI] [Google Scholar]
- Cawthon L.K. Primate factsheets: rhesus macaque (Macaca mulatta) taxonomy, morphology, and ecology. 2005. https://primate.wisc.edu/primate-info-net/pin-factsheets/pin-factsheet-rhesus-macaque/#evolution-ecology July 20.
- Chalise M.K. Primate census in different parts of Nepal. Journal of the University Campus TUTA, TU, Prospective on Higher Education. 2006;2(3):35–41. [Google Scholar]
- Chalise M.K., Bhattarai G.P., Pandey B. Ecology and behavior of Assamese monkeys in shivapuri nagarjun national park, Nepal. J. Nat. Hist. Mus. 2013;27:12–24. doi: 10.3126/jnhm.v27i0.14149. [DOI] [Google Scholar]
- Chapman C.A., Gillespie T.R., Goldberg T.L. Primates and the ecology of their infectious diseases: how will anthropogenic change affect host-parasite interactions? Evol. Anthropol. Issues News Rev. 2005;14(4):134–144. doi: 10.1002/evan.20068. [DOI] [Google Scholar]
- Chatterjee K.D. Cuha Ray Sree Saraswathy Press Ltd; 1976. Parasitology and Helminthology. [Google Scholar]
- Cibot M., Guillot J., Lafosse S., Bon C., Seguya A., Krief S. Nodular worm infections in wild non-human primates and humans living in the Sebitoli area (Kibale National Park, Uganda): do high spatial proximity favor zoonotic transmission? PLoS Neglected Trop. Dis. 2015;9(10) doi: 10.1371/journal.pntd.0004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordón G.P., Prados A.H., Romero D., Moreno M.S., Pontes A., Osuna A., Rosales M.J. Intestinal parasitism in the animals of the zoological garden “Peña Escrita” (Almuñecar, Spain) Vet. Parasitol. 2008;156(3–4):302–309. doi: 10.1016/j.vetpar.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Dogel V.A. 1964. General Parasitology. General Parasitology.https://www.cabdirect.org/cabdirect/abstract/19650802987 [Google Scholar]
- Fotedar R., Stark D., Beebe N., Marriott D., Ellis J., Harkness J. Laboratory Diagnostic techniques for Entamoeba species. Clin. Microbiol. Rev. 2007;20(3):511–532. doi: 10.1128/cmr.00004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie T.R., Lonsdorf E.V., Canfield E.P., Meyer D.J., Nadler Y., Raphael J., Pusey A.E., Pond J., Pauley J., Mlengeya T. Demographic and ecological effects on patterns of parasitism in eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe National Park, Tanzania. Am. J. Phys. Anthropol. 2010;143(4):534–544. doi: 10.1002/ajpa.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H.M. Doctoral dissertation, Massachusetts Institute of Technology; 2003. The Effects of Carpet Dye on the Bagmati River. [Google Scholar]
- Hilser H. An assessment of primate health in the Sabangau peat-swamp forest, Central Kalimantan, Indonesian Borneo. Cover By. 2011:5. [Google Scholar]
- Hsu M.J., Kao C.C., Agoramoorthy G. Interactions between visitors and formosan macaques (Macaca cyclopis) at shou‐Shan nature park, taiwan. Am. J. Primatol.: Official Journal of the American Society of Primatologists. 2009;71(3):214–222. doi: 10.1002/ajp.20638. [DOI] [PubMed] [Google Scholar]
- Huffman M.A., Nahallage C.A.D., Hasegawa H., Ekanayake S., De Silva L., Athauda I.R.K. Preliminary survey of the distribution of four potentially zoonotic parasite species among primates in Sri Lanka. J. Natl. Sci. Found. Sri Lanka. 2013;41(4) doi: 10.4038/jnsfsr.v41i4.6246. [DOI] [Google Scholar]
- Jaiswal A.K., Sudan V., Kanojiya D., Sachan A., Shanker D. A pilot study on gastrointestinal parasites of monkeys (Macaca mulatta) of Mathura-Vrindavan areas, India. J. Vet. Parasitol. 2014;28(1):66–68. [Google Scholar]
- Jha A., Chalise M.K., Shrestha R.M., Karki K. Intestinal parasitic investigation in temple rhesus monkeys of Kathmandu. Initiation. 2011;4:1–7. doi: 10.3126/init.v4i0.5530. [DOI] [Google Scholar]
- Jones-Engel L., Engel G.A., Schillaci M.A., Froehlich J., Paputungan U., Kyes R.C. Prevalence of enteric parasites in pet macaques in Sulawesi, Indonesia. Am. J. Primatol. 2004;62(2):71–82. doi: 10.1002/ajp.20008. [DOI] [PubMed] [Google Scholar]
- Jones-Engel L., Engel G.A., Schillaci M.A., Lee B., Heidrich J., Chalise M., Kyes R.C. Considering human–primate transmission of measles virus through the prism of risk analysis. Am. J. Primatol. 2006;68(9):868–879. doi: 10.1002/ajp.20294. [DOI] [PubMed] [Google Scholar]
- Jones-Engel L., Engel G.A., Heidrich J., Chalise M., Poudel N., Viscidi R., Barry P.A., Allan J.S., Grant R., Kyes R. Temple monkeys and health implications of commensalism, Kathmandu, Nepal. Emerg. Infect. Dis. 2006;12(6):900. doi: 10.3201/eid1206.060030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun M.M., Begum N., Mamun M.A.A., Mondal M.M.H., Azam M.S.U. Coprological study of gastrointestinal parasites of captive animals at Rangpur Recreational Garden and Zoo in Bangladesh. J. Threat. Taxa. 2014;6(8):6142–6147. doi: 10.11609/JoTT.o3093.6142-7. [DOI] [Google Scholar]
- Kouassi R.Y.W., McGraw S.W., Yao P.K., Abou-Bacar A., Brunet J., Pesson B., Bonfoh B., N’goran E.K., Candolfi E. Diversity and prevalence of gastrointestinal parasites in seven non-human primates of the Taï National Park, Côte d'Ivoire. Parasite. 2015;22 doi: 10.1051/parasite/2015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E.-M., Mätz-Rensing K., Stahl-Hennig C., Makoschey B., Hunsmann G., Kaup F.-J. Intestinal manifestations of experimental SIV-infection in rhesus monkeys (Macaca mulatta): a histological and ultrastructural study. J. Vet. Med. - Ser. B. 1997;44(1–10):501–512. doi: 10.1111/j.1439-0450.1997.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Levecke B., Dorny P., Geurden T., Vercammen F., Vercruysse J. Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet. Parasitol. 2007;148(3–4):236–246. doi: 10.1016/j.vetpar.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Li M., Zhao B., Li B., Wang Q., Niu L., Deng J., Gu X., Peng X., Wang T., Yang G. Prevalence of gastrointestinal parasites in captive non-human primates of twenty-four zoological gardens in China. J. Med. Primatol. 2015;44(3):168–173. doi: 10.1111/jmp.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-H., Yao Q., Dong H.-P., Wang S.-S., Chen R.-R., Song J.-K., Yan W.-C., Zhao G.-H. Molecular characterization of Balantioides coli in pigs from Shaanxi province, northwestern China. Parasitol. Res. 2020;119(9):3075–3081. doi: 10.1007/s00436-020-06800-6. [DOI] [PubMed] [Google Scholar]
- Mahapatra P.S., Puppala S.P., Adhikary B., Shrestha K.L., Dawadi D.P., Paudel S.P., Panday A.K. Air quality trends of the Kathmandu Valley: a satellite, observation and modeling perspective. Atmos. Environ. 2019;201:334–347. doi: 10.1016/j.atmosenv.2018.12.043. [DOI] [Google Scholar]
- Mariadoss A., Naresh B., Sakthivel P., Sujeetha A.R.P. Damages by non-human primates and management strategies in agroecosystem. Innovative Farming. 2019;4(1) Article 1. [Google Scholar]
- Martinez Arbizu P. 2020. pairwiseAdonis: pairwise multilevel comparison using adonis. R package version 0.4. [Google Scholar]
- Moffat T. Diarrhea, respiratory infections, protozoan gastrointestinal parasites, and child growth in Kathmandu, Nepal. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2003;122(1):85–97. doi: 10.1002/ajpa.10258. [DOI] [PubMed] [Google Scholar]
- Mogaji H.O., Dedeke G.A., Bada B.S., Bankole S., Adeniji A., Fagbenro M.T., Omitola O.O., Oluwole A.S., Odoemene N.S., Abe E.M., Mafiana C.F., Ekpo U.F. Distribution of ascariasis, trichuriasis and hookworm infections in ogun state, southwestern Nigeria. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0233423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monecke S., Roberts M.C., Braun S.D., Diezel C., Müller E., Reinicke M., Linde J., Joshi P.R., Paudel S., Acharya M., Chalise M.K., Feßler A.T., Hotzel H., Khanal L., Koju N.P., Schwarz S., Kyes R.C., Ehricht R. Sequence analysis of novel Staphylococcus aureus lineages from wild and captive macaques. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms231911225. Online: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munene E., Otsyula M., Mbaabu D.A.N., Mutahi W.T., Muriuki S.M.K., Muchemi G.M. Helminth and protozoan gastrointestinal tract parasites in captive and wild-trapped African non-human primates. Vet. Parasitol. 1998;78(3):195–201. doi: 10.1016/S0304-4017(98)00143-5. [DOI] [PubMed] [Google Scholar]
- Mutani A., Rhynd K., Brown G. A preliminary investigation on the gastrointestinal helminths of the Barbados green monkey. Cercopithecus aethiops sabaeus. 2003;45:193–195. doi: 10.1590/S0036-46652003000400003. Revista do Instituto de Medicina Tropical de São Paulo. [DOI] [PubMed] [Google Scholar]
- Napit R., Manandhar P., Poudel A., Rajbhandari P.G., Watson S., Shakya S., Pradhan S.M., Sharma A.N., Chaudhary A., Johnson C.K., Mazet J.K. Novel strains of Campylobacter cause diarrheal outbreak in Rhesus macaques (Macaca mulatta) of Kathmandu Valley. PLoS One. 2023;18(3) doi: 10.1371/journal.pone.0270778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obanda V., Maingi N., Muchemi G., Ng’ang’a C.J., Angelone S., Archie E.A. Infection dynamics of gastrointestinal helminths in sympatric non-human primates, livestock and wild ruminants in Kenya. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0217929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey B.D., Thapa L.B., Sherchand J.B., Rimal N., Bhattarai A., Morita K. Etiology of diarrhoea among adult patients during the early monsoon period in Kathmandu, Nepal. Japanese Journal of Tropical Medicine and Hygiene. 2002;30(2):133–137. doi: 10.2149/tmh1973.30.133. [DOI] [Google Scholar]
- Parmar S.M., Jani R.G., Mathakiya R.A. Study of parasitic infections in non-human primates of Gujarat state, India. Vet. World. 2012;5(6):362. doi: 10.5455/vetworld.2012.362-364. [DOI] [Google Scholar]
- Paudel N. Prevalence of intestinal helminth parasites in Macaca mulatta and their unmanaged consequences in Nepal. Journal of Multidisciplinary Sciences. 2020;2(2):1–7. doi: 10.33888/jms.2020.221. [DOI] [Google Scholar]
- Pedersen A.B., Davies T.J. Cross-species pathogen transmission and disease emergence in primates. EcoHealth. 2009;6(4):496–508. doi: 10.1007/s10393-010-0284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhrel G., Maharjan M. Gastro-intestinal parasites of Assamese macaque (Macaca assamensis hodgson, 1840) in shivapuri nagarjun national park, Kathmandu, Nepal. Journal of Institute of Science and Technology. 2014;19(2):53–57. doi: 10.3126/jist.v19i2.13852. [DOI] [Google Scholar]
- Ponce-Gordo F., García-Rodríguez J.J. Balantioides coli. Res. Vet. Sci. 2021;135:424–431. doi: 10.1016/j.rvsc.2020.10.028. [DOI] [PubMed] [Google Scholar]
- Risely A., Müller‐Klein N., Schmid D.W., Wilhelm K., Clutton‐Brock T.H., Manser M.B., Sommer S. Climate change drives loss of bacterial gut mutualists at the expense of host survival in wild meerkats. Global Change Biol. 2023;29(20):5816–5828. doi: 10.1111/gcb.16877. [DOI] [PubMed] [Google Scholar]
- Roberts M.C., Joshi P.R., Greninger A.L., Melendez D., Paudel S., Acharya M., et al. The human clone ST22 SCC mec IV methicillin-resistant Staphylococcus aureus isolated from swine herds and wild primates in Nepal: is man the common source? FEMS Microbiol. Ecol. 2018;94(5):fiy052. doi: 10.1093/femsec/fiy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M.C., Joshi P.R., Monecke S., Ehricht R., Müller E., Gawlik D., Diezel C., Braun S.D., Paudel S., Acharya M., Khanal L. Staphylococcus aureus and methicillin resistant S. aureus in Nepalese primates: resistance to antimicrobials, virulence, and genetic lineages. Antibiotics. 2020;9(10):689. doi: 10.3390/antibiotics9100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá R.M., Petrášová J., Pomajbíková K., Profousová I., Petrželková K.J., Sousa C., Cable J., Bruford M.W., Modrý D. Gastrointestinal symbionts of chimpanzees in Cantanhez national park, Guinea-bissau with respect to habitat fragmentation: GI symbionts in Guinea-bissau chimpanzees. Am. J. Primatol. 2013;75(10):1032–1041. doi: 10.1002/ajp.22170. [DOI] [PubMed] [Google Scholar]
- Sapkota B. Department of Zoology; 2020. Population Status and Threats to Survival of Rhesus Monkey Macaca mulatta (Zimmermann, 1780) in Bajrabarahi Area, Lalitpur Nepal [PhD Thesis.https://elibrary.tucl.edu.np/handle/123456789/10700 [Google Scholar]
- Sapkota B., Adhikari R.B., Regmi G.R., Bhattarai B.P., Ghimire T.R. Diversity and prevalence of gut parasites in urban macaques. Applied Science and Technology Annals. 2020;1(1):34–41. doi: 10.3126/asta.v1i1.30270. [DOI] [Google Scholar]
- Schmidt G.D., Roberts L.S. CV Mosby Company, 11830 Westline Industrial Drive; St. Louis: 1977. Foundations of Parasitology.https://www.cabdirect.org/cabdirect/abstract/19782901950 Missouri 63141. [Google Scholar]
- Schurer J.M., Ramirez V., Kyes P., Tanee T., Patarapadungkit N., Thamsenanupap P., Trufan S., Grant E.T., Garland-Lewis G., Kelley S., Nueaitong H., Kyes R.C., Rabinowitz P. Long-tailed macaques (Macaca fascicularis) in urban landscapes: gastrointestinal parasitism and barriers for healthy coexistence in northeast Thailand. Am. J. Trop. Med. Hyg. 2019;100(2):357–364. doi: 10.4269/ajtmh.18-0241. 10.4269%2Fajtmh.18-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirima C., Bizet C., Hamou H., Červená B., Lemarcis T., Esteban A., Peeters M., Mpoudi Ngole E., Mombo I.M., Liégeois F., Petrželková K.J. Soil-transmitted helminth infections in free-ranging non-human primates from Cameroon and Gabon. Parasites Vectors. 2021;14(1):1–18. doi: 10.1186/s13071-021-04855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulsby E.J.L. Vol. 291. 1982. https://www.cabdirect.org/cabdirect/abstract/19682902735 (Helminths. Arthropods and Protozoa of Domesticated Animals). [Google Scholar]
- Tabasshum T., Mukutmoni M., Begum A. Occurrence of gastrointestinal helminths in captive rhesus macaques (Macaca mulatta) Bangladesh J. Zool. 2018;46(2):231–237. doi: 10.3329/bjz.v46i2.39065. [DOI] [Google Scholar]
- Thawait V.K., Maiti S.K., Dixit A.A. Prevalence of gastro-intestinal parasites in captive wild animals of Nandan Van Zoo, Raipur, Chhattisgarh. Vet. World. 2014;7(7):448–451. doi: 10.14202/vetworld.2014.448-451. [DOI] [Google Scholar]
- Toft J.D. The pathoparasitology of nonhuman primates: a review. Primates. 1986:571–679. doi: 10.1007/978-1-4612-4918-4_45. [DOI] [Google Scholar]
- Turgeon G., Kutz S.J., Lejeune M., St-Laurent M.H., Pelletier F. Parasite prevalence, infection intensity and richness in an endangered population, the Atlantic-Gaspésie caribou. Int. J. Parasitol.: Parasites and Wildlife. 2018;7(1):90–94. doi: 10.1016/j.ijppaw.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac A.M., Conboy G.A., Little S.E., Reichard M.V. John Wiley & Sons; 2021. Veterinary Clinical Parasitology.https://www.wiley.com/go/zajac/parasitology [Google Scholar]
- Zanzani S., Gazzonis A., Epis S., Manfredi M. Study of the gastrointestinal parasitic fauna of captive non-human primates (Macaca fascicularis) Parasitol. Res. 2016;115 doi: 10.1007/s00436-015-4748-9. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Liu K., Wang C., Luo J., Lu J., He H. Molecular characterization of Entamoeba spp. in wild taihangshan macaques (Macaca mulatta tcheliensis) in China. Acta Parasitol. 2019;64(2):228–231. doi: 10.2478/s11686-019-00026-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.