Abstract
The cellular stress response (SR) is a phylogenetically conserved protection mechanism that involves inhibition of protein synthesis through recruitment of translation factors such as eIF4G into insoluble stress granules (SGs) and blockade of proinflammatory responses by interruption of the signaling pathway from tumor necrosis factor alpha (TNF-α) to nuclear factor-κB (NF-κB) activation. However, the link between these two physiological phenomena has not been clearly elucidated. Here we report that eIF4GI, which is a scaffold protein interacting with many translation factors, interacts with TRAF2, a signaling molecule that plays a key role in activation of NF-κB through TNF-α. These two proteins colocalize in SGs during cellular exposure to stress conditions. Moreover, TRAF2 is absent from TNFR1 complexes under stress conditions even after TNF-α treatment. This suggests that stressed cells lower their biological activities by sequestration of translation factors and TRAF2 into SGs through a protein-protein interaction.
Virtually all organisms, from prokaryotes to humans, are capable of mounting the stress response (SR) for cellular survival under stress conditions. Pretreatment of an organism with a sublethal stress can protect that organism from subsequent lethal shock responses to a more severe stress. For instance, high-temperature preconditioning reduces acute lung injury, septic shock, and liver damage caused by ischemia reperfusion injury in mice, resulting in a dramatic increase in survival rate (19, 30, 40). These phenomena are attributed to the inhibition of the proinflammatory nuclear factor-κB (NF-κB) signal transduction pathway by hyperthermia preconditioning (30, 31, 44). Hsp70 was suggested to be responsible for heat-induced protection against tumor necrosis factor alpha (TNF-α)-induced lethal inflammatory shock (32, 38, 46, 47), although inhibition of TNF signaling was also demonstrated in the absence of Hsp70 expression in cells preconditioned with heat (30, 44) or sodium arsenite (SA) treatments (18).
At the cellular level, discrete cytoplasmic foci called stress granules (SGs) are formed in environmentally stressed eukaryotic cells (1, 2, 21, 23, 24, 26, 29, 36, 41), perhaps through the action of translation factor eIF2α (24, 29). In heat-, thapsigargin-, or arsenite-treated cells, the SGs contain most of the components of the translational 48S preinitiation complex (i.e., small, but not large, ribosomal subunits, eIF3, eIF4E, eIF4G, eIF2, and eIF2B), other RNA-binding proteins such as T-cell-restricted intracellular antigens-1 (TIA-1) and T-cell-restricted intracellular antigen-related protein (TIAR), and untranslated mRNAs (1, 2, 23, 24, 26). As a consequence, mRNA translation is generally inhibited under stress conditions (14, 43). However, the relationship between the translational blockage and the anti-inflammatory response has not been clearly elucidated in previous work (13). Here we demonstrate a molecular link between these two physiological phenomena.
MATERIALS AND METHODS
Plasmid construction.
To obtain the specific cDNA sequence of eIF4GI, cDNA corresponding to amino acids (aa) 1 to 370 of eIF4GI was amplified from Chang liver cDNA library (6) by nested PCR using four primers: 5′CAAGCGACACAAATGAACAC3′ and 5′CTCTGGGCTTGACTCCACCTC3′ for the first round of PCR and 5′AGGTTACCCGGGAACCATGAACACGCCTTCTCAGC3′ and 5′AGGTTACTGCAGTTACAGATCTTCAGATGGGAC3′ for the second round of PCR. The amplified cDNA fragment and plasmid pSK(−) were digested with SmaI and PstI and then ligated together to construct pSK-eIF4GI(1-370). The sequence of eIF4GI was confirmed by sequencing. To construct pSK-eIF4GI(full length), pSK-eIF4GI(1-370) was treated with EcoRI. To generate eIF4GI, a full-length eIF4GI clone, plasmids pSK-eIF4GI (1-370) and pSK-HFCI (provided by Robert E. Rhoads, Louisiana State University) were treated with EcoRI and then ligated together. To construct pGBT9-eIF4GI(182-589), pSK-HFCI treated with XmaI-Klenow was inserted into pGBT9 treated with SmaI. To construct pGBT9-eIF4GI(182-415), pGBT9-eIF4GI(182-589) was treated with AflII-BamHI-Klenow and then self-ligated. To construct pGBT9-eIF4GI(415-589), pGBT9-eIF4GI(182-589) was treated with AflII-Klenow-SalI and the inserted into pGBT9 treated with BamHI-Klenow-SalI. To construct pRSET-A-eIF4GI(182-549), pSK-HFCI was treated with EcoRI-EcoNI-Klenow and then inserted into pRSET-A treated with NheI-Klenow-PvuI.
To construct the plasmid pGBT9-eIF4GI series used in the Y2H assays shown in Fig. 1D, cDNAs corresponding to the indicated amino acids were amplified from pSK-eIF4GI by PCRs using proper pairs of primers. The amplified cDNA fragments and pGBT9 were digested with EcoRI and BamHI and then ligated together to generate a series of plasmids. The clones were confirmed by DNA sequencing. To construct pEBG-eIF4GIΔC(1-509), pSK-eIF4GI(full length) was treated with XmaI-EcoNI-Klenow and then inserted into pEBG-Mnk1 (provided by Robert J. Schneider, New York University) treated with BamHI-Klenow. To construct pEBG-eIF4GI(full length), pSK-eIF4GI(full length) was treated with XbaI-HindIII-Klenow and then inserted into pEBG-Mnk1 treated with BamHI-Klenow. To construct pEBG, pEBG-Mnk1 was treated with BamHI-Klenow and self-ligated. To construct pEBG-eIF4GI(293-303), PCR was performed with oligonucleotides 5′CGGGATCCCGATACAAATGGCCGTAGAAGCC3′ and 5′CCATCGATGGCTAGATGGGGGTTGAGGCTTCTA3′. The amplified DNA fragment and pEBG were digested with BamHI-ClaI and then ligated together. To construct pEBG-eIF4GI(293-303) mut, PCR was performed with the two oligonucleotides 5′CGGGATCCCGATACAAATGTCTGTAGAAGAA3′ and 5′CCATCGATGGCTAGATGGGGGTTGATTCTTCTA3′. The amplified DNA fragment and pEBG were digested with BamHI-ClaI and then ligated together. Note that the underlined regions in the oligonucleotides can anneal each other. To construct pEGFP-CI-eIF4GI, pSK-eIF4GI(full length) was treated with XbaI-KpnI-Klenow and then inserted into pEGFP-CI treated with HindIII-Klenow.
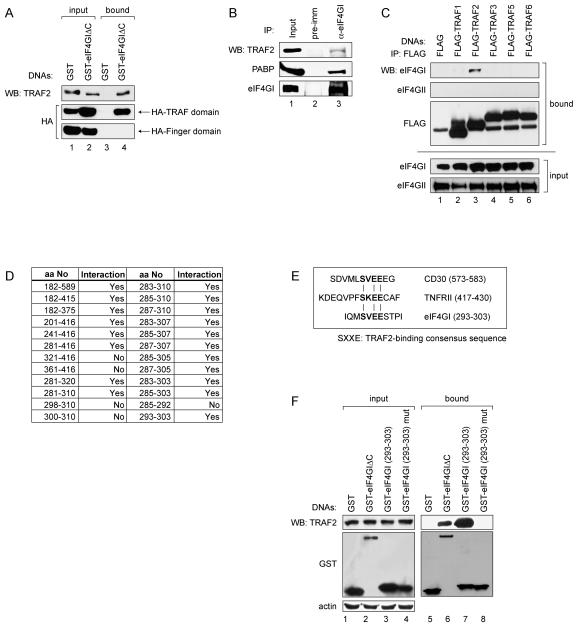
FIG. 1.
eIF4GI interacts with TRAF2. (A) 293T cells were transfected with plasmids expressing GST (lanes 1 and 3), GST-tagged eIF4GIΔC (lanes 2 and 4), and the HA-tagged TRAF and Finger domains of TRAF2 (panels labeled “HA-Finger domain” and “HA-TRAF domain”). The input panels show proteins in the cell extract before immunoprecipitation (1/50 of lysatens used in immunoprecipitation). Lanes 3 and 4 show proteins precipitated by GST-resin. WB, Western blotting. (B) HeLa cell extracts were subjected to immunoprecipitation using a rabbit preimmune serum (lane 2) or anti-eIF4GI antibody (lane3) and then analyzed by WB with antibodies against TRAF2 (panel labeled “TRAF2”), PABP (panel labeled “PABP”), and eIF4GI (panel labeled “eIF4GI”). (C) 293T cells were transfected with plasmids expressing FLAG or FLAG-tagged TRAF1, TRAF2, TRAF3, TRAF5, or TRAF6. Cell lysates were subjected to immunoprecipitation using an anti-FLAG monoclonal antibody and then analyzed by WB using anti-eIF4GI or eIF4GII antibodies. (D) Y2H analysis of TRAF2-binding region in eIF4GI. The boxed numbers indicate the amino acid number in eIF4GI included in the bait vector. Positive and negative results in the Y2H analyses are indicated by “Yes” and “No,” respectively. (E) TRAF2-binding motifs in CD30, TNFRII, and eIF4GI. (F) 293T cells were transfected with plasmids expressing GST (lanes 1 and 5), GST-tagged eIF4GIΔC (lanes 2 and 6), GST-eIF4GI (293-303) (lanes 3 and 7), and mutant GST-eIF4GI (293-303) containing an AVEA sequence instead of SVEE in the consensus motif (lanes 4 and 8). Lanes 1 to 4 and 5 to 8 show proteins in the cell extract before and after precipitation with GST-resin, respectively.
To obtain a cDNA clone of eIF4GII, nested PCR was performed using a Chang liver cDNA library (6). Four primers, 5′ACGCTGGTATACCCTCAAAC3′ and 5′TGATAATTTTTCGCTAACTCC3′ for the first round of PCR and 5′AGGTTACCCGGGAACCATGAATTCACAACCTCAAAC3′ and 5′AGGTTACCCGGGTTAATTAATTAGGTTAGTACTAG3′ for the second round of PCR, were used to generate nucleotides encoding amino acids 1 to 357 of eIF4GII. The amplified cDNA and plasmid pSK(−) were digested with SmaI and then ligated together to construct pSK-eIF4GII(1-357). 5′ CTGTCACTGCAGCTAGCGAC3′ and 5′CCGGAAATTCACTGTGTTAC3′ were used to generate nucleotides encoding aa 209 to 834 of eIF4GII, which was digested with NheI and NcoI and then ligated into NheI-NcoI-digested pSK(−) to construct pSK-eIF4GII(209-834). 5′GGCTTACGCAAACATGTGTC3′ and 5′TCCCCGCGGGGATTAGTTATCCTCAGACTC3′ were used to generate nucleotides encoding aa 808 to 1585 of eIF4GII, which was digested with NcoI and KspI and then ligated into NcoI-KspI-digested pSK(−) to construct pSK-eIF4GII(808-1585). To construct a full-length eIF4GII clone, pSK-eIF4GII, nucleotides generated from both NheI-NcoI-digested pSK-eIF4GII(209-834) and NcoI-KspI-digested pSK-eIF4GII(808-1585) were inserted into NheI-KspI-digested pSK-eIF4GII(1-357). The clone was confirmed by DNA sequencing. To construct pRSET-A-eIF4GII(197-545), nested PCR was performed using pSK-eIF4GII. 5′CGCGGATCCGCGGGGACTGTGGAGAGCGCTCATC3′ and 5′CCGGAATTCCGGTCAATCTTGTTCAGATTCAAGTAC3′ were used to generate nucleotides encoding aa 197 to 545 of eIF4GII, which was digested with BamHI and EcoRI and then inserted into BamHI-EcoRI-digested pRSET-A.
Plasmid pAD/GAL4-2-1-TRAF2 is the original clone isolated from the Chang liver cDNA library. To construct pTM1-TRAF2, pAD/GAL4-2-1-TRAF2 was treated with BamHI-SalI-Klenow and then inserted into pTM1 treated with StuI-NcoI-Klenow. To construct pCMV-HA-TRAF2, pTM1-TRAF2 was treated with NcoI-SalI-Klenow and then inserted into pCMV-HA treated with EcoRI-SmaI-Klenow. To construct pAD/GAL4-2-1-Finger domain, pAD/GAL4-2-1-TRAF2 was treated with BamHI-Klenow and then self-ligated. To construct pAD/GAL4-2-1-TRAF domain, pSK-TRAF domain was treated with BamHI-EcoRV and then inserted into pAD/GAL4-2-1-TRAF2 treated with SalI-Klenow-BamHI. To construct pSK-TRAF domain, pAD/GAL4-2-1-TRAF2 was treated with DraIII-BstXI-T4 polymerase and then inserted into pSK treated with SmaI. pRK-FLAG-TRAF1, pRK-FLAG-TRAF2, pRK-FLAG-TRAF3, pRK-FLAG-TRAF5, and pRK-FLAG-TRAF6 were provided by D. V. Goeddel of Tularik Inc. To construct pDsRed-TRAF2, pRK-FLAG-TRAF2 was treated with HindIII-Klenow-BamHI and then inserted into pDsRed-CI treated with XbaI-Klenow-BamHI.
Antibodies and chemicals.
Antibody against eIF4GI and eIF4GII was prepared in our lab (see below). TRAF2 was purchased from BD Pharmingen, FLAG was purchased from Sigma, actin was purchased from ICN, hsp27 was purchased from StressGen, and phospho-eIF2α was purchased from Cell Signaling Technology. Antibodies against hemagglutinin (HA), TIA-1, TNFR1, lamin A/C, hsp70, eIF3η, eIF4AII, DAP5, p65, and RIP were purchased from Santa Cruz. Antibodies against PABP and PTB4 were gifts of Gideon Dreyfuss of Howard Hughes Medical Institute, and glutathione S-transferase (GST) antibody was a gift of Sung Ho Ryu of POSTECH. Human recombinant TNF-α was purchased from Roche. Puromycin, sodium arsenite, cadmium chloride, magnesium chloride, hydrogen peroxide, and carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) were purchased from Sigma.
Cell cultures and transient transfection.
293T, HeLa, and COS-7 cells were grown in the presence of 6.0% CO2 in Dulbecco's modified Eagle's medium (Gibco) supplemented with antibiotics (penicillin, 100 U/ml; streptomycin, 100 μg/ml) and 10% fetal bovine serum (HyClone) at 37°C. Transient transfection was performed as described elsewhere (6). Heat shock of cells was carried out at 44°C for indicated times unless otherwise stated.
Y2H screening.
Yeast two-hybrid (Y2H) screening was performed by Panbionet Corp. (www.panbionet.com) using the yeast strain PBN204 (MATα ura3-52 his3-200 ade2-101 trp-901 leu2-3,112 gal4Δ gal80Δ ura3::kanMX6-pGAL1-URA3 pGAL1-lacZ ade2::pGAL2-ADE2).
Protein expression and purification.
Plasmid pRSET-A-eIF4GI(182-549) and pRSET-A-eIF4GII(197-545) was transformed into Escherichia coli strain BL21(DE3) pLysS (Novagen) by the use of heat shock techniques. Cultivation of E. coli and purification of His-tagged eIF4GI and eIF4GII proteins [His-eIF4GI (182-549) and His-eIF4GII(197-545)] were performed similarly to a method described elsewhere (5). The proteins were eluted from Ni-nitrilotriacetic acid columns with a 60-to-200 mM imidazole gradient. Additional Mono-Q column chromatography was performed to further purify proteins. The proteins were eluted from columns with a 240-to-500 mM NaCl gradient.
Preparation of anti-eIF4GI and -eIF4GII polyclonal antibodies.
A New Zealand white rabbit was immunized with 100 μg of the purified recombinant protein His-eIF4GI(182-549) and His-eIF4GII(197-545) by subcutaneous injection with complete Freud's adjuvant before and after a 14-day interval.
Fluorescence microscopy.
After transfection of DNAs, cells were grown on coverslips coated with 0.2% gelatin for 48 h and then washed three times with phosphate-buffered saline (PBS). The cells were fixed with 3.5% (wt/vol) paraformaldehyde (Sigma) at room temperature (RT) for 12 min. After being washed three times with PBS, the cells were permeabilized with 0.1% Triton X-100 at RT for 2 min and then washed three times with PBS. The samples were soaked in blocking solution (PBS containing 1% bovine serum albumin) for 30 min at RT and then incubated with primary antibodies for 1 h at RT. After being washed with PBS, the samples were treated with Hoechst 33258 for 2 min at RT and washed again with PBS three times. Samples were treated with rhodamine tetramethyl isocyanate-conjugated and/or fluorescein isothiocyanate-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.) for 1 h at RT. Finally, the coverslips were washed three times with PBS, placed on a glass slide, and then sealed with transparent nail polish. The fluorescent images were captured with a cooled charge-coupled device camera and a Zeiss (Jena, Germany) Axioplan microscope. Data were processed using Adobe Photoshop software (Mountain View, Calif.). The microscopic observations of proteins were performed as described elsewhere (6).
GST pull-down assay.
293T cells transfected with DNAs were lysed by soaking in Triton lysis buffer (0.1% Triton X-100, 25 mM HEPES [pH 7.5], 150 mM NaCl, 10% glycerol, 1 mM dithiothreitol, 0.1 mM EDTA, 2 mM sodium orthovanadate, 1 μg of aprotinin/ml, 1 μg of antipain/ml, 1 μg of bestatin/ml, 1 μg of pepstatin A/ml, 1 mM phenylmethylsulfonyl fluoride). Lysates were clarified by centrifugation at 14,000 × g at 4°C for 15 min and then incubated with 20 μl of glutathione-Sepharose 4B at 4°C for 4 h. Precipitated proteins were washed three times with the lysis buffer, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred to a nitrocellulose membrane.
Immunoprecipitation.
293T cells and HeLa cells were lysed using the Triton lysis buffer. The lysates were clarified by centrifugation at 14,000 × g for 15 min. Anti-FLAG monoclonal antibody (4 μg), rabbit polyclonal serum against eIF4GI (20 μl), or rabbit preimmune serum (20 μl) was incubated with 20 μl of protein G- or protein A-agarose overnight in 1 ml of PBS at 4°C. Lysates were precleared with 10 μl of protein G- or A-agarose at 4°C for 3 h. Then antibodies conjugated with protein G- or A-agarose were incubated with the precleared lysates at 4°C for 4 h. Precipitates were washed three times with lysis buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunoprecipitation of TNFR1 complex was performed as described elsewhere (49). Briefly, HeLa cells were lysed at 4°C for 45 min in 1 ml of lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 30 mM NaF, 2 mM sodium pyrophosphate, 1 μg of aprotinin/ml, 1 μg of antipain/ml, 1 μg of bestatin/ml, 1 μg of pepstatin A/ml, 1 mM phenylmethylsulfonyl fluoride). Cellular debris was removed by centrifugation performed twice at 10,000 × g for 5 min. The cell lysates were precleared with 50 μl of protein A-Sepharose beads, incubated at 4°C for 2 h with 2 μg of anti-p55 TNF receptor monoclonal antibody, and then mixed with 40 μl of protein G-Sepharose slurry (Pharmacia) (1:1 with PBS) and incubated for another 2 h. The Sepharose beads were washed twice with 1 ml of lysis buffer, twice with 1 ml of high-salt (1 M NaCl) lysis buffer, and twice more with 1 ml of lysis buffer.
Luciferase assay.
Reporter plasmid pNF-κB-Luc (Stratagene) (0.5 μg) was transfected into 293T cells by electroporation together with indicated expression vectors and transfection control plasmid pRLCMV-Luc (Promega) (0.1 μg). Luciferase assays were performed with a dual luciferase assay kit (Promega) per the manufacturer's instructions. Firefly luciferase activity values were normalized by Renilla luciferase activity values that reflect transfection efficiency and general cellular activities.
RESULTS
eIF4GI interacts with TNF-α receptor-associated factor 2 (TRAF2).
To understand the molecular basis of the stress response, we first sought to identify proteins interacting with eIF4GI, which is a component of SGs and a scaffold protein required for the assembly of translational initiation factors (10, 16, 27, 35). Yeast two-hybrid (Y2H) analysis was performed using the N-terminal part of eIF4GI (aa 182 to 589) according to the largest eIF4G identified by Byrd et al. (10) as bait, since no protein was previously known to interact with this region. The Y2H results showed that human TNF-α receptor associated factor 2 (TRAF2) interacted with the N-terminal part of eIF4GI (17 out of 23 positive clones obtained by Y2H analysis). To determine the regions required for this interaction, Y2H analysis was further performed using serially deleted eIF4GI and TRAF2 gene sequences in the bait and prey vectors, respectively. The TRAF domain of TRAF2 (about 200 amino acids at the C-terminal end) and the N-terminal region of eIF4GI (aa 182 to 415) were found to be required for the interaction (data not shown). The TRAF domain is well conserved among TRAF family members and is essential for the formation of TRAF homo- and heterodimers (9). When a Y2H analysis was performed using TRAF2 and the N-terminal part of eIF4GII (aa 1 to 357), which is an eIF4GI isoform sharing about 50% amino acid identity (17), no protein-protein interaction was detected (data not shown). These results indicate that eIF4GI but not eIF4GII specifically interacts with TRAF2, likely because the two differ at the TRAF2-binding site of eIF4GI (17) (see below). The interaction between TRAF2 and eIF4GI was further confirmed by biochemical methods. A plasmid expressing the N-terminal region of eIF4GI (aa 1 to 549) fused with GST (GST-eIF4GIΔC) was transfected into 293T cells, and a GST pull-down assay was performed (Fig. 1). Endogenous TRAF2 was pulled down by GST-eIF4GIΔC (aa 1 to 549) (Fig. 1A; lane 4 on the top panel) but not by GST alone (Fig. 1A; lane 3 on the top panel). To confirm the interaction between eIF4GI and the TRAF domain of TRAF2 in mammalian cells, plasmids expressing the HA-tagged TRAF or Finger domains of TRAF2 were cotransfected with plasmids expressing GST-eIF4GIΔC. When cell extracts were precipitated with glutathione-Sepharose 4B, the HA-TRAF domain was coprecipitated, but the HA-Finger domain was not (Fig. 1A; lane 4 on the middle and the bottom panels).
To confirm the interaction between endogenous eIF4GI and TRAF2 under normal conditions (37°C), HeLa cell extracts were immunoprecipitated with an anti-eIF4GI antibody (Fig. 1B). Endogenous TRAF2 was coprecipitated, albeit weakly (see below), by the anti-eIF4GI antibody (Fig. 1B, lane 3 on the top panel), and the poly(A)-binding protein (PABP), which is known to make a complex with eIF4GI, was also coprecipitated by the anti-eIF4GI antibody (Fig. 1B; lane 3 on the middle panel). These results indicate that endogenous TRAF2 and eIF4GI make a complex in HeLa cell under normal conditions.
There are many TRAF2-related proteins belonging to the TRAF family. All members of the TRAF family have a conserved TRAF domain that mediates protein-protein interactions (9, 20). Accordingly, we examined whether the TRAF domains of various TRAF2 homologues interact with eIF4GI. GST-eIF4GIΔC was coexpressed with FLAG-tagged TRAF1, TRAF2, TRAF3, TRAF5, or TRAF6, and GST pull-down assays were performed. Interestingly, only TRAF2 coprecipitated with eIF4GI (data not shown). Conversely, 293T cells were transfected with constructs expressing FLAG-tagged TRAF1, TRAF2, TRAF3, TRAF5, and TRAF6, and immunoprecipitation was performed with an anti-FLAG monoclonal antibody. Endogenous eIF4GI was coprecipitated by the anti-FLAG antibody when FLAG-TRAF2 was ectopically expressed in the cells (Fig. 1C; lane 3 on the first panel). Interestingly, TRAF1, the TRAF family protein showing the highest homology with TRAF2 (4, 9), showed weak binding with eIF4GI (Fig. 1C; lane 2 on the first panel), perhaps suggesting that TRAF1 interacts weakly with eIF4GI. Alternatively, TRAF1 might have coprecipitated with eIF4GI via indirect binding through the TRAF1-TRAF2 interaction (4, 9). eIF4GI did not coprecipitate with TRAF3, TRAF5, or TRAF6 (Fig. 1C; lanes 4 to 6 on the first panel). Interaction between eIF4GII and TRAF2 was not observed by Y2H analysis (data not shown). Coincident with this, eIF4GII was not coprecipitated with any of TRAF members by immunoprecipitation (Fig. 1C; lanes 1 to 6 on the second panel). Taken together, these results indicate that eIF4GI specifically interacts with TRAF2.
We then used the Y2H system to further analyze the TRAF2-binding region of eIF4GI (aa 182 to 415) (Fig. 1D). Y2H analysis revealed that 11 amino acids of eIF4GI (aa 293 to 303) not present in eIF4GII were sufficient for the interaction with TRAF2. This seems to explain why eIF4GII does not interact with TRAF2. Interestingly, the TRAF2-interacting motif SXXE, which is known to be essential for interaction with TRAF2 (37), resides in the center of the 11 amino acids. The same SXXE motif sequence (SVEE) is found both in eIF4GI and in CD30, which is also known to interact with TRAF2 (8) (bold characters in Fig. 1E). An artificial mutation in the consensus sequence of eIF4GI (from SVEE to AVEA) hampered the interaction between eIF4GI and TRAF2 (Fig. 1F), indicating that the SVEE sequence plays a key role in the interaction between eIF4GI and TRAF2.
TRAF2 migrates to stress granules (SGs) under heat stress conditions.
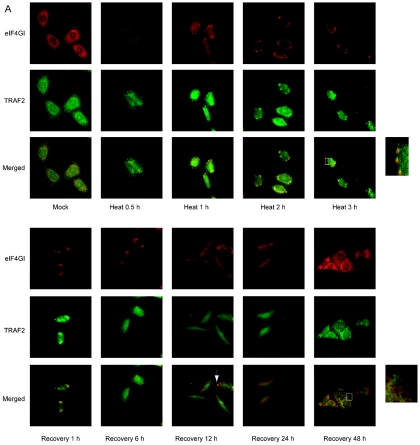
It is known that under heat stress conditions, TNF signaling is blocked (30, 31, 44) and mRNA translation is generally inhibited by the sequestration of translation factors into SGs (1, 2, 12, 23, 24, 26, 29). However, the link between these two physiological phenomena has not been clearly elucidated. To investigate the relationship between translation and TNF signaling, the subcellular localization patterns of endogenous eIF4GI and TRAF2 proteins were investigated by immunocytochemistry under normal (37°C) and heat stress (44°C) conditions. A polyclonal antibody against eIF4GI and a monoclonal antibody against TRAF2 were used in the immunocytochemical analyses (Fig. 2). At 37°C, eIF4GI was localized mainly in the cytoplasm and partially in the nucleus of HeLa cell as previously described by McKendrick et al. (33), whereas TRAF2 proteins showed a punctate pattern in the cytoplasm elsewhere (4, 15). Our subcellular localization data indicated that most of the TRAF2 and eIF4GI proteins distributed to separate regions of the cell at 37°C (Fig. 2A [“Mock”]). As the localization pattern of eIF4GI was previously shown to change under stress conditions (1, 12, 29), we further analyzed the subcellular distribution of eIF4GI and TRAF2 under heat stress conditions. Following cellular incubation at 44°C for 0.5 h, some of the eIF4GI proteins were found in distinct regions of the cell known as SGs localized around the nucleus (Fig. 2A [“Heat 0.5 h”]), as previously described (1, 2, 12, 21, 23, 24, 26, 29, 36, 41). Interestingly, the TRAF2 proteins also migrated to the SGs following heat treatment. A large portion of the eIF4GI and cytoplasmic TRAF2 proteins colocalized in the SGs after heat treatment for 0.5 h, as shown by the yellow dots in the merged image, although some of the eIF4GI and TRAF2 proteins, especially nuclear TRAF2 (34), remained in distinct regions, as shown by red and green signals, respectively (Fig. 2A [“Heat 0.5 h”]). Most of the cytoplasmic eIF4GI and TRAF2 proteins coincided with each other in the cells heat treated for longer than 1 h (Fig. 2A [“Heat 1 h” and “Heat 3 h”]; also see the enlarged portion on the panel labeled “Heat 3 h”). Curiously, some of eIF4GI and TRAF2 proteins remained in the nucleus even after heat treatment for 3 h (Fig. 2A [“Heat 3 h”]). The nuclear TRAF2 was suggested to play a role in regulation of transcription distinct from cytoplasmic TRAF2 that mediates signal transduction (34).
FIG. 2.
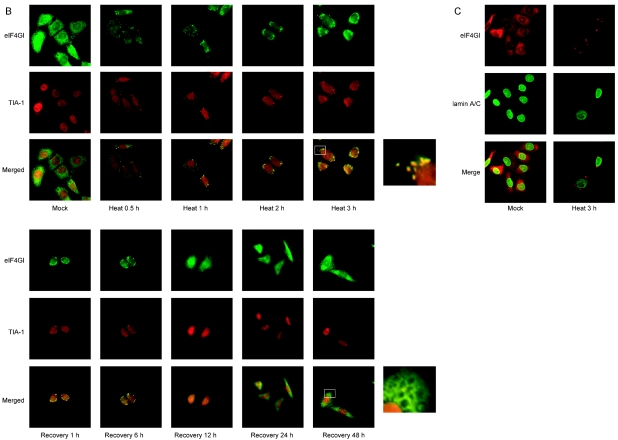
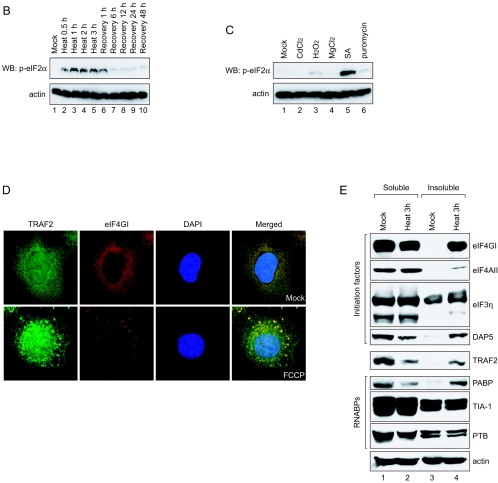
TRAF2 and eIF4GI migrate to SGs under heat stress conditions. (A) HeLa cells were heat treated at 44°C and then allowed to recover at 37°C for the indicated times. After treatments, cells were fixed, permeabilized, and treated with primary antibodies (an anti-eIF4GI polyclonal antibody and an anti-TRAF2 monoclonal antibody) and secondary antibodies (a rhodamine-conjugated donkey anti-rabbit immunoglobulin G and a fluorescein isothiocyanate-conjugated donkey anti-mouse immunoglobulin G). The localization patterns of eIF4GI and TRAF2 are shown in red and green, respectively. The boxes on the right side of the panels labeled “Heat 3 h” and “Recovery 48 h” show enlarged parts of pictures indicated by white boxes. (B) The same sets of samples shown in panel A were visualized with an anti-TIA-1 and an anti-eIF4GI antibody. (C) Distribution patterns of lamin A/C and eIF4GI before (Mock) and after (Heat 3 h) heat treatment were visualized with an anti-lamin A/C and an anti-eIF4GI antibody.
The recovery patterns of these proteins were monitored by shifting the temperature to 37°C for indicated times after heat treatment at 44°C for 3 h (Fig. 2A [“Recovery 1 h” to “Recovery 48 h”]). After 1 h of recovery, most of the TRAF2 and eIF4GI proteins were still in the SGs but were more diffusely distributed compared with our findings under the stress conditions (compare the panels labeled “Recovery 1 h” with the panels labeled “Heat 3 h” in Fig. 2A). As cells were recovered from heat stress for longer times, the TRAF2 and eIF4GI proteins were spread throughout the cytoplasm in most of cells (Fig. 2A [“Recovery 6 h” to “Recovery 24 h”]) except for a few cases in which the two proteins were still found in SGs (Fig. 2A; indicated by a white arrow in the bottom row of the panels labeled “12 h”). The cell morphology and the distribution pattern of TRAF2 proteins returned to normal 48 h postrecovery, when the distinct punctate patterns of TRAF2 proteins could be observed (Fig. 2A; see in particular the enlarged portion on the panels labeled “Recovery 48 h”).
The identity of the granules formed by heat stress was confirmed by immunocytochemistry with pairs of antibodies against eIF4GI versus TIA-1 (Fig. 2B), eIF4GI versus eIF3η (data not shown), and eIF4GI versus eIF4AII (data not shown) (1), which are known to migrate to SGs during heat stress. TIA-1 proteins were localized mostly in the nucleus at 37°C, but a part of them migrated to the SGs in the cytoplasm where some of eIF4G proteins migrated after heat treatment for 0.5 h. Cytoplasmic eIF4GI and TIA-1 were almost completely colocalized at the SGs after heat treatment for 3 h. SGs were clearly observed by 6 h postrecovery, as seen by eIF4GI and TRAF2 results (panels labeled “Recovery 1 h” and “Recovery 6 h” in Fig. 2B). SGs disappeared from most of cells by 12 h postrecovery, and eIF4GI and TIA-1 showed normal distribution patterns 48 h postrecovery.
To confirm that the integrity of cells remained intact under the heat stress conditions, immunocytochemical analyses were performed with antibodies against nuclear lamin A/C and eIF4GI (Fig. 2C). The nuclei of cells remained intact by 3 h post-heat treatment, as indicated by the distribution patterns of lamin A/C that were confined to the nuclei (Fig. 2C; panels “lamin A/C”). The lamin A/C and eIF4GI proteins were localized at distinct regions 3 h post-heat treatment (panels labeled “Heat 3 h” in Fig. 2C). These results clearly demonstrate that TRAF2 and eIF4GI proteins congregate in SGs under heat stress conditions and that these proteins return to separate subcellular regions upon cellular return to the nonstress temperature of 37°C.
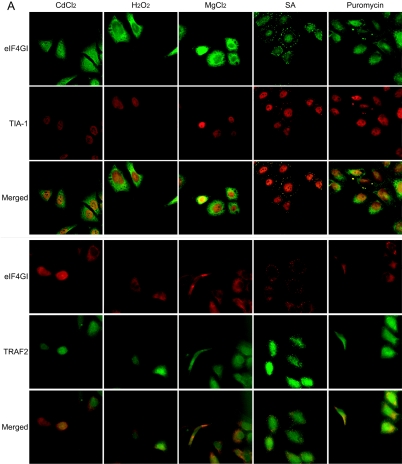
Migration of TRAF2 to SGs was further investigated by observing distribution patterns of TRAF2, eIF4GI, and TIA-1 in the cells treated with various stress-inducing agents (heavy metal ions cadmium chloride [CdCl2] and SA, oxidative stress inducer hydrogen peroxide [H2O2], concentrated divalent ion magnesium chloride [MgCl2], and polysome destabilizer puromycin) (22, 28) (Fig. 3). SA, a well-known heat shock protein (HSP) inducer (1), is often used to induce SG formation (26). SGs were detected in SA-treated cells (Fig. 3A; panels labeled “SA”), as described by Kedersha and Anderson (23). In these cells, cytoplasmic eIF4GI and TIA-1 were colocalized to the SGs. Cytoplasmic TRAF2 was also localized to the SGs, as indicated by colocalization of eIF4GI and TRAF2 on the panels labeled “SA” in Fig. 3A. Curiously, puromycin treatment induced SG formation in some, but not all, cells where cytoplasmic eIF4GI and TIA-1 were colocalized (Fig. 3A; panels labeled “Puromycin”). Cytoplasmic TRAF2 also migrated to the SGs in the cells containing SGs (Fig. 3A; panels labeled “Puromycin”). Other stress-inducing chemicals (CdCl2, H2O2, and MgCl2) did not induce SG formation or TRAF2 migration. These data suggest that stress per se does not induce SG formation or TRAF2 migration.
FIG. 3.
Effects of heat shock inducers on localization of eIF4GI and TRAF2. (A) HeLa cells grown on coverslips were treated with cadmium chloride (500 μM, 30 min), hydrogen peroxide (1 mM, 30 min), magnesium chloride (400 μM, 30 min), sodium arsenite (400 μM, 30 min), and puromycin (20 mg/ml, 6 h). Green fluorescence indicates endogenous TRAF2 (top panel) or eIF4GI (bottom panel), and red fluorescence indicates endogenous eIF4GI (top panel) or TIA-1 (bottom panel), respectively. Endogenous proteins were visualized as described in the legend to Fig. 2. (B) HeLa cells were heat treated as described in the legend to Fig. 2A and then analyzed by WB with antibodies against phospho-eIF2α and actin. (C) HeLa cells were treated with various stress-inducing agents and then analyzed by WB with antibodies against phospho-eIF2α and actin. (D) HeLa cells were mock treated (top panels) or treated with 10 μM of FCCP for 90 min (bottom panels) and then subjected to immunocytochemistry using anti-TRAF2 and -eIF4GI antibodies. TRAF2 and eIF4GI are shown in green and red, respectively. The nucleus is shown in blue by DAPI (4′,6′-diamidino-2-phenylindole) staining. (E) Solubility of proteins under normal and stress conditions. HeLa cells were mock treated (lanes 1 and 3) or heat treated for 3 h (lanes 2 and 4). Fractionation of the HeLa cell extract was performed as described elsewhere (10) with the addition of pretreatment with cross-linking agent 1,5-difluoro-2,4-dinitrobenzene (Sigma). The soluble (lanes 1 and 2) and insoluble (lanes 3 and 4) fractions were analyzed by Western blotting with antibodies against eIF4GI (panel labeled “eIF4GI”), PABP (panel labeled “PABP”), and TRAF2 (panel labeled “TRAF2”).
SG formation was suggested to be related to the phosphorylation of eIF2α subunits and/or to the reduced availability of eIF2α-GTP-tRNAiMet (23, 24, 26). Therefore, we monitored phosphorylation levels of eIF2α subunits under various stress conditions (Fig. 3B and C). Phosphorylation of eIF2α subunits was strongly induced by heat treatment and remained at high level by 1 h postrecovery (lanes 2 to 6 in Fig. 3B). The phosphorylation level of eIF2α subunits returned to normal after 6 h postrecovery (lanes 7 to 10 in Fig. 3B). SA, a strong SG former, strongly induced phosphorylation of eIF2α subunits (Fig. 3C, lane 5). Puromycin, a weak SG former, weakly induced phosphorylation of eIF2α subunits (Fig. 3C, lane 6). H2O2 did not induce SG formation, even though it induced weak phosphorylation of eIF2α subunits, in similarity to puromycin results (Fig. 3C, lane 3). The molecular basis of the discrepancy in SG formation by the weak inducers of the eIF2α phosphorylation (puromycin and H2O2) remains to be elucidated. Other stress-inducing chemicals (CdCl2 and MgCl2) did not induce phosphorylation of eIF2α (Fig. 3C, lanes 2 and 4). These results suggest that the phosphorylation of eIF2α subunits is related to SG formation and that weak phosphorylation of eIF2α subunits occasionally induces SG formation.
The effect of eIF2α phosphorylation on the migration of TRAF2 to SGs was further investigated by using an ionophore FCCP that induces SG formation without phosphorylation of eIF2α (24). As shown on the bottom panel in Fig. 3D, TRAF2 and eIF4G1 comigrated to SGs upon treatment with FCCP. This indicates that the formation of SG, rather than the phosphorylation of eIF2α, is required for the colocalization of TRAF2 and eIF4G1 to SGs irrespective of the causes of SG formation. This phenomenon provides an explanation for the long-lasting enigma of how the depletion of the mitochondrial electron transport eliminates the cytotoxic and gene-inductive effects of TNF (42).
The migration of TRAF2 to SGs was further investigated by measuring the solubility of TRAF2 and translation factors under normal and heat stress conditions. Upon heat treatment for 3 h, large proportions of translation factors eIF4GI, eIF4AII, and DAP5 were accumulated in the insoluble fraction of cell extracts where components of SGs are enriched (Fig. 3E; lanes 4 in panels labeled “eIF4GI,” “eIF4AII,” and “DAP5”), as previously shown by Cuesta et al. (12). Similarly, a large proportion of TRAF2 proteins were also enriched in the insoluble fraction upon heat treatment (Fig. 3E, lane 4 on panel TRAF2). Conversely, the level of TRAF2 in soluble fraction was reduced when cells were treated with heat (Fig. 3E; lane 2 on panel labeled “TRAF2”). Redistribution of TRAF2 to the insoluble subcellular components has been observed in the cells containing constitutively active receptors (3) and the cells treated with agents blocking mitochondrial activity (11). The distribution pattern of TRAF2 during heat treatment was very similar to that of PABP that augments translation through an interaction with the poly(A) tail of mRNA (compare panels labeled “TRAF2” and “PABP” in Fig. 3E). On the other hand, solubility of other RNA-binding proteins (TIA-1 and PTB) was not changed by the heat treatment (panels labeled “TIA-1” and “PTB”). It is noteworthy that the solubility of TIA-1, which partially migrates to SGs, was not changed during heat treatment. The dynamic nature of TIA-1 in the SGs is likely to contribute to the maintenance of solubility of TIA-1 during heat treatment (25).
Taken together, these localization and solubility data strongly indicate that TRAF2, a key TNF-signaling molecule, is sequestered into SGs under SG-forming conditions.
TRAF2 is not associated with the TNFR1 complex under heat stress conditions.
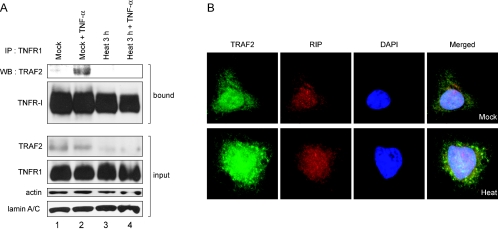
Following treatment with TNF-α, cells form a complex called the “TNFR1-mediated-signal transduction complex,” which is composed of TNFR1, adaptor TRADD, kinase RIP1, and TRAF2 (49). To determine whether TRAF2 exists in the TNFR1 complex when cells were activated by TNF-α, immunoprecipitation was performed with monoclonal antibody against TNFR1. Prior to treatment with TNF-α, TRAF2 was weakly coprecipitated with TNFR1 (Fig. 4A [lane 1 on panel labeled “TRAF2”]). TRAF2 was strongly coimmunoprecipitated with TNFR1 when cells were treated TNF-α at 37°C, as previously described by Zhang et al. (49) (Fig. 4A [lane 2 on panel labeled “TRAF2”]), but TRAF2 was not coimmunoprecipitated with TNFR1 with or without TNF-α treatment when cells were cultivated at 44°C for 3 h (Fig. 4 [lanes 4 and 3 on panels labeled “bound”]). The level of TNFR1 on the cell surfaces remained the same even under the heat stress conditions, as analyzed by fluorescence-activated cell sorter (data not shown). Together, these results indicate that TRAF2, which is associated with SGs under stress conditions, cannot participate in TNFR1 complex formation. The reduced levels of TRAF2 in the input panels (Fig. 4A [lanes 3 and 4 on panel labeled “TRAF2”]) are attributed to insolubility of TRAF2 under stress conditions (Fig. 3D); only soluble fractions can be analyzed in immunoprecipitation assays.
FIG. 4.
TRAF2 forms different complexes under normal and stress conditions. (A) HeLa cells were mock treated (lanes 1 and 2) or heat treated for 3 h (lanes 3 and 4) and then treated with 100 ng of human TNF-α/ml for 1 h (lanes 2 and 4). The samples in lanes 1 and 3 were not treated with TNF-α. After TNF-α treatment, cells were washed with cold PBS and then subjected to immunoprecipitation with a monoclonal anti-TNFR1 antibody. Control proteins (actin and lamin A/C) are shown in panels actin and lamin A/C. (B) HeLa cells were mock treated (top panels) or heat-treated for 1 h (bottom panels) and then subjected to immunocytochemistry using anti-RIP and anti-TRAF2 antibodies. TRAF2 and RIP are shown in green and red, respectively. The nucleus is shown in blue by DAPI staining.
TNFR1 complex is composed of several adaptor proteins such as TRADD, TRAF2, RIP, and FADD. An immunocytochemical experiment was performed to investigate whether these proteins are also associated with SGs under heat shock conditions. RIP, a receptor-interacting protein critical for activation of transcription factor NF-κB (49), was not observed at SGs during heat shock (Fig. 4B, bottom panels). This indicates that not all the components of TNFR complex migrate to SGs for blocking NF-κB signal transduction during heat stress (see below).
Interaction between eIF4GI and TRAF2 blocks TNF signaling.
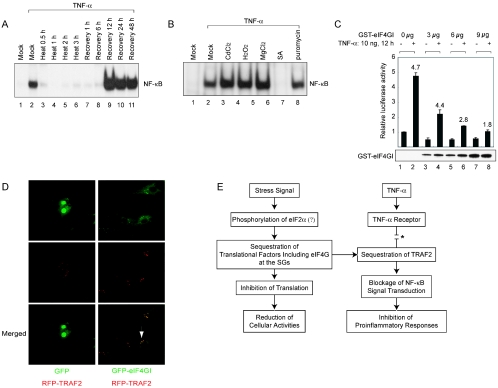
The effect of the sequestration of TRAF2 into SGs during heat treatment on TNF signaling pathway was monitored by measuring the level of activated NF-κB in the nucleus by using an electrophoresis mobility shift assay (EMSA) as described by Hong et al. (18a) (Fig. 5A and B). The EMSA was performed by using a DNA probe corresponding to an NF-κB-binding element and nuclear extracts of HeLa cells with or without TNF-α treatment. Following TNF-α treatment, activated NF-κB was detected in the nuclear fraction of the cell extracts, as indicated by NF-κB complex formation (Fig. 5A, lane 2). In contrast, the nuclear NF-κB complex was not observed when cells were pretreated with heat for 0.5 h or longer (Fig. 5A, lanes 3 to 6). The blockade of TNF signaling pathway remained by 6 h of recovery (Fig. 5A, lanes 7 and 8). The TNF signaling was restored by 12 h of recovery (Fig. 5A, lanes 9 to 11). Curiously, increased levels of nuclear NF-κB complexes of the heat-treated and recovered cells were observed compared with the mock-treated cell results (compare lanes 9 to 11 to lane 2). The molecular basis of this augmented signaling reaction remains to be elucidated.
FIG. 5.
eIF4GI-TRAF2 interaction blocks TNF signaling. (A) HeLa cells were treated as described in the legend to Fig. 2A and then further treated with 100 ng of TNF-α/ml at 37°C for 1 h. EMSA was performed using 32P-labeled oligonucleotides corresponding to the NF-κB-responsive element of the c-IAP2 promoter (17). (B) HeLa cells were treated as described in the legend to Fig. 3A and then further treated with 100 ng of TNF-α/ml at 37°C for 1 h. EMSA was performed as described for panel A. (C) 293T cells were cotransfected with pNF-κB-Luc (Stratagene), which encodes the firefly luciferase gene under the control of a NF-κB response element, and various amounts of plasmid pEBG-eIF4GI, which produces GST-eIF4GI. Total amounts of effector plasmids were kept the same (9 μg) in all experiments by adjusting the amounts of control plasmid pEBG that was cotransfected with plasmid pEBG-eIF4GI. Control plasmid pRLCMV-Luc (Promega) was also cotransfected to control for transfection efficiency. After 48 h, cells were incubated in serum-free medium for 3 h and then further incubated for 12 h in the presence (lanes 2, 4, 6, and 8) and absence (lanes 1, 3, 5, and 7) of 10 ng of TNF-α/ml. Three independent experiments were performed under each set of conditions. The bottom panel shows GST-eIF4GI polypeptide detected by Western blotting with an anti-GST antibody. (D) COS-7 cells were cotransfected with plasmids expressing GFP- and RFP-tagged TRAF2 (RFP-TRAF2) or GFP-tagged eIF4GI (GFP-eIF4GI) and RFP-TRAF2. (E) A hypothetical scheme for the modulation of translation and TNF signaling. The asterisk indicates that the interaction between TRAF2 and TNF-α receptor can be blocked by the sequestration of TRAF2 into SGs through an interaction with eIF4GI.
The effects of other stress-inducing agents (CdCl2, H2O2, MgCl2, SA, and puromycin) on TNF signaling were also monitored by the EMSA. TNF signaling was completely blocked by SA, which strongly induces SG formation. On the other hand, TNF signal transduction, at least in part, was observed in the cells treated with other stress inducers (CdCl2, H2O2, MgCl2, and puromycin) (lanes 2, 3, 4, 5, 6, and 8 in Fig. 5B). These data suggest that the SG formation is tightly related to the blockage of TNF signaling.
The effect of eIF4GI on the function of TRAF2 was investigated by cotransfection of plasmids expressing full-length eIF4GI fused with GST (GST-eIF4GI) and a reporter gene (firefly luciferase) under the control of NF-κB response element. At 48 h posttransfection, cells were incubated with serum-free medium for 1 h and then treated with 10 ng of TNF-α for 12 h. The levels of reporter gene expression decreased as GST-eIF4GI levels increased in the cells (Fig. 5C, lanes 1, 3, 5, and 7), likely due to reduced basal level activation of NF-κB by the overexpressed eIF4GI. About fivefold induction of NF-κB reporter activity was observed following TNF-α treatment of 293T cells (compare lane 2 to lane 1 in Fig. 5C). Dramatic changes were observed in the induction level of activated NF-κB following TNF-α treatment in cells expressing GST-eIF4GI. The induction levels of the NF-κB reporter gradually decreased with eIF4GI expression in a dose-dependent manner, as indicated by the numbers above the bars in Fig. 5C (compare lanes 4, 6, and 8 to lane 2). This inhibition of NF-κB activation is likely due to the interaction of TRAF2 with eIF4GI (Fig. 1). We speculate that this phenomenon mimics the activity of eIF4GI under stress conditions. In fact, some of the overexpressed eIF4GI proteins were localized to large speckles (Fig. 5D [green signals on panels GFP-eIF4GI with RFP-TRAF2]). TRAF2 proteins fused with red fluorescent protein (RFP) (RFP-TRAF2) were also detected in speckles (Fig. 5D [red signals on panels GFP-eIF4GI with RFP-TRAF2]) when coexpressed with eIF4GI; TRAF2 and eIF4GI colocalized in these speckles (Fig. 5D [indicated by an arrow on panel GFP-eIF4GI with RFP-TRAF2]). This indicates that TRAF2, at least in part, colocalizes with eIF4GI even at 37°C when eIF4GI is overexpressed. This eIF4GI and TRAF2 interaction seems to block NF-κB activation in cells overexpressing eIF4GI (Fig. 5C).
DISCUSSION
Here we report the sequestration of TRAF2 into SGs under stress conditions. These observations provide a possible explanation for the protection of high-temperature-pretreated mice from proinflammatory response triggering acute lung injury, septic shock, or liver damage caused by ischemia reperfusion (19, 30, 39, 40). We propose that heat preconditioning of cells leads to TRAF2 sequestration and subsequent blockade of TNF-α-mediated NF-κB proinflammatory signaling (Fig. 5E). Environmental stresses and other signals lead to formation of SGs triggered by eIF2α phosphorylation and/or by arrest of the translational preinitiation complexes (1, 2, 12, 23, 24, 26, 29). Translational factors including eIF4GI and RNA-binding proteins such as TIA-1 and TIAR are sequestered into SGs (1, 2, 12, 23, 24, 26, 29), leading to general repression of mRNA translation and decreases in many cellular activities due to inhibited gene expression. Interestingly, a poorly understood mechanism allows continued or even increased translation of some mRNAs, such as those for the HSPs, under stress conditions. This stress-specific regulation of gene expression allows cells to recover from stress damage and minimizes the potential risks from aberrant gene expression during stress conditions (1, 14, 43). We found that the TRAF2, a signaling molecule, is also sequestered into the SGs, possibly through a protein-protein interaction with eIF4GI (Fig. 1). Endogenous eIF4GI interacts with TRAF2 under normal conditions, albeit weakly (Fig. 1B and C), and overexpressed TRAF2 and eIF4GI partially colocalize at 37°C (Fig. 5D), suggesting that eIF4GI interacts weakly with TRAF2 even under normal conditions. The interaction between eIF4GI and TRAF2 seems to be strengthened during stress conditions, as indicated by the strong colocalization (Fig. 2) and coenrichment of these proteins in the insoluble fraction (Fig. 3D) under heat stress conditions. The sequestration of TRAF2 into SGs results in the blockage of TRAF2-mediated signaling processes such as TNF signaling, which subsequently reduces activation of NF-κB (Fig. 4 and 5A to 5C). As a consequence, proinflammatory responses, which are responsible for lethal stress responses such as septic shock (7, 45, 48), are nullified when organisms are preconditioned with sublethal stresses.
Sequestration of TRAF2 into SGs breaks the positive-feedback loop of proinflammatory response through TNF-α and NF-κB. This prevents propagation of the inflammatory response to neighboring cells and tissues and protects organisms from systemic inflammation. The sequestration of TRAF2 to the SGs may not be the only mechanism functioning in the blockage of the positive-feedback loop of proinflammatory response. The sequestration of TRAF2 and other safety checking mechanisms are likely to function in balancing pro- and anti-inflammatory responses in a concerted manner. Investigations into the mechanism of sequestration of TRAF2 into SGs may provide a clue for a better understanding of the protection of organisms from environmental stresses and may help researchers to find novel approaches for protecting against damaging proinflammatory responses.
Acknowledgments
We thank David V. Goeddel of Tularik Inc. (San Francisco) for providing the FLAG-tagged TRAF1, TRAF2, TRAF3, TRAF5, and TRAF6 plasmid vectors. We also thank Sung Ho Ryu (Division of Molecular and Life Sciences, Pohang University of Science and Technology) and Jee Hee Youn (Department of Anatomy, College of Medicine, Hanyang University) for their valuable suggestions and critical review of the manuscript.
This work was supported in part by grants NRL (M10204000018-03J0000-01410) and MCBRG(M10106000056-03B4500-01010) from MOST, grants 02-PJ2-PG1-CH16-0002 and 405-VN02-0702-0008 from KHIDI, grant NCRC(R15-2004-033-01001-0) from KRF, and a grant from POSCO.
REFERENCES
- 1.Anderson, P., and N. Kedersha. 2002. Stressful initiations. J. Cell Sci. 115:3227-3234. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, P., and N. Kedersha. 2002. Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation. Cell Stress Chaperones 7:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arch, R. H., R. W. Gedrich, and C. B. Thompson. 2000. Translocation of TRAF proteins regulates apoptotic threshold of cells. Biochem. Biophys. Res. Commun. 272:936-945. [DOI] [PubMed] [Google Scholar]
- 4.Arron, J. R., Y. Pewzner-Jung, M. C. Walsh, T. Kobayashi, and Y. Choi. 2002. Regulation of the subcellular localization of tumor necrosis factor receptor-associated factor (TRAF)2 by TRAF1 reveals mechanisms of TRAF2 signaling. J. Exp. Med. 196:923-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Back, S. H., J. E. Kim, J. Rho, B. Hahm, T. G. Lee, E. E. Kim, J. M. Cho, and S. K. Jang. 2000. Expression and purification of an active, full-length hepatitis C viral NS4A. Protein Expr. Purif. 20:196-206. [DOI] [PubMed] [Google Scholar]
- 6.Back, S. H., Y. K. Kim, W. J. Kim, S. Cho, H. R. Oh, J.-E. Kim, and S. K. Jang. 2002. Translation of polioviral mRNA is inhibited by cleavage of polypyrimidine tract-binding proteins executed by polioviral 3Cpro. J. Virol. 76:2529-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes, P. J., and M. Karin. 1997. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336:1066-1071. [DOI] [PubMed] [Google Scholar]
- 8.Boucher, L. M., L. E. Marengere, Y. Lu, S. Thukral, and T. W. Mak. 1997. Binding sites of cytoplasmic effectors TRAF1, 2, and 3 on CD30 and other members of the TNF receptor superfamily. Biochem. Biophys. Res. Commun. 233:592-600. [DOI] [PubMed] [Google Scholar]
- 9.Bradley, J. R., and J. S. Pober. 2001. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20:6482-6491. [DOI] [PubMed] [Google Scholar]
- 10.Byrd, M. P., M. Zamora, and R. E. Lloyd. 2002. Generation of multiple isoforms of eukaryotic translation initiation factor 4GI by use of alternate translation initiation codons. Mol. Cell. Biol. 22:4499-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandel, N. S., P. T. Schumacker, and R. H. Arch. 2001. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J. Biol. Chem. 276:42728-42736. [DOI] [PubMed] [Google Scholar]
- 12.Cuesta, R., G. Laroia, and R. J. Schneider. 2000. Chaperone hsp27 inhibits translation during heat shock by binding eIF4G and facilitating dissociation of cap-initiation complexes. Genes Dev. 14:1460-1470. [PMC free article] [PubMed] [Google Scholar]
- 13.DeMeester, S. L., T. G. Buchman, and J. P. Cobb. 2001. The heat shock paradox: does NF-kappaB determine cell fate? FASEB J. 15:270-274. [DOI] [PubMed] [Google Scholar]
- 14.Duncan, R. F., and J. W. Hershey. 1989. Protein synthesis and protein phosphorylation during heat stress, recovery, and adaptation. J. Cell Biol. 109:1467-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, X., M. L. Gaeta, L. A. Madge, J. H. Yang, J. R. Bradley, and J. S. Pober. 2001. Caveolin-1 associates with TRAF2 to form a complex that is recruited to tumor necrosis factor receptors. J. Biol. Chem. 276:8341-8349. [DOI] [PubMed] [Google Scholar]
- 16.Gingras, A. C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913-963. [DOI] [PubMed] [Google Scholar]
- 17.Gradi, A., H. Imataka, Y. V. Svitkin, E. Rom, B. Raught, S. Morino, and N. Sonenberg. 1998. A novel functional human eukaryotic translation initiation factor 4G. Mol. Cell. Biol. 18:334-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hershko, D. D., B. W. Robb, E. S. Hungness, G. Luo, and P. O. Hasselgren. 2002. Arsenite stabilizes IkappaBalpha and prevents NF-kappaB activation in IL-1 beta-stimulated Caco-2 cells independent of the heat shock response. J. Cell Biochem. 84:687-698. [DOI] [PubMed] [Google Scholar]
- 18a.Hong, S. Y., W. H. Yoon, J. H. Park, S. G. Kang, J. H. Ahn, and T. H. Lee. 2000. Involvement of two NF-κB binding elements in tumor necrosis factor alpha-, CD40-, and Epstein-Barr virus latent membrane protein 1-mediated induction of the cellular inhibitor of apoptosis protein 2 gene. J. Biol. Chem. 275:18022-18028. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss, R., I. Nunnally, S. Lindquist, J. Taulien, G. Perdrizet, and I. Karl. 1993. Hyperthermia protects mice against the lethal effects of endotoxin. Am. J. Physiol. 265:R1447-R1457. [DOI] [PubMed] [Google Scholar]
- 20.Inoue, J., T. Ishida, N. Tsukamoto, N. Kobayashi, A. Naito, S. Azuma, and T. Yamamoto. 2000. Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp. Cell Res. 254:14-24. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov, P. A., E. M. Chudinova, and E. S. Nadezhdina. 2003. Disruption of microtubules inhibits cytoplasmic ribonucleoprotein stress granule formation. Exp. Cell Res. 290:227-233. [DOI] [PubMed] [Google Scholar]
- 22.Jacquier-Sarlin, M. R., and B. S. Polla. 1996. Dual regulation of heat-shock transcription factor (HSF) activation and DNA-binding activity by H2O2: role of thioredoxin. Biochem. J. 318:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kedersha, N., and P. Anderson. 2001. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans. 30:963-969. [DOI] [PubMed] [Google Scholar]
- 24.Kedersha, N., S. Chen, N. Gilks, W. Li, I. J. Miller, J. Stahl, and P. Anderson. 2002. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 13:195-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kedersha, N., M. R. Cho, W. Li, P. W. Yacono, S. Chen, N. Gilks, D. E. Golan, and P. Anderson. 2000. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151:1257-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kedersha, N. L., M. Gupta, W. Li, I. Miller, and P. Anderson. 1999. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147:1431-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keiper, B. D., W. Gan, and R. E. Rhoads. 1999. Protein synthesis initiation factor 4G. Int. J. Biochem. Cell Biol. 31:37-41. [DOI] [PubMed] [Google Scholar]
- 28.Kim, D., S. Somji, S. H. Garrett, M. A. Sens, D. Shukla, and D. A. Sens. 2001. Expression of hsp 27, hsp 60, hsc 70, and hsp 70 by immortalized human proximal tubule cells (HK-2) following exposure to heat shock, sodium arsenite, or cadmium chloride. J. Toxicol. Environ. Health Part A 63:475-493. [DOI] [PubMed] [Google Scholar]
- 29.Kimball, S. R., R. L. Horetsky, D. Ron, L. S. Jefferson, and H. P. Harding. 2003. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am. J. Physiol. Cell Physiol. 284:C273-C284. [DOI] [PubMed] [Google Scholar]
- 30.Malhotra, V., T. Eaves-Pyles, K. Odoms, G. Quaid, T. P. Shanley, and H. R. Wong. 2002. Heat shock inhibits activation of NF-kappaB in the absence of heat shock factor-1. Biochem. Biophys. Res. Commun. 291:453-457. [DOI] [PubMed] [Google Scholar]
- 31.Malhotra, V., and H. R. Wong. 2002. Interactions between the heat shock response and the nuclear factor-kappaB signaling pathway. Crit. Care Med. 30:S89-S95. [PubMed] [Google Scholar]
- 32.Marber, M. S., R. Mestril, S. H. Chi, M. R. Sayen, D. M. Yellon, and W. H. Dillmann. 1995. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J. Clin. Investig. 95:1446-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKendrick, L., E. Thompson, J. Ferreira, S. J. Morley, and J. D. Lewis. 2001. Interaction of eukaryotic translation initiation factor 4G with the nuclear cap-binding complex provides a link between nuclear and cytoplasmic functions of the m(7) guanosine cap. Mol. Cell. Biol. 21:3632-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min, W., J. R. Bradley, J. J. Galbraith, S. J. Jones, E. C. Ledgerwood, and J. S. Pober. 1998. The N-terminal domains target TNF receptor-associated factor-2 to the nucleus and display transcriptional regulatory activity. J. Immunol. 161:319-324. [PubMed] [Google Scholar]
- 35.Morino, S., H. Imataka, Y. V. Svitkin, T. V. Pestova, and N. Sonenberg. 2000. Eukaryotic translation initiation factor 4E (eIF4E) binding site and the middle one-third of eIF4GI constitute the core domain for cap-dependent translation, and the C-terminal one-third functions as a modulatory region. Mol. Cell. Biol. 20:468-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nover, L., K. D. Scharf, and D. Neumann. 1983. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol. Cell. Biol. 3:1648-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park, Y. C., V. Burkitt, A. R. Villa, L. Tong, and H. Wu. 1999. Structural basis for self-association and receptor recognition of human TRAF2. Nature 398:533-538. [DOI] [PubMed] [Google Scholar]
- 38.Plumier, J. C., B. M. Ross, R. W. Currie, C. E. Angelidis, H. Kazlaris, G. Kollias, and G. N. Pagoulatos. 1995. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J. Clin. Investig. 95:1854-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan, A. J., S. W. Flanagan, P. L. Moseley, and C. V. Gisolfi. 1992. Acute heat stress protects rats against endotoxin shock. J. Appl. Physiol. 73:1517-1522. [DOI] [PubMed] [Google Scholar]
- 40.Saad, S., M. Kanai, M. Awane, Y. Yamamoto, T. Morimoto, W. Isselhard, T. Minor, H. Troidl, K. Ozawa, and Y. Yamaoka. 1995. Protective effect of heat shock pretreatment with heat shock protein induction before hepatic warm ischemic injury caused by Pringle's maneuver. Surgery 118:510-516. [DOI] [PubMed] [Google Scholar]
- 41.Scharf, K. D., H. Heider, I. Hohfeld, R. Lyck, E. Schmidt, and L. Nover. 1998. The tomato Hsf system: HsfA2 needs interaction with HsfA1 for efficient nuclear import and may be localized in cytoplasmic heat stress granules. Mol. Cell. Biol. 18:2240-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulze-Osthoff, K., R. Beyaert, V. Vandevoorde, G. Haegeman, and W. Fiers. 1993. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 12:3095-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scorsone, K. A., R. Panniers, A. G. Rowlands, and E. C. Henshaw. 1987. Phosphorylation of eukaryotic initiation factor 2 during physiological stresses which affect protein synthesis. J. Biol. Chem. 262:14538-14543. [PubMed] [Google Scholar]
- 44.Shanley, T. P., M. A. Ryan, T. Eaves-Pyles, and H. R. Wong. 2000. Heat shock inhibits phosphorylation of I-kappaBalpha. Shock 14:447-450. [DOI] [PubMed] [Google Scholar]
- 45.Tang, G., Y. Minemoto, B. Dibling, N. H. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-kappaB target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 46.Trost, S. U., J. H. Omens, W. J. Karlon, M. Meyer, R. Mestril, J. W. Covell, and W. H. Dillmann. 1998. Protection against myocardial dysfunction after a brief ischemic period in transgenic mice expressing inducible heat shock protein 70. J. Clin. Investig. 101:855-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Molle, W., B. Wielockx, T. Mahieu, M. Takada, T. Taniguchi, K. Sekikawa, and C. Libert. 2002. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity 16:685-695. [DOI] [PubMed] [Google Scholar]
- 48.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, S. Q., A. Kovalenko, G. Cantarella, and D. Wallach. 2000. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity 12:301-311. [DOI] [PubMed] [Google Scholar]