Abstract
The semaphorins are a large family of proteins involved in the patterning of both the vascular and the nervous systems. In order to analyze the function of the membrane-bound semaphorin 5A (Sema5A), we generated mice homozygous for a null mutation in the Sema5a gene. Homozygous null mutants die between embryonic development days 11.5 (E11.5) and E12.5, indicating an essential role of Sema5A during embryonic development. Mutant embryos did not show any morphological defects that could account for the lethality of the mutation. A detailed analysis of the vascular system uncovered a role of Sema5A in the remodeling of the cranial blood vessels. In Sema5A null mutants, the complexity of the hierarchically organized branches of the cranial cardinal veins was decreased. Our results represent the first genetic analysis of the function of a class 5 semaphorin during embryonic development and identify a role of Sema5A in the regional patterning of the vasculature.
Two of the most important processes during embryonic development are the establishment of the cardiovascular system and the establishment of the nervous system. Both are characterized by the stereotypic organization of their branching pattern that is established, at least in part, by similar mechanisms and positional cues. One family of proteins that play a role in shaping both systems are the semaphorins (8, 15). They constitute a large family of secreted and membrane-bound proteins that share a conserved semaphorin domain and can be subdivided into seven classes based on the presence of class-specific carboxy-terminal domains. Although the majority of the vertebrate semaphorins are integral membrane proteins (class 4 to 6), only the secreted class 3 semaphorins have been analyzed functionally in some detail. In vitro, Sema3 proteins act as potent repellents for axons (15). The analysis of mice deficient for Sema3a and Sema3f confirmed that they play an important role in wiring the nervous system (7, 37, 44). Sema3A promotes the fasciculation of peripheral axons and is necessary for the guidance of olfactory sensory axons in the olfactory bulb (7, 39, 40, 44). In addition, these mutants revealed a role of semaphorins during the development of the cardiovascular system. Sema3A-deficient mice show vascular and cardiac defects characterized by a right ventricular hypertrophy and a dilated right atrium (7, 21, 41). Sema3c knockout animals die perinatally due to the improper separation of the cardiac outflow tract and interruption of the aortic arch (14). The analysis of receptors for the class 3 semaphorins confirmed the dual role of these proteins in cardiovascular and neuronal development. The minimal receptor for the class 3 semaphorins consists of neuropilin-1 (Nrp-1) or -2 as the ligand-binding subunit and a member of the plexin family (plexin-A1 to -A4 or plexin-D1) as the signal-transducing subunit (15, 18). The phenotypes of animals deficient in Nrp-1 and Sema3A show very similar defects in the peripheral nervous system, and the phenotypes of those deficient in Sema3F, Nrp-2, and plexin-A3 show defects in the central nervous system (9, 11, 17, 27). The cardiovascular phenotypes of neuropilin-deficient mice have been more difficult to interpret because neuropilins also act as low-affinity receptors for VEGFA165 (42). Thus, although the cardiac defects of the Nrp-1, Sema3c, and Plxnd1 mutants are strikingly similar, the vascular abnormalities of the Nrp-1 and -2 knockout mice were considered a consequence of impaired VEGF signaling (18, 21, 26, 43). However, the presence of vascular defects in Sema3a and Plxnd1 mutants and the disruption of the semaphorin receptor plexin-D1 in the out of bonds mutant in zebrafish argue in favor of a direct function of semaphorins in the development of the vascular system (45).
In contrast to the class 3 semaphorins, very little is known about the function of the mammalian membrane-bound semaphorins. The requirement of Sema6D for cardiac looping and ventricular ballooning and its effects on endothelial cell migration suggest that the involvement in cardiovascular development is not restricted to the Sema3 proteins (46). The class 5 semaphorins are unique as they include both vertebrate and invertebrate homologues (5). The mammalian genome contains two members of this class, Sema5a and -5b (originally named SemF and SemG), which show largely complementary expression patterns (1). They are characterized by the presence of seven type 1 thrombospondin repeats in their extracellular domain. As the type 1 repeats of thrombospondin-1 and -2 promote neurite outgrowth (33, 35), it is possible that Sema5A and -5B may exert different biological responses through their semaphorin domain and thrombospondin repeats (1).
In order to address its physiological function, we inactivated Sema5a in embryonic stem (ES) cells. Here we show that Sema5a is an essential gene as homozygous Sema5a mutant mice die at mid-gestation. The mutants do not show any morphological defects that could explain the embryonic lethality. Our analysis uncovered a role of Sema5A in the remodeling of the cranial large-diameter vessels that sprout from the cardinal veins. Our results represent the first genetic analysis of the physiological function of Sema5A and reveal a role of semaphorins in the regional patterning of the vascular system.
MATERIALS AND METHODS
Construction of the targeting vector.
Two overlapping clones from the Sema5a locus were isolated by screening a 129/sv λfixII library (Stratagene) with a Sema5a cDNA probe. Three overlapping fragments spanning 10.2 kb were isolated, subcloned into the pBluescript-SK vector, and analyzed by sequencing and restriction mapping. The clones contained five exons of the Sema5a gene (exons 4 to 8) encoding amino acids (aa) 130 to 270. A targeting vector was constructed by replacing the region containing exons 4 and 5 with the neomycin selection cassette. The selection cassette was flanked by 7.2 kb (SacI-KpnI fragment in Fig. 1A) and 1.2 kb (XhoI fragment in Fig. 1A) of homologous sequences. The latter was generated by PCR using the following primers: 5′-CCCCTCGAGGTCTTTGAGTCACCCCTGAGC-3′ and 5′-CCCTCGAGAGAGACAGAGACAGTGAGACC-3′. A PGK-tk-negative selection cassette was placed at the 5′ end of the construct.
FIG. 1.
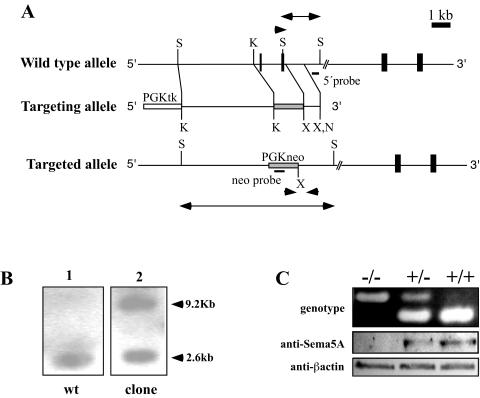
Generation of Sema5a knockout mice. (A) Exons 4 and 5 of the Sema5a locus were replaced by the PGK-neomycin resistance cassette (PGKneo; gray box). Schematic representations of the wild-type allele (top), the targeting vector (middle), and the mutant allele (bottom) are shown. The probes (5′ probe, neo probe) used for screening ES cells are indicated by black bars. Arrowheads represent the primers used to genotype Sema5a mutant mice. Arrows indicate the restriction fragments detected by the 5′ probe after digestion with SacI. Essential restriction enzyme sites are indicated by vertical bars. (B) Genomic DNA was isolated from the ES cell clone used to generate mutant mice, digested with SacI, and analyzed by Southern blotting with the 5′ probe. The wild-type (wt) and targeted alleles are represented by restriction fragments of 2.6 and 9.2 kb, respectively. (C) Western blot analysis of membrane fractions prepared from wild-type, heterozygous, and homozygous mutant embryos. No Sema5A protein was detected in extracts from homozygous mice. Detection of β-actin showed that comparable amounts of protein were loaded in each lane. K, KpnI; N, NotI; X, XhoI; S, SacI.
Isolation of targeted ES cells and generation of chimeras.
R1 ES cells (kindly provided by Andreas Nagy) were electroporated with 15 μg of linearized targeting vector by a single pulse of 800 mV and 3 μF with a Gene Pulser (Bio-Rad). Cells were cultured on irradiated G418-resistant primary mouse embryo fibroblasts. Selection with G418 and ganciclovir began after 24 and 48 h, respectively. We screened 144 doubly resistant colonies for homologous recombination by Southern blotting using the 5′ external probe (Fig. 1A and B). Three positive clones were identified, and the integration of a single fragment was confirmed with the Neo probe (Fig. 1B). One clone was used for the generation of aggregation chimeras. One high-percentage male chimera was crossed to wild-type NMRI mice. The offspring was genotyped by PCR using the following primers: F-3889 (5′-GGCAAGCTGTGGGTAGCAGAATGT-3′), F-3890 (5′-GAGCCTACATGTGTCTATGACCCA-3′), and PGK-2940 (5′-GGAATGTGTGCGAGGCCAGAGGT-3′). The phenotypic analysis of Sema5a mutants was performed on mice with a 129/Sv/NMRI mixed genetic background.
Western blot.
Membrane proteins from embryonic development day 10.5 (E10.5) mouse embryos were isolated by differential centrifugation as described previously (23). Six micrograms of protein was used for each sample, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes (Schleicher and Schuell). Western blots were developed with the Vectastain kit (Vector Laboratories) according to the manufacturer's instructions, using anti-Sema5A (1:2,000) (38) and anti-β-actin (1:2,000; Chemicon) antibodies.
Histological methods.
Embryos were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) and processed for paraffin embedding. Microtome sections were stained with hematoxylin and eosin according to standard procedures.
Immunofluorescence.
Embryos were fixed in 4% paraformaldehyde in PBS and cryoprotected in 30% sucrose-PBS. The cryosections (12 μm) were washed in PBS, blocked for 1 h in 3% normal goat serum-0.3%Triton X-100-1% bovine serum albumin in PBS (blocking buffer), and incubated overnight with anti-PECAM antibody diluted 1:50 in blocking buffer. The sections were washed five times in PBS and incubated with Alexa594-conjugated anti-rat secondary antibody and Hoechst-33258 (Molecular Probes) diluted in blocking buffer. After five washes in PBS followed by one wash in double-distilled water, the sections were mounted with an aqueous mounting medium (DAKO). The sections were analyzed with a Zeiss axiophot microscope equipped with a Hamatsu charge-coupled device camera, and images were analyzed with Adobe Photoshop and Deneba Canvas.
Whole-mount immunohistochemistry.
Embryos were fixed in 4% paraformaldehyde in PBS, dehydrated through a graded methanol series, and bleached for 4 h in 5% H2O2-methanol. After rehydration, embryos were washed three times in 1× PBS-3% instant milk powder-0.1% Triton X-100 (PBS-MT) and incubated with the primary antibody diluted in PBS-MT. After five washes with PBS-MT, the embryos were incubated with horseradish peroxidase-conjugated secondary antibody (Chemicon), washed five times with PBS-MT, and developed with 3′,3′-diaminobenzidine (DAB; Sigma). The reaction was stopped by rinsing the embryos three times in PBS. Rat anti-PECAM (1:50; Pharmingen) and the antineurofilament antibody 2H3 (1:5, ascites supernatant; Developmental Studies Hybridoma Bank, Iowa University) were used as primary antibodies. For quantitative analysis, the complexity index (Ci) of the cardinal vein branches was calculated according to the formula Ci = [Σn(Bno) × n(Bno)]/Σn(Bno), where n(Bno) indicates the number of branches of the order Bn.
In situ hybridization.
Nonradioactive in situ hybridization on paraffin sections was performed as described previously (30) with probes specific for ANF (50), Cx40 (47), Nkx2.5 (28), Bmp4 (51), Bmp10 (32), and Sema5b and Sema5a (1).
RESULTS
Generation of Sema5a null mice.
To investigate its physiological function, we generated Sema5a null mice by replacing exons 4 and 5 with a neomycin selection cassette (Fig. 1A). This mutation should result in a Sema5a null allele by introducing a stop codon after aa 121. The resulting protein fragment contains the first 110 aa of the semaphorin domain and is unlikely to retain any biological activity. An aberrant splicing event that joins exons 3 and 6 would result in a frameshift mutation that generates a stop codon after aa 141. Three correctly targeted ES cell clones were identified by Southern blotting, and one of them was used to generate chimeras by morula aggregation (Fig. 1B). A high percentage of chimeras transmitted the mutation through the germ line. Heterozygous mutants were viable and healthy, but homozygous Sema5a−/− mice died between E11.5 and E12.5 (Table 1). The level of Sema5A protein in wild-type, heterozygous, and mutant mice was assessed by Western blots of membrane protein fractions prepared from E10.5 embryos with an antibody directed against the cytoplasmic tail of Sema5A (Fig. 1C). The amount of Sema5A protein was reduced in heterozygous animals, and no protein was detectable in homozygous mutants, confirming that the Sema5a mutant allele represents a null mutation.
TABLE 1.
Genotypes of Sema5A mouse offspring at weaning and different embryonic stagesa
| Stage | % (no.) of animalsb
|
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| P28 | 31 (45) | 69 (88) | 0 |
| E15.5 | 28 (19) | 72 (33) | 0 |
| E12.5 | 29 (20) | 71 (34) | 0 |
| E11 | 27 (25) | 48 (37) | 25 (24) |
| E10 | 25 (26) | 58 (46) | 27 (20) |
| E9 | 13 (6) | 70 (19) | 17 (7) |
Heterozygous Sema5A mice were mated, and the offspring were genotyped by PCR at weaning and at different embryonic stages.
The absolute number of animals genotyped at each embryonic stage is indicated in parentheses.
Neuronal projections in Sema5a null mice are normal.
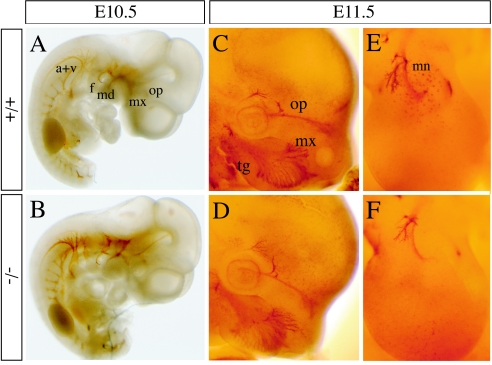
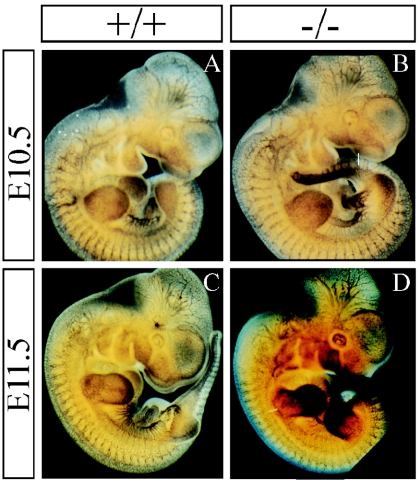
Until E12.5, Sema5a null embryos were indistinguishable from their wild-type and heterozygous littermates, with no obvious abnormalities or difference in size. Heartbeat was observed in all freshly dissected embryos. Based on the function of semaphorins in axon guidance and the embryonic expression pattern of Sema5a (1), we analyzed the development of axonal trajectories by whole-mount staining with an antineurofilament (2H3) antibody in E10.5 and E11.5 Sema5a mutants. No abnormalities were detected in the projections analyzed. In E10.5 wild-type and homozygous mutant embryos, the maxillary, mandibular, and ophthalmic components of the trigeminal nerve and the facial nerve were tightly fasciculated and followed their normal trajectories (Fig. 2A and B). By E11.5, they begin to innervate their target area in a pattern that was indistinguishable in wild-type and mutant embryos (Fig. 2C and D). The organization of the vagus and accessory nerves also appeared normal in Sema5a−/− animals. The innervation of the limb buds by sensory and motor neurons at E11.5 was also analyzed (Fig. 2E and F). At this stage, Sema5a expression is restricted to a stripe at the base of the fore- and hindlimb buds, suggesting that it may play a role in the organization of the plexus formed by the motor axons at this position before they innervate the limb buds (1). However, axons projected normally into the developing limb buds in all animals analyzed.
FIG. 2.
Development of the nervous system in Sema5a null mutants. Wild-type and Sema5a−/− E10.5 (A and B) and E11.5 (C to F) embryos were stained with an antineurofilament antibody. In wild-type (A and C) and homozygous mutant (B and D) embryos, the ophthalmic (op), maxillary (mx), facial (f), and mandibular (md) nerves extended normally and were tightly fasiculated. At E11.5, motor nerve bundles (mn) began to innervate the limb buds (E and F) normally in both wild-type and homozygous mutant embryos. a+v, accessory and vagal nerve.
Development of extraembryonic tissues in Sema5a−/− mutants.
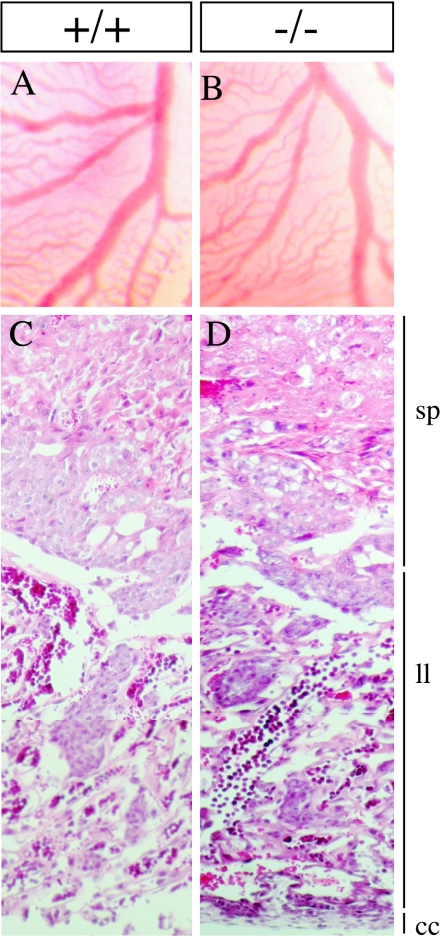
Lethality at mid-gestation often results from abnormalities in extraembryonic tissues and/or defects in cardiac development and function (2, 6, 29). Freshly dissected yolk sacs from mutant E11.5 embryos were indistinguishable from those of their wild-type counterparts (Fig. 3A and B). No hemorrhage was detected in the mutants, and the typical, highly branched, network of hierarchically organized vessels was observed in both wild-type and Sema5a−/− yolk sacs. Paraffin sections showed a normal development of the placenta (Fig. 3C and D). The thicknesses of the spongiotrophoblast and labyrinthine layers as well as the chorionic plate were comparable in wild-type and mutant embryos. The maternal and embryonic blood vessels were intermingled properly in the labyrinthine layer of the null mutants. The proper vascularization of the yolk sac and the normal morphology of the placenta suggest that the supply of oxygen and nutrients was not impaired in mutants. Thus, Sema5a−/− embryos do not die because of an abnormal development of extraembryonic tissues.
FIG. 3.
Development of extraembryonic tissues in Sema5a null mutants. Bright-field images of freshly dissected yolk sacs from E11 wild-type (A) and mutant (B) embryos showed pervasive vascularization by highly branched vessels in both cases. Hematoxylin-eosin-stained sections of E11 placentas from wild-type (C) and mutant (D) embryos showed a normal organization of the spongiotrophoblast (sp) and labyrinthine layers (ll) and the chorionic plate (cc).
Cardiac development in the Sema5a mutant embryos.
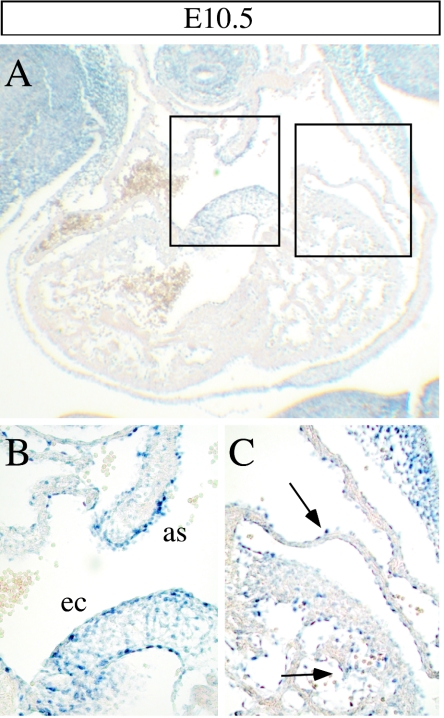
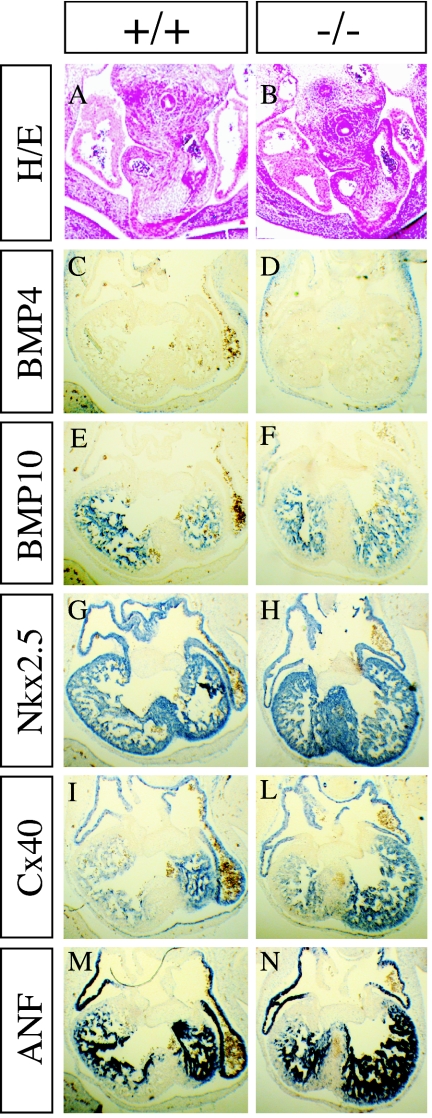
Between E8.5 and E13, the nonpartitioned heart tube develops into the mature four-chambered organ, due to the septation of the embryonic atrium and ventricles (29). Many mutations affecting this process result in embryonic lethality. In order to determine if Sema5A plays a role in these events, we analyzed its expression pattern in the hearts of E10.5 embryos by in situ hybridization. The Sema5a transcript was detected in the developing atrial septum and endocardial cushions (Fig. 4A and B) and, at a lower level, in the atrial and ventricular endocardium (Fig. 4C). Its expression pattern suggested that Sema5A might play a role in chamber morphogenesis and atrioventricular septation. Therefore, we focused our analysis on these two processes by monitoring the expression of different markers of cardiac development in E10.5 wild-type and knockout embryos by in situ hybridization (12). The expression of Bmp4 (Fig. 5C and D) was used as marker for the formation of the endocardial cushion and atrioventricular septation (24, 31). The transcripts for BMP10 (10, 32), the homeobox transcription factor Nkx2.5 (Fig. 4G to H), the gap junction protein Cx40 (Fig. 5I to L) (13), and the hormone ANF (Fig. 5M to N) (50) were selected as markers for chamber morphogenesis. In all cases, no differences were detected between wild-type and homozygous Sema5a mutant embryos, indicating that cardiogenesis is not affected by a mutation of Sema5a. In addition, no histological abnormalities were detected in the outflow tract of homozygous Sema5a mutant embryos (Fig. 5A and B). We also analyzed the expression pattern of Sema5b in wild-type and Sema5a knockout embryos in order to determine if a compensatory effect of Sema5b might explain the normal cardiac and neuronal development of the Sema5a mutants. No differences were observed in the expression pattern of Sema5b between wild-type and knockout embryos in all tissues analyzed (see Fig. S1 in the supplemental material).
FIG. 4.
Expression of Sema5a in the embryonic heart. Twelve-micrometer paraffin sections of E10.5 wild-type embryos were hybridized with digoxigenin-labeled RNA probes specific for Sema5a (A). A higher magnification of the areas marked by boxes is shown in panels B and C. The highest level of Sema5a mRNA was detected in the atrial septum (as) and endocardial (ec) cushion (A and B). Sema5a was also expressed in the atrial and ventricular endocardium, as indicated by arrows (C).
FIG. 5.
Cardiac development in Sema5a mutants. Twelve-micrometer paraffin sections of E10.5 wild-type (A, C, E, G, I, and M) and mutant (B, D, F, H, L, and N) embryos were stained with hematoxylin and eosin (H/E) (A and B) to analyze the morphology of the outflow tract or hybridized with digoxigenin-labeled RNA probes specific for Bmp4 (C and D), Bmp10 (E and F), Nkx2.5 (G and H), Cx40 (I and L), and Anf (M and N) as markers for the differentiation and growth of endocardial cushions (C and D) and ventricles and atria (E to N). The morphology of the outflow tract and the expression pattern of all markers were similar in mutant and wild-type embryos.
Vascular development in Sema5a mutants.
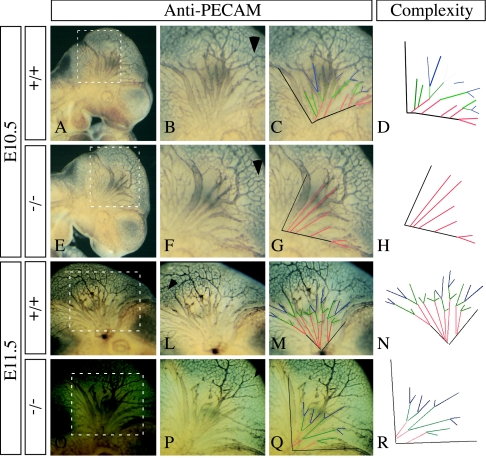
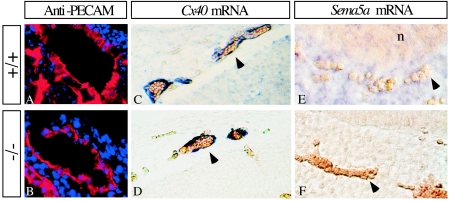
Many mutations affecting blood vessel formation result in embryonic lethality (36). We analyzed the vasculature of E10.5 and E11.5 embryos by whole-mount staining with the anti-PECAM antibody. The overall structures of the vascular system were similar in wild-type and homozygous mutant embryos at E10.5 and E11.5 (Fig. 6). For example, no abnormalities were detected in the organization of the intersomitic blood vessels or in the vascularization of the limb buds (Fig. 6). Thus, vasculogenesis and angiogenesis are largely normal in Sema5a−/− animals. However, upon closer examination, a decrease in the number of large-diameter vessels was observed in the cranial region of E10.5 mutant embryos (Fig. 7A to H). In developing embryos, the sprouting of branches from the cardinal veins results in the formation of a hierarchical network of large-diameter vessels in the medial region of the head. In contrast, the organization of the capillary network in the more dorsal and peripheral region of the head appeared normal (arrowheads in Fig. 7B and F). To determine the nature of the defect, the branching pattern of the large vessels was analyzed in wild-type and knockout embryos. While the number of primary branches sprouting from the cardinal veins was unaltered in the mutants at E10.5, these vessels did not form additional branches and failed to establish their normal hierarchical organization (Fig. 7D). This difference in the complexity of the branching pattern was observed in all the mutants analyzed (n = 10) but varied in its severity. The complexity of the branching pattern in wild-type, heterozygous, and homozygous mutant embryos was quantified by calculating a complexity index (Ci) that assigns different weights to primary, secondary, and additional, higher order branches. The complexity of the branching pattern in homozygous mutants was significantly decreased compared to heterozygous and wild type embryos (Ci for wild-type embryos, 2.15, n = 7, standard deviation of the mean [SD] = 0,05; Ci for heterozygous embryos, 2.07, n = 8, SD = 0,12; Ci for knockout embryos, 1.07, n = 10, SD 0,13). PECAM staining revealed that the reduction in complexity persisted also in E11.5 embryos (Fig. 6I to R). The phenotype was even more pronounced at this stage due to a reduction in the number of primary branches, making it unlikely that the phenotype resulted from a developmental delay. High levels of Sema5a transcript were detected in the mesoderm surrounding the cranial vessels (Fig. 8E). However, anti-PECAM immunofluorescent staining of vascular endothelial cells in cross-sections of the cardinal veins of E10.5 wild-type and mutant embryos did not reveal any obvious abnormality in vessel morphology (Fig. 8A and B). Also, the expression pattern of Cx40 in the large cranial vessels of E10.5 wild-type and homozygous mutant embryos was normal (Fig. 8C and D).
FIG. 6.
Sema5a null mutants have a largely normal vascular system. The vascular system of E10.5 (A and B) and E11.5 (C and D) wild-type (A and C) and homozygous Sema5a mutant (B and D) embryos was analyzed by whole-mount immunohistochemistry with an anti-PECAM antibody. No obvious morphological abnormalities could be detected in the vascular system.
FIG. 7.
Branching of large vessels is impaired in the cranial region of Sema5a null mutants. The cranial vasculature of E10.5 (A to H) and E11.5 (I to R) wild-type (A to D and I to M) and homozygous Sema5a mutant (E to H and O to R) embryos was analyzed by whole-mount immunohistochemistry with an anti-PECAM antibody. Panels B, C, F, G, L, M, P, and Q show a higher magnification of the marked areas. While the capillary network in the peripheral regions of the head (arrowheads in B, F, L, and P) was not affected in mutant embryos, the branching pattern of the large vessels (B, F, L, and P) was less complex in Sema5a−/− embryos (D, H, N, and R). The branching pattern of the large vessels is outlined in panels C, D, G, H, M, N, Q, and R. Sema5a mutants showed a decrease in the number of secondary (green lines) and tertiary (blue lines) branches. The cardinal veins and the primary branches are represented by black and red lines, respectively.
FIG. 8.
Sema5A is expressed in the mesoderm surrounding the cranial vessels, and their morphology is normal in the null mutants. Cryosections (A and B) and paraffin sections (C, D, E and F) of E10.5 wild-type (A, C, and F) and homozygous Sema5a mutant (B, D and E) embryos were stained with an anti-PECAM antibody (red in A and B) to visualize vascular endothelial cells and with a digoxigenin-labeled probes specific for Cx40 (C and D) and Sema5a (E and F) mRNAs by in situ hybridization. No differences were detectable between the PECAM and Cx40 stainings in wild-type and homozygous mutant embryos. Strong expression of Sema5a was detected in the mesoderm surrounding the vessels in wild-type embryos (E), while no signal was detectable in homozygous mutants (F). The nuclei (blue in panels A and B) were stained with Hoechst-33258. Arrowheads indicate the erythrocytes in the vessels; n, neuroepithelium.
DISCUSSION
Here we show that Sema5A is essential for embryonic development as mice homozygous for a Sema5a null mutation die between E11.5 and E12.5. The development of the extraembryonic tissues and the cardiovascular system, whose impairment is often responsible for the lethality of mutations at this stage, was normal in Sema5a mutants. In addition, no defects were detected in the development of the axonal trajectories, showing that Sema5A is not required for axonal pathfinding at this stage. While the reason for the lethality remained unclear, our genetic analysis revealed a role of Sema5A in regulating the vascular branching pattern. We observed abnormalities in the branches originating from the cardinal veins in the head of Sema5a mutants but not in other parts of the vascular system. This phenotype indicates a specific function of Sema5A in the refinement of this subtype of vessels.
Sema5a mutants show no defect in the peripheral nervous system.
The expression pattern of Sema5a and the established functions of semaphorins in neuronal development suggested that Sema5A may act as an axon guidance signal. However, our analysis did not identify any defects in the embryonic nervous system of Sema5a null mice. Thus, Sema5A is not essential for this process at the embryonic stages analyzed. It is possible that, similar to the defects in the vascular system, only a small subset of axons is affected, while the overall structure is unimpaired. It is unlikely that the absence of a severe phenotype is due to a functional redundancy with the only other class 5 member. The expression pattern of Sema5B is complementary to and largely nonoverlapping with Sema5A (see reference 1 and this work), and it is unaltered in the Sema5a mutants. Sema5A is involved in retinal axon pathfinding and regeneration, and expression of Sema5A is altered in Pax6 knockout mice that show defects in the development of the axonal connections in the forebrain (19, 25, 34). The embryonic lethality of the Sema5a mutants precluded an analysis of these late processes.
Normal development of extraembryonic tissues and heart in Sema5a mutants.
So far, we have been unable to determine the reason for the embryonic lethality of the Sema5a mutation. Our analysis of extraembryonic tissues and cardiac development did not reveal any abnormalities. The histology of the placenta and the vascularization of the yolk sac were normal. Thus, deficiencies in the supply of the embryo with nutrients and oxygen cannot be the reason for embryonic lethality. The absence of an evident developmental delay in Sema5a−/− embryos supports this conclusion.
Sema5a is expressed in the atrial and ventricular myocardium as well as in the endocardial cushions and the atrial septum. However, the formation and differentiation of cardiac tissues are normal in Sema5a mutants. Both the morphology of the heart and its differentiation, as visualized by the expression of markers for the myocardium (Nkx2.5, Anf, Cx40, and Bmp10), and the endocardial cushions (Bmp4) were similar in mutants and wild-type embryos, both qualitatively and quantitatively. Thus, Sema5a null mutants do not die because of a severe developmental defect in cardiogenesis. At present, we cannot rule out subtle physiological defects in the chamber myocardium, affecting contractility or intercellular coupling. Subtle impairment of cardiac function, together with an increased resistance to the blood flow that probably results from the vascular defects, may explain the embryonic lethality of the mutation.
Role of Sema5A during vascular development.
Our analysis of the vascular system revealed a role of Sema5A in the refinement of the cranial large vessels. It is unlikely that these subtle vascular abnormalities are a consequence of defects in extraembryonic tissues because both the yolk sac and the placenta develop normally in the mutants.
The most interesting feature of the Sema5a mutants is the unique regional specificity of the vascular defects. The branches of the anterior cardinal veins were the only type of vessels with detectable abnormalities. The phenotype implies that Sema5A is not involved in the differentiation of vessels in general, but in the regional patterning of the vasculature. The organization of the vascular system is achieved by controlling the position and angle of branches in different areas of the embryo (e.g., the sprouting of intersomitic vessels and the vascularization of different organs by the main vessels). This stereotyped branching requires signals providing positional information. Few of these signals have been identified so far, reflecting the limited understanding of the positional cues that coordinate, together with members of the VEGF and angiopoietin protein families, the remodeling of the primary capillary plexus into the mature vascular system (36).
The cranial cardinal veins are closely connected to the capillary plexus at E9.5. This homogenous network of dilated vessels is remodeled between E9.5 and E12.5 in a region-specific manner. Branches sprout rostrally from the cardinal veins to form a system of hierarchically organized vessels. In contrast, in the more ventral and rostral regions of the head, the remodeling of the plexus leads to the formation of a highly branched network of vessels that does not appear to be hierarchically organized. How this differential patterning is regulated is unknown. Strikingly, only the large vessels were affected in the Sema5a mutants, while the vascular network in the ventral and rostral regions of the head appeared normal. This observation, together with the lack of a phenotype in the vascular system of E9.5 embryos (data not shown), suggests that Sema5A is not necessary to initiate the differential remodeling of the vasculature in the head but to complete the development of the large vessels that branch from the cardinal veins.
Sema5A provides another example for an axonal guidance signal that plays a role in vascular patterning (8). Repulsive cues provided by different families of proteins guide the sprouting of the intersomitic vessels. In mouse mutants lacking ephrin-B2, intersomitic sprouts extend incorrectly into the surrounding somites (3). In vitro, Sema3A acts as a chemorepellent for vascular endothelial cells. Genetic elimination of the Sema3A receptor neuropilin-1 results in vascular abnormalities that appear dependent on impaired VEGF signaling (21, 26). However, inactivation of the Sema3a gene results in abnormal vessel development, at least in some genetic backgrounds (41). In zebrafish, inactivation of the semaphorin receptor plexin-D1 results in the formation of intersomitic vessels in aberrant positions (45). Similar abnormalities are detected also in mice deficient for plexin-D1 and its ligand-encoding gene, Sema3e (18, 22), suggesting that this ligand-receptor pair, like Sema5A, is required during the angiogenic remodeling of specific subtypes of vessels.
The cellular basis for the effects of Sema5A during vascular development.
High levels of Sema5A transcript are observed in the mesoderm surrounding the cranial vessels (this work and reference 1), pointing to a signaling function of Sema5A in the development of these vessels. While this work was in progress, plexin-B3 was reported to be a functional receptor for Sema5A (4). We did not detect expression of PlxnB3 in the E11.5 embryo by in situ hybridization (data not shown), suggesting that other receptors for Sema5A mediate its function at this stage. The nature of the signal provided by Sema5A to the branches of the cardinal veins remains to be determined. The decreased number of branches in Sema5A-deficient embryos could be caused by either a failure in the formation of vascular sprouts, their growth, or their stabilization. These processes involve endothelial cell migration, proliferation, and interaction with the extracellular matrix and the support cells (16, 49). Each of these steps may be regulated by Sema5A. The decrease in the number of primary branches in Sema5a mutants at E11.5 suggests that a defect in the stabilization of vessels is the main cause of the phenotype.
Drosophila Sema5c is involved in tumorigenesis, where it is required for the activation of the Dpp signaling pathway (48). Another member of the transforming growth factor β (TGF-β) superfamily, TGF-β, plays an important role in vascular morphogenesis and in the establishment and maintenance of vessel wall integrity (20). This observation raises the possibility that TGF-β and Sema5A cooperate during the angiogenic remodeling of the cranial vessels. However, inactivation of TGF-β and its receptors results in severe defects during vascular development, revealing a general requirement for TGF-β during the development of the circulatory system. Thus, additional work is required to determine if there is an interaction between the Sema5A and TGF-β pathways.
In summary, we have shown that Sema5A is essential for embryonic development and is necessary for the refinement of the cranial of large vessels. The Sema5A knockout mice will be a useful model to dissect the mechanisms that control the regional patterning of the vasculature and to further define the multiple functions of semaphorins during embryonic development.
Supplementary Material
Acknowledgments
We thank Corrie de Gier-de Vries, Gisela Pott, and Maria Wenning for expert technical assistance.
The 2H3 antibody developed by Thomas M. Jessel was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biological Sciences, University of Iowa, Iowa City. This work was supported by grants from the DFG to A.W.P.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adams, R. H., H. Betz, and A. W. Püschel. 1996. A novel class of murine semaphorins with homology to thrombospondin is differentially expressed during early embryogenesis. Mech. Dev. 57:33-45. [DOI] [PubMed] [Google Scholar]
- 2.Adams, R. H., A. Porras, G. Alonso, M. Jones, K. Vintersten, S. Panelli, A. Valladares, L. Perez, R. Klein, and A. R. Nebreda. 2000. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6:109-116. [PubMed] [Google Scholar]
- 3.Adams, R. H., G. A. Wilkinson, C. Weiss, F. Diella, N. W. Gale, U. Deutsch, W. Risau, and R. Klein. 1999. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 13:295-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artigiani, S., P. Conrotto, P. Fazzari, G. F. Gilestro, D. Barberis, S. Giordano, P. M. Comoglio, and L. Tamagnone. 2004. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 5:710-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahri, S. M., W. Chia, and X. Yang. 2001. Characterization and mutant analysis of the Drosophila sema 5c gene. Dev. Dyn. 221:322-330. [DOI] [PubMed] [Google Scholar]
- 6.Barak, Y., M. C. Nelson, E. S. Ong, Y. Z. Jones, P. Ruiz-Lozano, K. R. Chien, A. Koder, and R. M. Evans. 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585-595. [DOI] [PubMed] [Google Scholar]
- 7.Behar, O., J. A. Golden, H. Mashimo, F. J. Schoen, and M. C. Fishman. 1996. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383:525-528. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet, P. 2003. Blood vessels and nerves: common signals, pathways and diseases. Nat. Rev. Genet. 4:710-720. [DOI] [PubMed] [Google Scholar]
- 9.Chen, H., A. Bagri, J. A. Zupicich, Y. Zou, E. Stoeckli, S. J. Pleasure, D. H. Lowenstein, W. C. Skarnes, A. Chedotal, and M. Tessier-Lavigne. 2000. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron 25:43-56. [DOI] [PubMed] [Google Scholar]
- 10.Chen, H., S. Shi, L. Acosta, W. Li, J. Lu, S. Bao, Z. Chen, Z. Yang, M. D. Schneider, K. R. Chien, S. J. Conway, M. C. Yoder, L. S. Haneline, D. Franco, and W. Shou. 2004. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development 131:2219-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng, H. J., A. Bagri, A. Yaron, E. Stein, S. J. Pleasure, and M. Tessier-Lavigne. 2001. Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron 32:249-263. [DOI] [PubMed] [Google Scholar]
- 12.Christoffels, V. M., P. E. Habets, D. Franco, M. Campione, F. de Jong, W. H. Lamers, Z. Z. Bao, S. Palmer, C. Biben, R. P. Harvey, and A. F. Moorman. 2000. Chamber formation and morphogenesis in the developing mammalian heart. Dev. Biol. 223:266-278. [DOI] [PubMed] [Google Scholar]
- 13.Delorme, B., E. Dahl, T. Jarry-Guichard, I. Marics, J. P. Briand, K. Willecke, D. Gros, and M. Theveniau-Ruissy. 1995. Developmental regulation of connexin 40 gene expression in mouse heart correlates with the differentiation of the conduction system. Dev. Dyn. 204:358-371. [DOI] [PubMed] [Google Scholar]
- 14.Feiner, L., A. L. Webber, C. B. Brown, M. M. Lu, L. Jia, P. Feinstein, P. Mombaerts, J. A. Epstein, and J. A. Raper. 2001. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 128:3061-3070. [DOI] [PubMed] [Google Scholar]
- 15.Fiore, R., and A. W. Püschel. 2003. The function of semaphorins during nervous system development. Front. Biosci. 8:s484-s499. [DOI] [PubMed] [Google Scholar]
- 16.Gerhardt, H., and C. Betsholtz. 2003. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 314:15-23. [DOI] [PubMed] [Google Scholar]
- 17.Giger, R. J., J. F. Cloutier, A. Sahay, R. K. Prinjha, D. V. Levengood, S. E. Moore, S. Pickering, D. Simmons, S. Rastan, F. S. Walsh, A. L. Kolodkin, D. D. Ginty, and M. Geppert. 2000. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron 25:29-41. [DOI] [PubMed] [Google Scholar]
- 18.Gitler, A. D., M. M. Lu, and J. A. Epstein. 2004. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev. Cell 7:107-116. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg, J. L., M. E. Vargas, J. T. Wang, W. Mandemakers, S. F. Oster, D. W. Sretavan, and B. A. Barres. 2004. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J. Neurosci. 24:4989-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goumans, M. J., F. Lebrin, and G. Valdimarsdottir. 2003. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc. Med. 13:301-307. [DOI] [PubMed] [Google Scholar]
- 21.Gu, C., E. R. Rodriguez, D. V. Reimert, T. Shu, B. Fritzsch, L. J. Richards, A. L. Kolodkin, and D. D. Ginty. 2003. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5:45-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu, C., Y. Yoshida, J. Livet, D. V. Reimert, F. Mann, J. Merte, C. E. Henderson, T. M. Jessell, A. L. Kolodkin, and D. D. Ginty. 18 November 2004, posting date. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science 307:265-268. [Online.] doi:10.1146/science.110546. [DOI] [PubMed] [Google Scholar]
- 23.Huttner, W. B., W. Schiebler, P. Greengard, and P. De Camilli. 1983. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol. 96:1374-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiao, K., H. Kulessa, K. Tompkins, Y. Zhou, L. Batts, H. S. Baldwin, and B. L. Hogan. 2003. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 17:2362-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, L., G. Lopez-Bendito, P. Gruss, A. Stoykova, and Z. Molnar. 2002. Pax6 is required for the normal development of the forebrain axonal connections. Development 129:5041-5052. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki, T., T. Kitsukawa, Y. Bekku, Y. Matsuda, M. Sanbo, T. Yagi, and H. Fujisawa. 1999. A requirement for neuropilin-1 in embryonic vessel formation. Development 126:4895-4902. [DOI] [PubMed] [Google Scholar]
- 27.Kitsukawa, T., M. Shimizu, M. Sanbo, T. Hirata, M. Taniguchi, Y. Bekku, T. Yagi, and H. Fujisawa. 1997. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 19:995-1005. [DOI] [PubMed] [Google Scholar]
- 28.Lyons, I., L. M. Parsons, L. Hartley, R. Li, J. E. Andrews, L. Robb, and R. P. Harvey. 1995. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 9:1654-1666. [DOI] [PubMed] [Google Scholar]
- 29.Moorman, A. F., and V. M. Christoffels. 2003. Cardiac chamber formation: development, genes, and evolution. Physiol. Rev. 83:1223-1267. [DOI] [PubMed] [Google Scholar]
- 30.Moorman, A. F., A. C. Houweling, P. A. de Boer, and V. M. Christoffels. 2001. Sensitive nonradioactive detection of mRNA in tissue sections: novel application of the whole-mount in situ hybridization protocol. J. Histochem. Cytochem. 49:1-8. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima, Y., T. Yamagishi, S. Hokari, and H. Nakamura. 2000. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP). Anat. Rec. 258:119-127. [DOI] [PubMed] [Google Scholar]
- 32.Neuhaus, H., V. Rosen, and R. S. Thies. 1999. Heart specific expression of mouse BMP-10 a novel member of the TGF-beta superfamily. Mech. Dev. 80:181-184. [DOI] [PubMed] [Google Scholar]
- 33.O'Shea, K. S., L. H. Liu, and V. M. Dixit. 1991. Thrombospondin and a 140 kd fragment promote adhesion and neurite outgrowth from embryonic central and peripheral neurons and from PC12 cells. Neuron 7:231-237. [DOI] [PubMed] [Google Scholar]
- 34.Oster, S. F., M. O. Bodeker, F. He, and D. W. Sretavan. 2003. Invariant Sema5A inhibition serves an ensheathing function during optic nerve development. Development 130:775-784. [DOI] [PubMed] [Google Scholar]
- 35.Osterhout, D. J., W. A. Frazier, and D. Higgins. 1992. Thrombospondin promotes process outgrowth in neurons from the peripheral and central nervous systems. Dev. Biol. 150:256-265. [DOI] [PubMed] [Google Scholar]
- 36.Rossant, J., and L. Howard. 2002. Signaling pathways in vascular development. Annu. Rev. Cell. Dev. Biol. 18:541-573. [DOI] [PubMed] [Google Scholar]
- 37.Sahay, A., M. E. Molliver, D. D. Ginty, and A. L. Kolodkin. 2003. Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J. Neurosci. 23:6671-6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwamborn, J. C., R. Fiore, D. Bagnard, J. Kappler, C. Kaltschmidt, and A. W. Püschel. 2004. Semaphorin 3A stimulates neurite extension and regulates gene expression in PC12 cells. J. Biol. Chem. 279:30923-30926. [DOI] [PubMed] [Google Scholar]
- 39.Schwarting, G. A., C. Kostek, N. Ahmad, C. Dibble, L. Pays, and A. W. Püschel. 2000. Semaphorin 3A is required for guidance of olfactory axons in mice. J. Neurosci. 20:7691-7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarting, G. A., D. Raitcheva, J. E. Crandall, C. Burkhardt, and A. W. Püschel. 2004. Semaphorin 3A-mediated axon guidance regulates convergence and targeting of P2 odorant receptor axons. Eur. J. Neurosci. 19:1800-1810. [DOI] [PubMed] [Google Scholar]
- 41.Serini, G., D. Valdembri, S. Zanivan, G. Morterra, C. Burkhardt, F. Caccavari, L. Zammataro, L. Primo, L. Tamagnone, M. Logan, M. Tessier-Lavigne, M. Taniguchi, A. W. Püschel, and F. Bussolino. 2003. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424:391-397. [DOI] [PubMed] [Google Scholar]
- 42.Soker, S., S. Takashima, H. Q. Miao, G. Neufeld, and M. Klagsbrun. 1998. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92:735-745. [DOI] [PubMed] [Google Scholar]
- 43.Takashima, S., M. Kitakaze, M. Asakura, H. Asanuma, S. Sanada, F. Tashiro, H. Niwa, J. Miyazaki Ji, S. Hirota, Y. Kitamura, T. Kitsukawa, H. Fujisawa, M. Klagsbrun, and M. Hori. 2002. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc. Natl. Acad. Sci. USA 99:3657-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taniguchi, M., S. Yuasa, H. Fujisawa, I. Naruse, S. Saga, M. Mishina, and T. Yagi. 1997. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron 19:519-530. [DOI] [PubMed] [Google Scholar]
- 45.Torres-Vazquez, J., A. D. Gitler, S. D. Fraser, J. D. Berk, N. P. Van, M. C. Fishman, S. Childs, J. A. Epstein, and B. M. Weinstein. 2004. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev. Cell 7:117-123. [DOI] [PubMed] [Google Scholar]
- 46.Toyofuku, T., H. Zhang, A. Kumanogoh, N. Takegahara, F. Suto, J. Kamei, K. Aoki, M. Yabuki, M. Hori, H. Fujisawa, and H. Kikutani. 2004. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 18:435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Kempen, M. J., J. L. Vermeulen, A. F. Moorman, D. Gros, D. L. Paul, and W. H. Lamers. 1996. Developmental changes of connexin40 and connexin43 mRNA distribution patterns in the rat heart. Cardiovasc. Res. 32:886-900. [DOI] [PubMed] [Google Scholar]
- 48.Woodhouse, E. C., A. Fisher, R. W. Bandle, B. Bryant-Greenwood, L. Charboneau, E. F. Petricoin III, and L. A. Liotta. 2003. Drosophila screening model for metastasis: semaphorin 5c is required for l(2)gl cancer phenotype. Proc. Natl. Acad. Sci. USA 100:11463-11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yancopoulos, G. D., S. Davis, N. W. Gale, J. S. Rudge, S. J. Wiegand, and J. Holash. 2000. Vascular-specific growth factors and blood vessel formation. Nature 407:242-248. [DOI] [PubMed] [Google Scholar]
- 50.Zeller, R., K. D. Bloch, B. S. Williams, R. J. Arceci, and C. E. Seidman. 1987. Localized expression of the atrial natriuretic factor gene during cardiac embryogenesis. Genes Dev. 1:693-698. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, H., and A. Bradley. 1996. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122:2977-2986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.