Abstract
The Csk tyrosine kinase negatively regulates the Src family kinases Lck and Fyn in T cells. Engagement of the T-cell antigen receptor results in a removal of Csk from the lipid raft-associated transmembrane protein PAG/Cbp. Instead, Csk becomes associated with an ∼72-kDa tyrosine-phosphorylated protein, which we identify here as G3BP, a phosphoprotein reported to bind the SH3 domain of Ras GTPase-activating protein. G3BP reduced the ability of Csk to phosphorylate Lck at Y505 by decreasing the amount of Csk in lipid rafts. As a consequence, G3BP augmented T-cell activation as measured by interleukin-2 gene activation. Conversely, elimination of endogenous G3BP by RNA interference increased Lck Y505 phosphorylation and reduced TCR signaling. In antigen-specific T cells, endogenous G3BP moved into a intracellular location adjacent to the immune synapse, but deeper inside the cell, upon antigen recognition. Csk colocalization with G3BP occurred in this “parasynaptic” location. We conclude that G3BP is a new player in T-cell-antigen receptor signaling and acts to reduce the amount of Csk in the immune synapse.
The molecular mechanisms of T-cell-antigen receptor (TCR) signal transduction and T-cell activation have been intensely studied during the past decade. It has become evident that several protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs) play crucial roles (reviewed in references 33 and 38). The earliest known biochemical response to TCR ligation is an increased phosphorylation of a number of cellular proteins on tyrosine residues (21, 25). Pharmacological agents that prevent this phosphorylation block T-cell activation altogether (37, 26), whereas inhibitors of PTPs mimic TCR ligation and cause T-cell activation (44, 52) and prevent reversion of activated T cells to a resting phenotype (22).
Biochemical and genetic evidence indicates that the Src-family PTK Lck plays a crucial receptor-proximal role in TCR signaling (31), even in T cells that lack CD4 or CD8 (55). Although the molecular mechanism for the TCR-Lck connection is unclear, it seems that Lck responds to TCR stimulation with a rapid increase in its phosphorylation of tyrosines within the immunoreceptor tyrosine-based activation motifs (ITAMs) of the CD3 and ζ subunits of the TCR. Once phosphorylated, these motifs serve to recruit a second type of cytoplasmic PTK, ZAP-70 (8, 23), which is subsequently activated by direct phosphorylation at Y493 in its activation loop by Lck (7). Due to the presence of 10 ITAMs in the TCR complex, up to 10 ZAP-70 molecules may cluster on the fully phosphorylated receptor. Once activated by Lck, ZAP-70 autophosphorylates, presumably in trans, to create docking sites for SH2 domain-containing signaling proteins (41). The Src family PTKs are also responsible for recruitment and activation of the cytoplasmic Tec-related kinases Itk/Emt and Txk/Rlk (16, 19), which are directly involved in phosphorylation and activation of phospholipase Cγ1 (29, 48). It also appears that Src family PTKs have numerous other substrates, including cytoskeletal proteins, adapters, and other signaling molecules.
Given the central role of Src family PTKs, particularly Lck in T-cell activation, it seems obvious that these kinases must be extraordinarily tightly regulated to ensure that T cells respond appropriately to antigen. Indeed, Lck is regulated at all available levels from transcription and translation to multiple posttranslational modifications and controlled subcellular location. Perhaps the best studied regulation is the phosphorylation of an inhibitory tyrosine in the C terminus of Lck, Y505 (reviewed in reference 32). Mutation of this residue results in a constitutively active form of Lck, which can transform fibroblasts (2, 30). In T cells, Y505 is phosphorylated by the Csk PTK (4) and dephosphorylated by the CD45 PTP (34, 36, 45). It has been estimated that ca. 50% of Lck molecules are Y505 phosphorylated under physiological conditions in T cells (53), with a relatively slow turnover (43). In agreement with the notion that the balance between CD45 and Csk is important (35), most CD45-negative T cells fail to respond to TCR stimulation (6, 28, 49), whereas increased CD45 expression, e.g., in memory T cells (50), correlates with increased sensitivity to TCR ligation. Conversely, overexpression of Csk very efficiently reduces TCR signaling (9, 58), whereas a dominant-negative Csk augments it (56). In addition, a two- to threefold activation of Csk is used as a physiological mechanism for immunosuppression by cyclic AMP-inducing stimuli (58).
Csk is a 50-kDa cytoplasmic PTK comprised of Src homology 3 (SH3) and SH2 domains and a catalytic kinase domain (39, 47), but it differs from other nonreceptor PTKs in that it lacks N-terminal membrane docking motifs, tyrosine phosphorylation sites, and C-terminal regulatory sequences. Csk has a highly specialized and unique function as a general negative regulator of all Src family kinases (32, 40). Csk is expressed in all examined cell types but is particularly abundant in hematopoietic cells.
An important advance in our understanding of Csk regulation was the recent discovery of a transmembrane molecule, termed PAG (5) or Cbp (27), which specifically binds Csk through its SH2 domain. PAG/Cbp resides in lipid rafts and is phosphorylated on tyrosine in resting T cells (5, 56), thus anchoring a portion of Csk in the subcellular compartment that is enriched in Src family kinases. Upon TCR triggering, PAG/Cbp is rapidly dephosphorylated by an unknown PTP, resulting in dissociation of Csk (56). This apparently allows lipid raft-located Lck and Fyn to remain active longer and to phosphorylate ITAMs and other molecules. After ca. 10 min (in primary T cells), however, PAG/Cbp is rephosphorylated and Csk begins to return to the lipid rafts. This coincides with the downturn of tyrosine phosphorylation. The importance of this mechanism is perhaps best illustrated by the consequences of expression of a Csk-SH3-SH2 protein (lacking kinase domain), which will compete with endogenous Csk for binding to PAG/Cbp (56). This truncated protein caused a striking increase in basal and induced levels of tyrosine phosphorylation, which also lasted longer than in controls. The protein also augmented NFAT/AP-1 reporter gene activation (56).
Here we address the question of where Csk goes when it dissociates from PAG/Cbp and leaves the lipid rafts. We have identified another ligand for Csk, termed G3BP, which appears to be anchored at some distance from the immune synapse. The time course of Csk binding to G3BP is similar to the time course of Csk dissociation from PAG/Cbp upon TCR stimulation. In agreement with the notion that G3BP may sequester a portion of Csk away from the immune synapse, we found that expression of G3BP reduced Lck phosphorylation at Y505 and improved T-cell activation.
MATERIALS AND METHODS
Antibodies, proteins, and cells.
The antiphosphotyrosine (anti-PTyr) monoclonal antibody (MAb) 4G10 was from Upstate Biotechnology, Inc. (Lake Placid, N.Y.), and the 12CA5 MAb, which recognizes the influenza virus hemagglutinin (HA) epitope tag, was from Boehringer Mannheim (Indianapolis, Ind.). Anti-Lck-phosphoY505 was from Cell Signaling Technology, Inc. (Beverly, Mass.). The anti-TCR MAb C305 was from the American Type Culture Collection and was used as a culture supernatant. The anti-Flag MAb and the antiactin were from Sigma (St. Louis, Mo.). The anti-Lck and the polyclonal anti-Csk used for the immunoblotting were from Santa Cruz (Santa Cruz, Calif.). The anti-CD3 used for the immunofluorescence staining was from Becton Dickinson (San Diego, Calif.). The anti-G3BP MAb was described recently (54). A polyclonal rabbit antiserum (αCskC) directed against Csk was generated against a synthetic peptide corresponding to the last 30 amino acids of Csk conjugated to keyhole limpet hemocyanin. An MAb to PAG was kindly provided by Vaclac Horejsi.
Plasmids and site-directed mutagenesis.
The cDNA for human G3BP (54) was subcloned into the pEF5HA vector, a newer version of the pEF/HA vector (60), which adds a 9-amino-acid HA tag to the N terminus of the insert. The same cDNA was also subcloned into pEF4/His/EGFP, a new version of the pEF4/His vector from Invitrogen, into which we added the 720-bp enhanced green fluorescent protein (EGFP) insert. The expression plasmids for the Csk, Lck, Fyn, Itk/Emt, Syk, ZAP-70, Bcr-Abl, and Jak2 kinases were as before (12, 42, 61, 65). Csk expression plasmids with or without HA tag were used. The expression plasmids for PAG/Cbp and PEP were as described previously (17, 56). glutathione S-transferase (GST)-Csk, GST-Csk-SH2-SH3, GST-Csk-SH3, GST-Csk-SH2, and GST-G3BP-N (corresponding to amino acid residues 1 to 206) were produced by using the pGEX-2T prokaryotic expression vector (Pharmacia, Uppsala, Sweden). Site-directed mutagenesis was carried out by PCR by using the QuikChange kit (Stratagene, San Diego, Calif.) as recommended by the manufacturer. All mutations were verified by sequencing.
Cells and transfections.
Jurkat T leukemia cells were kept at logarithmic growth in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, nonessential amino acids, and 100 U of penicillin G and streptomycin/ml. The cells were transiently transfected with a total of 10 μg of DNA by electroporation with one 65-ms pulse at 230 V. Empty vector was added to control samples to make a constant amount of DNA in each sample.
Human peripheral blood and T cells were negatively selected directly from the whole blood by using the Rosette Sep T cell Depletion Cocktails (Stem Cell Technologies, Vancouver, British Columbia, Canada). The purity of the cell populations was over 90% as determined by fluorescence-activated cell sorting. CD8+ OT-I T cells and SigOVA257-264MEC/B7.1 cells were prepared as described previously (59). COS cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. These cells were transfected by using Lipofectamine (QIAGEN, Valencia, Calif.) according to the manufacturer's instructions.
Isolation and identification of G3BP.
A total of 4 μg of the fusion protein was mixed with 1 ml of a lysate of ∼50 × 106 pervanadate-treated (100 μM, 2 min) Jurkat cells in 20 mM Tris-HCl (pH 7.5)-150 mM NaCl-5 mM EDTA-10 μg of aprotinin and leupeptin-1 mM Na3VO4, followed by incubation for 1 h on ice. Next, 20 μl of glutathione-Sepharose 4B was added; after 1 h the beads were pelleted by centrifugation and washed five times in lysis buffer, and the bound proteins were eluted in sodium dodecyl sulfate (SDS) sample buffer and analyzed by anti-PTyr immunoblotting. A parallel lane was used to excise the band corresponding to the 72-kDa proteins. The filter piece was washed three times in deionized water, followed by incubation at 37°C with 1 μg of TPCK (tolylsulfonyl phenylalanyl chloromethyl ketone)-treated trypsin in 50 mM NH4HCO3 for 3 h and then with a second addition of trypsin overnight. The supernatant was lyophilized twice, desalted in a ZipTip, and mixed with 5 μl of α-cyanohydroxycinnaminic acid matrix. Then, 1 μl was spotted onto a target and analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) spectrometry.
In vitro phosphorylation and tryptic peptide mapping. The phosphorylation reaction contained 2 μg of GST-G3BP-ΔC, 100 ng of recombinant Lck in 25 μl of 50 mM HEPES (pH 7.5), 150 mM NaCl, 10 mM MnCl2, 1 mM Na3VO4, 10 μCi of [γ-32P]ATP, and 10 μM ATP. After 30 min at 30°C, the proteins were resolved on SDS gels and transferred onto nitrocellulose filters. Phospho-G3BP was localized by autoradiography, excised, and digested with trypsin as described previously (1, 4, 61, 61). The resulting peptides were separated in two dimensions by thin-layer electrophoresis at pH 1.9, followed by ascending chromatography.
Immunoprecipitation and Western blotting.
Immunoprecipitation was performed as described previously (17, 51, 63, 64). Proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) on 10 or 12% gels and transferred onto nitrocellulose filters. All antibodies was used at a 1:1,000 dilution, except for 4G10 anti-PTyr MAb at a dilution of 1:3,000, and the blots were developed by a standard alkaline phosphatase method or by using an enhanced chemiluminescence (Amersham, Arlington Heights, Ill.) technique according to the manufacturer's instructions.
Cell conjugation and confocal microscopy.
These procedures were performed as described previously (1). Briefly, the adherent SAMBOK cells (used as antigen-presenting cells [APC]) were seeded at 100,000 cells per chamber slide and cultured overnight. The next day, the slides were washed twice with medium to remove nonadherent cells or cell debris. Then, 200,000 OT-I cells were added to the monolayer of APC in 1 ml of medium, and the chamber slides were centrifuged at 500 × g for 30 s to allow the two cells populations to make contact. After various times at 37°C, the cells were carefully washed twice with warm phosphate-buffered saline (PBS) and fixed in ice-cold acetone. After permeabilization in 0.1% saponin-0.02% NaN3 in PBS for 10 min, the cells were incubated with the primary antibodies for 1 h. After three washes in 0.01% saponin in PBS, the primary antibodies were revealed by using Alexa 594 goat anti-mouse and Alexa 488 goat anti-rabbit (Molecular Probes). The stained cells were mounted with ProLong antifade kit (Molecular Probes) and then viewed under a confocal laser scanning microscopy MRC-1024 (Bio-Rad). The stained cells were mounted with Vectashield mounting medium containing DAPI (4′,6′-diamidino-2-phenylindole; H-1200; Vector Laboratories, Burlingame, Calif.). For the 3-dimensional reconstructions, 40 serial z sections were taken at 0.25-μm increments. The serial sections were then processed by using the software program Velocity (Velocity2 Pro Image3, LLC).
Luciferase assays.
Luciferase assays were performed as described previously (1, 24, 51, 62). Briefly, 2 × 107 cells were transfected 2 μg of NFAT/AP-1-luc or IL-2-luc, together with empty pEF/HA vector alone or G3BP plasmids and 0.5 μg of Renilla luciferase as a transfection efficiency control. After stimulation for 6 h, the luciferase activity was measured in an automatic luminometer by using a dual luciferase kit from Promega and according to the instructions of the manufacturer. The activity of Renilla luciferase, which varied <20% between samples, was used for normalization of results.
Subcellular fractionation and isolation of lipid rafts.
A total of 2 × 107 cells were resuspended in ice-cold hypotonic buffer (42 mM KCl, 10 mM HEPES [pH 7.4], 5 mM MgCl2, 1 mM Na3VO4, 10 μg each of aprotinin and leupeptin/ml) and incubated on ice for 10 min. Cells were then sheared by five passes through a 30-gauge needle. The lysates were centrifuged at 200 × g for 10 min to remove the nuclei, which were washed twice in lysis buffer and then resuspended in 20 mM HEPES-KOH (pH 7.9), 20 mM NaCl, 10 mM NaF, 0.2% Triton X-100, 1 mM EDTA, 25% glycerol, and 10 μg each of aprotinin and leupeptin/ml. Lysates were then vortexed, incubated on ice for 15 min, and centrifuged at 13,000 × g for 15 min, and the supernatant containing the nuclear proteins was collected. The supernatants from the first low-speed centrifugation were collected and centrifuged at 13,000 × g for 60 min at 4°C. The supernatant (cytosol) was collected, and the pellet was resuspended in 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 1 mM Na3VO4, and 10 μg each of aprotinin and leupeptin/ml, followed by vortexing for 5 min at 4°C and centrifugation at 13,000 × g for 60 min. The supernatant represents the detergent soluble particulate fraction, and the pellet (i.e., the detergent-insoluble fraction) was solubilized in 1% SDS. Each sample (nuclei, cytosol, and detergent-soluble and detergent-insoluble fractions) was diluted in Laemmli buffer for analysis by SDS-PAGE and immunoblotting.
Isolation of lipid rafts or glycolipid-enriched membrane microdomains was performed as described in detail elsewhere (66). Cells were homogenized in 1 ml of ice-cold lysis buffer (50 mM HEPES [pH 7.4], 100 mM NaCl, 5 mM EDTA, 1% Triton X-100, 10 mM sodium pyrophosphate, 1 mM Na3VO4, 50 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and 10 μg each of leupeptin, antipain, pepstatin A, and chymostatin/ml) by 10 pestle strokes in a Dounce homogenizer, loaded at the bottom of a 40 to 5% sucrose gradient, and centrifuged at 200,000 × g for 20 h. Next, 0.4-ml fractions were collected from the top and analyzed by immunoblotting
siRNA preparation and cells transfection.
The two small interfering RNA (siRNA) duplex sequences targeting G3BP were 5′-CUG CCA CAC CAA GAU UCG CdTdT (sense) and dTdTG ACG GUG UGG UUC UAA GCG-5′ (antisense), termed siRNA #1, and 5′-ACC ACC UCA UGU UGU UAA AdTdT (sense) and dTdTU UUA ACA ACA UGA GGU GGU-5′ (antisense), termed siRNA #2. Fluorescein-labeled luciferase GL2 siRNA duplex 5′-fluorescein-CGU ACG CGG AAU ACU UCG AdTdT (sense) and dTdTG CAU GCG CCU UAU GAA GCU-5′ (antisense) was used as a control. All of the siRNA duplexes were synthesized by Dharmacon Research, Inc. (Lafayette, Colo.), and were desalted and gel purified. A total of 2 × 107 Jurkat cells were transfected with 1 μM G3BP siRNA and 100 μM control siRNA or with 100 μM control siRNA alone. Cells positive for fluorescein were sorted by fluorescence-activated cell sorting 24 h after transfection and used for experiments 48 h after transfection.
RESULTS
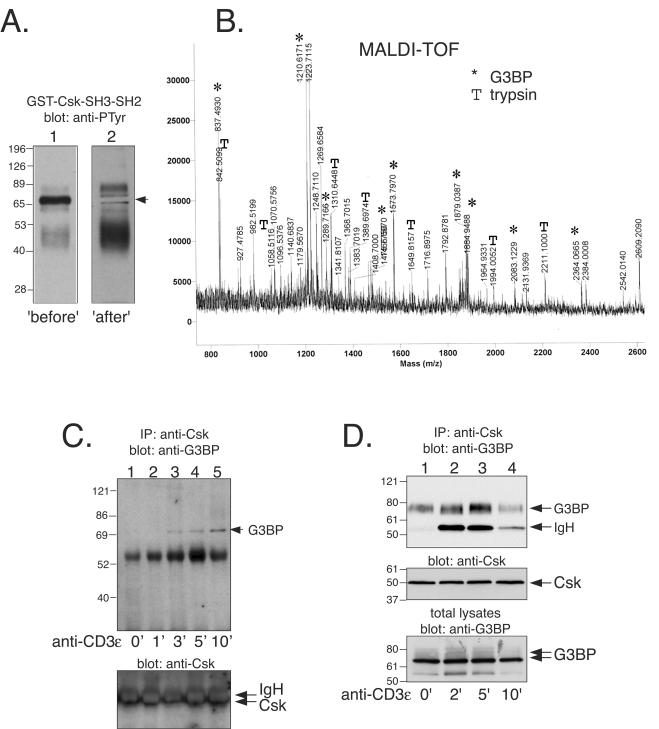
Identification of the Csk-associated 72-kDa phosphoprotein. We reported some time ago (42) that Csk forms a complex with a 72- to 75-kDa tyrosine-phosphorylated protein upon TCR triggering. To identify this protein, we prepared a detergent lysate of 5 × 107 Jurkat T cells treated for 2 min with 100 μM pervanadate and incubated it with 4 μg of GST-Csk-SH3-SH2 for 1 h on ice. The fusion protein was precipitated with glutathione-Sepharose beads, washed extensively, and resolved by SDS-PAGE. Two sample lanes of the gel were transferred to nitrocellulose, and one of them was immunoblotted with anti-PTyr to localize Csk-SH3-SH2 binding phosphoproteins. A major band at ∼72 kDa was detected (Fig. 1A, “before”). With this blot as a guide, we cut out the corresponding region of the parallel lane. The remaining filter was then immunoblotted with anti-PTyr to verify that the correct band had been excised (Fig. 1A, “after”). The excised filter piece was then washed and treated with two additions of 1 μg of TPCK-treated trypsin in 50 mM NH4HCO3, lyophilized, desalted in a ZipTip, and mixed with 5 μl of α-cyanohydroxycinnaminic acid matrix. Then, 1 μl was spotted onto a target and analyzed by MALDI-TOF spectrometry, which gave a good set of peptide peaks (Fig. 1B). Several of these peptides corresponded to well known trypsin autolysis products (indicated by “T” in Fig. 1B). The remaining peaks were used in a database search by using the ProFound (Rockefeller) software, which yielded RasGAP-SH3-binding protein (G3BP) (46) as a hit with nine matching peptides (indicated by asterisks in Fig. 1B), a sequence coverage of 28%, and a very high probability score. G3BP is a 466-amino-acid protein, which runs as a protein of ca. 72 kDa on SDS gels.
FIG. 1.
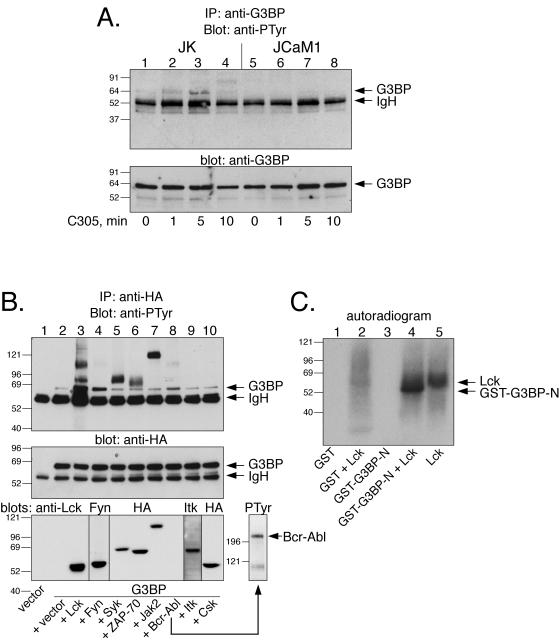
Identification of the 72-kDa Csk-associated phosphoprotein as G3BP. (A) Anti-PTyr immunoblots of material precipitated by a GST-Csk-SH3-SH2 protein (“before”). The second lane represents a parallel second sample, from which the region corresponding to the 72-kDa band was cut out with the help of the first lane, and the remaining filter was then blotted with anti-PTyr to verify that the correct band had been excised (“after”). The second filter is darker because it contained four times more material. (B) Mass spectrogram of the trypsin digest of the 72-kDa protein band from panel A. Peptide peaks derived from G3BP (✽) and trypsin (T) are indicated. (C) Anti-G3BP immunoblot of anti-Csk immunoprecipitates from untreated Jurkat T cells (lane 1) and from cells treated with OKT3 for 1 min (lane 2), 3 min (lane 3), 5 min (lane 4), or 10 min (lane 5). (D) Anti-G3BP immunoblot of anti-Csk immunoprecipitates from untreated normal human T lymphocytes (lane 1) and from cells treated with OKT3 and F(ab′)2 fragments for 2 min (lane 2), 5 min (lane 3), or 10 min (lane 4). The immunoglobulin heavy chain seen in lanes 2 to 4 is from OKT3.
To verify that the identification was correct, Csk was immunoprecipitated from Jurkat T cells stimulated with anti-CD3ɛ MAbs for various periods of time and immunoblotted with an anti-G3BP MAb (54). Although Csk did not coprecipitate G3BP from resting cells, a 72-kDa band appeared within a few minutes of stimulation to reach a maximum in ca. 10 min (Fig. 1C). Thus, in Jurkat T cells G3BP does form a physical complex with Csk in an inducible manner and with the same time course as that of the 72-kDa phosphoprotein (42). A similar result was obtained with normal human T lymphocytes, but the time course was skewed to the left: some G3BP was present in Csk immunoprecipitates from resting cells, and maximal amounts were seen at 5 min. In these cells, there was also a clear shift to slower-migrating forms, suggesting that Csk-bound G3BP was hyperphosphorylated. We conclude that G3BP associates with Csk both in Jurkat T cells and in normal human T lymphocytes.
G3BP reduces Lck phosphorylation at Y505 and keeps Csk from lipid rafts.
The principal function of Csk in T cells is to phosphorylate Lck at Y505 (4) and thereby inhibit TCR-induced T-cell activation (9, 58). To directly determine whether G3BP would affect this function of Csk, we expressed GFP or a GFP-G3BP fusion protein in Jurkat T cells, sorted out the green cells, and analyzed them for Lck phosphorylation at Y505 by immunoblotting with a phospho-Y505 specific antibody. These experiments (Fig. 2A) clearly showed that there was less phosphate on Lck Y505 in the presence of G3BP, suggesting that association of G3BP with Csk prevented Csk from phosphorylating Lck. In agreement with this notion, we found that G3BP reduced the amount of Csk in lipid raft fractions of T cells upon TCR triggering (Fig. 2B). In control cells, the amount of Csk in lipid rafts was reduced to approximately half at the 5-min time point, whereas in G3BP-expressing cells the decrease was much more dramatic (compare lanes 4 and 1 in the lower panels of Fig. 2B). G3BP had no effect on the amount of Csk in lipid rafts in resting cells (compare lanes 4 in the upper and lower panels of Fig. 2B), and there were no significant changes in the amounts of detergent soluble or cytosolic Csk in these experiments. Lipid raft fractionation (Fig. 2C) also showed that endogenous G3BP is present in very small amounts in lipid rafts in resting T cells and completely vanishes from this location upon TCR stimulation to accumulate in fractions 3 to 5 instead, which contains less buoyant material. These results demonstrate that G3BP can sequester Csk and keep it away from lipid rafts (where Lck is located) after TCR stimulation.
FIG. 2.
G3BP decreases Csk function and sequesters Csk away from lipid rafts. (A) The top panel shows an anti-Lck-phospho-Y505 immunoblot of lysates from Jurkat T cells transfected with GFP (lane 1) or GFP-G3BP (lane 2) and sorted for green fluorescence. The middle panel shows anti-Lck immunoblotting of the same filter to verify equal loading. The bottom panel shows anti-G3BP immunoblots of the same lysates. Note that endogenous G3BP is present in both lanes, but the transfected GFP-G3BP is only seen in lane 2. (B) The upper panel shows anti-Csk immunoblot of C305-treated (lanes 1 to 3) or resting (lanes 4 to 6) control Jurkat T cells fractionated into buoyant detergent-insoluble (lipid rafts), detergent-soluble fractions (soluble), or detergent-free soluble (cytosol) fractions. The lower panel shows anti-Csk immunoblot of a similarly fractionation of G3BP-transfected cells. Equal amounts of protein were loaded in each lane between the upper and lower panels. (C) The top panel shows anti-G3BP immunoblots of lipid raft fractionation of resting Jurkat T cells. Lipid rafts are in fractions 1 and 2. The second to fifth panels show the same experiment with cells stimulated through the TCR for 5, 10, 30, and 60 min. The sixth and seventh panels show immunoblots with anti-PAG, anti-Lck, and anti-Csk antibodies of the same fractions as in the top panel. The seventh panel is a longer exposure (without anti-Lck) to show Csk better. The bottom panel shows an anti-LAT blot of the same fractions as a lipid raft marker. (D) The upper panel shows an anti-Lck-phospho-Y506 immunoblot of lysates from Jurkat T cells transfected with control siRNA (lane 1) or G3BP siRNA (lane 2) together with a fluorescent RNA oligonucleotide and sorted for green fluorescence. The lower panel shows anti-G3BP immunoblots of the same lysates.
Since overexpression of G3BP may have nonphysiological effects on Csk, we decided to use the technology of RNA interference (14) to reduce the levels of endogenous G3BP. A double-stranded RNA oligonucleotide was designed that specifically targets G3BP mRNA and causes its degradation. In HeLa cells, this oligonucleotide causes a >80% decrease in immunoreactive G3BP protein, whereas control oligonucleotides did not (not shown). The expression of G3BP was also reduced in Jurkat T cells cotransfected with a fluorescein-conjugated control RNA duplex and sorted out for green fluorescence (Fig. 2D, lower panel). Immunoblotting of these cells with the phospho-Y505 specific antibody showed that the loss of G3BP resulted in increased phosphorylation of Lck at Y505. Csk levels were unchanged (data not shown). We conclude that endogenous G3BP indeed sequesters a portion of Csk from the vicinity of Lck.
G3BP augments TCR signaling and elimination of G3BP by RNAi reduces TCR signaling.
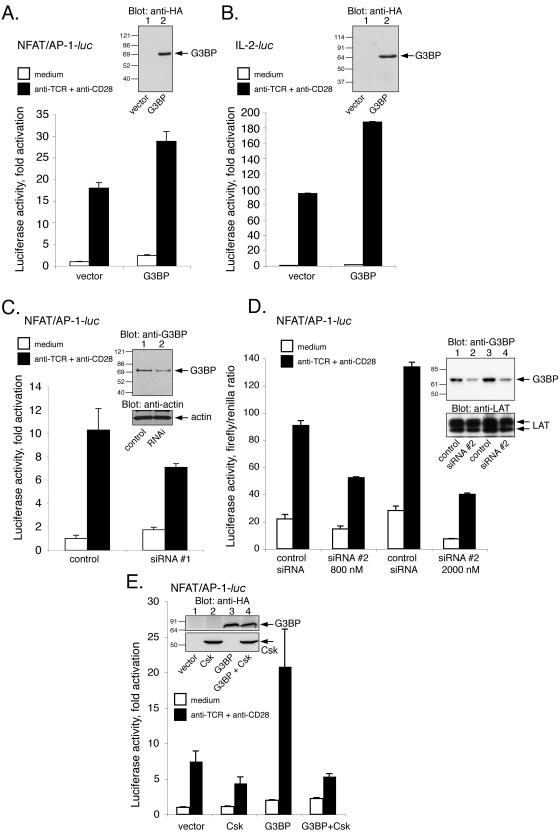
To determine whether the sequestering of Csk away from Lck and the reduced phosphorylation of Lck at Y505 have further consequences for TCR-driven T-cell activation, we measured the transactivation of the interleukin-2 (IL-2) gene. First, Jurkat T cells were cotransfected with a luciferase reporter gene driven by the NFAT/AP-1 element from the 5′ IL-2 promoter and either empty pEF/HA vector or the G3BP expression plasmid. At 2 days after transfection, the cells were stimulated for 6 h with anti-TCR plus anti-CD28 MAbs and lysed, and the activity of induced luciferase was measured. These experiments repeatedly showed that expression of G3BP significantly augmented the response (Fig. 3A). Similar results were obtained with the entire 5′ IL-2 promoter (Fig. 3B). In the experiment shown in Fig. 3B, IL-2 transactivation was 94-fold in controls and 188-fold in the presence of G3BP. These results were obtained in five independent experiments.
FIG. 3.
Stimulatory role of G3BP in TCR signaling. (A) Luciferase activity of lysates from Jurkat T cells transfected with a luciferase reporter driven by a NFAT/AP-1 element from the 5′ IL-2 promoter alone or together with the G3BP expression plasmid. Prior to lysis, the cells were either left untreated or stimulated for 6 h with anti-TCR (C305) plus anti-CD28 MAbs, as indicated. The inset shows an anti-HA immunoblot of the same lysates. The results are given as the fold induction versus unstimulated samples with reporter alone and represent the means of triplicate determinations. Error bars show the standard deviations of the values. Very similar results were obtained in two additional independent experiments. (B) Similar assay with a luciferase reporter containing the entire 5′ IL-2 gene promoter. The results are given as the fold induction versus unstimulated samples with reporter alone and represent the means of triplicate determinations. Error bars show the standard deviations of the values. Very similar results were obtained in one additional independent experiment. (C) Luciferase activity of lysates from Jurkat T cells transfected with the same NFAT/AP-1 luciferase reporter as in panel A plus either a control RNA or the G3BP-specific RNA interference oligonucleotide (RNAi). Three days later the cells were either left untreated or stimulated for 6 h with anti-TCR (C305) plus anti-CD28 MAbs, as indicated. The results are given as the fold induction versus unstimulated samples with reporter alone and represent the means of triplicate determinations. Error bars show the standard deviations of the values. Very similar results were obtained in another independent experiment. The insert shows an anti-G3BP immunoblot of lysates of Jurkat cells transfected with fluorescein-conjugated control RNA duplex alone (control) or together with a G3BP-specific RNA duplex (RNAi) and then sorted out for green fluorescence. The lower panel is an antiactin blot to verify equal loading. (D) Same experiment as in panel C with a different G3BP-specific siRNA used at 800 nM and 2 μM. The insert is a G3BP immunoblot (upper panel) and anti-LAT blot (lower panel) as a loading control. (E) Luciferase activity of lysates from Jurkat T cells transfected with the same NFAT/AP-1 luciferase reporter as in panel A plus either Csk, G3BP, or both. The insert shows an anti-HA immunoblot of the same lysates to demonstrate the expression of Csk and G3BP. The upper half represents a longer exposure.
Conversely, when Jurkat T cells were cotransfected with the G3BP-specific siRNA oligonucleotide and the IL-2 gene promoter luciferase plasmid, it was clear that the subsequent TCR-induced gene activation was reduced (Fig. 3C) compared to the control cells, which were transfected with an irrelevant RNA oligonucleotide. A second siRNA oligonucleotide had the same effect (Fig. 3D). The cells did not show any change in control Renilla luciferase activity, morphology, or viability. Thus, endogenous G3BP appears to exert a positive influence on TCR signaling, and exogenous G3BP can augment the response even further. These data are in good agreement with a Csk-sequestering function of G3BP.
Csk can neutralize the stimulatory effect of G3BP on TCR signaling.
To obtain further evidence that the positive effect of G3BP on TCR signaling is related to the ability of G3BP to sequester Csk, we tested whether additional Csk would neutralize the stimulatory effect of G3BP. Indeed, when Csk and G3BP were cotransfected, the positive effect of G3BP vanished (Fig. 3E). This result supports the notion that G3BP augments TCR signaling through its binding of Csk.
“Parasynaptic” localization of G3BP in antigen-specific T cells.
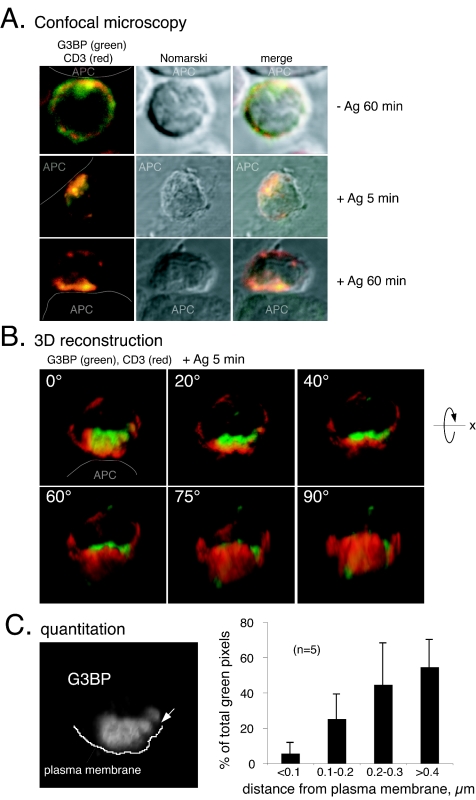
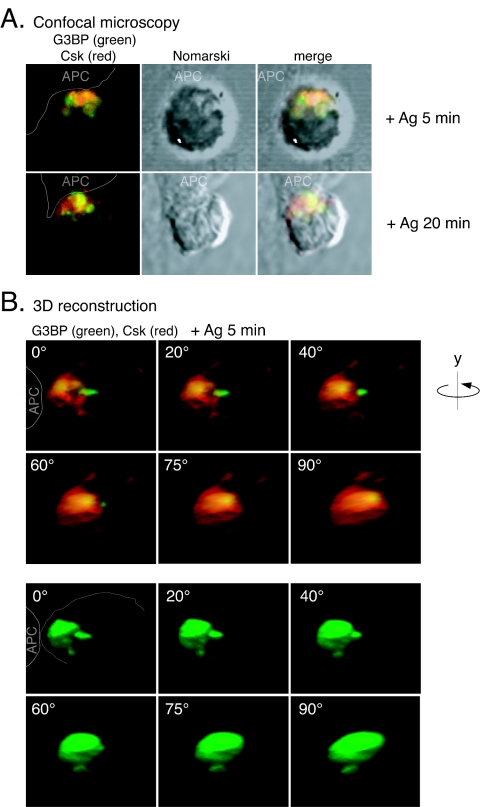
To further examine the notion that G3BP can keep Csk away from its Src family substrates, we decided to directly visualize endogenous G3BP and examine its subcellular location during antigen recognition and the formation of an immune synapse between a T-cell and an APC. Freshly isolated OVA257-264/Kb-specific CD8+ T cells from OT-I TCR transgenic mice (20) were overlaid on adherent antigen-expressing SigOVA257-264MEC/B7.1 cells (SAMBOK) (59) at 37°C for various times, fixed, and stained for endogenous G3BP and TCR/CD3 (Fig. 4) or Csk (Fig. 5). Before stimulation, the TCR was quite evenly distributed over the surface of the cells, whereas G3BP was cytoplasmic. Upon contact with APC, the distribution changed in that much of both G3BP and Csk in the T cells became concentrated toward the APC. The two molecules differed, however, in their proximity to the immune synapse: whereas much of Csk was at the plasma membrane (Fig. 5), G3BP never became associated with the cell surface (Fig. 4 and 5). Overlays of the two colors and three-dimensional reconstructions of 40 serial z-sections of the T cells showed that G3BP accumulated in the cytoplasm adjacent to the immune synapse but clearly deeper inside the cell by ca. 0.5 to 1 μm (Fig. 4B and 5B). A quantitation (Fig. 4C) of this location revealed that only ∼5% of G3BP was within 100 nm from the plasma membrane, whereas ∼25% of G3BP remained 100 to 200 nm deeper in, and the majority of G3BP was between 200 and 400 nm from the surface. This organization became evident within 5 min, became clear at 20 min, and lasted for many hours. Although it is not surprising that extracellularly labeled TCR/CD3 cover intracellular G3BP (Fig. 4), it is highly significant that intracellular Csk is so obviously layered on top of intracellular G3BP, which is restricted to deeper regions of the cytoplasm. This is particularly evident in the three-dimensional reconstructions when rotated 90° to show the stained molecules from the direction of the APC (Fig. 5B). We refer to the type of location displayed by G3BP as “parasynaptic” (“para” meaning “adjacent to”), and it represents a novel pattern of higher order organization of signaling molecules in the immune synapse. Most importantly, colocalization of Csk and G3BP occurred in the outermost half of the G3BP containing area, which is well separated from the plasma membrane. Thus, the portion of Csk that binds G3BP is physically separated from the pool of Csk that is associated with lipid rafts, PAG/Cbp, and thereby with the Src family PTKs that mediate TCR signaling.
FIG. 4.
Parasynaptic localization of G3BP during antigen recognition. (A) Location of G3BP (green) and CD3 (red) in CD8+ OT-I T cells overlaid on control BOK cells (−Ag) or OVA-presenting SAMBOK APC (+Ag) and viewed under the confocal microscope as described in Materials and Methods. The second panel in each row is a Nomarski differential phase-contrast image of the same T-cell, and the last panel is an overlay of the two first panels. (B) Quantitation of G3BP location in relation to the plasma membrane in immune synapses. The data are given as a percentage of the total G3BP pixels within 100-nm-wide zones from the plasma membrane and represent the average ± the standard deviations from five different images, including the two three-dimensional reconstructions shown in panel B and in Fig. 5. (C) Three-dimensional reconstruction of a representative OT-I T-cell stained for G3BP (green) and CD3 (red) in contact with a SAMBOK cell (white arrow at the contact site). The reconstructed cell is gradually rotated counterclockwise around the x axis from panel to panel, so that the bottom right hand panel represents the direction from which the APC would see the T cell. Note that G3BP accumulates at the T-cell-APC contact site but remains ∼0.5 μm from the cell surface.
FIG. 5.
Parasynaptic localization of G3BP and partial colocalization with Csk during antigen recognition. (A) Location of G3BP (green) and Csk (red) in CD8+ OT-I T cells overlaid on OVA-presenting SAMBOK APC and viewed under the confocal microscope. The second panel in each row is a Nomarski differential phase-contrast image of the same T-cell, and the last panel is an overlay of the two first panels. (B) Three-dimensional reconstruction of a representative OT-I T-cell stained for G3BP (green) and Csk (red) in contact with a SAMBOK cell (white arrow at contact site). The reconstructed cell is gradually rotated counterclockwise around the y axis, so that the bottom right-hand panel represents the direction from which the APC would see the T cell. Note that G3BP accumulates at the T-cell-APC contact site but remains deeper inside the cell than Csk.
Csk binds G3BP via SH1, SH2, and SH3 interactions.
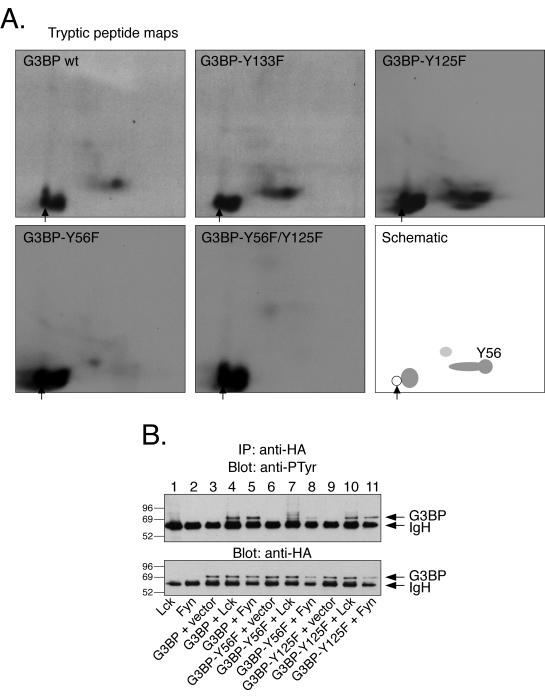
To clarify the molecular mechanisms of TCR-induced complex formation between G3BP and Csk, we undertook a series of experiments in vitro and in cells. First, we established that G3BP becomes tyrosine phosphorylated in response to TCR triggering in Jurkat T cells (Fig. 6A) but not in the Lck-negative JCaM1 cell line (Fig. 6A, lanes 5 to 8). G3BP was also readily tyrosine phosphorylated in COS cells coexpressing Lck or Fyn, and perhaps to a low extent by Bcr-Abl (Fig. 6B). Other PTKs, including Csk, did not phosphorylate G3BP. Tyrosine phosphorylation of G3BP was also observed in the ZAP-70-negative P116 and LAT-negative JCaM2 sublines of Jurkat, JCaM1 (not shown). Lck also readily phosphorylated recombinant G3BP in vitro (Fig. 6C). By tryptic peptide mapping of recombinant G3BP or its tyrosine-to-phenylalanine mutants, we determined that the principal in vitro phosphorylation site is Y56 (Fig. 7A). When the G3BP-Y56F mutant was expressed in COS cells, together with Lck or Fyn, subsequent anti-PTyr blots showed that is was much less phosphorylated than the wild-type G3BP (Fig. 7B). In contrast, all other mutants showed no change or only small reductions (lanes 10 and 11 and data not shown). Since the Y56F mutant still contained a small amount of PTyr, we created a series of double and triple mutants of Y56F and Y56F/Y125F in combination with all other tyrosines. However, all of these mutants still reacted with the anti-PTyr to the same low extent as the Y56F single mutant (not shown). These results suggest that Y56 is the major phosphorylation site and that there may be a low amount of phosphate at multiple other sites. Y56 resides in a putative Csk-SH2 binding motif (YGQK). G3BP also contains several Pro-rich motifs that could bind the Csk SH3 domain.
FIG. 6.
Tyrosine phosphorylation of G3BP by Src family PTKs. (A) The upper panel shows an anti-PTyr immunoblot of G3BP immunoprecipitated with the anti-G3BP MAb from Jurkat T leukemia cells (lanes 1 to 4) or the Lck-negative JCaM1 variant of Jurkat (lanes 5 to 8) stimulated with the C305 anti-TCR MAb for the indicated times. The lower panel shows equal amounts of G3BP in each lane verified by MAb anti-G3BP immunoblotting. (B) The upper panel shows an anti-PTyr immunoblot of G3BP immunoprecipitated with the anti-HA epitope tag antibody from COS cells cotransfected with the indicated kinases. The lower panel shows equal amounts of G3BP in each lane verified by anti-HA immunoblotting of the same filter. (C) Autoradiogram of GST (lanes 1 and 2) or GST-G3BP-N (lanes 3 and 4) incubated with recombinant Lck in the presence of [γ-32P]ATP for 30 min and resolved by SDS-PAGE. Lane 5 shows Lck alone.
FIG. 7.
Mapping of the major tyrosine phosphorylation site in G3BP. (A) Tryptic peptide maps of G3BP or G3BP mutants phosphorylated in vitro by recombinant Lck as in Fig. 2C. The bottom right-hand panel is a schematic view of the major peptides and the spot that contains Y56. Note that this spot is missing in the maps of the Y56F and Y56F/Y125F mutants. A sample origin is indicated by an arrow. (B) The upper panel shows an anti-PTyr immunoblot of G3BP immunoprecipitated with the anti-HA epitope tag antibody from COS cells cotransfected with G3BP mutants and Lck or Fyn, as indicated. The lower panel shows equal amounts of G3BP in each lane verified by anti-HA immunoblotting of the same filter.
GST fusion proteins of full-length Csk, as well as its SH3, SH2, and tandem SH3-SH2 regions, all bound G3BP (not shown), with clearly the strongest binding seen with full-length Csk. The SH2 domain bound significant amounts of G3BP only after pervanadate treatment of the cells, suggesting that tyrosine phosphorylation of G3BP was needed. In contrast, the SH3 domain bound G3BP best from untreated T cells. We conclude that the interaction between Csk and G3BP occurs through multiple contacts involving all three domains of Csk and that the strongest binding by far is seen with full-length Csk. The implications of this mode of binding include the notion that Csk bound to G3BP presumably is unable to interact with other ligands for its SH3 or SH2 domains and thus probably cannot act on Src family kinases. In agreement with this notion, we have not observed any coimmunoprecipitation between G3BP and either PEP or PAG/Cbp (data not shown).
DISCUSSION
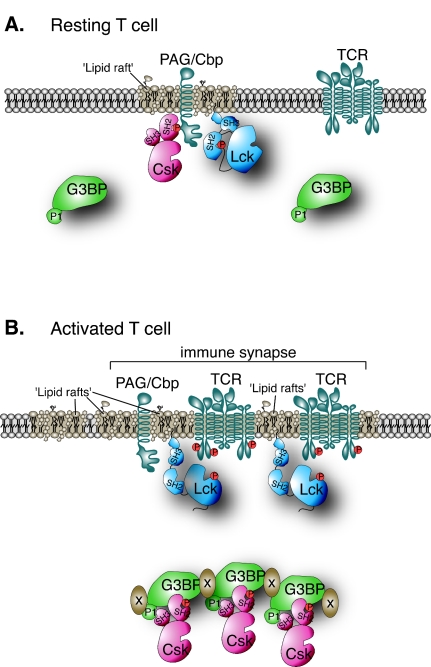
We have identified an inducibly tyrosine phosphorylated 72-kDa Csk-associated protein in T cells as G3BP, a protein originally cloned by virtue of its binding to the SH3 domain of Ras-GAP (46). G3BP coimmunoprecipitated with Csk from both Jurkat T leukemia cells and from normal primary human T lymphocytes. We have obtained evidence that the association between Csk and G3BP in primary antigen-specific mouse T cells occurs at a physically distinct subcellular location close to, but separate from, the immune synapse. We suggest that this may serve as a means to keep Csk segregated from the Src-family PTKs that transmit signals from antigen-triggered TCRs. This model is presented schematically in Fig. 8 and predicts that G3BP plays a positive role in TCR signaling by reducing the Csk-mediated inhibition of TCR-associated kinases. Indeed, we find that loss of endogenous G3BP resulted in decreased TCR signaling and that expression of G3BP reduced Lck phosphorylation at Y505 and augmented T-cell activation as measured by TCR/CD28-induced gene transactivation. The positive effect of G3BP was eliminated by increasing the amount of Csk. This growth-stimulatory function of G3BP is in agreement with its increased expression in human malignancies and stimulation of entry into S phase (18). We also find that G3BP expression becomes higher in proliferating T cells (not shown).
FIG. 8.
Schematic model of G3BP function in T-cell activation. (A) In a resting T cell, much of G3BP is diffusely distributed throughout deeper cytosolic regions, whereas the functionally important portion of Csk is at the plasma membrane bound to PAG/Cbp in lipid rafts adjacent to the targets for Csk, Lck, and Fyn. (B) Upon activation of the T cell, some of Csk is released from PAG/Cbp and binds to G3BP, which becomes enriched in the deeper cytoplasmic regions facing the APC in the polarized T cell. Thus, the portion of Csk bound to G3BP is kept away from the plasma membrane of the immune synapse.
Our model for G3BP function may well be an oversimplification of a more complex reality. Csk not only phosphorylates the C-terminal negative regulatory tyrosine of Lck and Fyn but also affects Src family PTKs indirectly by forming a physical complex with the PEP PTP (10), which dephosphorylates the positive regulatory tyrosines in Lck (17) and Fyn (11), ZAP-70, and perhaps other substrates. Since it appears that G3BP binds to Csk via multiple contacts, including the SH3 domain of Csk, it seems likely that G3BP competes with PEP for binding to Csk. Therefore, it may be that G3BP also serves to break up Csk-PEP complexes. Since Csk may play a role in targeting PEP to its Src family PTK substrates (or vice versa), the net effect of this function of G3BP may be to prevent inhibition of TCR-associated kinases not only by Csk but also by PEP. In this scenario, Csk exists in an equilibrium between PEP association and G3BP association in a manner that determines how large a fraction of the Csk pool that can access Src family PTKs at the plasma membrane. This equilibrium appears to shift toward G3BP upon TCR triggering. The reasons for this shift may include the tyrosine phosphorylation of G3BP, exposure of proline-rich motifs, increased expression of G3BP, and/or its translocation to a parasynaptic subcellular location. Together, these changes may allow Lck and Fyn to undergo a burst of activity (13, 57) and a prolonged elevation in basal activity.
It is also fully possible that G3BP has additional functions, as signaling molecules often do. G3BP was originally identified as a protein able to bind the SH3 domain of RasGAP, which, like Csk, is thought to be a negative regulator of signaling. The consequences of binding to RasGAP are unknown, but could also serve to keep RasGAP away from plasma membrane-associated Ras. G3BP has also been reported to associate with a ubiquitin-specific protease USP10 (54), an enzyme that specifically removes ubiquitin from other proteins and thereby rescues them from targeted proteolysis by the 26S proteasome. Binding of G3BP inhibited the activity of USP10 (54). It is not known yet if Csk and USP10 bind G3BP in a mutually exclusive manner. If so, Csk binding would release and activate USP10 and thereby affect the ubiquitination status of proteins involved in TCR signaling or receptor recycling. Finally, nuclear localization and induced nuclear import of G3BP has been reported (3). The N terminus of G3BP contains an NTF2 domain, which may interact with the Ran GTPase in nuclear pores, whereas the C terminus of G3BP has homology with ribonucleoproteins (46) and has RNase activity (15). Together, all of these findings suggest that the TCR-induced tyrosine phosphorylation of G3BP and its association with Csk may fulfill multiple functions, which we suggest include the segregation of a pool of Csk to reduce its inhibitory effect on the Src family PTKs that initiate TCR signaling and T-cell activation. In addition, G3BP may carry out other tasks with or without the aid of Csk in T lymphocytes.
A novel aspect of our study is the identification of an spatial organization of TCR signaling proteins not only in the plane of the membrane but also perpendicular to it. We refer to this anchoring of molecular components inside the cell at a distance from the plasma membrane upon formation of an immune synapse as a parasynaptic location. It may be significant that the staining for endogenous G3BP in Fig. 4 and 5 clearly reveals a partly organized structure resembling a cluster of bodies or vesicles. Since much of G3BP is poorly soluble in detergent-containing buffers, it seems likely that this structure is associated with cytoskeletal elements and perhaps endocytic or lysosomal vesicles involved in downmodulation or recycling of surface molecules. Alternatively, since the T-cell becomes polarized toward the APC, G3BP may be directed from the Golgi apparatus and/or the trans-Golgi network. These possibilities will have to be addressed experimentally. In either case, our findings indicate that the TCR-associated signaling molecules in the immune synapse are organized into a three-dimensional machinery of higher complexity than previously demonstrated.
Acknowledgments
This study was supported by fellowships from Rotary International, the Van Beirs Foundation, the Centre Anticancereux pres L'Universite de Liege, and the Spanish Ministry of Education and Culture and by grants CA81261 (S.P.S.) and AI53585, AI35603, AI55741, CA96949, and AI48032 (T.M.) from the National Institutes of Health. T.V. is supported by a postdoctoral fellowship from the Norwegian Cancer Society.
REFERENCES
- 1.Alonso, A., S. Rahmouni, S. Williams, M. van Stipdonk, L. Jaroszewski, A. Godzik, R. T. Abraham, S. P. Schoenberger, and T. Mustelin. 2003. Tyrosine phosphorylation of VHR by ZAP-70. Nat. Immunol. 4:44-48. [DOI] [PubMed] [Google Scholar]
- 2.Amrein, K., and B. M. Sefton. 1988. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc. Natl. Acad. Sci. USA 85:4247-4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes, C. J., F. Li, M. Mandal, Z. Yang, A. A. Sahin, and R. Kumar. 2002. Heregulin induces expression, ATPase activity, and nuclear localization of G3BP, a Ras signaling component, in human breast tumors. Cancer Res. 62:1251-1255. [PubMed] [Google Scholar]
- 4.Bergman, M., T. Mustelin, C. Oetken, J. Partanen, N. A. Flint, K. E. Amrein, M. Autero, P. Burn, and K. Alitalo. 1992. The human p50csk tyrosine kinase phosphorylates Lck at Tyr-505 and downregulates its catalytic activity. EMBO J. 11:2919-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brdicka, T., D. Pavlistova, A. Leo, E. Bruyns, V. Korinek, P. Angelisova, J. Scherer, A. Shevchenko, I. Hilgert, J. Cerny, K. Drbal, Y. Kuramitsu, B. Kornacker, V. Horejsi, and B. Schraven. 2000. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adapter protein, binds the protein tyrosine kinase csk and is involved in regulation of T-cell activation. J. Exp. Med. 191:1591-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahir McFarland, E. D., T. R. Hurley, J. T. Pingel, B. M. Sefton, A. Shaw, and M. L. Thomas. 1993. Correlation between Src family member regulation by the protein-tyrosine-phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proc. Natl. Acad. Sci. USA 90:1402-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, A. C., M. Dalton, R. Johnson, G. H. Kong, T. Wang, R. Thoma, and T. Kurosaki. 1995. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 14:2499-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, A. C., M. Iwashima, C. W. Turck, and A. Weiss. 1992. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR ζ chain. Cell 71:649-662. [DOI] [PubMed] [Google Scholar]
- 9.Chow, L. M. L., M. Fournel, D. Davidson, and A. Veillette. 1993. Negative regulation of T-cell receptor signalling by the tyrosine kinase p50csk. Nature 365:156-160. [DOI] [PubMed] [Google Scholar]
- 10.Cloutier, J. F., and A. Veillette. 1996. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 15:4909-4918. [PMC free article] [PubMed] [Google Scholar]
- 11.Cloutier, J. F., and A. Veillette. 1999. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J. Exp. Med. 189:111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couture, C., G. Baier, C. Oetken, S. Williams, D. Telford, A. Marie-Cardine, G. Baier-Bitterlich, S. Fischer, P. Burn, A. Altman, and T. Mustelin. 1994. Activation of p56lck by p72syk through physical association and N-terminal tyrosine phosphorylation. Mol. Cell. Biol. 14:5249-5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danielian, S., A. Alcover, L. Polissard, M. Stefanescu, O. Acuto, S. Fischer, and R. Fagard. 1992. Both T-cell receptor (TcR)-CD3 complex and CD2 increase the tyrosine kinase activity of p56lck: CD2 can mediate TcR-CD3-independent and CD45-dependent activation of p56lck. Eur. J. Immunol. 22:2915-2921. [DOI] [PubMed] [Google Scholar]
- 14.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 15.Gallouzi, I. E., F. Parker, K. Chebli, F. Maurier, E. Labourier, I. Barlat, J. P. Capony, B. Tocque, and T. Tazi. 1998. A novel phosphorylation-dependent RNase activity of GAP-SH3 binding protein: a potential link between signal transduction and RNA stability. Mol. Cell. Biol. 18:3956-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson, S., A. August, D. Branch, B. Dupont, and G. M. Mills. 1996. Functional LCK Is required for optimal CD28-mediated activation of the TEC family tyrosine kinase EMT/ITK. J. Biol. Chem. 271:7079-7083. [DOI] [PubMed] [Google Scholar]
- 17.Gjörloff-Wingren, A., M. Saxena, S. Williams, D. Hammi, and T. Mustelin. 1999. Characterization of TCR-induced receptor-proximal signaling events negatively regulated by the protein tyrosine phosphatase PEP. Eur. J. Immunol. 29:3845-3854. [DOI] [PubMed] [Google Scholar]
- 18.Guitard, E., F. Parker, F., R. Millon, J. Abecassis, and B. Tocque. 2001. G3BP is overexpressed in human tumors and promotes S phase entry. Cancer Lett. 162:213-221. [DOI] [PubMed] [Google Scholar]
- 19.Heyeck, S. D., H. M. Wilcox, S. C. Bunnell, and L. J. Berg. 1997. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J. Biol. Chem. 272:25401-25408. [DOI] [PubMed] [Google Scholar]
- 20.Hogquist, K. A., M. A. Gavin, and M. J. Bevan. 1993. Positive selection of CD8+ T cells induced by major histocompatibility complex binding peptides in fetal thymic organ culture. J. Exp. Med. 177:1469-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsi, E. D., J. N. Siegel, Y. Minami, E. T. Luong, R. D. Klausner, and L. E. Samelson. 1989. T-cell activation induces rapid tyrosine phosphorylation of a limited number of cellular substrates. J. Biol. Chem. 264:10836-10842. [PubMed] [Google Scholar]
- 22.Iivanainen, A. V., C. Lindqvist, T. Mustelin, and L. C. Andersson. 1990. Phosphotyrosine phosphatases are involved in reversion of T lymphoblastic proliferation. Eur. J. Immunol. 20:2509-2512. [DOI] [PubMed] [Google Scholar]
- 23.Iwashima, M., B. A. Irving, N. S. C. van Oers, A. C. Chan, and A. Weiss. 1994. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science 263:1136-1139. [DOI] [PubMed] [Google Scholar]
- 24.Jascur, T., J. Gilman, and T. Mustelin. 1997. Involvement of phosphatidylinositol 3-kinase in NFAT activation in T cells. J. Biol. Chem. 272:14483-14488. [DOI] [PubMed] [Google Scholar]
- 25.June, C. H., M. C. Fletcher, J. A. Ledbetter, and L. E. Samelson. 1990. Increases in tyrosine phosphorylation are detected before phospholipase C activation after T-cell receptor stimulation. J. Immunol. 144:1591-1598. [PubMed] [Google Scholar]
- 26.June, C. H., M. C. Fletcher, J. A. Ledbetter, G. L. Schieven, J. N. Siegel, A. F. Phillips, and L. E. Samelson. 1990. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc. Natl. Acad. Sci. USA 87:7722-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawabuchi, M., Y. Satomi, T. Takao, Y. Shimonishi, S. Nada, K. Nagai, A. Tarakhovsky, and M. Okada. 2000. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 404:999-1003. [DOI] [PubMed] [Google Scholar]
- 28.Koretzky, G. A., J. Picus, M. L. Thomas, and A. Weiss, A. 1990. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature 346:66-68. [DOI] [PubMed] [Google Scholar]
- 29.Liu, K. Q., S. C. Bunnell, C. B. Gurniak, and L. J. Berg. 1998. T-cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J. Exp. Med. 187:1721-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marth, J. D., J. A. Cooper, C. S. King, S. F. Ziegler, D. A. Tinker, R. W. Overell, E. G. Krebs, and R. Perlmutter. 1988. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck) Mol. Cell. Biol. 8:540-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molina, T. J., K. Kishihara, D. P. Siderowski, W. van Ewijk, A. Narendran, E. Timms, A. Wakeham, C. J. Paige, K.-U. Hartman, A. Veillette, D. Davidson, and T. W. Mak. 1992. Profound block in thymocyte development in mice lacking p56lck. Nature 357:161-164. [DOI] [PubMed] [Google Scholar]
- 32.Mustelin, T. 1994. Src family tyrosine kinases in leukocytes. R. G. Landes Co., Austin, Tex.
- 33.Mustelin, T., R. T. Abraham, C. E. Rudd, A. Alonso, and J. J. Merlo. 2002. Protein tyrosine phosphorylation in T-cell signaling. Front. Biosci. 7:918-969. [DOI] [PubMed] [Google Scholar]
- 34.Mustelin, T., and A. Altman. 1990. Dephosphorylation and activation of the T-cell tyrosine kinase pp56lck by the leukocyte common antigen (CD45) Oncogene 5:809-813. [PubMed]
- 35.Mustelin, T., and P. Burn. 1993. Regulation of src-family tyrosine kinases in lymphocytes. Trends Biochem. Sci. 18:215-220. [DOI] [PubMed] [Google Scholar]
- 36.Mustelin, T., K. M. Coggeshall, and A. Altman. 1989. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc. Natl. Acad. Sci. USA 86:6302-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mustelin, T., K. M. Coggeshall, N. Isakov, and A. Altman. 1990. Tyrosine phosphorylation is required for T-cell antigen receptor-mediated activation of phospholipase C. Science 247:1584-1587. [DOI] [PubMed] [Google Scholar]
- 38.Mustelin, T., G.-S. Feng, N. Bottini, A. Alonso, N. Kholod, D. Birle, J. J. Merlo, and H. Huynh. 2002. Protein tyrosine phosphatases. Front. Biosci. 7:85-142. [DOI] [PubMed] [Google Scholar]
- 39.Nada, S., M. Okada, A. MacAuley, J. A. Cooper, and H. Nakagawa. 1991. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature 351:69-72. [DOI] [PubMed] [Google Scholar]
- 40.Nada, S., T. Yagi, H. Takeda, T. Tokunaga, H. Nakagawa, Y. Ikawa, M. Okada, and S. Aizawa. 1993. Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell 73:1125-1135. [DOI] [PubMed] [Google Scholar]
- 41.Neumeister, E. N., Y. Zhu, S. Richard, C. Terhorst, A. C. Chan, and A. S. Shaw. 1995. Binding of ZAP-70 to phosphorylated T-cell receptor zeta and eta enhances its autophosphorylation and generates specific binding sites for SH2 domain-containing proteins. Mol. Cell. Biol. 15:3171-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oetken, C., C. Couture, M. Bergman, N. Bonnefoy-Bérard, S. Williams, K. Alitalo, K., P. Burn, and T. Mustelin, T. 1994. TCR/CD3-triggering causes activation of the p50csk tyrosine kinase and engagement of its SH2 domain. Oncogene 9:1625-1631. [PubMed] [Google Scholar]
- 43.Oetken, C., M. von Willebrand, A. Marie-Cardine, T. Pessa-Morikawa, A. Stähls, S. Fischer, and T. Mustelin. 1994. Induction of hyperphosphorylation and activation of the p56lck protein tyrosine kinase by phenylarsine oxide, a phosphotyrosine phosphatase inhibitor. Mol. Immunol. 31:1295-1302. [DOI] [PubMed] [Google Scholar]
- 44.O'Shea, J. J., D. W. McVivar, T. L. Bailey, C. Burns, and M. J. Smyth. 1992. Activation of human peripheral blood T lymphocytes by pharmacological induction of protein-tyrosine phosphorylation. Proc. Natl. Acad. Sci. USA 89:10306-10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostergaard, H. L., D. A. Shackelford, T. R. Hurley, P. Johnson, R. Hyman, B. M. Sefton, and I. S. Trowbridge. 1989. Expression of CD45 alters phosphorylation of the Lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc. Natl. Acad. Sci. USA 86:8959-8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker, F., F. Maurier, I. Delumeau, M. Duchesne, D. Faucher, L. Debussche, A. Dugue, F. Schweighoffer, and B. Tocque. 1996. A Ras-GTPase-activating protein SH3-domain-binding protein. Mol. Cell. Biol. 16:2561-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Partanen, J., E. Armstrong, M. Bergman, T. P. Mäkelä, H. Hirvonen, K. Huebner, and K. Alitalo. 1991. Cyl encodes a putative cytoplasmic tyrosine kinase lacking the conserved tyrosine autophosphorylation site (Y416src). Oncogene 6:2013-2018. [PubMed] [Google Scholar]
- 48.Perez-Villar, J. J., and S. B. Kanner. 1999. Regulated association between the tyrosine kinase Emt/Itk/Tsk and phospholipase-C γ1 in human T lymphocytes. J. Immunol. 163:6435-6441. [PubMed] [Google Scholar]
- 49.Pingel, J. T., and M. L. Thomas. 1989. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell 58:1055-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanders, M. E., M. W. Makgoba, and S. Shaw. 1988. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol. Today 9:195-199. [DOI] [PubMed] [Google Scholar]
- 51.Saxena, M., S. Williams, K. Taskén, and T. Mustelin. 1999. Crosstalk between cAMP-dependent kinase and MAP kinase through hematopoietic protein tyrosine phosphatase (HePTP). Nat. Cell Biol. 1:305-311. [DOI] [PubMed] [Google Scholar]
- 52.Secrist, J. P., L. A. Burns, L. Karnitz, G. A. Koretzky, and R. T. Abraham. 1993. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J. Biol. Chem. 268:5886-5893. [PubMed] [Google Scholar]
- 53.Sefton, B. M. 1990. The Lck tyrosine protein kinase. Oncogene 6:683-686. [PubMed] [Google Scholar]
- 54.Soncini, C., I. Berdo, and G. Draetta. 2001. Ras-GAP SH3 binding protein (G3BP) is a modulator of USP10, a novel human ubiquitin specific ligase. Oncogene 20:3869-3879. [DOI] [PubMed] [Google Scholar]
- 55.Straus, D. B., and A. Weiss. 1992. Genetic evidence for the involvement of the Lck tyrosine kinase in signal transduction through the T-cell antigen receptor. Cell 70:585-593. [DOI] [PubMed] [Google Scholar]
- 56.Torgersen, K. M., T. Vang, H. Abrahamsen, S. Yaqub, V. Horejsi, B. Schraven, B. Rolstad, T. Mustelin, and K. Taskén. 2001. Release from tonic inhibition of T-cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J. Biol. Chem. 276:29313-29318. [DOI] [PubMed] [Google Scholar]
- 57.Tsygankov, A. Y., B. M. Bröker, J. Fargnoli, J. A. Ledbetter, and J. B. Bolen. 1992. Activation of tyrosine kinase p60fyn following T-cell antigen receptor cross-linking. J. Biol. Chem. 267:18259-18262. [PubMed] [Google Scholar]
- 58.Vang, T., K. M. Torgersen, V. Sundvold, S. Saxena, F. O. Levy, B. S. Skälhegg, V. Hansson, T. Mustelin, and K. Taskén. 2001. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T-cell receptor. J. Exp. Med. 193:497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Stipdonk, M. J., E. E. Lemmens, and S. P. Schoenberger. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2:423-429. [DOI] [PubMed] [Google Scholar]
- 60.von Willebrand, M., T. Jascur, N. Bonnefoy-Bérard, H. Yano, A. Altman, Y. Matsuda, and T. Mustelin. 1996. Inhibition of phosphatidylinositol 3-kinase blocks TCR/CD3-induced activation of the mitogen-activated kinase Erk2. Eur. J. Biochem. 235:828-835. [DOI] [PubMed] [Google Scholar]
- 61.von Willebrand, M., S. Williams, M. Saxena, J. Gilman, P. Tailor, T. Jascur, G. P. Amarante-Mendes, D. R. Green, and T. Mustelin. 1998. Modification of phosphatidylinositol 3-kinase SH2 domain binding properties by Abl- or Lck-mediated tyrosine phosphorylation at Tyr-688. J. Biol. Chem. 273:3994-4000. [DOI] [PubMed] [Google Scholar]
- 62.Virolle, T., E. D. Adamson, V. Baron, D. Birle, D. Mercola, T. Mustelin, and I. de Belle. 2001. PTEN is directly transactivated in vivo by Egr-1 during irradiation-induced signalling. Nat. Cell Biol. 3:1124-1128. [DOI] [PubMed] [Google Scholar]
- 63.Wang, X., A. Gjörloff-Wingren, M. Saxena, N. Pathan, J. C. Reed, and T. Mustelin. 2000. The tumor suppressor PTEN regulates T-cell survival and antigen receptor signaling by acting as a phosphatidylinositol 3-phosphatase. J. Immunol. 164:1934-1939. [DOI] [PubMed] [Google Scholar]
- 64.Wang, X., H. Huynh, H., A. Gjörloff-Wingren, E. Monosov, M. Stridsberg, M. Fukuda, and T. Mustelin. 2002. Enlargement of secretory vesicles by protein tyrosine phosphatase PTP-MEG2 in RBL mast cells and Jurkat T cells. J. Immunol. 168:4612-4619. [DOI] [PubMed] [Google Scholar]
- 65.Williams, S., C. Couture, J. Gilman, T. Jascur, M. Deckert, A. Altman, and T. Mustelin. 1997. Reconstitution of TCR-induced Erk2 kinase activation in Lck-negative JCaM1 cells by Syk, but not Zap. Eur. J. Biochem. 245:84-90. [DOI] [PubMed] [Google Scholar]
- 66.Zhang, W. J., J. Sloan-Lancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T-cell receptor to cellular activation. Cell 92:83-92. [DOI] [PubMed] [Google Scholar]