Abstract
3-Phosphoinositide-dependent kinase 1 (PDK1) phosphorylates the activation loop of a number of protein serine/threonine kinases of the AGC kinase superfamily, including protein kinase B (PKB; also called Akt), serum and glucocorticoid-induced kinase, protein kinase C isoforms, and the p70 ribosomal S6 kinase. PDK1 contains a carboxyl-terminal pleckstrin homology domain, which targets phosphoinositide lipids at the plasma membrane and is central to the activation of PKB. However, PDK1 subcellular trafficking to other compartments is not well understood. We monitored the posttranslational modifications of PDK1 following insulin-like growth factor 1 stimulation. PDK1 underwent rapid and transient phosphorylation on S396, which was dependent upon plasma membrane localization. Phosphorylation of S396 was necessary for nuclear shuttling of PDK1, possibly through its influence on an adjacent nuclear export sequence. Thus, mitogen-stimulated phosphorylation of PDK1 provides a means for directed PDK1 subcellular trafficking, with potential implications for PDK1 signaling.
3-Phosphoinositide-dependent kinase 1 (PDK1) is a serine/threonine kinase belonging to the AGC superfamily of protein kinases (reviewed in references 31, 44, and 48). PDK1 was identified as the upstream activation loop kinase of protein kinase Bα (PKBα; also known as Akt1), which is essential for the activation of PKB (1, 39, 42). Activation of the lipid kinase phosphoinositide 3-kinase (PI3K) is critical for the activation of PKB by PDK1 and has been studied extensively in recent years because it is a key mediator of biological responses downstream of insulin and other tyrosine kinase receptors, regulating survival, cell cycle control, protein translation, and glucose metabolism (14, 21, 26, 52).
Since PDK1's discovery as a PKB kinase, the stable of PDK1 targets has expanded to include other AGC kinases, including protein kinase C (PKC) isoforms (5, 13, 15, 27, 40), the p70 and p90 ribosomal S6 kinases (S6K and RSK) (2, 19, 23, 36), and the serum- and glucocorticoid-induced kinases (SGKs) (7, 25, 34). This establishes PDK1 as a central activator of multiple signaling pathways coupled to a large number of growth-promoting stimuli. Importantly, many of the signaling pathways upon which PDK1 acts are characterized by alterations during human pathologies. In cancer, disruption of the apoptosis machinery is a critical event and occurs at multiple levels, including the direct regulation of apoptosis proteins, alteration in energy metabolism, and the control of protein synthesis. PDK1 interfaces with each of these cell regulatory networks through kinases such as PKB and therefore could be a point of therapeutic intervention. Indeed, the regulation of PDK1 and the identification of small molecule inhibitors is the subject of intense focus (17).
PDK1 contains a carboxyl-terminal pleckstrin homology (PH) domain that binds to the lipid products of PI3K, PI-3,4,5-P3, and PI-3,4-P2. The precise role of these lipid species in the activation of PDK1 and the phosphorylation of its substrates has been recently investigated. One likely function is plasma membrane shuttling (3, 4, 16, 38). When localized to the plasma membrane, but not other cell compartments, PDK1 is able to phosphorylate PKB efficiently (4, 38). However, other substrates of PDK1 are activated normally under conditions where the lipid-binding function of the PH domain has been disrupted (29). Thus, the functional significance of the PH domain of PDK1 may be relevant only for a subset of PDK1 targets. These targets, including PKB, are likely to be important for the antiapoptotic and antioncogenic potential of the PI3K pathway, since tumor suppressors such as the phosphatidylinositol 3′-phosphatase PTEN act to down-regulate signaling from PI3K to PDK1 and PKB (41, 43).
The location-specific activity of PDK1 may reflect a substrate conformational change that occurs at a permissible location. For PKB, membrane localization confers a permissive change that promotes activation loop phosphorylation by PDK1. This likely involves a change in the PKB structure accompanying PH domain interaction with membrane lipids (29, 30, 46), as well as phosphorylation of S473 within a C-terminal hydrophobic motif (38). All PDK1 substrates identified so far and validated in genetic models contain this conserved hydrophobic motif, which is usually located approximately 170 amino acids downstream of the activation loop. Additionally, substrates of PDK1, including S6K, RSK, and SGK, require hydrophobic motif phosphorylation to serve as a PDK1 docking site, thereby increasing PDK1-substrate interaction and activation loop phosphorylation (6-9, 18, 19, 51). Membrane-localized adapter proteins, including Grb14, may also contribute to activation of PDK1 (24). For PKB and other AGC kinases, the regulation of the hydrophobic motif phosphorylation is tightly controlled by distinct signaling pathways. In this regard, PDK1 likely phosphorylates other targets, depending on location-specific hydrophobic motif phosphorylation.
PDK1 is restricted from the nucleus in mammalian cells. In a recent study, treatment of cells with leptomycin-B, an inhibitor of the nuclear export receptor CRM1, promoted nuclear accumulation of PDK1 (28). Mutation or deletion of a CRM1-binding nuclear export sequence (NES) between amino acids 379 and 388 also promoted nuclear accumulation, which was similar to the effects of leptomycin-B (28). In addition, mitogens, including insulin, induced the shuttling of PDK1 within the nucleus (28). The mechanism controlling insulin-induced PDK1 nuclear shuttling remains unknown but is of interest, because it could define a new and previously undiscovered mechanism for nuclear PDK1 substrate phosphorylation.
We investigated whether a posttranslational event associated with PDK1 is necessary for the nuclear shuttling of PDK1 following growth factor receptor activation. Our results demonstrate that the PH domain of PDK1 is necessary for nuclear translocation. This suggests that localization to the plasma membrane primes PDK1 for accelerated nuclear import or suppression of nuclear export. We found that a serine-rich motif between S389 and S396 of PDK1, directly proximal to the putative NES region, undergoes rapid and transient phosphorylation following growth factor receptor activation in a Ras-, PI3K-, and PH domain-specific manner. A primary site of phosphorylation was localized to S396. The phosphorylation of S396 and its proximity to the NES region suggest that phosphorylation may regulate cellular trafficking of PDK1.
MATERIALS AND METHODS
Cell culture.
HEK 293 and MCF-7 cells were obtained from the American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium (DMEM) medium supplemented with 10% fetal calf serum and antibiotics at 37°C, 5% CO2, and humidity. PTEN−/− mouse embryonic fibroblast (MEF) cells were a gift from Vuc Stambolic and were maintained in DMEM supplemented with 10% fetal calf serum and antibiotics at 37°C, 5% CO2, and humidity.
Reagents.
Antibodies used were anti-PKB/AKT, anti-phospho-specific T308 PKB/AKT, anti-phospho-specific S473 PKB/AKT, anti-PDK1, and anti-phospho-specific S241 PDK1 (all from Cell Signaling Technology). Antihemagglutinin (anti-HA) epitope was from Sigma. Anti-myc epitope 9E10 was from Santa Cruz Biotechnology. Recombinant SGK S422D was obtained from Upstate Biotechnology. LY294002 was obtained from Sigma-Aldrich.
Site-directed mutagenesis.
The various site mutants of PDK1 were generated with the Quickchange kit (Stratagene). Mutations were sequence verified.
cDNA transfections.
HEK 293, PTEN−/− MEF, and MCF-7 cells were plated onto 35-mm-diameter dishes at 80% confluency and transfected with 100 to 500 ng of cDNA with Lipofectamine 2000 (Gibco-BRL) following the manufacturer's protocol. The following day, the transfection medium was removed and replaced by complete DMEM. At 18 h prior to treatments, cells were washed and serum starved in DMEM without fetal calf serum.
Confocal microscopy.
Cells were plated on glass coverslips and transfected with 100 ng of cDNA with Liptofectamine 2000. Cells were fixed in 3% paraformaldehyde for 15 min at room temperature, blocked with 0.5% bovine serum albumin-phosphate-buffered saline solution; incubated with anti-PDK1, anti-myc 9E10, or anti-HA primary antibodies where indicated for 2 h; washed; and then incubated with Alexa-488- or Alexa-564-conjugated secondary antibodies (Molecular Probes) for 1 h. Coverslips were mounted onto slides with Fluoromount-G (Southern Biotechnologies, Inc.). Immunofluorescence-stained cells were visualized with a Zeiss LSM510 confocal microscope, and images were captured with LSM software, version 2.3.
Cell lysis and immunoblotting.
Cells were lysed in 50 mM Tris-HCl, (pH 7.4), 0.5% Triton X-100, 25 mM NaF, 25 mM β-glycerophosphate, 5 mM EDTA, 1 μg of microcystin LR/ml, and protease inhibitors. Portions of the lysates were boiled with sodium dodecyl sulfate (SDS)-containing sample buffer and fractionated by SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were transferred to a polyvinylidene difluoride membrane, blocked in 5% skim milk for 30 min, and probed with the appropriate antibody overnight at 4°C. Secondary decoration with horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies was performed at room temperature for 1 h. Proteins were visualized by ECL according to the manufacturer's protocol (Amersham).
Metabolic labeling.
HEK 293 cells were plated onto 100-mm-diameter dishes at 80% confluency and transfected with 100 to 500 ng of cDNA with Lipofectamine 2000 (Gibco-BRL) following the manufacturer's protocol. After 24 h, cells were washed in phosphate-free medium and then placed in phosphate-free DMEM medium buffered with 10 mM HEPES (pH 7.4) with 1 mCi of 32P-labeled orthophosphate/ml at 37°C for 4 h, followed by stimulation with insulin-like growth factor 1 (IGF-1) (100 ng/ml) for 15 min. PDK1 was immunoprecipitated from detergent-solubilized lysates and fractionated on a 8% acrylamide gel with an acrylamide/bisacrylamide ratio of 118:1 and dried under heat and vacuum. 32P-labeled PDK1 was detected by autoradiography and quantified either by excision from the gel followed by liquid scintillation counting or by use of a PhosphorImager (Molecular Dynamics).
Tryptic digestion, two-dimensional (2D) phosphopeptide mapping, and phosphoamino acid analysis.
32P metabolically labeled PDK1 from various conditions and isolated as described above was excised from the gel and digested with 10 μg of tosylphenylalanyl chloromethyl ketone-treated trypsin (Promega)/ml in 50 mM (NH4)HCO3 (pH 7.8) overnight at 37°C. Gel fragments were pelleted by centrifugation, and the remaining supernatant was transferred to clean tubes and dried under a vacuum. Peptides were washed with diminishing volumes of water and resuspended in 5 μl of electrophoresis buffer [1% (NH4)2CO3; pH 8.8]. Electrophoresis was performed with 200-μm-thick microcrystalline cellulose plates (Kodak) at 1,000 V at 7°C for 60 min. The plates were chromatographed in the second dimension in chromatography buffer (n-butanol-pyridine-acetic acid-water, 32.5:25:5:20). Plates were dried, and phosphopeptides were visualized with a PhosphorImager (Molecular Dynamics). If cold synthetic phosphopeptides were also run, these were visualized with ninhydrin staining. Phosphoamino acid analysis was performed by scraping the visualized phosphopeptides into glass reaction vessels and treatment with 500 μl of 6 N HCl heated to 105°C for 60 min. The HCl was removed under vacuum, and the phosphoamino acids were washed with diminishing volumes of water. Separation was performed on cellulose plates with buffer consisting of 0.5% pyridine and 5% acetic acid at 1,000 V at 10°C for 45 min. 32P-labeled phosphoamino acids were detected by autoradiography. In each of the samples, 1 μg of a mixture of phospho-l-serine, phospho-l-threonine, and phospho-l-tyrosine was also added that was visualized by ninhydrin staining.
RESULTS
IGF-1-induced phosphorylation of expressed and endogenous PDK1.
We have previously observed that endogenous PDK1 was absent from nuclei in MCF-7 cells under serum starvation conditions (Fig. 1A) but was present following a 30′ treatment with IGF-1 (Fig. 1A), consistent with a recent report (28). Leptomycin-B was a potent inducer of nuclear PDK1, indicating that PDK1 is actively exported by a CRM1-dependent mechanism (Fig. 1A). The specificity of the PDK1 antibody used in these experiments was confirmed by control staining of PDK1−/− embryonic stem cells (data not shown). Finally, we noted that endogenous PDK1 isolated from the nucleus migrated at higher weights compared with cytoplasmic PDK1, which could be indicative of hyperphosphorylation (Fig. 1B).
FIG. 1.
IGF-1-stimulated nuclear shuttling of endogenous PDK1. (A) MCF-7 cells were plated onto glass coverslips and serum starved for 18 h. Cells were either stimulated with IGF-1 for 30 min or treated with leptomycin-B (50 nM) for 3 h. Cells were fixed in 3% paraformaldehyde, stained with anti-PDK1 (Cell Signaling), and counterstained with Alexa-488-conjugated anti-rabbit immunoglobulin G (IgG) (Molecular Probes). DNA was stained with DAPI (4′,6′-diamidino-2-phenylindole). Images were collected on a Zeiss LSM 510 confocal microscope. (B) MCF-7 cells growing in complete medium were fractionated into cytoplasmic (C) and nuclear (N) fractions as described in Materials and Methods. Portions of each fraction were resolved by SDS-PAGE and immunoblotted with anti-PDK1 (Cell Signaling).
The observation that nuclear PDK1 could be hyperphosphorylated suggested that phosphorylation of PDK1 participates in nuclear shuttling. Thus, we wanted to further characterize PDK1 phosphorylation to define its role in nuclear shuttling. We first began by monitoring the phosphorylation status of cytoplasmic PDK1 during receptor tyrosine kinase signaling. When PDK1 was expressed in HEK 293 cells, it migrated as a single band during sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 2A). Stimulation of the cells with IGF-1 for a short period of time caused a decrease in mobility of PDK1, which suggested phosphorylation. Treatment of PDK1 with calf alkyline phosphatase (CIP) reduced the doublet to a single fast-migrating band, also consistent with phosphorylation. The change in mobility caused by CIP suggests loss of multiple phosphorylation sites. PDK1 has previously been shown to be constitutively phosphorylated on at least five serine residues (S25, S241, S393, S396, and S410) (12), but our results suggest that signaling pathways activated by IGF-1 can further increase the degree of PDK1 phosphorylation on these or other sites.
FIG. 2.
IGF-1-stimulated PDK1 mobility shift. (A) HEK 293 cells were transfected with 500 ng of myc-PDK1 or empty vector for 24 h. Cells were starved for 18 h in serum-free medium and then stimulated with IGF-1 (100 ng/ml) or vehicle alone for 15 min. Cells were harvested into ice-cold lysis buffer and clarified by centrifugation, as described in Materials and Methods. PDK1 was immunoprecipitated with anti-myc 9E10 antibody, washed, and resuspended in CIP buffer (New England Biolabs). CIP (10 U) was added where indicated, and samples were incubated for 10 min at room temperature. Reactions were terminated by the addition of SDS sample buffer and boiling for 5 min. PDK1 was fractionated by SDS-PAGE and immunoblotted with 9E10 antibody. (B) HEK 293 cells were transfected with myc-PDK1 for 24 h, followed by serum starvation for 18 h, and then treated with IGF-1 (100 ng/ml) for the indicated times. Cells were lysed in ice-cold lysis buffer, and portions of the lysates were fractionated by SDS-PAGE, followed by immunoblotting with 9E10 anti-myc antibody. Samples were also probed with anti-phospho-T308 PKB antibody (Cell Siganling). (C) HEK 293 cells were serum starved for 18 h, followed by treatment with IGF-1 for the indicated times. Cells werelysed, and solubilized proteins were fractionated by SDS-PAGE. Endogenous PDK1 was detected with an anti-PDK1 antibody (Cell Signaling). Samples were also probed with an anti-phospho-S473 PKB antibody (Cell Signaling). (D) HEK 293 cells were transfected with 500 ng of myc-PDK1 for 24 h and then serum starved for 18 h. Cells were then treated where indicated with LY294002 for 15 min, followed by stimulation with IGF-1 (100 ng/ml) where indicated. myc-PDK1 was immunoprecipitated, fractionated by SDS-PAGE, and detected with anti-myc 9E10 antibody. (E) FLAG-PDK1 (3×; 200 ng) and p110-CAAX (200 ng) or empty vector was cotransfected into HEK 293 cells for 24 h. Cells were serum starved for 18 h and then stimulated with IGF-1 (100 ng/ml) for 15 min. Cells were lysed in ice-cold lysis buffer and fractionated by SDS-PAGE. Membranes were probed with anti-FLAG (M2 monoclonal; Sigma) or anti-p110 (Upstate). (F and G) 293 cells were cotransfected with myc-PDK1 (200 ng) and either RasN17, RasV12, or empty vector (200 ng each) where indicated for 24 h. Cells were serum starved for 18 h and then treated with LY294002 (25 μM) in the lanes indicated, followed by stimulation with IGF-1 (100 ng/ml) for 15 min where indicated. myc-PDK1 was immunoblotted as described above. (H) MCF-7 cells were serum starved overnight and treated with staurosporine (100 nM) for 10 min. Cells were stimulated with IGF-1 (100 ng/ml) for 15 min and then lysed in ice-cold lysis buffer. Clarified lysates were fractionated by SDS-PAGE, and endogenous PDK1 was detected with anti-PDK1 antibody (Cell Signaling). Phospho-T308, S473, and total PKB (also from Cell Signaling) were also detected.
IGF-1 was able to induce a mobility shift of ectopically expressed PDK1 within 2 min of stimulation (Fig. 2B). We next examined endogenous PDK1 to determine if this protein, which is present at much lower levels than ectopically expressed PDK1, experiences a similar elevation of phosphorylation in response to IGF-1. Stimulation of HEK 293 cells with IGF-1 for various times led to a rapid mobility shift of endogenous PDK1 along a time scale similar to that of PKB activation, including T308 and S473 phosphorylation (Fig. 2C). The mobility shift of endogenous PDK1 was observed in a number of different cell lines in response to growth factor stimulation, including NIH 3T3 (data not shown) and MCF-7 (Fig. 2H). Collectively these results indicate that both exogenously expressed and endogenous PDK1 is rapidly phosphorylated downstream of RTK signaling.
PI3K activity and the PH domain of PDK1 are necessary for PDK1 phosphorylation and nuclear shuttling.
We next focused on the conditions necessary for the effect of IGF-1 signaling on PDK1 phosphorylation. PDK1 contains a high-affinity lipid-binding PH domain which binds to phosphatidylinositol-3,4,5-trisphosphate (PIP3) and PI(3,4)P2, the direct products of PI3K. We speculated that the phosphorylation of PDK1 might involve IGF-1-induced PI3K activity. We tested this by blocking PI3K with an inhibitor prior to IGF-1 treatment and then measuring the mobility of PDK1 (Fig. 2D). PI3K inhibition prevented the shift in mobility of both expressed and endogenous PDK1 (Fig. 2D). Another PI3K inhibitor, wortmannin, produced identical results (data not shown). In a reciprocal experiment, coexpression of PDK1 with an isoprenylated p110 subunit of PI3K, which generates PIP3 independently of RTK activation, caused a constitutive shift in mobility (Fig. 2E). Thus, the PDK1 phosphorylation event(s) is coupled with the activity of PI3K.
The proto-oncogene Ras is a regulator of PI3K activity through an interaction between GTP-loaded Ras and the p110 subunit of PI3K (37). We asked whether IGF-1 stimulation of PDK1 phosphorylation involved Ras. Coexpression of PDK1 with the dominant-negative mutant of Ras (N17) interfered with the extent of IGF-1-induced mobility shift of PDK1 (Fig. 2F). In contrast, coexpression with the GTP-bound RasV12 mutant resulted in constitutive elevated levels of PDK1 phosphorylation (Fig. 2G). These results show that PI3K and its direct activator Ras are able to modulate the phosphorylation state of PDK1.
The IGF-1-stimulated mobility shift of endogenous PDK1 was blocked by staurosporine, a broadly specific protein kinase inhibitor (Fig. 2H). This suggests that PDK1 phosphorylation may be mediated by a staurosporine-sensitive kinase, which includes PDK1 itself (22), and members of the PKC family (45). The inhibition of PDK1 band shift by staurosporine correlated with inhibition of PKB phosphorylation on T308, but not S473, which appeared to be enhanced by staurosporine. Other inhibitors of signaling pathways, such as the MEK inhibitor PD98059 and the p38 inhibitor SB203580, had no effect on PDK1 mobility shift and phosphorylation (data not shown).
Next, we labeled cells with 32P to measure the degree of PDK1 phosphorylation directly. IGF-1 stimulation caused an approximately 2.5-fold increase in 32P labeling of expressed PDK1 (Fig. 3A). Blocking PI3K completely inhibited the elevation of PDK1 phosphorylation, in agreement with the role of PI3K in regulating PDK1 mobility shift. In contrast, rapamycin, an inhibitor of mTOR and p70S6K, had no effect on the phosphorylation of PDK1 (Fig. 3B).
FIG. 3.
PI3K and PH domain requirement for PDK1 phosphorylation. (A and B) HEK 293 cells were transfected with 500 ng of myc-PDK1 for 24 h and then serum starved for 18 h. Cells were labeled with [32P]orthophosphate (1 mCi/ml) in phosphate-free medium for 4 h, followed by treatment with LY294002 (25 mM) or rapamycin (100 nM) for 30 min. Following treatment with IGF-1 for 15 min and solubilization in ice-cold lysis buffer, PDK1 was immunoprecipitated with anti-myc 9E10 antibody and fractionated by SDS-PAGE. (C) Cells were transfected with wild-type PDK1 or R474A-PDK1, labeled, and stimulated with IGF-1 as described above (top). A portion of the immunoprecipitate was used to probe total PDK1 (bottom). (D) MCF-7 cells were plated onto glass coverslips and transfected with myc-PDK1 or myc-R474A-PDK1 for 24 h. Cells were then serum starved for 18 h and treated with IGF-1 for 15 min. Cells were fixed in 3% paraformaldehyde, stained with anti-myc 9E10 antibody, and counterstained with Alexa-488-conjugated anti-mouse antibody (Molecular Probes). Samples were visualized by confocal microscopy.
Since PI3K activity was necessary for PDK1 phosphorylation, we wanted to determine if this was due to plasma membrane localization through phospholipid binding with PDK1 or if some other PI3K-dependent function was responsible. For example, PI3K may contribute to the activation of kinases that phosphorylate cytoplasmic PDK1, rather than modulating its subcellular location. As well, LY294002 and wortmannin may inhibit other cellular targets besides PI3K that may be involved in PDK1 phosphorylation. To test this, we introduced a point mutation in the PH domain of PDK1, which renders it incapable of binding to phospholipids. The R474A mutant has been previously reported to remain cytosolic following PI3K activation, compared with wild-type PDK1, which translocates to the plasma membrane (3). In response to IGF-1, the R474A mutant was refractory to mobility shift, and it showed no increase in total phosphorylation during 32P labeling (Fig. 3C). Laser scanning microscopy comparing the wild type and R474A-PDK1 confirmed that the PH domain mutant remained cytosolic (Fig. 3D). This microscopy also revealed noticeably less R474A-PDK1 staining in the nucleus following IGF-1 stimulation than with wild-type PDK1, suggesting that PH domain lipid binding is a requirement for nuclear PDK1 shuttling (Fig. 3D). Thus, consistent with a role for PI3K in subcellular targeting, PDK1 requires a functional PIP3-binding PH domain to undergo IGF-1-induced phosphorylation.
Membrane localization is important for PDK1 phosphorylation.
We considered that if membrane localization brings PDK1 into the proximity of upstream kinases or places it in a position where trans-autophosphorylation can occur, then artificially targeting PDK1 to the plasma membrane should lead to high-level phosphorylation. We added the amino-terminal Src myristoylation sequence to R474A PDK1 to determine whether this modification had an effect on PDK1 phosphorylation. Confocal microscopy verified that myristoylated (myr)-R474A-PDK1 was predominantly plasma membrane localized, with very little present in the cytoplasm and none detectable in the nucleus (Fig. 4A). When examined by immunoblotting, myr-R474A-PDK1 migrated as a single band and more slowly than nonmyristoylated, wild-type PDK1 (Fig. 4A). Thus, anchoring PDK1 to the plasma membrane caused a constitutive decrease in mobility, signifying an elevated degree of basal phosphorylation. This was confirmed when we isolated myr-R474A PDK1 from 32P-labeled cells. Compared with wild-type PDK1, membrane-targeted PDK1 exhibited a greater level of phosphorylation (Fig. 4B). Confocal microscopy confirmed that myr-R474A PDK1 was largely plasma membrane localized (Fig. 4C).
FIG. 4.
Myristoylation of PDK1 increases phosphorylation. (A) Myc-PDK1 or myr-R474A-PDK1 was transfected into HEK 293 cells for 24 h, followed by 18 h of serum starvation and treatment with IGF-1 for 15 min. Lysates were fractionated by SDS-PAGE, and PDK1 was detected with anti-PDK1 antibody (Cell Signaling Technologies). (B) HEK 293 cells transfected with myc-PDK1 or myr-R474A-PDK1 were labeled as described in the legend to Fig. 3 and stimulated with IGF-1 for 15 min. PDK1 was detected by autoradiography (top) and a portion of the immunoprecipitate was probed for total PDK1 (bottom). (C) myc-PDK1 or myr-R474A-PDK1 were transfected into MCF-7 cells growing on glass coverslips. After 24 h, cells were fixed in 3% paraformaldehyde, and PDK1 was stained with anti-PDK1 antibody (Cell Signaling Technologies) and counterstained with Alexa-488-conjugated anti-rabbit IgG (Molecular Probes). Confocal images were captured with a Zeiss LSM 510 microscope. (D) HEK 293cells transfected with myc-PDK1 or myr-R474A-PDK1 were harvested and fractionated into cytoplasmic (C) or membrane (M) fractions as described in Materials and Methods. Volumes of the lysates corresponding to equal cell equivalents were fractionated by SDS-PAGE and immunoblotted with anti-PDK1 antibody (Cell Signaling Technologies). (E) myc-PDK1, myc-R474A-PDK1, or myr-R474A-PDK1 was expressed in HEK 293 cells for 24 h, followed by serum starvation for 18 h. Cells were labeled for 4 h with [32P]orthophosphate (1 mCi/ml), stimulated with IGF-1 (100 ng/ml) where indicated, and solubilized in ice-cold lysis buffer. PDK1 was immunoprecipitated with anti-myc 9E10 antibody and resolved by SDS-PAGE. 32P-labeled PDK1 was excised from the gel and digested with trypsin (Calbiochem) overnight at 37°C as described in Materials and Methods. The dried tryptic peptides were spotted onto cellulose plates and separated in the first dimension by electrophoresis in buffer (pH 1.9), dried, and separated in the second dimension with phosphochromatography buffer. 32P-labeled peptides were visualized by PhosphorImager analysis (Molecular Dynamics). (F) The tyrptic peptide labeled A was scraped from the cellulose plate and digested with 6 N constantly boiling HCl at 100°C for 20 min. The hydrolyzed amino acids were washed several times, separated by electrophoresis chromatography on cellulose plates in buffer (pH 1.9), and visualized by autoradiography. The migration of phosphoserine, phosphothreonine, and phosphotyrosine was determined by comigration with cold phosphoamino acids and visualized by ninhydrin staining.
To further confirm that the localization of PDK1 at the plasma membrane influences its degree of phosphorylation, we performed subcellular fractionation of cells expressing myr-R474A-PDK1. The fraction of myr-R474A-PDK1 isolated from the cell cytosol was evenly distributed between slow- and fast-migrating forms (Fig. 4D). myr-R474A-PDK1 isolated from the membrane fraction, however, was composed mostly of the slow-migrating (phosphorylated) form. Thus, the subcellular location of myr-R474A-PDK1 strongly correlated with an increased stoichiometry of phosphorylation on one or more sites.
To identify the site(s) of IGF-1-induced phosphorylation, we next isolated 32P-labeled PDK1 from cells treated with IGF-1 and performed tryptic digestion and separation of the peptides by electrophoresis-chromatography on cellulose plates (Fig. 4E). A number of peptides from PDK1 became phosphorylated during the labeling period of the experiment, agreeing with earlier published work identifying five sites of serine phosphorylation (12). Upon stimulation with IGF-1, only certain residues significantly increased in radioactivity, while the majority remained at a constant level. Furthermore, 2D maps derived from 32P-labeled R474A PDK1 showed that the primary sites of phosphorylation were no longer increased by IGF-1 stimulation (Fig. 4E). In contrast, myristoylation of R474A-PDK1 led to constitutive phosphorylation on these same sites as well as some additional sites, consistent with the greatly elevated degree of phosphorylation of myr-R474A-PDK1 seen in the results shown in Fig. 4A.
One of the labeled peptides found in each map comigrated with a synthetic phosphopeptide derived from the tryptic fragment containing S241. This residue is the critical activation loop serine present in other AGC kinases and is the functional equivalent to T308 of PKBα. However, unlike the process that normally occurs in PKB, S241 phosphorylation did not change following receptor activation. Immunoblotting with a phosphospecific S241 antibody showed that S241 is constantly phosphorylated (Fig. 5A). This antibody recognizes S241 specifically, since neither the S241A PDK1 mutant (Fig. 5A) nor wild-type PDK1 treated with alkaline phosphatase was immunoreactive. Thus, phosphorylation of S241 did not increase following IGF-1 receptor activation, in agreement with several previous studies of S241 phosphorylation (12, 49). As well, an IGF-1-stimulated mobility shift was identical between wild-type and S241A PDK1 (Fig. 5A), indicating that S241 is dispensable for the phosphorylation of PDK1 on the other site(s).
FIG. 5.
PDK1 phosphorylation does not require S241 or kinase activity. (A) myc-PDK1 or myc-S241A-PDK1 was expressed in HEK 293 cells for 24 h, serum starved for 18 h, and stimulated where indicated with IGF-1 for 15 min. PDK1 was immunoprecipitated with anti-myc 9E10 antibody, fractionated by SDS-PAGE, and immunoblotted with anti-S241 antibody (Cell Signaling) (top) and anti-PDK1 (Cell Signaling) (bottom). (B) HEK 293 cells were transfected with myc-PDK1, myc-R474A-PDK1, myc-S241-PDK1, or empty vector for 24 h; serum starved for 18 h; and then stimulated with IGF-1 for 15 min. PDK1 was immunoprecipitated with anti-myc 9E10 antibody and assayed for activity in vitro with S422D SGK as substrate as described in Materials and Methods. Reactions were terminated by addition of 2× SDS sample buffer and boiling. Proteins were resolved by SDS-PAGE, transferred to polyvinylidene difluoride, and visualized by autoradiography. (C) myc-PDK1 and myc-K111A-PDK1 were transfected into HEK 293 cells and labeled with [32P]orthophosphate as described in the legend to Fig. 3. (D) Kinase activity of myc-PDK1 and myc-K111A-PDK1 was measured as in the data shown in panel B. (E) Tryptic peptide mapping was performed on 32P-labeled PDK1 isolated from panel C.
We next mutated a critical lysine residue in PDK1 involved in ATP binding to generate a catalytically inactive form, to determine if PDK1 phosphorylation was a consequence of cis-autophosphorylation. We isolated PDK1 from 32P-labeled 293 cells expressing wild-type and K111A PDK1 (Fig. 5C). Although K111A PDK1 was unable to phosphorylate recombinant SGK in vitro, K111A PDK1 experienced a significant increase in phosphorylation upon stimulation with IGF-1, similar to wild-type PDK1 (Fig. 5C). 2D tryptic maps derived from this experiment showed a pattern of phosphorylation similar to that of wild-type PDK1, including phosphorylation of spot A (Fig. 5E).
IGF-1-stimulated phosphorylation of S396.
The high level of constitutive phosphorylation of PDK1 probably accounts for the relatively small increase in total radioactivity upon IGF-1 treatment. One of the peptides shown on the 2D maps increased significantly with stimulation and might account for the relatively high level of stoichiometry required for a mobility shift. This peptide was subjected to phosphoamino acid analysis, which revealed serine phosphorylation (Fig. 4F). We individually replaced each of the potential phosphoserine residues previously reported (12) with alanine. S396A-PDK1 failed to undergo an IGF-1-stimulated mobility shift (Fig. 6A). As well, radiolabeled tryptic peptide A was missing from maps derived from S396A-PDK1 (Fig. 6B). S396D substitution resulted in a constitutive mobility shift (Fig. 6C). The constitutive mobility shift of S396D compared with that of wild-type PDK1 was not reduced by treatment with alkaline phosphatase (data not shown), indicating that this was a site of IGF-1-induced phosphorylation. Quantitatively, phosphorylation on S396 accounted for most of the increased radioactivity induced by IGF-1 stimulation (Fig. 6D). These results indicate that S396 is transiently phosphorylated following IGF-1 stimulation.
FIG. 6.
PDK1 phosphorylation at S396 following IGF-1 stimulation. (A) myc-PDK1 or myc-S396A-PDK1 was transfected into 293 cells for 24 h, serum starved for 18 h, and stimulated with IGF-1 or pervanadate (PV) for 15 min. PDK1 was immunoprecipitated with anti-myc 9E10 antibody and resolved by SDS-PAGE. Immunoblotting was performed with anti-PDK1 (top) or anti-phosphotyrosine 4G10 (bottom) antibody. (B) HEK 293 cells were transfected with myc-PDK1 or myc-S396A-PDK1 and labeled with 3[32P]orthophosphate for 4 h. Cells were stimulated with IGF-1 for 15 min and solubilized in ice-cold lysis buffer. PDK1 was immunoprecipitated with anti-myc 9E10 antibody, digested with trypsin, and resolved by 2D chromatography as described in the legend to Fig. 4 and Materials and Methods. (C) HEK 293 cells transiently expressing myc-PDK1, myc-S396A-PDK1, or myc-S396D-PDK1 were stimulated with IGF-1 for 15 min. Lysates were fractionated by SDS-PAGE, and PDK1 was detected by immunoblotting with anti-PDK1 antibody (Cell Signaling). (D) HEK 293 cells were transfected with myc-PDK1 or myc-S396A-PDK1, followed by labeling with [32P]orthophosphate for 4 h and stimulation with IGF-1. myc-PDK1 was immunoprecipitated with anti-myc 9E10 antibody and fractionated by SDS-PAGE. 32P-labeled PDK1 was visualized and quantitated with a PhosphorImager (Molecular Dynamics). Error bars represent the standard deviation of three independent experiments.
A recent report described the tyrosine phosphorylation of PDK1 on three sites (Y9, Y373, and Y376) and regulation of PDK1 activity in response to pervanadate treatment (20, 33). Other reports have shown that members of the Src family of tyrosine kinases phosphorylate PDK1 in vitro (35). We noted that Y373 and Y376 are proximal to the putative NES (amino acids 379 to 388) and thus could also play a role in the regulation of PDK1 nuclear translocation under some conditions. We were unable to directly observe tyrosine phosphorylation of PDK1 following IGF-1 stimulation, in contrast to pervanadate, which was a potent stimulator of tyrosine phosphorylation (Fig. 6A). We also noted that pervanadate induced a mobility shift of both wild-type and S396A-PDK1, although the latter was a step lower, indicating the absence of a phosphorylation event. This suggests that pervanadate induces the phosphorylation of PDK1 on S396 as well as tyrosine residues. It remains possible that growth factors normally increase tyrosine phosphorylation of PDK1, but this is below the level of detection or specificity of the anti-phosphotyrosine antibody 4G10.
The next set of experiments examined whether PDK1 could autophosphorylate on S396 in vitro. We transfected and isolated PDK1 from growth factor-starved 293 cells and performed an in vitro kinase reaction with [γ-32P]ATP. The labeled protein was digested with trypsin, followed by 2D phosphopeptide mapping. The maps of Fig. 7 show that PDK1 autophosphorylates on several residues. One site of autophosphorylation is likely T513 (spot c), based on comparison of our 2D phosphopeptide maps to those generated by Wick et al., who also recently showed that this residue of murine PDK1 is autophosphorylated in vitro (50). Another spot, labeled “b,” migrated very close to the S396-containing peptide and also underwent strong autophosphorylation. This peptide was weakly labeled during in vivo experiments, and its level of phosphorylation did not change with IGF-1 treatment. The S396-containing peptide (labeled “a”) underwent weak autophosphorylation compared with in vivo, IGF-1-stimulated phosphorylation. This spot was absent from autophosphorylated S396A-PDK1, while T513 and spot b were unaffected (Fig. 7A). Thus, PDK1 can autophosphorylate on S396 in vitro, albeit weakly.
FIG. 7.
2D tryptic mapping of in vitro-phosphorylated PDK1. (A) myc-PDK1 was expressed and immunoprecipitated from serum-starved HEK 293 cells. [γ-32P]ATP and MnCl2 were added, and samples were incubated at 30°C for 30 min. Reactions were stopped by the addition of 2× SDS sample buffer and boiling. PDK1 was fractionated by SDS-PAGE, excised from the gel, and digested with trypsin as described in Materials and Methods. Peptides were separated by chromatography and visualized with a PhosphorImager. The image on the left shows an in vivo-labeled PDK1 digestion. Spots labeled a, b, and c indicate areas of phosphorylation. (B) Comparison of rat, mouse, and human PDK1 sequence alignment surrounding the polyserine motif and S396. S393 has also been reported to be a phosphorylation site and is indicated. Potential phosphorylation sites carboxyl terminal to S396 are not conserved between species.
These results, along with those shown in Fig. 5, prohibit a mechanism involving cis-autophosphorylation of S396. In agreement, we note that the activation loop mutant S241A PDK1 displayed a mobility shift equivalent to that of wild-type PDK1 in response to IGF-1 (Fig. 5). Therefore, phosphorylation of catalytically inactive PDK1 occurs through trans-autophosphorylation by endogenous PDK1 or by a distinct serine kinase.
Phosphorylation of S396 regulates nuclear shuttling of PDK1.
So far, we have demonstrated that IGF-1 stimulation promotes transient phosphorylation of PDK1 on S396. Under these conditions, PDK1 also shuttles to the nucleus. The impact of nuclear PDK1 was assessed by examining two downstream targets, PKB and the FOXO3a transcription factor. Coexpression of PKB with wild-type PDK1 led to a similar pattern of staining in the cytoplasm (Fig. 8A). We also mutated PDK1 within the NES region (L380 and F383 both replaced by serine), which had been reported to disrupt nuclear export (28). Strikingly, when we coexpressed PKB with the NES mutant of PDK1 (mNES-PDK1), we found that a significant fraction of PKB colocalized with mNES-PDK1 in the nucleus. This suggests that nuclear PDK1 may influence the nuclear shuttling of PKB, but the mechanism remains unknown. However, these results demonstrate that PKB and PDK1 can colocalize in the nucleus.
FIG. 8.
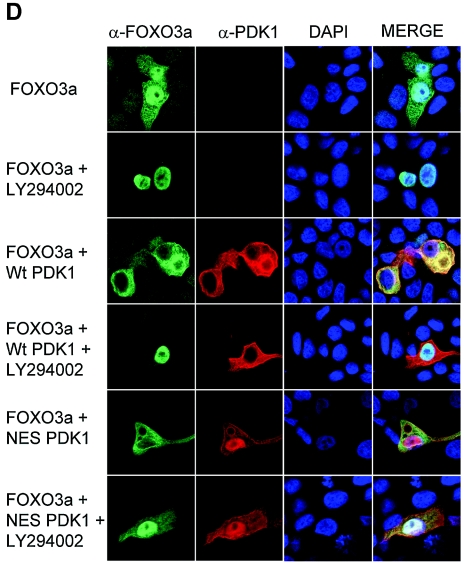
Nuclear PDK1 colocalizes with PKB and inhibits FOXO3a transcriptional activity and nuclear localization. (A) Wild-type PDK1 or mNES-PDK1 (50 ng) was cotransfected with HA-PKB (50 ng) on glass coverslips for 24 h. PDK1 and PKB were stained with anti-PDK1 (Cell Signaling Technologies) and anti-HA antibodies and visualized by confocal microscopy. (B) Cells were transfected with forkhead transcription factor FKHRL1 (FOXO3a; 25 ng), pGL2-Luciferase FOXO reporter plasmid (100 ng), and the indicated concentrations of wild-type PDK1. β-Galactosidase was coexpressed as a control for transfection. After 24 h of luciferase activity was measured and normalized to β-galactosidase activity. (C) Wild-type PDK1 or mNES-PDK1 at the indicated amounts (in nanograms) was cotransfected with FKHRL1 (FOXO3a; 25 ng) and the FOXO-responsive luciferase reporter plasmid (100 ng). After 24 h, luciferase activity was measured and normalized to β-galactosidase activity (top). Portions of the reserved lysates were immunoblotted for PDK1 (bottom). (D) FOXO3a and either empty vector, wild-type PDK1, or mNES-PDK1 where indicated were cotransfected into MCF-7 cells plated on glass coverslips. After 24 h, cells were treated for 2 h with LY294002 (25 μM) where indicated. Cells were stained with anti-FKHR and anti-PDK1 (both from Cell Signaling Technologies) and visualized by confocal microscopy.
We next asked how nuclear PDK1 might affect a direct target of PKB. FOXO3a is phosphorylated by PKB and other AGC kinases to cause its cytoplasmic redistribution. This reduces the transcriptional activity of FOXO3a and thus leads to a decrease in FOXO3a-transcribed genes. We coexpressed FOXO3a with a luciferase reporter plasmid containing the FOXO element from the IGFBP gene, along with wild-type PDK1 or mNES-PDK1. Increasing concentrations of wild-type PDK1 steadily reduced the transcriptional activity of FOXO3a, indicating that the elevated levels of PDK1 could repress FOXO3a (Fig. 8B). At about the 50% inhibitory concentrations, we compared wild-type PDK1 and mNES-PDK1. The mNES-PDK1 was significantly more potent at reducing FOXO3a activity than wild-type PDK1 (Fig. 8C). Immunoblotting of whole-cell lysates showed that wild-type PDK1 and mNES-PDK1 were expressed equally. These data indicate that mNES-PDK1 is more potent than wild-type PDK1 in suppressing FOXO3a activity.
We examined FOXO3a subcellular localization by confocal microscopy. FOXO3a was partially nuclear and cytoplasmic in serum-starved cells (Fig. 8D). Treatment of cells with LY294002, an inhibitor of PI3K, caused a complete translocation of FOXO3a to the nucleus, indicating that PI3K is necessary to maintain FOXO3a in the cytoplasm. We next coexpressed FOXO3a with PDK1 or mNES-PDK1. Both were able to cause a complete shift of FOXO3a from the nucleus to the cytoplasm (Fig. 8D). This agrees with our results with the FOXO3a report assay that wild-type PDK1 and mNES-PDK1 can suppress FOXO3a transcriptional activity. We also treated cells with LY294002 to block PI3K activity. Under these conditions, wild-type PDK1 was ineffective at maintaining a cytoplasmic redistribution of FOXO3a and was entirely nuclear (Fig. 8D). mNES-PDK1, on the other hand, was still able to maintain some FOXO3a in the cytoplasm, indicating that mNES-PDK1 can signal to FOXO3a in the absence of PI3K activity (Fig. 8D).
We next focused on the mechanism of the nuclear shuttling of PDK1 and whether this involved S396 phosphorylation, since S396 lies close to the putative CRM1-binding NES between amino acids 379 and 388. The HEK 293 cells we had used to characterize the phosphorylation of PDK1 were unsuitable for laser scanning microscopy. To test the effects of IGF-1 on nuclear accumulation of PDK1 and the role of S396, we utilized several other growth factor-sensitive cell lines, including PTEN−/− MEFs and the human mammary gland adenocarcinoma cell line MCF-7. In these cells, treatment with platelet-derived growth factor (PDGF) or IGF-1 stimulated a robust molecular weight band shift of endogenous PDK1 (data not shown), indicating that the signaling pathway upstream of PDK1 phosphorylation remained intact.
Ectopic transfection of cells with wild-type or S396A-PDK1 and visualization with laser scanning microscopy again revealed that PDK1 was mostly cytoplasmic, with very little nuclear staining for either (Fig. 9A). Leptomycin-B had the same effect on transfected PDK1 as did endogenous protein, causing a significant accumulation in the nucleus (Fig. 9A). Expression of mutant mNES-PDK1 revealed a pattern similar to that of leptomycin-B treatment, with a significant fraction of mNES-PDK1 localized to the nucleus (Fig. 9B). These results indicate that S396 phosphorylation is dispensable for the nuclear import of PDK1, but it could still regulate PDK1 export.
FIG. 9.
Effect of leptomycin-B and NES mutation of PDK1 on nuclear shuttling. (A) MCF-7 cells were plated on glass coverslips and transfected with myc-PDK1 or myc-S396A-PDK1 for 24 h. Cells were then treated with leptomycin-B for 3 h where indicated. Cells were fixed in 3% paraformaldehyde and stained with anti-myc 9E10 antibody, followed by Alexa-488-conjugated anti-mouse IgG (Molecular Probes). Images were collected with a Zeiss LSM 510 confocal microscope. (B) mNES-PDK1 or mNES-PDK1 containing various secondary mutations was transfected into MCF-7 cells growing on glass coverslips for 24 h. Cells were fixed with 3% paraformaldehyde, stained with anti-myc 9E10, and visualized as described above.
We also tested several other mutants in combination with the NES mutations. Both the PH domain mutant R474A mNES-PDK1 and kinase-dead mutant K111A mNES-PDK1 were similar in distribution to the NES mutations alone (Fig. 9B). We also noted the absence of plasma membrane localization with the R474A/NES mutation compared with the other proteins, consistent with the results shown in Fig. 4. Together these results suggest that neither lipid binding nor kinase activity is essential for PDK1 to accumulate in the nucleus if nuclear export is disrupted.
We next asked how S396 phosphorylation might impact nuclear shuttling of PDK1. PDK1 distribution was assessed in PTEN−/− MEFs treated with PDGF. Wild-type PDK1 showed an elevation in nuclear staining by 30 min that was sustained at 90 min (Fig. 10A). In contrast to wild-type PDK1, the S396A mutant PDK1 did not undergo increased nuclear accumulation following PDGF treatment. Thus, in PTEN−/− MEFs, signaling through the PDGF receptor caused nuclear accumulation of PDK1 that was sensitive to S396 mutation. We repeated this experiment with MCF-7 cells, and similar results were observed following IGF-1 stimulation for 30 min (Fig. 10B). We also generated green fluorescent protein (GFP)-PDK1 and mutants to assess the nuclear shuttling capability of this fusion protein. Similar to myc-PDK1, GFP-PDK1 underwent nuclear accumulation following IGF-1 stimulation in MCF-7 cells (Fig. 10C). This shift in nuclear GFP-PDK1 was abolished by mutation of S396 to alanine (Fig. 10C).
FIG. 10.
Nuclear shuttling of PDK1 requires S396. (A) PTEN−/− MEFs were plated on glass coverslips and transfected with myc-PDK1 or myc-S396A-PDK1 (100 ng) for 24 h, followed by serum starvation for 18 h. Cells were stimulated with PDGF (50 ng/ml) for the indicated times and fixed with 3% paraformaldehyde. Cells were stained with anti-PDK1 (Cell Signaling Technologies) and Alexa-488 conjugated to anti-rabbit IgG and examined by laser scanning confocal microscopy. (B) MCF-7 cells growing on glass coverslips were transfected with myc-PDK1 or myc-S396A-PDK1 (each, 100 ng) and stimulated with IGF-1 for 30 min. PDK1 was visualized as described for panel A. (C) Enhanced GFP-PDK1 or GFP-S396A-PDK1 was transfected into MCF-7 cells plated on glass coverslips. Transfected cells were serum starved for 18 h and then treated with IGF-1 (100 ng/ml) for 30 min. Cells were fixed with 3% paraformaldehyde and visualized by laser-scanning confocal microscopy. (D) Quantitation of nuclear PDK1 staining from a sample of MCF-7 cells treated as in panel B. Total nuclear fluorescence was determined with Zeiss LSM software, and the average value for the indicated number of cells examined is shown. The asterisk indicates significant difference with a P value of <0.0001 as determined with Student's paired t test.
Quantitatively, the amount of PDK1 shuttling to the nucleus was quite small compared with the amount detected in the nucleus in unstimulated cells. Analysis of nuclear staining of >50 MCF-7 cells showed that the amount of nuclear PDK1 was generally <2-fold smaller than the levels of PDK1 in serum-starved cells (Fig. 10D). Also, we could not detect a difference between the basal levels of nuclear wild-type PDK1 and S396A-PDK1 in serum-starved cells. This suggested to us that wild-type, non-S396-phosphorylated PDK1 accumulates to some extent in the nucleus, compared with high levels in the cytoplasm, perhaps due to overexpression of the protein. Alternatively, S396 phosphorylation could prime additional phosphorylation of the polyserine (S389-S396) motif, and this might still occur with the S396A mutant, but to a lesser extent.
DISCUSSION
PDK1 is the activation loop kinase of numerous protein kinases of the AGC kinase superfamily, including isoforms of the PKC family (32), S6K, RSK, SGK, and the three isoforms of PKB (31). The involvement of PDK1 in these distinct signaling modules points to an important and central role for PDK1 in cell signaling.
The regulation of PDK1 activity and specificity is becoming better understood. The kinase itself appears to be constitutively active, and the rate-limiting step for substrate phosphorylation appears to be dependent upon substrate conformation, which may include hydrophobic motif phosphorylation. Other means for regulating PDK1 could include subcellular targeting. Upon growth factor stimulation, PDK1 phosphorylates PKB efficiently at the plasma membrane, but it does not stimulate SGK phosphorylation localized to endosomes under the same conditions. Thus, while the PH domain of PDK1 is clearly required for the colocalization of PDK1 and PKB at the plasma membrane, other mechanisms could exist to target PDK1 to other subcellular locations to phosphorylate other substrates, including some that may be present in the nucleus. We embarked on this study to examine whether the subcellular targeting of PDK1 could be controlled by receptor signaling by directly regulating its phosphorylation.
We observed rapid and transient phosphorylation of PDK1 on S396 following IGF-1 stimulation. Previously, PDK1 was expertly mapped and shown to be phosphorylated on S396, as well as S393, S25, S241, and S410 (12). The total level of PDK1 phosphorylation did not change with IGF-1 stimulation in that study. It is possible that the increase in S396 phosphorylation that we observe could be masked behind the total phosphorylation on the other four serine residues. As well, the tryptic peptide containing S396 is 50 amino acids long and S396 is near the C terminus; thus, in the study by Casamayor et al. (12) additional digestion with Asp-N protease and 20 cycles of solid-phase sequencing were required to reach S396. Changes in phosphorylation levels could be difficult to detect, considering the low efficiency of this procedure. The IGF-1-induced phosphorylation of S396 was more apparent when the protein was analyzed by 2D tryptic mapping. The stoichiometry of IGF-1-induced S396 phosphorylation was high, since the molecular weight band shift of PDK1 following stimulation was completely lost in the S396A-PDK1 mutant. It is also possible that S396 phosphorylation primes additional phosphorylation events in the proximal polyserine motif between S389 and S396 and that the collective phosphorylation of these residues results in the band shift.
Translocation to the plasma membrane was a critical element of this process, because phosphorylation was sensitive to either an inactivating mutation in the PH domain or chemical inhibition of PI3K. Coexpression of PDK1 with a constitutively active, membrane-targeted PI3K (p110-CAAX) was sufficient to induce phosphorylation of PDK1. Artificially targeting PDK1 to the membrane via myristoylation was also sufficient to induce S396 phosphorylation, as well as other sites not yet characterized. Thus, S396 phosphorylation is tightly regulated in parallel with receptor tyrosine kinase signaling and PI3K activation. The catalytic activity of PDK1 does not appear to be regulated by S396 phosphorylation. Evidence for this is the in vivo phosphorylation of two substrates of PDK1, PKB and S6K. Both experienced phosphorylation on their activation loop residues by PDK1 and S396A-PDK1 (data not shown). This observation is consistent with the earlier characterization of PDK1 phosphorylation mutants by Alessi and colleagues (12).
IGF-1 and PDGF stimulated the nuclear shuttling of PDK1 in MCF-7 and PTEN−/− MEF cell lines. This process could involve transient phosphorylation of S396, because a S396A mutant PDK1 did not demonstrate similar nuclear retention in response to IGF-1 or PDGF. The basal level of nuclear PDK1 was very low under serum starvation conditions and increased by a small degree with receptor activation. These observations agree with the primary report of nuclear shuttling by Lim and coworkers (28). Thus, even under potent receptor tyrosine kinase signaling, PDK1 remains extranuclear to a large extent. This suggests that nuclear PDK1 must be actively exported by a mechanism suppressed by growth factor stimulation; even under optimal conditions, only a small fraction is retained in the nucleus. The stoichiometry of PDK1 phosphorylation was lower for ectopically expressed PDK1 than endogenous PDK1, which may account for the relatively small increase in nuclear PDK1. In this respect, endogenous PDK1 was observed to undergo nuclear accumulation to a greater degree than exogenously expressed PDK1.
Our studies also show that the PH domain of PDK1 is necessary for PDK1 nuclear shuttling (Fig. 4) but does not appear to be required under conditions where nuclear export is blocked. The NES mutant of PDK1 exhibited similar nuclear shuttling between the wild type and the PH domain mutant R474A, even though membrane localization was disrupted for this mutant (Fig. 9). Significantly, this suggests that PDK1 is not recruited to the nucleus through binding with a nuclear phosphoinositide pool but probably enters through some other mechanism.
The mechanism of S396 phosphorylation and how this regulates nuclear shuttling are of interest. IGF-1 stimulated the phosphorylation of S396 of kinase-dead PDK1, suggesting that S396 is targeted by a distinct serine kinase. Additionally, the phosphorylation of PDK1 was sensitive to the broadly specific protein kinase inhibitor staurosporine. The activity of the potential S396 kinase could be greatest at the plasma membrane, or PDK1 could be in favorable conformation in that location. Alternatively, it is possible that S396 phosphorylation occurs within the nucleus. In this scenario, PI3K activity and lipid binding with the PH domain are required for nuclear import of PDK1. Then, in the nucleus, PDK1 undergoes phosphorylation on S396, which suppresses CRM1-mediated export and promotes accumulation. Activity of the nuclear S396 kinase could therefore dictate the extent and duration of nuclear PDK1, balanced with dephosphorylation by S396-specific phosphatases.
Both hypotheses would predict that nuclear PDK1 is phosphorylated on S396 and possibly other sites to a greater extent in the nucleus than in the cytoplasm. In agreement with this, PDK1 isolated from the nuclear fraction was found to be hyperphosphorylated. Also, we have noticed that the mNES-PDK1 mutant migrates at a higher molecular weight than wild-type PDK1, although it is not clear yet if this shift is due to hyperphosphorylation of mNES-PDK1.
The physiological relevance of signal-directed nuclear targeting of PDK1 could be the phosphorylation of nuclear substrates. The FOXO family of transcription factors undergoes multisite phosphorylation in a complex mechanism of cytoplasmic-nuclear shuttling and transcriptional inactivation (11, 47). Three sites of phosphorylation are regulated by AGC kinases, including PKB and SGK (10), which are downstream targets of PDK1. One function of these sites is to sequester FOXO proteins to the cytoplasm through docking with 14-3-3 proteins, which may function to mask nuclear import mechanisms. Other phosphorylation sites may stabilize the association of the nuclear export complex of CRM1 and Ran, facilitating nuclear export. Increased levels of nuclear PDK1 may increase the activity of the AGC kinase-dependent phosphorylation of FOXO and promote nuclear export. In support of this hypothesis, we observed colocalization of the nuclear mNES-PDK1 mutant and nuclear PKB, which coincided with a marked decrease in FOXO transcriptional activity (Fig. 8), suggesting that mNES-PDK1 is more potent at inducing FOXO phosphorylation and nuclear exclusion. Future work will examine whether the subtle difference in nuclear shuttling of PDK1 resulting from S396 phosphorylation plays a role in transcription factor regulation.
In conclusion, the mechanism of nuclear-cytoplasmic shuttling of PDK1 may involve phosphorylation of S396, through signaling pathways activated by IGF and related growth factor receptors. One effect of S396 phosphorylation could be disruption of the CRM1-PDK1 complex that occurs prior to nuclear export. Activation of PI3K and a functional PH domain is necessary for nuclear shuttling, but the mechanism for nuclear import of PDK1 is not yet clear. Growth factors could promote S396 phosphorylation or might prime PDK1 for nuclear entry through a distinct mechanism, where it becomes a target for S396 phosphorylation, leading to nuclear accumulation. It is also possible that S396 phosphorylation primes other serine residues in the polyserine motif for phosphorylation, which could also regulate nuclear export. This mechanism could promote increased activity of PDK1 towards various nuclear targets, including PKB and FOXO transcription factors, and consequently alter gene expression.
.
Acknowledgments
M.P.S. was supported by a Canadian Institutes of Heath Research (CIHR) postdoctoral fellowship. This work was supported by grants to J.R.W. from the CIHR and the National Cancer Institute of Canada. J.R.W. acknowledges Senior Scientist support from CIHR and an International Scholarship from the Howard Hughes Medical Institute.
REFERENCES
- 1.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7:261-269. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., M. T. Kozlowski, Q. P. Weng, N. Morrice, and J. Avruch. 1998. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol. 8:69-81. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, K. E., J. Coadwell, L. R. Stephens, and P. T. Hawkins. 1998. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr. Biol. 8:684-691. [DOI] [PubMed] [Google Scholar]
- 4.Andjelkovic, M., S. M. Maira, P. Cron, P. J. Parker, and B. A. Hemmings. 1999. Domain swapping used to investigate the mechanism of protein kinase B regulation by 3-phosphoinositide-dependent protein kinase 1 and Ser473 kinase. Mol. Cell. Biol. 19:5061-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balendran, A., G. R. Hare, A. Kieloch, M. R. Williams, and D. R. Alessi. 2000. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 484:217-223. [DOI] [PubMed] [Google Scholar]
- 6.Biondi, R. M. 2004. Phosphoinositide-dependent protein kinase 1, a sensor of protein conformation. Trends Biochem. Sci. 29:136-142. [DOI] [PubMed] [Google Scholar]
- 7.Biondi, R. M., A. Kieloch, R. A. Currie, M. Deak, and D. R. Alessi. 2001. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 20:4380-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biondi, R. M., D. Komander, C. C. Thomas, J. M. Lizcano, M. Deak, D. R. Alessi, and D. M. van Aalten. 2002. High resolution crystal structure of the human PDK1 catalytic domain defines the regulatory phosphopeptide docking site. EMBO J. 21:4219-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biondi, R. M., and A. R. Nebreda. 2003. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem. J. 372:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunet, A., J. Park, H. Tran, L. S. Hu, B. A. Hemmings, and M. E. Greenberg. 2001. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell. Biol. 21:952-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgering, B. M., and G. J. Kops. 2002. Cell cycle and death control: long live Forkheads. Trends Biochem. Sci. 27:352-360. [DOI] [PubMed] [Google Scholar]
- 12.Casamayor, A., N. A. Morrice, and D. R. Alessi. 1999. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem. J. 342:287-292. [PMC free article] [PubMed] [Google Scholar]
- 13.Chou, M. M., W. Hou, J. Johnson, L. K. Graham, M. H. Lee, C. S. Chen, A. C. Newton, B. S. Schaffhausen, and A. Toker. 1998. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr. Biol. 8:1069-1077. [DOI] [PubMed] [Google Scholar]
- 14.Downward, J. 2004. PI 3-kinase, Akt and cell survival. Semin. Cell Dev. Biol. 15:177-182. [DOI] [PubMed] [Google Scholar]
- 15.Dutil, E. M., A. Toker, and A. C. Newton. 1998. Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1). Curr. Biol. 8:1366-1375. [DOI] [PubMed] [Google Scholar]
- 16.Filippa, N., C. L. Sable, B. A. Hemmings, and E. Van Obberghen. 2000. Effect of phosphoinositide-dependent kinase 1 on protein kinase B translocation and its subsequent activation. Mol. Cell. Biol. 20:5712-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fresno Vara, J. A., E. Casado, J. de Castro, P. Cejas, C. Belda-Iniesta, and M. Gonzalez-Baron. 2004. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 30:193-204. [DOI] [PubMed] [Google Scholar]
- 18.Frodin, M., T. L. Antal, B. A. Dummler, C. J. Jensen, M. Deak, S. Gammeltoft, and R. M. Biondi. 2002. A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation. EMBO J. 21:5396-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frodin, M., C. J. Jensen, K. Merienne, and S. Gammeltoft. 2000. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 19:2924-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grillo, S., T. Gremeaux, A. Casamayor, D. R. Alessi, Y. Le Marchand-Brustel, and J. F. Tanti. 2000. Peroxovanadate induces tyrosine phosphorylation of phosphoinositide-dependent protein kinase-1 potential involvement of src kinase. Eur. J. Biochem. 267:6642-6649. [DOI] [PubMed] [Google Scholar]
- 21.Hanada, M., J. Feng, and B. A. Hemmings. 2004. Structure, regulation and function of PKB/AKT—a major therapeutic target. Biochim. Biophys. Acta 1697:3-16. [DOI] [PubMed] [Google Scholar]
- 22.Hill, M. M., M. Andjelkovic, D. P. Brazil, S. Ferrari, D. Fabbro, and B. A. Hemmings. 2001. Insulin-stimulated protein kinase B phosphorylation on Ser-473 is independent of its activity and occurs through a staurosporine-insensitive kinase. J. Biol. Chem. 276:25643-25646. [DOI] [PubMed] [Google Scholar]
- 23.Jensen, C. J., M. B. Buch, T. O. Krag, B. A. Hemmings, S. Gammeltoft, and M. Frodin. 1999. 90-kDa ribosomal S6 kinase is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase-1. J. Biol. Chem. 274:27168-27176. [DOI] [PubMed] [Google Scholar]
- 24.King, C. C., and A. C. Newton. 2004. The adaptor protein Grb14 regulates the localization of 3-phosphoinositide dependent kinase-1 (PDK-1). J. Biol. Chem. 279:37518-37527. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi, T., and P. Cohen. 1999. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem. J. 339:319-328. [PMC free article] [PubMed] [Google Scholar]
- 26.Lawlor, M. A., and D. R. Alessi. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903-2910. [DOI] [PubMed] [Google Scholar]
- 27.Le Good, J. A., W. H. Ziegler, D. B. Parekh, D. R. Alessi, P. Cohen, and P. J. Parker. 1998. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281:2042-2045. [DOI] [PubMed] [Google Scholar]
- 28.Lim, M. A., C. K. Kikani, M. J. Wick, and L. Q. Dong. 2003. Nuclear translocation of 3′-phosphoinositide-dependent protein kinase 1 (PDK-1): a potential regulatory mechanism for PDK-1 function. Proc. Natl. Acad. Sci. USA 100:14006-14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McManus, E. J., B. J. Collins, P. R. Ashby, A. R. Prescott, V. Murray-Tait, L. J. Armit, J. S. Arthur, and D. R. Alessi. 2004. The in vivo role of PtdIns(3,4,5)P(3) binding to PDK1 PH domain defined by knockin mutation. EMBO J. 23:2071-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milburn, C. C., M. Deak, S. M. Kelly, N. C. Price, D. R. Alessi, and D. M. van Aalten. 2003. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem. J. 375:531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mora, A., D. Komander, D. M. van Aalten, and D. R. Alessi. 2004. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell Dev. Biol. 15:161-170. [DOI] [PubMed] [Google Scholar]
- 32.Newton, A. C. 2003. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J. 370:361-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, J., M. M. Hill, D. Hess, D. P. Brazil, J. Hofsteenge, and B. A. Hemmings. 2001. Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity. J. Biol. Chem. 276:37459-37471. [DOI] [PubMed] [Google Scholar]
- 34.Park, J., M. L. Leong, P. Buse, A. C. Maiyar, G. L. Firestone, and B. A. Hemmings. 1999. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 18:3024-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prasad, N., R. S. Topping, D. Zhou, and S. J. Decker. 2000. Oxidative stress and vanadate induce tyrosine phosphorylation of phosphoinositide-dependent kinase 1 (PDK1). Biochemistry 39:6929-6935. [DOI] [PubMed] [Google Scholar]
- 36.Pullen, N., P. B. Dennis, M. Andjelkovic, A. Dufner, S. C. Kozma, B. A. Hemmings, and G. Thomas. 1998. Phosphorylation and activation of p70s6k by PDK1. Science 279:707-710. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Viciana, P., and J. Downward. 2001. Ras activation of phosphatidylinositol 3-kinase and Akt. Methods Enzymol. 333:37-44. [DOI] [PubMed] [Google Scholar]
- 38.Scheid, M. P., P. A. Marignani, and J. R. Woodgett. 2002. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol. Cell. Biol. 22:6247-6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheid, M. P., and J. R. Woodgett. 2003. Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett. 546:108-112. [DOI] [PubMed] [Google Scholar]
- 40.Sonnenburg, E. D., T. Gao, and A. C. Newton. 2001. The phosphoinositide-dependent kinase, PDK-1, phosphorylates conventional protein kinase C isozymes by a mechanism that is independent of phosphoinositide 3-kinase. J. Biol. Chem. 276:45289-45297. [DOI] [PubMed] [Google Scholar]
- 41.Stambolic, V., A. Suzuki, J. L. de la Pompa, G. M. Brothers, C. Mirtsos, T. Sasaki, J. Ruland, J. M. Penninger, D. P. Siderovski, and T. W. Mak. 1998. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95:29-39. [DOI] [PubMed] [Google Scholar]
- 42.Stephens, L., K. Anderson, D. Stokoe, H. Erdjument-Bromage, G. F. Painter, A. B. Holmes, P. R. Gaffney, C. B. Reese, F. McCormick, P. Tempst, J. Coadwell, and P. T. Hawkins. 1998. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279:710-714. [DOI] [PubMed] [Google Scholar]
- 43.Stocker, H., M. Andjelkovic, S. Oldham, M. Laffargue, M. P. Wymann, B. A. Hemmings, and E. Hafen. 2002. Living with lethal PIP3 levels: viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science 295:2088-2091. [DOI] [PubMed] [Google Scholar]
- 44.Storz, P., and A. Toker. 2002. 3′-Phosphoinositide-dependent kinase-1 (PDK-1) in PI 3-kinase signaling. Front. Biosci. 7:d886-d902. [DOI] [PubMed] [Google Scholar]
- 45.Tamaoki, T., H. Nomoto, I. Takahashi, Y. Kato, M. Morimoto, and F. Tomita. 1986. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem. Biophys. Res. Commun. 135:397-402. [DOI] [PubMed] [Google Scholar]
- 46.Thomas, C. C., M. Deak, D. R. Alessi, and D. M. van Aalten. 2002. High-resolution structure of the pleckstrin homology domain of protein kinase b/akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr. Biol. 12:1256-1262. [DOI] [PubMed] [Google Scholar]
- 47.Tsai, W. C., N. Bhattacharyya, L. Y. Han, J. A. Hanover, and M. M. Rechler. 2003. Insulin inhibition of transcription stimulated by the forkhead protein Foxo1 is not solely due to nuclear exclusion. Endocrinology 144:5615-5622. [DOI] [PubMed] [Google Scholar]
- 48.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346:561-576. [PMC free article] [PubMed] [Google Scholar]
- 49.Wick, M. J., F. J. Ramos, H. Chen, M. J. Quon, L. Q. Dong, and F. Liu. 2003. Mouse 3-phosphoinositide-dependent protein kinase-1 undergoes dimerization and trans-phosphorylation in the activation loop. J. Biol. Chem. 278:42913-42919. [DOI] [PubMed] [Google Scholar]
- 50.Wick, M. J., K. R. Wick, H. Chen, H. He, L. Q. Dong, M. J. Quon, and F. Liu. 2002. Substitution of the autophosphorylation site Thr516 with a negatively charged residue confers constitutive activity to mouse 3-phosphoinositide-dependent protein kinase-1 in cells. J. Biol. Chem. 277:16632-16638. [DOI] [PubMed] [Google Scholar]
- 51.Yang, J., P. Cron, V. Thompson, V. M. Good, D. Hess, B. A. Hemmings, and D. Barford. 2002. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol. Cell 9:1227-1240. [DOI] [PubMed] [Google Scholar]
- 52.Yang, Z. Z., O. Tschopp, A. Baudry, B. Dummler, D. Hynx, and B. A. Hemmings. 2004. Physiological functions of protein kinase B/Akt. Biochem. Soc. Trans. 32:350-354. [DOI] [PubMed] [Google Scholar]