We present the first oxidative physiology values of two species of conservation need, Arctic terns and long-tailed jaegers. Both migrate long distances after nesting on tundra, a habitat impacted by climate change. Terns exhibited better oxidative integration than jaegers, although physiology was not related to migration metrics within either species.
Keywords: migration, oxidative stress, seabirds
Abstract
Arctic ecosystems are changing rapidly. The tundra supports nesting migratory seabirds that spend most of their year over the ocean. Migrations are demanding, but it is unclear how physiological capability may equip organisms to respond to their changing environments. For two migratory seabird species nesting in Alaska, USA, the Arctic tern (n = 10) and the long-tailed jaeger (n = 8), we compared oxidative physiology and aerobic capacity measured during incubation and we recorded individual movement paths using electronic tracking tags. Within species, we hypothesized that individuals with longer-distance migrations would show higher oxidative stress and display better aerobic capacity than shorter-distance migrants. We examined blood parameters relative to subsequent fall migration in jaegers and relative to previous spring migration in terns. We present the first measurements of oxidative stress in these species and the first migratory movements of long-tailed jaegers in the Pacific Ocean. Arctic terns displayed positive correlation of oxidative variables, or better integration than jaegers. Relative to physiological sampling, pre-breeding northward migration data were available for terns and post-breeding southward data were available for jaegers. Terns reached a farther maximum distance from the colony than jaegers (16 199 ± 275 km versus 10 947 ± 950 km) and rate of travel northward (447 ± 41.8 km/day) was positively correlated with hematocrit, but we found no other relationships. In jaegers, there were no relationships between individuals’ physiology and southward rate of travel (193 ± 52.3 km/day) or migratory distance. While it is not clear whether the much longer migrations of the terns is related to their better integration, or to another factor, our results spark hypotheses that could be evaluated through a controlled phylogenetic study. Species with better integration may be less susceptible to environmental factors that increase oxidative stress, including thermal challenges or changes in prey distribution as the Arctic climate changes rapidly.
Introduction
Climate change is altering the composition, structure and functioning of terrestrial and marine ecosystems in ways that may be abrupt and irreversible (IPCC, 2021). Arctic and alpine tundra are among the most rapidly changing terrestrial ecosystems (Post et al., 2009; Anderegg and Diffenbaugh, 2015) and in the oceans, many species have already shifted their migration patterns and distributions (IPCC, 2021). Arctic seabirds link these two changing biomes through long-distance migrations from tundra nesting habitat to marine post-breeding habitats. Migration and nesting are physiologically demanding periods of avian annual cycles, but for many Arctic species, baseline information on organismal physiology remains unknown.
The physiological capability of an animal has the potential to dramatically affect the way it uses habitat, including foraging and reproductive decisions (Costa and Sinervo, 2004; Madliger et al., 2018). Calls have been made for greater consideration of physiology in conservation, including the need to investigate how physiological metrics may assist in predicting organismal sensitivity to global change (Cooke et al., 2021). As climate change continues to alter oceans and tundra habitats, such information is important for understanding how potential ecosystem changes may impact the health, foraging and reproductive success of animals like long-distance migratory seabirds that capitalize on both systems. Oxidative stress has been identified as a tool in the conservation physiology “toolbox” for predicting and monitoring responses to environmental change (Madliger et al., 2018). It is understood that oxidative stress affects many aspects of an animal's health, but additional data are needed to predict how it may affect an animal's response to a changing environment (Beaulieu and Costantini, 2014; Cooper-Mullin and McWilliams, 2016; Skrip and McWilliams, 2016; Costantini et al., 2019). Thus, quantifying oxidative status of organisms in relation to life stages is an important first step (Beaulieu and Costantini, 2014).
Oxidative stress is an imbalance between the damaging by-products of aerobic metabolism (reactive oxygen species, ROS) and the molecules that can protect cells from these damaging by-products (antioxidants) (Sies, 1991; Jones, 2006; Beaulieu and Costantini, 2014; Costantini, 2016) and is a consequence of requiring oxygen for aerobic metabolism. This requirement leads to the generation of oxidizing agents, collectively called reactive oxygen species (Finkel and Holbrook, 2000; Costantini et al., 2010) that can damage cellular components including membrane lipids, proteins and nucleic acids (reviewed in (Costantini, 2016). However, antioxidant molecules can in turn, minimize or prevent oxidative damage (Costantini et al., 2010; Cooper-Mullin and McWilliams, 2016). Antioxidants take both enzymatic and non-enzymatic forms and may include vitamins and uric acid, a byproduct of protein catabolism (Cohen and McGraw, 2009).
There are four contributors to oxidative stress: 1) the production of free radicals (unstable biochemical species with one or more unpaired electrons, 2) the damage they cause, including the production of subsequent molecules that further oxidative damage, 3) antioxidant conditions and 4) repair mechanisms (Costantini, 2008; Monaghan et al., 2009). Measuring all four aspects is extremely difficult in free-ranging animals, and it is rare to find data on free radical production and repair mechanisms. ROS, in our study measured as organic hydroperoxides, are considered to be biomolecules that have been damaged by free radicals and reflect a measure of damage because they are the products of oxidative damage (Costantini, 2016). They may then also participate in an oxidative cascade by continuing to produce more free radicals, as well as damage other cellular components, such as lipids, proteins and nucleic acids (Costantini et al., 2010; Skrip and McWilliams, 2016).
The components of oxidative stress are dynamic. Organisms experiencing fluctuations in the production of ROS may counteract with antioxidants, which can be obtained through diet, or produced endogenously (Cooper-Mullin and McWilliams, 2016). The dynamic nature of oxidative stress means that it may not be in balance at all times, and if ROS production is not matched by antioxidants, organisms may experience oxidative stress (Costantini and Verhulst, 2009; Monaghan et al., 2009). The way in which these components interact can be evaluated through integration, which is the concept that the components of a biological system will demonstrate some inter-relationships related to functionality (Ravasz et al., 2002; Cohen et al., 2012). Integration is often viewed as a network and evaluated via correlation matrices of physiological variables (Mitteroecker and Bookstein, 2007; Cohen and McGraw, 2009). Comparing the inter-relationships between oxidative physiology components across species can help provide insight into the management of their oxidative physiology (Cohen et al., 2012; Costantini et al., 2013). Life history variables (reproduction, senescence, survival) can be affected if organisms are not adept at balancing resources (energetic or non-energetic) dedicated to addressing oxidative status (Alonso-Alvarez et al., 2006, 2017; Monaghan et al., 2009; Costantini et al., 2016).
The exact time frame over which oxidative perturbations may be detected and linked to environmental or life history variables remains uncertain. Some studies have detected oxidative stress perturbations on the order of 18 to 24 hours (migrating bats (Costantini et al., 2019)) to several weeks after thermal challenges (bats (Beaulieu et al., 2020)) to years (lifespan in seabirds (Costantini and Dell’Omo, 2015)). Therefore, oxidative stress values may reflect acute challenges or be integrative of longer term challenges.
Multiple studies of breeding birds have demonstrated links between oxidative stress and current or future reproduction. Studies in songbirds have demonstrated disruptions in oxidative homeostasis relative to clutch size, lay date and provisioning effort (Guindre-Parker et al., 2013; Costantini et al., 2016; Fowler and Williams, 2017; Pap et al., 2018). In seabirds, (Scopoli's shearwater and European shags) measurements of oxidative damage in the chick rearing period were linked to shorter lifespans and reduced survival (Costantini and Dell'Omo, 2015; Herborn et al., 2016). In addition to the effect of reproduction, oxidative stress can also be affected by food availability (Crommenacker et al., 2011), fasting (Schull et al., 2016), aging, stress (Hau et al., 2015), inflammation and exercise (Powers and Jackson, 2008; Nikolaidis et al., 2012).
Aerobic capacity refers to potential for oxygen uptake (Bech and Klaassen, 1996). Oxygen transport involves many factors, including oxygen intake in the lungs, transport through the circulatory system and utilization by muscles (Wagner, 1996; Williams et al., 2012). Red blood cells are critical, as they contain the pigment hemoglobin, which binds to oxygen to carry in through the vascular system to the tissues. Although not the only determinants of aerobic capacity, hematocrit or packed red blood cell volume (Yap et al., 2018) and hemoglobin (Calbet et al., 2006) are key determinants of oxygen transport and can affect the ability to sustain intense workloads (Williams, 2012), such as migration. These metrics can vary in birds, due to some combination of environmental effects and physiological variables, such as reproduction (Williams, 2012).
Organisms undergoing long distance migration may be at risk for increased oxidative stress (Cooper-Mullin and McWilliams, 2016). Fuel quality and antioxidant content before and during stopovers (periods of rest or refueling during migration) may affect oxidative stress levels (Pierce and McWilliams, 2014; Skrip and McWilliams, 2016). Migrating bats incur oxidative stress due to flight (Costantini et al., 2019) and migrating birds, highly dependent on lipid to fuel their flight, may face an unavoidable cost of oxidative damage (Skrip et al., 2015). The occurrence of oxidative damage and ability to access antioxidants could limit the flexibility of foraging/migration strategies (Jenni-Eiermann et al., 2014). However, more data are needed to link oxidative stress to foraging or migration performance. In non-migratory species, flight performance has been linked to oxidative stress (Costantini, 2008; Larcombe et al., 2008; Costantini et al., 2013; DeMoranville et al., 2022), but quantifying oxidative stress during migratory flight is challenging because animals are difficult to access mid-migration unless at a stopover site, especially for species that migrate over oceans. Studies in songbirds at their stopover sites showed increased antioxidants (blackbirds; (Eikenaar et al., 2017)), increased oxidative damage (garden warblers; (Skrip et al., 2015)) or both increased oxidative damage and antioxidants (European robins (Jenni-Eiermann et al., 2014)). Conversely, Northern bald ibis showed no oxidative changes during flight (Bairlein et al., 2015). Seabirds are underrepresented in field studies linking oxidative stress with movement metrics (foraging or migration), with only penguin oxidative stress linked to foraging (Beaulieu et al., 2010; Colominas-Ciuró et al., 2017, 2021).
In this study, we quantified oxidative stress and aerobic capacity of two species of highly migratory seabirds during the incubation period: long-tailed jaegers (Stercorarius longicaudus, ~ 350 g, also called long-tailed skuas) and Arctic terns (Sterna paradisaea, ~ 100 g). We were also interested in relating individual migration metrics to their oxidative physiology and explored this by following the migrations and foraging movements of terns and jaegers over the Pacific and Southern Oceans using electronic tags. Both species are relatively small and highly acrobatic seabirds that make long-distance migrations to the Southern Hemisphere from their Arctic and sub-Arctic breeding habitats, have a circumpolar breeding distribution and in some places, breed sympatrically (Harrison et al., 2021; Wong et al., 2021). Both Arctic terns and long-tailed jaegers are listed as “species of greatest conservation need” within Alaska with Arctic terns being listed for every bioregion in the state (Alaska Dept of Fish and Game, 2015). Long-tailed jaegers are also considered by the State of Alaska to be both sentinel species (indicators of environmental change) and stewardship species (species with a high percentage of their North American or global populations in Alaska). We were interested in physiological measures of to inform multiple aspects of Arctic seabird conservation and management including 1) establishing first measures for the oxidative physiology of these two species to inform future biomarker monitoring in rapidly changing Arctic and ocean habitats; 2) collecting measures that could be incorporated into population health assessments in lieu of direct demographic information; 3) providing foundational analyses for generating broader hypotheses about the relationship between integration, migration capability and predicted responses to environmental challenges across seabird species.
Recent papers linking oxidative physiology to population health note that in situations where populations or ecosystems may be difficult to monitor, but sentinel individuals are relatively easy to catch, physiological measures could be bioindicators of population health if incorporated into conservation planning (Adams and Ham, 2011; Beaulieu et al., 2013; Bergman et al., 2019). No firm population estimates exist for jaegers, and for terns, population estimates for interior Alaska are especially deficient (U.S. Fish and Wildlife Service, 2009). Terns are known to redistribute colonies from year-to-year, or to skip breeding when conditions are poor (Egevang and Frederiksen, 2011; Mallory et al., 2017; Scopel and Diamond, 2018). This diffuse nesting structure, and potential for redistribution, makes population monitoring difficult and expensive to obtain, even via a sampling approach, across the vast and remote Alaskan Arctic.
While most jaeger nesting habitat in Alaska is low-lying coastal tundra, there is an isolated inland breeding population of long-tailed jaegers in montane tundra in Denali National Park (where 6 of our 8 birds were sampled). Despite being located within a National Park, this population is difficult to survey due to the remote nature and large size of the Park, making the detection of declines or distribution shifts very difficult (McIntyre, 2007). Jaegers are important predators in the Arctic food web (Gilg et al., 2003; Krebs et al., 2003). They consume small mammals (voles and lemmings), shorebirds and songbirds. Structured interviews of those with 20 to 51 years of experience in the Park noted declines of long-tailed jaegers and American golden-plovers (a jaeger prey/host species) within the Park (Meeker et al., 2022). Climate change effects, including elevation-dependent warming (Pepin et al., 2015), include a pattern of woody plant encroachment on Arctic tundra in Alaska (Terskaia et al., 2020; Raiho et al., 2022), including moving higher in elevation to the montane tundra jaeger and shorebirds use as nesting habitat in the park. Thus, transition of tundra to shrub and woody vegetation, increased tourism, effects to prey species and general warming of the Arctic, all have the potential to disrupt both species’ nesting success and in turn, Arctic and sub-Arctic food webs. Seabirds in general are characterized by having low reproductive rates (Dobson and Jouventin, 2007) and are vulnerable to cumulative impacts that may reduce adult survival or otherwise decrease reproductive rates (Sydeman et al., 2021). Given similarities in many aspects of the two species life histories, and shared threats to their nesting habitat, collecting baseline information of these species' physiological status during their nesting season is essential.
Away from nesting habitats, both species switch to an oceanic lifestyle. The migrations of Arctic terns include the longest recorded animal migration of 80 000 km round-trip (Egevang et al., 2010) from the Arctic tundra to foraging grounds off Antarctica. Most populations in the Atlantic Ocean use the same migratory routes (Wong et al., 2021) while in the Pacific Ocean, birds migrating from Alaskan breeding grounds largely stayed in the eastern Pacific Ocean during migration (McKnight et al., 2013; Duffy et al., 2014). No studies of the oxidative physiology of Arctic terns have been published, although Costantini (2008) speculated on the potential relationship between extreme migration, longevity and flight related oxidative stress in the species. The mitochondrial DNA of Arctic terns was recently sequenced (Skujina et al., 2019) and may provide insight into metabolic adaptations, but no relationships have been elucidated as of yet.
Atlantic Ocean migration patterns of long-tailed jaeger populations are well studied. Birds from breeding grounds in the central Canadian Arctic (Harrison et al., 2021), and Greenland and northern Scandinavia make annual migrations to west and southern Africa (Gilg et al., 2013; van Bemmelen et al., 2017) and can cover as much as 900 km/day (Sittler et al., 2011). However, there are no migration studies of long-tailed jaegers that use the Pacific Ocean; distribution data show sightings as far south as New Zealand and Chile (Furness, 1987; Wiley and Lee, 2020). No information is currently available about long-tailed jaeger oxidative physiology.
Birds like Arctic terns and long-tailed jaegers that fly great distances may have a physiological oxidative cost, which may carry over into other life history phases; although the timing of these physiological signals’ persistence is uncertain. Our goals were to 1) establish baseline incubation oxidative stress and aerobic capacity of two seabird species that link the Arctic region to marine habitats through long migrations, but that we hypothesize differ in their migration distance, 2) evaluate the integration of each species’ physiological capacity and 3) test the hypothesis that within species, individual migration distance and rate of travel are positively correlated with their incubation oxidative stress.
Methods
Field methods
We captured adult Arctic terns and long-tailed jaegers in Alaska, USA during the late-incubation period of nesting (mid-June to early July 2018) with manually triggered bownet traps (Bub, 1991) set at nests. The eggs remained in the nest during processing and were not harmed. Typically, upon capture of one member of the nest, the mate would take its place incubating the eggs while we processed the captured individual. We recorded standard morphometrics (mass, flattened wing chord, diagonal tarsus, bill and total head plus bill), collected three breast feathers for DNA sexing (Animal Genetics, Tallahassee, FL, USA) and took blood samples (see below). All birds were fitted with a USGS Bird Banding Laboratory metal band.
Ten Arctic terns (four females, two males, four unknown) were captured in coastal tundra near Alpine, Alaska, USA. Full migration details are reported in Wong et al. (2021). Six individuals were previously captured in 2017 and tracked with 0.65 g archival light-level geolocators (Migrate Technology Ltd, Cambridge, UK, model W65). Tags were wound with self-amalgamating tape and attached to a Darvic PVC color-band on the leg with a miniature UV-resistant zip-tie. The final attachment weighed 1 g total mass (<2% of the body weight of the tern). Upon re-capture in 2018, we removed geolocators and color-bands, recorded morphometrics as described above and took feather and blood samples. We also newly captured and sampled an additional four individuals—these birds were banded with USGS metal bands upon capture but no previous tracking devices were carried by these birds, and no new devices were deployed. These birds were sampled to obtain additional baseline physiology measures.
Eight jaegers (four nesting pairs, four males, and four females) were captured, sampled and fitted with satellite tags. We captured six individuals in sub-Arctic alpine tundra habitat in Denali National Park and Preserve, Alaska, USA (63.1148° N, 151.1926° W) and two individuals in tundra habitat of the Arctic coastal plain near Alpine Alaska, USA (70.3281° N, 150.9775° W). The long-tailed jaeger nest from Alpine, Alaska had an unwitnessed depredation prior to capture; upon capture we first saw the nest contained only eggshell fragments. Both birds continued to exhibit incubation behavior by sitting on the nest as if eggs were still present. We attached 5-g Argos solar-powered satellite tags (Microwave Telemetry Inc. Columbia, MD USA; actual tag mass, 4.48–4.57 g; mean = 4.5 g) to the birds using a leg-loop harness (Mallory and Gilbert, 2008) made of either 4.7625 mm tubular Teflon (n = 5) Ribbon or 2.54 mm Spectra (n = 3)), both from Bally Ribbon Mills, and secured with aluminum crimps. Tags were duty cycled to transmit for 10 hours, and to charge for 48 hours, but were also programmed to remain transmitting when fully charged and thus often transmitted daily. The final attachment weighed a mean of 4.8 to 6.0 g (1.7–2.1% of the body weight of the birds). We assessed wing and leg mobility prior to release. These tags are not recovered and remain on the bird until the harness material degrades, the tag is shed due to weight changes, the tag stops functioning or charging, or the bird dies. Tags often cease transmission over ocean and fate is typically unknown.
We sampled blood (~ 0.5 mL) from the brachial vein and collected into a heparinized microcentrifuge tube after birds were fitted with bands and tags and prior to release. Samples were stored on ice until return to the field station. Upon return to the field station, we measured hematocrit, aliquoted whole blood to hemoglobin reagents (see below) and separated plasma by centrifuge (LW Scientific ZipCombo) at 12 000 rpm for 5 minutes. Plasma samples were frozen at −20°C until shipped on dry ice, where they were stored at −80°C until laboratory analysis. Hemoglobin samples were protected from light and refrigerated until analysis. Processing time from capture to blood sampling prior to release ranged from 32 to 60 minutes and we assessed the effect of holding time on oxidative variables by looking for correlations between oxidative physiology variables and time to sampling. There is variability in previous studies regarding the effect of restraint on oxidative variables. In domestic chickens, antioxidants in some birds increased dramatically after 1 hour of restraint, but in other birds decreased dramatically (Cohen et al., 2007). Oxidative stress appears to be stable (no variation) for over 30 minutes of restraint based on studies of barn swallows, garden warblers, snow buntings and house sparrows (Costantini et al., 2007; Guindre-Parker et al., 2013; Huber et al., 2017). Restrained bats showed no change over 77 minutes of restraint (Beaulieu et al., 2020). King penguins showed variation in oxidative stress after 30 minutes of capture, but overall did not support the idea that acute stress has strong effects on oxidative damage (Stier et al., 2019).
After release, we monitored birds visually to assess flight and monitored the return to the nest. All nests continued to be incubated after capture with the exception of the jaeger nest that had been depredated prior to capture. We do not have nest success (hatch and fledging) for most nests because we did not want to disturb the nest site further and in some cases we departed the field site prior to hatch.
Trapping, handling, marking and processing birds was done under permits from the Alaska Department of Fish and Game (Alaska Scientific permit 17-162 and 18-156 to A.-L. Harrison), the U.S. Geological Survey Bird Banding Laboratory (permit 09700 to P.P. Marra). Animal care protocols were approved by the Institutional Animal Care and Use Committees of the National Zoological Park (protocol 15-33 and 18-06, P.I. A.-L. Harrison) and the National Park Service (AKR_DENA_Harrison_Jaegers_2018.A2, P.I. A.-L. Harrison).
Laboratory analyses
We analysed a set of variables indicative of aerobic capacity (hematocrit and hemoglobin), oxygen metabolites (plasma reactive oxygen metabolites) and antioxidants (plasma non-enzymatic antioxidant levels and plasma uric acid). We measured hematocrit (% packed cell volume), hemoglobin (g·dl−1), plasma non-enzymatic antioxidant levels (μmol HClO mL−1; OXY), plasma reactive oxygen metabolites (mg H2O2 dL−1; ROM) and plasma uric acid (mg∙dL−1) as described in (Fowler et al., 2018). Briefly, hematocrit was measured following centrifugation for 3 minutes at 7500 g, using digital calipers (± 0.01 mm). We then determined the percentage of packed red cell volume to total column height (plasma plus packed red cell volume). Sample size for hematocrit: terns n = 10, jaegers n = 8. We measured hemoglobin (g∙dL−1 whole blood) using the cyanomethemoglobin method (Drabkin and Austin, 1932) modified for use with a microplate spectrophotometer, using 5 μL whole blood diluted in 1.25-mL Drabkin’s reagent (D5941 Sigma-Aldrich) with absorbance measured at 540 nm (following (Wagner et al., 2008b)). Pointe Scientific standards were used (H7506-STD). Mean intra-assay coefficient of variation was 1.8% and mean inter-assay coefficient of variation was 1.3%. Sample size for hemoglobin: terns n = 10, jaegers n = 7. Uric acid (mg∙dL−1) was measured with the Bioassay Quantichrom assay (DIUA-250) following the manufacturer’s guidelines. Samples were assayed in duplicate using 5-μL sample volume following 1:3 dilution with dd H2O. All samples were measured in one plate and mean intra-assay coefficient of variation was 4.9%. Sample size for uric acid: terns n = 8, jaegers n = 7.
For plasma reactive oxygen metabolites and plasma non-enzymatic antioxidants, we used oxidative stress assays that have been previously validated for avian sampling (Costantini, 2016; Skrip and McWilliams, 2016). Reactive oxygen metabolites (mg H2O2 dL−1; ROM) were measured using the dROM kit from Diacron International, Italy (catalog number MC001), modified after (Guindre-Parker et al., 2013). All samples were measured in one plate and mean intra-assay coefficient of variation was 4.4%. Sample size for ROM: terns n = 8, jaegers n = 6. Total non-enzymatic antioxidant titers (μmol HClO mL−1; OXY) were determined using the OXY kit from Diacron International, Italy (catalog number MC434), modified after (Guindre-Parker et al., 2013). All samples were measured in one plate and mean intra-assay coefficient of variation was 7.8%. Sample size for OXY: terns n = 9, jaegers n = 7. Uric acid’s antioxidant capacity is not captured by the OXY assay and thus it is not “double” counted. The OXY assay does not need to be corrected for uric acid levels, it is distinct from the antioxidant capacity of uric acid (Costantini, 2011).
Movement path estimation
We used movement models that account for the error associated with each tag type to convert raw data collected by electronic tags into location estimates. Differences in tag types necessitated different methods for processing their movement data. Light-level and sea-surface temperature data recorded by geolocator tags carried by Arctic terns were processed using the R statistical computing program (R Core Team, 2018) packages TwGeos (Lisovski, 2016) and SGAT (Wotherspoon et al., 2013); full details in (Wong et al., 2021). When light-level is the same across the globe (for example, during periods of the equinox), or does not vary during a day (for example, during periods of 24-hour daylight at the poles), locations cannot be resolved. Therefore, Arctic tern migration paths are truncated; movements in the Arctic Circle and a period of the Southern Ocean residency for some individuals were unavailable.
Argos satellite data for long-tailed jaegers were processed using a continuous-time movement model (Jonsen et al., 2020) following details in (Harrison et al., 2021) and implemented in package foieGras for R.
Differences in sampling frequency of electronic tags can greatly affect distance estimates of a movement path (Noonan et al., 2019). Because we were interested in comparing migration distances, we standardize model predictions to one position per day across tag types (the finest time frame available for geolocator devices).
Timing of physiological sampling relative to timing of migration data
This study was part of a larger study quantifying migration patterns in tundra breeding birds. Due to field logistics for the larger study goals, we had limited control over the sampling opportunities relative to tag deployment or recovery. Although both species were sampled for physiological variables during the same year and breeding stage (2018, incubation), the timing of physiological sampling relative to the timing of tag attachment differed between species. For Arctic terns, physiological sampling occurred during a recapture-event when archival tags were removed from the bird and the data were downloaded from the tag. Thus, the movement record is from the year prior to physiological sampling. For long-tailed jaegers, samples were taken at time of satellite tag attachment (which then transmitted remotely indefinitely and was not removed in the future) and we recorded movement data during the year after physiological sampling. For each species, we chose the migration period closest in time to physiological sampling to relate individual physiology with individual migration metrics. For terns we analysed the northward (spring) portion of the migration track, just prior to its arrival at the breeding colony. Thus, relationships between migration metrics and physiology may reflect the effect of pre-breeding migration on breeding physiology this species. For jaegers we analysed the southward migration, after the bird left the breeding colony in the fall, allowing us to evaluate the effect of breeding physiology on post-breeding migration. Many bird species differ in their rates of travel and migration distances between the northbound and southbound migrations due to seasonal variation in the environment (for example, prevailing winds or environmental perturbations such as El Niño Southern Oscillation) and timing of key life history events relative to the migration (for example, migrating to select a nest site and find a mate vs. migrating after breeding). In Arctic terns, for example, the pre-breeding migration was faster and more direct than the post-breeding migration in the Pacific Ocean (Wong et al., 2021) which could have different effects on tern physiology. Given that we analysed different migratory periods for the two species, we could not make comparisons between the species regarding the relationship between individual physiology and their migration metrics.
Migration metrics
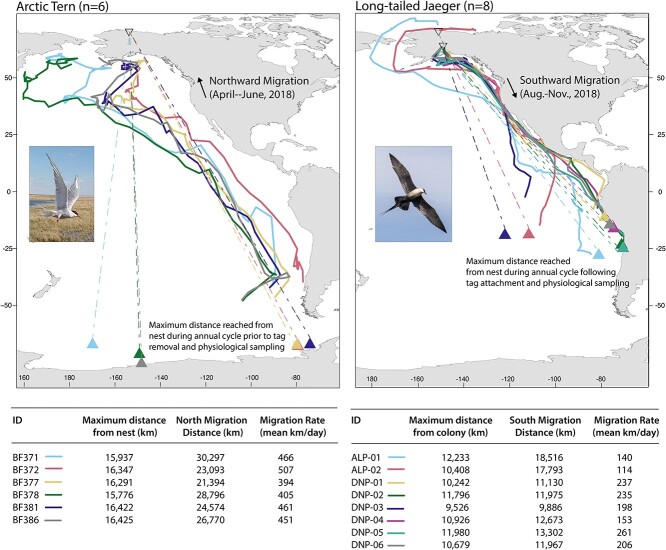
Complete, full-annual-cycle movement paths were available for jaegers (satellite-tracked), but not for terns (Figure 1, note dotted line portions of tern movement paths) due to limitations of light-level geolocators discussed above. We selected movement metrics that could be estimated from both technologies: 1) maximum distance reached from colony, 2) the distance of the migratory portion of the movement path, 3) rate of travel of the migratory portion of the movement path (migration distance divided by the time to cover the distance).
Figure 1.
Migratory metrics used to relate to physiological variables measured during incubation for two seabird species tracked with electronic tags from nesting grounds in Alaska, USA. Lines indicate periods of migration (fast, directed movement omitting breeding and over-wintering periods, but including migratory stopovers). The northward, pre-breeding migration of Arctic tern and southward, post-breeding migration of long-tailed jaeger are shown. Triangles indicate the location at which the bird reached a straight-line maximum distance from their nest site in the tracked year. See Supplementary Material, Fig. S1 for full-annual cycle movement paths.
Maximum distance from colony was calculated as the farthest distance recorded by each bird from the known nest site. For Arctic terns, although a portion of their non-breeding period was unavailable due to 24-hour polar sun, positions in the Southern Ocean were documented for all individuals and relative differences between terns and jaegers were apparent (see Results).
To estimate the distance and rate of travel of the migratory portion of the movement paths, we first needed to delineate that portion. For terns, the migratory portion of the pathway was previously calculated in (Wong et al., 2022). For long-tailed jaegers, we applied a change point analysis to a time series of net squared displacement to segment the track into breeding, migratory and non-breeding periods of the annual cycle. Net squared displacement (NSD) is the distance from colony of each location, squared and has been shown to increase rapidly during periods of fast, directed travel like migration behavior (Bunnefeld et al., 2011). When the bird is resident to a place (in a home range or stationary period, as in a breeding location), NSD plateaus. We used the geodist package in R to calculate NSD using the square of vincenty distances from colony, estimated for an ellipsoid Earth. A change point analysis, implemented with the changepoint package in R, identifies changes in the rate of change of NSD and suggests segment breaks, which we then manually reviewed and assigned to a period of the annual cycle based on date. A worked example is shown in Supplementary Material, Fig. S1. Brief stopovers during migration were included in the migratory segment. For each migration segment, we calculated the migration distance as the sum of vincenty distances between each daily location. We estimated the daily rate of migration as the migration distance divided by its duration.
Statistical treatments
Normality and equality of variance between species were assessed for all variables (see Table 1 for list), and the R statistical computing program (2018) used for statistical analyses.
Table 1.
Mean (±standard deviation) values of physiological variables and migration metrics measured in incubating Arctic terns (n = 10) and long-tailed jaegers (n = 8) captured in Alaska, USA
| Arctic tern | Long-tailed jaeger | |
|---|---|---|
| Mass (g)* | 98.9 ± 8.9 | 303.9 ± 17.3 |
| Hematocrit (%) | 45.7 ± 5.2 | 46.6 ± 4.7 |
| Hemoglobin (g.dl−1) | 14.1 ± 2.7 | 14.8 ± 3.2 |
| Uric acid (mg.dL−1) | 36.5 ± 17.0 | 27.8 ± 14.9 |
| Antioxidants (OXY, μmol HClO ml−1) | 291.6 ± 35.4 | 262.2 ± 55.8 |
| Reactive Oxygen Metabolites (ROM, mg H2O2 dl−1) | 2.7 ± 1.0 | 1.0 ± 0.4 |
| Wing chord (mm)* | 273.7 ± 6.2 | 305.1 ± 9.6 |
| Max distance from colony (km)* | 16 199 ± 275 | 10 947 ± 950 |
| Migration only distance (km) | 25 821 ± 3417^ | 13 405 ± 3107# |
| Rate of migration travel (km/day) | 447 ± 41.8^ | 193 ± 52.3# |
*indicates statistically significant difference between species (p < 0.001).
Physiology samples: Arctic terns (n = 10), long-tailed jaegers (n = 8). Migration metrics: Arctic terns (n = 6), long-tailed jaegers (n = 8).
^: spring migration
#: fall migration
To test for differences in baseline physiological values between species, we adjusted for mass by including it as a covariate in ANCOVA, after (Packard and Boardman, 1999) and (Skrip et al., 2015).
To explore relationships among physiological variables within species as a measure of integration, we used Pearson’s correlations to create correlation matrices. After identifying significant correlations of physiological variables (p < 0.05) within species, we further explored using linear regression to extract slopes and include mass as a covariate. We also used principal components analysis (prcomp in R) to combine physiological variables into a composite for exploring relationships to migration metrics (after (Hõrak and Cohen, 2010)). For some birds (terns, n = 3; jaegers, n = 2), low blood volume prevented us from performing all assays on all individuals. Because PCA requires all individuals to have values for all variables, these individuals were omitted from analysis resulting in a final sample size for PCA of terns n = 7 and jaegers n = 6.
To compare maximum distances between species we used a Welch's ranked t-test (maximum distances were not normally distributed, nor were variances equal between the groups). Within species, to test the hypothesis whether migration distance or rate was related to oxidative stress we used linear regression with mass as a covariate. Additionally, we explored whether the extracted principal components were related to migration variables. For Arctic terns, there was only 1 nest from which both birds were sampled for physiology values. For all other nests, the individual is the only representative in our dataset for the nest, thus nest ID is not included as a covariate. For jaegers, we sampled physiology variables from all individuals from all 4 nests, however we do not have a sample size that will permit the addition of nest ID as a covariate.
Results
We report the first values of incubation oxidative stress and aerobic capacity variables in Arctic terns and long-tailed jaegers. When accounting for differences in mass between terns and jaegers, there were no statistically significant differences in any physiological variables between species (Table 1, Figure 2). The maximum distance recorded from breeding colony (year before sampling for terns, year after sampling for jaegers) was significantly farther for Arctic terns (16 199 ± 275 km) than for long-tailed jaegers (10 947 ± 950 km) (Table 1; Figure 1, Welch's ranked t-test t = 6.97, df = 16, p < 0.001). Additionally, terns were significantly lighter in mass (t = −30.5, df = 9.9, p < 0.001, 98.9 ± 8.9 g vs 303.9 ± 17.3 g) and had significantly smaller flattened wing chords (t = −8.0, df = 11, p < 0.00, 273.7 ± 6.2 mm vs 305.1 ± 9.6 mm) and mass and wing chord were highly correlated (R = 0.91, p < 0.001). Although the migration tracks were not compared statistically between species due to the different timing (northward vs southward), values for migration-only distance and rate of travel duration migration are reported in Table 1.
Figure 2.
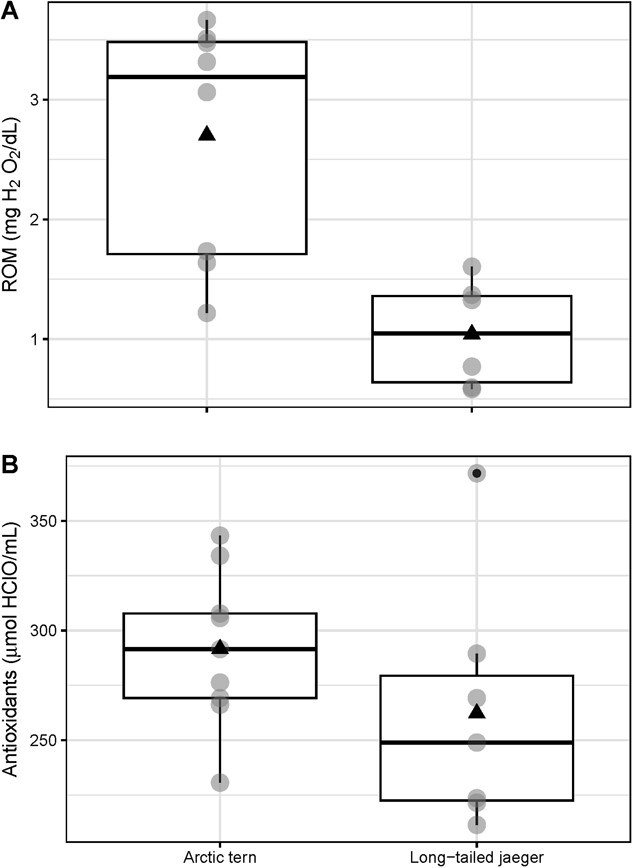
Variability in oxidative physiology between species. A: Reactive Oxygen Metabolites (ROM), B: Antioxidants (OXY) and C: Arctic terns and long-tailed jaegers captured in Alaska, USA. Horizontal lines indicate the median. Triangle (▲) indicates the mean. Apparent differences between distribution of raw values are not significant when mass is included as a covariate (see Table 1).
Integration and relationships among physiological variables:
There was no significant relationships between physiological measures and our range of handling times (32–60 minutes) prior to sampling, or between sexes or nest ID. We therefore did not include sex or nest parameters in our models. There were notable differences across species in correlation patterns. Within jaegers, there were no significant correlations between physiological variables (Table 2). In contrast, within terns, antioxidants (OXY) were significantly and positively correlated with ROM and uric acid (Table 2). Because the correlation matrix (Table 2) indicated that different species had different inter-relationships of some variables, we used linear regression to look closer at how the variable relationships differed in the species.
Table 2.
Correlation coefficients of physiological variables measured for incubating Arctic terns and long-tailed jaegers captured in Alaska, USA. (italics/bold = p < 0.05)
| Arctic tern | Long-tailed jaeger | |||||||
|---|---|---|---|---|---|---|---|---|
| Hb | OXY | ROM | Uric Acid | Hb | OXY | ROM | Uric Acid | |
| Hematocrit | 0.57 | −0.14 | −0.23 | −0.50 | −0.29 | −0.54 | −0.13 | −0.62 |
| Hemoglobin (Hb) | 0.18 | −0.11 | −0.19 | 0.09 | 0.34 | −0.08 | ||
| Antioxidants (OXY) | 0.86 | 0.90 | 0.42 | 0.69 | ||||
| Reactive oxygen metabolites (ROM) | 0.95 | 0.78 | ||||||
We found different relationships between uric acid and OXY in the different species, however the correlative approach does not account for animal mass. We thus further evaluated this relationship using linear regression with mass as a covariate. We found antioxidants were significantly related to uric acid in Arctic terns (F(2,5) = 10.76, p = 0.02, adjusted R2 = 0.74, Figure 3A). In contrast, long-tailed jaegers did not exhibit a significant relationship between uric acid and OXY when mass was included as a covariate. We explored removing a potential outlier, the high jaeger OXY value (371 μmol HClO∙mL−1), but strength of the relationship decreased even further, confirming the lack of relationship between jaeger OXY and uric acid.
Figure 3.
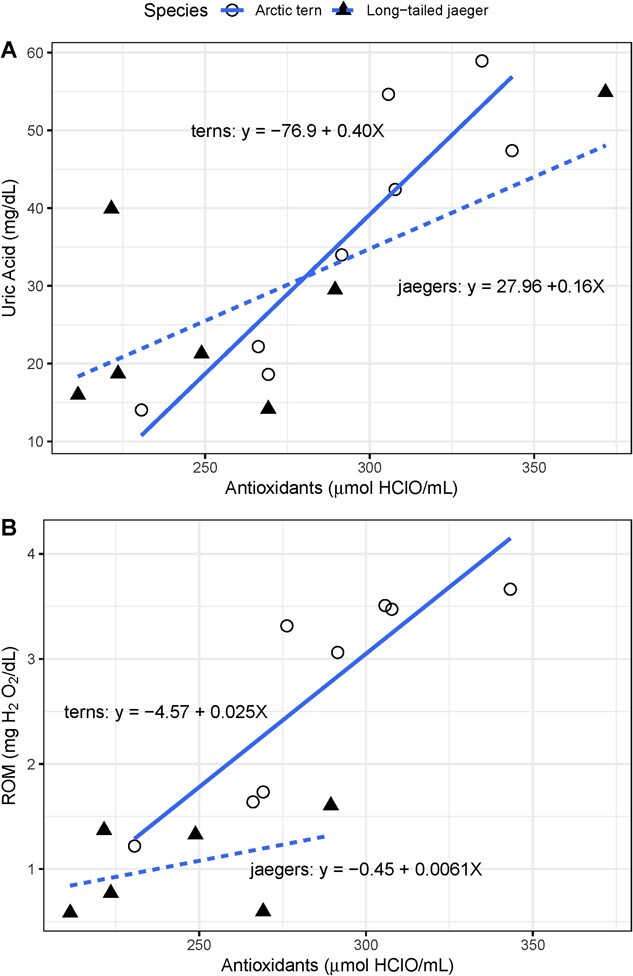
A) Relationship between physiological variables measured in incubating Arctic tern and long-tailed jaegers captured in Alaska, USA. Within terns, uric acid and antioxidants were significantly and positively related (adjusted R2 = 0.74, F2,5 = 10.76, p = 0.02), while in jaegers, there was no significant relationship. B) In terns, antioxidants were significantly related to ROM (reactive oxygen metabolites) (adjusted R2 = 0.64, F2,5 = 7.2, p = 0.03), but not in jaegers.
Although mean OXY and ROM values were statistically equivalent between species, within species they were significantly correlated in terns only (Table 2). Within each species, with mass included as a covariate, OXY significantly predicted ROM for terns (F2,5 = 7.2, p = 0.03, adjusted R2 = 0.64, Figure 2B), but not jaegers.
To further explore the differences in integration indicated by the previous analyses, we performed principal components analysis within each species. In terns, the first component accounted for 59.1% of the variation, and the second component accounted for 34.1%. Uric acid (−0.58), ROM (−0.56) and OXY (−0.52) loaded negatively, while Hb and Hct loaded positively. For jaegers, the first component carried 40% of the variation and the second 26.6%. The relationships among oxidative variables were not as clear or as strong in jaegers compared to terns. ROM loaded strongly (−0.68), as did uric acid (−0.53), but OXY did not and Hb was the third highest loading (−0.31), in the opposite direction of the terns.
Relationship between migration distance and physiology
For Arctic terns, there were no significant relationships between maximum distance individuals reached from colony and oxidative physiology (p > 0.05, Figure 4A). During their northbound spring migration prior to physiological sampling, the birds covered 25 821 ± 3417 km over a duration of 58.1 ± 9.1 days. The rate of travel was 447 ± 41.8 km/day (Table 1). Spring migration distance was also not related to tern oxidative physiology. However, we found a significant positive relationship between spring rate of migration (distance traveled/day) and hematocrit when controlling for mass (whole model: F(2,3) = 10.7, p = 0.04. For hematocrit alone: F(1,3) = 20.6, p = 0.02, y = 105.3 + 5.9x. Mass was not a significant predictor). When the first principal component of all the tern physiological variables was extracted and used as a variable for comparison to migration metrics, there was no relationship to distance or rate of travel (p < 0.05). Similarly, the extracted first principal component for Arctic tern ‘oxidative variables only’ was not related to migration distance or rate of travel (p < 0.05).
Figure 4.
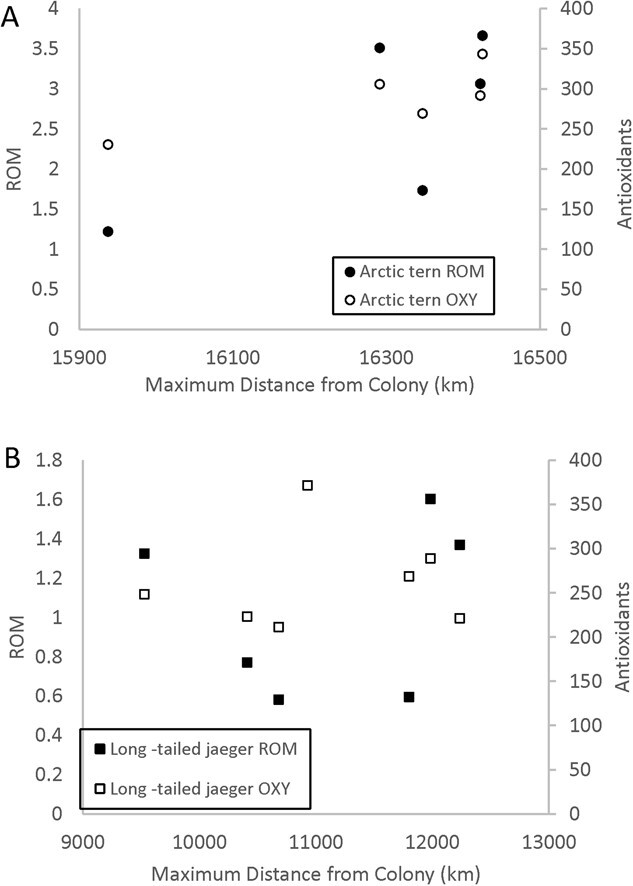
Maximum distance from colony is not related to either OXY or ROM. Individual OXY and ROM matched samples at the individual maximum distance from the colony.
For long-tailed jaegers there were no significant relationship between maximum distance reached from colony and oxidative physiology (p > 0.05, Figure 4B). There was a significant negative relationship between jaeger hemoglobin and maximum distance, but one high hemoglobin value (21.7 g∙dL−1) drove the relationship, which disappeared when the outlier was excluded. Extracted principal components from all jaeger physiology variables showed no relationship to maximum distance reached from colony (p > 0.05). Additionally, we explored collapsing only the oxidative stress metrics (OXY, ROM and uric acid) for long-tailed jaegers. When ‘oxidative status variables only’ were used, the first component explained 63.1% of the variation and the second 33.3%. However, when we assessed the extracted components for relationships to maximum distance, none were found (p > 0.05). During their southbound fall migration following physiological sampling, jaegers flew 13 405 ± 3107 km over 78.5 ± 42.5 days, resulting in a travel rate of 193 ± 52.3 km/day (Table 1). Neither the individual variables, nor the extractions from the PCA showed a relationship to any physiology metrics. Similarly, the fall (southbound) rate of migration (distance traveled/day) was unrelated to the physiological variables we sampled.
Discussion
Arctic Terns and long-tailed jaegers undertake physiologically demanding migrations of tens of thousands of kilometers each year (Egevang et al., 2010; Harrison et al., 2021; Wong et al., 2021). In this study, we established first measures for the oxidative physiology of these two species to inform future biomarker monitoring of population health in the context of rapidly changing Arctic and ocean habitats. We provided foundational analyses for generating broader hypotheses about the relationship between integration, migration capability and potential responses to environmental challenges across seabird species. Contrary to our predictions, individuals that traveled farthest from their breeding site did not have higher individual oxidative stress during incubation for either species. Although our relatively small sample size does not permit generalizable inferences, our results suggest that Arctic terns may have better integration than long-tailed jaegers. Oxidative stress occurs when ROM increase, but antioxidants do not increase concomitantly (Costantini and Verhulst, 2009; Monaghan et al., 2009). In captive zebra finches undergoing high intensity exercise, a reduction in biochemical integration is likely linked to a reduced resistance to oxidative stress (Costantini et al., 2013). Although only an experimental manipulation would fully indicate responsiveness, we interpret the current correlation of ROM and antioxidants in terns to indicate an ability to avoid oxidative stress. Species with better integration capabilities may be better prepared to manage their response to oxidative challenges, while species with less adept integration, such as long-tailed jaegers, have the potential to be more severely impacted by scenarios that could trigger more oxidative stress, such as increased temperatures and habitat and prey availability fluctuations due to climate change.
For our sample of individuals from two breeding species, mass-corrected physiological values were statistically similar across species. Within each species, we found more correlations among physiological variables, indicating more integration, in terns compared to jaegers. Arctic terns showed a stronger positive relationship between their antioxidants and ROM, than did long-tailed jaegers. Terns also demonstrated a significant positive relationship between antioxidants and ROM, whereas the positive pattern we found in jaegers was not significant. Additionally, there was a significant positive relationship between tern uric acid and other antioxidants (OXY). These relationships were not present in jaegers. Uric acid is a by-product of protein metabolism, but also serves an additional antioxidant function (Tsahar et al., 2006; Cohen and McGraw, 2009).
Dietary protein intake can affect uric acid values (Alan and McWilliams, 2013), but we do not feel that these integration pattern differences are due only to dietary differences. While we do not have specific diet for these individuals, generally, long-tailed jaeger breeding success is linked to their small rodent prey, including several species of voles and lemmings, their primary prey during the nesting season (Gilg et al., 2003; Barraquand et al., 2014). Conversely, Arctic terns eat primarily fish (Pratte et al., 2017). Collared lemming protein content (dry matter) is ~ 57% (Batzli and Esseks, 1992) and sand lance, a possible prey item for terns (Robertson et al., 2014) are 61–62% protein (Robards et al., 2000). Thus, although Arctic terns and long-tailed jaegers have different prey types, the general protein content is likely to be similar. This is reflected in the statistically similar baseline uric acid values between the species. We interpret the additional positive correlations between uric acid, OXY, and ROM in Arctic terns to indicate that they may be responding more effectively to the generation of ROM.
In addition to establishing baseline incubation physiological values, a goal of our study was to investigate whether differences in migration metrics were related to oxidative stress patterns. Neither species demonstrated a relationship between individual oxidative physiology and the migration metrics we evaluated. We note that within species, differences in migration distances across individuals are small compared to the absolute differences in migration distances across species (Table 1) and that the longer distance migrant, Arctic terns, had higher integration among oxidative physiology variables.
There are, however, multiple caveats in interpreting the differences we observed across species. While our small sample size limits generalizability of our conclusions, these first values suggest interesting hypotheses to explore in future studies with larger samples. The two species we studied are within the same order (Charadriiformes, (Chesser et al., 2021)), but are not within the same family. Garland and Adolph (1994) and Garland et al. (2005) caution against inferring evolutionary adaptations in studies comparing only two species and we cannot suggest that the difference in oxidative physiology we observed is an evolutionary adaptation to the much longer absolute migration distances of terns. There are not enough data on oxidative stress in different families of seabirds to rule out a phylogenetic influence on the patterns we describe. Sampling of additional families in this order across a range of migratory strategies and analysed with a phylogenetically independent contrast approach would aid in interpreting whether phylogeny and/or migratory strategy is a factor in explaining the oxidative physiology patterns we observe. The difference we found between species in integration is interesting and useful for helping us identify species more vulnerable to oxidative challenges, even if we do not yet know why the difference exists.
In migrating songbirds, fat mass has a positive relationship to ROM, and we encourage future studies to account for mass in oxidative studies, after (Skrip et al., 2015). The two species in this study differed significantly in mass (Table 1). We accounted for mass by including it as a covariate in our linear analyses (Packard and Boardman, 1999). After this treatment, there was no statistical significance of oxidative physiology variables differing between groups. Whether mass differences between the species is a major factor beyond our statistical controls is unknown. We do not have specific lipid mass, but within each group, mass and ROM were not correlated. Additionally, the birds in this study displayed the opposite trend as (Skrip et al., 2015)—smaller birds, terns, had higher ROM (Table 1). It seems unlikely that the higher mass of jaegers would be obscuring patterns—the expectation would be that the larger-bodied bird would have higher ROM simply from having more lipid to be oxidatively damaged. Instead, we found that smaller birds (terns) generated more ROM, and the correlative patterns of OXY and uric acid as antioxidants may be a response to that.
Another challenge to address is the temporal resolution regarding the time frame between sampling in incubation and migratory travel. How long would a physiological signal persist that would be relevant to indicate a relationship between a migratory trip they completed prior to breeding? Or to affect their post-breeding migration? Point samples of oxidative stress during the breeding season has been linked to future fecundity and survival in several avian species (Costantini and Dell’Omo, 2015; Herborn et al., 2016; Fowler and Williams, 2017) and in bats, oxidative stress signatures are detectable two weeks after a thermal challenge (Beaulieu et al., 2020), indicating that it is possible for signals to persist several weeks or months removed from the sampling time. The limits of temporal resolution for linking oxidative stress to other variables is an area physiological ecologists continue to explore. The better integration in Arctic terns, as indicated by correlations among ROM, antioxidants and uric acid (Costantini et al., 2011, 2013), may indicate the capacity for greater responsiveness to oxidative challenges encountered in migration, although we cannot control for all variables. Increased oxidative stress may be an inevitable consequence of migration (Cooper-Mullin and McWilliams, 2016) and the tighter relationship between non-enzymatic antioxidants and uric acid may indicate the potential to counteract increased ROM as a consequence of flight. Reproduction may also impact oxidative stress (Alonso-Alvarez et al., 2017), thus we cannot rule out that the better integration may also reflect the species’ differing responses to reproductive effort. While our samples are from incubation, we cannot be certain how well they reflect the level of oxidative stress seabirds experience mid-migratory flight. We did not find that the prior migratory effort, as we quantified it, in individual birds carried over to the oxidative stress in their breeding season (represented by the Arctic tern northward spring migration relative to their incubation physiology). We also did not find that individual oxidative stress measured in the incubation phase seemed to affect the individual migratory effort expended post-breeding, as reflected in long-tailed jaegers. In other species, mechanisms regulating integration capacity may persist across different life history stages and that integration may differ among species and be responsive to selective pressures (Costantini et al., 2013). It is unknown whether the correlation between ROM and antioxidants displayed in incubation in Arctic terns would persist to other situations. Only experimental trials manipulating the exercise intensity would be able to clarify whether the integration in terns persists. However, the positive relationship between ROM and antioxidants in Arctic terns (indicative of a managed response to oxidative stress) (Costantini and Verhulst, 2009), may give some indication that they have the capacity to respond to future situations (such as migration or thermal stress) that may increase ROM levels with matching increases in antioxidants. Although without an experimental manipulation of ROM levels and measurement of the concomitant antioxidant response, we cannot definitively predict how jaegers might respond to a challenge increasing ROM, the lack of a correlation between ROM and antioxidants in the current study does not provide strong evidence that if ROM were to increase, that antioxidants would increase as well.
Our work informs the discussion of the temporal resolution for detecting individual oxidative stress signatures and their relationship to individual migratory performance metrics. As the field of wildlife oxidative physiology develops, timing of sampling is an area that is crucial to refine (Costantini, 2019). Individual relationships to migratory metrics (maximum distance, migration distance or migration rate) were not detected in our sample and across the range of migration distances exhibited within species. The individual variability of migratory metrics, relative to the overall distance traveled was not high, which may be an additional factor in an ability to detect relationships at the scale of inference. The temporal resolution of sampling is a variable that must be considered as physiological ecologists continue to refine timing of sampling necessary for detection of patterns relative to behavioral variables.
Plasma is a commonly used matrix for field studies of avian oxidative stress ((Costantini, 2016) and see review below). We acknowledge that plasma may not be a comprehensive representation of all oxidative stress in all tissues (Costantini, 2019), but is recommended for free-ranging animals for which terminal sampling is not appropriate (Monaghan et al., 2009; Costantini, 2019). Sampling multiple tissues would undoubtedly provide a more complete picture, however, we were limited in our ability to sample other tissues and terminal sampling was not an option. Additionally, although factors such as preservation techniques and slight changes in methodology may introduce variability, we compare multiple studies that have collected samples similarly and used the same assays in avian systems. Compared to studies that used similar assays for plasma, our ROM values for Arctic terns and long-tailed jaegers were slightly higher than Scopoli’s shearwater (Costantini and Dell’Omo, 2015), canaries (Costantini et al., 2016) and kestrel nestlings (Costantini and Dell’Omo, 2006). Tern and jaeger ROM values in our study were similar to European starling ROM values (Fowler et al., 2018), and in line with breeding Adelie and Gentoo penguins (Beaulieu et al., 2013), but somewhat lower than foraging Adelie penguins (Beaulieu et al., 2010) and bats (Costantini et al., 2019; Beaulieu et al., 2020). The range of antioxidant values we found is similar to Scopoli's shearwater (Costantini and Dell’Omo, 2015), European starlings (Fowler et al., 2018), bats (Costantini et al., 2019) and breeding Gentoo and Adelie penguins (Beaulieu et al., 2013), but antioxidants in both terns and jaegers in our study were slightly higher than levels found in foraging Adelie penguins (Beaulieu et al., 2010). Arctic tern and long-tailed jaeger uric acid values are comparable to songbirds (European starlings (Fowler et al., 2018) and common blackbirds (Eikenaar et al., 2017)) as well as migratory shorebirds (bar-tailed godwit (Landys et al., 2005). Few other studies have reported oxidative stress in seabirds. Breeding thick billed murres show considerable interannual variability, but minimal differences between males and females in oxidative damage. Interestingly, the uric acid measurements in murres were tremendously higher than our measurements (36000–57 000 micromolar in murres compared to our converted values of ~ 2200 micromolar) (Lin et al., 2022). The authors suggest conditions prior to the breeding season may affect oxidative status. Hoffman et al., 2011 measured the relationship between mercury contaminant load and oxidative stress in Forster’s and Caspian terns, but they used different assays and different tissues (brain, kidney and liver) than this study, preventing direct comparison (Hoffman et al., 2011).
Hematocrit and hemoglobin as indicators of aerobic capacity have been linked to behavioral and ecological parameters in birds (Wagner et al., 2008b; Williams, 2012). For example, foraging efficiency and dive duration of Macaroni penguins was linked to hematocrit (Crossin et al., 2015). Our hematocrit values were a bit lower than songbird (incubating starlings 53% (Fowler et al., 2018) or migratory shorebird (bar-tailed godwit 47–51% (Landys-Ciannelli et al., 2002) hematocrit. However, our values are comparable with those reported for another marine bird and a congeneric of long-tailed jaeger, the great skua, at 45% to 46% (Kalmbach et al., 2004). Our study provides the first records of hematocrit in long-tailed jaegers and for Arctic terns, only hematocrit in chicks has previously been reported (<40%) (Bech and Klaassen, 1996). Previous studies have found differences in hematocrit due to egg production in songbirds (Williams et al., 2004; Wagner et al., 2008a). However, we found no differences between males and females, and without other values to compare to, we assumed they have recovered from any anemia due to egg production by our incubation sampling time period. The only pattern we found with aerobic capacity variables was Arctic tern spring migration rate (km/day) and hematocrit. This strong positive relationship confirms the importance of aerobic capacity for high intensity exercise.
A variety of uncontrolled variables in in vivo ecological studies has the potential to obscure clear relationships (Cohen et al., 2012) and in these free ranging birds we were unable to control for many of the parameters suggested by (Hõrak and Cohen, 2010). For example, we could not take repeated samples of the same individual, or control for age (although all birds were nesting adults), diet or activity prior to capture. Controlled, manipulative studies show that age and prior stress can affect oxidative stress (Marasco et al., 2017; Majer et al., 2019). However, our study contributes new information to our understanding of oxidative balance in long distance migrating seabirds. Future studies attempting to detect individual physiological signals of migration should either work on a sampling time frame closer to arrival from migration or attempt to collect additional variables such as age.
The relevance of physiology is not always immediately apparent to conservation practitioners, but the physiological tolerance of organisms to changing environments is important to understanding threats to biodiversity (Visser, 2008; Cooke and O’Connor, 2010; Cooke et al., 2021). We feel our data provide baseline values and some first insights into potential physiological flexibility. Physiological biomarkers, like oxidative stress, have been identified as important to building a ‘tool box’ to predict responses to conservation challenges using physiology (Madliger et al., 2018; Cooke et al., 2021), but the establishment of baselines is an important first step. As we begin to understand more about how environmental changes may affect oxidative challenges for organisms, it is important to determine their capacity to respond to such changes. Cooke et al. (2021) identified the potential for physiological tolerance as an important theme in conservation physiology.
Long-tailed jaegers are considered sentinel species of environmental change in Alaska (U.S. Fish and Wildlife Service, 2009), and a substantial proportion of their global population is thought to be in the State, although population estimates are unavailable for most of their Alaskan distribution (U.S. Fish and Wildlife Service, 2009). Tundra breeding jaeger populations are at risk as tundra conversion (encroachment of changing habitat upward into the tundra) occurs (Terskaia et al., 2020; Raiho et al., 2022). Changing plant communities (Elmendorf et al., 2012; Bjorkman et al., 2018), alterations in the small rodent populations (prey for long-tailed jaegers (Gilg et al., 2003; Barraquand et al., 2014)) and expanding ranges for other avian species (Elmhagen et al., 2015) are just some of the shifts associated with climate change. Thus, new challenges related to predation, competition and exposure to new physiological and ecotoxicological stimuli such as pollutants are likely to present themselves (Gilg et al., 2012). Physiology (including stress response and immunity) have been shown to mediate avian populations' resilience to disturbance in forest communities (Messina et al., 2018). Thermal challenges (for example, increased temperatures) are also likely to cause oxidative stress in animals (Andersson et al., 2018; Beaulieu et al., 2020).
Management frameworks, such as the climate change vulnerability assessment (CCVA), have emerged to attempt to consolidate many variables affecting organisms in order to assess components such as exposure, sensitivity, and adaptive capacity (Foden et al., 2019; Thurman et al., 2022). The adaptive capacity refers to the ability of a species to adjust to climate change and includes data on 36 different attributes; among them data on physiological tolerance (Thurman et al., 2020). Existing knowledge gaps for species is a serious limitation of using an adaptive capacity framework for setting climate informed conservation goals for species (Thurman et al., 2020). Managers attempting to predict whether species may be vulnerable to projected climate scenarios must have data available on these attributes. Our data do not provide a silver bullet to predicting how jaegers and terns will be affected by climate change, but our data suggest a hypothesis that better integration in terns indicates that terns are more able to manage increases in oxidative challenges. As climate change presents environmental challenges that are likely to lead to increases in oxidative stress, terns maybe more flexible to accommodate these changes, while jaegers may be less able to respond to the physiological challenges that will likely accompany environmental change. We feel our study contributes data that could be used in management frameworks such as CCVAs and physiological tolerances, enabling managers to better prioritize threats and potential responses of tundra breeding birds.
Additionally, as we provide the first measurements of oxidative physiology in these species, we provide baseline data that conservation practitioners can use as a reference point to compare subsequent oxidative physiology values in terns or jaegers. As future population trends are monitored, in concert with oxidative physiology values, this will further inform our understanding of oxidative physiology as a biomarker and its relationship to demographic parameters. Although there are always caveats with baseline data due to the difficulty of sampling free ranging animals, our data provide the first measurements as the Arctic environment is expected to change rapidly with climate change (IPCC, 2021). Our hope is that the knowledge about potential vulnerabilities will prove useful as conservation practitioners must incorporate many variables in a complex decision-making process that requires prioritization among species and habitat management decisions in the face of rapid environmental change. If the scientific community hopes to detect oxidative stress responses to accelerating climate change in seabirds that incubate in Arctic and alpine tundra, we must start to contribute to longitudinal data sets for future comparisons.
Supplementary Material
Acknowledgements
We are indebted to the following for help with logistics coordination and finding, capturing and processing birds: Ellie Heywood, Ari Haunschild, Mary Lewandoswki, Robyn McGhee, Carol McIntyre, Avery Meeker, Laura Phillips, Emily Williams, Arliss Winship, Mark Mallory and Marie Auger-Méthé.
Author Contribution
MF and ALH conceived of research and collected field data; ALH and JW analysed movement data; MF performed laboratory and statistical analysis; MF, ALH and JW wrote and revised the manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare.
Funding
Support and funding for field deployments was provided by the Smithsonian Migratory Bird Center’s Migratory Connectivity Project, the ConocoPhillips Charitable Investments Global Signature Program, ConocoPhillips Alaska, Denali National Park and Preserve and the Marine Geospatial Ecology Lab at Duke University, with funding from the Global Ocean Biodiversity Initiative. Funding for laboratory analyses was provided by the Springfield College Faculty Research Grant to MF.
Data availability
Mendeley Data, V1, doi: 10.17632/mfvk9zw68d.1
Supplementary material
Supplementary material is available at Conservation Physiology online.
Contributor Information
Melinda A Fowler, Department of Biology/Chemistry. Springfield College, 263 Alden Street, Springfield, MA 01109 USA.
Joanna B Wong, Institute for the Oceans and Fisheries, University of British Columbia, Vancouver, BC, V6T 1Z4, Canada; Department of Bird Migration, Swiss Ornithological Institute, 6204 Sempach, Switzerland.
Autumn-Lynn Harrison, Smithsonian‘s National Zoo and Conservation Biology Institute, Migratory Bird Center, 3001 Connecticut Avenue, NW, Washington, DC. 20008 USA.
References
- Adams SM, Ham KD (2011) Application of biochemical and physiological indicators for assessing recovery of fish populations in a disturbed stream. Environ Manag 47: 1047–1063. 10.1007/s00267-010-9599-7. [DOI] [PubMed] [Google Scholar]
- Alan RR, McWilliams SR (2013) Oxidative stress, circulating antioxidants, and dietary preferences in songbirds. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 164: 185–193. 10.1016/j.cbpb.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Alaska Dept of Fish and Game (2015) Alaska wildlife action plan
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Chastel O, Sorci G (2006) An experimental manipulation of life-history trajectories and resistance to oxidative stress. Evolution 60: 1913–1924. [PubMed] [Google Scholar]
- Alonso-Alvarez C, Canelo T, Romero-Haro AA (2017) The oxidative cost of reproduction: theoretical questions and alternative mechanisms. Bioscience 67: 258–270. 10.1093/biosci/biw176. [DOI] [Google Scholar]
- Anderegg WRL, Diffenbaugh NS (2015) Observed and projected climate trends and hotspots across the National Ecological Observatory Network regions. Front Ecol Environ 13: 547–552. 10.1890/150159. [DOI] [Google Scholar]
- Andersson MN, Nilsson J, Nilsson J-Å, Isaksson C (2018) Diet and ambient temperature interact to shape plasma fatty acid composition, basal metabolic rate and oxidative stress in great tits. J Exp Biol 221: jeb186759. 10.1242/jeb.186759. [DOI] [PubMed] [Google Scholar]
- Bairlein F, Fritz J, Scope A, Schwendenwein I, Stanclova G, Dijk G, Meijer HAJ, Verhulst S, Dittami J (2015) Energy expenditure and metabolic changes of free-flying migrating northern bald ibis. PloS One 10: e0134433–e0134433. 10.1371/journal.pone.0134433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraquand F, Høye TT, Henden J-A, Yoccoz NG, Gilg O, Schmidt NM, Sittler B, Ims RA (2014) Demographic responses of a site-faithful and territorial predator to its fluctuating prey: long-tailed skuas and arctic lemmings. Journal of Animal Ecology 83: 375–387. 10.1111/1365-2656.12140. [DOI] [PubMed] [Google Scholar]
- Batzli GO, Esseks E (1992) Body fat as an indicator of nutritional condition for the brown lemming. J Mammal 73: 431–439. 10.2307/1382080. [DOI] [Google Scholar]
- Beaulieu M, Costantini D (2014) Biomarkers of oxidative status: missing tools in conservation physiology. Conservation Physiology 2: cou014. 10.1093/conphys/cou014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu M, Ropert-Coudert Y, Le Maho Y, Ancel A, Criscuolo F (2010) Foraging in an oxidative environment: relationship between δ13C values and oxidative status in Adélie penguins. Proceedings of the Royal Society B: Biological Sciences 277: 1087–1092. 10.1098/rspb.2009.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu M, Thierry AM, Gonzalez-Acuna D, Polito MJ (2013) Integrating oxidative ecology into conservation physiology. Conservation Physiology 1: cot004. 10.1093/conphys/cot004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu M, Touzalin F, Dool SE, Teeling EC, Puechmaille SJ (2020) Timescale and colony-dependent relationships between environmental conditions and plasma oxidative markers in a long-lived bat species. Conservation Physiology 8. 10.1093/conphys/coaa083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech C, Klaassen M (1996) Blood hemoglobin content and metabolic performance of Arctic tern chicks Sterna paradisaea. Journal of Avian Biology 27: 112–117. 10.2307/3677140. [DOI] [Google Scholar]
- Bemmelen R, Moe B, Hanssen SA, Schmidt NM, Hansen J, Lang J, Sittler B, Bollache L, Tulp I, Klaassen Ret al. (2017) Flexibility in otherwise consistent non-breeding movements of a long-distance migratory seabird, the long-tailed skua. Mar Ecol Prog Ser 578: 197–211. 10.3354/meps12010. [DOI] [Google Scholar]
- Bergman JN, Bennett JR, Binley AD, Cooke SJ, Fyson V, Hlina BL, Reid CH, Vala MA, Madliger CL (2019) Scaling from individual physiological measures to population-level demographic change: case studies and future directions for conservation management. Biol Conserv 238: 108242. 10.1016/j.biocon.2019.108242. [DOI] [Google Scholar]
- Bjorkman AD, Myers-Smith IH, Elmendorf SC, Normand S, Rüger N, Beck PSA, Blach-Overgaard A, Blok D, Cornelissen JHC, Forbes BCet al. (2018) Plant functional trait change across a warming tundra biome. Nature 562: 57–62. 10.1038/s41586-018-0563-7. [DOI] [PubMed] [Google Scholar]
- Bub H (1991) Bird Trapping and Bird Banding. Cornell University Press, Ithaca [Google Scholar]
- Bunnefeld N, Börger L, Moorter B, Rolandsen CM, Dettki H, Solberg EJ, Ericsson G (2011) A model-driven approach to quantify migration patterns: individual, regional and yearly differences. Journal of Animal Ecology 80: 466–476. 10.1111/j.1365-2656.2010.01776.x. [DOI] [PubMed] [Google Scholar]
- Calbet JAL, Lundby C, Koskolou M, Boushel R (2006) Importance of hemoglobin concentration to exercise: acute manipulations. Respir Physiol Neurobiol 151: 132–140. 10.1016/j.resp.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Chesser RT, Billerman SM, Burns KJ, Cicero C, Dunn JL, Hernandez-Banos BE, Kratter AW, Lovette IJ, Mason NA, Rasmussen PC, et al. (2021) Check-list of North American birds American Ornithological Society. http://checklist.aou.org/taxa.
- Cohen A, Klasing K, Ricklefs R (2007) Measuring circulating antioxidants in wild birds. Comp Biochem Physiol B Biochem Mol Biol 147: 110–121. 10.1016/j.cbpb.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Cohen AA, Martin LB, Wingfield JC, McWilliams SR, Dunne JA (2012) Physiological regulatory networks: ecological roles and evolutionary constraints. Trends Ecol Evol 27: 428–435. 10.1016/j.tree.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Cohen AA, McGraw KJ (2009) No simple measures for antioxidant status in birds: complexity in inter- and intraspecific correlations among circulating antioxidant types. Functional Ecology 23: 310–320. 10.1111/j.1365-2435.2009.01540.x. [DOI] [Google Scholar]
- Colominas-Ciuró R, Bertellotti M, D’Amico V, Carabajal E, Benzal J, Vidal V, Motas M, Santos M, Coria N, Barbosa A (2021) Diet, antioxidants and oxidative status in pygoscelid penguins. Mar Ecol Prog Ser 665: 201–216. 10.3354/meps13651. [DOI] [Google Scholar]
- Colominas-Ciuró R, Santos M, Coria N, Barbosa A (2017) Reproductive effort affects oxidative status and stress in an Antarctic penguin species: an experimental study. PloS One 12: e0177124. 10.1371/journal.pone.0177124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Bergman JN, Madliger CL, Cramp RL, Beardall J, Burness G, Clark TD, Dantzer B, Barrera E, Fangue NAet al. (2021) One hundred research questions in conservation physiology for generating actionable evidence to inform conservation policy and practice. Conservation Physiology 9. 10.1093/conphys/coab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, O’Connor CM (2010) Making conservation physiology relevant to policy makers and conservation practitioners. Conserv Lett 3: 159–166. 10.1111/j.1755-263X.2010.00109.x. [DOI] [Google Scholar]
- Cooper-Mullin C, McWilliams SR (2016) The role of the antioxidant system during intense endurance exercise: lessons from migrating birds. J Exp Biol 219: 3684–3695. 10.1242/jeb.123992. [DOI] [PubMed] [Google Scholar]
- Costa DP, Sinervo B (2004) Field physiology: physiological insights from animals in nature. Annu Rev Physiol 66: 209–238. 10.1146/annurev.physiol.66.032102.114245. [DOI] [PubMed] [Google Scholar]
- Costantini D (2008) Oxidative stress in ecology and evolution: lessons from avian studies. Ecol Lett 11: 1238–1251. 10.1111/j.1461-0248.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- Costantini D (2011) On the measurement of circulating antioxidant capacity and the nightmare of uric acid. Methods in Ecology and Evolution 2: 321–325. 10.1111/j.2041-210X.2010.00080.x. [DOI] [Google Scholar]
- Costantini D (2016) Oxidative stress ecology and the d-ROMs test: facts, misfacts and an appraisal of a decade’s work. Behav Ecol Sociobiol 70: 809–820. 10.1007/s00265-016-2091-5. [DOI] [Google Scholar]
- Costantini D (2019) Understanding diversity in oxidative status and oxidative stress: the opportunities and challenges ahead. J Exp Biol 222: jeb194688. 10.1242/jeb.194688. [DOI] [PubMed] [Google Scholar]
- Costantini D, Cardinale M, Carere C (2007) Oxidative damage and anti-oxidant capacity in two migratory bird species at a stop-over site. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 144: 363–371. 10.1016/j.cbpc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Costantini D, Casasole G, AbdElgawad H, Asard H, Eens M (2016) Experimental evidence that oxidative stress influences reproductive decisions. Functional Ecology 30: 1169–1174. 10.1111/1365-2435.12608. [DOI] [Google Scholar]
- Costantini D, Dell’Omo G (2006) Environmental and genetic components of oxidative stress in wild kestrel nestlings (Falco tinnunculus). J Comp Physiol B 176: 575–579. 10.1007/s00360-006-0080-0. [DOI] [PubMed] [Google Scholar]
- Costantini D, Dell’Omo G (2015) Oxidative stress predicts long-term resight probability and reproductive success in Scopoli’s shearwater (Calonectris diomedea). Conservation Physiology 3: 1–7. 10.1093/conphys/cov024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D, Lindecke O, Pētersons G, Voigt CC (2019) Migratory flight imposes oxidative stress in bats. Current Zoology 65: 147–153. 10.1093/cz/zoy039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D, Monaghan P, Metcalfe NB (2011) Biochemical integration of blood redox state in captive zebra finches (Taeniopygia guttata). J Exp Biol 214: 1148–1152. 10.1242/jeb.053496. [DOI] [PubMed] [Google Scholar]
- Costantini D, Monaghan P, Metcalfe NB (2013) Loss of integration is associated with reduced resistance to oxidative stress. J Exp Biol 216: 2213–2220. 10.1242/jeb.083154. [DOI] [PubMed] [Google Scholar]
- Costantini D, Rowe M, Butler MW, McGraw KJ (2010) From molecules to living systems: historical and contemporary issues in oxidative stress and antioxidant ecology. Functional Ecology 24: 950–959. 10.1111/j.1365-2435.2010.01746.x. [DOI] [Google Scholar]
- Costantini D, Verhulst S (2009) Does high antioxidant capacity indicate low oxidative stress? Functional Ecology 23: 506–509. 10.1111/j.1365-2435.2009.01546.x. [DOI] [Google Scholar]
- Crommenacker J v d, Komdeur J, Burke T, Richardson DS (2011) Spatio-temporal variation in territory quality and oxidative status: a natural experiment in the Seychelles warbler (Acrocephalus sechellensis). Journal of Animal Ecology 80: 668–680. 10.1111/j.1365-2656.2010.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin GT, Takahashi A, Sakamoto KQ, Trathan PN, Williams TD (2015) Habitat selection by foraging macaroni penguins correlates with hematocrit, an index of aerobic condition. Mar Ecol Prog Ser 530: 163–176. 10.3354/meps11320. [DOI] [Google Scholar]
- DeMoranville KJ, Carter WA, Pierce BJ, McWilliams SR (2022) Flight and dietary antioxidants influence antioxidant expression and activity in a migratory bird. Integrative Organismal Biology 4: obab035. 10.1093/iob/obab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson FS, Jouventin P (2007) How slow breeding can be selected in seabirds: testing Lack’s hypothesis. Proc R Soc B 274: 275–279. 10.1098/rspb.2006.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabkin DL, Austin JH (1932) Spectrophotometric studies: I. spectrophotometric constants for common hemoglobin derivatives in human, dog, and rabbit blood. J Biol Chem 98: 719–733. 10.1016/S0021-9258(18)76122-X. [DOI] [Google Scholar]
- Duffy DC, McKnight A, Irons DB (2014) Trans-andean passage of migrating arctic terns over Patagonia. Marine Ornithology 41: 155–159. [Google Scholar]
- Egevang C, Frederiksen M (2011) Fluctuating breeding of Arctic terns (Sterna paradisaea) in Arctic and high-Arctic colonies in Greenland. Waterbirds 34: 107–111. 10.1675/063.034.0114. [DOI] [Google Scholar]
- Egevang C, Stenhouse IJ, Phillips RA, Petersen A, Fox JW, Silk JR (2010) Tracking of Arctic terns Sterna paradisaea reveals longest animal migration. Proc Natl Acad Sci U S A 107: 2078–2081. 10.1073/pnas.0909493107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenaar C, Källstig E, Andersson MN, Herrera-Dueñas A, Isaksson C (2017) Oxidative challenges of avian migration: a comparative field study on a partial migrant. Physiol Biochem Zool 90: 223–229. 10.1086/689191. [DOI] [PubMed] [Google Scholar]
- Elmendorf SC, Henry GHR, Hollister RD, Björk RG, Boulanger-Lapointe N, Cooper EJ, Cornelissen JHC, Day TA, Dorrepaal E, Elumeeva TGet al. (2012) Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nature Climate Change 2: 453–457. 10.1038/nclimate1465. [DOI] [Google Scholar]
- Elmhagen B, Kindberg J, Hellström P, Angerbjörn A (2015) A boreal invasion in response to climate change? Range shifts and community effects in the borderland between forest and tundra. Ambio 44: 39–50. 10.1007/s13280-014-0606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247. 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Foden WB, Young BE, Akçakaya HR, Garcia RA, Hoffmann AA, Stein BA, Thomas CD, Wheatley CJ, Bickford D, Carr JAet al. (2019) Climate change vulnerability assessment of species. WIREs Climate Change 10: e551. 10.1002/wcc.551. [DOI] [Google Scholar]
- Fowler MA, Paquet M, Legault V, Cohen AA, Williams TD (2018) Physiological predictors of reproductive performance in the European Starling (Sturnus vulgaris). Front Zool 15: 45. 10.1186/s12983-018-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler MA, Williams TD (2017) A physiological signature of “cost of reproduction” associated with parental care. American Naturalist 190: 762–773. 10.1086/694123. [DOI] [PubMed] [Google Scholar]
- Furness RW (1987) The Skuas. T & AD Poyser [Google Scholar]
- Garland T, Adolph SC (1994) Why not to do two-species comparative studies: limitations on inferring adaptation. Physiol Zool 67: 797–828. 10.1086/physzool.67.4.30163866. [DOI] [Google Scholar]
- Garland T, Bennett AF, Rezende EL (2005) Phylogenetic approaches in comparative physiology. J Exp Biol 208: 3015–3035. 10.1242/jeb.01745. [DOI] [PubMed] [Google Scholar]
- Gilg O, Hanski I, Sittler B (2003) Cyclic dynamics in a simple vertebrate predator-prey community. Science 302: 866–868. 10.1126/science.1087509. [DOI] [PubMed] [Google Scholar]
- Gilg O, Kovacs KM, Aars J, Fort J, Gauthier G, Grémillet D, Ims RA, Meltofte H, Moreau J, Post Eet al. (2012) Climate change and the ecology and evolution of Arctic vertebrates. Ann N Y Acad Sci 1249: 166–190. 10.1111/j.1749-6632.2011.06412.x. [DOI] [PubMed] [Google Scholar]
- Gilg O, Moe B, Hanssen SA, Schmidt NM, Sittler B, Hansen J, Reneerkens J, Sabard B, Chastel O, Moreau Jet al. (2013) Trans-equatorial migration routes, staging sites and wintering areas of a high-Arctic avian predator: the long-tailed Skua (Stercorarius longicaudus). PloS One 8: e64614. 10.1371/journal.pone.0064614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindre-Parker S, Baldo S, Gilchrist HG, Macdonald CA, Harris CM, Love OP (2013) The oxidative costs of territory quality and offspring provisioning. J Evol Biol 26: 2558–2565. 10.1111/jeb.12256. [DOI] [PubMed] [Google Scholar]
- Harrison A-L, Woodard P, Mallory ML, Rausch J (2021) Sympatrically breeding congeneric seabirds (Stercorarius spp.) from Arctic Canada migrate to four oceans. Ecol Evol 12: e8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau M, Haussmann MF, Greives TJ, Matlack C, Costantini D, Quetting M, Adelman JS, Miranda AC, Partecke J (2015) Repeated stressors in adulthood increase the rate of biological ageing. Front Zool 12: 4. 10.1186/s12983-015-0095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herborn K, Daunt F, Heidinger BJ, Granroth-Wilding HMV, Burthe SJ, Newell MA, Monaghan P (2016) Age, oxidative stress exposure and fitness in a long-lived seabird. Functional Ecology 30: 913–921. 10.1111/1365-2435.12578. [DOI] [Google Scholar]
- Hoffman DJ, Eagles-Smith CA, Ackerman JT, Adelsbach TL, Stebbins KR (2011) Oxidative stress response of Forster’s terns (Sterna forsteri) and Caspian terns (Hydroprogne caspia) to mercury and selenium bioaccumulation in liver, kidney, and brain. Environ Toxicol Chem 30: 920–929. 10.1002/etc.459. [DOI] [PubMed] [Google Scholar]
- Hõrak P, Cohen A (2010) How to measure oxidative stress in an ecological context: methodological and statistical issues. Functional Ecology 24: 960–970. 10.1111/j.1365-2435.2010.01755.x. [DOI] [Google Scholar]
- Huber N, Fusani L, Ferretti A, Mahr K, Canoine V (2017) Measuring short-term stress in birds: comparing different endpoints of the endocrine-immune interface. Physiol Behav 182: 46–53. 10.1016/j.physbeh.2017.09.017. [DOI] [PubMed] [Google Scholar]
- IPCC (2021) Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Synthesis Report. Cambridge University Press [Google Scholar]
- Jenni-Eiermann S, Jenni L, Smith S, Costantini D (2014) Oxidative stress in endurance flight: an unconsidered factor in bird migration. PloS One 9: e97650. 10.1371/journal.pone.0097650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP (2006) Redefining oxidative stress. Antioxid Redox Signal 8: 1865–1879. 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Jonsen ID, Patterson TA, Costa DP, Doherty PD, Godley BJ, Grecian WJ, Guinet C, Hoenner X, Kienle SS, Robinson PWet al. (2020) A continuous-time state-space model for rapid quality control of Argos locations from animal-borne tags. Movement Ecology 8: 31. 10.1186/s40462-020-00217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach E, Griffiths R, Crane JE, Furness RW (2004) Effects of experimentally increased egg production on female body condition and laying dates in the great skua Stercorarius skua. Journal of Avian Biology 35: 501–514. 10.1111/j.0908-8857.2004.03271.x. [DOI] [Google Scholar]
- Krebs CJ, Danell K, Angerbjörn A, Agrell J, Berteaux D, Bråthen KA, Danell Ö, Erlinge S, Fedorov V, Fredga Ket al. (2003) Terrestrial trophic dynamics in the Canadian Arctic. Can J Zool 81: 827–843. 10.1139/z03-061. [DOI] [Google Scholar]
- Landys MM, Piersma T, Guglielmo CG, Jukema J, Ramenofsky M, Wingfield JC (2005) Metabolic profile of long-distance migratory flight and stopover in a shorebird. Proceedings of the Royal Society B: Biological Sciences 272: 295–302. 10.1098/rspb.2004.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landys-Ciannelli MM, Jukema J, Piersma T (2002) Blood parameter changes during stopover in a long-distance migratory shorebird, the bar-tailed godwit Limosa lapponica taymyrensis. Journal of Avian Biology 33: 451–455. 10.1034/j.1600-048X.2002.03051.x. [DOI] [Google Scholar]
- Larcombe SD, Tregaskes CA, Coffey JS, Stevenson AE, Alexander L, Arnold KE (2008) The effects of short-term antioxidant supplementation on oxidative stress and flight performance in adult budgerigars Melopsittacus undulatus. J Exp Biol 211: 2859–2864. 10.1242/jeb.017970. [DOI] [PubMed] [Google Scholar]
- Lin Y, Patterson A, Jimenez AG, Elliott K (2022) Altered oxidative status as a cost of reproduction in a seabird with high reproductive costs. Physiol Biochem Zool 95: 35–53. 10.1086/717916. [DOI] [PubMed] [Google Scholar]
- Lisovski S (2016) R Package TwGeos: : Basic data processing for light based geolocation archival tags
- Madliger CL, Love OP, Hultine KR, Cooke SJ (2018) The conservation physiology toolbox: status and opportunities. Conservation Physiology 6. 10.1093/conphys/coy029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer AD, Fasanello VJ, Tindle K, Frenz BJ, Ziur AD, Fischer CP, Fletcher KL, Seecof OM, Gronsky S, Vassallo BGet al. (2019) Is there an oxidative cost of acute stress? Characterization, implication of glucocorticoids and modulation by prior stress experience. Proceedings of the Royal Society B: Biological Sciences 286: 20191698. 10.1098/rspb.2019.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory ML, Boadway KA, Davis SE, Maftei M, Diamond AW (2017) Breeding biology of Arctic terns (Sterna paradisaea) in the Canadian high Arctic. Polar Biology 40: 1515–1525. 10.1007/s00300-016-2072-1. [DOI] [Google Scholar]
- Mallory ML, Gilbert CD (2008) Leg-loop harness design for attaching external transmitters to seabirds. Marine Ornithology 36: 183–188. [Google Scholar]
- Marasco V, Stier A, Boner W, Griffiths K, Heidinger B, Monaghan P (2017) Environmental conditions can modulate the links among oxidative stress, age, and longevity. Mech Ageing Dev 164: 100–107. 10.1016/j.mad.2017.04.012. [DOI] [PubMed] [Google Scholar]
- McIntyre C (2007) Apparent Changes in Abundance and Distribution of Birds in Denali National Park. In Alaska Park Science, pp. 62–65
- McKnight A, Allyn AJ, Duffy DC, Irons DB (2013) ‘Stepping stone’ pattern in Pacific Arctic tern migration reveals the importance of upwelling areas. Mar Ecol Prog Ser 491: 253–264. 10.3354/meps10469. [DOI] [Google Scholar]
- Meeker AL, Marzluff JM, Gardner B (2022) Historical avifaunal change and current effects of hiking and road use on avian occupancy in a high-latitude tundra ecosystem. Ibis 164: 423–436. 10.1111/ibi.13034. [DOI] [Google Scholar]
- Messina S, Edwards DP, Eens M, Costantini D (2018) Physiological and immunological responses of birds and mammals to forest degradation: a meta-analysis. Biol Conserv 224: 223–229. 10.1016/j.biocon.2018.06.002. [DOI] [Google Scholar]
- Mitteroecker P, Bookstein F (2007) The conceptual and statistical relationship between modularity and morphological integration. Syst Biol 56: 818–836. 10.1080/10635150701648029. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12: 75–92. 10.1111/j.1461-0248.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Nikolaidis MG, Kyparos A, Spanou C, Paschalis V, Theodorou AA, Vrabas IS (2012) Redox biology of exercise: an integrative and comparative consideration of some overlooked issues. J Exp Biol 215: 1615–1625. 10.1242/jeb.067470. [DOI] [PubMed] [Google Scholar]
- Noonan MJ, Fleming CH, Akre TS, Drescher-Lehman J, Gurarie E, Harrison A-L, Kays R, Calabrese JM (2019) Scale-insensitive estimation of speed and distance traveled from animal tracking data. Movement Ecology 7: 35. 10.1186/s40462-019-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard GC, Boardman TJ (1999) The use of percentages and size-specific indices to normalize physiological data for variation in body size: wasted time, wasted effort? Comp Biochem Physiol A Mol Integr Physiol 122: 37–44. 10.1016/S1095-6433(98)10170-8. [DOI] [Google Scholar]
- Pap PL, Vincze O, Fülöp A, Székely-Béres O, Pătraș L, Pénzes J, Vágási CI (2018) Oxidative physiology of reproduction in a passerine bird: a field experiment. Behav Ecol Sociobiol 72: 18. 10.1007/s00265-017-2434-x. [DOI] [Google Scholar]
- Pepin N, Bradley RS, Diaz HF, Baraer M, Caceres EB, Forsythe N, Fowler H, Greenwood G, Hashmi MZ, Liu XDet al. (2015) Elevation-dependent warming in mountain regions of the world. Nature Climate Change 5: 424–430. 10.1038/nclimate2563. [DOI] [Google Scholar]
- Pierce BJ, McWilliams SR (2014) The fat of the matter: how dietary fatty acids can affect exercise performance. Integrative and Comparative Biology 54: 903–912. 10.1093/icb/icu098. [DOI] [PubMed] [Google Scholar]
- Post E, Forchhammer MC, Bret-Harte MS, Callaghan TV, Christensen TR, Elberling B, Fox AD, Gilg O, Hik DS, Høye TTet al. (2009) Ecological dynamics across the Arctic associated with recent climate change. Science 325: 1355–1358. 10.1126/science.1173113. [DOI] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276. 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratte I, Boadway KA, Davis SE, Maftei M, Mallory ML (2017) Diet dichotomy between two migrant seabirds breeding near a high Arctic polynya. R Soc Open Sci 4: 160982. 10.1098/rsos.160982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018) R: A language and environment for statistical computing
- Raiho AM, Scharf HR, Roland CA, Swanson DK, Stehn SE, Hooten MB (2022) Searching for refuge: a framework for identifying site factors conferring resistance to climate-driven vegetation change. Diversity and Distributions 28: 793–809. 10.1111/ddi.13492. [DOI] [Google Scholar]
- Ravasz E, Barabási AL, Somera AL, Mongru DA, Oltvai ZN (2002) Hierarchical organization of modularity in metabolic networks. Science 297: 1551–1555. 10.1126/science.1073374. [DOI] [PubMed] [Google Scholar]
- Robards MD, Anthony JA, Rose GA, Piatt JF (1999) Changes in proximate composition and somatic energy content for Pacific sand lance (Ammodytes hexapterus) from Kachemak Bay, Alaska relative to maturity and season. J Exp Mar Biol Ecol 242: 245–258. 10.1016/S0022-0981(99)00102-1. [DOI] [Google Scholar]
- Robertson GS, Bolton M, Grecian WJ, Wilson LJ, Davies W, Monaghan P (2014) Resource partitioning in three congeneric sympatrically breeding seabirds: foraging areas and prey utilization. The Auk 131: 434–446. 10.1642/AUK-13-243.1. [DOI] [Google Scholar]
- Schull Q, Viblanc VA, Stier A, Saadaoui H, Lefol E, Criscuolo F, Bize P, Robin J-P (2016) The oxidative debt of fasting: evidence for short- to medium-term costs of advanced fasting in adult king penguins. J Exp Biol 219: 3284–3293. 10.1242/jeb.145250. [DOI] [PubMed] [Google Scholar]
- Scopel LC, Diamond AW (2018) Predation and food–weather interactions drive colony collapse in a managed metapopulation of Arctic terns (Sterna paradisaea). Can J Zool 96: 13–22. 10.1139/cjz-2016-0281. [DOI] [Google Scholar]
- Sies H (1991) Oxidative stress: from basic research to clinical application. Am J Med 91: S31–S38. 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- Sittler B, Aebischer A, Gilg O (2011) Post-breeding migration of four long-tailed Skuas (Stercorarius longicaudus) from North and East Greenland to West Africa. Journal of Ornithology 152: 375–381. 10.1007/s10336-010-0597-6. [DOI] [Google Scholar]
- Skrip MM, Bauchinger U, Goymann W, Fusani L, Cardinale M, Alan RR, McWilliams SR (2015) Migrating songbirds on stopover prepare for, and recover from, oxidative challenges posed by long-distance flight. Ecol Evol 5: 3198–3209. 10.1002/ece3.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrip MM, McWilliams SR (2016) Oxidative balance in birds: an atoms-to-organisms-to-ecology primer for ornithologists. J Field Ornithol 87: 1–20. 10.1111/jofo.12135. [DOI] [Google Scholar]
- Skujina I, Healey AJE, Becquevort S, Shaw PW, McMahon R, Morgan C, Evans C, Taylor R, Hegarty M, McKeown NJ (2019) The complete mitochondrial genome of record-breaking migrant Arctic tern (Sterna paradisaea). Mitochondrial DNA Part B 4: 2738–2739. 10.1080/23802359.2019.1644225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier A, Schull Q, Bize P, Lefol E, Haussmann M, Roussel D, Robin J-P, Viblanc VA (2019) Oxidative stress and mitochondrial responses to stress exposure suggest that king penguins are naturally equipped to resist stress. Sci Rep 9: 8545. 10.1038/s41598-019-44990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydeman WJ, Schoeman DS, Thompson SA, Hoover BA, García-Reyes M, Daunt F, Agnew P, Anker-Nilssen T, Barbraud C, Barrett Ret al. (2021) Hemispheric asymmetry in ocean change and the productivity of ecosystem sentinels. Science 372: 980–983. 10.1126/science.abf1772. [DOI] [PubMed] [Google Scholar]
- Terskaia A, Dial RJ, Sullivan PF (2020) Pathways of tundra encroachment by trees and tall shrubs in the western Brooks Range of Alaska. Ecography 43: 769–778. 10.1111/ecog.05015. [DOI] [Google Scholar]
- Thurman LL, Gross JE, Mengelt C, Beever EA, Thompson LM, Schuurman GW, Hoving CL, Olden JD (2022) Applying assessments of adaptive capacity to inform natural-resource management in a changing climate. Conserv Biol 36: e13838. 10.1111/cobi.13838. [DOI] [PubMed] [Google Scholar]
- Thurman LL, Stein BA, Beever EA, Foden W, Geange SR, Green N, Gross JE, Lawrence DJ, LeDee O, Olden JDet al. (2020) Persist in place or shift in space? Evaluating the adaptive capacity of species to climate change. Front Ecol Environ 18: 520–528. 10.1002/fee.2253. [DOI] [Google Scholar]
- Tsahar E, Arad Z, Izhaki I, Guglielmo C (2006) The relationship between uric acid and its oxidative product allantoin: a potential indicator for the evaluation of oxidative stress in birds. J Comp Physiol B 176: 653–661. 10.1007/s00360-006-0088-5. [DOI] [PubMed] [Google Scholar]
- U.S. Fish and Wildlife Service (2009) Alaska Seabird Conservation Plan
- Visser ME (2008) Keeping up with a warming world; assessing the rate of adaptation to climate change. Proceedings: Biological Sciences 275: 649–659. 10.1098/rspb.2007.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EC, Prevolsek JS, Wynne-Edwards KE, Williams TD (2008a) Hematological changes associated with egg production: estrogen dependence and repeatability. J Exp Biol 211: 400–408. 10.1242/jeb.011205. [DOI] [PubMed] [Google Scholar]
- Wagner EC, Stables CA, Williams TD (2008b) Hematological changes associated with egg production: direct evidence for changes in erythropoiesis but a lack of resource dependence? J Exp Biol 211: 2960–2968. 10.1242/jeb.017897. [DOI] [PubMed] [Google Scholar]
- Wagner PD (1996) Determinants of maximal oxygen transport and utilization. Annu Rev Physiol 58: 21–50. 10.1146/annurev.ph.58.030196.000321. [DOI] [PubMed] [Google Scholar]
- Wiley RH, Lee DS (2020) Long-tailed Jaeger (Stercorarius longicaudus). In Billerman SM, ed, Birds of the World. Cornell Lab of Ornithology, NY [Google Scholar]
- Williams TD (2012) Physiological Adaptations for Breeding in Birds. Princeton University Press, Princeton, NJ [Google Scholar]
- Williams TD, Challenger WO, Christians JK, Evanson M, Love O, Vezina F (2004) What causes the decrease in haematocrit during egg production? Functional Ecology 18: 330–336. 10.1111/j.0269-8463.2004.00829.x. [DOI] [Google Scholar]
- Williams TD, Fronstin RB, Otomo A, Wagner E (2012) Validation of the use of phenylhydrazine hydrochloride (PHZ) for experimental manipulation of haematocrit and plasma haemoglobin in birds.(report). Ibis 154: 21–29. 10.1111/j.1474-919X.2011.01195.x. [DOI] [Google Scholar]
- Wong JB, Lisovski S, Alisauskas RT, English W, Harrison A-L, Kellett DK, Maftei M, Nagy-MacArthur A, Ronconi RA, Smith PAet al. (2022) Variation in migration behaviors used by Arctic terns (Sterna paradisaea) breeding across a wide latitudinal gradient. Polar Biology 45: 909–922. 10.1007/s00300-022-03043-2. [DOI] [Google Scholar]
- Wong JB, Lisovski S, English W, Giroux M-A, Harrison A-L, Kellett D, Lecomte N, Maftei M, Nagy-MacArthur A, Ronconi Ret al. (2021) Shared migration routes among Arctic terns: implications for international management. Mar Ecol Prog Ser 671: 191–206. 10.3354/meps13779. [DOI] [Google Scholar]
- Wotherspoon S, Sumner M, Lisovski S (2013) R Package SGAT: Solar/satellite geolocation for animal tracking
- Yap KN, Dick MF, Guglielmo CG, Williams TD (2018) Effects of experimental manipulation of hematocrit on avian flight performance in high- and low-altitude conditions. J Exp Biol 221: jeb191056. 10.1242/jeb.191056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.