Abstract
p300 and CBP are general transcriptional coactivators implicated in different cellular processes, including regulation of the cell cycle, differentiation, tumorigenesis, and apoptosis. Posttranslational modifications such as phosphorylation are predicted to select a specific function of p300/CBP in these processes; however, the identification of the kinases that regulate p300/CBP activity in response to individual stimuli and the physiological significance of p300 phosphorylation have not been elucidated. Here we demonstrate that the cardiotoxic anticancer agent doxorubicin (adriamycin) induces the phosphorylation of p300 in primary neonatal cardiomyocytes. Hyperphosphorylation precedes the degradation of p300 and parallels apoptosis in response to doxorubicin. Doxorubicin-activated p38 kinases α and β associate with p300 and are implicated in the phosphorylation-mediated degradation of p300, as pharmacological blockade of p38 prevents p300 degradation. p38 phosphorylates p300 in vitro at both the N and C termini of the protein, and enforced activation of p38 by the constitutively active form of its upstream kinase (MKK6EE) triggers p300 degradation. These data support the conclusion that p38 mitogen-activated protein kinase regulates p300 protein stability and function in cardiomyocytes undergoing apoptosis in response to doxorubicin.
p300 and its homologue CREB-binding protein (CBP) are general transcriptional coactivators that control many biological activities such as cellular growth, cellular differentiation, tumorigenesis, and apoptosis (see references 10, 19, 20, and 59 for reviews). The well-studied transcriptional regulating properties of p300/CBP are exerted through multiple mechanisms. p300/CBP act as bridging proteins and connect different sequence-specific transcription factors to the general transcription elements. Histone/factor acetyltransferase (HAT/FAT) enzymatic activity endows p300/CBP with the capacity to activate transcription by influencing chromatin structure and function through acetylation of nucleosomal histones and other transcription factors (10, 20). However, little information is available on the mechanisms that regulate p300/CBP function, including posttranscriptional events such as phosphorylation.
Phosphorylated p300 can be detected in both quiescent and proliferating cells, although the overall levels of p300 phosphorylation fluctuate along with cell cycle progression and in response to extracellular cues (69). For instance, in undifferentiated F9 cells, p300 is found in the hypophosphorylated form, and induction of differentiation on treatment with retinoic acid or infection with adenovirus E1A correlates with p300 hyperphosphorylation (39). Phosphorylation and ubiquitination of p300 also influence its ability to interact with E1A and simian virus 40 T antigen (5). T antigen binds to the hypophosphorylated form of p300 and might prevent p300/CBP phosphorylation, while E1A stimulates p300/CBP phosphorylation, probably through cyclin-cyclin-dependent kinase (CDK) complexes (18). p300/CBP can be phosphorylated by cyclin E-Cdk2 (1, 52), and cyclin E-Cdk2 phosphorylation of CBP stimulates its intrinsic HAT activity, which is likely to activate the expression of S-phase genes repressed in early G1 (1). However, this model has been challenged by other studies showing that E1A inhibits p300/CBP and PCAF HAT in vitro (9, 25).
In keeping with the notion that phosphorylation of p300/CBP can convert environmental cues into transcription information, p300/CBP contains several different phosphorylation sites available to extracellular signal activated kinases including protein kinase A, calcium/calmodulin-dependent protein kinase IV, protein kinase C, and AMP-activated protein kinase (see references 10 and 20 for reviews).
A number of residues potentially phosphorylatable by mitogen-activated protein kinases (MAPKs)—that is, proline-directed serines and threonines—are present and evolutionarily conserved in p300 and CBP. MAPKs respond to numerous stimuli and regulate a vast array of cellular processes including gene expression, cell movement, metabolism, and apoptosis (43). Among the MAPK, p38 proteins play important roles in inflammatory stress responses and in differentiation (33, 54). Recent evidence suggests that the function of p300/CBP can be regulated by MAPK (32). MAPK signaling also regulates hypertrophic growth in response to developmental signals or physiologic stimuli (8, 77) and controls apoptosis, although both proapoptotic and antiapoptotic roles have been ascribed to p38 MAPK (see references 6, 14, and 66 for reviews).
p300 is essential for heart development (58, 73), regulates cardiac cell-specific gene transcription (see reference 71 for a review), and plays a critical role in cardiac hypertrophy through its HAT activity (24, 70). The transcription of cardiac cell-specific genes is suppressed by the antineoplastic anthracycline doxorubicin (adriamycin), which leads to degenerative cardiomyopathy and heart failure (31). While uncovering the fundamental mechanisms responsible for the disregulation of transcription mediated by doxorubicin, we found that the agent induces a selective down-regulation of cardiac transcription factor mRNAs as well as a selective loss of p300 protein mediated by the proteasome (53). In this study, we investigate the mechanisms of p300 degradation by doxorubicin. We provide evidence that the doxorubicin-activated p38 pathway contributes to p300 degradation in cardiomyocytes undergoing apoptosis induced by anthracyclines. This new mode of regulation of p300 function may well be critical for the onset and the development of the cardiomyopathy induced by the chemotherapeutic agent and may be a general mechanism of regulating p300 activity.
MATERIALS AND METHODS
Reagents and antibodies.
Doxorubicin, H7, staurosporine, SB 202190, and SB 203580 were purchased from Calbiochem. The proteasome inhibitors MG-132 was purchased from Peptides International Inc. The anti-p300 polyclonal antibody (N-15), antiphosphothreonine (H-2), anti-β-catenin (E-5), anti-pp38 (D-8), anti-p53 polyclonal antibody, HA probe, and anti-GATA-4 polyclonal antibody were purchased from Santa Cruz. Secondary antibodies were from Amersham.
Primary neonatal cardiomyocyte isolation, and cell culture.
Neonatal rat cardiac myocytes from 2- to 3-day-old Sprague-Dawley rats were prepared as previously described (53). Briefly, cells were obtained by trypsinization after gentle mechanical disruption. The cells were washed, preplated to reduce non-myocardial cell contamination, and maintained at 37°C with 5% CO2 in modified Eagle's medium containing 5% calf serum, 2 mM glutamine, 1% penicillin-streptomycin, and 1% 5-bromodeoxyuridine. The medium was replaced every 2 days.
Treatment with doxorubicin and MG-132.
The cardiomyocytes were maintained for the indicated times in doxorubicin-free medium or in medium supplemented with 1 μM doxorubicin. Cardiomyocytes were treated with the proteasome inhibitor MG-132 for the indicated times and at the indicated concentrations. Control cells were treated with the vehicle solvent dimethyl sulfoxide.
Western blot analysis.
Nuclear extracts were prepared as previously described (53). Briefly, the cells were washed and scraped in lysis buffer (20 mM HEPES [pH 7.6], 20% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 10 mM NaCl) supplemented with freshly prepared protease and phosphate inhibitors (PPI) (1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, leupeptin and pepstatin at 10 μg/ml, and aprotinin at 100 μg/ml). They were lysed in a Dounce homogenizer. After centrifugation at 3000 rpm for 15 min, the supernatant fraction was discarded and the pellet was resuspended in cold nuclear extract buffer (20 mM HEPES [pH 7.6], 20% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 500 mM NaCl) supplemented with PPI. Cellular debris were removed by centrifugation at 12,000 rpm for 15 min at 4°C, and the supernatant containing nuclear proteins was assayed for protein (Bradford method). Equal amounts of nuclear proteins were electrophoresed on 4, 4 to 10%, or 4 to 20% Tris-glycine gels (Invitrogen) and transferred to nitrocellulose membranes (Hybond ECL; Amersham). The membranes were blocked for 30 min at room temperature in TBS (10 mM Tris-HCl [pH 8], 150 mM NaCl)-5% nonfat dry milk-0.05% Tween 20 and incubated with the indicated primary antibody overnight at 4°C. Incubation with a secondary antibody (anti-mouse or anti-rabbit immunoglobulin G [IgG]; Amersham) was carried out for 1 h at room temperature. After the membrane was washed, the antigen-antibody reaction was visualized with chemiluminescent reagent (Amersham).
Alkaline phosphatase and phosphatase inhibitor treatment.
Nuclear extracts (30- to 50-μg portions) were prepared from control cardiomyocytes and were incubated at 37°C for 30 min with calf intestine alkaline phosphatase (CIAP) in a buffer containing 50 mM Tris-HCl (pH 8) in the absence or presence of phosphatase inhibitors including 10 mM sodium phosphatase, 15 mM pyrophosphate, 5 mM sodium fluoride, and 0.1 mM sodium orthovanadate. The reaction product was then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 4% Tris-glycine gel (Invitrogen). After transfer, the membrane was blocked for 30 min at room temperature in TBS and incubated overnight at 4°C with an anti-p300 antibody (N-15; Santa Cruz). After incubation with a secondary antibody, the antigen-antibody reaction was visualized by cheminoluminescence.
Immunoprecipitation.
Endogenous p300 was immunoprecipitated from 100-μg portions of nuclear extracts with 4 μg of anti-p300 antibody (N-15) for 2 h at 4°C in a buffer containing 20 mM NaH2PO4 (pH 7.8), 160 mM NaCl, 0.1% NP-40, 5 mM EDTA, 1 mM dithiothreitol, supplemented with freshly made PPI. Phosphoproteins were immunoprecipitated with antiphosphothreonine (H-2; Santa Cruz) or control IgG (Santa Cruz). The extracts were then preadsorbed on protein A/G PLUS agarose (Santa Cruz) for 2 h at 4°C with rocking and washed three times. The agarose resin was recovered by centrifugation and resuspended in 20 μl of SDS loading dye. Samples were then analyzed by SDS-PAGE on 4 to 10 or 4 to 20% Tris-glycine gradient gels. After transfer, the membranes were blocked and incubated overnight at 4°C with primary antibodies. After the secondary reaction, the immunocomplexes were detected by chemiluminescence as described above.
Metabolic labeling and immunoprecipitation.
Neonatal rat cardiomyocytes were maintained in phosphate-free medium for 4 to 5 h and were then labeled with [32P]orthophosphate (ICN; 5 mCi/10 μl). The cells were maintained for 15 h in phosphate- free medium containing 5% dialyzed fetal bovine serum or in the same medium supplemented with 1 μM doxorubicin. Cardiomyocytes were also treated with 1 μM doxorubicin or cotreated with doxorubicin and 30 μM MG-132 for 21 h. After being labeled, the cells were extensively washed and lysed in RIPA buffer (Santa Cruz) supplemented with freshly made PPI and inhibitors (20 mM NaF, 20 mM β-glycerophosphate, 20 mM sodium orthovanadate, and 1 μM okadaic acid). Endogenous p300 was immunoprecipitated with an anti-p300 antibody (N-15), and was preadsorbed on protein A/G PLUS agarose. Cell extracts were also immunoprecipitated with a control IgG antibody. After being washed, the agarose resin was recovered by centrifugation and resuspended in SDS-loading dye. The samples were analyzed by SDS-PAGE on 4 to 12% Tris-glycine gradient gels. After transfer, the membranes were blocked and incubated with anti-p300 primary antibody. Immunocomplexes were detected by chemiluminescence. Phosphorylation of p300 was visualized by autoradiography after exposure of the membrane at −80°C.
Kinase inhibitor treatment.
Neonatal cardiomyocytes plated in 10-cm dishes were maintained for 12 h in regular medium or in medium supplemented with 1 μM doxorubicin. Control and doxorubicin-treated cardiomyocytes were also cotreated with the serine/threonine inhibitor H7 (3 μM), staurosporine (1 nM), or the p38 MAPK inhibitor SB202190 or SB203580 at the indicated concentrations for the indicated time. After treatment, nuclear extracts were prepared and the p300 protein level was analyzed by SDS-PAGE on a 4 to 12% Tris-glycine gradient gel. After overnight transfer, the membranes were probed with an anti-p300 antibody and immune complexes were detected by chemiluminescence as described above. The membranes were then stripped and reprobed with anti-β catenin antibody.
Immunofluorescence.
Primary neonatal cardiomyocytes were plated on coverslides in six-well dishes at a density of 0.5 × 106 cells/well. They were exposed to 1 μM doxorubicin for the indicated times. After fixation in paraformaldehyde, the cells were permeabilized and stained with anti-p300 antibody (N-15; dilution 1:100), anti-pp38 (dilution 1:50) or anti-p53 (dilution 1:50) followed by fluorescein isothiocyanate-conjugated anti-rabbit or anti-mouse IgG (Sigma; dilution, 1:100). The cover slides were mounted with Vectashield mounting media (Vector Laboratories). When indicated, the mounting medium also contained 4′,6-diamidino-2-phenylindole (DAPI) for visualization of the nuclei. Signals were observed by confocal microscopy (LSM 510; Zeiss).
Generation of recombinant p300 proteins by homologous recombination and amplification in Sf9 insect cells.
p300 full length (amino acids 1 to 2414), p300(965-1810), p300(671-1196), and p300(1135-2414) constructs were cotransfected with linearized baculovirus genome DNA into Sf9 cells. All the p300 deletion constructs were inserted downstream of a Flag epitope to facilitate the detection and purification of the proteins. The recombinant baculoviruses containing p300 deletion constructs were then amplified at a multiplicity of infection of 0.1 in Sf9 insect cells. Recombinant proteins were produced by infection of BTI-TN-5B1-4 insect cells at a multiplicity of infection of 5 to 20. At 3 days postinfection, the cells were harvested and cell extracts were prepared by a 45-min incubation of the cell pellets in a lysis buffer (10% glycerol, 20 mM Tris-HCl [pH 9], 0.2 mM EDTA, 0.2% Tween 20, 0.5 M KCl) on ice followed by centrifugation at 40,000 × g for 45 min.
Purification of recombinant p300 proteins with anti-Flag-M2 agarose affinity gels.
After amplification, the recombinant p300 proteins were purified using anti-Flag-M2 agarose affinity gels (Sigma). Briefly, at 72 h after infection, the Sf9 cells were harvested on ice and centrifuged at 1,600 to 2,000 rpm at 4°C in a Sorvall RT7 plus centrifuge (RTH250 rotor). After being washed with cold phosphate-buffered saline, the cells were dissolved in extraction buffer (10% glycerol, 20 mM Tris-HCl [pH 8], 0.2mM EDTA, 0.1% Tween 20, 0.5 M KCl, β-mercaptoethanol) supplemented with PPI. After freezing-thawing cycles, the extracts were shaken at 4°C for 30 min and were then centrifuged at 15,000 rpm at 4°C for 25min. A 1-ml volume of extract was then mixed with 100 μl of prewashed M2 agarose resin and incubated at 4°C overnight with shaking. The resin was then washed five times with extraction buffer and once with elution/storage buffer (10% glycerol, 20 mM Tris-HCl [pH 8], 0.2 mM EDTA, 0.1% Tween 20, 0.25 M KCl, β-mercaptoethanol) supplemented with PPI. The proteins were eluted by incubation for 1 h at 4°C with 100 μl of Flag peptide (100 ng/μl). After centrifugation at 3,000 × g for 30 s, the supernatant containing the purified recombinant p300 proteins was stored at −80°C.
In vitro kinase assay.
Recombinant p38 β protein (Calbiochem) was incubated in a kinase buffer (20 mM HEPES [pH 7.6], 10 mM MgCl2, 20 mM β-glycerolphosphate, 20 μM ATP). Reactions were initiated by addition of 1 μg of purified recombinant p300 proteins or glutathione S-transferase (GST)-PHAS-1 used as a positive control and 10 μCi of [γ-32P]ATP in 25 μl of kinase buffer. Reactions were carried out for 20 min at 30°C, and the products were analyzed by SDS-PAGE on a 4 to 12% gradient gel (Invitrogen). The gels were stained with Coomassie blue and destained; after the gels were dried, the reaction was visualized by autoradiography.
Adenoviral infection.
Adenoviral infections were performed as described previously (55). Briefly, neonatal primary cardiomyocytes were infected with an adenovirus expressing a hemagglutinin (HA)-tagged MKK6EE (Ad-MKK6EE-HA) (30) or with a control adenovirus expressing the green fluorescent protein (Ad-GFP) at identical titers. At 12 h postinfection, the cells were transferred into medium with or without SB202190 (5 μM) or SB203580 (5 μM); 24 h later, they were placed for another 30 h in fresh medium supplemented with p38 inhibitors with or without doxorubicin. Nuclear extracts were prepared as described above. Equal amounts of nuclear proteins were separated by SDS-PAGE on a 10% Tris-glycine gel. After transfer the proteins to nitrocellulose membranes, p300 was detected by a Western blot assay using an anti-p300 specific antibody. The membrane was then stripped and reprobed with an HA probe to measure the levels of Ad-MKK6EE and with β-catenin. In parallel, cardiomyocytes plated on coverslides were infected with serial dilutions of Ad-MKK6EE-HA or Ad-GFP and were processed for immunofluorescence using an HA antibody (Santa Cruz) as described above.
TUNEL assay.
Apoptosis was detected by using the in situ cell death detection kit, fluorescein (Roche). Cardiomyocytes were plated on coverslides in six-well dishes at a density of 0.5 × 106 cells/well. Untreated cardiomyocytes or cardiomyocytes treated with 1 μM doxorubicin for 24 h were subjected to a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay. Briefly, cells were fixed in 3.7% paraformaldehyde. After permeabilization, each well was incubated with 50 μl of TUNEL reaction mixture for 1 h at 37°C in the dark. As a positive control, permeabilized primary cardiomyocytes were incubated with DNase I (final concentration, 1 μg/μl) for 10 min at 25°C to induce DNA strand breaks. After the cells were washed three times with phosphate-buffered saline, coverslides were mounted with Vectashield mounting medium containing DAPI (Vector Laboratories). Signals were observed by confocal microscopy. An average of 100 nuclei were counted in each of 10 different fields on each slide. The apoptotic index (percentage of apoptotic nuclei) was calculated as (number of apoptotic nuclei by TUNEL assay/number of nuclei by DAPI staining) × 100%.
Quantitative reverse transcriptase PCR assay.
Total RNA was prepared with TriZol reagent (Invitrogen) from neonatal rat cardiac myocytes untreated, treated with 1 μM doxorubicin, or infected with Ad-GFP or Ad-MKK6EE. Relative quantitative reverse transcriptase PCR was carried out as described previously (53), using the RETROscript and QuantumRNA kits (Ambion). Briefly 2 μg of total RNA was used for first-strand cDNA synthesis, using 100 U of Moloney murine leukemia virus reverse transcriptase. Random primers were used in a 20-μl reaction volume in the presence of 10 μM deoxynucleoside triphosphates and of reverse transcription buffer (10 mM Tris-Cl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2) and [32P]dCTP. PCR amplifications were carried out using gene-specific primers under conditions of linear range. To compensate for differences in RNA quality and random tube-to-tube variation, rRNA primers were used as an internal control. The factors that we wanted to amplify were less abundant than the rRNA. To amplify them in the same linear range as the internal control, 18S primers were mixed with competimers. PCR products were separated on 5% acrylamide gels and quantitated with a STORM scanner (Molecular Dynamics). Results were normalized relative to 18S RNA expression. The sequence of the primers used were as follows: β-myosin heavy chain (β-MHC) sense, 5′-CCAACACCAACCTGTCCAA-3′, and antisense, 5′-ACTCTTCATTCAGGCCCTTG-3′; cardiac troponin I (cTnI) sense, 5′-GATGGAAGCGATGCGGCTG-3′, and antisense, 5′-GCATAGGTCCTGAAGCTCTTC-3′; GATA- 4 sense, 5′-TGTGCCAACTGCCAGACTAC-3′, and antisense, 5′-GCATCTCTTCACTGCTGCTG-3′.
RESULTS
p300 phosphorylation in primary neonatal cardiomyocytes.
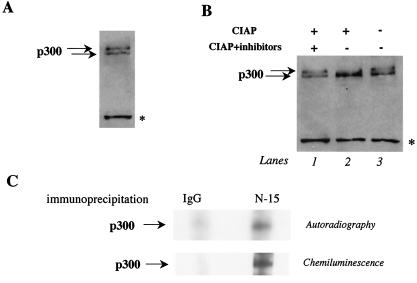
When we analyzed the mobility of p300 protein in untreated neonatal cardiomyocytes by Western blot analysis on a 4% Tris-glycine gel, we found that p300 migrated as two bands, probably reflecting differently charged molecules (Fig. 1A). Since p300 had previously been reported to be phosphorylated in vitro and in vivo, we hypothesized that these changes could reflect phosphorylation and dephosphorylation of p300 rather than degradation products. To establish this, we analyzed p300 mobility from nuclear extracts treated with CIAP or cotreated with CIAP and phosphatase inhibitors (39). In vitro phosphatase treatment of cardiac nuclear extracts resulted in a discrete shift of the slower-migrating form of p300 to the faster-migrating species of p300 (Fig. 1B, lane 2). This shift was inhibited by treatment with phosphatase inhibitors (lane 3), confirming that changes in phosphorylation-dephosphorylation of the protein account for the vast majority of the mobility differences observed. To fully establish that p300 is phosphorylated in cardiomyocytes in vivo, cells were labeled with [32P]orthophosphate and the phosphorylation status of p300 was determined by autoradiography after immunoprecipitation of p300 from the cells. Phosphorylation of p300 could be detected in untreated cardiomyocytes (Fig. 1C, upper panel). Proteins immunoprecipitated with a control IgG antibody gave no detectable signal confirming the specificity of the p300 signal. A cheminoluminescence reaction was performed after autoradiography and confirmed that the detected band corresponded to p300 protein (Fig. 1C, lower panel). These results demonstrate that a significant proportion of p300 is phosphorylated in primary neonatal cardiomyocytes. Interestingly, the phosphorylation of p300 observed in primary neonatal cardiomyocytes is unrelated to cell cycle progression as previously reported, since the cells used in our study are terminally differentiated.
FIG. 1.
p300 phosphorylation in primary neonatal cardiomyocytes in culture. (A) p300 has slow- and fast-mobility forms in cardiomyocytes. Nuclear extracts were prepared from control cardiomyocytes maintained in cultured for 1 week and were analyzed by Western blotting on a 4% Tris-glycine gel. After transfer of the proteins, the membranes were incubated with anti-p300 antibody overnight at 4°C and p300 mobility was visualized by chemiluminescence. The asterisk represents nonspecific binding of the primary antibody. (B) The slower-migrating form of p300 is eliminated by CIAP treatment. Cardiac nuclear extracts were prepared from control cardiomyocytes and were incubated with CIAP in the absence or presence of phosphatase inhibitors. p300 mobility was then analyzed by Western blotting on a 4% Tris-glycine gel as described for panel A. (C) p300 is phosphorylated in cardiomyocytes. Primary neonatal cardiomyocytes were maintained in medium without phosphate and then labeled with [32P]orthophosphate. Total-cell lysates were immunoprecipitated with an anti-p300 or a control IgG antibody. The proteins were resolved on a 10% Tris-glycine gel. Phosphorylated p300 was visualized by autoradiography, and total p300 protein was visualized by chemiluminescence.
Doxorubicin treatment induces phosphorylation-dependent degradation of p300 in cardiomyocytes.
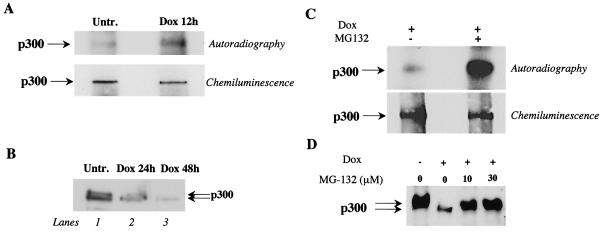
We have previously established that doxorubicin-treated cardiomyocytes express normal levels of p300 mRNA and that the failure of doxorubicin-treated cells to maintain adequate levels of p300 protein is due to a selective increase in degradation of the coactivator within 36 h (53). We next asked whether doxorubicin treatment increases p300 phosphorylation, and the possible relationship between hyperphosphorylation and degradation of p300 by the ubiquitin-proteasome pathway in cardiomyocytes exposed to doxorubicin. To investigate this possibility, we measured the amount of phosphorylated p300 in control cardiomyocytes and in cardiomyocytes treated with doxorubicin for 12 h, just prior to the degradation of the protein. Basal levels of phosphorylated p300 were detected in control cells, as shown in Fig. 1. The level of phosphorylated p300 was significantly increased 12 h after doxorubicin exposure (Fig. 2A, upper panel). We did not observe any change in the phosphorylation state of the protein at earlier time points of drug treatment (data not shown). These results suggest that doxorubicin-induced degradation of p300 coincides with its enhanced phosphorylation.
FIG. 2.
Doxorubicin induces phosphorylation of p300 in cardiomyocytes in culture, and inhibition of the proteasome activity reduces degradation of phosphorylated p300. (A) Primary cardiomyocytes were maintained in medium without phosphate and then labeled with [32P]orthophosphate with or without 1 μM doxorubicin (Dox). Total-cell lysates were prepared, and p300 was immunoprecipitated with an anti-p300 antibody. Phosphorylated p300 was visualized by autoradiography, and p300 protein was visualized by chemiluminescence. (B) Western blot analysis of nuclear extracts prepared from untreated and doxorubicin-treated primary cardiomyocytes for 24 and 48 h. (C) Primary cardiomyocytes were maintained in medium without phosphate and then labeled with [32P]orthophosphate for 15 hr in medium supplemented with 1 μM doxorubicin or in medium containing 1 μM doxorubicin plus 10 μM MG-132. Total-cell lystates were prepared, and p300 was immunoprecipitated with anti-p300 antibody. Phosphorylated p300 and total p300 were visualized as described for panel A. (D) Primary cardiocytes were treated with doxorubicin for 48 h or cotreated with doxorubicin and the proteasome inhibitor MG-132 at 10 and 30 μM. Nuclear extracts were prepared and separated on 4% Tris-glycine gel, and p300 protein was visualized with an anti-p300 antibody by cheminoluminescence.
We next established the relationship between p300 phosphorylation and degradation in doxorubicin-treated cardiomyocytes. Western blot analysis of control nuclear extracts on an SDS-4% polyacrylamide gel revealed both the slow-migrating and the fast-migrating species of p300 (Fig. 2B, lane 1). After doxorubicin treatment, the slow-migrating form disappeared and only the fast-migrating species of p300, corresponding to hypophosphorylated p300, was detected (lanes 2 and 3). Similar results were obtained from immunoprecipitation experiments (data not shown). These results suggest that the slow-migrating form of p300, corresponding to phosphorylated or hyperphosphorylated p300, is preferentially degraded after doxorubicin treatment, leading to the accumulation of the hypophosphorylated form of p300. To confirm that phosphorylation and degradation are causally linked, we treated cardiomyocytes with doxorubicin and compared the phosphorylation of p300 in the absence and presence of the proteasome inhibitor MG-132. Pretreatment of cardiomyocytes with MG-132, followed by doxorubicin exposure, resulted in the accumulation of the phosphorylated form of p300 (Fig. 2C, upper panel). The amount of p300 immunoprecipitated was similar under both conditions (lower panel). The results of these experiments support the view that enhanced phosphorylation of p300 induced by doxorubicin precedes proteasome degradation of the coactivator. If phosphorylation and proteasome-mediated degradation of p300 are linked events, inhibition of the proteasome should reverse the selective loss of the phosphorylated form of the protein. To test this hypothesis, cardiomyocytes were treated with doxorubicin for 48 h or with the combination of doxorubicin plus the proteasome inhibitor MG-132. p300 mobility was subsequently analyzed by Western blotting. Exposure of cardiomyocytes to doxorubicin resulted in the preferential degradation of the slow-migrating form of the protein, corresponding to hyperphosphorylated p300 as expected (Fig. 2D, second lane from left). In the presence of increasing concentrations of proteasome inhibitor, the slow-migrating form of p300 accumulated (two right lanes). We conclude from this experiment that the proteasome preferentially degrades the hyperphosphorylated fraction of p300.
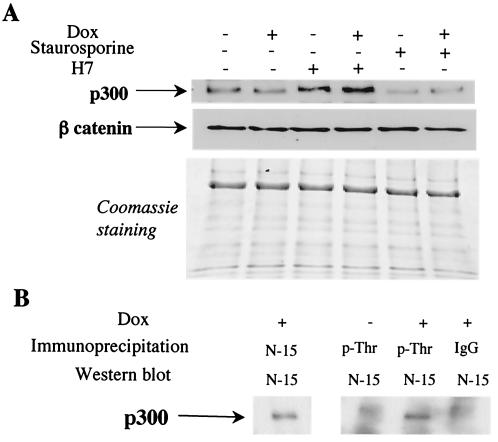
A serine/threonine inhibitor stabilizes p300 protein levels in cardiomyocytes, and doxorubicin treatment induces threonine phosphorylation of p300.
Protein kinases are critical components of many signaling pathways and are activated on treatments of cells with doxorubicin (2, 40, 61). We next attempted to identify which protein kinase(s) might be induced in the response to doxorubicin and might lead to the phosphorylation-dependent degradation of p300. In a preliminary analysis, we tested the effects of two general kinase inhibitors, H7 and staurosporine, on the stability of p300 protein in cardiomyocytes in culture. We used noncytotoxic concentrations of these agents known to inhibit protein kinase C, myosin light-chain kinase, and protein kinase A. Treatment of cardiomyocytes with staurosporine had no effect on levels of p300 in control or in doxorubicin-treated cells (Fig. 3A). However, treatment of the cells with H-7 resulted in a pronounced increase in the p300 protein level in control cardiocytes and also in cells exposed to doxorubicin (Fig. 3A). This effect was specific, since H7 did not change the levels of β-catenin (Fig. 3A). These results suggest that a specific serine/threonine inhibitor stabilizes p300 protein levels in cardiomyocytes in culture.
FIG. 3.
A serine/threonine inhibitor stabilizes the p300 protein level, and doxorubicin (Dox) treatment induces threonine phosphorylation of p300 in cardiomyocytes. (A) Primary neonatal cardiomyocytes were treated with 1 nM staurosporine or 3 μM H-7 and maintained in normal medium or in doxorubicin-containing medium for 12 h. The same amounts of cellular proteins were separated on 4 to 20% Tris-glycine gradient gels. After transfer, p300 was visualized with a specific antibody. The membrane was stripped and reprobed with a β-catenin antibody. The proteins remaining in the gel after transfer were stained with Coomassie brilliant blue, also used as a loading control. (B) Cardiomyocytes were maintained in normal medium or in medium supplemented with 1 μM doxorubicin. After 16 h, nuclear extracts were prepared and immunoprecipitated with the indicated antibodies, IgG, anti-p300, and antiphosphothreonine. After separation of the proteins on a 4 to 12% Tris-glycine gradient get, p300 was visualized by Western blot analysis.
To identify the amino acid residues of p300 that become phosphorylated on doxorubicin treatment, cardiomyocytes were maintained in normal medium or treated with Dox for a short time to prevent the total degradation of the protein. Endogenous proteins phosphorylated on threonine residues were immunoprecipitated with phosphospecific antibodies, and p300 in the precipitates was detected by Western blot analysis using an anti-p300 antibody. Phosphothreonine p300 could easily be detected in cardiomyocytes treated with doxorubicin but not in control cardiomyocytes (Fig. 3B).
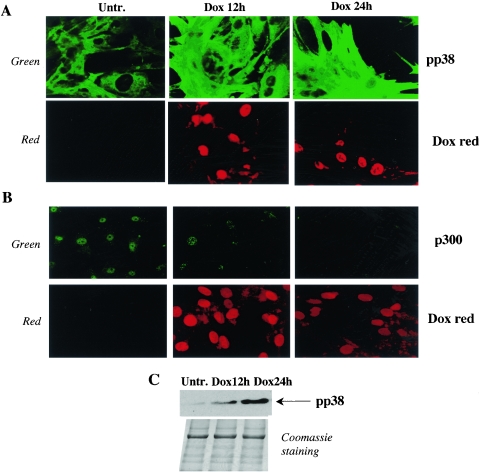
p38 is activated in cardiomyocytes treated with doxorubicin, and a p38 specific inhibitor stabilizes p300 protein and prevents doxorubicin-induced p300 degradation.
A number of reasons suggest that p38 MAPK is a potential candidate as a doxorubicin-activated kinase of p300. First, p38 members phosphorylate their substrates on serine and threonine residues, and our results show that doxorubicin induces threonine phosphorylation of p300 (Fig. 3B). Second, p38 is strongly activated by cellular stress similar to the stress engendered by doxorubicin, such as heat or osmotic shock, UV irradiation, cytokine exposure, or decrease oxygen tension (27, 46, 56). Third, a p38 specific inhibitor is known to block Dox-induced apoptosis in cardiomyocytes (35). To investigate the role of p38 MAPK, we first measured the levels of activated p38 (phosphorylated p38) in doxorubicin-treated cardiocytes by indirect immunofluorescence, using antibodies specific to the phosphorylated form of p38 (pp38). As shown in Fig. 4A, doxorubicin strongly induced p38 phosphorylation after 12 h of treatment and an even stronger activation was detected after 24 h of drug exposure. Importantly, this activation correlated the simultaneous degradation of p300 (Fig. 4B). Increased levels of pp38 were also observed by Western blot analysis after 12 h and 24 h of doxorubicin treatment (Fig. 4C).
FIG. 4.
Doxorubicin activates pp38 in cardiomyocytes. Primary neonatal cardiomyocytes were plated on coverslides and maintained in normal medium (Untr.) or treated with 1 μM doxorubicin for 12 or 24 h (Dox 12 h, Dox 24 h). (A and B) The cells were incubated with pp38(A) or p300 (B) antibodies as described in Materials and Methods. They were then examined by immunofluorescence confocal microscopy using a Zeiss microscope at ×40. The green color visualizes endogenous pp38 and p300 proteins. The red color visualizes cardiac nuclei stained with doxorubicin. (C) Primary neonatal cardiomyocytes were maintained in normal medium or treated with doxorubicin for 12 or 24 h. Equal amounts of cell extracts were run on a 4 to 20% Tris-glycine gradient gel, and pp38 levels were determined by Western blot analysis. The proteins remaining in the gel after transfer were stained with Coomassie brilliant blue.
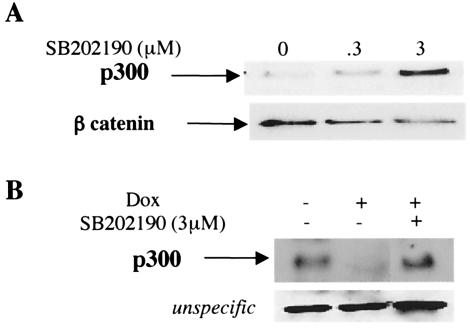
To demonstrate that activation of p38 is a signal for the proteasome degradation of p300, we measured p300 protein levels in cardiomyocytes treated with a p38-specific inhibitor, SB202190 (45). To avoid nonspecific effects of the inhibitor, only concentrations of 5 μM or less were used. Treatment of the cells with the p38 inhibitor resulted in a stabilization of p300 protein in cardiomyocytes. β-Catenin levels were used for the loading control (Fig. 5A). Exposure of cardiomyocytes to doxorubicin for 30 h resulted in p300 degradation. Importantly, cotreatment of the cells with the p38 inhibitor prevented p300 degradation (Fig. 5B). These results strongly suggest that the loss of the cofactor p300 in cardiomyocytes treated with doxorubicin is mediated by serine/threonine phosphorylation of p300 and involves the p38 MAPK.
FIG. 5.
Suppression of doxorubicin-induced p300 degradation by inhibition of p38 MAPK. (A) Primary cardiomyocytes were treated with various concentrations of SB 202190, and p300 protein levels were determined by Western blot analysis with an anti-p300 antibody. The same blot was stripped and reprobed with a β-catenin antibody used as a loading control. (B) Cardiomyocytes were maintained in the presence or absence of doxorubicin (Dox) and SB202190 for 30 h. After transfer of the proteins, the membrane was incubated with an anti-p300 antibody and chemiluminescence was performed.
p38 phosphorylates p300 in vitro.
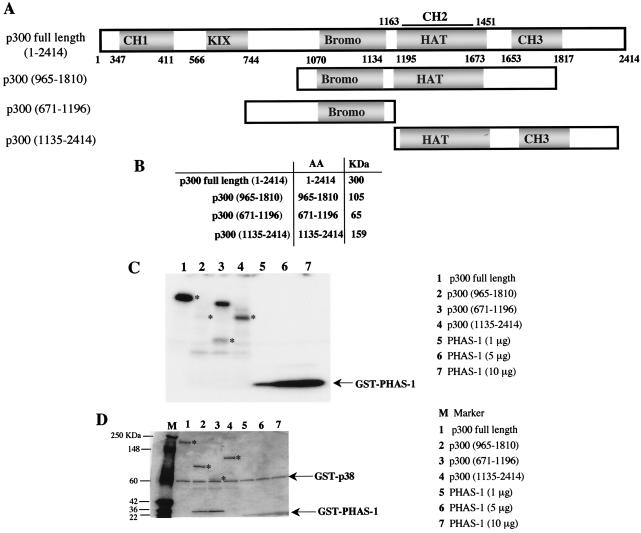
Next we tested the ability of activated p38 to directly phosphorylate p300 in vitro as a substrate by using recombinant p38β and various segments of p300 proteins. p300 full-length (amino acids 1 to 2414), p300(965-1810), p300(671-1196), and p300(1135-2414) were generated by homologous recombination and amplified in Sf9 insect cells (Fig. 6A). Recombinant proteins of the correct size (Fig. 6B) as determined by Coomassie blue staining (Fig. 6D) were purified with anti-Flag-M2 agarose affinity gels. Similar quantities of recombinant p300 polypeptides were used for the in vitro kinase assays, and recombinant PHAS-1 was used as a positive control for phosphorylation by p38 (Fig. 6C). Recombinant p38 efficiently phosphorylates full-length p300 and p300(1135-2414), whereas p300(965-1810) and p300(671-1196) showed no and low phosphorylation, respectively (Fig. 6C). These results indicate that amino acids located at the N-terminal and C-terminal domains of p300 are potential phosphorylatable residues for p38 kinases.
FIG. 6.
p38 phosphorylates p300 in vitro. (A) Schematic representation of recombinant p300 proteins generated by homologous recombination of Sf9 cells. (B) Predicted sizes of the recombinant proteins. p300 full length (amino acids [AA] 1 to 2414), p300(965-1810), p300(671-1196), and p300(1135-2414) were amplified in Sf9 cells and purified with anti-Flaf-M2 agarose affinity gels as described in Materials and Methods. (C) In vitro kinase assay performed with 1 μg of recombinant p300 proteins and recombinant. GST-p38β. GST-PHAS-1 (1, 5 and 10 μg) was used as a positive control for phosphorylation by p38. The reactions product were analyzed on a 4 to 12% Tris-glycine gel, and phosphorylation of p300 was visualized by autoradiography after a short exposure of the gel at −80°C. The stars indicates the phosphorylated recombinant p300 proteins. (D) Before being dried, the gel was stained with Coomassie brilliant blue to ensure that the recombinant p300 proteins were of the expected size. M represents protein markers (Invitrogen), and asterisks indicate the migration of the recombinant p300 proteins.
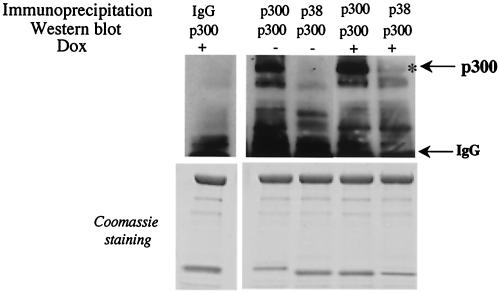
The ability of certain MAPKs to phosphorylate substrates is sometimes mediated by direct interaction with specific protein-protein docking domains (34, 72). To determine whether p300 and p38 stably interact in vivo, we attempted to immunoprecipitate the endogenous proteins. The binding of p38 to p300 was not detected in control cardiomyocytes, whereas a small fraction of p300 was found to physically interact with p38 in doxorubicin-treated cells (Fig. 7). The detection of such small amounts of p38 bound to p300 in doxorubicin-treated cells suggests that stable complex formation is at best transient, a result consistent with the results of other studies (72).
FIG. 7.
Interaction of p300 and p38 in cardiomyocytes in vivo. Primary neonatal cardiomyocytes were maintained for 12 h in normal medium or in medium supplemented with 1 μM doxorubicin (Dox). Nuclear extracts were prepared, and equal amounts of nuclear proteins were immunoprecipitated with anti-p300, anti-pp38, or control IgG antibodies. Immunoprecipitates were run on 4 to 12% Tris-glycine gradient gels, and after the transfer, the membranes were probed with anti-p300 antibody. After incubation with secondary antibodies, the associated proteins were visualized by chemiluminescence. The residual proteins in the gel that did not transfer were stained with Coomassie blue to ensure that equal amounts of cellular proteins were used.
Activation of p38 by constitutive active MAPK kinase 6 (MKK6EE) promotes p300 degradation and cardiac hypertrophy.
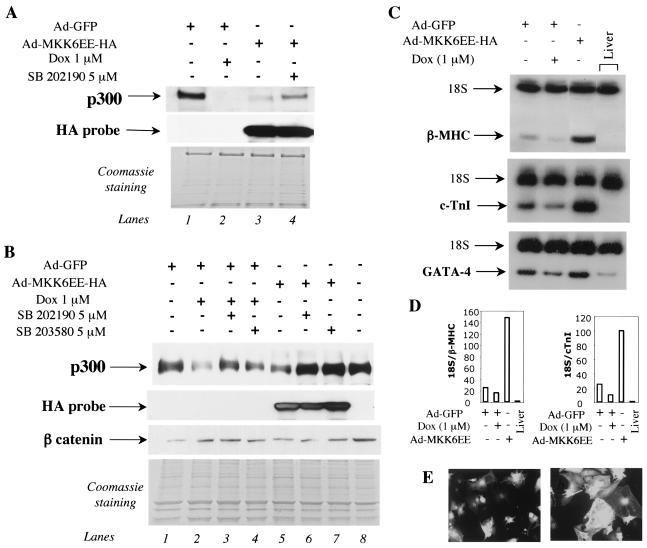
To demonstrate the direct involvement of p38 MAPK in regulating p300 protein stability, we tested whether activation of the p38 MAPK pathway would decrease p300 protein levels in cardiomyocytes in culture. To achieve a homogenous expression of the p38 activator MKK6EE in all the cells, we infected primary cardiomyocytes with an adenovirus expressing a HA-tagged MKK6EE protein or a GFP-encoding adenovirus, as a control. Treatment of the cells with 1 μM doxorubicin decreased p300 protein levels as expected (Fig. 8A and B, compare lanes 1 and 2). Infection of cardiomyocytes with MKK6EE adenovirus but not GFP adenovirus also resulted in a decrease of p300 protein expression (Fig. 8A, compare lanes 1 and 3; Fig. 8B, compare lanes 1 and 5). Addition of a specific inhibitor of p38 α and β, SB202190, abrogated the effect of MKK6EE (Fig. 8A, compare lanes 3 and 4; Fig. 8B, compare lanes 5 and 6). Another p38-specific inhibitor SB203580, also eliminated the effect of MKK6EE on p300 stability (Fig. 8B, compare lanes 5 and 7). As shown in Fig. 5B, treatment of cardiomyocytes with both p38 inhibitors inhibited p300 degradation by Dox (Fig. 8B, compare lane 2 with lanes 3 and 4). Expression of the activated form of MKK6EE in cardiomyocytes was verified by Western blot analysis using an HA probe (Fig. 8A, second panel, lanes 3 and 4; Fig. 8B, second panel, lanes 5 to 7). We ascertained that both GFP and MKK6EE viral preparations had identical titers by infecting primary cardiomyocytes with serial dilutions of viruses and by comparing the number of GFP-positive cells and HA-positive cells using indirect immunofluorescence (data not shown).
FIG. 8.
Enforced activation of p38 MAPK pathway by MKK6 enhances p300 degradation and induces cardiac hypertrophy. (A and B) Primary neonatal cardiomyocytes were infected with a control GFP adenovirus (Ad-GFP) (lanes 1 and 2 of panel A; lanes 1 to 4 of panel B) or with adenovirus expressing a HA-MKK6EE gene (Ad-MKK6EE-HA) (lanes 3 and 4 of panel A; lanes 5 to 7 of panel B) with identical titers, as described previously (55). At 12 h postinfection, cells infected with Ad-MKK6EE-HA were maintained for 30 h in normal medium (lane 3 in panel A; lane 5 in panel B) or in medium supplemented with 5 μM SB 202190 (lane 4 in panel A; lane 6 in panel B) or SB203580 (lane 7 in panel B). Cells were infected with the control Ad-GFP and treated with 1 μM doxorubicin (Dox) for 30 h (lane 2 in panel A; lanes 2 to 4 in panel B). Nuclear extracts were prepared, and nuclear proteins were separated by SDS-PAGE on a 4 to 12% Tris-glycine gradient gel. p300 protein levels were determined by Western blot analysis as described earlier. After the membrane was stripped, expression in the infected cardiomyocytes of exogenous MKK6EE and of β-catenin were measured by Western blot analysis. The residual proteins in the gel that did not transfer were stained with Coomassie brilliant blue, which showed that equal amounts of nuclear proteins were analyzed. (C) Primary cardiomyocytes plated in 10-cm dishes were infected with Ad-GFP or Ad-MKK6EE-HA. At 12 h postinfection, half of the cells infected with Ad-GFP were treated with 1 μM doxorubicin. Cells infected with Ad-MKK6 were maintained in normal medium. At 30 h later, total RNA was extracted and β-MHC, cTnI, and GATA-4 transcripts were measured by quantitative RT-PCR. 18S RNA mixed with competimers was used as an internal control. This experiment was done three times, with three independent preparations of cardiomyocytes. (D) Quantitation of the RT-PCR for β-MHC, cTnI, and GATA-4 in cardiomyocytes infected with Ad-GFP and Ad-MKK6EE. (E) Cell enlargement after infection with Ad-MKK6EE. Cardiomyocytes plated on coverslides were infected with Ad-GFP or Ad-MKK6EE-HA. At 4 days postinfection, GFP-postive cells were observed by confocal microscopy as described in the text. Cells expressing activated MKK6 were visualized by confocal microscopy after indirect immunofluorescence with an HA antibody.
In cultured cardiomyocytes, activated MKK6 induces cell hypertrophy (50, 65, 75), whereas in the animal, inhibition of p38 MAPK leads to a hypertrophic phenotype (8). Therefore, we investigated whether infection with Ad-MKK6EE results in hypertrophic growth in our isolated cardiomyocytes by measuring two hypertrophic markers, the mRNAs for β-myosin heavy chain (β-MHC) and cardiac troponin I (cTnI) by quantitative RT-PCR. Activated MKK6 induced hypertrophy, as evidenced by a strong up-regulation of β-MHC and cTnI mRNA (Fig. 8C and D). Hypertrophy of the cells was also obvious by comparing the cell sizes of low-confluence cultures infected with Ad-GFP or Ad-MKK6 after performing immunofluorescence with an HA antibody. Compared to cells infected with Ad-GFP, the Ad-MKK6EE-infected cells were much larger (Fig. 8E). Taken together, these results show that selective activation of the p38 MAPK by its upstream direct activator—MKK6—leads to cardiac hypertrophy and regulates p300 protein stability.
p300 degradation parallels apoptosis and stabilization of the tumor suppressor p53 in cardiomyocytes treated with doxorubicin.
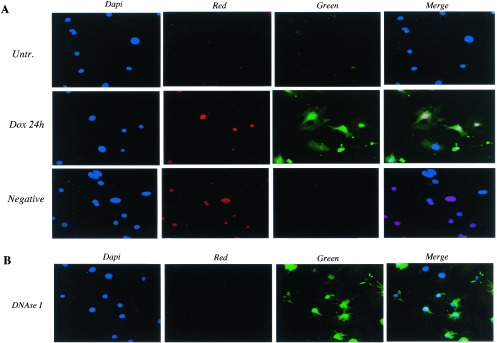
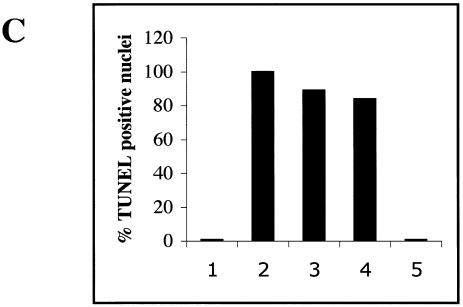
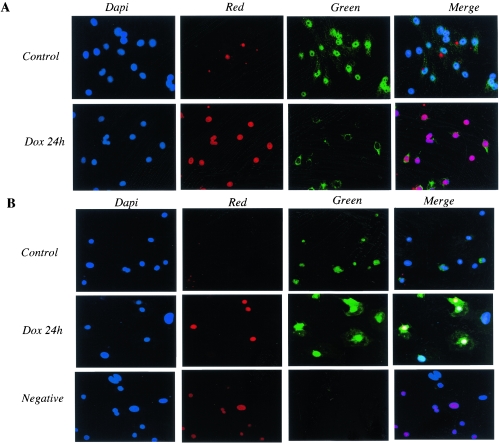
In many cell types, p300 is an integral component of mechanisms involved in the apoptotic pathway. Apoptosis is one mechanism accounting for the cardiotoxic effect of doxorubicin both in vitro and in vivo (4, 35). This represents a potential paradox, since in cardiomyocytes p300 is rapidly degraded in response to doxorubicin and p53 levels rise (53). To clarify the role of p300 in doxorubicin-mediated apoptosis in cardiomyocytes, we examined whether the degradation of p300 following doxorubicin exposure paralleled apoptosis and stabilization of the p53 protein. Doxorubicin treatment of cardiomyocytes for 12 h (data not shown) and 24 h led to a dramatic increase of apoptosis (Fig. 9A, green). DNase I treatment was used as a positive control for the TUNEL assay (Fig. 9B). We observed 89% ± 8.5% apoptotic nuclei after 12 h of doxorubicin exposure and 84% ± 7.5% after 24 h (Fig. 9C). Interestingly, apoptosis was induced in every cardiomyocyte that showed positive nuclear staining for doxorubicin (nuclei stained in red in Fig. 9A). The induction of apoptosis correlated with the degradation of p300 (Fig. 10A). It also paralleled the stabilization of the p53 protein (Fig. 10B) and the induction of p38 MAPK in response to doxorubicin but not following MKK6EE expression (data not shown). Therefore, we conclude that cell death by apoptosis occurs in primary cardiomyocytes treated with doxorubicin with similar kinetics to that of p300 degradation and stabilization of p53.
FIG. 9.
p300 degradation in cardiomyocytes treated with doxorubicin parallels apoptosis. (A) Primary neonatal cardiomyocytes plated on coverslides were maintained in normal medium (Untr.) or in medium supplemented with 1 μM doxorubicin for 24 h (Dox 24 h) and were subjected to the TUNEL assay as described in Materials and Methods. The cells were then mounted with DAPI-containing medium to visualize the nuclei. Apoptotic cells were analyzed by confocal microscopy and were detected by fluorescein (Green). Nuclei stained in red represent doxorubicin intercalation in the nuclei of cardiomyocytes (Red). (B) For a positive control, we used cardiomyocytes permeabilized and then incubated with DNase 1 to induce DNA strand breaks. (C) Percentage of apoptotic nuclei in untreated cells (bar 1), in DNase I-treated cells (bar 2), in cells treated for 15 h (bar 3) and 24 h (bar 4), with doxorubicin and in the negative control (bar 5).
FIG. 10.
p300 degradation and stabilization of the p53 protein after doxorubicin treatment. Primary neonatal cardiomyocytes plated on coverslides were maintained in normal medium (control) or in medium supplemented with 1 μM doxorubicin for 24 h (Dox 24 h). p300 (A) and p53 (B) protein levels were measured by indirect immunofluorescence using fluorescein-conjugated secondary antibodies (Green). Red staining represents Dox intercalation into the nuclei of cardiomyocytes (Red). DAPI staining of cardiac nuclei is in blue (Dapi).
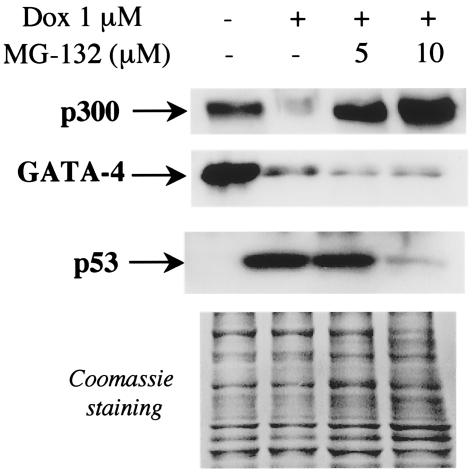
Inhibition of p300 degradation does not prevent the loss of GATA-4 protein induced by doxorubicin.
Recently, Aries et al. demonstrated that the transcription factor GATA-4 is an essential survival factor and is rapidly depleted after doxorubicin treatment (3). Therefore, we asked whether the loss of GATA-4 following doxorubicin exposure, which results in apoptosis, is due to the depletion of p300. To this end, we prevented p300 degradation by cotreatment of cardiomyocytes with doxorubicin and the proteasome inhibitor MG-132 and investigated the effect on GATA-4 protein levels. Treatment of cardiomyocytes with doxorubicin decreased both p300 protein levels and GATA-4 protein levels (Fig. 11), as expected. Exposure of doxorubicin-treated cells to MG-132 prevented p300 degradation, as previously described (53), but did not prevent GATA-4 depletion. These results indicate that GATA-4 depletion following doxorubicin treatment is not mediated by loss of p300. They also suggest that lack of GATA-4 deprives cells of a survival factor while depletion of p300 might lead to apoptosis by affecting the transcription of antiapoptotic genes belonging to a parallel pathway.
FIG. 11.
Loss of GATA-4 following doxorubicin exposure is not mediated by depletion of p300 protein. Primary neonatal rat cardiomyocytes were isolated and maintained in normal medium in medium supplemented with 1 μM doxorubicin (Dox), or in medium containing doxorubicin and MG-132. After 48 h, nuclear proteins were extracted and p300, GATA-4, and p53 protein levels were measured by Western blot analysis using specific antibodies. The residual proteins in the gel that did not transfer were stained with Coomassie brilliant blue, which showed that similar amounts of nuclear proteins were analyzed. This experiment was repeated three times with three independent preparations of cardiomyocytes.
DISCUSSION
The observations reported here describe for the first time that p300 stability is regulated by phosphorylation through the activation of the p38 pathway in response to the DNA-damaging agent doxorubicin (Fig. 12). These conclusions are supported by the findings that increased phosphorylation of p300 preceded p300 degradation induced by doxorubicin, that a serine/threonine kinase inhibitor or specific p38 blockade was sufficient to stabilize p300 protein levels in doxorubicin-treated cardiomyocytes in vivo, and that p38β could phosphorylate p300 in vitro. Furthermore, enforced activation of p38 by constitutively activated MKK6 (MKK6EE) decreased p300 protein levels, which was reversed by the addition of p38 inhibitors.
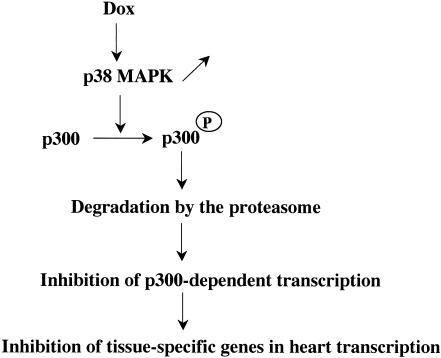
FIG. 12.
Model of p38-mediated degradation of p300. On treatment with doxorubicin (Dox), neonatal cardiomyocytes up-regulate the activity of p38, which targets the N-terminal and C-terminal regions of p300 for phosphorylation. Phosphorylated p300 is then targeted for degradation by the proteasome and is rapidly depleted in these cells. As a result, p300-dependent transcription is decreased, leading to a further repression of cardiac transcription.
p300 is a key component of the transcription machinery and integrates multiple intracellular signals, allowing appropriate levels of gene expression in response to physiological cues. Understanding the mode of regulation of p300 function is critical to determining how cells use transcriptional complexes to mediate specific transcription responses. Depending on the type of extra- or intracellular cues, p300 promotes the expression of genes implicated in a variety of responses, often mutually exclusive, such as proliferation, cell cycle arrest, differentiation, transformation, senescence, and apoptosis. One possible explanation of such diverse activities is that distinct cellular pools of p300, possibly reflecting different posttranslational modifications, execute discrete programs by selectively promoting the expression of genes devoted to specific cellular functions. For instance, signal-dependent degradation of a particular fraction of cellular p300 (e.g., the p300 molecules phosphorylated at specific sites) can be envisioned as a mechanism that would effect the activation of genes driving a particular cellular program. In this regard, the effect of doxorubicin on cardiac cells might be mediated by modifications of p300, possibly leading to the depletion of a specific fraction of p300. While this hypothesis is so far just a matter of conjecture, our finding that the degradation of p300 on exposure to doxorubicin correlates with activation of the p38 MAPK pathway leads us to propose that p300 stability can be regulated by a doxorubicin-activated p38 pathway. Doxorubicin induces several kinase activities in cardiomyocytes (40), including p44/42 MAPK (ERK1/ERK2), p38 MAPK, and JNK kinase (2). Whereas induction of the inhibitor of basic helix-loop-helix transcription factors Id by doxorubicin suppresses the expression of myofibrillar (and other) genes, thus abetting myofiber degeneration, general inhibition of kinase activities in cardiomyocytes treated with doxorubicin reduces myofiber degeneration (61). Such data suggest that myofiber degeneration induced by doxorubicin is mediated via phosphorylation-dependent mechanisms and raise the question whether MAPKs other than p38 contribute to the stability of p300 protein.
Cellular stress as induced by radical oxygen species, hypoxia, and proinflammatory cytokines are known activators of p38 MAPK in various cell types (33) including cardiomyocytes (60). Elevated p38 activity is found in several pathological forms of cardiac stress including ischemia (7) and also correlated with apoptosis (reviewed in reference 60 and 74). Experiments performed with isolated cardiomyocytes showed that p38 and its upstream kinases MKK3 and MKK6 are effectors of cardiac hypertrophy (50, 65, 75). However, activated MKK3 and MKK6 in the hearts of transgenic animals did not lead to a hypertrophic phenotype (47). In fact, reduced p38 signaling in the heart in vivo by expression of dominant-negative mutants of p38α, MKK3, or MKK6 induced cardiac hypertrophy (8, 77). Our data confirmed data, previously obtained with isolated cardiomyocytes, showing that expression of MKK6 induces markers of cardiac hypertrophy. Based on these results and on published data, we can hypothesize that activation of p38 initially induces cardiac hypertrophy and that additional or sustained activation of the p38 pathway then leads to cell death through degradation of p300. Cardiac hypertrophy is characterized by an adaptive hypertrophic response during which activation of gene expression results in increased expression of contractile proteins and reexpression of embryonic genes. After this phase, apoptosis may serve as the mechanism for the transition from hypertrophy to heart failure. Indeed, there is evidence that distinct members of p38 MAPK play opposing roles, with p38α being involved in cell death and p38β being involved in cardiac hypertrophy (65). Such a model could explain how activation of p38 MAPK might contribute to the initial phase of hypertrophy and how, as the hypertrophic stimuli persist, the balance between hypertrophy and apoptosis is disrupted and cell death occurs. Another possibility is that activation of p38 MAPK may produce cardiac hypertrophy through a p300-independent pathway. This possibility and the hypothesis that p300 degradation by activated p38 might be part of the molecular events leading to many pathological situations in heart muscle should be investigated.
p300/CBP contains several different phosphorylation sites that are targets of extracellular signal-activated kinases. Various reports have focused on functional consequences for phosphorylation at specific residues (11, 28, 29, 62, 68). Our findings now establish p300 as a substrate for p38 kinase. We identified two regions in p300 targeted by the kinase, one located at the N terminal and the other located at the C terminal of the molecule. The N-terminal region of p300 binds to both the tumor suppressor p53 and MDM2, a nuclear protein that possesses ubiquitin ligase activity. The complex formed by p300, p53, and MDM2 regulates p53 stability (22). In keeping with this observation, we can speculate that phosphorylation of the N-terminal region of p300 by p38 might disturb MDM2 binding, thus enabling MDM2 to engender proteasome-mediated degradation of p300. This hypothesis might represent one of many effects that occur following phosphorylation by p38; it should be tested in the future.
Our data obtained so far support the notion that p38 phosphorylates p300 in vivo. First, p38 phosphorylates p300 in vitro. Second, p38 is activated in cardiac cells after treatment with doxorubicin. Third, p300 is phosphorylated in vivo. Fourth, blockade of p38 stabilizes p300 protein and activation of p38 MAPK leads to p300 degradation. However, it remains a possibility that one or more steps in the pathway are indirect. The identification of the exact amino acids phosphorylated by p38 and whether those sites are relevant to p300 degradation in vivo will more firmly establish p300 as a direct in vivo target of p38. Although it is difficult to define these amino acids because of the presence of over 50 (serine/threonine)-proline residues at the N and C terminal of p300, such studies are undergoing.
The ability of some MAPKs to phosphorylate their substrates is sometimes mediated by direct interaction with specific docking domains (34, 72). However, we found only a small fraction of p38 bound to p300 in the nuclei of cardiomyocytes exposed to doxorubicin. Interestingly, p300 and p38 share the ability to bind the same sequence-specific transcription factors, MEF2A and MEF2C (26). This allows the possibility that in cardiomyocytes, p300, p38, and MEF2 might transiently associate into one complex, although the dynamic and functional consequences of such putative interactions remain to be investigated.
The observation that activation of the p38 MAPK pathway and degradation of p300 are coupled might appear paradoxical at first glance. On the one hand, we have shown that doxorubicin inhibits myogenic differentiation in skeletal muscle cells (41) and interferes with the function of tissue-specific transcription factors (42, 53). On the other hand, studies have assigned a promyogenic role to the MAPK pathway, since p38 MAPK stimulates the transcriptional activity of MEF2 by phosphorylating conserved residues of its transactivation domain (26, 36, 51, 55, 67). Furthermore, pharmacological inhibitors of p38 block cellular differentiation (16, 67, 76). This paradox might be explained by opposite effects of MAPK inhibitors on cells that are proliferating and cells that are terminally differentiated such as the primary cardiomyocytes used in our study. Moreover, it should be emphasized that, while p38 activation by MKK6EE is selective and channeled toward specific responses, doxorubicin induces p38 in a context of a broader response, including the activation of several proapoptotic genes (e.g., p53). Finally, this paradox might also be attributed to different degrees of MAPK activation in differentiating muscle cells versus cells treated with doxorubicin. Thus we can hypothesize that doxorubicin results in the activation of p38 beyond physiological levels, leading to p300 degradation and repression of transcription. It will be of interest to determine in vivo whether specific p38 inhibitors can prevent the inhibition of cardiac cell-specific gene transcription in cardiomyocytes treated with doxorubicin and can limit or delay the onset of the cardiomyopathy.
There is increasing evidence that cardiomyocyte apoptosis is an important contributor to the pathophysiology of the adverse cardiac effects of doxorubicin both in vitro and in vivo (4, 17, 35). Despite these observations, the molecular mechanisms of doxorubicin-induced apoptosis remain unexplored. The role of apoptosis in doxorubicin toxicity has particular relevance for our present study since one important regulator of apoptosis is p300. In noncardiac cells, p300 coactivates the tumor suppressor gene p53, regulates p53-inducible genes such as bax and mdm2 (23, 48), and plays a critical role in p53 mediated-apoptosis (21). Doxorubicin induces the acetylation of p73, a close relative of p53, and potentiates the apoptotic function of p73 by enhancing its ability to activate the transcription of proapoptotic genes (15). These observations are consistent with a proapoptotic role of p300 in noncardiac cells. In contrast, analysis of the effects of adenovirus E1A mutants and the introduction of E1A genes in cardiac cells suggests an antiapoptotic role of p300 (38, 49). Our data demonstrate that activation of p38 by doxorubicin leads to p300 degradation, which parallels apoptosis as well as stabilization of the tumor suppressor protein, p53. Furthermore, the induction of apoptosis by doxorubicin in cardiomyocytes is mediated at least in part by activation of p38, since p38α and p38β blockade significantly reduces cell death by apoptosis (35). A study recently published by Kawamura et al. supports an antiapoptotic function of p300 in heart muscle, since overexpression of p300 has a protective effect against doxorubicin-induced apoptosis in mice (37). We also observed a small decrease in the level of p300 protein in differentiated C2C12 cells treated with 1 μM doxorubicin (53). Interestingly, the loss of the coactivator correlates with activation of programmed cell death. Latella et al. have recently demonstrated that treatment of differentiated myotubes with concentrations of doxorubicin ranging from 0.5 to 1 μM results in serine 18 phosphorylation, stabilization of p53, and cell death (44). These findings, together with our own data, suggest that depletion of p300 following exposure to doxorubicin is mediated by DNA damage-activated p38 kinases and that the p300 depletion, together with activation of proapoptotic pathways (e.g., p53), plays an essential role in cardiomyocyte apoptosis.
Apoptosis induced by doxorubicin is mediated, at least in part, by a loss of the GATA-4 transcription factor (3). We found that blockade of p300 degradation by inhibition of the proteasome did not prevent the loss of GATA-4. This result suggests that doxorubicin can lead to apoptotic events by mechanisms other than p300 degradation by activated p38 pathways. GATA-4 depletion and apoptosis induced by doxorubicin can be limited in vitro and in animals by treatment with the α1-adrenergic agonist phenylephrine (3). Phenylephrine is also a strong activator of p38 MAPK and induces cardiac hypertrophy (12, 13, 63, 64). However, the protective effect of phenylephrine on doxorubicin-induced apoptosis is seen only at low concentrations of the drug, in the absence of any sign of cardiac hypertrophy (3). At such low doses of phenylephrine, we found no modification of p300 protein expression (not shown).
CBP/p300 is also degraded during neuronal apoptosis, due to caspase and calpain targeting. Also in this case, p300/CBP overexpression has a protective effect in an in vitro model of K+-deprived neuron apoptosis, with neuroprotection being mediated by the HAT domain (57). However, under survival conditions, overexpression of CBP/p300 in neurons can induce apoptosis (57). Similar mechanisms might apply to cardiomyocytes, which, like neurons, are terminally differentiated cells, suggesting that in these cells a fine balance of p300 might be required for cell survival. Since p300 degradation after doxorubicin exposure inhibits cardiac cell-specific transcription, the loss of the coactivator might also prevent the expression of genes with antiapoptotic function. The observation that doxorubicin decreases levels of bcl-2 proteins and that bcl-2 expression levels are higher in transgenic mice overexpressing p300 supports this view (37). An alternative explanation is that doxorubicin might direct the remaining pool of p300 toward the activation of proapoptotic genes. These possibilities are not necessarily mutually exclusive and remain to be investigated.
Acknowledgments
C.P. thanks members of the Kedes laboratory for helpful discussions.
This work was supported by grants from the National Institute of Health to L.K. C.P. was supported by a Beginning Grant-In-Aid from the American Heart Association, Western States Affiliate. P.L.P. was supported by a Beginning Grant-In-Aid from the American Heart Association and Muscular Dystrophy Association.
REFERENCES
- 1.Ait-Si-Ali, S., S. Ramirez, F. X. Barre, F. Dkhissi, L. Magnaghi-Jaulin, J. A. Girault, P. Robin, M. Knibiehler, L. L. Pritchard, B. Ducommun, D. Trouche, and A. Harel-Bellan. 1998. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature 396:184-186. [DOI] [PubMed] [Google Scholar]
- 2.Arai, M., A. Yoguchi, T. Takizawa, T. Yokoyama, T. Kanda, M. Kurabayashi, and R. Nagai. 2000. Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca(2+)-ATPase gene transcription. Circ. Res. 86:8-14. [DOI] [PubMed] [Google Scholar]
- 3.Aries, A., P. Paradis, C. Lefebvre, R. J. Schwartz, and M. Nemer. 2004. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc. Natl. Acad. Sci. USA 101:6975-6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arola, O. J., A. Saraste, K. Pulkki, M. Kallajoki, M. Parvinen, and L. M. Voipio-Pulkki. 2000. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 60:1789-1792. [PubMed] [Google Scholar]
- 5.Avantaggiati, M. L., M. Carbone, A. Graessmann, Y. Nakatani, B. Howard, and A. S. Levine. 1996. The SV40 large T antigen and adenovirus E1a oncoproteins interact with distinct isoforms of the transcriptional co-activator, p300. EMBO J. 15:2236-2248. [PMC free article] [PubMed] [Google Scholar]
- 6.Bishopric, N. H., P. Andreka, T. Slepak, and K. A. Webster. 2001. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr. Opin. Pharmacol. 1:141-150. [DOI] [PubMed] [Google Scholar]
- 7.Bogoyevitch, M. A., J. Gillespie-Brown, A. J. Ketterman, S. J. Fuller, R. Ben-Levy, A. Ashworth, C. J. Marshall, and P. H. Sugden. 1996. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ. Res. 79:162-173. [DOI] [PubMed] [Google Scholar]
- 8.Braz, J. C., O. F. Bueno, Q. Liang, B. J. Wilkins, Y. S. Dai, S. Parsons, J. Braunwart, B. J. Glascock, R. Klevitsky, T. F. Kimball, T. E. Hewett, and J. D. Molkentin. 2003. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Investing. 111:1475-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarti, D., V. Ogryzko, H. Y. Kao, A. Nash, H. Chen, Y. Nakatani, and R. M. Evans. 1999. A viral mechanism for inhibition of p300 and PCAF acetyltransferase activity. Cell 96:393-403. [DOI] [PubMed] [Google Scholar]
- 10.Chan, H. M., and N. B. La Thangue. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114:2363-2373. [DOI] [PubMed] [Google Scholar]
- 11.Chawla, S., G. E. Hardingham, D. R. Quinn, and H. Bading. 1998. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science 281:1505-1509. [DOI] [PubMed] [Google Scholar]
- 12.Chien, K. R., K. U. Knowlton, H. Zhu, and S. Chien. 1991. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 5:3037-3046. [DOI] [PubMed] [Google Scholar]
- 13.Clerk, A., A. Michael, and P. H. Sugden. 1998. Stimulation of the p38 mitogen-activated protein kinase pathway in neonatal rat ventricular myocytes by the G protein-coupled receptor agonists, endothelin-1 and phenylephrine: a role in cardiac myocyte hypertrophy? J. Cell Biol. 142:523-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Communal, C., W. S. Colucci, and K. Singh. 2000. p38 mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against beta-adrenergic receptor-stimulated apoptosis. Evidence for Gi-dependent activation. J. Biol. Chem. 275:19395-19400. [DOI] [PubMed] [Google Scholar]
- 15.Costanzo, A., P. Merlo, N. Pediconi, M. Fulco, V. Sartorelli, P. A. Cole, G. Fontemaggi, M. Fanciulli, L. Schiltz, G. Blandino, C. Balsano, and M. Levrero. 2002. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol. Cell 9:175-186. [DOI] [PubMed] [Google Scholar]
- 16.Cuenda, A., and P. Cohen. 1999. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 274:4341-4346. [DOI] [PubMed] [Google Scholar]
- 17.Delpy, E., S. N. Hatem, N. Andrieu, C. de Vaumas, M. Henaff, C. Rucker-Martin, J. P. Jaffrezou, G. Laurent, T. Levade, and J. J. Mercadier. 1999. Doxorubicin induces slow ceramide accumulation and late apoptosis in cultured adult rat ventricular myocytes. Cardiovasc. Res. 43:398-407. [DOI] [PubMed] [Google Scholar]
- 18.Eckner, R., J. W. Ludlow, N. L. Lill, E. Oldread, Z. Arany, N. Modjtahedi, J. A. DeCaprio, D. M. Livingston, and J. A. Morgan. 1996. Association of p300 and CBP with simian virus 40 large T antigen. Mol. Cell. Biol. 16:3454-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano, A., and M. L. Avantaggiati. 1999. p300 and CBP: partners for life and death. J. Cell. Physiol. 181:218-230. [DOI] [PubMed] [Google Scholar]
- 20.Goodman, R. H., and S. Smolik. 2000. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14:1553-1577. [PubMed] [Google Scholar]
- 21.Grossman, S. R. 2001. p300/CBP/p53 interaction and regulation of the p53 response. Eur. J. Biochem. 268:2773-2778. [DOI] [PubMed] [Google Scholar]
- 22.Grossman, S. R., M. Perez, A. L. Kung, M. Joseph, C. Mansur, Z. X. Xiao, S. Kumar, P. M. Howley, and D. M. Livingston. 1998. p300/MDM2 complexes participate in MDM2-mediated p53 degradation. Mol. Cell 2:405-415. [DOI] [PubMed] [Google Scholar]
- 23.Gu, W., X. L. Shi, and R. G. Roeder. 1997. Synergistic activation of transcription by CBP and p53. Nature 387:819-823. [DOI] [PubMed] [Google Scholar]
- 24.Gusterson, R. J., E. Jazrawi, I. M. Adcock, and D. S. Latchman. 2003. The transcriptional co-activators CREB-binding protein (CBP) and p300 play a critical role in cardiac hypertrophy that is dependent on their histone acetyltransferase activity. J. Biol. Chem. 278:6838-6847. [DOI] [PubMed] [Google Scholar]
- 25.Hamamori, Y., V. Sartorelli, V. Ogryzko, P. L. Puri, H. Y. Wu, J. Y. Wang, Y. Nakatani, and L. Kedes. 1999. Regulation of histone acetyltransferases p300 and PCAF by the bHLH protein twist and adenoviral oncoprotein E1A. Cell 96:405-413. [DOI] [PubMed] [Google Scholar]
- 26.Han, J., Y. Jiang, Z. Li, V. V. Kravchenko, and R. J. Ulevitch. 1997. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386:296-299. [DOI] [PubMed] [Google Scholar]
- 27.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 28.Hardingham, G. E., S. Chawla, F. H. Cruzalegui, and H. Bading. 1999. Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron 22:789-798. [DOI] [PubMed] [Google Scholar]
- 29.Hu, S. C., J. Chrivia, and A. Ghosh. 1999. Regulation of CBP-mediated transcription by neuronal calcium signaling. Neuron 22:799-808. [DOI] [PubMed] [Google Scholar]
- 30.Huang, S., Y. Jiang, Z. Li, E. Nishida, P. Mathias, S. Lin, R. J. Ulevitch, G. R. Nemerow, and J. Han. 1997. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity 6:739-749. [DOI] [PubMed] [Google Scholar]
- 31.Ito, H., S. C. Miller, M. E. Billingham, H. Akimoto, S. V. Torti, R. Wade, R. Gahlmann, G. Lyons, L. Kedes, and F. M. Torti. 1990. Doxorubicin selectively inhibits muscle gene expression in cardiac muscle cells in vivo and in vitro. Proc. Natl. Acad. Sci. USA 87:4275-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janknecht, R., and A. Nordheim. 1996. MAP kinase-dependent transcriptional coactivation by E1k-1 and its cofactor CBP. Biochem. Biophys. Res. Commun. 228:831-837. [DOI] [PubMed] [Google Scholar]
- 33.Johnson, G. L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911-1912. [DOI] [PubMed] [Google Scholar]
- 34.Kallunki, T., T. Deng, M. Hibi, and M. Karin. 1996. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell 87:929-939. [DOI] [PubMed] [Google Scholar]
- 35.Kang, Y. J., Z. X. Zhou, G. W. Wang, A. Buridi, and J. B. Klein. 2000. Suppression by metallothionein of doxorubicin-induced cardiomyocyte apoptosis through inhibition of p38 mitogen-activated protein kinases. J. Biol. Chem. 275:13690-13698. [DOI] [PubMed] [Google Scholar]
- 36.Kato, Y., V. V. Kravchenko, R. I. Tapping, J. Han, R. J. Ulevitch, and J. D. Lee. 1997. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 16:7054-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawamura, T., K. Hasegawa, T. Morimoto, E. Iwai-Kanai, S. Miyamoto, Y. Kawase, K. Ono, H. Wada, M. Akao, and T. Kita. 2004. Expression of p300 protects cardiac myocytes from apoptosis in vivo. Biochem. Biophys. Res. Commun. 315:733-738. [DOI] [PubMed] [Google Scholar]
- 38.Kirshenbaum, L. A., and M. D. Schneider. 1995. Adenovirus E1A represses cardiac gene transcription and reactivates DNA synthesis in ventricular myocytes, via alternative pocket protein- and p300-binding domains. J. Biol. Chem. 270:7791-7794. [DOI] [PubMed] [Google Scholar]
- 39.Kitabayashi, I., R. Eckner, Z. Arany, R. Chiu, G. Gachelin, D. M. Livingston, and K. K. Yokoyama. 1995. Phosphorylation of the adenovirus E1A-associated 300 kDa protein in response to retinoic acid and E1A during the differentiation of F9 cells. EMBO J. 14:3496-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurabayashi, M., S. Dutta, R. Jeyaseelan, and L. Kedes. 1995. Doxorubicin-induced Id2A gene transcription is targeted at an activating transcription factor/cyclic AMP response element motif through novel mechanisms involving protein kinases distinct from protein kinase C and protein kinase A. Mol. Cell. Biol. 15:6386-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurabayashi, M., R. Jeyaseelan, and L. Kedes. 1993. Antineoplastic agent doxorubicin inhibits myogenic differentiation of C2 myoblasts. J. Biol. Chem. 268:5524-5529. [PubMed] [Google Scholar]
- 42.Kurabayashi, M., R. Jeyaseelan, and L. Kedes. 1994. Doxorubicin represses the function of the myogenic helix-loop-helix transcription factor MyoD. Involvement of Id gene induction. J. Biol. Chem. 269:6031-6039. [PubMed] [Google Scholar]
- 43.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 44.Latella, L., J. Lukas, C. Simone, P. L. Puri, and J. Bartek. 2004. Differentiation-induced radioresistance in muscle cells. Mol. Cell. Biol. 24:6350-6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee, J. C., S. Kumar, D. E. Griswold, D. C. Underwood, B. J. Votta, and J. L. Adams. 2000. Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology 47:185-201. [DOI] [PubMed] [Google Scholar]
- 46.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, and et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 47.Liao, P., D. Georgakopoulos, A. Kovacs, M. Zheng, D. Lerner, H. Pu, J. Saffitz, K. Chien, R. P. Xiao, D. A. Kass, and Y. Wang. 2001. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc. Natl. Acad. Sci. USA 98:12283-12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lill, N. L., S. R. Grossman, D. Ginsberg, J. DeCaprio, and D. M. Livingston. 1997. Binding and modulation of p53 by p300/CBP coactivators. Nature 387:823-827. [DOI] [PubMed] [Google Scholar]
- 49.Liu, Y., and R. N. Kitsis. 1996. Induction of DNA synthesis and apoptosis in cardiac myocytes by E1A oncoprotein. J. Cell Biol. 133:325-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nemoto, S., Z. Sheng, and A. Lin. 1998. Opposing effects of Jun kinase and p38 mitogen-activated protein kinases on cardiomyocyte hypertrophy. Mol. Cell. Biol. 18:3518-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ornatsky, O. I., D. M. Cox, P. Tangirala, J. J. Andreucci, Z. A. Quinn, J. L. Wrana, R. Prywes, Y. T. Yu, and J. C. McDermott. 1999. Post-translational control of the MEF2A transcriptional regulatory protein. Nucleic Acids Res. 27:2646-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perkins, N. D. 1997. Achieving transcriptional specificity with NF-kappa B. Int. J. Biochem. Cell Biol. 29:1433-1448. [DOI] [PubMed] [Google Scholar]
- 53.Poizat, C., V. Sartorelli, G. Chung, R. A. Kloner, and L. Kedes. 2000. Proteasome-mediated degradation of the coactivator p300 impairs cardiac transcription. Mol. Cell. Biol. 20:8643-8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puri, P. L., and V. Sartorelli. 2000. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J. Cell. Physiol. 185:155-173. [DOI] [PubMed] [Google Scholar]
- 55.Puri, P. L., Z. Wu, P. Zhang, L. D. Wood, K. S. Bhakta, J. Han, J. R. Feramisco, M. Karin, and J. Y. Wang. 2000. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 14:574-584. [PMC free article] [PubMed] [Google Scholar]
- 56.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 57.Rouaux, C., N. Jokic, C. Mbebi, S. Boutillier, J. P. Loeffler, and A. L. Boutillier. 2003. Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. EMBO J. 22:6537-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shikama, N. W. Lutz, R. Kretzschmar, N. Sauter, J. F. Roth, S. Marino, J. Wittwer, A. Scheidweiler, and R. Eckner. 2003. Essential function of p300 acetyltransferase activity in heart, lung and small intestine formation. EMBO J. 22:5175-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snowden, A. W., and N. D. Perkins. 1998. Cell cycle regulation of the transcriptional coactivators p300 and CREB binding protein. Biochem. Pharmacol. 55:1947-1954. [DOI] [PubMed] [Google Scholar]
- 60.Sugden, P. H., and A. Clerk. 1998. “Stress-responsive” mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ. Res. 83:345-352. [DOI] [PubMed] [Google Scholar]
- 61.Sussman, M. A., S. F. Hamm-Alvarez, P. M. Vilalta, S. Welch, and L. Kedes. 1997. Involvement of phosphorylation in doxorubicin-mediated myofibril degeneration. An immunofluorescence microscopy analysis. Circ. Res. 80:52-61. [DOI] [PubMed] [Google Scholar]
- 62.Swope, D. L., C. L. Mueller, and J. C. Chrivia. 1996. CREB-binding protein activates transcription through multiple domains. J. Biol. Chem. 271:28138-28145. [DOI] [PubMed] [Google Scholar]
- 63.Taigen, T., L. J. De Windt, H. W. Lim, and J. D. Molkentin. 2000. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA 97:1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thorburn, J., S. Xu, and A. Thorburn. 1997. MAP kinase- and Rho-dependent signals interact to regulate gene expression but not actin morphology in cardiac muscle cells. EMBO J. 16:1888-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, Y., S. Huang, V. P. Sah, J. Ross, Jr., J. H. Brown, J. Han, and K. R. Chien. 1998. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 273:2161-2168. [DOI] [PubMed] [Google Scholar]
- 66.Wang, Y., B. Su, V. P. Sah, J. H. Brown, J. Han, and K. R. Chien. 1998. Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J. Biol. Chem. 273:5423-5426. [DOI] [PubMed] [Google Scholar]
- 67.Wu, Z., P. J. Woodring, K. S. Bhakta, K. Tamura, F. Wen, J. R. Feramisco, M. Karin, J. Y. Wang, and P. L. Puri. 2000. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 20:3951-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu, L., R. M. Lavinsky, J. S. Dasen, S. E. Flynn, E. M. McInerney, T. M. Mullen, T. Heinzel, D. Szeto, E. Korzus, R. Kurokawa, A. K. Aggarwal, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1998. Signal-specific co-activator domain requirements for Pit-1 activation. Nature 395:301-306. [DOI] [PubMed] [Google Scholar]
- 69.Yaciuk, P., and E. Moran. 1991. Analysis with specific polyclonal antiserum indicates that the E1A-associated 300-kDa product is a stable nuclear phosphoprotein that undergoes cell cycle phase-specific modification. Mol. Cell. Biol. 11:5389-5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yanazume, T., K. Hasegawa, T. Morimoto, T. Kawamura, H. Wada, A. Matsumori, Y. Kawase, M. Hirai, and T. Kita. 2003. Cardiac p300 is involved in myocyte growth with decompensated heart failure. Mol. Cell. Biol. 23:3593-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yanazume, T., T. Morimoto, H. Wada, T. Kawamura, and K. Hasegawa. 2003. Biological role of p300 in cardiac myocytes. Mol. Cell. Biochem. 248:115-119. [DOI] [PubMed] [Google Scholar]
- 72.Yang, S. H., P. R. Yates, A. J. Whitmarsh, R. J. Davis, and A. D. Sharrocks. 1998. The Elk-1 ETS-domain transcription factor contains a mitogen-activated protein kinase targeting motif. Mol. Cell. Biol. 18:710-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao, T. P., S. P. Oh, M. Fuchs, N. D. Zhou, L. E. Ch'ng, D. Newsome, R. T. Bronson, E. Li, D. M. Livingston, and R. Eckner. 1998. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93:361-372. [DOI] [PubMed] [Google Scholar]
- 74.Yin, T., G. Sandhu, C. D. Wolfgang, A. Burrier, R. L. Webb, D. F. Rigel, T. Hai, and J. Whelan. 1997. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J. Biol. Chem. 272:19943-19950. [DOI] [PubMed] [Google Scholar]
- 75.Zechner, D., D. J. Thuerauf, D. S. Hanford, P. M. McDonough, and C. C. Glembotski. 1997. A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J. Cell. Biol. 139:115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zetser, A., E. Gredinger, and E. Bengal. 1999. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 274:5193-5200. [DOI] [PubMed] [Google Scholar]
- 77.Zhang, S., C. Weinheimer, M. Courtois, A. Kovacs, C. E. Zhang, A. M. Cheng, Y. Wang, and A. J. Muslin. 2003. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J. Clin. Investig. 111:833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]