Abstract
Regulation of the hematopoietic transcription factor PU.1 (Spi-1) plays a critical role in the development of white cells, and abnormal expression of PU.1 can lead to leukemia. We previously reported that the PU.1 promoter cannot induce expression of a reporter gene in vivo, and cell-type-specific expression of PU.1 in stable lines was conferred by a 3.4-kb DNA fragment including a DNase I hypersensitive site located 14 kb upstream of the transcription start site. Here we demonstrate that this kb −14 site confers lineage-specific reporter gene expression in vivo. This kb −14 upstream regulatory element contains two 300-bp regions which are highly conserved in five mammalian species. In Friend virus-induced erythroleukemia, the spleen focus-forming virus integrates into the PU.1 locus between these two conserved regions. DNA binding experiments demonstrated that PU.1 itself and Elf-1 bind to a highly conserved site within the proximal homologous region in vivo. A mutation of this site abolishing binding of PU.1 and Elf-1 led to a marked decrease in the ability of this upstream element to direct activity of reporter gene in myelomonocytic cell lines. These data suggest that a potential positive autoregulatory loop mediated through an upstream regulatory element is essential for proper PU.1 gene expression.
PU.1 is an Ets-family transcription factor which controls many critical genes important for the development of white cells within the hematopoietic system, in particular, myeloid and B cells (28, 32, 34, 57, 61). PU.1 regulates promoters of the granulocyte colony-stimulating factor, macrophage colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor receptors (21, 23, 58, 65), integrin proteins such as CD11b (43), extracellular matrix proteins (54), other transcription factors such as SCL (8), and many other genes that are expressed in myeloid cells (23, 61). PU.1 also regulates activities of immunoglobulin G (IgG) μ and κ enhancers that are essential for lymphoid commitment (39, 46). In addition, PU.1 controls gene expression by interacting with many factors such as c-Jun (7), IRF4/Pip (14, 46, 47), NF-interleukin 6 beta (IL-6β) (C/EBPβ) (36), C/EBPα (51), and GATA-1 (38, 52, 66, 67).
PU.1 plays a critical role in lineage development. Disruption of the PU.1 gene leads to a loss of myeloid and B-cell development (32, 57) and a block in differentiation of hematopoietic stem cells to common myeloid progenitors (CMP) and common lymphoid progenitors (CLP) (1, 12). Loss of PU.1 also leads to defective stem cell function (12, 56) and homing (15). Furthermore, PU.1 can direct myeloid development of multipotential cell lines (37). Part of its ability to induce myeloid and inhibit erythroid development is likely due to its inhibitory cross-talk with GATA-1, which leads to a block in erythroid development depending on the relative ratio of these two proteins (38, 52, 66, 67). The results of these and other studies demonstrate that regulated expression of PU.1 is very important for normal hematopoietic commitment and differentiation.
PU.1 is tightly regulated within the hematopoietic system. PU.1 is expressed in hematopoietic stem cells, as well as the multipotential progenitors CMP and CLP (1, 33). Interestingly, as each one of these progenitors differentiates, PU.1 is maintained or upregulated in some lineages and downregulated in others. For example, PU.1 is expressed in CMP; the level of expression increases in GMP and their progeny (granulocytes and monocytes) but decreases in megakaryocyte-erythrocyte progenitors (MEP) and their derivatives (megakaryocytes and erythroid cells) (1, 9, 22, 28, 33). Failure to downregulate PU.1 in erythroid cells leads to a block in erythroid development and leukemia. PU.1 itself was originally identified as the Spi-1 (for spleen focus-forming virus [SFFV] provirus integration-1) site in Friend virus-induced erythroleukemia (34, 44). Transgenic mice which maintain expression of PU.1 in erythroblasts induce erythroleukemia (35). Similarly, PU.1 is expressed in CLP and maintained in B cells but downregulated in T cells, and forced expression of PU.1 in early T cells can inhibit development of mature T-cell types (4, 5, 33, 59). Finally, “knockdown” mice in which PU.1 expression in the bone marrow was decreased to 20% of wild-type levels all develop a block in macrophage and B-cell development, genomic instability, and acute myeloid leukemia (AML) (53).
All of these studies indicate that control of PU.1 levels is critical for normal development and that dysregulation can lead to drastic consequences. Therefore, it is very important to elucidate the mechanisms of regulation of PU.1 gene expression in hematopoiesis. Previous in vitro transfection studies demonstrated that the PU.1 promoter is regulated by PU.1 itself, Sp1, and octamer factors in myeloid cells (9) as well as by Oct-2 and the B-cell-specific coactivator Bob-1 in B cells (10, 25). However, transgenic mice containing only the PU.1 promoter failed to express reporter genes in vivo, while a murine PU.1 P1 clone including the entire PU.1 gene locus and 35 kb each of 5′ and 3′ flanking sequences expressed exogenous PU.1 RNA in a manner similar to that of endogenous PU.1 in terms of both expression levels and cell type specificity (29). These data suggested that distal regulatory elements were necessary for expression of PU.1 in vivo. Experiments with stable cell lines demonstrated that a genomic fragment containing a DNase I hypersensitive site located 14 kb upstream of the PU.1 transcription start site conferred more than 100-fold-higher reporter gene expression in myeloid cell lines but not in a T-lymphoid-cell line (29). These results strongly suggested that an element necessary for proper in vivo expression is located within this kb −14 region. In this present study, we tested the ability of this kb −14 upstream regulatory element (URE) to direct in vivo expression in transgenic mice and demonstrate that a site within a conserved region which binds PU.1 is critical for URE activity.
MATERIALS AND METHODS
Transgenic mice.
To make the construct for transgenic expression of the Thy1.1 reporter (Fig. 1), we subcloned a human growth hormone N gene lacking the first 5′ 498 bp but including splice donor and acceptor sites and its polyadenylation signal into BamHI-NotI-digested pBluescript KSII(+) (Stratagene, La Jolla, Calif.). Subsequently, the murine PU.1 2.1-kb promoter fragment (9) and Thy1.1 cDNA (13) were subcloned into the SacI and BamHI sites, respectively. Finally, a HindIII 3.4-kb fragment including the murine PU.1 kb −14 URE was subcloned into a HindIII site (Fig. 1).
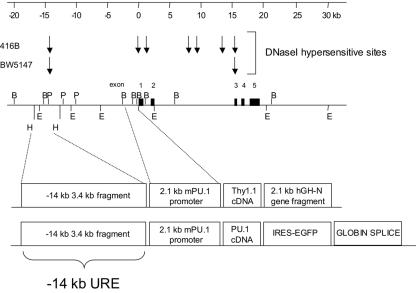
FIG. 1.
Map of the murine PU.1 gene transgenic constructs. The top diagram is a kilobase marker for reference, and below it are diagrams of the DNase I hypersensitive sites detected in the myeloid cell line 416B and T-cell line BW5147 as previously described (29). Diagrams of the murine PU.1 genomic locus, showing the location of the five coding exons and restriction sites for BamHI (B), HindIII (H), PstI (P), and EcoRI (E) and diagrams of the two transgenic constructs used in these studies are shown below. The first construct contains a 3.4-kb HindIII fragment from the kb −14 region, referred to in this paper as the kb −14 URE (upstream regulatory element), and a 2.1-kb PU.1 promoter fragment which includes 166 bp of 5′ untranslated region (9, 29), followed by a Thy1.1 cDNA as a reporter and a truncated 2.1-kb human growth hormone N gene (hGH-N). The second construct contains the identical PU.1 regulatory elements followed by the PU.1 cDNA, EGFP reporter, and a rabbit globin fragment to provide splice donor-acceptor sites and a polyadenylation signal.
For production of transgenic mice with the enhanced green fluorescent protein (EGFP) reporter, the same pBluescript vector containing the kb −14 URE, 2.1-kb PU.1 promoter, Thy1.1 cDNA, and truncated human growth hormone gene described above was linearized with NotI. Partial XhoI digestion was performed to remove the Thy1.1 cDNA and growth hormone gene, and the remaining plasmid was religated using a XhoI-NotI adaptor. A 1-kb fragment of the rabbit β-globin gene containing exon 2, intron 2, exon 3, and the polyadenylation signal was inserted into the SacII site of the transgenic construct by blunt-end ligation to create pBluescript-(−14 kb)URE/prom/β-globin. Next, the sequence encoding a murine PU.1 cDNA was released from p-Bluescript-PU.1 (28) and inserted into the XhoI-SacII sites of pIRES2-EGFP (Clontech Laboratories). A NotI adaptor was inserted into the XhoI site of this plasmid, and the PU.1iresEGFP expression cassette was released by NotI digestion and inserted into the NotI site of pBluescript-(−14 kb)URE/prom/β-globin to create pBluescript-(−14 kb)URE/prom/PU.1iresGFP/β-globin (Fig. 1, bottom construct).
DNA was linearized with NotI (for the Thy1.1 reporter construct) or PvuI (for the EGFP reporter) and injected into fertilized oocytes of FVB/N mice and implanted in uteri of pseudopregnant FVB/N mice according to standard procedures (13).
Genotyping of transgenic mice with Southern blot hybridization and PCR.
For genotyping of transgenic mice, tails were digested with 20 mM Tris HCl (pH 7.6)-200 mM NaCl-10 mM EDTA (pH 8.0) supplemented with 200 μg of proteinase K/ml for 14 h at 55°C. Genomic DNA was extracted with phenol-chloroform and precipitated with ethanol. After centrifugation, genomic DNA was suspended with 10 mM Tris HCl (pH 7.6)-1 mM EDTA. Genotyping was done by PCR and confirmed by Southern blot analysis. The primer set used for PCR genotyping of Thy1.1 reporter mice was 5′-GTCGCTCTCCTGCTCTCAGTCTTGC-3′ and 5′-GGGCTTGGAGGAGGGAGAGGGAAAG-3′, derived from the Thy1 cDNA. For Southern blot analysis, genomic DNAs were digested with PstI (New England Biolabs) and separated in 0.6% agarose gels, transferred to nylon membranes (Biotrans Plus; ICN, Costa Mesa, Calif.) with 0.4 M NaOH—1.5 M NaCl solution for 24 h, and immobilized with a UV cross-linker (Stratagene). A 550-bp murine Thy1.1 cDNA was used as a probe. EGFP reporter transgenic mice were genotyped by Southern blot analysis using NcoI-digested genomic tail DNA with probe P6, a 1.5-kb EcoRI-PstI fragment located within the 3.4-kb fragment including the kb −14 URE in the murine PU.1 gene (29). The transgenic fragment was detected as a 5-kb fragment in contrast to the 3-kb wild-type fragment detected with this probe.
FACS analysis of bone marrow cells from transgenic mice.
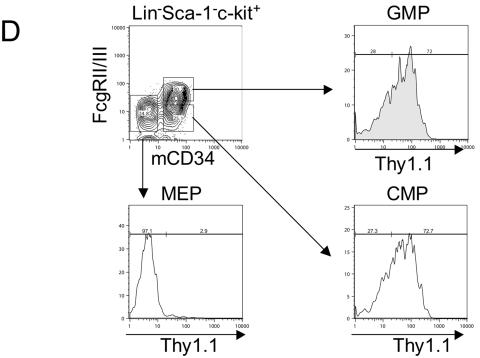
Bone marrow cells were flushed from femurs and tibias of 2- to 3-month-old transgenic mice, suspended with phosphate-buffered saline (PBS) with 2% fetal bovine serum (FBS), and filtered through 40-μm-pore-size nylon mesh (Becton Dickinson, Franklin Lakes, N.J.). One million cells of a single-cell suspension were stained with phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated anti-Thy1.1 antibody (BD Pharmingen, San Diego, Calif.) and FITC-conjugated Gr-1 (BD Pharmingen), B220, or Mac-1 (Caltag Laboratories, San Francisco, Calif.) antibody or PE-conjugated anti-TER119 antibody (BD Pharmingen). Cells were washed with PBS twice and resuspended in PBS with 2 μg of propidium iodide (Sigma, St. Louis, Mo.)/ml. Fluorescence-activated cell sorter (FACS) analysis was performed with a FACScan cytometer (Becton Dickinson, San Jose, Calif.). To check Thy1.1 expression in myeloid progenitors, we used five-color FACS analysis for CMP, GMP, and MEP with sorting methods described elsewhere (1). All progenitor populations were sorted or analyzed using a double-laser (488- and 350-nm Enterprise II plus 647-nm Spectrum) high-speed cell sorter (Moflo-MLS; Cytomation, Fort Collins, Colo.).
Nuclear cell extracts and in vitro translation of PU.1 protein.
A total of 4 × 106 cells were washed with PBS and resuspended in 10 mM HEPES-KOH (pH 7.9)-1.5 mM MgCl2-10 mM KCl-0.5 mM dithiothreitol-0.2 mM phenylmethylsulfonyl fluoride (PMSF) at 0°C. At 10 to 30 min after incubation, cells were collected by centrifugation and resuspended in 20 mM HEPES-KOH (pH 7.9)-25% glycerol-420 mM NaCl-1.5 mM MgCl2-0.2 mM EDTA-0.5 mM dithiothreitol-0.2 mM PMSF at 0°C. After 20 min of incubation, cell lysates were centrifuged and the supernatant was collected as nuclear extract. To synthesize murine PU.1 protein in vitro, a linearized plasmid that contained the murine PU.1 cDNA inserted into the EcoRI site of pBluescript KSII(+) was used as a template. PU.1 protein was translated with a TNT coupled reticulocyte lysate system (Promega) and T3 RNA polymerase.
ChIP assay for in vivo DNA binding.
Chromatin immunoprecipitation (ChIP) experiments were performed as previously described (16) with minor modifications. Cells (2 × 10 7 per IP) were grown in Dulbecco's modified Eagle's medium (DMEM)-10% FBS (416B), DMEM-10% FBS-3%Wehi-5% BHK medium (503, 503/PU.1), or RPMI medium-10% FBS (U937 and IB4). Cells were cross-linked with formaldehyde (0.5%) and agitated at room temperature for 10 min. Cross-linking was stopped by addition of glycine (0.125 M) at room temperature for 5 min. Cells were then pelleted by centrifugation for 5 min at 1,000 g, washed once with cold PBS and transferred to microcentrifuge tubes, and lysed in a 2× pellet volume with cell lysis buffer (10 mM Tris [pH 8], 10 mM NaCl, 0.2% NP-40, 1 mM PMSF, 1 μg of leupeptin and aprotinin/ml) and incubated on ice for 10 min.
The cell lysate was centrifuged at 2,500 rpm for 5 min at 4°C to recover the nuclei. The nuclei were lysed in 1.5 volumes of nuclei lysis buffer (50 mM Tris [pH 8], 10 mM EDTA, 1% SDS, 1 mM PMSF, 1 μg of aprotinin/ml, 1 μg of leupeptin/ml) and incubated on ice for 10 min. The chromatin was sonicated for 2 × 25 s with a 90% duty cycle and output setting 5 on a Branson Sonifier 450 apparatus. The supernatant containing soluble chromatin was transferred to microcentrifuge tubes and centrifuged at maximum speed for 10 min at 4°C. The chromatin was then precleared by the addition of 25 μg of normal rabbit IgG (Santa Cruz) and incubated with agitation for 1 h at 4°C, after which the chromatin plus IgG was transferred to a fresh microcentrifuge tube containing 100 μl of protein A agarose (Santa Cruz). The chromatin-bead slurry was incubated at 4°C for 2 h, and the supernatant was then divided into five tubes. One tube was saved for total input and was frozen immediately; the remaining four aliquots were used for immunoprecipitation with 10 μg of normal rabbit IgG (Santa Cruz), 5 μg of PU.1 antibody (Santa Cruz), 10 μg of Sp1 antibody (Santa Cruz), or 10 μg of Sp3 antibody (Santa Cruz) and incubated at 4°C for 2 h.
The lysate-antibody mixture was then transferred to new microcentrifuge tubes containing 30 μl of protein A agarose pellets and incubated overnight at 4°C. After centrifugation, the pellets were washed twice with IP Wash I (20 mM Tris [pH 8], 2 mM EDTA, 0.1% SDS, 1% Triton X-100, 1 mM PMSF, 1 mg of aprotinin/ml, 1 mg of leupeptin/ml), once with IP Wash II (10 mM Tris [pH 8], 0.25 M LiCl, 1 mM EDTA, 1% NP-40, 1% deoxycholate, 1 mM PMSF, 1 mg of aprotinin/ml, 1 mg of leupeptin/ml), and twice with Tris-EDTA. Beads were pelleted by centrifugation at 10,000 rpm for 30 s after each wash. The DNA-protein-antibody complexes were eluted twice with 150 μl of elution buffer (0.1 M NaHCO3, 1% SDS), and the supernatants were combined and transferred to a fresh tube. A total of 1 μg of RNase A and NaCl (0.3 M) was added to all samples, including the input sample, and the mixture was incubated at 67°C for 4 to 5 h. Proteinase K (0.24 mg/ml) was added at 45°C for 2 h. The samples were extracted twice with phenol-chloroform and ethanol precipitated in the presence of 10 μg of tRNA and 5 μg of glycogen. DNA was resuspended with 30 μl of water, and 2 μl of each sample was used for PCR analysis.
The total input sample was diluted 1/300 before being used as a template for PCR. To specifically isolate DNA fragments of <500 bp, the entire 30 μl of resuspended DNA was loaded on a 1% low-melting agarose gel and run for 30 min at 50 V, and those DNA fragments that were 500 bp or less in length were excised from the gel, purified according to a published technique (48), and used as a PCR template. For PCR the following oligonucleotides were used: for the PU.1 murine kb −14 URE distal (5′) homology region, 5′-TGCCTGAGCTTCAGAAGAGATCTG and 5′-ACGCACCTCTCGTTCCTGGTCC-3′; for the murine kb −14 proximal (3′) homology region, 5′-GCACACATGCTTCCTGTGGTGACT-3′ and 5′-CCATGTGCCCTAGCTGTCACCCCTA-3′; for the PU.1 human kb −14 URE distal (5′) homology region, 5′-TCTCTGGGTAGATGGGGGTACCTA-3′ and 5′-TCACTCACCTCTTGCTTCTGGTCC-3′; and for the human kb −14 URE 3′ homology region, 5-GCACACATGCTTCCTGTGGTGACT-3′ and 5′-CCACGTGCCCTGACTCCCCTCCTAGC-3′.
A 100-μl reaction mixture containing 150 ng of each primer, 2 μl of ChIP DNA, and PCR Supermix (Invitrogen) was subjected to 30 cycles of PCR at 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min followed by 10 min at 72°C. A total of 15 μl of the PCR was run on 1% agarose gels and visualized by ethidium bromide staining. For Elf-1, ChIP assays were performed as previously described (16). Antibodies against Elf-1 and GATA-2 were purchased from Santa Cruz Biotechnology Inc. Precipitates were analyzed by semiquantitative PCR (forward primer, 5′-CTAACCCCTGCACATGAAAGC-3′; reverse primer, 5′-TCTGGGCAGGGTCAGAGTG-3′). PCRs were stopped at cycle 29 when amplification was in log phase and analyzed by gel electrophoresis followed by Southern analysis. Input DNA represented a sample removed before immunoprecipitation and acted as a positive control. Rabbit IgG was used as the negative control. Severalfold enrichment was calculated as the ratio of band intensities to that of the IgG control band. Signal intensities were measured using a Hewlett-Packard phosphorimager.
EMSA for in vitro DNA binding.
Oligonucleotides 5′-GGTGACTGGGCGCTTCCTGTTTTCTCAGGC-3′ and 5′-GCCTGAGAAAACAGGAAGCGCCCAGTCACC-3′ were annealed for electrophoretic mobility shift assays (EMSA) of the murine PU.1 kb −14 URE PU.1 binding site. Annealed oligonucleotides were labeled with [γ-P32]ATP by the use of T4 polynucleotide kinase and incubated with nuclear extracts or in vitro-translated PU.1 protein in 10 mM HEPES (pH 7.8), 50 mM KCl, 1 mM dithiothreitol, 1 mM EDTA, and 5% glycerol for 15 min at 0°C. Reaction mixtures were separated with 6% polyacrylamide gels in 0.5× TBE buffer at 4°C. Gels were dried prior to autoradiography. Anti-PU.1, -SP1, -SP3, and -AML type 1 (AML1) antibodies (Santa Cruz) were used for supershift assays. For competition assays, the Ets competitor E18 oligonucleotides 5′-CCCCACTTCCGGTCTCGATC-3′ and 5′-GATCGAGACCGGAAGTGGGG-3′, which have been described previously (27, 63), were used.
Analysis of PU.1 kb −14 URE by stable transfection assays in cell lines.
Constructs including the 334-bp murine PU.1 promoter and 152 bp of 5′ untranslated sequence with or without the kb −14 HindIII 3.4-kb DNA fragment (URE) have been described elsewhere (29). These constructs are cloned into the pXP2 luciferase reporter vector (40). To introduce mutations in the PU.1 binding site in the proximal (3′) homology region of the kb −14 URE, PCR mutagenesis was performed. The 5′-flanking DNA fragment was amplified with the primer set 5′-TGGGTTCTCACTGACCCCTGACA-3′ and 5′-AGGCCGGTGCCTGAGAAAACATCGCGCGCCCAGTCACC-3′ (the mutation in the PU.1 site is underlined), and the 3′-flanking DNA fragment was amplified with the primer set 5′-CTTCCTGTGGTGACTGGGCGCGCGATGTTTTCTCAGGC-3′ and 5′-CGCTAGGCCTTGCTGAAGTAGTC-3′ with 25-cycle PCRs. The gel-purified PCR products (1 μl of each) were mixed with each other and then subjected to a four-cycle PCR. Then, primer set 5′-TGGGTTCTCACTGACCCCTGACA-3′ and 5′-CGCTAGGCCTTGCTGAAGTAGTC-3′ was added, and 25 more cycles of PCR were performed. All PCRs were performed with Pfx DNA polymerase (GIBCO BRL) that has 3′ to 5′ proofreading activity; the template was the pXP2 construct which contained the 334-bp murine PU.1 promoter and 152 bp of 5′ untranslated sequence and the kb −14 URE 3.4-kb DNA fragment. An expected 2.1-kb PCR product was obtained and subjected to AflII and XhoI digestion. The template DNA was digested with AflII and XhoI and replaced by the AflII- and XhoI-digested PCR product harboring the mutation in the PU.1 binding site within the kb −14 URE proximal homology region. The mutation in the PU.1 binding site and the sequence of the region spanning the AflII and XhoI sites were confirmed by DNA sequencing.
For isolation of stable transformants and luciferase assays, 416B cells were cultured with DMEM-10% FCS and transfected by Fugene 6 (Boehringer-Mannheim) with 2 μg of ScaI-digested linearized reporter gene and 0.2 μg of pGKneo per well in 6-well plates. Stable transformants were selected with 1 mg of G418/ml beginning 2 days after transfection for 2 weeks. Pools were made of stable transformants from each well, and three independent pools for each construct were isolated. Independent clones for each construct were also obtained by limiting dilution. Cells (2.5 × 104) of stable transformants were used for luciferase assays according to the protocol of the manufacturer (Promega). Cell lysates were quantitated with the Bradford protein assay (Bio-Rad Laboratories, Hercules, Calif.). Luciferase activity was standardized by dividing luciferase the gene copy number of each cell line, which was determined by Southern blot analysis using the murine PU.1 promoter 0.5-kb fragment as a probe.
RESULTS
The murine PU.1 kb −14 upstream regulatory region confers myeloid- and B-lymphocyte-lineage-specific expression in transgenic mice.
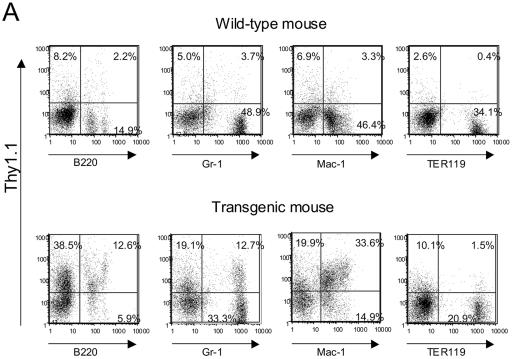
Previously, we reported that while the PU.1 promoter was unable to direct expression of a reporter gene in vivo, a murine genomic clone including 35 kb of 5′ upstream sequences could confer myeloid- and B-lymphoid-lineage-specific expression in transgenic mice and myeloid cell lines. Furthermore, we demonstrated that the URE, including a DNase I hypersensitive site located 14 kb 5′ of the transcription start site, could confer high-level reporter gene expression in myeloid cell lines but not in a T-cell line which does not express endogenous PU.1 (29). Therefore, to directly demonstrate that the kb −14 URE could direct reporter gene expression in vivo, we produced transgenic mouse lines which included the 3.4-kb fragment from the kb −14 URE, the 2.1-kb PU.1 promoter, and a Thy1.1 cDNA reporter, with a truncated human growth hormone gene to provide splice donor-acceptor sites and a polyadenylation signal (Fig. 1). We generated six transgenic founders and assessed Thy1.1 protein expression by flow cytometry. Progeny of three of the five founder lines demonstrated the presence of Thy1.1 reporter gene expression in myeloid cells and B lymphocytes (Fig. 2A); two other founder lines did not express the reporter, and a sixth did not pass the transgene through the germ line. A majority of the B220+ B cells and a majority of Mac-1+ myeloid cells expressed Thy1.1 protein on their surface. We also observed expression of the Thy1.1 reporter on cells which expressed Gr-1, which is highly expressed on granulocytes rather than monocytes/macrophages, whereas most TER119+ erythroid cells did not express the Thy1.1 protein.
FIG. 2.
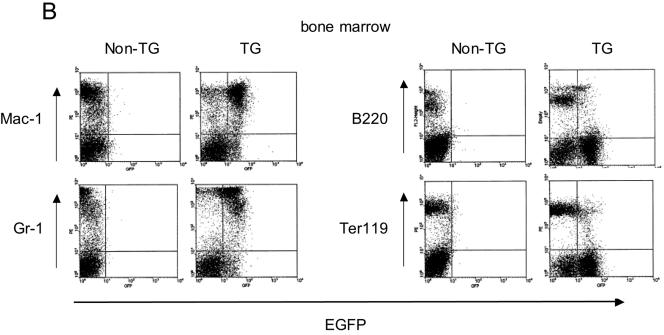
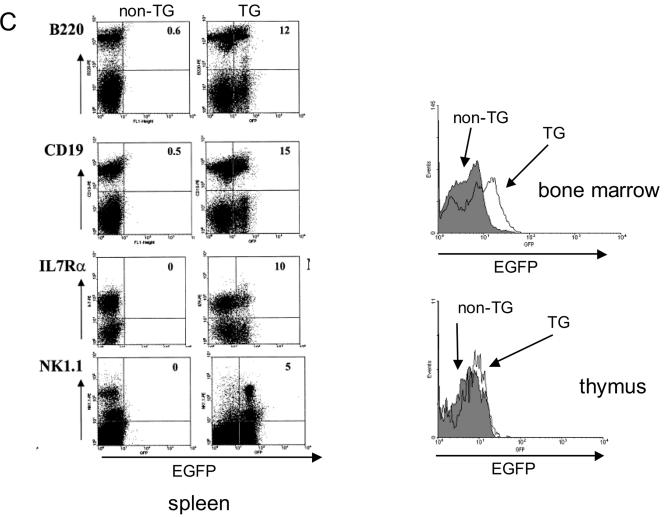
The kb −14 URE directs expression of reporter genes in myeloid and B cells in vivo. (A) Thy1.1 reporter gene expression in myeloid and B cells of mice harboring the PU.1 kb −14 URE and promoter construct depicted in Fig. 1. Bone marrow cells from a transgenic and a nontransgenic (“wild-type”) littermate were stained with PE-conjugated Thy1.1 antibody (for measuring reporter gene expression) and FITC-conjugated antibodies recognizing Gr-1 (stains granulocytes), Mac-1 (stains macrophages and granulocytes), or B220 (stains B cells). For analysis of erythroid cells with PE-conjugated TER119 antibody, bone marrow cells were stained with FITC-conjugated Thy1.1 antibody. Similar results were observed for two other independent founder lines. (B) EGFP reporter gene expression in bone marrow myeloid and B cells of transgenic (TG) mice harboring the transgenic construct depicted in the bottom diagram in Fig. 1. “Non-TG” refers to a nontransgenic littermate used as a control. (C) Left panels: EGFP reporter gene expression in B cells and NK cells in spleens of mice of the same founder line analyzed as described above (panel B), determined utilizing antibodies specific for B220 and CD19 (B cells), NK1.1 (natural killer cells), and IL-7 receptor α (early lymphoid progenitors). Right panels: EGFP reporter gene expression in total bone marrow (top panel) and thymus (bottom panel). EGFP expression in nontransgenic littermates is shown in the shaded area and for the EGFP transgenic mice is shown in the nonshaded area in both panels. (D) Thy1.1 reporter gene expression in myeloid progenitors CMP, GMP, and MEP for the same founder whose results are depicted in panel A was determined by five-color FACS analysis. Methods for characterizing CMP, GMP, and MEP have been described elsewhere (1).
Since endogenous Thy1.1 was expressed at high levels in the T cells of the strain of mice utilized in these studies, we could not assess the specificity of the URE in T cells. Therefore, we generated an additional founder line which contained the same PU.1 URE and promoter regulatory elements expressing an EGFP as a reporter (Fig. 1). Analysis of progeny of this second transgenic founder again demonstrated expression in myeloid cells at high levels in both Mac-1 and Gr-1 cells as well as at a lower level in B lymphocytes (Fig. 2B). Interestingly, we again observed a lesser amount of expression of the EGFP reporter in TER119 erythroid cells, perhaps reflecting the expression of the endogenous PU.1 gene in early erythroid cells (17, 33). Analysis of the spleen demonstrated EGFP reporter coexpression with B220, CD19, and IL-7 receptor α, consistent with expression in B cells (Fig. 2C, left panels). In addition, the reporter could be detected in cells expressing the natural killer (NK) marker NK1.1 (Fig. 2C). We could easily observe expression in total bone marrow but not in total thymus (Fig. 2C, right panels). Therefore, it appears that the kb −14 URE indeed confers cell-type-specific expression in mature cells in vivo in a pattern similar to that previously described for the endogenous PU.1 gene.
We next assessed the activity and specificity of the kb −14 URE in myeloid progenitor cell populations (1). We have previously reported that endogenous PU.1 is expressed in common myeloid progenitors (CMP) and granulocyte-macrophage progenitors (GMP) but not in MEP (1, 33). When we measured Thy1.1 reporter activity in progenitors, more than 70% of CMP and GMP expressed Thy1.1 protein, whereas less than 5% of MEP showed such expression (Fig. 2D). These data demonstrate that the PU.1 kb −14 upstream region and promoter can direct expression of the Thy1.1 reporter in a manner similar to that of endogenous murine PU.1 gene in both mature cells and in hematopoietic progenitors.
The kb −14 URE contains two highly conserved regions and is a target for SFFV integration.
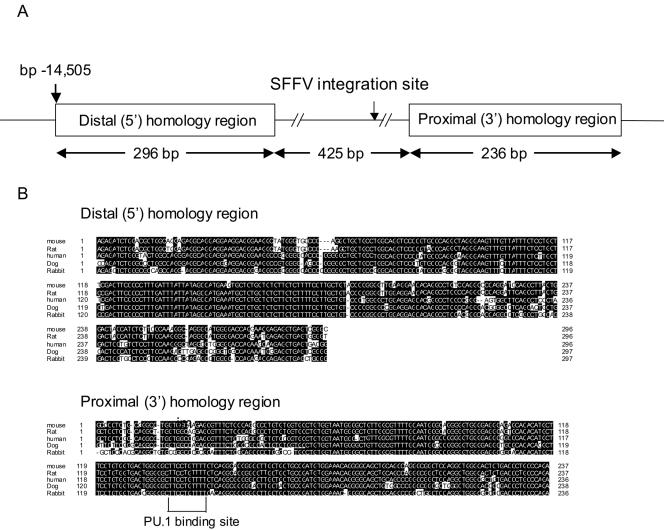
Computer homology searches for conserved genomic sequences have now been used in a number of instances to identify regulatory elements (19, 30). Therefore, we performed a homology search of the murine PU.1 kb −14 URE with human genomic sequences. Located centrally within the 3.5-kb HindIII fragment utilized in the transgenic studies (Fig. 1 and 2), we identified two highly conserved regions of 296 and 236 bp, respectively, separated by approximately 500 bp of nonconserved sequences (Fig. 3A). The sequences of these two conserved regions are more than 90% identical between mice and humans and are also highly conserved with those of rats, dogs, and rabbits (Fig. 3B). This high degree of conservation among five mammalian species strongly suggested that these two conserved regions might contain functionally important binding sites for transcription factors regulating PU.1. Interestingly, by comparison of the precise sequence adjacent to a major SFFV integration site with the sequence of the PU.1 URE, we found that the major SFFV integration site was located in the nonconserved 500-bp “spacer” sequences lying between these two homology regions (Fig. 3A).
FIG. 3.
The PU.1 kb −14 URE includes two highly homologous regions conserved in five mammalian species. (A) The diagram (not drawn to scale) represents the two homologous regions, which are separated by a 425-bp “spacer” in which there is no significant sequence similarity. The location of a major SFFV integration site is shown within this spacer. The two homologous regions and spacer lie centrally within the 3.4-kb HindIII fragment at kb −14 utilized in the transgenic constructs (Fig. 1). Bp 1 at the 5′ end of the distal homology region is 14,505 bp 5′ of the transcription start site (GenBank accession number AL691450) (9). (B) Sequence alignment of the kb −14 murine PU.1 URE with rat, human, dog, and rabbit PU.1 genomic sequences. Identical residues are shown in black. The PU.1 binding site identified by ChIP and EMSA analysis in Fig. 4 and 5 is enclosed in a bracket.
A complex including PU.1 binds to a site in the proximal homology region in vivo.
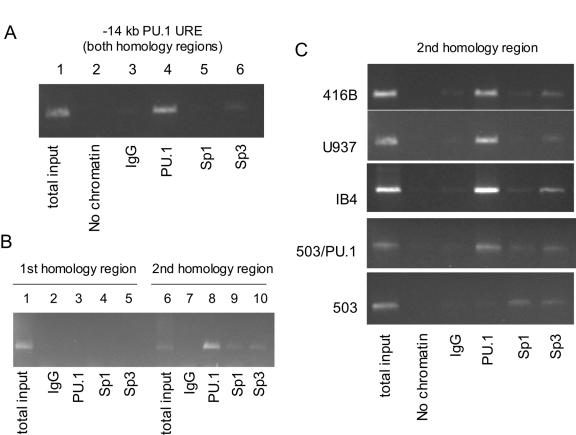
We previously reported that PU.1 appears to autoregulate its promoter in transient assays (9). However, as our subsequent studies have demonstrated that the promoter itself is insufficient to direct PU.1 expression in vivo, we postulated that PU.1 might also bind to the URE. To determine whether PU.1 binds to the kb −14 URE, we performed ChIP assays with this region. Analysis of the sequence suggested candidate binding sites for both PU.1 and Sp1 in the distal (3′) conserved region. Therefore, we utilized PU.1, Sp1, and Sp3 antibodies in ChIP assays to scan the URE. Binding to the kb −14 URE region was easily detectable utilizing PU.1 antibodies, while we could only detect weak binding of Sp3 when probing the entire URE (Fig. 4A). We identified a potential PU.1 binding site within the proximal conserved region (Fig. 3) on the basis of similarity to PU.1 binding sites we had identified in myeloid promoters (43, 65) which also matched sequences identified by PCR primer site selection (50). Because the lengths of DNA fragments in the ChIP assay after sonication of the DNA sample are approximately 200 to 1,000 bp, we could not separate the two conserved regions with the conventional ChIP assay whose results are shown in Fig. 4A. Therefore, we used gel fractionation and purification to analyze DNA fragments of <500 bp to separate these two conserved regions. In this case, we could detect binding with PU.1, Sp1, and Sp3 antibodies only within the proximal (3′) conserved region but not within the distal (5′) homologous region (Fig. 4B). Strong binding to this region with PU.1 antibodies could be detected in myeloid cells from both mice (416B) and humans (U937) as well as in a B-cell line (IB4) (Fig. 4C). To further demonstrate the requirement for PU.1 to detect binding to this site, we utilized the 503 cell line, an immature myeloid line derived from PU.1−/− embryos, as well as a line in which exogenous expression of PU.1 restored differentiation of these cells (503/PU.1) (3). While binding could be detected with Sp1 and Sp3 antibodies in both lines, binding with PU.1 antibodies was only detected in 503/PU.1 cells. These data demonstrate that PU.1 protein is in a complex binding to a site within the kb −14 URE, suggesting that autoregulation through this region might be important for expression of PU.1.
FIG. 4.
PU.1 is part of the complex which binds to the proximal (3′) homology region in vivo. (A) ChIP assay of the entire kb −14 URE region. Lane 1, total input of genomic DNA from the myeloid cell line 416B; lane 2, no chromatin; Lane 3, IgG (control) antibody; lane 4, anti-PU.1 antibody; lane 5, anti-Sp1 antibody; lane 6, anti-Sp3 antibody. Amplified products were visualized by ethidium bromide staining. (B) Chromatin immunoprecipitation assay of the kb −14 URE distal (5′) and proximal (3′) conserved regions by use of DNA fragments of <500 bp. Lanes 1 to 5, PCR was performed using primers which amplified the distal (5′) homologous region; lanes 6 to 10, PCR was performed using primers amplifying the proximal (3′) homologous region. Lanes 1 and 6, total input; lanes 2 and 7, IgG (control); lanes 3 and 8, anti-PU.1 antibody; lanes 4 and 9, anti-Sp1 antibody; lanes 5 and 10, anti-Sp3 antibody. (C) ChIP assay of the 3′ downstream conserved region in myeloid and lymphoid cell lines, including 416B (murine myeloid), U937 (human myeloid), IB4 (B cell), 503/PU.1 (a murine PU.1−/− line “rescued” after stable transfection with a PU.1 expression construct), and 503 (murine PU.1−/− line). Antibodies were the same as those used as described for Fig. 4B.
PU.1 binds to a specific site in the proximal (3′) conserved region.
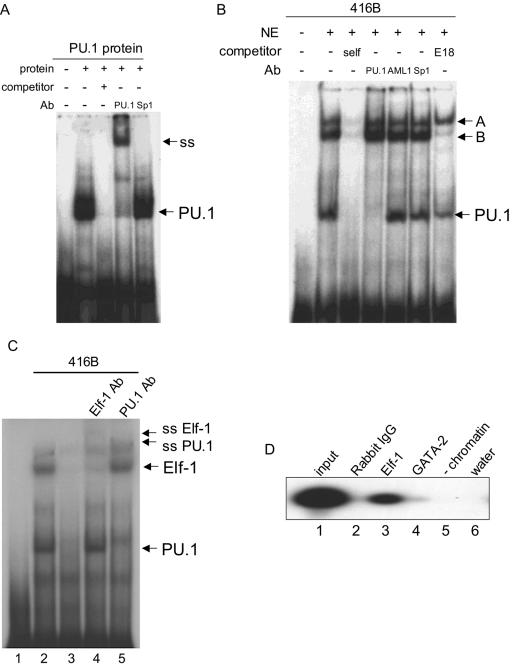
To precisely locate the PU.1 protein binding site, we performed EMSA. As noted above, sequence analysis demonstrated one candidate motif with the sequence AAAAGAGGAAG, in similarity to the results seen with other PU.1 binding motifs reported previously (43, 50, 54, 65). We first performed EMSA assays with an oligonucleotide spanning this potential PU.1 binding site. Using in vitro-translated PU.1 protein, we detected a single major shifted band (Fig. 5A). This band was supershifted with anti-PU.1 antibody but not with anti-Sp1 antibody, proving that PU.1 can bind to this site. We next performed EMSA assays with nuclear extracts from 416B cells, a murine myeloid progenitor line which we have used to test the function of the kb −14 URE (29). In addition to a complex which migrated with mobility similar to that of PU.1, we detected in 416B nuclear extracts at least two slower-migrating complexes, which we named complex A and complex B (Fig. 5B). All three complexes bind this site specifically, as excess unlabeled oligonucleotide probe competes efficiently with all.
FIG. 5.
PU.1, Elf-1, and an additional factor bind to a conserved sequence in the proximal (3′) homology region of the kb −14 PU.1 URE. (A) EMSA with in vitro-translated PU.1 protein and an oligonucleotide including a potential PU.1 binding site in the proximal (3′) homology region (Fig. 3 and 6A). “Competitor” refers to competition with itself. PU.1 and Sp1 refer to antibodies (Ab) used for supershift assays. The relative position of the PU.1 complex is shown on the right side of the panel (PU.1), as is the position of the complex supershifted with antibodies to PU.1 but not Sp1 (ss). The first lane contains probe only. (B) EMSA with nuclear extracts from myeloid 416B cells, demonstrating that PU.1 and two other complexes bind to this site. Antibodies recognizing PU.1, AML1, and Sp1 were used for supershifts. The fastest-migrating complex reacted with PU.1 antibody, while the two slower-migrating complexes, designated A and B, respectively, did not. In addition to an oligonucleotide containing itself (self), an additional oligonucleotide which competes with many Ets factors (but not with PU.1) (27, 63) was used in the last lane. Complex B, which competes with the E18 oligonucleotide, likely contains an Ets family member. (C) Complex B is Elf-1. EMSA was performed with 416B nuclear extracts. Lane 3 contains a self-competitor, and antibodies recognizing Elf-1 and PU.1 were used in supershift experiments in lanes 4 and 5, respectively. Arrows on the right side of the panel indicate the positions of complexes corresponding to PU.1 and Elf-1 as well as the positions of supershift complexes. (D) ChIP assays demonstrate binding of Elf-1, but not GATA-2, to the kb −14 URE region. Lane 1, total input of genomic DNA from the myeloid cell line 416B; lane 2, rabbit IgG (control) antibody; lane 3, anti-Elf-1 antibody; lane 4, anti-GATA-2 antibody; lane 5, no chromatin (control for immunoprecipitation and PCR amplification); lane 6, water (control for the final PCR amplification step). Amplified products were visualized by Southern blot analysis.
We next tried to assess the identity of the proteins in these three complexes. As shown in Fig. 5B, none of the three complexes could be supershifted with either anti-AML1 or anti-Sp1 antibodies. The faster-migrating complex supershifted efficiently when incubated with anti-PU.1 antibody, demonstrating that it contained PU.1. We then utilized an oligonucleotide (E18) that competes efficiently with many Ets family members other than PU.1 (27, 63). The E18 oligonucleotide competed with complex B but not complex A or PU.1, suggesting that complex B might include an Ets family transcription factor (Fig. 5B). To further understand the nature of complexes A and B, we performed EMSA using nuclear extracts from a variety of myeloid and lymphoid lines. As expected, we could detect PU.1 binding in myeloid lines (KG1a, HL-60, U937, and K562) and B-cell lines (BJAB and RAJI) but not in T-cell lines (Jurkat and MOLT4). No such specificity could be demonstrated for complexes A and B, which could be detected in all cell lines tested, although the abundance of complex A differed from cell line to cell line (data not shown).
To identify complex B, we then proceeded to test a large panel of Ets factor antibodies in supershift assays. Antibodies against a large panel of Ets factors, including Ets-1, Ets-2, Fli-1, Spi-B, ERG1/ERG2, NERF1, E2A, SAP-1A, Elk1, and PEA1, failed to induce a supershift of complex B, as did antibodies against RBP-Jk and ICSBP. However, use of antibodies to the Ets family member Elf-1 resulted in a loss of complex B and the appearance of a supershifted band (Fig. 5C), suggesting that at least part of complex B included Elf-1. To further verify that Elf-1 was part of a complex binding to this site in vivo, we performed ChIP assays with anti-Elf-1 antibodies. Antibodies to Elf-1 were able to react with chromatin including this site in 416B cells (Fig. 5D), supporting the notion that Elf-1 in addition to PU.1 binds to this element to regulate PU.1 expression. The relative enrichment of the PCR signal from the PU.1 URE with Elf-1 antibodies over those with GATA-2 antibodies or IgG was 15-fold. In summary, PU.1, Elf-1, and at least one unidentified factor (complex A), which is unlikely to be an Ets family member, bind to this site in myeloid and lymphoid cells.
The PU.1 binding site is required for activity of the kb −14 URE regulatory function in myeloid cells.
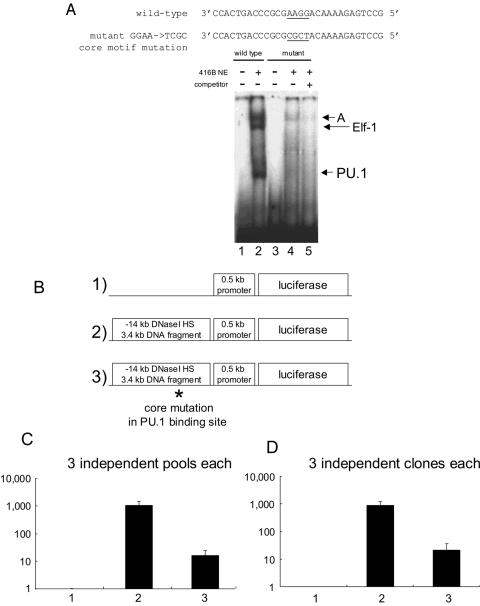
To demonstrate the functional role of the PU.1 binding site, we designed a mutation which would abolish binding of PU.1 to this element. This oligonucleotide contained mutations in the PU.1 binding core motif (GGAA to TCGC) and completely abolished PU.1 binding (Fig. 6A). This mutation also abolished binding of complex B containing Elf-1, demonstrating that both Ets proteins require the purine-rich core for DNA binding to this site. In contrast, binding by complex A was weakened but not abolished. Although we tried a number of other mutant oligonucleotides, we were unable to identify a sequence which discriminated between binding of PU.1 and binding of Elf-1 (data not shown).
FIG. 6.
Mutations of the PU.1 binding site in the proximal (3′) homology region which disrupt binding of PU.1 and Elf-1 abolish the activity of the kb −14 PU.1 URE. (A) Introduction of a mutation in the purine-rich core of the PU.1 binding site disrupts binding of PU.1 and Elf-1 to this site. A mutation was introduced into the core GGAA sequence by PCR mutagenesis. Sequences of the wild-type and mutant oligonucleotides are shown at the top. The purine-rich core (GGAA) which is changed to TCGC in the mutant is underlined. 416B nuclear extracts were used as a source of DNA binding proteins. The probes utilized in binding assays were wild-type oligonucleotide (lanes 1 to 2) and mutant oligonucleotide (lanes 3 to 5). Mutant oligonucleotide was used as a self-competitor for lane 5. (B) Constructs utilized for stable transformants of the 416B myeloid cell line: 1) the 334-bp murine PU.1 promoter and 152 bp of 5′ untranslated sequence in the luciferase vector pXP2 (“0.5 kb promoter”); 2) the PU.1 kb −14 URE (the 3.4-kb HindIII fragment) upstream of the 0.5 kb promoter; 3) the PU.1 kb −14 URE containing the mutation in the PU.1 site (GGAA → TCGC) shown in Fig. 6A. (C) Mutation of the PU.1 binding site results in a 100-fold decrease in URE activity. The luciferase activity of three independent pools of the 0.5-kb promoter only (lane 1), the 0.5-kb promoter with wild-type URE (lane 2), and the 0.5-kb promoter with a mutation in the PU.1 site of the URE (lane 3) is shown. The mean and standard deviation for each pool are shown. (D) Mutation of the PU.1 binding site reduces URE activity in individual clones of stably transfected 416B cells. The experiment is similar to that whose results are shown in Fig. 6C except that three independent clones were used for each construct. The mean and standard deviation for each clone are shown. Luciferase activity was normalized to transgene copy number measured by Southern blot analysis.
We next asked whether the binding of PU.1 and Elf-1 to this site was essential for the activity of the kb −14 URE in regulation of the PU.1 gene. We previously reported that a 3.4-kb fragment including this PU.1 kb −14 URE along with the 334-bp murine PU.1 promoter and 152 bp of 5′ untranslated sequence can augment reporter gene expression 100- to 300-fold in myeloid cell lines (416B and U937) but not in the T-lymphoid-cell line (BW5147) compared with that of the promoter and 5′ untranslated sequences alone. Therefore, we introduced the mutation (GGAA to TCGC) that abolished PU.1 and Elf-1 binding in EMSA (Fig. 6A) into a construct including the PU.1 kb −14 URE 3.4-kb HindIII fragment, the PU.1 promoter and 5′ untranslated region, and a luciferase reporter previously used in these assays (Fig. 6B) (29). We tested wild-type and mutant constructs, along with a construct including the promoter but not the URE, in stable transfectants of 416B myeloid cells (Fig. 6B). As we previously reported (29), addition of the kb −14 URE 3.4-kb fragment conferred 100- to 1,000-fold-higher luciferase expression in stably transfected 416B cells compared to the results seen with the PU.1 promoter alone (Fig. 6C and D). In contrast, mutations in the core motif of the PU.1/Elf-1 binding site decreased reporter activity 100-fold (Fig. 6C and D). The same results were obtained whether we analyzed three independent pools of clones (Fig. 6C) or three independent clones of each construct (Fig. 6D). These results show that this element binding PU.1 and Elf-1 is essential for the function of the PU.1 URE and that the PU.1 gene is positively regulated through this site located 14 kb upstream of the transcription start site.
DISCUSSION
Although there are examples of genes in which a few hundred base pairs of promoter sequence are able to confer regulated expression (18), it is becoming increasingly apparent that proper regulation of most genes requires a number of elements in addition to the promoter and that many of these are located tens of kilobases or more from the transcription start site. Therefore, a number of investigators have utilized a combination of methods to identify distal regulatory elements, including DNase I hypersensitivity assays, sequence homology searching, and the use of large genomic DNA fragments conferred by YAC, BAC, PAC, or P1 clones to generate transgenic animals, particularly when isolated elements have failed to work in vivo (6, 24, 30, 41, 42, 45, 49, 64).
In the case of PU.1, constructs including the promoter alone failed to express in vivo in transgenic models; in contrast, mice harboring a murine P1 clone including 35 kb of 5′ and 3′ flanking sequences expressed the exogenous PU.1 gene in a myeloid- and B-cell-specific manner similar to that of the endogenous gene (29). Subsequent DNase I hypersensitivity assays identified an element 14 kb upstream of the gene which conferred reporter gene activity more than 100-fold higher than that seen with the PU.1 promoter only in stably transfected myeloid lines. Interestingly, this element failed to show any activity in transient transfections, suggesting that its functions are dependent on chromatin context. Here we demonstrate that this element also confers reporter gene expression in transgenic mice; that the pattern of expression is restricted to granulocytes, macrophages, and B cells in a lineage-specific manner; and that proper expression is seen in myeloid but not in erythroid progenitors. Supporting the importance of this element, in other studies we have demonstrated that targeted disruption of the kb −14 URE in mice led to an 80% decrease in PU.1 gene expression in the bone marrow (53).
We and others have demonstrated that distal regulatory elements can be identified by sequence homology (19, 30). Therefore, we searched within the 3.4-kb upstream fragment for regions of homology and found two domains which were highly conserved in five different mammalian species, including mice and humans, for which genomic sequence information is available. Recent studies of the zebra fish PU.1 gene have revealed that a 7-kb upstream fragment can direct expression in vivo in transgenic fish. However, consistent with findings in other genes, although the coding sequences of mammalian and zebra fish PU.1 are conserved, the regulatory regions are not, and we could not find any sequence similarity between the mammalian PU.1 kb −14 URE and zebra fish genomic sequences, including this 7-kb fragment (K. Hsu and T. Look, unpublished observations).
PU.1 was originally identified as SFFV integration site 1 (Spi-1) (34, 44). The SFFV provirus integration sites span several kilobases approximately 14 kb 5′ of the PU.1 coding region (34, 44). By comparison of the precise sequences adjacent to known viral integration sites with the sequence of the PU.1 URE, we found one major SFFV integration site between these two homology regions (Fig. 3A). Because this kb −14 URE has an open chromatin structure according to the results of a DNase I hypersensitivity assay (29), it is understandable that this region was targeted by SFFV as an integration site. Integration of SFFV induces dysregulation of the PU.1 gene, and it is thought that the failure of PU.1 to downregulate during erythroid differentiation contributes to the erythroleukemia. These findings suggest that one mechanism by which the SFFV induces the maintenance of PU.1 expression in early erythroid cells could be by disruption of the kb −14 URE. Specifically, one hypothesis is that the distal (5′) conserved region is important for downregulation of PU.1 in erythroid cells, while the proximal (3′) element serves as a positive element. Integration of the SFFV in between these two elements might prevent negative regulation in erythroid cells; in addition, the viral LTR might serve to directly activate the PU.1 gene. Both mechanisms might participate in dysregulation of PU.1 in erythroid leukemia cells. Other reports have also described PU.1 downregulation in erythroid commitment (17, 63) and shown that failure to downregulate PU.1 would result in inhibition of the essential erythroid regulator GATA-1 (38, 52, 66, 67). Future experiments utilizing transgenic mice harboring either the distal or proximal region will be required to test the hypothesis that the distal conserved region plays a role in downregulation in erythroid cells.
Autoregulation may be a common feature of several transcriptional regulators to augment the relatively irreversible nature of differentiation processes in the hematopoietic system (60, 61). Of interest is that PU.1 and GATA-1 both potentially exhibit autoregulation, whereas at the same time interactions between these two mediate inhibition of each other's function (38, 52, 66, 67). Therefore, an attractive model is that reciprocal inhibition and autoregulation of these two regulators provides a mechanism for lineage choice during early blood stem cell and progenitor cell differentiation. Similar combinations of inhibition and autoregulation involving PU.1 and C/EBPα have been hypothesized to mediate the decision of myeloid progenitors to differentiate into either granulocytes or monocytes (11, 31, 51, 62).
As PU.1 is an important regulator of multiple hematopoietic lineages, an important question is what regulates the regulators. We previously reported a potential autoregulatory site in the PU.1 promoter in transient assays (9). Utilizing a combination of in vivo and in vitro assays, we identified a PU.1 binding site in the proximal (3′) conserved region of the kb −14 URE. A mutation which abolished binding by PU.1 led to a loss of function of the URE in myeloid cells. These results also suggest that PU.1 might be positively regulated by PU.1 itself, and there could be two positive autoregulatory sites in the PU.1 gene: one in the kb −14 URE and one in the promoter region. However, in addition to binding by PU.1 to this element, this same site bound at least two other proteins: Elf-1 and an unknown complex (complex A). While the role of Elf-1 in regulation of PU.1 expression has yet to be determined, it is of interest that we have shown that Elf-1 is very important in the function of enhancers which activate another hematopoietic transcription factor, SCL (20). If Elf-1 does indeed regulate both SCL and PU.1, then it may serve an important function in the initiation of expression of two very important hematopoietic regulators, raising the possibility that Elf-1 serves a common role in multiple hematopoietic lineages. In view of the fact that both Elf-1 and complex A bind to this site in addition to PU.1, additional experiments will be required to firmly establish autoregulation by PU.1 through this site.
Previously, we reported that the kb −14 URE has an open chromatin structure not only in myeloid cell lines but also in a T-lymphoid-cell line, even though this region could not activate reporter gene expression in the same T-cell line (29). However, EMSA analysis demonstrates that complexes A and B bind to this region in T-lymphoid-cell lines (Jurkat and Molt-4), perhaps contributing to the open chromatin structure. This raises the issue of what the biological meaning of these binding complexes in T cells might be. One possibility is that these complexes could be negative regulators of PU.1 expression and serve to shut off PU.1 gene expression in T-lymphoid commitment. It has been reported that constitutive expression of PU.1 induces a block in differentiation of T-lymphoid cells at the pro-T-cell stage, indicating the importance of downregulation of PU.1 in T-lymphoid commitment (4, 5, 26). Suppression of PU.1 gene expression by these slowly migrating complexes might be a mechanism of downregulation of PU.1 in T-lymphoid commitment. Identification of the second complex (complex A) will be necessary to prove this hypothesis.
As described above, the kb −14 URE and PU.1 promoter can confer reporter gene expression in transgenic mice and cell lines, whereas the PU.1 promoter by itself could not. However, the RNA expression level is quite low in transgenic mice with these constructs compared with that seen with the 91-kb P1 transgene which harbors the entire murine PU.1 gene and 35 kb each of 5′ and 3′ flanking sequence (data not shown) (29). In addition to this 91-kb transgene, we also made transgenic mice with a construct which lacks sequences from −35 kb to −20 kb upstream of the PU.1 transcription start site. The expression of transgenic RNA in these mice was approximately fivefold less than that of the original 91-kb construct (Y. Okuno and D. G. Tenen, unpublished data). These results suggest that there is at least one other element that augments the level of expression of PU.1 in addition to the kb −14 URE. In addition, it has been reported that Notch signaling increases PU.1 gene expression (55). We could not detect a binding site for RBP/Jk, a transcription factor downstream of Notch signaling pathways, in the kb −14 URE or demonstrate augmentation of URE activity following transfection of activated Notch (T. Schroeder and D. G. Tenen, unpublished data). One possibility is that other cis long-range elements located in the region between −35 and −20 kb relative to the PU.1 transcription start site have a binding site for RBP/Jκ. Alternatively, PU.1 has been reported to be regulated by elements within the first intron (2), which were not included in our transgenic constructs, and addition of these elements might be necessary for normal levels of PU.1 expression. Further analysis of the kb −14 URE and additional regulatory elements will be necessary to fully elucidate the mechanisms of PU.1 gene expression and subsequent consequences on hematopoietic differentiation and leukemia.
Acknowledgments
We thank Bruce Torbett for providing the 503 PU.1−/− cell line, Karl Hsu and Tom Look for analysis of zebra fish PU.1 genomic sequences, Timm Schroeder for discussions regarding Notch regulation of PU.1, Katharina Wagner for assistance in FACS analysis, Tony Green for encouragement and support, and members of the Tenen laboratory for thoughtful discussions.
This work was supported by Public Health Service grant CA41456 from the National Cancer Institute (D.G.T.); DK62064 from the National Institute of Diabetes and Digestive and Kidney Diseases (H.S.R.); DFG (German research foundation) research fellowship RO 2295/1-1 (F.R.); and a senior lecturer fellowship from the Leukemia Research Fund (B.G.).
REFERENCES
- 1.Akashi, K., D. Traver, T. Miyamoto, and I. L. Weissman. 2000. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404:193-197. [DOI] [PubMed] [Google Scholar]
- 2.Amaravadi, L., and M. J. Klemsz. 1999. DNA methylation and chromatin structure regulate PU.1 expression. DNA Cell Biol. 18:875-884. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, K. L., K. A. Smith, H. Perkin, G. Hermanson, C. G. Anderson, D. G. Jolly, R. A. Maki, and B. E. Torbett. 1999. PU.1 and the granulocyte- and macrophage colony-stimulating factor receptors play distinct roles in late-stage myeloid cell differentiation. Blood 94:2310-2318. [PubMed] [Google Scholar]
- 4.Anderson, M. K., G. Hernandez-Hoyos, R. A. Diamond, and E. V. Rothenberg. 1999. Precise developmental regulation of Ets family transcription factors during specification and commitment to the T cell lineage. Development 126:3131-3148. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, M. K., A. H. Weiss, G. Hernandez-Hoyos, C. J. Dionne, and E. V. Rothenberg. 2002. Constitutive expression of PU.1 in fetal hematopoietic progenitors blocks T cell development at the pro-T cell stage. Immunity 16:285-296. [DOI] [PubMed] [Google Scholar]
- 6.Antoch, M. P., E. J. Song, A. M. Chang, M. H. Vitaterna, Y. Zhao, L. D. Wilsbacher, A. M. Sangoram, D. P. King, L. H. Pinto, and J. S. Takahashi. 1997. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell 89:655-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behre, G., A. J. Whitmarsh, M. P. Coghlan, T. Hoang, C. L. Carpenter, D.-E. Zhang, R. J. Davis, and D. G. Tenen. 1999. C-Jun is a JNK-independent coactivator of the PU.1 transcription factor. J. Biol. Chem. 274:4939-4946. [DOI] [PubMed] [Google Scholar]
- 8.Bockamp, E. O., J. L. Fordham, B. Gottgens, A. M. Murrell, M. J. Sanchez, and A. R. Green. 1998. Transcriptional regulation of the stem cell leukemia gene by PU.1 and Elf-1. J. Biol. Chem. 273:29032-29042. [DOI] [PubMed] [Google Scholar]
- 9.Chen, H. M., D. Ray-Gallet, P. Zhang, C. J. Hetherington, D. A. Gonzalez, D.-E. Zhang, F. Moreau-Gachelin, and D. G. Tenen. 1995. PU.1 (Spi-1) autoregulates its expression in myeloid cells. Oncogene 11:1549-1560. [PubMed] [Google Scholar]
- 10.Chen, H. M., P. Zhang, H. S. Radomska, C. J. Hetherington, D.-E. Zhang, and D. G. Tenen. 1996. Octamer binding factors and their coactivator can activate the murine PU.1 (spi-1) promoter. J. Biol. Chem. 271:15743-15752. [DOI] [PubMed] [Google Scholar]
- 11.Dahl, R., J. C. Walsh, D. Lancki, P. Laslo, S. R. Iyer, H. Singh, and M. C. Simon. 2003. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPalpha ratio and granulocyte colony-stimulating factor. Nature Immunol. 4:1029-1036. [DOI] [PubMed] [Google Scholar]
- 12.Duprez, E., J. Iwasaki-Arai, K. Geary, C. Somoza, R. Murray, K. Akashi, and D. G. Tenen. 2002. PU.1 deficiency blocks the production of common myeloid and common lymphoid progenitors in both adult and fetal liver myelopoiesis. Blood 100:61a. [Google Scholar]
- 13.Dziennis, S., R. A. Van Etten, H. L. Pahl, D. L. Morris, T. L. Rothstein, C. M. Blosch, R. M. Perlmutter, and D. G. Tenen. 1995. The CD11b promoter directs high level expression of reporter genes in macrophages in transgenic mice. Blood 85:319-329. [PubMed] [Google Scholar]
- 14.Eisenbeis, C. F., H. Singh, and U. Storb. 1995. Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev. 9:1377-1387. [DOI] [PubMed] [Google Scholar]
- 15.Fisher, R. C., J. D. Lovelock, and E. W. Scott. 1999. A critical role for PU.1 in homing and long-term engraftment by hematopoietic stem cells in the bone marrow. Blood 94:1283-1290. [PubMed] [Google Scholar]
- 16.Forsberg, E. C., K. M. Downs, and E. H. Bresnick. 2000. Direct interaction of NF-E2 with hypersensitive site 2 of the β-globin locus control region in living cells. Blood 96:334-339. [PubMed] [Google Scholar]
- 17.Galson, D. L., J. O. Hensold, T. R. Bishop, M. Schalling, A. D. D'Andrea, C. Jones, P. E. Auron, and D. E. Housman. 1993. Mouse β-globin DNA-binding protein B1 is identical to a proto-oncogene, the transcription factor Spi-1/PU.1, and is restricted in expression to hematopoietic cells and the testis. Mol. Cell. Biol. 13:2929-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopalsrivastava, R., and J. Piatigorsky. 1994. Identification of a lens-specific regulatory region (Lsr) of the murine alpha b-crystallin gene. Nucleic Acids Res. 22:1281-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Göttgens, B., L. M. Barton, J. G. R. Gilbert, A. J. Bench, M. J. Sanchez, S. Bahn, S. Mistry, D. Grafham, A. McMurray, M. Vaudin, E. Amaya, D. R. Bentley, and A. R. Green. 2000. Analysis of vertebrate SCL loci identifies conserved enhancers. Nat. Biotechnol. 18:181-186. [DOI] [PubMed] [Google Scholar]
- 20.Göttgens, B., A. Nastos, S. Kinston, S. Piltz, E. C. M. Delabesse, M. Stanley, M. J. Sanchez, A. Ciau-Uitz, R. Patient, and A. R. Green. 2002. Establishing the transcriptional programme for blood: the SCL stem cell enhancer is regulated by a multiprotein complex containing Ets and GATA factors. EMBO J. 21:3039-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hohaus, S., M. S. Petrovick, M. T. Voso, Z. Sun, D.-E. Zhang, and D. G. Tenen. 1995. PU.1 (Spi-1) and C/EBPα regulate the expression of the granulocyte-macrophage colony-stimulating factor receptor α gene. Mol. Cell. Biol. 15:5830-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hromas, R., A. Orazi, R. S. Neiman, R. Maki, C. Van Beveren, J. Moore, and M. Klemsz. 1993. Hematopoietic lineage-restricted and stage-restricted expression of the ETS oncogene family member PU.1. Blood 82:2998-3004. [PubMed] [Google Scholar]
- 23.Iwama, A., P. Zhang, G. J. Darlington, S. R. McKercher, R. A. Maki, and D. G. Tenen. 1998. Use of RDA analysis of knockout mice to identify myeloid genes regulated in vivo by PU.1 and C/EBPα. Nucleic Acids Res. 26:3034-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman, R. M., C. T. Pham, and T. J. Ley. 1999. Transgenic analysis of a 100-kb human beta-globin cluster-containing DNA fragment propagated as a bacterial artificial chromosome. Blood 94:3178-3184. [PubMed] [Google Scholar]
- 25.Kistler, B., P. Pfisterer, and T. Wirth. 1995. Lymphoid- and myeloid-specific activity of the PU.1 promoter is determined by the combinatorial action of octamer and ets transcription factors. Oncogene 11:1095-1106. [PubMed] [Google Scholar]
- 26.Klemsz, M. J., M. Gaboli, R. Hromas, and P. P. Pandolfi. 1997. Expression of PU.1 in the thymus results in altered T cell development. Blood 90:22a. [Google Scholar]
- 27.Klemsz, M. J., R. A. Maki, T. Papayannopoulou, J. Moore, and R. Hromas. 1993. Characterization of the ets oncogene family member, fli-1. J. Biol. Chem. 268:5769-5773. [PubMed] [Google Scholar]
- 28.Klemsz, M. J., S. R. McKercher, A. Celada, C. Van Beveren, and R. A. Maki. 1990. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell 61:113-124. [DOI] [PubMed] [Google Scholar]
- 29.Li, Y., Y. Okuno, P. Zhang, H. S. Radomska, H. M. Chen, H. Iwasaki, K. Akashi, M. J. Klemsz, S. R. McKercher, R. A. Maki, and D. G. Tenen. 2001. Regulation of the PU.1 gene by distal elements. Blood 98:2958-2965. [DOI] [PubMed] [Google Scholar]
- 30.Loots, G. G., R. M. Locksley, C. M. Blankespoor, Z. E. Wang, W. Miller, E. M. Rubin, and K. A. Frazer. 2000. Identification of a coordinate regulator of interleukins 4, 13, and 5 by cross-species sequence comparisons. Science 288:136-140. [DOI] [PubMed] [Google Scholar]
- 31.McIvor, Z., S. Hein, H. Fiegler, T. Schroeder, C. Stocking, U. Just, and M. Cross. 2003. Transient expression of PU.1 commits multipotent progenitors to a myeloid fate whereas continued expression favors macrophage over granulocyte differentiation. Exp. Hematol. 31:39-47. [DOI] [PubMed] [Google Scholar]
- 32.McKercher, S. R., B. E. Torbett, K. L. Anderson, G. W. Henkel, D. J. Vestal, H. Baribault, M. Klemsz, A. J. Feeney, G. E. Wu, C. J. Paige, and R. A. Maki. 1996. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 15:5647-5658. [PMC free article] [PubMed] [Google Scholar]
- 33.Miyamoto, T., H. Iwasaki, B. Reizis, M. Ye, T. Graf, I. L. Weissman, and K. Akashi. 2002. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev. Cell 3:137-147. [DOI] [PubMed] [Google Scholar]
- 34.Moreau-Gachelin, F., A. Tavitian, and P. Tambourin. 1988. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature 331:277-280. [DOI] [PubMed] [Google Scholar]
- 35.Moreau-Gachelin, F., F. Wendling, T. Molina, N. Denis, M. Titeux, G. Grimber, P. Briand, W. Vainchenker, and A. Tavitian. 1996. Spi-1/PU.1 transgenic mice develop multistep erythroleukemias. Mol. Cell. Biol. 16:2453-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagulapalli, S., J. M. Pongubala, and M. L. Atchison. 1995. Multiple proteins physically interact with PU.1. J. Immunol. 155:4330-4338. [PubMed] [Google Scholar]
- 37.Nerlov, C., and T. Graf. 1998. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 12:2403-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nerlov, C., E. Querfurth, H. Kulessa, and T. Graf. 2000. GATA-1 interacts with the myeloid PU.1 transcription factor and represses PU.1 dependent transcription. Blood 95:2543-2551. [PubMed] [Google Scholar]
- 39.Nikolajczyk, B. S., J. A. Sanchez, and R. Sen. 1999. ETS protein-dependent accessibility changes at the immunoglobulin mu heavy chain enhancer. Immunity 11:11-20. [DOI] [PubMed] [Google Scholar]
- 40.Nordeen, S. K. 1988. Luciferase reporter gene vectors for analysis of promoters and enhancers. BioTechniques 6:454-458. [PubMed] [Google Scholar]
- 41.Okuno, Y., C. S. Huettner, H. S. Radomska, V. Petkova, H. Iwasaki, K. Akashi, and D. G. Tenen. 2002. Distal elements are critical for human CD34 expression in vivo. Blood 100:4420-4426. [DOI] [PubMed] [Google Scholar]
- 42.Okuno, Y., H. Iwasaki, C. S. Huettner, H. S. Radomska, D. A. Gonzalez, D. G. Tenen, and K. Akashi. 2002. Differential regulation of the human and murine CD34 genes in hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 99:6246-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pahl, H. L., R. J. Scheibe, D.-E. Zhang, H. M. Chen, D. L. Galson, R. A. Maki, and D. G. Tenen. 1993. The proto-oncogene PU.1 regulates expression of the myeloid-specific CD11b promoter. J. Biol. Chem. 268:5014-5020. [PubMed] [Google Scholar]
- 44.Paul, R., S. Schuetze, S. L. Kozak, C. A. Kozak, and D. Kabat. 1991. The Sfpi-1 proviral integration site of Friend erythroleukemia encodes the ets-related transcription factor PU.1. J. Virol. 65:464-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson, K. R., C. H. Clegg, C. Huxley, B. M. Josephson, H. S. Haugen, T. Furukawa, and G. Stamatoyannopoulos. 1993. Transgenic mice containing a 248-kb yeast artificial chromosome carrying the human beta-globin locus display proper developmental control of human globin genes. Proc. Natl. Acad. Sci. USA 90:7593-7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pongubala, J. M., S. Nagulapalli, M. J. Klemsz, S. R. McKercher, R. A. Maki, and M. L. Atchison. 1992. PU.1 recruits a second nuclear factor to a site important for immunoglobulin κ 3′ enhancer activity. Mol. Cell. Biol. 12:368-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pongubala, J. M., C. Van Beveren, S. Nagulapalli, M. J. Klemsz, S. R. McKercher, R. A. Maki, and M. L. Atchison. 1993. Effect of PU.1 phosphorylation on interaction with NF-EM5 and transcriptional activation. Science 259:1622-1625. [DOI] [PubMed] [Google Scholar]
- 48.Qian, L., and M. Wilkinson. 1991. DNA fragment purification: removal of agarose 10 minutes after electrophoresis. BioTechniques 10:736-738. [PubMed] [Google Scholar]
- 49.Radomska, H. S., A. B. Satterthwaite, T. C. Burn, I. A. Oliff, C. S. Huettner, and D. G. Tenen. 1998. Multiple control elements are required for expression of the human CD34 gene. Gene 222:305-318. [DOI] [PubMed] [Google Scholar]
- 50.Ray-Gallet, D., C. Mao, A. Tavitian, and F. Moreau-Gachelin. 1995. DNA binding specificities of Spi-1/PU.1 and Spi-B transcription factors and identification of a Spi-1/Spi-B binding site in the c-fes/c-fps promoter. Oncogene 11:303-313. [PubMed] [Google Scholar]
- 51.Reddy, V., A. Iwama, G. Iotzova, G. Schulz, A. Elsasser, R. K. Vangala, D. G. Tenen, W. Hiddemann, and G. Behre. 2002. The granulocytic inducer C/EBPα inactivates the myeloid master gene PU.1: possible role in lineage commitment decisions. Blood 100:483-490. [DOI] [PubMed] [Google Scholar]
- 52.Rekhtman, N., F. Radparvar, T. Evans, and A. I. Skoultchi. 1999. Direct interaction of hematopoietic transcription factors PU.1 and GATA-1: functional antagonism in erythroid cells. Genes Dev. 13:1398-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenbauer, F., K. Wagner, J. L. Kutok, H. Iwasaki, M. M. Le Beau, Y. Okuno, K. Akashi, S. Fiering, and D. G. Tenen. 2004. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat. Genet. 36:624-630. [DOI] [PubMed] [Google Scholar]
- 54.Rosenbauer, F., K. Wagner, P. Zhang, K. P. Knobeloch, A. Iwama, and D. G. Tenen. 2004. pDP4, a novel glycoprotein secreted by mature granulocytes, is regulated by transcription factor PU.1. Blood 103:4294-4301. [DOI] [PubMed] [Google Scholar]
- 55.Schroeder, T., H. Kohlhof, N. Rieber, and U. Just. 2003. Notch signaling induces multilineage myeloid differentiation and up-regulates PU.1 expression. J. Immunol. 170:5538-5548. [DOI] [PubMed] [Google Scholar]
- 56.Scott, E. W., R. C. Fisher, M. C. Olson, E. W. Kehrli, M. C. Simon, and H. Singh. 1997. PU.1 functions in a cell-autonomous manner to control the differentiation of multipotential lymphoid-myeloid progenitors. Immunity 6:437-447. [DOI] [PubMed] [Google Scholar]
- 57.Scott, E. W., M. C. Simon, J. Anastai, and H. Singh. 1994. The transcription factor PU.1 is required for the development of multiple hematopoietic lineages. Science 265:1573-1577. [DOI] [PubMed] [Google Scholar]
- 58.Smith, L. T., S. Hohaus, D. A. Gonzalez, S. E. Dziennis, and D. G. Tenen. 1996. PU.1 (Spi-1) and C/EBPα regulate the granulocyte colony-stimulating factor receptor promoter in myeloid cells. Blood 88:1234-1247. [PubMed] [Google Scholar]
- 59.Spain, L. M., A. Guerriero, S. Kunjibettu, and E. W. Scott. 1999. T cell development in PU.1-deficient mice. J. Immunol. 163:2681-2687. [PubMed] [Google Scholar]
- 60.Tenen, D. G. 2003. Disruption of differentiation in human cancer: AML shows the way. Nature Rev. Cancer 3:89-101. [DOI] [PubMed] [Google Scholar]
- 61.Tenen, D. G., R. Hromas, J. D. Licht, and D.-E. Zhang. 1997. Transcription factors, normal myeloid development, and leukemia. Blood 90:489-519. [PubMed] [Google Scholar]
- 62.Timchenko, N. A., D. R. Wilson, L. R. Taylor, S. Abdelsayed, M. Wilde, M. Sawadogo, and G. J. Darlington. 1995. Autoregulation of the human C/EBPα gene by stimulation of upstream stimulatory factor binding. Mol. Cell. Biol. 15:1192-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Voso, M. T., T. C. Burn, G. Wulf, B. Lim, G. Leone, and D. G. Tenen. 1994. Inhibition of hematopoiesis by competitive binding of the transcription factor PU.1. Proc. Natl. Acad. Sci. USA 91:7932-7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu, W., Z. Misulovin, H. Suh, R. R. Hardy, M. Jankovic, N. Yannoutsos, and M. C. Nussenzweig. 1999. Coordinate regulation of RAG1 and RAG2 by cell type-specific DNA elements 5′ of RAG2. Science 285:1080-1084. [DOI] [PubMed] [Google Scholar]
- 65.Zhang, D.-E., C. J. Hetherington, H. M. Chen, and D. G. Tenen. 1994. The macrophage transcription factor PU.1 directs tissue specific expression of the macrophage colony-stimulating factor receptor. Mol. Cell. Biol. 14:373-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang, P., G. Behre, J. Pan, A. Iwama, N. Wara-aswapati, H. S. Radomska, P. E. Auron, D. G. Tenen, and Z. J. Sun. 1999. Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc. Natl. Acad. Sci. USA 96:8705-8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, P., X. Zhang, A. Iwama, C. Yu, K. A. Smith, B. Mueller, S. Narravula, B. E. Torbett, S. H. Orkin, and D. G. Tenen. 2000. PU.1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood 96:2641-2648. [PubMed] [Google Scholar]