Abstract
The retinoblastoma gene, RB1, is one of the most frequently mutated genes in human cancer. Rb heterozygous mice develop pituitary tumors with 100% incidence, and the E2F transcription factors are required for this. To assess whether deregulated E2F activity is sufficient to induce pituitary tumors, we generated transgenic mice expressing an inducible E2F3 protein in the intermediate lobe of the pituitary gland. We found that short-term deregulation of E2F activity, similar to the earliest stages of Rb loss, is able to induce abnormal proliferation of otherwise quiescent melanotrophs. However, while long-term exposure to deregulated E2F activity results in hyperplasia of the intermediate lobe, it did not lead to tumor formation. In fact, melanotrophs become insensitive to sustained E2F stimulation and enter an irreversible senescence-like state. Thus, although deregulated E2F activity results in hyperproliferation, it is not sufficient to mimic loss of Rb, sustain proliferation of melanotrophs, and ultimately induce pituitary tumors. Similarly, we found that primary cells in tissue culture become insensitive to sustained E2F3 activation and undergo premature senescence in a pRB-, p16Ink4a-, and p19Arf-dependent manner. Thus, we conclude that deregulated E2F activity is not sufficient to fully mimic loss of Rb due to the engagement of a senescence response.
Deregulation of a functional retinoblastoma protein (pRB) pathway is a feature of the majority of human cancers (50). pRB itself is inactivated in approximately 25% of human tumors, whereas other tumors contain alterations in core members of the pRB pathway, including overexpression of cyclin D1 and CDK4 and inactivation of p16INK4A (43).
Ablation of Rb in the mouse leads to embryonic lethality due to placental and hematopoietic defects (5, 19, 22). Like humans, mice carrying a single Rb mutant allele are predisposed to tumors but develop endocrine tumors mainly affecting the pituitary and the thyroid glands (15). Pituitary tumors arising in Rb+/− mice originate in melanotroph cells of the intermediate lobe of the pituitary that have lost the wild-type Rb allele (33). Indeed, data obtained with both chimeric mice and pituitary-specific Rb knockout mice have shown that loss of Rb is an essential and limiting step for pituitary tumor development (28, 49, 51). Interestingly, inactivation of two of the upstream regulators of the pRB pathway, p18INK4c and p27KIP1, also results in pituitary tumors, highlighting the central role of pRB in pituitary homeostasis (11, 13, 21).
Due to the prominent role of RB1 as a tumor suppressor gene, it is essential to understand how pRB controls cell proliferation. pRB has been reported to interact with many cellular proteins, among which the best characterized are the E2F family of transcription factors (53). The E2Fs are key regulators of the G1-S transition, and their activity is required for normal cell proliferation in mouse primary cells (52). The ability of pRB to bind E2F is modulated by the phosphorylation state of pRB, which is controlled by the combined actions of cyclin-CDK and CDK inhibitors (2). When pRB is bound to the E2Fs, it regulates their transcriptional activity by blocking their transactivation properties and by recruiting repressor complexes to E2F-responsive promoters (2). Phosphorylation or loss of pRB therefore results in release of active E2Fs and derepression of E2F target genes.
Several results obtained in tissue culture cells and in mouse models have suggested that the E2Fs are key targets for pRB. For example, deletion of E2f1 or E2f3 delays the embryonic lethality of Rb-deficient embryos (47, 55). Moreover, loss of E2f1 and E2f3 has been shown to decrease the incidence of pituitary tumors in Rb+/− mice (53, 54). An open question, however, is whether deregulated E2F activity alone is able to mimic Rb loss. In tissue culture studies, ectopic E2F (E2F1-3) expression is sufficient to promote DNA replication in immortalized quiescent rodent fibroblasts in the absence of growth factors (9, 20, 27, 35, 41). However, the overexpression of the activating E2Fs is not sufficient to induce S phase in primary mouse embryo fibroblasts (MEFs) or human diploid fibroblasts with a functional pRB and p53 pathway (24), while acute loss of pRB induces cell cycle reentry in primary MEFs (37).
Deregulation of E2F activity has thus far been studied in vivo mainly in transgenic animals overexpressing E2F1 in tissues where little is known about the effect of Rb loss (6, 17, 34). E2F1 overexpression in vivo triggers apoptosis in target tissues, suggesting a strong selection against transgenic expressing cells (17). However, in certain tissues, such as the skin and the liver, E2F1 overexpression can also result in tumor formation (6, 34).
To assess whether increased E2F activity is able to mimic loss of pRB, we generated mice with deregulated E2F activity in the same cell type in which Rb loss results in tumorigenesis. We report here that deregulated E2F activity, similar to Rb loss, is able to induce proliferation of otherwise quiescent melanotrophs of the pituitary gland. Early abnormal proliferating cells in Rb+/− mice are the first evidence of malignant progression. However, the abnormal proliferating cells which arise due to deregulation of E2F activity do not result in malignant transformation but instead give rise to a hyperplastic phenotype. We demonstrate that melanotrophs, when exposed to continuous E2F deregulation, become insensitive to further E2F stimulation and ultimately enter an irreversible senescence-like state. Similarly, mouse and human primary cells show a biphasic response to deregulated E2F activity, responding to transient E2F deregulation by undergoing unscheduled proliferation and activating E2F target genes but being refractory to sustained E2F stimulation and ultimately undergoing premature senescence. We have found that senescence induced by sustained E2F3 activity is mediated by pRB, p19Arf, and p16Ink4a and thus collectively our results show that upon deregulated E2F activity, sustained abnormal proliferation is prevented by engagement of the senescence response mediated by the tumor suppressors pRB, p16Ink4a, and p19Arf.
MATERIALS AND METHODS
Generation of transgenic mice.
To generate the proopiomelanocortin (POMC)-estrogen receptor (ER)-E2F3 transgenic construct, the rat POMC promoter (14) was cloned in the pCI expression vector (Promega) into which the ER-E2F3 cDNA (48) was subsequently inserted. The POMC-ER-E2F3 fragment was excised from the parental plasmid and microinjected into the pronucleus of day 1-fertilized C57BL/6 embryos, and the resulting embryos were transferred into pseudopregnant foster mice. The resulting litters were screened for the presence of the transgene by Southern blot analysis with a radiolabeled rat POMC fragment as a probe.
Histology and immunohistochemical staining of mouse pituitary gland.
Pituitaries were obtained from transgenic and wild-type littermates, matched according to age, sex, and genetic background (mixed C57/129). Tamoxifen (Sigma) was injected at a dose of 1 mg/day (in sunflower oil). Tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained with hematoxylin and eosin. Primary antibodies used in this study were PCNA (Pharmingen); E2F3 (TFE31; Santa Cruz) (K. Helin, unpublished data); bromodeoxyuridine (Becton Dickinson); p16 (Santa Cruz H-156 and, with similar results, Santa Cruz M-156); and MCM2 (Santa Cruz N-19). All antibodies were used on paraffin-embedded sections microwaved (700 W, three times for 5 min) in 0.01 M citrate buffer (pH 6.0) for antigen retrieval. Detection was performed with the Envision kit (Dako) for mouse monoclonal antibodies and the Vectastain ABC kit (Vector Laboratories) for rabbit and goat polyclonal antibodies, according to the manufacturer's instructions. 4′,6′-Diamidino-2-phenylindole (DAPI) staining of pituitaries was performed on rehydrated paraffin sections for 4 min.
Cell culture and virus-mediated gene transfer.
MEFs were harvested from 13.5-day-old embryos. ARF heterozygous mice were mated to generate sibling-matched embryos. RBloxP/loxP MEFs were derived from homozygous RBloxP/loxP embryos. MEFs and TIG3-human telomerase reverse transcriptase cells were cultured in 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 50 U of penicillin per ml, 50 μg of streptomycin per ml, and 2 μg of l-glutamine per ml. pBabe-ER-E2F3 constructs were described previously (31, 48). 4-Hydroxy tamoxifen was added to the medium at 300 nM. Adeno-Cre stocks were purchased from the Gene Transfer core facility of the University of Iowa College of Medicine; 100 PFU was used per cell. Serial 3T3 cultivation was conducted as previously described (38).
Immunofluorescence.
Bromodeoxyuridine assays on MEFs were performed as previously described (18). Immunofluorescence was performed as previously described (31, 48). Detection of senescence-associated heterochromatic foci was performed as previously described (32).
Chromatin immunoprecipitation.
Chromatin immunoprecipitations were performed and analyzed essentially as described (12). Briefly, cells were cross-linked, harvested, and sonicated (average fragment length of 500 to 1,000 bp). The lysate was precleared and immunoprecipitated with polyclonal antibodies specific for E2F3 (2 μg of sc878, Santa Cruz), pRB (2 μg of sc50), or unrelated Flag antibody (2 μg of F3165, Sigma). Immune complexes were recovered with blocked protein A beads, the beads were washed thoroughly, then the complexes were eluted, cross-links were reversed, and material was recovered by phenol-chloroform extraction and ethanol precipitation. DNA was resuspended in 200 μl, and 7.5 μl was used per quantitative PCR reaction with 200 nM primers in 25 μl of SYBR Green reaction mix (Perkin Elmer). Primer sequences are available upon request.
Real-time quantitative PCR.
cDNA was generated by reverse transcription-PCR with the PE Applied Biosystems TaqMan reverse transcription reagents. Reactions were determined with the SYBR Green I detection chemistry system (Applied Biosystems, Foster City, Calif.), with an ABI Prism 7700 sequence detection system. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control gene for normalization. The sequences of the primers used are available upon request.
Western blotting.
For Western blotting, pituitary glands isolated from transgenic and wild-type mice were lysed in ELB (50 mM HEPES [pH 7.0], 250 mM NaCl, 0.1% NP-40) buffer. Protein lysates (25 μg) were separated on a sodium dodecyl sulfate-8% polyacrylamide gel and transferred to Immobilon-P (Millipore) by electroblotting. Immunoblotting was performed with the following antibodies: ER (Santa Cruz MC-20), pRB (Pharmingen G3-245), and p16 (Santa Cruz sc-1661).
RESULTS
Generation of ER-E2F3 transgenic mouse.
To assess whether deregulated E2F activity is sufficient to mimic Rb loss, we generated transgenic mice expressing E2F3 in the intermediate lobe of the pituitary gland. We used E2F3 due to its ability to induce quiescent cells to enter S phase more efficiently than other E2Fs and its reduced apoptotic potential (8). Transgenic mice expressing an inducible ER-E2F3 fusion protein were constructed (29, 31, 48). This inducible system enables us to closely mimic the developmental steps of pituitary tumorigenesis observed in Rb+/− mice and to avoid potential selection against transgenes during development.
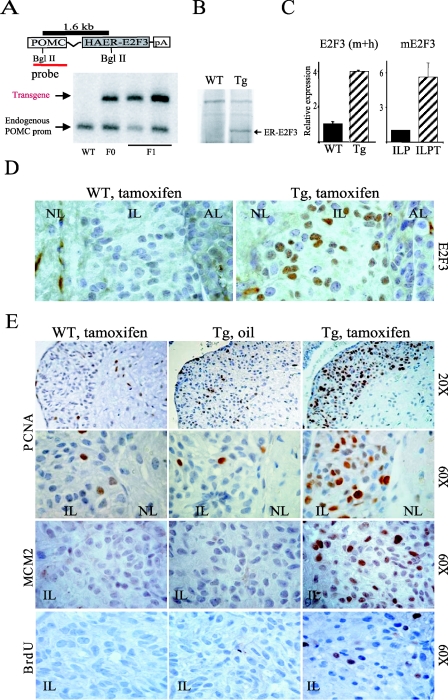
Expression of the ER-E2F3 protein was regulated by the proopiomelanocortin (POMC) promoter previously shown to be active in the corticonotroph and melanotroph cells of the pituitary gland (14, 23, 25). The presence of the transgene in the founder mouse and in its litter was determined by Southern blot analysis (Fig. 1A). The expression of ER-E2F3 was confirmed by immunoblotting on total pituitary extracts with an antibody to the estrogen receptor (Fig. 1B) and in situ by immunohistochemistry (Fig. 1D) with an antibody to E2F3. As expected, the transgene is expressed almost exclusively in melanotrophs of the pituitary gland, and by immunohistochemistry analysis, we estimated that more than 50% of the melanotroph cells in the intermediate lobe express the ER-E2F3 fusion protein (Fig. 1D and 3B).
FIG. 1.
POMC ER-E2F3 transgenic mice. (A) Schematic representation and detection of the ER-E2F3 transgenic construct. BglII restriction sites, the POMC probe, and the 1.6-kb BglII fragment are indicated. By Southern blot analysis, we detected a transgene-specific band (1.6 kb) and a band corresponding to the endogenous POMC gene, confirming the presence of the transgene in the founder mice (F0) and the F1 litter. (B) ER-E2F3 expression as determined by immunoblotting on total pituitary lysates with an estrogen receptor (ER)-specific antibody. (C) (Left panel) Relative expression of E2F3 mRNA in transgenic (dashed bar) and wild-type (black bar) pituitaries. (Right panel) Expression of murine E2f3 in a wild-type intermediate lobe (black bar) and in intermediate lobe pituitary tumors (ILPT) derived from Rb wild-type and mutant mice. (D) ER-E2F3 expression in situ. Immunohistochemistry with an antibody specific for human E2F3 on pituitaries derived from wild-type (WT) and transgenic (Tg) mice treated for 3 days with tamoxifen (+TAM). NL, neural lobe; IL, intermediate lobe; AL, anterior lobe. (E) ER-E2F3 is functional and induces unscheduled proliferation in vivo. PCNA and MCM2 expression and bromodeoxyuridine (BrdU) incorporation measured by immunohistochemistry in the intermediate lobe of wild-type (WT) animals treated with tamoxifen and transgenic animals (Tg) treated witheither tamoxifen or sunflower oil as a control .
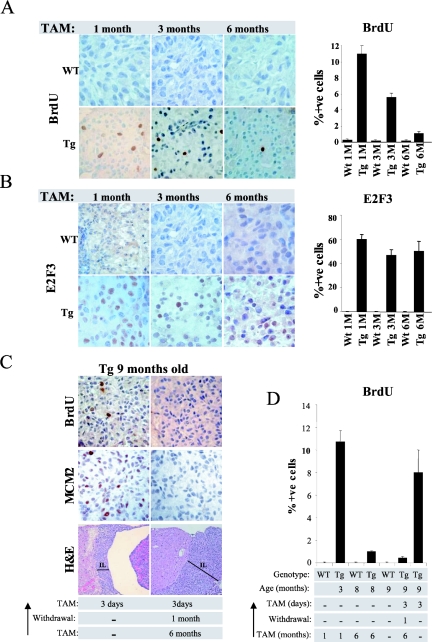
FIG. 3.
Melanotrophs become resistant to sustained increased E2F activity. (A) Decreased proliferation following sustained E2F activation. Bromodeoxyuridine incorporation measured by immunohistochemistry in the intermediate lobe of wild-type (WT) and transgenic (Tg) animals treated for the indicated time with daily tamoxifen (TAM) injections. The bar graph indicates the percentage of bromodeoxyuridine-positive melanotrophs in the intermediate lobe and the standard deviation of the mean. (B) Expression of ER-E2F3 protein is retained in tamoxifen-treated mice. Immunohistochemistry analysis of ER-E2F3 expression in the intermediate lobe of wild-type (WT) and transgenic (Tg) animals treated for the indicated times with daily tamoxifen injections. The bar graph indicates the percentage of E2F3-positive melanotrophs in the intermediate lobe and the standard deviation of the mean. (C) Sustained E2F activity triggers melanotrophs to become refractory to E2F stimulation. From the top: bromodeoxyuridine incorporation, MCM2 expression, and hematoxylin and eosin staining of melanotrophs after 3 days of tamoxifen treatment of 9-month-old transgenic mice either not previously exposed to tamoxifen (left panel) or treated for 6 months followed by 1 month of withdrawal (right panel). Note that the hyperplastic morphology of the intermediate lobe (bar) of the long-term-induced transgenic animals does not regress upon tamoxifen withdrawal (right panel). (D) Percentage of bromodeoxyuridine-positive melanotrophs. Treatment of 9-month-old transgenic animals leads to the same extent of S-phase induction achieved in 3-month-old transgenic animals (compare second lane from left with last on the right). Sustained E2F activity prevents S-phase induction in melanotrophs (fourth and sixth lanes from the left).
To evaluate the level of E2F3 overexpression in the pituitary gland, we designed PCR primers that recognize both human and mouse E2F3 cDNA. Using these primers, we estimate that the level of E2F3 overexpression in the pituitary gland of transgenic animals is approximately fourfold above the endogenous E2f3 levels (Fig. 1C; see Fig. S1 in the supplemental material). However, this difference is based on the transgene being expressed in all cells of the pituitary gland. Since this is not the case, we have estimated that the mean expression of E2F3 in POMC-expressing cells is approximately 50-fold higher than nonproliferating POMC-expressing cells (see Fig. S1 in the supplemental material). Importantly, the levels of E2f3 expression in pituitary tumors derived from pRB heterozygous mice are approximately 5-fold higher compared to what observed in a normal intermediate lobe (Fig. 1C), suggesting that ER-E2F3 is expressed at 10-fold higher levels in the transgenic mice compared to Rb-null pituitary tumors. Thus, we have generated transgenic mice expressing ER-E2F3 in cells from which pituitary tumors in Rb heterozygous mice originate.
ER-E2F3 fusion protein is inducible and functional.
To characterize the functionality of ER-E2F3 in vivo, we treated 3-month-old transgenic animals and control littermate wild-type animals for 3 days with either tamoxifen or, as a negative control, the carrier sunflower oil. Subsequently, the expression of the known E2F targets PCNA and MCM2 was assessed by immunohistochemistry. Tamoxifen treatment of transgenic mice leads to an increase in PCNA and MCM2 levels in more than 50% of the melanotrophs of the intermediate lobe, which is similar to the number of ER-E2F3-positive cells (Fig. 1E). In contrast, PCNA and MCM2 expression was not affected in control animals (Fig. 1E). Hence, we conclude that the transgenic mice express a fully functional and inducible E2F3 protein.
Ectopic E2F3 induction results in proliferation of quiescent melanotrophs.
Next we assessed whether ectopic E2F3 expression, like loss of pRB, results in the proliferation of otherwise quiescent melanotrophs. In agreement with a previous report (33), we did not detect any proliferating melanotrophs in the intermediate lobe of 3-month-old wild-type animals (Fig. 1E and data not shown). Moreover, we did not detect any proliferating melanotrophs in control mice, confirming the tight regulation of the ER-E2F3 fusion protein (Fig. 1E). Strikingly, activation of E2F3 induced proliferation in a high fraction of melanotrophs (14% of bromodeoxyuridine-positive cells after 3 h of labeling) in tamoxifen-treated transgenic mice, while no proliferation was observed in the controls (Fig. 1E and 3A). Thus, our data show that deregulation of E2F activity in melanotrophs of the intermediate lobe, like pRB loss, triggers unscheduled proliferation.
Deregulated E2F activity results in hyperplasia.
Loss of the second Rb allele in the majority of Rb heterozygous mice (>70%) takes place in a discrete number of cells within 2 months of age (33). In order to mimic the timing of the steps leading to tumorigenesis in the Rb+/− mice, we activated E2F3 by daily tamoxifen injections in 2-month-old transgenic mice (Fig. 2A). Prior to treatment, no morphological differences were observed in transgenic animals compared to wild-type animals (Fig. 2B). As controls, wild-type animals treated with tamoxifen and mice not treated with tamoxifen were included to monitor the potential effects arising from tamoxifen and the transgene integration site, respectively. A group of age-matched Rb+/− mice were also monitored during the experiment as positive controls.
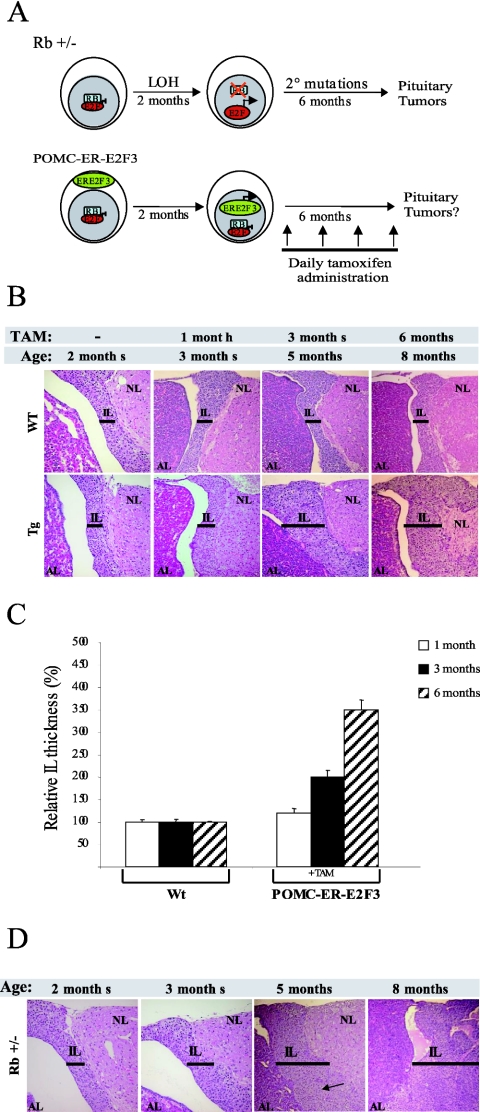
FIG. 2.
Deregulated E2F activity induces hyperplasia. (A) Experimental design. Schematic representation of tumorigenic steps leading to intermediate lobe tumors in Rb+/− mice (upper panel) and the experimental approach used in this study (lower panel). (B) Activation of E2F3 results in hyperplasia. Hematoxylin and eosin staining of wild-type and transgenic pituitaries treated for 0, 1, 3, and 6 months with daily injections of tamoxifen (TAM). Note the enlargement of the intermediate lobe in transgenic mice treated for 3 months and 6 months (bar). (C) Relative thickness of the intermediate lobe. The bar graph indicates the relative thickness of the intermediate lobe upontamoxifen treatment for the indicated times in transgenic animals relative to wild-type animals and the standard deviation of the mean. For each time point, the thickness of the intermediate lobe was measured in at least three different sections derived from five mice per genotype. The difference in size of the intermediate lobe in transgenic mice compared to wild-type (WT) mice is statistically significant upon 3 and 6 months of tamoxifen (TAM) treatment (Student t test; P = 0.0003 and P = 0.00001, respectively) (D) Neoplastic foci in Rb heterozygous mice. Pituitaries were stained as indicated for panel B. Note the appearance of neoplastic foci in 5-month-old mice and the progression towards tumor development in 8-month-old mice.
Mice were injected with tamoxifen until they were 8 months old, at which age the vast majority of Rb+/− mice have developed intermediate lobe tumors (28). Groups of 10 mice for each genotype and treatment were sacrificed after 1 month and 3 months of treatment, and 20 mice were analyzed after 6 months of treatment. The pituitaries of wild-type animals, either tamoxifen treated or untreated, appeared morphologically identical at all stages analyzed (data not shown). Thus, at the dose used in our experiments, tamoxifen treatment does not affect pituitary homeostasis. In addition, the pituitaries of nontreated transgenic animals were morphologically indistinguishable from the pituitaries of wild-type mice at all stages analyzed, demonstrating that the activity of the ER-E2F3 fusion protein is tightly controlled (data not shown).
We did not observe significant differences in survival between tamoxifen-treated ER-E2F3 transgenic mice and wild-type mice throughout the course of the experiment (data not shown). The pituitary glands of the transgenic mice showed an enlargement of the intermediate lobe after 1 month of treatment compared to control animals (Fig. 2B and C). This difference in morphology was more evident following 3 months of treatment in all the intermediate lobes analyzed (Fig. 2B and C).
Analysis of transgenic animals treated for 6 months further confirmed the hyperplastic phenotype (Fig. 2B and C). However, we found no evidence of macroscopic tumors or neoplastic foci in any (0 of 20) of the pituitaries analyzed from the tamoxifen-treated transgenic mice. Consistent with previous reports, we observed the first intermediate lobe tumors in Rb+/− animals by 5 months of age (matched control for transgenic mice treated for 3 months) (19, 22, 33). Moreover, in all the 8-month-old Rb+/− animals analyzed (matched controls for transgenic mice treated for 6 months), either macroscopic lesions or neoplastic foci were detected (Fig. 2D). These results strongly suggest that, although deregulation of E2F activity is sufficient to induce hyperplastic growth, it is not sufficient to mimic Rb loss leading to pituitary tumorigenesis.
Melanotrophs become refractory towards E2F stimulation.
To investigate why E2F deregulation does not lead to tumor formation despite being able to induce unscheduled proliferation ultimately resulting in hyperplastic growth, we analyzed the effect of E2F3 activation on melanotroph proliferation throughout the 6 months of treatment. We found no proliferation in intermediate lobe melanotrophs of wild-type mice by 3 months of age, and moreover, we did not detect any proliferating melanotrophs in control mice, confirming the absence of side effects of tamoxifen and the tight regulation of the ER-E2F3 fusion protein (Fig. 3A and data not shown).
In transgenic mice induced with tamoxifen for 1 month we found a significant fraction of melanotrophs undergoing proliferation (12% bromodeoxyuridine positive after a 3-h pulse-labeling), while no proliferation was observed in the controls (Fig. 3A). The total number of bromodeoxyuridine-positive cells decreased after 3 months of treatment, and by 6 months of treatment the percentage of bromodeoxyuridine-positive melanotrophs decreased to less than 1% (Fig. 3A). Importantly, E2F3 expression levels were unaffected throughout the period of tamoxifen induction, suggesting that the decrease in the number of proliferating cells was not due to a selection against melanotrophs expressing ER-E2F3 (Fig. 3B). Consistent with this, we detected only very few apoptotic melanotrophs (approximately 1%) by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining in treated animals (data not shown). Our data therefore suggest that melanotrophs become refractory to sustained deregulated E2F activity.
Sustained E2F activity induces cell cycle arrest.
One potential explanation for the inability of E2F to induce S phase in the later stages of the experiment could be that melanotrophs of older mice are less responsive to deregulated E2F activity than melanotrophs of younger mice. To test this, we induced E2F activity for 3 days in 9-month-old transgenic mice not previously exposed to tamoxifen. As controls for these experiments, we tested whether increased E2F3 activity could induce S phase in 9-month-old transgenic mice previously induced with tamoxifen for 6 months and “rested” for 1 month. Strikingly, deregulated E2F activity induces S-phase progression in 9-month-old transgenic mice not previously treated with tamoxifen but failed to do the same in transgenic mice previously induced for 6 months (Fig. 3C and D). Thus, the inability to respond to E2F stimulation after long-term induction of the transgene is not due to a refractory state of “aged” melanotrophs but is an event occurring due to prolonged exposure to deregulated E2F activity. Moreover, while induced E2F activity resulted in a robust induction of the E2F target gene MCM2, in 9-month-old transgenic mice not previously treated with tamoxifen, it failed to do so in mice previously exposed to sustained E2F activity (Fig. 3C). These data show that melanotrophs become permanently refractory to further mitogenic stimulation upon sustained E2F activity and that this correlates with the lack of induction of E2F target genes. Interestingly, 1 month of withdrawal had no effect on the morphology of the intermediate lobe of the transgenic animals, suggesting that this event is also irreversible (Fig. 3C).
Deregulated E2F activity results in a biphasic response in mouse primary cells.
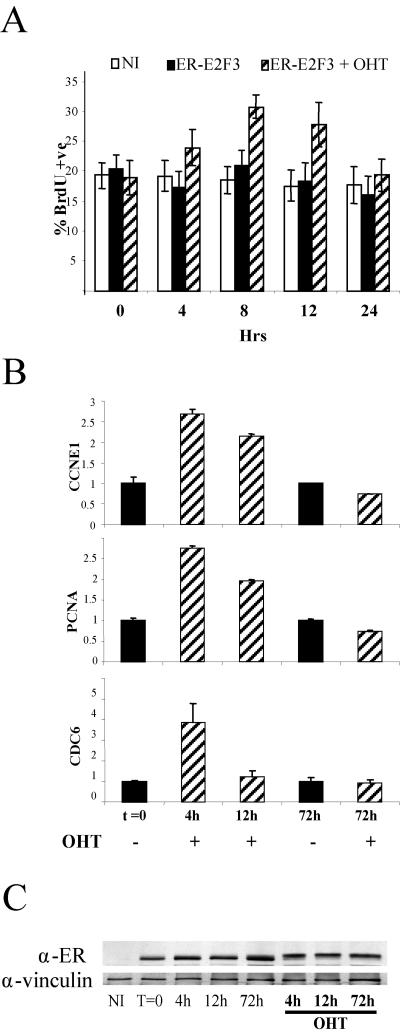
To understand if the engagement of a refractory state observed in vivo is a cell-autonomous response to deregulated E2F activity, we tested the outcome of transient and sustained E2F activity in mouse embryo fibroblast (MEFs). Induction of ER-E2F3 in growing early-passage MEFs resulted in a slight but significant (P < 0.005 at 8 h) increase in bromodeoxyuridine-positive cells within 8 h of induction (Fig. 4A). Increased E2F activity in proliferating mouse primary cells therefore promotes S-phase entry. Notably, however, the ability of E2F3 to induce proliferation is transient, because the levels of bromodeoxyuridine incorporation returned to the same levels seen in control cells 24 h after induction (Fig. 4A).
FIG. 4.
MEFs exhibit a biphasic response to E2F3 stimulation. (A) E2F3 induction enhances S-phase entry in cycling MEFs. The percentage of MEFs incorporating bromodeoxyuridine upon E2F3 induction is shown. NI, not infected; ER-E2F3, infected cells not induced;ER-E2F3 + OHT, infected cells induced with 4-hydroxy tamoxifen. (B) Transient induction of E2F targets upon E2F stimulation. Expression levels of the E2F targets PCNA, cyclin E1 (CCNE1), and CDC6 upon E2F3 induction was assessed by quantitative PCR, normalized against GAPDH, and the not-induced samples (t = 0) set as 1.0. (C) Expression of the ER-E2F3 fusion protein throughout the assay was assessed at the indicated times, and vinculin was used as a loading control.
Next we monitored the expression of known E2F target genes and found that, while a transient (4 h) ER-E2F3 induction resulted in the transcriptional activation of all genes tested, longer induction (>72 h) failed to do the same (Fig. 4B and data not shown). Importantly, the expression of ER-E2F3 was unaltered throughout the course of the experiment, excluding that the different response to E2F3 induction was due to loss of its expression (Fig. 4C). Our data therefore suggest that mouse primary cells, like melanotrophs, are not growth stimulated by sustained increased E2F activity.
Prolonged activation of E2F3 precludes the formation of transcriptionally active E2F3 complexes.
As shown in Fig. 5A, activation of E2F3 in human diploid fibroblasts also led to a transient induction of E2F target genes. The inability of deregulated E2F activity to maintain the activation of E2F target genes could be due either to the inaccessibility of responsive promoters to ER-E2F3 binding or to the inability of the chromatin-bound E2F to form active transcriptional complexes. To test this, we performed chromatin immunoprecipitation assays and found that, although the expression of E2F target genes was activated only transiently by E2F3 (Fig. 5A), ER-E2F3 was also significantly enriched on E2F target promoters 72 h after activation (Fig. 5B). Thus, ER-E2F3 is bound to E2F promoters after sustained activation but does not function as an activator of transcription.
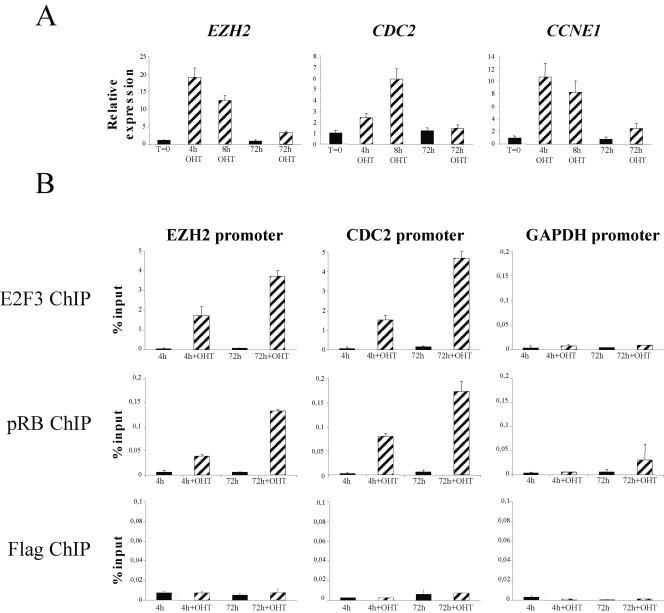
FIG. 5.
pRB is recruited to E2F dependent promoters in response to E2F3 activation. (A) Transient induction of E2F targets upon E2F3 activation. Expression levels of the E2F targets EZH2, CDC2, and cyclin E1 (CCNE1) after E2F3 activation was assessed by quantitative PCR, normalized against GAPDH, and not induced (t = 0) was set as 1.0. (B) ER-E2F3 binds to E2F-responsive promoters and recruits pRB upon activation. Enrichment, relative to the input, of the EZH2, CDC2, and GAPDH promoters following E2F3, pRB, or Flag chromatin immunoprecipitation (ChIP) is shown.
Since E2F3 forms active repressor complexes when bound to pRB, we assessed pRB binding to E2F target promoters after E2F3 activation. Consistent with previous reports (45), we found very little pRB associated with E2F target promoters in asynchronously growing cells without E2F activation. Surprisingly, pRB was recruited to promoters following as little as 4 h of E2F3 activation (Fig. 5B), and significantly, this enrichment increased upon sustained induction of E2F. This could suggest that pRB forms a repressive complex with E2F3 on the promoters of E2F target genes, preventing their sustained activation.
Since pRB can recruit inhibitory histone deacetylase activity to E2F-responsive promoters (44, 46), we analyzed the acetylation state of the promoters at various times after 4-hydroxy tamoxifen treatment. We did not detect any overall change in the level of acetylation (histone H3) at E2F target promoters (data not shown), which could reflect that changes on E2F target promoters occur at the level of a single nucleosome, as previously described (30), and not at all nucleosomes associated with promoters. Collectively, our data suggest that the inability of E2F to sustain the activation of E2F-responsive genes is not due to a reduced occupancy of E2F-responsive promoters by E2F3. In contrast, our data suggest either that E2F is unable to form activator complexes or that it switches to repressor complexes. Further work is required to uncover the molecular mechanisms leading to such a switch and will be the focus of our future analyses.
Sustained E2F activity induces senescence-like features in vivo.
Constitutive expression of E2F1 can trigger senescence-like features in human diploid fibroblasts (10). Interestingly, it was reported recently that specific heterochromatic structures, termed senescence-associated heterochromatic foci, form in cells undergoing “natural” replicative senescence as well as premature senescence and that the appearance of these structures correlate with epigenetic silencing of E2F target genes (32). We therefore hypothesized that cells enter a premature senescent state when exposed to sustained deregulated E2F activity, which could explain their refractory response to growth stimulation and transcriptional activation. Consistent with this hypothesis, we found that both mouse and human primary cells enter premature senescence (also called stasis) when exposed to sustained E2F3 activity and accumulate senescence markers such as senescence-associated beta-galactosidase activity in MEFs, senescence-associated heterochromatic foci, and p16Ink4a accumulation in human diploid fibroblasts (see Fig. S2 in the supplemental material).
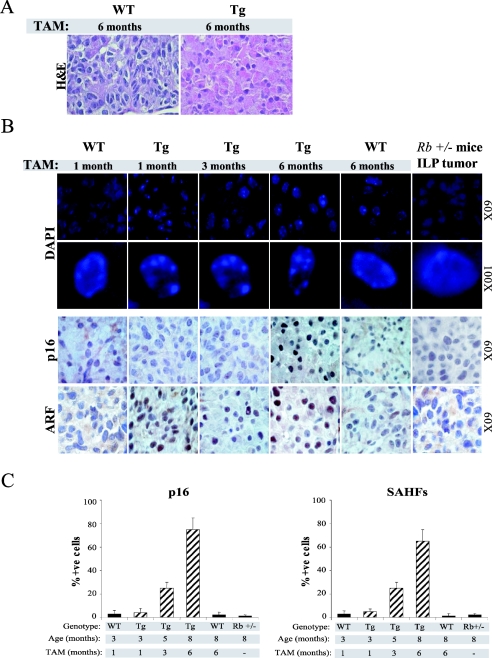
Next we tested whether sustained E2F activity resulted in senescence-like features in vivo. Interestingly, we observed a substantial change in the nucleoplasmic-cytoplasmic ratio of melanotrophs in transgenic mice treated with tamoxifen for 6 months. In wild-type animals, as well as transgenics treated for only a short period, melanotrophs are characterized by small cytoplasm and large nuclei (Fig. 6A and data not shown). In contrast, the nucleoplasmic-cytoplasmic ratio becomes smaller in long-term-treated transgenic melanotrophs (Fig. 6A). Similar morphological changes are observed in tissue culture when cells undergo senescence.
FIG. 6.
Sustained E2F activity induces pRB-dependent senescence-like features. (A) Morphological changes as a consequence of E2F activity. Hematoxylin- and eosin-stained intermediate lobe of wild-type and transgenic mice treated for 6 months with tamoxifen. Note the increased nucleoplasmic-cytoplasmic ratio in transgenic animals compared to the wild type. (B) Accumulation of senescence markers upon sustained E2F activity. Panels show staining of either pituitary gland sections derived from wild-type (WT) and transgenic (Tg) animals or pituitary tumors derived from Rb+/− mice. (Upper panel) Two magnifications of DAPI staining showing the formation of senescence-associated heterochromatic foci (SAHFs) in the intermediate lobe of transgenic animals upon sustained E2F activity. Note the formation of foci in transgenic animals treated for 6 months. (Lower panels) Immunohistochemistry analysis of p16Ink4a and p19Arf in the intermediate lobe. (C) Percentage of p16Ink4a and senescence-associated heterochromatic focus-positive melanotrophs in the intermediate lobe of wild-type and transgenic mice treated for the indicated times with tamoxifen (TAM) and in the intermediate lobe tumor of Rb+/− mice.
We next tested whether, as observed in human diploid fibroblasts, senescence-associated heterochromatic foci are formed in melanotrophs. Interestingly, we detected aberrant heterochromatic DNA structures in the melanotrophs of transgenic mice treated for 6 months which were absent in control animals (Fig. 6B and C). Moreover, heterochromatic DNA structures were also detectable in a small proportion of melanotrophs derived from transgenic mice treated for 3 months (Fig. 6B and C). The inverse correlation between the appearance of heterochromatic foci and the ability of melanotrophs to respond to E2F activity suggests that sustained E2F activity results in a senescence-like state.
To further substantiate this observation, we used two independent markers of senescence, p16Ink4a and p19Arf (26). p19Arf is highly expressed in the intermediate lobe of induced transgenic animals, consistent with its being a direct E2F target (Fig. 6B). We found that the accumulation of p16Ink4a, like senescence-associated heterochromatic foci, inversely correlates with the ability of melanotrophs to respond to E2F stimulation, strongly suggesting that prolonged E2F stimulation leads to senescence-like features in vivo. Notably, we did not detect any of these senescence markers in cells of intermediate lobe tumors derived from Rb+/− mice (Fig. 6B and C).
E2F-induced senescence is mediated by pRb, p16Ink4a, and p19Arf.
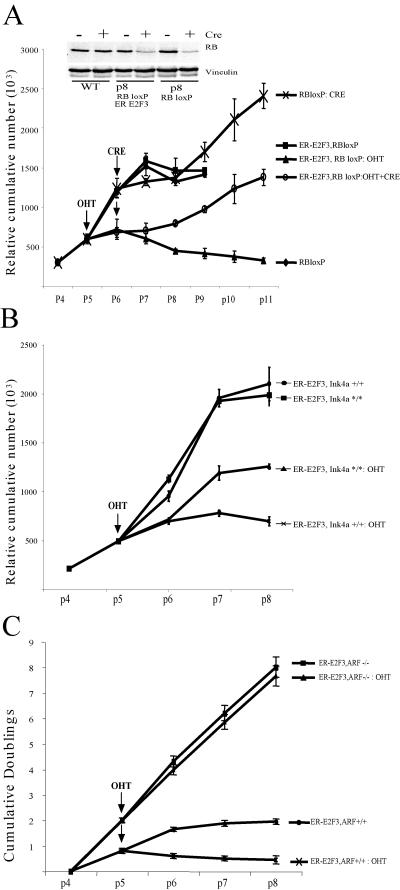
The vast majority of cancers harbor mutations in the pRB pathway, which result in deregulation of E2F activity. Since we found that deregulated E2F activity in the presence of pRb results in a senescence-like state, we reasoned that inactivation of pRb in this context would be sufficient to overcome this phenotype. Since pRb inactivation in the pituitary gland is, by itself, sufficient to induce pituitary tumors, we tested this hypothesis in MEFs derived from embryos containing floxed Rb alleles (49). As for wild-type MEFs, ER-E2F3 activation in Rb-LoxP MEFs results in significant inhibition of cell proliferation compared to the controls (not infected or ER-E2F3 infected but not induced) (Fig. 7A). In contrast, acute loss of pRb (as a consequence of Cre expression) is sufficient to prevent E2F3-induced senescence (Fig. 7A). We therefore conclude that deregulation of E2F activity results in pRB-dependent premature senescence.
FIG. 7.
E2F-induced senenescence is dependent on pRB, p16Ink4a and p19Arf. (A) E2F-induced senescence is pRB dependent. MEFs were passaged following a 3T3 protocol. Genotypes and treatments of cells were as follows: uninfected (RBloxP), ER-E2F3 infected (ER-E2F3, RB loxP), ER-E2F3 infected and induced with tamoxifen (ER-E2F3, RB loxP:OHT), Adeno-CRE infected (RB lox P: CRE), Adeno-CRE and ER-E2F3 infected and induced with tamoxifen (ER-E2F3, RB loxP: OHT and CRE). Tamoxifen administration and Adeno-CRE infection were performed at passage 5 and passage 6, respectively, as indicated by the arrows. (Inset) Protein expression levels of pRB in MEFs of the indicated genotype either infected with Adeno-CRE or not infected. The expression of vinculin was used as a loading control. (B) E2F3-induced senescence is partly dependent on p16Ink4a. MEFswere passaged following a 3T3 protocol. Genotypes and treatments of cells were as follows: wild-type, ER-E2F3 infected (ER-E2F3, Ink4+/+), wild-type ER-E2F3 infected and induced with tamoxifen (ER-E2F3, Ink4+/+:OHT), p16Ink4a null MEFs ER-E2F3 infected (ER-E2F3, Ink4*/*) p16Ink4a null MEFs ER-E2F3 infected and induced with tamoxifen (ER-E2F3, Ink4*/*: OHT). Tamoxifen administration was performed at the indicated passage (arrow). (C) E2F-induced senescence is ARF dependent. MEFs were passaged following a 3T3 protocol. Genotypes and treatments of cells as follows: wild-type, ER-E2F3 infected (ER-E2F3, ARF+/+), wild-type ER-E2F3 infected and induced with tamoxifen (ER-E2F3, ARF+/+: OHT), ARF−/− MEFs ER-E2F3 infected (ER-E2F3, ARF−/−), and ARF−/− MEFs ER-E2F3 infected and induced with tamoxifen (ER-E2F3, ARF+/+: OHT). Tamoxifen administration was performed at the indicated passage (arrow).
Additional proteins are likely to be involved in the induction of senescence upon E2F deregulation. We were particularly interested in determining the role of the products of the Ink4a locus, the tumor suppressors p19Arf and p16Ink4a, given their accumulation in melanotrophs and their role in senescence. Following ER-E2F3 activation, we found that while wild-type cells undergo a dramatic cell growth inhibition, p16Ink4a null cells continue to proliferate despite being partially growth impaired with respect to control noninduced cells (Fig. 7B; see Fig. S3 in the supplemental material). Importantly, wild-type and p16Ink4a null MEFs express similar levels of ER-E2F3 (see Fig. S3 in the supplemental material). Moreover, in agreement with the observation that p16Ink4a is a mediator of the senescence response induced by E2F3, p16Ink4a levels were increased in wild-type cells, and the number of senescence-associated β-galactosidase-positive cells decreased in p16Ink4a null MEFs after E2F3 activation (see Fig. S3 in the supplemental material). However, since p16Ink4a loss leads to only partial rescue of the senescence response, our results suggest that other genes are involved in E2F3-induced senescence. Consistent with this, activation of E2F3 in ARF null cells did not lead to premature senescence of the cells (Fig. 7C). Based on these results, we conclude that both the ARF-p53 pathway and the p16-pRB pathway regulate the induction of senescence triggered by increased E2F activity.
DISCUSSION
Previous work has shown the requirement for E2F in phenotypes associated with loss of pRb (53, 54). Here, we have shown that deregulated E2F activity mimics the earliest stages of pRb loss by inducing the proliferation of quiescent melanotrophs. Increased E2F activity, however, is not sufficient to completely mimic pRb loss, since it fails to induce tumors. Our data demonstrate that melanotrophs become refractory to further proliferation stimuli and acquire senescence-like features as a result of sustained E2F activation. Furthermore, we have shown that MEFs undergo premature senescence in response to increased E2F activity. The induction of senescence is dependent on pRB and is mediated by p19Arf and p16Ink4a (Fig. 8).
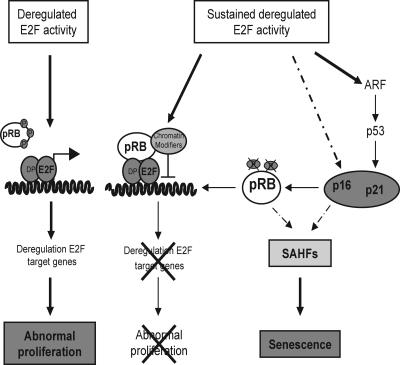
FIG. 8.
Model for the biphasic response to E2F deregulation. See text for discussion of the model.
Sustained E2F activity induces senescence-like features in vivo.
The first detectable phenotype of cells that have lost wild-type Rb is their ability to proliferate differently from those retaining a wild-type copy (33). We found that increased E2F activity, like pRb loss, is able to drive quiescent melanotrophs into S phase. Thus, deregulated E2F activity is indeed able to mimic the early consequences of Rb loss. However, the induction of proliferation due to increased E2F activity results in hyperplasia of the intermediate lobe followed by a senescence-like state, whereas pRb-deficient melanotrophs retain their ability to proliferate and ultimately give rise to highly invasive tumors. The question is why increased E2F activity does not lead to tumorigenesis.
One explanation could be that pRb, in addition to regulating E2F activity, also promotes differentiation and that increased E2F activity is unable to induce DNA replication in the differentiated gland. We excluded this possibility, showing that transient deregulation of E2F activity is sufficient to induce S phase regardless of the age of the mice and is therefore able to overcome the inhibition of a fully mature dopaminergic neural network (7, 16, 36). An alternative explanation, which is still to be tested, is that loss of pRb, unlike E2F deregulation, prevents the differentiation of certain cell types (e.g., stem cells) and that only this subpopulation of cells are capable of giving rise to tumors. A third possibility is that we only expressed E2F3a in our studies and not E2F3b, which recently was shown to repress transcription from the ARF promoter (1). This finding might suggest that increased E2F3a activity is unable to mimic the loss of pRb due to the inability of this protein to reduce ARF levels. Although we know that loss of ARF in tissue culture will prevent premature senescence, we do not believe that the lack of E2F3b mechanistically explains the lack of tumorigenesis because ectopic expression of E2F3b would, like that of the other E2Fs containing a transactivation domain (E2F1 through 2F5), lead to activation of transcription and not repression of transcription.
A fourth possibility, which we currently favor, is that increased E2F3 activity induces senescence in vivo and that pRB, in addition to regulating E2F, has an important checkpoint function. This suggestion is based on the accumulation of senescence-like features, such as failure to respond to mitogens, senescence-associated β-galactosidase activity, and accumulation of senescence-associated heterochromatic foci induced by E2F3 in primary cells. Moreover, we also found several senescence-like features in vivo, such as increased levels of the cell cycle inhibitor p16Ink4a, morphological alterations, and the formation of senescence-associated heterochromatic foci. Thus, we propose that the induction of senescence acts a tumor suppression mechanism able to restrain the growth of melanotrophs harboring deregulated E2F activity in the absence of pRB loss. Indeed, engagement of a senescence response upon chemotherapeutic treatment has previously been reported to act as a tumor suppressor mechanism able to block abnormal proliferation of mouse lymphomas (39).
E2F target genes do not respond to sustained activation of E2F.
Interestingly, both in vivo and in tissue culture, the proliferation response of cells to deregulated E2F activity correlates with induction of E2F target genes, while senescence induction correlates with a lack of E2F target genes. Our data showing that ER-E2F3 is constantly bound to E2F-responsive promoters demonstrate that it is not altered accessibility of promoters that determines the refractory state of these genes. Instead, our results suggest that E2F3 does not sustain the formation of activating complexes but most likely forms repressing complexes. Interestingly, pRB is recruited to E2F-responsive promoters upon activation of E2F3. It is somewhat surprising that recruitment of pRB is seen as early as 4 h after E2F3 activation, and it is unclear whether this represents a subset of cells in which the promoters are already repressed or whether a critical level of pRB is required to initiate repression of target genes. However, given the established role of pRB in the active repression of E2F target genes (3), these data suggest that pRB could play a crucial role in switching E2F3 activity from activator to repressor complexes through the recruitment of chromatin modifiers.
pRB and senescence: a feedback mechanism.
Sustained E2F activity leads to increased expression of p16Ink4a and to senescence-associated heterochromatic focus formation. Since p19Arf is an E2F-responsive gene, it is also induced by E2F3. Importantly, increased levels of p16Ink4a and p19Arf trigger a pRB-dependent G1 arrest, inducing hypophosphorylation of pRB (40), which are crucial events in senescence (4, 42). Senescence-associated heterochromatic focus formation occurs in primary cells undergoing “spontaneous” senescence or Ras-induced premature senescence (32). The appearance of these structures coincides with the formation of heterochromatin on E2F-responsive genes, rendering these refractory to increased E2F activity. Interestingly, the pRB pathway plays a crucial role in the formation of senescence-associated heterochromatic foci, and pRB has been shown to be recruited to E2F target promoters in senescent cells (32).
Strikingly, loss of pRB is sufficient to bypass the senescence induced by increased E2F activity, and thus pRB plays a crucial role in the mechanism of growth inhibition triggered by sustained deregulated E2F activity. Interestingly, we have also shown that the products of the Ink4a locus, p19Arf and p16Ink4a, both mediate E2F3-induced senescence, consistent with the accumulation of these proteins when E2F activity is increased. Importantly, the increased expression of p16Ink4a and p19Arf results in the accumulation of hypophosphorylated pRB, providing a negative feedback mechanism able to restrain E2F-induced proliferation.
In summary, we provide evidence for a dual role of pRB in restricting tumor formation, which is consistent with our previously published data showing a dual role of pRB in regulating the G1/S transition (24). First, pRB controls the activity of a number of cellular proteins, including the E2Fs, by directly binding to these. Second, if other genetic events should lead to aberrant activity of proteins associated with pRB (here exemplified by increased E2F activity), it exerts a checkpoint mechanism, rendering cells senescent.
Supplementary Material
Acknowledgments
We thank A. P. Bracken and J. C. Marine for critically reading the manuscript, S. Leung for technical expertise and advice, A. Berns for providing RbloxP mice and Ink4a*/* MEFs, T. Jacks for providing Rb mice, and C. J. Sherr for providing ARF mice.
This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Fondazione Italiana per la Ricerca sul Cancro (FIRC), and the Danish Ministry of Research. E.L.D. and D.P. were fellows of FIRC, and C.A. was a Marie Curie fellow of the European Union.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org.
REFERENCES
- 1.Aslanian, A., P. J. Iaquinta, R. Verona, and J. A. Lees. 2004. Repression of the Arf tumor suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev. 18:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attwooll, C., E. L. Denchi, and K. Helin. 2004. The E2F family: specific functions and overlapping interests. EMBO J. [DOI] [PMC free article] [PubMed]
- 3.Brehm, A., and T. Kouzarides. 1999. Retinoblastoma protein meets chromatin. Trends Biochem. Sci. 24:142-145. [DOI] [PubMed] [Google Scholar]
- 4.Brookes, S., J. Rowe, M. Ruas, S. Llanos, P. A. Clark, M. Lomax, M. C. James, R. Vatcheva, S. Bates, K. H. Vousden, D. Parry, N. Gruis, N. Smit, W. Bergman, and G. Peters. 2002. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. EMBO J. 21:2936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke, A., E. Maandag, M. van Roon, N. van der Lugt, M. van der Valk, M. Hooper, A. Berns, and H. te Riele. 1992. Requirement for a functional Rb-1 gene in murine development. Nature 359:328-330. [DOI] [PubMed] [Google Scholar]
- 6.Conner, E. A., E. R. Lemmer, M. Omori, P. J. Wirth, V. M. Factor, and S. S. Thorgeirsson. 2000. Dual functions of E2F-1 in a transgenic mouse model of liver carcinogenesis. Oncogene 19:5054-5062. [DOI] [PubMed] [Google Scholar]
- 7.Davis, M. D., W. Lichtensteiger, M. Schlumpf, and A. Bruinink. 1984. Early postnatal development of pituitary intermediate lobe control in the rat by dopamine neurons. Neuroendocrinology 39:1-12. [DOI] [PubMed] [Google Scholar]
- 8.DeGregori, J., G. Leone, A. Miron, L. Jakoi, and J. R. Nevins. 1997. Distinct roles for E2F proteins in cell growth control an apoptosis. Proc. Natl. Acad. Sci. USA 94:7245-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimri, G. P., E. Hara, and J. Campisi. 1994. Regulation of two E2F-related genes in presenescent and senescent human fibroblasts. J. Biol. Chem. 296:16180-16186. [PubMed] [Google Scholar]
- 10.Dimri, G. P., K. Itahana, M. Acosta, and J. Campisi. 2000. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol. Cell. Biol. 20:273-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fero, M. L., M. Rivkin, M. Tasch, P. Porter, C. E. Carow, E. Firpo, K. Polyak, L. H. Tsai, V. Broudy, R. M. Perlmutter, K. Kaushansky, and J. M. Roberts. 1996. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85:733-744. [DOI] [PubMed] [Google Scholar]
- 12.Frank, S. R., M. Schroeder, P. Fernandez, S. Taubert, and B. Amati. 2001. Binding of c-Myc to chromatin mediates mitogen-induced acetylation of histone H4 and gene activation. Genes Dev. 15:2069-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin, D. S., V. L. Godfrey, D. A. O'Brien, C. Deng, and Y. Xiong. 2000. Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol. Cell. Biol. 20:6147-6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammer, G. D., V. Fairchild-Huntress, and M. J. Low. 1990. Pituitary-specific and hormonally regulated gene expression directed by the rat proopiomelanocortin promoter in transgenic mice. Mol. Endocrinol. 4:1689-1697. [DOI] [PubMed] [Google Scholar]
- 15.Harrison, D. J., M. L. Hooper, J. F. Armstrong, and A. R. Clarke. 1995. Effects of heterozygosity for the Rb-1t19neo allele in the mouse. Oncogene 10:1615-1620. [PubMed] [Google Scholar]
- 16.Hindelang, C., J. M. Felix, F. M. Laurent, M. J. Klein, and M. E. Stoeckel. 1990. Ontogenesis of proopiomelanocortin gene expression and regulation in the rat pituitary intermediate lobe. Mol. Cell. Endocrinol. 70:225-235. [DOI] [PubMed] [Google Scholar]
- 17.Holmberg, C., K. Helin, M. Sehested, and O. Karlström. 1998. E2F-1 induced p53-independent apoptosis in transgenic mice. Oncogene 17:143-155. [DOI] [PubMed] [Google Scholar]
- 18.Humbert, P. O., R. Verona, J. M. Trimarchi, S. Dandapani, and J. A. Lees. 2000. E2f3 is critical for normal cellular proliferation. Genes Dev. 14:690-703. [PMC free article] [PubMed] [Google Scholar]
- 19.Jacks, T., A. Fazeli, E. Schmitt, R. Bronson, M. Goodell, and R. Weinberg. 1992. Effects of an Rb mutation in the mouse. Nature 359:295-300. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, D. G., J. K. Schwarz, W. D. Cress, and J. R. Nevins. 1993. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature 365:349-352. [DOI] [PubMed] [Google Scholar]
- 21.Kiyokawa, H., R. D. Kineman, K. O. Manova-Todorova, V. C. Soares, E. S. Hoffman, M. Ono, D. Khanam, A. C. Hayday, L. A. Frohman, and A. Koff. 1996. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85:721-732. [DOI] [PubMed] [Google Scholar]
- 22.Lee, E. Y.-H. P., C.-Y. Chang, N. Hu, Y.-C. J. Wang, C.-C. Lai, K. Herrup, W.-H. Lee, and A. Bradley. 1992. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359:288-294. [DOI] [PubMed] [Google Scholar]
- 23.Liu, B., G. D. Hammer, M. Rubinstein, M. Mortrud, and M. J. Low. 1992. Identification of DNA elements cooperatively activating proopiomelanocortin gene expression in the pituitary glands of transgenic mice. Mol. Cell. Biol. 12:3978-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lomazzi, M., M. C. Moroni, M. R. Jensen, E. Frittoli, and K. Helin. 2002. Suppression of the p53- or pRB-mediated G1 checkpoint is required for E2F-induced S-phase entry. Nat. Genet. 31:190-194. [DOI] [PubMed] [Google Scholar]
- 25.Low, M. J., B. Liu, G. D. Hammer, M. Rubinstein, and R. G. Allen. 1993. Post-translational processing of proopiomelanocortin (POMC) in mouse pituitary melanotroph tumors induced by a POMC-simian virus 40 large T antigen transgene. J. Biol. Chem. 268:24967-24975. [PubMed] [Google Scholar]
- 26.Lowe, S. W., and C. J. Sherr. 2003. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 13:77-83. [DOI] [PubMed] [Google Scholar]
- 27.Lukas, J., B. O. Petersen, K. Holm, J. Bartek, and K. Helin. 1996. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol. Cell. Biol. 16:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maandag, E. C. R., M. van der Valk, M. Vlaar, C. Feltkamp, J. O'Brien, M. van Roon, N. van der lugt, A. Berns, and H. te Riele. 1994. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 13:4260-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moroni, M. C., E. S. Hickman, G. Caprara, E. L. Denchi, E. Colli, F. Cecconi, H. Müller, and K. Helin. 2001. APAF-1 is a transcriptional target for E2F and p53. Nat. Cell Biol. 3:552-558. [DOI] [PubMed] [Google Scholar]
- 30.Morrison, A. J., C. Sardet, and R. E. Herrera. 2002. Retinoblastoma protein transcriptional repression through histone deacetylation of a single nucleosome. Mol. Cell. Biol. 22:856-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller, H., A. P. Bracken, R. Vernell, M. C. Moroni, F. Christians, E. Grassilli, E. Prosperini, E. Vigo, J. D. Oliner, and K. Helin. 2001. E2Fs regulate the expression of genes involved in differentiation, development, proliferation and apoptosis. Genes Dev. 15:267-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narita, M., S. Nunez, E. Heard, A. W. Lin, S. A. Hearn, D. L. Spector, G. J. Hannon, and S. W. Lowe. 2003. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113:703-716. [DOI] [PubMed] [Google Scholar]
- 33.Nikitin, A., and W. H. Lee. 1996. Early loss of the retinoblastoma gene is associated with impaired growth inhibitory innervation during melanotroph carcinogenesis in Rb+/- mice. Genes Dev. 10:1870-1879. [DOI] [PubMed] [Google Scholar]
- 34.Pierce, A. M., S. M. Fisher, C. J. Conti, and D. G. Johnson. 1998. Deregulated expression of E2F1 induces hyperplasia and cooperates with ras in skin tumor development. Oncogene 16:1267-1276. [DOI] [PubMed] [Google Scholar]
- 35.Qin, X.-Q., D. M. Livingston, W. G. Kaelin, and P. Adams. 1994. Deregulated E2F1 expression leads to S-phase entry and p53-mediated apoptosis. Proc. Natl. Acad. Sci. USA 91:10918-10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rene, F., C. Hindelang, P. Vuillez, M. Plante, M. J. Klein, J. M. Felix, and M. E. Stoeckel. 1993. Morphofunctional aspects of melanotrophic cells developing in situ and in vitro. Ann. N. Y. Acad. Sci. 680:89-110. [DOI] [PubMed] [Google Scholar]
- 37.Sage, J., A. L. Miller, P. A. Perez-Mancera, J. M. Wysocki, and T. Jacks. 2003. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424:223-228. [DOI] [PubMed] [Google Scholar]
- 38.Sage, J., G. J. Mulligan, L. D. Attardi, A. Miller, S. Chen, B. Williams, E. Theodorou, and T. Jacks. 2000. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 14:3037-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt, C. A., J. S. Fridman, M. Yang, S. Lee, E. Baranov, R. M. Hoffman, and S. W. Lowe. 2002. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109:335-346. [DOI] [PubMed] [Google Scholar]
- 40.Serrano, M., G. J. Hannon, and D. Beach. 1993. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366:704-707. [DOI] [PubMed] [Google Scholar]
- 41.Shan, B., and W.-H. Lee. 1994. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol. Cell. Biol. 14:8166-8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharpless, N. E., N. Bardeesy, K. H. Lee, D. Carrasco, D. H. Castrillon, A. J. Aguirre, E. A. Wu, J. W. Horner, and R. A. DePinho. 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413:86-91. [DOI] [PubMed] [Google Scholar]
- 43.Sherr, C. J. 1996. Cancer cell cycles. Science 274:1672-1677. [DOI] [PubMed] [Google Scholar]
- 44.Stevaux, O., and N. J. Dyson. 2002. A revised picture of the E2F transcriptional network and RB function. Curr. Opin. Cell Biol. 14:684-691. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi, Y., J. B. Rayman, and B. D. Dynlacht. 2000. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 14:804-816. [PMC free article] [PubMed] [Google Scholar]
- 46.Trimarchi, J. M., and J. A. Lees. 2002. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell. Biol. 3:11-20. [DOI] [PubMed] [Google Scholar]
- 47.Tsai, K. Y., Y. Hu, K. F. Macleod, D. Crowley, L. Yamasaki, and T. Jacks. 1998. Mutation of E2F1 suppresses apoptosis and inappropriate S-phase entry and extends survival of Rb-deficient mouse embryos. Mol. Cell 2:293-304. [DOI] [PubMed] [Google Scholar]
- 48.Vigo, E., H. Muller, E. Prosperini, G. Hateboer, P. Cartwright, M. C. Moroni, and K. Helin. 1999. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol. Cell. Biol. 19:6379-6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vooijs, M., M. van der Valk, H. te Riele, and A. Berns. 1998. Flp-mediated tissue-specific inactivation of the retinoblastoma tumor suppressor gene in the mouse. Oncogene 17:1-12. [DOI] [PubMed] [Google Scholar]
- 50.Weinberg, R. A. 1995. The retinoblastoma protein and cell cycle control. Cell 81:323-330. [DOI] [PubMed] [Google Scholar]
- 51.Williams, B. O., E. M. Schmitt, L. Remington, R. T. Bronson, D. M. Albert, R. A. Weinberg, and T. Jacks. 1994. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 13:4251-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu, L., C. Timmers, B. Maiti, H. I. Saavedra, L. Sang, G. T. Chong, F. Nuckolls, P. Giangrande, F. A. Wright, S. J. Field, M. E. Greenberg, S. Orkin, J. R. Nevins, M. L. Robinson, and G. Leone. 2001. The E2F1-3 transcription factors are essential for cellular proliferation. Nature 414:457-462. [DOI] [PubMed] [Google Scholar]
- 53.Yamasaki, L., R. Bronson, B. O. Williams, N. J. Dyson, E. Harlow, and T. Jacks. 1998. Loss of E2F-1 reduces tumorigenesis and extends the lifespan of Rb1(+/-) mice. Nat. Genet. 18:360-364. [DOI] [PubMed] [Google Scholar]
- 54.Ziebold, U., E. Y. Lee, R. T. Bronson, and J. A. Lees. 2003. E2F3 loss has opposing effects on different pRB-deficient tumors, resulting in suppression of pituitary tumors but metastasis of medullary thyroid carcinomas. Mol. Cell. Biol. 23:6542-6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziebold, U., T. Reza, A. Caron, and J. Lees. 2001. E2F3 contributes both to the inappropriate proliferation and to the apoptosis arising in Rb mutant embryos. Genes Dev. 15:386-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.