Abstract
Active lupus nephritis (LN) in pregnancy is strongly associated with poor maternal and fetal outcomes and, therefore, has implications on the planning, timing, and management. Prepregnancy evaluation is essential for all LN patients with childbearing potential to ensure pregnancies proceed in a safe and timely manner. Both maternal and fetal risks are communicated to patient during the evaluation. Stratification into different risk profile groups is then made based on disease activity and organ impairment severity. Patients with LN are generally divided into 3 main groups. Patients with LN who become pregnant receive treatments that are nonteratogenic and optimal for fetal and maternal outcomes. Throughout the pregnancy period, these patients are monitored closely under surveillance by a multidisciplinary team of clinicians. The management of patients with LN in pregnancy can be challenging both diagnostically (distinguishing LN from pre-eclampsia and determining the role and timing of kidney biopsy) and therapeutically (LN flares during pregnancy and managing a newly diagnosed LN during pregnancy).
Index Words: Lupus nephritis, pregnancy, evaluation, groups, maternal, fetal
Systemic lupus erythematosus (SLE) is a chronic, multisystem, autoimmune disorder affecting predominantly women, particularly those of childbearing age.1 Prepregnancy evaluation is vital for all patients with SLE with childbearing potential to maximize the chance of a successful pregnancy and minimize risks to both mother and baby.2 Data from 2 systematic reviews and meta-analyses have shown that active lupus nephritis (LN) is associated with poor pregnancy outcomes to both mother and baby.3,4 These outcomes include maternal LN flare, gestational hypertension, and pre-eclampsia as well as prematurity of the newborn, intrauterine growth retardation, and spontaneous miscarriages.
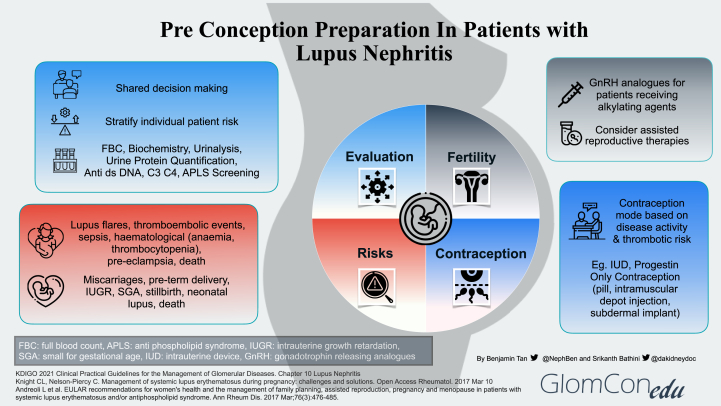
This review addresses the role of prepregnancy evaluation in patients with pre-existing LN and a suggested approach to pregnancy. Figure 1 summarizes the 4 main aspects to address during the preconception preparation of patients with LN.
Figure 1.
Preconception preparation in patients with lupus nephritis.
Prepregnancy Evaluation and Planning
Prepregnancy evaluation is imperative for all women with LN so that they can be stratified into different risk profile groups. A multidisciplinary approach is important in determining the best approach to care through shared decision making.5 Generally, women of childbearing age with LN can be divided into 3 groups, namely,6,7 inactive or quiescent LN, active LN disease, and LN with severe impairment of organ function and/or pre-existing severe organ damage.
An assessment of disease activity is made using clinical, laboratory (eg, biochemistry, urine analysis, urine protein quantification, and serologies), and histological parameters (eg, kidney biopsy).2 Commencing pregnancy with active disease is strongly correlated with adverse maternal and fetal outcomes.
Patients who have multiple spontaneous miscarriages (<10 weeks gestation) and a history of early fetal death (>10 weeks gestation), stillbirth, or premature birth <34 gestational weeks, will need to be screened for antiphospholipid syndrome (APS) antibodies which include lupus anticoagulant, anticardiolipin antibody and antibeta-2-glycoprotein 1 antibody.2,7 If a diagnosis of obstetrics APS is confirmed, anticoagulation using low molecular weight heparin will need to be commenced together with aspirin.2,7
Women with LN are strongly encouraged to undergo comprehensive prepregnancy planning to ensure disease remission as well as a bright prospect for a safe and successful pregnancy.2
Risks of Pregnancy in the Setting of Lupus Nephritis
Pregnancy may cause LN flares with progressive kidney disease. In addition, SLE itself may cause a variety of obstetric-related complications, notably hypertension, pre-eclampsia, and thromboembolic events, as well as fetal-related complications such as preterm delivery, miscarriages, intrauterine growth retardation, and congenital heart block.8
A recent systematic review and meta-analysis, looking at 16 studies comprising 1760 pregnancies, evaluated the outcomes of pregnancy in patients with LN over the last 2 decades. Gestational hypertension (odds ratio [OR], 5.65; 95% confidence interval [CI], 2.94-10.84), pre-eclampsia (OR, 2.84; 95% CI, 1.87-4.30), SLE flare (OR, 2.66; 95% CI, 1.51-4.70), LN flare (OR, 15.18; 95% CI, 5.89-39.14), and proteinuria (OR, 8.86; 95% CI 4.75-16.52) were among the major obstetrical outcomes observed in women with LN.4 In terms of fetal outcomes, pregnant patients with LN demonstrated a significant decrease in live births (OR, 0.62; 95% CI, 0.49-0.80) and a significant increase in preterm births (OR, 1.92; 95% CI, 1.49-2.49) as well as intrauterine fetal growth retardation (OR, 1.43; 95% CI, 1.08-1.91).4 These results were consistent with the findings from an earlier systematic review and meta-analysis.3
Preservation of Fertility
Given the inherent risks of infertility (eg, menstrual irregularities and premature ovarian failure) associated with cyclophosphamide, a mycophenolic acid analog (MPAA) based regimen is the preferred initial therapy for proliferative LN.9 It is also strongly recommended that all women of childbearing age with SLE be counseled about fertility issues, especially the adverse outcomes associated with increasing age and the use of alkylating agents.10 A risk evaluation and mitigating strategies program is being carried out when MPAA-based therapy is prescribed to support its safe use and promote awareness among health care professionals and patients on the teratogenic concerns of MPAA in pregnancy.11
If the patient is to receive an alkylating agent, then fertility preservation methods such as gonadotropin-releasing hormone analogs and oocyte cryopreservation should be considered.10 Assisted reproductive therapies, such as in vitro fertilization protocols and ovulation induction treatments, can also be considered in patients with stable or inactive disease.10
Contraception
To prevent unplanned pregnancies, especially during active disease and while receiving teratogenic medications, women with LN should be advised regarding contraception methods. Many options are available such as the intrauterine device (IUD), progestin-only contraception (POC) in the form of pills, intramuscular depot injections, or subdermal implants.10 The choice of contraception mode would ultimately depend on the patient’s preference taking into account the pros and cons of each option and balanced by the patient’s thrombotic risk profile.10 The risk of thrombosis is based on a history of active thrombotic episodes, current disease activity, general risk factors (eg, tobacco smoking, obesity, and family history of hormonal-dependent malignancies), as well as positive thrombophilia biomarkers, which include APS antibodies.
The copper IUD can be considered in all patients with LN without any gynecological contraindication, whereas the levonorgestrel-based IUD could be an option if the benefit of the released hormone (such as the reduction of excessive menstrual bleeding) outweighs the thrombotic risk. In patients with positive APS antibodies (with or without definite APS), a POC may be considered instead of estrogen-based contraception.9
It may be worthwhile to note that the IUD and POC are generally associated with a reduced risk of thromboembolism compared with estrogen-based contraception and, therefore, are used frequently in patients with LN.10 When considering the use of these nonestrogen–based contraceptions, one must be cognizant of the rare but potential side effects that may be experienced by the patient. The use of depot medroxyprogesterone acetate, an injectable POC, has been associated with decreased bone mineral density leading to osteoporosis as well as menstrual bleeding irregularities.12 Meanwhile, the use of an IUD is associated with a very small risk of pelvic-related infections.13 Etonogestrel-based implants are associated with a slightly higher theoretical risk of thromboembolism because of the nature of third-generation progestin.14 Barrier, pericoital, and withdrawal methods are generally avoided owing to high failure rates.
A Timing Approach to Pregnancy in Lupus Nephritis
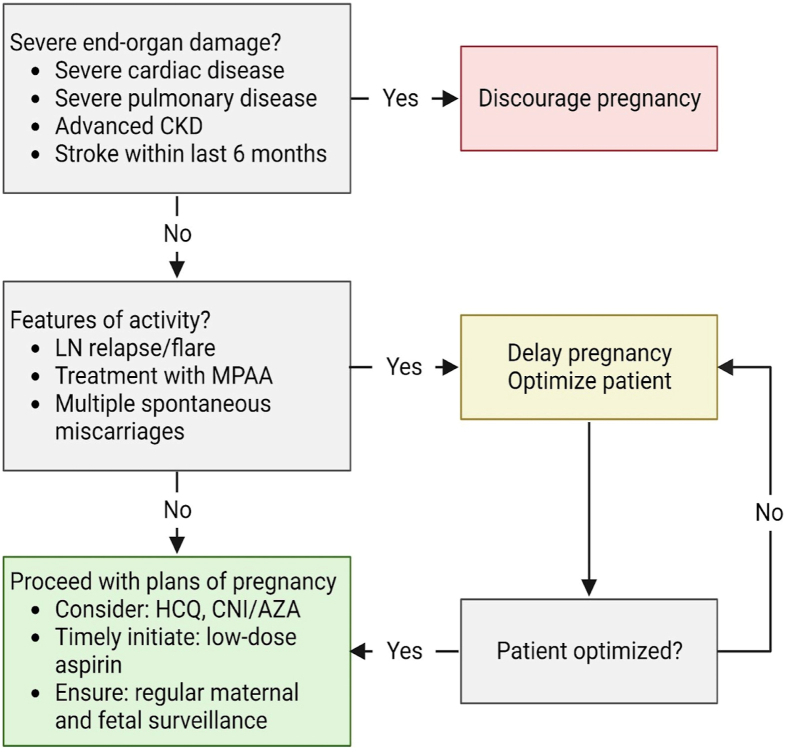
Stratification of women with LN into 3 main groups is the first step toward developing a systematic approach of management. This requires the ability to identify high-risk patients and to plan a safe and timely pregnancy tailored according to patients’ preferences and values. Figure 2 depicts a suggested approach after a comprehensive risk assessment is carried out.7
Figure 2.
An approach to pregnancy in lupus nephritis. Abbreviations: AZA, azathioprine; CKD, chronic kidney disease; CNI, calcineurin inhibitor; HCQ, hydroxychloroquine; LN, lupus nephritis; MPAA, mycophenolic acid analog.
LN with severe organ impairment
Patients with LN and concurrent severe organ impairment or damage (Group 3) should be counseled regarding the risks for disease progression that could lead to end-organ failure and pregnancy-related risks to both mother and baby. The LUMINA (Lupus in Minorities: Nature versus Nurture) study group evaluated predictors of postpartum damage accrual in a multiethnic cohort in the United States.15 The study confirmed findings that the effect on postpartum damage accrual is not because of pregnancy per se but rather a summation of contributing factors which include damage accrual before pregnancy, disease activity preconception, and duration of pregnancy.15
In light of these findings, women with LN and severe end-organ damage should be counseled regarding the high risks associated with pursuing pregnancy.7 They should be encouraged to consider alternative options such as adoption and surrogacy. Table 1 describes the various conditions in detail.7
Table 1.
Lupus Nephritis with Severe End-Organ Damage
| Conditions | Comment |
|---|---|
| Severe cardiac disease | Moderate to severe heart failure and severe valvulopathy with evidence of pulmonary hypertension |
| Severe pulmonary disease | Severe restrictive lung disease with pulmonary hypertension |
| Advanced chronic kidney disease | CKD stages 4-5 |
| Stroke | Stroke occurrence within the last 6 mo |
Abbreviations: CKD, chronic kidney disease.
Active LN
Patients with active LN disease (Group 2) are advised to wait for at least 6 months after LN is inactive before considering pregnancy to minimize adverse pregnancy outcomes to both mother and child.9,16 In a retrospective cohort study of pregnant women with lupus between 1990 and 2013, Tedeschi et al17 showed that the odds of developing LN were 80-fold higher (OR, 80; 95% CI, 8.9-723) if the pregnancy had been preceded by an episode of active nephritis in the 6 months before conception.17 A multicenter, cross-sectional study conducted in Italy showed that LN at conception, and even cases in remission, confer a higher risk of flare during pregnancy.18 Other studies show that the flare rates during pregnancy or postpartum among patients with pre-existing LN range between 30% and 60%.5
Delaying pregnancy plans for these conditions would ensure patients are well-optimized for successful pregnancy outcomes in the future. General preconception advice is also given, including a discussion of appropriate contraception methods (Refer Contraception Section).10
Stable LN in remission
Patients with inactive LN disease (Group 1) are informed that this is the safest (lowest risk) window or period to proceed with pregnancy planning.7 Before embarking on pregnancy, screening for antiphospholipid syndrome (APS) antibodies is strongly recommended for all SLE patients.10 Disease activity should be continuously monitored throughout pregnancy. On the basis of recommendations adapted from the Italian Study Group on Kidney Disease and Pregnancy, regular blood tests, including full blood count, kidney function, and electrolytes, should be taken every 4-6 weeks while urinalysis and urine protein quantification should be monitored every 2-4 weeks based on the level of proteinuria.6,19
It is imperative to discontinue teratogenic medication such as MPAA and transition to nonteratogenic therapy such as calcineurin inhibitor or azathioprine.9 A “wash-out” period of at least 3 months is advised for such patients.9 During this period, patients are monitored for disease activity, tolerance to nonteratogenic therapy and advised regarding contraception methods (refer “Contraception” Section). Patients can then proceed with pregnancy after the “wash-out” period, provided the disease remains clinically inactive.
If antihypertensive therapy is required, pregnancy-safe medications such as labetalol, nifedipine, and methyldopa should be considered.7 The decision to continue renin-angiotensin-aldosterone system (RAAS) blockade agents will depend on its perceived risk-benefit ratio as well as the patient’s kidney function, blood pressure (BP), and proteinuria levels before conception. If BP and proteinuria levels are not optimal, RAAS blockade therapy may be continued first and only stopped in the first trimester on conception.6 This avoids an unnecessarily prolonged cessation of these agents while waiting for conception to take place, which may take months to years. This decision is made, balancing the advantage of long-term renoprotection against the adverse fetal outcomes associated with the use of RAAS blockade medications in the second and third trimester.6 The approach requires careful patient education on the importance of confirming the pregnancy early and immediately stopping the drug.
The use of hydroxychloroquine (HCQ) is generally safe throughout pregnancy, although a recent study showed a small increase in the risk of congenital malformations among HCQ-exposed pregnancies.9,20 The significant benefit of HCQ in reducing the risk of flares during and after pregnancy needs to be weighed against the small increased risk of congenital malformations. An increased risk of flares has also been associated with the discontinuation or tapering of HCQ.21 Therefore, in light of this evidence, the Kidney Disease Improving Global Outcomes (KDIGO) recommends the use of HCQ or an equivalent antimalarial in patients with LN unless contraindicated. 9
The initiation of low-dose aspirin (<100mg) and calcium supplements is recommended before 16 weeks of gestation as prophylactic therapy against pre-eclampsia.9,22
Throughout the antepartum period, patients should undergo regular maternal and fetal surveillance by a multidisciplinary team consisting of a nephrologist, obstetrician, and rheumatologist.2,5 Besides the routine ultrasonographic surveillance during the first (11-14 weeks of gestation) and second trimester (20-24 weeks of gestation), pregnant patients with stable LN are recommended to have supplementary fetal surveillance in the third trimester (at monthly intervals from 28-34 weeks and then weekly intervals from 34 weeks gestation).2,10
Differentiating LN from Pre-Eclampsia
Distinguishing LN flare from pre-eclampsia, especially in the second and third trimesters, remains a challenge even for an experienced clinician, because both these conditions can have similar clinical presentations.23 These conditions can present with increased BP, raised serum creatinine, proteinuria, and edema. Even though they represent 2 separate pathological entities, these conditions are not mutually exclusive and may even be present concurrently in a patient.16,23 Differentiating both these conditions has therapeutic implications because the cornerstone management of pre-eclampsia is urgent delivery of the baby whereas LN flare is managed with immunosuppression.2
Table 2 summarizes the proposed features used to differentiate LN flare from pre-eclampsia.23,24 In general, the occurrence of LN can take place anytime throughout the pregnancy with supporting extrarenal features of lupus, whereas pre-eclampsia usually sets in midway of pregnancy from about 20 weeks of gestation. In terms of laboratory features, LN patients usually present with active urinary sediment, lupus autoantibody positivity, reduced complement levels, and normal serum uric acid levels. Pre-eclampsia is associated with high serum uric acid levels and low urinary calcium excretion.23 A recent systematic review and meta-analysis by Sanchez-Ramos et al involving almost 1200 females consistently underscored the association between reduced urinary calcium excretion and pre-eclampsia.25 Although the review did not elucidate the pathophysiologic process behind the association, hypocalciuria is thought to be because of increased renal tubular reabsorption of calcium during pre-eclampsia.26
Table 2.
| Features | Lupus Nephritis | Pre-eclampsia |
|---|---|---|
| Onset | Anytime during pregnancy | After 20 wk of pregnancy |
| Hypertension | Maybe present or absent | Present |
| Extrarenal SLE signs and symptoms | Present | Absent |
| Serum uric acid levels | <4.9mg/dL (low or normal) | >4.9mg/dL (high) |
| Complement levels | Maybe decreased or normal | Normal |
| Anti ds-DNA levels | Increased | Normal |
| Active urinary sediments | Present | Absent |
| Urinary calcium excretion | >195 mg/day | <195 mg/day |
| sFlt-1/PlGF ratio24 | Normal | Increased |
Abbreviations: SLE, systemic lupus erythematosus; DNA, deoxyribonucleic acid; sFlt-1, soluble fms-like tyrosine kinase 1; PlGF, placental growth factor.
Role and Timing of Kidney Biopsy During Pregnancy in Patients with LN
A kidney biopsy may be warranted in the pregnant patient only if the result is likely to influence the direction of care and management of the patient. Biopsy is usually considered in pregnancy when progressive kidney function impairment and/or severe proteinuria could interfere with pregnancy outcomes and when establishing a diagnosis is needed to determine treatment.6 The procedure should be considered possibly before 25 weeks of gestation age to reduce the risk of bleeding complications after biopsy, as alluded by Pontichelli et al27 in a recent review on kidney biopsy in pregnant women with LN.27 A systematic narrative review of nearly 200 kidney biopsies performed during pregnancy showed that the procedure is associated with a relatively small (2%) but increased risk of bleeding complication particularly around 25 weeks of gestation.28
LN flares during Pregnancy
Treatment of LN flares should not be delayed if it occurs during pregnancy.7 The direction of management should be guided based on several considerations, including the severity and extent of organ impairment, the duration of pregnancy during LN flare, and the benefit-risk ratio of continuing pregnancy while ensuring the mother’s wellbeing.2,16 The decision whether to continue pregnancy or not should be individualized in alignment with the patient’s values and preferences after an in-depth discussion between the doctor and patient.
In a young woman with severe LN flare during the early stages of pregnancy, the option of therapeutic abortion should be discussed. The main goal would be to bring the disease activity under control by early administration of immunosuppressive agents while avoiding the teratogenic side effects of the therapy.6 The control of disease activity at an early stage greatly increases the chance of a successful pregnancy outcome in the future.
If LN flare occurred at an advanced stage of pregnancy in a patient with prior difficulties to conceive, then treatment of the flare itself may be an option. Intravenous pulsed corticosteroids would be the first step followed by a reduced dose of oral prednisolone, azathioprine, and calcineurin inhibitor.2,6 The goal of treatment is the attempt to contain LN to allow pregnancy to proceed for as long as possible until the fetus achieves sufficient maturity for delivery. A close follow-up in the first 6 months postpartum is needed because of the high risk of LN flare during this period. 6
Diagnosis of LN During Pregnancy
LN flare may possibly be the first presentation of lupus during pregnancy. The diagnosis of LN during pregnancy is, in itself, rare and certainly poses a challenge, as symptoms of SLE tend to overlap with those of pregnancy.2,5 LN may be diagnosed during pregnancy based on a constellation of features, including clinical status, positivity of autoantibodies, hypocomplementemia, and in some cases, with a kidney biopsy, especially if the presentation is atypical.6
Managing a pregnant patient with newly diagnosed LN incorporates similar principles of treatment when managing patients with LN flares during pregnancy. (Refer to “LN Flares During Pregnancy” Section). Undergirding the management is the importance of shared decision making between both the patient and doctor. Decisions are made after weighing the risk-benefit ratio of continuing pregnancy as well as the efficacy and safety profile of treatment to both mother and baby.
Conclusion
A multidisciplinary model of care remains the cornerstone approach in ensuring accurate disease assessment and prognostication, identifying those at high risk, and mapping out a comprehensive and holistic prepregnancy plan for an optimal outcome of a safe and timely pregnancy. This includes shared decision making with patients, an effective 2-way risk communication process, fertility preservation measures, contraception where indicated, a systematic risk-stratification process, step-wise pharmacologic management, and regular maternal and fetal surveillance.
Article Information
Authors’ Full Names and Academic Degrees
Benjamin Tan, MB BCh BaO, MRCP, Paolo Nikolai So, MD, FPCP, Anoushka Krishnan, FRACP, Sol Carriazo, MD, Josélyn Reyes Bahamonde, MD, Tanuj Moses Lamech, DM (Nephrology), Mohamed Hassanein, MD, Edgar Lerma, MD, and Nasim Wiegley, MD on behalf of the GlomCon Editorial Team
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received April 10, 2023. Evaluated by 1 external peer reviewer, with direct editorial input from the Editor-in-Chief. Accepted in revised form July 16, 2023.
Footnotes
Complete author and article information provided before references.
References
- 1.Kaul A., Gordon C., Crow M.K., et al. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 2.Lightstone L., Hladunewich M.A. Lupus nephritis and pregnancy: concerns and management. Semin Nephrol. 2017;37(4):347–353. doi: 10.1016/j.semnephrol.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Smyth A., Oliveira G.H., Lahr B.D., Bailey K.R., Norby S.M., Garovic V.D. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010;5(11):2060–2068. doi: 10.2215/CJN.00240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J., Ma J., Zhang W.H., Di W. Management and outcomes of pregnancy with or without lupus nephritis: a systematic review and meta-analysis. Ther Clin Risk Manag. 2018;14:885–901. doi: 10.2147/TCRM.S160760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Germain S., Nelson-Piercy C. Lupus nephritis and renal disease in pregnancy. Lupus. 2006;15(3):148–155. doi: 10.1191/0961203306lu2281rr. [DOI] [PubMed] [Google Scholar]
- 6.Fakhouri F., Schwotzer N., Cabiddu G., et al. Glomerular diseases in pregnancy: pragmatic recommendations for clinical management. Kidney Int. 2023;103(2):264–281. doi: 10.1016/j.kint.2022.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Knight C.L., Nelson-Piercy C. Management of systemic lupus erythematosus during pregnancy: challenges and solutions. Open Access Rheumatol. 2017;9:37–53. doi: 10.2147/OARRR.S87828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman F.Z., Rahman J., Al-Suleiman S.A., Rahman M.S. Pregnancy outcome in lupus nephropathy. Arch Gynecol Obstet. 2005;271(3):222–226. doi: 10.1007/s00404-003-0574-x. [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Andreoli L., Bertsias G.K., Agmon-Levin N., et al. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. 2017;76(3):476–485. doi: 10.1136/annrheumdis-2016-209770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M., Rostas S., Gabardi S. Mycophenolate fetal toxicity and risk evaluation and mitigation strategies. Am J Transplant. 2013;13(6):1383–1389. doi: 10.1111/ajt.12238. [DOI] [PubMed] [Google Scholar]
- 12.Cromer B.A., Bonny A.E., Stager M., et al. Bone mineral density in adolescent females using injectable or oral contraceptives: a 24-month prospective study. Fertil Steril. 2008;90(6):2060–2067. doi: 10.1016/j.fertnstert.2007.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beerthuizen R.J. Pelvic inflammatory disease in intrauterine device users. Eur J Contracept Reprod Health Care. 1996 Sep;1(3):237–243. doi: 10.3109/13625189609150665. [DOI] [PubMed] [Google Scholar]
- 14.Keenan L., Kerr T., Duane M., Van Gundy K. Systematic review of hormonal contraception and risk of venous thrombosis. Linacre Q. 2018;85(4):470–477. doi: 10.1177/0024363918816683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade R.M., McGwin G., Jr., Alarcón G.S., et al. LUMINA study group. Predictors of post-partum damage accrual in systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (XXXVIII) Rheumatology (Oxford) 2006;45(11):1380–1384. doi: 10.1093/rheumatology/kel222. [DOI] [PubMed] [Google Scholar]
- 16.Dao K.H., Bermas B.L. Systemic lupus erythematosus management in pregnancy. Int J Womens Health. 2022;14:199–211. doi: 10.2147/IJWH.S282604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tedeschi S.K., Massarotti E., Guan H., Fine A., Bermas B.L., Costenbader K.H. Specific systemic lupus erythematosus disease manifestations in the six months prior to conception are associated with similar disease manifestations during pregnancy. Lupus. 2015;24(12):1283–1292. doi: 10.1177/0961203315586455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imbasciati E., Tincani A., Gregorini G., et al. Pregnancy in women with pre-existing lupus nephritis: predictors of fetal and maternal outcome. Nephrol Dial Transplant. 2009;24(2):519–525. doi: 10.1093/ndt/gfn348. [DOI] [PubMed] [Google Scholar]
- 19.Cabiddu G., Castellino S., Gernone G., et al. A best practice position statement on pregnancy in chronic kidney disease: the Italian Study Group on Kidney and Pregnancy. J Nephrol. 2016;29(3):277–303. doi: 10.1007/s40620-016-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clowse M.E.B., Eudy A.M., Balevic S., et al. Hydroxychloroquine in the pregnancies of women with lupus: a meta-analysis of individual participant data. Lupus Sci Med. 2022;9(1) doi: 10.1136/lupus-2021-000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almeida-Brasil C.C., Hanly J.G., Urowitz M., et al. Flares after hydroxychloroquine reduction or discontinuation: results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann Rheum Dis. 2022;81(3):370–378. doi: 10.1136/annrheumdis-2021-221295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmeyr G.J., Lawrie T.A., Atallah Á.N., Torloni M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2018;10(10):CD001059. doi: 10.1002/14651858.CD001059.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto Y., Aoki S. Systemic lupus erythematosus: strategies to improve pregnancy outcomes. Int J Womens Health. 2016;8:265–272. doi: 10.2147/IJWH.S90157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirashima C., Ogoyama M., Abe M., et al. Clinical usefulness of serum levels of soluble fms-like tyrosine kinase 1/placental growth factor ratio to rule out preeclampsia in women with new-onset lupus nephritis during pregnancy. CEN Case Rep. 2019;8(2):95–100. doi: 10.1007/s13730-018-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMaster K.M., Kaunitz A.M., Burbano de Lara P., Sanchez-Ramos L. A systematic review and meta-analysis of hypocalciuria in pre-eclampsia. Int J Gynaecol Obstet. 2017;138(1):3–11. doi: 10.1002/ijgo.12165. [DOI] [PubMed] [Google Scholar]
- 26.Taufield P.A., Ales K.L., Resnick L.M., Druzin M.L., Gertner J.M., Laragh J.H. Hypocalciuria in preeclampsia. N Engl J Med. 1987;316(12):715–718. doi: 10.1056/NEJM198703193161204. [DOI] [PubMed] [Google Scholar]
- 27.Moroni G., Calatroni M., Donato B., Ponticelli C. Kidney biopsy in pregnant women with glomerular diseases: focus on lupus nephritis. J Clin Med. 2023;12(5):1834. doi: 10.3390/jcm12051834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piccoli G.B., Daidola G., Attini R., et al. Kidney biopsy in pregnancy: evidence for counseling? a systematic narrative review. BJOG. 2013;120(4):412–427. doi: 10.1111/1471-0528.12111. [DOI] [PubMed] [Google Scholar]