Abstract
An extract from the marine alga Ulva lactuca was highly osmoprotective in salt-stressed cultures of Sinorhizobium meliloti 102F34. This beneficial activity was due to algal 3-dimethylsulfoniopropionate (DMSP), which was accumulated as a dominant compatible solute and strongly reduced the accumulation of endogenous osmolytes in stressed cells. Synthetic DMSP also acted as a powerful osmoprotectant and was accumulated as a nonmetabolizable cytosolic osmolyte (up to a concentration of 1,400 nmol/mg of protein) throughout the growth cycles of the stressed cultures. In contrast, 2-dimethylsulfonioacetate (DMSA), the sulfonium analog of the universal osmoprotectant glycine betaine (GB), was highly toxic to unstressed cells and was not osmoprotective in stressed cells of wild-type strains of S. meliloti. Nonetheless, the transport and accumulation of DMSA, like the transport and accumulation of DMSP and GB, were osmoregulated and increased fourfold in stressed cells of strain 102F34. Strikingly, DMSA was not toxic and became highly osmoprotective in mutants that are impaired in their ability to demethylate GB and DMSA. Furthermore, 2-methylthioacetate and thioglycolic acid (TGA), the demethylation products of DMSA, were excreted, apparently as a mechanism of cellular detoxification. Also, exogenous TGA and DMSA displayed similar inhibitory effects in strain 102F34. Thus, on the basis of these findings and other physiological and biochemical evidence, we infer that the toxicity of DMSA in wild-type strains of S. meliloti stems from its catabolism via the GB demethylation pathway. This is the first report describing the toxicity of DMSA in any organism and a metabolically stable osmoprotectant (DMSP) in S. meliloti.
In contrast to many bacterial species which use betaines (quaternary ammonium compounds) only as nonmetabolizable osmoprotectants (7, 11, 16, 38), Sinorhizobium meliloti (formerly Rhizobium meliloti) uses betaines that are produced by its host (Medicago sativa L.) either as growth substrates or as accumulated solutes which restore turgor in osmotically stressed cells (3, 17, 42, 45). The utilization of betaines in nutrition or in osmoregulation in S. meliloti 102F34 depends on the osmolarity of the growth medium. At low osmolarity, glycine betaine (N,N,N-trimethylglycine) (GB) and stachydrine (N,N-dimethylproline) are actively catabolized and strongly stimulate their own catabolism, which favors utilization of these compounds as carbon and nitrogen sources (17, 42). At high osmolarity, GB and stachydrine are accumulated as cytosolic osmolytes which strongly enhance the growth rate and cell yield of the free-living bacterium (osmoprotection) and partially restore nitrogen fixation activity in salt-stressed alfalfa seedlings nodulated by S. meliloti (3, 36). For these reasons, it has been proposed that the tolerance to salinity and the productivity of beneficial bacteria and important crops could be increased through genetic engineering by transferring the ability to accumulate GB to nonaccumulating species of agronomic and/or industrial interest (8, 16, 23, 27, 40).
The following mechanisms are involved in the accumulation of GB in S. meliloti grown at high osmolarities: (i) stimulated uptake from the growth medium; (ii) stimulated synthesis from imported choline; and (iii) reduced catabolism in stressed cells (3, 42). However, because GB catabolism continues at elevated osmolalities, intracellular GB levels fall as the supplied osmoprotectant is depleted from the growth medium. Consequently, an accumulation of endogenously synthesized osmolytes is required to supplant the catabolized betaine (45) and maintain a net positive turgor (cytoplasm/medium), which is the driving force for cell growth. Obviously, the synthesis of endogenous osmolytes requires induction of specific genes and is energetically more costly and less efficient than the rapid activation of a constitutive betaine uptake activity, which can be stimulated severalfold in response to sudden osmotic upshifts (3, 35, 43, 46). Thus, futile catabolism of GB in stressed cells of S. meliloti may very well reduce the ability of these cells to grow and survive during long periods of osmotic stress. Furthermore, it is noteworthy that S. meliloti is the only bacterial species to catabolize all common osmolytes (GB, stachydrine, ectoine, etc.) that it uses as osmoprotectants (3, 17, 45, 46).
The following two strategies can be used to obtain durable accumulation of highly beneficial osmoprotectants in salt-stressed cells of S. meliloti and possibly enhance their growth and survival in saline environments: (i) the genes encoding the catabolism of metabolizable osmolytes can be inactivated, and (ii) new osmoprotectants that are accumulated as nonmetabolizable osmolytes can be identified. The first strategy might not be the most judicious strategy, because betaines produced by alfalfa apparently play a role in symbiosis. For example, the stc genes, which encode the catabolism of stachydrine in S. meliloti, are required for proper nodulation of alfalfa (18). Also, the trc genes, which govern the catabolism of trigonelline (1-methylpyridinium-3-carboxylate or picolinic acid betaine), and the betBA genes, which encode the oxidation of choline to GB (i.e., the first two steps in the catabolism of choline), are expressed at all stages of the symbiosis between S. meliloti and alfalfa (9, 29). Thus, we focused on the second strategy and turned to marine algae as potential sources of nonmetabolizable osmolytes. These organisms contain high levels of various tertiary sulfonium compounds (TSCs) and quaternary ammonium compounds which act (or may act) as bacterial osmoprotectants (5, 10, 30, 39, 40).
Recently (34), we reported that an extract from the green alga Ulva lactuca stimulated the growth of S. meliloti wild-type strain 102F34 at high osmolarity. The extract contained two TSCs, 3-dimethylsulfoniopropionate (3-dimethylpropiothetin) (DMSP) and 2-dimethylsulfonioacetate (2-dimethylthetin) (DMSA) (Fig. 1), which displayed contrasting biological activities. Here, we describe the specific effects of the algal extract and several S-methylated analogs of DMSP and DMSA on the growth and physiology of salt-stressed and unstressed cultures of S. meliloti. Our data provide persuasive evidence that DMSP acts as a nonmetabolizable osmoprotectant in S. meliloti, but DMSA can be highly toxic. Also, this study provides new insight into the possible roles of DMSA, the sulfonium analog of the universal osmoprotectant GB (11, 16, 40), in functions other than osmoregulation.
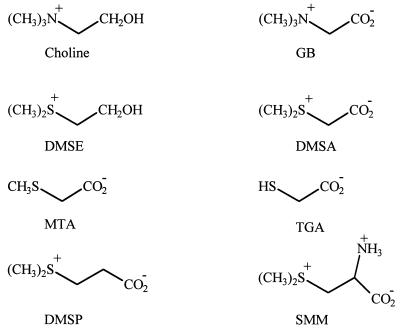
FIG. 1.
Chemical structures of the methylated onium compounds and the related sulfur analogs used in this study. SMM, S-methyl-l-methionine.
MATERIALS AND METHODS
Media and strains.
The carbon- and nitrogen-free mineral base of the media used in this study was S medium (42). Inocula were grown overnight in MSY medium, concentrated to an optical density at 570 nm (OD570) of 10 in S medium, and used at a 100-fold dilution. Unless otherwise indicated, cultures were grown aerobically at 30°C in lactate-aspartate-salts (LAS) minimal medium (45). Bacterial growth (cell density) was monitored spectrophotometrically at 570 nm. All growth experiments were performed in triplicate, and the errors were less than 10%. Protein contents were determined as described by Lowry et al. (24) by using bovine serum albumin as the standard.
S. meliloti 102F34 and RCR2011 (wild-type strains), as well as S. meliloti LTS23-1020, GMI766, and VP01, were used in this study. Strain LTS23-1020 (102F34 rif betA::Tn5) does not oxidize choline to GB because it is deficient in choline dehydrogenase activity (37). GMI766 [RCR2011 Δ(nod-nifA)766, Gbc−] does not catabolize GB because as-yet-unidentified gbc (GB catabolism) genes have been deleted in this strain (17). S. meliloti VP01 is a derivative of S. meliloti 102F34 which was isolated by an enrichment procedure after inoculation of strain 102F34 into LAS medium containing 1 mM phosphoniobetaine (the trimethylated phosphonium analog of GB), which is a toxic betaine that prevents the growth of wild-type strains of S. meliloti in LAS medium (32).
Seaweed extract.
The green intertidal alga U. lactuca L. was harvested at Paimpol (anse du Guilben) on the northern coast of Brittany, France, washed in seawater, and transported to the lab within 2 h. An aqueous algal extract was prepared by autoclaving (30 min, 120°C) 4 kg of fresh algae in distilled water. After centrifugation (10,000 × g, 15 min), the supernatant was evaporated to dryness and extracted in methanol (three times, 20 min each) with gyratory shaking. Then the methanolic supernatant (10,000 × g, 15 min) was evaporated, and the aqueous Ulva extract was reconstituted so that the soluble compounds extracted from 1.5 g of dry algal material were concentrated in 1 ml of distilled water. This extract was sterilized by filtration and used in bacterial osmoprotection bioassays at a 1,000-fold dilution. High-voltage paper electrophoresis and chromatographic analysis (3, 45) showed that the concentrated Ulva extract contained a major sulfonium compound, DMSP (concentration, about 0.5 M), and a lower concentration of DMSA (about 5 mM).
Unlabeled chemicals.
The chemical structures of the methylated onium compounds used in this study are shown in Fig. 1. 2-Dimethylsulfonioethanol (dimethylthioethanol) (DMSE) was synthesized as described by Ferger and du Vigneaud (14) by using iodomethane and 2-methylthioethanol. DMSA was synthesized in its chloride form from dimethylsulfide and 2-chloroacetic acid and was purified as described by Maw (26). DMSP was synthesized by condensation of dimethylsulfide and acrylate, as described by Le Berre and Delacroix (22). S-Methyl-l-methionine was a gift from F. Larher, University of Rennes 1, Rennes, France. All other chemicals were of the best commercial grade.
The chemical identities of the synthetic TSCs were determined by (i) paper chromatography and high-voltage electrophoresis, followed by spraying with Dragendorff’s reagent (3); (ii) infrared spectroscopy performed with a model 16PC FT-IR spectrometer (Perkin-Elmer, Norwalk, Conn.); and (iii) 1H and 13C nuclear magnetic resonance (NMR) spectroscopy. 1H and 13C NMR spectra were recorded in D2O at 300 and 75.4 MHz, respectively, with a Brüker model AC300P spectrometer. The spectral characteristics of the synthetic TSCs were compared to previously published values (5, 31).
Synthesis of radiochemicals.
[methyl-14C]DMSA (2 GBq mmol−1) and [1-14C]DMSA (2 GBq mmol−1) were synthesized at Isotopchim Laboratories (Ganagobie-Peyruis, France) and were purified in the laboratory. [methyl-14C]DMSA iodide was synthesized from [methyl-14C]iodomethane (2 GBq mmol−1) and methylthioacetic acid as described by Ferger and du Vigneaud (14). [1-14C]DMSA bromide was synthesized as described by Maw (26) by using [1-14C]bromoacetic acid (2 GBq mmol−1) and dimethylsulfide. [1-14C]DMSP (37 MBq mmol−1) was synthesized by using [1-14C]acrylic acid (37 MBq mmol−1) and dimethylsulfide according to the procedure of Le Berre and Delacroix (22), with the following minor modifications: the temperature of the reaction medium was kept at 16°C, and the chloride salt of DMSP was obtained by adding fuming HCl instead of dry gaseous HCl. The radiolabeled sulfoniums were purified by high-voltage paper electrophoresis (3) and were eluted in their chloride forms with 0.1 N HCl.
[methyl-14C]- and [1,2-14C]GB (2.07 and 0.27 GBq mmol−1, respectively) were prepared enzymatically from the corresponding [14C]cholines (NEN Research Products, Les Ulis, France) as described previously (3).
13C NMR spectral determination of intracellular osmolytes.
Cultures used in 13C NMR experiments were grown in LAS medium with or without 0.5 M NaCl, 1 mM DMSP, or the crude Ulva extract, which was used at a 1,000-fold dilution. Cytosolic osmolytes were extracted with 80% ethanol and samples for NMR analysis were prepared as described previously (45, 46). Natural-abundance 13C NMR spectra were recorded at 75.4 MHz in the Fourier transform mode by using a Brüker model AC300P spectrometer and 1,024 acquisitions. The osmolytes in the extracts were identified by comparing their chemical shifts with the chemical shifts of the synthetic compounds at pH 7.
Transport, accumulation, and catabolism of radiolabeled onium compounds.
The rates of uptake of 14C-labeled onium compounds were determined by using a filtration procedure (3, 46) and a saturating concentration (50 μM) of [1-14C]DMSP, [methyl-14C]DMSA, or [methyl-14C]GB. Initial rates of uptake were determined by performing a series of four filtrations over a 2- to 5-min period. Transport assays were repeated three times, and the errors were less than 6%.
The accumulation and catabolism of DMSP, DMSA, and GB were investigated as follows. Strains of S. meliloti were inoculated into low- or high-osmolarity LAS medium containing the desired 14C-labeled onium compound (approximately 3.7 kBq/ml), which was used either at a final concentration of 1 mM or without isotopic dilution, as specified below. The cultures were grown aerobically in sealed Erlenmeyer flasks in order to trap evolved 14CO2. Aliquots (1 to 2 ml) of these cultures were harvested periodically and extracted in ethanol as described by Talibart et al. (45). Radiolabeled cytosolic solutes were identified and quantified following electrophoretic and/or chromatographic analysis of the ethanol-soluble fractions (ESFs) and electronic autoradiography with a Packard InstantImager. The radioactivities of 14CO2 and aliquots of the ethanol-insoluble fractions (EIFs) were quantified by liquid scintillation counting (3, 45).
Cellular volumes were determined by measuring the differential distribution of 3H2O and [1-14C]inulin in the intra- and extracellular compartments, as described by Stock et al. (44).
The dimethylsulfonium [(CH3)2S+] moiety of DMSP is a good leaving group and may be lost spontaneously or under alkaline conditions (20, 30). Therefore, control experiments without cells were performed along with all metabolism studies in which radiolabeled DMSP or DMSA was supplied to S. meliloti. DMSA was chemically stable under our experimental conditions, but DMSP underwent limited spontaneous degradation to dimethylsulfide and acrylate (about 2 to 5% in 10 to 15 h). The data presented below were corrected to account for this factor. All experiments were replicated at least twice, and the errors were less than 12%.
RESULTS
An algal extract and synthetic DMSP display similar osmoprotective activities in S. meliloti 102F34 cultures.
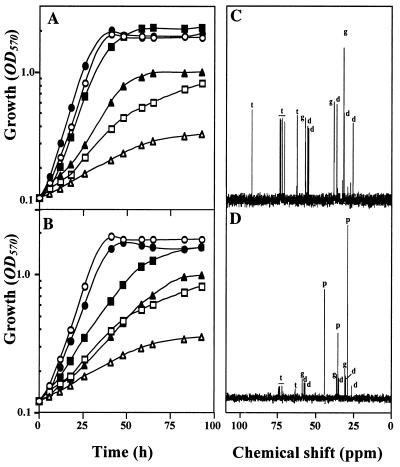
Bacterial osmoprotection bioassays were carried out at two salinity levels (0.5 and 0.65 M NaCl) by using each of the putative osmoprotectants at a concentration of 1 mM. GB, the most powerful osmoprotectant known to date for S. meliloti (3, 46), was used as a positive control. The crude aqueous extract from U. lactuca had no effect on the growth rate and final cell yield (as determined by OD570) of an unstressed culture of S. meliloti 102F34. However, it was highly osmoprotective in a culture grown under severe salt stress. After a short lag phase, the algal extract restored the growth of the cells cultivated in LAS medium containing 0.5 M NaCl to the level observed in the unstressed culture (Fig. 2A). Interestingly, a purified algal fraction that comigrated electrophoretically with synthetic DMSP could mimic almost quantitatively the beneficial effect of the algal extract (data not shown). Also, a high level of osmoprotection was obtained with 1 mM synthetic DMSP (Fig. 2B), but this compound was slightly less effective than the crude algal extract or 1 mM GB. Indeed, DMSP stimulated about twofold the growth rate of a stressed culture grown with 0.5 M NaCl and almost restored the final cell yield to the unstressed level (Table 1). DMSP was also less osmoprotective for cells cultured in the presence of 0.65 M NaCl, the highest salinity at which S. meliloti 102F34 can grow in the absence of exogenous osmoprotectants. In this case, DMSP brought the final cell yield of the culture to 50% of the unstressed level and stimulated the growth rate about 1.7-fold. Meanwhile, GB stimulated the growth rate 3.5-fold and almost fully restored the total cell yield of the culture grown in the presence of 0.65 M NaCl (Table 1). No further improvements in the growth rates and the cell yields of the stressed cultures were observed when the concentration of DMSP was increased to more than 1 mM (data not shown).
FIG. 2.
(A and B) Osmoprotective activity of an aqueous extract from U. lactuca (A) and synthetic DMSP (B) in S. meliloti 102F34 cultures. Growth was measured by determining OD570. Cultures were grown in LAS medium (circles), in LAS medium containing 0.5 M NaCl (squares), or in LAS medium containing 0.65 M NaCl (triangles). Open symbols, no algal extract or DMSP added; solid symbols, cultures supplemented with algal extract (A) or 1 mM DMSP (B). (C and D) Representative 13C NMR spectra from salt-stressed cultures of S. meliloti 102F34 grown to the early stationary phase in LAS medium containing 0.5 M NaCl (C) or in LAS medium containing 0.5 M NaCl and the U. lactuca extract or 1 mM DMSP (D). The resonances from the dipeptide N-acetylglutaminylglutamine amide (peaks d), l-glutamate (peaks g), trehalose (peaks t), and DMSP (peaks p) are indicated when these compatible solutes were detected in the extract(s). The spectra were obtained by using 1,500 OD570 units of cells and 1,024 acquisitions and are shown at the same scale so that direct visual comparisons can be made.
TABLE 1.
Comparative effects of tertiary sulfonium compounds and GB on the growth of S. meliloti 102F34
| NaCl concn in LAS medium (M) | Growth parameters in LAS medium containinga:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No addition
|
DMSP (1 mM)
|
GB (1 mM)
|
DMSA (1 mM)
|
DMSE (1 mM)
|
DMSE (1 mM)b
|
|||||||
| DT (h) | Maximum OD570 | DT (h) | Maximum OD570 | DT (h) | Maximum OD570 | DT (h) | Maximum OD570 | DT (h) | Maximum OD570 | DT (h) | Maximum OD570 | |
| 0 | 5.0 | 1.9 | 6.4 | 1.8 | 5.4 | 2.0 | 35 | 0.6 | 40 | 0.5 | 6.7 | 1.8 |
| 0.5 | 20 | 0.9 | 11 | 1.6 | 5.5 | 2.1 | 32 | 0.5 | 38 | 0.5 | 21 | 0.8 |
| 0.65 | 45 | 0.3 | 27 | 0.9 | 13 | 1.8 | 42 | 0.3 | NDc | ND | ND | ND |
The growth rate is expressed as the DT (in hours per generation), and the maximum OD570 is the optical density of the culture in the stationary phase. Each value is the mean from triplicate determinations, and the standard deviations were less than 10%.
Growth parameters of strain LTS23-1020 (betA mutant) in the presence of DMSE. The growth parameters of strain LTS23-1020 in LAS medium, in LAS plus 0.5 M NaCl, and in LAS containing 1 mM DMSA were similar to those observed when wild-type strain 102F34 was grown in the same media.
ND, not determined.
S-Methylmethionine (Fig. 1) is a TSC which functions as a natural precursor of osmotically accumulated DMSP in the halophyte Wollastonia biflora (20, 47). However, whether S-methylmethionine itself can play a role in bacterial osmoregulation is not known. Thus, S-methylmethionine and methionine (a monomethylated sulfur amino acid) were also included in the osmoprotection bioassays. Interestingly, these two compounds were not osmoprotective for either S. meliloti 102F34 or Escherichia coli MC4100 (data not shown).
Effect of exogenous DMSP on the osmolyte composition of stressed cultures of S. meliloti 102F34.
Natural-abundance 13C NMR spectroscopy was used to identify the organic osmolytes that accumulated in stressed cultures of S. meliloti 102F34 grown in the presence of the Ulva extract or synthetic DMSP. Moreover, to determine whether the accumulation of any exogenous osmolyte(s) was growth phase dependent (as observed with GB [45]), cells were harvested at three different stages of growth between the early exponential and stationary phases. No NMR-detectable solutes were detected in the unstressed cultures grown in the presence or absence of the algal extract or synthetic DMSP (data not shown). As expected (43, 46), stressed cells grown until the stationary phase in LAS medium containing 0.5 M NaCl (i.e., in the absence of algal extract or DMSP) accumulated three major osmolytes, l-glutamate, the dipeptide N-acetylglutaminylglutamine amide, and trehalose (Fig. 2C). Strikingly, the spectra obtained from stressed cultures of S. meliloti 102F34 grown with the Ulva extract or synthetic DMSP were always very similar at all stages of the growth cycles. A spectrum obtained from cells grown to the early stationary phase in the presence of 1 mM DMSP is shown in Fig. 2D. These stressed cells accumulated DMSP as a dominant cytosolic solute and minor amounts of the three endogenous osmolytes. These data demonstrate that DMSP (algal or synthetic) was durably accumulated as a dominant osmolyte which strongly reduced the accumulation of endogenous osmolytes in salt-stressed cells. They also suggest that DMSP, in contrast to GB (which is progressively catabolized by stressed cultures of S. meliloti 102F34 [45]), either acted as a nonmetabolizable osmolyte or had a very slow rate of turnover in this strain.
DMSA is toxic and does not act as an osmoprotectant in S. meliloti 102F34.
DMSA was also added as a putative osmoprotectant to S. meliloti 102F34 cultures because it was found in minor amounts in the Ulva extract and is very similar structurally to GB and DMSP (Fig. 1). Also, DMSA is highly osmoprotective in E. coli (10). Surprisingly, we observed that DMSA was highly toxic to an unstressed culture of S. meliloti 102F34. Indeed, the doubling time (DT) of this wild-type strain increased from 5 h in LAS medium to 35 h in LAS medium containing 1 mM DMSA (Table 1). Moreover, the final cell yield of the latter culture (maximum OD570, 0.6) was 3.2 times lower than that of the unstressed culture grown in LAS medium without DMSA (maximum OD570, 1.9). DMSA exhibited maximal toxicity at a concentration of 0.5 to 1 mM (data not shown). Also, as little as 1 mM DMSA was more inhibitory than the severe salt stress caused by 0.5 M NaCl, as judged by comparing the DTs and final optical densities of the corresponding cultures (Table 1). Furthermore, no significant changes in growth parameters (DT and maximum OD570) were observed when DMSA was added as a putative alleviator of salt stress to cultures grown in the presence of 0.5 or 0.65 M NaCl. In other words, DMSA, in contrast to DMSP and GB (Table 1), did not act as a beneficial osmoprotectant in S. meliloti 102F34. Interestingly, similar results (growth inhibition at low osmolarity and no growth improvement at high osmolarity) were also observed when DMSE, the sulfonium analog of choline (Fig. 1), was substituted for DMSA (Table 1).
The biological activities of DMSA and DMSE were also examined in cultures of S. meliloti LTS23-1020, a choline dehydrogenase-deficient derivative of 102F34 which cannot oxidize choline to GB (37). DMSA also inhibited the growth of LTS23-1020 at low osmolarity, but no growth inhibition by DMSE was observed in the unstressed mutant. Furthermore, as observed with choline (37), DMSE was also physiologically inert in the stressed mutant (Table 1). Thus, growth inhibition by DMSE in wild-type S. meliloti 102F34 most probably resulted from oxidation of DMSE to DMSA via the choline-GB (bet) pathway (37, 42). This interpretation is supported by the fact that strain LTS23-1020, in contrast to 102F34, also failed to oxidize DMSE to DMSA, as judged from an electrophoretic analysis of cytosolic extracts in parallel to the synthetic compounds (data not shown). It is also consistent with the fact that choline per se is not osmoprotective for S. meliloti and many other bacteria, unless it is oxidized to GB, which is the true osmoprotectant (7, 11, 27, 37).
Osmoregulated uptake and accumulation of DMSP and DMSA in S. meliloti 102F34.
The osmoprotective activities of methylated onium compounds are closely related to their chemical structures and the ability of stressed cells to transport and accumulate these solutes as cytosolic osmolytes (11, 19, 30, 38). Therefore, uptake and accumulation of [1-14C]DMSP and [methyl-14C]DMSA were examined to determine whether the contrasting biological activities of these compounds in S. meliloti 102F34 could be linked to differences in uptake and/or in their cytosolic levels. [methyl-14C]GB was used as a control in the transport assays (i.e., as an osmoprotectant which is readily transported and accumulated by stressed cells of S. meliloti 102F34) (3, 45). Unstressed cells grown in LAS medium took up DMSP, DMSA, and GB at rates of 6.1, 8.5, and 12 nmol/min/mg of protein, respectively (Table 2). Moreover, uptake of the three onium compounds was osmoregulated and was stimulated 2.5-, 3.8-, and 3.2-fold, respectively, in stressed cells cultured in LAS medium containing 0.5 M NaCl. Similar increases in uptake rates were also observed in stressed cells cultured in LAS medium containing 0.5 M KCl or 0.4 M K2SO4.
TABLE 2.
Effects of salt stress and unlabeled analogs on the uptake of [14C]DMSP, [14C]DMSA, and [14C]GB by S. meliloti 102F34a
| Onium suppliedb | Uptake rateb (nmol/min/mg of protein)
|
% Inhibition of uptake by unlabeled analogs at different competitor/substrate ratiosd
|
||||||
|---|---|---|---|---|---|---|---|---|
| GB
|
DMSA
|
DMSP
|
||||||
| Control culture | Stressed culturec | 1:1 | 10:1 | 1:1 | 10:1 | 1:1 | 10:1 | |
| [14C]DMSP | 6.1 | 15 | NDe | 99 | ND | 95 | ND | 80 |
| [14C]DMSA | 8.5 | 32 | 79 | 97 | 47 | 84 | 27 | 43 |
| [14C]GB | 12 | 38 | 50 | 80 | 24 | 56 | 10 | 22 |
Cultures were grown and uptake experiments were performed in LAS minimal medium (unstressed control culture) or in LAS containing 0.5 M NaCl (stressed culture).
Uptake rates were determined as described in the text in the early exponential phase of growth (OD570, 0.3).
Similar uptake rates were observed in stressed cultures grown in LAS medium containing isoosmotic concentrations of KCl (0.5 M) or K2SO4 (0.4 M).
The results are expressed as percentages of reduction compared with uninhibited transport rates which were observed in a stressed culture. Similar levels of inhibition were observed in unstressed (control) cells (data not shown).
ND, not determined.
Competition studies revealed that uptake of [14C]DMSP in stressed cells was inhibited more strongly by GB and DMSA than by unlabeled DMSP. Indeed, a 10-fold molar excess of GB or DMSA almost totally inhibited the uptake of [14C]DMSP, whereas the same level of unlabeled DMSP caused an 80% reduction in [14C]DMSP uptake (Table 2). Reciprocally, DMSP and DMSA were weaker inhibitors of [14C]GB uptake than unlabeled GB was. Indeed, a 10-fold molar excess of DMSP resulted in only a 22% reduction in [14C]GB uptake. Similarly GB uptake was inhibited only 24% by an equimolar concentration of DMSA. Thus, DMSP was about 10 times less effective than DMSA in inhibiting the uptake of [14C]GB (Table 2). Furthermore, GB was a stronger inhibitor of [14C]DMSA uptake (79 and 97% inhibition at inhibitor/substrate ratios of 1:1 and 10:1, respectively) than unlabeled DMSA was (47 and 84% inhibition, respectively). Conversely, DMSP was a weaker inhibitor of [14C]DMSA uptake (27 and 43% inhibition, respectively) than DMSA was. Together, the results of these competition studies are consistent. They indicate that GB, DMSA, and DMSP were apparently taken up via transporters which had a greater ability to transport GB and DMSA than DMSP (Table 2), a situation which was previously observed in E. coli (19, 33).
The cytosolic levels of GB and its two sulfonium analogs in S. meliloti 102F34 were readily determined by measuring the radioactivities of labeled compounds in the ESFs obtained from cultures which were grown in the presence of the radiolabeled compounds at a concentration of 1 mM. Because accumulation of GB is growth phase dependent in stressed cells of S. meliloti 102F34 (45), values were determined periodically over the growth cycles of the cultures. The data obtained from cells which were harvested in the early and late exponential phases are shown in Table 3. Strikingly, the levels of [14C]DMSP, [14C]DMSA, and [14C]GB were very similar and reached values of about 1,400 nmol/mg of protein (i.e., about 300 mM) in stressed cells which were harvested in the early exponential phase. Interestingly, at this early stage of growth, the cytosolic concentrations of [14C]DMSP, [14C]DMSA, and [14C]GB in stressed cells were 6.5-, 11.8-, and 4.7-fold higher, respectively, than the cytosolic concentrations in unstressed cells. Similar results were also observed in stressed cells grown in the presence of 0.5 M KCl or 0.4 M K2SO4 (data not shown). Then, because GB was rapidly catabolized (although at different rates [see below]) by the unstressed and stressed cultures, only low levels of [14C]GB were recovered in the cells at the end of the exponential phase (6 and 40 mM in unstressed and stressed cells, respectively) (Table 3). At this late stage of growth, the cytosolic levels of [14C]DMSP and [14C]DMSA were still very low in the unstressed cells (23 and 45 mM, respectively) but remained very high in the stressed cells (270 and 200 mM, respectively) (Table 3), and the levels in stationary-phase cells were similar (data not shown). In summary, accumulation of DMSP and DMSA, like accumulation of GB, was osmoregulated in S. meliloti 102F34. However, the two TSCs, unlike GB, were apparently accumulated as stable cytosolic osmolytes throughout the growth cycles of the stressed cultures of S. meliloti 102F34.
TABLE 3.
Osmoregulated accumulation of [14C]DMSP, [14C]DMSA, and [14C]GB in S. meliloti 102F34a
| 14C-labeled onium supplied | NaCl concn in LAS medium (M) | Amt of 14C-labeled onium recovered in the cells at:
|
|||
|---|---|---|---|---|---|
| Early exponential phase
|
Late exponential phase
|
||||
| nmol/mg of protein | mMb | nmol/mg of protein | mMb | ||
| DMSP | 0 | 300 | 46 | 150 | 23 |
| 0.5 | 1,460 | 300 | 1,300 | 270 | |
| GB | 0 | 140 | 22 | 40 | 6 |
| 0.5 | 1,300 | 260 | 200 | 40 | |
| DMSA | 0 | 400 | 60 | 300 | 45 |
| 0.5 | 1,400 | 280 | 1,000 | 200 | |
Cultures were grown in LAS medium or in LAS containing 0.5 M NaCl, and 1 mM [1-14C]DMSP, 1 mM [1-14C]DMSA, or 1 mM [1,2-14C]GB was added at the time of inoculation.
Concentrations were calculated based on estimates of cell volumes, which were determined as described by Stock et al. (44).
Analysis of the cellular extracts and the growth media from stressed and unstressed cultures grown in the presence of 1 mM [14C]DMSP revealed that the osmoprotectant supplied was always recovered quantitatively as [14C]DMSP in the cytosolic fractions (the ESFs) and in the growth media. No radioactivity was ever detected in the EIF and 14CO2 fractions from these cultures at any stage of the growth cycles. Similar results were also observed when [1-14C]DMSP was added without isotopic dilution (i.e., at a concentration of about 50 μM) to stressed and unstressed cultures of S. meliloti 102F34 and RCR2011 (data not shown). Finally, DMSP (10 mM) did not support the growth of these strains when it was added as a source of carbon and energy in combination with ammonia or urea (10 mM) as the nitrogen source (data not shown). Thus, the two wild-type strains of S. meliloti did not catabolize DMSP under any conditions.
Comparison of the fates of DMSA and GB in unstressed and stressed cultures of S. meliloti 102F34.
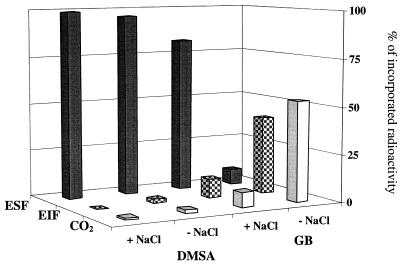
The very different patterns of accumulation of [14C]DMSA and [14C]GB in S. meliloti 102F34 (Table 3) strongly suggest that these compounds were catabolized at different rates when they were added at the physiologically active concentration of 1 mM (Table 1). Figure 3 shows the contrasting metabolism patterns of 1 mM [14C]GB and 1 mM [14C]DMSA in unstressed cultures of S. meliloti 102F34. At the end of a 4-h incubation with [1-14C]GB, 92% of the cellular radioactivity was recovered in 14CO2 and in the EIF. In contrast, only 5% of the 14C supplied was recovered in these fractions in cells which received 1 mM toxic [14C]DMSA (Fig. 3). Moreover, most (>93%) of the radiocarbon recovered in the cytosolic fractions (the ESFs) and in the growth media comigrated electrophoretically and chromatographically with synthetic [14C]DMSA (data not shown). Interestingly, addition of 0.5 M NaCl to LAS minimal medium had no significant effect on the catabolism of 1 mM [14C]DMSA; however, as reported previously (45), the catabolism of GB was strongly inhibited in stressed cells which were harvested at an early stage of exponential growth (Fig. 3).
FIG. 3.
Comparative fates of a physiologically active concentration (1 mM) of DMSA and GB in stressed and unstressed cultures of S. meliloti 102F34. Exponentially growing cells (OD570, 0.4) cultured in LAS medium containing 0.5 M NaCl (+ NaCl) or in LAS medium without NaCl (− NaCl) were fed 1 mM [1-14C]DMSA or 1 mM [1,2-14C]GB (3.7 kBq/ml) for 4 h, extracted in ethanol, and processed as described in the text. The distribution of 14C in the ESF, the EIF, and CO2 is expressed as percentages of total disintegrations per minute recovered from the cultures incubated with the substrates.
Toxicity of DMSA stems from its catabolism via the GB demethylation pathway.
The very close structural relatedness of DMSA and GB (Fig. 1) and the fact that these compounds were equally osmoprotective for E. coli and are mutually interchangeable as nonmetabolizable osmolytes in this species (10, 38) strongly suggested that the toxicity of 1 mM DMSA in S. meliloti 102F34 resulted from catabolism of this compound via the GB demethylation pathway (42), which is absent in E. coli (23). Thus, we monitored the fate of micromolar levels of [1,2-14C]GB, [methyl-14C]GB, [1-14C]DMSA, and [methyl-14C]DMSA in unstressed cultures of S. meliloti 102F34. The radioactive compounds were supplied without isotopic dilution (at a concentration of 1.8 or 13.9 μM) in order to increase their specific activities and to avoid the inhibitory effects of millimolar levels of DMSA on growth. Table 4 shows that the radioactivity of the cytosolic fractions (the ESFs) was always very low and did not exceed 4% of the 14C radioactivity supplied. Moreover, about 74% of the radiocarbon originating from [1,2-14C]GB and [methyl-14C]GB was recovered in the EIF and 14CO2. Meanwhile, labeling of these two fractions was negligible in cells grown in the presence of 1.8 μM [1-14C]DMSA, but was significant (27% of the 14C supplied) when the cells received 1.8 μM [methyl-14C]DMSA. These data are consistent with those presented in Table 3 and Fig. 3 and confirm that the patterns of DMSA and GB metabolism were remarkably different in S. meliloti 102F34.
TABLE 4.
Metabolism of micromolar levels of [14C]GB and [14C]DMSA by unstressed cultures of S. meliloti 102F34a
| 14C-labeled onium supplied | Amt of radioactivity supplied (103 dpm) | Amt of 14C recovered in medium (103 dpm) | Amt of 14C recovered in cellular fractions (103 dpm)
|
Total dpm recovered (103) | ||
|---|---|---|---|---|---|---|
| CO2 fraction | EIF | ESF | ||||
| [1,2-14C]GB (13.9 μM) | 224 | 37 (16)b,c | 67 (30) | 99 (44) | 5 (2) | 208 |
| [methyl-14C]GB (1.8 μM) | 222 | 37 (17)c | 104 (47) | 58 (26) | 4 (2) | 203 |
| [1-14C]DMSA (1.8 μM) | 228 | 210 (92)d | 1 (<1) | 1 (<1) | 10 (4) | 222 |
| [methyl-14C]DMSA (1.8 μM) | 244 | 163 (67)e | 37 (15) | 30 (12) | 6 (3) | 236 |
S. meliloti 102F34 was inoculated into LAS medium containing a 14C-labeled onium compound (3.7 kBq/ml). Cells were harvested after 14 h of incubation and extracted in ethanol as described in the text.
The numbers in parentheses are percentages of the radioactivity supplied.
More than 95% of the radioactivity recovered in the medium comigrated with [14C]GB during high-voltage electrophoresis and paper chromatography.
A total of 80% of the radiocarbon in the medium was recovered in [1-14C]MTA and in [1-14C]TGA, as determined by autoradiography with the Packard InstantImager following thin layer chromatography with CH2Cl2-ethanol (1:1, vol/vol).
A total of 70% of the label in the medium was recovered in [methyl-14C]MTA.
Analysis of the radiochemical compositions of the growth media provided further insight into the specific fates of DMSA and GB. At the end of the experiment, [14C]GB accounted for 95% of the residual radiocarbon (about 16% of the 14C supplied) remaining in the growth media of the cultures which received 13.9 μM [1,2-14C]GB or 1.8 μM [methyl-14C]GB. In sharp contrast, the extracellular radioactivity accounted for 92 and 67% of the radiocarbon which was added in the form of [1-14C]DMSA and [methyl-14C]DMSA, respectively (Table 4). However, it is particularly noteworthy that radioactive DMSA accounted for, at most, 19% of the 14C supplied to unstressed cultures of S. meliloti 102F34. In other words, DMSA was catabolized at a much higher rate when it was supplied in micromolar concentrations (this experiment) than when it was added at millimolar concentrations (Fig. 3). Moreover, the data in Table 4 also indicate that the catabolites of DMSA and GB had different fates in S. meliloti 102F34; GB was assimilated, whereas the catabolites of DMSA were excreted.
S. meliloti catabolizes GB via serial demethylations to N,N-dimethylglycine [(CH3)2NCH2COO−], sarcosine (CH3NHCH2COO−), and glycine (H3N+CH2COO−) (42). Catabolism of DMSA via this pathway should yield, successively, S-methylthioacetate (2-methylthioacetate) (MTA) and thioglycolic acid (2-mercaptoacetate) (TGA) (Fig. 1) (the sulfur analog of sarcosine and glycine, respectively). Obviously, [methyl-14C]MTA is the only demethylation product of DMSA that can be identified as a radiolabeled catabolite of [methyl-14C]DMSA, whereas both [14C]MTA and [14C]TGA can be recovered from [1-14C]DMSA. Therefore, it is extremely interesting that [methyl-14C]MTA accounted for 70% of the radiocarbon recovered in the growth medium and about one-half (47%) of the radioactivity supplied in the form of [methyl-14C]DMSA (Table 4). This indicates that one of the two radiocarbons of [methyl-14C]DMSA (the methyl group removed) was incorporated mainly into 14CO2 and the EIF, while the second radiocarbon was largely recovered in extracellular [methyl-14C]MTA. Obviously, this suggests that DMSA-derived [methyl-14C]MTA was excreted into the growth medium at a faster rate than that at which it was catabolized. Furthermore, no [methyl-14C]MTA was found in the cytoplasm of the cells (data not shown).
Similarly, catabolites of 1.8 μM [1-14C]DMSA that comigrated with MTA and TGA were found only in the growth medium of unstressed cells of S. meliloti 102F34. Unfortunately, we could not satisfactorily separate MTA and TGA and did not determine the amounts of these compounds. Nonetheless, these two compounds together accounted for 80% of the extracellular radiocarbon. Obviously, this finding and the fact that negligible amounts of 14C were recovered in the EIF and 14CO2 fractions (Table 4) provide further evidence that the demethylation products of DMSA are more actively excreted than catabolized by unstressed cells of S. meliloti 102F34. This interpretation is also supported by the results of an analysis of the radiochemical composition of the growth medium of a culture which received a physiologically active concentration (1 mM) of [methyl-14C]DMSA for 15 h; at the end of the experiment, [methyl-14C]DMSA and [methyl-14C]MTA accounted for 93 and 5% of the extracellular radioactivity, respectively. No [methyl-14C]MTA was detected in the cytoplasm of the cells from this culture (data not shown). Furthermore, excretion of DMSA-derived MTA and TGA is consistent with the fact that DMSA, MTA, and TGA (at concentrations of 1 to 10 mM), in contrast to GB and its demethylation products (42), were not used as growth substrates by S. meliloti 102F34 (data not shown). In summary, the radiochemical data described above indicate that S. meliloti 102F34 assimilated GB as a source of carbon and energy but excreted the demethylation products of DMSA, most probably as a mechanism for cellular detoxification of these compounds.
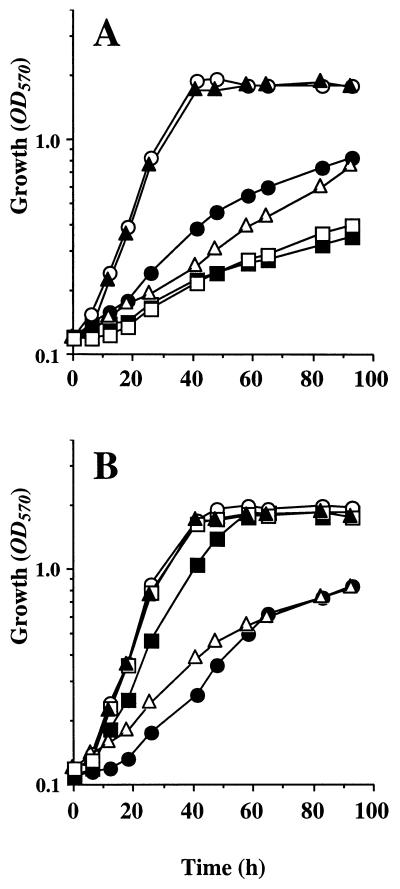
The possibility that the toxicity of DMSA in S. meliloti 102F34 resulted from demethylation of this compound was further examined by analyzing the growth responses of other strains of S. meliloti to DMSA and its demethylation products. First, 1 mM DMSA was added as a putative osmoprotectant to S. meliloti wild-type strains 102F34 and RCR2011 and two catabolic mutants (GMI766 and VP01) that do not use GB as a growth substrate (17, 32). As expected, DMSA strongly inhibited the growth of the two wild-type strains in low-osmolarity LAS medium and was not osmoprotective in these strains (Fig. 4A). In sharp contrast, DMSA had no inhibitory effect on the growth of GMI766 and VP01 in LAS medium, but acted as a powerful osmoprotectant when the mutants were grown in LAS medium containing 0.5 M NaCl (Fig. 4B). Furthermore, GMI766, which is not able to catabolize GB (17, 45), was also not able to catabolize [14C]DMSA. Meanwhile, VP01 was severely impaired in its ability to demethylate both [14C]GB and [14C]DMSA (data not shown).
FIG. 4.
Effects of DMSA and its demethylation products, MTA and TGA, on the growth of salt-stressed and unstressed cultures of S. meliloti. Unstressed cultures of wild-type strain 102F34 (A) and mutant strain VP01 (B) were grown in LAS medium with no additions (○) and in LAS medium containing 1 mM DMSA (□), 1 mM MTA (▴), or 1 mM TGA (▵). Salt-stressed cultures were grown in LAS medium containing 0.5 M NaCl (•) or in LAS medium containing 0.5 M NaCl and 1 mM DMSA (▪). The results obtained with wild-type strain RCR2011 and strain GMI766 were very similar to the results obtained with strains 102F34 and VP01, respectively (data not shown).
MTA and TGA (1 mM) were also added to cultures of the four strains of S. meliloti. MTA was physiologically inert in all of these strains; i.e., MTA was not toxic in low-osmolarity LAS medium and was not osmoprotective at high osmolarity (data not shown). In sharp contrast, TGA strongly inhibited the growth of the four strains in LAS minimal medium. Finally, TGA and DMSA exhibited similar inhibitory activities in unstressed cultures of wild-type strains RCR2011 and 102F34 (Fig. 4). In summary, the results of these growth assays provide further evidence that the acute toxicity of DMSA in wild-type strains of S. meliloti (32) was indirect and stemmed from the catabolism of this sulfonium compound via the GB demethylation pathway.
DISCUSSION
Contrasting biological activities of S-methylated compounds in S. meliloti 102F34 grown at low and high osmolarities.
We demonstrate in this report that S-methylated analogs of GB and DMSP (which are two common osmolytes in bacteria, marine algae, and plants [5, 11, 16, 39, 40]) exhibit contrasting biological activities in salt-stressed and unstressed cultures of S. meliloti. First, DMSP is a powerful osmoprotectant that strongly stimulates sinorhizobial growth at high salinities. Second, DMSE and DMSA, the sulfonium analogs of choline and GB (Fig. 1), are highly toxic to wild-type strains of S. meliloti grown at low salinities. Moreover, DMSE and DMSA are not osmoprotective in wild-type strains of S. meliloti, in contrast to DMSP (Fig. 2), choline, and GB (3). However, DMSE is physiologically inert in a mutant (LTS23-1020) that is deficient in choline dehydrogenase activity. Hence, its toxicity in the parental strain (102F34) stems from its oxidation to DMSA via the choline-GB (betAB) pathway (37, 42). To our knowledge, this is the first study to describe the acute toxicity of DMSA in any organism. Third, S-methylmethionine, a natural precursor of DMSP in the halophyte W. biflora (20, 47), is physiologically inert in S. meliloti 102F34 grown at low and high salinities. Likewise, neither methionine (a monomethylated amino acid) nor MTA displays any toxicity or any osmoprotective activity in S. meliloti 102F34. Interestingly, except for DMSA (see below), all of the S- and N-methylated analogs of GB and DMSP, which were not osmoprotective in S. meliloti 102F34 (3; this study), do not have a net neutral charge at physiological pH. Thus, they cannot accumulate to high concentrations without a proper counterion, which might also be less compatible with cellular functions than neutral organic osmolytes (11, 16, 40).
Toxicity of DMSA results from its demethylation via the GB pathway.
The toxicity of DMSA and its lack of effectiveness in osmoprotection in S. meliloti 102F34 were intriguing for several reasons: (i) nitrogenous betaines which are structurally more distantly related to GB than to DMSA (stachydrine, carnitine, trigonelline) all act as powerful osmoprotectants in S. meliloti (3); (ii) DMSA, on the one hand, and DMSP and GB, on the other hand, display remarkably different biological activities in S. meliloti 102F34, although these compounds have very similar structures, carry a net neutral charge at physiological pH, are transported at similar rates, and contribute to similar levels of turgor adjustment (i.e., are accumulated to similar levels) in stressed cells of S. meliloti 102F34; and (iii) in contrast, DMSP and DMSA can substitute for GB both as powerful osmoprotectants and as highly compatible solutes in E. coli (10, 30, 38). In short, DMSP, GB, and DMSA are mutually interchangeable as cytosolic osmolytes (i.e., they contribute equally to osmotic adjustment) in salt-stressed cells of E. coli and S. meliloti. Therefore, incompatibility of DMSA with cellular functions cannot be used to explain the lack of osmoprotective activity by this compound in S. meliloti 102F34.
Physiological and biochemical evidence presented in this paper supports the conclusion that the toxicity of DMSA in S. meliloti stems from the catabolism of this compound via the GB demethylation pathway (42). First, our results obtained with DMSA are highly reminiscent of the results obtained with phosphoniobetaine [(CH3)3P+CH2COO−] and arsenobetaine [(CH3)3As+CH2COO−], the phosphonium and arsonium analogs of DMSA and GB (32). Indeed, DMSA, like phosphoniobetaine and arsenobetaine, is toxic only in wild-type strains of S. meliloti, which actively catabolize GB and use it as a source of carbon and nitrogen. Furthermore, DMSA, like phosphoniobetaine, arsenobetaine, and GB, is physiologically inert in unstressed cultures of the following two mutants which do not use GB as a growth substrate: (i) strain GMI766, which catabolizes neither GB (17, 45) nor DMSA (this study); and (ii) strain VP01, a phosphoniobetaine-resistant mutant that is severely impaired in its ability to demethylate GB (32) and DMSA (this study). Moreover, it is particularly noteworthy that the phosphonio- and arsenobetaines, like DMSA and GB, are also very effective osmoprotectants in S. meliloti VP01 (32) and GMI766 (33). Thus, the physiological responses of these mutants to DMSA, phosphoniobetaine, and arsenobetaine unequivocally resemble those of E. coli (10, 32, 38) and several other bacterial species (33) that use GB and its three structural analogs only as very powerful osmoprotectants and nonmetabolizable osmolytes. Together, these data provide persuasive evidence that DMSA (like its phosphonium and sulfonium analogs [32]) acts as an osmoprotectant in S. meliloti, provided that it is not catabolized. Our data also confirm that the compatibility of an osmolyte is independent of the organism in which it accumulates (11, 16, 30).
Second, there is a very good correlation between the remarkably different metabolic patterns and the equally different biological activities of DMSA and GB in S. meliloti 102F34. Indeed, at a concentration of 1 mM, DMSA has its maximal inhibitory effect on growth and strongly suppresses (although indirectly [see below]) its own catabolism in this strain (Table 1 and Fig. 3). Consequently, DMSA cannot be used as a carbon source. In contrast, GB is catabolized at high rates and is assimilated as a building block by S. meliloti 102F34 (42, 45).
Third, the two-step demethylation of DMSA in S. meliloti 102F34 was expected to yield, successively, MTA and TGA. Thus, it is particularly noteworthy that exogenous DMSA and TGA similarly inhibit the growth of wild-type strains of S. meliloti in low-osmolarity medium. Obviously, only [methyl-14C]MTA can be identified as a radiolabeled demethylation product of [methyl-14C]DMSA (Fig. 1). Interestingly, DMSA-derived [methyl-14C]MTA was not recovered in the cells, but was excreted into the growth medium. Likewise, radioactive MTA and TGA, which are derived from [1-14C]DMSA, are predominantly excreted by S. meliloti 102F34 and account for most (80%) of the radiocarbon originating from this compound (Table 4). Clearly, this indicates that the efflux of demethylation products of DMSA occurs at a much faster rate than the catabolism of these compounds in the cells. This efflux is probably a mechanism for cellular detoxification of these compounds, both of which act as potent inhibitors of many enzymes (1, 2). MTA, in contrast to DMSA and TGA, has no inhibitory effect on sinorhizobial growth (Fig. 4). This result was unexpected because MTA is a metabolic intermediate in the demethylation of DMSA to TGA. However, the lack of toxicity of MTA is not necessarily surprising, because DMSA-derived MTA is excreted at high rates. Also, the different biological activities of MTA and TGA in S. meliloti may reflect the fact that TGA (which possesses a free —SH group) is chemically more reactive and is much more inhibitory to enzymes than MTA (1). Finally, these differences could also be related to differences in the net transport rates (influx minus efflux) of exogenously supplied MTA and TGA.
GB is a universal osmoprotectant which is widely distributed in nature and has attracted considerable research attention (11, 16, 27, 40). In contrast, there is very limited information on the presence and functions of its sulfonium analog. Indeed, DMSA has been found only in two marine algae, Digenea simplex and U. lactuca (31, 34). Surprisingly, no clear physiological functions for DMSA in microorganisms are known, except for its proven role in osmoregulation in E. coli (10, 38). However, DMSA was widely used in the 1950s as a synthetic analog of GB in biochemical research on betaine-homocysteine methyltransferase (BHMT), an enzyme that catalyzes the first demethylation of GB and the concomitant synthesis of methionine in animals (14, 25, 26). Interestingly, DMSA is also a substrate for BHMT in extracts from the soil bacterium Pseudomonas denitrificans, but its possible effects on bacterial growth have not been reported (51). In contrast, DMSA is metabolized to dimethylsulfide (CH3-S-CH3) and methanethiol (CH3-SH) by unspecified rumen bacteria (41, 52). There are diverse catabolic pathways for GB in bacteria (for a review, see reference 28). Hence, it should be particularly interesting to determine whether DMSA, phosphoniobetaine, and arsenobetaine are catabolized via similar routes and display any toxicity or osmoprotective activity in representative isolates belonging to different groups of GB-degrading bacteria.
DMSP is a nonmetabolizable osmoprotectant in S. meliloti.
Radiolabeled DMSP, in contrast to DMSA and GB, is never catabolized to any detectable extent either in stressed cells or in unstressed cells of wild-type strains of S. meliloti. Thus, considering that [14C]DMSP (i) was added at a high and low specific activities, (ii) is readily taken up over a wide range of substrate concentrations (from 50 μM to 1 mM), and (iii) can accumulate to high cytosolic levels (up to 0.3 M), we inferred that this compound was readily accessible to the sinorhizobial BHMT (in vivo) under all of our experimental conditions. Therefore, we concluded that DMSP is not a substrate for BHMT in S. meliloti 102F34. In other words, the high specificity of this enzyme most likely accounts for the fact that DMSP and DMSA display contrasting biological activities in wild-type strains of S. meliloti. In this respect, it is extremely interesting to note that different catabolic pathways for GB and DMSP often coexist in bacteria. For example, GB, unlike DMSP, is not used as a growth substrate by Pseudomonas doudoroffii ATCC 27123 and Alcaligenes-like strain M3A (12). Likewise, three gram-negative, aerobic, GB-demethylating bacteria obtained from a hypersaline lake use DMSP in different pathways (13); (i) strain ML-G, like S. meliloti 102F34, does not grow on DMSP, (ii) strain ML-D cleaves DMSP by using a DMSP lyase (to yield dimethylsulfide and acrylic acid), and (iii) strain MM-P demethylates DMSP to produce 3-methylthiopropionate and demethiolates 3-methylthiopropionate to produce methanethiol. However, it is not known whether DMSP and GB are demethylated by the same or different enzymes in strain MM-P or in the marine aerobic bacterium strain BIS-6 (13, 50). Finally, strains of a Desulfobacterium sp. provide further examples of the considerable diversity of catabolic pathways for DMSP and GB which coexist in bacteria. Indeed, these strains demethylate DMSP via a novel pathway involving a DMSP-tetrahydrofolate methyltransferase that does not accept GB as a substrate (21). Our data on S. meliloti provide further evidence that GB-demethylating bacteria do not necessarily catabolize DMSP and support the recent proposal that bacterial onium demethylases are highly specific for one type of methyl donor, either DMSP or GB (49), as well as (in wild-type strains of S. meliloti) the two-carbon carboxylic analogs DMSA (this study), phosphoniobetaine, and arsenobetaine (32).
Here we also provide the first evidence that an algal extract and DMSP, a common TSC in marine algae and halophytes (5, 39, 40), confer increased salinity tolerance to a bacterium of agricultural importance. Obviously, the beneficial activity of the Ulva extract in stressed cultures of S. meliloti is not due to a nutritional effect, because the extract has no effect on unstressed cultures. Synthetic DMSP is slightly less osmoprotective than the crude Ulva extract. However, DMSP is the only NMR-detectable osmolyte of algal origin to accumulate in stressed cells of S. meliloti (Fig. 2D). The higher level of activity of the Ulva extract might be attributable to less abundant algal osmolytes that do not accumulate to NMR-detectable levels. Alternatively, such algal solutes may not accumulate as cytosolic osmolytes in stressed cells, as shown for ectoine, an unusual osmoprotectant which stimulates the synthesis of endogenous osmolytes in S. meliloti (32).
The spectral and radiochemical data in this paper also demonstrate that DMSP functions as an ideal osmoprotectant in S. meliloti. Indeed, DMSP strongly stimulates sinorhizobial growth at high salinities, its uptake and its cytosolic concentration increase in proportion to the osmolarity of the growth medium, and it, unlike other sinorhizobial osmoprotectants (ectoine, GB and other betaines [3, 17, 45, 46]) is not catabolized by stressed cultures of S. meliloti. Consequently, exogenous DMSP is durably accumulated as a cytosolic osmolyte throughout the growth cycles of these cultures. These interesting findings may have practical applications in agriculture. Indeed, marine algae and algal extracts are used as fertilizers and have many beneficial effects on plant crops. Interestingly, some of these effects are attributed to algal betaines (4, 6). Additional studies should determine whether other algal extracts and DMSP enhance nitrogen fixation activity in salt-stressed alfalfa seedlings nodulated by S. meliloti, as observed with exogenous GB and stachydrine (35, 36). These studies should also determine whether the durable accumulation of DMSP in stressed cells (compared with the temporary accumulation of GB [45]) increases salinity and drought tolerance, as well as long-term survival of S. meliloti in commercial inocula and in the soil.
Finally, two distinct biosynthetic pathways for DMSP have been elucidated in the halophyte W. biflora and in the green marine algae Enteromorpha intestinalis (15, 47). Methionine is the precursor of DMSP in both pathways. In W. biflora, methionine is methylated to produce S-methylmethionine. Then, S-methylmethionine is converted to DMSP via DMSP aldehyde, which is a sulfonium analog of betaine aldehyde, the metabolic intermediate in the choline-GB pathway in S. meliloti (37, 42). Interestingly, plant betaine aldehyde dehydrogenases efficiently catalyze the oxidation of DMSP aldehyde to DMSP (48). Future studies should determine whether S. meliloti also catalyzes this oxidative step and/or any other reaction(s) in the two DMSP pathways (in particular, the conversion of S-methylmethionine to DMSP aldehyde). Thus, they should determine whether cloning of a whole pathway or of any specific missing step(s) is required to engineer DMSP biosynthesis and increase salinity tolerance in S. meliloti and other rhizobia.
ACKNOWLEDGMENTS
This research was funded by grants from the Centre National de la Recherche Scientifique (CNRS) and the University of Rennes 1 and by a doctoral fellowship to V.P. from the Ministère de l’Education Nationale et de la Recherche.
C. Rosenberg and F. Larher are acknowledged for providing strain GMI766 and synthetic S-methylmethionine, respectively.
REFERENCES
- 1.Baldwin D A, Egan T J, Marques H M. The effects of anions on the kinetics of reductive elimination of iron from monoferric transferrins by thiols. Biochim Biophys Acta. 1990;1038:1–9. doi: 10.1016/0167-4838(90)90002-w. [DOI] [PubMed] [Google Scholar]
- 2.Bauche F, Sabourault D, Giudicelli Y, Nordmann J, Nordmann R. Inhibition in vitro of acyl-CoA dehydrogenases by 2-mercaptoacetate in rat liver mitochondria. Biochem J. 1983;215:457–464. doi: 10.1042/bj2150457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard T, Pocard J-A, Perroud B, Le Rudulier D. Variations in the response of salt-stressed Rhizobium strains to betaines. Arch Microbiol. 1986;143:359–364. [Google Scholar]
- 4.Blunden G. Agricultural uses of seaweeds and seaweed extracts. In: Guiry M D, Blunden G, editors. Seaweed resources in Europe: uses and potential. Chichester, United Kingdom: John Wiley & Sons Ltd.; 1992. pp. 65–81. [Google Scholar]
- 5.Blunden G, Gordon S. Betaines and their sulphonio analogues in marine algae. Prog Physiol Res. 1986;4:39–80. [Google Scholar]
- 6.Blunden G, Jenkins T, Liu Y-W. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J Appl Phycol. 1997;8:535–543. [Google Scholar]
- 7.Boch J, Kempf B, Schmid R, Bremer E. Synthesis of the osmoprotectant glycine betaine in Bacillus subtilis: characterization of the gbsAB genes. J Bacteriol. 1996;178:5121–5129. doi: 10.1128/jb.178.17.5121-5129.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohnert H J, Jensen R G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996;14:89–97. [Google Scholar]
- 9.Boivin C, Camut S, Malpica C A, Truchet G, Rosenberg C. Rhizobium meliloti genes encoding catabolism of trigonelline are induced under symbiotic conditions. Plant Cell. 1990;2:1157–1170. doi: 10.1105/tpc.2.12.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers S T, Kunin C M, Miller D, Hamada A. Dimethylthetin can substitute for glycine betaine as an osmoprotectant molecule for Escherichia coli. J Bacteriol. 1987;169:4845–4847. doi: 10.1128/jb.169.10.4845-4847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 12.de Souza M P, Yoch D C. Purification and characterization of dimethylsulfoniopropionate lyase from an Alcaligenes-like dimethyl sulfide-producing marine isolate. Appl Environ Microbiol. 1995;61:21–26. doi: 10.1128/aem.61.1.21-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz M R, Taylor B F. Metabolism of methylated osmolytes by aerobic bacteria from Mono Lake, a moderately hypersaline, alkaline environment. FEMS Microbiol Ecol. 1996;19:239–247. [Google Scholar]
- 14.Ferger M F, du Vigneaud V. Oxidation in vivo of the methyl groups of choline, betaine, dimethylthetin, and dimethyl-β-propiothetin. J Biol Chem. 1950;185:53–57. [PubMed] [Google Scholar]
- 15.Gage D A, Rhodes D, Nolte K D, Hicks W A, Leustek T, Cooper A J L, Hanson A D. A new route for synthesis of dimethylsulphoniopropionate in marine algae. Nature. 1997;387:891–894. doi: 10.1038/43160. [DOI] [PubMed] [Google Scholar]
- 16.Galinski E A. Osmoadaptation in bacteria. Adv Microb Physiol. 1995;37:273–328. [PubMed] [Google Scholar]
- 17.Goldmann A, Boivin C, Fleury V, Message B, Lecoeur L, Maille M, Tepfer D. Betaine use by rhizosphere bacteria: genes essential for trigonelline, stachydrine, and carnitine catabolism in Rhizobium meliloti are located on pSym in the symbiotic region. Mol Plant Microbe Interact. 1991;4:571–578. doi: 10.1094/mpmi-4-571. [DOI] [PubMed] [Google Scholar]
- 18.Goldmann A, Lecoeur L, Message B, Delarue M, Schoonejans E, Tepfer D. Symbiotic plasmid genes essential to the catabolism of proline betaine, or stachydrine, are also required for efficient nodulation by Rhizobium meliloti. FEMS Microbiol Lett. 1994;115:305–311. [Google Scholar]
- 19.Gouesbet G, Jebbar M, Talibart R, Bernard T, Blanco C. Pipecolic acid is an osmoprotectant for Escherichia coli taken up by the general osmoporters ProU and ProP. Microbiology. 1994;140:2415–2422. doi: 10.1099/13500872-140-9-2415. [DOI] [PubMed] [Google Scholar]
- 20.James F, Nolte K D, Hanson A D. Purification and properties of S-adenosyl-l-methionine:l-methionine S-methyltransferase from Wollastonia biflora leaves. J Biol Chem. 1995;270:22344–22350. doi: 10.1074/jbc.270.38.22344. [DOI] [PubMed] [Google Scholar]
- 21.Jansen M, Hansen T A. Tetrahydrofolate serves as a methyl acceptor in the demethylation of dimethylsulfoniopropionate in cell extracts of sulfate reducing bacteria. Arch Microbiol. 1997;169:84–87. doi: 10.1007/s002030050545. [DOI] [PubMed] [Google Scholar]
- 22.Le Berre A, Delacroix A. L’addition des sels d’amines tertiaires aux composés éthyléniques électrophiles. III. Bétaïnes et sels quaternaires à partir d’acides α,β-insaturés. Bull Soc Chim Fr. 1973;7-8:2404–2408. [Google Scholar]
- 23.Le Rudulier D, Strøm A R, Dandekar A M, Smith L T, Valentine R C. Molecular biology of osmoregulation. Science. 1984;224:1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Maw G. Thetin-homocysteine transmethylase. The distribution of the enzyme, studied with the aid of trimethylsulfonium chloride as substrate. Biochem J. 1959;72:602–608. doi: 10.1042/bj0720602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maw G A. Thetin-homocysteine transmethylase. A preliminary manometric study of the enzyme from rat liver. Biochem J. 1956;63:116–124. doi: 10.1042/bj0630116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller K J, Wood J M. Osmoadaptation by rhizosphere bacteria. Annu Rev Microbiol. 1996;50:101–136. doi: 10.1146/annurev.micro.50.1.101. [DOI] [PubMed] [Google Scholar]
- 28.Oren A. Formation and breakdown of glycine betaine and trimethylamine in hypersaline environments. Antonie Leeuwenhoek. 1990;58:291–298. doi: 10.1007/BF00399342. [DOI] [PubMed] [Google Scholar]
- 29.Osteras M, Boncompagni E, Vincent N, Poggi M C, Le Rudulier D. Abstracts of the 11th International Congress on Nitrogen Fixation. Paris, France: Institut Pasteur; 1997. Expression analysis of the bet locus encoding the glycine betaine biosynthesis pathway in Sinorhizobium meliloti, abstr. 11.37; p. 128. [Google Scholar]
- 30.Paquet L, Rathinasabapathi B, Saini H, Zamir L, Gage D A, Huang Z-H, Hanson A D. Accumulation of the compatible solute 3-dimethylsulfoniopropionate in sugarcane and its relatives, but not other gramineous crops. Aust J Plant Physiol. 1994;21:37–48. [Google Scholar]
- 31.Patti A, Morrone R, Chillemi R, Piattelli M, Sciuto S. Thetines and betaines of the red alga Digenea simplex. J Nat Prod. 1993;56:432–435. [Google Scholar]
- 32.Pichereau V, Cosquer A, Gaumont A, Bernard T. Synthesis of trimethylated phosphonium and arsonium analogues of the osmoprotectant glycine betaine; contrasted biological activities in two bacterial species. Bioorg Med Chem Lett. 1997;7:2893–2896. [Google Scholar]
- 33.Pichereau, V., and T. Bernard. 1997. Unpublished data.
- 34.Pichereau V, Uguen P, Bernard T. An extract from the marine alga Ulva lactuca improves growth of Rhizobium meliloti under hyperosmotic conditions: role of methylated sulfonium compounds. Plant Physiol Suppl. 1996;111:74. [Google Scholar]
- 35.Pocard J-A. La glycine betaïne, effet osmoprotecteur, transport et métabolisme chez Rhizobium meliloti cultivéin vitro et en symbiose avec Medicago sativa L. Ph. D. thesis. Rennes, France: University of Rennes 1; 1987. [Google Scholar]
- 36.Pocard J-A, Bernard T, Goas G, Le Rudulier D. Restauration partielle, par la glycine bétaïne et la proline bétaïne, de l’activité fixatrice d’azote de jeunes plantes de Medicago sativa L. soumises à un stress hydrique. C R Acad Sci. 1984;298:477–480. [Google Scholar]
- 37.Pocard J-A, Vincent N, Boncompagni E, Smith L T, Poggi M-C, Le Rudulier D. Molecular characterization of the bet genes encoding glycine betaine synthesis in Sinorhizobium meliloti 102F34. Microbiology. 1997;143:1369–1379. doi: 10.1099/00221287-143-4-1369. [DOI] [PubMed] [Google Scholar]
- 38.Randall K, Lever M, Peddie B A, Chambers S T. Natural and synthetic betaines counter the effects of high NaCl and urea concentrations. Biochim Biophys Acta. 1996;1291:189–194. doi: 10.1016/s0304-4165(96)00057-8. [DOI] [PubMed] [Google Scholar]
- 39.Reed R H. Measurement and osmotic significance of β-dimethylsulphoniopropionate in marine macroalgae. Mar Biol Lett. 1983;34:173–181. [Google Scholar]
- 40.Rhodes D, Hanson A D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:357–384. [Google Scholar]
- 41.Salsbury R L, Merricks D L. Production of methanethiol and dimethyl sulfide by rumen micro-organisms. Plant Soil. 1975;43:191–209. [Google Scholar]
- 42.Smith L T, Pocard J-A, Bernard T, Le Rudulier D. Osmotic control of betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988;170:3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith L T, Smith G M. An osmoregulated dipeptide in stressed Rhizobium meliloti. J Bacteriol. 1989;171:4714–4717. doi: 10.1128/jb.171.9.4714-4717.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stock J B, Rauch B, Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977;252:7850–7861. [PubMed] [Google Scholar]
- 45.Talibart R, Jebbar M, Gouffi K, Pichereau V, Gouesbet G, Blanco C, Bernard T, Pocard J-A. Transient accumulation of glycine betaine and dynamics of endogenous osmolytes in salt-stressed cultures of Sinorhizobium meliloti. Appl Environ Microbiol. 1997;63:4657–4663. doi: 10.1128/aem.63.12.4657-4663.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talibart R, Jebbar M, Gouesbet G, Himdi-Kabbab S, Wroblewski H, Blanco C, Bernard T. Osmoadaptation in rhizobia: ectoine-induced salt tolerance. J Bacteriol. 1994;176:5210–5217. doi: 10.1128/jb.176.17.5210-5217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trossat C, Nolte K D, Hanson A D. Evidence that the pathway of dimethylsulfoniopropionate biosynthesis begins in the cytosol and ends in the chloroplast. Plant Physiol. 1996;111:965–973. doi: 10.1104/pp.111.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trossat C, Rathinasabapathi B, Hanson A D. Transgenically expressed betaine aldehyde dehydrogenase efficiently catalyzes oxidation of dimethylsulfoniopropionaldehyde and ω-aminoaldehydes. Plant Physiol. 1997;113:1457–1461. doi: 10.1104/pp.113.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Maarel M J E C, Jansen M, Haanstra R, Meijer W G, Hansen T A. Demethylation of dimethylsulfoniopropionate to 3-S-methylmercaptopropionate by marine sulfate-reducing bacteria. Appl Environ Microbiol. 1996;62:3978–3984. doi: 10.1128/aem.62.11.3978-3984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visscher P T, Taylor B F. Demethylation of dimethylsulfoniopropionate to 3-mercaptopropionate by an aerobic marine bacterium. Appl Environ Microbiol. 1994;60:4617–4619. doi: 10.1128/aem.60.12.4617-4619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White R F, Kaplan L, Birnbaum J. Betaine-homocysteine transmethylase in Pseudomonas denitrificans, a vitamin B12 overproducer. J Bacteriol. 1973;113:218–223. doi: 10.1128/jb.113.1.218-223.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zikakis J P, Salsbury R L. Metabolism of sulfur amino acids by rumen microorganisms. J Dairy Sci. 1969;52:2014–2019. doi: 10.3168/jds.S0022-0302(69)86888-8. [DOI] [PubMed] [Google Scholar]