Abstract
Rationale & Objective
Copeptin and Midrange pro-atrial natriuretic peptide (MR-pro-ANP) are associated with outcomes independently of N-terminal pro-brain natriuretic peptide (NT-pro-BNP) in patients with heart failure (HF). The value of these markers in patients with chronic kidney disease (CKD) has not been studied.
Study Design
Prospective cohort study.
Setting & Participants
A total of 4,417 patients enrolled in the German Chronic Kidney Disease (GCKD) study with an estimated glomerular filtration rate of 30-60 mL/min/1.73m2 or overt proteinuria (urinary albumin-creatinine ratio >300mg/g or equivalent).
Exposures
Copeptin, MR-pro-ANP, and NT-pro-BNP levels were measured in baseline samples.
Outcomes
Noncardiovascular death, cardiovascular (CV) death, major adverse CV event (MACE), and hospitalization for HF.
Analytical Approach
HRs for associations of Copeptin, MR-pro-ANP, and NT-pro-BNP with outcomes were estimated using Cox regression analyses adjusted for established risk factors.
Results
During a maximum follow-up of 6.5 years, 413 non-CV deaths, 179 CV deaths, 519 MACE, and 388 hospitalizations for HF were observed. In Cox regression analyses adjusted for established risk factors, each one of the 3 markers were associated with all the 4 outcomes, albeit the highest HRs were found for NT-pro-BNP. When models were extended to include all the 3 markers, NT-pro-BNP remained associated with all 4 outcomes. Conversely, from the 2 novel markers, associations remained only for Copeptin with non-CV death (HR, 1.62; 95% CI, 1.04-2.54 for highest vs lowest quintile) and with hospitalizations for HF (HR, 1.73; 95% CI, 1.08-2.75).
Limitations
Single-point measurements of Copeptin, MR-pro-ANP, and NT-pro-BNP.
Conclusions
In patients with moderately severe CKD, we confirm NT-pro-BNP to be strongly associated with all outcomes examined. As the main finding, the novel marker Copeptin demonstrated independent associations with non-CV death and hospitalizations for HF, and should therefore be evaluated further for risk assessment in CKD.
Plain-Language Summary
A blood sample–based biomarker that indicates high cardiovascular risk in a patient with kidney disease would help to guide interventions and has the potential to improve outcomes. In 4,417 patients of the German Chronic Kidney Disease study, we assessed the relationship of Copeptin, pro-atrial natriuretic peptide, and N-terminal pro-brain natriuretic peptide (NT-pro-BNP) with important outcomes over a follow-up period of 6.5 years. NT-pro-BNP was strongly associated with all of the 4 outcomes, including death unrelated to cardiovascular disease, death because of cardiovascular disease, a major cardiovascular event, and hospitalization for heart failure. Copeptin was associated with death unrelated to cardiovascular disease and hospitalization for heart failure. NT-pro-BNP and Copeptin are, therefore, promising candidates for a blood sample–based strategy to identify patients with kidney disease at high cardiovascular risk.
Index Words: chronic kidney disease, kidney diseases, cardiac diseases, biomarkers, heart failure, Copeptin, MR-pro-ANP, NT-pro-BNP
Chronic kidney disease (CKD) is characterized by a high burden of cardiovascular (CV) complications and is projected to become the fifth leading cause of death in 2040 worldwide.1 A biomarker-based strategy to identify those patients with CKD at greatest risk may help to improve outcomes, eg, by prompting further diagnostic investigations, such as cardiac imaging and by aiding to direct cardioprotective and renoprotective therapies.
Brain natriuretic peptide (BNP) is mainly released from the left ventricle of a diseased heart and causes natriuresis and vasodilation. BNP levels as well as levels of its inactive signaling peptide N-terminal pro-brain natriuretic peptide (NT-pro-BNP) are associated with long-term CV outcomes, perhaps even more strongly in patients with CKD than in those without CKD.2, 3, 4 However, interpretation of BNP and NT-pro-BNP levels in individuals with CKD is hampered by the fact that impaired kidney function can contribute to elevated levels.
Atrial natriuretic peptide (ANP), which promotes natriuresis and vasodilation similar to BNP, is mainly released from the cardiac atrium. It could be argued that this allows for more sensitive detection of structural and/or functional changes of the heart than NT-pro-BNP. An assay has been developed against the middle portion of the peptide (MR-pro-ANP), which has a longer half-life than intact ANP. MR-pro-ANP has already been shown to predict outcomes in patients with heart failure (HF).5
Arginine vasopressin (AVP) is secreted from the posterior pituitary gland in response to high plasma osmolality and in response to hypotension or circulatory underfilling. AVP promotes water reabsorption from the renal collecting duct and contributes to blood pressure (BP) regulation through vasoconstriction. Measurement of AVP is rather challenging due to its short half-life, and has, therefore, been replaced by the measurement of the much more stable Copeptin, released with AVP in a 1:1 fashion from pre-pro-AVP. Copeptin is elevated and predicts outcomes in patients with HF.6,7
To explore the potential of Copeptin and MR-pro-ANP in patients with CKD, we measured these novel markers along with the established marker NT-pro-BNP in baseline samples of the German Chronic Kidney Disease (GCKD) study, a large prospective observational cohort study. We examined the association of the 3 markers with kidney function at baseline, and with non-CV death, CV death, major adverse CV events (MACE), and hospitalization for HF during a maximum observation period of 6.5 years.
Methods
Study Design and Participants
The GCKD study is an ongoing prospective cohort study including 5,217 patients with moderately severe CKD. Details of the study design have been published.8 In brief, individuals aged 18-74 years were eligible if their estimated glomerular filtration rate (eGFR) was between 30 and 60 mL/min/1.73 m2 (stage G3, A1-3) or if their eGFR was > 60 mL/min/1.73 m2 in the presence of overt albuminuria (urinary albumin-creatinine ratio [UACR] > 300 mg/g or equivalent; stage G1-2, A3). Exclusion criteria were non-White ethnicity, solid organ or bone marrow transplantation, active malignancy within 24 months before screening, HF New York Heart Association Stage IV, legal guardianship, or inability to provide informed consent. The GCKD study was registered in a national database for clinical studies (Deutsches Register für Klinische Studien, DRKS 00003971), and was approved by the ethics committees of all participating centers. Written informed consent was obtained from each study participant. Routine laboratory parameters presented in this study were measured in central laboratories as reported previously.9 eGFR was calculated from serum creatinine by the CKD-epidemiology collaboration formula.10 Diabetes was defined by a prescription of antidiabetic medication or HbA1c > 6.5%. Presence of HF at baseline was assessed by medical history, ie, when the patient was aware that HF had been diagnosed previously. Preexisting CV disease (CVD) at baseline was defined as a history of nonfatal myocardial infarction, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty or stroke, and interventions at the carotid arteries (carotid endarterectomy and/or carotid balloon angioplasty, or stent implantation).
Exposure
Copeptin (C-terminal pro-Vasopressin [CT-pro-AVP]) was measured with an immunofluorescent assay in singlets (Kryptor, BRAHMS) with a limit of detection of 0.41 pmol/l and a limit of quantitation of 1.23 pmol/l. An intra-assay coefficient of variation (precision) and an interassay coefficient of variation (repeatability) of <8% and <10%, respectively, for Copeptin levels between 4 and 15 pmol/l, and <4% and <5%, respectively, for levels between 15 and 50 pmol/l have been reported. The 99th percentile in healthy participants was 13.5 pmol/l.11
MR-pro-ANP was measured with an immunofluorescent assay in singlets (Kryptor, BRAHMS), with a limit of detection of 2.1 pg/mL, an intra-assay coefficient of variation (precision) and an interassay coefficient of variation (repeatability) of <2.5% and ≤6.5%, respectively, in concentrations of 20-1000 pmol/l. The 97.5th percentile in healthy participants was 85.2 pg/mL according to the manufacturer's package insert.
NT-pro-BNP was measured with an immunoassay in singlets (Cobas, Roche Diagnostics, performed on an Elecsys 2010 system) with a limit of detection of 5 pg/mL, an intra-assay coefficient of variation (precision) of 4.6% at 44 pg/mL, and an interassay coefficient of variation (repeatability) of 1.9% at 64 pg/mL. The 97.5th percentile in healthy participants aged 55-64 years was 263 pg/mL according to the manufacturer's package insert.
From a total of 5,217 patients enrolled into the GCKD study, biomarker measurements of 475 (9.1%) patients did not satisfy quality control and were excluded. Quality controlled biomarker measurements together with outcome data were available for 4,471 (85.7%) patients. These data form the basis of the current analysis.
The biomarker measurements were performed at the Institute of Clinical Chemistry and Laboratory Medicine at the University Medicine Greifswald, Germany.
Outcome Assessment
Patients are interviewed yearly by trained personnel alternating face-to-face and telephone visits. During these encounters, data on any hospitalizations and clinical events since the last visit are collected in a structured way. This information is subsequently verified by obtaining hospital discharge letters and out-patient letters from the treating physician(s). A trained end point committee composed of up to 4 independent physicians continually extracts outcomes from these reports according to a prespecified end point catalogue.12 Information of deaths is also obtained from death certificates of civil registry offices. For the current analysis, outcomes were analyzed until 6.5 years after the patients’ individual baseline visit. Therefore, the maximum possible censoring time was 6.5 years. The end points analyzed for the current study were as follows: (1) non-CV death, (2) CV death (ie, death from myocardial infarction, coronary artery disease; sudden cardiac death; death because of other cardiac causes including decompensated HF, pulmonary embolism, or cardiac valve disease; death because of a cerebrovascular event), (3) MACE (a composite of nonfatal myocardial infarction or nonfatal ischemic or hemorrhagic stroke, but excluding CV death), and (4) hospitalization for HF.
Statistical Analysis
Copeptin, MR-pro-ANP, and NT-pro-BNP levels measured in the baseline samples were categorized a priori according to quintiles (Q1-Q5). Cox proportional hazards models were used to examine the associations of the 3 markers with non-CV death, CV death, MACE, and hospitalization for HF during follow-up. The supremum test, including a correction for multiple comparisons by the Bonferroni-Holm method, was used to examine the proportional hazards assumption.13 If patients did not complete the 6.5 year follow-up period, censoring was done at the time of the last follow-up, ie, when participants left the study (did not want to participate any more in follow-up visits) or were lost to follow-up. The hazard estimates obtained from our models were cause-specific because patients were censored at death if it was not part of the outcome of interest.
The resulting hazard ratios (HRs) are presented with 95% confidence intervals (CI). For each of the outcomes, we first examined univariable models with Copeptin, MR-pro-ANP, and NT-pro-BNP alone, and then models only adjusted for age and gender (model A). Subsequent multivariable models were additionally adjusted for risk factors selected based on clinical expertise of the authors, including age, gender, body mass index (BMI), systolic BP, low-density lipoprotein (LDL) cholesterol, diabetes mellitus, smoking, high-sensitive C-reactive protein (hs-CRP), eGFR, UACR, preexisting CVD, history of HF, CV medication (ie, use of statins, renin angiotensin inhibitors (RASi), antiplatelet agents, β-blockers, and mineralocorticoid receptor antagonists), and study site as a possible confounder (model B). The “final” multivariable models additionally included the other 2 biomarkers (model C). Results from overall Cox regression analyses are visualized by plotting cumulative incidence functions for each of the analyzed outcomes (derived from model A using the Aalen-Johansen estimator).14
All data were collected and managed using Askimed as a cloud-based web platform (https://www.askimed.com). Data extraction from Askimed was performed in February 2021. To this timepoint, 311 (5.9%) participants had prematurely left the study, and 188 (2.3%) participants had been lost to follow-up. All available data during their active participation, however, were used for this analysis. Two patients withdrew their study consent prohibiting use of any data collected. The data file for the subgroup analyses is provided in Item S1.
Statistical analyses were performed with SAS 9.4 2002-2012 by SAS Institute Inc.
Results
Distribution of Copeptin, MR-pro-ANP, and NT-pro-BNP Concentrations and Relationships with Clinical Parameters
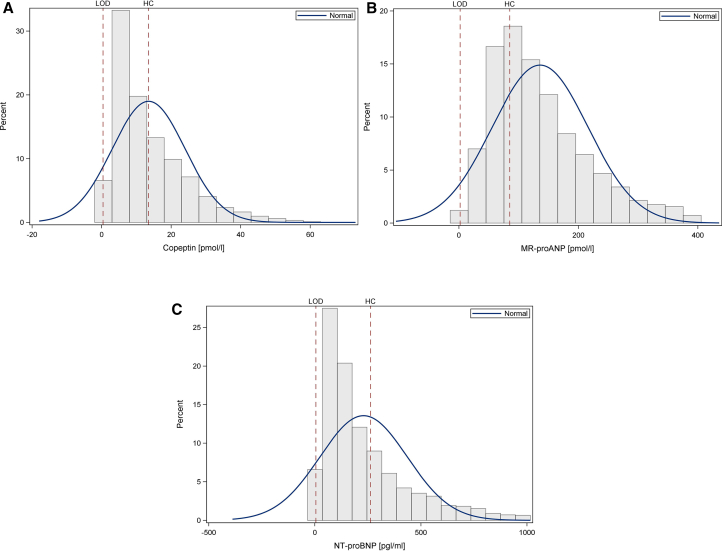
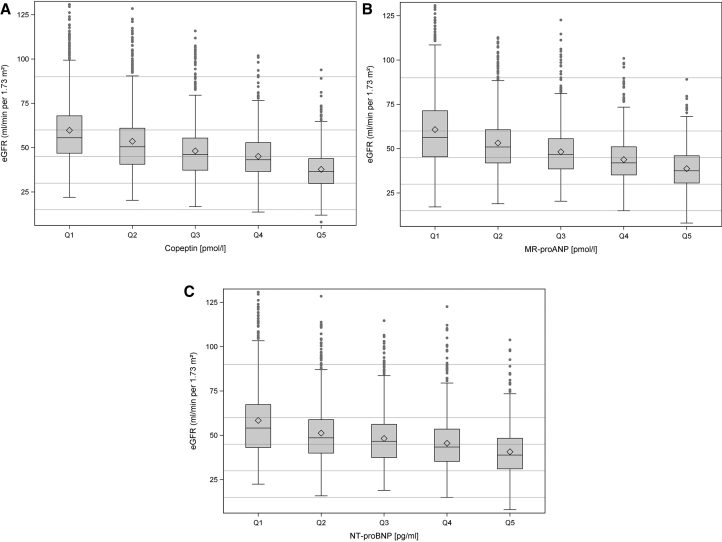
Fig 1A shows the distribution of Copeptin, MR-pro-ANP (Fig 1B), and NT-pro-BNP (Fig 1C) serum concentrations, respectively, together with information on limits of detection and cut-offs in healthy participants. For Copeptin, 39.2% of values were above the 99th percentile of healthy participants. For MR-pro-ANP, 70.6% of values were >97.5th percentile, and it was 38.0% for NT-pro-BNP. Demographics, clinical characteristics, and laboratory parameters according to Copeptin levels are shown in Table 1. The respective data for MR-pro-ANP and NT-pro-BNP quintiles are shown in Tables S1 and S2. Fig 2 shows eGFR values according to Copeptin (Fig 2A), MR-pro-ANP (Fig 2B), and NT-pro-BNP (Fig 2C) quintiles.
Figure 1.
(A) shows the distribution of Copeptin levels (limit of detection [LOD] = 0.41 pmol/l, 99th percentile cut-off in healthy individuals = 13.5 pmol/l), (B) shows the distribution of MR-pro-ANP levels (LOD = 2.1 pmol/l, 97.5th percentile cut-off in healthy individuals = 85.2 pmol/l), and (C) shows the distribution of NT-pro-BNP levels (LOD = 5 pg/mL, 97.5th percentile cut-off in healthy individuals = 263 pg/mL).
Table 1.
Demographics and Clinical Parameters at Baseline Stratified by Copeptin Quintiles
| Copeptin (pmol/l) | ||||||
|---|---|---|---|---|---|---|
| Total Cohort | Quintile 1 (≤4.7) | Quintile 2 (>4.7 - ≤8.1) | Quintile 3 (>8.1 - ≤13.2) | Quintile 4 (>13.2 - ≤21.4) | Quintile 5 (>21.4) | |
| N (%) | 4417 | 853 (19.3%) | 879 (19.9%) | 889 (20.1%) | 896 (20.3%) | 900 (20.4%) |
| Demographics | ||||||
| Age (y) | 61 ± 12 | 59.0 ± 12.3 | 59.7 ± 12.4 | 61.2 ± 10.8 | 61.7 ± 10.5 | 62.0 ± 11.3 |
| Male | 2,681 (61%) | 295 (35%) | 483 (55%) | 566 (64%) | 662 (74%) | 675 (75%) |
| Regional Center | ||||||
| AA – Aachen | 446 (10%) | 72 (8%) | 94 (11%) | 81 (9%) | 102 (11%) | 97 (11%) |
| BE – Berlin | 372 (8%) | 67 (7%) | 59 (7%) | 68 (8%) | 89 (10%) | 89 (10%) |
| ER – Erlangen | 801 (18%) | 160 (19%) | 173 (19%) | 174 (19%) | 149 (17%) | 145 (16%) |
| FR – Freiburg | 300 (7%) | 50 (6%) | 57 (6%) | 72 (8%) | 56 (6%) | 65 (7%) |
| HA – Hannover | 376 (9%) | 89 (10%) | 85 (10%) | 59 (7%) | 60 (7%) | 83 (8%) |
| HE – Heidelberg | 404 (9%) | 90 (11%) | 76 (9%) | 76 (9%) | 89 (10%) | 73 (8%) |
| JE – Jena | 575 (13%) | 107 (13%) | 96 (11%) | 126 (14%) | 126 (14%) | 120 (13%) |
| MÜ – München | 425 (10%) | 99 (12%) | 91 (10%) | 89 (10%) | 74 (8%) | 72 (8%) |
| WÜ – Würzburg | 718 (16%) | 119 (14%) | 148 (17%) | 144 (16%) | 151 (17%) | 156 (17%) |
| Laboratory Measures | ||||||
| eGFR (mL/min per 1.73 m2) | 49 ± 17 | 59.8 ± 19.2 | 53.6 ± 18.6 | 48.2 ± 15.5 | 45.1 ± 12.6 | 37.8 ± 11.1 |
| UACR (mg/g) | 47 (9-366) | 18.4 (6.8-160.0) | 37.6 (7.1-312.2) | 45.3 (9.0-356.8) | 64.8 (12.5-402.9) | 96.9 (17.7-614.9) |
| hs-CRP (mg/dL) | 2 (1-5) | 1.8 (0.8-3.9) | 2.1 (0.9-5.0) | 2.3 (1.1-5.0) | 2.6 (1.3-5.2) | 2.8 (1.3-6.6) |
| LDL cholesterol (mg/dL) | 113 (88-143) | 121.2 (96.4-147.8) | 116.6 (92.3-143.0) | 113.5 (87.1-143.8) | 109.2 (83.7-140.6) | 104.0 (82.4-135.8) |
| HDL cholesterol (mg/dL) | 48 (39-60) | 56.0 (45.2-67.6) | 49.5 (40.8-62.3) | 47.9 (39.6-60.4) | 45.5 (3687-55.7) | 44.1 (36.6-54.0) |
| CVD Risk Factors | ||||||
| Systolic BP (mm Hg) | 140 ± 21 | 137.6 ± 19.5 | 139.9 ± 19.9 | 140.4 ± 20.7 | 141.2 ± 20.4 | 139.8 ± 21.5 |
| Diastolic BP (mm Hg) | 79 ± 12 | 79.8 ± 10.8 | 79.9 ± 11.1 | 79.5 ± 12.1 | 78.9 ± 12.0 | 77.4 ± 12.7 |
| BMI (kg/m2) | 30 ± 6 | 28.2 ± 5.1 | 29.5 ± 5.7 | 30.4 ± 6.1 | 30.8 ± 5.9 | 31.0 ± 6.5 |
| Diabetes | 1,645 (37%) | 169 (20%) | 275 (31%) | 344 (39%) | 400 (45%) | 457 (51%) |
| Previous CVD | 1,180 (27%) | 143 (17%) | 206 (23%) | 252 (28%) | 256 (29%) | 323 (36%) |
| Heart Failure | 836 (19%) | 107 (13%) | 142 (16%) | 152 (17%) | 198 (22%) | 237 (26%) |
| Smoking history | ||||||
| never | 1,813 (41%) | 439 (51%) | 375 (43%) | 344 (39%) | 331 (37%) | 324 (36%) |
| former | 1,918 (44%) | 297 (35%) | 383 (44%) | 396 (45%) | 417 (47%) | 425 (47%) |
| current | 673 (15%) | 116 (14%) | 117 (13%) | 146 (16%) | 144 (16%) | 150 (17%) |
| Medication Use | ||||||
| RASi | 3,713 (84%) | 661 (77%) | 729 (83%) | 765 (86%) | 777 (87%) | 781 (87%) |
| Statins | 2,155 (49%) | 334 (39%) | 412 (47%) | 437 (49%) | 463 (52%) | 509 (57%) |
| Antiplatelet agents | 1,564 (36%) | 247 (29%) | 292 (33%) | 344 (39%) | 329 (37%) | 352 (39%) |
| Beta-blockers | 2,480 (57%) | 389 (46%) | 448 (51%) | 502 (56%) | 537 (60%) | 604 (67%) |
| MRA | 364 (8%) | 33 (4%) | 75 (9%) | 63 (7%) | 88 (10%) | 105 (12%) |
Note: Continuous variables are presented as mean (standard deviation) or median (interquartile range). Categorical variables are presented as number (percentage) in the overall study population.
Abbreviations: eGFR, estimated glomerular filtration rate; UACR, urinary albumin excretion rate; hs-CRP, high-sensitive C-reactive protein; LDL, low-density lipoprotein; HDL, high density lipoprotein; BP, blood pressure; BMI, body mass index; CVD, cardiovascular disease; RASi, renin angiotensin system inhibitor; MRA, mineralocorticoid receptor antagonist.
Figure 2.
Estimated glomerular filtration rate (eGFR) values according to quintiles of Copeptin (A), MR-pro-ANP (B), and NT-pro-BNP (C).
Associations of Copeptin, MR-pro-ANP and NT-pro-BNP with Outcomes
During a maximum follow-up of 6.5 years, 413 (%) non-CV deaths, 179 (%) CV deaths, 519 (%) MACE, and 388 (%) hospitalizations for HF occurred. In Cox regression, the supremum test did not indicate any deviation from the proportional hazards assumption (results not shown). Table 2 shows the HRs for the associations of quintiles of Copeptin, MR-pro-ANP, and NT-pro-BNP with the outcomes (lowest quintile as reference). We first entered either Copeptin, MR-pro-ANP, or NT-pro-BNP into the unadjusted models, then into age and gender adjusted model (model A), and then into the adjusted model (model B). For all the 4 outcomes studied, the HRs for Copeptin and MR-pro-ANP were lower than those for NT-pro-BNP.
Table 2.
Association of Copeptin, MR-pro-ANP and NT-pro-BNP with Outcomes
| Separate Models |
Model incl. Copeptin + MR-pro-ANP + NT-pro-BNP |
|||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted Model Aa | Adjusted Model Bb | Final Model Cb | |||
| Noncardiovascular Death, 413/4417 | ||||||
| Copeptin | ||||||
| HR per SDc increase | 1.31 [1.25-1.38] | 1.31 [1.24-1.39] | 1.19 [1.08-1.32] | 1.10 [1.00-1.22] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 1.40 [0.91-2.17] | 1.27 [0.82-1.96] | 1.02 [0.64-1.64] | 0.97 [0.60-1.55] | ||
| Q3 | 2.45 [1.65-3.63] | 2.10 [1.40-3.13] | 1.58 [1.02-2.44] | 1.45 [0.94-2.23] | ||
| Q4 | 2.86 [1.95-4.21] | 2.35 [1.58-3.49] | 1.53 [0.98-2.37] | 1.36 [0.88-2.11] | ||
| Q5 | 4.63 [3.20-6.69] | 3.70 [2.54-5.41] | 2.01 [1.30-3.14] | 1.62 [1.04-2.54] | ||
| MR-pro-ANP | ||||||
| HR per SDd increase | 1.63 [1.55-1.73] | 1.51 [1.42-1.60] | 1.41 [1.30-1.52] | 1.40 [1.24-1.57] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 1.14 [0.71-1.82] | 0.98 [0.62-1.58] | 0.87 [0.54-1.42] | 0.72 [0.44-1.18] | ||
| Q3 | 1.93 [1.26-2.95] | 1.45 [0.94-2.23] | 1.14 [0.72-1.80] | 0.75 [0.46-1.23] | ||
| Q4 | 2.81 [1.88-4.20] | 1.91 [1.26-2.89] | 1.47 [0.94-2.28] | 0.82 [0.50-1.34] | ||
| Q5 | 6.76 [4.65-9.84] | 4.20 [2.84-6.20] | 2.72 [1.76-4.20] | 1.08 [0.64-1.79] | ||
| NT-pro-BNP | ||||||
| HR per SDe increase | 1.22 [1.19-1.26] | 1.2 [1.16-1.24] | 1.14 [1.08-1.19] | 0.99 [0.92-1.07] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 1.94 [1.16-3.25] | 1.67 [1.00-2.80] | 1.80 [1.02-3.16] | 1.94 [1.08-3.48] | ||
| Q3 | 2.53 [1.55-4.15] | 2.06 [1.24-3.39] | 2.11 [1.22-3.66] | 2.24 [1.22-4.08] | ||
| Q4 | 4.46 [2.81-7.09] | 3.35 [2.10-5.38] | 2.99 [1.76-5.07] | 2.92 [1.60-5.31] | ||
| Q5 | 11.31 [7.28-17.58] | 7.59 [4.82-11.96] | 5.73 [3.38-9.68] | 4.78 [2.58-8.87] | ||
| Cardiovascular Death, 179/4417 | ||||||
| Copeptin | ||||||
| HR per SDb increase | 1.38 [1.30-1.48] | 1.39 [1.28-1.49] | 1.29 [1.12-1.48] | 1.14 [0.98-1.31] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 2.75 [1.22-6.17] | 2.29 [1.02-5.17] | 1.31 [0.56-3.02] | 1.11 [0.48-2.59] | ||
| Q3 | 3.24 [1.47-7.15] | 2.49 [1.12-5.54] | 1.31 [0.58-2.98] | 1.21 [0.52-2.76] | ||
| Q4 | 5.11 [2.39-10.92] | 3.68 [1.70-7.95] | 1.94 [0.88-4.30] | 1.60 [0.72-3.57] | ||
| Q5 | 11.55 [5.59-23.87] | 8.08 [3.86-16.87] | 2.91 [1.32-6.44] | 2.10 [0.94-4.71] | ||
| MR-pro-ANP | ||||||
| HR per SDc increase | 1.83 [1.70-1.96] | 1.68 [1.56-1.82] | 1.54 [1.38-1.71] | 1.33 [1.14-1.55] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 1.34 [0.60-3.03] | 1.17 [0.52-2.65] | 1.14 [0.50-2.57] | 1.09 [0.48-2.51] | ||
| Q3 | 2.12 [1.01-4.49] | 1.62 [0.76-3.44] | 1.26 [0.60-2.71] | 1.07 [0.46-2.46] | ||
| Q4 | 2.84 [1.38-5.83] | 1.92 [0.92-3.99] | 1.13 [0.52-2.42] | 0.72 [0.30-1.68] | ||
| Q5 | 12.10 [6.32-23.16] | 7.30 [3.74-14.24] | 3.70 [1.82-7.51] | 1.22 [0.54-2.80] | ||
| NT-pro-BNP | ||||||
| HR per SDd increase | 1.29 [1.25-1.33] | 1.28 [1.24-1.32] | 1.25 [1.18-1.32] | 1.12 [1.03-1.23] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 1.36 [0.57-3.24] | 1.19 [0.50-2.85] | 0.98 [0.40-2.35] | 0.99 [0.40-2.47] | ||
| Q3 | 1.13 [0.46-2.78] | 0.94 [0.38-2.34] | 0.66 [0.26-1.67] | 0.69 [0.26-1.87] | ||
| Q4 | 3.30 [1.56-6.98] | 2.56 [1.20-5.48] | 1.44 [0.66-3.18] | 1.53 [0.62-3.76] | ||
| Q5 | 16.73 [8.49-32.95] | 11.26 [5.60-22.69] | 4.99 [2.36-10.53] | 4.25 [1.70-10.55] | ||
| Major Adverse Cardiovascular Event, 519/4417 | ||||||
| Copeptin | ||||||
| HR per SDb increase | 1.27 [1.21-1.34] | 1.24 [1.16-1.32] | 1.10 [1.00-1.21] | 1.04 [0.96-1.15] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 1.52 [1.08-2.14] | 1.31 [0.94-1.85] | 1.05 [0.74-1.51] | 1.01 [0.70-1.46] | ||
| Q3 | 1.62 [1.15-2.26] | 1.29 [0.92-1.82] | 0.90 [0.62-1.29] | 0.87 [0.60-1.26] | ||
| Q4 | 2.41 [1.75-3.30] | 1.81 [1.30-2.51] | 1.28 [0.90-1.83] | 1.21 [0.86-1.73] | ||
| Q5 | 3.46 [2.55-4.69] | 2.54 [1.86-3.48] | 1.39 [0.96-2.01] | 1.23 [0.86-1.79] | ||
| MR-pro-ANP | ||||||
| HR per SDc increase | 1.53 [1.44-1.62] | 1.4 [1.32-1.49] | 1.25 [1.16-1.36] | 1.11 [1.00-1.24] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 1.01 [0.70-1.45] | 0.91 [0.62-1.31] | 0.80 [0.54-1.17] | 0.77 [0.52-1.13] | ||
| Q3 | 1.78 [1.28-2.47] | 1.44 [1.04-2.02] | 1.15 [0.80-1.63] | 0.99 [0.68-1.46] | ||
| Q4 | 2.06 [1.50-2.85] | 1.51 [1.08-2.11] | 1.00 [0.70-1.43] | 0.72 [0.48-1.08] | ||
| Q5 | 4.21 [3.12-5.68] | 2.83 [2.08-3.88] | 1.66 [1.16-2.36] | 0.87 [0.56-1.34] | ||
| NT-pro-BNP | ||||||
| HR per SDd increase | 1.25 [1.22-1.29] | 1.23 [1.2-1.27] | 1.17 [1.12-1.23] | 1.12 [1.04-1.20] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 1.38 [0.94-2.03] | 1.27 [0.86-1.87] | 1.08 [0.72-1.60] | 1.10 [0.72-1.67] | ||
| Q3 | 1.62 [1.12-2.35] | 1.45 [1.00-2.11] | 1.09 [0.74-1.61] | 1.14 [0.74-1.76] | ||
| Q4 | 2.93 [2.09-4.11] | 2.45 [1.72-3.47] | 1.61 [1.10-2.33] | 1.71 [1.12-2.62] | ||
| Q5 | 6.07 [4.41-8.36] | 4.56 [3.26-6.36] | 2.56 [1.76-3.72] | 2.58 [1.62-4.10] | ||
| Hospitalization for Heart Failure, 388/4417 | ||||||
| Copeptin | ||||||
| HR per SDb increase | 1.33 [1.27-1.40] | 1.35 [1.28-1.43] | 1.3 [1.18-1.43] | 1.12 [1.02-1.24] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 1.53 [0.97-2.41] | 1.42 [0.90-2.25] | 1.06 [0.66-1.72] | 0.96 [0.60-1.56] | ||
| Q3 | 2.53 [1.67-3.83] | 2.27 [1.48-3.47] | 1.47 [0.94-2.32] | 1.27 [0.80-2.01] | ||
| Q4 | 2.99 [1.99-4.51] | 2.60 [1.72-3.95] | 1.56 [0.98-2.46] | 1.29 [0.82-2.05] | ||
| Q5 | 5.53 [3.75-8.16] | 4.72 [3.18-7.02] | 2.36 [1.50-3.74] | 1.73 [1.08-2.75] | ||
| MR-pro-ANP | ||||||
| HR per SDc increase | 1.81 [1.71-1.92] | 1.68 [1.58-1.79] | 1.58 [1.46-1.71] | 1.33 [1.18-1.49] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 1.25 [0.70-2.24] | 1.08 [0.60-1.93] | 1.23 [0.68-2.25] | 1.03 [0.56-1.91] | ||
| Q3 | 3.01 [1.81-4.98] | 2.25 [1.36-3.76] | 2.08 [1.20-3.60] | 1.22 [0.68-2.20] | ||
| Q4 | 4.56 [2.81-7.40] | 3.11 [1.90-5.09] | 2.74 [1.60-4.69] | 1.20 [0.66-2.14] | ||
| Q5 | 12.34 [7.78-19.56] | 7.77 [4.84-12.48] | 6.03 [3.54-10.28] | 1.51 [0.84-2.75] | ||
| NT-pro-BNP | ||||||
| HR per SDd increase | 1.40 [1.35-1.45] | 1.38 [1.32-1.42] | 1.32 [1.26-1.37] | 1.16 [1.08-1.25] | ||
| Q1 | 1 (ref.) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Q2 | 2.78 [1.35-5.75] | 2.40 [1.16-4.97] | 1.87 [0.90-3.94] | 1.74 [0.80-3.76] | ||
| Q3 | 4.03 [2.01-8.07] | 3.30 [1.64-6.63] | 2.58 [1.26-5.26] | 2.23 [1.04-4.80] | ||
| Q4 | 10.06 [5.24-19.31] | 7.66 [3.96-14.81] | 5.21 [2.64-10.25] | 4.27 [2.02-8.99] | ||
| Q5 | 31.69 [16.81-59.74] | 21.96 [11.52-41.82] | 12.72 [6.5-24.88] | 9.03 [4.22-19.33] | ||
Note: Results are presented as hazard ratios with 95% confidence intervals given in parentheses.
Abbreviations: MR-pro-ANP, Midrange pro-atrial natriuretic peptide; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; SD, standard deviation; HR, hazard ratio; eGFR, estimated glomerular filtration rate; UACR, urinary albumin excretion rate; hs-CRP, high-sensitive C-reactive protein; LDL, low-density lipoprotein; HDL, high density lipoprotein; BP, blood pressure; BMI, body mass index; CVD, cardiovascular disease; RASi, renin angiotensin system inhibitor; MRA, mineralocorticoid receptor antagonist.
Model A: all models adjusted for age and gender.
Model B: All models adjusted for age, gender, BMI, systolic BP, LDL cholesterol, diabetes, eGFR, UACR, smoking, hs-CRP, preexisting CVD, history of heart failure, CV medication (ie, use of statins, RASi, antiplatelet agents, beta-blockers, and MRA) and regional center.
SD for Copeptin 11.7 pmol/l (whole population).
SD for MR-pro-ANP 109.04 pmol/l (whole population).
SD for NT-pro-BNP 1073.4 pg/mL (whole population).
Information on the HRs for all included variables of models A and B are provided in supplementary Table S3 for Copeptin, in Table S4 for MR-pro-ANP, and in Table S5 for NT-pro-BNP. As an example, Table S3 shows that in the extensively adjusted model B for Copeptin, we found that for the outcome non-CV death, independent associations were found for age, previous diagnosis of HF, diabetes, smoking, and statin use. For the outcome CV death, independent associations were found for age, gender, previous diagnosis of HF, diabetes, previous CVD, and use of MR antagonists. For the outcome MACE, independent associations were found for age, gender, diabetes, previous CVD, and use of antiplatelet agents. For the outcome hospitalizations for HF, independent associations were found for age, previous diagnosis of heart failure, BMI, diabetes, previous CVD, and use of beta-blockers.
Fig S1 shows the cumulative incidence functions of non-CV death (panel A), CV death (B), MACE (C), and hospitalizations for HF (D) for quintiles of Copeptin. Similar graphs were constructed for quintiles of MR-pro-ANP (Fig S2) and for quintiles of NT-pro-BNP (Fig S3). In agreement with the results of the Cox regression analyses, the estimated incidence proportion increased more steeply with increasing NT-pro-BNP levels compared with increasing Copeptin or MR-pro-ANP levels for all outcomes studied.
When all the 3 markers were entered simultaneously into the final adjusted models (last column in Table 2), NT-pro-BNP remained associated with all of the outcomes. From the 2 novel markers, only for Copeptin remained an association of the highest versus lowest quintile with non-CV death and hospitalizations for HF.
Discussion
In this large prospective study of patients with moderately severe CKD, we found the levels of Copeptin, MR-pro-ANP, and NT-pro-BNP strongly associated with kidney function. Thus, in this regard, there does not seem to be any advantage for the novel markers Copeptin and MR-pro-ANP as compared with NT-pro-BNP.
Further, all the 3 markers were associated with non-CV death, CV death, MACE, and hospitalizations for HF when considered individually. This was even the fact when the models were adjusted for a wide spectrum of established risk factors. When all the 3 markers were considered together in the models, only NT-pro-BNP remained significant for each end point. This confirms previous reports that NT-pro-BNP is strongly associated with outcomes in patients with CKD.2, 3, 4 From the 2 novel markers, Copeptin remained independently associated with non-CV death and hospitalizations for HF.
The observed association of Copeptin with non-CV events may be explained by detection of non-cardiac causes of circulatory ”underfilling,” such as liver disease. Notably, stress has been demonstrated to cause release of AVP or Copeptin,15 and, therefore, either stress itself, or medical conditions related with stress, could have contributed to the association of Copeptin with non-CV events. However, HF is not only associated with CV events, but also with an increased risk of non-CV events.16 Therefore, the detection of HF may have also contributed to the observed association between Copeptin and non-CV event rates. Finally, the independence of the association of Copeptin with hospitalizations for HF from MR-pro-ANP and NT-pro-BNP may be explained by the differential mechanisms that cause release of Copeptin (mainly circulatory underfilling) versus those that cause release of natriuretic peptides (atrial and ventricular “overfilling”).
A previous study has demonstrated an association of Copeptin levels with medium-term mortality in patients with CKD and coronary artery disease.17 Further, in patients with CKD including those on hemodialysis, Copeptin levels were shown to be associated with CV and other outcomes.18,19 However, natriuretic peptides were not measured in any of those studies. Our current data suggest that Copeptin levels might be useful for risk assessment in patients with CKD even independently of natriuretic peptides. Patients identified to be at high risk by such a biomarker-based strategy could benefit from further diagnostic investigations and from novel therapies including sodium-dependent glucose transporter-2 inhibitors or nonsteroidal mineralocorticoid receptor antagonists (MRA), which have shown to provide improved protection from kidney and CV events.20, 21, 22
Our study has several limitations and strengths. Limitations include the availability of biomarker measurements only from the baseline examination and that our findings may not be generalizable to other ethnicities. Further, we report only on associations. As a next step, prognostic studies should be performed, ideally including independent cohorts for validation. Particular strengths are the large sample size, the ability to adjust for a large number of relevant risk factors, and the central adjudication of outcomes.
In conclusion, we showed that Copeptin, MR-pro-ANP, and NT-pro-BNP are all associated with outcomes in patients with CKD, even when analyses are adjusted extensively for other risk factors. NT-pro-BNP outperformed the 2 other markers for all end points investigated. However, Copeptin demonstrated associations with non-CV death and hospitalization for HF independently of NT-pro-BNP levels, and should therefore be evaluated further for its value in the risk assessment of patients with CKD.
Article Information
German Chronic Kidney Disease (GCKD) Study Investigators
University of Erlangen-Nürnberg: Kai-Uwe Eckardt, Heike Meiselbach, Markus P. Schneider, Mario Schiffer, Hans-Ulrich Prokosch, Barbara Bärthlein, Andreas Beck, André Reis, Arif B. Ekici, Susanne Becker, Dinah Becker-Grosspitsch, Ulrike Alberth-Schmidt, Birgit Hausknecht, Anke Weigel; University of Freiburg: Gerd Walz, Anna Köttgen, Ulla T. Schultheiß, Fruzsina Kotsis, Simone Meder, Erna Mitsch, Ursula Reinhard; RWTH Aachen University: Jürgen Floege, Turgay Saritas; Charité, University Medicine Berlin: Elke Schaeffner, Seema Baid-Agrawal, Kerstin Theisen; Hannover Medical School: Hermann Haller, Jan Menne; University of Heidelberg: Martin Zeier, Claudia Sommerer, Johanna Theilinger; University of Jena: Gunter Wolf, Martin Busch, Rainer Paul; Ludwig-Maximilians University of München: Thomas Sitter; University of Würzburg: Christoph Wanner, Vera Krane, Antje Börner-Klein, Britta Bauer; Medical University of Innsbruck, Institute of Genetic Epidemiology: Florian Kronenberg, Julia Raschenberger, Barbara Kollerits, Lukas Forer, Sebastian Schönherr, Hansi Weissensteiner; University of Regensburg, Institute of Functional Genomics: Peter Oefner, Wolfram Gronwald; and University of Bonn, Institute of Medical Biometry, Informatics and Epidemiology, Medical Faculty: Matthias Schmid, Jennifer Nadal.
Authors’ Full Names and Academic Degrees
Markus P. Schneider, MD, Matthias Schmid, PhD, Jennifer Nadal, Vera Krane, MD, Turgay Saritas, MD, Martin Busch, MD, Ulla T. Schultheiss, MD, Heike Meiselbach, PhD, Nele Friedrich, MD, Matthias Nauck, MD, Jürgen Floege, MD, Florian Kronenberg, MD, Christoph Wanner, MD, and Kai-Uwe Eckardt, MD, on behalf of the GCKD study investigators
Authors’ Contributions
Research idea and study design: MPS, KUE; data acquisition: MPS, VK, TS, MB, UTS, HM, NF, MN, JF, FK, CW; data analysis/interpretation: MPS, MS, JN, KUE; statistical analysis: MS, JN; supervision or mentorship: MPS, KUE. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
The authors would like to gratefully acknowledge the financial support from BRAHMS (Hennigsdorf, Germany) for the Copeptin and MR-proBNP measurements, and from Roche (Basel, Switzerland) for the NT-proBNP measurements. The GCKD study is supported by the German Ministry of Education and Research (Bundesministerium für Bildung und Forschung, FKZ 01ER 0804, 01ER 0818, 01ER 0819, 01ER 0820, and 01ER 0821) and the KfH Foundation for Preventive Medicine (Kuratorium für Heimdialyse und Nierentransplantation e.V. – Stiftung Präventivmedizin), and corporate sponsors (www.gckd.org). The funders had no role in study design, data collection, analysis, reporting or in the decision to submit for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received January 28, 2023. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form July 9, 2023.
Footnotes
Complete author and article information provided before references.
Figure S1: Cumulative incidence of non-CV death (panel A), CV death (panel B), MACE (panel C), and hospitalizations for HF (panel D) according to Copeptin level quintile
Figure S2: Cumulative incidence of non-CV death (panel A), CV death (panel B), MACE (panel C), and hospitalizations for HF (panel D) according to MR-pro-ANP level quintile
Figure S3: Cumulative incidence of non-CV death (panel A), CV death (panel B), MACE (panel C), and hospitalizations for HF (panel D) according to NT-pro-BNP level quintile
Table S1: Demographics and Clinical Parameters at Baseline Stratified by MR-pro-ANP Quintiles.
Table S2: Demographics and Clinical Parameters at Baseline Stratified by NT-pro-BNP Quintiles.
Table S3: Model A and Model B for Copeptin (showing all included variables).
Table S4: Model A and Model B for MR-pro-ANP (showing all included variables).
Table S5: Model A and Model B for NT-pro-BNP (showing all included variables).
Contributor Information
Markus P. Schneider, Email: Markus.Schneider@uk-erlangen.de.
GCKD study investigators:
Kai-Uwe Eckardt, Heike Meiselbach, Mario Schiffer, Hans-Ulrich Prokosch, Barbara Bärthlein, Andreas Beck, André Reis, Arif B. Ekici, Susanne Becker, Dinah Becker-Grosspitsch, Ulrike Alberth-Schmidt, Birgit Hausknecht, Anke Weigel, Gerd Walz, Anna Köttgen, Ulla T. Schultheiß, Fruzsina Kotsis, Simone Meder, Erna Mitsch, Ursula Reinhard, Jürgen Floege, Turgay Saritas, Elke Schaeffner, Seema Baid-Agrawal, Kerstin Theisen, Hermann Haller, Jan Menne, Martin Zeier, Claudia Sommerer, Johanna Theilinger, Gunter Wolf, Martin Busch, Rainer Paul, Thomas Sitter, Christoph Wanner, Vera Krane, Antje Börner-Klein, Britta Bauer, Florian Kronenberg, Julia Raschenberger, Barbara Kollerits, Lukas Forer, Sebastian Schönherr, Hansi Weissensteiner, Peter Oefner, Wolfram Gronwald, Matthias Schmid, and Jennifer Nadal
Supplementary Material
Figure S1-S3; Table S1-S5.
Item S1
References
- 1.Foreman K.J., Marquez N., Dolgert A., et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392(10159):2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregg L.P., Adams-Huet B., Li X., et al. Effect modification of chronic kidney disease on the association of circulating and imaging cardiac biomarkers with outcomes. J Am Heart Assoc. 2017;6(7) doi: 10.1161/JAHA.116.005235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Kimmenade R.R., Januzzi J.L., Jr., Baggish A.L., et al. Amino-terminal pro-brain natriuretic peptide, renal function, and outcomes in acute heart failure: redefining the cardiorenal interaction? J Am Coll Cardiol. 2006;48(8):1621–1627. doi: 10.1016/j.jacc.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 4.Matsushita K., Sang Y., Ballew S.H., et al. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: the Atherosclerosis Risk in Communities study. Arterioscler Thromb vasc Biol. 2014;34(8):1770–1777. doi: 10.1161/ATVBAHA.114.303465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Haehling S., Jankowska E.A., Morgenthaler N.G., et al. Comparison of midregional pro-atrial natriuretic peptide with N-terminal pro-B-type natriuretic peptide in predicting survival in patients with chronic heart failure. J Am Coll Cardiol. 2007;50(20):1973–1980. doi: 10.1016/j.jacc.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Balling L., Kistorp C., Schou M., et al. Plasma copeptin levels and prediction of outcome in heart failure outpatients: relation to hyponatremia and loop diuretic doses. J Card Fail. 2012;18(5):351–358. doi: 10.1016/j.cardfail.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Dungen H.D., Tscholl V., Obradovic D., et al. Prognostic performance of serial in-hospital measurements of copeptin and multiple novel biomarkers among patients with worsening heart failure: results from the MOLITOR study. ESC Heart Fail. 2018;5(2):288–296. doi: 10.1002/ehf2.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckardt K.U., Barthlein B., Baid-Agrawal S., et al. The German chronic kidney disease (GCKD) study: design and methods. Nephrol Dial Transplant. 2012;27(4):1454–1460. doi: 10.1093/ndt/gfr456. [DOI] [PubMed] [Google Scholar]
- 9.Titze S., Schmid M., Kottgen A., et al. Disease burden and risk profile in referred patients with moderate chronic kidney disease: composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant. 2015;30(3):441–451. doi: 10.1093/ndt/gfu294. [DOI] [PubMed] [Google Scholar]
- 10.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. doi:10.7326%2F0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgenthaler N.G., Struck J., Alonso C., Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112–119. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 12.Steinbrenner I., Schultheiss U.T., Kotsis F., et al. Urine metabolite levels, adverse kidney outcomes, and mortality in CKD patients: a metabolome-wide association study. Am J Kidney Dis. 2021;78(5):669–677. doi: 10.1053/j.ajkd.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Lin D.Y., Wei L.J., Ying Z. Model-checking techniques based on cumulative residuals. Biometrics. 2002;58(1):1–12. doi: 10.1111/j.0006-341x.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 14.Beyersmann J., Scheike T.H. CRC Press; 2014. Classical regression models for competing risks. Handbook of Survival Analysis; pp. 157–178. [Google Scholar]
- 15.Siegenthaler J., Walti C., Urwyler S.A., Schuetz P., Christ-Crain M. Copeptin concentrations during psychological stress: the PsyCo study. Eur J Endocrinol. 2014;171(6):737–742. doi: 10.1530/EJE-14-0405. [DOI] [PubMed] [Google Scholar]
- 16.Parveen S., Zareini B., Arulmurugananthavadivel A., et al. Association between early detected heart failure stages and future cardiovascular and non-cardiovascular events in the elderly (Copenhagen Heart Failure Risk Study) BMC Geriatr. 2022;22(1):230. doi: 10.1186/s12877-022-02875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelbertz C., Brand E., Fobker M., Fischer D., Pavenstadt H., Reinecke H. Elevated copeptin is a prognostic factor for mortality even in patients with renal dysfunction. Int J Cardiol. 2016;221:327–332. doi: 10.1016/j.ijcard.2016.07.058. [DOI] [PubMed] [Google Scholar]
- 18.Fenske W., Wanner C., Allolio B., et al. Copeptin levels associate with cardiovascular events in patients with ESRD and type 2 diabetes mellitus. J Am Soc Nephrol. 2011;22(4):782–790. doi: 10.1681/ASN.2010070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krane V., Genser B., Kleber M.E., et al. Copeptin associates with cause-specific mortality in patients with impaired renal function: results from the LURIC and the 4D study. Clin Chem. 2017;63(5):997–1007. doi: 10.1373/clinchem.2016.266254. [DOI] [PubMed] [Google Scholar]
- 20.Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 21.The E.-K.C.G., Herrington W.G., Staplin N., et al. Empagliflozin in atients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakris G.L., Agarwal R., Anker S.D., et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1-S3; Table S1-S5.
Item S1