ABSTRACT
Healthcare systems worldwide are currently undergoing significant transformations in response to increasing costs, a shortage of healthcare professionals and the growing complexity of medical needs among the population. Value-based healthcare reimbursement systems are emerging as an attempt to incentivize patient-centricity and cost containment. From a technological perspective, the transition to digitalized services is intended to support these transformations. A Health Information System (HIS) is a technological solution designed to govern the data flow generated and consumed by healthcare professionals and administrative staff during the delivery of healthcare services. However, the exponential growth of digital capabilities and applied advanced analytics has expanded their traditional functionalities and brought the promise of automating administrative procedures and simple repetitive tasks, while enhancing the efficiency and outcomes of healthcare services by incorporating decision support tools for clinical management. The future of HIS is headed towards modular architectures that can facilitate implementation and adaptation to different environments and systems, as well as the integration of various tools, such as artificial intelligence (AI) models, in a seamless way. As an example, we present the experience and future developments of the European Clinical Database (EuCliD®). EuCliD is a multilingual HIS used by 20 000 nurses and physicians on a daily basis to manage 105 000 patients treated in 1100 clinics in 43 different countries. EuCliD encompasses patients’ follow-up, automatic reporting and mobile applications while enabling efficient management of clinical processes. It is also designed to incorporate multiagent systems to automate repetitive tasks, AI modules and advanced dynamic dashboards.
Keywords: artificial intelligence in healthcare, clinical decision support, digitalized healthcare, health information system, value-based healthcare
INTRODUCTION
The need for new paradigms in healthcare services
Healthcare systems in developed countries are facing unprecedented challenges due to the ageing of the population, and increasing prevalence of multi-comorbidity and chronic diseases, coupled with public expectation of more personalized care [1]. In parallel, healthcare spending has constantly risen well beyond inflation rates in most countries, and such increase was mainly driven by the rising complexity of the delivered services, rather than the medication cost [2, 3]. Furthermore, the healthcare workforce is shrinking worldwide, and the shortage of physicians and nurses is a challenge for the sustainability of the health sector in most developed countries [4, 5]. As the gap between doctors’ supply and service demand increases, the quality of care may drop due to a corresponding increase in error rate in clinical decisions [6]. The coronavirus disease 2019 (COVID-19) pandemic has further exacerbated such trends worldwide.
As a response to the increasing pressures on healthcare systems, healthcare organizations are transitioning from a provider-centric, episodic, centralized care model, towards integrated systems in which patients and healthcare staff interact even remotely, or asynchronously access digital healthcare services. Furthermore, emerging value-based funding systems incentivize patient-centricity and integrated care pathways while containing costs by remunerating measurable patients’ health outcomes rather than the volume of service provided. Value-based schemes rely on the systematic collection of data on health outcomes and promote efficient healthcare governance policies (https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0558). Digital technologies integrated in modern Health Information Systems (HIS) are the cornerstone of such transformations.
The new role of health information systems in the digital transformation of healthcare
European (https://digital-strategy.ec.europa.eu/en/policies/ehealth) and national (https://www.espon.eu/sites/default/files/attachments/1_Reponen_0.pdf) institutions have launched several initiatives to promote and regulate the digital transformation in the sector. Many institutions are maintaining clinical databases that enable efficient and accurate access to data. However, such systems are not typically designed to support healthcare governance, administrative workflows and appropriateness of care [7].
Modern HIS, exploiting new advancements in digital technology and artificial intelligence (AI), will be the cornerstone of new ways of delivering healthcare that are constructed around the concepts of patient-centricity, population-health management, decentralization and automation [8].
Patient-centricity relates to the principles of patient autonomy, self-determination and shared-decision-making. To this end, digital solutions will make health education for healthcare professional [9] and patients [10], self-care tools [11], structured collection of patient-reported outcomes [12] and telemedicine [13] affordable and widely available.
Population-health management is the process of improving health outcomes for a specific group of people by analyzing and managing their health-related data to identify and address risks and disparities within the population. Risk stratification tools, integrated into HIS, will be the cornerstone of population-health management approaches and will enable tailored, intensified interventions targeting high-risk patients in several therapeutic areas [14].
Decentralization in health systems entails the process of care delivery by distributed services. Over the last 50 years, healthcare has seen rapid decentralization across several domains. Many dialysis clinics have moved from inpatient to outpatient service facilities, as well as surgeries and primary services, which are now being delivered at retail locations. Digital services will further expand the availability and equity of healthcare while reducing waiting times and cost [15, 16]. Most recently, the COVID-19 pandemic has boosted decentralized services: as patients adhered to stay-at-home and social-distancing policies, facility-based visit volume has dropped precipitously [17]. In comparison, telehealth consultations have risen, even in sectors apparently more resistant to distant-care pathways, such as psychotherapy and mental health services [18].
Finally, new digital services may enable automation in healthcare by integrating AI modules. Although the most popular benefit of automation is probably the implementation of medical decision support systems [19], automation can help in many other tasks, such as data entry and knowledge abstraction from literature [20, 21], scheduling [22], billing [23], repetitive tasks in clinical workflows [24], data security or dashboard analytics [25], to name a few. However, among the many challenges to a wider uptake of AI-based algorithms in clinical practice [26–28], the lack of integration into HIS is one of the most prominent: modern health information system will be required to enable easy integration of digital applications based on AI and advanced analytics capabilities [29, 30].
EuCliD®, THE EUROPEAN CLINICAL DATABASE
Development and evolution of a comprehensive health information system for the management of patients with chronic kidney disease
EuCliD is the acronym for the European Clinical Database [31]: it was designed just as a multilingual and fully codified database to record and manage patients’ data collected by healthcare professionals while delivering healthcare in clinics belonging to the Fresenius Medical Care (FME) network [32]. In 2004, a new version of EuCliD was developed as an online web application. EuCliD functions were expanded to support all main clinical and administrative processes in the clinics. The tool was deployed with the two-fold objective of meeting data and process needs at a local level (i.e. daily use by clinical staff) and providing global data harmonization [33]. To this end, EuCliD was based on an medical ontology specifically devised to capture concepts and information flows relevant for end-stage kidney disease patients. EuCliD web is now implemented in roughly 1100 clinics, in 43 different countries in Europe, Middle East, Africa, Latin America and Asia-Pacific. In 2019, more than 100 000 active patients were managed with EuCliD by over 20 000 nurses and physicians daily. It collects millions of datapoints per day and provides real-time records of patients and dialysis machine data from each dialysis session, with automatic import of laboratory test results.
Main processes of EuCliD
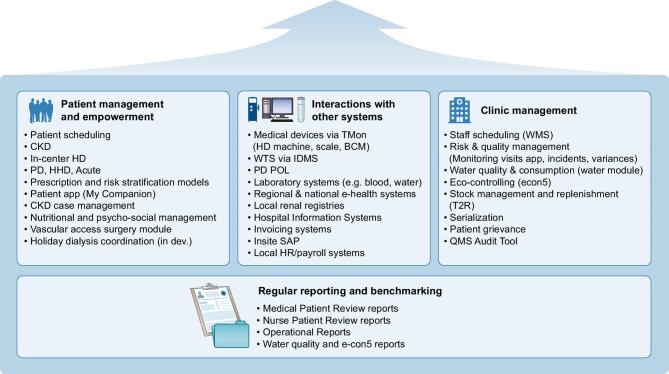
EuCliD is an HIS supporting most clinical and administrative processes for nephrology clinics. It encompasses sections optimized to support healthcare delivery and documentation for pre-dialysis care, vascular access management, medication management, referrals and diagnostic services, and dialysis administration, as well as administrative task modules (see Fig. 1). The latter include, for example, staff scheduling, risk analysis, quality and auditing, water quality and consumption, or stock management.
Figure 1:
Content structure and non-exhaustive summary of sections included in EuCliD.
EuCliD is connected with several devices such as dialysis machines, body composition monitors [34], and laboratory providers for automatic import of biochemical essay results. Such interfaces are used to acquire data and to expedite clinical workflows by enabling the healthcare professionals to interact with medical devices, monitor treatment in real time and manage their prescription through EuCliD. Data can automatically feed local billing systems and be exported to national registries and hospital systems.
Once clinical information is acquired, EuCliD generates customized clinical reports to support the management of a specific patient, an entire unit or even for the whole country network through the use of an integrated query builder that allows custom extraction of patient or clinical data according to specific requirements. The quality improvement program of the NephroCare FME dialysis network [35] relies heavily on EuCliD reporting system for all performance management activities based on key performance indicators (KPIs) [36].
The FME balanced scorecard (BSC) is one of such approaches implemented for continuous quality control of dialysis clinics. The BSC integrates a number of KPIs and it is generated on a monthly basis. The concept of BSC is not new [37], and it is a well-accepted methodology in the healthcare sector [38–41]. The FME BSC has been a key tool for quality improvement and continuous performance amelioration that has resulted in enhancement of care delivery over time [42]. Furthermore, by applying machine learning techniques to BSC data, it was possible to discover clusters of clinics with similar care pathways and reveal insights about temporal trajectories across these clusters [38, 39]. Such advanced analytics capabilities made it possible to assist clinicians and medical governance in ameliorating care pathways across the network.
Since September 2014, the FME EMEA NephroCare clinics network has introduced a new continuous quality improvement (CQI) policy called Medical Patient Review (MPR) based on the evaluation of 10 KPIs related to patients’ clinical status. MPR-CQI is a process of evaluation, planning and action aimed at improving patients’ clinical outcomes based on periodical audits carried out by the Chief Clinical Officer and Medical Directors in all clinics. At the operational level, the attending physician makes use of structured reports to identify, prioritize and address patients’ medical needs and to help facilitate initiation of appropriate medical actions for each individual patient. We have recently shown that improvement in KPIs occurring after the MPR-CQI policy implementation was associated with a 30% reduction in mortality risk. Such results were robust to adjustment for potential unmodifiable confounding factors [35]. Pizzarelli and Basile noted that CQI through MPR nudges physicians to perform better on quality metrics. Therefore, they suggested that these results should convince the nephrological community to implement quality improvement programs based on the systematic collection, retrieval and analysis of solid parameters whose correlation with hard clinical outcomes is well established [43]. The MPR program has been recently expanded by the introduction of an advanced benchmarking system based on 21 AI-based disease models which provides evidence to support a quality enhancement process based on rationale, realistic improvement expectation in modifiable intermediate endpoints. The advanced benchmarking system is built around a core Bayesian Network model predicting mortality risk 2 two years based on the 21 modifiable key performance indicators of the MPR and 5 non-modifiable factors (i.e. age, sex, Charlson's comorbidity index, body mass index and dialysis vintage). The core model is used to weight the importance of each modifiable factors on mortality risk and provide priority ratings to specific remediation actions for each KPI based on the observed target achievement rates. The core mortality risk model is complemented with 21 patient-level probabilistic models assessing the likelihood of target achievement given the 5 non-modifiable factors. Each of these models is then used to suggest reasonable improvement expectations based on the difference between observed target achievement rate and the predicted achievement rate given the case-mix of each center. The advanced benchmarking tool is used to prioritize potential areas of improvement in underperforming centers while highlighting good practices in overperforming centers. This new tool is currently being piloted in two European countries and results on target achievement and mortality risk reduction will be available in 2 years.
Furthermore, EuCliD hosts AI modules that provide health information and insight to healthcare professionals thus supporting their clinical decision-making. One notable example is the Anemia Control Module (ACM), a certified medical device based on artificial neural networks that suggests the optimal erythropoietin-stimulating agent and iron dosage based on clinical parameters directly abstracted from EuCliD. Currently, ACM is used in over 100 clinics in the NephroCare network and provided over 80 000 dosing suggestions during 2021. Use of ACM has been shown to strongly improve hemoglobin target achievement and reduce drug utilization by 25% to 50% [27, 29]. New AI modules, such as the Cardiovascular Literature-Based Risk Algorithm (CALIBRA [44]), the Prognostic Reasoning System for CKD Progression (PROGRES-CKD [45]) and the Arteriovenous Fistula Failure model (FFM [46]) to name a few, have been more recently developed using EuCliD data and are currently under evaluation before further clinical implementation.
It is worth noting that EuCliD, as an HIS, does not have a direct impact on health outcomes. However, EuCliD has made significant contributions to the field of nephrology since its deployment over 20 years ago, leading to numerous scientific publications. Furthermore EuCliD data contributed to the Monitoring Dialysis Outcomes (MONDO) cohort and the Recognizing Excellence and Optimizing Outcomes (ARO) I and II cohorts, two large multinational research initiatives [47, 48]. EuCliD is now available for scientific collaborations, commercial agreements with industry partners and epidemiological research by independent researchers through various data services and collaboration agreements. These services include advanced statistical analysis capabilities and the DataRoom, which is a fully anonymized and user-friendly tool. The DataRoom enables the extraction of data and facilitates complex analyses in a timely and straightforward manner. By utilizing the web interface, researchers can access datasets suitable for clinical studies or AI model development without directly sharing the data with the end-user. Subsequently, an analytical layer operates on the fully anonymized extracted dataset to produce statistical inferences and modeling studies.
Data privacy and security
Data privacy and cybersecurity are two central aspects to be considered when managing patient data. To address these needs, the design paradigm of EuCliD is developed according to security and implements General Data Protection Regulation requirements [49]. Data are encrypted and personal information like names or addresses are visible only by the personnel of the clinic. To protect EuCliD from threats like cyber-attacks or data exfoliation, several tools constantly monitor the systems and periodical penetration tests are performed.
THE NEAR-FUTURE EVOLUTION OF EuCliD
EuCliD has been successfully supporting FME clinics tool for almost 20 years, enhancing the original clinical database to a new paradigm that has improved clinical outcomes and increased efficiency [27, 29, 35]. In order to leverage on the latest available cloud technologies, and improve performances and user experience, a fully modular and scalable EuCliD application has been developed. The new EuCliD version will support the concepts of patient-centricity, automation and decentralization even further. EuCliD will encompass the full patient's journey from chronic kidney disease to transplant, providing a consistent user experience throughout all therapy pathways and disease transitions. Additionally, the new EuCliD is designed to connect to external HIS by the use of standardized ontologies (i.e. FIHR, International Classification of Diseases-11 coding, Anatomical Therapeutic Chemical, and other international coding standards), a crucial aspect to enable care coordination along the full patient journey even when treatment is performed outside the FME network of clinics.
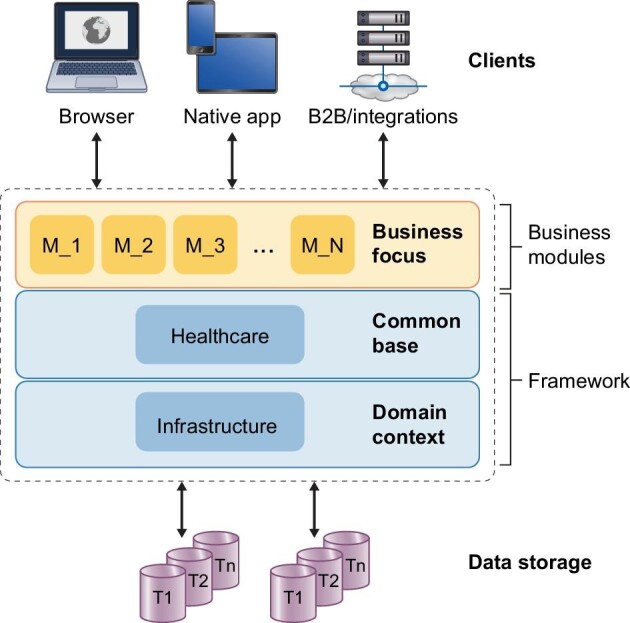
The new version of EuCliD has been designed to enable a native integration of AI-based solutions, by means of a modular architecture based on microservices. The different microservices are independent from an architectural point of view and they have the potential to become stand-alone applications. As a consequence, only the modules that are necessary for each specific clinical or administrative task can be triggered, thus increasing system efficiency. Importantly, the modular microservice-based architecture is cloud-based and needs no server infrastructure, which considerably lowers costs and infrastructural investments by the customer.
Patient-centricity is achieved by integrating different apps and channels for specific user groups, enabling a smoother user interconnectedness and delivery of digital services tailored to the needs of nurses, physicians, technicians and patients. A renewed user interface and applications optimized for tablet and smartphone use will enable bedside operations which facilitate medical decision-making while fostering patient–provider interactions. Since 2021, we introduced a new application enabling planned and momentary collection of electronic patients-reported outcomes (ePRO) at the point of care or at the patient’s home. The ePRO measure application is meant to be general and it enables the administration of any closed-answer questionnaire. Once ePRO measure are collected they are automatically recorded in the medical record of the patient and are used to inform medical decision-making and foster patient–physician communication. The application enables clinicians to select the most appropriate ePRO measure, assess results in real-time through a dedicated scoring dashboard, plan corrective actions and administer health education modules to the patients. The application is optimized to work with tablet or smartphone. In the future we will embed new functionalities allowing doctors plan treat-to-target or N-of-1 trial to personalize symptoms management, automated questionnaire selections based on patients profiling and computer adaptive testing based on item response theory.
One important innovation introduced in the new EuCliD version is an integrated multiagent platform [50], which enables development and deployment of live smart agents with the aim of enabling process automation, digital twins in real-time applications or for emulation purposes, such as in silico clinical trials or policy simulations. Smart agents refer to software that is specifically created to optimize, automate or assist doctors in clinical or administrative tasks. In healthcare, smart agents can be used to help with tasks such as patient monitoring, billing, appointment scheduling, medication management and population health management. These agents can be combined in a multi-agent network to represent intricate systems and can be utilized to simulate policies, evaluate how complex organizations respond to external shocks, or assess the potential impact of population health management strategies [50]. Multi-agent systems have the ability to simulate and map complex behavioral patterns and can be used to automate operations as well. The first EuCliD application of the multiagent platform is an appointment management tool, where agents, representing patients, doctors and clinic resources, autonomously look for an optimal scheduling of the clinic appointments (Fig. 2). The multiagent approach can also play a role in supporting users by automatizing and coordinating processes, deploying AI and identifying events which may trigger a given process, e.g. medical alerts. One of the most attractive characteristics of the agents is that they can learn not only their own task but also enhanced collective tasks, cooperating with other agents by means of different learning approaches, particularly, reinforcement learning [51].
Figure 2:
Example of a multiagent application within an HIS. Multiagent systems embedded in an HIS for the optimization of medical appointment and scheduling. The system automatically generates appointment proposal and generate visit schedule based on patient flow where digital agents, representing patients, doctors and clinic resources, autonomously look for an optimal scheduling of the clinic appointments.
CONCLUSIONS
Healthcare systems in developed countries are facing unprecedented challenges due to increasing costs, expanded demand for personalized services, raising clinical complexity and shortage of healthcare professionals. In order to respond to such pressures, public institutions and private healthcare providers are transitioning from a provider-centric, episodic, centralized care model, towards integrated systems in which patients and healthcare staff interact even remotely or asynchronously access digital healthcare services.
This review has presented the successful experience of EuCliD. On top of being a multilingual database able to work and adapt to the singularities of different locations, EuCliD allows monitoring and managing clinical processes in a straightforward way. This is reflected in the production of automatic reports and patients’ follow-up and the integration of AI-based tools that allow for fast prescription suggestions, automatic risk stratification or smart scheduling. The use of EuCliD has yielded improved clinical outcomes, an increased efficiency and a better user experience. This has been objectively measured in BSCs based on KPIs.
Despite large benefits, the wider and consistent adoption of complex HIS also depends on the quality of the user's experience, the impact on clinical workflows and the improvement in the general efficiency of the clinical operations in the clinic. EuCliD is not different from any other digital tool having the ambition to serve the needs of clinicians and patients—its adjustment to users’ needs and demands is a continuous, never-ending journey of adaptation and amelioration aimed at optimizing the human–software interaction.
PERSPECTIVE
Modern HIS will play a pivotal role in supporting the evolving healthcare governance, administrative and clinical workflows, and appropriateness of care. Through the experience of EuCliD we outlined the historical evolution of clinical databases into HIS and their potential future application incorporating advanced digital technologies and AI to promote patient-centricity, population-health management, automation and decentralization of healthcare.
The new EuCliD will be completely modular and cloud-based, thus allowing an easy integration of different tools, e.g. those based on AI and multiagents, and avoiding the need to set up servers on-site. This approach is fully focused on the final users, with specific apps for doctors, nurses and patients.
ACKNOWLEDGEMENTS
Authors thank John Larkin (GMO—Clinical Advanced Analytics, FMC-NA) for his thoughtful comments and revision.
Contributor Information
Carlo Barbieri, Global Digital Transformation and Innovation, Clinical Digital Center of Excellence, Fresenius Medical Care, Crema Italy.
Luca Neri, Global Medical Office, Clinical Advanced Analytics, Fresenius Medical Care, Crema Italy.
Stefano Stuard, Global Medical Office, Clinical and Therapeutic Governance, Fresenius Medical Care, Naples, Italy.
Flavio Mari, Global Digital Transformation and Innovation, Clinical Digital Center of Excellence, Fresenius Medical Care, Crema Italy.
José D Martín-Guerrero, Intelligent Data Analysis Laboratory, Department of Electronic Engineering, ETSE -UV, Universitat de València, Valencia, Spain.
CONFLICT OF INTEREST STATEMENT
C.B., L.N. and S.S. are employees at Fresenius Medical Care. J.D.M.-G. received research grants from Fresenius Medical Care in the past 10 years.
DATA AVAILABILITY STATEMENT
No new data were generated or analyzed in support of this research.
REFERENCES
- 1. Hadas L, Refael B, Tomer S.. Personalized health systems—past, present, and future of research development and implementation in real-life environment. Front Med 2019;6:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. OECD Chart: Health Spending, Total/Government/Compulsory/Voluntary, US Dollars/Capita, Annual, 2021. https://data.oecd.org/chart/6Oxi (19 July 2023, date last accessed). [Google Scholar]
- 3. Hajat C, Siegal Y, Adler-Waxman A.. Clustering and healthcare costs with multiple chronic conditions in a US study. Front Public Health 2021;8:607528. 10.3389/fpubh.2020.607528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murphy GT, Birch S, MacKenzie Aet al. . Simulating future supply of and requirements for human resources for health in high-income OECD countries. Hum Resour Health 2016;14:77. 10.1186/s12960-016-0168-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical radiology UK workforce census report. The Royal College of Radiologists, 2018, London, UK. https://www.rcr.ac.uk/clinical-radiology/rcr-clinical-radiology-census-report-2021/detailed-census-data-clinical-radiology (19 July 2023, date last accessed). [Google Scholar]
- 6. Sokolovskaya E, Shinde T, Ruchman RBet al. . The effect of faster reporting speed for imaging studies on the number of misses and interpretation errors: a pilot study. J Am Coll Radiol 2015;12:683–8. 10.1016/j.jacr.2015.03.040 [DOI] [PubMed] [Google Scholar]
- 7. Nørgaard M, Johnsen SP.. How can the research potential of the clinical quality databases be maximized? The Danish experience. J Intern Med 2016;279:132–40. 10.1111/joim.12437 [DOI] [PubMed] [Google Scholar]
- 8. Gopal G, Suter-Crazzolara C, Toldo Let al. . Digital transformation in healthcare – architectures of present and future information technologies. Clin Chem Lab Med 2019;57:328–35. 10.1515/cclm-2018-0658 [DOI] [PubMed] [Google Scholar]
- 9.Tudor Car L, Soong A, Kyaw BMet al. . Health professions digital education on clinical practice guidelines: a systematic review by Digital Health Education collaboration. BMC Med 2019;17:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rowland SP, Fitzgerald JE, Holme Tet al. . What is the clinical value of mHealth for patients? npj Digit Med 2020;3:4. 10.1038/s41746-019-0206-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karami Salaheddin Kola M, Jafari H, Charati JYet al. . Comparing the effects of teach-back method, multimedia and blended training on self-care and social support in patients with heart failure: a randomized clinical trial. J Educ Health Promot 2021;30:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schick-Makaroff K, Tate K, Molzahn A.. Use of electronic patient reported outcomes in clinical nephrology practice: a qualitative pilot study. Can J Kidney Health Dis 2019;30:2054358119879451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Augestad KM, Sneve AM, Lindsetmo R-O.. Telemedicine in postoperative follow-up of STOMa PAtients: a randomized clinical trial (the STOMPA trial). Br J Surg 2020;107:509–18. 10.1002/bjs.11491 [DOI] [PubMed] [Google Scholar]
- 14. Golas SB, Nikolova-Simons M, Palacholla Ret al. . Predictive analytics and tailored interventions improve clinical outcomes in older adults: a randomized controlled trial. npj Digit Med 2021;4:97. 10.1038/s41746-021-00463-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reddy TS, Kunwar A, Durgad Ket al. . Decentralization of India Hypertension Control Initiative services to maintain continuum of care for hypertensive patients during COVID-19 pandemic in Telangana. WHO South East Asia J Public Health 2021;10:49–58. [Google Scholar]
- 16. Panda B, Thakur HP.. Decentralization and health system performance – a focused review of dimensions, difficulties, and derivatives in India. BMC Health Serv Res 2016;16:561. 10.1186/s12913-016-1784-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arsenault-Lapierre G, Henein M, Gaid Det al. . Hospital-at-home interventions vs in-hospital stay for patients with chronic disease who present to the emergency department: a systematic review and meta-analysis. JAMA Netw Open 2021;4:e2111568. 10.1001/jamanetworkopen.2021.11568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharma A, Sasser T, Schoenfelder Gonzalez Eet al. . Implementation of home-based telemental health in a large child psychiatry department during the COVID-19 crisis. J Child Adolesc Psychopharmacol 2020;30:404–13. 10.1089/cap.2020.0062 [DOI] [PubMed] [Google Scholar]
- 19. Sutton RT, Pincock D, Baumgart DCet al. . An overview of clinical decision support systems: benefits, risks, and strategies for success. npj Digit Med 2020;3:17. 10.1038/s41746-020-0221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chowdhary KR (ed.). Natural language processing. In: Fundamentals of Artificial Intelligence. New Delhi, India: Springer, 2020. [Google Scholar]
- 21. Kaspar M, Fette G, Hanke Met al. . Automated provision of clinical routine data for a complex clinical follow-up study: a data warehouse solution. Health Inform J 2022;28:1–17. 10.1177/14604582211058081 [DOI] [PubMed] [Google Scholar]
- 22. Vega C, Gawron P, Lebioda Jet al. . Smart Scheduling (SMASCH): multi-appointment scheduling system for longitudinal clinical research studies. JAMIA Open 2022;5:ooac038. 10.1093/jamiaopen/ooac038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmed A, Abdullah U, Sawar MJ.. Software architecture of a learning apprentice system in medical billing. Proceedings of the World Congress on Engineering 2010, Vol I WCE 2010, London, UK. [Google Scholar]
- 24. Rosati S, Tralli A, Balestra G.. A multi-agent system for monitoring patient flow. Stud Health Technol Inform 2013;192:944. [PubMed] [Google Scholar]
- 25. Guidi G, Pettenati MC, Miniati Ret al. . Heart failure analysis dashboard for patient's remote monitoring combining multiple artificial intelligence technologies. Annu Int Conf IEEE Eng Med Biol Soc 2012;2012:2210–3. [DOI] [PubMed] [Google Scholar]
- 26. Varghese J. Artificial intelligence in medicine: chances and challenges for wide clinical adoption. Visc Med 2020;36:443–9. 10.1159/000511930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barbieri C, Molina M, Ponce Pet al. . An international observational study suggests that artificial intelligence for clinical decision support optimizes anemia management in hemodialysis patients. Kidney Int 2016;90:422–9. 10.1016/j.kint.2016.03.036 [DOI] [PubMed] [Google Scholar]
- 28. Stranieri A, Venkatraman S, Minicz Jet al. . Emerging point of care devices and artificial intelligence: prospects and challenges for public health. Smart Health 2022;24:100279. 10.1016/j.smhl.2022.100279 [DOI] [Google Scholar]
- 29. Bucalo ML, Barbieri C, Roca Set al. . The anaemia control model: does it help nephrologists in therapeutic decision-making in the management of anaemia? Nefrologia (Engl Ed) 2018;38:491–502. 10.1016/j.nefroe.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 30. Halamka J, Cerrato P.. Understanding the role of digital platfroms in technology readiness. Regen Med 2021;16:207–13. 10.2217/rme-2020-0135 [DOI] [PubMed] [Google Scholar]
- 31. Marcelli D, Kirchgessner J, Amato Cet al. . EuCliD (European Clinical Database): a database comparing different realities. J Nephrol 2001;14:S94–100. [PubMed] [Google Scholar]
- 32. Steil H, Amato C, Carioni Cet al. . EuCliD–a medical registry. Methods Inf Med 2004;43:83–8. [PubMed] [Google Scholar]
- 33. Richards N, Ayala JA, Cesare Set al. . Assessment of quality guidelines implementation using a continuous quality improvement programme. Blood Purif 2007;25:221–8. 10.1159/000101026 [DOI] [PubMed] [Google Scholar]
- 34. Borga M, West J, Bell JDet al. . Advanced body composition assessment: from body mass index to body composition profiling. J Investig Med 2018;66:887–95. 10.1136/jim-2018-000722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garbelli M, Ion Titapiccolo J, Bellocchio Fet al. . Prolonged patient survival after implementation of a continuous quality improvement programme empowered by digital transformation in a large dialysis network. Nephrol Dial Transplant 2022;37:469–76. 10.1093/ndt/gfab160 [DOI] [PubMed] [Google Scholar]
- 36. Stopper A, Amato C, Gioberge Set al. . Managing complexity at dialysis service centers across Europe. Blood Purif 2007;25:77–89. 10.1159/000096402 [DOI] [PubMed] [Google Scholar]
- 37. Kaplan RS, Norton DP.. The balanced scorecard—measures that drive performance. Harvard Bus Rev 1992;70:71–9. [PubMed] [Google Scholar]
- 38. Cattinelli I, Bolzoni E, Barbieri Cet al. . Use of self-organizing maps for balanced scorecard analysis to monitor the performance of dialysis clinic chains. Health Care Manag Sci 2012;15:79–90. 10.1007/s10729-011-9183-6 [DOI] [PubMed] [Google Scholar]
- 39. Martín-Guerrero JD, Marcelli D, Soria-Olivas Eet al. . Self-organising maps: a new way to screen the level of satisfaction of dialysis patients. Expert Syst Appl 2012;39:8793–8. 10.1016/j.eswa.2012.02.001 [DOI] [Google Scholar]
- 40. Inamdar N, Kaplan RS, Bower M.. Applying the balanced scorecard in healthcare provider organizations. J Healthc Manag 2002;47:195–6. [PubMed] [Google Scholar]
- 41. Zelman WN, Pink GH, Matthias CB.. Use of the balanced scorecard in health care. J Health Care Finance 2003;29:1–16. [PubMed] [Google Scholar]
- 42. Stopper A, Raddatz A, Grassmann Aet al. . Delivering quality of care while managing the interests of all stakeholders. Blood Purif 2011;32:323–30. 10.1159/000333829 [DOI] [PubMed] [Google Scholar]
- 43. Pizzarelli F, Basile C.. Do we have to rely on metric-based quality improvement strategies for the management of ESKD? Nephrol Dial Transplant 2022;37:397–9. 10.1093/ndt/gfab201 [DOI] [PubMed] [Google Scholar]
- 44. Neri L, Lonati C, Titapiccolo JIet al. . The cardiovascular literature-based risk algorithm (CALIBRA): predicting cardiovascular events in patients with non-dialysis dependent chronic kidney disease. Front Nephrol 2022;2:922251. 10.3389/fneph.2022.922251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bellocchio F, Lonati C, Ion Titapiccolo Jet al. . Validation of a novel predictive algorithm for kidney failure in patients suffering from chronic kidney disease: the Prognostic Reasoning System for Chronic Kidney Disease (PROGRES-CKD). Int J Environ Res Public Health 2021;18:12649. 10.3390/ijerph182312649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peralta R, Garbelli M, Bellocchio Fet al. . Development and validation of a machine learning model predicting arteriovenous fistula failure in a large network of dialysis clinics. Int J Environ Res Public Health 2021;18:12355. 10.3390/ijerph182312355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Usvyat LA, Haviv YS, Etter Met al. . The monitoring dialysis outcomes (MONDO) initiative. Blood Purif 2013;35:37–48. 10.1159/000345179 [DOI] [PubMed] [Google Scholar]
- 48. De Francisco ALM, Kim J, Anker SDet al. . An epidemiological study of hemodialysis patients based on the European Fresenius Medical Care hemodialysis network: results of the ARO study. Nephron Clin Pract 2010;118:c143–54. 10.1159/000319936 [DOI] [PubMed] [Google Scholar]
- 49. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation). [Google Scholar]
- 50. Wooldridge M. An Introduction to Multiagent Systems, 2nd edition. Chichester, UK: John Wiley & Sons Ltd, 2009. [Google Scholar]
- 51. Sutton RS, Barto AG.. Reinforment Learning: An Introduction, 2nd edition. Cambridge, MA:The MIT Press, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.