ABSTRACT
Background
Dialysis patients have been maintaining a high rate of cardiovascular morbidity and mortality. For this reason, it is to introduce necessary new technical advances in clinical practice. There is a relation between toxins retention and inflammation, mortality and morbidity. Medium cut-off (MCO) membranes are a new generation of membranes that allow the removal of a greater number of medium-sized molecules compared with high-flux hemodialysis (HF-HD), but retaining albumin. MCO membranes have an increased permeability and the presence of internal filtration. Because of these special properties, MCO generated a new concept of therapy called expanded HD (HDx). Until now, online hemodiafiltration (OL-HDF) has demonstrated its superiority, in terms of survival, compared with HF-HD. However, the comparison between OL-HDF and HDx remains an unsolved question.
Methods
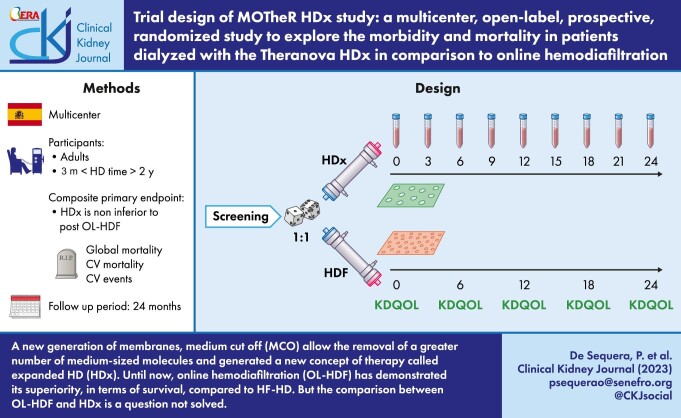
The MOTheR HDx study trial (NCT03714386) is an open-label, multicenter, prospective, 1:1 randomized, parallel-group trial designed to evaluate the efficacy and safety of HDx compared with OL-HDF in patients treated for dialysis in Spain for up to 36 months. The main endpoint is to determinate whether HDx is non inferior to OL-HDF at reducing the combined outcome of all-cause death and stroke (ischemic or hemorrhagic), acute coronary syndrome (angina and myocardial infarction), peripheral arterial disease (amputation or revascularization) and ischemic colitis (mesenteric thrombosis).
Results
The trial has already started.
Keywords: cardiovascular events, expanded hemodialysis (HDx), medium cut-off membranes (MCO), mortality, online hemodiafiltration (OL-HDF)
Graphical Abstract
Graphical Abstract.
INTRODUCTION
One of the primary functions of dialysis is the elimination of uremic toxins (UT). The kidney is the model to follow, capable of purifying continuously all types of UT without the elimination of albumin. Dialysis is far from emulating these functions, although the capability of blood purification has improved over the years.
Knowledge of UTs has also advanced in the last decades [1, 2]; now we know that their retention is associated with cardiovascular risk [3–5], the main cause of mortality in dialysis patients.
High rates of cardiovascular morbidity and mortality are maintained despite the fact that in recent years important technical advances have been made in dialysis therapies, and considerable effort has been made to minimize cardiovascular risk factors present in this group of patients [6–8]. Importantly, studies [9] have found associations between levels of large middle-molecule UTs and immune dysfunction and inflammation, as well as adverse outcomes. UTs are usually classified according to their molecular weight (MW) and whether they are bound to proteins, mainly albumin [1, 2]. Recently, some authors proposed to separate the middle-size UT into two groups: molecules of medium and high MW [10], setting the limit of these at 15 000 Da, according to different authors. Innovation in hemodialysis (HD) techniques and the introduction of new HD membranes allow the elimination of a greater number of medium-sized molecules as compared with conventional HD.
Although the comparative efficacy of hemodiafiltration (HDF) vs HD remains unproven for some authors [11], most randomized trials like On line Hemodiafiltration Study (ESHOL) have demonstrated the superiority, in terms of survival, of online HDF (OL-HDF) patients as compared with high-flux HD (HF-HD) and low-flux HD [12, 13]. Although this trial may have some limitations such as specific inclusion criteria and life censoring, we were able conclude that elimination of the UTs of higher MW and in a greater quantity is associated with a better prognosis of dialysis patients. A systematic review to compare the outcomes associated with the modalities of expanded hemodialysis (HDx) versus HF-HD and/or HDF in patients with end-stage kidney disease (ESKD) concluded that the efficacy and safety of HDF is supported by a robust evidence base that includes several randomized controlled trials. While HDx may offer benefits over HF-HD, long-term studies are required to compare HDx with high-volume OL-HDF [14].
During recent years, a new type of membrane has been developed, with a higher cut-off point, called medium cut-off (MCO), with the ability to remove high MW molecules, as is done by high cut-off (HCO) membranes used in myeloma, but capable of retaining albumin [15–18]. These MCO membranes are used to facilitate clearance of medium-sized toxins by forcing the internal filtration phenomenon [19].
The clearance of UT with these dialyzers has been compared with that of HF dialyzers and with OL-HDF [20–34]. HD-HF has similar or slightly inferior results regarding the elimination of molecules of low MW in contrast to OL-HDF and HDx. Regarding the middle molecules, post-dilution OL-HDF and HDx are superior to HF-HD.
This study aims to demonstrate the clinical benefits associated with the use of MCO membrane, with a greater pore size and a greater selectivity, which allows optimization of the elimination of larger molecules. HDx is the therapy enabled by this MCO membrane dialyzer, Theranova (Baxter©), which combines a high diffusive transport and a moderate amount of convective transport by backfiltration. The study will explore the long-term clinical impact of the greater clearance capacity on pre-dialysis plasma levels of middle molecules over time, as well as in regard to maintaining pre-dialysis plasma albumin.
ENDPOINTS
The primary objective is to determine whether HDx is non-inferior to post-dilution OL-HDF at reducing the combined outcome of all-cause death and stroke (ischemic or hemorrhagic), acute coronary syndrome (angina and myocardial infarction), peripheral arterial disease (amputation or revascularization) and ischemic colitis (mesenteric thrombosis) and in subjects in HD.
The secondary objective is to determine whether HDx is superior to post-dilution OL-HDF for the combined endpoint of all-cause death and stroke (ischemic or hemorrhagic), acute coronary syndrome, peripheral arterial disease (amputation or revascularization) and ischemic colitis (mesenteric thrombosis). These will be tested after the primary objective using a closed test procedure.
Other secondary objectives are to compare HDx and post-dilution OL-HDF with respect to rate of hospitalizations, all-cause death, cardiovascular mortality, safety and tolerance, efficacy and intradialytic hemodynamic stability.
The study was approved by the Ethical Committee of Comunidad de Madrid. (December 2018).
MATERIALS AND METHODS
Study design
This is an open-label, multicenter, prospective, 1:1 randomized, parallel-group study to evaluate the safety and efficacy of HDx compared with HDF in patients with ESKD in Spain for up to 36 months.
Study population
The study population was stable incident HD patients (without significant changes in technique or therapy) recruited from HD in-hospital units and related satellite centers in Spain.
Inclusion and exclusion criteria
Inclusion criteria:
ESKD patients.
Older than 18 years old.
HD therapy three times per week for >3 months and <24 months.
Exclusion criteria:
No informed consent provided.
Pregnant, breastfeeding or planning to become pregnant.
Active systemic diseases: liver cirrhosis, malignancy prior to enrollment and/or immunosuppressive therapy.
Scheduled for living-donor transplantation within the study period.
Inefficient dialysis: infra-dialysis dose (Kt/V <1.3), single needle dialysis and/or temporary non-tunneled catheter.
Patients with a significant residual renal function [defined as (urea clearance + creatinine clearance)/2 >5 mL/min].
Currently participating in another interventional clinical study or has participated in another interventional clinical study in the past 3 months that may interfere with this study.
Duration of study
The recruitment period is set to be 12 months. This could be prolonged if the number of patients needed is not achieved. The follow-up period will be 24 months.
Treatment procedures
HDx/post-dilution OL-HDF therapies must be implemented following current clinical practices guidelines and procedures at the hospital. No additional actions are required. Dialysis modality is kept during the follow-up. During this time, variations in other factors are expected in both groups (heparin doses, dry weight modifications, blood flow modification) according to an individualized clinical practice. These variables will be collected every 3 months.
General rules will be applied regarding dialysis prescription:
The dialysis prescription is structured and modified following current clinical practice guidelines.
Blood flow rate and treatment duration (4 h) will be maintained stable during the study observation period, unless clinical needs require a change.
Dialysis fluid flow rate: preferably to be at 500 mL/min.
Recommended target dialysate fluid temperature is 36°C and should not be modified. Dialysis fluid temperature will be the same as replacement volume for OL-HDF.
Biofeedback systems may be used at the discretion of the investigator.
A synthetic high-flux dialyzer will be used for OL-HDF and an MCO dialyzer for HDx (Theranova 400/500). Surface at the discretion of the investigator.
The length of dialysis sessions in each treatment modality will be not modified. When OL-HDF cannot be performed temporarily for technical reasons, affected patients will be treated with the same high-flux dialyzer. For OL-HDF patients, a minimum of 23 L/session of total convection volume (substitution + ultrafiltration) will be requested. Convection volume will be adjusted for body surface area [35].
Both OL-HDF and HDx will be performed with ultrapure dialysis fluids, defined as <0.1 CFU/mL and <0.03 EU/mL [according to Sociedad Española de Nefrología (S.E.N.) guidelines] [36]. The composition of dialysate should be the same in both groups.
The following parameters will be recorded at baseline and every 3 months: effective dialysis time, blood flow rate (Qb), dialysate flow rate (Qd), vascular access, convection volume, dry body weight, predialysis and postdialysis body weight, convective volume, and predialysis and postdialysis systolic and diastolic blood pressure and Kt.
Quality of life will be evaluated by the Kidney Disease Quality of Life (KDQOL) test recommended every 6 months following current clinical practice.
All medication will be prescribed following current clinical practice.
A database with the above information on study patients will be performed on line.
Assessments
The following laboratory predialysis data will be recorded at baseline and every 3 months: urea, creatinine, bicarbonate, sodium, potassium, C-reactive protein, uric acid, total proteins, albumin, prealbumin, calcium, phosphate, magnesium, intact parathyroid hormone, β2-microglobulin, hemoglobin, transferrin saturation index and ferritin.
Clinical events will be collected every 3 months: intradialytic symptoms (symptomatic hypotension, episodes of arrhythmia and thoracic pain), unexpected hospital admissions (cause and its duration), and withdrawals from the study and their cause. To calculate the number of dialysis sessions complicated by intradialytic symptoms and unexpected hospitalizations, all sessions preceding the quarterly visit will be considered.
The doses of antihypertensive drugs, erythropoiesis-stimulating agent (ESA), iron supplements, calcimimetics and vitamin D will be recorded at baseline and every 3 months.
Patient quality of life will be collected at baseline, 6, 12, 18 and 24 months by the KDQOL [37].
Safety assessments
Adverse events will be coded using MedDRA. All of them must be communicated following legal requirements.
Incidence of adverse events, adverse events of special interest, device-related adverse events, adverse events leading to withdrawal, and serious adverse events will be summarized by coded term, and percentage by treatment group and overall.
Adverse events of special interest are intradialytic events (death, symptomatic hypotension, episodes of arrhythmia and thoracic pain).
Statistics
Justification and sample size calculation
Noninferiority Trials have become a major tool for the evaluation of drugs, devices, biologics and other medical treatments. Therefore, it is possible to determine that a new treatment is not worse than the control treatment by an acceptably small amount, with a given degree of confidence. This is especially relevant when a treatment with placebo or with a no-treatment control in a study is not ethical, when an effective treatment has already been established [38]. This study wants to compare, from a no inferiority perspective, two approved therapies: HDx vs OL-HDF, trying to reproduce the ESHOL study where OL-HDF demonstrated superiority compared with conventional HD [12].
Sample size is based in the primary objective of the study: “The primary objective of this study is to determine whether HDx is non-inferior to online OL-HDF at reducing the combined outcome of all-cause death and stroke (ischemic or hemorrhagic) and acute coronary syndrome in subjects in HD.” The sample size is 350 patients per arm (700 both groups). We used the no inferiority test, with a statistical power 80%, type I error 0.025, incidence rate (IR) 0.33 events per patient during 2.46 years of follow-up: δ 1.25; IR 0.85; N per group 350.
Repeat events of the combined endpoint are controlled per patient with a negative binomial regression in the calculation of the confidence interval of the IR ratio. Regarding the hazard ratio, the date of the event is the date of the first event that occurred among those defined in the combined endpoint.
Randomization
Patients will be randomized 1:1 to HDx or OL-HDF three times a week. A central computerized random-generator will be utilized to allocate patients to each study group and randomization will be stratified by center. Figure 1 shows schematic overview of the MoTHER HDx study.
Figure 1:
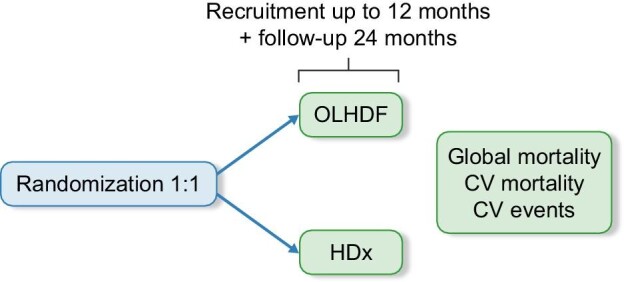
Schematic overview of the MoTHER HDx study.
Statistical methods
Proportions will be compared by chi-squared test with continuity correction or Fisher's exact test. The means of normally distributed variables are to be compared by Student's t-test. Patient survival will be evaluated by the Kaplan–Meier method, and comparisons between groups will be made by the log-rank test. Multivariable Cox regression analyses will be done to adjust for confounding variables. Two-sided significance tests will be used throughout, and a P-value <.05 will be considered significant. Further details of the planned statistical methods will be provided in the study statistical analysis plan (SAP). The purpose of the SAP is to further elaborate the statistical methods described in the protocol and describes analysis conventions to guide the statistical programming work. Any changes to the final SAP will be documented.
Unless otherwise noted, all analyses will be performed using SPSS© software International Business Machines Corp. (New Orchard Road, Armonk, NY, USA). All rights reserved.
Analysis sets
The intent-to-treat full analysis (ITT) will include all patients who have received at least one treatment with the study dialyzer.
The per-protocol-set (PPS) is defined as the set of all patients who complete at least 80% of their scheduled dialysis visits and who have a valid result for the primary endpoints.
All analyses will be performed on the ITT unless otherwise noted. Primary analyses will also be performed on the PPS.
Patients whose survival time would be unknown or uninterpretable will be excluded from the analysis.
Demographics and baseline characteristics
Continuous demographic and baseline characteristics will be summarized descriptively by treatment group and overall using sample size (N), mean, standard deviation (SD), median, minimum and maximum.
Categorical demographic and baseline characteristics will be summarized descriptively by treatment group and overall using frequencies and percentages.
Medical history (medical conditions or surgery items directly linked to the renal replacement therapy) and ESAs, phosphate binders and iron supplements that patients are taking at screening will be listed.
Primary assessments
Reduction on percentage of combined endpoint at 24 months. The difference in % change between the groups will be calculated, and an exploratory t-test will be performed for each therapy and evaluated at a significance level of .05. If the no inferiority is reached, we will explore as a secondary analysis the superiority.
Secondary assessments
SpKt/Vurea and other efficacy, safety and tolerance parameters for the full treatment period (up to 24 months) will be summarized descriptively by treatment group using sample size (N), mean and SD. Additionally, the incidence of patients having average spKt/V ≥1.3 will be summarized by counts and percentages. Average serum phosphorous levels will be summarized descriptively by treatment group using sample size (N), mean and SD for the baseline visit and for each month of dialysis treatment. Serum markers will be define at baseline with sample size (N), mean and standard deviation; and per each month of dialysis treatment.
The incidence of patients having average phosphorous level ≤5.5 mg/dL will be also summarized by treatment group, counts and percentages for the baseline visit and each month of dialysis treatment.
The following healthcare resource utilization metrics will be summarized by median and by frequencies and percentages:
Hospitalizations, all-cause death and cardiovascular death at 24 months.
Patient quality of life by KDQOL-SF 1.3 at baseline, 6, 12, 18 and 24 months.
Serum ferritin and transferrin saturation at baseline and every 3 months.
ESA responsiveness measured as erythropoietin resistance index at baseline, and every 3 months.
Hemoglobin levels at baseline and every 3 months.
ESA dosage, type, administration frequency and route at baseline, and every 3 months.
Intravenous iron dosage at baseline and every 3 months.
Safety assessments
Raw and “change from baseline” values of the routine serum chemistry/hematology assessments and vital signs, Week 12 and Week 24 using sample size (N), mean, SD, median, minimum and maximum.
Adverse events will be coded using MedDRA. Incidence of adverse events, adverse events of special interest, device-related adverse events, adverse events leading to withdrawal, and serious adverse events will be summarized descriptively by coded term and percentage by treatment group and overall at 24 months.
Interim analysis
An interim analysis is not planned for this study. If statistical power is not enough at 24 months, it is planned to extend patients’ follow-up for 12 months more.
Timeline
The trial has already started.
ACKNOWLEDGEMENTS
We thank all the involved centers and healthcare workers, especially the nurse team, for their implication in this project.
APPENDIX
The MOTheR collaborative network:
Hospital Universitario Infanta Leonor: Patricia de Sequera, María Teresa Jaldo, Marta Puerta, Laura Medina; Hospital General Universitario Gregorio Marañón: Almudena Vega, Soraya Abad, Nicolás Macías, Ana García; Hospital General Universitario de Guadalajara: Katia Pérez Del Valle, Concepción Álamo, Marta Sánchez Heras; Complejo Hospitalario de Orense: Elena Iglesias Lamas, Maria Crucio López; Hospital Universitario Ramón Y Cajal: Milagros Fernández Lucas, Nuria Rodríguez Mendiola, Martha Elizabeth Díaz Domínguez, Gloria Ruíz Roso; Hospital Clinic: Francisco Maduell, José Jesús Broseta, Marta Arias-Guillen, Lida María Rodas; Hospital Universitario Marqués De Valdecilla: Celestino Piñera, María Kislikova; Hospital Regional de Málaga: Elvira Esquivias; Hospital Universitario Virgen de la Macarena: Ana Isabel Martínez Puerto, Mercedes Salgueira; Hospital Universitario Puerto Real: Antonio Luis García Herrera, Carolina Lancho, Verónica De La Espada; Hospital Universitario Miguel Servet: Carmen Peralta Roselló; Hospital Universitario Son Espases: Joan Manuel Gascó Company; Complejo Hospitalario de Toledo: María Antonia García Rubiales, Marta Torres Guinea, Elena Pascual Pajares; Hospital Virgen de la Luz: Begoña Rincón Ruiz; Complejo Hospitalario de Canarias: Mª Del Sagrario García Rebollo, Beatriz Escamilla Cabrera; Hospital Nuestra Sra De La Candelaria: Nieves Del Castillo Rodríguez; Fundación Puigvert: Elisabeth Coll, Juan Manuel Diaz, María Jesús Lloret; Hospital Lucus Augusti: Alba García Enríquez; Hospital Universitario 12 de Octubre: Evangelina Mérida, María Fernández, Lucia Aubert; Hospital Universitario de Getafe: Laura Espinel Costoso; Hospital Universitario Príncipe De Asturias: Patricia Martínez Miguel, Hanane Bouarich, María Pérez Fernández; Fundación Hospital De Alcorcón: Eduardo Gallego, Enrique Gruss; Hospital Universitario Severo Ochoa: María Sánchez Sánchez, Juan Carlos Herrero Berrón; Hospital Universitario Infanta Sofía: Angel Gallego Villalobos; Hospital De Calahorra: Francisco Martín; Hospital de Zumárraga: Oihana Larrañaga, Teresa Visus; Hospital Universitario A Coruña: Teresa García Falcón, Carmen Pallares García; Hospital Universitario Insular de Gran Canaria: Mª Del Mar Lago; Complejo hospitalario de Navarra: Itziar Castaño, Joaquín Manrique; Clínica Universitaria de Navarra: Nuria Garcia-Fernandez; Hospital Universitario Reina Sofia: Raquel Ojeda López; Hospital de Vic: Eugenia Castellote, Bernat Guasch; Hospital Ernest Lluch: Samia Etaaboudi; Hospital Universitario Joan XXIII: Julia Garros Martínez, Lisset Josefina Pulido, Beatriz Fuentes Huertas; Hospital Universitario del Sureste: Beatriz Gil-Casares, Fernando Tornero, José María Bautista; Clínica Diálisis RTS: Jesús Guillermo Acosta Visbal, Yarelys León Sánchez, Ruth Amair Rojas; Hospital Público Da Mariña: Raquel Fernández Fernández, Walter López Alarcón; Hospital Universitario San Pedro: Antonio Gil Paraíso, Emma Huarte Loza; Hospital Can Misses: Rocío Vidal Morillo-Velarde; Hospital Universitario Puerta de Hierro: José María Portoles, Mª Rosario Llopez-Carratala; Complejo Hospitalario de Badajoz: Rosa M. Ruiz-Calero, Martin Hidalgo, Álvaro Álvarez; Hospital Inca: Antonio Francisco Planas, Mónica Mosquera; Hospital Araba: Oscar García Uriarte; Hospital Universitario Castellón: Alejandro Pérez Alba; Hospital San Carlos: Virginia López De La Manzanara; Hospital Universitario de León: Jorge Estifan; Hospital San Cecilio: Elena Hernández García, Ana Isabel Morales García; Hospital Gómez Ulla: José Carlos De La Flor Merino, Tania Linares Grávalos; Diaverum: Shaira Martínez-Vaquera, José Luis Pizarro León, Alejandro Jiménez Herrador, Leonardo Díaz Álvarez, Antonio Romero Alcántara, Juan De Dios Ramiro Moya, Lidia Diaz Gómez, Benaldina García Jiménez, Raúl Orihuela Vico, Rocío Leiva Alonso, Nathasha Carolina Nava Pérez, Carlos Jarava Mantecón, Marta Uvieli García Quiceno, Jesús Domínguez Bravo, Scarleth Elizabeth Flores Alvarenga, Manuel Antonio Martínez García, Paula Aledón Viñes, Gustavo Useche Bonilla, Ángel García Pérez, Brenda Henningsmeyer Utrera, Olga Martínez Pascual, María Otero Cupeiro, Verónica Pesqueira Cameselle, Marta Sanz Sainz, Yamila Saharaui Catalá, Antonio Marín Franco.
Notes
Members of the MOTheR collaborative network are listed in the Appendix.
Contributor Information
Patricia de Sequera, Nephrology Department, Hospital Universitario Infanta Leonor, Madrid, Spain.
Rafael Pérez-García, Nephrology Department, Hospital Universitario Infanta Leonor, Madrid, Spain.
Almudena Vega, Nephrology Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain.
Shaira Martínez-Vaquera, Diaverum, Spain.
Jesús Guillermo Acosta, Clínica Diálisis RTS, Murcia, Spain.
Katia Pérez Del Valle, Nephrology Department, Hospital Universitario de Guadalajara, Guadalajara, Spain.
Milagros Fernández-Lucas, Nephrology Department, Hospital Universitario Ramón y Cajal, Madrid, Spain.
María Antonia García-Rubiales, Nephrology Department, Hospital Universitario Puerto Real, Cádiz, Spain.
Antonio Luis García-Herrera, Nephrology Department, Complejo Hospitalario de Toledo, Toledo, Spain.
Elisabeth Coll, Nephrology Department, Fundación Puigvert, Barcelona, Spain.
Evangelina Mérida, Nephrology Department, Hospital Universitario 12 de Octubre, Madrid, Spain.
Patricia Martínez-Miguel, Nephrology Department, Hospital Universitario Alcalá de Henares, Madrid, Spain.
Itziar Castaño, Nephrology Department, Complejo Hospitalario de Navarra, Navarra, Spain.
Beatriz Gil-Casares, Nephrology Department, Hospital Universitario del Sureste, Madrid, Spain.
Julia Garro, Nephrology Department, Hospital Universitario Joan XXIII, Tarragona, Spain.
Francisco Maduell, Nephrology Department, Hospital Clinic, Barcelona, Spain.
the MOTheR collaborative network:
Patricia de Sequera, María Teresa Jaldo, Marta Puerta, Laura Medina, Almudena Vega, Soraya Abad, Nicolás Macías, Ana García, Katia Pérez Del Valle, Concepción Álamo, Marta Sánchez Heras, Elena Iglesias Lamas, Maria Crucio López, Milagros Fernández Lucas, Nuria Rodríguez Mendiola, Martha Elizabeth Díaz Domínguez, Gloria Ruíz Roso, Francisco Maduell, José Jesús Broseta, Marta Arias-Guillen, Lida María Rodas, Celestino Piñera, María Kislikova, Elvira Esquivias, Ana Isabel Martínez Puerto, Mercedes Salgueira, Antonio Luis García Herrera, Carolina Lancho, Verónica De La Espada, Carmen Peralta Roselló, Joan Manuel Gascó Company, María Antonia García Rubiales, Marta Torres Guinea, Elena Pascual Pajares, Begoña Rincón Ruiz, Mª Del Sagrario García Rebollo, Beatriz Escamilla Cabrera, Nieves Del Castillo Rodríguez, Elisabet Coll, Juan Manuel Diaz, María Jesús Lloret, Alba García Enríquez, Evangelina Mérida, María Fernández, Lucia Aubert, Laura Espinel Costoso, Patricia Martínez Miguel, Hanane Bouarich, María Pérez Fernández, Eduardo Gallego, Enrique Gruss, María Sánchez Sánchez, Juan Carlos Herrero Berrón, Angel Gallego Villalobos, Francisco Martín, Oihana Larrañaga, Teresa Visus, Teresa García Falcón, Carmen Pallares García, Mª Del Mar Lago, Itziar Castaño, Joaquín Manrique, Nuria Garcia-Fernandez, Raquel Ojeda López, Eugenia Castellote, Bernat Guasch, Samia Etaaboudi, Julia Garros Martínez, Lisset Josefina Pulido, Beatriz Fuentes Huertas, Beatriz Gil-Casares, Fernando Tornero, José María Bautista, Jesús Guillermo Acosta Visbal, Yarelys León Sánchez, Ruth Amair Rojas, Raquel Fernández Fernández, Walter López Alarcón, Antonio Gil Paraíso, Emma Huarte Loza, Rocío Vidal Morillo-Velarde, José María Portoles, Mª Rosario Llopez-Carratala, Rosa M Ruiz-Calero, Martin Hidalgo, Álvaro Álvarez, Antonio Francisco Planas, Mónica Mosquera, Oscar García Uriarte, Alejandro Pérez Alba, Virginia López De La Manzanara, Jorge Estifan, Elena Hernández García, Ana Isabel Morales García, José Carlos De La Flor Merino, Tania Linares Grávalos, Shaira Martínez-Vaquera, José Luis Pizarro León, Alejandro Jiménez Herrador, Leonardo Díaz Álvarez, Antonio Romero Alcántara, Juan De Dios Ramiro Moya, Lidia Diaz Gómez, Benaldina García Jiménez, Raúl Orihuela Vico, Rocío Leiva Alonso, Nathasha Carolina Nava Pérez, Carlos Jarava Mantecón, Marta Uvieli García Quiceno, Jesús Domínguez Bravo, Scarleth Elizabeth Flores Alvarenga, Manuel Antonio Martínez García, Paula Aledón Viñes, Gustavo Useche Bonilla, Ángel García Pérez, Brenda Henningsmeyer Utrera, Olga Martínez Pascual, María Otero Cupeiro, Verónica Pesqueira Cameselle, Marta Sanz Sainz, Yamila Saharaui Catalá, and Antonio Marín Franco
FUNDING
Spanish Nephrology Society foundation (SENEFRO) is the sponsor of the study. Baxter assumes the funding of the study as an independent research grant. Baxter guarantees the non-relation between selection processes, or any process that may influence the results of the study. Financial support will be independent of results of the study.
CONFLICT OF INTEREST STATEMENT
P.d.S. reports honorarium for conferences, consulting fees and advisory boards from Amgen, Astellas, AstraZeneca, Baxter, Braun, Fresenius Medical Care, GlaxoSmithKline, Nipro, Otsuka, Sandoz, Nipro and Vifor-Pharma. She is the present president of the Spanish Society of Nephrology (S.E.N.). R.P.-G. reports honorarium from Nipro. A.V. has received consultancy fees and lecture fees from Baxter, Braun and Astellas. P.M.-M. report honorarium for conferences and consulting fee from Nipro. M.F.-L. reports honorarium for conferences from Nipro. E.C. reports honorarium for conferences from Fresenius, Astellas and AstraZeneca, and studies from Baxter. I.C. reports honorarium for conferences from Braun, Palex and Vifor Pharma. F.M. has received consultancy fees and lecture fees from Baxter, Fresenius Medical Care, Medtronic, Nipro, Toray and Vifor. K.P.d.V., A.L.G.-H., J.G., M.A.G.-R., E.M. and B.G.-C. have no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
Research idea and study design was by P.d.S. and R.P.-G. Data acquisition was performed by A.V., S.M.V., J.G.A., K.P.d.V., M.F.-L., A.L.G.-H., M.A.G.-R., E.C., E.M., P.M.-M, I.C., B.G.-C, J.G. and F.M.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article itself.
REFERENCES
- 1. Vanholder R, De Smet R, Glorieux Get al. European Uremic Toxin Work Group (EUTox) . Review on uremic toxins: classification, concentration, and inter individual variability. Kidney Int 2003;63:1934–43. 10.1046/j.1523-1755.2003.00924.x [DOI] [PubMed] [Google Scholar]
- 2. Vanholder R, Pletinck A, Schepers Eet al. Biochemical and clinical impact of organic uremic retention solutes: a comprehensive update. Toxins (Basel) 2018;10:33. 10.3390/toxins10010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolley MJ, Hutchison CA. Large uremic toxins: an unsolved problem in end-stage kidney disease. Nephrol Dial Transplant 2018;33:6–11. 10.1093/ndt/gfy179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cozzolino M, Galassi A, Pivari Fet al. The cardiovascular burden in end-stage renal disease. Contrib Nephrol 2017;191:44–57. 10.1159/000479250 [DOI] [PubMed] [Google Scholar]
- 5. Dai L, Golembiewska E, Lindholm Bet al. End-stage renal disease, inflammation and cardiovascular outcomes. Contrib Nephrol 2017;191:32–43. 10.1159/000479254 [DOI] [PubMed] [Google Scholar]
- 6. Nordio M, Limido A, Maggiore Uet al. Italian Dialysis and Transplantation Registry . Survival in patients treated by long-term dialysis compared with the general population. Am J Kidney Dis 2012;59:819–28. 10.1053/j.ajkd.2011.12.023 [DOI] [PubMed] [Google Scholar]
- 7. Renal Spanish Registry (2021) presented in the LII Spanish Society Congress, 2022. https://www.senefro.org/contents/webstructure/MEMORIA_REER_2021_PRELIMINAR.pdf (December 2022, last date accessed). [Google Scholar]
- 8. Kalantar-Zadeh K, Ikizler TA, Block Get al. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis 2003;42:864–81. 10.1016/j.ajkd.2003.07.016 [DOI] [PubMed] [Google Scholar]
- 9. Maduell F, Moreso F, Pons Met al. ESHOL Study Group . High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013;24:487–97. 10.1681/ASN.2012080875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ronco C, Marchionna N, Brendolan Aet al. Expanded haemodialysis: from operational mechanism to clinical results. Nephrol Dial Transplant 2018;33:41–7. 10.1093/ndt/gfy202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vernooij RWM, Bots ML, Strippoli GFMet al. CONVINCE scientific committee. CONVINCE in the context of existing evidence on haemodiafiltration. Nephrol Dial Transplant 2022;37:1006–13. 10.1093/ndt/gfac019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maduell F, Moreso F, Mora-Macià Jet al. ESHOL study reanalysis: all-cause mortality considered by competing risks and time-dependent covariates for renal transplantation. Nefrologia 2016;36:156–63. 10.1016/j.nefro.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 13. Pérez-García R. On-line haemodiafiltration after the ESHOL study. Nefrologia 2014;34:139–44. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell CR, Hornig C, Canaud B.. Systematic review to compare the outcomes associated with the modalities of expanded hemodialysis (HDx) versus high-flux hemodialysis and/or hemodiafiltration (HDF) in patients with end-stage kidney disease (ESKD). Semin Dial 2023;36:86–106. https://doi.org/doi: 10.1111/sdi.13130 [DOI] [PubMed] [Google Scholar]
- 15. Boschetti-de-Fierro A, Voigt M, Storr Met al. Extended characterization of a new class of membranes for blood purification: the high cut-off membranes. Int J Artif Organs 2013;36:455–63. 10.5301/ijao.5000220 [DOI] [PubMed] [Google Scholar]
- 16. Boschetti-de-Fierro A, Voigt M, Storr Met al. MCO membranes: enhanced selectivity in high-flux class. Sci Rep 2015;5:18448. 10.1038/srep18448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cordeiro ISF, Cordeiro L, Wagner CSet al. High-flux versus high-retention-onset membranes: in vivo small and middle molecules kinetics in convective dialysis modalities. Blood Purif 2020;49:8–15. 10.1159/000502082 [DOI] [PubMed] [Google Scholar]
- 18. Belmouaz M, Bauwens M, Lecron J-Cet al. Protein loss and medium cut-off haemodialysis. Clin Kidney J 2021;14:460–1. 10.1093/ckj/sfz180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Canaud B. Recent advances in dialysis membranes. Curr Opin Nephrol Hypertens 2021;30:613–22. 10.1097/MNH.0000000000000744 [DOI] [PubMed] [Google Scholar]
- 20. Belmouaz M, Diolez J, Bauwens Met al. Comparison of hemodialysis with medium cut-off dialyzer and on-line hemodiafiltration on the removal of small and middle-sized molecules. Clin Nephrol 2018;89:50–6. [PubMed] [Google Scholar]
- 21. Boschetti-de-Fierro A, Beck W, Hildwein Het al. Membrane innovation in dialysis. Contrib Nephrol 2017;191:100–14. 10.1159/000479259 [DOI] [PubMed] [Google Scholar]
- 22. Kirsch AH, Lyko R, Nilsson LGet al. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant 2017;32:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirsch AH, Rosenkranz AR, Lyko Ret al. Effects of hemodialysis therapy using dialyzers with medium cut-off membranes on middle molecules. Contrib Nephrol 2017;191:158–67. 10.1159/000479264 [DOI] [PubMed] [Google Scholar]
- 24. Latosinska A, Hulko M, Speidel Ret al. Removal of cell-activating substances using dialyzers with various permeability profiles. Artif Organs 2018;42:78–87. 10.1111/aor.12952 [DOI] [PubMed] [Google Scholar]
- 25. García-Prieto A, Vega A, Linares Tet al. Evaluation of the efficacy of a medium cut-off dialyser and comparison with other high-flux dialysers in conventional haemodialysis and online haemodiafiltration. Clin Kidney J 2018;11:742–6. 10.1093/ckj/sfy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maduell F, Rodas L, Broseta JJet al. High-permeability alternatives to current dialyzers performing both high-flux hemodialysis and postdilution online hemodiafiltration. Artif Organs 2019;43:1014–21. 10.1111/aor.13480 [DOI] [PubMed] [Google Scholar]
- 27. Cho NJ, Park S, Islam MIet al. Long-term effect of medium cut-off dialyzer on middle uremic toxins and cell-free hemoglobin. PLoS One 2019;14:e0220448. 10.1371/journal.pone.0220448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim TH, Kim SH, Kim TYet al. Removal of large middle molecules via haemodialysis with medium cut-off membranes at lower blood flow rates: an observational prospective study. BMC Nephrol 2019;21:2. 10.1186/s12882-019-1669-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lim JH, Park Y, Yook JMet al. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Sci Rep 2020;10:7780. 10.1038/s41598-020-64622-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willy K, Girndt M, Voelkl Jet al. Expanded haemodialysis therapy of chronic haemodialysis patients prevents calcification and apoptosis of vascular smooth muscle cells in vitro. Blood Purif 2018;45:131–8. 10.1159/000484925 [DOI] [PubMed] [Google Scholar]
- 31. Maduell F, Broseta JJ, Rodríguez-Espinosa Det al. Efficacy and safety of the medium cut-off Elisio HX dialyzer. Blood Purif 2023;52:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ciceri P, Tettamanti G, Galassi Aet al. Pro-calcifying analysis of uraemic serum from patients treated with medium cut-off membrane in a prospective, cross-over study. Clin Kidney J 2020;14:1798–807. 10.1093/ckj/sfaa216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ciceri P, Cozzolino M.. Expanded haemodialysis as a current strategy to remove uremic toxins. Toxins (Basel) 2021;13:380. 10.3390/toxins13060380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cozzolino M, Magagnoli L, Ciceri Pet al. Effects of a medium cut-off (Theranova®) dialyser on haemodialysis patients: a prospective, cross-over study. Clin Kidney J 2019;14:382–9. 10.1093/ckj/sfz155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davenport A, Peters SA, Bots MLet al. HDF Pooling Project Investigators. Higher convection volume exchange with online hemodiafiltration is associated with survival advantage for dialysis patients: the effect of adjustment for body size. Kidney Int 2016;89:193–9. 10.1038/ki.2015.264 [DOI] [PubMed] [Google Scholar]
- 36. Pérez-García R, García Maset R, Gonzalez Parra Eet al. Guideline for dialysate quality of Spanish Society of Nephrology (second edition, 2015). Nefrologia 2016;36:1–52. [DOI] [PubMed] [Google Scholar]
- 37. Hays RD, Kallich JD, Mapes DLet al. Kidney Disease Quality of Life Short Form (KDQOL-SFTM), Version 1.3: A Manual for Use and Scoring. Santa Monica, CA: RAND Corporation, 1997. [Google Scholar]
- 38. Mauri L, D'Agostino RB Sr. Challenges in the design and interpretation of noninferiority trials. N Engl J Med 2017;377:1357–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article itself.