ABSTRACT
Background
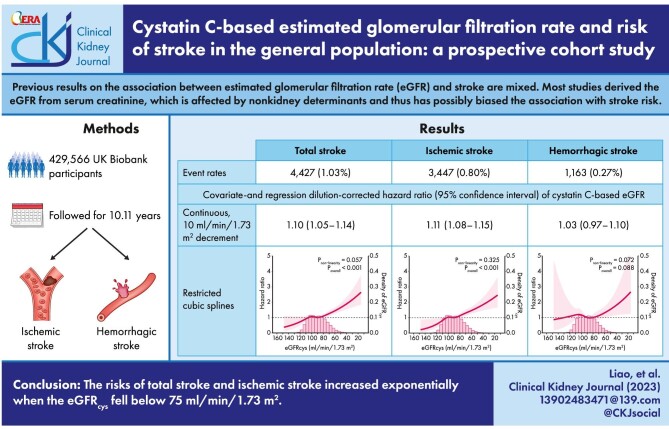
Previous results on the association between the estimated glomerular filtration rate (eGFR) and stroke are mixed. Most studies derived the eGFR from serum creatinine, which is affected by non-kidney determinants and thus has possibly biased the association with stroke risk.
Methods
In this cohort study, we included 429 566 UK Biobank participants (94.5% white, 54% women, age 56 ± 8 years) free of stroke at enrollment. The eGFRcys and eGFRcr were calculated with serum cystatin C and creatinine, respectively. Outcomes of interest were risk of total stroke and subtypes. We investigated the linear and nonlinear associations using Cox proportional hazards models and restricted cubic splines, corrected for regression dilution bias.
Results
During an average follow-up of 10.11 years, 4427 incident strokes occurred, among which 3447 were ischemic and 1163 were hemorrhagic. After adjustment for confounders, the regression dilution-corrected hazard ratios (95% confidence intervals) for every 10 mL/min/1.73 m2 decrement in eGFRcys were 1.10 (1.05–1.14) for total stroke and 1.11 (1.08–1.15) for ischemic stroke. A similar pattern was observed with eGFRcr, although the association was weaker. When either type of eGFR was below 75 mL/min/1.73 m2, the risks of total and ischemic stroke increased exponentially as eGFR decreased. A U-shaped relationship was witnessed if eGFRcr was used instead. There was a null association between eGFR and hemorrhagic stroke.
Conclusions
The risks of total stroke and ischemic stroke increased exponentially when the eGFRcys fell below 75 mL/min/1.73 m2.
Keywords: cystatin C, estimated glomerular filtration rate, regression dilution bias, stroke, UK Biobank
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Worldwide, stroke is the third leading cause of death and disability combined [1]. Many stroke survivors experience a range of disabilities, including physiological or psychological sequelae, which often affect their quality of life [2]. Stroke places an immense economic and social burden on public health; therefore, it is important to reduce the burden of stroke through primary prevention [1].
The decline in kidney function may contribute to the development of stroke by impairing cerebral autoregulation, remodeling the cerebral vasculature and reducing cerebral blood flow [3]. A meta-analysis of 33 prospective cohort studies proposed that a baseline estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 (also known as chronic kidney disease stage 3 to 5) was independently related to a higher risk of stroke [4]. Later, a subsequent meta-analysis including 63 cohort studies and 20 randomized controlled trials identified a linear relationship between eGFR and incident stroke [5]. However, in the Chronic Renal Insufficiency Cohort Study [6] and in a post hoc analysis of the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) trial [7], the eGFR was not associated with an increased risk of stroke after adjustment for confounders. In most previous reports, it is worth noting that the eGFR was calculated using creatinine-based formulas. However, serum creatinine measurement does not provide an accurate eGFR, particularly in those with preserved kidney function [8]. Serum cystatin C, the use of which has become prevalent in the last decade, superiorly captures the level of kidney function independent of age and muscle mass [9]. In addition, most studies relied on a single evaluation of the exposure at baseline and thus neglected the random measurement error and variability of the eGFR [10], leading to an underestimation of the underlying association due to regression dilution bias [11–13]. As such, the inaccuracy of creatinine and regression dilution bias altogether might have flawed prior reports of this kind.
In this study, we hypothesized that eGFR was independently associated with the risk of stroke in the general population. Herein, we investigated the linear and nonlinear associations of the cystatin C–based eGFR (eGFRcys) with the risk of total stroke and its subtypes in UK Biobank participants. Furthermore, we used repeated eGFRcys measurements to attenuate regression dilution bias. For comparison, we performed the analyses in parallel using the creatinine-based eGFR (eGFRcr).
MATERIALS AND METHODS
Study design and participants
This was a prospective cohort study of participants from the UK Biobank. Details of the UK Biobank appear in the Supplementary Methods. We included participants who provided written informed consent to the UK Biobank and had not withdrawn from the study by 9 August 2021; had no history of stroke at baseline; and had undergone serum cystatin C measurement. We excluded participants with incomplete data for the calculation of the eGFRcys, e.g. age or sex; those with malignant tumor or end-stage kidney disease prior to recruitment; and those who were diagnosed with acute kidney injury within 90 days prior to enrollment.
Exposures
All participants provided serum and random spot urine samples at baseline (2006–10), and a subsample of 15 245 participants provided additional samples as the repeated assessments between 2012 and 2013 (https://biobank.ctsu.ox.ac.uk/∼bbdatan/Repeat_assessment_doc_v1.0.pdf). In the main analysis, the GFR was estimated from cystatin C using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations [8]. In addition, we also examined the equations based on creatinine (eGFRcr) as well as a combination of both creatinine and cystatin C (eGFRcr-cys) [8]. Details of the measurement and calculation of the exposures, as well as covariates considered in this study, appear in the Supplementary Methods.
Outcomes
The primary outcome was any first incident stroke between baseline and 31 March 2019. The secondary outcomes were the risk of stroke subtypes, including ischemic and hemorrhagic strokes. Stroke events were ascertained from algorithmically defined outcomes obtained through algorithmic combinations of coded information from the UK Biobank's baseline assessment data collection (which included data from participants on their self-reported medical conditions, operations and medications) and data from hospital admissions (diagnoses and procedures) and death registries. Those who were lost to follow-up, died, dropped out or had no stroke on 31 March 2019 were censored.
Statistical analysis
First, we conducted a descriptive analysis by eGFRcys categories (>105, 90–105, 75–<90, 60–<75, <60 mL/min/1.73 m2, in accordance with previous studies) [14–17]. In addition, we visualized histograms of eGFRcys and eGFRcr and Kaplan‒Meier curves of survival probability according to the categories of eGFRcys and eGFRcr. We also calculated the incidence rate per 100 000 person-years of stroke and its two subtypes by both eGFRcys and eGFRcr categories.
Next, we explored the associations between eGFRcys and outcomes. Considering the large sample size of the UK Biobank, we did not perform data imputation before modeling. We used the Cox proportional hazards models as the main analysis to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for eGFRcys with the outcomes. Simultaneously, we repeated the analyses with eGFRcr to read the disparity with eGFRcys.
The eGFRcys and eGFRcr were entered into the model in three forms: a continuous form (per 10 mL/min/1.73 m2 decrement, no conversion); a binary form (<60 mL/min/1.73 m2 vs ≥60 mL/min/1.73 m2); and a multicategorical form (with the 90–105 mL/min/1.73 m2 category as the reference). The nonlinear association of eGFRcys and eGFRcr on a continuous scale with the outcomes were assessed by restricted cubic splines.
We constructed two types of models for each outcome: the crude model and the adjusted model. Covariates in the adjusted models included demographics, including age, sex, ethnicity, education and economic status (Townsend deprivation index); lifestyle factors, including smoking status, alcohol consumption and metabolic equivalents; physical measurements, including body mass index (BMI), systolic blood pressure, diastolic blood pressure; laboratory measurements, including glycated hemoglobin A1c, low-density lipoprotein, high-density lipoprotein, triglycerides, C-reactive protein and urine albumin-to-creatinine ratio; comorbid conditions, including hypertension, diabetes, cardiovascular diseases; and medications, including antihypertensive medications, hypoglycemic agents, lipid-lowering drugs and antiplatelet drugs.
Within-person variability and laboratory measurement errors (known as regression dilution bias) [11] of the exposure always lead to risk underestimation if the exposure was measured only once. Therefore, we used the McMahon–Peto method [18, 19] to correct the association estimates using the repeated cystatin C and creatinine measurements after 4.3 years (SD 0.9 years) since recruitment. We calculated the regression dilution ratios (RDRs) by dividing the difference in the mean eGFRcys and eGFRcr between the 5th and 1st quintiles in the repeat measurements by the equivalent mean differences in the baseline measurements. We divided the log HRs and standard errors in the crude and covariate-adjusted models by RDRs to obtain the corrected estimates.
We applied a series of sensitivity analyses to further test the robustness of the results. First, we considered any deaths prior to first stroke from baseline to 31 March 2019, as competing risks. As such, we confirmed all the analyses of eGFRcys with the Fine and Gray approach (subdistribution hazards models) as a substitute to manage the competing risks [20]. Second, to examine a particularly healthy subset, we assessed the association between the eGFRcys and the outcomes by excluding the participants who took certain medications or were diagnosed with cardiovascular diseases at baseline. Third, to minimize reverse causality, we excluded participants who developed stroke within 2 years after recruitment. Fourth, we confirmed the results by using eGFRcr-cys as the exposure [8]. Fifth, to further understand the relation between BMI and GFRcr, we excluded underweight participants with a BMI <18.5 kg/m2. Finally, we performed preplanned subgroup analyses to assess potential effect modifications between the eGFRcys and baseline characteristics.
All analyses were performed with R Statistical Software, version 4.0.3. All P-values were two-sided, and a P-value of <.05 was considered to indicate statistical significance. More information on the statistical analyses is provided in the Supplementary data.
RESULTS
Baseline characteristics
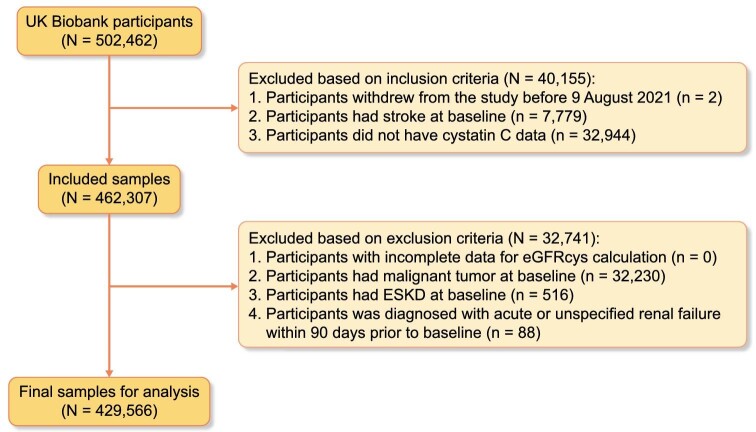
Of the 502 462 participants recruited, we included 462 307 participants meeting the inclusion criteria and further excluded 32 741 participants meeting the exclusion criteria. Thus, the study sample comprised 429 566 participants (Fig. 1). The distributions of eGFRcys and eGFRcr are depicted in Supplementary data, Fig. S1. Table 1 shows the baseline characteristics of participants in the five eGFRcys categories. The majority (94.5%) were of white ethnicity, and nearly half (54%) were women. The average age of the participants was 56 (SD 8) years. Overall, people with a lower eGFRcys level were older, with low education levels and unfavorable economic status. They were more likely to be smokers, physically inactive and obese, and also presented with more comorbid conditions.
Figure 1:
Flowchart of inclusion and exclusion. eGFRcys, estimated glomerular filtration rate based on serum cystatin C calculated with the Chronic Kidney Disease Epidemiology Collaboration equation.
Table 1:
Baseline characteristics of participants.
| eGFRcys (mL/min/1.73 m2) | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Missing value, n | Whole population | >105 | 90–105 | 75–<90 | 60–<75 | <60 |
| n (%) | 429 566 | 72 155 (16.80) | 141 078 (32.84) | 131 305 (30.57) | 67 316 (15.67) | 17 712 (4.12) | |
| Age, mean (SD), years | 0 | 56 (8) | 49 (6) | 55 (8) | 58 (7) | 61 (6) | 63 (5) |
| Women, n (%) | 0 | 232 182 (54) | 37 995 (53) | 82 026 (58) | 66 933 (51) | 35 645 (53) | 9583 (54) |
| Ethnicity, n (%) | 2040 | ||||||
| White | 403 862 (94.5) | 65 533 (91.3) | 132 955 (94.6) | 124 831 (95.5) | 63 982 (95.6) | 16 561 (94.1) | |
| Asian | 10 130 (2.4) | 1976 (2.8) | 3012 (2.1) | 2823 (2.2) | 1650 (2.5) | 669 (3.8) | |
| Black | 6934 (1.6) | 2368 (3.3) | 2301 (1.6) | 1455 (1.1) | 622 (0.9) | 188 (1.1) | |
| Others | 6600 (1.5) | 1927 (2.7) | 2215 (1.6) | 1584 (1.2) | 686 (1.0) | 188 (1.1) | |
| Educationa, n (%) | 5057 | ||||||
| Level 1 | 70 896 (17) | 4831 (7) | 17 412 (12) | 24 317 (19) | 18 009 (27) | 6327 (36) | |
| Level 2 | 71 919 (17) | 12 103 (17) | 23 948 (17) | 22 033 (17) | 11 000 (17) | 2835 (16) | |
| Level 3 | 140 920 (33) | 24 673 (35) | 47 070 (34) | 43 036 (33) | 21 086 (32) | 5055 (29) | |
| Level 4 | 140 774 (33) | 29 841 (42) | 51 326 (37) | 40 338 (31) | 16 124 (24) | 3145 (18) | |
| Townsend deprivation index, median (IQR) | 536 | –2.14 (–3.65, 0.53) | –2.04 (–3.62, 0.65) | –2.25 (–3.71, 0.26) | –2.22 (–3.68, 0.41) | –1.99 (–3.55, 0.83) | –1.33 (–3.24, 1.86) |
| Smoking status, n (%) | 2136 | ||||||
| Never | 235 834 (55) | 43 931 (61) | 81 426 (58) | 69 923 (54) | 32 767 (49) | 7787 (44) | |
| Previous | 146 318 (34) | 21 942 (31) | 46 784 (33) | 46 142 (35) | 24 642 (37) | 6808 (39) | |
| Current | 45 278 (11) | 5993 (8) | 12 305 (9) | 14 588 (11) | 9431 (14) | 2961 (17) | |
| Alcohol consumption, n (%) | 956 | ||||||
| Never | 33 833 (8) | 4256 (6) | 8991 (6) | 10 270 (8) | 7395 (11) | 2921 (17) | |
| Occasional | 96 737 (23) | 13 894 (19) | 28 555 (20) | 29 802 (23) | 18 677 (28) | 5809 (33) | |
| Frequent | 298 040 (70) | 53 825 (75) | 103 288 (73) | 90 952 (69) | 41 055 (61) | 8920 (51) | |
| Metabolic equivalents, median (IQR), min/week | 81 728 | 1779 (812, 3572) | 1884 (885, 3626) | 1853 (873, 3590) | 1760 (792, 3600) | 1653 (702, 3492) | 1386 (542, 3012) |
| BMI, n (%) | 1633 | ||||||
| <25 kg/m2 | 142 083 (33.2) | 33 859 (47.1) | 55 789 (39.7) | 36 796 (28.1) | 13 047 (19.5) | 2 592 (14.8) | |
| 25–≤30 kg/m2 | 182 274 (42.6) | 28 581 (39.7) | 59 774 (42.5) | 58 984 (45.1) | 28 679 (42.8) | 6 256 (35.6) | |
| >30 kg/m2 | 103 576 (24.2) | 9463 (13.2) | 25 103 (17.8) | 35 079 (26.8) | 25 228 (37.7) | 8703 (49.6) | |
| SBP, mean (SD), mmHg | 24 082 | 138 (19) | 131 (17) | 136 (18) | 140 (18) | 142 (19) | 142 (19) |
| DBP, mean (SD), mmHg | 24 079 | 82 (10) | 81 (10) | 82 (10) | 83 (10) | 83 (10) | 82 (11) |
| Hypertension, n (%) | 1 | 39 816 (9) | 2572 (4) | 8714 (6) | 12 716 (10) | 10 469 (16) | 5345 (30) |
| Diabetes, n (%) | 1871 | 21 538 (5) | 2635 (4) | 5294 (4) | 6142 (5) | 4717 (7) | 2750 (16) |
| Cardiovascular diseases, n (%) | 0 | 37 181 (9) | 2988 (4) | 8712 (6) | 12 052 (9) | 9147 (14) | 4282 (24) |
| Antihypertensive medications, n (%) | 0 | 91 462 (21) | 6568 (9) | 21 668 (15) | 29 941 (23) | 23 110 (34) | 10 175 (57) |
| Hypoglycemic agents, n (%) | 0 | 13 281 (3) | 1557 (2) | 3134 (2) | 3767 (3) | 3037 (5) | 1786 (10) |
| Lipid-lowering drugs, n (%) | 0 | 81 222 (19) | 7083 (10) | 21 514 (15) | 26 949 (21) | 18 465 (27) | 7211 (41) |
| Antiplatelet drugs, n (%) | 0 | 56 881 (13) | 5032 (7) | 14 431 (10) | 18 756 (14) | 13 329 (20) | 5333 (30) |
| Glycated hemoglobin A1c, median (IQR), mmol/mol | 22 133 | 35.2 (32.7, 37.8) | 33.7 (31.4, 36.1) | 34.8 (32.4, 37.2) | 35.5 (33.2, 38.1) | 36.4 (33.9, 39.2) | 37.6 (34.8, 41.3) |
| LDL, mean (SD), mmol/L | 1097 | 3.57 (0.86) | 3.40 (0.80) | 3.59 (0.84) | 3.64 (0.87) | 3.60 (0.91) | 3.37 (0.96) |
| HDL, mean (SD), mmol/L | 36 450 | 1.45 (0.38) | 1.50 (0.37) | 1.51 (0.39) | 1.43 (0.37) | 1.36 (0.35) | 1.27 (0.35) |
| Triglycerides, median (IQR), mmol/L | 613 | 1.48 (1.04, 2.14) | 1.19 (0.85, 1.79) | 1.37 (0.98, 1.99) | 1.58 (1.13, 2.24) | 1.73 (1.25, 2.41) | 1.86 (1.36, 2.58) |
| CRP, median (IQR), mg/L | 1205 | 1.31 (0.65, 2.72) | 0.84 (0.43, 1.74) | 1.08 (0.56, 2.20) | 1.46 (0.76, 2.88) | 2.03 (1.04, 3.95) | 2.82 (1.45, 5.64) |
| UACR, n (%) | 25 361 | ||||||
| <30 mg/g | 386 140 (95.5) | 64 787 (96.5) | 127 180 (96.4) | 119 686 (96.2) | 60 230 (94.2) | 14 257 (85.3) | |
| 30–<300 mg/g | 16 573 (4.1) | 2254 (3.4) | 4490 (3.4) | 4519 (3.6) | 3401 (5.3) | 1909 (11.4) | |
| ≥300 mg/g | 1492 (0.4) | 125 (0.2) | 228 (0.2) | 257 (0.2) | 324 (0.5) | 558 (3.3) | |
Level 1, none of the above; level 2, O-levels/GCSEs or equivalent or CSEs or equivalent; level 3, A-levels/AS-levels or equivalent or National Vocational Qualification or Higher National Diploma or Higher National Certificate or equivalent, or other professional qualifications; level 4, college or university degree.
SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFRcys, estimated glomerular filtration rate based on serum cystatin C calculated with the Chronic Kidney Disease Epidemiology Collaboration equation; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CRP, C-reactive protein; UACR, urine albumin-to-creatinine ratio.
Outcomes during follow-up
Of all the participants, 1101 (0.26%) were lost to follow-up or dropped out. During 10.11 years of mean follow-up, a total of 4427 (1.03%) incident strokes occurred, including 3447 (0.80%) ischemic and 1163 (0.27%) hemorrhagic cases. The incidence rates were 103.5, 80.6 and 27.1 per 100 000 person-years for total stroke, ischemic stroke and hemorrhagic stroke, respectively (Table 2).
Table 2:
Association between cystatin C–based eGFR and stroke with Cox proportional hazards models.
| Crude model | Fully adjusted model | |||||
|---|---|---|---|---|---|---|
| eGFRcys | Incidence rate, per 100 000 person-years | Events (%) | HRs (95% CIs) | RDR-adjusted HRs (95% CIs) | HRs (95% CIs) | RDR-adjusted HRs (95% CIs) |
| Total stroke | ||||||
| Continuous, 10 mL/min/1.73 m2 decrement | 103.5 | 4427 (1.03) | 1.39 (1.37–1.41) | 1.43 (1.41–1.47) | 1.09 (1.05–1.12) | 1.10 (1.05–1.14) |
| Binary | ||||||
| ≥60 mL/min/1.73 m2 | 94.4 | 3879 (0.94) | Reference | Reference | Reference | Reference |
| <60 mL/min/1.73 m2 | 327.9 | 548 (3.09) | 3.45 (3.16–3.77) | 3.89 (3.52–4.29) | 1.31 (1.14–1.50) | 1.34 (1.15–1.56) |
| Multicategory | ||||||
| >105 mL/min/1.73 m2 | 41.0 | 298 (0.41) | 0.55 (0.48–0.63) | 0.52 (0.45–0.60) | 0.82 (0.69–0.98) | 0.80 (0.67–0.98) |
| 90–105 mL/min/1.73 m2 | 74.5 | 1054 (0.75) | Reference | Reference | Reference | Reference |
| 75–<90 mL/min/1.73 m2 | 103.9 | 1358 (1.03) | 1.39 (1.29–1.51) | 1.44 (1.32–1.57) | 1.01 (0.91–1.12) | 1.01 (0.90–1.13) |
| 60–<75 mL/min/1.73 m2 | 177.3 | 1169 (1.74) | 2.37 (2.18–2.57) | 2.57 (2.35–2.82) | 1.24 (1.10–1.39) | 1.27 (1.11–1.43) |
| <60 mL/min/1.73 m2 | 327.9 | 548 (3.09) | 4.37 (3.94–4.84) | 5.03 (4.49–5.64) | 1.44 (1.23–1.69) | 1.49 (1.25–1.78) |
| Ischemic stroke | ||||||
| Continuous, 10 mL/min/1.73 m2 decrement | 80.6 | 3447 (0.80) | 1.45 (1.43–1.47) | 1.49 (1.47–1.54) | 1.10 (1.06–1.14) | 1.11 (1.08–1.15) |
| Binary | ||||||
| ≥60 mL/min/1.73 m2 | 72.5 | 2981 (0.72) | Reference | Reference | Reference | Reference |
| <60 mL/min/1.73 m2 | 278.5 | 466 (2.63) | 3.82 (3.46–4.21) | 4.34 (3.90–4.83) | 1.30 (1.12–1.51) | 1.33 (1.13–1.57) |
| Multicategory | ||||||
| >105 mL/min/1.73 m2 | 27.9 | 203 (0.28) | 0.53 (0.45–0.62) | 0.50 (0.42–0.59) | 0.81 (0.66–1.00) | 0.79 (0.63–1.00) |
| 90-105 mL/min/1.73 m2 | 52.8 | 747 (0.53) | Reference | Reference | Reference | Reference |
| 75–<90 mL/min/1.73 m2 | 81.8 | 1070 (0.81) | 1.55 (1.41–1.70) | 1.62 (1.46–1.79) | 1.08 (0.96–1.22) | 1.09 (0.96–1.24) |
| 60–<75 mL/min/1.73 m2 | 145.6 | 961 (1.43) | 2.75 (2.50–3.02) | 3.03 (2.73–3.36) | 1.29 (1.13–1.48) | 1.32 (1.14–1.54) |
| <60 mL/min/1.73 m2 | 278.5 | 466 (2.63) | 5.24 (4.67–5.88) | 6.14 (5.41–6.97) | 1.51 (1.26–1.80) | 1.57 (1.29–1.90) |
| Hemorrhagic stroke | ||||||
| Continuous, 10 mL/min/1.73 m2 decrement | 27.1 | 1163 (0.27) | 1.23 (1.20–1.28) | 1.27 (1.22–1.32) | 1.03 (0.97–1.09) | 1.03 (0.97–1.10) |
| Binary | ||||||
| ≥60 mL/min/1.73 m2 | 25.6 | 1054 (0.26) | Reference | Reference | Reference | Reference |
| <60 mL/min/1.73 m2 | 64.5 | 109 (0.62) | 2.51 (2.06–3.05) | 2.74 (2.20–3.40) | 1.25 (0.93–1.69) | 1.28 (0.92–1.78) |
| Multicategory | ||||||
| >105 mL/min/1.73 m2 | 14.1 | 103 (0.14) | 0.58 (0.46–0.72) | 0.55 (0.43–0.70) | 0.80 (0.60–1.07) | 0.78 (0.57–1.08) |
| 90–105 mL/min/1.73 m2 | 24.5 | 347 (0.25) | Reference | Reference | Reference | Reference |
| 75–<90 mL/min/1.73 m2 | 25.5 | 335 (0.26) | 1.04 (0.90–1.21) | 1.05 (0.89–1.23) | 0.82 (0.67–1.00) | 0.80 (0.64–1.00) |
| 60–<75 mL/min/1.73 m2 | 40.5 | 269 (0.40) | 1.65 (1.41–1.93) | 1.73 (1.45–2.06) | 1.08 (0.86–1.35) | 1.09 (0.85–1.39) |
| <60 mL/min/1.73 m2 | 64.5 | 109 (0.62) | 2.62 (2.11–3.25) | 2.87 (2.27–3.63) | 1.20 (0.86–1.67) | 1.22 (0.85–1.75) |
Covariates in the fully adjusted model included age, sex, ethnicity, education, Townsend deprivation index, smoking status, alcohol consumption, metabolic equivalents, BMI, systolic blood pressure, diastolic blood pressure, glycated hemoglobin A1c, low-density lipoprotein, high-density lipoprotein, triglycerides, C-reactive protein, urine albumin-to-creatinine ratio, hypertension, diabetes, cardiovascular diseases, antihypertensive medications, hypoglycemic agents, lipid-lowering drugs and antiplatelet drugs. The regression dilution ratio was 0.91.
The proportional hazards assumption was checked for all the models using statistical tests and graphical diagnostics based on the scaled Schoenfeld residuals. Time-dependent covariates were constructed in those where the proportional hazards assumption was violated (diastolic blood pressure in the models for total stroke; systolic blood pressure and diastolic blood pressure in the models for ischemic stroke; C-reactive protein in the models for hemorrhagic stroke). Variance inflation factor values were below 5 for all the models presented, indicating a low risk of multicollinearity.
eGFRcys, estimated glomerular filtration rate based on serum cystatin C calculated with the Chronic Kidney Disease Epidemiology Collaboration equation.
Association between eGFR and risk of stroke
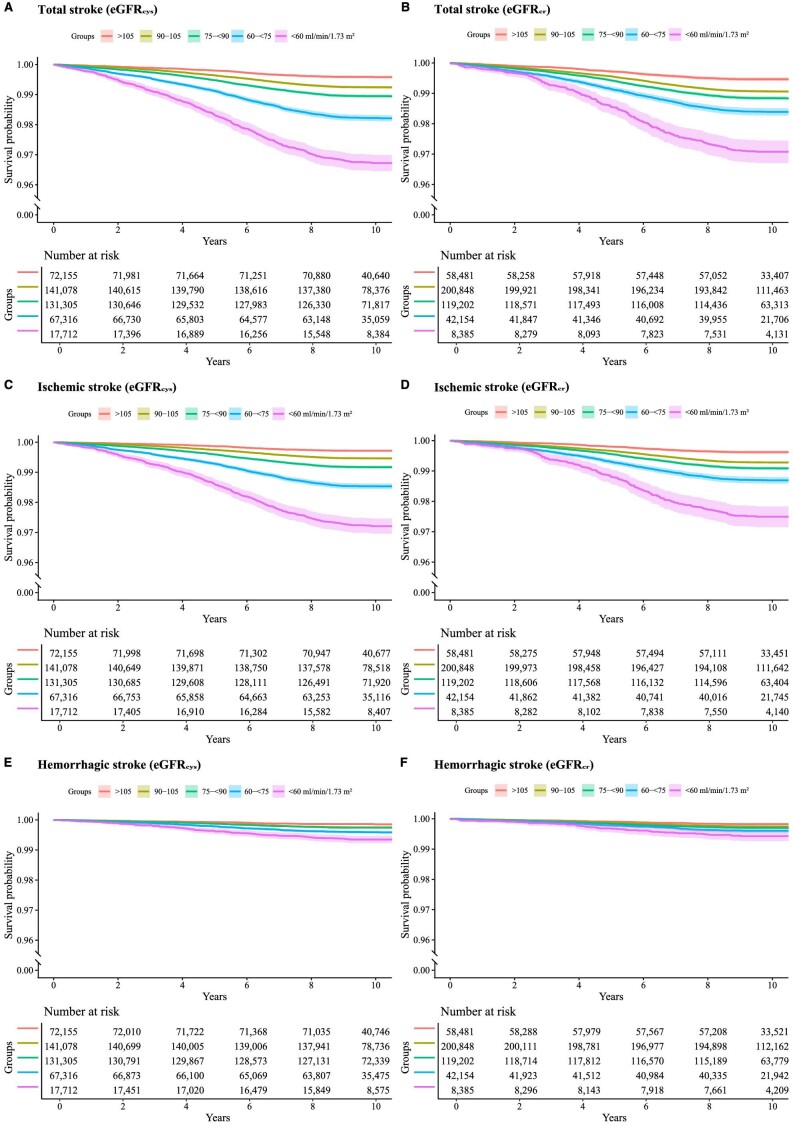
As shown in Fig. 2, Kaplan‒Meier curves indicate that people in the lower eGFRcys or eGFRcr categories were more likely to develop incident stroke, with the risks being obvious for total and ischemic strokes (Fig. 2A–D) but largely diminishing for hemorrhagic stroke (Fig. 2E and F). Differences in stroke risks appeared more noticeable when the participants were divided according to the eGFRcys.
Figure 2:
Kaplan‒Meier curves of survival probability by eGFR categories. Survival probability for primary (A and B for total stroke) and secondary outcomes (C and D for ischemic stroke; E and F for hemorrhagic stroke) according to the categories of eGFRcys and eGFRcr. The median follow-up period for total stroke was 10.11 (IQR 9.39–10.81) years, that for ischemic stroke was 10.11 (IQR 9.40–10.81) years and for hemorrhagic stroke was 10.11 (IQR 9.40–10.82) years. In the participants who experienced stroke, the median follow-up periods were 5.01 (IQR 2.98–6.62), 5.04 (IQR 3.01–6.66) and 4.92 (IQR 2.90–6.55) years for total stroke, ischemic stroke and hemorrhagic stroke, respectively. The median follow-up period for participants who did not develop stroke was 10.17 (IQR 9.48–10.84) years. eGFRcr, estimated glomerular filtration rate based on serum creatinine calculated with the Chronic Kidney Disease Epidemiology Collaboration equation; eGFRcys, estimated glomerular filtration rate based on serum cystatin C calculated with the Chronic Kidney Disease Epidemiology Collaboration equation; IQR, interquartile range.
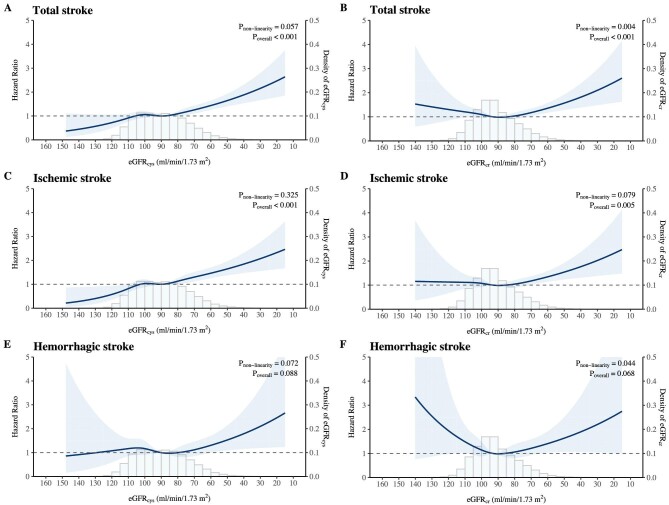
Table 2 shows that a lower eGFRcys was independently associated with an increased risk of total stroke and ischemic stroke but not with hemorrhagic stroke after adjustment for potential confounders. When subsequently corrected for RDRs, the associations became slightly stronger. Such relationships generally held for all three forms of eGFRcys entered. For each 10 mL/min/1.73 m2 decrement in the eGFRcys, the risk increased by 10% (adjusted HR 1.10, 95% CI 1.05–1.14) and 11% (adjusted HR 1.11, 95% CI 1.08–1.15) for total stroke and ischemic stroke, respectively. However, the risk was not significantly increased for hemorrhagic stroke (adjusted HR 1.03, 95% CI 0.97–1.10). The binary analyses confirmed the same relationships. The multicategory analyses coupled with restricted cubic spline (Fig. 3A) suggested a cubic relationship between the eGFRcys and the risk of total stroke might exist, although not statistically significant (P for nonlinearity = 0.057). When eGFRcys was below 75 mL/min/1.73 m2, stroke risk increased almost exponentially as eGFRcys decreased. However, when eGFRcys was above 75 mL/min/1.73 m2, the risk did not significantly differ but seemingly decreased as the eGFRcys exceeded 105 mL/min/1.73 m2. A similar mode of association was visualized for ischemic stroke (Fig. 3C) but not for hemorrhagic stroke (Fig. 3E).
Figure 3:
Restricted cubic splines for total stroke, ischemic stroke and hemorrhagic stroke. The adjusted restricted cubic splines were defined with five knots at the 5th, 27.5th, 50th, 72.5th and 95th percentiles, conditional on the median values of the covariates. HRs were adjusted for age, sex, ethnicity, education, Townsend deprivation index, smoking status, alcohol consumption, metabolic equivalents, BMI, systolic blood pressure, diastolic blood pressure, glycated hemoglobin A1c, low-density lipoprotein, high-density lipoprotein, triglycerides, C-reactive protein, urine albumin-to-creatinine ratio, hypertension, diabetes, cardiovascular diseases, antihypertensive medications, hypoglycemic agents, lipid-lowering drugs and antiplatelet drugs, and additionally corrected for regression dilution bias of the estimates. The regression dilution ratio was 0.91. The 95% CIs were derived by bootstrap resampling, the times of which were equal to the numbers of observations. Each point on the curve is the pointwise average HR. Shaded areas represent 95% CIs. The horizontal dashed line indicates an HR of 1. eGFRcr, estimated glomerular filtration rate based on serum creatinine calculated with the Chronic Kidney Disease Epidemiology Collaboration equation; eGFRcys, estimated glomerular filtration rate based on serum cystatin C calculated with the Chronic Kidney Disease Epidemiology Collaboration equation.
As shown in Table 3, the continuous analyses indicated that the associations of eGFRcr with total stroke (adjusted HR 1.05, 95% CI 1.01–1.10) and ischemic stroke (adjusted HR 1.06, 95% CI 1.01–1.11) were weaker than those of eGFRcys. Contrary to the results of eGFRcys, the multicategory analyses revealed that compared with the reference group, participants in the highest eGFRcr category (>105 mL/min/1.73 m2) paradoxically exhibited an increased risk of stroke: adjusted HRs (95% CIs) were 1.20 (0.98–1.50) for total stroke, 1.29 (1.00–1.67) for ischemic stroke and 1.09 (0.73–1.60) for hemorrhagic stroke, respectively. A U-shaped relationship was observed in the restricted cubic splines (Fig. 3B, D and F).
Table 3:
Association between creatinine-based eGFR and stroke with Cox proportional hazards models.
| Crude model | Fully adjusted model | |||||
|---|---|---|---|---|---|---|
| eGFRcr | Incidence rate, per 100 000 person-years | Events (%) | HRs (95% CIs) | RDR-adjusted HRs (95% CIs) | HRs (95% CIs) | RDR-adjusted HRs (95% CIs) |
| Total stroke | ||||||
| Continuous, 10 mL/min/1.73 m2 decrement | 103.5 | 4422 (1.03) | 1.28 (1.25–1.30) | 1.35 (1.33–1.39) | 1.04 (1.01–1.08) | 1.05 (1.01–1.10) |
| Binary | ||||||
| ≥60 mL/min/1.73 m2 | 99.9 | 4 187 (1.00) | Reference | Reference | Reference | Reference |
| <60 mL/min/1.73 m2 | 292.9 | 235 (2.80) | 2.92 (2.56–3.32) | 3.77 (3.20–4.44) | 1.31 (1.08–1.58) | 1.40 (1.10–1.76) |
| Multicategory | ||||||
| >105 mL/min/1.73 m2 | 52.6 | 310 (0.53) | 0.57 (0.51–0.64) | 0.50 (0.43–0.58) | 1.16 (0.98–1.39) | 1.20 (0.98–1.50) |
| 90–105 mL/min/1.73 m2 | 92.4 | 1853 (0.92) | Reference | Reference | Reference | Reference |
| 75–<90 mL/min/1.73 m2 | 115.0 | 1360 (1.14) | 1.24 (1.16–1.33) | 1.31 (1.20–1.43) | 0.99 (0.90–1.08) | 0.99 (0.88–1.10) |
| 60–<75 mL/min/1.73 m2 | 160.3 | 664 (1.58) | 1.72 (1.58–1.88) | 1.97 (1.76–2.19) | 1.16 (1.03–1.30) | 1.20 (1.04–1.38) |
| <60 mL/min/1.73 m2 | 292.9 | 235 (2.80) | 3.15 (2.75–3.61) | 4.15 (3.51–4.91) | 1.35 (1.11–1.64) | 1.45 (1.14–1.85) |
| Ischemic stroke | ||||||
| Continuous, 10 mL/min/1.73 m2 decrement | 80.6 | 3443 (0.80) | 1.32 (1.30–1.35) | 1.41 (1.37–1.45) | 1.05 (1.01–1.09) | 1.06 (1.01–1.11) |
| Binary | ||||||
| ≥60 mL/min/1.73 m2 | 77.3 | 3242 (0.77) | Reference | Reference | Reference | Reference |
| <60 mL/min/1.73 m2 | 250.1 | 201 (2.40) | 3.22 (2.79–3.71) | 4.27 (3.57–5.09) | 1.30 (1.06–1.60) | 1.38 (1.07–1.79) |
| Multicategory | ||||||
| >105 mL/min/1.73 m2 | 37.3 | 220 (0.38) | 0.53 (0.46–0.61) | 0.46 (0.38–0.54) | 1.23 (1.00–1.51) | 1.29 (1.00–1.67) |
| 90–105 mL/min/1.73 m2 | 70.7 | 1418 (0.71) | Reference | Reference | Reference | Reference |
| 75–<90 mL/min/1.73 m2 | 90.1 | 1066 (0.89) | 1.27 (1.17–1.38) | 1.35 (1.22–1.49) | 0.99 (0.90–1.10) | 0.99 (0.88–1.13) |
| 60–<75 mL/min/1.73 m2 | 129.7 | 538 (1.28) | 1.83 (1.65–2.02) | 2.11 (1.87–2.39) | 1.16 (1.01–1.32) | 1.20 (1.01–1.41) |
| <60 mL/min/1.73 m2 | 250.1 | 201 (2.40) | 3.52 (3.03–4.08) | 4.76 (3.96–5.72) | 1.34 (1.09–1.66) | 1.44 (1.11–1.88) |
| Hemorrhagic stroke | ||||||
| Continuous, 10 mL/min/1.73 m2 decrement | 27.1 | 1162 (0.27) | 1.19 (1.14–1.23) | 1.23 (1.18–1.30) | 1.02 (0.96–1.09) | 1.02 (0.95–1.11) |
| Binary | ||||||
| ≥60 mL/min/1.73 m2 | 26.5 | 1116 (0.27) | Reference | Reference | Reference | Reference |
| <60 mL/min/1.73 m2 | 56.8 | 46 (0.55) | 2.13 (1.58–2.86) | 2.55 (1.77–3.68) | 1.26 (0.84–1.90) | 1.33 (0.81–2.22) |
| Multicategory | ||||||
| >105 mL/min/1.73 m2 | 17.1 | 101 (0.17) | 0.69 (0.56–0.86) | 0.64 (0.49–0.83) | 1.07 (0.78–1.46) | 1.09 (0.73–1.60) |
| 90–105 mL/min/1.73 m2 | 24.8 | 498 (0.25) | Reference | Reference | Reference | Reference |
| 75–<90 mL/min/1.73 m2 | 29.8 | 354 (0.30) | 1.20 (1.05–1.38) | 1.25 (1.06–1.49) | 0.98 (0.82–1.17) | 0.98 (0.78–1.22) |
| 60–<75 mL/min/1.73 m2 | 39.1 | 163 (0.39) | 1.57 (1.32–1.88) | 1.75 (1.41–2.18) | 1.20 (0.95–1.51) | 1.25 (0.94–1.67) |
| <60 mL/min/1.73 m2 | 56.8 | 46 (0.55) | 2.28 (1.68–3.08) | 2.78 (1.91–4.04) | 1.30 (0.85–1.98) | 1.38 (0.82–2.33) |
Covariates in the fully adjusted model included age, sex, ethnicity, education, Townsend deprivation index, smoking status, alcohol consumption, metabolic equivalents, BMI, systolic blood pressure, diastolic blood pressure, glycated hemoglobin A1c, low-density lipoprotein, high-density lipoprotein, triglycerides, C-reactive protein, urine albumin-to-creatinine ratio, hypertension, diabetes, cardiovascular diseases, antihypertensive medications, hypoglycemic agents, lipid-lowering drugs and antiplatelet drugs. The regression dilution ratio was 0.81.
The proportional hazards assumption was checked for all the models using statistical tests and graphical diagnostics based on the scaled Schoenfeld residuals. Time-dependent covariates were constructed in those where the proportional hazards assumption was violated (diastolic blood pressure in the models for total stroke; systolic blood pressure, diastolic blood pressure, cardiovascular diseases in the models for ischemic stroke; C-reactive protein in the models for hemorrhagic stroke). Variance inflation factor values were below 5 for all the models presented, indicating a low risk of multicollinearity.
eGFRcr, estimated glomerular filtration rate based on serum creatinine calculated with the Chronic Kidney Disease Epidemiology Collaboration equation.
Sensitivity analyses and subgroup analyses
All the sensitivity analyses revealed consistent results with the main analysis. When the Fine and Gray approach was applied to account for competing risks, the results were altered little (Supplementary data, Table S1). Similarly, the findings remained constant to a large extent in the diverse scenarios that we tested (Supplementary data, Tables S2 and S3). In the subgroup analyses (Supplementary data, Fig. S2), we found no significant differences in the associations of eGFRcys with risk of total stroke across the prespecified strata, accompanied by null interaction between eGFRcys and all the stratified factors.
DISCUSSION
In this large cohort study, we observed a cubic relationship between the eGFRcys and the risk of total and ischemic strokes. The associations were generally stronger after the calibration of regression dilution bias and remained consistent in various sensitivity and subgroup analyses. However, when the eGFRcr was used as a proxy of kidney function, the mode of the associations appeared to be U-shaped. We found null association between eGFRcys and hemorrhagic stroke.
The robust nature of our results is apparent from the overall consistency of the results achieved using multiple approaches. Remarkably, in a community-based population, we found that the risk of stroke started to increase exponentially as the eGFR was below 75 mL/min/1.73 m2, which is significantly higher than the literature reported threshold of 60 mL/min/1.73 m2 [4]. As the relationship between a reduced eGFR and stroke risk is still debated, our results strengthen the evidence from several previous studies, including two systematic reviews [4, 5]. One meta-analysis including 284 672 participants and 7863 stroke events showed that the stroke risk increased among participants with an eGFRcr <60 mL/min/1.73 m2 (relative risk 1.43, 95% CI 1.31–1.57) [4]. Another meta-analysis including 2 156 147 participants with 30 392 stroke events revealed that each 10 mL/min/1.73 m2 decrement in eGFRcr increased the risk of stroke by 7% (relative risk 1.07, 95% CI 1.04–1.09) [5]. In contrast, another pooled analysis of four community-based studies reported null association between a low eGFRcr and stroke [21]. It is worth noting that in many publications, a single eGFR evaluated at baseline was merely entered into the models as a dichotomous variable (<60 mL/min/1.73 m2 vs ≥60 mL/min/1.73 m2) or as a continuous variable. In this regard, neither a potential nonlinear relationship nor the correction for regression dilution bias could be addressed. Our results indeed support the nonlinearity of the association between kidney function and stroke risk. Thus, the simplistic analysis strategy may have underestimated the true association.
Creatinine is not sufficiently sensitive to detect mild to moderate kidney function impairment (40–70 mL/min/1.73 m2) [9]. This possibly leads to misclassification as the reference group in the eGFRcr analysis. Earlier studies in the general population have been inevitably limited by these unpredictable pitfalls related to creatinine. Moreover, an apparently higher eGFRcr was related to stroke risks, a clinically counterintuitive phenomenon being presented for all three outcomes in our analysis. We observed distinct results between eGFRcys (or eGFRcys-cr) and eGFRcr mainly in the highest category (above 105 mL/min/1.73 m2), which likely reflects differences in the accuracy of the two measures of kidney function. Both the accuracy and precision of eGFRcr are compromised compared with those of eGFRcys and eGFRcys-cr, especially when kidney function is relatively normal [8]. Pseudo-elevated eGFRcr may mirror the loss of lean muscle mass and poor nutrition [22], all of which are associated with adverse outcomes [23].
Evidence for the association of impaired kidney function with hemorrhagic stroke risk is conflicting. We did not find a detrimental association between a lower eGFR and hemorrhagic stroke risk. In line with our results, a pooled analysis of four studies showed that a low eGFRcr-cys was significantly associated with an increased risk of ischemic stroke but not hemorrhagic stroke [17]. Recently, a nationwide cohort study in South Korea also reported null association between eGFRcr and the risk of hemorrhagic stroke [24]. The opposite result was reported in the Rotterdam Study using eGFRcr calculated by the Cockcroft–Gault equation [25]. Dissimilarities in these findings may be attributed to the case mix, the spectrum of kidney function, and the equations employed [26, 27]. Nevertheless, the relationship between kidney function and the risk of hemorrhagic stroke appears less clear than that for ischemic stroke and thus warrants further elucidation.
The kidneys and the brain vasculature have many mutual anatomical and functional properties [28] and share similar risk factors [29]. The putative homeostatic mechanisms of a lower eGFR and stroke include cerebral autoregulation, blood flow and vessel remodeling [3]. Impaired kidney function can lead to instability in the regulation of cerebral blood flow, which relies on a constant and adequate supply [30].
From a public health point of view, both stroke and kidney disease are noncommunicable diseases. The current study strengthened the evidence for the relationship between kidney function and stroke risks, and it was shown that screening for kidney function with serum cystatin C may help risk stratification for stroke in the general population. Moreover, our findings highlight that when individuals have an eGFR below 75 mL/min/1.73 m2 in routine laboratory tests, the risk of stroke should not be underestimated. With the Kidney Disease: Improving Global Outcomes (KDIGO) guideline promoting the measurement of cystatin C [31], more widespread use of cystatin C could expectedly reduce the bias of eGFR in studying adverse outcomes [32, 33].
This study has several limitations that should be considered. First, given the nature of observational studies, we can only suggest an association but cannot ascertain causality. Second, the participants who volunteered were mainly whites aged 40–69 years; therefore, the generalizability of our findings to other ethnic and age groups may be limited. Third, our estimated GFR relied on serum biomarkers rather than the gold standard method of measured GFR [34], which may introduce some uncertainty. Although cystatin C testing is available in many laboratories, it is more expensive than creatinine testing due to the cost of reagents [35] and may be influenced by non-kidney factors such as thyroid function [36, 37] or glucocorticoid use [38]. We were unable to adjust for these confounding factors. The inclusion of participants with such conditions may have overestimated the impact of eGFRcys on outcomes. Fourth, because GFR is estimated using a single measure of cystatin C or creatinine at baseline, we cannot provide GFR values at the time of the stroke or information on GFR progression over time. Finally, due to the lack of data on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification in the UK Biobank [39], we were unable to specify the relationship between eGFR and etiological types of stroke and thereby provide mechanistic insights [40].
In conclusion, we observed a nonlinear relationship between eGFRcys and the risk of total and ischemic strokes in this population-based study. The risks of stroke progressively increased as eGFRcys decreased below 75 mL/min/1.73 m2. In contrast, the eGFRcr-related results were likely to be biased. Our findings emphasize the importance of monitoring kidney function in patients and suggest that eGFRcys should be considered the preferred marker for assessing stroke risk.
Supplementary Material
ACKNOWLEDGEMENTS
We appreciate each participant attending the UK Biobank. This study was conducted using the UK Biobank Resource by Y.S., T.L. and Y.L. under project number 65814. We thank the funders that supported this study.
Contributor Information
Jinlan Liao, Renal Division, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China.
Fei Xiao, Renal Division, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China; Shantou University Medical College, Shantou, Guangdong Province, China.
Liuqiao Yang, BGI-Shenzhen, Shenzhen, Guangdong Province, China.
Yanling Wei, Clinical Research Academy, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China.
Congying Song, Clinical Research Academy, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China.
Jing Li, Renal Division, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China.
Sike Yu, BGI-Shenzhen, Shenzhen, Guangdong Province, China.
Yueqi Lu, BGI-Shenzhen, Shenzhen, Guangdong Province, China.
Jingwen Zhang, Renal Division, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China.
Liang Dai, Clinical Research Academy, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China.
Wei Liang, Renal Division, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China.
Tao Li, BGI-Shenzhen, Shenzhen, Guangdong Province, China.
Zuying Xiong, Renal Division, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China.
Yangfeng Wu, Peking University Clinical Research Institute, Peking University, Beijing, China.
Meg J Jardine, The George Institute for Global Health, University of New South Wales, Sydney, New South Wales, Australia; Concord Repatriation General Hospital, Sydney, New South Wales, Australia; Department of Medicine, Stanford Centre for Clinical Research, Stanford University School of Medicine, Stanford, CA, USA.
Juan Jesus Carrero, Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden; Division of Nephrology, Department of Clinical Sciences, Karolinska Institutet, Danderyd Hospital, Stockholm 182 88, Sweden.
Ying Shan, BGI-Shenzhen, Shenzhen, Guangdong Province, China; Clinical Research Academy, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China.
Xiaoyan Huang, Renal Division, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China; Clinical Research Academy, Peking University Shenzhen Hospital, Peking University, Shenzhen, Guangdong Province, China.
FUNDING
J.Liao, F.X., J.Z., J.Li, W.L., Z.X. and X.H. were supported by grants from the San-Ming Project of Medicine, Shenzhen (SZSM201812097); J.Liao was supported by the Medical Science and Technology Research Foundation of Guangdong Province (B2019025). T.L. was supported by the National Key Research and Development Program of China (grant No. 2020YFC2008002). Y.S. was supported by National Natural Science Foundation of China (82204148), and Scientific Research Foundation of Peking University Shenzhen Hospital (KYQD2022203). X.H. was supported by the Shenzhen Science and Technology Innovation Commission (grant No. JCYJ20200109140412476) and the Peking University Shenzhen Hospital (grant No. LCYJ2020001). The funders had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
AUTHORS’ CONTRIBUTIONS
X.H., J.Liao and Z.X. conceptualized the study; J.Liao and X.H. contributed to methodology; L.Y. and Y.S. had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; L.Y. contributed to visualization; X.H. and Y.S. contributed to validation; Y.S., X.H. and Z.X. provided supervision; Y.Wei, C.S., J.Li, S.Y., Y.L., J.Z., L.D., W.L. and T.L. provided resources; J.Liao and F.X. wrote the original draft; Y.Wu, M.J.J. and J.J.C. provided critical feedback on interpretation; and all authors reviewed and edited the manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study were obtained from the UK Biobank (https://www.ukbiobank.ac.uk/). The corresponding author will make the code used for all analyses available upon reasonable request.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
REFERENCES
- 1. Feigin VL, Stark BA, Johnson COet al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021;20:795–820. 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen Q, Cao C, Gong Let al. Health related quality of life in stroke patients and risk factors associated with patients for return to work. Medicine (Baltimore) 2019;98:e15130. 10.1097/MD.0000000000015130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghoshal S, Freedman BI.. Mechanisms of stroke in patients with chronic kidney disease. Am J Nephrol 2019;50:229–39. 10.1159/000502446 [DOI] [PubMed] [Google Scholar]
- 4. Lee M, Saver JL, Chang KHet al. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ 2010;341:c4249. 10.1136/bmj.c4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Masson P, Webster AC, Hong Met al. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 2015;30:1162–9. 10.1093/ndt/gfv009 [DOI] [PubMed] [Google Scholar]
- 6. Sandsmark DK, Messe SR, Zhang Xet al. Proteinuria, but not eGFR, predicts stroke risk in chronic kidney disease: Chronic Renal Insufficiency Cohort Study. Stroke 2015;46:2075–80. 10.1161/STROKEAHA.115.009861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ford I, Bezlyak V, Stott DJet al. Reduced glomerular filtration rate and its association with clinical outcome in older patients at risk of vascular events: secondary analysis. PLoS Med 2009;6:e1000016. 10.1371/journal.pmed.1000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inker LA, Schmid CH, Tighiouart Het al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–9. 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murty MS, Sharma UK, Pandey VBet al. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J Nephrol 2013;23:180–3. 10.4103/0971-4065.111840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee S, Park S, Kim Yet al. Impact of variability in estimated glomerular filtration rate on major clinical outcomes: a nationwide population-based study. PLoS One 2020;15:e0244156. 10.1371/journal.pone.0244156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clarke R, Shipley M, Lewington Set al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999;150:341–53. 10.1093/oxfordjournals.aje.a010013 [DOI] [PubMed] [Google Scholar]
- 12. Hutcheon JA, Chiolero A, Hanley JA.. Random measurement error and regression dilution bias. BMJ 2010;340:c2289. 10.1136/bmj.c2289 [DOI] [PubMed] [Google Scholar]
- 13. Berglund L. Regression dilution bias: tools for correction methods and sample size calculation. Ups J Med Sci 2012;117:279–83. 10.3109/03009734.2012.668143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chronic Kidney Disease Prognosis Consortium , Matsushita K, van der Velde Met al.Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsushita K, Mahmoodi BK, Woodward Met al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012;307:1941–51. 10.1001/jama.2012.3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahmoodi BK, Matsushita K, Woodward Met al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet North Am Ed 2012;380:1649–61. 10.1016/S0140-6736(12)61272-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mahmoodi BK, Yatsuya H, Matsushita Ket al. Association of kidney disease measures with ischemic versus hemorrhagic strokes: pooled analyses of 4 prospective community-based cohorts. Stroke 2014;45:1925–31. 10.1161/STROKEAHA.114.004900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MacMahon S, Peto R, Cutler Jet al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet North Am Ed 1990;335:765–74. 10.1016/0140-6736(90)90878-9 [DOI] [PubMed] [Google Scholar]
- 19. Papier K, Knuppel A, Perez-Cornago Aet al. Circulating insulin-like growth factor-I and risk of 25 common conditions: outcome-wide analyses in the UK Biobank study. Eur J Epidemiol 2022;37:25–34. 10.1007/s10654-021-00811-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Austin PC, Lee DS, Fine JP.. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–9. 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weiner DE, Tighiouart H, Amin MGet al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004;15:1307–15. 10.1097/01.ASN.0000123691.46138.E2 [DOI] [PubMed] [Google Scholar]
- 22. Baxmann AC, Ahmed MS, Marques NCet al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol 2008;3:348–54. 10.2215/CJN.02870707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim CH, Rhee TM, Woo Park Ket al. Association between low muscle mass and prognosis of patients with coronary artery disease undergoing percutaneous coronary intervention. J Am Heart Assoc 2021;10:e018554. 10.1161/JAHA.120.018554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oh CM, Park SK, Jung JYet al. Reduced glomerular filtration rate and risk of stroke: a nationwide cohort study in South Korea. J Atheroscler Thromb 2021;28:928–41. 10.5551/jat.56143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bos MJ, Koudstaal PJ, Hofman Aet al. Decreased glomerular filtration rate is a risk factor for hemorrhagic but not for ischemic stroke: the Rotterdam Study. Stroke 2007;38:3127–32. 10.1161/STROKEAHA.107.489807 [DOI] [PubMed] [Google Scholar]
- 26. Boehme AK, Esenwa C, Elkind MS.. Stroke risk factors, genetics, and prevention. Circ Res 2017;120:472–95. 10.1161/CIRCRESAHA.116.308398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Camacho E, LoPresti MA, Bruce Set al. The role of age in intracerebral hemorrhages. J Clin Neurosci 2015;22:1867–70. 10.1016/j.jocn.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 28. Marini S, Georgakis MK, Anderson CD.. Interactions between kidney function and cerebrovascular disease: vessel pathology that fires together wires together. Front Neurol 2021;12:785273. 10.3389/fneur.2021.785273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelly D, Rothwell PM.. Disentangling the multiple links between renal dysfunction and cerebrovascular disease. J Neurol Neurosurg Psychiatry 2020;91:88–97. 10.1136/jnnp-2019-320526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schiller A, Covic A.. Kidney and brain—a renal perspective of ‘Les Liaisons Dangereuses’. Nephrol Dial Transplant 2010;25:1370–3. 10.1093/ndt/gfq068 [DOI] [PubMed] [Google Scholar]
- 31. Levin A, Stevens PE, Bilous RWet al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 32. Shlipak MG, Matsushita K, Arnlov Jet al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 2013;369:932–43. 10.1056/NEJMoa1214234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lees JS, Welsh CE, Celis-Morales CAet al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat Med 2019;25:1753–60. 10.1038/s41591-019-0627-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Porrini E, Ruggenenti P, Luis-Lima Set al. Author correction: estimated GFR: time for a critical appraisal. Nat Rev Nephrol 2019;15:121. 10.1038/s41581-018-0105-4 [DOI] [PubMed] [Google Scholar]
- 35. Shlipak MG, Mattes MD, Peralta CA.. Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis 2013;62:595–603. 10.1053/j.ajkd.2013.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kimmel M, Braun N, Alscher MD.. Influence of thyroid function on different kidney function tests. Kidney Blood Press Res 2012;35:9–17. 10.1159/000329354 [DOI] [PubMed] [Google Scholar]
- 37. Xin C, Xie J, Fan Het al. Association between serum cystatin C and thyroid diseases: a systematic review and meta-analysis. Front Endocrinol 2021;12:766516. 10.3389/fendo.2021.766516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Knight EL, Verhave JC, Spiegelman Det al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 2004;65:1416–21. 10.1111/j.1523-1755.2004.00517.x [DOI] [PubMed] [Google Scholar]
- 39. Adams HP Jr, Bendixen BH, Kappelle LJet al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 40. Kelly DM, Li L, Rothwell PM;. Oxford Vascular Study. Etiological subtypes of transient ischemic attack and ischemic stroke in chronic kidney disease: population-based study. Stroke 2020;51:2786–94. 10.1161/STROKEAHA.120.030045 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study were obtained from the UK Biobank (https://www.ukbiobank.ac.uk/). The corresponding author will make the code used for all analyses available upon reasonable request.