ABSTRACT
Background
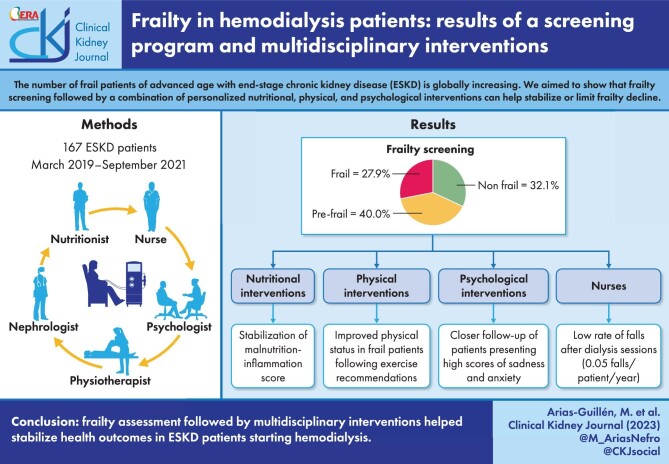
The number of frail patients of advanced age with end-stage kidney disease (ESKD) undergoing hemodialysis is increasing globally. Here we evaluated a frailty screening program of ESKD patients starting hemodialysis, and subsequent multidisciplinary interventions.
Methods
This was a prospective observational study of ESKD patients in a hemodialysis program. Patients were evaluated for frailty (Fried frail phenotype) before and after a 12-month period. Patients followed standard clinical practice at our hospital, which included assessment and multidisciplinary interventions for nutritional (malnutrition-inflammation score, protein-energy wasting), physical [short physical performance battery (SPPB)] and psychological status.
Results
A total of 167 patients (mean ± standard deviation age 67.8 ± 15.4 years) were screened for frailty, and 108 completed the program. At screening, 27.9% of the patients were frail, 40.0% pre-frail and 32.1% non-frail. Nutritional interventions (enrichment, oral nutritional supplements, intradialytic parenteral nutrition) resulted in stable nutritional status for most frail and pre-frail patients after 12 months. Patients following recommendations for intradialytic, home-based or combined physical exercise presented improved or stable in SPPB scores after 12 months, compared with those that did not follow recommendations, especially in the frail and pre-frail population (P = .025). A rate of 0.05 falls/patient/year was observed. More than 60% of frail patients presented high scores of sadness and anxiety.
Conclusions
Frailty screening, together with coordinated interventions by nutritionists, physiotherapists, psychologists and nurses, preserved the health status of ESKD patients starting hemodialysis. Frailty assessment helped in advising patients on individual nutritional, physical or psychological needs.
Keywords: end-stage kidney disease, frailty, hemodialysis, nutritional assessment, physical assessment
Graphical Abstract
Graphical Abstract.
INTRODUCTION
The aging of the population in developed countries, together with the improved management of chronic diseases, have increased the number and the age of patients with end-stage kidney disease (ESKD) undergoing hemodialysis. Recent technological advances in hemodialysis now offer greater flexibility and tolerance to treatment, allowing the inclusion of elderly patients with more comorbidities in hemodialysis programs [1, 2]. The aging of the population starting hemodialysis has resulted in an increase in the prevalence of frailty among these patients, a condition that has been defined as a state of impaired homeostasis reserve causing an increased vulnerability to stressor events, such as infections or surgery, which could lead to a disproportionate and cumulative decline in health [3]. Frailty has been considered a predictor of disability, hospitalization, falls, loss of mobility, cardiovascular disease and death [4]. While the incidence of frailty among older people without ESKD is 3%–6%, frailty increases dramatically among people with ESKD (15%–21%), and even more so among those on hemodialysis treatment (up to 73% depending on the tool used) [1].
Studies in the past decade have demonstrated that hemodialysis patients could benefit from preventive or therapeutic measures aiming to adapt the procedures and mitigate the risks associated with frailty [5–7]. For this reason, early frailty screening of these patients by healthcare personnel has been strongly recommended, allowing for the detection of those who are frail and most vulnerable to the development of adverse health events [1, 8, 9]. The goal should be to detect not only frail patients, but also the pre-frail patients, as the latter have an increased risk of becoming frail within 3 years. However, despite the major clinical and economic implications of frailty, screening is still not routinely performed in many hemodialysis units, in part because there is no consensus regarding the optimal tool to use [10]. The current methods for assessing frailty can be divided into those based on physical frailty, such as Fried's Frailty Phenotype [11], which focuses on functional assessment and is a good predictor of clinical events [12], and multidimensional ones, such as the Frailty Index [13]; other alternatives are the Clinical Frailty Scale [14], the Edmonton Frailty Scale [15] and the FRAIL scale [16], which have been validated for the hemodialysis population. The implementation of the use of these scales in daily clinical practice is not easy, as some are time-consuming and require auxiliary instruments.
In recent years there has been increased interest in the implementation of screening programs for frailty in hemodialysis patients [8, 9, 17, 18]. However, there are few studies analyzing outcomes of interventions aimed at slowing progression or even reversing frailty in hemodialysis patients after screening. Although the nutritional, physical and psychological needs of hemodialysis patients have been extensively studied [19–22], their effects on frailty progression are not well understood. The objective of this longitudinal observational study was to determine the impact of the implementation of a frailty screening program and the subsequent multidisciplinary interventions involving nutritionists, physiotherapists, psychologists and nurses, in a population of ESKD patients undergoing hemodialysis.
MATERIALS AND METHODS
This prospective observational study was conducted from March 2019 to September 2021 at the Clinic Hospital of Barcelona (Spain). The study's recruitment was planned to ensure a minimum follow-up of 12 months between the basal and final visits. All nutritional, physical and psychological interventions followed standard routine clinical practice at our hospital and were voluntary and prescribed at the discretion of the specialist. The conduct of this study did not alter standard practice in any way. All patients provided signed informed consent and the study was conducted in accordance with the Declaration of Helsinki and local regulations. To be included in the study the patients had to be >18 years of age, with stage 5 ESKD (eGFR <15 mL/min/1.73 m2) in a hemodialysis program, and with no hospital admissions for acute events in the month prior to inclusion.
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations including the Helsinki Declaration. The study was approved by the Clinic Hospital of Barcelona (Spain). The collection and analysis of data for this study was possible thanks to a document, evaluated, and approved by the HCB Ethics and Research Committee, signed by all patients for the use of data in review and observational studies (Reg. HCB/2018/1168). All patients provided informed consent prior to participating in the study.
Study procedures and assessments
The main assessment in this study was frailty as measured by the Fried index. Based on the five Fried frailty criteria (weight loss, exhaustion, low physical activity, slowness, weakness), the patients were divided into three phenotypes: non-frail (score 0), pre-frail (score 1–2) and frail (score 3–5) [11].
Upon inclusion in the study, the patients were assessed at the basal visit by a nutritionist, a physiotherapist and a psychologist. This multidisciplinary team evaluated and ranked each patient using well-known instruments (Table 1). These healthcare providers also prescribed interventions and monitored each patient.
Table 1:
Nutritional, physical and psychological evaluations and interventions conducted in routine clinical practice at our hospital.
| Patient color coding | |||
| Nutritional | |||
| Instrument | |||
| MIS | 0–5 | 6–8 | ≥9 |
| SGA | A | B | C |
| PEW | No | Yes/No | Yes |
| Interventiona | Diet diaryb | Diet diaryb | Diet diaryb |
| Enrichmentc | Enrichmentc | ||
| Oral nutritional supplementsd | |||
| IDPNe | |||
| Evaluation | After 1 year | Every 6–9 months | Every 3–6 months |
| Physical | |||
| Instrument | |||
| SPPB | >10 | 8–10 | <8 |
| DFRI | Low risk of falls | Medium risk of falls | High risk of falls |
| BADL | Autonomous | Moderate dependency | Strong dependency |
| Intervention | General recommendations for activity | IPE | Intradialytic strength training |
| HBE, IPE | Strength training (supervised or at home) | Interdialytic training in gym | |
| Monitored for risk of falls and dependency | General recommendations for physical activity | Bed safety measures (position, rails) | |
| Transferring to bed supervised | Close nurse supervision | ||
| Advise adequate shoes | All transfers supervised | ||
| Measures to avoid orthostatic hypotension | Home adaptation | ||
| Evaluate orthopedic dynamic measures (also at home) | |||
| Psychological | |||
| Cognitive | |||
| Instrument | |||
| SPMSQ | 0–3 | 4–7 | 8–10 |
| IADL | Autonomous | Moderate dependency | Strong dependency |
| Intervention | Monitoring | Neuropsychology | Derivation to neurologist |
| Evaluate neurocognitive stimulation | Information about Alzheimer Foundation | ||
| Evaluation | Yearly | Every 3–6 months | At neurologist's criteria |
| Emotional | |||
| Instrument | |||
| EED | 0–3 | 4–7 | 8–10 |
| Intervention | Monitoring | In-depth psychopathological interview | In-depth psychopathological interview |
| Group therapy | Evaluate HADS | ||
| Evaluate derivation to psychiatrist | |||
| Evaluation | Yearly | Every 3 months | Monthly |
Patients with correct nutritional test results underwent no intervention, but nurses and nutritionists monitored any developing changes periodically.
Diet diary: registry of all foods for a 3-day period (no dialysis day, dialysis day, holiday) in a prespecified form.
Recommendations to enrich the diet (personalized by nutritionist).
Nutritionally complete preparations of one or more nutrients specifically adapted to the needs of ESKD patients.
IDPN was administered during regularly scheduled dialysis sessions as a supplement (commonly three times per week), and requires patients to obtain some of their nutrients orally outside of dialysis time.
HADS, Hospital Anxiety and Depression Scale; PEW, protein-energy wasting; SGA, Subjective Global Assessment.
The nutritional status of each patient was evaluated with the Malnutrition-Inflammation Score (MIS) [23] and the Subjective Global Assessment [24]. Protein-energy wasting was defined according to the International Society of Renal Nutrition and Metabolism criteria [25] and calculated using the Nutrendial web application (www.nutrendial.cat) [26]. The MIS provides quantitative score of the nutritional status of the patient and its severity. The severity of the MIS is composed of four grades for each component which are ranked from 0 (normal) to 3 (very severe). The overall score of the 10 MIS components ranges from 0 to 30, with high scores indicating increased severity. The Subjective Global Assessment is a semiquantitative scoring system based on history and physical examination. The history evaluates five components separately: weight loss during the preceding 6 months, gastrointestinal symptoms, food intake, functional capacity and comorbidities. The physical examination consists of two components: loss of subcutaneous fat and muscle wasting. These components are classified in terms of the three major Subjective Global Assessment scores: A, well nourished; B, mild to moderate malnutrition; and C, severe malnutrition [24].
Functional status of the patients was evaluated by a physiotherapist with the Short Physical Performance Battery (SPPB) [27], and the nurses used the Downton Fall Risk Index (DFRI) [28], and the Basic Activities of Daily Living (BADL), based on the Barthel Index (BI) [29]. The SPPB combines the results of the balance tests, gait speed and sit-to-stand, and it has been used as a predictive tool for disability and monitoring of physical function in older people. The scores range from 0 (worst performance) to 12 (best performance). The DFRI is a self-reported instrument that detects the risk of falling on a scale of 0–11 (score of ≥3 indicates a patient at risk of falling). The BI measures performance in 10 variables describing BADL and mobility, with a higher number reflecting greater ability to function independently. The time taken and physical assistance required to perform each item was used in scoring (0–100). Once the patients had been assessed by the physiotherapist, they were asked to participate in some form of physical exercise: cardiovascular work during the hemodialysis session using a pedal exerciser (intradialytic physical exercise, IPE); combined cardiovascular and strength exercise during the hemodialysis session; or functional-type home-based exercise (HBE). Patients who were already exercising on their own were advised to continue with their individual programs. Falls of patients in hemodialysis were recorded by the nursing staff and scored electronically in the hospital database.
The cognitive status of the patients was evaluated by a psychologist with Pfeiffer's test [Short Portable Mental Status Questionnaire (SPMSQ)] [30], and the Lawton and Brody Instrumental Activities of Daily Life (IADL) scale [31, 32]. The SPMSQ score derives from the number of errors made by the patient on a 10-item list (as errors are coded as 1 and correct answers as 0, lower values indicate better cognitive performance). Items include tasks on orientation, memory and attention. The IADL assesses independent living skills in older adults and can be used in community or hospital settings. Patients were scored according to their highest level of functioning in each of eight domains. A summary score ranges from 0 (low function, dependent) to 8 (high function, independent).
The emotional status of the patients was evaluated with the Questionnaire to Assess Emotional Distress in Renal Patients undergoing Dialysis (EED) [33]. The EED includes five questions with different response formats (dichotomized, Likert scales and open questions) to assess sadness, anxiety, concerns, resources to cope with illness, external signs of distress, and other considerations. The score ranges from 0 to 10.
Following the evaluations, the patients were allocated into three groups (color coded green, orange and red) in each assessment (nutritional, functional, psychological), and given specific interventions (see below) depending on their nutritional, physical, or psychological status (Table 1). After 12 months undergoing hemodialysis, the patients were assessed again by the nutritionist and the physiotherapist, using the same instruments. Due to the limitations imposed by coronavirus disease 2019 (COVID-19) pandemic, the evaluation by the psychologist had to be postponed, which precluded assessments with a 12-month interval.
Statistical methods
A descriptive statistical analysis was performed for all variables. Continuous variables were described by the number of valid cases, mean, standard deviation (SD), median, 25th and 75th percentiles (P25–P75), and range. Categorical variables were described by means of absolute and relative frequencies of each category over the total of valid values (N).
Analysis of variance (ANOVA), the chi-square test or Fisher's exact test were used for comparisons of categorical variables. In the case of continuous variables, the Student's t-test or the Mann–Whitney U-test was used. For longitudinal comparisons, the repeated-measures t-test was used. For all comparisons, a two-tailed statistical significance α of 0.05 was applied.
Statistical analyses were performed using the SAS (Statistical Analysis System) program, version 9.3 or later on Windows platform.
RESULTS
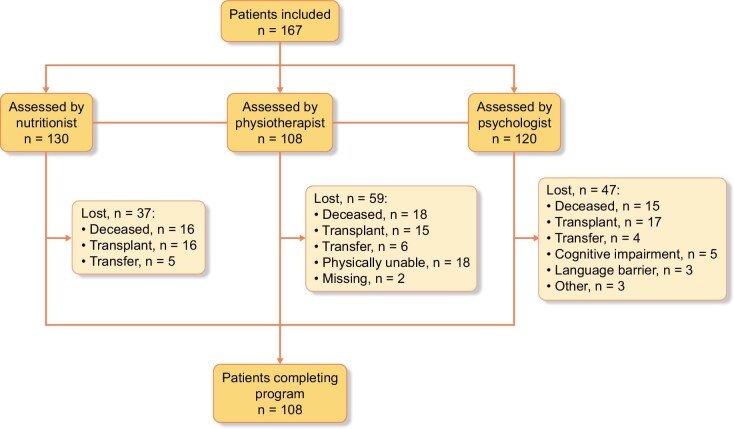
A total of 167 patients were included in the study and 107 patients completed all assessments after 12 months (Fig. 1). The mean ± SD age was 67.8 ± 15.4 years and 67.1% were male (Table 2); 80.8% were entering the hemodialysis program and 19.2% were already in hemodialysis when the study started. The main causes of ESKD were nephrosclerosis in 41 patients (24.6%), diabetes in 26 (15.6%), glomerulonephritis in 24 (14.4%) and polycystic kidney disease in 16 (9.6%). The main comorbidities associated to ESKD were hypertension in 146 patients (87.4%) and diabetes [type 1 in 10 patients (6.0%) and type 2 in 56 patients (33.5%)]. The mean time in dialysis was 50.6 ± 48.9 months (range 3–312), with a mean daily dose of 2.3 ± 0.6 Kt/V. A total of 23 patients died during the study, and 28 underwent a renal transplant.
Figure 1:
Patient disposition.
Table 2:
Population baseline characteristics.
| Variable | N = 167 |
|---|---|
| Age, years, mean ± SD | 67.8 ± 15.4 |
| Sex, male, n (%) | 112 (67.1) |
| Hypertension, n (%) | 146 (87.4) |
| Diabetes, n (%) | 66 (39.5) |
| Dialysis dose, Kt/V, mean ± SD | 2.3 ± 0.6 |
| Time on dialysis, months, mean ± SD | 50.6 ± 48.9 |
| Charlson indexa, mean ± SD | 6.6 ± 2.7 |
| Social assessment scaleb, mean ± SD | 8.8 ± 3.3 |
| BADL scalec, n (%) | |
| Total dependency | 7 (4.2) |
| Severe dependency | 6 (3.6) |
| Moderate dependency | 33 (19.8) |
| Little dependency | 17 (10.2) |
| Independent | 104 (62.3) |
| IADL scaled, n (%) | |
| Total dependency | 9 (5.4) |
| Severe dependency | 6 (3.6) |
| Moderate dependency | 21 (12.6) |
| Little dependency | 29 (17.4) |
| Independent | 102 (61.1) |
| Fall riskd, n (%) | |
| High | 18 (10.8) |
| Medium | 52 (31.1) |
| Low | 97 (58.1) |
| Cognitive impairmentf, n (%) | |
| No impairment | 144 (86.2) |
| Mild | 10 (6.0) |
| Moderate | 9 (5.4) |
| Severe | 4 (2.4) |
| Emotional distressg, mean ± SD | |
| Sadness | 3.48 ± 2.96 |
| Anxiety | 3.14 ± 2.74 |
According to the Fried frailty phenotypes, of 165 patients with data available at the baseline visit, 53 (32.1%) were non-frail, 66 (40.0%) were pre-frail and 46 (27.9%) were frail (Fig. 2). In the final visit there were 101 patients with data available, and of these 28 (27.7%) were non-frail, 33 (32.7%) were pre-frail and 40 (39.6%) were frail. No significant changes were observed overall among the three Fried phenotypes between the two timepoints (P = .1377).
Figure 2:
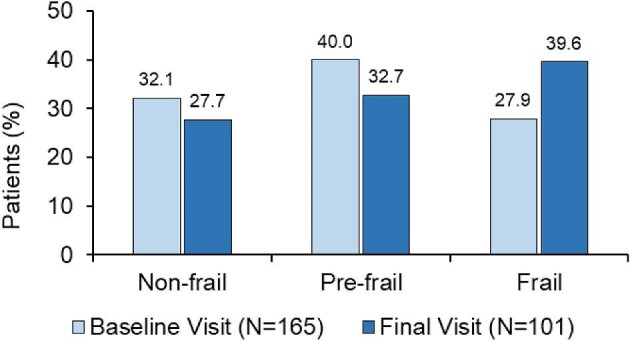
Fried phenotypes at the baseline and final visits.
A nutritional assessment was conducted on 157 patients in the baseline visit and 130 in the final visit. Overall, no significant differences were observed in the MIS mean score or the proportions of patients in each of the categories for the MIS, Subjective Global Assessment or protein-energy wasting during the study period (Table 3). Also, mean values for albumin, cholesterol and transferrin did not change significantly in this patient population. When classified according to Fried phenotypes, no significant changes were observed for MIS, albumin, cholesterol or transferrin (Table 4). Patients were classified according to nutritional intervention and Fried phenotype, for baseline and final visits, revealing a higher need for nutritional intervention (dietary enrichment or supplements) in pre-frail and frail patients compared with non-frail patients (Table 5). The proportion of patients requiring these nutritional interventions was reduced between baseline and final visits.
Table 3:
Nutritional and functional assessments at the baseline and final visits.
| Variable | Baseline visit | Final visit | P a |
|---|---|---|---|
| Nutritional | N = 157 | N = 130 | |
| MIS, mean ± SD | 6.69 ± 3.66 | 6.22 ± 4.23 | .312 |
| MIS, N (%) | |||
| 0–5 | 68 (43.3) | 70 (53.8) | .171 |
| 6–8 | 52 (33.1) | 32 (24.6) | |
| ≥9 | 37 (23.6) | 28 (21.5) | |
| SGA, N (%) | |||
| A | 77 (49.0) | 61 (46.9) | .938 |
| B | 73 (46.5) | 63 (48.5) | |
| C | 7 (4.5) | 6 (4.6) | |
| PEW, N (%) | |||
| Yes | 30 (19.1) | 16 (12.3) | .118 |
| No | 127 (80.9) | 114 (87.7) | |
| Albumin, mean ± SD | 3.86 ± 0.39 | 3.92 ± 0.38 | .191 |
| Cholesterol, mean ± SD | 158.42 ± 37.31 | 156.74 ± 36.21 | .701 |
| Transferrin, mean ± SD | 175.58 ± 34.79 | 175.54 ± 33.06 | .991 |
| Functional | N = 149 | N = 109 | |
| SPPB score, mean ± SD | 9.0 ± 2.8 | 8.9 ± 2.8 | .829 |
| Functional categories, n (%) | |||
| >10 | 63 (42.3) | 37 (33.9) | .317 |
| 8–10 | 42 (28.2) | 39 (35.8) | |
| <8 | 44 (29.5) | 33 (30.3) |
Chi-square test.
PEW, protein-energy wasting; SGA, Subjective Global Assessment.
Table 4:
Differences between final and basal scores in mean nutritional and functional parameters according to Fried phenotype.
| Variable | Total | Non-frail | Pre-frail | Frail | P a |
|---|---|---|---|---|---|
| MIS | −0.39 ± 4.01 (N = 130) | −1.24 ± 2.48 (N = 42) | −0.11 ± 4.04 (N = 55) | 0.21 ± 5.30 (N = 33) | .238 |
| Albumin | 0.05 ± 0.39 (N = 130) | 0.15 ± 0.25 (N = 42) | 0.00 ± 0.31 (N = 55) | −0.02 ± 0.59 (N = 33) | .101 |
| Cholesterol | −3.79 ± 28.20 (N = 129) | 0.90 ± 24.00 (N = 41) | −2.16 ± 28.42 (N = 55) | −12.33 ± 31.46 (N = 33) | .113 |
| Transferrin | −1.54 ± 30.00 (N = 130) | 1.19 ± 25.39 (N = 42) | −1.09 ± 29.54 (N = 55) | −5.76 ± 36.06 (N = 33) | .606 |
| SPPB | −0.33 (1.76) | −0.08 (1.19) | −0.80 (2.02) | 0.39 (1.79) | .025 |
ANOVA test. The P-value shows whether there is a difference in the evolution in each of the three groups between basal and final visits.
Table 5:
Differences in Fried phenotype according to nutritional intervention.
| Baseline visit | Final visit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nutritional intervention | Total | NA | Non-frail | Pre-frail | Frail | NA | Non-frail | Pre-frail | Frail |
| Monitoring | 82 (100) | 1 (1.2) | 36 (43.9) | 35 (42.7) | 10 (12.2) | 37 (45.1) | 17 (20.7) | 16 (19.5) | 12 (14.6) |
| Diet diary | 41 (100) | 1 (2.4) | 12 (29.3) | 18 (43.9) | 10 (24.4) | 9 (22.0) | 9 (22.0) | 13 (31.7) | 10 (24.4) |
| Diet diary + enrichment | 16 (100) | 1 (6.3) | 9 (56.3) | 6 (37.5) | 7 (43.8) | 1 (6.3) | 2 (12.5) | 6 (37.5) | |
| Diet diary + supplements | 23 (100) | 3 (13.0) | 4 (17.4) | 16 (69.6) | 11 (47.8) | 1 (4.3) | 2 (8.7) | 9 (39.1) | |
| IDPN | 4 (100) | 1 (25.0) | 3 (75.0) | 1 (25.0) | 3 (75.0) | ||||
All data are presented as N (%).
NA, not available.
The functional assessment (SPPB) was evaluated for 149 patients at the baseline visit and 109 at the final visit (Table 3). No statistically significant differences were observed between the two timepoints, or between the number of patients with low, moderate or normal physical status (P = .317). However, when the change between baseline and final functional status was evaluated against the Fried phenotypes, a significant change was found in SPPB scores, which decreased slightly in non-frail (−0.08 ± 1.19, N = 40) and pre-frail patients (−0.80 ± 2.02, N = 49), and increased in frail patients (0.39 ± 1.79, N = 18) (P = .025).
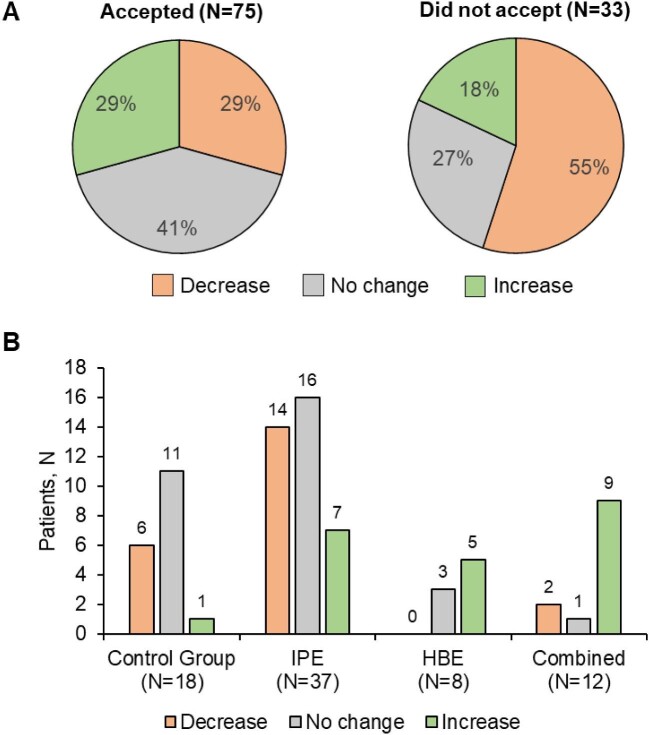
Of 159 patients, 20 (12.6%) patients were advised to start home-based exercise, 80 (50.3%) intradialytic exercise and 25 (15.7%) a combination of home-based exercise and intradialytic exercise, and 33 (20.8%) to continue their routine physical exercise (1 patient did not receive a recommendation for medical reasons). However, of 108 patients assessed, only 75 (69.4%) accepted to follow the physical exercise proposed, while 33 (30.5%) refused to do any exercise or could not do any for other reasons (e.g. underlying pathologies, difficulty in understanding the regimen, anatomical characteristic incompatible with the continued execution of the program). Of all the patients who agreed to participate in any of the proposed physical exercise modalities, 29% had an improvement in their final SPPB score compared with baseline, in 41% it remained the same, while in 29% there was a decrease (Fig. 3A). Of the patients who declined to participate and did not perform any type of prescribed exercise, in 18% the SPPB score increased, in 27% there was no change and in 55% the SPPB score decreased. Of the patients who accepted the recommendations for the exercise program, 7 patients out of 37 (18.9%) with IPE showed an improvement in SPPB scores (Fig. 3B).
Figure 3:
Changes in SPPB scores (decrease, no change, increase) according to the acceptance by the patients of the recommendations by the physiotherapist (A) and, in those who accepted, the type of exercise program followed (B).
A total of 24 falls were registered during the study period (9 falls in the year 2019; 9 in 2020; and 5 in 2021), or about 0.05 falls/patient/year. Most falls were observed in the post-dialysis period.
A total of 120 patients were evaluated by the psychologist. Of these, 38 (31.7%) were non-frail, 53 (44.2%) were pre-frail and 28 (23.3%) were frail. Frail patients scored higher in the EED for sadness and anxiety (4.4 ± 2.9 and 4.4 ± 2.9, respectively) than patients who were pre-frail (3.3 ± 3.1 and 2.7 ± 2.5) or non-frail (3.1 ± 2.6 and 2.8 ± 2.8). A total of 61 patients (50.8%) presented an EED score ≥4 and most of these (>60%) were frail (Fig. 4). The psychology intervention focused on these patients and 59 of them initiated a variety of interventions, with a mean of 4.2 visits per patient per year. Among them, nine patients received psychiatric treatment, six for major depressive disorder and three for substance disorder.
Figure 4:
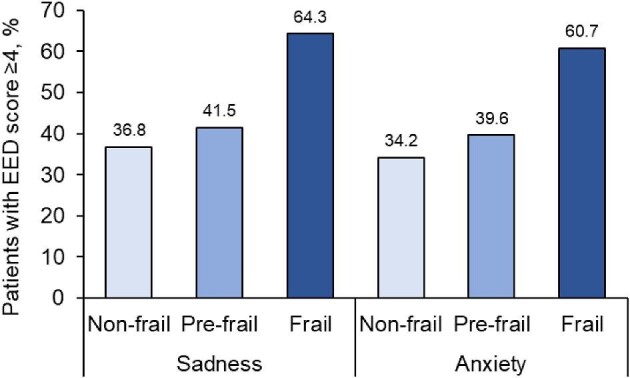
Psychological assessment according to Fried phenotype. Percentage of patients with EED score ≥4 (range 0–10) in the psychological evaluation. A total of 120 patients were assessed (non-frail = 38, pre-frail = 53, frail = 28). As indicated in Materials and methods, no 12-month evaluation was possible due to the limitations imposed by the COVID-19 pandemic.
DISCUSSION
In this observational study we described the effects of the implementation of a frailty screening program in a population of ESKD patients initiating hemodialysis, and the effects of multidisciplinary interventions involving nutritionists, physiotherapists, psychologists and nurses. The screening of patients starting hemodialysis showed that about a third of them could be considered frail, and another third pre-frail. The high prevalence of frailty in this patient population is consistent with previous reports [1, 6, 8], and highlights the need for improved awareness and evaluation of the special needs of this group of patients. Worse frailty scores in ESKD patients have been associated with worse outcomes [34], and frailty assessment could be used to inform clinical decisions and to improve counselling to patients and caregivers. In our study, the frailty assessment was used to guide a multidisciplinary group of healthcare specialists to offer individualized recommendations to the patients with the goal of improving nutritional, physical and psychological outcomes.
We found a high prevalence of patients with nutritional deficiency in our cohort, as 57% of patients presented MIS scores ≥6. After 12 months of follow-up, most patients (53%) had MIS scores 0–5, suggesting that the interventions to stabilize or improve nutrition were at least partially successful. A small decrease (6.8%) in patients who were at risk for or already catalogued with protein-energy wasting was observed. Different types of nutritional interventions [food enrichment, oral nutritional supplements, intradialytic parenteral nutrition (IDPN)] were qualitatively evaluated, and it is likely that they contributed to improve MIS and protein-energy wasting results, especially in women and older patients. Our results suggest that frail and pre-frail patients required a more substantial nutritional intervention than non-frail patients. Recent work has shown that protein-rich foods/supplements should be used to improve nutritional status in ESKD patients in hemodialysis, achieving the greatest impact in those patients with the poorest baseline nutritional status [22]. A randomized study demonstrated that IDPN can help increase serum albumin, body weight, spontaneous oral intake and MIS in patients in hemodialysis [35]. A meta-analysis of randomized clinical trials studying the effects of oral nutritional supplements and IDPN in patients with maintenance dialysis therapy has shown that, although more studies are needed, modest improvement in nutritional status can be observed [36]. In our view, a periodic nutritional assessment is essential to evaluate the patient's condition, as early nutritional intervention reduces possible complications later.
Complementary to nutritional recommendations, physical activity interventions can also be implemented to prevent the loss of muscle mass and strength. In our study we observed a gain in functionality in frail patients, although globally, the SPPB score slightly decreased during the 12-month follow-up. At our hospital, both the physiotherapist and the nursing team aimed to convey to the patients the benefits of maintaining physical activity, not only during the dialysis sessions but also at their homes. Patients who accepted the recommendation to do physical exercise as part of their treatment obtained better results (70% presented improved or stable SPPB scores) compared with patients who did not accept the recommendation (50% improved or stable SPPB scores). It should be noted that during hemodialysis, the exercise was only aerobic, a form of exercise which is usually very well accepted and maintained. Combined cardiovascular and strength exercise seems to be the most effective, as seven out of nine patients who followed this recommendation presented an increase in SPPB scores. Most patients continuing their physical exercise routines (control group) or starting physical exercise at home were able to successfully maintain or increase SPPB scores. Prior studies have shown that exercise during hemodialysis can induce modest improvements in physical functioning and muscle strength [37–39], although the evidence from large randomized studies is limited and inconsistent [40].
Recent studies have concluded that focus on the identification of patients at risk, comprehensive assessment, and the implementation of prevention programs are required to reduce falls [41]. A global incidence of 0.85–1.60 falls per patient/year in hemodialysis patients has been reported [42]. In this study a remarkably low rate of 0.05 falls per patient/year was observed. At our hospital, frailty screening and the associated categorization of patients according to frailty allowed nurses to focus resources on those patients at highest risk of falling. Some patients received help for home tasks and advice on how to facilitate movement. As previous studies show that falls usually occur after dialysis sessions [43], a period at which the patient is most vulnerable, the nursing staff in charge of helping the patients was increased specifically at those periods.
Overall, although the results of our study showed that there was an increase in the percentage of frail patients after 12 months, the data suggest that the nutritional and physical interventions helped to avoid further deterioration of pre-frail and frail patients. In addition, the implementation of the frailty screening program led to major improvements in the management of the hemodialysis unit, the optimization of the nurses’ workloads and more efficient allocation of resources. Similar projects have been implemented elsewhere, with generally positive results [7, 8]. In recent years, the paradigm of quality of care for patients with ESKD has changed, making necessary the introduction of models that include evaluation of frailty in clinical practice to improve resource management. The healthcare models of ESKD patients need to move from an approach segmented by specialties to a more integrated vision which considers the social situation and the patient's experience of their illness and their family context. As frailty in hemodialysis is considered a predictor of adverse outcomes such as increased hospitalization, loss of mobility, comorbidities and decreased survival [1, 4, 44–47], it is urgent to clarify the reasons that contribute to frailty decline and establish protocols aimed at mitigating them. A recent comparative study of frailty scales and clinical outcomes has shown that the Fried Frailty Phenotype used in this study was significantly associated with clinical events such as hospitalizations, fractures and/or all-cause mortality [12].
Some limitations of our study must be considered when interpreting the data. Firstly, patients were not randomized into control and intervention groups, as it seemed unethical not to treat the patients with severe malnutrition or limited physical function after the detection at screening. However, although this fact limits the overall conclusions, the results of the study still provided us with better understanding of the patients’ nutritional and physical needs, as well as the necessary interventions to mitigate them. Secondly, some groups of patients might have been over- or underrepresented, as the inclusion criteria were unrestrictive to allow for a broad representation of ESKD patients in hemodialysis at our hospital. This is especially significant because most of the study was carried out during the COVID-19 pandemic, which caused a huge stress in the healthcare system and forced many patients to alter their habits. For example, the limitation of mobility due to the lockdown worsened the functional capacity of the frail population.
The main strengths of our study are the number of patients evaluated and the integrative approach to healthcare, which aimed to describe numerous interventions by different specialists in the same cohort. Although clinical trials focused on specific interventions in selected patients are necessary, our study highlights the importance of frailty screening in the general population of hemodialysis patients and the diversity of measures that can be adopted for their care.
In conclusion, the implementation of a program of assessment of frailty, together with the coordinated action of a multidisciplinary team of nutritionists, physiotherapists, psychologists and nurses, improved the health outcomes for ESKD patients at the Barcelona Hospital Clinic. Frailty assessment helped in informing patients of their prognosis at the dialysis initiation, and advising on individual nutritional, physical or psychological needs. Counseling is especially important in light of the current aging of the population, which leads to increased numbers of elderly ESKD patients in need of hemodialysis. For this reason, health professionals should include frailty assessment in their clinical practice and incorporate strategies that meet the needs of this fragile patient population.
ACKNOWLEDGEMENTS
The authors wish to thank all the patients who participated in this study, and the nursing team who collaborated in data collection and curation, and to the medical team. Francisco López de Saro (Trialance SCCL) provided medical writing assistance in the development of this manuscript.
Contributor Information
Marta Arias-Guillén, Department of Renal Transplantation and Nephrology, Hospital Clínic, Barcelona, Spain.
Bárbara Romano, Department of Endocrinology and Nutrition, Hospital Clínic, Barcelona, Spain.
Anna Yuguero-Ortiz, Department of Renal Transplantation and Nephrology, Hospital Clínic, Barcelona, Spain; Department of Orthopedic Surgery and Traumatology, Hospital Clínic, Barcelona, Spain.
Ana López-Lazcano, Clinical Health Psychology Section, Psychiatry and Clinical Psychology Service, Institute of Neurosciences, Hospital Clínic, Barcelona, Spain.
Sonia Guerrero, Department of Renal Transplantation and Nephrology, Hospital Clínic, Barcelona, Spain.
Vanesa Villegas, Department of Renal Transplantation and Nephrology, Hospital Clínic, Barcelona, Spain.
Mar Martínez, Department of Renal Transplantation and Nephrology, Hospital Clínic, Barcelona, Spain.
Nuria Clemente, Department of Renal Transplantation and Nephrology, Hospital Clínic, Barcelona, Spain.
Miquel Gómez, Laboratori Experimental de Nefrologia i Trasplantament (LENIT), Fundació Clínic per la Recerca Biomèdica (FCRB), Hospital Clínic de Barcelona, Barcelona, Spain.
Lida Rodas, Department of Renal Transplantation and Nephrology, Hospital Clínic, Barcelona, Spain.
José Jesús Broseta, Department of Renal Transplantation and Nephrology, Hospital Clínic, Barcelona, Spain.
Marta Quintela, Department of Renal Transplantation and Nephrology, Hospital Clínic, Barcelona, Spain.
Francisco Maduell, Department of Renal Transplantation and Nephrology, Hospital Clínic, Barcelona, Spain.
Beatriu Bayés, Department of Renal Transplantation and Nephrology, Hospital Clínic, Barcelona, Spain.
AUTHORS’ CONTRIBUTIONS
M.A.-G. and M.Q. conceptualized and designed the study. M.A.-G. collected the data and contributed to its analysis and interpretation. M.A.-G., F.M. and B.B. wrote-up the manuscript. B.R., A.Y.-O. and A.L.-L. conducted the investigation. B.R., A.Y.-O., A.L.-L., M.G. and J.J.B. analyzed and interpretated the data. S.G., V.V., M.M., N.C. and L.R. contributed towards data collection. All authors read and approved the final manuscript.
FUNDING
The authors did not receive funding to conduct this study.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
CONFLICT OF INTEREST STATEMENT
The authors declare no financial support for the project. F.M. has received consultancy fees and lecture fees from Baxter, Fresenius Medical Care, Medtronic, Nipro, Toray and Vifor. The other authors declare no conflicts of interest.
REFERENCES
- 1. Lee H-J, Son Y-J.. Prevalence and associated factors of frailty and mortality in patients with end-stage renal disease undergoing hemodialysis: a systematic review and meta-analysis. Int J Environ Res Public Health 2021;18:3471–83. 10.3390/ijerph18073471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith G, Avenell A, Band MMet al. Associations between frailty, physical performance, and renal biomarkers in older people with advanced chronic kidney disease. Eur Geriatr Med 2021;12:943–52. 10.1007/s41999-021-00478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clegg A, Young J, Iliffe Set al. Frailty in elderly people. Lancet 2013;381:752–62. 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morley JE, Vellas B, Abellan van Kan Get al. Frailty Consensus: a call to action. J Am Med Dir Assoc 2013;14:392–7. 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johansen KL, Chertow GM, Jin Cet al. Significance of frailty among dialysis patients. J Am Soc Nephrol 2007;18:2960–7. 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 6. Chowdhury R, Peel NM, Krosch Met al. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr 2017;68:135–42. 10.1016/j.archger.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 7. Young HML, Ruddock N, Harrison Met al. Living with frailty and haemodialysis: a qualitative study. BMC Nephrol 2022;23:260–72. 10.1186/s12882-022-02857-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nixon AC, Brown J, Brotherton Aet al. Implementation of a frailty screening programme and Geriatric Assessment Service in a nephrology centre: a quality improvement project. J Nephrol 2021;34:1215–24. 10.1007/s40620-020-00878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zanotto T, Mercer TH, van der Linden MLet al. Screening tools to expedite assessment of frailty in people receiving haemodialysis: a diagnostic accuracy study. BMC Geriatr 2021;21:411–21. 10.1186/s12877-021-02356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson BM, Qasim M, Correa Get al. Correlations, agreement and utility of frailty instruments in prevalent haemodialysis patients: baseline cohort data from the FITNESS study. Clin Kidney J 2022;15:145–52. 10.1093/ckj/sfab137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fried LP, Tangen CM, Walston Jet al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 12. Imamura K, Yamamoto S, Suzuki Yet al. Comparison of the association between six different frailty scales and clinical events in patients on hemodialysis. Nephrol Dial Transplant 2023;38:455–62. 10.1093/ndt/gfac047. [DOI] [PubMed] [Google Scholar]
- 13. Rockwood K, Song X, MacKnight Cet al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rockwood K, Mitnitski A.. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011;27:17–26. 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 15. Rolfson DB, Majumdar SR, Tsuyuki RTet al. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006;35:526–9. 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chao C-T, Hsu Y-H, Chang P-Yet al. Simple self-report FRAIL scale might be more closely associated with dialysis complications than other frailty screening instruments in rural chronic dialysis patients. Nephrology (Carlton) 2015;20:321–8. 10.1111/nep.12401. [DOI] [PubMed] [Google Scholar]
- 17. van Loon IN, Goto NA, Boereboom FTJet al. Frailty screening tools for elderly patients incident to dialysis. Clin J Am Soc Nephrol 2017;12:1480–8. 10.2215/CJN.11801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumarasinghe AP, Chakera A, Chan Ket al. Incorporating the Clinical Frailty Scale into routine outpatient nephrology practice: an observational study of feasibility and associations. Intern Med J 2021;51:1269–77. 10.1111/imj.14892. [DOI] [PubMed] [Google Scholar]
- 19. Wilkinson TJ, McAdams-DeMarco M, Bennett PNet al. Global Renal Exercise Network . Advances in exercise therapy in predialysis chronic kidney disease, hemodialysis, peritoneal dialysis, and kidney transplantation. Curr Opin Nephrol Hypertens 2020;29:471–9. 10.1097/MNH.0000000000000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoshino J. Renal rehabilitation: exercise intervention and nutritional support in dialysis patients. Nutrients 2021;13:1444. 10.3390/nu13051444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noor H, Reid J, Slee A.. Resistance exercise and nutritional interventions for augmenting sarcopenia outcomes in chronic kidney disease: a narrative review. J Cachexia Sarcopenia Muscle 2021;12:1621–40. 10.1002/jcsm.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hendriks FK, Kooman JP, van Loon LJC.. Dietary protein interventions to improve nutritional status in end-stage renal disease patients undergoing hemodialysis. Curr Opin Clin Nutr Metab Care 2021;24:79–87. 10.1097/MCO.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalantar-Zadeh K, Kopple JD, Block Get al. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 2001;38:1251–63. 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 24. Kalantar-Zadeh K, Kleiner M, Dunne Eet al. A modified quantitative subjective global assessment of nutrition for dialysis patients. Nephrol Dial Transplant 1999;14:1732–8. 10.1093/ndt/14.7.1732. [DOI] [PubMed] [Google Scholar]
- 25. Fouque D, Kalantar-Zadeh K, Kopple Jet al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 2008;73:391–8. 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 26. Arias-Guillén M, Collado S, Coll Eet al. Prevalence of protein-energy wasting in dialysis patients using a practical online tool to compare with other nutritional scores: results of the Nutrendial study. Nutrients 2022;14:3375. 10.3390/nu14163375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guralnik JM, Simonsick EM, Ferrucci Let al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 28. Downton J. Falls in the Elderly. London, UK: Edward Arnold, 1993. [Google Scholar]
- 29. Mahoney FI, Barthel DW.. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- 30. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975;23:433–41. 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 31. Lawton MP, Brody EM.. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 32. Graf C. The Lawton instrumental activities of daily living scale. Am J Nurs 2008;108:52–62; quiz 62–3. 10.1097/01.NAJ.0000314810.46029.74. [DOI] [PubMed] [Google Scholar]
- 33. García-Llana H, Rodríguez-Rey R, Rollán de la Sota MJet al. Desarrollo de un instrumento para la evaluación del malestar emocional para pacientes renales en diálisis. Enferm Nefrol 2016;19:349–57. 10.4321/S2254-28842016000400006. [DOI] [Google Scholar]
- 34. Pugh J, Aggett J, Goodland Aet al. Frailty and comorbidity are independent predictors of outcome in patients referred for pre-dialysis education. Clin Kidney J 2016;9:324–9. 10.1093/ckj/sfv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kittiskulnam P, Banjongjit A, Metta Ket al. The beneficial effects of intradialytic parenteral nutrition in hemodialysis patients with protein energy wasting: a prospective randomized controlled trial. Sci Rep 2022;12:4529. 10.1038/s41598-022-08726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu PJ, Ma F, Wang QYet al. The effects of oral nutritional supplements in patients with maintenance dialysis therapy: a systematic review and meta-analysis of randomized clinical trials. PLoS One 2018;13:e0203706. 10.1371/journal.pone.0203706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manfredini F, Mallamaci F, D'Arrigo Get al. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol 2017;28:1259–68. 10.1681/ASN.2016030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferrari F, Helal L, Dipp Tet al. Intradialytic training in patients with end-stage renal disease: a systematic review and meta-analysis of randomized clinical trials assessing the effects of five different training interventions. J Nephrol 2020;33:251–66. 10.1007/s40620-019-00687-y. [DOI] [PubMed] [Google Scholar]
- 39. Bündchen DC, Sousa H, Afreixo Vet al. Intradialytic exercise in end-stage renal disease: an umbrella review of systematic reviews and/or meta-analytical studies. Clin Rehabil 2021;35:812–28. 10.1177/0269215520986784. [DOI] [PubMed] [Google Scholar]
- 40. Jeong JH, Biruete A, Tomayko EJet al. Results from the randomized controlled IHOPE trial suggest no effects of oral protein supplementation and exercise training on physical function in hemodialysis patients. Kidney Int 2019;96:777–86. 10.1016/j.kint.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Young HML, Ruddock N, Harrison Met al. The impact of falls: a qualitative study of the experiences of people receiving haemodialysis. Int J Environ Res Public Health 2022;19:3873. 10.3390/ijerph19073873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shirai N, Inoue T, Ogawa Met al. Relationship between nutrition-related problems and falls in hemodialysis patients: a narrative review. Nutrients 2022;14:3225. 10.3390/nu14153225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pérez-Gurbindo I, Angulo Carrere MT, Arribas Cobo Pet al. Haemodialysis patients have worse postural balance with an associated risk of falls. Nefrologia (Engl Ed) 2020;40:655–63. 10.1016/j.nefroe.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 44. Clark D, Matheson K, West Bet al. Frailty severity and hospitalization after dialysis initiation. Can J Kidney Health Dis 2021;8:205435812110233. 10.1177/20543581211023330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garcia-Canton C, Rodenas A, Lopez-Aperador Cet al. Frailty in hemodialysis and prediction of poor short-term outcome: mortality, hospitalization and visits to hospital emergency services. Ren Fail 2019;41:567–75. 10.1080/0886022X.2019.1628061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oki R, Hamasaki Y, Tsuji Set al. Clinical frailty assessment might be associated with mortality in incident dialysis patients. Sci Rep 2022;12:17651. 10.1038/s41598-022-22483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Soldati A, Poggi MM, Azzolino Det al. Frailty index and adverse outcomes in older patients in haemodialysis. Arch Gerontol Geriatr 2022;101:104673. 10.1016/j.archger.2022.104673. [DOI] [PubMed] [Google Scholar]
- 48. Charlson ME, Pompei P, Ales KLet al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 49. Cabrera González D, Menéndez Caicoya A, Fernández Sánchez Aet al. Evaluación de la fiabilidad y validez de una escala de valoración social en el anciano. Aten Primaria 1999;23:434–40. [PubMed] [Google Scholar]
- 50. García González JV, Díaz Palacios E, Salamea García Aet al. An evaluation of the feasibility and validity of a scale of social assessment of the elderly. Aten Primaria 1999;23:434–40. [PubMed] [Google Scholar]
- 51. Arenas MD, Alvarez-Ude F, Angoso Met al. Functional dependency evaluation of hemodialysis patients: a multicentric study. Nefrologia 2006;26:600–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding author upon reasonable request.