ABSTRACT
Background and aims
Patients on hemodialysis (HD) or peritoneal dialysis (PD) often have insufficient energy and protein intake, resulting in poor nutritional status and adverse outcomes. Oral nutritional supplements (ONSs) are the most commonly used to increase such patients’ energy and protein intakes.
Methods
In this systematic review and meta-analysis, we analyzed studies on nutritional status, inflammatory markers, and electrolyte levels in patients on dialysis receiving ONSs. We searched four electronic databases from inception until 31 December 2022, for randomized controlled trials comparing ONS treatment versus placebo or routine care.
Results
22 studies with 1185 patients on dialysis were included in our meta-analysis. Compared with the control group, the ONS group exhibited significantly increased serum albumin levels [1.26 g/l (95%CI, 0.50–2.02, P < 0.0001; I2 = 80.4%)], body mass indexes (BMIs) [0.30 kg/m2 (95%CI, 0.09–0.52, P = 0.005; I2 = 41.4%)], and handgrip strength (HGS) [0.96 kg (95%CI, 0.07–1.84, P = 0.034; I2 = 41.4%)] from baseline to the end of intervention. No significant differences were observed between the groups in lean body mass, phase angle, C-reactive protein, and serum phosphorus and potassium levels. In terms of improving albumin, the subgroup analyses show that ONS use seems to be more inclined to three variations: HD patients, short-term use, and non-intradialytic supplementation.
Conclusion
In conclusion, ONS use can improve the nutritional status of patients on dialysis in terms of their serum albumin, BMI, and HGS without significant effects on serum phosphorus, potassium, and C-reactive protein levels. However, it remains uncertain whether these results translate to improvement in clinically relevant outcomes. Large-scale high-quality studies are still required in this population.
Keywords: albumin, chronic kidney disease, hemodialysis, nutritional status, oral nutritional supplements, peritoneal dialysis
INTRODUCTION
Hemodialysis (HD) and peritoneal dialysis (PD) are the two common forms of dialysis therapy for end-stage renal disease. Metabolic waste that is typically produced by food intake can be depurated by dialysis. Patients with chronic kidney disease (CKD) undergoing HD or PD are often prescribed dietary restrictions—in particular, restrictions involving foods rich in sodium, potassium, and phosphorus. In addition, the intake of energy and/or protein in dialysis patients is often reduced due to gastrointestinal symptoms such as nausea or anorexia. Thus, patients on dialysis commonly develop malnutrition and protein-energy wasting (PEW) [1–3]. PEW, a state of multiple metabolic and nutritional abnormalities resulting from a combination of insufficient intake, metabolic acidosis, uremic toxins, inflammation, and hypercatabolism [4] also causes poor quality of life and increases the risk of adverse outcomes [5]. Given the influence of such energy wasting on patients with CKD or those undergoing dialysis, the International Society of Renal Nutrition and Metabolism introduced the term PEW in 2008 to describe the state of decreased body stores of protein and energy.
The latest Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (2020) state that adequate protein and energy intake in the regular diet is critical for patients on HD and PD [6]. However, the energy and protein intake from regular meals is generally lower than that of the recommended amount for these patients [7–9]. This low intake is a critical factor in the etiology of PEW in patients with CKD, especially those undergoing maintenance dialysis therapy (MDT) [7, 8]. Consequently, considerable research effort has been directed toward developing effective strategies for maintaining or improving the nutritional status of patients on dialysis, with the most common approach being providing food and nutritional supplements [10].
Several observational studies have indicated that consuming oral nutritional supplements (ONSs) or extra snacks improves nutritional status in terms of albumin or anthropometric measures in patients on dialysis [11–14]. Moreover, some studies have reported that ONS use is associated with improved outcomes in patients on HD [15–18]. In dialysis patients where oral dietary intake from regular meals cannot maintain adequate nutritional status, nutritional supplementation, administered orally, enterally, or parenterally, is shown to be effective in replenishing protein and energy stores [19]. In clinical practice, the ONS use is the preferred pathway. However, many problems related to this practice, such as postprandial hypotension, gastrointestinal symptoms, and reduced treatment efficiency, have led to the favorability of its implementation being debated and to inconsistencies in the policies related to in-center nutrition within dialysis clinics [20, 21]. Therefore, randomized controlled trials (RCTs) are required to clarify the risk–benefit profile of ONS use in patients on dialysis and to determine whether ONS use can improve the prognosis of these patients by improving their nutritional status.
No consensus exists on the type, time of initiation, or duration of use of enteral nutrition or nutritional supplementation for patients on MDT. Although our previous meta-analysis of this topic [22] included many RCTs [23–38], most had a small sample size and were of low quality. Moreover, considerable heterogeneity was noted among these studies. These factors led to a very low level of evidence for ONS use improving the nutritional status of patients on dialysis [22]. Similarly, a recent meta-analysis concluded that protein-based ONS use can effectively improve the nutritional status in terms of serum albumin in patients with CKD requiring dialysis, albeit with high heterogeneity among the included studies [39]. Six relevant RCTs have been published [40–45] since our previous meta-analysis [22], and one had a large sample size (N = 240) [42]. Therefore, we conducted an updated systematic review and meta-analysis of RCTs to further quantitatively evaluate the effect of ONS use versus routine or placebo care on patients on dialysis.
MATERIALS AND METHODS
Search strategy
The present meta-analysis was conducted and reported on the basis of the PRISMA guidelines [46]. We searched the PubMed, Embase, ClinicalTrials.gov, and Cochrane Library databases for eligible studies from inception to 31 December 2022 (all databases were retrieved using ‘age >18 years’ as a filter). Studies investigating the association between ONS use and the nutritional status, with the studies’ data including those on serum albumin levels, body mass index (BMI), lean body mass, handgrip strength (HGS), electrolyte levels, and inflammation levels, of adult patients on dialysis were retrieved using the following search terms: dialysis, hemodialysis, haemodialysis, hemofiltrition, peritoneal dialysis, renal replacement therapy, chronic renal failure, end-stage renal disease, chronic kidney disease, CKD, nutrition supplement*, nutritional support, oral nutritional supplement, ONS, oral supplement*, nutrient*, macronutrients, calorie supplement*, energy supplement*, protein supplement*, and amino acid supplement*. The complete search strategy is presented in Supplementary Table S1. In addition, we manually searched the reference lists of the retrieved articles for other potentially relevant studies.
Inclusion and exclusion criteria
We selected RCTs in which patients on dialysis (HD or PD) were administered ONSs as the intervention. The other inclusion criteria were as follows: (i) included a comparison of the effects of oral non-protein (carbohydrates or fat/lipids) or protein/amino acid or energy-based mixed nutritional supplements with or without micronutrients with those of standard care with or without placebo care; (ii) reported at least one of the following: BMI, lean body mass (measured using dual-energy X-ray absorptiometry or bioelectrical impedance), HGS, phase angle, serum albumin levels, phosphorus level, potassium level, and C-reactive protein (CRP) level; (iii) used a commercial or a noncommercial ONS that provided calories; and (iv) included only patients older than 18 years. The details are presented in Table 1.
Table 1:
Summary of inclusion and exclusion criteria applied during evaluation of studies for review.
| Selection criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Adults studies (age ≥18 years) | Animal data |
| Nutrition status (either well nourished or malnourished) Patients on dialysis (of any type) |
Dialysis patients with HIV infection or acute infection | |
| Intervention Comparison |
All studies using oral nutritional supplements with any macronutrient (carbohydrate, fat, or protein/amino acid) Setting in hospital or community (outpatient or home) Placebo, routine care, or no supplementation |
Feeds only given non-caloric nutrients or Beta-hydroxy beta-methylbutyrate (HMB) or concomitantly given keto acid or keto analogs Without control group |
| Outcome measures Study type |
Serum albumin level; BMIs; fat-free mass or lean body mass; handgrip strength; phase angle; Electrolytes (serum potassium and phosphate); C-reactive protein Randomized controlled trials |
Studies without any predetermined outcome measure Non-randomized studies |
We excluded RCTs that did not report mean [standard deviation (SD)] changes in BMI, lean body mass, HGS, phase angle, serum albumin levels, phosphorus levels, potassium levels, and CRP for the intervention and control groups. In addition, abstracts without full articles, reviews, and case reports were excluded.
Data extraction
Two independent reviewers (L.P.J. and G.J.Y.) extracted data from the full texts of the eligible studies. Disagreements were resolved through discussion with a third reviewer (Y.K.). The following data were extracted: the name of the first author, publication year, sample size of each comparison group, duration of interventions, study population, intervention modality in case and control groups, and participant characteristics (BMI, lean body mass, HGS, phase angle, serum albumin levels, phosphorus levels, potassium levels, and CRP before and after the intervention). If the trial was a crossover study, the outcomes at the end of the first phase (before the crossover) were analyzed.
Data synthesis and statistical analysis
The changes in each outcome were reported as differences between mean values before and after the intervention. If the means and SDs of the changes from baseline were specified in the papers, they were directly used. If not, the mean changes in the observed parameters were calculated by subtracting the baseline values from the values after the intervention, and the SD of the difference was calculated as follows [47]:
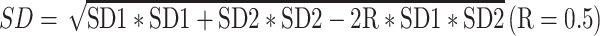 |
If a two-arm design was used to implement interventions of the same nature, the two arms were merged using the following method [22]:
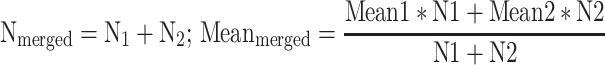 |
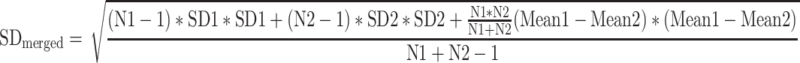 |
Meta-analysis was performed using STATA v.12.0 (Stata, College Station, TX, USA) and Review Manager v.5.3 (Cochrane Collaboration). The effects of the intervention are presented as mean differences (MDs) or standardized MDs, when appropriate. The heterogeneity between studies was evaluated using the I2 index. If I2 > 0%, a random-effects model was used, and subgroup analysis was further required to identify the source of the heterogeneity. If not, the fixed-effects model was applied. Statistical significance was defined as two-tailed P < 0.05. Publication bias was assessed using funnel plots and the Egger test.
RESULTS
Study characteristics
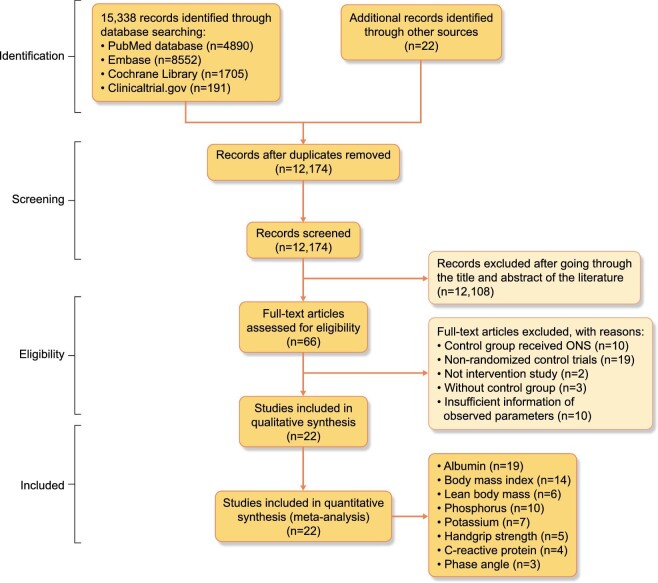
Figure 1 illustrates the flowchart of the study selection. The initial search yielded 15 338 entries. After removing duplicate entries and excluding irrelevant studies through title and abstract review, we retrieved the full texts of 66 studies for evaluation. Finally, 22 studies were included in the meta-analysis [23–38, 40–45]. The characteristics of the included studies are summarized in Table 2.
Figure 1:
Flowchart of study selection.
Table 2:
Characteristics of the studies included in the meta-analysis.
| Reference (published year) | Patients (n) in each group | Population description | Intervention modality of case group | Intervention modality of control group | Study design and duration | Main outcomes measures in analysis |
|---|---|---|---|---|---|---|
| Tomayko EJ et al. (2015) [23] | Case (11 and 12); control (15) | MHD patients, treatment for ≥3 months, ≥3 days/week, nutritional status was not stated | 27 g whey or 27 soy protein within beverage, consumed within 15 mins of dialysis; intradialytic supplementation | 2 g non-caloric powder within beverage, consumed within 15 mins of dialysis | Randomized, controlled, blinded; 6 mo |
Albumin, phosphorus, and potassium |
| Calegari A et al. (2011) [24] | Case (9); control (6) | Malnourished HD patients (SGA > 15 points) | Non-industrialized nutrition supplement (Thick mixed food), 355 kcal, 53% of carbohydrate, 10 g of protein, and 15 g of lipid; intradialytic supplementation | Routine nutritional guidance | Randomized, controlled, non-blinded, crossover; 3 mo (first phase) |
BMI, LBM, albumin, phosphorus, and potassium |
| Bolasco P et al. (2011) [25] | Case (15); control (14) | HD patients, thrice-weekly albumin <3.5 g/dl and BMI > 20 kg/m2, nutritional status was not stated | 12 g amino acid powder dissolved in water; non-intradialytic supplementation | No intervention | Randomized, controlled, non-blinded;3 mo | BMI, phase angle, LBM, albumin, and CRP |
| Tabibi H et al. (2010) [26] | Case (18); control (18) | Continuous ambulatory PD; nutritional status was not stated | 28 g packets of raw textured soy flour (containing 14 g of soy protein); non-intradialytic supplementation | Usual diet without consumption of soy-containing products | Randomized, controlled, non-blinded;8 weeks | Albumin |
| Imani H et al. (2009) [27] | Case (18); control (18) | Continuous ambulatory PD; nutritional status was not stated | 28 g packets of raw textured soy flour (containing 14 g of soy protein); non-intradialytic supplementation | Usual diet without consumption of soy-containing products | Randomized, controlled, non-blinded;8 weeks | Phosphorus |
| Fouque D et al. (2008) [28] | Case (37); control (29) | MHD patients, albumin <40 g/l and BMI <30 kg/m2; mildly nourished | 250 ml Renilon 7.5 daily, 500 kcal, containing 18.75 g protein and 15 mg phosphotus; non-intradialytic supplementation | Standard care | Multicenter, randomized, open-label, controlled; 3 mo |
BMI |
| González-Espinoza L et al. (2005) [29] | Case (13); control (15) | Continuous ambulatory PD for at least 1 month; malnourished | 22 g of high biological-value protein (egg albumin) daily; non-intradialytic supplementation | Conventional nutritional counseling | Randomized, open-label, controlled; 6 mo |
Albumin, phosphorus, and potassium |
| Morretti HD et al. (2009) [30] | Case (31); Control (18) | HD and PD patients; nutritional status was not stated | 15 g liquid hydrolyzed collagen protein (3 times per week for HD patients and 7 times per week for PD patients); non-intradialytic supplementation | No supplement | Randomized,controlled, non-blinded, crossover; 6 mo |
Albumin |
| Sohrabi Z et al. (2016) [31] | Case (23): control (23) | Regular HD patients with malnutrition | 15 g whey protein without vitamin E (three times per week); intradialytic supplementation | No intervention | Randomized, controlled, non-blinded;8 weeks | BMI, LBM, albumin, and phosphorus |
| Hung SC et al. (2009) [32] | Case (20); control (21) | Nondiabetic HD patients; malnourished | Daily use of one can of a commercially ONS (475 kcal, contained 16.6 g protein, 22.7 g fat, and 52.8 g carbohydrate); non-intradialytic supplementation | Without supplementation | Prospective, randomized, controlled, non-blinded; 12 weeks | BMI and albumin |
| Rattanasompattikul M et al. (2013) [33] | Case (22); Control (21) | MHD patients with Alb <40 g/l; nutritional status was not stated | 19 g protein combined with fish oil, borage oil, beta-carotene, vitamin C and E, zinc, and selenium; intradialytic supplementation | Placebo | Randomized, double-blind, controlled; 16 weeks |
Albumin, CRP, phosphorus, and potassium |
| Teixidó-Planas J et al. (2005) [34] | Case (35); control (30) | PD patients; nutritional status was not stated | 200 ml (200 kcal) of an oral supplement with mixed nutrients; non-intradialytic supplementation | Standard care | randomized, open-label, controlled; 12 mo |
Albumin and LBM |
| Sahathevan S, et al. (2018) [35] |
Case (37); control (37) |
Malnourished PD patients, with Alb <40 g/l and BMI < 24.0 kg/m2 | 27.4 g whey protein powder ingested post-meal plus dietary counseling; non-intradialytic supplementation | dietary counseling only | Randomized, controlled, open-label 6 mo |
Albumin, HGS, BMI, LBM, and phosphorus |
| Allman MA et al. (1990) [36] | Case (9)/Control (12) | Regular HD patients for >3 months; nutritional status was not stated | 100–150 g glucose-polymer (400–600 kcal) plus water-soluble vitamin; non-intradialytic supplementation | No energy supplement | Randomized controlled, non-blinded;6 mo | BMI, LBM, and albumin |
| Eustace JA et al. (2000) [37] | HD: Case (14); control (15) PD: Case (9); Control (9) |
HD and PD patients, albumin <3.8 g/dl); nutritional status was not stated | Daily 10.8 g EAA with meals; non-intradialytic supplementation | Placebo in appearance to the EAA tablets | Randomized, double-blind, controlled; 3 mo |
BMI, HGS and albumin |
| Sharma M et al. (2002) [38] | Case (16 and 10); control (14) | Malnourished; regular thrice-weekly MHD patients (for at least 1 month) and BMI <20 kg/m2 and albumin <4.0 g/dL | Standard home-prepared ONS: (500 kcal and 15 g protein) versus CKD-specific ONS (Reno care Ⅱ, Criticare, Mumbai, India: 500 kcal and 15 g protein); non-intradialytic supplementation | Dietary counseling no specific post-HD supplement | Randomized, controlled, non-blinded;1 mo | BMI, albumin, potassium and phosphorus |
| Jeong JH, et al. (2019) [40] | Case (45); control (44) | Regular HD patients for ≥3 months; nutritional status was not stated | 30 g whey protein mixed in 4–6 ounces of water; intradialytic supplementation | ∼150 g of a non-nutritive beverage during dialysis session | Randomized controlled, non-blinded; 12 mo | BMI, LBM, CRP, and albumin |
| Limwannata P et al. (2021) [41] | Case A (28); case B (30); control (28) | Regular HD patients for ≥3 months; Malnourished HD patients (Alb <38 g/l and energy intake <25 kcal/kg/day and protein intake <1 g/kg/day) | Case A: 370 kcal sachets of NEPRO (16.63 g whey protein, 33 g carbohydrate, and 19.76 g fat); Case B: 370 kcal sachets of ONCE dialyze (16.98 g whey protein, 41.19 g carbohydrate, and 16.45 g fat); non-intradialytic supplementation | Diet counseling without supplements | Randomized, non-blind, controlled; 30 days | BMI, HGS, albumin, phosphorus, and potassium |
| Yang Y et al. (2021) [42] | Case (120); Control (120) | Regular HD patients for >3 months; nutritional status was not stated | 60 ml (300 kcal) of an oral supplement once per day, and fat provides 97% of the total energy; non-intradialytic supplementation | Usual diet without supplements | Randomized, non-blind, controlled; 12 weeks |
BMI, phase angle, HGS, and albumin |
| Wen L et al. (2022) [43] | Case (52); control (52) | Regular HD patients for >3 months; malnourished(7-point SGA ≤ 5) | Non-protein calorie jelly, each serving contained 5.4 g of fat and 22.5 g of carbohydrate, twice a day; non-intradialytic supplementation | Usual diet without supplements | Randomized controlled, non-blinded; 6 mo | BMI, HGS, albumin, and phosphorus |
| Murtas S et al. (2022) [44] | Case (11); Control (11) | Regular HD patients for >3 months; nutritional status was not stated | Sachets of amino acid mixture. A total of 31.5 g of amino acids were given weekly; non-intradialytic supplementation | Sachets of placebo | Randomized, double-blind, controlled; 6 mo |
phase angle, Albumin, BMI, phosphorus, CRP, and potassium |
| Qin A et al. (2022) [45] | Case (20); Control (21) | Regular HD patients for ≥3 months; patients with PEW | 30 ml of an ONS, twice a day (supplied 300 kcal per day; 2.0 g of carbohydrates, and 26.9 g of lipids); non-intradialytic supplementation | dietary recommendations without supplements | Randomized, non-blind, controlled; two mo | Phosphorus |
MHD, maintenance hemodialysis; HD, hemodialysis; PD, peritoneal dialysis; HGS, handgrip strength; LBM, lean body mass; SGA, subjective global assessment; PEW, protein-energy wasting; ONS, oral nutritional supplement.
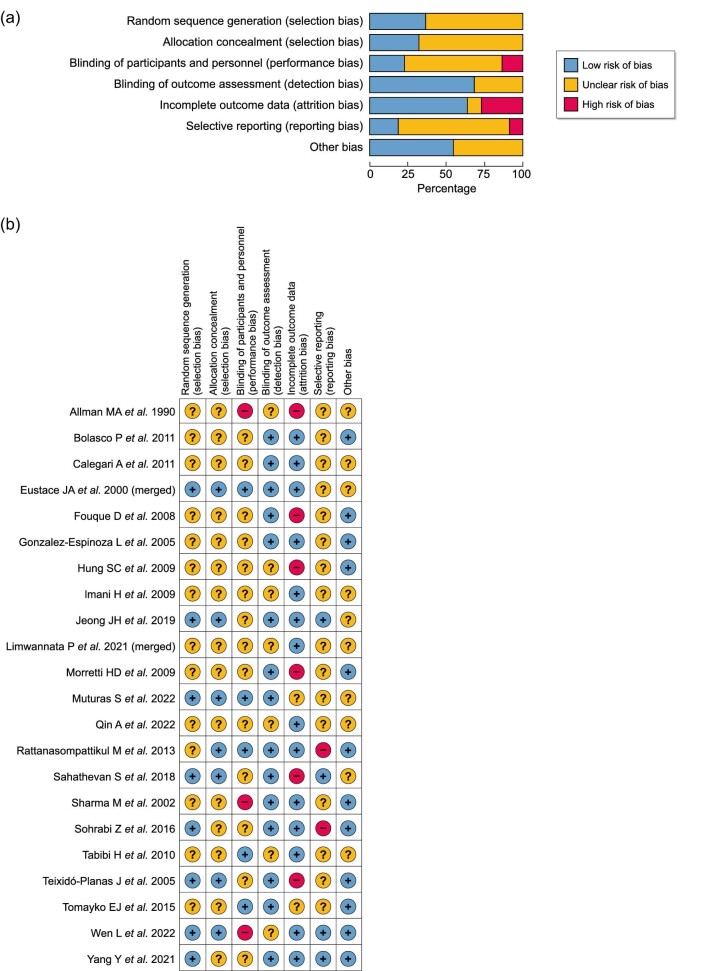
Quality assessment and risk of bias findings
A quality assessment of the included studies was performed with reference to the Cochrane Handbook for Systematic Reviews of Interventions (Fig. 2A)and B). The risk of bias assessment involved the following domains: random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, blinding of the outcome assessment, incomplete outcome data, selective outcome reporting, and other bias.
Figure 2:
(a) Risk of bias in seven domains for all included studies. (b) Risk of bias assessment across seven domains for each included study.
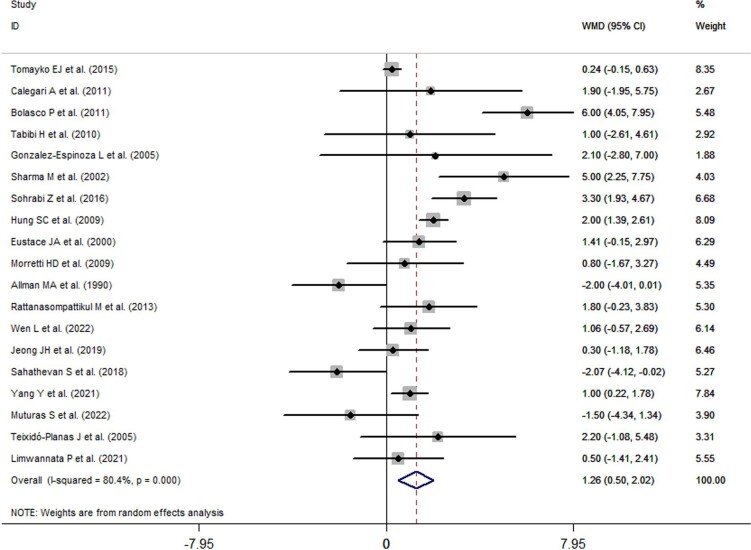
Overall effects of ONS use on serum albumin levels
The overall effects of ONS use on serum albumin levels are presented in Fig. 3. Nineteen trials reported changes in serum albumin levels before and after the intervention. Serum albumin levels significantly increased by 1.26 g/l (95% CI: 0.50–2.02, P = 0.001) in the ONS groups compared with the control groups. A significant degree of heterogeneity was observed (I2 = 80.4%, P < 0.001). Subsequently, a subgroup analysis was performed.
Figure 3:
Forest plots depicting the overall effect of ONS use on serum albumin levels.
Subgroup analysis of ONS use on serum albumin levels
Subgroup analyses were conducted on suspected variables, including the type of dialysis, intervention duration, supplementation timing, nutritional status, and type of ONS. The results are presented in Table 3.
Table 3:
Results of subgroup analyses of the effects of ONS use on serum albumin levels.
| Serum albumin (g/l) | |||||
|---|---|---|---|---|---|
| Subgroup | Effect size | 95% CI | I 2 | P value | |
| Type of dialysis | Hemodialysis (n = 14) | 1.51 | 0.65, 2.37 | 84.6% | 0.001 |
| PD (n = 5) | 0.21 | −1.58, 2.01 | 39.4% | 0.815 | |
| Intervention duration | <6 months (n = 10) | 2.32 | 1.38, 3.27 | 73.4% | <0.0001 |
| ≥6 months (n = 9) | −0.03 | −0.82, 0.76 | 42.9% | 0.939 | |
| Supplementation timing | Intradialytic (n = 5) | 1.38 | −0.03, 2.79 | 79.8% | 0.055 |
| Not intradialytic (n = 14) | 1.21 | 0.21, 2.21 | 78.4% | 0.018 | |
| Nutritional status | malnourished (n = 8) | 1.63 | 0.39, 2.88 | 73.5% | 0.01 |
| Malnourishment not specified (n = 11) | 1.01 | 0.05, 1.96 | 78.2% | 0.039 | |
| Type of ONS | Protein/amino acid (n = 11) | 1.24 | 0.02, 2.45 | 83.1% | 0.046 |
| Non-protein or mixed (n = 8) | 1.26 | 0.25, 2.27 | 70.2% | 0.014 | |
ONS, oral nutritional supplements; CI, confidence interval.
On the basis of the type of dialysis, the patients were divided into an HD group [23–25, 31–33, 36–38, 40–44] and a PD group [26, 29, 34, 35, 37]. The results revealed that ONS use significantly increased the serum albumin levels in patients on HD (1.51 g/l, 95% CI: 0.65–2.37, I2 = 84.6%, P = 0.001) but not in patients on PD.
On the basis of their intervention durations, the included studies were divided into a long-term intervention group (≥6 months) [23, 29, 30, 34–36, 40, 43, 44] and a short-term intervention group (<6 months) [24–26, 31–33, 37, 38, 41, 42]. This subgroup analysis indicated that short-term ONS use increased serum albumin levels (2.32 g/l, 95% CI: 1.38–3.27, I2 = 73.4%, P < 0.001) but long-term ONS use did not.
When the included studies were divided into intradialytic [23, 24, 31, 33, 40] and non-intradialytic [25, 26, 29, 30, 32, 34–38, 41–44] ONS groups on the basis of their supplementation timing, we observed that ONS significantly increased serum albumin levels in the non-intradialytic group (1.21 g/l, 95% CI: 0.21–2.21, I2 = 78.4%, P < 0.018) but not in the intradialytic group.
Other subgroup analyses indicated that ONS improved serum albumin levels in patients on dialysis with malnourished [24, 29, 31, 32, 35, 38, 41, 43] or unspecified malnourished status [23, 25, 26, 30, 33, 34, 36, 37, 40, 42, 44] and in those who were given protein/amino acid [23, 25, 26, 29–31, 33, 35, 37, 40, 44] or non-protein or mixed ONSs [24, 32, 34, 36, 38, 41–43].
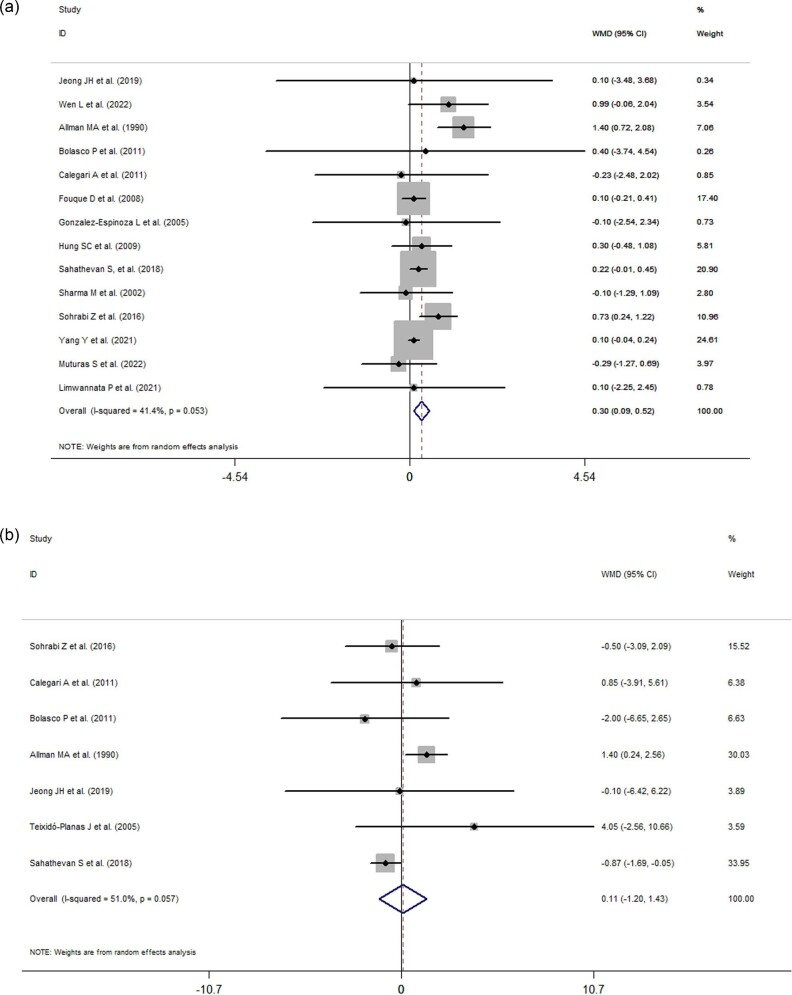
Effects of ONS use on BMI and lean body mass
The effects of ONS use on the BMIs and lean body masses of patients on dialysis are presented in Fig. 4A)and B. Of the included studies, 14 and 7 trials reported results related to or changes in BMI and lean body mass, respectively. When statistically pooled, the changes in BMI and lean body mass were 0.30 kg/m2 (95% CI: 0.09 to 0.52, I2 = 41.4%, P = 0.005), and 0.11 kg (95% CI: −1.20 to 1.43, I2 = 51%, P = 0.868), respectively, indicating that ONS use significantly increased the BMI but not lean body mass of patients on MDT.
Figure 4:
(a) Forest plots depicting the effect of ONS use on BMIs. (b) Forest plots depicting the effect of ONS use on lean body mass.
Effects of ONS use on handgrip strength and phase angle
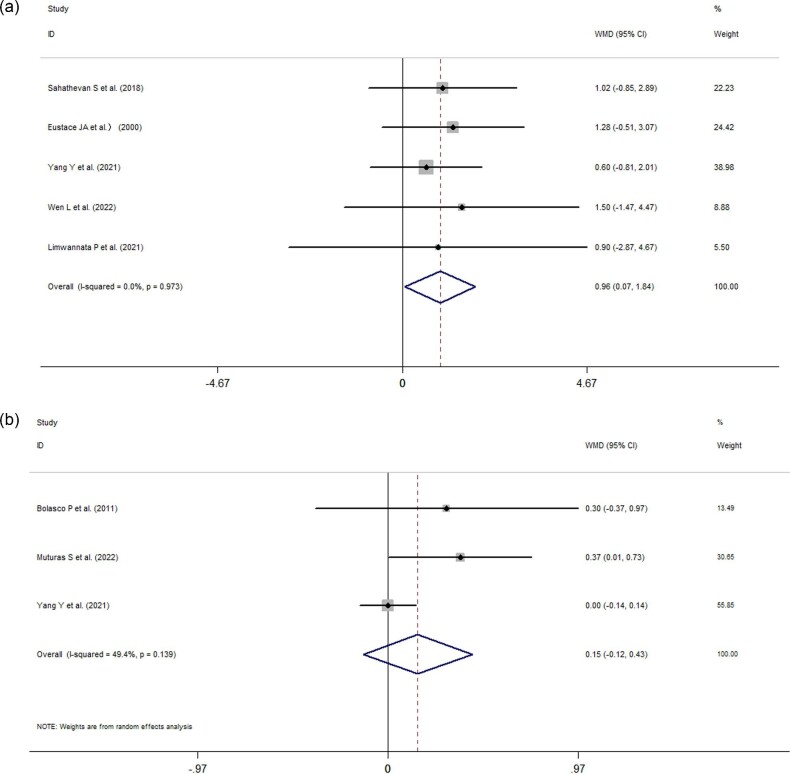
Five studies reported changes in HGS, and three studies described changes in phase angle, respectively. The pooled data indicated that ONS use changed HGS by 0.96 kg (95% CI: 0.07 to 1.84, P = 0.034, I2 = 0%, P = 0.973; Fig. 5A) and the phase angle by 0.15° (95% CI: −0.12 to 0.43, I2 = 49.4%, P = 0.274; Fig. 5B).
Figure 5:
(a) Forest plots depicting the effect of ONS use on handgrip strength. (b) Forest plots depicting the effect of ONS use on the phase angle.
Effects of ONS on electrolyte levels
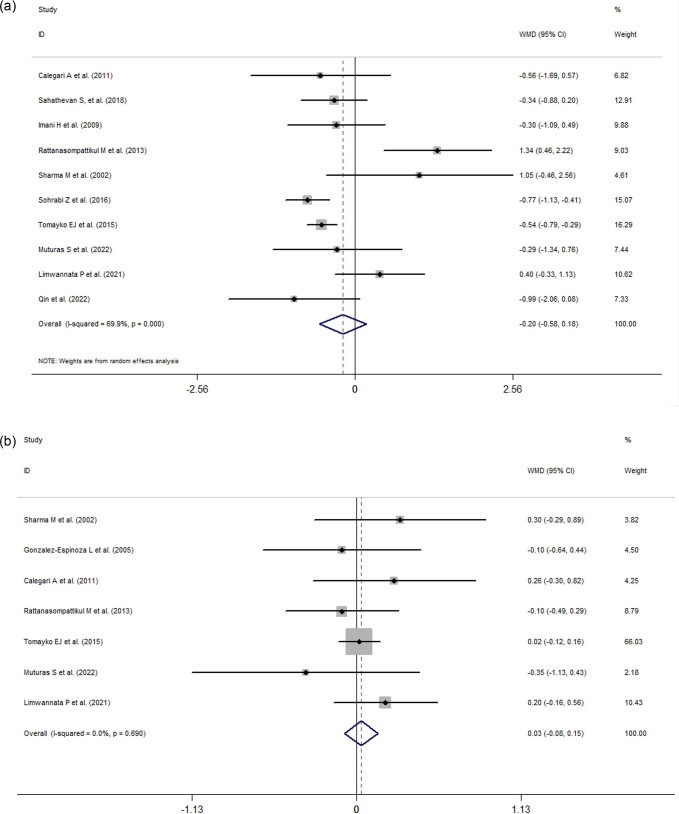
Ten studies reported the effects of ONS use on serum phosphorus, and seven studies showed changes in potassium levels, respectively. Analyses of the pooled data revealed that ONS use did not significantly influence the serum phosphorus levels (−0.20 mg/dl, 95% CI: −0.58 to 0.18, P = 0.306, I2 = 69.9%; Fig. 6A) or serum potassium levels (0.03 mmol/l, 95% CI: −0.08 to 0.15, I2 = 0%, P = 0.56; Fig. 6B) of patients on dialysis.
Figure 6:
(a) Forest plots depicting the effect of ONS use on serum phosphorus levels. (b) Forest plots depicting the effect of ONS use on serum potassium levels.
Effects of ONS use on CRP levels
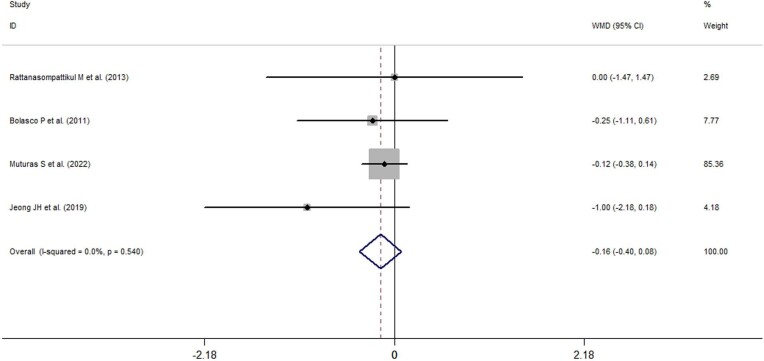
Data on the effects of ONS use on CRP levels were available only in three studies, and quantitative analysis indicated that ONS use did not significantly influence the CRP levels (−0.16 mg/l, 95% CI: −0.40 to 0.08, I2 = 49.4%, P = 0.191) of patients on dialysis (Fig. 7).
Figure 7:
Forest plots depicting the effect of ONS use on C-reactive protein levels.
Sensitivity analysis
Considerable heterogeneity was observed in the effects of ONS use on albumin levels, which was one of the main observation parameters, despite subgroup analyses being conducted on potential variables. Although we sequentially omitted individual trials during sensitivity analysis, no single trial was determined to influence the albumin level outcomes (data not shown). The included studies were divided into high-quality (4–7 points) and low-quality (1–3 points) studies on the basis of their modified Jadad scale scores [48]. Low-quality studies [24–32, 34–36, 38, 40, 41, 43, 45] were excluded, and only five high-quality trials remained [23, 33, 37, 42, 44]. However, one of these studies was excluded because the unit albumin was presented in was “mg/dl,” which was likely erroneous [23]. These steps reduced the heterogeneity to 21.8%, and pooled analysis indicated that ONS use increased serum albumin levels by 1.01 g/l (95% CI: 0.17–1.85, P = 0.019).
Publication bias
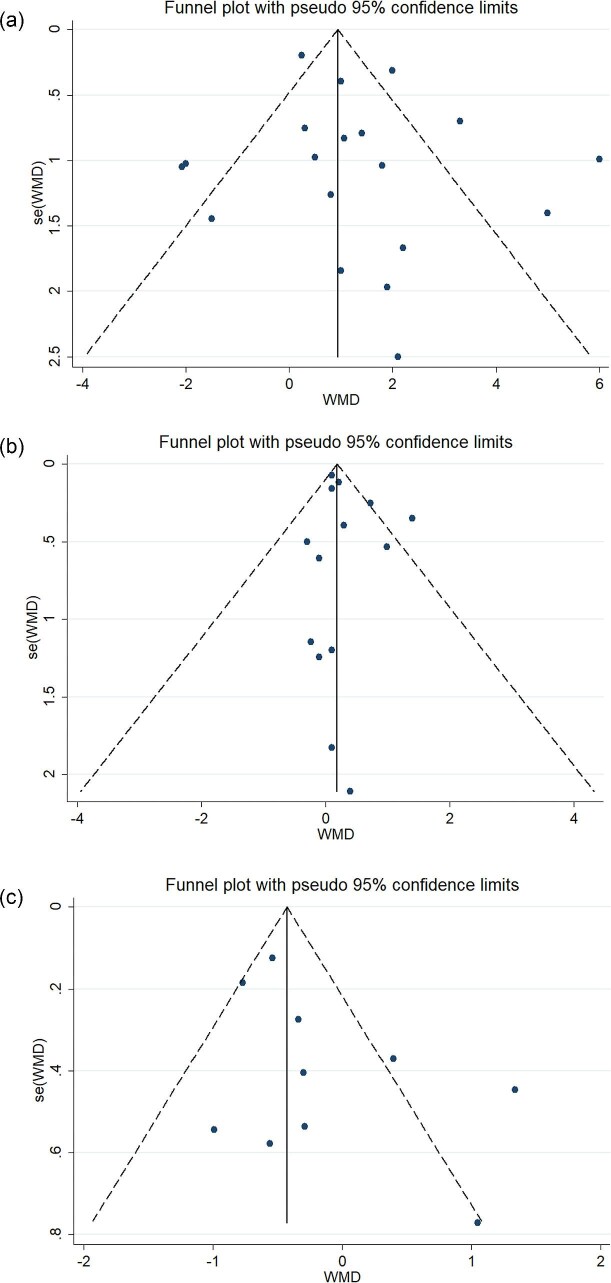
Potential publication bias was detected using funnel plots and the Egger test. The funnel plots of the effects of ONS use on serum albumin levels (Fig. 8A), BMI (Fig. 8B), and phosphorus levels (Fig. 8C) present an approximately symmetric pattern. The Egger test results also indicated no publication bias for the effects of ONS use on serum albumin levels (t = 0.84, P = 0.361), BMI (t = 1.04, P = 0.319), and phosphorus levels (t = 1.77, P = 0.114). Funnel plots were not created for the other variables (lean body mass, the phase angle, handgrip, potassium levels, and CRP levels) because the number of relevant studies that included the variables was <10.
Figure 8:
(a) Funnel plot for serum albumin levels. (b) Funnel plot for BMIs. (c) Funnel plot for serum phosphorus levels.
DISCUSSION
We previously conducted a similar meta-analysis in which we quantitatively analyzed the effect of ONS use on serum albumin levels, BMI, and electrolyte levels. However, the quality of the analyzed evidence was very low [22]. Our current, updated systematic review and meta-analysis includes six additional RCTs assessing the effects of ONS use on the nutritional status, electrolyte levels, and inflammation of patients on dialysis. The main findings support that ONS administration can improve the nutritional status of patients on dialysis in terms of their albumin levels, BMIs, and handgrip strength without significantly altering their serum phosphorus and potassium levels. However, ONS use did not significantly affect lean body mass, the phase angle, and CRP levels.
PEW represents the progressive loss of bodily reserves of protein and energy fuels (body muscle and fat mass) and is common in patients on dialysis. A recent meta-analysis reported that the prevalence of PEW in patients on MDT was 28%–54% [4] and that its risk factors include inadequate dietary intake and additional nutrient loss (such as loss of amino acids, peptides, vitamins, trace elements, and glucose) during dialysis [7, 8, 49, 50]. However, the definition of PEW when applied for patients on dialysis is neither clear nor universally agreed on [51].
A low serum albumin level is a commonly used marker and an essential component of the diagnostic criteria of PEW [5] and can predict mortality in patients with MDT (HD or PD) [6, 52]. De Mutsert et al. [53] observed that a 1-g/dl decrease in the serum albumin level was associated with a 47% increased risk of mortality in patients on HD and 38% increased risk of mortality in patients on PD. According to Araujo et al. [54], a serum albumin concentration <3.5 g/dl was associated with higher odds of mortality in patients who had been on HD for >10 years. In addition, the sensitivity of measuring the serum albumin concentration to predict the outcomes of patients with CKD is high, and its granularity is as little as ≤2 g/l [55, 56]. By contrast, a prospective cohort study including patients on PD and HD reported that serum albumin levels cannot predict mortality risk and were not correlated with lean tissue index [57]. These discrepancies may be due to the differences in study population and durations of the studies. However, the latest KDOQI guidelines state that serum albumin may be used as a predictor of hospitalization and mortality for adults with CKD 5D on HD, with lower levels associated with a higher risk [6].
ONS use is common in malnourished patients in clinical settings or receiving family care, and it can be easily implemented to compensate an inadequate energy and protein intake from the diet. For adults with CKD of 3–5 stage at risk of or with PEW, the latest KDOQI guidelines suggest a minimum 3-month trial of ONS administration to improve nutritional status if dietary energy and protein intake does not meet nutritional requirements [6]. The results of our present meta-analysis and those of two previous reviews [39, 58] verify that ONS administration increases serum albumin levels. However, insufficient comparable data preclude the performance of a meta-analysis of its effects on mortality. Given the importance of albumin levels in predicting mortality risk, our results to some extent indicate that ONS use may improve the outcomes of patients on dialysis because it leads to increased albumin levels. However, notable heterogeneity was identified in both our current and previous meta-analyses regarding the overall effects of ONS use on serum albumin levels. Many factors, such as the type of dialysis, type or source of ONSs, patient's nutritional status, duration of intervention, patient compliance, supplementation timing, patient age, quality of the study, or patient comorbidities, may be involved in the generation of heterogeneity. Similar to the previous review [39], we conducted subgroup analyses of various potential factors, including the type of dialysis, duration of intervention, supplementation timing, types of ONSs, and nutritional status. However, heterogeneity remained in most of our subgroup analyses. In addition, we observed that non-intradialytic supplementation but not intradialytic supplementation significantly improved albumin levels. By contrast, many studies have reported that intradialytic nutrition can improve the nutritional status, inflammation, and biochemical measures (including albumin levels) of patients on HD [59]. Therefore, whether intradialytic nutrition is superior to providing similar support outside of dialysis treatment is worth exploring [59]. When stratified by study quality, the heterogeneity of the studies decreased, and the pooled effect on albumin remained significant, indicating that the heterogeneity was mainly the result of the differences in study quality.
For patients on dialysis, dietary restrictions are commonly imposed to limit potassium and phosphorus intake and prevent fluid overload [51]. Therefore, whether ONS use increases electrolyte disorders should be investigated. Among the studies assessing the effect of ONS use on phosphorus [23, 24, 27, 31, 33, 35, 38, 41, 44, 45], only two demonstrated significant changes in the phosphorus levels between the ONS and control groups after intervention [31, 46]; the pooled data indicated that ONS use had no significant influence on the serum phosphorus levels. We also discovered negative results related to potassium levels in the literature [23, 24, 29, 33, 38, 41, 44]. Our findings regarding phosphorus and potassium levels were in agreement with those of two systematic reviews [39, 58], indicating that ONS use has a very low probability of causing electrolyte disorders related to phosphorus and potassium in patients on MDT. However, the pooled results for phosphorus levels had high heterogeneity. Thus, the implementation of ONS treatment in clinical practice should be based on patients’ specific conditions.
The nutritional status of patients with CKD should be monitored regularly. In adults with CKD 1–5D, dual-energy X-ray absorption remains the gold standard for measuring body composition [6]. Simple anthropometric parameters are also often used to reflect the nutritional status of patients on dialysis. Many studies have investigated the association of BMI with mortality in patients on HD and PD. BMI seems to play a different role in predicting the mortality risks for patients on HD and PD. Most studies have consistently reported a higher risk of mortality for patients on HD who were underweight and a lower risk for patients on HD who were overweight or obese [6, 60–62], whereas conflicting results have been reported for the morality in patients on PD [6, 63, 64]. The KDOQI 2020 guidelines indicate that underweight status (based on BMI) can be used as a predictor of higher mortality in patients on PD, whereas in patients on HD, a high BMI is paradoxically associated with a more favorable outcome [6]. In the present study, our result regarding BMI was inconsistent with that of the review by Mah et al. [39], who reported that oral protein-based supplements (versus placebo or no treatment) did not significantly improve the BMI of patients on dialysis. The difference in these results related to BMI may have arisen because of the following reasons. (i) The types of ONSs in the two reviews were different. In their review, Mah et al. [39] included only studies using protein-based ONSs, whereas we included studies using both protein-based and non-protein ONSs. (ii) Mah et al. included only papers published before 2020 [39], whereas we included five studies published in the last 2 years [41–45]. In addition, the effects of ONS use on lean body mass were analyzed in our study and that of Mah et al., and we observed similar negative results to those reported in Mah et al. [39]. The results regarding BMI and lean body mass of our study indicate that ONS use improves fat mass in patients on dialysis.
Unlike other reviews [22, 39, 58], we analyzed the effects of ONS use on HGS and the phase angle. Patients on MDT often have low physical performance, which is associated with a high mortality rate, and HGS may be used as an indicator of protein-energy status, functional status, and all-cause mortality in patients on HD and PD [6, 65–67]. Notably, our study discovered that the HGS of patients on dialysis can be significantly improved using ONSs, indicating that ONS use may improve the muscle function of patients on dialysis. In addition, Mah et al. [39] found that protein-based ONSs may result in a higher serum prealbumin and mid-arm muscle circumference, especially in malnourished patients, suggesting that ONS use may be more beneficial for malnourished dialysis patients in terms of muscle mass. In our meta-analysis, only three studies reported the effects of ONS use on the phase angle [25, 42, 44], and the results they have reported are conflicting. A quantitative analysis of these studies did not reveal that ONS use improves the phase angle. In addition, neither the current review nor the review of Mah et al. has reported that ONS use can improve the CRP level. Moreover, the review of Mah et al. analyzed the effect of ONS use on interleukin-6 and obtained a negative result [39].
No consensus has been arrived at with respect to the type, time of initiation, or duration of use of enteral nutrition or nutritional supplementation for patients on MDT. Furthermore, few meta-analyses of this topic have been conducted. The strengths of our study are as follows: (i) compared with our previous review, this updated meta-analysis offers a more robust analysis of the effects of ONS use on the parameters reflecting the nutritional status of patients on dialysis, including HGS, lean body mass, and the phase angle, and it analyzed the marker of inflammation, which we failed to analyze in our previous study. (ii) Extensive subgroup analyses on the effects of ONS on serum albumin levels were conducted in this review, and the potential source of heterogeneity in the studies in this field was identified. Publication bias was analyzed not only by using funnel plots but also by using the Egger test. (iii) We summarized the suitable subgroups, durations, timings, and types of ONS interventions implemented for patients on dialysis, which could guide future research on this topic.
This study has several limitations. First, obvious heterogeneity was observed in the meta-analysis of the overall effects of ONS use on serum albumin levels. To investigate and minimize the source of heterogeneity, we performed many subgroup analyses. However, the heterogeneity remained after the subgroup analyses based on the type of dialysis, duration of intervention, supplementation timing, types of ONSs, and nutritional status were performed. Furthermore, we grouped the included studies by quality and observed that the heterogeneity mainly arose from the studies’ uneven quality. Therefore, our results should be interpreted cautiously, and ONS treatment should be suggested for patients on dialysis on the basis of the specific conditions of the individuals. Second, ∼70% of the included studies had small sample sizes (n < 60), and >70% were of low quality, according to the modified Jadad scale [48]. These factors decrease the overall quality of the meta-analysis. Finally, we could not determine the effects of ONS use on the mortality risk or quality of life of patients on dialysis due to the current limited studies. Although our study investigated the effects of ONSs on inflammatory markers, only CRP was reported, and no other markers such as interleukin-6 were reported.
CONCLUSION
Our meta-analysis revealed that ONS use did not significantly affect phosphorus and potassium levels, lean body mass, the phase angle, and CRP levels but did significantly increase serum albumin levels, BMI, and HGS in patients on MDT. Thus, ONS use may improve the nutritional status and muscle function of patients on dialysis without aggravating electrolyte disturbances, such as those related to phosphorus and potassium. Large-scale, well-designed studies are warranted to verify these findings.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Zong Xin Wang for helping us with the statistical analysis.
Contributor Information
Peng Ju Liu, Department of Clinical Nutrition, Department of Health Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, PR China.
Jiayu Guo, Department of Clinical Nutrition, Department of Health Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, PR China.
Yu Zhang, Department of Clinical Nutrition, Department of Health Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, PR China.
Fang Wang, Department of Clinical Nutrition, Department of Health Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, PR China.
Kang Yu, Department of Clinical Nutrition, Department of Health Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, PR China.
Funding
This study was supported by the National Key R&D Program of China (2022YFF1100604/2022YFF1100601).
Authors’ Contributions
Conceptualization was done by Y.K. and L.P.J.; methodology was developed by W.F., G.J., and Z.Y.; formal analysis was carried out by G.J., and L.P.J.; data were curated by G.J. and Z.Y.; the original draft preparation and writing were done by L.P.J., G.J., and Y.K.; review and editing of drafts was done by Y.K., L.P.J., and W.F.; supervision was carried out by Y.K. and Z.Y.; and funding was acquired by Y.K. and L.P.J. All authors have read and agreed to the published version of the manuscript.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Conflict of interest statement
None declared.
REFERENCES
- 1. Bergstrom J. Nutrition and mortality in hemodialysis. J Am Soc Nephrol 1995;6:1329–41. 10.1681/ASN.V651329 [DOI] [PubMed] [Google Scholar]
- 2. Kang DH, Kang EW, Choi SRet al. Nutritional problems of Asian peritoneal dialysis patients. Perit Dial Int 2003;23:S58–S64. 10.1177/089686080302302s13 [DOI] [PubMed] [Google Scholar]
- 3. Carrero JJ, Thomas F, Nagy Ket al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the International Society of Renal Nutrition and Metabolism. J Ren Nutr 2018;28:380–92. 10.1053/j.jrn.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 4. Obi Y, Qader H, Kovesdy CPet al. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care 2015;18:254–62. 10.1097/MCO.0000000000000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oliveira EA, Zheng R, Carter CEet al. Cachexia/protein energy wasting syndrome in CKD: causation and treatment. Semin Dial 2019;32:493–9. 10.1111/sdi.12832 [DOI] [PubMed] [Google Scholar]
- 6. Ikizler TA, Burrowes JD, Byham-Gray LDet al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 update. Am J Kidney Dis 2020;76:S1–S107. 10.1053/j.ajkd.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 7. Rocco MV, Paranandi L, Burrowes JDet al. Nutritional status in the HEMO Study cohort at baseline. Hemodialysis. Am J Kidney Dis 2002;39:245–56. 10.1053/ajkd.2002.30543 [DOI] [PubMed] [Google Scholar]
- 8. Wang AY, Sanderson J, Sea MMet al. Important factors other than dialysis adequacy associated with inadequate dietary protein and energy intakes in patients receiving maintenance peritoneal dialysis. Am J Clin Nutr 2003;77:834–41. 10.1093/ajcn/77.4.834 [DOI] [PubMed] [Google Scholar]
- 9. Song Y, March DS, Biruete Aet al. A comparison of dietary intake between individuals undergoing maintenance hemodialysis in the United Kingdom and China. J Ren Nutr 2022;32:224–33. 10.1053/j.jrn.2021.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ikizler TA, Cano NJ, Franch Het al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the international society of renal nutrition and metabolism. Kidney Int 2013;84:1096–107. 10.1038/ki.2013.147 [DOI] [PubMed] [Google Scholar]
- 11. Hassan K. Does whey protein supplementation improve the nutritional status in hypoalbuminemic peritoneal dialysis patients? Ther Apher Dial 2017;21:485–92. 10.1111/1744-9987.12552 [DOI] [PubMed] [Google Scholar]
- 12. Sezer S, Bal Z, Tutal Eet al. Long-term oral nutrition supplementation improves outcomes in malnourished patients with chronic kidney disease on hemodialysis. JPEN J Parenter Enteral Nutr 2014;38:960–5. 10.1177/0148607113517266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li J, Hou G, Sun Xet al. A low-cost, intradialytic, protein-rich meal improves the nutritional status in Chinese hemodialysis patients. J Ren Nutr 2020;30:e27–e34. 10.1053/j.jrn.2019.03.084 [DOI] [PubMed] [Google Scholar]
- 14. Małgorzewicz S, Gałęzowska G, Cieszyńska-Semenowicz Met al. Amino acid profile after oral nutritional supplementation in hemodialysis patients with protein-energy wasting. Nutrition 2019;57:231–6. 10.1016/j.nut.2018.06.013 [DOI] [PubMed] [Google Scholar]
- 15. Weiner DE, Tighiouart H, Ladik Vet al. Oral intradialytic nutritional supplement use and mortality in hemodialysis patients. Am J Kidney Dis 2014;63:276–85. 10.1053/j.ajkd.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 16. Lacson E Jr, Wang W, Zebrowski Bet al. Outcomes associated with intradialytic oral nutritional supplements in patients on maintenance hemodialysis: a quality improvement report. Am J Kidney Dis 2012;60:591–600. 10.1053/j.ajkd.2012.04.019 [DOI] [PubMed] [Google Scholar]
- 17. Leonberg-Yoo AK, Wang W, Weiner DEet al. Oral nutritional supplements and 30-day readmission rate in hypoalbuminemic maintenance hemodialysis patients. Hemodialysis Int 2019;23:93–100. 10.1111/hdi.12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benner D, Brunelli SM, Brosch Bet al. Effects of oral nutritional supplements on mortality, missed dialysis treatments, and nutritional markers in hemodialysis patients. J Ren Nutr 2018;28:191–6. 10.1053/j.jrn.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 19. Ikizler TA, Cano NJ, Franch Het al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int 2013;84:1096–107. 10.1038/ki.2013.147 [DOI] [PubMed] [Google Scholar]
- 20. Benner D, Burgess M, Stasios Met al. In-center nutrition practices of clinics within a large hemodialysis provider in the United States. Clin J Am Soc Nephrol 2016;11:770–5. 10.2215/CJN.09270915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kistler B, Benner D, Burgess Met al. To eat or not to eat-international experiences with eating during hemodialysis treatment. J Ren Nutr 2014;24:349–52. 10.1053/j.jrn.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 22. Liu PJ, Ma F, Wang QYet al. The effects of oral nutritional supplements in patients with maintenance dialysis therapy: a systematic review and meta-analysis of randomized clinical trials. PLoS ONE 2018;13:e0203706. 10.1371/journal.pone.0203706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tomayko EJ, Kistler BM, Fitschen PJet al. Intradialytic protein supplementation reduces inflammation and improves physical function in maintenance hemodialysis patients. J Ren Nutr 2015;25:276–83. 10.1053/j.jrn.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 24. Calegari A, Barros EG, Veronese FVet al. Malnourished patients on hemodialysis improve after receiving a nutritional intervention. J Bras Nefrol 2011;33:394–401. 10.1590/S0101-28002011000400002 [DOI] [PubMed] [Google Scholar]
- 25. Bolasco P, Caria S, Cupisti Aet al. A novel amino acids oral supplementation in hemodialysis patients: a pilot study. Ren Fail 2011;33:1–5. 10.3109/0886022X.2010.536289 [DOI] [PubMed] [Google Scholar]
- 26. Tabibi H, Imani H, Hedayati Met al. Effects of soy consumption on serum lipids and apoproteins in peritoneal dialysis patients: a randomized controlled trial. Perit Dial Int 2010;30:611–8. 10.3747/pdi.2009.00161 [DOI] [PubMed] [Google Scholar]
- 27. Imani H, Tabibi H, Atabak Set al. Effects of soy consumption on oxidative stress, blood homocysteine, coagulation factors, and phosphorus in peritoneal dialysis patients. J Ren Nutr 2009;19:389–95. 10.1053/j.jrn.2009.01.020 [DOI] [PubMed] [Google Scholar]
- 28. Fouque D, McKenzie J, de Mutsert Ret al. Use of a renal-specific oral supplement by haemodialysis patients with low protein intake does not increase the need for phosphate binders and may prevent a decline in nutritional status and quality of life. Nephrol Dial Transplant 2008;23:2902–10. 10.1093/ndt/gfn131 [DOI] [PubMed] [Google Scholar]
- 29. Gonzalez-Espinoza L, Gutierrez-Chavez J, del Campo FMet al. Randomized, open label, controlled clinical trial of oral administration of an egg albumin-based protein supplement to patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 2005;25:173–80. 10.1177/089686080502500212 [DOI] [PubMed] [Google Scholar]
- 30. Moretti HD, Johnson AM, Keeling-Hathaway TJ. Effects of protein supplementation in chronic hemodialysis and peritoneal dialysis patients. J Ren Nutr 2009;19:298–303. 10.1053/j.jrn.2009.01.029 [DOI] [PubMed] [Google Scholar]
- 31. Sohrabi Z, Eftekhari MH, Eskandari MHet al. Intradialytic oral protein supplementation and nutritional and inflammation outcomes in hemodialysis: a randomized controlled trial. Am J Kidney Dis 2016;68:122–30. 10.1053/j.ajkd.2016.02.050 [DOI] [PubMed] [Google Scholar]
- 32. Hung SC, Tarng DC. Adiposity and insulin resistance in nondiabetic hemodialysis patients: effects of high energy supplementation. Am J Kidney Nutr 2009;90:64–9. [DOI] [PubMed] [Google Scholar]
- 33. Rattanasompattikul M, Molnar MZ, Lee MLet al. Anti-inflammatory and anti-oxidative nutrition in hypoalbuminemic dialysis patients (AIONID) study: results of the pilot-feasibility, double-blind, randomized, placebo-controlled trial. J Cachexia Sarcopenia Muscle 2013;4:247–57. 10.1007/s13539-013-0115-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teixido-Planas J, Ortiz A, Coronel Fet al. Oral protein-energy supplements in peritoneal dialysis: a multicenter study. Perit Dial Int 2005;25:163–72. 10.1177/089686080502500211 [DOI] [PubMed] [Google Scholar]
- 35. Sahathevan S, Se CH, Ng Set al. Clinical efficacy and feasibility of whey protein isolates supplementation in malnourished peritoneal dialysis patients: a multicenter, parallel, open-label randomized controlled trial. Clin Nutr ESPEN 2018;25:68–77. 10.1016/j.clnesp.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 36. Allman MA, Stewart PM, Tiller DJet al. Energy supplementation and the nutritional status of hemodialysis patients. Am J Clin Nutr 1990;51:558–62. 10.1093/ajcn/51.4.558 [DOI] [PubMed] [Google Scholar]
- 37. Eustace JA, Coresh J, Kutchey Cet al. Randomized double-blind trial of oral essential amino acids for dialysis-associated hypoalbuminemia. Kidney Int 2000;57:2527–38. 10.1046/j.1523-1755.2000.00112.x [DOI] [PubMed] [Google Scholar]
- 38. Sharma M, Rao M, Jacob Set al. A controlled trial of intermittent enteral nutrient supplementation in maintenance hemodialysis patients. J Ren Nutr 2002;12:229–37. 10.1053/jren.2002.35300 [DOI] [PubMed] [Google Scholar]
- 39. Mah JY, Choy SW, Roberts MAet al. Oral protein-based supplements versus placebo or no treatment for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev 2020;5:CD012616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jeong JH, Biruete A, Tomayko EJet al. Results from the randomized controlled IHOPE trial suggest no effects of oral protein supplementation and exercise training on physical function in hemodialysis patients. Kidney Int 2019;96:777–86. 10.1016/j.kint.2019.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Limwannata P, Satirapoj B, Chotsriluecha Set al. Effectiveness of renal-specific oral nutritional supplements compared with diet counseling in malnourished hemodialysis patients. Int Urol Nephrol 2021;53:1675–87. 10.1007/s11255-020-02768-5 [DOI] [PubMed] [Google Scholar]
- 42. Yang Y, Qin X, Chen Jet al. The effects of oral energy-dense supplements on nutritional status in nondiabetic maintenance hemodialysis patients: a randomized controlled trial. Clin J Am Soc Nephrol 2021;16:1228–36. 10.2215/CJN.16821020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wen L, Tang C, Liu Yet al. Effects of oral non-protein calorie supplements on nutritional status among maintenance hemodialysis patients with protein-energy wasting: a multi-center randomized controlled trial. Food Funct 2022;13:8465–73. 10.1039/D1FO03791A [DOI] [PubMed] [Google Scholar]
- 44. Murtas S, Aquilani R, Fiori Get al. Effects of a novel amino acid formula on nutritional and metabolic status, anemia and myocardial function in thrice-weekly hemodialysis patients: results of a six-month randomized double-blind placebo-controlled pilot study. Nutrients 2022;14:3492. 10.3390/nu14173492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qin A, Tan J, Hu Wet al. Oral energy supplementation improves nutritional status in hemodialysis patients with protein-energy wasting: a pilot study. Front Pharmacol 2022;13:839803. 10.3389/fphar.2022.839803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liberati A, Altman DG, Tetzlaff Jet al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Follmann D, Elliott P, Suh Iet al. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769–73. 10.1016/0895-4356(92)90054-Q [DOI] [PubMed] [Google Scholar]
- 48. Jadad AR, Moore RA, Carroll Det al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 49. Ikizler TA, Flakoll PJ, Parker RAet al. Amino acid and albumin losses during hemodialysis. Kidney Int 1994;46:830–7. 10.1038/ki.1994.339 [DOI] [PubMed] [Google Scholar]
- 50. Combarnous F, Tetta C, Cellier CCet al. Albumin loss in on-line hemodiafiltration. Int J Artif Organs 2002;25:203–9. 10.1177/039139880202500306 [DOI] [PubMed] [Google Scholar]
- 51. Piccoli GB, Lippi F, Fois Aet al. Intradialytic nutrition and hemodialysis prescriptions: a personalized stepwise approach. Nutrients 2020;12:785. 10.3390/nu12030785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mehrotra R, Duong U, Jiwakanon Set al. Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with hemodialysis. Am J Kidney Dis 2011;58:418–28. 10.1053/j.ajkd.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Mutsert R, Grootendorst DC, Boeschoten EWet al. Subjective global assessment of nutritional status is strongly associated with mortality in chronic dialysis patients. Am J Clin Nutr 2009;89:787–93. 10.3945/ajcn.2008.26970 [DOI] [PubMed] [Google Scholar]
- 54. Araujo IC, Kamimura MA, Draibe SAet al. Nutritional parameters and mortality in incident hemodialysis patients. J Ren Nutr 2006;16:27–35. 10.1053/j.jrn.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 55. Kalantar-Zadeh K, Kilpatrick RD, Kuwae Net al. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant 2005;20:1880–8. 10.1093/ndt/gfh941 [DOI] [PubMed] [Google Scholar]
- 56. Lacson E Jr, Ikizler TA, Lazarus JMet al. Potential impact of nutritional intervention on end-stage renal disease hospitalization, death, and treatment costs. J Ren Nutr 2007;17:363–71. 10.1053/j.jrn.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 57. Mathew S, Abraham G, Vijayan Met al. Body composition monitoring and nutrition in maintenance hemodialysis and CAPD patients–a multicenter longitudinal study. Ren Fail 2015;37:66–72. 10.3109/0886022X.2014.964147 [DOI] [PubMed] [Google Scholar]
- 58. Stratton RJ, Bircher G, Fouque Det al. Multinutrient oral supplements and tube feeding in maintenance dialysis: a systematic review and meta-Analysis. Am J Kidney Dis 2005;46:387–405. 10.1053/j.ajkd.2005.04.036 [DOI] [PubMed] [Google Scholar]
- 59. Kistler BM, Benner D, Burrowes JDet al. Eating during hemodialysis treatment: a consensus statement from the International Society of Renal Nutrition and Metabolism. J Ren Nutr 2018;28:4–12. 10.1053/j.jrn.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 60. Wiesholzer M, Harm F, Schuster Ket al. Initial body mass indexes have contrary effects on change in body weight and mortality of patients on maintenance hemodialysis treatment. J Ren Nutr 2003;13:174–85. 10.1016/S1051-2276(03)00091-8 [DOI] [PubMed] [Google Scholar]
- 61. Yen TH, Lin JL, Lin-Tan DTet al. Association between body mass and mortality in maintenance hemodialysis patients. Ther Apher Dial 2010;14:400–8. 10.1111/j.1744-9987.2010.00818.x [DOI] [PubMed] [Google Scholar]
- 62. Chazot C, Gassia JP, Di Benedetto Aet al. Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrol Dial Transplant 2009;24:2871–6. 10.1093/ndt/gfp168 [DOI] [PubMed] [Google Scholar]
- 63. Ahmadi SF, Zahmatkesh G, Streja Eet al. Association of body mass index with mortality in peritoneal dialysis patients: a systematic review and meta-analysis. Perit Dial Int 2016;36:315–25. 10.3747/pdi.2015.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aslam N, Bernardini J, Fried Let al. Large body mass index does not predict short-term survival in peritoneal dialysis patients. Perit Dial Int 2002;22:191–6. 10.1177/089686080202200205 [DOI] [PubMed] [Google Scholar]
- 65. Lee YH, Kim JS, Jung SWet al. Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol 2020;21:166. 10.1186/s12882-020-01831-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang AY, Sea MM, Ho ZSet al. Evaluation of handgrip strength as a nutritional marker and prognostic indicator in peritoneal dialysis patients. Am J Clin Nutr 2005;81:79–86. 10.1093/ajcn/81.1.79 [DOI] [PubMed] [Google Scholar]
- 67. Vogt BP, Borges MCC, Goés CRet al. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin Nutr 2016;35:1429–33. 10.1016/j.clnu.2016.03.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.