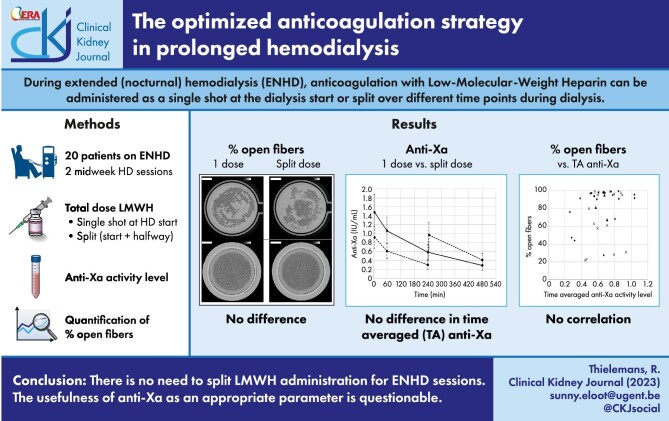
ABSTRACT
Background
During extended (nocturnal) hemodialysis (ENHD), the dose of low-molecular-weight heparin (LMWH) can be administered as a single injection or as a divided dose over different time points. Our hypothesis was that a single injection might be sufficient to maintain dialyzer fiber patency. In addition, we investigated whether the biochemical clotting parameter anti-Xa accurately predicts fiber blocking.
Methods
Our hypothesis was tested in 20 stable patients on ENHD in a random cross-over setting during two consecutive midweek sessions. The regular total dose of LMWH (i.e. enoxaparin, Clexane® 40–100 mg, Sanofi, Belgium) was either given (i) in a single injection at the dialysis start or (ii) divided over two injections, at the start and halfway the dialysis session. Blood samples were taken from the arterial blood line at different time points to determine plasma anti-Xa activity levels. Post-dialysis, the rinsed and dried hemodialyzers were scanned with a reference micro-computed tomography (µCT) scanning technique, and non-blocked fibers were counted in a central cross-section of the dialyzer outlet potting (ImageJ, NIH, USA).
Results
The percentage of open fibers in the dialyzers after a single injection of LMWH [91 (61–96)%] versus divided administration [94 (79–98)%] was not different. Time averaged anti-Xa activity levels were clinically not significantly different between both sessions. Anti-Xa activity levels correlated with the administered anticoagulation doses normalized for body weight, but not with the percentages open fibers in the dialyzers.
Conclusion
Our results indicate that there is no need to administer enoxaparin over two injections for ENHD up to 8 h. The usefulness of monitoring anti-Xa levels to predict fiber patency, assessed by µCT, can be questioned, but further clinical trials are needed.
Keywords: anticoagulation, anti-Xa, chronic hemodialysis, fiber clotting, nocturnal dialysis
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Anticoagulation remains necessary in hemodialysis to avoid clotting of the extracorporeal dialysis circuit. Unfractionated heparin (UFH) and low-molecular-weight heparins (LMWH) are both effective and have similar safety profiles [1–3]. In hemodialysis patients with no additional risk for bleeding or coagulation, UFH is administered as a bolus at the dialysis start, followed by a continuous infusion. Actual dosing is mostly titrated by using standing order protocols, based on clotting parameters such as activated partial thromboplastin time (aPTT) or activated clotting time. Use of UFH enhances safety as it allows rapid monitoring, but it also substantially increases the burden on the dialysis nursing staff. On the other hand, LMWHs are administered as a bolus at the start of the dialysis session. In most centers, routine monitoring of coagulation parameters is usually not done. Heparin dosing schemes, based on the type of LMWH, its half-life and dialysability, patient's body weight, and the length of the dialysis session are applied. For patients on extended (nocturnal) hemodialysis (ENHD), there is at present no hard evidence on whether it is preferable to administer one large dose of heparin at the beginning of the session, or whether an additional bolus after 4 h [4–6] should be injected. Another relevant clinical question is whether administration of an additional dose after 4 h is unpractical and does not disturb sleep during night dialysis.
Multiple surrogate markers are available to monitor the coagulation activity and fiber patency during ENHD, either during or after hemodialysis. Biochemical markers, dialysis parameters and visual scoring of the dialyzer and/or the venous drip chamber can be used [7–10], but none of these tools objectively quantifies coagulation correctly at the level of fiber patency. Consequently, determining dose and timing of LMWH based on these data might lead to erroneous results. Micro-computed tomography (µCT) scanning was recently recognized as the gold standard to quantify post-dialysis fiber patency and is thus recommended to investigate dose and timing of LMWH anticoagulation in patients on ENHD [11, 12].
The main purpose of this randomized cross-over study was to objectively quantify possible differences in post-dialysis dialyzer fiber patency by using µCT. This parameter was measured at the end of an ENHD session with a single dose of LMWH administered at the dialysis start versus one in which the heparin dose was divided over two time points. As second question, we investigated whether the anti-Xa activity level correlated accurately with fiber patency.
MATERIALS AND METHODS
Patients
This single-center cross-over study included 20 stable maintenance ENHD patients who had stable dialysis sessions and no change in anticoagulation dose during the past 4 weeks. The patients had no known coagulation disorder and no active inflammation or malignancy, and had a well-functioning vascular access.
The protocol adhered to the Declaration of Helsinki, was approved by the institutional research committee (Ethical Committee—Ghent University Hospital, BC 11018–B6702021001093-12/2021), and was registered at www.clinicaltrials.gov (NCT05204810-12/09/2021—“Optimisation of Anticoagulation in Patients on Nocturnal Hemodialysis”). Written informed consent was obtained from all included patients.
Dialysis and anticoagulation
In one session, routine total regular dose of LMWH was administered divided over two time points, i.e. at the start and halfway the dialysis session. In the second session, the total dose was administered at the start of the dialysis. The total dose was determined by clinical expertise as is usual practice in our unit. The two study dialysis sessions were performed at midweek with a 1-week interval and the different anticoagulation strategies were applied in random order. Patients received their total regular dose LMWH (i.e. enoxaparin, Clexane®, Sanofi, Belgium), either as one injection at the dialysis start (i.e. 40, 60, 80 or 100 mg) or with the total dose divided over the start and halfway the dialysis session (i.e. 20 + 20 mg, 40 + 20 mg, 40 + 40 mg, 60 + 40 mg). In all cases, anticoagulation was injected in the outlet blood line.
Dialyses were performed on 5008 dialysis machines (Fresenius Medical Care, Bad Homburg, Germany) with patient's standard dialyzer and dialysis settings. Ultrafiltration rates were set according to the patient's interdialytic weight gain and clinical status.
Randomization (https://www.randomizer.org/) was performed by the study coordinator. Each patient served as his/her own control.
Blood sampling and laboratory
Blood was sampled from the arterial blood line at 5 min after administration of the heparin at the dialysis start, 1 h after start, halfway dialysis just before and 5 min after the eventual second anticoagulation administration, and just before the end of the dialysis session. Blood samples were immediately sent to the Routine Laboratory of the Ghent University Hospital where anti-Xa activity levels were determined by a chromogenic assay (STA®-Liquid Anti-Xa assay, Stago, Asnières-sur-Seine, France).
µCT scanning and coagulation quantification
At the end of the study session, a standard rinsing procedure of the hemodialyzer was performed with exact 300 mL rinsing solution (i.e. online dialysis fluid). Next, the hemodialyzer was dried for at least 20 h, applying continuous mild positive pressure ventilation simultaneously in blood and dialysate compartment. Dialyzer fiber blocking was visualized in the dialyzer outlet potting using a reference µCT scanning technique with a resolution of 25 µm, as described previously [12].
The raw projection data is reconstructed into 2D visualizations using the Octopus Reconstruction software package. Non-blocked fibers were counted in the central cross-section of the dialyzer outlet potting, using an open-source platform for biological-image analysis (ImageJ 1.51 H, NIH, Bethesda, MD, USA). Three different thresholds were used to define the surface area of an open fiber: i.e. 50%, 70% and 90% of the cross-section of a non-used fiber. Comparing the number of non-blocked fibers in the tested dialyzer with the total number of fibers in a non-used dialyzer, as measured from scanning three non-used dialyzers, provides an objective estimate of the percentage of fiber blocking.
Statistical analysis
Statistical analyses were performed using SPSS (version 27, SPSS Inc., Chicago, IL, USA). Continuous variables were summarized as mean ± standard deviation or median [25th percentile (pct); 75th pct]. To compare different related variables, paired t-tests and Wilcoxon Signed Ranks tests were performed, and to relate different parameters, Spearman correlations were completed.
RESULTS
Relevant demographic and clinical data at baseline of the investigated patient population are summarized in Table 1. The 20 included patients (age 54.3 ± 16.5; 19 male) were dialyzed twice for 477 ± 22 min and 476 ± 22 min, with no difference between the two sessions in blood flow (twice 184 ± 16 mL/min), dialysate flow (twice 300 mL/min) and ultrafiltration [2.3 (0.8; 2.8) L and 2.6 (1.3; 2.8) L]. Patients were dialyzed with FX800 CorDiax dialyzer (Fresenius Medical Care, Bad Homburg, Germany) (n = 10), Theranova 400 dialyzer (Baxter, USA) (n = 8) and ATA™ Solacea™ 19H dialyzer (Nipro, Osaka, Japan) (n = 2). There were no patient dropouts during the investigational period, scheduled dialysis duration and flow settings were maintained in both test sessions, and no adverse or bleeding events were recorded.
Table 1:
Demographic and clinical data of the patient population at baseline.
| Gender (M/F) | 19 M/1 F |
| Age (years) | 54.3 ± 16.5 |
| Dry body weight (kg) | 77.9 ± 16.2 |
| Dialysis vintage (months) | 27.4 (16.5; 66.6) |
| Renal disease | IgA nephropathy (n = 4); renal cell carcinoma (n = 4); autosomal dominant polycystic kidney disease (n = 2); interstitial nephritis (n = 2); diabetic nephropathy (n = 1); lithium nephropathy (n = 1); HIV-associated nephropathy (n = 1); CAKUT (n = 1); focal segmental glomeruloscleroses (n = 1); bilateral reflux (n = 1); granulomatosis with polyangiitis (n = 1); nephronophthisis (n = 1) |
| Vascular access | Arterio-venous fistula (n = 14); central venous catheter (n = 6) |
| Anticoagulation dose | Enoxaparin 40 mg (n = 1); 60 mg (n = 9); 80 mg (n = 10); 100 mg (n = 1) |
| Platelet inhibitors | Acetylsalicylic acid: 80 mg (n = 6); 100 mg (n = 1) |
| Hb (g/dL) | 12.2 (10.9; 12.5) |
| Platelet count (10³/µL) | 213 ± 74 |
| aPTT (s) | 36.9 (34.6; 40.7) |
| INR (–) | 0.96 (0.92; 1.02) |
| AT (%) | 88.6 ± 10.5 |
| CRP (mg/L) | 7.0 (3.2; 12.3) |
Data are presented as mean ± standard deviation or median (25th pct; 75th pct).
M: male; F: female; CAKUT: congenital anomalies of the kidney and urinary tract; IgA: immunoglobulin A; Hb: hemoglobin; INR: international normalized ratio; AT: antithrombin; CRP: C-reactive protein.
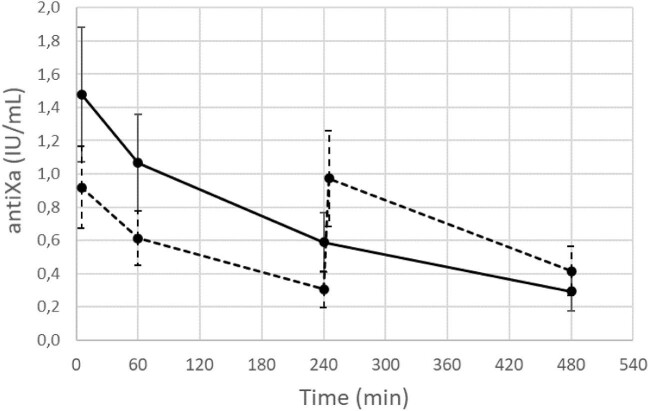
Anti-Xa activity levels during both dialysis sessions are shown in Fig. 1. Time-averaged anti-Xa activity levels were higher for the session with a single anticoagulation administration (i.e. 0.69 ± 0.20 versus 0.61 ± 0.17; P < .001), a difference not considered clinically significant. The maximum measured anti-Xa activity levels were reached 5 min after start of dialysis in all sessions with a single administration and in 9/20 sessions with two smaller dose administrations of anticoagulation. These levels were significantly higher in the single versus split administration (i.e. 1.48 ± 0.41 versus 1.03 ± 0.28; P < .001). Minimum activity levels, as observed at the end versus halfway the dialysis session with single versus split dose, were not different between both dialysis sessions (i.e. 0.29 ± 0.12 versus 0.31 ± 0.11), while anti-Xa activity levels at the dialysis end were lower in the single versus split dose (i.e. 0.29 ± 0.12 versus 0.41 ± 0.15; P < .001).
Figure 1:
Anti-Xa activity levels (IU/mL) during the session with the administration of the total dose of anticoagulation in a single shot at the dialysis start (full line) versus a total dose split over the dialysis start and halfway the dialysis session (dashed line).
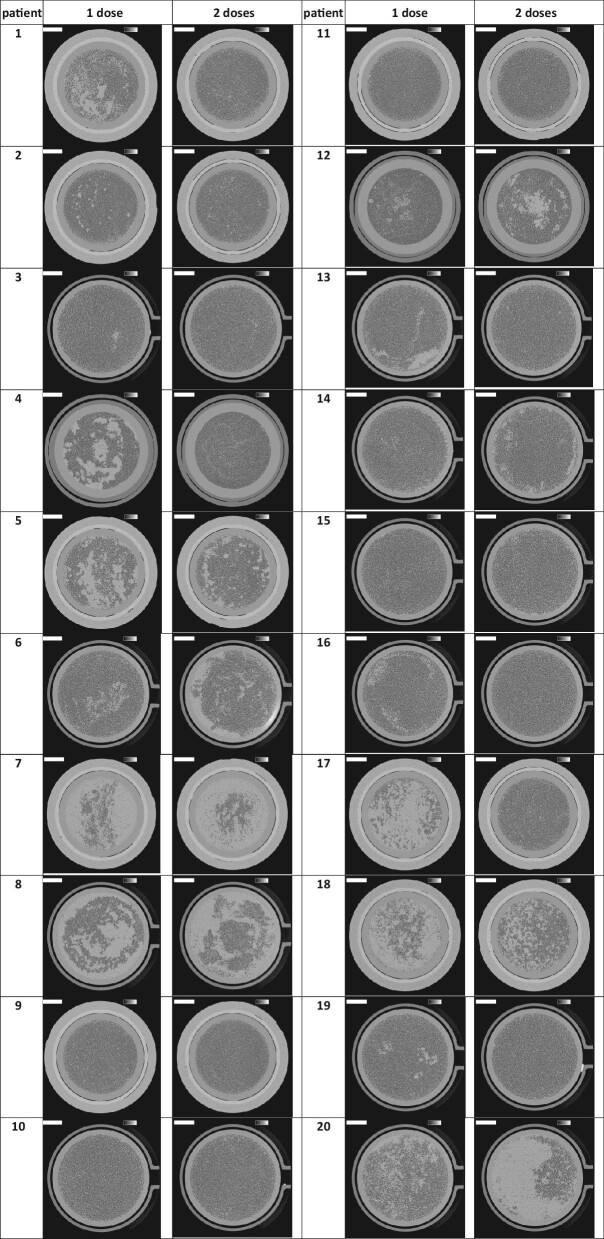
Cross-sections of the dialyzer outlet potting for both experimental dialysis sessions per patient are presented in Fig. 2. The lumens of open fibers are visualized as black dots. The number of open fibers in three non-used FX800 CorDiax dialyzers was 13 051 ± 1, in Solacea™ dialyzers 12 087 ± 4, and in Theranova dialyzers 12 852 ± 1. Table 2 shows the percentage of open fibers in both test sessions and for the different thresholds of open fiber area (i.e. 50%, 70% and 90%). The relative percentage of open fibers in the dialyzers was not different between the sessions with one versus two administrations, and this was irrespective of the type of dialyzer.
Figure 2:
Cross-sections halfway the outlet potting in 20 patients and two dialysis test sessions. The lumens of open fibers are visualized as black dots. The greyscale represents the local linear attenuation coefficient in the range from 0 to 0.5 cm−1 and the scale bar denotes 10 mm.
Table 2:
Percentage open fibers after the session with one and two injections (n = 20) for the thresholds of 50%, 70% and 90% open fiber area.
| Open fiber area | One injection | Two injections | P-value |
|---|---|---|---|
| 50% | 92 (63; 97) | 95 (79; 98) | .255 |
| 70% | 91 (61; 96) | 94 (79; 98) | .235 |
| 90% | 55 (45; 62) | 61 (49; 79) | .344 |
Data are median (25th pct; 75th pct).
All anti-Xa time-averaged, maximum and minimum activity levels correlated, as expected, with the administered anticoagulation doses normalized for body weight (R = 0.74, 0.66 and 0.54, respectively; all P < .001). Percentage of open fibers was not correlated with the time-averaged and maximum anti-Xa (R = 0.26; P = .11 and R = 0.09; P = .57, respectively), and correlated with a very poor prevalence power with minimum anti-Xa (R = 0.37; P = .02) (Fig. 3).
Figure 3:
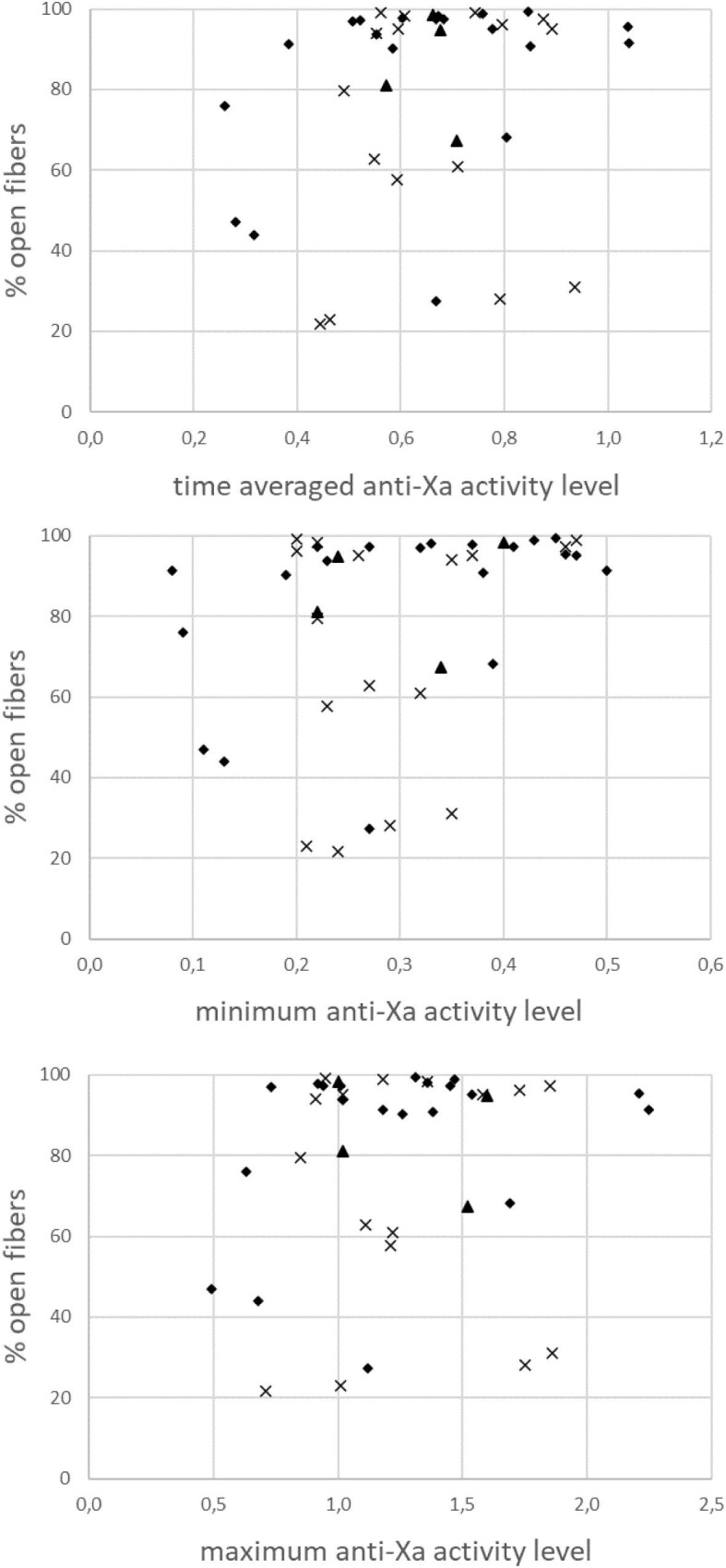
The percentage open fibers in relation with the time-averaged and intradialytic minimum and maximum anti-Xa activity levels in the FX800 CorDiax (rhombs), Solacea (triangles) and Theranova dialyzers (crosses).
DISCUSSION
The present study investigated the difference in fiber patency after a maintenance ENHD session with either a single predialysis anticoagulation dose versus a session where the same total anticoagulation dose was administered over two time points with a 4-h interval. The main findings of this study are that the number of patent dialyzer fibers between these two sessions was not different. Accordingly, opting for the more practical single injection application can be defended. Also, the kinetics of the biochemical parameter anti-Xa correlated with the dose and strategy of the anticoagulation administrations, but not with the number of open fibers. Its usefulness to predict fiber patency should thus be questioned.
While split anticoagulation dosing with LMWH during ENHD has been accepted as standard care, the present findings show that a single anticoagulation administration at the start of a dialysis session is not contraindicated. With such a single dose protocol, working load on the dialysis nursing staff is reduced, and the risk of an omitted second dose decreased.
Different protocols on administration of LMWH in ENHD can be found in the literature. Bugeja et al. applied a protocol of two equal doses of tinzaparin in ENHD patients, one at dialysis start and one after 4 h [4]. This approach resulted in undetectable anti-Xa levels before the start of the next dialysis session, indicating absence of accumulation. The same authors also described the absence of access-related bleeding events post-dialysis. According to pharmacodynamic kinetic data of anti-Xa levels, sufficient anticoagulation was provided during the whole dialysis session. Buitenwerf et al. assessed nadroparin following a protocol with 100% dose at start and 50% dose after 4 h [5]. This paper documented that LMWH accumulation is unlikely in the setting of fortnightly nocturnal HD, while anti-Xa levels appeared to be in the therapeutic range. Verhave et al. compared dalteparin and nadroparin, both with two administrations [6]. A greater proportion of patients reached target levels with two doses of dalteparin compared with two doses of nadroparin, whereas the latter also resulted in prolonged anti-Xa activity and measurable anticoagulation up to the next dialysis session. However, in none of these three studies was actual fiber clotting assessed.
Continuous infusion of nadroparin preceded by a single bolus resulted in residual anticoagulation after dialysis as based on anti-Xa activity, so the continuous infusion was stopped 2 h before treatment [13, 14]. No data on objective fiber patency or clotting were provided, but no differences in clearance or visual scoring of thrombus formation between continuous infusion of LMWH and unfractionated heparin were reported. Based on the evaluation of clotting using a visual scale and through activity levels of anti-Xa, a single dose of dalteparin seemed only to be effective when higher doses were applied (5000–7500 units) [15]. No attempts for split administration were made in this study.
Despite several studies have correlated dialyzer patency with anti-Xa levels, no consensus was obtained on the optimal target value of anti-Xa during hemodialysis. Different results and suggestions can be found in the literature, varying from striving for a target range (0.5–1, 0.2–0.6 IU/mL) [6, 16], to minimum (0.2, 0.35, 0.4 IU/mL) [17–19] as well as to maximum (0.5 IU/mL) [4] target levels at the end of the dialysis session. One can imagine that these inconsistent recommendations are at least partially induced by the fear for either over-anticoagulation (and thus bleeding risk) or for under-anticoagulation (and thus clotting risk).
The place of biomarkers to quantify dialyzer patency is still unclear. At the level of the individual patient visual scoring is not reliable to quantify patency because of its disappointing point prevalence power in relation to a gold standard technique for quantifying patency (i.e. µCT scanning) [12]. Indeed, a recent study revealed that clotting is not a linear process, with the greatest loss of patent fibers and dialyzer extraction occurring during the second half of dialysis. These results imply that the fiber blocking process has only a minor impact on total solute removal of, at least, small solutes like urea (i.e. urea reduction ratio) [20].
The present study objectivated fiber blocking by µCT imaging of the dialyzers post-dialysis and hoped to be able to define more reliable anti-Xa targets. As in other studies [18], time-averaged anti-Xa activity levels correlated well with the administered anticoagulation dose. Any strong correlation between fiber patency and the measured time-averaged, minimum or maximum anti-Xa activity levels was not found. Indeed, while the effectiveness of LMWH therapy is at present mainly monitored by assessing plasma anti-Xa activity the clinical relevance of such anti-Xa activity measurements is questionable.
We noted a high fiber patency rate, i.e. 12/20 and 13/20 dialyzers showed >90% open fibers for a single, respectively split anticoagulation dose. Whereas these results might suggest that a substantial part of the patients are likely somewhat over-anticoagulated, bleeding complications or prolonged oozing after decannulation were not reported in our patient population.
This study has some limitations, i.e. the rather small number of patients and high number of patent dialyzers made it impossible to draw hard conclusions about the usefulness of anti-Xa as biochemical marker for fiber patency as assessed by µCT. However, although the study was performed in only 20 patients, the present protocol allowed to use each patient as his own control over the two experimental sessions, increasing the power of this study.
In conclusion, according to our data, administration of a total dose of enoxaparin in ENHD sessions up to 8 h either as a single injection or as a split dose does not impact fiber clotting. For practical reasons a single injection of enoxaparin may be preferred in dialysis patients. Anti-Xa activity levels did not correlate with the objectively measured percentage of open fibers as assessed by µCT. Further clinical trials are needed to define the usefulness of anti-Xa as an appropriate biochemical parameter for assessing dialyzer fiber patency.
ACKNOWLEDGEMENTS
The authors are indebted to the dialysis nurses, Ann Drieghe, Pascaline De Clercq, Naomi Noët, Emily Termont, Nele Van Nuffel, Christophe Naessens, Nimal Kiribandage, Angelique Lapage and Sabien Inion, for their help during the clinical study, and Norbert Lameire for the extensive revision of our paper.
Contributor Information
Raïsa Thielemans, Nephrology Department, Ghent University Hospital, Ghent, Belgium.
Floris Vanommeslaeghe, Nephrology Department, Ghent University Hospital, Ghent, Belgium.
Iván Josipovic, Centre for X-ray Tomography (UGCT), Physics and Astronomy, Ghent University, Ghent, Belgium.
Filip De Somer, Cardiac Surgery, Ghent University Hospital, Ghent, Belgium.
Katrien Devreese, Laboratory Clinical Chemistry and Hematology, Ghent University Hospital, Ghent, Belgium.
Matthieu Boone, Centre for X-ray Tomography (UGCT), Physics and Astronomy, Ghent University, Ghent, Belgium.
Wim Van Biesen, Nephrology Department, Ghent University Hospital, Ghent, Belgium.
Sunny Eloot, Nephrology Department, Ghent University Hospital, Ghent, Belgium.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article.
REFERENCES
- 1. Davenport A. Review article: low-molecular-weight heparin as an alternative anticoagulant to unfractionated heparin for routine outpatient haemodialysis treatments. Nephrology (Carlton) 2009;14:455–61. 10.1111/j.1440-1797.2009.01135.x [DOI] [PubMed] [Google Scholar]
- 2. Lim W, Cook DJ, Crowther MA.. Safety and efficacy of low molecular weight heparins for hemodialysis in patients with end-stage renal failure: a meta-analysis of randomized trials. J Am Soc Nephrol 2004;15:3192–206. 10.1097/01.ASN.0000145014.80714.35 [DOI] [PubMed] [Google Scholar]
- 3. Nadarajah L, Fan S, Forbes Set al. Major bleeding in hemodialysis patients using unfractionated or low molecular weight heparin: a single-center study. Clin Nephrol 2015;84:274–9. 10.5414/CN108624 [DOI] [PubMed] [Google Scholar]
- 4. Bugeja A, Harris S, McCormick Bet al. Safety and efficacy of tinzaparin anticoagulation during nocturnal hemodialysis. Am J Nephrol 2019;50:255–61. 10.1159/000502506 [DOI] [PubMed] [Google Scholar]
- 5. Buitenwerf E, Risselada AJ, van Roon ENet al. Effect of nadroparin on anti-Xa activity during nocturnal hemodialysis. BBA Clin 2015;3:276–9. 10.1016/j.bbacli.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verhave G, Weijmer MC, van Jaarsveld BC.. Anticoagulation with dalteparin and nadroparin in nocturnal haemodialysis. Neth J Med 2015;73:270–5. [PubMed] [Google Scholar]
- 7. Dorsch O, Krieter DH, Lemke HDet al. A multi-center, prospective, open-label, 8-week study of certoparin for anticoagulation during maintenance hemodialysis—the membrane study. BMC Nephrol 2012;13:50. 10.1186/1471-2369-13-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hofbauer R, Moser D, Frass Met al. Effect of anticoagulation on blood membrane interactions during hemodialysis. Kidney Int 1999;56:1578–83. 10.1046/j.1523-1755.1999.00671.x [DOI] [PubMed] [Google Scholar]
- 9. Klingel R, Schaefer M, Schwarting Aet al. Comparative analysis of procoagulatory activity of haemodialysis, haemofiltration and haemodiafiltration with a polysulfone membrane (APS) and with different modes of enoxaparin anticoagulation. Nephrol Dial Transplant 2004;19:164–70. 10.1093/ndt/gfg459 [DOI] [PubMed] [Google Scholar]
- 10. Ziai F, Benesch T, Kodras Ket al. The effect of oral anticoagulation on clotting during hemodialysis. Kidney Int 2005;68:862–6. 10.1111/j.1523-1755.2005.00468.x [DOI] [PubMed] [Google Scholar]
- 11. Claudel SE, Miles LA, Murea M.. Anticoagulation in hemodialysis: a narrative review. Semin Dial 2021;34:103–15. 10.1111/sdi.12932 [DOI] [PubMed] [Google Scholar]
- 12. Vanommeslaeghe F, Van Biesen W, Dierick Met al. Micro-computed tomography for the quantification of blocked fibers in hemodialyzers. Sci Rep 2018;8:2677. 10.1038/s41598-018-20898-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong SS, Lau WY, Chan PKet al. Low-molecular weight heparin infusion as anticoagulation for haemodialysis. Clin Kidney J 2016;9:630–5. 10.1093/ckj/sfw049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong SS, Lau WY, Ng MLet al. Clinical study on low-molecular weight heparin infusion as anticoagulation for nocturnal home haemodialysis. Nephrology 2018;23:317–22. 10.1111/nep.12995 [DOI] [PubMed] [Google Scholar]
- 15. Huang SS, Qi K, Louzada Met al. Using dalteparin in quotidian and nocturnal hemodialysis patients: a prospective study. Hemodial Int 2020;24:195–201. 10.1111/hdi.12805 [DOI] [PubMed] [Google Scholar]
- 16. Coene KLM, Dekker MJE, Kerskes Met al. Practical value of anti-Xa activity in the evaluation of extracorporeal circuit anticoagulation during haemodialysis: results of a cross-sectional single-centre study. Nephron 2017;137:205–11. 10.1159/000479390 [DOI] [PubMed] [Google Scholar]
- 17. Polkinghorne KR, McMahon LP, Becker GJ.. Pharmacokinetic studies of dalteparin (Fragmin), enoxaparin (Clexane), and danaparoid sodium (Orgaran) in stable chronic hemodialysis patients. Am J Kidney Dis 2002;40:990–5. 10.1053/ajkd.2002.36331 [DOI] [PubMed] [Google Scholar]
- 18. Sagedal S, Hartmann A, Sundstrom Ket al. A single dose of dalteparin effectively prevents clotting during haemodialysis. Nephrol Dial Transplant 1999;14:1943–7. 10.1093/ndt/14.8.1943 [DOI] [PubMed] [Google Scholar]
- 19. Tao M, Zheng D, Liang Xet al. Evaluation of the anticoagulant effect of low-molecular-weight heparins based on the anti-Xa level during haemodialysis. Nephrology 2020;25:723–9. 10.1111/nep.13697 [DOI] [PubMed] [Google Scholar]
- 20. Vanommeslaeghe F, Josipovic I, Boone Met al. Impact of intradialytic fiber clotting on dialyzer extraction and solute removal: a randomized cross-over study. Sci Rep 2022;12:5717. 10.1038/s41598-022-09696-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.