ABSTRACT
Background
Genetic causes are increasingly recognized in patients with focal segmental glomerulosclerosis (FSGS), but it remains unclear which patients should undergo genetic study. Our objective was to determine the frequency and distribution of genetic variants in steroid-resistant nephrotic syndrome FSGS (SRNS-FSGS) and in FSGS of undetermined cause (FSGS-UC).
Methods
We performed targeted exome sequencing of 84 genes associated with glomerulopathy in patients with adult-onset SRNS-FSGS or FSGS-UC after ruling out secondary causes.
Results
Seventy-six patients met the study criteria; 24 presented with SRNS-FSGS and 52 with FSGS-UC. We detected FSGS-related disease-causing variants in 27/76 patients (35.5%). There were no differences between genetic and non-genetic causes in age, proteinuria, glomerular filtration rate, serum albumin, body mass index, hypertension, diabetes or family history. Hematuria was more prevalent among patients with genetic causes. We found 19 pathogenic variants in COL4A3–5 genes in 16 (29.3%) patients. NPHS2 mutations were identified in 6 (16.2%) patients. The remaining cases had variants affecting INF2, OCRL, ACTN4 genes or APOL1 high-risk alleles. FSGS-related genetic variants were more common in SRNS-FSGS than in FSGS-UC (41.7% vs 32.7%). Four SRNS-FSGS patients presented with NPHS2 disease-causing variants. COL4A variants were the most prevalent finding in FSGS-UC patients, with 12 patients carrying disease-causing variants in these genes.
Conclusions
FSGS-related variants were detected in a substantial number of patients with SRNS-FSGS or FSGS-UC, regardless of age of onset of disease or the patient's family history. In our experience, genetic testing should be performed in routine clinical practice for the diagnosis of this group of patients.
Keywords: FSGS, hereditary diseases, nephrotic syndrome, podocytopathy, steroid-resistant
INTRODUCTION
Focal segmental glomerulosclerosis (FSGS) is a histological lesion seen on light microscopy, characterized by the presence of sclerosis in parts (segmental) of at least one glomerulus (focal). A primary disease or an adaptive phenomenon that results in podocyte injury and depletion may evolve into this histological pattern. According to the etiology, FSGS lesion has been classified into immunological, genetic and secondary forms. The latest version of the KDIGO guidelines includes FSGS of undetermined cause (FSGS-UC) to define patients with this histological pattern but whose etiology is unknown [1]. FSGS-UC is essentially a diagnosis of exclusion in patients in which primary, secondary or genetics causes have been ruled out.
Although FSGS is considered a podocyte disorder, genetic forms of FSGS result from pathogenic mutations in genes related to interaction between the podocyte and the basement membrane [2, 3]. The clinical presentation of genetic FSGS is extremely variable, from adult-onset mild disease to perinatal nephrotic syndrome. The causative gene determines the age of onset. Thus, disease-causing mutations in genes related to podocyte cytoskeleton or slit diaphragm (NPHS1, NPHS2, LAMB2…) are predominantly found in childhood [4–7], while pathogenic genetic variants associated to type 4A collagen (COL4A) genes represent most cases in adulthood [8, 9]. In the adult population, a genetic cause has been established in 8%–26% of cases, although it is possible that this number is underestimated [8–10]. The clinical and histological characteristics that seem to better predict the genetic etiology are the absence of response to immunosuppressive medication, the absence of diffuse podocyte foot process effacement in the renal biopsy, and normal serum albumin despite nephrotic proteinuria [3, 11, 12]. Nevertheless diffuse foot process effacement by itself may not be able to differentiate primary FSGS from genetic forms of FSGS [13]. Genetic testing is recommended for early-onset forms, especially those resistant to steroids. However, in the adult population, establishing the criteria for a genetic study continues to be a challenge [14]. The recommendations of the KDIGO guidelines for genetic testing also includes family history, features suggestive of a syndromic disease, aiding in diagnosis, limiting immunosuppression exposure, determining the risk of recurrent disease in kidney transplantation and for risk assessment in living kidney donor candidates, and prenatal diagnoses. Despite the guideline recommendations, the decision to perform a genetic test, in routine clinical practice remains complex. As in other hereditary nephropathies, most of the genetic FSGS do not have an obvious family history, FSGS-UC forms rarely receive immunosuppression, and lastly the optimum approach is to perform the etiological diagnosis at the beginning of the evaluation and not in the pre-transplant study [15, 16].
In this study, we report the prevalence of genetic variants in adult-onset FSGS according to clinical presentation, particularly, adult-onset steroid-resistant nephrotic syndrome (SRNS) and adult-onset FSGS-UC.
MATERIALS AND METHODS
Patients
This study was a retrospective multicenter cohort study performed in 18 Spanish and Portuguese hospitals. Patients were eligible if they had biopsy-proven FSGS and SRNS or FSGS-UC. SRNS-FSGS was defined as protein excretion higher than 0.3 g per 24 h after 16 weeks of prednisone treatment. FSGS-UC patients were defined as those with proteinuria of any range with normal serum albumin levels in whom a secondary cause of FSGS had been ruled out. Low birth weight, morbid obesity, any cause of reduction of renal mass, reflux nephropathy, sickle cell disease, any advanced kidney disease with substantial loss of nephrons, sleep apnea, cyanotic congenital heart disease, renal artery stenosis, malignant hypertension, cholesterol emboli, viral infections such as human immunodeficiency virus, parvovirus B19, cytomegalovirus or hepatitis C virus, hemophagocytic syndrome, and medications such as ledipasvir, sofosbuvir, mammalian target of rapamycin (mTOR) inhibitors (prior to biopsy), calcineurin inhibitors (prior to biopsy), anthracyclines, heroin, lithium, interferon, anabolic steroids and pamidronate, were considered possible causes of secondary FSGS.
Clinical data, biopsy reports and laboratory data for the 2 years following biopsy were carefully reviewed. Those patients that presented a potential secondary or primary cause of FSGS (i.e. abrupt onset, response to immunosuppression) were excluded from the study.
Relevant medical data and family history information was collected. Patients with first- to third-degree relatives with proteinuria and/or renal failure were defined as familial cases; otherwise, they were defined as sporadic cases.
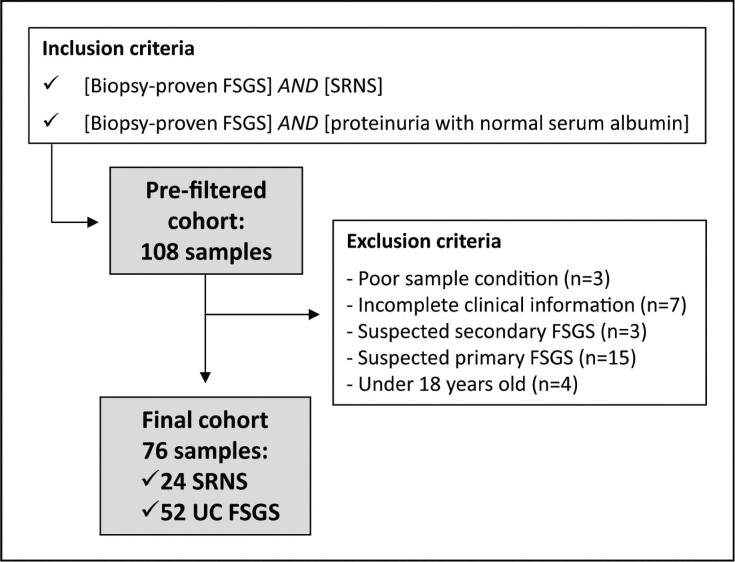
We excluded patients under 18 years at the time of biopsy and patients with FSGS due to a secondary cause (Fig. 1). Laboratory data at the time of kidney biopsy and the following 2 years were collected, as well as the biopsy reports. The study was approved by local ethics committees, and all participants provided written informed consent.
Figure 1:
Screening flowchart. Patient flow through the study. Screening population included all adult patients with biopsy-proven FSGS and SRNS or FSGS-UC. Reasons for screening failures were classified as follows: poor sample condition (n = 3), incomplete clinical information (n = 7), suspected secondary FSGS (n = 3), suspected primary FSGS (n = 15) and under 18 years old at the time of biopsy (n = 4).
Genetics
DNA was extracted from peripheral blood (leukocytes) received from each patient with the commercial kits GenEXTM Genomic Kit (GeneAllTM, Seoul, Korea) and QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany), following manufacturer’s guidelines. Quality and concentration of the obtained DNA was checked with Nanodrop (ThermoScientific, Rochester, NY, USA).
We employed targeted next-generation sequencing (NGS) technology to study of the main genes known to cause FSGS. This subset of genes is part of a custom panel of genes designed for targeting the main genes associated with glomerular pathologies (Supplementary data, Table S1). The exons, splice sites and flanking regions of these candidate genes were captured and amplified using the “TruSeq DNA Library Prep for Enrichment” kit (Illumina, San Diego, CA, USA) with the “xGen hybridization capture of DNA libraries” kit (IDT, Integrated DNA technologies, www.idtdna.com), and sequenced on the Next Seq 500 Illumina platform (Illumina, San Diego, CA, USA). All DNA handling and sequencing procedures were completed at NefroCHUS or the Galician Public Foundation for Genomic Medicine (FPGMX).
Raw sequencing reads were processed according to GATK best practice guidelines [17, 18]. Reads were aligned to the human reference genome (GRCh37) using bwa version 0.7.17-r1188. Low-quality reads were removed from the primary data set using fastpversion0.20 [19]. Variants were called using GATK version 4.1.9 [17, 18], Pindel version 0.2.5b9 [20] and ExomeDepth version 1.1.15 [21], following their corresponding best practice guidelines. Variants were annotated with an in-house annotation pipeline merging SnpEff's functional gene annotation with ANNOVAR annotations to retrieve population frequencies (1000 Genomes Project, gnomAD and an in-house database among others), functional prediction scores (SIFT, CADD, etc.) or clinical information (ClinVar, OMIM, etc.).
Variants were classified independently by two geneticists specialized in hereditary kidney diseases according to the American College of Medical Genetics (ACMG) guidelines and recent amendments [22]. Disease-causing variants were defined as those that were classified as “pathogenic” or “likely pathogenic” and that were explicative of the patient's nephropathy. A bibliographic search of each diagnostic variant was carried out using different search tools (PubMed, ClinVar and the Human Gene Mutation Database) to determine whether they had been previously reported. Segregation analysis was carried out by Sanger sequencing.
Statistical analysis
Statistical significance was determined by the two-sample t-test (two-tailed), Fisher or χ2-tests (two-tailed) and Mann–Whitney U tests using SPSS version 21 (IBM, Armonk, NY, USA). P < .05 was considered to be statistically significant.
Comparison of our genetic findings with other comparable published works
A bibliographic search was carried out to identify studies that met the following criteria: (i) cohort of patients clinically diagnosed with FSGS with inclusion criteria similar to ours; (ii) genetic study performed by NGS based on glomerular candidate gene panels.
RESULTS
Baseline characteristics
A total of 108 patients from 104 families were assessed for eligibility, of whom 32 patients (29.6%) were excluded from the study. Main reasons for screening failures were not meeting the inclusion criteria (n = 22), and inadequate clinical, laboratory or biopsy data (n = 10). The final study cohort in this study consisted of 76 adult patients biopsied between 1991 and 2019 (Fig. 1).
Baseline characteristics of the genetically studied cohort are shown in Table 1. Overall, mean age was 40.8 ± 12.1 years, 52 participants (68.4%) were male and 93.4% were Caucasian. Of the 76 patients included in the study, 52 belonged to the FSGS-UC group (68.4%), and 24 to the SRNS-FSGS group (31.6%). Electron microscopy examination was available only in 9 (11.8%) patients. The mean estimated glomerular filtration rate (eGFR) at the time of biopsy was 77 ± 34 mL/min/1.73 m2. Median 24-h urine protein excretion was 4.1 g/day (IQR 1.6–5.0 g/day). More than half of the patients (56.6%) were hypertensive, and 7 patients (9.2%) were diabetic. Mean body mass index (BMI) was 26.7 ± 4.9 kg/m2. No patients presented with histological lesions of diabetes, hypertension or any other superimposed kidney disease on renal biopsy. Fifty-five (72.4%) patients were on angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker prior to their renal biopsy. Family history of any kind of chronic kidney disease was present in 34 (44.7%) patients.
Table 1:
The clinical picture: baseline characteristics of the global cohort and according to biopsy criteria.
| Global cohort | SRNS-FSGS | FSGS-UC | |
|---|---|---|---|
| N (%) | N = 76 | N = 24 (31.6) | N = 52 (68.4) |
| Genetic finding, % | 35.5 | 41.7 | 32.6 |
| Age (years), mean ± SD | 40.4 ± 12.1 | 38.4 ± 11.5 | 40.8 ± 12.5 |
| Male sex, % | 68.4 | 66.6 | 69.2 |
| BMI (kg/m2), mean ± SD | 26.7 ± 4.9 | 26.3 ± 4.9 | 26.9 ± 4.9 |
| Hypertension, % | 56.6 | 37.7 | 65.4 |
| Diabetes mellitus, % | 9.2 | 12.5 | 7.7 |
| Family history, % | 44.7 | 33.3 | 50 |
| RAS blockade, % | 72.4 | 66.7 | 75.0 |
| Immunosuppression, % | 48.70 | 100 | 26.9 |
| Glucocorticoids, % | 48.70 | 100.0 | 26.9 |
| CNI, % | 31.60 | 66.7 | 15.4 |
| MMF, % | 15.80 | 29.1 | 9.6 |
| mTor inhibitors, % | 1.30 | 4.2 | 0 |
| Rituximab, % | 5.20 | 16.6 | 0 |
| eGFR CKD-EPI (mL/min), mean ± SD | 77.6 ± 34. 0 | 73.1 ± 37.2 | 79.5 ± 32.6 |
| Serum albumin (g/dL), mean ± SD | 3.6 ± 0.8 | 2.8 ± 0.7 | 4.0 ± 0.6 |
| Proteinuria (g/day), mean ± SD | 4.1 ± 3.5 | 7.1 ± 4.4 | 2.7 ± 1.3 |
| Hematuria, % | 60.5 | 70.8 | 55.8 |
All data refer to the time of kidney biopsy.
RAS: renin–angiotensin system; eGFR CKD-EPI: estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration; CNI: calcineurin inhibitor; MMF: mycophenolate mofetil.
Most of the patients received some type of immunosuppressive treatment, without response. Since this is a retrospective study, with patients treated in 18 different centers over almost 30 years, the immunosuppression regimens were heterogeneous. Immunosuppression was more prevalent in the SRNS-FSGS group than in the FSGS-UC group (100% vs 27%). All nephrotic patients received steroids, 66.6% calcineurin inhibitors, 29.2% mycophenolate mofetil, 2.9% mTOR inhibitors and 16.6% rituximab.
In the non-nephrotic group, 26.9% of patients received steroids, 15.4% calcineurin inhibitors and 9.6% mycophenolate mofetil. Nine (17.3%) patients were treated with two or more immunosuppressive agents. Seventeen patients in the genetic cause group had been under immunosuppressive treatment. None of them had a complete remission and in seven patients a partial remission was achieved.
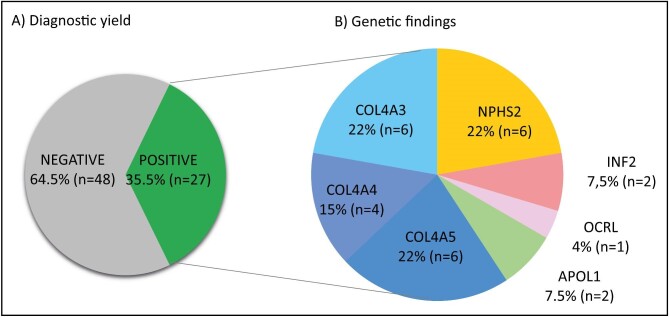
Genetic findings in the global FSGS cohort
The detection rate of disease-causing mutations in the global cohort was 35.5% (27 of 76 patients, Fig. 2). The median time from kidney biopsy to the genetic diagnosis was 5.8 (2.8–10.6) years. Of these patients, 11 (40.8%) had an autosomal dominant disease, 9 (33.3%) an autosomal recessive disease (compound heterozygous variants), 6 (2.2%) an X-linked disease and 1 (3.7%) had two pathogenic mutations—one in COL4A4 and another in ACTN4. Table 2 and Supplementary data, Table S2 show a comprehensive list of the genetic findings. Patients with variants of uncertain significance (VUS) where the segregation analysis was not possible or lacking segregation were considered negative for genetic testing.
Figure 2:
Diagnostic yield and genetic findings. Section (A) shows the diagnostic yield obtained. Section (B) summarizes the genetic findings, grouped by gene. One patient had a dual diagnosis (see Table 2).
Table 2:
List of diagnostic variants.
| Potential causing variant | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | Sex | Age at biopsy (years) | Potential causal gene | cDNA and protein change | State (inheritance) | Type of variant | ACMG classa | dbSNP ID | Ref. (PMID) |
| SRNS-FSGS patients (based on biopsy) | |||||||||
| 9 | M | 19 | NPHS2 | c.862G > A (p.A288T) | Het (AR/AD) | Missense | LP | rs1490010141 | 20 947 785 |
| c.686G > A (p.R229Q) | Het (AR/AD) | Missense | VUS-LP | rs61747728 | 30 241 959, 24 509 478 | ||||
| 48 | M | 34 | NPHS2 | c.855_856del (p.R286TfsTer17) | Het (AR/AD) | Frameshift | P | rs749740335 | 24 742 477 |
| c.686G > A (p.R229Q) | Het (AR/AD) | Missense | VUS-LP | rs61747728 | 30 241 959, 24 509 478 | ||||
| 95 | M | 30 | NPHS2 | c.973C > T (p.H325Y) | Het (AR/AD) | Missense | LP | rs551511369 | 19 406 966 |
| c.686G > A (p.R229Q) | Het (AR/AD) | Missense | VUS-LP | rs61747728 | 30 241 959, 24 509 478 | ||||
| 99 | F | 43 | NPHS2 | c.928G > A (p.E310K) | Het (AR/AD) | Missense | P | NA | 19 145 239 |
| c.686G > A (p.R229Q) | Het (AR/AD) | Missense | VUS-LP | rs61747728 | 30 241 959, 24 509 478 | ||||
| 4 | F | 28 | COL4A3 | c.3044G > A (p.G1015E) | Het (AR/AD) | Missense | P | rs121912826 | 11 961 012 |
| c.4421T > C (p.L1474P) | Het (AR/AD) | Missense | HYPOMb | rs200302125 | 26 346 198 | ||||
| 31 | M | 34 | COL4A3 | c.3044G > A (p.G1015E) | Het (AR/AD) | Missense | P | rs121912826 | 11 961 012 |
| c.4421T > C (p.L1474P) | Het (AR/AD) | Missense | HYPOMb | rs200302125 | 26 346 198 | ||||
| 36 | M | 48 | COL4A4 | c.801_802del (p.Y268Ter) | Het (AR/AD) | Nonsense | P | NA | NA |
| ACTN4 | c.751C > T (p.R251W) | Het (AD) | Missense | VUS-LP | rs898478281 | NA | |||
| 3 | M | 56 | COL4A5 | c.3088G > A (p.G1030S) | Hemiz (XLD) | Missense | P | rs104886210 | 27 627 812 |
| 63 | M | 29 | APOL1 | G1: c.1024A > G, c.1152T > G (p.S342G, p.I384M) | Het (Risk Fact.) | Missense | Risk factor | G1 (rs73885319, rs60910145) | 25 168 832 |
| G2: c.1164_1169del (p.N388_Y389del) | Het (Risk Fact.) | In-frame deletion | Risk factor | G2 (rs71785313) | 25 168 832 | ||||
| 17 | M | 36 | OCRL | c.2035T > C (p.C679R) | Hemiz. (XLR) | Missense | LP | NA | NA |
| FSGS-UC patients (based on biopsy) | |||||||||
| 65 | M | 23 | NPHS2 | c.928G > A (p.E310K) | Het (AR/AD) | Missense | P | NA | 19 145 239 |
| c.686G > A (p.R229Q) | Het (AR/AD) | Missense | VUS-LP | rs61747728 | 30 241 959, 24 509 478 | ||||
| 69 | F | 34 | NPHS2 | c.714G > T (p.R238S) | Het (AR/AD) | Missense | P | rs748812981 | 15 253 708 |
| c.561G > A (p.M187I) | Het (AR/AD) | Missense | LP | NA | 20 947 785 | ||||
| 66 | F | 39 | INF2 | c.344T > A (p.I115N) | Het (AD) | Missense | LP | NA | NA |
| 79 | F | 59 | INF2 | c.202T > C (p.F68L) | Het (AD) | Missense | LP | NA | NA |
| 6 | F | 59 | COL4A3 | c.1918G > A (p.G640R) | Het (AR/AD) | Missense | P | rs200672668 | 11 134 255 |
| 55 | M | 51 | COL4A3 | c.388G > C (p.G130R) | Het (AR/AD) | Missense | P | NA | NA |
| c.4981C > T (p.R1661C) | Het (AR/AD) | Missense | LP | rs201697532 | 25 229 338 | ||||
| 64 | M | 54 | COL4A3 | c.3463G > A (p.G1155S) | Het (AR/AD) | Missense | P | rs774583962 | NA |
| 84 | M | 27 | COL4A3 | c.3751G > A (p.G1251S) | Het (AR/AD) | Missense | P | NA | 28 632 965 |
| 5 | M | 45 | COL4A4 | c.3488G > A (p.G1163D) | Het (AR/AD) | Missense | LP | NA | NA |
| SRNS-FSGS patients (based on biopsy) | |||||||||
| 19 | M | nk | COL4A4 | c.4764T > G (p.C1588W) | Het (AR/AD) | Missense | LP | NA | 24 052 634 |
| 75 | M | 39 | COL4A4 | c.755G > A (p.G252D) | Het (AR/AD) | Missense | P | NA | 33 532 864 |
| 21 | M | 36 | COL4A5 | (c.1032 + 4A > C) | Hemiz (XLD) | Likely splicing effect | LP | NA | NA |
| 56 | M | 46 | COL4A5 | (c.465 + 4dup) | Hemiz (XLD) | Likely splicing effect | LP | NA | NA |
| 59 | M | 50 | COL4A5 | (c.465 + 4dup) | Hemiz (XLD) | Likely splicing effect | LP | NA | NA |
| 73 | M | 40 | COL4A5 | c.4579T > A (p.C1527S) | Hemiz (XLD) | Missense | LP | rs755766520 | NA |
| 81 | F | 47 | COL4A5 | c.1799G > T (p.G600V) | Het (XLD) | Missense | P | NA | NA |
| 37 | M | 18 | APOL1 | G1: c.1024A > G, c.1152T > G (p.S342G, p.I384M) | Het (risk fact.) | Missense | Risk factor | G1 (rs73885319, rs60910145) | 25 168 832 |
| G2: c.1164_1169del (p.N388_Y389del) | Het (risk fact.) | In-frame deletion | Risk factor | G2 (rs71785313) | 25 168 832 | ||||
Variants classified in May 2023.
See ref. [22] (https://pubmed.ncbi.nlm.nih.gov/25741868/).
This variant can be considered hypomorphic (44). In association with another variant of COL4A3, as is the case in both patients, it may be associated with autosomal recessive Alport syndrome and kidney failure.
Het: heterozygous; Hemiz: hemizygous; AD: autosomal dominant; AR: autosomal recessive; nk: not known; P: pathogenic; LP: likely pathogenic; HYPOM: hypomorphic.
Ten relatives of six probands were received to carry out a study of carriers for examining co-segregation (probands IDs: 17; 37; 66; 75; 79; and 81; Supplementary data, Table S3). For family FSGS_MAD21 (proband 37), the co-segregation study rules out that the variant identified in the COL4A3 gene, initially classified as VUS, is related to the pathology, but it does not discard the implication of APOL1 risk haplotypes. For the other five families, the study of carriers in relatives supports the involvement of the proposed variant in the pathology.
Collagen 4A3–5 variants were the most frequent molecular diagnoses, with 19 pathogenic or probably pathogenic mutations identified in 16 (59.3%) patients (Fig. 2 and Table 2). There were six patients with COL4A5 pathogenic or likely pathogenic variants (NM_033380.3: p.G1030S; c.1032 + 4A > C; c.465 + 4dup; p.C1527S; p.G600V), four patients with COL4A4 pathogenic or likely pathogenic variants (NM_000 092: p.Y268Ter; p.G1163D; p.C1588W; p.G252D), one of them with an additional pathogenic or likely pathogenic in a second candidate gene (ACTN4, NM_004924.6: p.R251W), and six patients with COL4A3 pathogenic or likely pathogenic mutations (NM_000 091: p.G1015E; p.L1474P; p.G130R; p.R1661C; p.G640R; p.G1155S; p.G1251S), three of them in heterozygous state and three in compound heterozygous state. Compound heterozygote pathogenic or likely pathogenic variants identified in NPHS2 gene (NM_014 625: p.R238S; p.M187I; p.A288T; p.R286TfsTer17; p.E310K; p.H325Y; p.R229Q) accounted for six (22.2%) cases. In the remaining cases we found pathogenic or likely pathogenic variants affecting several other candidate genes (INF2, NM_022 489: p.I115N, p.F68L; OCRL, NM_000276.4: p.C679R). Two patients of African ancestry presented the two APOL1 high-risk alleles, G1 and G2 [23, 24].
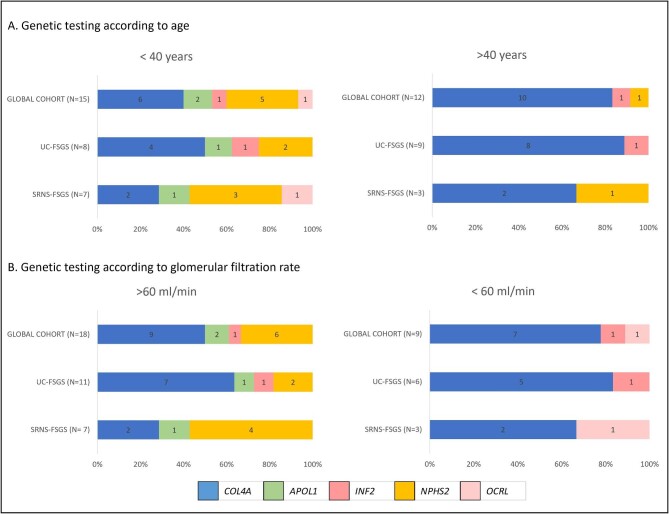
At the time of the biopsy, there were no differences between patients with a genetic diagnosis and patients with a negative result in terms of age, proteinuria, GFR, serum albumin, BMI, hypertension, diabetes, electron microscopy findings or positive family history (Table 3). Hematuria was more prevalent among patients with an identified genetic cause (P = .008; Table 3). There was no difference in eGFR decline between genetic and idiopathic groups during the follow-up period. Patients with COL4A3–5 mutations were older at the age of clinical diagnosis (44 vs 31 years, P = .015) than those with pathogenic variants in NPHS2 gene (Fig. 3A). Patients who combined COL4A3 or COL4A4 with other diagnostic variants were a median of 10 years younger, although the difference was not statistically significant. Four out of 16 (25%) patients with COL4A variants presented with chronic kidney failure but all NPHS2 patients showed eGFR above 60 mL/min/1.73 m2 (mean eGFR 69 vs 117 mL/min/1.73 m2, P = .005) (Fig. 3B). Furthermore, proteinuria was lower in patients with COL4A variants compared with NPHS2 variants (2.7 vs 6.8 g/day, P = .021). Interestingly, we found no differences in prevalence of hematuria between COL4A and non-COL4A patients.
Table 3:
Baseline characteristics of the global cohort and according to biopsy criteria and genetic finding.
| Biopsy criterion | Global cohort | SRNS-FSGS | FSGS-UC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genetic finding | NEG | POS | NEG | POS | NEG | POS | ||||
| N (%) | 49 (64.5) | 27 (35.5) | P-value | 14 (58.3) | 10 (41.7) | P-value | 35 (67.3) | 17 (32.7) | P-value | |
| Age (years), mean ± SD | 40.4 ± 12.1 | 40.3 ± 12.6 | 39.6 ± 11.5 | .80 | 39.9 ± 12.2 | 36.4 ± 10.6 | .47 | 40.5 ± 12.9 | 41.5 ± 11.9 | .79 |
| Male sex, % | 68.4 | 65.3 | 74.1 | .43 | 57.1 | 80 | .39 | 68.6 | 70.6 | .082 |
| BMI (kg/m2), mean ± SD | 26.7 ± 4.9 | 27.0 ± 4.8 | 26.3 ± 5.1 | .55 | 27.4 ± 5.3 | 24.8 ± 4.1 | .21 | 26.8 ± 4.7 | 27.2 ± 5.7 | .82 |
| Hypertension, % | 56.6 | 61.2 | 48.1 | .27 | 50 | 20 | .21 | 65.7 | 64.7 | .94 |
| Diabetes mellitus, % | 9.2 | 10.2 | 7.4 | .65 | 21 | 0 | .24 | 5.7 | 11.8 | .59 |
| Family history, % | 44.70 | 44.9 | 44.4 | .97 | 21.4 | 50 | .20 | 54.3 | 41.2 | .38 |
| RAS blockade, % | 72.4 | 73.5 | 70.4 | .78 | 64.3 | 70 | .65 | 77.1 | 70.6 | .43 |
| Immunosuppression, % | 48.70 | 45 | 52 | .46 | 100 | 100 | 25.0 | 29.40 | .41 | |
| eGFR CKD-EPI (mL/min), mean ± SD | 77.6 ± 34. 0 | 76.2 ± 33.3 | 80.0 ± 35.9 | .67 | 77.8 ± 33.7 | 81.6 ± 42.1 | .38 | 79.1 ± 32.9 | 74.3 ± 32.9 | 1 |
| Serum albumin (g/dL), mean ± SD | 3.6 ± 0.8 | 3.6 ± 0.9 | 3.6 ± 0.7 | .78 | 2.5 ± 0.7 | 2.9 ± 0.6 | .41 | 4.0 ± 0.6 | 3.9 ± 0.4 | .65 |
| Proteinuria (g/day), mean ± SD | 4.1 ± 3.5 | 4.1 ± 3.2 | 4.2 ± 4.2 | .60 | 7.1 ± 5.6 | 7.0 ± 5.6 | .77 | 2.9 ± 1.8 | 2.3 ± 1.5 | .25 |
| Hematuria, % | 60.5 | 49.0 | 81.5 | .008 | 50 | 100 | .019 | 48.6 | 70.6 | .18 |
All data refer to the time of kidney biopsy.
P-values are derived from Fisher's two-tailed test, χ2 two-tailed test or Mann–Whitney U tests for comparison of characteristics in patients with positive vs negative genetic test results. P < .05 was considered to be statistically significant.
RAS: renin–angiotensin system; eGFR CKD-EPI: estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration; CNI: calcineurin inhibitor; MMF: mycophenolate mofetil; NEG: no disease-causing variant identified; POS: disease-causing variant identified.
Figure 3:
Genetic testing according to age (A) and glomerular filtration rate. (B) according to the inclusion criteria (whole cohort, FSGS-UC or SRNS-FSGS).
Comparison between positive vs negative genetic patients with SRNS-FSGS or FSGS-UC based on KDIGO guidelines inclusion criteria
The presence of pathogenic or likely pathogenic genetic variants related to FSGS was more common in the SRNS-FSGS group (41.7% vs 32.7%), although this difference was not statistically significant (Table 3). The distribution of genetic findings among 10 patients with SRNS-FSGS was: four patients (40%) with two NPHS2 variants (compound heterozygotes), one patient with the two high risk haplotypes of APOL1 gene in heterozygosity (G1 and G2 haplotypes), one patient with a hemizygous OCRL variant and four patients (40%) with variants in the collagen 4A genes: 1 patient (male) presented with a COL4A5 variant, two patients with two compound heterozygous variants at COL4A3, and another patient with one COL4A4 variant and another ACTN4 variant (Fig. 3A). COL4A3–5 variants were the most prevalent finding in the FSGS-UC group, with 12 patients carrying one or two variants in these genes (76.4%). In particular, there were four patients with COL4A3 variants (one heterozygotes, three compound heterozygous), three with a COL4A4 variant (all heterozygote) and five with a COL4A5 variant (four hemizygous males and one heterozygous female).
We stratified our cohort according to the KDIGO guidelines for indications for genetic diagnosis (patients with family history of kidney disease, syndromic features, early-onset or disease resistant to immunosuppressive therapy). All patients with SRNS-FSGS are included in these recommendations, and FSGS-UC patients were stratified according to having or not indications for genetic diagnosis. In accordance with these recommendations, 71% of patients with FSGS-UC would be candidates for genetic testing. However, we did not find differences between the prevalence of genetic causes among those who presented some criteria for genetic testing (29.7%) compared with those who did not (29.7 vs 40%, P = .474).
Table 3 shows clinical characteristics in patients with positive vs negative genetic testing results in SRNS-FSGS and FSGS-UC groups. No significant clinical differences were found between FSGS-UC and SRNS-FSGS patients. There were four cases with SRNS-FSGS among Alport patients, and two patients with NPHS2 variants presented with FSGS-UC. This reinforces the need to genetically diagnose those patients with such a disease profile, in order to properly classify them.
DISCUSSION
Genetic testing in FSGS has usually been reserved for familial cases, patients with specific phenotypes and children with SRNS-FSGS [5, 25–29]. However, our results support that adult-onset genetic FSGS is frequent in both SRNS-FSGS and FSGS-UC, even in the absence of a family history or resistance to immunosuppressive therapies.
In a large cohort of CKD patients, Groopman et al. [30], showed that 62% of patients with pathogenic mutations in COL4A3–5 did not have clinical diagnoses of the nephropathies classically associated with these genes, and 16% of patients had been diagnosed of FSGS. In a cohort of adult disease onset and a high likelihood for hereditary FSGS, Braunisch et al. [31] identified a monogenic cause in 29% of patients, after performing whole-exome sequencing.
In our cohort of SRNS-FSGS, we found candidate disease-causing variants in 41.7% of all patients, even though family history of CKD was not an inclusion criterion. Moreover, among the patients with SRNS-FSGS and positive genetic diagnosis, only 50% had a family history of renal disease. Therefore, we consider that the absence of family history should not preclude genetic study in FSGS presenting with SRNS. Variant penetrance, the presence of other genetic variants and environmental factors would explain the different phenotypes among relatives with the same pathogenic mutation.
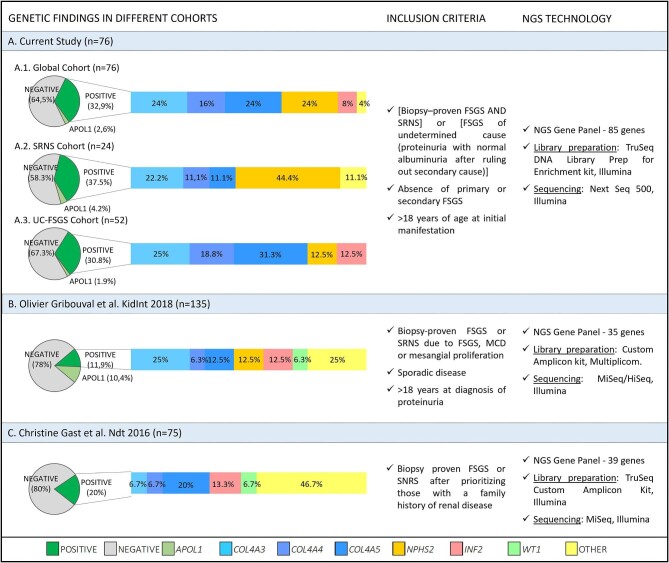
To contextualize our results with what has been published to date, we set out to compare our findings with those of other studies comparable to ours—namely studies including FSGS cohorts with criteria similar to ours, and in which an NGS strategy based on glomerular candidate gene panels had been used. After reviewing the literature, we found two articles that met our criteria (Fig. 4). In the study by Gribouval et al. [8] where the spotlight was extended, considering sporadic SRNS and/or FSGS, pathogenic mutations were identified in 11.8% of patients, and an additional 10.4% of patients carried APOL1 high-risk alleles. Gast et al. [9] analysed a cohort of 75 FSGS probands by means of a custom NGS panel of 39 candidate genes. They found definitely or probably pathogenic mutations in 15 of them (20%). In our series, only patients without secondary FSGS were included, which could explain the high percentage of disease-causing mutations.
Figure 4:
Comparison of cohorts. This figure shows schematically our study and that of two other comparable cohorts; particularly, we show for each study the inclusion criteria considered, the NGS strategy employed, and the genetic results obtained. Section (A) refers to our cohort. The genetic results are represented both for the global cohort and broken down into the two considered subgroups: the FSGS-UC cohort and the SRNS-FSGS cohort. Section (B) shows the results of Gribouval et al. [8]. Section (C) shows the results of Gast et al. [9].
The 2021 KDIGO guidelines proposes a new classification of FSGS that includes a new category, FSGS of undetermined cause [1]. It covers patients whose presentation is similar to secondary FSGS: proteinuria with normal serum albumin and in which a secondary etiology has not been identified. Our study explores the genetic etiology in this specific subgroup. In our cohort, 35.5% of the patients had an underlying pathological genetic cause that could be responsible for their clinical and histological picture.
Our results are consistent with other series which reported type 4 collagen mutations to be the most frequent disease-causing mutations in adult FSGS population [8, 9, 32–34].
In our study, this distribution is modified according to the clinical characteristics, with genetic variants related to collagen type 4 being the most frequent in FSGS-UC and those related to the slit diaphragm the most frequent in SRNS-FSGS. However, both variants were detected in both groups. COL4A5 variants with X-linked inheritance have been attributed to be the driver in 85% of cases of Alport disease. Nevertheless, recent studies have highlighted the relevance of COL4A4 and COL4A3 mutations [35–42]. We found 71.4% of Alport disease patients to be secondary to COL4A3 and COL4A4 variants. Our cohort showed the wide phenotypic spectrum of COL4A3–5 associated FSGS, ranging from SRNS in early adulthood to non-nephrotic proteinuria in middle age. As described in other studies, hematuria was not always present [9, 37]. We found that all patients with monogenic COL4A3 or -A4 variants were hypertensive and none of them presented with nephrotic proteinuria. Of particular interest is that patients with compound heterozygous variants at COL4A4 genes, or one heterozygous variant in one of these genes with an additional variant in another gene (ACTN4), seemed to have an earlier onset and three out of four presented with SRNS-FSGS.
The genetic approach could contribute significantly to shortening the diagnostic process, and allow focus on targeted treatments.
The clinical phenotype is not helpful in most cases. As mentioned above, not all patients with collagen 4 variants presented hematuria and nor did patients with the rest of the variants present the full spectrum of associated syndromes. Indiscriminate empirical treatment of FSGS with steroids should be avoided unless the suspicion of a primary FSGS is very high. In our series, among patients with a potential causative variant, five patients (50%) with SRNS-FSGS received additional immunosuppression, and 5 (29%) patients with non-nephrotic proteinuria had been under unnecessary immunosuppressive treatment.
FSGS reflects irreversible lesions in the kidney, therefore familial screening and presymptomatic care of affected relatives is of utmost importance. Genetic testing enables genetic counseling and preimplantation genetic diagnostics.
As a limitation of this study, our cohort consisted mainly of Caucasian adults, thus the genetic diagnostic yield or the genetic findings cannot be extrapolated to other populations. Patient inclusion in this study was based on clinical criteria, as electron microscopy was not available in many cases. We believe that changes in podocyte effacement or glomerular basement membrane might improve the efficiency of genetic testing indication. Although our selection criteria did not prioritize familial cases, 45% of patients reported a family history of kidney disease. Importantly, this variable did not differ between patients with and without pathogenic genetic variants.
Some studies have tested non-glomerular genes in patients with FSGS [31, 34]. The Toronto GN Registry cohort [33] reported CAKUT (congenital anomalies of the kidney and urinary tract) genes in 5% of patients with a histological diagnosis of FSGS. We restricted our study to 84 genes known to be causative of genetic FSGS.
It is possible that the variants detected are not the only cause of the disease and that additional metabolic, hypertensive or other factors were involved to develop the fully expressed phenotype. However, we believe that this does not diminish the importance of determining a possible genetic origin, which would aid in limiting unnecessary immunosuppression, studying other pathologies of the syndrome and an early detection of affected relatives.
Miao et al. [43] detected a monogenic variant in 33.3% patients with secondary FSGS with known causes. Our study excluded patients with secondary causes for FSGS, and selected those in whom underlying genetic findings were more likely to be present, therefore we cannot rule out a genetic cause in secondary FSGS.
Targeted NGS analysis does not exclude the presence of variants in unexamined regions (introns, regulatory regions or regions not accessible to hybridization), nor large deletions, insertions or inversions that cannot be detected by the technique used.
In conclusion, genetic testing is a minimally invasive diagnostic procedure that helps to determine the origin of the patient's disease, provides guidance on prognosis and future treatments, and enables family screening. Our experience supports the implementation of genetic testing in routine clinical practice for the diagnosis of patients with SRNS-FSGS or FSGS-UC, regardless of the patient's age at disease onset or family history of kidney disease.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all patients and their families for participating in this study and the Biobank of Hospital Universitario Fundación Alcorcón. We also acknowledge the staff at all participating sites and the support provided by the GLOSEN group.
Contributor Information
Ana María Tato, Department of Nephrology, Hospital Universitario Fundación Alcorcón, Alcorcón, Spain.
Noa Carrera, Laboratorio de Nefroloxía (No. 11), Grupo de Xenética e Bioloxía do Desenvolvemento das Enfermidades Renais, Instituto de investigación sanitaria de Santiago de Compostela – IDIS, Santiago de Compostela, Spain.
Maria García-Murias, Laboratorio de Nefroloxía (No. 11), Grupo de Xenética e Bioloxía do Desenvolvemento das Enfermidades Renais, Instituto de investigación sanitaria de Santiago de Compostela – IDIS, Santiago de Compostela, Spain.
Amir Shabaka, Department of Nephrology, Hospital Universitario Fundación Alcorcón, Alcorcón, Spain.
Ana Ávila, Department of Nephrology, Hospital Universitario Doctor Peset, Valencia, Spain.
María Teresa Mora Mora, Department of Nephrology, Hospital Universitario Juan Ramón Jiménez, Huelva, Spain.
Cristina Rabasco, Department of Nephrology, Hospital Universitario Reina Sofía, Córdoba, Spain.
Karina Soto, Department of Nephrology, Hospital Fernando Fonseca, Lisbon, Portugal.
Francisco Jose de la Prada Alvarez, Department of Nephrology, Hospital Universitario Virgen Macarena, Sevilla, Spain.
Loreto Fernández-Lorente, Department of Nephrology, Hospital Virgen del Camino, Pamplona, Spain.
Antolina Rodríguez-Moreno, Department of Nephrology, Hospital Universitario Clínico San Carlos, Madrid, Spain.
Ana Huerta, Department of Nephrology, Hospital Universitario Puerta de Hierro, Majadahonda, Spain.
Carmen Mon, Department of Nephrology, Hospital Universitario Severo Ochoa, Leganés, Spain.
Clara García-Carro, Department of Nephrology, Hospital Vall d'Hebrón, Barcelona, Spain.
Fayna González Cabrera, Department of Nephrology, Hospital Universitario de Gran Canaria Doctor Negrín, Gran Canaria, Spain.
Juan Antonio Martín Navarro, Department of Nephrology, Hospital Universitario Infanta Leonor, Madrid, , Spain.
Ana Romera, Department of Nephrology, Hospital de Ciudad Real, Ciudad Real, Spain.
Eduardo Gutiérrez, Department of Nephrology, Hospital Universitario Doce de Octubre, Madrid, Spain.
Javier Villacorta, Department of Nephrology, Hospital Universitario Ramón y Cajal, Madrid, Spain.
Alberto de Lorenzo, Department of Nephrology, Hospital HM San Chinarro, Madrid, Spain.
Beatriz Avilés, Department of Nephrology, Hospital Costa del Sol, Marbella, Spain.
Miguel Angel Garca-González, Laboratorio de Nefroloxía (No. 11), Grupo de Xenética e Bioloxía do Desenvolvemento das Enfermidades Renais, Instituto de investigación sanitaria de Santiago de Compostela – IDIS, Santiago de Compostela, Spain; Fundación Pública Galega de Medicina Xenómica-SERGAS, Complexo Hospitalario de Santiago de Compostela, Santiago de Compostela, Spain.
Gema Fernández-Juárez, Department of Nephrology, Hospital Universitario La Paz, Madrid, Spain; Instituto de Investigación de la Paz (IdIPAZ), Madrid, Spain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest to disclose.
AUTHORS’ CONTRIBUTIONS
Research idea and study design: N.C., G.F.-J., M.A.G.-G., A.M.T. Data acquisition: A.A., B.A., G.F.-J., L.F.-L., C.G.-C., E.G., F.G.C., A.H., A.L., J.A.M.N., C.M., M.T.M.M., F.J.P.A., C.R., A.R.-M., A.R., A.S., K.S., A.M.T., J.V. Data analysis/interpretation: N.C., G.F.-J., M.A.G.-G., A.M.T., M.G.-M., A.S. Statistical analysis: A.M.T. Supervision or mentorship: G.F.-J., M.A.G.-G. Each author contributed significant intellectual content during the drafting or revision of the manuscript and agrees to be personally responsible for his or her own contributions and to ensure that questions concerning the accuracy or completeness of any part of the work, even those in which the author was not directly involved, are appropriately investigated and resolved, including with bibliographic documentation if appropriate.
FUNDING
This work was supported by grants from the Fundación Íñigo Álvarez de Toledo, the Instituto de Salud Carlos III (PI18/00 378 under FIS/FEDER and RD21/0005/0020-RICORS funds to M.A.G.-G.; RD21/10 005/0001-RICORS to G.F.-J.) and the Xunta de Galicia (IN607B-2016/020 to M.A.G.-G., funded by the European Union “NextGenerationEU” Facility for recovery and resilience). None of the funders had any role in study design, data collection, analysis, reporting or the decision to submit for publication.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Rovin BH, Adler SG, Barratt Jet al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 2021;100:S1–276. 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 2. Shabaka A, Tato Ribera A, Fernández-Juárez G.. Focal segmental glomerulosclerosis: state-of-the-art and clinical perspective. Nephron 2020;144:413–27. 10.1159/000508099 [DOI] [PubMed] [Google Scholar]
- 3. De Vriese AS, Sethi S, Nath KAet al. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol 2018;29:759–74. 10.1681/ASN.2017090958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Santín S, Bullich G, Tazón-Vega Bet al. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 2011;6:1139–48. 10.2215/CJN.05260610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trautmann A, Bodria M, Ozaltin Fet al. Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin J Am Soc Nephrol 2015;10:592–600. 10.2215/CJN.06260614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sadowski CE, Lovric S, Ashraf Set al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 2015;26:1279–89. 10.1681/ASN.2014050489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang F, Zhang Y, Mao Jet al. Spectrum of mutations in Chinese children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 2017;32:1181–92. 10.1007/s00467-017-3590-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gribouval O, Boyer O, Hummel Aet al. Identification of genetic causes for sporadic steroid-resistant nephrotic syndrome in adults. Kidney Int 2018;94:1013–22. 10.1016/j.kint.2018.07.024 [DOI] [PubMed] [Google Scholar]
- 9. Gast C, Pengelly RJ, Lyon Met al. Collagen (COL4A) mutations are the most frequent mutations underlying adult focal segmental glomerulosclerosis. Nephrol Dial Transplant 2016;31:961–70. 10.1093/ndt/gfv325 [DOI] [PubMed] [Google Scholar]
- 10. Laurin LP, Lu M, Mottl AKet al. Podocyte-associated gene mutation screening in a heterogeneous cohort of patients with sporadic focal segmental glomerulosclerosis. Nephrol Dial Transplant 2014;29:2062–9. 10.1093/ndt/gft532 [DOI] [PubMed] [Google Scholar]
- 11. Hommos MS, De Vriese AS, Alexander MPet al. The incidence of primary vs secondary focal segmental glomerulosclerosis: a clinicopathologic study. Mayo Clin Proc 2017;92:1772–81. 10.1016/j.mayocp.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sethi S, Zand L, Nasr SHet al. Focal and segmental glomerulosclerosis: clinical and kidney biopsy correlations. Clin Kidney J 2014;7:531–7. 10.1093/ckj/sfu100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishizuka K, Miura K, Hashimoto Tet al. Degree of foot process effacement in patients with genetic focal segmental glomerulosclerosis: a single-center analysis and review of the literature. Sci Rep 2021;11:12008. 10.1038/s41598-021-91520-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sambharia M, Rastogi P, Thomas CP.. Monogenic focal segmental glomerulosclerosis: a conceptual framework for identification and management of a heterogeneous disease. Am J Med Genet C Semin Med Genet 2022;190:377–98. 10.1002/ajmg.c.31990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Becherucci F, Landini S, Palazzo Vet al. A clinical workflow for cost-saving high-rate diagnosis of genetic kidney diseases. J Am Soc Nephrol 2023;34:706–20. 10.1681/ASN.0000000000000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Snoek R, van Jaarsveld RH, Nguyen TQet al. Genetics-first approach improves diagnostics of ESKD patients <50 years old. Nephrol Dial[HL]:AU: Please check edits made to publication details in ref [16] are correct Transplant 2022;37:349–57. [DOI] [PubMed] [Google Scholar]
- 17. McKenna A, Hanna M, Banks Eet al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010;20:1297–303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DePristo MA, Banks E, Poplin Ret al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 2011;43:491–8. 10.1038/ng.806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen S, Zhou Y, Chen Yet al. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018;34:i884–90. 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ye K, Schulz MH, Long Qet al. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics 2009;25:2865–71. 10.1093/bioinformatics/btp394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plagnol V, Curtis J, Epstein Met al. A robust model for read count data in exome sequencing experiments and implications for copy number variant calling. Bioinformatics 2012;28:2747–54. 10.1093/bioinformatics/bts526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richards S, Aziz N, Bale Set al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friedman DJ, Pollak MR.. APOL1 nephropathy: from genetics to clinical applications. Clin J Am Soc Nephrol 2021;16:294–303. 10.2215/CJN.15161219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kopp JB, Nelson GW, Sampath Ket al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011;22:2129–37. 10.1681/ASN.2011040388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lovric S, Ashraf S, Tan Wet al. Genetic testing in steroid-resistant nephrotic syndrome: when and how? Nephrol Dial Transplant 2016;31:1802–13. 10.1093/ndt/gfv355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lipska BS, Iatropoulos P, Maranta Ret al. Genetic screening in adolescents with steroid-resistant nephrotic syndrome. Kidney Int 2013;84:206–13. 10.1038/ki.2013.93 [DOI] [PubMed] [Google Scholar]
- 27. Giglio S, Provenzano A, Mazzinghi Bet al. Heterogeneous genetic alterations in sporadic nephrotic syndrome associate with resistance to immunosuppression. J Am Soc Nephrol 2015;26:230–6. 10.1681/ASN.2013111155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park E, Lee C, Kim NKDet al. Genetic study in Korean pediatric patients with steroid-resistant nephrotic syndrome or focal segmental glomerulosclerosis. J Clin Med 2020;9:2013. 10.3390/jcm9062013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lata S, Marasa M, Li Yet al. Whole-exome sequencing in adults with chronic kidney disease: a pilot study. Ann Intern Med 2018;168:100. 10.7326/M17-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Groopman EE, Marasa M, Cameron-Christie Set al. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med 2019;380:142–51. 10.1056/NEJMoa1806891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Braunisch MC, Riedhammer KM, Herr PMet al. Identification of disease-causing variants by comprehensive genetic testing with exome sequencing in adults with suspicion of hereditary FSGS. Eur J Hum Genet 2021;29:262–70. 10.1038/s41431-020-00719-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bullich G, Domingo-Gallego A, Vargas Iet al. A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney Int 2018;94:363–71. 10.1016/j.kint.2018.02.027 [DOI] [PubMed] [Google Scholar]
- 33. Yao T, Udwan K, John Ret al. Integration of genetic testing and pathology for the diagnosis of adults with FSGS. Clin J Am Soc Nephrol 2019;14:213–23. 10.2215/CJN.08750718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang M, Chun J, Genovese Get al. Contributions of rare gene variants to familial and sporadic FSGS. J Am Soc Nephrol 2019;30:1625–40. 10.1681/ASN.2019020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Voskarides K, Damianou L, Neocleous Vet al. COL4A3/COL4A4 mutations producing focal segmental glomerulosclerosis and renal failure in thin basement membrane nephropathy. J Am Soc Nephrol 2007;18:3004–16. 10.1681/ASN.2007040444 [DOI] [PubMed] [Google Scholar]
- 36. Pierides A, Voskarides K, Athanasiou Yet al. Clinico-pathological correlations in 127 patients in 11 large pedigrees, segregating one of three heterozygous mutations in the COL4A3/COL4A4 genes associated with familial haematuria and significant late progression to proteinuria and chronic kidney disease from focal segmental glomerulosclerosis. Nephrol Dial Transplant 2009;24:2721–9. [DOI] [PubMed] [Google Scholar]
- 37. Furlano M, Martínez V, Pybus Met al. Clinical and genetic features of autosomal dominant Alport syndrome: a case series. Am J Kidney Dis 2021;78:560–70.e1. 10.1053/j.ajkd.2021.02.326 [DOI] [PubMed] [Google Scholar]
- 38. Matthaiou A, Poulli T, Deltas C.. Prevalence of clinical, pathological and molecular features of glomerular basement membrane nephropathy caused by COL4A3 or COL4A4 mutations: a systematic review. Clin Kidney J 2020;13:1025–36. 10.1093/ckj/sfz176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Malone AF, Phelan PJ, Hall Get al. Rare hereditary COL4A3/COL4A4 variants may be mistaken for familial focal segmental glomerulosclerosis. Kidney Int 2014;86:1253–9. 10.1038/ki.2014.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kamiyoshi N, Nozu K, Fu XJet al. Genetic, clinical, and pathologic backgrounds of patients with autosomal dominant Alport Syndrome. Clin J Am Soc Nephrol 2016;11:1441–9. 10.2215/CJN.01000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gibson J, Fieldhouse R, Chan MMYet al. Prevalence estimates of predicted pathogenic COL4A3–COL4A5 variants in a population sequencing database and their implications for Alport syndrome. J Am Soc Nephrol 2021;32:2273–90. 10.1681/ASN.2020071065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mastrangelo A, Madeira C, Castorina Pet al. Heterozygous COL4A3/COL4A4 mutations: the hidden part of the iceberg? Nephrol Dial Transplant 2022;37(12):2398–407. 10.1093/ndt/gfab334 [DOI] [PubMed] [Google Scholar]
- 43. Miao J, Pinto e Vairo F, Hogan MCet al. Identification of genetic causes of focal segmental glomerulosclerosis increases with proper patient selection. Mayo Clin Proc 2021;96:2342–53. 10.1016/j.mayocp.2021.01.037 [DOI] [PubMed] [Google Scholar]
- 44. Savige J, Huang M, Croos Dabrera MSet al. Genotype-phenotype correlations for pathogenic COL4A3COL4A5 Variants in X-Linked, Autosomal Recessive, and Autosomal Dominant Alport Syndrome. Front Med (Lausanne) 2022;9. https://doi.org: 10.3389/fmed.2022.865034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.