Abstract
The skin, the largest organ in the body, undergoes age-related changes influenced by both intrinsic and extrinsic factors. The primary external factor is photoaging which causes hyperpigmentation, uneven skin surface, deep wrinkles, and markedly enlarged capillaries. In the human dermis, it decreases fibroblast function, resulting in a lack of collagen structure and also decreases keratinocyte function, which compromises the strength of the protective barrier. In this study, we found that treatment with γ-aminobutyric acid (GABA) had no toxicity to skin fibroblasts and GABA enhanced their migration ability, which can accelerate skin wound healing. UVB radiation was found to significantly induce the production of matrix metalloproteinase 1 (MMP-1), but treatment with GABA resulted in the inhibition of MMP-1 production. We also investigated the enhancement of filaggrin and aquaporin 3 in keratinocytes after treatment with GABA, showing that GABA can effectively improve skin moisturization. In vivo experiments showed that oral administration of GABA significantly improved skin wrinkles and epidermal thickness. After the intake of GABA, there was a significant decrease observed in the increase of skin thickness measured by calipers and erythema. Additionally, the decrease in skin moisture and elasticity in hairless mice exposed to UVB radiation was also significantly restored. Overall, this study demonstrates the potential of GABA as functional food material for improving skin aging and moisturizing.
Keywords: γ-Aminobutyric acid, Aging, Collagen, MMP-1, Filaggrin, AQP3
INTRODUCTION
Skin is the largest organ in the body, comprising 16% of total body weight, and it serves as a first line of defense and maintains essential chemical substances and nutrients. It has been established that the skin undergoes age-related changes due to a combination of intrinsic factors, which are naturally occurring over time, and extrinsic factors, which result from external stressors such as UV radiation and micro-particles. (Kim and Park, 2016) These factors can lead to dryness or inflammation of the skin’s epidermis, a decline in fibroblast proliferation and function in the skin’s dermis, and a loss of elasticity and the formation of wrinkles (Fisher et al., 2002). Keratinocytes, which constitute the majority of epidermal cells, are distinguished by their synthesis of cytokeratin and the presence of desmosomes. They are also tightly bound to each other to form a strong physical and chemical barrier (Warskulat et al., 2004). The dermis also contains abundant fibroblasts, which play a key role in many of the skin’s physiological reactions by producing connective tissue in the dermis and connecting other cells (Ito et al., 2007). Chronological aging is associated with epidermal atrophy and a reduction in the number of fibroblasts and collagen in the dermis, which are the main histological changes in the skin (Di Cagno et al., 2010). Intrinsically aged skin is characterized by a decrease in epidermal turnover, a phenomenon that is associated with shrinkage in the spinous layer (Kim and Park, 2016).
Gamma-aminobutyric acid (GABA), a non-protein amino acid, is naturally produced by the body (Olsen and DeLorey, 1999). GABA is found in large amounts in vertebrates, plants, and microbes, and it is the primary inhibitory neurotransmitter in the adult brain. It also has immune system-inhibitory properties (Plante et al., 2012). GABA is packed into synaptic vesicles in the nervous system and then released into the synaptic cleft, where it diffuses to the target receptors on the postsynaptic surface. GABA has a molecular weight of only 103, making it able to pass through the skin and bind to GABA receptors in skin cells. It has been found to play a role in several skin functions: (1) the production of hyaluronic acid (Warskulat et al., 2004); (2) the ability of normal human keratinocytes to maintain cell volume homeostasis under UV radiation (Warskulat et al., 2004); and (3) the ability of dermal fibroblasts to survive when exposed to oxidative stress (Ito et al., 2007).
The matrix metalloproteinases (MMPs), a family of zinc-containing proteases, are responsible for the degradation of most extracellular matrix proteins (Nelson et al., 2000; Sternlicht and Werb, 2001). Research on skin aging and MMPs has significantly increased in the past 20 years. Studies have demonstrated that UVB radiation leads to increased expression and activation of MMP in human skin. (Fisher et al., 1996). UV radiation has also been shown to stimulate the mitogen-activated protein kinase (MAPK) signaling pathway (Kozák et al., 2003) and activate various growth factor and cytokine receptors on the cell surface, upregulating nuclear factor kappa B (NF-kB) and transcription factor activator protein-1 (AP-1), which consists of c-Jun and c-Fos proteins, on the cell surface (Cheng et al., 2012). Combining MMP-1, MMP-3, and MMP-9 results in the majority of type I and type III cutaneous collagen degradation (Quan et al., 2009; Kim et al., 2011). Anti-aging therapies for the skin have been shown to decrease the expression of MMPs, increase collagen synthesis, reduce the concentration of epidermal melanin, and decrease collagen breakdown in the dermis through epidermal hyperplasia and thickening (Griffiths et al., 2005).
Filaggrin is essential for skin barrier function and is metabolized into natural moisturizing factors (NMFs), which are responsible for retaining water and maintaining an acidic pH in the skin (Rawlings and Harding, 2004). Keratinocytes in the epidermis form the top layer through differentiation from the basal layer to the stratum corneum (Schoop et al., 1999). Each layer has different calcium concentrations. Higher concentrations of calcium in the granular layer cause hyaline cutin granules to release their contents, exposing profilaggrin for processing and fragmentation into active filaggrin monomers (Vičanová et al., 1998).
Aquaporins (AQPs) are integral membrane proteins that facilitate osmotically-driven water transport across cell plasma membranes through the formation of water-selective pores (Verkman, 2005). In mammals, there are 13 known AQPs (AQP0-AQP12) which can be divided into two categories based on their permeability. AQP 3, 7, 9, and 10, referred to as “aquaglyceroporins,” are capable of transporting water as well as glycerol and potentially other small solutes. AQP 1, 2, 4, 5, and 8 primarily function as water-selective transporters. (Hara-Chikuma and Verkman, 2008). The most well-researched and established AQP in the skin is AQP3, which promotes water transport and aids in cell migration, as well as speeding up the healing of skin wounds. AQP3-promoted glycerol transport, based on glycerol’s moisturizing properties, is also important for cell proliferation and the hydration and elasticity of the skin (Frigeri et al., 1995).
This study examined whether GABA regulates cell proliferation and migration, and collagen synthesis in human fibroblasts, and evaluated the anti-aging and moisturizing effects of GABA and lactic acid bacterium fermented rice germ extract (LFRGE) on human keratinocytes. It was investigated in this study how GABA and LFRGE regulate MMP-1 expression in UVB-irradiated human fibroblasts and how they affect filaggrin synthesis and AQP3 level in normal human keratinocytes. Finally the anti-aging and moisturizing effects of LFRGE were evaluated in animal model using UVB-irradiated hairless mice.
MATERIALS AND METHODS
Cell culture
Normal Human Dermal Fibroblast (HDF) and HaCaT, human immortalized keratinocyte cell line, were purchased from the American Type Culture Collection (ATCC) (VA, USA). They were maintained in Dulbecco’s Modified Essential Medium (Gibco, NY, USA) containing 10% Fetal bovine serum (Welgene, Gyeongsan, Korea), and 1% Penicillin-Streptomycin (Welgene) in 5% CO2 incubator.
Preparation of GABA and LFRGE
GABA (NLT 95%) was purchased from MH2 Biochemical Co., Ltd. (Eumseong, Korea). LFRGE (ca. 15% GABA w/w) was provided by EVERSPRING Co., Ltd. (Seongnam, Korea). LFRGE was manufactured as follows. Water extract of rice germ is fermented by Lactobacillus sakei. After fermentation, the fermented extract is heat-treated to inactivate bacteria which are removed to yield cell-free-filtrate solution. The solution is then standardized to GABA content prior to spray drying to obtain LFRGE finally.
Cell proliferation
In a 96-well plate, HDF cells were seeded at a density of 1×104 cells per well. After 24-h incubation at 37°C, HDF cells were treated with GABA and LFRGEs for 48 or 72 h. And then the media were replaced with 10% EZ-cytox reagent after being washed twice with DPBS (Daeil Lab Service, Seoul, Korea). The absorbance was determined using a microplate reader (Tecan, Mannedorf, Switzerland) at a wavelength of 450 nm after an hour of incubation at 37°C.
Enzyme-Linked Immunosorbent Assay (ELISA)
HDF cells were seeded at a density of 1.5×104 cells/well in a 48 well plate. The supernatant media and cells were collected after treatment. The test for the procollagen and MMP-1 was performed according to the manufacturer’s instructions (R&D Systems, MN, USA). HDF cells were treated with drugs via 48 h and supernatant media were used for analyzing secreted procollagen. And HDF cells were exposed to UVB irradiation at 30 mJ/cm2. The UVB source was six fluorescent lamps (TL 20 W/12 RS SLV, wavelength 290 to 390 nm, peak emission 315 nm; Philips, Amsterdam, Netherlands), and the UVB irradiation intensity was measured with a UV meter (VARIOCONTROL ver. 2.03, Waldmann, Villingen-Schwenningen, Germany). A BCA Protein Assay Kit (Thermos Scientific, Rockford, IL, USA) was used to measure the protein content of the cell, with bovine serum albumin serving as the reference.
Scratch assay
HDF cells were seeded at a density of 2.5×105 cells/well in 12 well plate. Incubated at 5% CO2 and scratched the cells with 200p tips and washed with DPBS 2 times. GABA was treated with DMEM with 0% FBS and DMEM with 2% FBS was used as a positive control. Taken cell photo after treatment with GABA and analysis with the ImageJ software (ImageJ, RRID:SCR 003070, http://rsb.info.nih.gov/ij).
q-PCR
Total RNA was extracted using RNAiso Plus (Takara, Shiaga, Japan) according to the manufacturer’s instructions. cDNA was synthesized using RevertAid™ Reverse Transcriptase (Thermos Scientific). Quantitative polymerase chain reaction analysis was performed using FastStart Essential DNA Probes Master (Roche, Basel, Switzerland) with CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA).
Western blot
Cells were washed twice in DPBS and protein was extracted using RIPA lysis buffer containing Protease inhibitor cocktail (Sigma-Aldrich, MO, USA) and 1mM PMSF (Sigma-Aldrich). Equal amounts of protein were separated by NuPAGE™ 12% Bis-Tris Gel (Invitrogen, CA, USA) and transferred to PVDF membrane. After blocked 5% BSA in TBS-T, they were incubated overnight with anti-Filaggrin antibody (Santa-Cruz) and anti-b-actin antibody (Santa-Cruz, CA, USA) and then incubated with a Horse-radish peroxidase-conjugated anti-mouse secondary antibody (Bio-Rad).
Experimental animals
Six-week-old female specific pathogen free (SPF) mice were purchased from Central Lab. Animal Inc. (Seoul, Korea). The hairless mice acclimated to the environment for 1 week before starting the experiment, and then were divided into 3 groups with 10 mice in each group. Body weights were measured on the day before dosing and ranked body weights were used to randomize groups. The rearing environment was maintained under controlled temperature (23 ± 3°C) and humidity (55 ± 15%) and automatic lighting (12-h light and dark cycle), with illuminance/noise/ammonia concentration below 268 Lux/55.1 dB/5 ppm. During purification, quarantine, dosing and observation, accept stainless steel cages (200 W×260 D×130 Hmm). During the purification period, administration period and post-administration observation period, the number of animals in each breeding box was no more than 5. Feed was provided to hairless mice (Orient Bio, Seongnam, Korea). Experimental animal care and management was based on the “Guide for the Care and Use of Laboratory Animals,” and all experiments were approved by the Institutional Animal Care and Use Committee of Gyeonggi Institute of Science and Technology (Suwon, Korea).
UVB irradiation
Irradiation was performed using six fluorescent lamps (TL 20 W/12RS SLV, wavelength 290 to 390 nm, peak emission 315 nm; (Philips)) 3 times a week. UV irradiation was conducted as follows: week 1, 1 MED; week 2, 2 MED, week 3, 3 MED; week4~12, 4 MED. The irradiation dose was adjusted to form appropriate wrinkles as skin condition. Approximately, 130 MED were irradiated. It is measured using a Waldmann UV meter (VARIOCONTROL ver. 2.03, Waldmann). Oral administration of LFRGE was performed using a gavage technique delivering it into the stomach using a blunt-ended needle at a dosage of 15 mg/kg.b.w./day as GABA along with UV irradiation.
Symptoms analysis
All animals were observed once a day for general symptoms, on the day of UV exposure, hourly at the end of the investigation and thereafter until 6 h, and general symptom observations continued until the end of the test. Once a week, every Monday, at a certain time (03:00 pm), the body weight of all animals was measured. Shots were taken with the SMZ1500 (Nikon, Tokyo, Japan) at 4, 8 and 12 weeks. Moisture content was measured at 4, 8 and 12 weeks with a Corneometer (Courage & Khazaka, Mathias Brüggen, Germany). A close-up of the animal’s back was taken with a digital camera (EOS 600D, Canon, Tokyo, Japan), and a replica was obtained using a silicone polymer (SILFLO impression material, Flexico, London, England) to analyze and evaluate the degree of wrinkles.
Visual observation of skin and analysis of skin cast
For the skin mold, fix the incident angle of light at 20 degrees, take a picture of the wrinkle shadow contrast image using the Visioline program, and then use the computer analysis system Skin Visiometer SV600 software (Courage & Khazaka) The area, number, length, and depth of wrinkles were measured and wrinkles were evaluated.
Histopathological analysis
At the end of the test, the dorsal skin was removed and fixed with formalin. Dehydrate with paraffin, then embed in paraffin, use microtome to create sections of 5 μm or shorter, and deparaffinize again with alkane and xylene. After staining with Hematoxylin & eosin, the stained tissue was photographed using a Nikon ECLIPSE Ti-E inverted fluorescence microscope (Nikon), and analyzed using NIS-Element BR 3.0 software (Nikon).
Skin hydration, elasticity, and erythema evaluation
Skin hydration and elasticity were measured on the dorsal skin of the mice using Corneometer (Courage & Khazaka) and Cutometer (Courage & Khazaka). Erythema induced by UV irradiation was evaluated using a spectrophotometer (KONICA MINOLTA, Tokyo, Japan). All experiments were performed at weeks 4, 8, and 12.
Skin thickness
In order to measure skin thickness, an electronic caliper (Teclock, Nagano, Japan) was used. All experiments were performed at weeks 4, 8, and 12.
Statistical analysis
Using SPSS as a statistical program, significant F-value items in ANOVA are tested for significance at the (p<0.05) level using the Tukey test.
RESULTS
GABA promotes HDFn cell proliferation
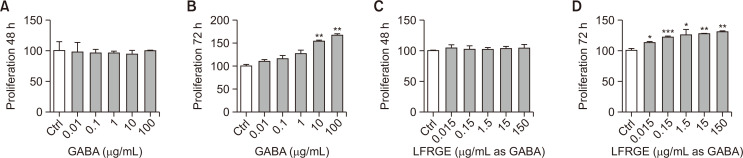
Based on the role of fibroblasts in the skin, we first examined the proliferation of fibroblasts after treatment with different concentrations of GABA. The results of the cell viability assay showed that HDFn cells showed no toxicity when treated with different concentrations (0.01-100 μg/mL) of GABA for 48 h (Fig. 1A). After GABA treatment for 72 h, HDFn cells proliferated in a concentration-dependent manner. Compared to the control group, HDFn cells proliferated by about 60% when treated with 100 μg/mL of GABA (Fig. 1B). HDFn cells treated with different concentrations of LFRGE (0.015-150 μg/mL as GABA) for 48 h and 72 h showed similar results. After LFRGE treatment for 72 h, HDFn cells proliferated in a concentration-dependent manner. Compared to the control group, HDFn cells proliferated by about 25% when treated with 150 μg/mL as GABA of LFRGE (Fig. 1C, 1D).
Fig. 1.
Cell proliferation assay of HDFn. (A, B) HDFn were treated with GABA for 48 h or 72 h. (C, D) HDFn were treated with LFRGE for 48 h or 72 h. n=4. *p<0.05, **p<0.01, ***p<0.001.
GABA promotes procollagen synthesis
One of the defining features of aging skin is the alteration in the quantity and organization of collagen fibers. (Moloney et al., 1992). In order to determine the effect of GABA on collagen production in human dermal fibroblasts (HDFn), ELISA was used to measure procollagen content. TGF-β, a known regulator of cellular matrix production, was used as a positive control. The results showed that treatment with GABA for 48 h significantly increased procollagen content in a concentration-dependent manner by approximately 20% at a GABA concentration of 100 μg/mL (Fig. 2).
Fig. 2.
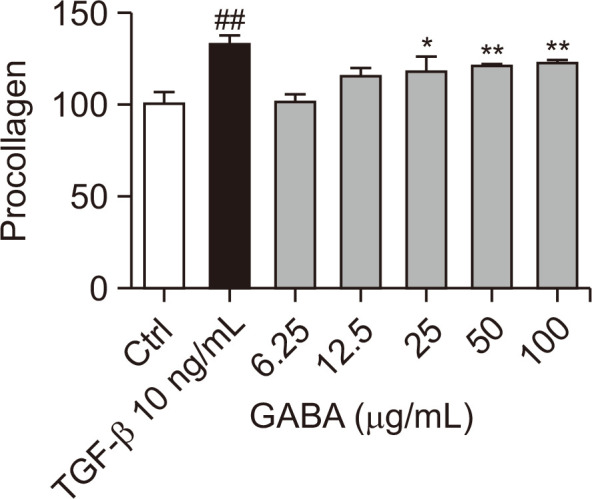
The procollagen content of HDFn was detected by ELISA experiment. The content of procollagen in cells was measured after GABA treatment for 48 h. The sample concentration was used after 1/2 serial dilution based on the highest concentration in the cell proliferation experiment. n=4, *p<0.05, ##p<0.01, **p<0.01.
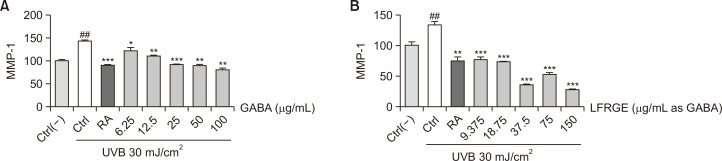
GABA and LFRGE inhibit MMP-1 synthesis which increased by UVB exposure
Increased activity of matrix metalloproteinase-1 (MMP-1) leads to degradation of type I collagen fibers in the skin, causing loss of elasticity and the formation of wrinkles. Retinoic acid (RA) has been demonstrated to alleviate UV-induced skin damage through the suppression of MMP-1 expression. (Moloney et al., 1992; Griffiths et al., 1993; Fisher et al., 1998). To investigate the effect of various treatments on MMP-1 activity in human dermal fibroblasts (HDFn), ELISA was used. UVB irradiation at a dose of 30 mJ/cm2 resulted in an approximately 50% increase in MMP-1 content in HDFn cells, while treatment with GABA inhibited this increase in a concentration-dependent manner (Fig. 3A). Similarly, treatment with LFRGE also inhibited the increase of MMP-1 induced by UVB (Fig. 3B). At the lowest concentration tested, the inhibitory effect of LFRGE on MMP-1 was almost equivalent to that of RA.
Fig. 3.
GABA and LFRGEs reduced MMP-1 level increased by UV irradiation. (A) Different concentrations of GABA or (B) LFRGEs or RA 1 μM were treated for 24 h, then irradiated with 30 mJ/cm2 UVB, and the expression of MMP-1 was detected after 48 h. ELISA was used to detect the expression of MMP-1. n=4, ##p<0.01 compared with normal cells, *p<0.05, **p<0.01, ***p<0.001 compared with UVB-irradiated cells.
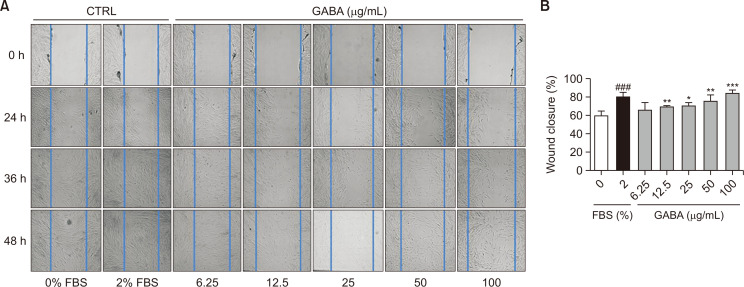
The effect of GABA on accelerating wound healing
Skin fibroblasts are crucial for maintaining skin homeostasis and function. Changes in their mobility during aging can impact skin wound healing, and the ability of aging skin to heal wounds is significantly reduced (Stringa et al., 2000). To investigate the effect of GABA on the mobility of human dermal fibroblasts (HDFn), cell scratch experiments were performed. Cell culture medium containing 2% fetal bovine serum (FBS) was used as a positive control, while cell culture medium without FBS was used for sample processing. After scratching cells at 0, 24, 36, and 48 h, photographs were taken (Fig. 4A) and analyzed using ImageJ software (Fig. 4B). The data showed that, after 36 h of scratching, the positive control group had a 20% increase in healing ability compared to the control group, while GABA treatment enhanced cell migration in a concentration-dependent manner. At a GABA concentration of 100 μg/mL, cell movement speed even surpassed that of the positive control.
Fig. 4.
The effects of GABA on wound healing. The cell movement of HDFn after treatment with different concentrations of GABA was detected by cell scratch test. (A) Specified time points of cell scratch experiment after sample processing. Pictures of cells viewed under a microscope. (B) Quantitation of the data shown in (A). n=3, *p<0.05, **p<0.01, ***p<0.001, ###p<0.001.
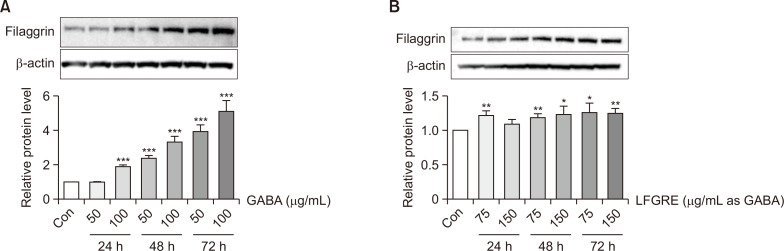
GABA and LFRGE upregulated Filaggrin protein level in human keratinocyte cells
Given the role of filaggrin in promoting collagen synthesis and its relationship to skin wrinkle formation, we measured filaggrin protein expression following GABA and LFRGE treatment. As shown in Fig. 5, treatment of HaCaT cells with various concentrations of GABA and LERGE for 24, 48, and 72 h resulted in a significant increase in filaggrin expression compared to the control group. After treatment with 100 μg/mL GABA for 24 h, filaggrin expression increased by approximately 75%, and after treatment with GABA for 48 h and 72 h, filaggrin expression increased by 200-400% in a concentration-dependent manner (Fig. 5A). Treatment with LFRGE also resulted in an approximately 25% increase in filaggrin expression compared to the control group (Fig. 5B).
Fig. 5.
GABA and LFRGE promote filaggrin synthesis in HaCaT cells. (A, B) Determination of protein expression changes of filaggrin after GABA or LFRGEs by western blot. n=3. *p<0.05, **p<0.01, ***p<0.001.
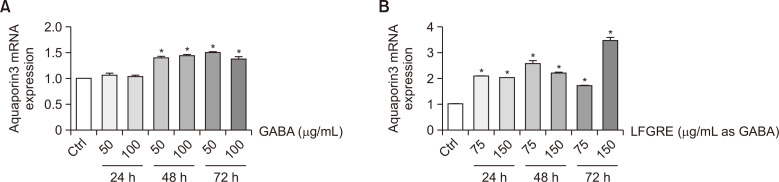
GABA and LFRGE enhanced AQP3 level
Aquaporin 3 (AQP3) plays a role in the differentiation and proliferation of keratinocytes and impacts skin hydration. To investigate the effect of GABA and LFRGE on AQP3 expression, HaCaT cells were treated with GABA and LFRGE at two different concentrations for 24-72 h. The results showed that mRNA levels of AQP3 were increased by GABA treatment at 48 h and 72 h (Fig. 6A). mRNA levels of AQP3 were significantly increased after treatment with LFRGE at 24, 48, and 72 h, with the highest expression observed after 72 h at a concentration of 150 μg/mL as GABA (Fig. 6B). These findings suggest that treatment with GABA or LFRGE can enhance skin hydration through modulation of AQP3 expression.
Fig. 6.
Effects of GABA and LFRGE on AQP3 level in HaCaT cells. (A, B) q-PCR assay for AQP3 mRNA level after GABA or LFRGE treatment. n=3. *p<0.05.
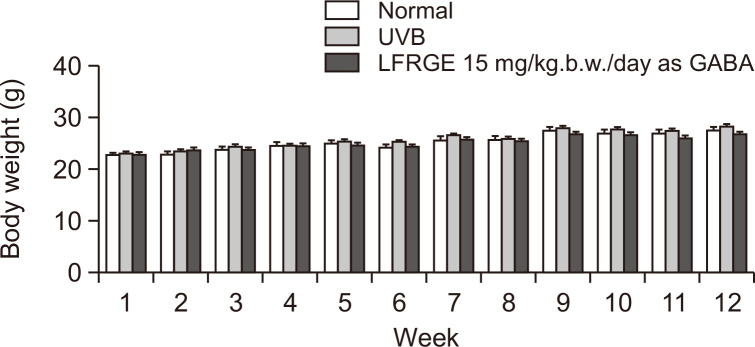
Symptom changes caused by oral administration of LFRGE in hairless mice
During the study period, no abnormal clinical symptoms resulting from oral administration of LFRGE were observed in any of the test groups. Body weight also showed normal gain with no statistically significant difference between groups (Fig. 7).
Fig. 7.
Body weight changes in hairless mice. Data are indicated as mean ± SD of 10 mice in each group.
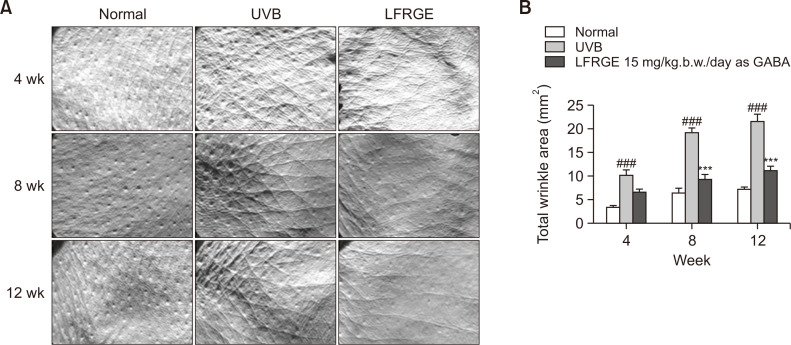
The effect of oral administration of LFRGE on wrinkles caused by using Replica
To assess the effect of LFRGE on UV-induced wrinkle formation, a hairless mouse animal model was used and the presence of wrinkles was evaluated through photographs of the animal’s back. Improvement of wrinkles was visually confirmed by using the replica (Fig. 8A), and it was observed that hairless mice treated with LFRGE had significantly less wrinkles compared to UVB-irradiated mice (Fig. 8B).
Fig. 8.
Effects of LFRGE intake on wrinkle formation in UVB-irradiated hairless mice. (A) Photographs of dorsal skin of hairless mice after UVB irradiation/no irradiation. The UVB irradiation dose is 80 mJ/cm2, and after oral administration of 15 mg/kg.b.w./day as GABA of LFRGE, the skin wrinkles are significantly improved under the naked eye. (B) Skin Visiometer SV600 software analysis results of skin wrinkle data. n=10, ###p<0.001 compared with normal mice, ***p<0.001 compared with UVB-irradiated mice.
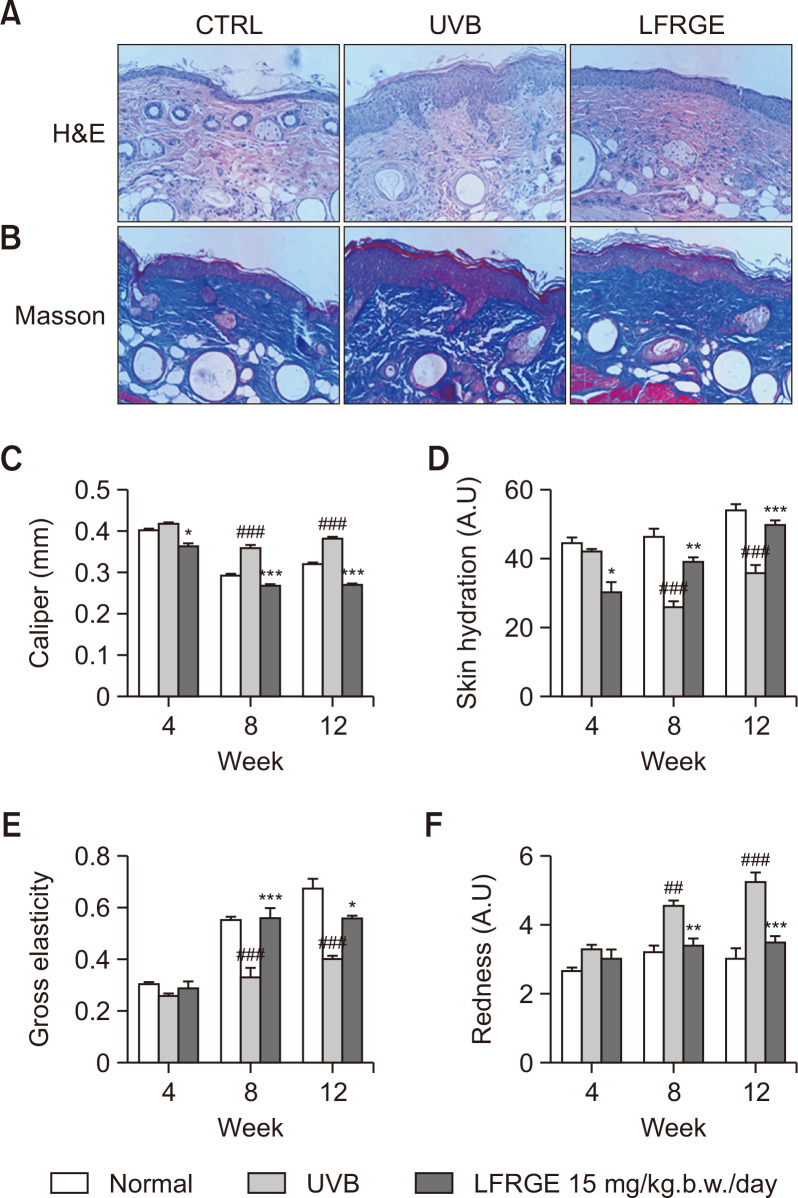
Oral administration of LFRGE recovers skin moisture content and elasticity that decreased by UV irradiation
Exposure to ultraviolet (UV) radiation can damage the skin’s permeability barrier, leading to decreased skin moisture and elasticity. Oral administration of LFRGE significantly decreased epidermal thickness and promotes collagen fibers regeneration compared to UVB irradiated group, visualized in histological sections with H&E and Masson trichrome staining (Fig. 9A, 9B). Skin thickness was measured with calipers (Fig. 9C). Analysis of the skin showed that the skin moisture (Fig. 9D) and elasticity (Fig. 9E) were significantly reduced, and redness (Fig. 9F) and skin thickness (Fig. 9C) were increased by UVB irradiation. However, oral administration of LFRGE to mice significantly improved the reduction of skin moisture and elasticity, and the increasing redness and thickness caused by UVB irradiation.
Fig. 9.
Effects of LFRGE intake on skin tissue in UVB-irradiated hairless mice. Skin tissue sections were stained with H&E (A) and Masson’s trichrome staining (B). Skin thickness was measured with calipers (C). After oral administration of LFRGE to hairless mice, the loss of hydration (D) and elasticity (E) was significantly improved and the erythema (F) decreased. n=10, ##p<0.01, ###p<0.001 compared with normal mice. *p<0.05, **p<0.01, ***p<0.001 compared with UVB-irradiated mice.
DISCUSSION
Telomere shortening, matrix metalloproteinases and signaling pathways, oxidative stress, vascular alterations, numerous skin age-associated cytokines, and several other environmental stressors are the main causes of skin aging (Kim and Park, 2016). It has been reported that topical retinoids, cosmeceuticals, combination cytokines and growth factors, neurotoxins and dermal fillers, and laser light technology devices can all be used to produce molecular anti-aging therapies (Kim and Park, 2016). Changes in cell physiology and morphology, rather than just a decline in cell proliferation, are characteristics of aging (Lago and Puzzi, 2019). Our research shows that GABA and LFRGE can significantly promote the proliferation of HDF cells and delay the aging process of the skin, and help to alleviate the aging of epidermal cells and promote moisturizing effects.
Dermal fibroblasts produce type I procollagen, which is converted into collagen through proteolytic mechanisms (Varani et al., 2006). Our research results showed that type I procollagen production in HDF can be effectively induced by GABA. Matrix metalloproteinases (MMPs) and their natural inhibitors, tissue inhibitors of metalloproteinases (TIMPs), often regulate collagen breakdown. MMP-1 has been suggested to initiate collagen fragmentation (Fisher et al., 2009). Studies using mutant MMP-1 isoforms that autoactivate in human dermal fibroblast cultures support the idea that active MMP-1 causes collagen fiber breakdown during skin aging and ultrastructural changes (Xia et al., 2013). Our findings demonstrate that GABA and LFRGE has an inhibitory effect on MMP-1 production, which can improve skin elasticity and wrinkles.
Elderly skin is often thin and fragile, more susceptible to bruising, and less able to heal wounds (Fisher et al., 2008). GABA treatment effectively enhances the mobilization of fibroblasts, which can improve wound healing and maintain smooth skin.
Filaggrin deficiency has a significant impact on the arrangement of keratin filaments in the cytoskeleton and the composition of the cornified envelope (CE), resulting in a decrease in the amount of hyaline stratum corneum particles, a significant decrease in the amount of natural moisturizing factor (NMF) and skin hydration, and an alkalinization of the skin’s pH (Armengot-Carbo et al., 2015). The increased activity of certain proteases at higher pH also promotes the release of pro-inflammatory mediators from keratinocytes, triggering an inflammatory reaction mediated by T helper type 2 (Th2) cells even in the absence of allergens (Gruber et al., 2011). Our experimental results demonstrate that GABA and LFRGE can enhance filaggrin expression, improving the proper formation and function of the skin barrier.
The membranes of various epithelial cells express AQP3 extensively, with high levels in keratinocytes (Bollag et al., 2020), colon (Matsuzaki et al., 1999) and the basolateral membrane of renal collecting duct cells (Ecelbarger et al., 1995). AQPs also differ in their ability to transport various substances such as urea, glycerol, H2O2, ions, and gases (Geng and Yang, 2017). In addition to transport, some AQPs also regulate other biological functions of the epithelium, such as cell migration, morphogenesis, and angiogenesis (Nico and Ribatti, 2010). AQP3 plays a significant role in the control of several physiological and pathological processes. Our research demonstrates that GABA and LFRGE can enhance skin hydration through the activation of AQP3 in cells.
Exposure to UV radiation can harm skin cells, resulting in skin cancer and premature aging. UVBs have shorter wavelengths that can penetrate and damage the outermost layer of the skin. In hairless mice, LFRGE effectively improved UVB-induced skin wrinkles and dehydration, and increased skin elasticity at a dose that did not affect mouse growth.
Our results showed that GABA and LFRGE have a positive impact on improving skin aging, which was also confirmed in in vivo experiments. The effectiveness of LFRGE as a material for improving skin aging and moisturization was established.
REFERENCES
- Armengot-Carbo M., Hernández-Martín Á., Torrelo A. The role of filaggrin in the skin barrier and disease development. Actas Dermosifiliogr. 2015;106:86–95. doi: 10.1016/j.ad.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Bollag W. B., Aitkens L., White J., Hyndman K. A. Aquaporin-3 in the epidermis: more than skin deep. Am. J. Physiol. Cell Physiol. 2020;318:C1144–C1153. doi: 10.1152/ajpcell.00075.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. Y., Hsieh H. L., Hsiao L. D., Yang C. M. PI3-K/Akt/JNK/NF-κB is essential for MMP-9 expression and outgrowth in human limbal epithelial cells on intact amniotic membrane. Stem Cell Res. 2012;9:9–23. doi: 10.1016/j.scr.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Di Cagno R., Mazzacane F., Rizzello C. G., De Angelis M., Giuliani G., Meloni M., De Servi B., Gobbetti M. Synthesis of γ-aminobutyric acid (GABA) by Lactobacillus plantarum DSM19463: functional grape must beverage and dermatological applications. Appl. Microbiol. Biotechnol. 2010;86:731–741. doi: 10.1007/s00253-009-2370-4. [DOI] [PubMed] [Google Scholar]
- Ecelbarger C. A., Terris J., Frindt G., Echevarria M., Marples D., Nielsen S., Knepper M. A. Aquaporin-3 water channel localization and regulation in rat kidney. Am. J. Physiol. Renal Physiol. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- Fisher G. J., Datta S. C., Talwar H. S., Wang Z. Q., Varani J., Kang S., Voorhees J. J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Fisher G. J., Kang S., Varani J., Bata-Csorgo Z., Wan Y., Datta S., Voorhees J. J. Mechanisms of photoaging and chronological skin aging. AMA Arch. Derm. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Fisher G. J., Quan T., Purohit T., Shao Y., Cho M. K., He T., Varani T., Kang S., Voorhees J. J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009;174:101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G. J., Talwar H. S., Lin J., Lin P., McPhillips F., Wang Z., Li X., Wan Y., Kang S., Voorhees J. J. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. JCI Insight. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G. J., Varani J., Voorhees J. J. Looking older: fibroblast collapse and therapeutic implications. JAMA Dermatol. 2008;144:666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri A., Gropper M. A., Umenishi F., Kawashima M., Brown D., Verkman A. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J. Cell Sci. 1995;108:2993–3002. doi: 10.1242/jcs.108.9.2993. [DOI] [PubMed] [Google Scholar]
- Geng X., Yang B. Transport characteristics of aquaporins. Adv. Exp. Med. Biol. 2017;969:51–62. doi: 10.1007/978-94-024-1057-0_3. [DOI] [PubMed] [Google Scholar]
- Griffiths C. E. M., Maddin S., Wiedow O., Marks R., Donald A. E., Kahlon G. Treatment of photoaged skin with a cream containing 0.05% isotretinoin and sunscreens. J. Dermatolog. Treat. 2005;16:79–86. doi: 10.1080/09546630510027732. [DOI] [PubMed] [Google Scholar]
- Griffiths C., Russman A. N., Majmudar G., Singer R. S., Hamilton T. A., Voorhees J. J. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) N. Engl. J. Med. 1993;329:530–535. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- Gruber R., Elias P. M., Crumrine D., Lin T. K., Brandner J. M., Hachem J. P., Presland R. B., Fleckman P., Janecke A. R., Sandilands A., McLean W. I., Fritsch P. O., Mildner M., Tschachler E., Schmuth M. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am. J. Pathol. 2011;178:2252–2263. doi: 10.1016/j.ajpath.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M., Verkman A. S. Roles of aquaporin-3 in the epidermis. J. Investig. Dermatol. 2008;128:2145–2151. doi: 10.1038/jid.2008.70. [DOI] [PubMed] [Google Scholar]
- Ito K., Tanaka K., Nishibe Y., Hasegawa J., Ueno H. GABA-synthesizing enzyme, GAD67, from dermal fibroblasts: evidence for a new skin function. Biochim. Biophys. Acta Gen. Subj. 2007;1770:291–296. doi: 10.1016/j.bbagen.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Kim J., Lee C. W., Kim E. K., Lee S. J., Park N. H., Kim H. S., Kim H. K., Char K. H., Jang Y. P., Kim J. W. Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. J. Ethnopharmacol. 2011;137:427–433. doi: 10.1016/j.jep.2011.04.072. [DOI] [PubMed] [Google Scholar]
- Kim M., Park H. J. Molecular Mechanisms of the Aging Process and Rejuvenation. InTech; Manila, Philippines: 2016. Molecular mechanisms of skin aging and rejuvenation; p. 450. [DOI] [Google Scholar]
- Kozak I., Klisenbauer D., Juhas T. UV-B induced production of MMP-2 and MMP-9 in human corneal cells. Physiol. Res. 2003;52:229–234. doi: 10.33549/physiolres.930272. [DOI] [PubMed] [Google Scholar]
- Lago J. C., Puzzi M. B. The effect of aging in primary human dermal fibroblasts. PLoS One. 2019;14:e0219165. doi: 10.1371/journal.pone.0219165.597a01bca1d34fdfa9489aabe2cd77e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki T., Suzuki T., Koyama H., Tanaka S., Takata K. Water channel protein AQP3 is present in epithelia exposed to the environment of possible water loss. J. Histochem. Cytochem. 1999;47:1275–1286. doi: 10.1177/002215549904701007. [DOI] [PubMed] [Google Scholar]
- Moloney S. J., Edmonds S. H., Giddens L. D., Learn D. B. The hairless mouse model of photoaging: evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. J. Photochem. Photobiol. 1992;56:505–511. doi: 10.1111/j.1751-1097.1992.tb02194.x. [DOI] [PubMed] [Google Scholar]
- Nelson A. R., Fingleton B., Rothenberg M. L., Matrisian L. M. Matrix metalloproteinases: biologic activity and clinical implications. J. Clin. Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- Nico B., Ribatti D. Aquaporins in tumor growth and angiogenesis. Cancer Lett. 2010;294:135–138. doi: 10.1016/j.canlet.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., DeLorey T. M. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th ed. 1999. GABA and glycine. [DOI] [Google Scholar]
- Plante D. T., Jensen J. E., Schoerning L., Winkelman J. W. Reduced γ-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: a link to major depressive disorder? Neuropsychopharmacology. 2012;37:1548–1557. doi: 10.1038/npp.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T., Qin Z., Xia W., Shao Y., Voorhees J. J., Fisher G. J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009;14:20–24. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings A. V., Harding C. R. Moisturization and skin barrier function. Dermatol. Ther. 2004;17:43–48. doi: 10.1111/j.1396-0296.2004.04S1005.x. [DOI] [PubMed] [Google Scholar]
- Schoop V. M., Fusenig N. E., Mirancea N. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J. Invest. Dermatol. 1999;112:343–353. doi: 10.1046/j.1523-1747.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- Sternlicht M. D., Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringa E., Knauper V., Murphy G., Gavrilovic J. Collagen degradation and platelet-derived growth factor stimulate the migration of vascular smooth muscle cells. J. Cell Sci. 2000;113:2055–2064. doi: 10.1242/jcs.113.11.2055. [DOI] [PubMed] [Google Scholar]
- Varani J., Dame M. K., Rittie L., Fligiel S. E., Kang S., Fisher G. J., Voorhees J. J. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman A. S. More than just water channels: unexpected cellular roles of aquaporins. J. Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- Vičanová J., Boelsma E., Mommaas A. M., Kempenaar J. A., Forslind B., Pallon J., Egelrud T., Koerten H. K., Ponec M. Normalization of epidermal calcium distribution profile in reconstructed human epidermis is related to improvement of terminal differentiation and stratum corneum barrier formation. J. Invest. Dermatol. 1998;111:97–106. doi: 10.1046/j.1523-1747.1998.00251.x. [DOI] [PubMed] [Google Scholar]
- Warskulat U., Reinen A., Grether-Beck S., Krutmann J., Häussinger D. The osmolyte strategy of normal human keratinocytes in maintaining cell homeostasis. J. Invest. Dermatol. 2004;123:516–521. doi: 10.1111/j.0022-202X.2004.23313.x. [DOI] [PubMed] [Google Scholar]
- Xia W., Hammerberg C., Li Y., He T., Quan T., Voorhees J. J., Fisher G. J. Expression of catalytically active-matrix metalloproteinase-1 in dermal fibroblasts induces collagen fragmentation and functional alterations that resemble aged human skin. Aging Cell. 2013;12:661–671. doi: 10.1111/acel.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]