Abstract
Several lines of evidence suggest that GATA6 has an integral role in controlling development of the mammalian liver. Unfortunately, this proposal has been impossible to address directly because mouse embryos lacking GATA6 die during gastrulation. Here we show that the early embryonic deficiency associated with GATA6-knockout mice can be overcome by providing GATA6-null embryos with a wild-type extraembryonic endoderm with the use of tetraploid embryo complementation. Analysis of rescued Gata6−/− embryos revealed that, although hepatic specification occurs normally, the specified cells fail to differentiate and the liver bud does not expand. Although GATA6 is expressed in multiple tissues that impact development of the liver, including the heart, septum transversum mesenchyme, and vasculature, all are relatively unaffected by loss of GATA6, which is consistent with a cell-autonomous requirement for GATA6 during hepatogenesis. We also demonstrate that a closely related GATA factor, GATA4, is expressed transiently in the prehepatic endoderm during hepatic specification and then lost during expansion of the hepatic primordium. Our data support the proposal that GATA4 and GATA6 are functionally redundant during hepatic specification but that GATA6 alone is available for liver bud growth and commitment of the endoderm to a hepatic cell fate.
In mice, hepatic development begins at the six- to eight-somite stage of embryogenesis (E8.0) in response to inductive signals from the heart and septum transversum, including fibroblast growth factors and bone morphogenetic proteins, as well as the presence of endothelial cells (9, 15, 17, 24, 40, 45). In addition to growth factors, gene-targeting studies have implicated several transcription factors in controlling distinct aspects of hepatogenesis (8-10). These include the homeobox factor Hex, which is necessary for morphogenesis and growth of the liver bud; Prox1, which is required for hepatoblast migration; and HNF4, which is essential for hepatocyte differentiation and the epithelial transformation of the liver (1, 19, 22, 23, 33, 41).
Molecular and genetic analyses have suggested that the GATA factors may also act to control hepatogenesis (2, 36, 38, 39, 44, 46, 47). The GATA family of zinc finger transcription factors currently consists of six members in mammals (34). GATA1, -2, and -3 appear to act primarily in hematopoietic cells while GATA4, -5, and -6 are expressed in a diverse array of tissues (25). In vivo footprinting analyses of pluripotent embryonic gut endoderm revealed that GATA4 bound the Albumin (Alb1) enhancer prior to the onset of albumin expression in the primary liver bud, implying a role for GATA4 in regulating the onset of hepatic mRNA expression (2, 3, 5, 7, 15). Genetic evidence supporting a requirement for GATA factors in development of the gastrointestinal tract and its derivatives, including the liver, has emerged from studies with multiple model systems: GATA5 has been implicated in gut and liver development in zebra fish (37, 38), END1 is required for endoderm development in Caenorhabditis elegans (46, 47), SERPENT is essential for gut development in Drosophila melanogaster (36, 39), and GATA6 contributes to branching morphogenesis of the lungs (18, 43).
Of the known GATA proteins, GATA6 is a particularly appealing candidate for a factor that may contribute toward liver development because GATA6 regulates expression of the nuclear hormone receptor HNF4 (16, 22, 27, 33). Moreover, development of Gata6−/− embryos arrests during gastrulation with a phenotype that is very similar to that associated with Hnf4−/− embryos, which is consistent with GATA6 acting upstream of HNF4 during development (4, 20, 27). This early embryonic lethality, which is believed to be a consequence of extraembryonic endoderm dysfunction, has so far prevented the use of Gata6−/− embryos for any analyses of the role of GATA6 during liver development. However, extraembryonic endoderm deficiencies can be rescued by generating embryos from embryonic stem (ES) cells by using tetraploid embryo complementation (12, 22). We therefore attempted to address whether GATA6 is required for hepatogenesis by generating embryos from Gata6−/− ES cells by the tetraploid embryo complementation approach. We found that Gata6−/− ES cell-derived embryos survive until E10.5, definitively demonstrating that embryonic lethality in GATA6-knockout embryos is a consequence of loss of GATA6 function in the extraembryonic endoderm as suggested previously (20, 27). Analyses of the developing liver in these embryos show that GATA6 is dispensable for hepatic specification but essential for liver bud expansion and for normal expression of hepatic mRNAs within the nascent hepatoblasts. We also examined heart, septum transversum mesenchyme, and vascular development in Gata6−/− ES cell-derived embryos because they are the source of paracrine signals that are necessary for liver development. In contrast to the liver, however, we found that development of these tissues is not significantly affected by the absence of GATA6.
MATERIALS AND METHODS
Generation of targeted ES cell lines and embryos.
Gata6+/− and Gata6−/− R1 ES cells were produced by standard procedures with a previously described GATA6 targeting construct (26), shown in Fig. 1. All cells were confirmed to have the predicted genotype by Southern blot analysis (Fig. 1). Embryos were generated directly from ES cells by tetraploid embryo complementation as described previously (12, 29). Pregnant mare serum gonadotropin used in superovulation was obtained from A. F. Parlow at the National Hormone and Peptide Program.
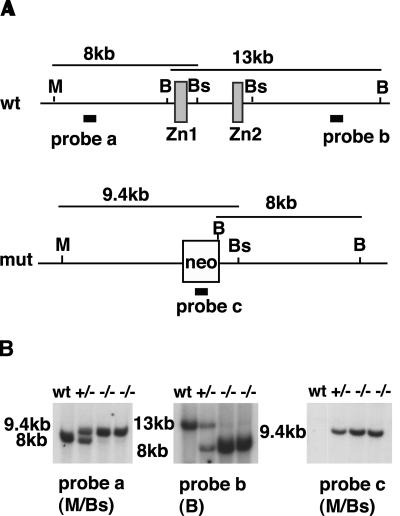
FIG. 1.
Generation of Gata6−/− ES cells. (A) Schematic showing that Gata6 exons (boxes), which encode the DNA binding domain (Zn), were replaced with a neomycin phosphotransferase cassette (neo) by using the targeting vector described by Morrisey et al. (27). Relative positions of probes and the sizes of the BamHI (B), BstZ171 (Bs), and MluI (M) restriction enzyme products that they identify are shown. (B) Southern blots confirming the genotype of ES cells containing wild-type (Wt), heterozygous (+/−), and homozygous (−/−) mutant alleles of Gata6. The size of restriction fragments, shown in kilobases (kb), was calculated by comparison to a DNA ladder. wt, wild type; mut, mutant.
RT-PCR.
Foregut endoderm was dissected from six- to eight-somite-stage embryos and hearts were dissected from E9.5 embryos in ice-cold phosphate-buffered saline under a dissecting microscope with tungsten needles. RNA was prepared from individual samples with Qiagen's RNeasy minikit and processed for reverse transcription-PCR (RT-PCR) as described previously (12). The following oligonucleotide primer sequences were used in amplifications: Hprt, AGCGCAAGTTGAATCTGC and AGCGACAATCTACCAGAG; Gata6, ATGGCGTAGAAATGCTGAGG and TGAGGTGGTCGCTTGTGTAG; Gata4, TGGCCGACGTGGGAGCAT and CGGCGGGAAGCGGACAG; Hand1, AACCTCAACCCCAAAAGCC and GGAAGGGAAAGGAAGGGAAAG; Hand2, TACCAGCTACATCGCCTAC and TCTTTCTTCCTCTTCTCCTC; Nkx2-5, CGCCGCCTCCGCCAACAGCAACT and GGGCGACGGCAAGACAACCAG; Srf, AGATCCCTGTCTCTGCAGTTCAGC and GCGTGGCATCCAGGTTCA; Myocd, CTGTGTGGAGTCCTCAGGTCAAACC and GATGTGCTGCGGGCTCTTCAG; Ncx1, CCATCTTCGGAATGTCAATG and CCATCTTCGGAATGTCAATG; αMHC, CTGCTGGAGAGGTTATTCCTCG and GGAAGAGTGAGCGGCGCATCAAGG; βMHC, GCCAACACCAACCTGTCCAAGTTC and TGCAAAGGCTCCAGGTCTGAGGGC; cardiac alpha actin, AGAGTATGATGAGGCAGGC and ATGACTGATGAGAGATGGGG; Mlc2a, AGGCACAACGTGGCTCTTCT and AGCTGGGAATAGGTCTCCTTCA; Mlc2v, GGAGGGCAACGGCACGGTCAT and AAGGCGAGCACAGGTAGGGTAAGC; Anf, GAGAGACGGCAGTGCTTCTAGGC and CGTGACACACCACAAGGGCTTAGG; skeletal actin, CGCGACATCAAAGAGAAGCT and GGGCGATGATCTTGATCTTC; Fgf1, CGGAAAGTGCGGGCGAAGTG and ACCGGGAGGGGCAGAAACAAGA; Bmp2, GGGACCCGCTGTCTTCTAGTGTTGC and TGAGTGCCTGCGGTACAGATCTAGCA; Bmp4, TCTAGAGGTCCCCAGAAGCAGCTGC and GCATTCGGTTACCAGGAATCATGGTG; Afp, TCGTATTCCAACAGGAGG and AGGCTTTTGCTTCACCAG; Alb1, CTTAAACCGATGGGCGATCTCACT and CCCCACTAGCCTCTGGCAAAAT; Hnf4, CTTCCTTCTTCATGCCAG and ACACGTCCCCATCTGAAG; Rbp, ATCCAGTGGTCATCGTTTCCTCGCT and GAACTTCGACAAGGCTCGTTTCTCTGG; Ttr, CTCACCACAGATGAGAAG and GGCTGAGTCTCTCAATTC.
In situ hybridization.
Embryos were embedded in paraffin. Sections were cut at 5 to 7 μm and were processed for in situ hybridization with 33P-labeled antisense RNA probes as described elsewhere (11, 35). Digital dark-field images were inverted and overlaid on bright-field images by using Adobe Photoshop. Experimental and control images were processed identically in all cases.
Immunohistochemistry.
Immunohistochemistry was performed on paraformaldehyde-fixed paraffin sections as described previously (33) with a 1/500 dilution of primary antibodies recognizing HNF4 (Santa Cruz, catalog no. sc-6556), GATA4 (Santa Cruz, catalog no. sc-1237), or platelet endothelial cell adhesion molecule (PECAM; CD31; BD PharMingen).
RESULTS
Gata6−/− ES cell-derived embryos complete gastrulation.
Development of Gata6−/− embryos is blocked during gastrulation, and the embryos are unable to generate a fully differentiated extraembryonic endoderm (20, 27). In addition, they fail to express mRNAs normally present in this endoderm including Hnf4 (27). These data suggest that the early embryonic lethality associated with loss of GATA6 is a consequence of defective extraembryonic endoderm function, as has previously been demonstrated for Hnf4−/− embryos (12). Tetraploid embryo complementation has been used successfully to generate embryos from ES cells and to circumvent deficiencies in the extraembryonic endoderm function (12, 29). In this approach, tetraploid embryos contribute cells to the extraembryonic endoderm while the fetus is derived solely from ES cells (30). We therefore used this procedure to test whether the gastrulation arrest associated with the GATA6-null phenotype was due to extraembryonic endoderm defects as suggested by the analyses of Gata6-knockout embryos (20, 27). A Gata6−/− allele was generated in R1 ES cells according to the same strategy used to produce Gata6-knockout mice (27), and Gata6−/− cells were selected by growth of Gata6+/− cells in a high concentration of G418 (Fig. 1A) (28). The genotype of all ES cells used in this study was confirmed by Southern blot analyses of genomic DNA (Fig. 1B). As presented in Fig. 2, post-gastrulation-stage E9.5 and E10.5 embryos could be produced from both control Gata6+/− (n = 160) and experimental Gata6−/− (n = 180) ES cells, and embryos of both genotypes exhibited characteristics of normal developmental patterning (Fig. 2A). The genotype of embryos was confirmed by PCR analysis of genomic DNA. No wild-type Gata6 allele was detected in fetuses derived from Gata6−/− ES cells (Fig. 2B and C), which was expected because the Gata6+/+ tetraploid cells contribute to the extraembryonic endoderm only in such chimeric embryos (12, 30). Although Gata6+/− ES cell-derived embryos developed normally through E11.5, by this same embryonic stage Gata6−/− ES cell-derived embryos lacked tissue integrity and were readsorbing. The latest time point at which living Gata6−/− ES cell-derived embryos could be recovered was E10.5. While rescued Gata6−/− embryos had clearly progressed through gastrulation, they were consistently smaller than control embryos (Fig. 2A). However, although they were relatively diminutive, the rate of development was indistinguishable between control and experimental embryos; somite number, appearance of limb buds, heart looping, and neural tube closure were the same between Gata6+/− and Gata6−/− ES cell-derived embryos isolated at E9.5 and E10.5. The cause of death or why Gata6−/− ES cell-derived embryos are smaller has not been established. However, the extent of cell proliferation, determined by anti-phosphohistone H3 immunohistochemistry, was similar between Gata6+/− and Gata6−/− ES cell-derived embryos at E9.5 (data not shown). In addition, the level of apoptotic cell death, determined by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL), was also generally similar between control and mutant embryos (data not shown). In some GATA6-null embryos (three of five) there appeared to be a qualitative increase in TUNEL positive staining around the region of the presumptive liver bud compared to control embryos; however, this increase in TUNEL staining was variable, and we were unable to generate reproducible quantitative results. The finding that E10.5 embryos can routinely be generated from Gata6−/− ES cells by tetraploid embryo complementation while GATA6-knockout embryos arrest during gastrulation definitively demonstrates that GATA6 expression is required in the extraembryonic endoderm to maintain an environment that supports gastrulation of the epiblast.
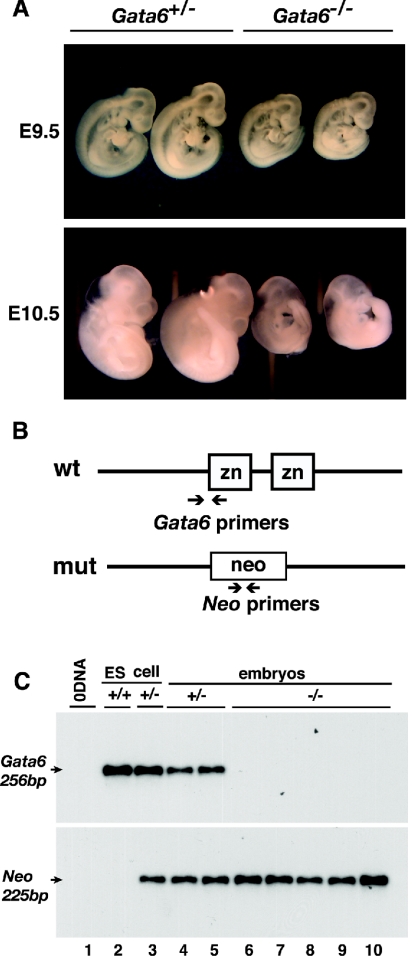
FIG. 2.
Embryos generated from Gata6−/− ES cells by tetraploid embryo complementation complete gastrulation. (A) Micrographs of E9.5 and E10.5 embryos generated from either Gata6+/− or Gata6−/− ES cells. (B) Schematic showing position of primers relative to the Gata6 wild-type allele and null allele in which exons encoding the zinc finger domains of GATA6 were replaced by Neo; note that one of the Gata6 oligonucleotides contained sequences encoding a portion of the first zinc finger that were deleted in Gata6−/− ES cells. wt, wild type; mut, mutant. (C) PCR analysis with primers that amplify Gata6 genomic DNA or the Neo gene confirms that Gata6 was present in control Gata6+/+ and Gata6+/− ES cells and embryos (lanes 2 to 5) but was absent from embryos derived from Gata6−/− ES cells (lanes 6 to 10) or in reaction mixtures lacking template (0 DNA, lane 1).
Development of the liver is blocked at E9.5 in Gata6−/− ES cell-derived embryos.
To determine whether GATA6 was required for development of the liver, we compared formation of the liver bud in histological sections of embryos at E9.5 and E10.5 that were generated from Gata6+/− or Gata6−/− ES cells. All analyses were performed on embryos with similar numbers of somites to ensure that they were at equivalent developmental stages—the appearance of new somite pairs occurs approximately every 2 h in the mouse and allows high-resolution timing of the developmental stage of embryos between E8.5 and E10.5. Figures 3A and C show that in control embryos the liver bud could easily be identified by E9.5 as a distinct outgrowth of the ventral foregut and, by E10.5, that hepatoblasts had delaminated from the primary liver bud and had migrated into the surrounding septum transversum mesenchyme. In contrast to controls, development of the liver in Gata6−/− ES cell-derived embryos was severely perturbed. At E9.5, the liver bud consisted of a small rudimentary outgrowth of endoderm that had failed to expand by E10.5 (Fig. 3B and D). Indeed, at E10.5, the liver bud was virtually indiscernible and there was no indication that the hepatoblasts had delaminated from the foregut.
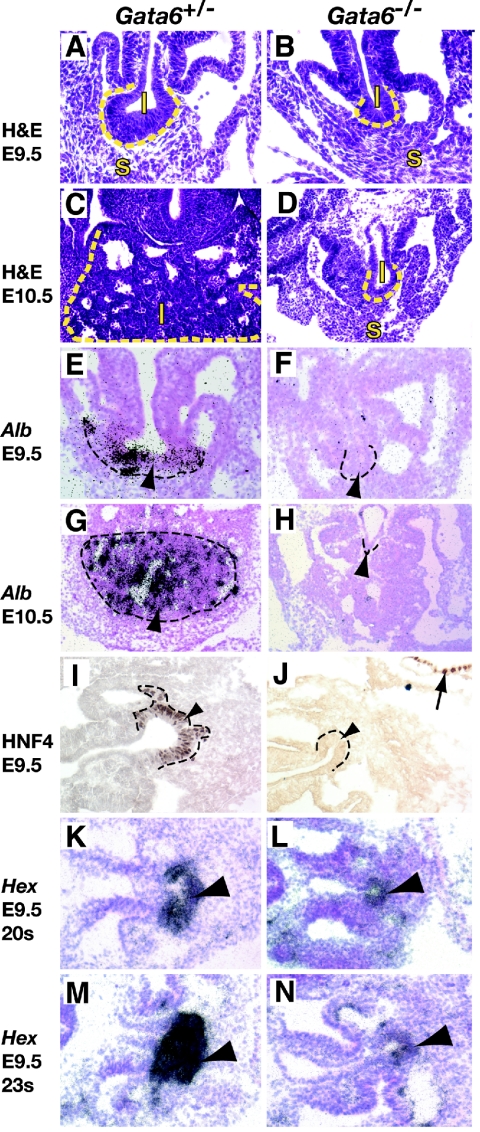
FIG. 3.
The liver bud fails to expand and establish normal expression of hepatic markers in the absence of GATA6. Somite-matched embryos isolated at either E9.5 (A, B, E, F, and I to N) or E10.5 (C, D, G, and H) were generated from Gata6+/− (A, C, E, G, I, K, and M) or Gata6−/− (B, D, F, H, J, L, and N) ES cells. (A to D) Hematoxylin- and eosin-stained transverse sections of embryos showing the developing liver (l; outlined by dashes) and septum transversum mesenchyme (s). (E to H) In situ hybridization analysis identifying Albumin mRNA (dark grains) in control but not Gata6−/− liver buds(arrowheads). (I and J) Immunohistochemistry identifying HNF4 protein, which can be seen as brown staining, in nuclei of control but not GATA6-null liver primordia (arrowheads). Note the presence of HNF4 in the extraembryonic endoderm (arrow). (K to N) In situ hybridization to detect Hex mRNA (dark grains) in the liver bud (arrowhead) of Gata6+/− (K and M) or Gata6−/− (L and N) E9.5 embryos containing 20 (K and L) or 23 (M and N) somite pairs. Experimental and control embryos are shown at the same magnification.
These data suggested that there was a failure in the ability of the ventral endoderm to follow a normal hepatic developmental program. To address this directly, control and mutant embryos were stained for expression of characteristic markers of hepatic development. Figures 3E to H show that in situ hybridization analyses identified Alb1 mRNA within the developing liver of control embryos at E9.5 and E10.5 while in mutant embryos Alb1 mRNA was undetectable. Previous work had demonstrated that GATA6 regulates expression of the hepatocyte differentiation factor HNF4 (16, 27). As shown in Fig. 3I, HNF4 protein can be identified, by immunohistochemistry, in the nucleus of endodermal cells within the liver bud at E9.5. In Gata6−/− embryos, however, although HNF4 can be identified in the visceral endoderm that acts as a positive control for antibody staining, no expression of HNF4 is found within the liver bud (Fig. 3J).
Immediately following inductive signaling from surrounding tissues, the nascent hepatic cells initiate the process of differentiation. The onset of differentiation is rapid, and levels of hepatic mRNAs increase quickly, which presumably reflects commitment of the endoderm to a hepatic fate. Radioactive in situ hybridization, shown in Fig. 3K and M, shows that expression of Hex mRNA, which encodes a homeobox transcription factor essential for liver development, increases significantly between 20 and 23 somites in control E9.5 embryos (1, 19, 23). In contrast, although Hex mRNA could be detected in Gata6−/− ES cell-derived embryos, the level was lower than that seen in controls, and moreover, between 20 and 23 somites Hex mRNA levels failed to increase in the absence of GATA6 (Fig. 3L and N). The fact that albumin and HNF4 were undetectable and that Hex mRNAs failed to increase in GATA6-null embryos demonstrates that GATA6 is required for the liver bud to mature and commit to a normal hepatic developmental program.
Hepatic specification occurs in Gata6−/− ventral endoderm.
The finding that Hex mRNA was present in the endoderm of Gata6−/− embryos suggested that hepatic development had been induced within the ventral foregut. However, Hex mRNA is also expressed in the developing ventral pancreas, and so it seemed possible that the identification of Hex transcripts reflected development of the pancreas rather than liver. To definitively address whether hepatic specification had occurred in the absence of GATA6, we determined the extent of liver gene expression in ventral endoderm isolated from experimental and control E8.0 embryos containing six to eight somite pairs by RT-PCR. It is at this developmental stage that inductive cues acting on the endoderm specify hepatic cell fate (15). Figure 4A shows that, as expected, Gata6 mRNA could be detected by RT-PCR in control ventral endoderm but was not detected in Gata6−/− endoderm. Importantly, this result confirmed the absence of any GATA6-positive tetraploid embryo-derived extraembryonic endoderm, which could potentially confound the analysis. Examination of steady-state levels of mRNAs encoding alpha-fetoprotein (Afp), albumin, HNF4, retinol binding protein (Rbp4), and transthyretin (Ttr), all of which are expressed in hepatoblasts, revealed that all were identified at comparable levels between control and Gata6−/− ventral endoderm isolates. The fact that Alb mRNA could be detected by RT-PCR in the ventral endoderm of E8.0 Gata6−/− embryos but not by in situ hybridization at E9.5 likely reflects the relative sensitivity of the two assays. These data show that GATA6 is dispensable for hepatic specification and that Gata6−/− ventral endoderm is competent to initiate a program of liver gene expression.
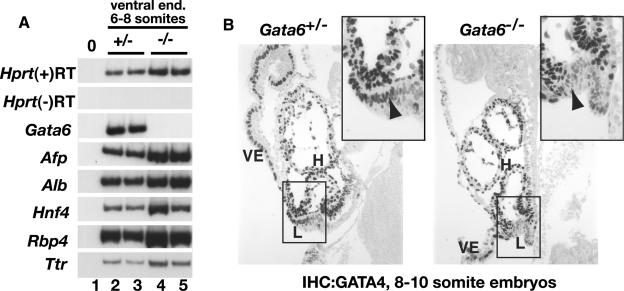
FIG. 4.
GATA6 is dispensable for specification of the hepatic endoderm. (A) RT-PCR analysis of hepatic mRNAs in ventral endoderm isolated from Gata6+/− (lanes 2 and 3) or Gata6−/− embryos (lanes 4 and 5) containing six to eight somite pairs. Amplification of Hprt was used as a loading control, and omission of reverse transcriptase from the RT-PCR and amplifications lacking template (lane 1) confirmed the absence of contaminating DNA. (B) GATA4 protein can be identified by immunohistochemistry (IHC) as nuclear staining in the presumptive hepatic endoderm (arrowhead) of both Gata6+/− and Gata6−/− E8.5 embryos containing 8 to 10 somite pairs. Insets show high-resolution images of boxed areas. VE, extraembryonic visceral endoderm; H, developing heart; L, presumptive hepatic endoderm.
Given the clear absence of liver development at E9.5, the finding that expression of hepatic mRNAs within the ventral endoderm during the earliest stages of liver development (E8.5) was unaffected by loss of GATA6 seemed surprising. GATA factors, however, have similar DNA binding characteristics, and both GATA6 and GATA4 are expressed in endoderm-derived tissues, suggesting that they may functionally overlap (26, 31). We therefore tested whether GATA4 was present in the ventral endoderm of embryos lacking GATA6 during hepatic specification. Figure 4B shows that GATA4 was indeed detected by immunohistochemistry in the presumptive hepatic ventral endoderm of both control and Gata6−/− E8.5 ES cell-derived embryos containing 8 to 10 somites, suggesting that GATA4 has the potential to compensate for loss of GATA6 during hepatic specification.
GATA4 is transiently expressed in the early ventral endoderm and may complement loss of GATA6 during hepatic specification.
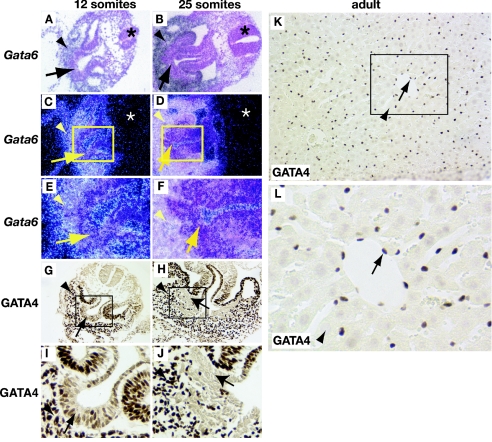
If GATA4 and GATA6 have redundant roles in controlling liver gene expression during hepatic specification, it raises the question of why GATA4 is apparently unable to compensate for loss of GATA6 during later stages of hepatic development at E9.5 when the liver bud is expanding. With this in mind we examined expression of GATA4 and GATA6 in embryos containing 12 somites (E8.5), which had just completed hepatic specification, and in embryos containing 25 somites, in which hepatoblasts were migrating from the expanding liver bud. Figures 5A, C, E, G, and I show that, by 12 somites, GATA4 and -6 continue to be expressed in the specified hepatic endoderm of the liver bud as well as in the surrounding septum transversum mesenchyme. By 25 somites Gata6 mRNA was identified in the expanding hepatoblast cells, although at levels lower than those found in the septum transversum (Fig. 5B, D, and F). This is consistent with previously published immunostaining data that show GATA6 protein also present in hepatoblasts of E14.5 livers (31). However, strikingly and in contrast to Gata6, GATA4 expression is abruptly extinguished in hepatoblasts as the cells delaminate from the specified hepatic endoderm coincident with the stage at which loss of GATA6 results in a hepatic phenotype (Fig. 5H and J). This low to undetectable expression of GATA4 in hepatic parenchymal cells is also seen in adult livers. Figures 5K and L show that expression of GATA4 is most predominant in the endothelial cells surrounding the sinusoidal capillaries and large veins of the adult liver and in contrast is difficult to detect by immunohistochemistry in the hepatocytes. In situ hybridization studies showed that Gata4 mRNA was also undetectable in hepatoblasts in embryos at E9.5 (data not shown), suggesting that regulation was likely at the level of transcription. This dynamic expression of GATA4 in the early ventral endoderm is consistent with the proposal that GATA4 and GATA6 have redundant functions during hepatic specification but that only GATA6 is available to control expansion of the liver bud and commitment of hepatoblasts to express the normal profile of hepatic mRNAs.
FIG. 5.
Expression of GATA4 but not GATA6 is extinguished in migrating hepatoblasts. Embryos containing 12 (A, C, E, G, and I) or 25 (B, D, F, H, and J) somite pairs and adult livers (K and L) were isolated and processed for in situ hybridization to detect Gata6 mRNA (A to F) or for immunohistochemistry to detect GATA4 protein (G to L). Both GATA4 protein (brown nuclear staining; G and I) and Gata6 mRNA (A, C, and E) can be detected in the septum transversum mesenchyme (arrowheads) and liver bud (arrows) of 12-somite-pair embryos. At 25 somite pairs, Gata6 mRNA and GATA4 protein were detected in septum transversum mesenchyme (arrowheads; B, D, F, H, and J). In the liver bud (arrows; B, D, F, H, and J), however, the presence of Gata6 mRNA could be identified but GATA4 protein was undetectable. GATA4 protein was also detected predominantly in the endothelial cells (arrows) but not in hepatocytes (arrowheads) of adult livers (K and L). Panels E, F, I, J, and L show high-resolution images of boxed areas in panels C, D, G, H, and K, respectively. Both bright-field (A and B) and corresponding dark-field (C to F) images of Gata6 in situ hybridization analyses are presented. The presence of mRNA can be detected as bright silver grains in dark-field images; note the absence of staining in neural tubes (asterisks).
GATA6 is dispensable for early development of the vasculature, septum transversum, and heart.
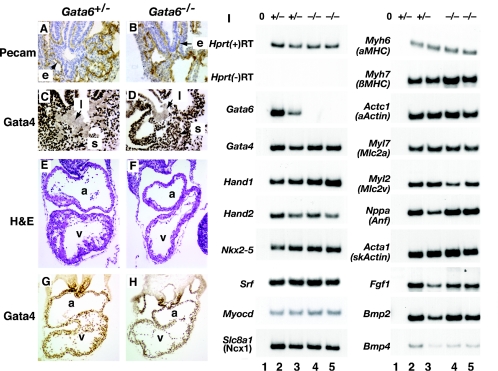
A combination of genetic and molecular analyses has identified the developing heart, septum transversum mesenchyme, and vascular tissue as sources of signals required for specification and expansion of the hepatic primordium in mice (21). Many of these tissues also express GATA6, raising the possibility that the block in development of the liver in the absence of GATA6 could be caused by defective development of these nonhepatic tissues. Figures 6A to D show immunohistochemical staining of control and Gata6−/− E9.5 ES cell-derived embryos for PECAM, which is expressed in endothelial cells, and for GATA4, which is highly expressed in the septum transversum mesenchyme at the level of the liver bud. Both endothelial cells and septum transversum mesenchyme were seen to surround the hepatic bud regardless of GATA6's presence or absence. The finding that septum transversum mesenchyme was still present in the absence of GATA6 was interesting given that we had previously shown that Gata4−/− embryos fail to form septum transversum mesenchyme as well as the related proepicardium (42).
FIG. 6.
GATA6 is dispensable for early development of the heart, septum transversum meschyme, and vasculature. (A and B) Immunohistochemistry (brown staining) identifies PECAM-positive endothelial cells (e; arrows) surrounding the presumptive hepatic endoderm in both control and GATA6-null embryos at E9.5. (C and D) Septum transversum mesenchyme that expresses GATA4 can be identified surrounding the presumptive hepatic bud (arrow) in both Gata6+/− and Gata6−/− E9.5 embryos by immunohistochemistry. (E to H) Hematoxylin- and eosin-stained sections of Gata6+/− and Gata6−/− fetal hearts at E9.5 finds no substantive differences in the morphology of endocardium or myocardium, which both express GATA4 protein as determined by immunohistochemistry (brown nuclear staining) (G and H). l, liver; s, septum transversion; a, atrium; v, ventricle. (I) RT-PCR analysis of cardiac mRNAs in hearts isolated from Gata6+/− (lanes 2 and 3) or Gata6−/− (lanes 4 and 5) E9.5 embryos. Amplification of Hprt was used as a loading control, omission of reverse transcriptase [(−)RT] from the RT-PCR and amplifications lacking template (lane 1) confirmed the absence of contaminating DNA, and PCR with Gata6 primers confirmed that only control embryos expressed Gata6 mRNA. All gene names use official nomenclature with common names below in parentheses where appropriate.
It has also been proposed that the GATA factors have crucial roles in controlling cardiac development (32). Since cardiogenesis is essential for liver development, we determined whether development of the heart was affected by loss of GATA6. Figures 6E and F show hematoxylin- and eosin-stained sections through the cardiac region of E9.5 control and Gata6−/− ES cell-derived embryos. Both embryos appeared to have developed comparably, containing differentiated cardiomyocytes and endocardial cells. In a minority of cases, the extent of ventricular trabeculation was subtly reduced in the absence of GATA6; however, many Gata6−/− ES cell-derived embryos appeared normal in this regard. The role of GATA factors in controlling cardiac gene expression has been studied intensely, and so we compared steady-state levels of several cardiomyocyte mRNAs by RT-PCR in hearts isolated from E9.5 embryos. As shown in Fig. 6I, expression of all cardiac mRNAs was similar in both control and Gata6−/− ES cell-derived hearts. Importantly, these mRNAs included those encoding Fgf1, Bmp2, and Bmp4, which have previously been shown to regulate development of the liver (17, 40). The observation that cardiac gene expression was unaffected by loss of GATA6 could be due to maintenance of GATA4 expression in the Gata6−/− cardiomyocytes. In support of GATA4 and GATA6 having redundant functions in the heart, GATA4 protein could indeed be detected in Gata6−/− cardiomyocytes by immunohistochemistry, and Gata4 mRNA was identified in GATA6-null hearts by RT-PCR (Fig. 6G to I).
DISCUSSION
Our production of post-gastrulation-stage embryos from Gata6−/− ES cells, through tetraploid embryo complementation, definitively establishes that GATA6-knockout mice arrest during early embryonic development due to defects in extraembryonic endoderm function as was proposed previously (20, 27). A direct role for GATA6 acting as a regulator of extraembryonic endoderm development is also supported by loss-of-function studies of F9 embryonal carcinoma cells. In these experiments, the ability of F9 cells to form parietal endoderm in culture was suppressed when expression of GATA4 and GATA6 was simultaneously reduced using a short interfering RNA approach (14). In addition, forced expression of GATA6 in ES cells induced these pluripotent cells to adopt an extraembryonic endoderm fate (13). Together these results convincingly argue that GATA6 is a central regulator of extraembryonic endoderm differentiation.
The availability of Gata6−/− embryos that develop to E10.5 has also allowed us to demonstrate an essential role for GATA6 in controlling development of the mammalian liver. Although analysis of cell-type-specific knockouts of GATA6 will be necessary to definitively establish whether this factor acts cell autonomously during hepatogenesis, the observation that development of the heart, septum transversum, and embryonic vasculature appears relatively normal in Gata6−/− embryos is consistent with GATA6 acting within the endoderm. Molecular and biochemical studies also support a cell-autonomous role for GATA factors in controlling liver development. Analyses of the Albumin gene's transcriptional regulatory elements have uncovered several transcription factors that appear to have roles in hepatic development (2, 15). Of these factors, FoxA and GATA4 have been shown to interact with the Albumin enhancer in pluripotent definitive endoderm prior to the onset of Albumin gene expression and hepatic specification (15). GATA4 and FoxA are unusual in that they can recognize their respective binding sites and form stable complexes in the context of compacted chromatin, which is normally recalcitrant to transcription factor binding (5, 7). Importantly, binding of either FoxA or GATA4 opens compacted chromatin, and FoxA appears to have the capacity to reposition nucleosomes around the Albumin enhancer (5, 7). Based on these observations, it has been proposed that FoxA and GATA4 may act as potentiators of endodermal differentiation (44). In this scheme, binding of these factors would remodel local areas of chromatin around the transcriptional regulatory elements of liver genes in the pluripotent endoderm, rendering them capable of binding transcriptional activators. This would maintain these genes in a competent but inactive state. If transcriptional activators were induced in response to inductive signals, they would then have access to the transcriptionally competent genes and initiate differentiation through activation of gene expression. Most of the studies that have led to this model have focused on GATA4 as the factor that acts in conjunction with FoxA to mediate hepatic competency of the endoderm (5-7). However, GATA binding sites can be recognized by GATA4, -5, and -6, suggesting that any of these could potentially act as pioneer factors.
Our data show that, as development proceeds, GATA6 alone is necessary for expansion of the liver bud and commitment of the endoderm to a hepatic cell fate. However, based on the finding that hepatic mRNA expression is unaltered in the absence of GATA6 in E8.5 embryos containing six to eight somites, hepatic specification of Gata6−/− ventral endoderm appears to be intact. This may seem surprising given the compelling molecular evidence that supports a role for the GATA factors in regulating competency and, as a consequence, hepatic specification. However, examination of the expression of GATA4 within the endoderm of both wild-type and Gata6−/− embryos offers a possible explanation of why GATA6 is dispensable for hepatic specification. We have demonstrated that GATA4 can be detected in the ventral endoderm prior to specification of the hepatic lineage. This expression continues within the presumptive nascent hepatic cells until the 12- to 14-somite stage of development (∼E9.0). However, after this developmental time point GATA4 is rapidly lost from the cells within the nascent hepatic endoderm as the liver bud expands, although expression remains within dorsal domains of the foregut. In contrast to GATA4, Gata6 mRNA can be detected within the presumptive hepatic endoderm throughout these early stages of hepatogenesis. Based on these analyses we speculate that GATA4 compensates for loss of GATA6 during hepatic specification but that GATA6 alone is present to control subsequent stages of hepatic development. These results also suggest that GATA factors not only act during competency and specification stages but continue to be required as the liver bud is expanding. These proposals may ultimately be answered by the generation of embryos lacking both GATA4 and GATA6.
In addition to regulating development of the liver, GATA6 has also been implicated in heart development (32). Our studies of rescued GATA6-null embryos revealed at most only subtle changes in cardiac morphology; in some embryos formation of trabeculae within the developing ventricular regions appeared to be slightly reduced compared to controls. Although these data show that GATA6 is dispensable for early heart formation, it is possible that GATA6 is required for some physiological aspects of fetal cardiovascular function, which could conceivably explain the relatively small size of GATA6-null embryos. GATA4 is also expressed in the developing heart, and we have previously reported that GATA4-null embryos have an extremely thin ventricular myocardium and complete absence of the proepicardium (42). This did not appear to be the case for Gata6−/− ES cell-derived embryos in which development of both proepicardium and myocardium was generally indistinguishable from control embryos. In addition, as was the case for Gata4−/− embryos, GATA6-null embryos presented no alteration in cardiac gene expression, including expression of GATA4. Comparing the phenotypes presented by Gata4−/− and Gata6−/− ES cell-derived embryos, it is clear that the relationship between GATA4, GATA6, and their regulation of development is complex; in some cases, for example in hepatic specification and cardiac gene expression, there appears to be redundancy in function between GATA4 and GATA6, while in others, for example in development of the septum transversum and proepicardium, one factor appears to have a dominant role. Whether these factors act in a redundant or nonredundant manner may be explained by subtle differences in the timing of expression of the two factors in a given tissue during development or by differences in the binding of GATA4 and GATA6 to specific partners, such as Fog proteins and other coactivators. At the moment these issues are difficult to address; however, they may be resolved in the future by the availability of conditional alleles or by the use of specific short interfering RNAs.
In summary, we have demonstrated that providing Gata6−/− embryos with a wild-type extraembryonic endoderm allows these embryos to proceed through gastrulation. Examination of these rescued Gata6−/− embryos reveals that, although they are smaller, development of many tissues occurs normally, with the exception of the liver, which arrests shortly after formation of the primary hepatic bud. While several transcription factors have been shown to influence hepatocyte differentiation and maturation of the liver, GATA6 can now be classified as one of a very few that are essential for the earliest stages of hepatic development. The future challenges that arise from this study clearly include defining the molecular mechanisms through which GATA6 controls development of the hepatic primordium.
Acknowledgments
We thank Paula Traktman, Lisa Cirillo, Michele Battle, and Gena Konopka for critically reading the manuscript and Ravi Misra for advice on primers for PCR on cardiac mRNA.
This work was supported by NIH NIDDK grants DK55743, DK60064, and DK66226 and an AHA postdoctoral fellowship 0120668Z to A.J.W.
REFERENCES
- 1.Bort, R., J. P. Martinez-Barbera, R. S. Beddington, and K. S. Zaret. 2004. Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development 131:797-806. [DOI] [PubMed] [Google Scholar]
- 2.Bossard, P., and K. S. Zaret. 1998. GATA transcription factors as potentiators of gut endoderm differentiation. Development 125:4909-4917. [DOI] [PubMed] [Google Scholar]
- 3.Cascio, S., and K. S. Zaret. 1991. Hepatocyte differentiation initiates during endodermal-mesenchymal interactions prior to liver formation. Development 113:217-225. [DOI] [PubMed] [Google Scholar]
- 4.Chen, W. S., K. Manova, D. C. Weinstein, S. A. Duncan, A. S. Plump, V. R. Prezioso, R. F. Bachvarova, and J. E. Darnell, Jr. 1994. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 8:2466-2477. [DOI] [PubMed] [Google Scholar]
- 5.Cirillo, L. A., F. R. Lin, I. Cuesta, D. Friedman, M. Jarnik, and K. S. Zaret. 2002. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell 9:279-289. [DOI] [PubMed] [Google Scholar]
- 6.Cirillo, L. A., C. E. McPherson, P. Bossard, K. Stevens, S. Cherian, E. Y. Shim, K. L. Clark, S. K. Burley, and K. S. Zaret. 1998. Binding of the winged-helix transcription factor HNF3 to a linker histone site on the nucleosome. EMBO J. 17:244-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cirillo, L. A., and K. S. Zaret. 1999. An early developmental transcription factor complex that is more stable on nucleosome core particles than on free DNA. Mol. Cell 4:961-969. [DOI] [PubMed] [Google Scholar]
- 8.Costa, R. H., V. V. Kalinichenko, A. X. Holterman, and X. Wang. 2003. Transcription factors in liver development, differentiation, and regeneration. Hepatology 38:1331-1347. [DOI] [PubMed] [Google Scholar]
- 9.Duncan, S. A. 2003. Mechanisms controlling early development of the liver. Mech. Dev. 120:19-33. [DOI] [PubMed] [Google Scholar]
- 10.Duncan, S. A. 2000. Transcriptional regulation of liver development. Dev. Dyn. 219:131-142. [DOI] [PubMed] [Google Scholar]
- 11.Duncan, S. A., K. Manova, W. S. Chen, P. Hoodless, D. C. Weinstein, R. F. Bachvarova, and J. E. Darnell, Jr. 1994. Expression of transcription factor HNF-4 in the extraembryonic endoderm, gut, and nephrogenic tissue of the developing mouse embryo: HNF-4 is a marker for primary endoderm in the implanting blastocyst. Proc. Natl. Acad. Sci. USA 91:7598-7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan, S. A., A. Nagy, and W. Chan. 1997. Murine gastrulation requires HNF-4 regulated gene expression in the visceral endoderm: tetraploid rescue of HNF-4−/− embryos. Development 124:279-287. [DOI] [PubMed] [Google Scholar]
- 13.Fujikura, J., E. Yamato, S. Yonemura, K. Hosoda, S. Masui, K. Nakao, J. Miyazaki, and H. Niwa. 2002. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 16:784-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Futaki, S., Y. Hayashi, T. Emoto, C. N. Weber, and K. Sekiguchi. 2004. Sox7 plays crucial roles in parietal endoderm differentiation in F9 embryonal carcinoma cells through regulating Gata-4 and Gata-6 expression. Mol. Cell. Biol. 24:10492-10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gualdi, R., P. Bossard, M. Zheng, Y. Hamada, J. R. Coleman, and K. S. Zaret. 1996. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 10:1670-1682. [DOI] [PubMed] [Google Scholar]
- 16.Hatzis, P., and I. Talianidis. 2001. Regulatory mechanisms controlling human hepatocyte nuclear factor 4α gene expression. Mol. Cell. Biol. 21:7320-7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung, J., M. Zheng, M. Goldfarb, and K. S. Zaret. 1999. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science 284:1998-2003. [DOI] [PubMed] [Google Scholar]
- 18.Keijzer, R., M. van Tuyl, C. Meijers, M. Post, D. Tibboel, F. Grosveld, and M. Koutsourakis. 2001. The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development. Development 128:503-511. [DOI] [PubMed] [Google Scholar]
- 19.Keng, V. W., H. Yagi, M. Ikawa, T. Nagano, Z. Myint, K. Yamada, T. Tanaka, A. Sato, I. Muramatsu, M. Okabe, M. Sato, and T. Noguchi. 2000. Homeobox gene Hex is essential for onset of mouse embryonic liver development and differentiation of the monocyte lineage. Biochem. Biophys. Res. Commun. 276:1155-1161. [DOI] [PubMed] [Google Scholar]
- 20.Koutsourakis, M., A. Langeveld, R. Patient, R. Beddington, and F. Grosveld. 1999. The transcription factor GATA6 is essential for early extraembryonic development. Development 126:723-732. [PubMed] [Google Scholar]
- 21.Lemaigre, F., and K. S. Zaret. 2004. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr. Opin. Genet. Dev. 14:582-590. [DOI] [PubMed] [Google Scholar]
- 22.Li, J., G. Ning, and S. A. Duncan. 2000. Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha. Genes Dev. 14:464-474. [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez Barbera, J. P., M. Clements, P. Thomas, T. Rodriguez, D. Meloy, D. Kioussis, and R. S. Beddington. 2000. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development 127:2433-2445. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto, K., H. Yoshitomi, J. Rossant, and K. S. Zaret. 2001. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294:559-563. [DOI] [PubMed] [Google Scholar]
- 25.Molkentin, J. D. 2000. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J. Biol. Chem. 275:38949-38952. [DOI] [PubMed] [Google Scholar]
- 26.Morrisey, E. E., H. S. Ip, M. M. Lu, and M. S. Parmacek. 1996. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev. Biol. 177:309-322. [DOI] [PubMed] [Google Scholar]
- 27.Morrisey, E. E., Z. Tang, K. Sigrist, M. M. Lu, F. Jiang, H. S. Ip, and M. S. Parmacek. 1998. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 12:3579-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortensen, R. M., D. A. Conner, S. Chao, A. A. T. Geisterfer-Lowrance, and J. G. Seidman. 1992. Production of homozygous mutant ES cells with a single targeting construct. Mol. Cell. Biol. 12:2391-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy, A., and J. Rossant. 1993. Production of completely ES cell-derived fetuses, p. 147-179. In A. Joyner (ed.), Gene targeting: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 30.Nagy, A., J. Rossant, R. Nagy, W. Abramow-Newerly, and J. C. Roder. 1993. Derivation of completely cell culture-derived mice from early passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90:8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemer, G., and M. Nemer. 2003. Transcriptional activation of BMP-4 and regulation of mammalian organogenesis by GATA-4 and -6. Dev. Biol. 254:131-148. [DOI] [PubMed] [Google Scholar]
- 32.Parmacek, M. S., and J. M. Leiden. 1999. GATA transcription factors and cardiac development, p. 291-306. In R. P. Harvey and N. Rosenthal (ed.), Heart development. Academic Press, San Diego, Calif.
- 33.Parviz, F., C. Matullo, W. D. Garrison, L. Savatski, J. W. Adamson, G. Ning, K. H. Kaestner, J. M. Rossi, K. S. Zaret, and S. A. Duncan. 2003. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat. Genet. 34:292-296. [DOI] [PubMed] [Google Scholar]
- 34.Patient, R. K., and J. D. McGhee. 2002. The GATA family (vertebrates and invertebrates). Curr. Opin. Genet. Dev. 12:416-422. [DOI] [PubMed] [Google Scholar]
- 35.Rausa, F. M., H. Ye, L. Lim, S. A. Duncan, and R. H. Costa. 1998. In situ hybridization with 33P-labeled RNA probes for determination of cellular expression patterns of liver transcription factors in mouse embryos. Methods 16:29-41. (Erratum, 16:359-360.) [DOI] [PubMed] [Google Scholar]
- 36.Rehorn, K. P., H. Thelen, A. M. Michelson, and R. Reuter. 1996. A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development 122:4023-4031. [DOI] [PubMed] [Google Scholar]
- 37.Reiter, J. F., J. Alexander, A. Rodaway, D. Yelon, R. Patient, N. Holder, and D. Y. Stainier. 1999. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 13:2983-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiter, J. F., Y. Kikuchi, and D. Y. Stainier. 2001. Multiple roles for Gata5 in zebrafish endoderm formation. Development 128:125-135. [DOI] [PubMed] [Google Scholar]
- 39.Reuter, R. 1994. The gene serpent has homeotic properties and specifies endoderm versus ectoderm within the Drosophila gut. Development 120:1123-1135. [DOI] [PubMed] [Google Scholar]
- 40.Rossi, J. M., R. N. Dunn, B. L. M. Hogan, and K. S. Zaret. 2001. Distinct mesodermal signals, including BMPs from septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 15:1998-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sosa-Pineda, B., J. T. Wigle, and G. Oliver. 2000. Hepatocyte migration during liver development requires Prox1. Nat. Genet. 25:254-255. [DOI] [PubMed] [Google Scholar]
- 42.Watt, A. J., M. A. Battle, J. Li, and S. A. Duncan. 2004. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc. Natl. Acad. Sci. USA 101:12573-12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, H., M. M. Lu, L. Zhang, J. A. Whitsett, and E. E. Morrisey. 2002. GATA6 regulates differentiation of distal lung epithelium. Development 129:2233-2246. [DOI] [PubMed] [Google Scholar]
- 44.Zaret, K. 1999. Developmental competence of the gut endoderm: genetic potentiation by GATA and HNF3/fork head proteins. Dev. Biol. 209:1-10. [DOI] [PubMed] [Google Scholar]
- 45.Zaret, K. S. 2002. Regulatory phases of early liver development: paradigms of organogenesis. Nat. Rev. Genet. 3:499-512. [DOI] [PubMed] [Google Scholar]
- 46.Zhu, J., T. Fukushige, J. D. McGhee, and J. H. Rothman. 1998. Reprogramming of early embryonic blastomeres into endodermal progenitors by a Caenorhabditis elegans GATA factor. Genes Dev. 12:3809-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu, J., R. J. Hill, P. J. Heid, M. Fukuyama, A. Sugimoto, J. R. Priess, and J. H. Rothman. 1997. end-1 encodes an apparent GATA factor that specifies the endoderm precursor in Caenorhabditis elegans embryos. Genes Dev. 11:2883-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]