Abstract
Although signaling by death receptors involves the recruitment of common components into their death-inducing signaling complexes (DISCs), apoptosis susceptibility of various tumor cells to each individual receptor differs quite dramatically. Recently it was shown that, besides caspase-8, caspase-10 is also recruited to the DISCs, but its function in death receptor signaling remains unknown. Here we show that expression of caspase-10 sensitizes MCF-7 breast carcinoma cells to TRAIL- but not tumor necrosis factor (TNF)-induced apoptosis. This sensitization is most obvious at low TRAIL concentrations or when apoptosis is assessed at early time points. Caspase-10-mediated sensitization for TRAIL-induced apoptosis appears to be dependent on caspase-3, as expression of caspase-10 in MCF-7/casp-3 cells but not in caspase-3-deficient MCF-7 cells overcomes TRAIL resistance. Interestingly, neutralization of TRAIL receptor 2 (TRAIL-R2), but not TRAIL-R1, impaired apoptosis in a caspase-10-dependent manner, indicating that caspase-10 enhances TRAIL-R2-induced cell death. Furthermore, whereas processing of caspase-10 was delayed in TNF-treated cells, TRAIL triggered a very rapid activation of caspase-10 and -3. Therefore, we propose a model in which caspase-10 is a crucial component during TRAIL-mediated apoptosis that in addition actively requires caspase-3. This might be especially important in systems where only low TRAIL concentrations are supplied that are not sufficient for the fast recruitment of caspase-8 to the DISC.
Apoptosis is a fundamental process essential for normal tissue homeostasis and development that plays a physiological role in deletion of activated lymphocytes at the end of an immune response and in elimination of virus-infected or oncogenically transformed cells (25, 31, 47). Hence, dysregulation of apoptosis may be directly involved in several human diseases including degenerative and autoimmune diseases, neoplasia, and immunodeficiency syndromes. Based on their tremendous cytotoxic potential, death ligands such as tumor necrosis factor (TNF), CD95 ligand (CD95L), and TRAIL (TNF-related apoptosis-inducing ligand) were considered promising cancer therapeutic agents. This view was supported by the finding that, in contrast to chemotherapeutic drugs and radiation therapy that usually require the p53 tumor suppressor gene, death ligands can induce apoptosis independently of p53. However, severe toxic side effects preclude TNF and CD95L from use in systemic anticancer therapy (8). On the other hand, a totally different picture emerged with the death ligand TRAIL. Although identified by sequence homology to CD95L and TNF, TRAIL was found to selectively kill tumor cells, whereas normal cells were left unharmed by this cytokine (50, 51). This was also confirmed by in vivo studies as systemic administration of TRAIL suppressed tumor growth in SCID mice and nonhuman primates without being toxic to normal tissues (2, 52). In addition, in mouse models, TRAIL was used successfully in locoregional treatment of glioblastoma xenografts (37) and was shown to be necessary for natural killer cell-mediated suppression of liver metastasis (40, 44).
These data appear particularly surprising in view of the assumption that different death receptors induce apoptosis via similar mechanisms recruiting identical signaling components such as the adaptor molecule Fas-associated death domain (FADD) and procaspase 8 to their respective death-inducing signaling complexes (DISCs) (5, 27, 38, 51, 53). Even more puzzling were the observations that various tumor cells are resistant to apoptosis induction by TNF or CD95L but sensitive towards TRAIL (3, 29, 35, 46). Susceptibility to TRAIL-induced apoptosis was originally believed to be influenced by the expression levels of two additional TRAIL receptors (TRAIL-R3/DcR1/TRID and TRAIL-R4/DcR2/TRUNDD) (1, 14, 51). These receptors are not able to transduce apoptotic signals due to the lack of a functional intracellular death domain, but they still bind TRAIL and thereby act as so-called decoy receptors. However, in subsequent studies, no correlation was found between the expression of either of these decoy receptors and TRAIL sensitivity, indicating the possible existence of additional signaling components that distinguish death receptor-mediated pathways (50).
One of these components was postulated to be caspase-10, the second death effector domain (DED)-containing initiator caspase besides caspase-8 (49). Four different splice variants of caspase-10 have been reported (caspase-10a/Mch4, caspase- 10b/FLICE2, caspase-10c and caspase-10d) that are abundantly expressed in fetal tissues, suggesting a role in embryonic development (12, 32, 49). In addition, based on sequence homology to caspase-8, caspase-10 has been proposed to be involved in death receptor signaling; however, studies investigating this hypothesis yielded conflicting results. For instance, several reports documented that caspase-8, but not caspase-10, is the crucial initiator caspase in death receptor signaling, as various caspase-8-deficient tumor cells were resistant to CD95L and TRAIL although caspase-10 was expressed in these cells (19, 21, 45). On the other hand, caspase-10 mutations have recently been associated with a rare immunological disorder, called autoimmune lymphoproliferative syndrome, a disease that was originally believed to be restricted to mutations in the CD95 receptor-ligand system (56). These caspase-10-inactivating mutations were proposed to be causative for the disease (autoimmune lymphoproliferative syndrome type II), as mature dendritic cells and activated peripheral T cells from these patients were resistant to TRAIL when compared to the TRAIL-sensitive phenotype of healthy control cells. These caspase-10 mutations did not affect apoptosis susceptibility to TNF or CD95L, indicating again a crucial role for caspase-10 specifically in TRAIL signaling. Other apoptosis-inactivating caspase-10 mutations were also found in several primary tumors as well as in tumor cell lines of different origin (15, 34, 39).
The first evidence for a more specific role of caspase-10 was provided by a study demonstrating that TRAIL-induced apoptosis was blocked by a dominant-negative caspase-10b but not by a dominant-negative caspase-8 mutant, although the latter efficiently blocked death by ligation of TNF receptor 1 (TNF-R1) (33, 49). Although initially not detected due to unknown reasons or the unavailability of a specific caspase-10 antibody (4, 23, 42), the recruitment of caspase-10 to death receptor-signaling complexes has now been confirmed by several independent studies (24, 41, 55). However, whereas two of these studies demonstrated that both caspase-8 and caspase-10 signal apoptosis through either the CD95 or the TRAIL death receptors independently from each other (24, 55), the other report postulated that caspase-10 cannot substitute for caspase-8 (41). Thus, although there is no doubt that caspase-10 is recruited to death receptor-signaling complexes, its role, if any, appears to be redundant for the function of caspase-8. Neither of these studies, however, provided a detailed comparison of death receptor-induced apoptosis and caspase activation in the absence or presence of these initiator caspases. In addition, as the carboxy-terminal half of caspase-10 that is involved in substrate recognition and binding differs greatly from that of caspase-8, different roles for both caspases in death receptor-induced apoptosis are conceivable (12, 49).
Therefore, we compared apoptosis signaling by TRAIL and TNF in MCF-7 breast carcinoma cells in the presence and absence of caspase-10. We found that expression of caspase-10 accelerated TRAIL-induced but not TNF-induced apoptosis in a remarkable caspase-3-dependent manner. Sensitization by caspase-10 was most evident when low cytokine doses were used or when apoptosis signaling and induction were assessed at early time points. Our results further indicate that caspase- 10 is a proximal caspase activated in TRAIL signaling, at least under low dose treatment, an observation that might be particularly important in systems in which only low, but physiologically relevant, TRAIL concentrations are available.
MATERIALS AND METHODS
Cell lines, reagents, and antibodies.
HeLa H21 cervical carcinoma cells (16) and MCF-7 breast carcinoma cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 10 mM glutamine, 100 U of penicillin /ml, and 0.1 mg of streptomycin/ml (all from PAA Laboratories, Linz, Austria). MCF-7/casp-3 cells stably expressing caspase-3 (18) and the various MCF-7 transfectants stably expressing caspase-10a or caspase-10b in the absence or presence of caspase-3 were maintained in the same medium supplemented with either 400 μg of neomycin/ml (Invitrogen, Karlsruhe, Germany), 200 μg of hygromycin/ml (Gibco), or a combination of both. The monoclonal antibodies directed against caspase-3 and caspase-8 were from R&D Systems (Wiesbaden, Germany) and from BioCheck (Münster, Germany), respectively. The monoclonal caspase-10 antibody (clone 4C1) was from MBL International (Nagoya, Japan). The caspase-3 substrate DEVD-AMC (N-acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin) and the caspase-8 substrate IETD-AMC (N-acetyl-Ile-Glu-Thr-Asp-aminomethylcoumarin) were from Biomol (Hamburg, Germany). Human recombinant TNF with a specific activity of 4 × 107 U/mg of protein was obtained from Knoll AG (Ludwigshafen, Germany). Leucine zipper-tagged TRAIL (LZ-TRAIL) was a gift from Henning Walczak (Heidelberg, Germany). His-tagged TRAIL and the monoclonal antibodies recognizing the four TRAIL receptors were from Alexis (Lausen, Switzerland).
cDNAs and reverse transcription (RT)-PCR.
The cDNAs encoding the CASP-10a and -10b isoforms were obtained by PCR from Jurkat cells and from human spleen cDNA (Clontech), respectively, by using the Pfu DNA polymerase. The following oligonucleotide primers were used: 5CASP-10a/b (5′-AAA GGA TCC GCT AGC ATG AAA TCT CAA GGT CAA CAT TGG TAT TCC-3′), 3CASP-10a (5′-TTT CTC GAG TAT TGA AAG TGC ATC CAG GGG CAC-3′), and 3CASP-10b (5′-AAA GAA TTC CTA GGA AAC GCT GCT CCA CCT GCG-3′). The PCR products were cloned into the plasmid pIRES1hyg (CASP-10b), an expression vector encoding a hygromycin resistance gene, or into the expression plasmid pcDNA4zeo (CASP-10a/b), carrying the Zeocin resistance gene. Note that the pIRES1hyg/CASP-10b cDNA used to generate the main MCF-7/casp-3/casp-10b clone that was used throughout this study contained the original CASP-10 stop codon resulting in the expression of an untagged caspase-10b protein. The various MCF-7 clones described in Fig. 7 were generated with the pcDNA4zeo/CASP-10a/b cDNAs and express caspase-10-Myc/His fusion proteins. No obvious difference was observed between tagged and untagged caspase- 10 proteins. The PCR products were fully sequenced and confirmed to be wild type. For detection and nucleotide sequence analysis of CASP-10b cDNA from various cell lines, total cellular RNA was purified using an RNAeasy kit (QIAGEN, Hilden, Germany). RNA (5 μg) was reverse transcribed using Superscript II (Life Technologies), and the cDNA was amplified with the above-mentioned primers.
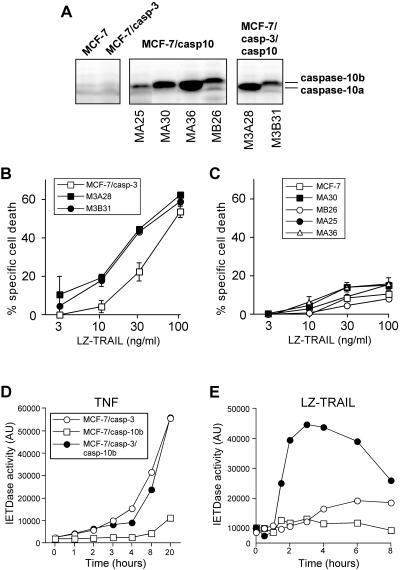
FIG. 7.
Expression of caspase-10a and caspase-10b sensitizes MCF-7/casp-3, but not caspase-3-deficient MCF-7 cells, to TRAIL-induced apoptosis. (A) Western blot analyses for the expression levels of caspase-10a (55 kDa) and caspase-10b (59 kDa) in the indicated cell lines. (B) Cell death assessment of MCF-7/casp-3 cells and MCF-7/casp-3 cells stably expressing caspase-10a (M3A28) or caspase-10b (M3B31) that were treated with the indicated concentrations of TRAIL. Cell death was assessed after 24 h by the crystal violet assay. One representative experiment out of two performed in triplicate is shown. (C) Cell death assessment of MCF-7 cells and MCF-7 cells stably expressing caspase-10a (MA25, MA30, and MA36) or caspase- 10b (MB26) that were treated with the indicated concentrations of TRAIL. Cell death was assessed after 24 h by the crystal violet assay. One representative experiment out of two performed in triplicate is shown. (D and E) Assessment of the caspase-8 (IETDase) activity in extracts of MCF-7/casp-3, MCF-7/casp-10b, and MCF-7/casp-3/casp-10b cells that were treated for the indicated times with TNF or TRAIL at a concentration of 25 ng/ml. AU, arbitrary units.
Stable transfection of MCF-7 and MCF-7/casp-3 cells.
Caspase-3-deficient and -proficient MCF-7 cells were stably transfected with the above-described expression constructs by using the SuperFect reagent (QIAGEN). Forty hours posttransfection, cells were trypsinized and reseeded in selection medium containing 600-μg/ml concentrations of the appropriate antibiotic. Individual clones were picked and further cultured in 200-μg/ml concentrations of the appropriate antibiotic. To exclude the introduction of mutations in caspase-10 during the generation of the stable clones, the cDNAs encoded by the plasmid were reisolated from the single clones and sequenced. No mutations were found in the clones used.
Preparation of cell extracts and Western blotting.
Cell extracts were prepared as described previously (17). To confirm equal loadings, protein concentrations were determined with the Bio-Rad protein assay. Subsequently, proteins were separated under reducing conditions on a sodium dodecyl sulfate-polyacrylamide gel and electroblotted onto a polyvinylidene difluoride membrane (Amersham, Braunschweig, Germany). Following incubation with the various antibodies, the proteins were visualized by enhanced chemiluminescent staining using ECL reagents (Amersham Biosciences, Freiburg, Germany).
Measurement of cell death.
Cell death was assessed by microscopic examination or was measured with the standard TNF cytotoxicity assay (crystal violet assay) that is based on the staining of viable cells (16). Briefly, cells (2 × 105/ml) were seeded into 96-well microtiter plates in 100 μl of culture medium. Cells were incubated with the death stimuli for the indicated times at 37°C, and viable cells were stained with 20% methanol containing 0.5% crystal violet and solubilized in 33% acetic acid. The absorbance was measured at an optical density of 590 nm (A590). Percent specific cell death is defined as 100 − (A590 of test well × 100/A590 of untreated well). Each experiment was performed independently at least three times and an individual experiment was carried out in triplicate.
Fluorescence-activated cell sorter analyses.
Cells were mechanically detached, washed with phosphate-buffered saline (PBS), and resuspended in PBS containing 1% bovine serum albumin. Cells were incubated for 60 min at room temperature with the monoclonal antibodies against TRAIL-R1 (HS101), TRAIL-R2 (HS202), or control mouse immunoglobulin G1 (mIgG1) at 10 μg/ml. Cells were washed twice in PBS, resuspended in PBS containing 1% bovine serum albumin, and incubated with R-phycoerythrin-conjugated goat anti-mouse IgG (1:100; Jackson ImmunoResearch) for 30 min at room temperature. After extensive washing with PBS, receptor expression was determined by flow cytometric analyses using the FL2-histogram profile. All flow cytometric analyses were performed on a FACSCalibur (Becton Dickinson, Heidelberg, Germany) with the CellQuest analysis software.
siRNA design and transient transfection.
The small interfering RNAs (siRNAs) against caspase-8 and caspase-10 were designed using three independent selection programs from Dharmacon (Lafayette, Colo.), Ambion, and from the Genomics Institute of the Novartis Research Foundation (siRNA Picker) and applying published standard criteria. The selected sequence for caspase-8 was 5′-AA-GGGUCAUGCUCUAUCAGAU-dTdT-3′. The two caspase-10 siRNA duplexes, 5′-AA-AGGAAGCCGAGUCGUAUCA-dTdT-3′ and 5′-AA-GAACUCCUCUAUAUCAUAC-dTdT-3′ , did not suppress expression of any of the caspase-10 isoforms, whereas the caspase-10 siRNA with the sequence 5′-AAAAAUAAGCAUGCAGGUAGU-dTdT-3′ suppressed only caspase-10b expression, without influencing expression of the other caspase-10 isoforms. Therefore, the siSMART-pool against caspase-10 was purchased from Dharmacon (catalog no. M-004402-01). As a control for the specificity of the siRNA-mediated gene suppression, we used siRNA duplexes against luciferase (siGL3) with the following sequence: 5′-AA-CTTACGCTGAGTACTTCGA-dTdT-3′.
siRNAs were delivered by Dharmacon as double-stranded RNA oligonucleotides and transfected into cells by using LipofectAMINE 2000 (Invitrogen) at a final concentration of 25 nM siRNA plus 4 μl of LipofectAMINE 2000/105 cells/ml in a six-well plate format with a final volume of 2 ml. Forty-eight hours after the siRNA treatment, cells were incubated with TRAIL and assayed either by Western blotting for caspase-8 and caspase-10 expression or with the fluorometric assay for caspase-3 (DEVDase) activity (see below).
Fluorometric assay of caspase activity.
Caspase activity was determined as described previously (11). Briefly, cell lysates were incubated with 50 μM fluorogenic caspase-3 substrate DEVD-AMC or the caspase-8 substrate IETD-AMC in 200 μl of buffer containing 50 mM HEPES (pH 7.4), 100 mM NaCl, 10% sucrose, 0.1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, and 10 mM dithiothreitol. The release of aminomethylcoumarin was measured by fluorometry using an excitation wavelength of 360 nm and an emission wavelength of 475 nm.
RESULTS
Tumor cells display different sensitivities towards TNF- and TRAIL-induced apoptosis.
It is well documented that apoptosis induction by the various death receptors, including TNF-R1, relies on the recruitment of FADD and caspase-8 into their respective DISCs, implying redundant signaling pathways. However, several tumor cell lines are resistant to TNF or CD95L but sensitive to apoptosis induction by TRAIL (3, 29, 35, 46). These observations indicate that different mechanisms or additional signaling components must exist for the individual death receptor pathways. In an attempt to understand these differences, we initially compared apoptosis susceptibilities of HeLa H21 cervical carcinoma cells and MCF-7 breast carcinoma cells to TNF and TRAIL. In the absence of protein biosynthesis inhibitors such as cycloheximide, HeLa H21 cells are resistant to TNF (16) but can be easily killed by TRAIL in a dose-dependent manner (Fig. 1A and B). On the other hand, MCF-7 cells that are devoid of caspase-3 due to the functional deletion of the CASP-3 gene (18) respond to these death receptor ligands in exactly the opposite way. They are sensitive towards TNF but remarkably do not undergo apoptosis in response to TRAIL, even when high doses of up to 100 ng/ml of this cytokine are used. Higher TRAIL concentrations, however, also yielded apoptosis in MCF-7 cells (data not shown). Interestingly, expression of caspase-3 in MCF-7 cells restored, at least partially, TRAIL sensitivity, indicating that, in contrast to TNF, caspase-3 appears to be required for TRAIL signaling. Notably, when compared to the parental MCF-7 and the HeLa H21 cells, MCF-7/casp-3 cells were killed by TRAIL with an intermediate efficiency. In particular, whereas approximately 17 and 37% of H21 cells were killed by TRAIL concentrations as low as 12.5 and 25 ng/ml, respectively, apoptosis of MCF-7/casp-3 cells was only observed when higher TRAIL doses starting from 50 ng/ml were used (Fig. 1B). Together, these results therefore indicate that, in the cell lines used, TNF and TRAIL most likely utilize different signaling pathways and/or components.
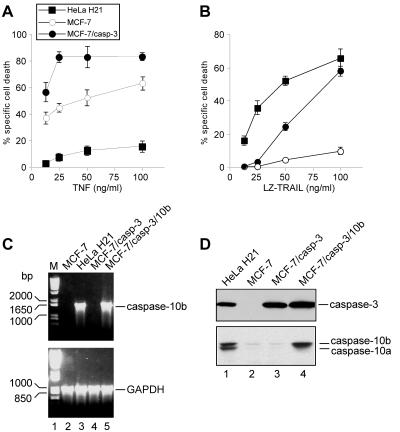
FIG. 1.
Different apoptosis susceptibilities of caspase-10-deficient and -proficient tumor cells to TNF and TRAIL. HeLa H21, MCF-7, and MCF-7/casp-3 cells were incubated with the indicated concentrations of TNF (A) or LZ-TRAIL (B). After 24 h, cell death was assessed by the crystal violet assay, a cytotoxicity assay that is based on the staining of viable cells (16). One representative experiment out of three performed in triplicate is shown. (C) RT-PCR analysis for caspase 10b mRNA expression. Reverse-transcribed GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA was included as a control. (D) Western blots demonstrating the expression levels of caspase- 3 (upper panel) and the two caspase-10 isoforms (caspase-10a and caspase-10b) (lower panel) in the various tumor cell lines.
Thus, we wondered whether MCF-7 cells, in addition to caspase-3, also lack other important signaling components that might be required for an efficient apoptosis induction by TRAIL. One of these components might be the initiator caspase-10 that was recently found to be recruited into the TNF, CD95, and TRAIL signaling complexes following stimulation with their respective ligands (24, 28, 41). Indeed, caspase-10 expression analyses supported our assumption, as at least two isoforms of this caspase, namely, caspase-10a and caspase-10b, were abundantly expressed in TRAIL-sensitive HeLa H21 cells but were conspicuously absent in both MCF-7 lines (Fig. 1D). This finding was also confirmed by RT-PCR analysis that even demonstrated the complete lack of caspase-10 mRNA transcripts in MCF-7 and MCF-7/casp-3 cells (Fig. 1C).
Expression of caspase-10b sensitizes MCF-7/casp-3/casp-10b cells to TRAIL-induced but not TNF-induced apoptosis.
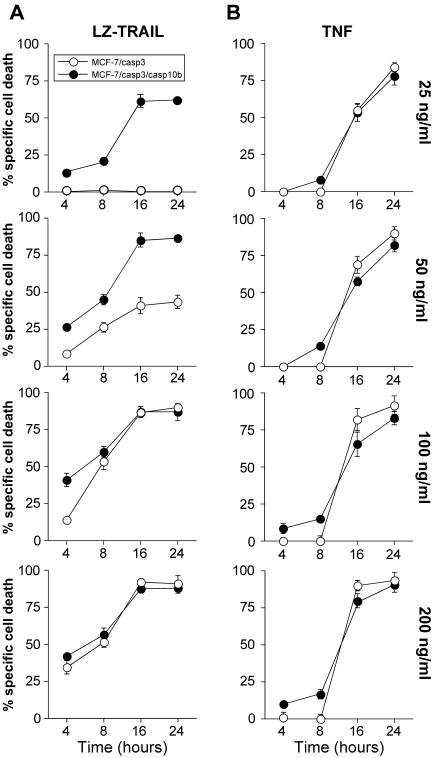
To examine a possible role for caspase-10 in TRAIL signaling, MCF-7/casp-3 cells were stably transfected with an expression vector encoding caspase-10b, as this isoform was previously suggested to be involved in TRAIL killing (49). Several clones were analyzed by RT-PCR and Western blotting and one clone was chosen initially for further experiments (Fig. 1C and D). First we compared TRAIL- and TNF-induced apoptosis in caspase-10-deficient and -proficient MCF-7/casp-3 cells. We found that the presence of caspase-10b specifically accelerated TRAIL-induced apoptosis in a remarkable dose- and time-dependent manner, whereas the response of both MCF-7 clones to various TNF concentrations did not differ significantly (Fig. 2). After 16 h, more than 60% of MCF-7/casp-3/casp-10b cells were killed by only 25 ng of TRAIL/ml, a concentration that was not sufficient to induce apoptosis in caspase-10-deficient MCF-7/casp-3 cells. In agreement with Fig. 1B, apoptosis induction of MCF-7/casp-3 cells was only achieved with TRAIL concentrations starting from 50 ng/ml (41%), a dose that resulted in the death of more than 85% of their caspase-10b-expressing derivatives. Furthermore, although apoptosis of MCF-7/casp-3/casp-10b cells was consistently observed at an earlier time point regardless of the TRAIL concentration applied, both cell lines showed a similar response to this cytokine at later time points when higher TRAIL concentrations of 100 or 200 ng/ml were used. In contrast, the presence of caspase-10b did not significantly affect the response of MCF-7/casp-3/casp-10b cells to TNF (Fig. 2) even when TNF doses as low as 1 ng/ml were used that are barely capable of inducing apoptosis in either cell line (data not shown). These results indicate that caspase-10b might be an essential component of the TRAIL but not of the TNF signaling pathway.
FIG. 2.
Increased TRAIL sensitivity of MCF-7/casp-3/casp-10b cells. Caspase-10-deficient MCF-7/casp-3 and caspase-10b-expressing MCF-7/casp-3/casp-10b cells were incubated with the indicated concentrations of either LZ-TRAIL (A) or TNF (B). After the indicated times, cell death was assessed by the crystal violet assay. One representative experiment out of four performed in triplicate is shown.
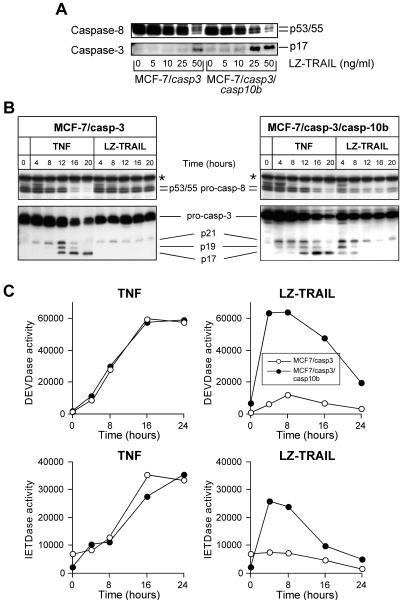
Consistent with these observations, treatment of MCF-7/casp-3/casp-10b cells with 25 ng of TRAIL/ml was sufficient to induce processing of caspase-3 and caspase-8, as evidenced by the appearance of the active p17 caspase-3 subunit and by the decrease of the p53/p55 procaspase-8 levels, respectively (Fig. 3A). In contrast, both events were only triggered in MCF-7/casp-3 cells by using TRAIL at a concentration of 50 ng/ml. Kinetic analyses further confirmed these results and showed that treatment with a single dose of 25 ng of TRAIL/ml resulted in the processing of caspase-8 and caspase-3 in MCF-7/casp-3/casp-10b cells but not in caspase-10-deficient MCF-7/casp-3 cells (Fig. 3B). Processing of both caspases directly correlated with their activation profile, as demonstrated by the cleavage of the fluorogenic caspase-3 and caspase-8 substrates DEVD-AMC and IETD-AMC, respectively (Fig. 3C). Please note that in MCF-7/casp-3/casp-10b cells both caspases reached their maximum activity after a 4-h treatment with 25 ng of TRAIL/ml and declined thereafter, whereas only a marginal caspase-3 and caspase-8 activity was observed in similarly treated MCF-7/casp-3 cells. In contrast, a significant activation of caspase-3 and caspase-8 by 25 ng of TNF/ml was only achieved at later time points (between 8 and 12 h), which was, however, similar in both cell lines (Fig. 3B and C). Thus, expression of caspase-10b appears to sensitize these cells to TRAIL-induced apoptosis by reducing the threshold of TRAIL concentrations required to activate caspase-3 and caspase-8.
FIG. 3.
Accelerated processing and activation of caspase-8 and caspase-3 in MCF-7/casp-3/casp-10b cells, specifically in TRAIL-mediated signaling. (A) Western blot analyses for the status of the caspase-8 proform (upper panel) and the active p17 subunit of caspase-3 (lower panel) in extracts of MCF-7/casp-3 and MCF-7/casp-3/casp-10b cells treated for 4 h with the indicated concentrations of TRAIL. (B) Western blot analyses for the status of the caspase-8 proform (upper panels) and caspase-3 (lower panels) in extracts of MCF-7/casp-3 (left panels) and MCF-7/casp-3/casp-10b cells (right panels) treated for the indicated times with TNF or TRAIL at a concentration of 25 ng/ml. The protein band that is marked with an asterisk is of unknown origin and serves as a loading control. (C) Caspase-3 (DEVDase) and caspase-8 (IETDase) enzymatic activities in extracts of MCF-7/casp-3 and MCF-7/casp-3/casp-10b cells treated for the indicated times with TNF or TRAIL at a concentration of 25 ng/ml.
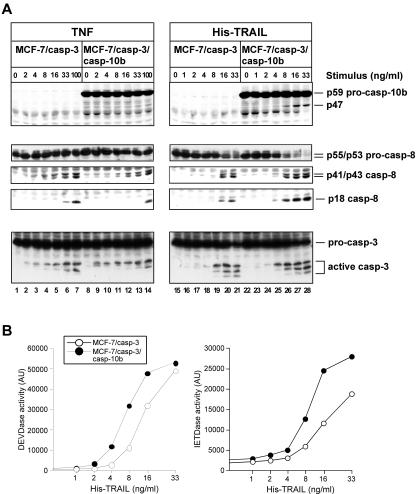
His-tagged TRAIL also accelerates caspase-3 and caspase-8 processing in a caspase-10-dependent manner.
As TRAIL signaling, at least in some cases (50), can depend on the TRAIL preparations used, we performed similar experiments using a His-tagged TRAIL preparation. Initial experiments revealed that His-TRAIL displays an approximately three- to fourfold higher activity than LZ-TRAIL in our system (data not shown). This might be explained by the fact that His-TRAIL tends to form higher-molecular-weight aggregates, rendering it more potent than LZ-TRAIL, which only forms trimers (50). Nevertheless, dose-response analyses confirmed the results obtained before (Fig. 3) and revealed that caspase-10b-expressing MCF-7/casp-3/casp-10b cells required much lower TRAIL concentrations to efficiently activate caspases than did caspase- 10b-deficient MCF-7/casp-3 cells (Fig. 4A). Whereas processing of caspase-3 and caspase-8 into their active subunits was readily detectable in MCF-7/casp-3/casp-10b cells by using as little as 4 ng of His-TRAIL/ml, a two- to fourfold higher concentration of His-TRAIL was required to achieve comparable effects in caspase-10b-deficient MCF-7/casp-3 cells (Fig. 4A, right panels). Noteworthy, even higher His-TRAIL concentrations such as 8 or 16 ng/ml were less effective in caspase- 10-deficient MCF-7/casp-3 cells, as judged by the lower levels of active caspase-3 and caspase-8 fragments and a less processed procaspase-8 compared to those in MCF-7/casp-3/casp-10b cells (Fig. 4A, right panels). We also analyzed the activation profile of caspase-10. Similar to the processing of caspase-8, activation of caspase-10b starts with the proteolytic cleavage of the small p12 subunit, resulting in the appearance of an intermediate p47 product that consists of the prodomain and the large (p17) catalytic subunit. Caspase-10b processing into the p47 fragment was only observed in His-TRAIL-stimulated MCF-7/casp-3/casp-10b cells (Fig. 4A, upper right panel) but not when the cells were treated with TNF (Fig. 4A, upper left panel). In addition, both cell lines responded in a similar dose-dependent manner to TNF with the processing of caspase-8 and caspase-3 (Fig. 4A, left panels), supporting our hypothesis that caspase-10 might play a role in TRAIL but not in TNF signaling.
FIG. 4.
Accelerated caspase activation in MCF-7/casp-3/casp-10b cells is also induced by His-tagged TRAIL. (A) Western blot analyses for processing of caspase-10, caspase-8, and caspase-3 in MCF-7/casp-3 and MCF-7/casp-3/casp-10b cells treated with the indicated concentrations of TNF or His-TRAIL. Because TRAIL signaling proceeds with faster kinetics, TRAIL-treated cells were harvested after 4 h, whereas extracts of TNF-stimulated cells were prepared after 8 h. (B) The same extracts that were used in panel A for immunoblotting were analyzed for caspase- 3 (DEVDase) and caspase-8 (IETDase) enzymatic activity. AU, arbitrary units.
Analyses of the caspase activation profiles using the fluorogenic substrates confirmed the results obtained by immunoblotting and showed, over the entire concentration range, an activation of both caspase-3 and caspase-8 in His-TRAIL-stimulated MCF-7/casp-3/casp-10b cells that was reproducibly stronger than in similarly treated MCF-7/casp-3 cells (Fig. 4B). Again, TNF induced a similar degree of caspase-3 and caspase-8 activation in both cell lines at all concentrations tested (data not shown).
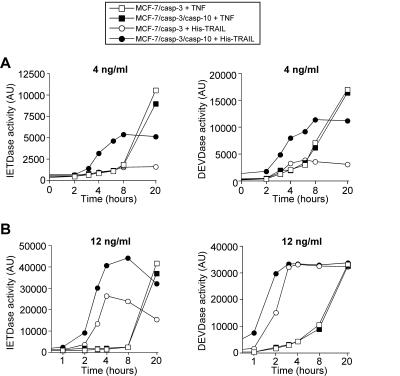
Caspase-10 did not only lower the threshold of caspase activation but also the kinetics of their activation profile in response to His-TRAIL. Similar to that observed with LZ-TRAIL, caspase-3 and caspase-8 activation occurred much faster and with a greater efficiency in MCF-7/casp-3/casp-10b cells than in their caspase-10-deficient counterparts (Fig. 5). This was particularly evident at a low His-TRAIL concentration (Fig. 5A) but also when the cells were treated with His-TRAIL at a concentration that is able to induce caspase activation in the absence of caspase-10 (Fig. 5B). Again, the kinetics of caspase-3 and caspase-8 activation in response to TNF were remarkably similar in both cell lines (Fig. 5). Altogether, these results indicate that the sensitizing effect of caspase-10 is also evident with TRAIL versions that have different capabilities of receptor oligomerization.
FIG. 5.
Kinetics of caspase activation in caspase-10-expressing and -deficient MCF-7/casp-3 cells. MCF-7/casp-3 and MCF-7/casp-3/casp-10b cells were treated with TNF or His-TRAIL at concentrations of 4 ng/ml (A) or 12 ng/ml (B). After the indicated time points, cell extracts were prepared and analyzed for caspase-3 (DEVDase) and caspase-8 (IETDase) activity. AU, arbitrary units.
Expression of caspase-10b does not alter the expression levels of the TRAIL receptors.
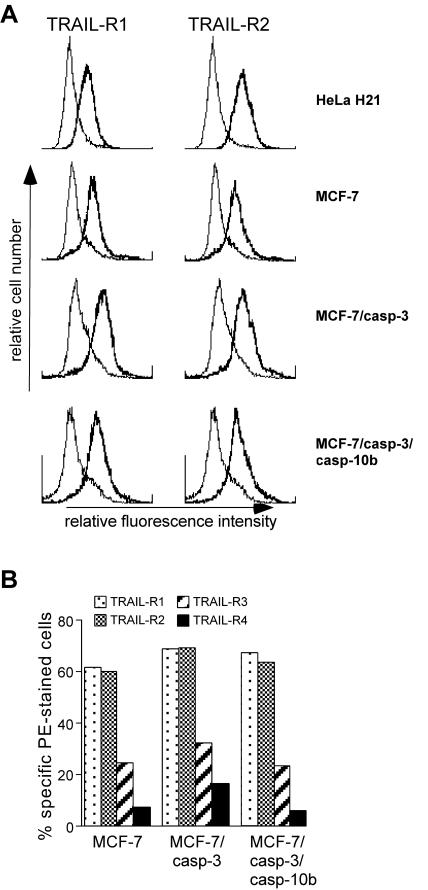
TRAIL signaling is mediated by two receptors, TRAIL-R1 and TRAIL-R2, which upon stimulation initiate DISC formation (14, 27). In addition, many cells also express two so-called decoy receptors (TRAIL-R3 and TRAIL-R4) that are unable to transduce signals due to the lack of a functional intracellular death domain. To investigate whether introduction of the CASP-10 gene resulted in an alteration of TRAIL receptor expression, which then might explain the observed sensitization effect, the various cell lines including the TRAIL-sensitive HeLa H21 cell line were analyzed by surface staining for the expression of the individual TRAIL receptors. However, TRAIL-R1 and TRAIL-R2 were almost equally expressed within one cell line and, more importantly, their expression levels did not differ significantly between MCF-7, MCF-7/casp-3, and MCF-7/casp-3/casp-10b cells (Fig. 6A). In addition, no significant differences between these cells were observed with regard to the expression levels of TRAIL-R3 and TRAIL-R4 that were, however, not as abundantly expressed as the two apoptosis-inducing TRAIL receptors (Fig. 6B). Together, these data indicate that the observed acceleration of TRAIL signaling in MCF-7/casp-3/casp-10b cells is not caused by an altered expression of any of the TRAIL receptors.
FIG. 6.
Similar expression levels of agonistic and antagonistic TRAIL receptors in HeLa H21, MCF-7, MCF-7/casp-3, and MCF-7/casp-3/casp-10b cells. (A) Fluorescence-activated cell sorter analysis showing expression of TRAIL-R1 (left panels, thick line) and TRAIL-R2 (right panels, thick line) of the indicated cell lines. The thin line represents the IgG control. (B) Quantitative analysis of the expression of the individual TRAIL receptors as indicated by the number of specifically stained phycoerythrin (PE)-positive cells.
Both caspase-10 isoforms, 10a and 10b, sensitize caspase- 3-proficient, but not caspase-3-deficient, MCF-7 cells to TRAIL-induced apoptosis.
Next, we examined the possibility that the observed acceleration of TRAIL signaling by caspase- 10b is due to a clonal artifact. In addition, we wanted to determine whether caspase-10a can substitute for caspase-10b and whether either isoform can accelerate TRAIL killing also in caspase-3-deficient MCF-7 cells. For this purpose, we generated MCF-7 and MCF-7/casp-3 clones that stably express either caspase-10a or caspase-10b in the absence or presence, respectively, of caspase-3. Several individual clones were selected and analyzed for their expression levels of the two caspase-10 isoforms by immunoblotting (Fig. 7A). Both caspase-10b- and caspase-10a-expressing MCF-7/casp-3 clones, M3B31 and M3A28, respectively, were significantly more sensitive towards TRAIL-induced apoptosis than were caspase-10-deficient MCF-7/casp-3 cells (Fig. 7B). This observation not only confirms our previous results but also demonstrates that acceleration of TRAIL-induced apoptosis can be mediated by either caspase-10 isoform. Interestingly, caspase-3-deficient MCF-7 cells expressing caspase-10a or caspase-10b were as resistant to TRAIL killing as parental MCF-7 cells (Fig. 7C), implying the requirement for caspase-3 in this process. On the other hand, when expressed in MCF-7/casp-3 cells, the apoptosis sensitizing effect of the caspase-10 isoforms was only observed in TRAIL signaling but not in TNF signaling. When compared to their respective parental MCF-7 lines, the caspase-10-expressing MCF-7 clones were killed by TNF to the same extent in the absence or presence of caspase-3 (data not shown).
So far, our data not only provide strong evidence that caspase-10 specifically enhances TRAIL-induced apoptosis, they also suggest that caspase-3 might be required for this effect (Fig. 1B and 7C). This view was further supported by our finding that 25 ng of LZ-TRAIL/ml, a concentration that is not sufficient to activate caspase-8 in MCF-7 (data not shown) or MCF-7/casp-3 cells, also failed to activate this initiator caspase in the caspase-3-deficient but caspase-10b-proficient MCF-7/casp-10b clone (Fig. 7E). As shown before (Fig. 3C), activation of caspase-8 by this concentration of TRAIL was only achieved in MCF-7/casp-3/casp-10b cells, a cell line in which both caspases are present (Fig. 7E). In contrast, TNF treatment induced comparable levels of caspase-8-like activity in both MCF-7/casp-3 and MCF-7/casp-3/casp-10b cells, whereas activation of caspase-8 in parental MCF-7 (data not shown) and MCF-7/casp-10b cells was only observed at a later time point (Fig. 7D). Similar results were obtained when the processing of caspase-8 was assessed by Western blot analyses (data not shown). These results demonstrate that only caspase-3, but not caspase-10, is required for TNF-induced caspase-8 activation which is in line with our observation that caspase-10 specifically accelerates TRAIL-induced, but not TNF-induced, apoptosis. Also note in this experiment that in contrast to TNF, TRAIL signaling proceeded with much faster kinetics, as evidenced by the early induction of caspase-8-like proteolytic activity (Fig. 7E).
Modulation of caspase-10 expression affects TRAIL-R2 signaling.
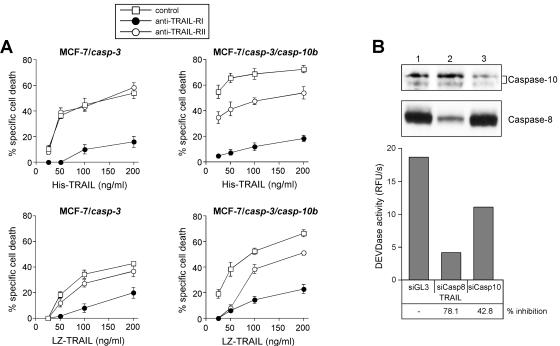
To analyze whether a particular TRAIL receptor is involved in the sensitizing activity of caspase-10, we performed cell death assays in the absence or presence of neutralizing antibodies to either TRAIL-R1 or TRAIL-R2. TRAIL-induced apoptosis was strongly inhibited in both cell lines regardless of the presence of caspase-10 when TRAIL-R1 was blocked by the neutralizing antibodies (Fig. 8A). In contrast, blocking TRAIL-R2 had no or only a marginal effect on TRAIL killing of caspase-10-deficient MCF-7/casp-3 cells but was able to block, at least partially, apoptosis of MCF-7/casp-3/casp-10b cells (Fig. 8A). As this was observed reproducibly with both TRAIL preparations, His-TRAIL and LZ-TRAIL, these data provide evidence that the sensitizing effect of caspase-10 is mediated via TRAIL-R2.
FIG. 8.
(A) TRAIL-R2 signaling is enhanced by caspase-10. Crystal violet-based cell death assessment of His-TRAIL (upper panels)- and LZ-TRAIL (lower panels)-induced apoptosis in the presence or absence of neutralizing antibodies to TRAIL-R1 and TRAIL-R2 (each 10 μg/ml). (B) Down-regulation of caspase-10 expression impairs TRAIL signaling in HeLa cells. HeLa cells were transiently transfected with either the control luciferase (GL3) siRNA (lane 1) or with caspase-8 (lane 2) or caspase-10 (lane 3) siRNA duplexes. Forty-eight hours following transfection, cells were treated for 1 h with TRAIL (0.1 μg/ml) and assayed by Western blotting for caspase-8 and caspase-10 expression (upper panel). The same lysates were assayed for DEVDase activity using the fluorometer in the kinetic mode (lower panel). RFU, relative fluorescence units.
So far, our results were obtained from overexpression studies in MCF-7 cells. In order to also verify the sensitizing role of caspase-10 in another cellular model system, we asked whether suppression of caspase-10 expression would impair TRAIL-induced caspase activation in HeLa cells. To this end, we employed the SMART-pool siRNA technology, as three individual caspase-10 siRNAs could not suppress expression of all caspase-10 isoforms and hence had no effect on TRAIL signaling (data not shown). However, when the SMART-pool caspase-10 siRNA was transiently transfected into HeLa cells, a significant reduction of the detectable caspase-10 isoforms was observed which correlated well with a 42.8% inhibition of TRAIL-induced DEVDase activity (Fig. 8B, lane 3). This inhibition was even more pronounced when the cells were treated with the caspase-8 siRNA (Fig. 8B, lane 2). Together, these results are in line with our previous data and show that caspase-10 is crucial for TRAIL signaling and that the sensitizing effect of caspase-10 is not only restricted to the MCF-7 cell system but can also be observed in HeLa cells.
Processing of caspase-10 is an early event in TRAIL-induced, but not in TNF-induced, apoptosis.
Next we performed DISC analyses using low TRAIL and TNF concentrations. However, all our attempts to precipitate DISC components failed (data not shown). This is most likely due to the fact that all DISC analyses are usually performed with microgram amounts of either ligand or receptor antibodies (24, 41). Hence, it is possible that very low nanogram levels of cytokines as used in the present study are not sufficient to pull down any of these components. A similar observation was also made earlier (M. Sprick, personal communication).
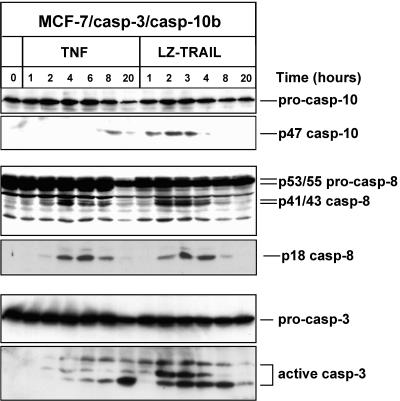
Nevertheless, we finally analyzed the kinetics of caspase activation in TNF- or TRAIL-treated MCF-7/casp-3/casp-10b cells. Treatment of MCF-7/casp-3/casp-10b cells with TRAIL resulted in an early activation of caspase-10b which was already detectable 1 h after stimulation, as demonstrated by the appearance of the p47 intermediate fragment (Fig. 9). Caspase-3 processing following TRAIL treatment occurred at approximately the same time, whereas activation of caspase-8, as evidenced by the appearance of the p41/43 fragments, was first detectable after 2 h. In contrast, when the cells were treated with TNF, caspase-10b activation was only observed at 8 h, a time point at which caspase-8 and caspase-3 were already activated (Fig. 9). Thus, although different affinities of the antibodies might account for some of the observed differences in the kinetics, our results indicate that activation of caspase-10 in TNF signaling occurs downstream of caspase-8 and caspase-3, whereas under the same conditions caspase-10 is a proximal caspase recruited to and processed at the TRAIL DISC.
FIG. 9.
Early processing of caspase-10 in TRAIL signaling but not in TNF signaling. Western blot analyses for the status of caspase-10, caspase-8, and caspase-3 in extracts of MCF-7/casp-3/casp-10b cells treated for the indicated times with TNF or TRAIL at a concentration of 25 ng/ml.
DISCUSSION
Although intensively studied recently, the role of caspase-10 in death receptor signaling remains obscure. Whereas a number of studies indicate that caspase-10 might be crucial for apoptosis signaling (29, 30, 33, 49, 56), other reports suggest that caspase-10 is not important or even not involved in signaling by death receptor ligands (4, 19, 41, 42). More recent reports, however, postulate that caspase-10 signals apoptosis through different death receptor systems (24, 28, 55). Moreover, the finding that inactive caspase-10 mutant proteins impaired apoptosis signaling by all death receptors (56) indicates that the various death receptors recruit identical signaling components including caspase-10 into their DISCs. However, the question remains why some tumor cell lines display different apoptosis susceptibilities to these death receptors (3, 29, 35, 46). Many different mechanisms can account for this phenomenon, including the expression of decoy receptors or other antiapoptotic proteins such as FLIP (FLICE-inhibitory protein) or members of the Bcl-2 and IAP (inhibitors of apoptosis protein) families (1, 50). However, in several studies, no correlation could be found between apoptosis susceptibility and expression of either of these proteins (10, 22, 30, 35, 58), implying the existence of additional mechanisms that affect death receptor pathways (54).
The results presented here provide strong evidence that caspase-10 might be a critical DISC component distinguishing death receptor-mediated signaling events especially at low cytokine concentrations. TRAIL-induced but not TNF-induced apoptosis and caspase activation were accelerated in a time- and dose-dependent manner in the presence of this initiator caspase. Furthermore, caspase-10 processing was found to be an early event in TRAIL signaling that was first observed 1 h following stimulation. In contrast, processing of caspase-10 induced by TNF was only evident at 8 h, a time point at which caspase-3 and caspase-8 were already activated. Thus, it appears that caspase-10 functions as an initiator caspase in TRAIL signaling but not in TNF signaling, at least in MCF-7 cells. Like caspase-10, caspase-3 was also found to be activated early (1 h) in TRAIL-induced apoptosis of MCF-7/casp-3/casp-10 cells, which is consistent with the role of caspase-10 as an initiator caspase capable of activating caspase-3 (12). On the other hand, processing and activation of caspase-8 induced by low TRAIL concentrations in these cells was only evident after 1.5 to 2 h, suggesting that under these conditions (low TRAIL concentrations) caspase-10 might be an important initiator caspase processed at the TRAIL DISC. Supportive evidence for our finding includes a recent study suggesting that caspase-10 is involved in spontaneous, but not in TNF-induced, apoptosis of neutrophils (13).
Our findings are consistent with a biophysical study performed with fluorescent resonance energy transfer analyses that revealed an interaction of FADD DED with caspase-10 DED that was much stronger than with caspase-8 DED (55). In addition, the TRAIL DISC of BJAB cells was shown to contain higher caspase-10 levels than the CD95 DISC, whereas either DISC recruited almost similar amounts of caspase-8 following stimulation with their respective ligands (41). It seems surprising that such a preferential recruitment of caspase-10 was not detected in more recent studies that thoroughly compared the composition of the CD95 and TRAIL DISCs (24, 41). It should be noted, however, that DISC analyses are generally performed with microgram concentrations of the respective death ligands. Thus, the reason for the missed detection of a preferential caspase-10 recruitment is probably due to a technical detail that makes is impossible to perform DISC analyses with such low cytokine concentrations as the nanogram range that was used in the present study (data not shown; also M. Sprick, personal communication).
Our data also suggest that the caspase-10-mediated sensitization to TRAIL-induced apoptosis which is not restricted to the MCF-7 system (impaired TRAIL signaling in HeLa cells transfected with caspase-10 siRNA) most likely proceeds via TRAIL-R2, as only neutralizing antibodies to this receptor impaired TRAIL killing in a caspase-10-dependent manner. This view is supported by recent DISC analyses demonstrating a substantially reduced recruitment of caspase-10, but not of caspase-8, in the presence of neutralizing TRAIL-R2 antibodies, whereas blocking of TRAIL-R1 reduced the recruited levels of both caspases to similar amounts (41). Based on the aforementioned finding that FADD interacts much more strongly with caspase-10 than with caspase-8 (55), it appears plausible that especially under the latter conditions in which almost equal amounts of caspase-8 and caspase-10 are recruited to the TRAIL-R2 DISC, the presence of caspase-10 might influence TRAIL sensitivity.
As mentioned above, caspase-8 activation with low LZ- or His-TRAIL concentrations (25 and 4 ng/ml, respectively) was only achieved in MCF-7/casp-3/casp-10b but not in caspase- 10-deficient MCF-7/casp-3 cells. This result suggests that either caspase-10 facilitates the recruitment of caspase-8 to the DISC or activation of caspase-8, at least under the conditions applied, is mediated downstream of the TRAIL DISC. As caspase-10 and caspase-8 are not able to activate each other in proximity (7), and because expression of caspase-10 in caspase- 3-deficient MCF-7 cells did not result in an enhanced caspase- 8 activation, this might argue for the second hypothesis. Thus, it appears that in our system using low TRAIL concentrations, caspase-3 is responsible for the DISC-independent activation of caspase-8, an event that was also observed recently in drug-induced apoptosis (11, 57). Such a scenario would be consistent with our finding that caspase-3 is essential for efficient TRAIL killing, as expression of this effector caspase alone was sufficient to increase TRAIL sensitivity of MCF-7 cells, at least partially. Moreover, the acceleration of TRAIL killing due to the expression of caspase-10 was only observed in the presence of caspase-3. Finally, the recent observation that Bax-deficient cells are completely resistant to TRAIL-induced apoptosis (9, 20, 26) not only suggests the requirement of mitochondrial events but might also explain the necessity for the presence of caspase-3. This is further emphasized by recent findings demonstrating that overexpression of Bcl-2 or Bcl-xL inhibited TRAIL-induced apoptosis, whereas this was only partially achieved with a dominant-negative caspase-8 construct (36).
There are also several other possibilities that could explain our observation of a preferential involvement of caspase-10 in TRAIL signaling but not in TNF signaling. During revision of the manuscript it was reported that, in contrast to the TRAIL receptors, apoptotic TNF-R1 signaling proceeds via two sequential complexes (28). Thus, following TNF stimulation, a first complex is formed at the cell membrane, which contains TNF-R1, the adapter protein TRADD, and the NF-κB activating signaling components RIP and TRAF2. Upon dissociation of TNF-R1 from complex I, a second different complex is formed in the cytosol, which recruits the apoptotic machinery such as FADD and caspase-8. Although caspase-10 was also found in the complex II, it is conceivable that caspase-8 and caspase-10 have different accessibilities for either the membrane-bound TRAIL receptor complexes or the cytosolic apoptosis-signaling complex of TNF. A more selective recruitment of both initiator caspases could also be affected by different signaling components in the signaling complexes as well as by still-unknown posttranslational modifications that have been found to occur. In addition, in some cell types, caspase-8 but not caspase-10 is localized at mitochondria or other subcellular compartments (6, 43; also our own unpublished observations), which might also differentially control the recruitment of both caspases to membrane versus cytosolic signaling complexes.
Together, our data provide strong evidence that especially under conditions where only limited TRAIL concentrations are present or when apoptosis is assessed at early time points, caspase-10 is crucial for TRAIL-induced apoptosis. Although abundantly expressed in fetal tissues (32), caspase-10 was found to be frequently mutated or down-regulated in multiple tumor cell lines, including breast cancer lines, further supporting our conclusion (15, 24, 34, 39, 55). Interestingly, a recent report demonstrated that TRAIL, but not CD95L, induced the activation of caspase-10 in colon carcinoma cells, which correlated well with the loss of the mitochondrial membrane potential and release of cytochrome c occurring with much faster kinetics in TRAIL- than in CD95L-induced apoptosis (48). However, it remains to be further elucidated whether the early caspase-10 recruitment also distinguishes TRAIL- from CD95-induced signaling.
Acknowledgments
We thank H. Walczak for the generous gift of LZ-TRAIL.
This work was supported by grants from the Interdisciplinary Center of Clinical Research of the University of Münster and the Deutsche Krebshilfe.
REFERENCES
- 1.Ashkenazi, A., and V. M. Dixit. 1999. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11:255-260. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi, A., R. C. Pai, S. Fong, S. Leung, D. A. Lawrence, S. A. Marsters, C. Blackie, L. Chang, A. E. McMurtrey, A. Hebert, L. DeForge, I. L. Koumenis, D. Lewis, L. Harris, J. Bussiere, H. Koeppen, Z. Shahrokh, and R. H. Schwall. 1999. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 104:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basile, J. R., V. Zacny, and K. Münger. 2001. The cytokines tumor necrosis factor-α (TNF-α) and TNF-related apoptosis-inducing ligand differentially modulate proliferation and apoptotic pathways in human keratinocytes expressing the human papillomavirus-16 E7 oncoprotein. J. Biol. Chem. 276:22522-22528. [DOI] [PubMed] [Google Scholar]
- 4.Bodmer, J.-L., N. Holler, S. Reynard, P. Vinciguerra, P. Schneider, P. Juo, J. Blenis, and J. Tschopp. 2000. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat. Cell Biol. 2:241-243. [DOI] [PubMed] [Google Scholar]
- 5.Chan, F., K.-M., R. M. Siegel, and M. J. Lenardo. 2000. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity 13:419-422. [DOI] [PubMed] [Google Scholar]
- 6.Chandra, D., G. Choy, X. Deng, B. Bhatia, P. Daniel, and D. G. Tang. 2004. Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol. Cell. Biol. 24:6592-6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, M., and J. Wang. 2002. Initiator caspases in apoptosis signaling pathways. Apoptosis 7:313-319. [DOI] [PubMed] [Google Scholar]
- 8.Daniel, P. T., T. Wieder, I. Sturm, and K. Schulze-Osthoff. 2001. The kiss of death: promises and failures of death receptors and ligands in cancer therapy. Leukemia 15:1022-1032. [DOI] [PubMed] [Google Scholar]
- 9.Deng, Y. T., Y. Lin, and X. Wu. 2002. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 16:33-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eggert, A., M. A. Grotzer, T. J. Zuzak, B. R. Wiewrodt, R. Ho, N. Ikegaki, and G. M. Brodeur. 2001. Resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Cancer Res. 61:1314-1319. [PubMed] [Google Scholar]
- 11.Engels, I. H., A. Stepczynska, C. Stroh, K. Lauber, C. Berg, R. Schwenzer, H. Wajant, R. U. Jänicke, A. G. Porter, C. Belka, M. Gregor, K. Schulze-Osthoff, and S. Wesselborg. 2000. Caspase-8/FLICE functions as an executioner caspase in anticancer drug-induced apoptosis. Oncogene 19:4563-4573. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes-Alnemri, T., R. C. Armstrong, J. Krebs, S. M. Srinivasula, L. Wang, F. Bullrich, L. C. Fritz, J. A. Trapani, K. J. Tomaselli, G. Litwack, and E. S. Alnemri. 1996. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc. Natl. Acad. Sci. USA 93:7464-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goepel, F., P. Weinmann, J. Schymeinsky, and B. Walzog. 2004. Identification of caspase-10 in human neutrophils and its role in spontaneous apoptosis. J. Leukoc. Biol. 75:836-843. [DOI] [PubMed] [Google Scholar]
- 14.Griffith, T. S., and D. H. Lynch. 1998. TRAIL: a molecule with multiple receptors and control mechanisms. Curr. Opin. Immunol. 10:559-563. [DOI] [PubMed] [Google Scholar]
- 15.Harada, K., S. Toyooka, N. Shivapurkar, A. Maitra, A. M. Reddy, H. Matta, K. Miyajima, C. F. Timmons, G. E. Tomlinson, D. Mastrangelo, R. J. Hay, P. M. Chaudhary, and A. F. Gazdar. 2002. Deregulation of caspase-8 and -10 expression in pediatric tumors and cell lines. Cancer Res. 62:5897-5901. [PubMed] [Google Scholar]
- 16.Jänicke, R. U., F. H. H. Lee, and A. G. Porter. 1994. Nuclear c-Myc plays an important role in the cytotoxicity of tumor necrosis factor alpha in tumor cells. Mol. Cell. Biol. 14:5661-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jänicke, R. U., X. Y. Lin, F. H. H. Lee, and A. G. Porter. 1996. Cyclin D3 sensitizes tumor cells to tumor necrosis factor-induced, c-Myc-dependent apoptosis. Mol. Cell. Biol. 16:5245-5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jänicke, R. U., M. L. Sprengart, M. R. Wati, and A. G. Porter. 1998. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 273:9357-9360. [DOI] [PubMed] [Google Scholar]
- 19.Juo, P., C. J. Kuo, J. Yuan, and J. Blenis. 1998. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 8:1001-1008. [DOI] [PubMed] [Google Scholar]
- 20.Kandasamy, K., S. M. Srinivasula, E. S. Alnemri, C. B. Thompson, S. J. Korsmeyer, J. L. Bryant, and R. K. Srivastava. 2003. Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res. 63:1712-1721. [PubMed] [Google Scholar]
- 21.Kawahara, A., Y. Ohsawa, H. Matsumura, Y. Uchiyama, and S. Nagata. 1998. Caspase-independent cell killing by Fas-associated protein with death domain. J. Cell Biol. 143:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keogh, S. A., H. Walczak, L. Bouchier-Hayes, and S. J. Martin. 2000. Failure of Bcl-2 to block cytochrome c redistribution during TRAIL-induced apoptosis. FEBS Lett. 471:93-98. [DOI] [PubMed] [Google Scholar]
- 23.Kischkel, F. C., D. A. Lawrence, A. Chuntharapai, P. Schow, K. J. Kim, and A. Ashkenazi. 2000. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 12:611-620. [DOI] [PubMed] [Google Scholar]
- 24.Kischkel, F. C., D. A. Lawrence, A. Tinel, H. LeBlanc, A. Virmani, P. Schow, A. Gazdar, J. Blenis, D. Arnott, and A. Ashkenazi. 2001. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J. Biol. Chem. 276:46639-46646. [DOI] [PubMed] [Google Scholar]
- 25.Krammer, P. H. 2000. CD95's deadly mission in the immune system. Nature 407:789-795. [DOI] [PubMed] [Google Scholar]
- 26.LeBlanc, H., D. Lawrence, E. Varfolomeev, K. Totpal, J. Morlan, P. Schow, S. Fong, R. Schwall, D. Sinicropi, and A. Ashkenazi. 2002. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat. Med. 8:274-281. [DOI] [PubMed] [Google Scholar]
- 27.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 28.Micheau, O., and J. Tschopp. 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114:181-190. [DOI] [PubMed] [Google Scholar]
- 29.Mitsiades, N., V. Poulaki, S. Tseleni-Balafouta, D. A. Koutras, and I. Stamenkovic. 2000. Thyroid carcinoma cells are resistant to Fas-mediated apoptosis but sensitive to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 60:4122-4129. [PubMed] [Google Scholar]
- 30.Mitsiades, N., V. Poulaki, C. Mitsiades, and M. Tsokos. 2001. Ewing's sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. Cancer Res. 61:2704-2712. [PubMed] [Google Scholar]
- 31.Nagata, S. 1997. Apoptosis by death factors. Cell 88:355-365. [DOI] [PubMed] [Google Scholar]
- 32.Ng, P. W., A. G. Porter, and R. U. Jänicke. 1999. Molecular cloning and characterization of two novel pro-apoptotic isoforms of caspase-10. J. Biol. Chem. 274:10301-10308. [DOI] [PubMed] [Google Scholar]
- 33.Pan, G., J. Ni, Y.-F. Wei, G. Yu, R. Gentz, and V. M. Dixit. 1997. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277:815-818. [DOI] [PubMed] [Google Scholar]
- 34.Park, W. S., J. H. Lee, M. S. Shin, J. Y. Park, H. S. Kim, J. H. Lee, Y. S. Kim, S. N. Lee, W. Xiao, C. H. Park, S. H. Lee, N. J. Yoo, and J. Y. Lee. 2002. Inactivating mutations of the caspase-10 gene in gastric cancer. Oncogene 21:2919-2925. [DOI] [PubMed] [Google Scholar]
- 35.Petak, I., L. Douglas, D. M. Tillman, R. Vernes, and J. A. Houghton. 2000. Pediatric rhabdomyosarcoma cell lines are resistant to Fas-induced apoptosis and highly sensitive to TRAIL-induced apoptosis. Clin. Cancer Res. 6:4119-4127. [PubMed] [Google Scholar]
- 36.Petak, I., R. Vernes, K. S. Szucs, M. Anozie, K. Izeradjene, L. Douglas, D. M. Tillman, D. C. Phillips, and J. A. Houghton. 2003. A caspase-8-independent component in TRAIL/Apo-2L-induced cell death in human rhabdomyosarcoma cells. Cell Death Differ. 10:729-739. [DOI] [PubMed] [Google Scholar]
- 37.Roth, W., S. Isenmann, U. Naumann, S. Kugler, M. Bahr, J. Dichgans, A. Ashkenazi, and M. Weller. 1999. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem. Biophys. Res. Commun. 265:479-483. [DOI] [PubMed] [Google Scholar]
- 38.Schulze-Osthoff, K., D. Ferrari, M. Los, S. Wesselborg, and M. E. Peter. 1998. Apoptosis signaling by death receptors. Eur. J. Biochem. 254:439-459. [DOI] [PubMed] [Google Scholar]
- 39.Shin, M. S., H. S. Kim, C. S. Kang, W. S. Park, S. Y. Kim, S. N. Lee, J. H. Lee, J. Y. Park, J. J. Jang, C. W. Kim, S. H. Kim, J. Y. Lee, N. J. Yoo, and S. H. Lee. 2002. Inactivating mutations of CASP10 gene in non-Hodgkin lymphomas. Blood 99:4094-4099. [DOI] [PubMed] [Google Scholar]
- 40.Smyth, M. J., E. Cretney, K. Takeda, R. H. Wiltrout, L. M. Sedger, N. Kayagaki, H. Yagita, and K. Okumura. 2001. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon γ-dependent natural killer cell protection from tumor metastasis. J. Exp. Med. 193:661-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sprick, M. R., E. Rieser, H. Stahl, A. Grosse-Wilde, M. A. Weigand, and H. Walczak. 2002. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. EMBO J. 21:4520-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sprick, M. R., M. A. Weigand, E. Rieser, C. T. Rauch, P. Juo, J. Blenis, P. H. Krammer, and H. Walczak. 2000. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12:599-609. [DOI] [PubMed] [Google Scholar]
- 43.Stegh, A. H., B. C. Barnhart, J. Volkland, A. Algeciras-Schimnich, N. Ke, J. C. Reed, and M. E. Peter. 2002. Inactivation of caspase-8 on mitochondria of Bcl-xL-expressing MCF7-Fas cells: role for the bifunctional apoptosis regulator protein. J. Biol. Chem. 277:4351-4360. [DOI] [PubMed] [Google Scholar]
- 44.Takeda, K., Y. Hayakawa, M. J. Smith, N. Kayagaki, N. Yamaguchi, S. Kakuta, Y. Iwakura, H. Yagita, and K. Okumura. 2001. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 7:94-100. [DOI] [PubMed] [Google Scholar]
- 45.Teitz, T., T. Wei, M. B. Valentine, E. F. Vanin, J. Grenet, V. A. Valentine, F. G. Behm, A. T. Look, J. M. Lahti, and V. J. Kidd. 2000. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat. Med. 6:529-535. [DOI] [PubMed] [Google Scholar]
- 46.Thomas, W. D., and P. Hersey. 1998. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J. Immunol. 161:2195-2200. [PubMed] [Google Scholar]
- 47.Thompson, C. B. 1995. Apoptosis in the pathogenesis and treatment of disease. Science 267:1456-1462. [DOI] [PubMed] [Google Scholar]
- 48.Velthuis, J. H., K. M. Rouschop, H. J. De Bont, G. J. Mulder, and J. F. Nagelkerke. 2002. Distinct intracellular signaling in tumor necrosis factor-related apoptosis-inducing ligand- and CD95 ligand-mediated apoptosis. J. Biol. Chem. 277:24631-24637. [DOI] [PubMed] [Google Scholar]
- 49.Vincenz, C., and V. M. Dixit. 1997. Fas-associated death domain protein interleukin-1β-converting enzyme 2 (FLICE2), an ICE/Ced-3 homologue, is proximally involved in CD95- and p55-mediated death signalling. J. Biol. Chem. 272:6578-6583. [DOI] [PubMed] [Google Scholar]
- 50.Wajant, H., K. Pfizenmaier, and P. Scheurich. 2002. TNF-related apoptosis inducing ligand (TRAIL) and its receptors in tumor surveillance and cancer therapy. Apoptosis 7:449-459. [DOI] [PubMed] [Google Scholar]
- 51.Walczak, H., and P. H. Krammer. 2000. The CD95 (Apo1/Fas) and the TRAIL (Apo-2L) apoptosis systems. Exp. Cell Res. 256:58-66. [DOI] [PubMed] [Google Scholar]
- 52.Walczak, H., R. E. Miller, K. Ariail, B. Gliniak, T. S. Griffith, M. Kubin, W. Chin, J. Jones, A. Woodward, T. Le, C. Smith, P. Smolak, R. G. Goodwin, C. T. Rauch, J. C. Schuh, and D. H. Lynch. 1999. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 5:157-163. [DOI] [PubMed] [Google Scholar]
- 53.Wallach, D., A. V. Kovalenko, E. E. Varfolomeev, and M. P. Boldin. 1998. Death-inducing functions of ligands of the tumor necrosis factor family: a Sanhedrin verdict. Curr. Opin. Immunol. 10:279-288. [DOI] [PubMed] [Google Scholar]
- 54.Wallach, D., T. U. Arumugam, M. P. Boldin, G. Cantarella, K. A. Ganesh, Y. Goltsev, T. M. Goncharov, A. V. Kovalenko, A. Rajput, E. E. Varfolomeev, and S. Zhang. 2002. How are the regulators regulated? The search for mechanisms that impose specificity on induction of cell death and NF-kappaB activation by members of the TNF/NGF receptor family. Arthritis Res. 4(Suppl. 3):S189-S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, J., H. J. Chun, W. Wong, D. M. Spencer, and M. J. Lenardo. 2001. Caspase-10 is an initiator caspase in death receptor signaling. Proc. Natl. Acad. Sci. USA 98:13884-13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, J., L. Zheng, A. Lobito, F. K.-M. Chang, J. Dale, M. Sneller, X. Yao, J. M. Puck, S. E. Straus, and M. J. Lenardo. 1999. Inherited human caspase-10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell 98:47-58. [DOI] [PubMed] [Google Scholar]
- 57.Wieder, T., F. Essmann, A. Prokop, K. Schmelz, K. Schulze-Osthoff, R. Beyaert, B. Dorken, and P. T. Daniel. 2001. Activation of caspase-8 in drug-induced apoptosis of B-lymphoid cells is independent of CD95/Fas receptor-ligand interaction and occurs downstream of caspase-3. Blood 97:1378-1387. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, X. D., A. Franco, K. Myers, C. Gray, T. Nguyen, and P. Hersey. 1999. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 59:2747-2753. [PubMed] [Google Scholar]