Abstract
Aims
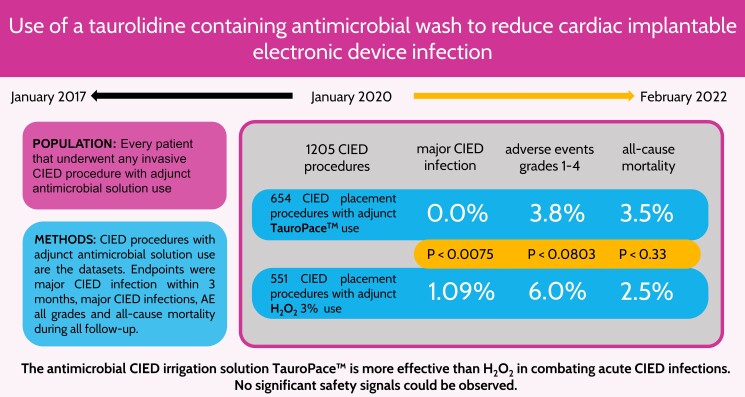
TauroPace (Tauropharm, Bavaria Germany), a taurolidine solution for combating cardiac implantable electronic device (CIED) infection, was compared with a historical control of 3% hydrogen peroxide (H2O2) in a prospective observational study.
Methods and results
The device pocket was irrigated, and all hardware accessible within (leads, suture sleeves, pulse generator) was wiped with H2O2, TauroPace, or taurolidine in a galenic formulation during any invasive CIED procedure at the study centre. Only CIED procedures covered by TauroPace or H2O2 from 1 January 2017 to 28 February 2022 were included for analysis. Patients who underwent >1 procedure were censored for the last treatment group and reassigned at the next procedure. The primary endpoint was major CIED infection within 3 months. The secondary endpoints were CIED infection beyond 3 months, adverse events potentially related to the antimicrobial solutions, CIED system, procedure, and death, till the end of follow-up. TauroPace covered 654 procedures on 631 patients, and H2O2 covered 551 procedures on 532 patients. The TauroPace group had more patient risk factors for infection than the H2O2 group (P = 0.0058) but similar device and procedure-specific risk factors (P = 0.17). Cardiac implantable electronic device infection occurred in 0/654 (0%) of the TauroPace group and 6/551 (1.1%) of the H2O2 group (P = 0.0075). Death occurred in 23/654 (3.5%) of the TauroPace group and 14/551 (2.5%) of the H2O2 group (P = 0.33). Non-infection related adverse events were rarer in the TauroPace (3.8%) than the H2O2 (6.0%) group (P = 0.0802).
Conclusion
TauroPace is safe but more effective than H2O2 in reducing CIED infection.
Clinical trial registration
ClinicalTrials.gov Identifier: NCT05576194
Keywords: Cardiac implantable electronic device, Infection, Taurolidine, Tauropace, Hydrogen peroxide
Graphical Abstract
Graphical Abstract.
What’s new?
The first clinical study researching a novel taurolidine containing antimicrobial solution, designed to prevent cardiac implantable electronic device (CIED) infections.
Compares a commercially available hydrogen peroxide 3% solution to the taurolidine containing antimicrobial solution.
Assesses the safety of the intervention with the antimicrobial solutions.
Introduces a novel bundle of techniques to enhance outcome after CIED procedures.
Introduction
Cardiac implantable electronic devices (CIEDs) are implanted in ≍1.2–1.4 million patients globally per annum.1 CIED infection rate is rising faster than the expansion in procedural volume.2,3 This may be due to CIED patients having more co-morbidities,2,3 receiving more complex systems,3,4 living longer, and requiring revisional procedures.4,5 In one ultra-long-term study,6 the cumulative probabilities of CIED infection were 6.2% at 15 years and 11.7% at 25 years, and 2.6, 2.7, and 24.1% after the first, second, and third procedures at 15 years. The hazard ratio (HR) for infection was 3.91 for pulse generator replacement and 3.08 for system upgrade. CIED infection generally requires complete explantation of all hardware.7–9 Lead extraction is a majorly invasive procedure associated with significant morbidities and potential mortality and requires specialist training and equipment.1 Contemporary studies put the financial costs of treating CIED infection at up to US$77 397 per patient and up to US$362 606 per patient with sepsis.10,11 Even after CIED infection has clinically been ‘cured’, patients continue to suffer excess mortality (≍50% higher relative risk) for at least 3 more years.12 Only pre-operative antibiotics13–15 and an antibiotic-eluting envelope16–20 but not instillation of antiseptic and antibiotic solutions into the pocket21–24 have been shown to be effective against CIED infection in randomized clinical trials and are recommended in the current guidelines.7
TauroPace™ (TP, Tauropharm, Bavaria, Germany) contains dissolved taurolidine, a derivative of the non essential amino-sulfonic acid taurine that breaks down in vivo to release chemically reactive species which destroy pathogens, impede surface adhesion, neutralize endotoxin and exotoxin, and promote wound healing.25–35 TP is versatile in its application and can be used to wash the outer surfaces of medical devices, flush their inner lumens, or irrigate the surgical site. The safety and efficacy of TP in combating CIED infection was compared against a historical control using 3% hydrogen peroxide (H2O2) for the same purpose in a prospective observational study.
Materials and methods
Study design
The study was a prospective observational registry comparing the incidences of CIED infection and other adverse events after any CIED procedure (de novo implantation, revision, upgrade or downgrade with or without lead involvement, generator replacement, system explantation with or without lead extraction) using TP as intra-operative antimicrobial solution adjunct (AMSA) against a historical control using H2O2 for the same purpose at a single centre. The CIED procedures and not the patients were the sampling units. Patients who underwent >1 CIED procedure during observation were deemed to have been censored for the initial treatment group and re-classified as new data units (with or without cross-over to the other treatment group) at the time of the second CIED procedure.
Study population
An AMSA was used intra-operatively in all CIED procedures at the study centre. Three agents had been used: H2O2, taurolidine in a galenic formulation, and TP. Initially, the AMSA choice was at the operator’s discretion. After January 2020, only TP was used. The TP group included all CIED procedures covered with TP: those performed from January 2020 to February 2022 were prospectively followed; those performed in 2019 (when TP was first introduced into the study centre) were retrospectively analysed. The H2O2 group included all CIED procedures covered with H2O2 from January 2017 to December 2019 and were retrospectively analysed. CIED procedures covered by galenic taurolidine were excluded from analysis as the agent has become commercially unavailable.
Procedural details
Multiple measures were incorporated into the standard of care at the study centre to minimize the risk of CIED infection and adverse events, in accordance with the best practices recommended in guidelines.7 Adjunct antibiotic-eluting mesh envelopes or similar devices are not used at the centre.
Pre-operative preparation
All patients had a physical examination, routine blood tests, an electrocardiogram (ECG), and an echocardiogram. Any pre-existent CIEDs were interrogated. Antibiotic prophylaxis (either cefuroxime 1500 mg or vancomycin 20 mg/kg) was given to all patients within 60–120 min before the commencement of the surgical procedure as a single dose as per the current guidelines.7 All procedures were performed in an electrophysiology laboratory with laminar airflow and restricted access during the procedure. Safety restraints were applied to the patient’s torso, legs, and arms under informed consent. Excessive chest hair was removed with an electric clipper as per the current guidelines.7 Defibrillation patches were applied to the patient’s chest in the anterior-posterior positions. Three ECG electrodes were applied to the patient’s limbs as far away from the surgical site as possible.
Anaesthesia and sedation
Local anaesthetic agents (e.g. ropivacaine 3 mg/kg plus clonidine 100 µg to extend action duration) were injected into anatomical fascial planes under ultrasound guidance and sterile conditions separately before the patient’s skin was prepared for the main CIED procedure. For a pre-muscular pocket, 15 cc of local anaesthetic agents was injected between the pectoralis major and minor (PECS I block).36 For a sub-muscular pocket, another 15 cc of local anaesthetic agents was injected between the pectoralis minor and the serratus anterior (PECS II block).37 For an inter-muscular pocket needed for subcutaneous implantable cardioverter defibrillator (S-ICD) procedures, an extra 15 cc of local anaesthetic agents was injected laterally between the serratus anterior and latissimus dorsi (serratus plane block).38 The parasternal intercostal spaces were instilled with the local anaesthetic agents if the procedure involved the S-ICD electrode. For sedation, the patient might receive no drugs, oral lorazepam, intravenous midazolam, or general anaesthesia (S-ICD procedures only).
Skin preparation
The patient’s skin from the mid-axillary line to the contralateral sternal border, both shoulders and upper arms, and the neck up to the earlobes were cleaned with an anti-septic solution (exclusively povidone iodine; chlorhexidine not used). The non-cleaned areas of the patient’s body were then covered with self-adhesive drapes. Adhesive iodophor-impregnated incision foils were not used. Skin preparation was exclusively performed by the physician operator, who double-gloved for the step and discarded the outer gloves afterwards. All surgical gowns, drapes, and gloves were disposable single-use.
Intra-operative use of antimicrobial solution adjunct
The AMSA was applied intra-operatively by: (i) immersing the hardware in the AMSA; (ii) using a swab saturated with the AMSA to wipe, wrap, and handle the hardware; (iii) flushing sheath/catheter lumens (TP only); and (iv) irrigating the surgical site with the AMSA. For S-ICD procedures, only TP was used as the AMSA. For all other types of CIED procedures, either TP or H2O2 was used. A total of 100 cc of AMSA were used per procedure.
For de novo implantation, the pocket was irrigated with the AMSA after sheath insertion into the target vein. The new lead was wiped with an AMSA swab before insertion. The suture sleeve was slid up and down the lead body through AMSA swab to ensure complete coverage of all surfaces. After the lead tip had been satisfactorily deployed, the sleeve was moved into position for fixation through an AMSA swab. After the sleeve had been fixed, the lead body, sleeve, sutures, and adjacent tissues were irrigated with 5 cc of the AMSA. The pulse generator was either immersed in the AMSA or wiped/wrapped with an AMSA swab. The connector pin and the pulse generator were handled through AMSA swabs during connection. The tip of the torque wrench was dipped in the AMSA before engaging the set screw. The pulse generator and attached leads were inserted into the pocket through AMSA swabs. The pocket (and all the hardware within) was irrigated with the AMSA and closed, employing a continuous absorbable suture for the deep layer (hypothesis: interrupted suture promotes suture granuloma formation) and continuous subdermal non-absorbable suture for the dermal layer. Interrupted suture with adjunct adhesive strips was rarely used.
For any CIED revision procedure (replacement, upgrade, downgrade), the incision was made cranial to the pre-existent pulse generator to avoid damaging the hardware within. The pocket was irrigated with the AMSA as soon as it was opened in case there was any potential dormant bacterial colonization.39 The pulse generator was mobilized and extracted out of the pocket through an AMSA swab. The torque wrench was dipped in the AMSA before and discarded immediately after use. The connector pins and accessible segments of the pre-existent leads were wiped with AMSA swabs. The fibro-collagenous capsule of the pocket was not removed (i.e. no attempt at capsulectomy as per the current guidelines7) and irrigated with 5 cc of the AMSA instead. Any new leads and pulse generator were prepared and handled with the AMSA as for de novo implantation. A new torque wrench pre-dipped in the AMSA was used to connect the connector pins of all leads (old or new) to the pulse generator. Pulse generator handling and pocket irrigation were for de novo implantation.
S-ICD implantation was performed exclusively with the two-incision technique and TP as the AMSA.40 The S-ICD lead was prepared and handled with TP as for a transvenous lead. The tunnelling tools (long and short) were wiped with TP swabs, and the introducer sheaths flushed with TP before insertion. After the long tunnelling tool had been passed between the axillary and the xiphoid incisions, the lead was attached to the long tunnelling tool with a suture and pulled out of the xiphoid incision under a TP swab. The shock coil segment of the lead was wrapped with a TP swab before placement. After the tunnelling tool sheath assembly had been inserted into the intended parasternal position, the tunnelling tool was withdrawn under a TP swab holding the sheath in place. The sheath lumen was flushed with another 5 cc of TP. The S-ICD lead was unwrapped and inserted into the sheath, displacing the TP into the surrounding subcutaneous tunnel. The sheath was peeled under a TP swab holding the lead in place. The suture sleeve was fixed. The xiphoid incision was irrigated with 5 cc of TP and then closed. A suture was attached to the fascia of the serratus anterior for pulse generator attachment. The pocket was irrigated with 5 cc of TP. The S-ICD pulse generator was prepared, handled, and connected to the lead as for a transvenous CIED. The pocket was irrigated with 50 cc of TP and then closed with a specific skin closure device (ZIP skin closure, Stryker) after the fascial incision plane had been closed with interrupted absorbable suture in the deep wound ground.
Post-operative care
After the wound had been closed, the surgical site was re-cleaned with povidone iodine. A sterile surgical dressing was applied to cover the wound. Non-sterile pressure dressings were applied on top of the sterile dressing as necessary. Before discharge, the CIED and the wound were checked. The patient was instructed to monitor the surgical wound, not to shower directly over the wound (waterproof dressings provided by the hospital) for 14 days and not to take a full body bath, swim, carry heavy loads, or exercise for 6 weeks.
Follow-up
All patients were routinely reviewed in outpatient clinic at 1, 3, 6, and 12 months post-procedure, unless the procedure was a straightforward pulse generator replacement, in which case the first review would be at 3 months. Thereafter, patients would be reviewed annually. Whenever patients did not attend their clinic appointments, their CIEDs would be assessed remotely, and their clinical status ascertained from their primary cardiologists or general practitioners. If there were any concerns about a patient’s well-being or CIED, the patient would be contacted to attend clinic in person for complete assessment.
Operators’ experience
The CIED procedures were performed by four operators. For the purpose of the study, 3 operators who had each performed >1000 CIED procedures of all complexity before January 2017 were regarded as ‘experienced’, and the remaining operator who performed <50 procedures per year and <200 procedures over 3 years were regarded as ‘inexperienced’ and a potential procedure-related risk factor.
Endpoints and event ascertainment
The primary endpoint was ‘major’ CIED infection (as defined by the Novel 2019 CIED infection criteria7) within 3 months of the last CIED procedure. The secondary endpoints were death from any cause (a competing risk for major CIED infection) and adverse events attributable to the CIED procedure, AMSA, or the CIED system up to the last follow-up. A data safety committee comprising three investigators (S.B., B.B., and H.B.) adjudicated on all endpoint events. Suspected CIED infection was investigated as per the current guidelines: physical examination, ultrasound scan of the pocket (if no overt hardware protrusion), trans-thoracic and/or trans-oesophageal echocardiography, full blood count, and a minimum of three sets of blood cultures drawn from different sites.7 If management involved CIED re-intervention (including system explantation and lead extraction), swabs and tissue specimens from within the pocket were sent for microbiology examination.
Statistical analysis
In order to generate hypotheses from this consecutive case series, the while-on-treatment strategy was followed to handle intercurrent events. Patients were analysed as treated and only until an intercurrent event changed treatment or prevented observation of the endpoint. The primary estimand was the difference of proportions (RD for risk difference) of patients with a major CIED infection while alive and unrevised within 3 months between CIED washed with H2O2 and CIED washed with TP. A secondary estimand was the HR of rates of major CIED infections while alive, unrevised and followed-up between CIED washed with H2O2 and CIED washed with TP. Revision and death were considered as censoring observations, accordingly. Only when cumulative incidence functions were estimated, major CIED infection and death were considered as competing risks. Because of the observational character of the clinical cohort study and the obvious propensity for estimates to be biased by the earlier treatment policy and cohort effects like the SARS-CoV-2 pandemic, estimates were adjusted. As there is evidence for numerous risk factors for major CIED infections, and as very few such events could be observed, adjustment was for the number of risk factors present at treatment. The calculation of 95% confidence intervals (CIs) was done by methods based on the score function. Other estimands were estimated using the principle of balancing weights.41 To that end, the propensity to be in one of the cohorts was estimated by logistic regression on patient, CIED, and procedure-related risk factors in their metric form, if any, e.g. procedure duration and age (see supplementary material online, TableS1). The average treatment effect in the untreated (ATU) standardizes on the population treated with H2O2, and the average treatment effect of the treated (ATT) standardizes on the population who received TP. The average treatment effect of the eligible standardizes on the union of both cohorts, while the average treatment effect in the overlap (ATO) standardizes on patients with a risk profile that actually occurred in both cohorts. The ATO answers the question that would be better answered by a randomised controlled trial. Secondary endpoints were analysed similarly. Exploratory analyses were logistic regressions. A set of risk factors was screened for predictive value by including them in the model one at a time, sorting them by P-value and highlighting small P-values in a Bonferroni–Holm procedure.42 This was restricted to mortality, as there were too few events for the primary endpoint. Subgroup analyses were restricted to frequency tables by the low numbers of events. The software R (version 4) was used for statistical analyses.
Oversight
The study was approved by the ethics committee of the statutory health commission. The principal investigator (S.B.) and a study monitor (cardiologist) oversaw the conduct of the entire study and verified all data at source. All prospectively enrolled patients provided written informed consent for their participation. Consent was not required from patients whose data were retrospectively and anonymously analysed after initial acquisition under Article 27(4) of the Bavarian State Hospital Law.
Results
Study population
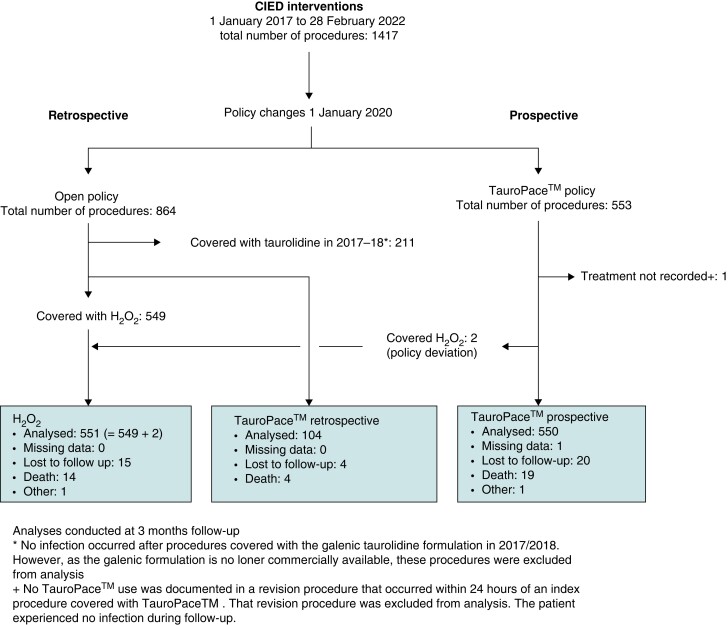
The TP group includes 654 procedures: 550 procedures (on 534 distinct patients) from the TP only policy period and 104 procedures (on 100 distinct patients; 9 procedures were early revisions crossed over from the H2O2 group within 3 months) from the open AMSA policy period (Figure 1). The H2O2 group includes 551 procedures: 549 procedures (on 531 distinct patients) from the open AMSA policy period and 2 procedures (on 2 distinct patients) from the TP only policy period (i.e. policy deviation). One procedure from the TP only policy period was excluded from analysis because the AMSA used was not recorded during revision within 24 h. (No CIED infection was observed during follow-up.) The 211 procedures covered by galenic taurolidine (used in the study centre from 2015 to 2018) during the open AMSA policy period were excluded from analysis as the agent was no longer commercially available. However, no infection was observed in the galenic taurolidine group during routine clinical follow-up outside the study. The baseline characteristics are mostly balanced between the two study groups (Table 1). The entire study population had a mean age of 73.3 (16–99) years and a 64%:36% male:female ratio.
Figure 1.
Enrolment, treatment, and follow-up of the CIED procedures. CIED, cardiac implantable electronic device.
Table 1.
Patient, procedure, and CIED-related risks for CIED infection as median (quartiles) and as counted for adjusted analyses sorted by risk
| H2O2 | TP | Difference | 95% CI | P-value | |
|---|---|---|---|---|---|
| N = 551 | N = 654 | ||||
| Duration | 45 (30; 70) | 41 (30; 51) | 4 | 2–7 | <0.001 |
| Lockdown procedure | 0 (0%) | 61 (9%) | |||
| CIED | 0.0011 | ||||
| PPM | 311 (56%) | 345 (53%) | |||
| ICD | 119 (22%) | 117 (18%) | |||
| CRT-P | 26 (5%) | 48 (7%) | |||
| CRT-D | 93 (17%) | 135 (21%) | |||
| S-ICD | 0 (0%) | 9 (1%) | |||
| CCM | 2 (0%) | 0 (0%) | |||
| Age (years) | 77 (69; 82) | 76 (67; 82) | 1 | 0–2 | 0.155 |
| BMI | 27.3 (24.2; 30.8) | 27.5 (24.8; 30.5) | −0.155 | −0.777 to 0.293 | 0.350 |
| CIED procedure | 0.452 | ||||
| New placement | 385 (70%) | 434 (66%) | |||
| Downgrade | 5 (1%) | 9 (1%) | |||
| Upgrade | 37 (7%) | 61 (9%) | |||
| Generator replacement | 95 (17%) | 117 (18%) | |||
| Early revision | 29 (5%) | 33 (5%) | |||
| Leads implanted | 2 (1; 2) | 2 (1; 2) | 0 | 0–0 | 0.508 |
| Risk factors | 4 (3; 5) | 4 (3; 6) | 0 | −1 to 0 | 0.0042 |
| Patient risk factors | 3 (2; 4) | 3 (2; 5) | 0 | 0–0 | 0.0058 |
| Immunosuppression | 29 (5.26%) | 78 (11.9%) | −6.66% | −9.83 to −3.55% | 6 × 10−5 |
| Pocket haematoma | 16 (2.9%) 9 revised | 37 (5.66%) 6 revised | −2.75% | −5.11 to −0.45% | 0.0202 |
| Dialysis | 4 (0.726%) | 14 (2.14%) | −1.41% | −2.93 to −0.05% | 0.0437 |
| Diabetes | 162 (29.4%) | 227 (34.7%) | −5.31% | −10.5 to 0.00% | 0.0498 |
| Skin disease | 32 (5.81%) | 52 (7.95%) | −2.14% | −5.03 to 0.77% | 0.146 |
| Age <65 | 102 (18.5%) | 140 (21.4%) | −2.89% | −7.39 to 1.66% | 0.212 |
| Male gender | 342 (62.1%) | 428 (65.4%) | −3.37% | −8.83 to 2.07% | 0.225 |
| Neoplasia | 78 (14.2%) | 108 (16.5%) | −2.36% | −6.42 to 1.76% | 0.259 |
| Heart failure | 358 (65%) | 405 (61.9%) | 3.05% | −2.42 to 8.47% | 0.274 |
| Acute renal failure | 48 (8.71%) | 67 (10.2%) | −1.53% | −4.85 to 1.84% | 0.365 |
| Anticoagulation/dual anti-platelet therapy | 349 (63.3%) | 427 (65.3%) | −1.95% | −7.39 to 3.47% | 0.483 |
| Chronic renal insufficiency | 230 (41.7%) | 283 (43.3%) | −1.53% | −7.11 to 4.08% | 0.593 |
| Chronic obstructive pulmonary disease | 47 (8.53%) | 59 (9.02%) | −0.491% | −3.69 to 2.79% | 0.764 |
| Previous implant infection | 6 (1.09%) | 6 (0.917%) | 0.171% | −1.05 to 1.54% | 0.765 |
| Procedure/CIED risks | 0 (0; 1) | 1 (0; 1) | −1 | 0–0 | 0.171 |
| Inexperienced | 17 (3.09%) | 149 (22.8%) | −19.7% | −23.3–−16.2% | 7.8 × 10−12 |
| Procedure >59 min | 169 (30.7%) | 121 (18.5%) | 12.2% | 7.31–17.1% | 4.1 × 10−4 |
| Leads abandoned | 26 (4.72%) | 53 (8.1%) | −3.39% | −6.19 to −0.60% | 0.0181 |
| Temporary pacing | 7 (1.27%) | 17 (2.6%) | −1.33% | −3.01 to 0.29% | 0.069 |
| revision, any | 151 (27.4%) | 151 (23.1%) | 4.32% | −0.60 to 9.27% | 0.085 |
| >2 leads handled | 41 (7.44%) | 62 (9.48%) | −2.04% | −5.19 to 1.16% | 0.207 |
BMI, body mass index; CCM, cardiac contractility modulation; CIED, cardiac implantable electronic device; CRT-D, implantable cardioverter defibrillator able to deliver cardiac resynchronization therapy; CRT-P, permanent pacemaker able to deliver cardiac resynchronization therapy; H2O2, hydrogen peroxide 3%; ICD, implantable cardioverter defibrillator; PPM, permanent pacemaker; S-ICD, subcutaneous implantable cardioverter defibrillator; TP, TauroPace.
Follow-up
The entire follow-up exceeded 36 months (median 15 months). Twenty-four patients in the TP group and 15 patients in the H2O2 group were lost to follow-up during the course of the study (Figure 1). However, 96.7% of the study population completed at least 3 months of follow-up.
Patient risk factors
The TP group had a higher prevalence of four patient risk factors for CIED infection than the H2O2 group (Table 1), including immunosuppression (medications such as corticosteroid, methotrexate, mycophenolic acid or diseases such as acquired immunodeficiency syndrome, connective tissue disease, vasculitis), haematoma, dialysis, and diabetes. The higher incidence of haematoma in the TP group (6%) than in the H2O2 group (3%) might be due to closer prospective surveillance of the former as the prevalence of anticoagulation/anti-platelet therapy was similar and the majority (84%) of haematoma in the TP group was minor and spontaneously resolved. Only 16% (6/37) required revision in the TP group, compared with 56% (9/16) in the H2O2 group. (Three of the nine revisions from the H2O2 group were covered with TP.) The differences in the prevalence of dialysis and diabetes become statistically insignificant after adjusting for multiple testing. Other known patient factors for CIED infection were balanced between the two groups.
Procedure and device-related risk factors
CIED types and CIED procedure types were similar between the two groups (Table 1). The procedure duration was shorter in the TP group (mean 46.6 min; median 41 min) than in the H2O2 (mean 50.0 min; median 45 min) group. Inexperienced operator was a much more prominent procedural risk factor in the TP group (22.8%) than in the H2O2 group (3.1%). The joint distribution of patient and procedure and device-related risk factors is shown in supplementary material online, Figure S1. The distributions of these numbers conditional on treatment group are shown in supplementary material online, Figure S2.
Primary endpoint
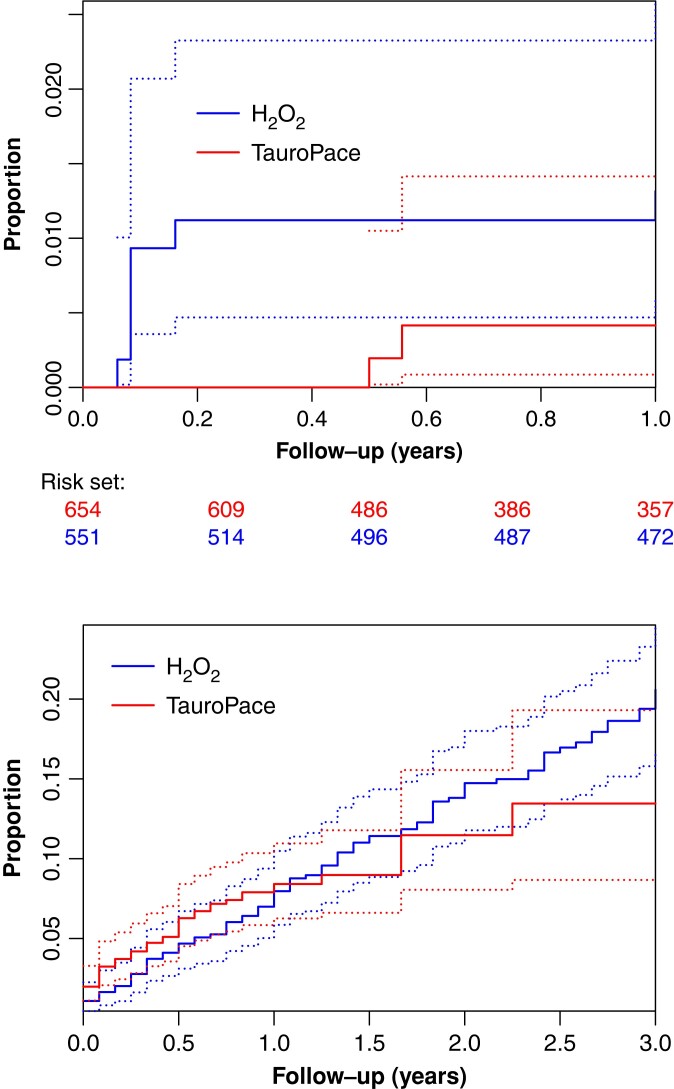
CIED infection was less common in the TP group than in the H2O2 group over the entire follow-up (Figure 2). In the first 3 months after the last procedure, the TP group experienced no major CIED infection (i.e. resulting in invasive revision, system explantation, chronic antibiotic treatment, or death), whereas the H2O2 group saw six major CIED infections (Table 2), a statistically significant absolute risk difference of 1.09% (95% CI 0.4–2.35%, P = 0.0075).
Figure 2.
Cumulative incidence curves for major CIED infection (top) and death (bottom) by cohort (H2O2, TauroPace™) with pointwise CIs (broken lines) as unadjusted competing risk estimates. CIED, cardiac implantable electronic device; CI, confidence interval.
Table 2.
Major CIED infections (3 months follow-up)
| Sex | Age | Device | Procedure time (minutes) | Time to onset (days) | Pathogen | Risk factors |
|---|---|---|---|---|---|---|
| Male | 81 | CRT-D | 164 | 22 | Staphylococcus epidermidis | Haematoma, renal insuff., oral anticoagulation, neoplasia, heart insuff., long procedure, male gender, long procedure |
| Male | 61 | ICD | 50 | 25 | Staphylococcus epidermidis | Haematoma, heart insuff., male gender, younger age |
| Male | 81 | VVI-R | 47 | 12 | Staphylococcus aureus | Haematoma, early revision due to lead dislodgement, skin disorder, COPD, heart insuff., oral anticoagulation (Afib), Diabetes, male gender |
| Male | 83 | VVI-R | 52 | 59 | Staphylococcus aureus | Chronic renal insufficiency, oral anticoagulation (Afib), heart insuff., diabetes, male gender |
| Female | 83 | DDD-R | 31 | 7 | Staphylococcus epidermidis, ConS | Acute on chronic renal failure, malignancy, upgrade revision |
| Female | 73 | CRT-D | 92 | 12 | Staphylococcus epidermidis | Chronic renal insuff., heart insuff. Diabetes, oral anticoagulation, long procedure |
All major CIED infections occurred in the H2O2 group.
Afib, atrial fibrillation; ConS; Coagulase-negative Staphylococci; COPD, chronic obstructive pulmonary disease; CRT-D, implantable cardioverter defibrillator able to deliver cardiac resynchronization therapy; DDD-R, dual-chamber rate modulated permanent pacemaker; ICD, implantable cardioverter defibrillator; insuff., insufficiency; VVI-R, single chamber (ventricular pacing) rate modulated permanent pacemaker.
Secondary endpoints
Over the remainder of the follow-up, the TP group had 3 major CIED infections and the H2O2 group had altogether 9 (Kaplan–Meier estimated event rate, 0.459 and 1.63%, respectively; HR, 0.41; 95% CI: 0.10–1.56; HR adjusted for number of risk factors, 0.37; 95% CI: 0.09–1.41, Table 3). The average treatment effect estimated with balancing weights (Table 4) strengthens this result. Major CIED infection was managed by complete system explantation. In only one major CIED infection in the TP group, could no pathogen be identified from device or pocket. Three common organisms were isolated from the remaining 2 (one lead related) and 9 (3 lead related) CIED infections in the TP and H2O2 groups.
Table 3.
Cox regressions from outcome on CIED AMSA
| Outcome | Adjusted for number of | HR (TauroPace™ over H2O2) | 95% CI |
|---|---|---|---|
| Major CIED infection | – | 0.408 | 0.107–1.56 |
| Major CIED infection | Risk factors | 0.365 | 0.095–1.41 |
| Mortality | – | 0.985 | 0.686–1.41 |
| Mortality | Patient risk factors | 0.894 | 0.621–1.29 |
Shown are estimates of HRs using cause-specific haards with and without adjustments.
AMSA, antimicrobial solution adjunct; CIED, cardiac implantable electronic device; HR, hazard ratio.
Table 4.
Cox regressions from outcome on CIED AMSA weighted by different functions of the propensity to belong to a cohort
| Estimand | Population | Question: should TP be … | HR* |
|---|---|---|---|
| (95% CI) | |||
| With respect to major CIED infection | |||
| ATU | Receiving H2O2 | … withheld from those who got it? | 0.451 (0.103–1.98) |
| ATT | Receiving TP | … extended to those who got H2O2? | 0.545 (0.113–2.62) |
| ATE | Union of both | … used in all eligible? | 0.495 (0.115–2.14) |
| ATO | Overlap of both | … used if undecided between the two? | 0.34 (0.069–1.68) | |
| With respect to all-cause mortality | |||
| ATU | Receiving H2O2 | … withheld from those who got it? | 0.846 (0.535–1.34) |
| ATT | Receiving TP | … extended to those who got H2O2? | 0.985 (0.658–1.47) |
| ATE | Union of both | … be used in all eligible? | 0.916 (0.619–1.35) |
| ATO | Overlap of both | … used if undecided between the two? | 1.07 (0.745–1.54) | |
Shown are estimates of HRs using cause-specific hazards with and without adjustments.
AMSA, antimicrobial solution adjunct; AT, average treatment effect; CIED, cardiac implantable electronic device; TP, TauroPace; U, ‘untreated’ (the H2O2 group); T, ‘treated’ (the TP group); E, ‘eligible’ (union of the H2O2 and TP groups); O, ‘overlap’ (intersection of the H2O2 and TP groups); HR, hazard ratio; CI, confidence interval.
System revisions were necessary in 29 (5%) patients in the H2O2 group and 33 (5%) patients in the TP group. The complication rates are in line with those reported in the current literature.18
Serious adverse effects attributable to either TP or H2O2 were not found. Adverse effects attributable to the CIED or procedure were fewer in the TP group than the H2O2 group (3.8 vs. 6.0%, difference 2.2%, 95% CI: −0.26 to 4.79%, P = 0.0803), but the difference did not reach statistical significance. Screening of the seven CIED and procedure-related risk factors for major CIED infection did not yield any statistically significant association with the adverse effects attributable to CIED or procedure (see supplementary material online, Table S2).
Death within 3 months post-index procedure occurred in 23/654 (3.5%) of the TP group and 14/551 (2.5%) in the H2O2 group (difference −1%, 95% CI: −2.98 to +1.05%, P = 0.33). Screening of the 15 patient risk factors for major CIED infection (see supplementary material online, Table S3) reveals renal impairment as the main driver for mortality. Screening was repeated with survival times explained by patient, procedure and device risk factors for the different estimands (see supplementary material online, Tables S3a to S3d). Prospective recruitment for the TP group coincided with the worldwide COVID-19 pandemic, which might also partially account for the higher mortality rate in the TP group.
Discussion
Cardiac implantable electronic device (CIED) infection is a major drawback of CIED therapy. Recent epidemiological data show a worrying trend towards higher incidence and potential under-detection of CIED infection.43,44 Certain patient-specific and procedure- or device-related risk factors predispose to CIED infection.11,45,46 Various measures have been explored to reduce CIED infection,24,47,48 but none has been consistently shown to be effective.23,49 The current guidelines do not recommend and even discourage antibiotic or antiseptic pocket irrigation during CIED procedures,7,43 but the clinical practice is well established and still commonly practised despite the absence of conclusive evidence for clinical benefit.18,47
TauroPace is designed to disinfect CIED hardware and the surgical site (pocket) and could be used as an AMSA during CIED procedures. The active ingredient of TP is taurolidine, a derivative of the non-essential amino acid taurine. In vivo, taurolidine breaks down into taurine amide and finally taurine and water. N-Methylol groups released during the process chemically react with the amino and hydroxyl groups of susceptible molecules in the cell wall of pathogens and certain toxins, denaturing the endotoxins and polysaccharide/lipopolysaccharide components in the cell wall of pathogens and deactivating susceptible exotoxins. These chemical changes stop pathogens from adhering to the surfaces of both inanimate objects and organic tissues.50,51 Even established biofilms could become disrupted.52 Clinical evidence showing the safety and efficacy of taurolidine in preventing infection of both organic and inanimate objects placed into the human body (including directly into the bloodstream) is rapidly accumulating.53–60 TauroPace has been successfully used to salvage infected CIEDs and prevent explantation.61–63
The TP group included patients at an elevated risk of death from co-morbidities, probably aggravated by the COVID pandemic. However, major CIED infection rate was lower in the TP group than in the H2O2 group throughout the study duration. The rates of major CIED infection beyond 1 year post-procedure of 1.63% for the H2O2 group and 0.46% for the TP group are comparable with or better than those reported in randomised controlled trials,17,18 without any concomitant rise in the complication (e.g. lead dislocation or malfunction, threshold elevation, haematoma, the twiddler syndrome) or system revision rates. In fact, when compared with the contemporary outcome results from other centres in the literature, the overall complication rate at the study centre was lower.18,64 This reflects a generally high performance standard at the study centre, even under extended and intensified scrutiny.
No adverse effects (e.g. allergy, anaphylaxis) was observed with the intra-operative use of TP adjunct in >600 CIED procedures over 4 years. The use of TP adjunct shortened the procedure duration by 4 min compared with H2O2. Moreover, the general procedure duration recorded at the study centre was also shorter than those reported in the current literature.18,64
Based on the study results, the number-needed-to-treat (NNT) with TP to prevent one major CIED infection compared with H2O2 is 92 (=1/1.09%). Given the high cost of treating major CIED infection, the relatively low NNT and cost of TP appears to justify its routine use in all CIED procedures, without the need to target selectivey cases deemed to be at extraordinarily high risk.65 This is a major clinical bonus for patients and operators alike.
Limitations
Comparing a prospectively and consecutively enrolled cohort with a retrospectively evaluated historic control may introduce biases. Physicians performed better when enrolling patients in a prospective manner. Inexperienced operators performed the CIED procedure faster during prospective enrolment in the study.
Intensity of surveillance might introduce bias. Compared with the retrospective H2O2 group, haematoma was observed more frequently but led to revision less frequently in the prospective TP group. Infections are hard to adjudicate retrospectively after death. However, undetected CIED infections in the retrospective H2O2 group would strengthen the superiority of TP.
Patient selection was unlikely to have influenced the outcome in the prospective cohort, as all procedures were covered with TP and included in analysis. In the retrospective cohort, patients deemed to be most at risk of CIED infection or death appear to have been treated with galenic taurolidine rather than H2O2. Such a bias would strengthen the superiority of TP.
The retrospective cohort was operated by two of the three senior physicians, who used H2O2. The third physician had started using galenic taurolidine before the start date of the retrospective observation period in January 2017. Operators’ skills might have been a confounding factor. However, 2/3 of the extract procedures in the TP group were performed by the early TP adopter before the policy change in January 2020.
Major CIED infections were rare, which makes it difficult to ascertain treatment effects.
Follow-up was censored at 3 months in many patients, especially in the TP group (enrolment started later). Differential follow-up might introduce bias to cumulative incidence functions in competing risks analysis, but not for cause-specific estimates of HR.
Conclusions
Intra-operative TP is more effective than H2O2 in reducing acute CIED infection in an observational study. No significant safety issues have been observed with TP use during all follow-up. The clinical utility of TP in reducing CIED infection deserves validation in large randomized trials.
Supplementary Material
Contributor Information
Stefan Borov, Department of Cardiology, Klinikum Freising, Alois-Steinecker-Straße 18, Freising 85354, Germany; Medical Faculty, Christian-Albrechts University, Christian-Albrechts-Platz 4, Kiel 24118, Germany.
Benito Baldauf, Medical Faculty, Christian-Albrechts University, Christian-Albrechts-Platz 4, Kiel 24118, Germany; Institute of Life Science, Hochschule Bremerhaven, An der Karlstadt 8, Bremerhaven 27568, Germany.
Jana Henke, Medical Faculty, Christian-Albrechts University, Christian-Albrechts-Platz 4, Kiel 24118, Germany.
Herribert Pavaci, Krankenhaus Landshut Achdorf, Achdorfer Weg 3, Landshut 84036, Germany.
Arben Perani, Krankenhaus Landshut Achdorf, Achdorfer Weg 3, Landshut 84036, Germany.
Bernhard Zrenner, Krankenhaus Landshut Achdorf, Achdorfer Weg 3, Landshut 84036, Germany.
Josef Dietl, Krankenhaus Landshut Achdorf, Achdorfer Weg 3, Landshut 84036, Germany.
Julinda Mehilli, Krankenhaus Landshut Achdorf, Achdorfer Weg 3, Landshut 84036, Germany.
Ernest W Lau, Department of Cardiology, Royal Victoria Hospital, Grosvenor Road, Belfast BT12 6BA, UK.
Reinhard Vonthein, Institut für Medizinische Biometrie und Statistik, Universität zu Lübeck, Ratzeburger Allee 160, Lübeck 23562, Germany.
Hendrik Bonnemeier, Medical Faculty, Christian-Albrechts University, Christian-Albrechts-Platz 4, Kiel 24118, Germany; Institute of Life Science, Hochschule Bremerhaven, An der Karlstadt 8, Bremerhaven 27568, Germany; Department of Cardiology, Helios Klinikum Cuxhaven, Altenwalder Ch 10, Cuxhaven 27474, Germany; Department of Cardiology, Helios Klinikum Wesermarsch, Mildred-Scheel-Straße 1, Nordenham 26954, Germany.
Supplementary material
Supplementary material is available at Europace online.
Funding
S.B.: research grant through his employer from the manufacturer of TauroPace™. B.B.: consultant/advisor: manufacturer of TauroPace™, Kimal plc, Kapamed, Crosstec, Bioline Supply, MCM Ag, Tauro-Implant, Medival SRL, Spectranetics, Abbott. J.M.: received a research grant through her employer from the manufacturer of TauroPace™. R.V.: research grant through his employer from the manufacturer of TauroPace™. Tauropharm funded open access for this manuscript.
Data availability
Upon reasonable request, the data are shared by the corresponding author without limitations.
References
- 1. Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo Ret al. . 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017;14:e503–51. [DOI] [PubMed] [Google Scholar]
- 2. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RTet al. . 16-year Trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011;58:1001–6. [DOI] [PubMed] [Google Scholar]
- 3. Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010;33:414–9. [DOI] [PubMed] [Google Scholar]
- 4. Olsen T, Jørgensen OD, Nielsen JC, Thøgersen AM, Philbert BT, Johansen JB. Incidence of device-related infection in 97 750 patients: clinical data from the complete Danish device-cohort (1982–2018). Eur Heart J 2019;40:1862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge Ret al. . Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation 2010;122:1553–61. [DOI] [PubMed] [Google Scholar]
- 6. Dai M, Cai C, Vaibhav V, Sohail MR, Hayes DL, Hodge DOet al. . Trends of cardiovascular implantable electronic device infection in 3 decades: a population-based study. JACC Clin Electrophysiol 2019;5:1071–80. [DOI] [PubMed] [Google Scholar]
- 7. Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MGet al. . European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace 2020;22:515. [DOI] [PubMed] [Google Scholar]
- 8. Sandoe JA, Barlow G, Chambers JB, Gammage M, Guleri A, Howard Pet al. . Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother 2015;70:325. [DOI] [PubMed] [Google Scholar]
- 9. Perrin T, Deharo JC. Therapy and outcomes of cardiac implantable electronic devices infections. Europace 2021;23:iv20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greenspon AJ, Eby EL, Petrilla AA, Sohail MR. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. Pacing Clin Electrophysiol 2018;41:495–503. [DOI] [PubMed] [Google Scholar]
- 11. Sohail MR, Eby EL, Ryan MP, Gunnarsson C, Wright LA, Greenspon AJ. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large US payer database 1-year post implantation. Circ Arrhythm Electrophysiol 2016;9:e003929. [DOI] [PubMed] [Google Scholar]
- 12. Sohail MR, Henrikson CA, Jo Braid-Forbes M, Forbes KF, Lerner DJ. Increased long-term mortality in patients with cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol 2015;38:231–9. [DOI] [PubMed] [Google Scholar]
- 13. de Oliveira JC, Martinelli M, Nishioka SA, Varejão T, Uipe D, Pedrosa AAet al. . Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009;2:29–34. [DOI] [PubMed] [Google Scholar]
- 14. Da Costa A, Kirkorian G, Cucherat M, Delahaye F, Chevalier P, Cerisier Aet al. . Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation 1998;97:1796–801. [DOI] [PubMed] [Google Scholar]
- 15. Malagù M, Vitali F, Brieda A, Cimaglia P, De Raffele M, Tazzari Eet al. . Antibiotic prophylaxis based on individual infective risk stratification in cardiac implantable electronic device: the PRACTICE study. Europace 2022;24:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sohail MR, Corey GR, Wilkoff BL, Poole JE, Mittal S, Kennergren Cet al. . Clinical presentation, timing, and microbiology of CIED infections: an analysis of the WRAP-IT trial. JACC Clin Electrophysiol 2021;7:50–61. [DOI] [PubMed] [Google Scholar]
- 17. Mittal S, Wilkoff BL, Kennergren C, Poole JE, Corey R, Bracke FAet al. . The world-wide randomized antibiotic envelope infection prevention (WRAP-IT) trial: long-term follow-up. Heart Rhythm 2020;17:1115–22. [DOI] [PubMed] [Google Scholar]
- 18. Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss Eet al. . Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019;380:1895–905. [DOI] [PubMed] [Google Scholar]
- 19. Chaudhry U, Borgquist R, Smith JG, Mörtsell D. Efficacy of the antibacterial envelope to prevent cardiac implantable electronic device infection in a high-risk population. Europace 2022;24:1973–80. [DOI] [PubMed] [Google Scholar]
- 20. Ziacchi M, Biffi M, Iacopino S, di Silvestro M, Marchese P, Miscio F. et al. Reducing infections through cardiac device envelope: insight from real world data. The REINFORCE project. Europace 2023. doi: 10.1093/europace/euad224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lakkireddy D, Pillarisetti J, Atkins D, Biria M, Reddy M, Murray Cet al. . IMpact of pocKet rEvision on the rate of InfecTion and other CompLications in patients rEquiring pocket mAnipulation for generator replacement and/or lead replacement or revisioN (MAKE IT CLEAN): a prospective randomized study. Heart Rhythm 2015;12:950–6. [DOI] [PubMed] [Google Scholar]
- 22. Kang FG, Liu PJ, Liang LY, Lin YQ, Xie SL, He Yet al. . Effect of pocket irrigation with antimicrobial on prevention of pacemaker pocket infection: a meta-analysis. BMC Cardiovasc Disord 2017;17:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krahn AD, Longtin Y, Philippon F, Birnie DH, Manlucu J, Angaran Pet al. . Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol 2018;72:3098–109. [DOI] [PubMed] [Google Scholar]
- 24. Apel D, Brunelli M, El-Gahry M, Geller JC, Lauer B, Ohlow MA. Effect of intraoperative local application of 3% hydrogen peroxide on pocket infections following cardiac implantable electronic device implantation: an observational study. Indian Pacing Electrophysiol J 2018;18:159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Browne MK, Leslie GB, Pfirrmann RW. Taurolin, a new chemotherapeutic agent. J Appl Bacteriol 1976;41:363–8. [DOI] [PubMed] [Google Scholar]
- 26. Pfirrmann RW, Leslie GB. The anti-endotoxin activity of Taurolin in experimental animals. J Appl Bacteriol 1979;46:97–102. [DOI] [PubMed] [Google Scholar]
- 27. Gidley MJ, Sanders JK, Myers ER, Allwood MC. The mode of antibacterial action of some ‘masked’ formaldehyde compounds. FEBS Lett 1981;127:228–32. [PubMed] [Google Scholar]
- 28. Gorman SP, McCafferty DF, Woolfson AD, Jones DS. Electron and light microscopic observations of bacterial cell surface effects due to taurolidine treatment. Lett Appl Microbiol 1987;4:103–9. [Google Scholar]
- 29. Blenkharn JI. Sustained anti-adherence activity of taurolidine (Taurolin) and noxythiolin (Noxyflex S) solutions. J Pharm Pharmacol 1988;40:509–11. [DOI] [PubMed] [Google Scholar]
- 30. Jacobi CA, Menenakos C, Braumann C. Taurolidine—a new drug with anti-tumor and anti-angiogenic effects. Anticancer Drugs 2005;16:917–21. [DOI] [PubMed] [Google Scholar]
- 31. Caruso F, Darnowski JW, Opazo C, Goldberg A, Kishore N, Agoston ESet al. . Taurolidine antiadhesive properties on interaction with E. coli; its transformation in biological environment and interaction with bacteria cell wall. PLoS One 2010;5:e8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dinçer S, Babül A, Erdoğan D, Ozoğul C, Dinçer SL. Effect of taurine on wound healing. Amino Acids 1996;10:59–71. [DOI] [PubMed] [Google Scholar]
- 33. Değim Z, Celebi N, Sayan H, Babül A, Erdoğan D, Take G. An investigation on skin wound healing in mice with a taurine-chitosan gel formulation. Amino Acids 2002;22:187–98. [DOI] [PubMed] [Google Scholar]
- 34. Ashkani-Esfahani S, Zarifi F, Asgari Q, Samadnejad AZ, Rafiee S, Noorafshan A. Taurine improves the wound healing process in cutaneous leishmaniasis in mice model, based on stereological parameters. Adv Biomed Res 2014;3:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu L, Zhang Q, Li Y, Song W, Chen A, Liu Jet al. . Collagen sponge prolongs taurine release for improved wound healing through inflammation inhibition and proliferation stimulation. Ann Transl Med 2021;9:1010–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blanco R. The ‘PECS block’: a novel technique for providing analgesia after breast surgery. Anaesthesia 2011;66:847–8. [DOI] [PubMed] [Google Scholar]
- 37. Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of PECS II (modified PECS I): a novel approach to breast surgery. Rev Esp Anestesiol Reanim 2012;59:470–5. [DOI] [PubMed] [Google Scholar]
- 38. Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia 2013;68:1107–13. [DOI] [PubMed] [Google Scholar]
- 39. Kleemann T, Becker T, Strauss M, Dyck N, Weisse U, Saggau Wet al. . Prevalence of bacterial colonization of generator pockets in implantable cardioverter defibrillator patients without signs of infection undergoing generator replacement or lead revision. Europace 2010;12:58–63. [DOI] [PubMed] [Google Scholar]
- 40. Knops RE, Olde Nordkamp LR, de Groot JR, Wilde AA. Two-incision technique for implantation of the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2013;10:1240–3. [DOI] [PubMed] [Google Scholar]
- 41. Li H, Wang C, Chen W-C, Lu N, Song C, Tiwari Ret al. . Estimands in observational studies: some considerations beyond ICH E9 (R1). Pharm Stat 2022;21:835–44. [DOI] [PubMed] [Google Scholar]
- 42. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70. [Google Scholar]
- 43. Traykov V, Bongiorni MG, Boriani G, Burri H, Costa R, Dagres Net al. . Clinical practice and implementation of guidelines for the prevention, diagnosis and management of cardiac implantable electronic device infections: results of a worldwide survey under the auspices of the European Heart Rhythm Association. Europace 2019;21:1270–9. [DOI] [PubMed] [Google Scholar]
- 44. Rennert-May E, Chew D, Lu S, Chu A, Kuriachan V, Somayaji R. Epidemiology of cardiac implantable electronic device infections in the United States: a population-based cohort study. Heart Rhythm 2020;17:1125–31. [DOI] [PubMed] [Google Scholar]
- 45. Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med 2011;171:1821. [DOI] [PubMed] [Google Scholar]
- 46. Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015;17:767–77. [DOI] [PubMed] [Google Scholar]
- 47. Zheng Q, Di Biase L, Ferrick KJ, Gross JN, Guttenplan NA, Kim SGet al. . Use of antimicrobial agent pocket irrigation for cardiovascular implantable electronic device infection prophylaxis: results from an international survey. Pacing Clin Electrophysiol 2018;41:1298–306. [DOI] [PubMed] [Google Scholar]
- 48. Palraj BR, Farid S, Sohail MR. Strategies to prevent infections associated with cardiovascular implantable electronic devices. Expert Rev Med Devices 2017;14:371–81. [DOI] [PubMed] [Google Scholar]
- 49. Lakkireddy D, Valasareddi S, Ryschon K, Basarkodu K, Rovang K, Mohiuddin SMet al. . The impact of povidone-iodine pocket irrigation use on pacemaker and defibrillator infections. Pacing Clin Electrophysiol 2005;28:789–94. [DOI] [PubMed] [Google Scholar]
- 50. Branger B, Marion K, Bergeron E, Perret C, Zabadani B, Reboul Pet al. . Using detachment-promoting agents for the prevention of chronic peritoneal dialysis-associated infections. Artif Organs 2008;32:918–24. [DOI] [PubMed] [Google Scholar]
- 51. Traub WH, Leonhard B, Bauer D. Taurolidine: in vitro activity against multiple-antibiotic-resistant, nosocomially significant clinical isolates of Staphylococcus aureus, Enterococcus faecium, and diverse Enterobacteriaceae. Chemotherapy 1993;39:322–30. [DOI] [PubMed] [Google Scholar]
- 52. Pirracchio L, Joos A, Luder N, Sculean A, Eick S. Activity of taurolidine gels on ex vivo periodontal biofilm. Clin Oral Investig 2018;22:2031–7. [DOI] [PubMed] [Google Scholar]
- 53. Zeriouh M, Sabashnikov A, Patil NP, Schmack B, Zych B, Mohite PNet al. . Use of taurolidine in lung transplantation for cystic fibrosis and impact on bacterial colonization. Eur J Cardiothorac Surg 2018;53:603–9. [DOI] [PubMed] [Google Scholar]
- 54. Vernon-Roberts A, Lopez RN, Frampton CM, Day AS. Meta-analysis of the efficacy of taurolidine in reducing catheter-related bloodstream infections for patients receiving parenteral nutrition. JPEN J Parenter Enteral Nutr 2022;46:1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goulet O, Breton A, Coste ME, Dubern B, Ecochard-Dugelay E, Guimber Det al. . Pediatric Home Parenteral Nutrition in France: a six years national survey. Clin Nutr 2021;40:5278–87. [DOI] [PubMed] [Google Scholar]
- 56. Neusser MA, Bobe I, Hammermeister A, Wittmann U. A 2% taurolidine catheter lock solution prevents catheter-related bloodstream infection (CRBSI) and catheter dysfunction in hemodialysis patients. Br J Nurs 2021;30:S24–32. [DOI] [PubMed] [Google Scholar]
- 57. Wouters Y, Causevic E, Klek S, Groenewoud H, Wanten GJA. Use of catheter lock solutions in patients receiving home parenteral nutrition: a systematic review and individual-patient data meta-analysis. JPEN J Parenter Enteral Nutr 2020;44:1198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Daoud DC, Wanten G, Joly F. Antimicrobial locks in patients receiving home parenteral nutrition. Nutrients 2020;12:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gabriel JA. Catheter lock solutions to prevent CVAD-related infection. Br J Nurs 2020;29:S25–9. [DOI] [PubMed] [Google Scholar]
- 60. Clark JE, Graham N, Kleidon T, Ullman A. Taurolidine-citrate line locks prevent recurrent central line-associated bloodstream infection in pediatric patients. Pediatr Infect Dis J 2019;38:e16–8. [DOI] [PubMed] [Google Scholar]
- 61. Borov S, Baldauf B, Lau EW, Bonnemeier H. Salvage of infected cardiac implantable electronic device with taurolidine—a case report. Cardiothorac Surg 2022;30:7. [Google Scholar]
- 62. Giaccardi M, Baldauf B, Lau EW, Borov S, Bonnemeier H. Salvage of cardiac implantable electronic device pocket infection with skin erosion in frail 92-year-old. J Cardiovasc Dev Dis 2022;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weichsel J, Baldauf B, Bonnemeier H, Lau EW, Dittrich S, Cesnjevar R. Eradication of ventricular assist device driveline infection in paediatric patients with taurolidine. J Cardiovasc Dev Dis 2022;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kirkfeldt RE, Johansen JB, Nohr EA, Jørgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014;35:1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Frausing M, Johansen JB, Afonso D, Jørgensen OD, Olsen T, Gerdes C. et al. Cost-effectiveness of an antibacterial envelope for infection prevention in patients undergoing cardiac resynchronization therapy reoperations in Denmark. Europace 2023;25:euad159. doi: 10.1093/europace/euad159 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon reasonable request, the data are shared by the corresponding author without limitations.