Abstract
Objectives
Highly active antiretroviral therapy (HAART) has decreased morbidity and mortality among HIV/AIDS-infected patients; however, many patients experience treatment failure. The present study aims to evaluate HIV-infected patients' clinical and immunological profiles with first-line antiretroviral treatment (ART) failure (immunological and clinical) at tertiary care hospitals in Northeast India and explore related treatment failure factors.
Methods
The hospital-based observational study was conducted among HIV-infected patients with first-line ART failure attending a tertiary care hospital from July 1, 2019, to June 30, 2020. The type of first-line ART failure was defined as a clinical, immunological, or virological failure as decided by the State AIDS Clinical Expert Panel (SACEP) meeting. Data were analyzed with Windows MS Excel (Microsoft Corporation, Redmond, Washington) and Statistical Package for the Social Sciences (SPSS) version 21 (IBM Corp., Armonk, NY).
Results
Among the 90 HIV-infected patients experiencing first-line ART treatment failure, the majority, 38 (42.2%), were in the age group of 30-40 years, 64 (71.1%) were males, and 70 (77.8%) were of average weight. Tuberculosis was the most typical opportunistic infection, affecting 11 (12.2%) patients. Most patients (38.9%) were initially presented at clinical stage 3. Maximum failures were experienced by patients with baseline CD4 ranging from 100-200 cells/mm3, with 38 (42.2%) patients, and by patients on efavirenz (64.5%) and tenofovir-based regimens (56.6%). Failures occurred more for 24-30 months and were common among patients with adherence below 90%.
Conclusion
Treatment failure was more common among young male patients and those with normal body mass index (BMI). Low baseline CD4 count and poor adherence were influential in the occurrence of treatment failure. First-line ART failure was higher in tenofovir- and efavirenz-based regimens.
Keywords: adherence, virological failure, immunological failure, treatment failure, antiretroviral treatment
Introduction
With cases documented from almost every nation, HIV infection is a global pandemic. The HIV epidemic has spread worldwide, with each wave having distinct characteristics. HIV is primarily spread through sexual contact, blood and blood products, and by infected mothers to their infants.
HIV infection can have various clinical effects. The clinical effects may be from a primary infection-related acute clinical illness through an extended asymptomatic period to advanced disease.
With an HIV/AIDS prevalence rate of 0.3%, India ranks 80th globally. It has 2.1 million individuals living with the disease, according to the 2018 Joint United Nations Program on HIV/AIDS (UNAIDS) report [1]. Antiretroviral therapy (ART) has been made available at no cost at all ART centers as part of India's National ART Program, established on April 1, 2004, under the National AIDS Control Organization (NACO). Despite reducing morbidity and mortality with the introduction of HAART, many patients fail to achieve a sustained virologic response to therapy, leading to treatment failure.
Treatment failure is an inadequate response to treatment or a lack of a sustained response based on clinical, immunological, or virological criteria [2]. Monitoring viral loads has evolved into the gold standard of care for determining ART efficacy [3]. Failure or delayed identification of a failing regimen may lead to drug resistance, toxicity, increased morbidity, and mortality [4,5]. However, it has been difficult for regions with inadequate resources to diagnose treatment failure correctly and promptly.
The present study was undertaken to study the clinical and immunological profile of first-line ART failure patients and to explore any relationship of treatment failure with the type of ART, duration of first-line ART, and adherence among HIV-infected patients with treatment failure (immunological and clinical) to first-line ART attending tertiary care hospital of north-east India.
Materials and methods
The current hospital-based observational study was conducted among HIV-infected patients with treatment failure (immunological and clinical) to first-line ART attending ART plus center and outdoor patient department (OPD) and indoor patient department (IPD) of the Department of Medicine at the Gauhati Medical College and Hospital (GMCH) during the period July 1, 2019, to June 30, 2020.
Inclusion and exclusion criteria
HIV/AIDS patients aged above 15 years with first-line ART treatment failure (immunological and clinical) willing to participate in the study after six months of treatment were included. HIV-infected patients under 15 years, those with first-line ART duration of less than six months, ART-naive patients, and patients on or failing second-line ART were excluded from the study. HIV-2 patients were also not included. Patients unwilling to participate in the study were also excluded.
Sample size
The sample size was determined based on the following formula:
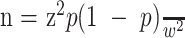
where n is the minimum sample size, w is the estimated error (0.05), p is the estimated prevalence (6%), and z = 1.96 by assuming a 95% confidence interval, and the sample size estimated is 90.
The type of first-line ART failure was defined as a clinical, immunological, or virological failure as decided by the State AIDS Clinical Expert Panel (SACEP) meeting based on the Guidelines of Treatment Failure of NACO (NACO-2018) as follows.
Clinical Failure
A new or recurrent WHO stage 4 condition after at least six months of ART is considered a clinical failure.
Immunological Failure
A fall of CD4 count to pre-therapy baseline (or below), a 50% fall from on-treatment peak value if known, or persistent CD4 levels below 100 cells/microliter after six to 12 months of treatment is considered an immunological failure.
Virological Failure
Plasma viral load > 1000 copies/ml is considered a virological failure.
Adherence calculation
Adherence was calculated as per the formula mentioned below:
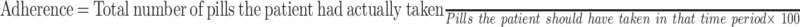
which is also equal to:
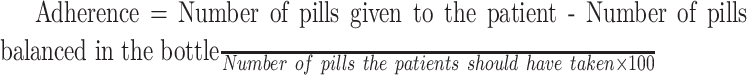
Data were collected on the patient's demographics, medical history, clinical examination, and laboratory parameters in a preformed and pretested proforma.
Statistical analysis
Data were analyzed with Windows MS Excel (Microsoft Corporation, Redmond, Washington) and Statistical Package for the Social Sciences (SPSS) version 21 (IBM Corp., Armonk, NY). The distribution of categorical data was described as count and percentages, while continuous data were presented as mean and standard deviation (SD). Association between categorical variables was tested using a chi-square test, considering a p-value < 0.05 as significant.
Ethical issues
Ethical approval was obtained from the Ethics Committee of Gauhati Medical College, Guwahati, with Ref. #: MC/190/2007/Pt-II/MAR-2019/PG/54. Before collecting the data, informed consent was taken from the patients. Complete privacy was maintained for the patient’s data as per the NACO guideline.
Results
A total of 90 HIV-infected patients with treatment failure (immunological and clinical) to first-line ART attending ART Plus Center and OPD and IPD of medicine were included in this study.
Among the first-line ART failure patients (immunological and clinical), the maximum failure rates were seen in the 30-40 age group in 38 (42.2) patients. The mean age of failure was 37.72 years. Out of the 90 patients, 64 (71%) were males with a male-to-female ratio of 2.46:1. The median BMI was 21.94, with 70 (77.8%) out of 90 patients being of average weight (Table 1).
Table 1. Demographic profile of HIV/AIDS patients with first-line ART failure.
HIV: Human immunodeficiency virus; AIDS: Acquired immunodeficiency syndrome; ART: Antiretroviral therapy.
| Characteristics | Categories | Number of patients (%) |
| Age group (years) | 10-20 | 5 (5.6) |
| 20-30 | 14 (15.5) | |
| 30-40 | 38 (42.2) | |
| 40-50 | 25 (27.8) | |
| 50 and above | 8 (8.9) | |
| >60 | 0 (0.0) | |
| Sex | Male | 64 (71.1) |
| Female | 26 (28.9) | |
| BMI | Underweight (<18.5) | 7 (7.8) |
| Regular (18.5-24.9) | 70 (77.8) | |
| Overweight (25–29.9) | 13 (14.4) | |
| Obese (≥30) | 0 (0.0) |
The data showed high mean serum transaminase levels among the patients. The details are shown in Table 2.
Table 2. Clinical profile of HIV/AIDS patients with first-line ART failure.
HIV: Human immunodeficiency virus; AIDS: Acquired immunodeficiency syndrome; ART: Antiretroviral therapy; ESR: Erythrocyte sedimentation rate; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase.
| Clinical characteristics | Mean ± SD |
| Hemoglobin (g/dl) | 11.04 ± 1.61 |
| Total count | 6329.48 ± 2299.39 |
| Platelet count (in lacs) | 2.00 ± 0.95 |
| ESR | 47.88 ± 30.79 |
| AST (units/liter) | 35.83 ± 13.37 |
| ALT (units/liter) | 41.32 ± 16.19 |
| Creatinine | 0.72 ± 0.46 |
As shown in Table 3, 71 (78.9%) out of 90 patients had no opportunistic infections. Pulmonary (7/90 patients) and extrapulmonary (4/90 patients) tuberculosis (12.2%) was the most common opportunistic infection, followed by bacterial and viral pneumonia (4.4%).
Table 3. Distribution of opportunistic infections among ART failure patients.
ART: Antiretroviral therapy.
| Opportunistic infections | Number of patients (%) |
| None | 71 (78.9) |
| Pulmonary tuberculosis | 7 (7.8) |
| Extrapulmonary tuberculosis | 4 (4.4) |
| Bacterial pneumonia | 2 (2.2) |
| Pneumocystis carinii pneumonia | 2 (2.2) |
| Oral candidiasis | 2 (2.2) |
| Esophageal candidiasis | 1 (1.1) |
| Extrapulmonary cryptococcosis | 1 (1.1) |
Of the 90 patients, 37 (41.1%) had presented with treatment failure initially at clinical stage 3 in the present study. The median baseline CD4 was 195.58. Maximum failures were experienced by patients with baseline CD4 ranging from 100-200 cells/mm3 in 38 (42.2%) patients, followed by those with baseline CD4 200 cells/mm3 and above (Table 4).
Table 4. Immunological profile of the patients.
| Variables | Categories | Number of patients (%) |
| Clinical stage at baseline | Stage 1 | 33 (36.7) |
| Stage 2 | 12 (13.3) | |
| Stage 3 | 37 (41.1) | |
| Stage 4 | 8 (8.9) | |
| Baseline CD4 count (cells/mm3) | <100 | 25 (27.8) |
| 100-200 | 38 (42.2) | |
| ≥200 | 27 (30.0) |
Among 90 treatment failure patients, all patients had an immunological failure. Virological failure was observed in 72 (80.0%) cases (Table 5).
Table 5. Type of failure.
| Type of failure | Number of patients (%) |
| Immunological | 90 (100.0) |
| Clinical | 8 (8.9) |
| Virological | 72 (80.0) |
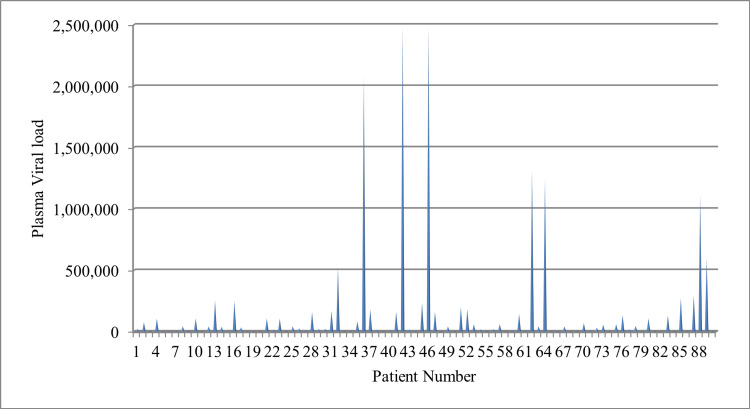
The median viral load was 139646 copies/milliliter. Among the 90 ART failure patients, 44 (48.9%) had a viral load between 1,000-100,000 copies/milliliter, and 28(31.1%) had a viral load above 100,000 copies/milliliter (Figure 1).
Figure 1. Plasma viral load of HIV/AIDS patients with first-line ART failure (clinical and immunological).
HIV: Human immunodeficiency virus; AIDS: Acquired immunodeficiency syndrome; ART: Antiretroviral therapy.
Among the 90 first-line ART failure patients (immunological and clinical), the maximum failure rates were observed with the TLE regimen in 49 (54.5%) patients, followed by the ZLN regimen in 30 (33.3%) patients. TLN regimen had the lowest failure rate (2.2%). Maximum failures were seen in the duration of 24-30 months. The median time of failure was 37 months. Almost 19% (17/90 patients) of failures occurred after 48 months of treatment. Maximum failure was seen in the patients (38/90 patients) with adherence below 90% (Table 6).
Table 6. Regimen, duration, and adherence of first-line ART.
ART: Antiretroviral therapy; ALE: Abacavir + Lamivudine + Efavirenz; TLE: Tenofovir + Lamivudine + Efavirenz; TLN: Tenofovir + Lamivudine + Nevirapine; ZLE: Zidovudine + Lamivudine + Efavirenz; ZLN: Zidovudine + Lamivudine + Nevirapine.
| Variables | Categories | No of patients (%) |
| ART regimen | ALE | 6 (6.7) |
| TLE | 49 (54.5) | |
| TLN | 2 (2.2) | |
| ZLE | 3 (3.3) | |
| ZLN | 30 (33.3) | |
| Duration of first-line ART (months) | 6-12 | 0 (0.0) |
| 12-18 | 10 (11.1) | |
| 18-24 | 16 (17.8) | |
| 24-30 | 38 (42.2) | |
| 30-36 | 5 (5.6) | |
| 36-42 | 2 (2.2) | |
| 42-48 | 2 (2.2) | |
| >48 | 17 (18.9) | |
| Adherence (%) | >95 | 30 (33.3) |
| 90-95 | 22 (24.4) | |
| <90 | 38 (42.2) |
The factors significantly associated with time to treatment failure after ART initiation were age at ART initiation, plasma viral load, and adherence (p-value < 0.05). A significant linear trend (p-value < 0.05) was observed between age at ART initiation and time to treatment failure, indicating a delay in treatment failure (>24 months) among those aged more than 30 years. ART failure occurred significantly early (≤42 months) among those having plasma viral load >1000 copies/milliliter (p-value < 0.05). Poor treatment adherence was also considerably associated with early treatment failure (≤42 months). The details are shown in Table 7.
Table 7. Factors associated with time to ART failure.
ART: Antiretroviral therapy.
* p-value for Chi-square for trend.
| Factors | Categories | Time to ART failure | p-value for Chi-square | ||
| 24 months | 24-42 months | >42 months | |||
| Age group (years) | 10-20 | 4 (12.1) | 1 (2.6) | 0 (0.0) | 0.03* |
| 20-30 | 8 (24.2) | 6 (15.8) | 0 (0.0) | ||
| 30-40 | 9 (27.3) | 18 (47.4) | 11 (57.9) | ||
| 40-50 | 9 (27.3) | 11 (28.9) | 5 (26.3) | ||
| >50 | 3 (9.1) | 2 (5.3%) | 3 (15.8) | ||
| Sex | M | 25 (75.8) | 28 (73.7) | 11 (57.9) | 0.35 |
| F | 8 (24.2) | 10 (26.3) | 8 (42.1) | ||
| Baseline CD4 | <100 | 9 (27.3) | 13 (34.2) | 4 (21.1) | 0.52 |
| 100-200 | 13 (39.4) | 13 (34.2) | 11 (57.9) | ||
| >200 | 11 (33.3) | 12 (31.6) | 4 (21.1) | ||
| Plasma viral load | <1000 | 3 (9.1) | 5 (13.2) | 10 (52.6) | <0.01 |
| 1000-100000 | 18 (54.5) | 19 (50.0) | 7 (36.8) | ||
| >100000 | 12 (36.4) | 14 (36.8) | 2 (10.5) | ||
| Adherence | >95 | 8 (24.2) | 7 (18.4) | 15 (78.9) | <0.01 |
| 90-95 | 5 (15.2) | 13 (34.2) | 4 (21.1) | ||
| <90 | 20 (60.6) | 18 (47.4) | 0 (0.0) | ||
Discussion
Managing first-line ART failure is a primary concern for HIV programs. A higher risk of morbidity and mortality is linked to continuing a failing first-line treatment regimen. Additionally, the emergence of medication resistance restricts the future development of effective and tolerable regimens. The present study was undertaken to study the clinical and immunological profiles of first-line ART failure patients and to explore any relationship between treatment failure with the type of ART, duration of first-line ART, and adherence.
The maximum number of patients with first-line ART failure (immunological and clinical) was found to be in the age group of 30-40 years (42.2%), with a mean failure age of 37.72 years. The finding suggests that older patients are less likely to fail the first-line regimen. Similar studies observed a lower incidence of treatment failure among the older age group after adherence adjustment for therapy [6]. A significant association between virologic failure and age below 40 was also documented [7,8]. However, according to a few other researches, aging is significantly linked to treatment failure, which may be because older age groups have deficient immune reconstitution mechanisms [9]. The male preponderance observed among the first-line ART failure patients in the current study with a male-to-female ratio of 2.46:1 agrees with some other literature [10].
In contrast to our observations, a recent systematic review observed that female children were at higher risk of first-line ART failure than male children [11]. However, a systematic review of observational studies published between January 1998 and November 2013 revealed no sex difference in virologic or immunologic treatment outcomes [12]. The majority (77.8%) of ART failure patients had normal BMIs with a median BMI of 21.9, which agrees with other studies [6,13,14]. The biochemical investigations showed high mean serum transaminase levels among ART failure patients. Various studies have reported hepatotoxicity as a significant concern among patients on ART [14,15]. Opportunistic infections were present in 21.1% of ART failure patients. Tuberculosis (12.2%) was the most common opportunistic infection, followed by bacterial and viral pneumonia (4.4%). Several studies have reported tuberculosis as the most common opportunistic infection among HIV/AIDS patients in India [16].
Most of the ART failure patients were presented initially at clinical stage 3. Several authors have reported a preponderance of ART failure patients with advanced baseline clinical stage [17,18]. A recent long-term follow-up study has identified advanced clinical staging as an independent predictor of treatment failure among HIV patients [19]. The median baseline CD4 of the patients was 195.58, which agrees with similar studies [20]. Maximum failures were experienced by patients with baseline CD4 ranging from 100 to 200 cells/mm3 (42.2%). CD4 count is the strongest predictor of subsequent disease progression and survival. An inverse relationship between the CD4 count at baseline and the risk of disease progression and treatment failure was reported by various researchers [20-22].
Among 90 treatment failure patients, all patients had an immunological failure (100%), 72 had a virological failure (80.0%), and only eight had a clinical failure (8.9%). Previous studies on treatment failure were primarily prospective, including failure and responder cases. Hence, the present study could not draw a conclusive parallel comparison of failure type.
However, in a study conducted among 54 patients with first-line treatment failure (immunological and clinical), 48 (88.9%) had immunological failure, six (11.1%) had clinical failure, and 37 (68.5%) had virological failure [23].
In our study among nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) drug regimens, most (56.6%) of the first-line ART failure patients were on a tenofovir-based regimen. While among non-nucleoside reverse transcriptase inhibitor drugs (NNRTIs), first-line ART failure was mostly encountered among patients on an efavirenz-based regimen (64.5%). Previous studies have primarily compared the effectiveness between efavirenz-based HAART and protease inhibitor (PI)-based HAART, suggesting a consistent result of higher effectiveness of the efavirenz-based regimen [24]. The higher incidence of failure rates with triple NRTI regimen than with dual-class regimen (NRTI + NNRTI) is documented [2]. A recent study reported a significant association between including a PI in the first regimen and switching to a second-line treatment [22]. As the use of a PI-based regimen as first-line HAART is limited to exceptional circumstances as per NACO guidelines and infection with HIV-2 has been excluded from the present study, any case of treatment failure with a PI-based regimen could not be identified. Several studies have reported more failure in patients on stavudine and zidovudine-based NRTI regimens than tenofovir-based regimens, whereas NNRTIs mainly reported treatment failures among patients on nevirapine-based regimens in those studies [2,16,25]. In the present study, there were no patients on a stavudine-based regimen. This was because stavudine was phased out as the first-line ARV due to its toxicities, and there has been a gradual major switch to tenofovir due to its better safety profile.
Among the first-line ART failure patients (immunological and clinical), maximum failure was seen in the duration of 24-30 months, with a median time of failure of 37 months. With the early and prompt diagnosis per NACO operational guidelines, the median time of switching to the second line has been delayed as observed in the present study, which agrees with another Indian study [25]. Also, with the increasing focus on the enhanced adherence counseling (EAC) and the choice of well-designed ARV regimens, the median time of first-line ART failure has improved.
Adherence is the most significant patient-enabled predictor of treatment outcomes for the patients on HAART. WHO recommends at least 95% adherence to ART to avoid the emergence of resistant strains. In our study, among the first-line ART failure patients (immunological and clinical), maximum failure was seen in the patients with adherence of <90%. These findings were almost like another study. Previous research has identified inadequate adherence as an independent predictor of first-line ART treatment failure [26]. A recent study from India stated that high costs, alcoholism, choosing non-allopathic drugs, and depression were the most frequent causes of inadequate adherence and treatment termination. Missed doses were attributed to various factors, including feeling well, depression, forgetfulness, and a busy schedule [27]. The remarkable improvements in HIV-related health indices could eventually be negated by nonadherence. Due to high viral mutation rates and short drug half-lives in nonadherent patients, resistant virus strains emerge, significantly narrowing the range of available antiviral treatments. Therefore, even if adherence is increased, poor adherence may encourage the selection of mutants that are no longer sensitive in addition to immediate increases in plasma HIV RNA.
The global HIV response, with coordinated political and financial commitment, has made substantial progress toward its goals of reaching critical populations and other marginalized groups, removing barriers to treatment caused by poverty, integrating communities in service delivery and decision-making processes, and placing the right to health at the forefront [28]. Enhancement of pandemic preparedness and response has been a topic of discussion across the globe as a result of the recent COVID-19 pandemic and the expectation of future pandemic threats. The HIV response is an excellent representation of a worldwide health endeavor that has succeeded in significantly advancing pandemic preparedness and response [29]. Although the number of new HIV infections and AIDS-related fatalities has significantly declined since the epidemic's peak, there has not been much improvement in reducing new infections in the last decade. [28] Minimizing stigma among HIV-infected patients and those who are at risk of the disease, expanding the HIV workforce, reducing detrimental socioeconomic determinants of health, and recommitting and reinvesting in health are the priority areas that are to be considered globally for HIV elimination strategies [30].
Limitation
The results of this observational study, which was limited to one ART center, may only apply to some. To draw a firm conclusion, further prospective research with a bigger sample size in a larger community and several ART centers is required.
Conclusions
Younger male patients with normal BMIs had a higher propensity for treatment failure. Low baseline CD4 count and poor adherence were influential in the occurrence of treatment failure. Compared to other NRTIs and NNRTIs, the incidence of first-line ART failure was higher with tenofovir- and efavirenz-based regimens. The median time of first-line ART failure has been postponed due to the development of newer, more potent ARV medications.
The authors have declared that no competing interests exist.
Author Contributions
Acquisition, analysis, or interpretation of data: Putul Mahanta Sr., Anindita Phukan, Chiranjita Phukan, Diganta Buragohain Sr.
Critical review of the manuscript for important intellectual content: Putul Mahanta Sr., Anindita Phukan, Chiranjita Phukan, Swaroop K. Baruah Sr., Diganta Buragohain Sr.
Supervision: Putul Mahanta Sr., Chiranjita Phukan, Swaroop K. Baruah Sr.
Concept and design: Anindita Phukan, Chiranjita Phukan, Swaroop K. Baruah Sr.
Human Ethics
Consent was obtained or waived by all participants in this study. Gauhati Medical College and Hospital issued approval MC/190/2007/Pt-II/MAR-2019/PG/54
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.UNAIDS data 2018. [ Jul; 2023 ]. 2023. https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf
- 2.Predictors of first-line antiretroviral therapy failure amongst HIV-infected adult clients at Woldia Hospital, Northeast Ethiopia. Babo YD, Alemie GA, Fentaye FW. PLoS One. 2017;12:0. doi: 10.1371/journal.pone.0187694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The role of point-of-care viral load monitoring in achieving the target of 90% suppression in HIV-infected patients in Nigeria: study protocol for a randomized controlled trial. Meloni ST, Agbaji O, Chang CA, et al. BMC Infect Dis. 2019;19:368. doi: 10.1186/s12879-019-3983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.First-line antiretroviral treatment failure and associated factors in HIV patients at the University of Gondar Teaching Hospital, Gondar, Northwest Ethiopia. Ayalew MB, Kumilachew D, Belay A, et al. HIV AIDS (Auckl) 2016;8:141–146. doi: 10.2147/HIV.S112048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. Boender TS, Kityo CM, Boerma RS, et al. J Antimicrob Chemother. 2016;71:2918–2927. doi: 10.1093/jac/dkw218. [DOI] [PubMed] [Google Scholar]
- 6.Developing a predictive risk model for first-line antiretroviral therapy failure in South Africa. Rohr JK, Ive P, Horsburgh CR, et al. J Int AIDS Soc. 2016;19:20987. doi: 10.7448/IAS.19.1.20987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virological and immunological failure of HAART and associated risk factors among adults and adolescents in the Tigray region of Northern Ethiopia. Hailu GG, Hagos DG, Hagos AK, Wasihun AG, Dejene TA. PLoS One. 2018;13:0. doi: 10.1371/journal.pone.0196259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Determinants of virological failure among patients on highly active antiretroviral therapy in University of Gondar Referral Hospital, Northwest Ethiopia: a case-control study. Bayu B, Tariku A, Bulti AB, Habitu YA, Derso T, Teshome DF. HIV AIDS (Auckl) 2017;9:153–159. doi: 10.2147/HIV.S139516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevalence and factors associated with treatment failure during antiretroviral therapy Atbobo-Dioulasso university teaching hospital (Burkina Faso)(2008-2013) Zoungrana J, Poda A, Sondo KA, Diallo I, Bado G, Oudrago AS. Austin J HIV AIDS Res. 2016;3:1027. [Google Scholar]
- 10.Predictors of treatment failure among adult antiretroviral treatment (ART) clients in Bale Zone Hospitals, South Eastern Ethiopia. Haile D, Takele A, Gashaw K, Demelash H, Nigatu D. PLoS One. 2016;11:0. doi: 10.1371/journal.pone.0164299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.First line antiretroviral treatment failure and its association with drug substitution and sex among children in Ethiopia: systematic review and meta-analysis. Masresha SA, Alen GD, Kidie AA, Dessie AA, Dejene TM. Sci Rep. 2022;12:18294. doi: 10.1038/s41598-022-22237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sex differences in HIV outcomes in the highly active antiretroviral therapy era: a systematic review. Castilho JL, Melekhin VV, Sterling TR. AIDS Res Hum Retroviruses. 2014;30:446–456. doi: 10.1089/aid.2013.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Association of body mass index with immune recovery, virological failure and cardiovascular disease risk among people living with HIV. Han WM, Jiamsakul A, Jantarapakde J, et al. HIV Med. 2021;22:294–306. doi: 10.1111/hiv.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prevalence of hepatotoxicity among HIV-infected patients in Ethiopia: a systematic review and meta-analysis. Mohammed O, Alemayehu E, Bisetegn H, et al. BMC Infect Dis. 2022;22:826. doi: 10.1186/s12879-022-07838-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Risk factors for alanine aminotransferase elevations in a prospective cohort of HIV-infected Tanzanian adults initiating antiretroviral therapy. Mugusi SF, Sando D, Mugusi FM, Hawkins C, Aboud S, Fawzi WW, Sudfeld CR. J Int Assoc Provid AIDS Care. 2019;18:2325958219884939. doi: 10.1177/2325958219884939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Recent trends in the spectrum of opportunistic infections in human immunodeficiency virus infected individuals on antiretroviral therapy in South India. Shahapur PR, Bidri RC. J Nat Sci Biol Med. 2014;5:392–396. doi: 10.4103/0976-9668.136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prevalence and associated factors of treatment failure among HIV/AIDS patients on HAART attending University of Gondar Referral Hospital Northwest Ethiopia. Ayele G, Tessema B, Amsalu A, Ferede G, Yismaw G. BMC Immunol. 2018;19:37. doi: 10.1186/s12865-018-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Immunologic and clinical failure of antiretroviral therapy in people living with human immunodeficiency virus within two years of treatment. Asgedom SW, Maru M, Berihun B, Gidey K, Niriayo YL, Atey TM. Biomed Res Int. 2020;2020:5474103. doi: 10.1155/2020/5474103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Predictors of first-line antiretroviral treatment failure among children on antiretroviral therapy at the University of Gondar comprehensive specialised hospital, North-west, Ethiopia: a 14-year long-term follow-up study. Wondifraw EB, Tebeje NB, Akanaw W, Chanie ES. BMJ Open. 2022;12:0. doi: 10.1136/bmjopen-2022-064354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virological treatment failure among adult HIV/AIDS patients from selected hospitals of North Shoa Zone, Amhara Region, Ethiopia. Derseh BT, Shewayerga B, Dagnew Mekuria A, Admasu Basha E. Infect Drug Resist. 2020;13:4417–4425. doi: 10.2147/IDR.S280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prognosis of HIV patients receiving antiretroviral therapy according to CD4 counts: a long-term follow-up study in Yunnan, China. Ren L, Li J, Zhou S, et al. Sci Rep. 2017;7:9595. doi: 10.1038/s41598-017-10105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rate and determinants of treatment response to different antiretroviral combination strategies in subjects presenting at HIV-1 diagnosis with advanced disease. Esposito A, Floridia M, d'Ettorre G, et al. BMC Infect Dis. 2011;11:341. doi: 10.1186/1471-2334-11-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Predictors of failure of first-line antiretroviral therapy in HIV-infected adults: Indian experience. Rajasekaran S, Jeyaseelan L, Vijila S, Gomathi C, Raja K. AIDS. 2007;21:0–53. doi: 10.1097/01.aids.0000279706.24428.78. [DOI] [PubMed] [Google Scholar]
- 24.Efavirenz versus protease inhibitors in patients with HIV: a systematic review and meta-analysis. Nduaguba SO, Okoh C, Barner JC, et al. AIDS Rev. 2021;23:103–114. doi: 10.24875/AIDSRev.20000098. [DOI] [PubMed] [Google Scholar]
- 25.A study of the profile of patients failing first-line NACO recommended ART. Mittal M, Chand P, Mall AK. Int J Adv Med Sci. 2018;5:1256. [Google Scholar]
- 26.Determinants of first-line antiretroviral treatment failure among adult patients on treatment in Mettu Karl Specialized Hospital, South West Ethiopia; a case control study. Zenu S, Tesema T, Reshad M, Abebe E. PLoS One. 2021;16:0. doi: 10.1371/journal.pone.0258930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.What influences adherence among HIV patients presenting with first-line antiretroviral therapy failure (ART failure)? A retrospective, cross-sectional study from a private clinic in Nagpur, India. Shanmukhappa SC, Abraham RR, Huilgol P, et al. J Family Med Prim Care. 2020;9:6217–6223. doi: 10.4103/jfmpc.jfmpc_1155_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Advancing global health and strengthening the HIV response in the era of the sustainable development goals: the International AIDS Society-Lancet Commission. Bekker LG, Alleyne G, Baral S, et al. Lancet. 2018;392:312–358. doi: 10.1016/S0140-6736(18)31070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leveraging the HIV response to strengthen pandemic preparedness. Collins C, Isbell MT, Karim QA, Sohn AH, Beyrer C, Maleche A. PLOS Glob Public Health. 2023;3:0. doi: 10.1371/journal.pgph.0001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Is the USA on track to end the HIV epidemic? Guilamo-Ramos V, Thimm-Kaiser M, Benzekri A. Lancet HIV. 2023;10:0–6. doi: 10.1016/S2352-3018(23)00142-X. [DOI] [PubMed] [Google Scholar]