Abstract
Retinopathy of prematurity (ROP), a blinding condition affecting preterm infants, is an interruption of retinal vascular maturation that is incomplete when born preterm. Although ROP demonstrates delayed onset following preterm birth, representing a window for therapeutic intervention, there are no curative or preventative measures available for this condition. The in utero environment, including placental function, is increasingly recognized for contributions to preterm infant disease risk. The current study identified a protective association between acute placental inflammation and preterm infant ROP development using logistic regression, with the most significant association found for infants without gestational exposure to maternal preeclampsia and those with earlier preterm birth. Expression analysis of proteins with described ROP risk associations demonstrated significantly decreased placental high temperature requirement A serine peptidase-1 (HTRA-1) and fatty acid binding protein 4 protein expression in infants with acute placental inflammation compared with those without. Within the postnatal peripheral circulation, HTRA-1 and vascular endothelial growth factor-A demonstrated inverse longitudinal trends for infants born in the presence of, compared with absence of, acute placental inflammation. An agnostic approach, including whole transcriptome and differential methylation placental analysis, further identify novel mediators and pathways that may underly protection. Taken together, these data build on emerging literature showing a protective association between acute placental inflammation and ROP development and identify novel mechanisms that may inform postnatal risk associations in preterm infants.
Retinopathy of prematurity (ROP) is a blinding condition of prematurity characterized by disruption in normal retinal vascular development, resulting in a spectrum of anatomic aberrancy and visual impairment. ROP represents a significant health burden, collectively accounting for 40% of all childhood blindness worldwide. More importantly, ROP demonstrates increasing incidence with greater survival of early preterm infants.1, 2, 3 Despite delayed disease onset following preterm birth, representing a therapeutic window, there are no curative or preventative measures available for ROP. Although birth weight (BW) of <1500 g, prematurity of <32 weeks, and postnatal oxygen exposure confer independent ROP risk, these factors are not sufficiently modifiable to prevent ROP.4,5 Greater insight into risk pathomechanisms and protective paradigms, particularly those occurring before development of clinical disease, will underly future efforts to facilitate normal retinal maturation and prevent significant ocular and visual morbidity associated with ROP.
The in utero and placental environment is increasingly recognized for contribution to both preterm infant disease susceptibility and disease protection.6, 7, 8, 9, 10 While the underlying rationale is not clear, changes in blood flow and, thus, nutrient and oxygen delivery are postulated, as is direct modification of the inflammasome evidenced by differential cytokine profiling in preterm infant cord blood samples relative to placental histology.11,12 The role of placental histopathologic diagnoses to ROP development is debated in the literature. For example, early studies suggest that acute placental inflammation in the setting of chorioamnionitis is associated with increased ROP risk.13 However, recent work demonstrates no association14 or a protective association within distinct preterm populations.15 Clarity on the relationship between acute placental inflammation and preterm infant ROP development may allow for improved disease stratification and screening as well as insight into preclinical disease mechanisms.
The current study demonstrated a significant inverse association between acute placental inflammation and ROP using logistic regression within a discovery placental histology cohort. This association was evident when controlling for confounding effects of known ROP risk associations [namely, gestational age (GA), most marked at early gestations and within preterm infants without preeclamptic gestations]. The study explored potential molecular pathomechanisms underlying these associations within a second recruited preterm infant replication cohort. A candidate approach was adopted to demonstrate significant differences in placental high temperature requirement A serine peptidase-1 (HTRA-1) and fatty acid binding protein 4 (FABP4) protein expression between tissues characterized by acute inflammation compared with those without. Inverse longitudinal trends were identified for HTRA-1 and vascular endothelial growth factor (VEGF)-A within the peripheral circulation of infants born preterm in the setting of acute placental inflammation compared with those without, within this same replication cohort. Finally, Illumina-based whole transcriptome and methylation placental tissue analysis was used to identify a subset of genes and pathways that demonstrate differential expression and/or modification relative to both ROP and placental inflammation clinical outcomes.
These findings support recent work suggesting a protective relationship between acute placental inflammation and preterm infant ROP risk, as well as a disparate ROP risk association for preterm infants with or without gestational exposure to maternal preeclampsia. Significant RNA, protein, and DNA modifications were observed within placental tissues correlating with both acute placental inflammation and ROP clinical outcomes using both a candidate approach centered on established ROP risk mediators and an agnostic approach, identifying novel associations. More importantly, many identified placental associations remained associated within the preterm infant systemic circulation, adding relevance to their roles in postnatal disease development. Taken together, these data contribute to a framework of understanding toward informing the molecular pathobiology underlying placental inflammatory protection from ROP and eventual preventive therapies for ROP.
Materials and Methods
The discovery cohort consisted of 182 placental tissue samples from preterm births (BW, ≤1500 g; and GA, ≤32 weeks’ gestation) at the University of Utah (Salt Lake City, UT) between 2010 and 2020.
Clinical Biorepository Placental Histology Analysis
Placental tissue prepared on histologic slides and hematoxylin and eosin stained were identified within an existing clinical biorepository at the University of Utah; all samples meeting inclusion criteria (births with BW ≤1500 g and GA ≤32 weeks’ gestation) were analyzed. Histologic samples were independently reviewed for infectious acute placental inflammation (chorioamnionitis) by an expert placental pathologist (J.C.) using standardized criteria for presence, absence, and severity of acute placental inflammation.16 The severity of infectious acute placental inflammation histology was characterized using gold standard Amsterdam consensus grading criteria denoting acute maternal (less severe) versus infant-level (more severe) inflammation.16 Inflammatory features not consistent with infectious acute placental inflammation, such as immune or idiopathic inflammatory lesions, as defined by Redline,16 were excluded from analysis; infants were also excluded if full clinical data were not available. Clinical data were collected from the medical chart with appropriate institutional review board approval (institutional review board number 88171; Principal Investigator, L.A.O.), including maternal preeclampsia diagnosis, infant ROP diagnosis, BW, GA, and sex. The relationships between inflammatory histology, presence or absence of ROP, GA, sex, and BW were determined by univariate and multivariate analysis, as indicated and as previously published.4
Replication Cohort Consisting of a Recruited Patient Cohort Biorepository
Infants were recruited from two neonatal intensive care units at the University of Utah or the Medical University of South Carolina (Charleston, SC). Infants were included if they were born at <31 weeks’ gestational age and <1250 g BW, meeting ROP screening guidelines at their respective centers, and were eligible for blood collection. All studies were performed with institutional review board approval at either the University of Utah or Medical University of South Carolina and adhered to the Declaration of Helsinki. Patient recruitment is as previously published.17 In brief, the parent or legal guardian for eligible infants was provided with informed consent before enrollment. Inclusion and exclusion criteria for preeclampsia and ROP diagnoses are informed by American Academy of Pediatrics, American College of Obstetricians and Gynecologists, and International Classification of ROP guidelines and assessed on the basis of clinical diagnoses made by the clinical care team.18, 19, 20, 21 Placental tissue was collected from University of Utah cohort patients only within ≤1 hour following preterm delivery in a standardized manner and flash frozen or paraffin embedded. Cord or peripheral blood was collected from infants at both institutions. Infant cord blood was collected at the time of preterm delivery when deemed appropriate by the clinical care team. Preterm infant peripheral blood was collected between 35 and 37 weeks’ gestational age. Per the standardized protocol, up to 2 mL of infant blood was collected and plasma was immediately separated, accessioned, and stored at −80°C. Epidemiologic, demographic, and clinical data, including preeclampsia, maternal age, preterm labor, maternal antibiotic or steroid administration, race/ethnicity, infant sex, infant gestational age, and birth weight, were collected for each enrolled patient and stored in a secure REDCap database, as previously published.4,17
Outcome Measures for Human Studies
The primary outcome variables were presence or absence of any ROP or placental inflammation, as previously defined.16,19 The diagnosis and severity of infectious acute placental inflammation were determined from the medical chart and verified using gold standard diagnostic criteria by an expert placental pathologist (J.C.).16 ROP zone, stage, and severity were documented according to the international classification of ROP.19, 20, 21, 22 Secondary outcome measures included ROP severity and need for treatment. ROP severity was represented by the greatest degree of severity documented during the patient's clinical course, as determined by indirect ophthalmoscopy provided during clinical care.
Placental Immunofluorescence Analysis
Average integrated density was determined for six high-power fields for paraffin-imbedded human placental tissues obtained from the described replication cohort. Placental villi (fetal tissue) within each high-power field were identified using DAPI nuclei labeling. Fluorescent staining from either FABP4 (Novus Biologicals, Centennial, CO; catalog number AF3150) or HTRA-1 (Thermo Fisher, Waltham, MA; number PA5-11412) was measured using a standardized gain on the EVOS imaging system (Thermo Fisher). Fluorescence was measured using the Cy5 filter for both proteins with an Alexa647 fluorophore-conjugated secondary. Average fluorescence was determined for six high-power fields within fetal tissue (villi) per patient and then normalized to background fluorescence using non-specific channel fluorescence. The statistical relationship between total fluorescence and clinical data, including presence, absence, or severity of placental inflammation and ROP development, was done using a paired t-test.
Regression Analysis for Association
A univariate analysis was performed to determine the significance of described and proposed ROP risk variables, including BW, GA, maternal preeclampsia, sex, and acute placental inflammation, with ROP development. Variables with significance ≥0.3 in univariate analysis were analyzed using logistic regression, as previously reported.4,23 Statistical analysis was performed using R studio version 2022.07.1 (https://www.rstudio.com/products/rstudio/download). For variables with P ≤ 0.05 in multivariate analysis, the results are designated as statistically significant. CIs were calculated to indicate the direction of the effect. If the CI does not include the numerical term 0, a statistically significant result is assumed. Effect size was determined using odds ratio calculations.
HTRA-1 and VEGF-A Plasma Protein Analysis
Plasma from patients within the replication cohort described above was analyzed and consisted of the following: acute inflammation, n = 13; control, n = 17; ROP, n = 14; and control, n = 16. Expression of each candidate protein was measured in infant plasma using either enzyme-linked immunosorbent assay (HTRA-1; LSBio, Seattle, WA; number LS-F11673) or Luminex (VEGF-A; Luminex, Austin, TX) when a clinical test was available through the ARUP clinical laboratory (Salt Lake City, UT; https://www.aruplab.com; cited May 19, 2020). Measurement was performed at two time points, within cord blood collected at the time of preterm birth and within peripheral blood collected within the GA window of 35 to 37 weeks. All enzyme-linked immunosorbent assay samples were run in triplicate, and concentrations (ng/μL) were calculated relative to standard. Average values for each condition were compared for statistical significance using a traditional two-tailed t-test.
Infinium MethylationEPIC BeadChip
DNA was extracted from fetal surface placental tissues corresponding to patients within the replication cohort, as described above (acute inflammation, n = 9; and control, n = 7), using the Qiagen (Hilden, Germany) AllPrep system. DNA quantity was assessed with the QuantiFluor dsDNA System (Promega, Madison, WI) on the Synergy HT plate reader (BioTek Instruments, Inc., Winooski, VT), and the highest yield samples were normalized to 250 ng in 30 μL. The samples then underwent bisulfite conversion using the Zymo EZ DNA Methylation Kit (Zymo Research Corp., Irvine, CA) with a genomic DNA input of 250 ng. The recommended modification to the protocol using alternative incubation conditions for the Illumina, Inc. (San Diego, CA), assays was performed. On bisulfite conversion completion, samples were sent to the University of Utah DNA Sequencing and Genomics Core Facility (Salt Lake City, UT) for Infinium HD Methylation using the Illumina MethylationEPIC kit scanning on the iScan instrument and raw data export.
Whole Transcriptome Placental Analysis
Whole transcriptome expression analysis was performed on fetal surface placental tissues using the Illumina Stranded Total RNA Kit with Ribo-Zero Plus, per the standard protocol and as published previously24,25 in collaboration with the University of Utah Huntsman Cancer Institute's High-Throughput Genomics and Bioinformatic Analysis Shared Resource Core. Tissue was obtained from the replication cohort for infants relative to ROP development (ROP, n = 14; and control, n = 16) and presence of acute placental inflammation (placental inflammation, n = 13; and control, n = 17). RNA was extracted from a 5 × 5-mm fetal surface placental tissue section, which was obtained within 30 minutes of delivery and flash frozen, as noted above, using the Qiagen AllPrep RNA/DNA extraction system.
Bioinformatic Analysis
Differential Methylation Analysis
The samples were processed on an Infinium MethylationEPIC BeadChip Array, as described above. The IDAT files were loaded into the minfi package in R and normalized using the functional normalization algorithm.26 Differentially methylated regions (DMRs) were identified relative to placental inflammation using the bumphunter package.27 R was used to further plot methylation differences and fold changes from significant genes with DMRs.
Whole Transcriptome Analysis
Human genome and gene feature files were downloaded from Ensembl release 104, (https://useast.ensembl.org/info/genome/genebuild/index.html) and a reference database was generated using STAR version 2.7.9a (https://www.encodeproject.org/software/star) with splice junctions optimized for 150-bp reads.28 Optical duplicates were removed from the paired end FASTQ files using clumpify version 38.34, and reads were trimmed of adapters using cutadapt 1.16.29 The trimmed reads were aligned to the reference database using STAR in two-pass mode to output a binary alignment map (BAM) file sorted by coordinates. Mapped reads were assigned to annotated genes using featureCounts version 1.6.3.30 Raw gene counts were filtered to remove features with 0 counts and <10 reads in every sample, and differentially expressed genes (DEGs) were identified using a 5% false discovery rate with DESeq2 version 1.30.1.31 Pathways were analyzed using the fast gene set enrichment package32 (gene set enrichment analysis)33 to identify significantly enriched pathways within DEGs from placental tissues characterized by acute inflammation or from infants who developed ROP using the Hallmark database.
Results
Characteristics of Discovery Cohort
The study identified births within the ROP GA and BW risk window, including infants born before 32 weeks and weighing <1500 g, in an existing placental biobank consisting of deliveries between 2010 and 2020 (n = 182) (Table 1). Clinical data for included infants were collected from the medial record, including BW, GA, sex, maternal preeclampsia status, and infant ROP screening data. Given prior studies demonstrating disparate ROP risk associations for acute placental inflammation in preeclamptic compared with non-preeclamptic gestations,15 the population was sub-stratified by this gestational criterion. Identified samples were independently reviewed for placental inflammation by an expert placental pathologist (J.C.) using standardized criteria for presence, absence, and severity of infectious acute placental inflammation, as noted above.16 As demonstrated in Table 1, GA but not BW differed significantly between these subgroups, as did development of ROP, severity of ROP, and presence of acute placental inflammation.
Table 1.
Placental Biobank Patient Characteristics
| Characteristic | Preterm infant cohort | Preeclamptic cohort | Non-preeclamptic cohort | P value |
|---|---|---|---|---|
| Total, N | 182 | 66 | 116 | |
| Average gestational age, weeks | 28 | 28.9 | 27.1 | <0.001 |
| Average birth weight, g | 988.5 | 995 | 982 | 0.80 |
| Male sex, n (%) | 85 (47) | 34 (51) | 51 (44) | 0.32 |
| ROP, n (%) | 100 (55) | 29 (44) | 71 (61) | 0.03 |
| Type 1 ROP, n (%) | 12 (7) | 1 (2) | 11 (9) | 0.03 |
| Placental inflammation, n (%) | 66 (36) | 3 (5) | 63 (54) | 0.04 |
Gestations characterized by preterm birth of <32 weeks and birth weight of <1500 g were identified within an existing clinical biorepository between the years 2010 and 2020. Clinical information was collected, including gestational age, birth weight, sex, preeclampsia, and ROP presence and severity. Acute placental inflammation histology was independently characterized by an expert placental pathologist (J.C.).
ROP, retinopathy of prematurity.
Acute Placental Inflammation Is Inversely Associated with Presence and Severity of ROP
To determine the association between acute placental inflammation and preterm infant ROP development, a univariate analysis was performed (Table 2), consisting of established and proposed ROP risk variables, followed by a regression analysis that included all variables with P ≤ 0.3 in univariate analysis. Regression analysis demonstrated statistically significant inverse associations with preterm infant ROP development for GA and acute placental inflammation, as denoted in Table 3. Statistical significance was defined as P ≤ 0.05 in multivariate analysis with a CI that does not include the numerical term 0. Effect size was determined using odds ratio calculations.
Table 2.
Univariate Analysis for Association with ROP Development
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Birth weight | 0.996 | 0.994–0.997 | 5.48×10−9 |
| Gestational age | 0.35 | 0.25–0.46 | 6.56×10−12 |
| Preeclampsia | 0.50 | 0.27–0.91 | 0.0253 |
| Acute placenta inflammation | 1.71 | 0.92–3.23 | 0.0931 |
| Male sex | 1.61 | 0.89–2.92 | 0.115 |
Univariate analysis was performed to determine association between acute placental inflammation with ROP development. Established ROP risk variables, including birth weight, gestational age, and preeclampsia, were also queried; association with male sex was determined to assess for sex as a biological variable.
OR, odds ratio; ROP, retinopathy of prematurity.
Table 3.
Logistic Regression Demonstrates Significant Inverse Associations for GA and Acute Placental Inflammation
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Birth weight | 0.998 | 0.997–1.000 | 0.0917 |
| GA | 0.34 | 0.23–0.48 | 7.15×10−9 |
| Preeclampsia | 0.79 | 0.28–2.25 | 0.6626 |
| Acute placenta inflammation | 0.28 | 0.089–0.83 | 0.0251 |
| Male sex | 1.70 | 0.73–4.04 | 0.2251 |
Logistic regression was performed for all univariate factors with P ≤ 0.3. Only GA and acute placental inflammation maintained significance. Significance was defined as P ≤ 0.05 in multivariate analysis and CIs not including the numerical term 0. Inverse association is determined by the OR.
GA, gestational age; OR, odds ratio.
The Inverse Association between Presence and Severity of Acute Placental Inflammation Is More Marked at Earlier Gestations
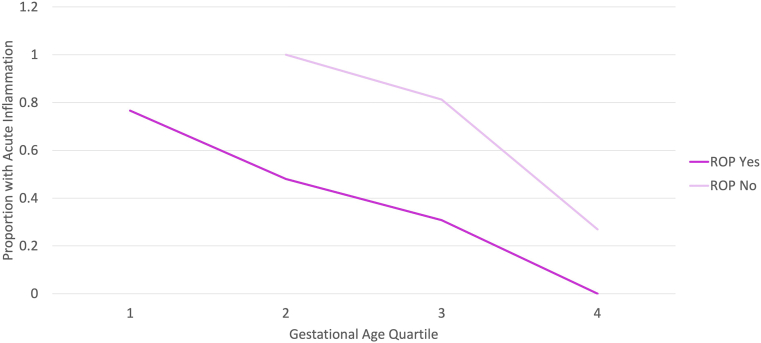
To determine whether there was a GA range for which ROP risk was most significantly associated with the presence of acute placental inflammation, the relationship between these variables was examined within four GA quartiles. The first GA quartile represented births <26 weeks; the second GA quartile represented births <27.6 weeks; the third GA quartile represented births <28.9 weeks; and fourth GA quartile represented births >29 weeks. As indicated in Figure 1, the inverse association between ROP development and acute placental inflammation was present for all GA quartiles; however, there was a trend toward a greater association for preterm infants born at earlier gestational ages.
Figure 1.
The presence of acute placental inflammation is associated with reduced preterm infant retinopathy of prematurity (ROP) development within all gestational ages (GAs). ROP development within each of four gestational age quartiles was examined relative to presence or absence of acute placental inflammation. First GA quartile, <26 weeks; second GA quartile, <27.6 weeks; third GA quartile, <28.9 weeks; and fourth GA quartile, >29 weeks. ROP development is inversely associated (P = 0.008) with the presence of acute placental inflammation within all quartiles. This was most marked for earlier GA birth quartiles.
ROP Development Is Inversely Associated with Both Maternal and Fetal Inflammatory Response and Most Significant for Infants Without Gestational Exposure to Preeclampsia
Acute placental inflammation represents an infectious etiology originating within the cervical region and ascending to involve the placental tissues. Thus, severity can be represented by the degree to which the infection has ascended within the placenta. The maternal surface, anatomically closer to the infection source, represents limited placental spread. Involvement of the fetal surface represents advanced infectious spread within the placenta. Using this metric for severity, the relationship between preterm infant development of ROP and the severity of acute placental inflammation was examined. As noted in Table 4, both maternal and fetal surface inflammatory responses were inversely associated with infant ROP development, although this correlation was more significant with increasing severity of inflammation. The study sought to determine whether the association was present in all gestations within the ROP risk window compared with those with the absence of gestational preeclampsia. Although a significant inverse association was observed between maternal and fetal surface inflammatory response and preterm infant ROP development within both cohorts, the associations were more significant within the non-preeclamptic cohort (Table 4).
Table 4.
Association between the Severity of Acute Placental Inflammation and ROP Development Differs by Gestational Preeclampsia
| Variable | Full cohort |
Non-preeclamptic groups |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Maternal inflammatory response | 0.52 | 0.31–0.84 | 0.011 | 0.34 | 0.14–0.68 | 0.0060 |
| Fetal inflammatory response | 0.45 | 0.25–0.77 | 0.0048 | 0.34 | 0.15–0.74 | 0.0039 |
The presence or absence of maternal inflammatory response (less severity) or fetal inflammatory response (greater severity) was correlated with development of ROP for infants while controlling for the significance of gestational age within the full cohort or non-preeclamptic cohorts. As denoted by P value and OR, all associations were inverse. Both severities of acute placental inflammation were found to significantly associate with ROP development, although there was a slight trend toward greater significance with increasing severity.
OR, odds ratio; ROP, retinopathy of prematurity.
HTRA-1 and FABP4 Proteins Are Significantly Decreased in Placenta from Infants with Placental Inflammation Compared with Control
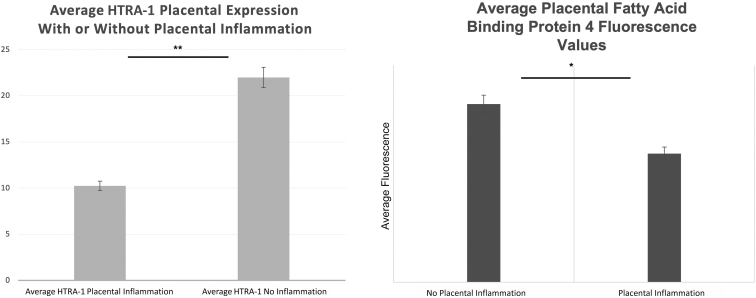
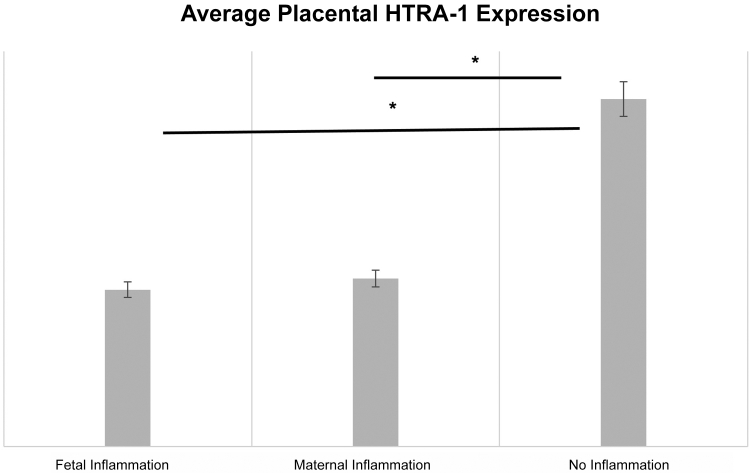
A candidate approach was adopted to examine the potential biochemical etiology for the observed inverse association between infant ROP development and acute placental inflammation. Placental protein levels for the serine protease HTRA-1 and FABP4 were measured using semiquantitative immunofluorescence. These factors were selected as they both play roles in placental inflammation, preeclampsia, and ROP development in humans and animal models.17,34, 35, 36, 37, 38, 39, 40, 41, 42 Expression of each protein was examined independently within a subset of placental specimens from the replication cohort. Average villous tissue fluorescence from six high-power fields was compared for infants with (n = 25) and without (n = 25) acute placental inflammation, as noted above. Protein levels of both HTRA-1 and FABP4 were significantly decreased in placental tissues with acute placental inflammation compared with those without (Figure 2). Furthermore, as demonstrated in Figure 3, there was a slightly more marked association between decreased placental HTRA-1 protein expression for placental tissues characterized by fetal surface inflammation (n = 12) versus maternal surface inflammation (n = 13) when comparing with control placental tissues without inflammation (n = 25).
Figure 2.
Placental fatty acid binding protein 4 (FABP4) and high temperature requirement A serine peptidase-1 (HTRA-1) proteins are decreased in tissues with inflammation compared with those without. Placental sections from paraffin-embedded tissues corresponding to those from our placental biorepository patient cohort were labeled with either HTRA-1 (left) or FABP (right) antibodies. Total fluorescence values were averaged over six high-power fields (HPFs) and normalized to background fluorescence from a non-specific channel for the same HPF. HPFs were selected for villous and, therefore, fetal surface placenta only. Statistical comparisons were performed using a two-tailed t-test. ∗P < 0.05, ∗∗P < 0.01.
Figure 3.
Placental high temperature requirement A serine peptidase-1 (HTRA-1) protein expression is decreased for both fetal and maternal surface inflammation compared with tissue without inflammation. Placental tissues were labeled with HTRA-1 antibodies, and total fluorescence in six high-power fetal tissue fields was averaged and then normalized to background fluorescence. Statistical associations were determined using a two-tailed t-test. n = 12 placental tissues with fetal inflammation; n = 13 placental tissues with maternal inflammation; n = 25 placental tissues with no inflammation. ∗P < 0.05.
HTRA-1 and VEGF-A Longitudinal Postnatal Plasma Protein Levels Are Significantly Different for Infants with Acute Placental Inflammation Compared with Those Without
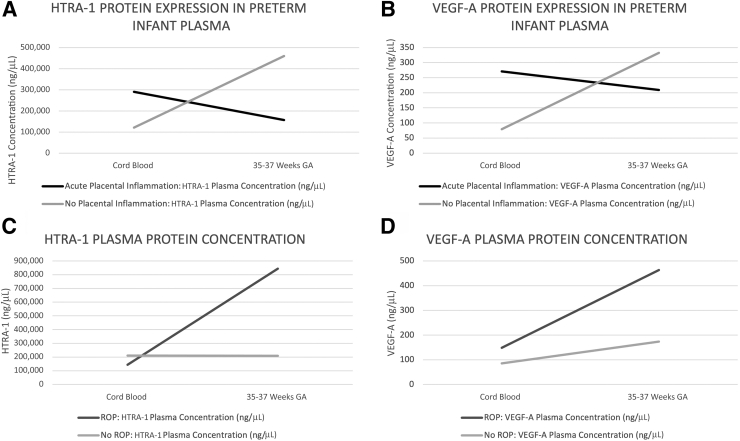
Candidate mediators within the postnatal circulation were examined for relevance to the period of ROP development. Increased expression of both the serine protease HTRA-1 and angiogenic growth factor VEGF-A has been associated with pathomechanisms underlying ROP development postnatally.17,43,44 Thus, the study sought to determine whether there were significant differences in plasma expression of these established risk mediators relative to the more novel risk modifier, acute placental inflammation. Expression of each protein was examined in preterm infant plasma at two time points, at preterm birth within cord blood, and during the gestational age window characterized by ROP pathogenesis, GA 35 to 37 weeks. This was done in the replication cohort, consisting of recruited preterm infants within the ROP GA and BW risk window from two institutions, as noted above. HTRA-1 expression was measured using enzyme-linked immunosorbent assay, and VEGF-A expression was measured using Luminex, as previously described.17 As shown in Figure 4, A and B, both HTRA-1 and VEGF-A expression were increased within cord blood from infants born in the setting of acute placental inflammation compared with those without. The inverse was true at GA 35 to 37 weeks as infants born preterm in the absence of placental inflammation demonstrated elevated HTRA-1 and VEGF-A, whereas those born in the presence of placental inflammation demonstrated reduced VEGF-A and HTRA-1 plasma expression (Figure 4). When analyzed relative to preterm infant ROP development, as shown in Figure 4, C and D, infants who developed ROP demonstrated a greater degree of increase in both HTRA-1 and VEGF-A at 35 to 37 weeks’ GA than infants who did not develop ROP.
Figure 4.
Vascular endothelial growth factor (VEGF)-A and high temperature requirement A serine peptidase-1 (HTRA-1) plasma concentrations differ longitudinally for preterm infants having gestations with or without acute placental inflammation or retinopathy of prematurity (ROP) development. Plasma was isolated from cord or peripheral blood samples, and HTRA-1 (A and C) or VEGF-A (B and D) protein was analyzed using Luminex (VEGF-A) or enzyme-linked immunosorbent assay (HTRA-1). Peripheral blood samples were collected between postnatal weeks 35 and 37, corresponding to the gestational age (GA) range during which ROP disease typically is present. Data were stratified by presence or absence of acute placental inflammation or development of ROP, and average values were plotted longitudinally for each outcome measure.
Whole Transcriptome Analysis Identifies Significantly Differentially Expressed Genes Relative to Placental Inflammation
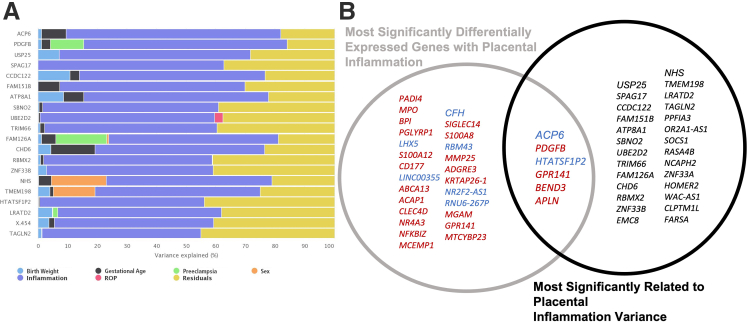
An agnostic analysis approach was adopted to identify novel mediators underlying the inverse risk association between infectious acute placental inflammation and ROP development. Whole transcriptome gene expression analysis was performed on fetal surface placental tissue from preterm infants within the replication cohort [(acute inflammation, n = 13; control, n = 17) (ROP, n = 14; control, n = 16)]. DEGs relative to placental inflammation or postnatal ROP development were identified. DEGs were generated, as previously published, by filtering raw gene counts to remove features with 0 counts and <10 reads in every sample and identifying differentially expressed genes using a 5% false discovery rate (false discovery rate, <5%) with DESeq2 version 1.30.1.24,31 After correction for multiple testing, 35 genes were identified with significant differential expression relative to placental inflammation, and 234 genes were identified relative to infant ROP development (adjusted P ≤ 0.05). Furthermore, variance analysis identified 33 genes within the transcriptome data that demonstrated expression variance significantly related to placental inflammation rather than other queried variables, including birth weight, gestation age, sex, ROP development, or maternal preeclampsia (Figure 5A). Venn overlap between genes with inflammation-specific variance and inflammation DEGs demonstrated six genes (Figure 5B) that account for inflammation variance, but for which expression-level changes also significantly correlated with acute placental inflammation. Finally, four significant DEGs were identified, shared between placental inflammation and infant ROP development, including LHX5, ACAP1, SIGLEC14, and ADGRE3. Of these genes, LHX5 and ADGRE3 demonstrated inverse gene expression changes relative to inflammation (increased expression for ADGRE3; and decreased expression for LHX5) versus ROP development (decreased expression for ADGRE3; and increased expression for LHX5). ACAP1 and SIGLEC14 demonstrated increased expression in both ROP and placental inflammation clinical backgrounds.
Figure 5.
Genes with expression variance most related to placental inflammation. A: Variance analysis identified genes within the whole transcriptome data set that demonstrated expression variance most significantly related to placental inflammation (denoted in blue) as opposed to other queried variables, including birth weight, gestational age, retinopathy of prematurity (ROP), preeclampsia, or sex. B: Venn diagram modeling for significant placental inflammation–related differentially expressed genes (gray circle) and genes with variance most related to placental inflammation (black circle). Up-regulated genes are denoted in red, and down-regulated genes are denoted in blue. As seen in the Venn overlap, six genes are both significantly related to placental inflammation and gene expression is most significantly related to placental inflammation.
Pathway Analysis Demonstrates Inversely Enriched Pathways for Placental Inflammation Compared with ROP Development
To examine the global differences in placental gene expression, the pathway analysis was performed using fast gene set enrichment package32 (gene set enrichment analysis).33 The Hallmark database was used to identify significantly enriched pathways within DEGs from placental tissues characterized by acute inflammation or from infants who developed ROP. As shown in Figure 6, several pathways were identified demonstrating inverse enrichment for acute placental inflammation compared with ROP phenotypes, including the following: coagulation, epithelial-to-mesenchymal transition, E2F targets, G2M checkpoint, and MYC targets version 2.
Figure 6.
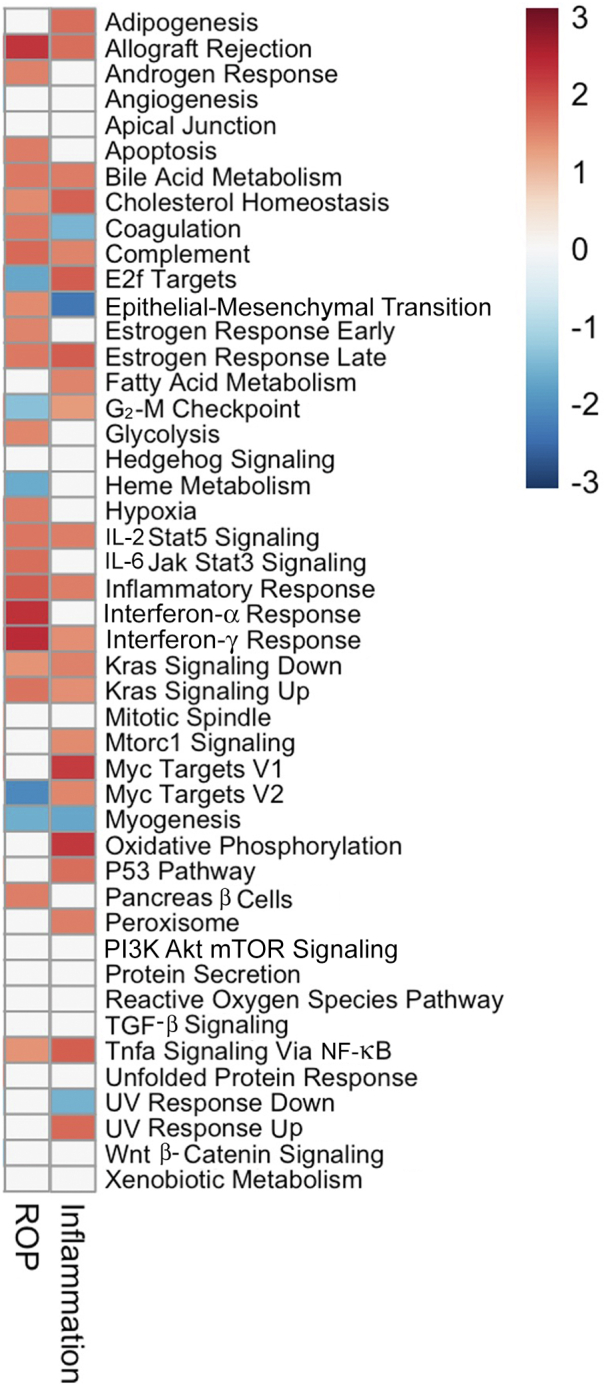
Gene set enrichment analysis (GSEA) demonstrates inverse pathway enrichment for placental tissues characterized by acute inflammation compared with those associated with preterm infant retinopathy of prematurity (ROP) development. GSEA was performed on differentially expressed genes significantly associated with either the ROP or acute placental inflammation clinical outcomes within whole transcriptome data. Jak, Janus kinase; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase; TGF-β, transforming growth factor-β.
Differential Methylation Analysis Demonstrates DMRs Corresponding to Placental Inflammation with Relevance to Whole Transcriptome Data
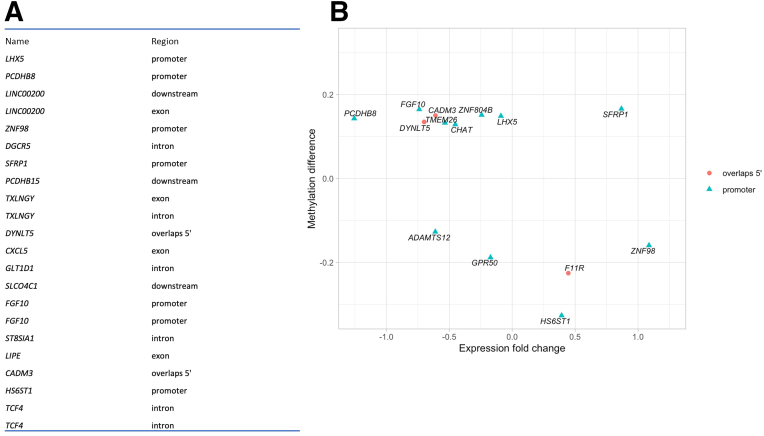
To determine the role for DNA modification in placental gene expression, DNA from fetal surface placental tissues within the recruited patient cohort was analyzed (acute inflammation, n = 9; and control, n = 7). This analysis was based on the recent precedent for enriched differential placental DNA methylation within inflammatory genes relative to preterm infant ROP phenotype.45 It identified 2209 differentially methylated regions with an area P < 0.1. Plotting the distances to the 5′ end of the nearest transcript identified differentially methylated regions within promoter, 5′ untranslated region, exonic, downstream, and intronic regions; most regions were hypermethylated, as denoted in Table 5. To determine the potential relevance of these data to the whole transcriptome data set, 225 genes with a false discovery rate <90% were identified. Combining the 2209 DMRs to the whole transcriptome data resulted in 1626 unique genes with DMRs, 1101 genes expressed in the whole transcriptome data, and 62 DMRs associated with genes with a false discovery rate <90%. Figure 7 denotes the overlapping DMR with greatest significance (Figure 7A) and DMR within promoter or 5′ untranslated regions that demonstrates significant overlap in the whole transcriptome data (Figure 7B).
Table 5.
DMRs Correlating with Acute Placental Inflammation
| DMR region | DMRs hypomethylated, N | DMRs hypermethylated, N | % Hypermethylated |
|---|---|---|---|
| Upstream | 85 | 219 | 72 |
| Promoter | 102 | 276 | 73 |
| 5′ UTR | 38 | 57 | 60 |
| Exon | 87 | 239 | 73.3 |
| Intron | 266 | 460 | 63.4 |
| Downstream | 123 | 257 | 67.6 |
Fetal surface placental DNA from the recruited patient cohort was analyzed for DMR using Infinium MethylationEPIC BeadChip. Both hypomethylated and hypermethylated DMRs were identified, although hypermethylated regions were statistically more common, as evidenced in the percentage hypermethylated.
DMR, differentially methylated region; UTR, untranslated region.
Figure 7.
Differentially methylated DNA regions significantly correlating with placental inflammation. DNA was extracted from placental tissues corresponding to patients within our recruited patient cohort. Analysis for differentially methylated regions (DMRs) was performed using the Infinium MethylationEPIC BeadChip; data were stratified by the presence or absence of acute placental inflammation. A: Significant DMRs within promoter, 5′ untranslated region (UTR), exonic, downstream, and intronic regions were identified; those with greatest association are listed. B: There is significant interaction between DMRs within promoter and 5′ UTR regions and significant differentially expressed genes within whole transcriptome data.
Discussion
Herein, the study demonstrated an inverse association between preterm infant ROP development and the presence of acute placental inflammation using logistic regression. This inverse association was most significant for gestations without maternal preeclampsia and was best measured when controlling for the collinearity of GA. Finally, candidate protein associations and identify novel potential mediators were identified using a transcriptome-level agnostic approach, which may underly decreased ROP risk in the setting of placental inflammation.
Disparate associations have been reported between acute placental inflammation and ROP development. The current work demonstrating a protective relationship, more marked within a non-preeclamptic population, supports recent findings within a fetal growth–restricted population, lacking preeclamptic gestational exposure.15 Also consistent with this group’s findings, the association between acute placental inflammation and ROP development was best determined when controlling for the confounding significance of GA, as noted in the logistic regression. Indeed, as noted in Tables 2 and 3, a significant inverse association between ROP development and acute placental inflammation was seen only when accounting for the role of GA. Therefore, the current work suggests that the disparate associations reported between placental inflammation and ROP development may be related to confounding effects of GA, or different risk associations based on gestational exposures, such as preeclampsia.
The candidate approach herein examined differential protein expression within placental tissues as well as preterm infant cord or peripheral blood. At the placental level, significant protein expression differences were identified in HTRA-1 and FABP4 within placental tissues with or without acute inflammation using semiquantitative immunofluorescence. Specifically, decreased expression of both proteins within acute placental inflammation was observed. Certainly, reduced HTRA-1 and FABP4 have been associated with reduced ROP risk in humans and in animal models.17,34 This is the first time these changes have been found within the placenta and relative to inflammatory histology. However, it is possible that the relationship to placental inflammation and preterm infant ROP risk is based on these established risk relationships. Herein, the expectation when moving the candidate analysis to the peripheral circulation was that the cord blood would mirror protein changes identified within the placenta. Therefore, it was surprising to see increased HTRA-1 as well as VEGF-A within preterm infant cord blood, as increased levels of each protein are associated with ROP disease.17,44 However, longitudinal analysis demonstrated that, for infants born in the setting of placental inflammation, plasma HTRA-1 and VEGF-A expression decreased during gestational weeks 35 to 37, during which time ROP disease typically develops. The opposite was true for infants born in the absence of placental inflammation; in these preterm infants, an increase in plasma HTRA-1 and VEGF-A was observed. This suggests a differential risk postnatal longitudinal course relative to gestational exposures and supports the hypothesis that gestational environments characterized by acute infectious placental inflammation modify established ROP risk variables in a way that reduces ROP development. Indeed, this is consistent with a protective role for placental inflammation in ROP risk as infants who developed ROP in this same cohort demonstrated a greater degree of protein increase at 35 to 37 weeks than infants who did not develop ROP. Thus, within this replication cohort, decreased levels of plasma HTRA-1 and VEGF-A within the 35 to 37 weeks’ gestational age window correlated with presence of placental inflammation in utero and absence of ROP development postnatally.
The agnostic transcriptome-level analysis identified numerous DEGs within placental tissues relative to both ROP and acute placental inflammation outcome measures. There was an overlap between these DEGs as well as within enriched pathways represented by these DEGs. More importantly, the pathways with inversely enriched significance for placental inflammation compared with preterm infant ROP development, including coagulation, epithelial-to-mesenchymal transition, E2F targets, G2M checkpoint, and MYC targets version 2, have been shown to play significant roles in ROP pathogenesis46 and retinal function.47,48
Finally, the study identified numerous placental DNA DMRs that were significantly associated with placental inflammation. The vast majority of these were hypermethylated and, thus, expected to inhibit transcription and silence expression. These findings build on recently published work demonstrating significant DMR placental DNA within inflammatory pathways correlating with ROP outcomes.45 The analysis overlying the differentially methylated data set with the whole transcriptome analysis supported these findings. For example, LIM homeobox family 5 (LHX5) was identified within the DEG as significantly associated with placental inflammation as well as ROP development, notably with inverse expression that is decreased in placental inflammation and increased in ROP clinical outcomes. LHX5 gene was identified as a significantly hypermethylated region relative to placental inflammation. Thus, this analysis may have identified a novel mediator of ROP risk as well as started to define the mechanism for regulation within the placenta.
Limitations of this study lay the foundation for future studies. Additional work is needed to clarify the effect of antenatal maternal exposures (eg, to antibiotics and steroids) on this described protective association. Within the reported discovery and replication cohorts, an average of 83.2% of women received steroid and/or antibiotic treatment antenatally. This is common in the setting of preterm labor or suspected infection. However, the role for these interventions in shaping the placental microenvironment and associated ROP risk is important and requires further study. In addition, the candidate study suggested that placental inflammation can influence expression of established ROP risk mediators, although causation is not fully elucidated. Further mechanistic interrogation of this hypothesis as well as novel mediators identified within the agnostic analysis is needed to determine the full complement of molecular mediators underlying the protective relationship between acute placental inflammation and ROP development.
Taken together, this work demonstrates a protective association between infectious placental inflammation and preterm infant ROP development. It also built on recent work demonstrating that this protective association differs relative to other gestational exposures, such as maternal preeclampsia.15 This understanding may lead to improved ROP risk stratification for preterm infants, although it also may shed light on potential mediators of ROP risk or protection. Therefore, both a candidate analysis, examining established ROP mediators within placental and peripheral tissues with disparate placental inflammation, as well as an agnostic analysis aimed at identification of novel molecular mediators of preclinical ROP pathogenesis, were explored. Notably, differing longitudinal courses were identified for established risk factors within the peripheral circulation relative to the presence or absence of placental inflammation. Additionally, novel placental gene expression changes correlating with placental inflammation and ROP development were identified. Although, going forward, individual genes may be important mediators to study, this study identified inverse pathway enrichment within placental tissues characterized by acute inflammation or preterm infant ROP development and DMRs with a role in placental inflammation and overlap with ROP DEGs. The genes and pathways identified herein have described roles in ROP and retinal cell biology, indicating that they may have relevance to ROP development. Future work will add to these findings to clarify mechanisms underlying risk associations and work toward utilizing this knowledge for advancement of preventive therapeutics.
Footnotes
Supported by the National Eye Institute1K08EY031800-03 (L.A.O.); NIH core grant EY014800; and a Research to Prevent Blindness (New York, NY) unrestricted grant (Department of Ophthalmology and Visual Sciences, University of Utah).
Disclosures: None declared.
Contributor Information
Leah A. Owen, Email: leah.owen@hsc.utah.edu.
Margaret M. DeAngelis, Email: mmdeange@buffalo.edu.
Author Contributions
L.A.O., M.M.D., and J.C. conceptualized the study; L.A.O., C.Z., C.C., M.M.D., K.S., and C.S. performed experiments; L.A.O., M.M.D., K.S., and L.D.K. validated the study; L.A.O., L.C., B.W., C.Z., M.M.D., and J.C. analyzed data; M.M.D., L.A.O., and L.D.K. obtained resources; M.M.D., L.A.O., and J.C. curated data; M.M.D., L.A.O., C.F., C.C.Y., and J.C. wrote the original draft; M.M.D., L.A.O., and J.C. supervised the study; M.M.D. and L.A.O. administered the project; and L.A.O. acquired funding.
References
- 1.Owen L.A., Hartnett M.E. Current concepts of oxygen management in retinopathy of prematurity. J Ophthalmic Vis Res. 2014;9:94–100. [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll L., Owen L.A. Current evidence and outcomes for retinopathy of prematurity prevention: insight into novel maternal and placental contributions. Explor Med. 2020;1:4–26. doi: 10.37349/emed.2020.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J., Stahl A., Hellstrom A., Smith L.E. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr. 2011;23:173–178. doi: 10.1097/MOP.0b013e3283423f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owen L.A., Morrison M.A., Hoffman R.O., Yoder B.A., DeAngelis M.M. Retinopathy of prematurity: a comprehensive risk analysis for prevention and prediction of disease. PLoS One. 2017;12:e0171467. doi: 10.1371/journal.pone.0171467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slidsborg C., Jensen A., Forman J.L., Rasmussen S., Bangsgaard R., Fledelius H.C., Greisen G., la Cour M. Neonatal risk factors for treatment-demanding retinopathy of prematurity: a Danish national study. Ophthalmology. 2016;123:796–803. doi: 10.1016/j.ophtha.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Sharma A., Sood B.G., Qureshi F., Xin Y., Jacques S.M. Chronic inflammatory placental lesions correlate with bronchopulmonary dysplasia severity in extremely preterm infants. Pediatr Dev Pathol. 2021;24:430–437. doi: 10.1177/10935266211013625. [DOI] [PubMed] [Google Scholar]
- 7.Mestan K.K., Check J., Minturn L., Yallapragada S., Farrow K.N., Liu X., Su E., Porta N., Gotteiner N., Ernst L.M. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta. 2014;35:570–574. doi: 10.1016/j.placenta.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Çakir U., Yildiz D., Kahvecioğlu D., Okulu E., Alan S., Erdeve Ö., Heper A.O., Atasay B., Arsan S. Placenta, secret witness of infant morbidities: the relationship between placental histology and outcome of the premature infant. Turk Patoloji Derg. 2019;35:28–35. doi: 10.5146/tjpath.2018.01443. [DOI] [PubMed] [Google Scholar]
- 9.Catov J.M., Scifres C.M., Caritis S.N., Bertolet M., Larkin J., Parks W.T. Neonatal outcomes following preterm birth classified according to placental features. Am J Obstet Gynecol. 2017;216:411.e1–411.e14. doi: 10.1016/j.ajog.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Franklin A., Yallapragada S., Birkett R., Grobman W., Ernst L.M., Mestan K. The impact of placental pathology discordance in multiple gestation pregnancies on bronchopulmonary dysplasia-associated pulmonary hypertension. Pulm Circ. 2020;10 doi: 10.1177/2045894020910674. 2045894020910674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zein H., Mohammad K., Leijser L.M., Brundler M.A., Kirton A., Esser M.J. Cord blood cytokine levels correlate with types of placental pathology in extremely preterm infants. Front Pediatr. 2021;9:136. doi: 10.3389/fped.2021.607684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scifres C.M., Parks W.T., Feghali M., Caritis S.N., Catov J.M. Placental maternal vascular malperfusion and adverse pregnancy outcomes in gestational diabetes mellitus. Placenta. 2017;49:10–15. doi: 10.1016/j.placenta.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Chen M.L., Allred E.N., Hecht J.L., Onderdonk A., VanderVeen D., Wallace D.K., Leviton A., Dammann O., ELGAN Study Placenta microbiology and histology and the risk for severe retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2011;52:7052–7058. doi: 10.1167/iovs.11-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitra S., Aune D., Speer C.P., Saugstad O.D. Chorioamnionitis as a risk factor for retinopathy of prematurity: a systematic review and meta-analysis. Neonatology. 2014;105:189–199. doi: 10.1159/000357556. [DOI] [PubMed] [Google Scholar]
- 15.Park J.Y., Park C.W., Moon K.C., Park J.S., Jun J.K., Lee S.J., Kim J.H. Retinopathy of prematurity in infants without fetal growth restriction is decreased with the progression of acute histologic chorioamnionitis: new observation as a protective factor against retinopathy of prematurity. Placenta. 2021;104:161–167. doi: 10.1016/j.placenta.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Redline R.W. Classification of placental lesions. Am J Obstet Gynecol. 2015;213(Suppl):S21–S28. doi: 10.1016/j.ajog.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 17.Owen L.A., Shirer K., Collazo S.A., Szczotka K., Baker S., Wood B., Carroll L., Haaland B., Iwata T., Katikaneni L.D., DeAngelis M.M. The serine protease HTRA-1 is a biomarker for ROP and mediates retinal neovascularization. Front Mol Neurosci. 2020;13:605918. doi: 10.3389/fnmol.2020.605918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ACOG practice bulletin no. 33: diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 19.Chiang M.F., Quinn G.E., Fielder A.R., Ostmo S.R., Paul Chan R.V., Berrocal A., Binenbaum G., Blair M., Peter Campbell J., Capone A., Chen Y., Dai S., Ells A., Fleck B.W., Good W.V., Elizabeth Hartnett M., Holmstrom G., Kusaka S., Kychenthal A., Lepore D., Lorenz B., Martinez-Castellanos M.A., Özdek Ş., Ademola-Popoola D., Reynolds J.D., Shah P.K., Shapiro M., Stahl A., Toth C., Vinekar A., Visser L., Wallace D.K., Wu W.C., Zhao P., Zin A. International classification of retinopathy of prematurity, third edition. Ophthalmology. 2021;128:e51–e68. doi: 10.1016/j.ophtha.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Committee for the Classification of Retinopathy of Prematurity The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 21.An international classification of retinopathy of prematurity: the committee for the classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 22.Good W.V., Hardy R.J. The multicenter study of early treatment for retinopathy of prematurity (ETROP) Ophthalmology. 2001;108:1013–1014. doi: 10.1016/s0161-6420(01)00540-1. [DOI] [PubMed] [Google Scholar]
- 23.Owen L.A., Morrison M.A., Ahn J., Woo S.J., Sato H., Robinson R., Morgan D.J., Zacharaki F., Simeonova M., Uehara H., Chakravarthy U., Hogg R.E., Ambati B.K., Kotoula M., Baehr W., Haider N.B., Silvestri G., Miller J.W., Tsironi E.E., Farrer L.A., Kim I.K., Park K.H., DeAngelis M.M. FLT1 genetic variation predisposes to neovascular AMD in ethnically diverse populations and alters systemic FLT1 expression. Invest Ophthalmol Vis Sci. 2014;55:3543–3554. doi: 10.1167/iovs.14-14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S., Lowe A., Dharmat R., Lee S., Owen L.A., Wang J., Shakoor A., Li Y., Morgan D.J., Hejazi A.A., Cvekl A., DeAngelis M.M., Zhou Z.J., Chen R., Liu W. Generation, transcriptome profiling, and functional validation of cone-rich human retinal organoids. Proc Natl Acad Sci U S A. 2019;116:10824–10833. doi: 10.1073/pnas.1901572116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen L.A., Kowalewski A.A., Lessnick S.L. EWS/FLI mediates transcriptional repression via NKX2.2 during oncogenic transformation in Ewing's sarcoma. PLoS One. 2008;3:e1965. doi: 10.1371/journal.pone.0001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortin J.P., Triche T.J., Hansen K.D. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics. 2017;33:558–560. doi: 10.1093/bioinformatics/btw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaffe A.E., Murakami P., Lee H., Leek J.T., Fallin M.D., Feinberg A.P., Irizarry R.A. Bump hunting to identify differentially methylated regions in epigenetic epidemiology studies. Int J Epidemiol. 2012;41:200–209. doi: 10.1093/ije/dyr238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12. [Google Scholar]
- 30.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 31.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korotkevich G., Sukhov V., Budin N., Shpak B., Artyomov M.N., Sergushichev A. Fast gene set enrichment analysis. bioRxiv. 2021 doi: 10.1101/060012. [Preprint] doi: [DOI] [Google Scholar]
- 33.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saint-Geniez M., Ghelfi E., Liang X., Yu C., Spencer C., Abend S., Hotamisligil G., Cataltepe S. Fatty acid binding protein 4 deficiency protects against oxygen-induced retinopathy in mice. PLoS One. 2014;9:e96253. doi: 10.1371/journal.pone.0096253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Y., Peng H., Wang P., Wang H., Dong M. Increased expression of fatty acid binding protein 4 in preeclamptic placenta and its relevance to preeclampsia. Placenta. 2016;39:94–100. doi: 10.1016/j.placenta.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Yan J.Y., Wang X.J. Expression and significance of adipocyte fatty acid-binding protein in placenta, serum and umbilical cord blood in preeclampsia. Zhonghua Fu Chan Ke Za Zhi. 2010;45:885–890. [PubMed] [Google Scholar]
- 37.Pan X., Jin X., Wang J., Hu Q., Dai B. Placenta inflammation is closely associated with gestational diabetes mellitus. Am J Transl Res. 2021;13:4068–4079. [PMC free article] [PubMed] [Google Scholar]
- 38.Trojnar M., Patro-Małysza J., Kimber-Trojnar Ż., Leszczyńska-Gorzelak B., Mosiewicz J. Associations between fatty acid-binding protein 4–a proinflammatory adipokine and insulin resistance, gestational and type 2 diabetes mellitus. Cells. 2019;8:227. doi: 10.3390/cells8030227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Z., Lin V., May A., Che B., Xiao X., Shaw D.H., Su F., Wang Z., Du H., Shaw P.X. HTRA1 synergizes with oxidized phospholipids in promoting inflammation and macrophage infiltration essential for ocular VEGF expression. PLoS One. 2019;14:e0216808. doi: 10.1371/journal.pone.0216808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao S.M., Crowley M., Louie S., Delgado O., Buchanan N., Stefanidakis M., Jaffee B. HtrA1 regulates the subretinal infiltration of microglia cells in response to bacterial lipopolysaccharides (LPS) and aging in mice. Invest Ophthalmol Vis Sci. 2013;54:3666. [Google Scholar]
- 41.Ahamed W., Yu R.M.C., Pan Y., Iwata T., Barathi V.A., Wey Y.S., Tun S.B.B., Qiu B., Tan A., Wang X., Cheung C.M.G., Wong T.Y., Yanagi Y. HTRA1 regulates subclinical inflammation and activates proangiogenic response in the retina and choroid. Int J Mol Sci. 2022;23:10206. doi: 10.3390/ijms231810206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell R.A., Campbell H.D., Bircher J.S., de Araujo C.V., Denorme F., Crandell J.L., Rustad J.L., Monts J., Cody M.J., Kosaka Y., Yost C.C. Placental HTRA1 cleaves α1-antitrypsin to generate a NET-inhibitory peptide. Blood. 2021;138:977–988. doi: 10.1182/blood.2020009021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong L., Nian H., Shao Y., Zhang Y., Li Q., Yi Y., Tian F., Li W., Zhang H., Zhang X., Wang F., Li X. PTB-associated splicing factor inhibits IGF-1-induced VEGF upregulation in a mouse model of oxygen-induced retinopathy. Cell Tissue Res. 2015;360:233–243. doi: 10.1007/s00441-014-2104-5. [DOI] [PubMed] [Google Scholar]
- 44.Aiello L.P., Pierce E.A., Foley E.D., Takagi H., Chen H., Riddle L., Ferrara N., King G.L., Smith L.E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulka C.M., Dammann O., Santos H.P., VanderVeen D.K., Smeester L., Fichorova R., O'Shea T.M., Fry R.C. Placental CpG methylation of inflammation, angiogenic, and neurotrophic genes and retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2019;60:2888–2894. doi: 10.1167/iovs.18-26466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh C., Benos A., Grenell A., Rao S., Anand-Apte B., Sears J.E. Hyperoxia inhibits proliferation of retinal endothelial cells in a Myc-dependent manner. Life. 2021;11:614. doi: 10.3390/life11070614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou M., Geathers J.S., Grillo S.L., Weber S.R., Wang W., Zhao Y., Sundstrom J.M. Role of epithelial-mesenchymal transition in retinal pigment epithelium dysfunction. Front Cell Dev Biol. 2020;8:501. doi: 10.3389/fcell.2020.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang N., Eckert K.A., Zomorrodi A.R., Xin P., Pan W., Shearer D.A., Weisz J., Maranus C.D., Clawson G.A. Down-regulation of HtrA1 activates the epithelial-mesenchymal transition and ATM DNA damage response pathways. PLoS One. 2012;7:e39446. doi: 10.1371/journal.pone.0039446. [DOI] [PMC free article] [PubMed] [Google Scholar]