Abstract
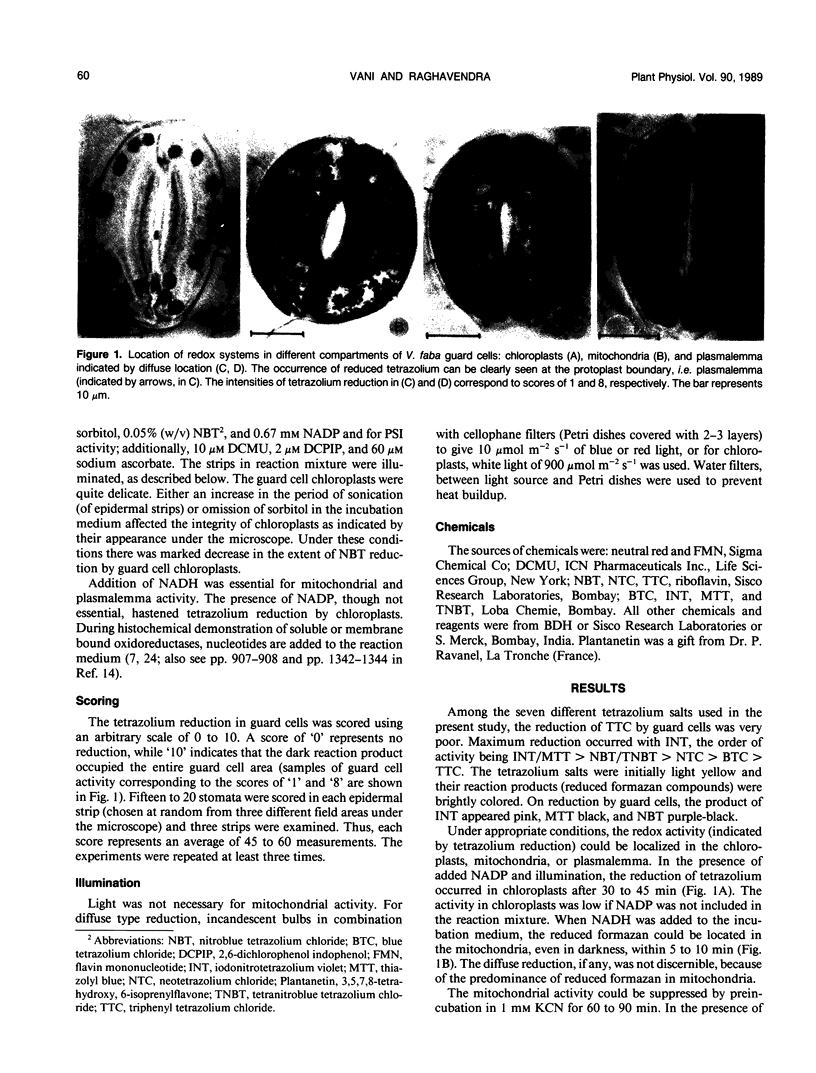
The stomata in the abaxial epidermis of Vicia faba were examined for the location of redox systems using tetrazolium salts. Three distinct redox systems could be demonstrated: chloroplast, mitochondrial, and plasmalemma. The chloroplast activity required light and NADP. Mitochondrial activity required added NADH and was suppressed by preincubation with KCN. The plasmalemma redox system in guard cells also required NADH, but was insensitive to KCN and was stimulated by blue light. The involvement of an NADH dehydrogenase in the blue light stimulated redox system in guard cells was suggested by the sensitivity to plantanetin, an inhibitor of NADH dehydrogenase. The redox system of mitochondria was the most active followed by that of plasmalemma. The activity of chloroplasts was the least among the three redox systems. The plasmalemma mediated tetrazolium reduction was stimulated by exogenous flavins and suppressed by Kl or phenylacetate, inhibitors of flavin excitation. We therefore conclude that an NADH-dependent, flavin mediated electron transport system, sensitive to blue light, operates in the plasmalemma of guard cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crane F. L., Sun I. L., Clark M. G., Grebing C., Löw H. Transplasma-membrane redox systems in growth and development. Biochim Biophys Acta. 1985 Aug 1;811(3):233–264. doi: 10.1016/0304-4173(85)90013-8. [DOI] [PubMed] [Google Scholar]
- Horwitz B. A., Gressel J. Elevated riboflavin requirement for postphotoinductive events in sporulation of a trichoderma auxotroph. Plant Physiol. 1983 Jan;71(1):200–204. doi: 10.1104/pp.71.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. B. The use of phenazine methosulfate for improving the histochemical localization of ketose reductase (L-iditol:NAD oxidoreductase, or sorbitol dehydrogenase). J Histochem Cytochem. 1967 Apr;15(4):207–215. doi: 10.1177/15.4.207. [DOI] [PubMed] [Google Scholar]
- Lin W. Further Characterization on the Transport Property of Plasmalemma NADH Oxidation System in Isolated Corn Root Protoplasts. Plant Physiol. 1984 Feb;74(2):219–222. doi: 10.1104/pp.74.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra A. S. Energy Supply for Stomatal Opening in Epidermal Strips of Commelina benghalensis. Plant Physiol. 1981 Feb;67(2):385–387. doi: 10.1104/pp.67.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanel P., Tissut M., Douce R. Platanetin: A Potent Natural Uncoupler and Inhibitor of the Exogenous NADH Dehydrogenase in Intact Plant Mitochondria. Plant Physiol. 1986 Feb;80(2):500–504. doi: 10.1104/pp.80.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K., Zeiger E. Cyclic and Noncyclic Photophosphorylation in Isolated Guard Cell Chloroplasts from Vicia faba L. Plant Physiol. 1985 Jun;78(2):211–214. doi: 10.1104/pp.78.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noorden C. J., Butcher R. G. Histochemical localization of NADP-dependent dehydrogenase activity with four different tetrazolium salts. J Histochem Cytochem. 1984 Sep;32(9):998–1004. doi: 10.1177/32.9.6747280. [DOI] [PubMed] [Google Scholar]