Abstract
Background
Idiopathic scoliosis is a three‐dimensional deformity of the spine. The most common form is diagnosed in adolescence. While adolescent idiopathic scoliosis (AIS) can progress during growth and cause a surface deformity, it is usually not symptomatic. However, in adulthood, if the final spinal curvature surpasses a certain critical threshold, the risk of health problems and curve progression is increased.
Objectives
To evaluate the efficacy of bracing for adolescents with AIS versus no treatment or other treatments, on quality of life, disability, pulmonary disorders, progression of the curve, and psychological and cosmetic issues.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, five other databases, and two trials registers up to February 2015 for relevant clinical trials. We also checked the reference lists of relevant articles and conducted an extensive handsearch of grey literature.
Selection criteria
Randomized controlled trials (RCTs) and prospective controlled cohort studies comparing braces with no treatment, other treatment, surgery, and different types of braces for adolescent with AIS.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration.
Main results
We included seven studies (662 participants). Five were planned as RCTs and two as prospective controlled trials. One RCT failed completely, another was continued as an observational study, reporting also the results of the participants that had been randomized.
There was very low quality evidence from one small RCT (111 participants) that quality of life (QoL) during treatment did not differ significantly between rigid bracing and observation (mean difference (MD) ‐2.10, 95% confidence interval (CI) ‐7.69 to 3.49). There was very low quality evidence from a subgroup of 77 adolescents from one prospective cohort study showing that QoL, back pain, psychological, and cosmetic issues did not differ significantly between rigid bracing and observation in the long term (16 years).
Results of the secondary outcomes showed that there was low quality evidence that rigid bracing compared with observation significantly increased the success rate in 20° to 40° curves at two years' follow‐up (one RCT, 116 participants; risk ratio (RR) 1.79, 95% CI 1.29 to 2.50). There was low quality evidence that elastic bracing increased the success rate in 15° to 30° curves at three years' follow‐up (one RCT, 47 participants; RR 1.88, 95% CI 1.11 to 3.20).
There is very low quality evidence from two prospective cohort studies with a control group that rigid bracing increases the success rate (curves not evolving to 50° or above) at two years' follow‐up (one study, 242 participants; RR 1.50, 95% CI 1.19 to 1.89) and at three years' follow‐up (one study, 240 participants; RR 1.75, 95% CI 1.42 to 2.16). There was very low quality evidence from a prospective cohort study (57 participants) that very rigid bracing increased the success rate (no progression of 5° or more, fusion, or waiting list for fusion) in adolescents with high degree curves (above 45°) (one study, 57 adolescents; RR 1.79, 95% CI 1.04 to 3.07 in the intention‐to‐treat (ITT) analysis).
There was low quality evidence from one RCT that a rigid brace was more successful than an elastic brace at curbing curve progression when measured in Cobb degrees in low degree curves (20° to 30°), with no significant differences between the two groups in the subjective perception of daily difficulties associated with wearing the brace (43 girls; risk of success at four years' follow‐up: RR 1.40, 1.03 to 1.89). Finally, there was very low quality evidence from one RCT (12 participants) that a rigid brace with a pad pressure control system is no better than a standard brace in reducing the risk of progression.
Only one prospective cohort study (236 participants) assessed adverse events: neither the percentage of adolescents with any adverse event (RR 1.27, 95% CI 0.96 to 1.67) nor the percentage of adolescents reporting back pain, the most common adverse event, were different between the groups (RR 0.72, 95% CI 0.47 to 1.10).
Authors' conclusions
Due to the important clinical differences among the studies, it was not possible to perform a meta‐analysis. Two studies showed that bracing did not change QoL during treatment (low quality), and QoL, back pain, and psychological and cosmetic issues in the long term (16 years) (very low quality). All included papers consistently showed that bracing prevented curve progression (secondary outcome). However, due to the strength of evidence (from low to very low quality), further research is very likely to have an impact on our confidence in the estimate of effect. The high rate of failure of RCTs demonstrates the huge difficulties in performing RCTs in a field where parents reject randomization of their children. This challenge may prevent us from seeing increases in the quality of the evidence over time. Other designs need to be implemented and included in future reviews, including 'expertise‐based' trials, prospective controlled cohort studies, prospective studies conducted according to pre‐defined criteria such as the Scoliosis Research Society (SRS) and the international Society on Scoliosis Orthopedic and Rehabilitation Treatment (SOSORT) criteria. Future studies should increase their focus on participant outcomes, adverse effects, methods to increase compliance, and usefulness of physiotherapeutic scoliosis specific exercises added to bracing.
Plain language summary
Braces for idiopathic scoliosis in adolescents
Review question
We reviewed the evidence about the effect of bracing on pulmonary disorders (lung diseases), disability, back pain, quality of life, and psychological and cosmetic issues in adolescent with idiopathic scoliosis. We found seven studies. We looked at randomized controlled trials (RCTs) and prospective controlled cohort studies (CCTs).
Background
Scoliosis is a condition where the spine is curved in three dimensions (from the back the spine appears to be shaped like an 's' and the trunk is deformed). It is often idiopathic, which means the cause is unknown. The most common type of scoliosis is generally discovered around 10 years of age or older, and is defined as a curve that measures at least 10° (called a Cobb angle; measured on x‐ray). Because of the unknown cause and the age of diagnosis, it is called adolescent idiopathic scoliosis (AIS).
While there are usually no symptoms, the appearance of AIS frequently has a negative impact on adolescents. Increased curvature of the spine can present health risks in adulthood and in older people. Braces are one intervention that may stop further progression of the curve. They generally need to be worn full time, with treatment lasting until the end of growth (most frequently, from a minimum of two to four/five years). However, bracing for this condition is still controversial, and questions remain about how effective it is.
Study characteristics
This review included seven studies, with a total of 662 adolescents of both genders. AIS from 15° to more than 45° curves were considered. Elastic, rigid (polyethylene), and very rigid (polycarbonate) braces were studied. The evidence is current to October 2013. Funding sources were not reported or external governmental or scientific agencies.
Key results
We did not find any results on pulmonary disorders and disability. Quality of life was not affected during brace treatment (very low quality evidence); quality of life, back pain, and psychological and cosmetic issues did not change in the long term (very low quality evidence). Rigid bracing seems effective in 20° to 40° curves (low quality evidence), elastic bracing in 15° to 30° curves (low quality evidence), and very rigid bracing in high degree curves above 45° (very low quality evidence); rigid was more successful than an elastic bracing (low quality evidence), and a pad pressure control system did not increase results (very low quality evidence). No specific harms were reported.
Primary outcomes such as pulmonary disorders, disability, back pain, psychological and cosmetic issues, and quality of life should be better evaluated in the future. Side effects, as well as the usefulness of exercises and other adjunctive treatments to bracing should be studied too.
Quality of the evidence
The evidence was moderate to very low quality. Reason for downgrading were evidence coming from few randomized trials with few participants and many lost at follow‐up or from observational prospective controlled studies. An issue in the field of AIS is the high rate of failure of RCTs, since parents want to choose with physicians the preferred treatment for their children. Thus, it is challenging to obtain high quality evidence in this field.
Summary of findings
Summary of findings for the main comparison. Brace compared with observation (randomized controlled trial) for idiopathic scoliosis in adolescents.
| Brace compared with observation (randomized controlled trial) for idiopathic scoliosis in adolescents | ||||||
| Patient or population: adolescents with idiopathic scoliosis Settings: Intervention: brace Comparison: observation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Observation (RCT) | Brace | |||||
| Quality of life PedsQL scores1 Follow‐up: 2 years | The mean quality of life in the control groups was 83.0 ± 13.2 (0‐100)2 | The mean quality of life in the intervention groups was 2.1 lower (7.69 lower to 3.49 higher) | ‐ | 111 (1 study) | ⊕⊝⊝⊝ very low3,4 | Higher scores indicating a better quality of life |
| Risk of success Curves remaining below 50° Follow‐up: 2 years | Study population | RR 1.79 (1.29 to 2.5) | 116 (1 study) | ⊕⊕⊝⊝ low5 | ‐ | |
| 415 per 1000 | 744 per 1000 (536 to 1000) | |||||
| Moderate | ||||||
| 415 per 1000 | 743 per 1000 (535 to 1000) | |||||
| Pulmonary disorders, disability, back pain, psychological issues, and cosmetic issues Subjective | Study population | Not estimable | 0 (0) | See comment | None of the included studies assessed these outcomes | |
| See comment | See comment | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Any adverse event Number of participants reporting at least 1 adverse event | See comment | See comment | Not estimable | 0 (0) | See comment | None of the included studies assessed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PedsQL: Pediatric Quality of Life Inventory; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 PedsQL, a generic quality‐of‐life instrument used in studies of acute and chronic illness (Varni 2001; Varni 2003).2 Scores range from 0 to 100, with higher scores indicating a better quality of life. 3 Unclear risk of selection bias for allocation concealment. 4 Only one study with 111 participants. 5 Only one study with 116 participants.
Summary of findings 2. Bracing compared with observation (cohort studies) for idiopathic scoliosis in adolescents.
| Brace compared with observation (cohort studies) for idiopathic scoliosis in adolescents | ||||||
| Patient or population: adolescents with idiopathic scoliosis Settings: Intervention: brace Comparison: observation (cohort studies) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Observation (cohort studies) | Brace | |||||
| Quality of life PedsQL score1 Follow‐up: 2 years | The mean quality of life in the control groups was 83.3 ± 13.3 (0‐100)2 | The mean quality of life in the intervention groups was 0.1 higher (3.9 lower to 4.1 higher) | ‐ | 236 (1 study) | ⊕⊝⊝⊝ very low3 | Higher scores indicating a better quality of life |
| Risk of success curves remaining below 50° Follow‐up: 2 years | 479 per 1000 | 719 per 1000 (570 to 906) | RR 1.5 (1.19 to 1.89) | 242 (1 study) | ⊕⊝⊝⊝ very low4 | Highly clinically relevant |
| Any adverse event number of participants with at least 1 adverse event Follow‐up: 2 years | 427 per 1000 | 542 per 1000 (410 to 713) | RR 1.27 (0.96 to 1.67) | 242 (1 study) | ⊕⊝⊝⊝ very low4 | ‐ |
| Pulmonary disorders, disability, back pain, psychological issues, and cosmetic issues subjective or objective | See comment | See comment | Not estimable | 0 (0) | See comment | None of the included studies assessed these outcomes |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PedsQL: Pediatric Quality of Life Inventory; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 PedsQL, a generic quality‐of‐life instrument used in studies of acute and chronic illness (Varni 2001; Varni 2003). 2 Scores range from 0 to 100, with higher scores indicating a better quality of life. 3 Only one observational study with 236 participants.4 Only one observational study with 242 participants.
Summary of findings 3. Brace and exercise compared with observation in high degree curves (cohort study) for idiopathic scoliosis in adolescents.
| Brace and exercise compared with observation in high degree curves (cohort study) for idiopathic scoliosis in adolescents | ||||||
| Patient or population: adolescents with idiopathic scoliosis Settings: Intervention: brace and exercise Comparison: observation in high degree curves (cohort study) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Observation in high degree curves (Cohort study) | Brace and exercise | |||||
| Quality of life | See comment | See comment | Not estimable | 0 (0) | See comment | None of the included studies assessed this outcome |
| Risk of success no progression over 50°, no fusion, no waiting list for fusion | Study population | RR 1.79 (1.04 to 3.07) | 57 (1 study) | ⊕⊝⊝⊝ very low1,2 | ‐ | |
| 444 per 1000 | 796 per 1000 (462 to 1000) | |||||
| Moderate | ||||||
| 444 per 1000 | 795 per 1000 (462 to 1000) | |||||
| Any adverse event number of participants with at least 1 adverse event | See comment | See comment | Not estimable | 0 (0) | See comment | None of the included studies assessed this outcome |
| Pulmonary disorders, disability, back pain, psychological issues, and cosmetic issues subjective or objective | Study population | Not estimable | 0 (0) | See comment | None of the included studies assessed these outcomes | |
| See comment | See comment | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Lost at follow‐up (19.3%), unbalanced between groups: 7.7% in the experimental group, 44.4% in the control group. 2 Only one study with 57 participants.
Summary of findings 4. Rigid versus elastic brace (randomized controlled trial) for idiopathic scoliosis in adolescents.
| Rigid versus elastic brace (randomized controlled trial) for idiopathic scoliosis in adolescents | ||||||
| Patient or population: adolescents with idiopathic scoliosis Settings: Intervention 1: rigid brace Intervention 2: elastic brace | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rigid versus elastic brace (RCT) | |||||
| Quality of life | See comment | See comment | Not estimable | 0 (0) | See comment | None of the included studies assessed this outcome |
| Risk of success curves remaining below 50° Follow‐up: 4 years | 682 per 1000 | 955 per 1000 (702 to 1000) | RR 1.4 (1.03 to 1.89) | 43 (1 study) | ⊕⊕⊝⊝ low1 | ‐ |
| Any adverse event number of participants with at least 1 adverse event | See comment | See comment | Not estimable | 0 (0) | See comment | None of the included studies assessed this outcome |
| Pulmonary disorders, disability, back pain, psychological issues, and cosmetic issues subjective or objective | See comment | See comment | Not estimable | 0 (0) | See comment | None of the included studies assessed these outcomes |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 only one study with 43 participants.
Background
Description of the condition
Scoliosis is a three‐dimensional deformity of the spine and the trunk (Negrini 2012). The most common form is idiopathic scoliosis (70% to 80% of cases) (Hresko 2013; Negrini 2012). Adolescent idiopathic scoliosis (AIS) is discovered at 10 years of age or older (Hresko 2013), and is defined as a curve of at least 10°, measured on a standing radiograph using the Cobb technique (Negrini 2012). While the prevalence of AIS is 0.9% to 12% in the general population (Grivas 2006), almost 10% of people diagnosed with AIS will require some form of treatment. Furthermore, up to 0.1% of the population is at risk of surgery (Lonstein 2006; Parent 2005). A severe form of AIS is more commonly found in females (80% to 90%). Typically, AIS does not cause any health problems during growth (except for extreme cases). However, the resulting surface deformity frequently has a negative impact on adolescents that can give rise to quality of life (QoL) issues and in the most severe cases, psychological disturbances (Freidel 2002a; Freidel 2002b; MacLean 1989; Reichel 2003). Adolescents are generally treated in an attempt to halt the progressive nature of the deformity. No treatments succeed in full correction to a normal spine, and even reduction of the deformity is difficult (Danielsson 2001a; Lonstein 2006). If scoliosis surpasses a critical threshold, usually considered to be 30° Cobb, at the end of growth, the risk of health problems in adulthood increases significantly (Lonstein 2006; Negrini 2006a; Weinstein 2003). Problems include reduced QoL, disability, pain, increased cosmetic deformity, functional limitations, pulmonary problems, and possible progression during adulthood (Danielsson 2001a; Danielsson 2003a; Danielsson 2003b; Grivas 2008; Mayo 1994; Negrini 2006a; Pehrsson 1992; Pehrsson 2001; Vasiliadis 2008; Weinstein 2003). Because of this, management of scoliosis also includes the prevention of secondary problems associated with the deformity (Negrini 2006b).
Description of the intervention
Treatment options for the prevention of AIS progression include exercises, bracing, and surgery (Fusco 2011; Lenssinck 2005; Negrini 2003; Negrini 2005; Negrini 2008a; Negrini 2009a; Negrini 2012; Rigo 2006; Romano 2008; Romano 2012; Romano 2013; Rowe 1997). Bracing can be defined as the application of external corrective forces to the trunk. This is usually achieved through rigid supports, but elastic bands are also used (Coillard 2003). Treatment commences when the curve is diagnosed as progressive or exceeds a threshold, which is considered to be above 20° Cobb, usually between 25° and 30° (Lonstein 2006; Negrini 2005; Richards 2005). Braces should generally be worn full‐time (at least 20 hours per day) with treatment usually lasting from a minimum of two to four or five years, until the end of bone growth (Katz 2001; Landauer 2003; Rahman 2005; SRS 2006). All this causes a significant impact on the lives of children and adolescents (Climent 1999; Noonan 1997; Odermatt 2003; Ugwonali 2004; Vasiliadis 2006).
How the intervention might work
The mechanical forces and the external and proprioceptive inputs of bracing can reduce unnatural loading and asymmetrical movements and improve neuromuscular control. This facilitates proper spinal growth, neuromotor re‐organization, and change of motor behaviours (Castro 2003; Coillard 2002; Grivas 2008; Lupparelli 2002; Negrini 2006c; Odermatt 2003; Smania 2008; Stokes 2006).
Why it is important to do this review
Currently, the bracing of adolescents with AIS is controversial. It is considered standard treatment in continental Europe, but not in many centres of the UK, US, and elsewhere (Altaf 2013; Hresko 2013). Bracing has been widely criticized because there is a paucity of evidence regarding its benefits (Dickson 1999a; Dickson 1999b; Dolan 2007a; Dolan 2007b; Goldberg 1993). Moreover, bracing has been linked to reduced QoL and increased psychological issues (Climent 1999; Fällström 1986; Noonan 1997; Ugwonali 2004; Vasiliadis 2006). To date, reviews on braces have been mainly narrative, have not considered the key issue of evaluating the methodological quality of the studies in the review, and have not included all existing studies (Dolan 2007b; Lenssinck 2005; Rowe 1997). Our previous Cochrane review was based on only two studies and found inconclusive evidence (Negrini 2010a). An update of this review will help clinicians to decide whether the sacrifices required by children to wear braces are indeed worthwhile.
Objectives
To evaluate the efficacy of bracing for adolescents with AIS versus no treatment or other treatments, on quality of life, disability, pulmonary disorders, progression of the curve, and psychological and cosmetic issues.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) and prospective controlled cohort studies.
Types of participants
We included all participants who were 10 years of age or older (until the end of bone growth) when diagnosed as having AIS. We included only studies in which bone maturity was evaluated by the Risser sign, wrist radiographs, or both. We excluded studies in which participants presented with any type of secondary scoliosis (congenital, neurological, metabolic, post‐traumatic, etc.) diagnosed according to the Scoliosis Research Society (SRS) (SRS 2006), and the international Society on Scoliosis Orthopedic and Rehabilitation Treatment (SOSORT) (Negrini 2012), criteria.
Types of interventions
We included all types of rigid, semi‐rigid, and elastic braces (defined as devices to apply external corrective forces to the spine and trunk), worn for a specific number of hours per day for a specific number of years. We considered all possible control interventions and comparisons.
Types of outcome measures
Primary outcomes
Pulmonary disorders, disability, back pain, QoL, and psychological and cosmetic issues. We included only validated measures of study outcomes, and we assessed minimal clinically important differences on a case‐by‐case basis.
Secondary outcomes
Clinical and radiographic parameters (Negrini 2006a; Negrini 2012). Very short (any result before the end of bone growth), intermediate (results at the end of bone growth), and long‐term (results in adulthood) outcomes. Progression of scoliosis was measured by:
Cobb angle in degrees (absolute values);
number of participants who had progressed by more than 5° Cobb (radiographic measurement error, considered as the minimal clinically important difference) (Negrini 2012);
risk of success, defined in terms of participants that at the end of treatment were neither treated surgically (fused) nor surpassing specific thresholds considered clinically meaningful (45° or 50°, or both) (Negrini 2012; Richards 2005);
Adverse effects, as outlined in identified trials.
Search methods for identification of studies
Electronic searches
For this update, we searched the following electronic databases to 17 and 18 February 2015 to identify relevant studies:
the Cochrane Register of Controlled Trials (CENTRAL, The Cochrane Library, which includes Cochrane Back Review Group Trials Register; Issue 1 of 12, January 2015);
MEDLINE (Ovid SP, 1946 to February week 2 2015);
MEDLINE In‐Process & Other Non‐Indexed Citations (Ovid SP, 13 February 2015);
EMBASE (Ovid SP, 1980 to week 7 2015);
Cumulative Index to Nursing and Allied Health Literature (CINAHL; EBSCO, 1981 to 18 February 2015);
PsycINFO (Ovid SP, 2002 to February week 2 2015);
Physiotherapy Evidence Database (PEDro);
Cochrane Back Review Group Trials Register (Reference Manager and Cochrane Register of Studies (CRS));
ClinicalTrials.gov;
World Health Organization (WHO) International Clinical Trials Registry Platform (WHO ICTRP);
PubMed.
As with the original review, we used the search strategies recommended by the Cochrane Back Review Group for the identification of RCTs (Furlan 2009), and adapted them to include cohort studies. The Cochrane Back Review Group Trials Search Co‐ordinator developed the strategies and used a combination of controlled vocabulary terms (e.g. MeSH terms) and keywords to describe methodology, disorders, and treatment. These methods were consistent with the Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Appendix 1, Appendix 2, and Appendix 3 show the strategies for each database.
Searching other resources
We also included the following strategies:
reference lists of all relevant papers;
main electronic sources of ongoing trials (National Research Register, meta‐Register of Controlled Trials; Clinical Trials);
grey literature, including conference proceedings, PhD theses, and unpublished work conducted by manufacturers that were likely to contain trials relevant to the review;
contacted investigators and authors in this field for information on unpublished or incomplete trials.
All searches included non‐English language studies. When considered likely to meet inclusion criteria, we translated studies published in languages other than English.
Appendix 4 and Appendix 5 show the sources handsearched and the years considered.
Data collection and analysis
Selection of studies
Two review authors (JBS, NC) independently evaluated the search results by reading the titles; two other review authors (TB, TM) independently reviewed the abstracts of the remaining papers. We obtained potentially relevant studies in full text and two review authors (TK, FZ) independently assessed them for inclusion. None of the papers was reviewed by any of the authors who may have written the original papers. At all stages, we resolved disagreements through discussion. The lead review author (SN) solved any persisting disagreements.
Data extraction and management
We prepared a standardized data extraction form, which we used to extract data from the included papers. Two review authors (SM, FZ) independently extracted data on the population, study characteristics, and results added to Review Manager 5.3 (RevMan 2012). We discussed any disagreements, and consulted the lead review author (SN) if disagreements persisted. We summarized key findings in a narrative format and assessed for inclusion in a meta‐analysis where possible.
Clinical relevance of results
The review authors assessed each trial for its clinical relevance by using the five questions outlined by Shekelle 1994 , and recommended by the Cochrane Back Review Group (Furlan 2009; Appendix 6). We assessed all important outcomes for each comparison. The main conclusions were clinical, because our main aim was to give clinicians state‐of‐the‐art information, according to relevant studies on this issue.
Assessment of risk of bias in included studies
We assessed the risk of bias of RCTs and controlled clinical trials (CCTs) in this review using the 12 criteria recommended by the Cochrane Back Review Group (Furlan 2009; Higgins 2011), as outlined in Appendix 7. We used the Newcastle‐Ottawa Scale (NOS scale) to assess the prospective cohort studies with a control group (Wells 2008). The NOS scale assesses three broad areas: selection bias, attrition bias, and detection bias. See Appendix 8 for details. For each included study, each type of bias was rated as high, low, or unclear and entered into the risk of bias table.
Two review authors, one with methodological expertise and one with content expertise, independently assessed the risk of bias of the included studies. The review authors resolved any disagreements by discussion, including input from a third independent review author if required. Risk of bias assessment was not blinded to trial authors, institution, or journal.
Measures of treatment effect
We analysed dichotomous outcomes by calculating the risk ratio (RR) for each trial, with the uncertainty in each result expressed with 95% confidence intervals (CI). We analysed continuous outcomes by calculating the mean difference (MD) or the standardized mean difference (SMD) with 95% CI.
Data synthesis
Meta‐analysis was not performed because the retrieved studies were too heterogeneous with regards to the study design, types of comparisons, populations included, and braces applied (elastic, rigid, very rigid). Therefore, we did not perform the pre‐planned investigations of heterogeneity, sensitivity analysis excluding studies with high risk of bias, and subgroup analysis for studies at low risk of bias. We assessed the overall quality of the evidence for each outcome. We used an adapted GRADE approach, as recommended by the Cochrane Back Review Group (Furlan 2009).
Factors that may decrease the quality of the evidence are study design and risk of bias, inconsistency of results, indirectness (not generalizable), imprecision (sparse data), and other factors (e.g. reporting bias and publication bias). The quality of the evidence for a specific outcome was downgraded by a level, according to the performance of the studies against these five factors.
High quality evidence: there are consistent findings among at least 75% of RCTs with low risk of bias, consistent, direct, and precise data and no known or suspected publication biases. Further research is unlikely to change either the estimate or our confidence in the results.
Moderate quality evidence: one of the domains is not met. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality evidence: two of the domains are not met. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality evidence: three of the domains are not met. We are very uncertain about the results.
No evidence: no RCTs were identified that addressed this outcome.
Results
Description of studies
Results of the search
We found 2479 titles with the electronic search (Figure 1), 13 studies with the handsearch, and 40 titles by searching Conference Proceedings and websites. After removing duplicates, we screened 859 titles and excluded 706 based on titles and 10 after reviewing the abstracts. We retrieved 143 full texts. We excluded 135 studies, one of which because we were unable to retrieve the full paper (Wessberg 2011). We wrote to the principal investigators but they did not respond. Both Coillard 2012 and Lusini 2013 agreed to send the final versions of articles that were under review for publication. Lusini 2013 has since been published. This resulted in seven included studies, two of which were reported in the original version of this review. Two studies added to Studies awaiting classification (Guo 2014; Wiemann 2014).
1.
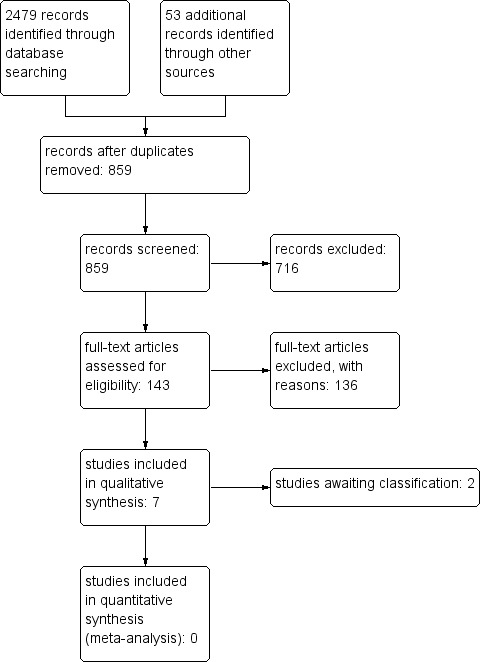
Study flow diagram.
Included studies
Seven articles met our inclusion criteria: five were planned as RCTs (Bunge 2008; Coillard 2012; Lou 2012; Weinstein 2013a; Wong 2008), and two as prospective controlled trials (Lusini 2013; Nachemson 1995). Two RCTs failed due to very low recruitment of participants (Bunge 2010; Weinstein 2013a).
The RCT by Weinstein 2013a focused on 25° to 40° curves. Unfortunately, 64.7% of adolescents refused to participate and 21% of adolescents and their parents rejected randomization; other adolescents were lost for numerous reasons. The final percentage of participants that could be allocated to the randomized arm was 10.6%, including 0.9% that crossed over groups. Due to this low inclusion rate, the authors extended the inclusion criteria to include adolescents with 20° curves. In addition, they transformed the study into a prospective controlled trial, including a randomized arm. This study was considered both as a prospective non‐randomized study with the all sample (Weinstein 2013a), and as randomized trial considering only the sub‐sample that was randomized (Weinstein 2013b).
Bunge 2010 aimed to recruit adolescents and compare braces with observation only; the study failed completely during the recruitment phase; so we excluded it from further consideration.
Thus, we included four randomized controlled trials/arms (Coillard 2012; Lou 2012; Weinstein 2013b; Wong 2008), and three prospective controlled trials (Lusini 2013; Nachemson 1995; Weinstein 2013a). One controlled prospective paper had a follow‐up at 16 years in a sub‐group of adolescents (Nachemson 1995).
Nachemson 1995 was a worldwide collaboration including hospitals from two continents; they observed two groups of clinicians, where the first group believed in the effectiveness of treatment with a brace, and the second group firmly believed that a brace was ineffective and thus managed people with careful observation; two centres of this last group treated adolescents with lateral electrical surface stimulation.
Types of treatments and comparisons: Braces included elastic bands (Coillard 2012; Wong 2008), rigid (polyethylene) (Lou 2012; Nachemson 1995; Weinstein 2013a; Weinstein 2013b; Wong 2008), and very rigid (polycarbonate) thoraco‐lumbo‐sacral orthosis (Lusini 2013). Two studies compared bracing with observation (Coillard 2012; Weinstein 2013a; Weinstein 2013b), one study compared bracing plus physiotherapeutic‐specific scoliosis exercises versus observation (Lusini 2013). One study compared rigid bracing with observation or electrical stimulation (Nachemson 1995). Two studies compared two different types of braces: rigid versus an elastic soft brace (Wong 2008), and two different rigid braces with the same number of hours wearing the brace every day (Lou 2012).
Duration of the trials: the duration was different among all included studies, with the range being between one and five years. Coillard 2012 had a follow‐up at five years post‐randomization, Lou 2012 had follow‐up at three years, and Lusini 2013 had follow‐up at two to nine years. In Nachemson 1995, after being treated until maturity (up to four years), a subset of all Swedish adolescents were followed up for 16 years after treatment (range 10.9 to 19.4 years), including a braced (Malmö; 41 participants) and observed (Göteborg; 65 participants) group.
Participants: 662 participants were included, of these 483 were treated with a brace, 133 observed, and 46 were prescribed a control treatment different from bracing (electrical stimulation) (Appendix 9). Studies were not completely homogeneous in terms of population characteristics. The mean age was approximately 12.5 years for all studies except Lusini 2013 (mean age above 14 years). In most studies, Cobb degrees were between 20° and 40°, apart from the studies of Coillard 2012 (15° to 30°) and Lusini 2013 (greater than 45°). The two studies evaluating elastic bracing focused on low degree curves (15° to 30° (Coillard 2012), and 20° to 30° (Wong 2008), while those using very rigid bracing focused on very high degree curves greater than 45° (Lusini 2013). Lou 2012 described neither the Cobb angles nor the age of the participants.
Outcomes: of the primary outcomes considered in this review, only QoL modifications due to bracing were considered by three papers: Weinstein 2013b used the PedsQL score (Varni 2001; Varni 2003), Nachemson 1995 used the SRS22 (Asher 2003a; Asher 2003b) and the 36‐item Short Form (SF=36) (Ware 1992; Wiklund 1991), and Wong 2008 used a purpose‐designed questionnaire. All the studies focused on the secondary outcome, scoliosis progression.
Countries in which the studies were conducted: one RCT was conducted in Hong Kong (Wong 2008), two in Canada (Coillard 2012; Lou 2012), and one was a multicentre study conducted in the US and Canada (Weinstein 2013b). One prospective cohort study was a multinational study conducted in three centres in the UK, four centres in the US, one centre in Canada, and two centres in Sweden (Nachemson 1995). The other prospective study was performed in Italy (Lusini 2013).
Excluded studies
We excluded 136 papers for the following main reasons: 45 were retrospective, 37 were prospective but without concurrent controls, and 53 were excluded for other reasons. Bunge 2008 was an RCT, but was excluded from the final analysis because of the low numbers of participants that agreed to participate and be randomized.
Risk of bias in included studies
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We judged the method of random sequence generation as low risk of bias in two RCTs (Coillard 2012; Weinstein 2013b). Random sequence generation was unclear in the other two RCTs (Lou 2012; Wong 2008). The allocation concealment was at low risk of bias in one RCT (Coillard 2012), and unclear in the remaining studies. It was at high risk of bias in the observational studies.
Blinding
Neither the RCTs nor the prospective cohort studies could be blinded for participants and providers because of the type of intervention assessed (brace). The risk of detection bias was high for all the studies for subjective outcomes (e.g. QoL or disability) and low for objective outcomes (e.g. Cobb degrees or scoliosis progression). The outcome assessor was not blinded in Coillard 2012, and was blinded in Weinstein 2013a, whereas blinding of the assessor was not reported in all other studies. Consequently, for subjective outcomes (e.g. self reported pain), we judged the risk of detection bias to be high for Coillard 2012, low for Weinstein 2013a, and unclear in the other studies, For objective outcomes, we rated detection bias as low because they are unlikely to be biased by lack of blinding.
Incomplete outcome data
Three RCTs reported no drop‐outs (Lou 2012; Weinstein 2013b; Wong 2008). We judged Coillard 2012 at high risk of attrition bias because there was a high rate of drop‐outs and this was unbalanced between groups. In two of the prospective cohort studies, the percentage of loss at follow‐up was unbalanced between groups (21% in the experimental group and 7% in the control group in Nachemson 1995; 7.7% in the experimental group and 44% in the control group in Lusini 2013). However, Lusini 2013 performed an intention‐to‐treat (ITT) analysis with worst‐case analysis considering loss at follow‐up as a failure for the outcome 'improvement', and as a success for the outcome 'scoliosis progression/fusion'. Consequently, we judged this study to be at low risk of attrition bias. We judged the Weinstein 2013a paper at low risk of bias because there was no loss at follow‐up.
Selective reporting
All studies were free of selective reporting.
Other potential sources of bias
In terms of group similarity at baseline, in two RCTs, groups were similar for the main prognostic factors (Coillard 2012;Wong 2008), in one RCT, no information was reported about the baseline characteristics of participants (Lou 2012). In one prospective cohort study, the brace group had more participants with severe scoliosis, fewer participants with imbalance, and fewer participants with menarche at baseline compared with the electrical stimulation or observation‐only groups (Nachemson 1995). Bunge 2010, Lusini 2013, and Weinstein 2013a reported no information about the similarity or differences of participants at baseline.
Two of the observational studies did not adjust for the most important confounding factors. Weinstein 2013a used propensity scores to reduce the effect of treatment selection bias, so we judged this study at low risk of bias due to confounding. Two studies did not report information on compliance and co‐interventions. Weinstein 2013a assessed compliance by temperature monitor data and self reported diary, so we judged it as being at low risk of bias due to non‐compliance. The timing of outcome assessment was similar across groups in all studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
1. Brace versus observation (randomized controlled trials)
Primary outcome measures
Pulmonary disorders, disability, back pain, psychological issues, and cosmetic issues
No studies assessed pulmonary disorders, disability, back pain, psychological issues, and cosmetic issues.
Quality of life
Two years' follow‐up:Weinstein 2013b (111 participants) found that the mean PedsQL did not differ significantly between bracing and observation (MD ‐2.10, 95% CI ‐7.69 to 3.49; Analysis 1.1).
1.1. Analysis.
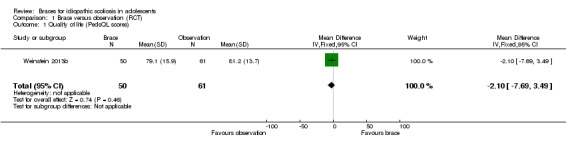
Comparison 1 Brace versus observation (RCT), Outcome 1 Quality of life (PedsQL scores).
Secondary outcome measures
Progression of scoliosis
Two years' follow‐up:Weinstein 2013b found the rate of success (curves remaining below 50°) was 38/51 in the brace group and 27/65 in the observation group (RR 1.79, 95% CI 1.29 to 2.50; Analysis 1.2). The results were in favour of brace.
1.2. Analysis.
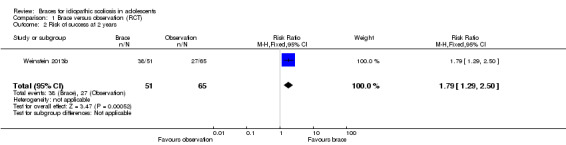
Comparison 1 Brace versus observation (RCT), Outcome 2 Risk of success at 2 years.
Three years' follow‐up:Coillard 2012 reported the rate of success (correction or stabilization, i.e. 5° or less curve progression) as 21/26 in the brace group and 9/21 in the control group(RR 1.88, 95% CI 1.11 to 3.20; Analysis 1.3). The results were in favour of brace.
1.3. Analysis.

Comparison 1 Brace versus observation (RCT), Outcome 3 Risk of success at 3 years.
Five years' follow‐up:Coillard 2012 found the rate of success was 19/26 in the brace group and 12/21 in the control group (RR 1.28, 95% CI 0.83 to 1.98; Analysis 1.4). There was no significant difference between groups.
1.4. Analysis.
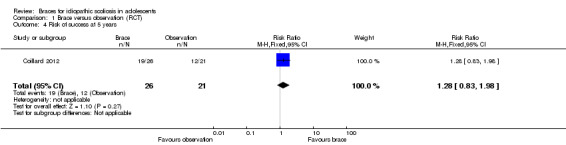
Comparison 1 Brace versus observation (RCT), Outcome 4 Risk of success at 5 years.
Participants with curves exceeding 45º at maturity:Coillard 2012 found that 3/21 (14.3%) participants in the control group and 3/26 (11.5%) participants in the treated group had Cobb angles that exceeded 45° at the end of study. Weinstein 2013b found that 13/51 participants in the brace group and 38/65 in the observation group reached 50° or more at the end of growth.
Participants who had undergone surgery or received a recommendation for surgery:Coillard 2012 reported that 3/21 (14.3%) immature participants required surgical fusion while in the trial. The mean curve magnitude at the beginning of the treatment in this particular group was 27° (range 20° to 30º) and they all had a Risser sign of 0. In the treated group, 2/26 (7.7%) immature participants were recommended surgery during the study and 1/26 treated participant was recommended surgery after three years following the end of treatment.
Adverse events
No studies assessed adverse events.
2. Brace versus observation or electrical stimulation (prospective cohort studies)
Primary outcome measures
Pulmonary disorders and disability
No studies assessed pulmonary disorders, and disability.
Quality of life, back pain, and psychological and cosmetic issues
Two years' follow‐up:Weinstein 2013a (236 participants) reported that the mean PedsQL for all participants included in the study did not differ significantly between bracing and observation (MD 0.10, 95% CI ‐3.90 to 4.10; Analysis 2.1).
2.1. Analysis.
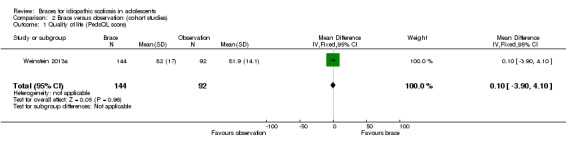
Comparison 2 Brace versus observation (cohort studies), Outcome 1 Quality of life (PedsQL score).
Long‐term (16 years) follow‐up: the Swedish cohort of Nachemson 1995 reported 16 years' follow‐up with 40 participants in the observation group and 37 participants in the brace group. Using the SRS22, they found no differences between groups for each of the sub‐scales and the total score (mean (SD); pain: 4.3 (0.7) with observation versus 4.4 (0.6) with brace; P value = 0.94; self image/appearance: 3.9 (0.8) with observation versus 3.9 (0.7) with brace; P value = 0.98; function/activity: 4.5 (0.5) with observation versus 4.5 (0.5) with brace; P value = 0.60; mental health: 4.1 (0.7) with observation versus 4.1 (0.7) with brace; P value = 0.93; satisfaction with management: 3.7 (1.0) with observation versus 3.8 (0.9) with brace; P value = 0.45; total score: 4.1 (0.5) with observation versus 4.2 (0.4) with brace; P value = 0.91).
Similarly, there were no differences using the SF‐36 (mean observation versus brace; physical functioning 94.5 (95% CI 91.9 to 97.1) versus 94.9 (95% CI 92.1 to 97.1); P value = 0.80; role physical: 93.1 (95% CI 87.3 to 98.9) versus 91.9 (95% CI 84.8 to 97.7); P value = 0.94; bodily pain: 75.0 (95% CI 67.4 to 82.5) versus 68.1 (95% CI 60.2 to 74.5); P value = 0.19; general health: 83.7 (95% CI 74.6 to 88.2) versus 79.8 (95% CI 75.1 to 83.6); P value = 0.15; vitality: 69.9 (95% CI 63.3 to 76.1) versus 68.2 (95% CI 61.6 to 73.7); P value = 0.78; social functioning: 91.9 (95% CI 86.7 to 97.0) versus 89.5 (95% CI 83.3 to 94.6); P value = 0.34; emotional aspects: 90.0 (95% CI 82.5 to 97.5) versus 86.5 (95% CI 76.5 to 94.6); P value = 0.79; mental health: 83.5 (95% CI 78.9 to 88.1) versus 81.3 (95% CI 76.2–85.4); P value = 0.51).
Secondary outcome measures
Progression of scoliosis
Two years' follow‐up:Weinstein 2013a examined rate of success (curves not evolving to 50° or above) among 146 braced and 96 observed participants. The rate of success was in favour of the bracing group (RR 1.50, 95% CI 1.19 to 1.89; Analysis 2.2).
2.2. Analysis.
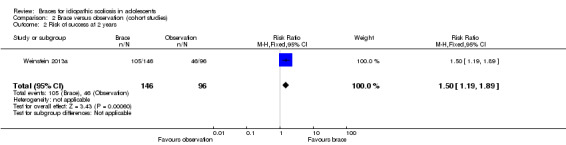
Comparison 2 Brace versus observation (cohort studies), Outcome 2 Risk of success at 2 years.
Three years' follow‐up:Nachemson 1995 reported that the success rates (defined as less than 6° increase of the curve) were 80% (95% CI 66% to 88%) for bracing, 46% (95% CI 25% to 56%) for observation, and 39% (95% CI 19% to 59%) for electrical stimulation. When comparing brace with observation, the results favoured the brace group (240 participants; RR 1.75, 95% CI 1.42 to 2.16; Analysis 2.3).
2.3. Analysis.

Comparison 2 Brace versus observation (cohort studies), Outcome 3 Risk of success at 3 years.
Four years' follow‐up:Nachemson 1995 reported that the success rates were 74% (95% CI 52% to 84%) for bracing, 34% (95% CI 16% to 49%) for observation, and 33% (95% CI 12% to 60%) for electrical stimulation (log‐rank test P value < 0.0001). When comparing brace with observation, the results favoured the brace group (240 participants; RR 2.22, 95% CI 1.70 to 2.90; Analysis 2.4). A worst‐case analysis for the bracing group in which the 23 participants who dropped out from the brace arm were considered to have had failed treatment, maintained a highly significant success in preventing progression of 6° or more until skeletal maturity (log‐rank test P value < 0.0005).
2.4. Analysis.
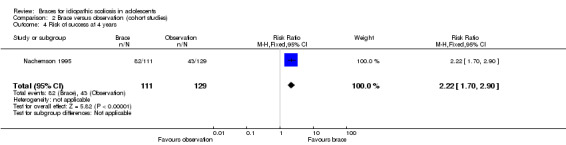
Comparison 2 Brace versus observation (cohort studies), Outcome 4 Risk of success at 4 years.
Long‐term (16 years) follow‐up:Nachemson 1995 found that participants braced or observed progressed more than 5° (range 5° to 21°). This progression meant that braced participants returned to the pre‐treatment levels (31.9° now versus 33.0° at start). Observed participants (excluding 11 who were braced and six who were fused during growth because of failure) showed an overall progression from the start of treatment of 6.4° (range 5° to 14°).
Adverse events
Two years' follow‐up:Weinstein 2013a found no difference between groups in the percentage of participants with any adverse event (RR 1.27, 95% CI 0.96 to 1.67; Analysis 2.5) and in the percentage of participants reporting back pain (which was the most common adverse event) (RR 0.72, 95% CI 0.47 to 1.10; Analysis 2.6). One serious adverse event, a hospitalization for anxiety and depression, was reported in one participant who wore a brace. Adverse events involving the skin under the brace were reported in 12/146 (8%) participants who wore a brace.
2.5. Analysis.

Comparison 2 Brace versus observation (cohort studies), Outcome 5 Any adverse event.
2.6. Analysis.
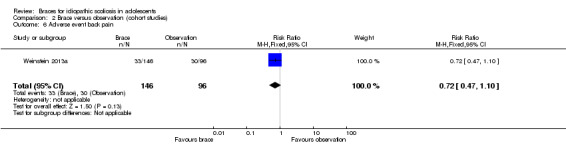
Comparison 2 Brace versus observation (cohort studies), Outcome 6 Adverse event back pain.
3. Brace and exercise versus observation in high‐degree curves (prospective cohort study)
Primary outcome measures
Pulmonary disorders, disability, back pain, psychological issues, cosmetic issues, and quality of life
The study did not assess pulmonary disorders, disability, back pain, psychological issues, cosmetic issues, and QoL.
Secondary outcome measures
Progression of scoliosis
Two to nine years' follow up:Lusini 2013 reported that the rate of success (no progression of 5° or more, no fusion, or no waiting list for fusion) was 25/33 in the brace group and 0/10 in observation group in the per‐protocol analysis (RR 15.21, 95% CI 1.00 to 230.23; Analysis 3.2) and 31/39 in the brace group and 8/18 in the observation group in the ITT analysis (RR 1.79, 95% CI 1.04 to 3.07; Analysis 3.3). The results were in favour of brace.
3.2. Analysis.
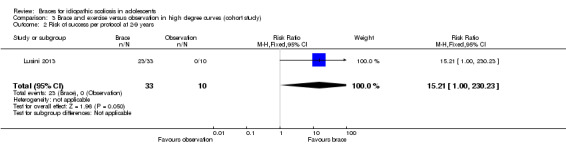
Comparison 3 Brace and exercise versus observation in high degree curves (cohort study), Outcome 2 Risk of success per protocol at 2‐9 years.
3.3. Analysis.

Comparison 3 Brace and exercise versus observation in high degree curves (cohort study), Outcome 3 Risk of success intention to treat at 2‐9 years.
Adverse events
The study did not assess adverse events.
4. Smart brace versus standard rigid brace (randomized controlled trial)
Primary outcome measures
Pulmonary disorders, disability, back pain, psychological issues, cosmetic issues, and quality of life
The study did not assess pulmonary disorders, disability, back pain, psychological issues, cosmetic issues, and QoL.
Secondary outcome measures
Progression of scoliosis
Lou 2012 (12 participants) found no significant difference between the Smart brace and the standard rigid brace. The Cobb angles (mean ± SD) were: pre‐brace 33 ± 6° with Smart brace versus 33 ± 6° with standard rigid brace; in brace: 20 ± 5° with Smart brace versus 21 ± 4° with standard rigid brace; three years after: 35 ± 7° with Smart brace versus 38 ± 9° with standard rigid brace. The in‐brace correction (% of initial Cobb angle) was 38 ± 3% with Smart brace versus 36 ± 5% with standard rigid brace.
Five years' follow‐up: risk of progression (mean ± SD): 60.2 ± 27% with Smart versus 63.4 ± 27% with standard rigid brace. At the end of treatment, the Cobb angle progressed by (mean ± SD) 2.2 ± 1.2° with Smartbrace versus 4.8 ± 8° with standard rigid brace.
Adverse events
The study did not assess adverse events.
Compliance
The participants in the Smart brace group were more likely to wear their brace at the prescribed level during day time activity compared with the standard rigid group (67% with Smart brace versus 54% with standard rigid brace).
5. Rigid brace versus elastic brace (randomized controlled trial)
Primary outcome measures
Pulmonary disorders, disability, back pain, psychological issues, and cosmetic issues
The study did not assess pulmonary disorders, disability, back pain, psychological issues, and cosmetic issues.
Quality of life
While the rigid brace caused significantly more problems with heat (85% with rigid brace versus 27% with elastic brace), as well as difficulties with donning and doffing, the participants using the elastic braces had difficulties with toileting (Wong 2008).
Secondary outcome measures
Progression of scoliosis
Four years' follow‐up:Wong 2008 found that, in participants with 20° to 30° Cobb angle before skeletal maturity, a rigid brace showed better results than an elastic brace (SpineCor) (risk of success defined as no progression more than 5°: RR 1.40, 95% CI 1.03 to 1.89; Analysis 4.2).
4.2. Analysis.
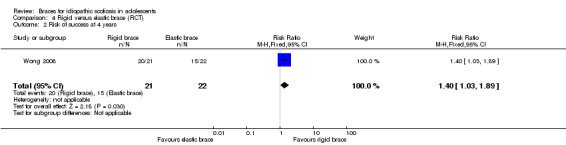
Comparison 4 Rigid versus elastic brace (RCT), Outcome 2 Risk of success at 4 years.
Adverse events
Wong 2008 did not assess adverse events.
Discussion
Summary of main results
Despite a comprehensive search of published and unpublished literature, we found only seven studies (one failed), which included 662 participants.
We did not find any results on pulmonary disorders and disability. There was moderate quality evidence from one small RCT (111 participants) that QoL did not differ significantly between rigid bracing and observation (Weinstein 2013b); QoL, back pain, and psychological and cosmetic issues did not change in the long term (16 years) (very low quality evidence) (Nachemson 1995). All included papers were consistent in showing that bracing prevented progression (secondary outcome): rigid bracing in 20° 40° curves (moderate quality evidence) (Nachemson 1995; Weinstein 2013a; Weinstein 2013b), elastic bracing in 15° to 30° curves (low quality evidence) (Coillard 2012), very rigid bracing in high degree curves above 45° (very low quality evidence) (Lusini 2013); rigid was more successful than elastic bracing (low quality evidence) (Wong 2008), and a pad pressure control system did not increase results (very low quality evidence) (Lou 2012). Nevertheless, due to the strength of evidence (from low to very low quality), further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
No specific harms have been reported. The high rate of failure of RCTs demonstrated the huge difficulties in performing RCTs in a field where parents reject randomization of their children: this questions the possibility of consistently increasing the strength of the actual evidence.
Overall completeness and applicability of evidence
The current evidence about brace treatment for AIS is of low to very low quality. Until now, four RCTs have been performed, two comparing two types of braces (Wong 2008; Lou 2012), and two comparing braces versus observation (Coillard 2012; Weinstein 2013b). In Coillard 2012 and Wong 2008, participants had a range of pathology below the most frequent indications for bracing (Negrini 2012), 15‐30° (Coillard 2012), and 20° to 30º (Wong 2008). On the contrary, in the classical range of 25° to 40° curves (Negrini 2012; Richards 2005), the implementation of RCTs is challenging. The members of one of the main scientific societies in the field, the SRS, which consists mainly of orthopaedic surgeons, were found to be in equipoise on bracing (Dolan 2007b), and were able to plan an RCT (Weinstein 2013b); conversely, members of the second main society, the conservative experts of SOSORT, rejected the possibility of performing an RCT (Negrini 2009b; Negrini 2012; Negrini 2014); they found this possibility comparable to an RCT on parachutes (Smith 2003). Despite these professional positions, the strongest argument against the possibility of performing RCTs comes from the reality that most parents (70% to 80% of cases) will not allow their children to be randomized. This was the main reason for failure of the two best efforts performed in recent years (Bunge 2008; Negrini 2014; Weinstein 2013a). In fact, while the Dutch RCT failed completely (Bunge 2010), the US trial (Weinstein 2013a), financed with more than USD 5 million by the US Government through the 'National Institute of Arthritis and Musculoskeletal and Skin Diseases', has finally been changed from an RCT to a CCT (Weinstein 2013a). In 2013, the ethical committee requested that the study be stopped due to the evident success of bracing (Weinstein 2013a), and for this reason, it was also possible to report the RCT data. Therefore, the probability of new, future RCTs of bracing versus observation is low. Clinicians in this field will rely on the current low quality evidence for many years to come. Bunge, the main Dutch researcher (an epidemiologist) concluded, "it is harder to perform a RCT that abolishes or postpones a treatment than a RCT that adds a new treatment" (Bunge 2010). Nevertheless, RCTs comparing different types or designs of braces (Lou 2012; Wong 2008), or different approaches have already been done and will presumably be performed in the future.
Apart from the research design used by Alf Nachemson (Nachemson 1995), the SRS Bracing Committee proposed another possible study design to address the methodological criteria for bracing studies (Richards 2005). Compliance and the standard of bracing should also be considered (Grivas 2012; Negrini 2009b). In fact, the wide range of results in brace studies (Dolan 2007a) usually leads to a discussion on the methodology of the study and the type of brace used, but the quality of bracing and participants' management should also be considered (Grivas 2012; Negrini 2009b). These have been addressed by the Society on Scoliosis Orthopaedic and Rehabilitation Treatment (SOSORT) with the Guidelines on "Standards of management of idiopathic scoliosis with corrective braces in daily clinics and in clinical research" (Negrini 2009b). The SRS and SOSORT criteria for bracing should be considered for the methodological and management standards to be followed in future research studies, and will allow meta‐analysis to be performed on solid methodological criteria.
Other fields to be explored are the importance of compliance and methods to increase compliance (Donzelli 2012; Katz 2010); the possible usefulness of physiotherapeutic scoliosis (specific or not) exercises (Negrini 2012; Zaina 2009); means to reduce the impact of bracing on participants, even if according to our results there is low quality evidence that it is not different from observation alone (Weinstein 2013a).
Clinical relevance
All included studies strongly mimic the clinical reality (high ecological and external validity). Two studies included only females, which reflects the fact that the majority (80% to 90%) of people with AIS are female (Nachemson 1995; Wong 2008). In fact, the limit of the current evidence comes from the difficulty previously discussed in performing a classical RCT (high internal, but usually low external validity).
Generally in the literature, and specifically in the retrieved studies within this review, outcomes other than Cobb degrees are barely considered. This reflects physicians' attitudes that during growth, their focus is on avoiding or at least curbing curve progression (secondary aim) to prevent future problems of QoL, disability, back pain, etc. (primary aims). This approach comes from the fact that scoliosis is progressive during growth, and if the curves surpass 30° Cobb at the end of growth, the risk of health problems in adulthood increases. Consequently, results reported in this review are clinically relevant, according to the current focus in the literature on Cobb degrees as the primary outcome. Nevertheless, the lack of focus on secondary adverse effects of treatment, as well as the absence of long‐term, primary outcome results (QoL, disability, pain) must be stressed and addressed in future studies.
No major risks of the intervention have been reported in the literature, apart from skin problems and anxiety (Weinstein 2013a), hot during summer with rigid bracing and difficulties in toileting with the elastic braces, that is, minor adverse effects (Wong 2008).
Quality of the evidence
Overall, the quality of evidence in favour of bracing alone or bracing plus exercise compared to observation or electrical stimulation is from low to very low quality. The included studies for these comparisons were two RCTs with only 47 and 116 participants. One RCT was at high risk of attrition bias, the other trial was at unclear risk of selection bias. The other included studies were three prospective cohort studies, two of which had a high attrition rate and no adjustment for potential confounding factors. In addition, the evidence for comparisons of different types of braces is low: only two RCTs with very small sample size and a high or unclear risk of bias across all domains of bias.
Note that since 80% to 90% of people with AIS are female, the inclusion of one study of only females was not considered to be a source of indirectness (Nachemson 1995;Wong 2008).
Potential biases in the review process
The strength of the review is the extensive and comprehensive searches conducted, including many different sources in many languages. Another strength is its high ecological validity, due to the real‐life situations considered in the studies. The main weakness of the review is the absence of strong studies in this field that do not make it possible to reach firm conclusions. Nevertheless, results among the studies included are fairly coherent. Two authors of this review were also authors of one of the primary studies (Lusini 2013); this paper was evaluated by the other review authors.
Agreements and disagreements with other studies or reviews
The previous Cochrane review was based on two studies only (Negrini 2010a; Negrini 2010b). In recent years, a number of well‐designed studies have been conducted, and as a whole, the current evidence is much stronger than that presented in the original review.
One "evidence‐based review" looked at entirely different outcomes from those considered here: the "rate of surgery" (failure of treatment) in braced groups ranged between 1.4% and 41% (Dolan 2007a). This paper was based on retrospective comparative studies, and on retrospective and prospective case series results, all of which we excluded from the current review. Furthermore, only papers in English were considered, while those adding exercises to bracing were excluded. It was not possible to obtain a good uniformity of methods and outcomes among papers, even if sub‐group analysis was attempted. These problems could be overcome following the SRS criteria for bracing studies (Richards 2005). Moreover, excluding papers that add exercises to bracing should not be done in the future, because, according to SOSORT criteria (Negrini 2009b), this is a management criterion to increase compliance. In fact, papers including exercises report very low surgery rates (2% to 7% for efficacy analysis, 10% to 14% for worst ‐case analysis), comparable to the best results in the bracing papers reported above (Maruyama 2003; Negrini 2008b; Negrini 2009a; Rigo 2003; Weiss 2003).
Authors' conclusions
Implications for practice.
Due to the important clinical differences among the studies, it was not possible to perform a meta‐analysis. We found no studies reporting pulmonary disorders and disability; one study showed that bracing did not change quality of life (QoL) during treatment (moderate quality evidence); QoL, back pain, and psychological and cosmetic issues did not change in the long term (16 years) (very low quality). All included papers were consistent in showing that bracing avoided progression (secondary outcome). Due to the strength of evidence (from moderate to very low, owing to the methodological quality of the studies), a good estimate of the effect remains uncertain. The high rate of failure of randomized controlled trials (RCTs) demonstrates the huge difficulties in performing RCTs in a field where parents reject randomization of their children: this questions the possibility of consistently increasing the strength of the actual evidence.
Implications for research.
Due to the difficulties in performing RCTs in this field, “expertise‐based" trials, where people are randomized to centres acting according to their preferred protocols, are a possible option. Together with controlled prospective trials, another option is studies conducted according to the SRS (Richards 2005) and SOSORT (Negrini 2009b) criteria for bracing to allow comparability, such as prospective multicentre cohort studies or prospective case series of participants treated and not treated. Other similar criteria for different populations would be important to allow future meta‐studies to be performed.
Moreover, any future study should significantly widen their focus on participant outcomes (not just radiographic outcomes of scoliosis progression) as well as adverse effects, so that balanced conclusions may be generated. Other fields to be explored are the importance of compliance and methods to increase compliance; the possible usefulness of physiotherapeutic exercises as well as means to reduce the impact of bracing on participants.
What's new
| Date | Event | Description |
|---|---|---|
| 19 February 2015 | New search has been performed | The literature search has been updated. 5 more studies incorporated and 2 studies added to Studies awaiting classification (Guo 2014; Wiemann 2014). |
| 27 February 2014 | New citation required and conclusions have changed | 5 new papers have been added: 3 RCTs (Bunge 2008, Lou 2012, Coillard 2012) and two prospective controlled trials (Lusini 2013, Weinstein 2013b). Weinstein 2013b also included a randomized arm (Weinstein 2013a). Since the last version of the review was published the quality of the evidence increased from very low to a range from moderate to very low. It was concluded that results were consistently in favour of bracing. |
Acknowledgements
We wish to thank all the Cochrane Back Group Editors, and particularly Vicki Pennick (first edition), Teresa Marin (second edition), and Rachel Couban, for their work and continuous help. Specifically, Rachel Couban who performed all the electronic searches.
Appendices
Appendix 1. MEDLINE and EMBASE search strategies
MEDLINE and MEDLINE Non‐Indexed and In‐Process Citations
Last searched 17 February 2015
1 Comparative Study/
2 exp Evaluation Studies/
3 exp Follow‐Up Studies/
4 exp Prospective Studies/
5 exp Cross‐Over Studies/
6 exp Epidemiologic Studies/
7 exp Case‐Control Studies/
8 exp Cohort Studies/
9 exp Cross‐Sectional Studies/
10 (cohort adj (study or studies)).mp.
11 cohort analy$.mp.
12 (follow up adj (study or studies)).mp.
13 (observational adj (study or studies)).mp.
14 longitudinal.mp.
15 retrospective.mp.
16 cross sectional.mp.
17 control$.mp.
18 prospective$.mp.
19 volunteer.mp.
20 or/1‐19
21 randomized controlled trial.pt.
22 controlled clinical trial.pt.
23 randomized.ab,ti.
24 placebo.ab,ti.
25 drug therapy.fs.
26 randomly.ab,ti.
27 trial.ab,ti.
28 groups.ab,ti.
29 or/21‐27
30 (animals not (humans and animals)).sh.
31 29 not 30
32 Animals/
33 Humans/
34 32 not (32 and 33)
35 29 not 34
36 20 not 34
37 35 or 36 or 31
38 exp Spinal Diseases/
39 exp Scoliosis/
40 scoliosis.mp.
41 or/38‐40
42 exp Braces/
43 brace$.mp.
44 bracing.mp.
45 exp Orthotic Devices/
46 exp Orthopedic Equipment/
47 limit 46 to yr="1902 ‐ 1975"
48 or/42‐45
49 47 or 48 (
50 exp Adolescent/
51 adolescen$.mp.
52 50 or 51
53 41 and 48 and 52
54 37 and 53
55 limit 54 to yr=2013‐2015
56 limit 54 to ed=20131009‐20150217
57 55 or 56
EMBASE
Last searched 17 February 2015. For this search, the animal study filter was updated and line 51 was changed from 34 and 51 to 34 or 51. See previous strategy below.
1 exp Clinical Study/
2 exp Case Control Study/
3 exp Family Study/
4 exp Longitudinal Study/
5 exp Retrospective Study/
6 exp Prospective Study/
7 exp Cohort Analysis/
8 (cohort adj (study or studies)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
9 (case control adj (study or studies)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
10 (follow up adj (study or studies)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
11 (observational adj (study or studies)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
12 (epidemiologic$ adj (study or studies)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
13 (cross sectional adj (study or studies)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
14 exp Comparative Study/
15 evaluation study.mp.
16 follow‐up study.mp. or exp Follow Up/
17 Crossover Procedure/
18 prospective$.mp.
19 exp VOLUNTEER/
20 or/1‐19
21 Clinical Article/
22 exp Clinical Study/
23 Clinical Trial/
24 Controlled Study/
25 Randomized Controlled Trial/
26 Major Clinical Study/
27 Double Blind Procedure/
28 Multicenter Study/
29 Single Blind Procedure/
30 Phase 3 Clinical Trial/
31 Phase 4 Clinical Trial/
32 crossover procedure/
33 placebo/
34 or/21‐33
35 allocat$.mp.
36 assign$.mp.
37 blind$.mp.
38 (clinic$ adj25 (study or trial)).mp.
39 compar$.mp.
40 control$.mp.
41 cross?over.mp.
42 factorial$.mp.
43 follow?up.mp.
44 placebo$.mp.
45 prospectiv$.mp.
46 random$.mp.
47 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
48 trial.mp.
49 (versus or vs).mp.
50 or/35‐49
51 34 or 50
52 20 or 51
53 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
54 human/ or normal human/ or human cell/
55 53 and 54
56 53 not 55
57 52 not 56
58 exp SPINE/
59 exp Spine Disease/
60 exp SCOLIOSIS/
61 exp Idiopathic Scoliosis/
62 scoliosis.mp.
63 or/58‐62
64 exp Brace/
65 brace$.mp.
66 bracing.mp.
67 exp ORTHOTICS/
68 exp orthopedic equipment/
69 or/64‐68
70 Adolescent/
71 adolescen#.mp.
72 70 or 71
73 63 and 69 and 72
74 57 and 73
75 limit 74 to yr=2013‐2015
76 limit 74 to em=201340‐201507
77 75 or 76
Previous search strategy for 2012 and 2013
1 exp Clinical Study/
2 exp Case Control Study/
3 exp Family Study/
4 exp Longitudinal Study/
5 exp Retrospective Study/
6 exp Prospective Study/
7 exp Cohort Analysis/
8 (cohort adj (study or studies)).mp.
9 (case control adj (study or studies)).mp.
10 (follow up adj (study or studies)).mp.
11 (observational adj (study or studies)).mp.
12 (epidemiologic$ adj (study or studies)).mp.
13 (cross sectional adj (study or studies)).mp.
14 exp Comparative Study/
15 evaluation study.mp.
16 follow‐up study.mp. or exp Follow Up/
17 Crossover Procedure/
18 prospective$.mp.
19 exp VOLUNTEER/
20 or/1‐19
21 Clinical Article/
22 exp Clinical Study/
23 Clinical Trial/
24 Controlled Study/
25 Randomized Controlled Trial/
26 Major Clinical Study/
27 Double Blind Procedure/
28 Multicenter Study/
29 Single Blind Procedure/
30 Phase 3 Clinical Trial/
31 Phase 4 Clinical Trial/
32 crossover procedure/
33 placebo/
34 or/21‐33
35 allocat$.mp.
36 assign$.mp.
37 blind$.mp.
38 (clinic$ adj25 (study or trial)).mp.
39 compar$.mp.
40 control$.mp.
41 cross?over.mp.
42 factorial$.mp.
43 follow?up.mp.
44 placebo$.mp.
45 prospectiv$.mp.
46 random$.mp.
47 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
48 trial.mp.
49 (versus or vs).mp.
50 or/35‐49
51 34 and 50
52 20 or 51
53 Human/
54 Nonhuman/
55 exp ANIMAL/
56 Animal Experiment/
57 54 or 55 or 56
58 53 not 57
59 52 not 57
60 58 or 59
61 exp SPINE/
62 exp Spine Disease/
63 exp SCOLIOSIS/
64 exp Idiopathic Scoliosis/
65 scoliosis.mp.
66 or/61‐65
67 exp Brace/
68 brace$.mp.
69 bracing.mp.
70 exp ORTHOTICS/
71 exp orthopedic equipment/
72 or/67‐71
73 Adolescent/
74 adolescen#.mp.
75 73 or 74
76 66 and 72 and 75
77 52 and 76
Appendix 2. CENTRAL and CINAHL search strategies
CENTRAL
Last searched 17 February 2015.
#1 MeSH descriptor: [Scoliosis] this term only
#2 scoliosis
#3 #1 or #2
#4 MeSH descriptor: [Braces] this term only
#5 braces in Trials
#6 bracing in Trials
#7 #4 or #5 or #6
#8 #3 and #7
#9 #8 Publication Year from 2013 to 2015, in Trials
CINAHL
Last searched 18 February 2015.
S14 S13 Limiters ‐ Published Date: 20131001‐20150231
S13 S12 and S9 and S5
S12 S11 or S10
S11 adolescen*
S10 (MH "Adolescence+")
S9 S8 or S7 or S6
S8 "bracing*"
S7 "brace*"
S6 (MH "Orthoses+")
S5 S4 or S3 or S2 or S1
S4 "scoliosis"
S3 (MH "Scoliosis")
S2 (MH "Spinal Diseases+")
S1 (MH "Spine+")
Appendix 3. PsycINFO, PEDro, Back Group Trials Register, clinical trials registries, and PubMed search strategies
PsycINFO
Last searched 17 February 2015.
scoliosis.mp.
braces.mp.
bracing.mp.
2 or 3
1 and 4
limit 5 to yr=2013‐2015
PEDro
Last searched 17 February 2015. For this search, the method section was left blank. In the previous searches in 2012 and 2013, the method section was limited to clinical trial.
Abstract & Title: scoliosis
AND
Method: left blank
AND
Published since: 2013
Back Group's Trials Register
Cochrane Register of Studies (CRS)
Last searched 18 February 2015. The purpose of this search was to identify studies not in CENTRAL, therefore only studies not in CENTRAL and dated 2013 and onward were selected.
#1 (scoliosis AND brac*) AND (INREGISTER)
Reference Manager
2012: All non‐indexed text fields: (scoliosis AND brac*), published since 2008
ClinicalTrials.gov
Last searched 17 February 2015.
Search term: scoliosis
AND
Intervention: brace or bracing
AND received from 10/10/2013 to 02/17/2015
WHO ICTRP
Last searched 17 February 2015.
Title: brace or bracing
AND
Condition: scoliosis
Date of registration is between 01/10/2013‐17/02/2015
PubMed
Last searched 17 February 2015.
((((braces or bracing))) AND scoliosis) AND ("2013/10/01"[Date ‐ Publication] : "3000"[Date ‐ Publication])
Appendix 4. Journals handsearched
| Journal | Language | From | To |
| Acta Orthopaedica and Traumatologica Hellenica | Greek | 1948 | 2013 |
| Annales Academiae Medicae Silesiensis | Polish | 1997 | 2013 |
| Annales de Kinésithérapie | French | 1978 | 2007 |
| Cahiers de Kinésithérapie | French | 1978 | 1997 |
| Chinesiologia Scientifica | Italian | 1978 | 2013 |
| Chirurgia Narzadow Ruchu i Ortopedia Polska | Polish | 1997 | 2013 |
| Fizjoterapia | Polish | 1993 | 2013 |
| Fizjoterapia Polska | Polish | 2001 | 2013 |
| Ginnastica Medica, Medicina Fisica e Riabilitazione | Italian | 1953 | 2013 |
| Journal of Japanese Orthopaedic Association | Japanese | 1963 | 1995 |
| Journal of Japanese Scoliosis Research Society | Japanese | 1988 | 2006 |
| Journal of Japanese Spine Society | Japanese | 1990 | 2007 |
| Kinésithérapie Scientifique | French | 1978 | 2007 |
| Kultura Fizyczna | Polish | 1997 | 2013 |
| Kwartalnik Ortopedyczny | Polish | 1991 | 2013 |
| Medycyna Manualna | Polish | 1997 | 2013 |
| Ortopedia Traumatologia Rehabilitacja | Polish | 1999 | 2013 |
| Postepy Rehabilitacji | Polish | 1997 | 2013 |
| Rehabilitacja Medyczna | Polish | 1997 | 2013 |
| Rehabilitacja w Praktyce | Polish | 2006 | 2013 |
| Résonances Européennes Du Rachis | French | 1994 | 2010 |
Appendix 5. Conference proceedings handsearched
| Society | Language | From | To | Single years |
| American Physical Therapy Association | English | ‐ | ‐ | 1991; 1992 |
| Back Pain Society | English | ‐ | ‐ | 1990 |
| British Scoliosis Society | English | ‐ | ‐ | 1992; 1999; 2000; 2006 |
| Chartered Society of Physiotherapists | English | ‐ | ‐ | 1994; 1999; 2000; 2006 |
| European Spinal Defomities Society | English | ‐ | ‐ | 1994 |
| Groupe Europeen Kinesitherapique de travail de scoliose | French | ‐ | ‐ | 1991; 1992 |
| International Research Society of Spinal Deformities published in the research into spinal deformities series | English | 1996 | 2013 | ‐ |
| Phillip Zorab Symposium | English | ‐ | ‐ | 1979 |
| Polskie Towarzystwo Ortopedyczne i Traumatologiczne (Polish Orthopedic and Traumatologic Society) | Polish | 1978 | 2006 | ‐ |
| Quebec Scoliosis Society | French/English | ‐ | ‐ | 1994 |
| Scoliosis Research Society ‐ SRS Meeting abstracts | English | 2001 | 2012 | ‐ |
| Società Italiana di chirurgia vertebrale ‐ GIS | Italian | 1978 | 2012 | ‐ |
| Society on Scoliosis Orthoapedic and Rehabilitation Treatment ‐ SOSORT Meeting abstracts | English | 2003 | 2013 | ‐ |
| Surface Topography and Spinal Deformity meetings | English | 1980 | 1994 | ‐ |
| World Confederation of Physical Therapy | English | ‐ | ‐ | 1991; 1995 |
Appendix 6. Assessment of clinical relevance
1. Are the patients described in detail so that you can decide whether they are comparable to those that you see in your practice?
2. Are the interventions and treatment settings described well enough so that you can provide the same for your patients?
3. Were all clinically relevant outcomes measured and reported?
4. Is the size of the effect clinically important?
5. Are the likely treatment benefits worth the potential harms?
Appendix 7. Criteria for risk of bias assessment for randomized controlled trials (RCTs) and controlled clinical trials (CCTs)
Random sequence generation (selection bias)
Selection bias (biased allocation to interventions) due to inadequate generation of a randomized sequence
There is a low risk of selection bias if the investigators describe a random component in the sequence generation process such as: referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random).
There is a high risk of selection bias if the investigators describe a non‐random component in the sequence generation process, such as: sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number; or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or a series of tests, or availability of the intervention.
Allocation concealment (selection bias)
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment
There is a low risk of selection bias if the participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomization); sequentially numbered drug containers of identical appearance; or sequentially numbered, opaque, sealed envelopes.
There is a high risk of bias if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers), assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered), alternation or rotation, date of birth, case record number, or other explicitly unconcealed procedures.
Blinding of participants
Performance bias due to knowledge of the allocated interventions by participants during the study
There is a low risk of performance bias if blinding of participants was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of personnel/care providers (performance bias)
Performance bias due to knowledge of the allocated interventions by personnel/care providers during the study
There is a low risk of performance bias if blinding of personnel was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of outcome assessor (detection bias)
Detection bias due to knowledge of the allocated interventions by outcome assessors
There is low risk of detection bias if the blinding of the outcome assessment was ensured and it was unlikely that the blinding could have been broken; or if there was no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding, or:
for participant‐reported outcomes in which the particpant was the outcome assessor (e.g. pain, disability): there is a low risk of bias for outcome assessors if there is a low risk of bias for participant blinding (Boutron 2005);
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between participants and care providers (e.g. co‐interventions, length of hospitalization, treatment failure), in which the care provider is the outcome assessor: there is a low risk of bias for outcome assessors if there is a low risk of bias for care providers (Boutron 2005);
for outcome criteria that are assessed from data from medical forms: there is a low risk of bias if the treatment or adverse effects of the treatment could not be noticed in the extracted data (Boutron 2005).
Incomplete outcome data (attrition bias)
Attrition bias due to amount, nature, or handling of incomplete outcome data
There is a low risk of attrition bias if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardized difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if drop‐outs are very large, imputation using even 'acceptable' methods may still suggest a high risk of bias) (van Tulder 2003). The percentage of withdrawals and drop‐outs should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but arbitrary, not supported by literature) (van Tulder 2003).
Selective reporting (reporting bias)
Reporting bias due to selective outcome reporting
There is low risk of reporting bias if the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way, or if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
There is a high risk of reporting bias if not all of the study's pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Group similarity at baseline (selection bias)
Bias due to dissimilarity at baseline for the most important prognostic indicators.
There is low risk of bias if groups are similar at baseline for demographic factors, value of main outcome measure(s), and important prognostic factors (examples in the field of back and neck pain are duration and severity of complaints, vocational status, percentage of people with neurological symptoms) (van Tulder 2003).
Co‐interventions (performance bias)
Bias because co‐interventions were different across groups
There is low risk of bias if there were no co‐interventions or they were similar between the index and control groups (van Tulder 2003).
Compliance (performance bias)
Bias due to inappropriate compliance with interventions across groups
There is low risk of bias if compliance with the interventions was acceptable, based on the reported intensity/dosage, duration, number and frequency for both the index and control intervention(s). For single‐session interventions (e.g. surgery), this item is irrelevant (van Tulder 2003).
Intention‐to‐treat‐analysis
There is low risk of bias if all randomized participants were reported/analysed in the group to which they were allocated by randomization.
Timing of outcome assessments (detection bias)
Bias because important outcomes were not measured at the same time across groups
There is low risk of bias if all important outcome assessments for all intervention groups were measured at the same time (van Tulder 2003).
Other bias
Bias due to problems not covered elsewhere in the table
There is a low risk of bias if the study appears to be free of other sources of bias not addressed elsewhere (e.g. study funding).
Appendix 8. Criteria for the risk of bias assessment of observational studies
Selection bias
Representativeness of the exposed cohort: assess whether the sample is truly representative of the average adolescents with scoliosis; somewhat representative of the average adolescents with scoliosis; selected group of adolescents with scoliosis; no description of the derivation of the cohort. This item was added in the 'Risk of bias' table as 'other source of bias'.
Selection of the non‐exposed cohort: assess whether the sample has been drawn from the same community as the exposed cohort; drawn from a different source/community, "no description of the derivation of the non exposed cohort'. This item was added in the 'Risk of bias' table as 'other source of bias'.
Ascertainment of exposure: information in the study was obtained from a secure record (e.g. clinical records); structured interview; written self report; no description. This item was added in the 'Risk of bias' table as 'other source of bias'.
Comparability of cohorts on the basis of the design or analysis: either exposed and non‐exposed participants must be matched in the design or confounders (or both) must be adjusted for in the analysis. Statements of no differences between groups or that differences were not statistically significant are not sufficient for establishing comparability. If the risk ratio for the exposure of interest is adjusted for the confounders listed, then the groups will be considered to be comparable on each variable used in the adjustment. Were most important prognostic factors matched? Yes/No. Were unmatched important prognostic factors adjusted for? Yes/No. This item was assessed in the 'Risk of bias' table under the item 'group similar at baseline'.
Attrition bias
Complete follow‐up: assess if: all participants accounted for; participants lost to follow‐up unlikely to introduce bias (lost to follow‐up 5%); participants lost to follow‐up greater than 5% and description provided of those lost. This item was assessed in the 'Risk of bias' table under the item 'incomplete outcome data'.
Detection bias
Independent blind assessment: independent or blind assessment stated in the paper, or confirmation of the outcome by reference to secure records (x‐rays, medical records, etc.), record linkage, or self report; or no blinding; no description. This item was assessed in the 'Risk of bias' table under the item 'blinding of outcome assessor'.
Appendix 9. Clinical characteristics of the included studies
| Coillard | Lou | Lusini | Nachemson | Weinstein* | Wong | Total | ||
| Type of study | RCT | RCT | QRCT | QRCT | QRCT | (RCT arm) | RCT | ‐ |
| Population | 68 | 12 | 57 | 240 | 242 | 116 | 43 | 662 |
| Total braced | 36 | 12 | 39 | 111 | 242 | 51 | 43 | 483 |
| Brace active | 36 | 6 | 39 | 111 | 146 | 51 | 22 | 360 |
| Brace control | ‐ | 6 | ‐ | ‐ | 96 | ‐ | 21 | 123 |
| Observation | 32 | ‐ | 18 | 83 | ‐ | 65 | ‐ | 133 |
| Electrical stimulation | ‐ | ‐ | ‐ | 46 | ‐ | ‐ | ‐‐ | 46 |
| Gender | ||||||||
| Males | 7 | 2 | 11 | 0 | 24 | 15 | 0 | 44 |
| Females | 40 | 10 | 46 | 240 | 221 | 101 | 43 | 600 |
| Age | ||||||||
| Mean | 12.02 | 12.05 | 15.03 | 12.07 | 12.07 | 12.07 | 12.05 | ‐ |
| SD | 02.02 | 01.07 | 01.10 | 01.01 | 01.01 | 00.08 | ‐ | |
| Min | 10 | 10 | 10 | 10 | 10 | 10 | 10.06 | ‐ |
| Max | ND | ND | ND | 15 | 15 | 15 | 13.08 | ‐ |
| Bone age | ||||||||
| Risser min | 0 | NR | 0 | 0 | 0 | 0 | 0 | ‐ |
| Risser max | 2 | NR | 4 | 4 | 2 | 2 | 2 | ‐ |
| Cobb degrees | ||||||||
| Mean | 21 | 33 | 52.5 | ‐ | 30.4 | 30.5 | 24.3 | ‐ |
| SD | 4.5 | 6 | NR | ‐ | 6 | 6 | 2.7 | ‐ |
| Min | 15 | NR | 45 | 20 | 20 | 20 | 20 | ‐ |
| Max | 30 | NR | 93 | 35 | 40 | 40 | 30 | ‐ |
| max: maximum; min: minimum; ND: not defined; NR: information not retrievable in the study; QRCT: Quasi RCT, i.e. prospective controlled trial; RCT: randomized controlled trial; SD: standard deviation. | ||||||||
| * The entire study by Weinstein is a QRCT, since it includes 2 arms, 1 RCT, the other QRCT. | ||||||||
Data and analyses
Comparison 1. Brace versus observation (RCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life (PedsQL scores) | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐7.69, 3.49] |
| 2 Risk of success at 2 years | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.29, 2.50] |
| 3 Risk of success at 3 years | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.11, 3.20] |
| 4 Risk of success at 5 years | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.83, 1.98] |
| 5 Any adverse event | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Brace versus observation (cohort studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life (PedsQL score) | 1 | 236 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐3.90, 4.10] |
| 2 Risk of success at 2 years | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.19, 1.89] |
| 3 Risk of success at 3 years | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.42, 2.16] |
| 4 Risk of success at 4 years | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.70, 2.90] |
| 5 Any adverse event | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.96, 1.67] |
| 6 Adverse event back pain | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.47, 1.10] |
Comparison 3. Brace and exercise versus observation in high degree curves (cohort study).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Risk of success per protocol at 2‐9 years | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.21 [1.00, 230.23] |
| 3 Risk of success intention to treat at 2‐9 years | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.04, 3.07] |
| 4 Any adverse event | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Rigid versus elastic brace (RCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Risk of success at 4 years | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.03, 1.89] |
| 3 Any adverse event | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bunge 2010.
| Methods | Multicentre randomized controlled trial | |
| Participants | Girls and boys aged 8‐15 years whose diagnosis of AIS has been established by an orthopaedic surgeon, who have not yet been treated by bracing or surgery, and for whom further growth of physical height is still expected based on medical examination and maturation characteristics (Risser sign) established by X‐ray | |
| Interventions |
Experimental: Boston brace worn every day 12‐23 hours. Participants are usually advised to attend physiotherapy for muscle training and to correct body posture. Physiotherapy alone is not expected to prevent further progression of the curvature. Therefore, participants were free to choose whether they would attend physiotherapy. Although some orthopaedic surgeons prefer to keep people in the hospital for a few days to allow them to become used to wearing the brace, others do not. The orthopaedic surgeons were allowed to apply their own protocol concerning this hospital admission Control: people in the control group were not initially braced during the 2‐year study, unless their curvature shows more than 10° progression compared with the Cobb angle at inclusion. In this case, the orthopaedic surgeon, participants, and their parents could decide to start brace treatment. The participants in the control group were allowed to attend physiotherapy if they want to, because physiotherapy alone would not prevent further progression of the curvature |
|
| Outcomes | Progression in Cobb angle Health‐related quality of life |
|
| Notes | Study failed in the recruitment phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Information not reported |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Blinding of participants and orthopaedic surgeons for treatment was not possible for the type of intervention |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Blinding of participants and orthopaedic surgeons for treatment was not possible for the type of intervention but outcome were unlikely to be biased by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Quote: "to ensure blinding of the primary outcome, the randomization status of the participants will not be disclosed to these two orthopedic surgeons, who judge the patient's Xrays" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Quote: "To ensure blinding of the primary outcome, the randomization status of the participants will not be disclosed to these two orthopedic surgeons, who judge the patient's Xrays" |
| Incomplete outcome data (attrition bias) were drop‐out reported and equal between groups? | Unclear risk | Not applicable. Study failed in the recruitment phase |
| Incomplete outcome data (attrition bias) were all randomized participants analyzed in the group to which they were allocated? | Unclear risk | Not applicable. Study failed in the recruitment phase |
| Selective reporting (reporting bias) | Unclear risk | Not applicable. Study failed in the recruitment phase |
| Groups similar at baseline | Unclear risk | Not applicable. Study failed in the recruitment phase |
| Co‐interventions | High risk | The participants in the control group were allowed to attend physiotherapy if they want to |
| Compliance with intervention | Unclear risk | Not applicable. Study failed in the recruitment phase |
| Similar outcome timing | Low risk | Every 4 months for both groups |
| Representativeness of the exposed cohort | Unclear risk | Not applicable |
| Selection of the non‐exposed cohort | Unclear risk | Not applicable |
| Ascertainment of exposure | Unclear risk | Not applicable |
Coillard 2012.
| Methods | Randomized controlled trial | |
| Participants | 68 participants diagnosed with AIS and with a Cobb angle 15‐30°. Mean age: 12.2 years Radiological confirmation of absence of significant pathological malformation of the spine. All participants had no prior treatment for scoliosis All participants had a suspected high risk of progression: 1. family history of scoliosis or other well knows prognostic factors (Risser, age, menstruation status, etc.) or 2) confirmed progression (Cobb angle increase of 5° in the last 6 months), or both |
|
| Interventions |
Experimental: Dynamic SpineCor brace orthosis, which uses a specific Corrective Movement dependant of the type of the curve. The curve specific Corrective Movement is performed and the orthosis is applied according to definitions contained in the SpineCor Assistant Software. All the health providers need to complete a 2‐phase training course before fitting the SpineCor orthosis In order to obtain the neuromuscular integration, the orthosis must maintain and amplify the corrective movement over time. The orthosis must be worn 20 hours a day for a minimum of 18 months to create a neuromuscular integration of the Corrective Movement through active bio‐feedback (36 participants) Control: no treatment (32 participants) |
|
| Outcomes | Percentage of participants who had ≤ 5° curve progression and the percentage of participants who had ≥ 6° progression Percentage of participants who had surgery recommendation/under gone before skeletal maturity Percentage of participants with curves > 45º at maturity |
|
| Notes | Follow‐up: 5 years post randomization | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "an independent controller based in Sainte‐Justine Hospital in Montreal assigned the patients to the control and treated group based on a random computer generated number table" |
| Allocation concealment (selection bias) | Low risk | Quote: "an independent controller based in Sainte‐Justine Hospital in Montreal assigned the patients to the control and treated group based on a random computer generated number table" |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Blinding of participants not possible for the type of interventions compared (brace vs. no treatment) |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Blinding of participants not possible for the type of interventions compared (brace vs. no treatment), but outcomes unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | High risk | Quote: "the measurements were done without being blinded to the treatment or control group status" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) were drop‐out reported and equal between groups? | High risk | 21 (15 (47%) from the control group and 6 (17%) from the brace group) participants were lost due to withdrawal from the study |
| Incomplete outcome data (attrition bias) were all randomized participants analyzed in the group to which they were allocated? | High risk | Only per‐protocol analysis performed |
| Selective reporting (reporting bias) | Low risk | Results from all pre‐specified outcomes were adequately reported |
| Groups similar at baseline | Low risk | No significant difference at baseline |
| Co‐interventions | Unclear risk | Information not reported |
| Compliance with intervention | Unclear risk | Information not reported |
| Similar outcome timing | Low risk | |
| Representativeness of the exposed cohort | Unclear risk | Not applicable |
| Selection of the non‐exposed cohort | Unclear risk | Not applicable |
| Ascertainment of exposure | Unclear risk | Not applicable |
Lou 2012.
| Methods | Randomized controlled trial | |
| Participants | 12 participants, 10 girls, mean age 12.5 ± 1.7 years, no further description | |
| Interventions |
Experimental: Smart brace for 12 months and then rigid standard brace for 12 months. Smart brace was a standard brace with a microcomputer system, a force transducer, and an air‐bladder control system. The force transducer and air bladder were embedded at the main pressure pad area to control the interface pressure. When the mean pad pressure was less than the target range over a period of 15 minutes the microcomputer system directed air to be pumped into the bladder. Similarly, when the mean pad pressure was greater than the target range over a period of 15 minutes, the microcomputer system caused air to be released from the bladder. The pressure control was to maintain the interface pressure at the prescribed level during daily activities (6 participants) Control: standard rigid brace for 24 months (6 participants) |
|
| Outcomes | Cobb angle Risk of progression Brace wear time Quality of brace wear |
|
| Notes | Follow‐up: 3 years after the brace treatment was finished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information reported |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No information reported; outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) were drop‐out reported and equal between groups? | Low risk | No lost at follow‐up |
| Incomplete outcome data (attrition bias) were all randomized participants analyzed in the group to which they were allocated? | Low risk | No information reported |
| Selective reporting (reporting bias) | Low risk | Results from all pre‐specified outcomes were adequately reported |
| Groups similar at baseline | Unclear risk | No information reported |
| Co‐interventions | Unclear risk | No information reported |
| Compliance with intervention | Low risk | No significant difference between groups |
| Similar outcome timing | Low risk | |
| Representativeness of the exposed cohort | Unclear risk | Not applicable |
| Selection of the non‐exposed cohort | Unclear risk | Not applicable |
| Ascertainment of exposure | Unclear risk | Not applicable |
Lusini 2013.
| Methods | Prospective controlled cohort study | |
| Participants | 57 participants with AIS, at least 1 curve of ≥ 45°, Risser stage 0‐4, aged >above 10 years, first evaluation between 1 March 2003 and 31 December 2010, surgical intervention refused | |
| Interventions |
Experimental: full‐time brace treatment. Participants who arrived in the institute for the first time in 2003 and 2004 were treated with either a Risser cast followed by the Lyon brace, or only the Lyon brace if they refused a cast; from 2005, participants were treated with the Sforzesco brace. Braces had to be worn full‐time (24 hours per day for the Risser cast, 23 hours for the Lyon/Sforzesco brace for the first year, followed by a 1‐hour reduction for 6 months, and then a weaning of 2 hours every 6 months. Physiotherapy‐specific exercises were prescribed systematically to all participants, which were to be performed twice a week. Participants were prescribed Scientific Exercises Approach to Scoliosis exercises to be followed up and updated regularly in the institute (every 3 months ‐ exercised then performed autonomously at home or followed by a trainer) (39 participants) Control: treatment not accepted or came for a second opinion only (18 participants) |
|
| Outcomes | Percentage of participants to have radiographically improved above the measurement error (5°). We considered the main curve (if there was > 1 curve, both were considered main curves if their difference was less than 11° Cobb) and the maximum curve. Treatment success (improvement of ≥ 5°) Treatment failure (either progression of ≥ 5°, or fusion) Clinical and radiographic results: TRACE for aesthetics, Cobb degrees, angle of trunk rotation, and plumb‐line distances for the sagittal plane Compliance |
|
| Notes | Both per‐protocol (treatment completers) and ITT analysis (all participants enrolled, including drop‐outs) performed length of follow‐up: 2‐9 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort study |
| Allocation concealment (selection bias) | High risk | Prospective cohort study |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Blinding of participants not possible for the type of interventions compared (brace vs. no treatment) |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Blinding of participants not possible for the type of interventions compared (brace vs. no treatment) but outcomes unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | High risk | Information about blinding of outcome assessor not reported but he was probably not blinded |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information about blinding of outcome assessor not reported but he was probably not blinded; but outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) were drop‐out reported and equal between groups? | High risk | 11 participants lost at follow‐up (19.3%), unbalanced between groups: 7.7% in the experimental group, 44.4% in the control group |
| Incomplete outcome data (attrition bias) were all randomized participants analyzed in the group to which they were allocated? | Low risk | ITT analysis with worst‐case analysis considering lost at follow‐up as failure when the outcome "improvement" was addressed and as success when the outcome "scoliosis progression/fusion" was addressed |
| Selective reporting (reporting bias) | Low risk | Results from all pre‐specified outcomes have been adequately reported |
| Groups similar at baseline | Unclear risk | Data not reported |
| Co‐interventions | High risk | Physiotherapy prescribed only to the experimental group |
| Compliance with intervention | Unclear risk | Information not reported |
| Similar outcome timing | Unclear risk | Information not reported |
| Representativeness of the exposed cohort | Low risk | Sample representative of the mean population with scoliosis |
| Selection of the non‐exposed cohort | Low risk | Drawn from the same cohort |
| Ascertainment of exposure | Low risk | Clinical records |
Nachemson 1995.
| Methods | Multicentre multinational prospective cohort trial. 8 centres enrolled; included only physicians who firmly believed in effectiveness of bracing or who firmly believed that bracing was ineffective. Each physician consecutively enrolled all participants who met the inclusion criteria and prescribed only 1 treatment | |
| Participants | 240 girls with AIS; mean age 12.7 years; Cobb angle 30‐35°: 42% in the observation group and 65% in the brace group; Cobb angle 20‐29°: 58% in the observation group and 35% in the brace group; menarche at baseline: 57% in the observation group and 41% in the brace group; imbalance: 46% in the observational group and 25% in the brace group | |
| Interventions |
Experimental: plastic brace worn for at least 16 hours per day (111 girls) Control: observation only (who received the electrical stimulation referred to in the text) (129 girls) |
|
| Outcomes | Failure of treatment as measured by an increase of the curve of ≥ 6°, noted on 2 consecutive roentgenograms performed every 4 months before menarche and every 6 months after menarche | |
| Notes | Length of follow‐up: 16 years after maturity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort study |
| Allocation concealment (selection bias) | High risk | Prospective cohort study |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Blinding of participants not possible for the type of interventions compared (brace vs. no treatment) |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Blinding of participants not possible for the type of interventions compared (brace vs. no treatment) but outcomes unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No subjective outcomes measured |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Roentgenograms read by providers, but objective outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) were drop‐out reported and equal between groups? | High risk | 7% lost at follow‐up in the control group; 21% lost at follow‐up in the experimental group Comment: percentage unbalanced between groups, but worst‐case analysis performed |
| Incomplete outcome data (attrition bias) were all randomized participants analyzed in the group to which they were allocated? | High risk | Quote: "the patients lost at follow‐up were included in the survivorship analysis for the time they were in the study" Quote: "the 23 patients who dropped out from the brace group were analysed in the worst‐case analysis and considered as treatment failure" Comment: only the participants who dropped out from the experimental group were included in the worst‐case analysis |
| Selective reporting (reporting bias) | Low risk | Results from all pre‐specified outcomes have been adequately reported |
| Groups similar at baseline | High risk | Comparability of cohorts on the basis of the design or analysis: more participants with severe scoliosis (30‐35° in the brace group (65% with brace vs. 42% with observation); fewer participants with imbalance in the brace group (25% with brace vs. 46% with observation); menarche at baseline: 41% with brace vs. 57% with observation No adjustment for most important confounding factors Comment: differences at the baseline were in favour of the control group |
| Co‐interventions | Unclear risk | Not reported |
| Compliance with intervention | Unclear risk | Not reported |
| Similar outcome timing | Low risk | All participants received a roentgenogram every 4 months before menarche and every 6 months after menarche |
| Representativeness of the exposed cohort | Low risk | Truly representative of the average adolescents with scoliosis |
| Selection of the non‐exposed cohort | High risk | Drawn from a different source |
| Ascertainment of exposure | Low risk | Secure record (e.g. clinical records) |
Weinstein 2013a.
| Methods | Multicentre study with a randomized cohort and a preference cohort | |
| Participants | 242 adolescents at high risk of AIS progression. 116 adolescents (48%) in the randomized cohort and 126 (52%) adolescents in the preference cohort. The 2 cohorts differed significantly at baseline with respect to sex distribution, the interval between the diagnosis of scoliosis and trial enrolment, the person who first noticed the scoliosis, and the largest degree of apical vertebral rotation Mean age: 12.7 ± 1 years; girls: 91.3%; Cobb angle: 30.4 ± 6.0°; Risser grade 0: 69.2%, 1: 26.7%, 2: 11.2%, 3: 2.1%, 4‐5: 0.8%. Thoracic curve: 24.6%, thoracolumbar 13.2%, lumbar 3.7, double major 28.5%, double thoracic 9.1%, thoracic and thoracolumbar 13.6%, triple 7.5% |
|
| Interventions |
Experimental: brace: rigid thoracolumbosacral orthosis, prescribed to be worn for a minimum of 18 hours per day. Participating centres prescribed the type of brace used in their normal clinical practice (146 adolescents) Control: observation, no specific treatment (96 adolescents) |
|
| Outcomes | Curve progression of ≥ 50°Treatment failure Skeletal maturity without this degree Treatment success (skeletal maturity defined as a Risser grade of 4 for girls or 5 for boys) Quality of life (Pediatric Quality of Live Inventory: PedsQL) |
|
| Notes | Length of follow‐up: mean 23 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Observational study: participants chose the preferred treatment |
| Allocation concealment (selection bias) | High risk | Observational study: participants chose the preferred treatment |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Blinding of participants and providers not possible for the type of the interventions compared (brace vs. no intervention) |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Blinding of participants and providers not possible for the type of the interventions compared (brace vs. no intervention) but outcomes unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Quote: "all radiographic evaluations and outcome determinations were made at the central coordinating centre by two readers who were unaware of the treatment assignment and the treatment received" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Quote: "all radiographic evaluations and outcome determinations were made at the central coordinating centre by two readers who were unaware of the treatment assignment and the treatment received" |
| Incomplete outcome data (attrition bias) were drop‐out reported and equal between groups? | Low risk | No lost at follow‐up |
| Incomplete outcome data (attrition bias) were all randomized participants analyzed in the group to which they were allocated? | Low risk | No lost at follow‐up |
| Selective reporting (reporting bias) | Low risk | Results from all pre‐specified outcomes have been adequately reported |
| Groups similar at baseline | Low risk | Quote: "propensity scores will be used to reduce the effect of treatment selection bias (due to nonrandomized treatment assignment and/or crossover) in the estimation of the treatment effect" |
| Co‐interventions | Unclear risk | Information not reported |
| Compliance with intervention | Low risk | There were no significant between‐group differences at baseline, except for the comparisons of sex in the 2 study cohorts (P value = 0.02) Quote: "patients in the bracing arm completed a 2‐week brace wear diary between each follow‐up visit. Moreover temperature monitor data (date, time stamps, and temperature) were downloaded at least every 6 months by the research coordinator. Temperatures 82.4° or greater 72 indicated that the brace was being worn" |
| Similar outcome timing | Low risk | Every 6 months |
| Representativeness of the exposed cohort | Low risk | The sample was truly representative of the average adolescents with scoliosis |
| Selection of the non‐exposed cohort | Low risk | The sample has been drawn from the same community as the exposed cohort |
| Ascertainment of exposure | High risk | Self report data |
Weinstein 2013b.
| Methods | Randomized cohort of a multicentre study including also a preference cohort | |
| Participants | 116 adolescents at high risk of AIS progression; mean age: 12.7 ± 1; girls: 87%; Cobb angle: 30.5 ± 6.0°; Risser grade 0: 61%, 1: 22%, 2: 15%, 3: 2%, 4: 1%. Thoracic curve 22%, thoracolumbar 15%, lumbar 3%, double major 33%, double thoracic 5%, thoracic and thoracolumbar 17%, triple 6% | |
| Interventions |
Experimental: brace: rigid thoracolumbosacral orthosis, prescribed to be worn for a minimum of 18 hours per day. Participating centres prescribed the type of brace used in their normal clinical practice (51 participants) Control: observation: no specific treatment (65 participants) |
|
| Outcomes | Curve progression of ≥ 50° Treatment failure Skeletal maturity without this degree Treatment success (skeletal maturity defined as a Risser grade of 4 for girls or 5 for boys) Quality of life (Pediatric Quality of Live Inventory: PedsQL) |
|
| Notes | Length of follow‐up: mean 23 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated assignment stratified according to curve type ( thoracic vs. all others) |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Blinding of participants and providers not possible for the type of the interventions compared (brace vs. no intervention) |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | Blinding of participants and providers not possible for the type of the interventions compared (brace vs. no intervention) but outcomes unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | Quote: "all radiographic evaluations and outcome determinations were made at the central coordinating centre by two readers who were unaware of the treatment assignment and the treatment received" |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Quote: "all radiographic evaluations and outcome determinations were made at the central coordinating centre by two readers who were unaware of the treatment assignment and the treatment received" |
| Incomplete outcome data (attrition bias) were drop‐out reported and equal between groups? | Low risk | No lost at follow‐up |
| Incomplete outcome data (attrition bias) were all randomized participants analyzed in the group to which they were allocated? | Low risk | No lost at follow‐up |
| Selective reporting (reporting bias) | Low risk | Results from all pre‐specified outcomes have been adequately reported |
| Groups similar at baseline | Low risk | There were no significant between‐group differences at baseline |
| Co‐interventions | Unclear risk | Information not reported |
| Compliance with intervention | Low risk | Quote: "patients in the bracing arm completed a 2‐week brace wear diary between each follow‐up visit. Moreover temperature monitor data (date, time stamps, and temperature) were downloaded at least every 6 months by the research coordinator. Temperatures 82.4 ° or greater 72 indicated that the brace was being worn" |
| Similar outcome timing | Low risk | Every six months |
| Representativeness of the exposed cohort | Unclear risk | Not applicable |
| Selection of the non‐exposed cohort | Unclear risk | Not applicable |
| Ascertainment of exposure | Unclear risk | Not applicable |
Wong 2008.
| Methods | Randomized controlled trial | |
| Participants | 43 female adolescents diagnosed with progressive scoliosis. Mean age 12.5 years; mean menarche at 12.7 years. Mean Risser's sign 0.4; mean AP Cobb angle: 24.3° | |
| Interventions |
Experimental: dynamic orthosis named 'SpineCor' worn for 23 hours per day (22 adolescents) Control: conventional rigid spinal orthosis worn 23 hours per day (21 adolescents) |
|
| Outcomes | Adolescents acceptance assessed by feedback questionnaire with 16 questions in visual analogue scale Progression of scoliosis as measured by percentage of participants without documented progression and still managed with the original treatment |
|
| Notes | Length of follow‐up: 18 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not reported Quote: "Forty‐three subjects were recruited and randomly assigned to two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Blinding of providers not possible for the type of interventions compared (rigid brace vs. dynamic SpineCor brace) |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | Blinding of providers not possible for the type of interventions compared (rigid brace vs. dynamic SpineCor brace) |
| Blinding of outcome assessment (detection bias) subjective outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Information not reported; probably not blinded; outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) were drop‐out reported and equal between groups? | Low risk | No drop‐outs |
| Incomplete outcome data (attrition bias) were all randomized participants analyzed in the group to which they were allocated? | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Results from all pre‐specified outcomes have been adequately reported |
| Groups similar at baseline | Low risk | Groups comparable for mean age, age at menarche, Risser's sign, AP Cobb angle, apical vertebral r otation degrees, Trunk listing |
| Co‐interventions | Unclear risk | Information about co‐intervention not reported |
| Compliance with intervention | Unclear risk | Information about compliance not reported |
| Similar outcome timing | Low risk | All participants received radiographs after the first month and then every 3 months; all participants completed a feedback questionnaire at 3rd, 9th and 18th months of intervention |
| Representativeness of the exposed cohort | Unclear risk | Not applicable |
| Selection of the non‐exposed cohort | Unclear risk | Not applicable |
| Ascertainment of exposure | Unclear risk | Not applicable |
AIS: adolescent idiopathic scoliosis; ITT: intention to treat.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allington 1996 | Retrospective |
| Andersen 2006 | Follow‐up retrospective non‐controlled study |
| Avellanet 2006 | Case report |
| Bassett 1986 | Retrospective |
| Bassett 1987 | Retrospective |
| Becchetti 1990 | Not controlled |
| Bernard 2005 | Retrospective |
| Bowen 2001 | Prospective with retrospective control group |
| Brox 2012 | Prospective uncontrolled study |
| Bullmann 2004 | Prospective no control group |
| Bunge 2007 | Retrospective |
| Bunnell 1980 | Prospective without control group |
| Carman 1985 | Retrospective |
| Carr 1980 | Follow‐up retrospective not controlled study |
| Cassella 1991 | Review |
| Castro 2003 | Not controlled |
| Charlopain 1998 | Retrospective |
| Cheung 2007 | Retrospective |
| Coillard 1999 | Not controlled |
| Coillard 2003 | Not controlled |
| Coillard 2007 | Not controlled |
| Cottalorda 2005 | No end growth results |
| D'Amato 2001 | Prospective with literature control group |
| Danielsson 2001a | Follow‐up with healthy control group |
| Danielsson 2001b | Follow‐up of retrospective study |
| Danielsson 2006 | Follow‐up with no relevant data |
| Den Boer 1999 | Prospective controlled with historical cohort |
| Dickson 1999a | Review |
| Dobosiewicz 2006 | Not controlled |
| Durham 1990 | Retrospective not controlled |
| Dziri 1991 | Retrospective not controlled |
| Ebenbichler 1994 | Review |
| Edmonsson 1977 | Follow‐up not controlled |
| El Sayyad 1994 | Randomized controlled trial including juvenile and adolescent idiopathic scoliosis (6‐16 years) |
| Emans 1986 | Retrospective not controlled |
| Feise 2005 | Not relevant topic |
| Fernandez‐Feliberti 1995 | Prospective controlled including both juvenile and adolescent idiopathic scoliosis (8‐15 years old) |
| Fisher 1987 | Prospective with retrospective control group. Controls were matched to pa rticipants |
| Fällström 1986 | Follow‐up with no relevant data |
| Gabos 2004 | Retrospective |
| Gammon 2010 | Retrospective study |
| Geissele 1991 | Not relevant topic |
| Gepstein 2002 | Retrospective controlled study |
| Goldberg 1981 | Retrospective |
| Gore 1981 | Screening, not controlled |
| Green 1986 | Retrospective, not controlled |
| Griffet 1996 | Not controlled |
| Griffet 2000 | Not relevant topic |
| Grivas 2003 | Retrospective with literature control group. Included also 2 participants < 10 years |
| Haefeli 2006 | Retrospective follow up |
| Hanks 1998 | Retrospective |
| Hassan 1983 | Not controlled |
| Hensinger 2007 | Editorial |
| Hopf 1985 | Case series |
| Howard 1998 | Retrospective |
| Janicki 2007 | Retrospective |
| Kahanovitz 1982 | Not controlled |
| Karol 2001 | Not controlled |
| Katz 1997 | Retrospective |
| Keiser 1976 | Retrospective |
| Kohashi 1996 | Not relevant topic |
| Korovessis 2000 | Prospective not controlled |
| Kotwicki 2002 | Retrospective not controlled |
| Kumano 1992 | Not controlled |
| Little 2000 | Retrospective |
| Lonstein 1994 | Retrospective |
| Lou 2004 | Not controlled |
| Lou 2005 | Not controlled |
| Mellencamp 1977 | Retrospective |
| Miller 1984 | Retrospective |
| Minami 1982 | Not controlled |
| Miyasaki 1980 | Not controlled |
| Moe 1970 | Retrospective |
| Mollon 1984 | No primary research paper |
| Montgomery 1989 | Retrospective controlled |
| Montgomery 1990 | Retrospective |
| Mouilleseaux 1984 | No primary research paper |
| Mounier 1984 | No primary research paper |
| Negrini 2007 | Prospective with retrospective control group |
| Noonan 1996 | Juvenile participants |
| O'Donnell 1988 | Retrospective |
| O'Neill 2005 | Retrospective |
| Park 1977 | Retrospective |
| Peltonen 1988 | Not controlled |
| Peterson 1995 | Prospective not relevant |
| Pham 2007 | Retrospective |
| Piazza 1990 | Retrospective |
| Price 1990 | Prospective not controlled |
| Price 1997 | Not controlled |
| Rahman 2005 | Prospective not controlled |
| Rigo 2003 | Literature control group |
| Roach 1998 | Retrospective |
| Robinson 1996 | Juvenile scoliosis |
| Rosso 1998 | Not controlled |
| Rowe 1997 | Meta‐analysis |
| Schmitt 1987 | Juvenile and adolescent idiopathic scoliosis (7‐16 years old) |
| Schraudebach 1974 | Juvenile and adolescent idiopathic scoliosis |
| Scoloveno 1990 | Retrospective |
| Shirado 1995 | Not relevant topic |
| Skaggs 1996 | Letter to the editor |
| Spoonamore 2004 | Retrospective |
| Tonseth 2005 | Retrospective |
| Trivedi 2001 | Retrospective not controlled |
| Upadhyay 1995 | Not controlled |
| Van Rhijn 2002 | Not controlled |
| Van Rhijn 2003 | Retrospective |
| Veldhuizen 2002 | Not controlled |
| Vijvermans 2004 | Retrospective |
| Watanabe 2005 | Not relevant topic |
| Weigert 2006 | Retrospective |
| Weiss 2003 | Retrospective |
| Weiss 2005 | Case series |
| Weiss 2006 | No brace treatment |
| Wessberg 2011 | Incomplete data, only congress abstract |
| Wever 2002 | Not controlled |
| Wiley 2000 | Retrospective |
| Willers 1993 | Follow‐up not controlled |
| Yamauchi 1986 | Retrospective follow‐up |
| Ylikoski 1989 | Not controlled |
| Yrjonen 2006 | Prospective with retrospective control group |
Characteristics of studies awaiting assessment [ordered by study ID]
Guo 2014.
| Methods | Randomized Controlled Trial according to SRS standardized criteria |
| Participants | 34 females, 10‐14 years of age |
| Interventions | SpineCor elastic brace versus rigid brace |
| Outcomes | 35.0% progressed in SpineCor versus 5.6% in Rigid brace (P = 0.026). At the 4 years follow‐up after skeletally maturity, 29.4% of successfully treated by rigid brace showed progression, versus 38.5% in SpineCor (P > 0.05). For both groups, the primary curves were slightly improved at the time of brace weaning, but additionally increased at the latest follow‐up |
| Notes |
Wiemann 2014.
| Methods | Randomized (by location) Controlled Trial |
| Participants | 37 females, Risser 0, Codd degrees 15‐25 |
| Interventions | nighttime Charleston bending brace versus observation |
| Outcomes | All patients in the observation group progressed to fulltime bracing threshold. In the nighttime bracing group, 29% of the patients did not progress to 25 degrees primary curve magnitude. Rate of progression to surgical magnitude was similar in the 2 groups. |
| Notes |
Differences between protocol and review
We changed the criteria to assess methodological quality of included studies from that described in the protocol to conform to the recommended methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and to the requirements of Review Manager 5 (RevMan 2012).
Contributions of authors
Substantial contributions to conception and design: Negrini S, Minozzi S.
Study search and selection: Bettany‐Saltikov J, Chockalingam N, Grivas TB, Kotwicki T, Maruyama T, Negrini S, Romano M, Zaina F.
Methodological assessment: Minozzi S, Negrini S.
Acquisition/abstraction of data: Minozzi S, Zaina F, Bettany‐Saltikov J, Chockalingam N.
Data analysis: Negrini S, Minozzi S, Zaina F.
Interpretation of data: Negrini S, Grivas TB, Kotwicki T, Maruyama T, Romano M, Vasiliadis ES.
Drafting the article: Negrini S, Minozzi S.
Revising it critically for important intellectual content: Negrini S, Grivas TB, Kotwicki T, Maruyama T, Romano M.
Final approval of the version to be published: Negrini S, Minozzi S, Bettany‐Saltikov J, Zaina F, Chockalingam N, Grivas TB, Kotwicki T, Maruyama T, Romano M.
Declarations of interest
Negrini S and Romano M are stakeholders of ISICO (Italian Scientific Spine Institute), Milan, Italy.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bunge 2010 {published data only}
- Bunge EM, Habbema JD, Koning HJ. A randomised controlled trial on the effectiveness of bracing patients with idiopathic scoliosis: failure to include patients and lessons to be learnt. European Spine Journal 2010;19(5):747‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge EM, Koning HJ. Bracing patients with idiopathic scoliosis: design of the Dutch randomized controlled treatment trial. BMC Musculoskeletal Disorders 2008;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coillard 2012 {published data only}
- Coillard C, Circo A, Rivard C. A prospective randomized study of the natural history of idiopathic scoliosis versus treatment with the SpineCor brace. Scoliosis 2012;7(Suppl 1):O24. [PubMed] [Google Scholar]
Lou 2012 {published data only}
- Lou E, Hill D, Raso J, Donauer A, Moreau M, Mahood J, et al. Smart brace versus standard rigid brace for the treatment of scoliosis: a pilot study. Research into Spinal Deformities 2012;8:338‐41. [PubMed] [Google Scholar]
Lusini 2013 {published data only}
- Lusini M, Donzelli S, Minnella S, Zaina F, Negrini S. Brace treatment is effective in idiopathic scoliosis over 45°: an observational prospective cohort controlled study. Spine Journal 2014;14(9):1935‐9. [DOI] [PubMed] [Google Scholar]
- Lusini M, Donzelli S, Zaina F Negrini S. Brace treatment is effective in idiopathic scoliosis over 45°: a prospective controlled study. Scoliosis 2013;8(suppl 1):O35. [DOI] [PubMed] [Google Scholar]
Nachemson 1995 {published data only}
- Danielsson AJ, Hasserius R, Ohlin A, Nachemson AL. A prospective study of brace treatment versus observation alone in adolescent idiopathic scoliosis. A follow‐up of 16 years after maturity. Spine 2007;32(20):2198‐207. [DOI] [PubMed] [Google Scholar]
- Danielsson AJ, Hasserius R, Ohlin A, Nachemson AL. Body appearance and quality of life in adult patients with adolescent idiopathic scoliosis treated with a brace or under observation alone during adolescence. Spine 2012;37(9):755‐62. [DOI] [PubMed] [Google Scholar]
- Danielsson AJ, Hasserius R, Ohlin A, Nachemson AL. Health‐related quality of life in untreated versus brace‐treated patients with adolescent idiopathic scoliosis: a long‐term follow‐up. Spine 2010;35(2):199‐205. [DOI] [PubMed] [Google Scholar]
- Nachemson AL, Peterson LE. Effectiveness of treatment with a brace in girls who have adolescent idiopathic scoliosis. A prospective, controlled study based on data from the Brace Study of the Scoliosis Research Society. Journal of Bone & Joint Surgery. American Version 1995;77(6):815‐22. [DOI] [PubMed] [Google Scholar]
Weinstein 2013a {published data only}
- Weinstein SL, Dolan LA, Wright JG, Dobbs MB. Effects of bracing in adolescents with idiopathic scoliosis. New England Journal of Medicine 2013;369(16):1512‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SL, Dolan LA, Wright JG, Dobbs MB. Design of the Bracing in Adolescent Idiopathic Scoliosis Trial (BrAIST). SPINE 2013;38(21):1382‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinstein 2013b {published data only}
- Weinstein SL, Dolan LA, Wright JG, Dobbs MB. Effects of bracing in adolescents with idiopathic scoliosis. New England Journal of Medicine 2013;369(16):1512‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2008 {published data only}
- Wong MS, Cheng JC, Lam TP, Ng BK, Sin SW, Lee‐Shum SL, et al. The effect of rigid versus flexible spinal orthosis on the clinical efficacy and acceptance of the patients with adolescent idiopathic scoliosis. Spine 2008;33(12):1360‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allington 1996 {published data only}
- Allington NJ, Bowen JR. Adolescent idiopathic scoliosis: treatment with the Wilmington brace. A comparison of full‐time and part‐time use. Journal of Bone & Joint Surgery ‐ American Volume 1996;78(7):1056‐62. [PubMed] [Google Scholar]
Andersen 2006 {published data only}
- Andersen MO, Christensen SB, Thomsen K. Outcome at 10 years after treatment for adolescent idiopathic scoliosis. Spine 2006;31(3):350‐4. [DOI] [PubMed] [Google Scholar]
Avellanet 2006 {published data only}
- Avellanet M, Gonzalez VMA, Saenz A, Hijos M‐E. Is it too late start orthopedic treatment for idiopathic scoliosis with Risser scores of 4?. Annales de Readaptation et de Medecine Physique 2006;49(9):659‐62. [DOI] [PubMed] [Google Scholar]
Bassett 1986 {published data only}
- Bassett GS, Bunnell WP, MacEwen GD. Treatment of idiopathic scoliosis with the Wilmington brace. Results in patients with a twenty to thirty‐nine‐degree curve. Journal of Bone and Joint Surgery 1986;68(4):602‐5. [PubMed] [Google Scholar]
Bassett 1987 {published data only}
- Bassett GS, Bunnell WP. Influence of the Wilmington brace on spinal decompensation in adolescent idiopathic scoliosis. Clinical Orthopaedics and Related Research 1987;223:164‐9. [PubMed] [Google Scholar]
Becchetti 1990 {published data only}
- Becchetti S, Senes FM, Pinelli G. Maguelone plaster braces for the reduction of scoliotic curves: methods and results. Italian Journal of Orthopaedics & Traumatology 1990;16(1):53‐60. [PubMed] [Google Scholar]
Bernard 2005 {published data only}
- Bernard JC, Jemni S, Schneider M, Boussard D, Saillard V, Bard R, et al. Evaluation of the efficacy of a carbon brace ("Corset monocoque carbone respectant la respiration" [CMCR]) preserving lung capacity to treat idiopathic scoliosis in children and adolescents: a retrospective study of 115 patients. Annales de Readaptation et de Medecine Physique 2005;48(9):637‐49. [DOI] [PubMed] [Google Scholar]
Bowen 2001 {published data only}
- Bowen JR, Keeler KA, Pelegie S. Adolescent idiopathic scoliosis managed by a nighttime bending brace. Orthopedics 2001;24(10):967‐70. [DOI] [PubMed] [Google Scholar]
Brox 2012 {published data only}
- Brox JI, Lange JE, Gunderson RB, Steen H. Good brace compliance reduced curve progression and surgical rates in patients with idiopathic scoliosis. European Spine Journal 2012;21(10):1957‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bullmann 2004 {published data only}
- Bullmann V, Halm HF, Lerner T, Lepsien U, Hackenberg L, Liljenqvist U. Prospective evaluation of braces as treatment in idiopathic scoliosis. Zeitschrift fur Orthopadie und Ihre Grenzgebiete 2004;142(4):403‐9. [DOI] [PubMed] [Google Scholar]
Bunge 2007 {published data only}
- Bunge EM, Juttmann RE, Kleuver M, Biezen FC, Koning HJ, NESCIO group. Health‐related quality of life in patients with adolescent idiopathic scoliosis after treatment: short‐term effects after brace or surgical treatment. European Spine Journal 2007;16(1):83‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bunnell 1980 {published data only}
- Bunnell WP, MacEwen GD, Jayakumar S. The use of plastic jackets in the non‐operative treatment of idiopathic scoliosis. Preliminary report. Journal of Bone & Joint Surgery 1980;62(1):31‐8. [PubMed] [Google Scholar]
Carman 1985 {published data only}
- Carman D, Roach JW, Speck G, Wenger DR, Herring JA. Role of exercises in the Milwaukee brace treatment of scoliosis. Journal of Pediatric Orthopedics 1985;5(1):65‐8. [DOI] [PubMed] [Google Scholar]
Carr 1980 {published data only}
- Carr WA, Moe JH, Winter RB, Lonstein JE. Treatment of idiopathic scoliosis in the Milwaukee brace. Journal of Bone & Joint Surgery 1980;62(4):599‐612. [PubMed] [Google Scholar]
Cassella 1991 {published data only}
- Cassella MC, Hall JE. Current treatment approaches in the nonoperative and operative management of adolescent idiopathic scoliosis. Physical Therapy 1991;71(12):897‐909. [DOI] [PubMed] [Google Scholar]
Castro 2003 {published data only}
- Castro FP, Jr. Adolescent idiopathic scoliosis, bracing, and the Hueter‐Volkmann principle. Spine Journal 2003;3(3):180‐5. [DOI] [PubMed] [Google Scholar]
Charlopain 1998 {published data only}
- Charlopain P, Biot B, Fauchet R. Lyonnais orthopedic treatment: long‐term results (237 patients). Annales de Readaptation et de Medecine Physique 1998;41(3):147‐53. [Google Scholar]
Cheung 2007 {published data only}
- Cheung KMC, Cheng EYL, Chan SCW, Yeung KWK, Luk KDK. Outcome assessment of bracing in adolescent idiopathic scoliosis by the use of the SRS‐22 questionnaire. International Orthopaedics 2007;31(4):507‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coillard 1999 {published data only}
- Coillard C, Leroux MA, Zabjek KF, Rivard CH. The reducibility of idiopathic scoliosis during non‐operative treatment. Annales de Chirurgie 1999;53(8):781‐91. [PubMed] [Google Scholar]
Coillard 2003 {published data only}
- Coillard C, Leroux MA, Zabjek KF, Rivard CH. SpineCor ‐ a non‐rigid brace for the treatment of idiopathic scoliosis: post‐treatment results. European Spine Journal 2003;12(2):141‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coillard 2007 {published data only}
- Coillard C, Vachon V, Circo AB, Beausejour M, Rivard CH. Effectiveness of the SpineCor brace based on the new standardized criteria proposed by the scoliosis research society for adolescent idiopathic scoliosis. Journal of Pediatric Orthopaedics 2007;27(4):375‐9. [DOI] [PubMed] [Google Scholar]
Cottalorda 2005 {published data only}
- Cottalorda J, Kohler R, Garin C, Genevois P, Lecante C, Berge B. Orthoses for mild scoliosis: a prospective study comparing traditional plaster mold manufacturing with fast, non‐contact, 3‐dimensional acquisition. Spine 2005;30(4):399‐405. [DOI] [PubMed] [Google Scholar]
D'Amato 2001 {published data only}
- D'Amato CR, Griggs S, McCoy B. Nighttime bracing with the Providence brace in adolescent girls with idiopathic scoliosis. Spine 2001;26(18):2006‐12. [DOI] [PubMed] [Google Scholar]
Danielsson 2001a {published data only}
- Danielsson AJ, Nachemson AL. Radiologic findings and curve progression 22 years after treatment for adolescent idiopathic scoliosis: comparison of brace and surgical treatment with matching control group of straight individuals. Spine 2001;26(5):516‐25. [DOI] [PubMed] [Google Scholar]
Danielsson 2001b {published data only}
- Danielsson AJ, Wiklund I, Pehrsson K, Nachemson AL. Health‐related quality of life in patients with adolescent idiopathic scoliosis: a matched follow‐up at least 20 years after treatment with brace or surgery. European Spine Journal 2001;10(4):278‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Danielsson 2006 {published data only}
- Danielsson AJ, Romberg K, Nachemson AL. Spinal range of motion, muscle endurance, and back pain and function at least 20 years after fusion or brace treatment for adolescent idiopathic scoliosis: a case‐control study. Spine 2006;31(3):275‐83. [DOI] [PubMed] [Google Scholar]
Den Boer 1999 {published data only}
- Boer WA, Anderson PG, Limbeek J, Kooijman MA. Treatment of idiopathic scoliosis with side‐shift therapy: an initial comparison with a brace treatment historical cohort. European Spine Journal 1999;8(5):406‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickson 1999a {published data only}
- Dickson RA. Spinal deformity ‐ adolescent idiopathic scoliosis: nonoperative treatment. Spine 1999;24(24):2601‐6. [DOI] [PubMed] [Google Scholar]
Dobosiewicz 2006 {published data only}
- Dobosiewicz K, Durmala J, Czernicki K, Piotrowski J. Radiological results of Dobosiewicz method of three‐dimensional treatment of progressive idiopathic scoliosis. Studies in Health Technology & Informatics 2006;123:267‐72. [PubMed] [Google Scholar]
Durham 1990 {published data only}
- Durham JW, Moskowitz A, Whitney J. Surface electrical stimulation versus brace in treatment of idiopathic scoliosis. Spine 1990;15(9):888‐92. [DOI] [PubMed] [Google Scholar]
Dziri 1991 {published data only}
- Dziri C, Delarque A, Conil JL, Kraenzler R, Costes O, Bardot P. Results of short‐term treatment with CTM braces in a series of 25 patients with idiopathic scoliosis. Annales de Readaptation et de Medecine Physique 1991;34(1):41‐6. [Google Scholar]
Ebenbichler 1994 {published data only}
- Ebenbichler G, Liederer A, Lack W. Idiopathic scoliosis and non‐operative scoliosis therapy. Wiener Medizinische Wochenschrift 1994;144(24):593‐604. [PubMed] [Google Scholar]
Edmonsson 1977 {published data only}
- Edmonsson AS, Morris JT. Follow‐up study of Milwaukee brace treatment in patients with idiopathic scoliosis. Clinical Orthopaedics & Related Research 1977;126:58‐61. [PubMed] [Google Scholar]
El Sayyad 1994 {published data only}
- Sayyad M, Conine TA. Effect of exercise, bracing and electrical surface stimulation on idiopathic scoliosis: a preliminary study. International Journal of Rehabilitation Research 1994;17(1):70‐4. [DOI] [PubMed] [Google Scholar]
Emans 1986 {published data only}
- Emans JB, Kaelin A, Bancel P, Hall JE, Miller ME. The Boston bracing system for idiopathic scoliosis. Follow‐up results in 295 patients. Spine 1986;11(8):792‐801. [DOI] [PubMed] [Google Scholar]
Fällström 1986 {published data only}
- Fällström K, Cochran T, Nachemson A. Long‐term effects on personality development in patients with adolescent idiopathic scoliosis. Influence of type of treatment. Spine 1986;11(7):756‐8. [DOI] [PubMed] [Google Scholar]
Feise 2005 {published data only}
- Feise RJ, Donaldson S, Crowther ER, Menke JM, Wright JG. Construction and validation of the scoliosis quality of life index in adolescent idiopathic scoliosis. Spine 2005;30(11):1310‐5. [DOI] [PubMed] [Google Scholar]
Fernandez‐Feliberti 1995 {published data only}
- Fernandez‐Feliberti R, Flynn J, Ramirez N, Trautmann M, Alegria M. Effectiveness of TLSO bracing in the conservative treatment of idiopathic scoliosis. Journal of Pediatric Orthopedics 1995;15(2):176‐81. [PubMed] [Google Scholar]
Fisher 1987 {published data only}
- Fisher DA, Rapp GF, Emkes M. Idiopathic scoliosis: transcutaneous muscle stimulation versus the Milwaukee brace. Spine 1987;12(10):987‐91. [DOI] [PubMed] [Google Scholar]
Gabos 2004 {published data only}
- Gabos PG, Bojescul JA, Bowen JR, Keeler K, Rich L. Long‐term follow‐up of female patients with idiopathic scoliosis treated with the Wilmington orthosis. Journal of Bone & Joint Surgery 2004;86(9):1891‐9. [DOI] [PubMed] [Google Scholar]
Gammon 2010 {published data only}
- Gammon SR, Mehlman CT, Chan W, Heifetz J, Durrett G, Wall EJ. A comparison of thoracolumbosacral orthoses and SpineCor treatment of adolescent idiopathic scoliosis patients using the Scoliosis Research Society standardized criteria. Journal of Pediatric Orthopedics 2010;30(6):531‐8. [DOI] [PubMed] [Google Scholar]
Geissele 1991 {published data only}
- Geissele AE, Kransdorf MJ, Geyer CA, Jelinek JS, Dam BE. Magnetic resonance imaging of the brain stem in adolescent idiopathic scoliosis. Spine 1991;16(7):761‐3. [DOI] [PubMed] [Google Scholar]
Gepstein 2002 {published data only}
- Gepstein R, Leitner Y, Zohar E, Angel I, Shabat S, Pekarsky I, et al. Effectiveness of the Charleston bending brace in the treatment of single‐curve idiopathic scoliosis. Journal of Pediatric Orthopedics 2002;22(1):84‐7. [PubMed] [Google Scholar]
Goldberg 1981 {published data only}
- Goldberg C, Dowling F, Blake NS, Regan BF. A retrospective study of Cotrel dynamic spinal traction in the conservative management of scoliosis. Irish Medical Journal 1981;74(12):363‐5. [PubMed] [Google Scholar]
Gore 1981 {published data only}
- Gore DR, Passehl R, Sepic S, Dalton A. Scoliosis screening: results of a community project. Pediatrics 1981;67(2):196‐200. [PubMed] [Google Scholar]
Green 1986 {published data only}
- Green NE. Part‐time bracing of adolescent idiopathic scoliosis. Journal of Bone & Joint Surgery 1986;68(5):738‐42. [PubMed] [Google Scholar]
Griffet 1996 {published data only}
- Griffet J, Thevenot J, Barral F. GTB orthoses in the treatment by lumbar hyperlordosis of idiopathic scoliosis. Annales de Readaptation et de Medecine Physique 1996;39(2):117‐22. [Google Scholar]
Griffet 2000 {published data only}
- Griffet J, Leroux MA, Badeaux J, Coillard C, Zabjek KF, Rivard CH. Relationship between gibbosity and Cobb angle during treatment of idiopathic scoliosis with the SpineCor brace. European Spine Journal 2000;9(6):516‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grivas 2003 {published data only}
- Grivas TB, Vasiliadis E, Chatziargiropoulos T, Polyzois VD, Gatos K. The effect of a modified Boston brace with anti‐rotatory blades on the progression of curves in idiopathic scoliosis: aetiologic implications. Pediatric Rehabilitation 2003;6(3‐4):237‐42. [DOI] [PubMed] [Google Scholar]
Haefeli 2006 {published data only}
- Haefeli M, Elfering A, Kilian R, Min K, Boos N. Nonoperative treatment for adolescent idiopathic scoliosis: a 10‐ to 60‐year follow‐up with special reference to health‐related quality of life. Spine 2006;31(3):355‐66. [DOI] [PubMed] [Google Scholar]
Hanks 1998 {published data only}
- Hanks GA, Zimmer B, Nogi J. TLSO treatment of idiopathic scoliosis. An analysis of the Wilmington jacket. Spine 1988;13(6):626‐9. [PubMed] [Google Scholar]
Hassan 1983 {published data only}
- Hassan I, Bjerkreim I. Progression in idiopathic scoliosis after conservative treatment. Acta Orthopaedica Scandinavica 1983;54(1):88‐90. [DOI] [PubMed] [Google Scholar]
Hensinger 2007 {published data only}
- Hensinger RN, Thompson GH. Orthotic management in adolescent idiopathic scoliosis: leveling the playing field. Journal of Pediatric Orthopedics 2007;27(4):367‐8. [DOI] [PubMed] [Google Scholar]
Hopf 1985 {published data only}
- Hopf C, Heine J. Long‐term results of conservative treatment of scoliosis with the Cheneau brace. Zeitschrift fur Orthopadie und Ihre Grenzgebiete 1985;123(3):312‐22. [DOI] [PubMed] [Google Scholar]
Howard 1998 {published data only}
- Howard A, Wright JG, Hedden D. A comparative study of TLSO, Charleston, and Milwaukee braces for idiopathic scoliosis. Spine 1998;23(22):2404‐11. [DOI] [PubMed] [Google Scholar]
Janicki 2007 {published data only}
- Janicki JA, Poe‐Kochert C, Armstrong DG, Thompson GH. A comparison of the thoracolumbosacral orthoses and providence orthosis in the treatment of adolescent idiopathic scoliosis: results using the new SRS inclusion and assessment criteria for bracing studies. Journal of Pediatric Orthopedics 2007;27(4):369‐74. [DOI] [PubMed] [Google Scholar]
Kahanovitz 1982 {published data only}
- Kahanovitz N, Levine DB, Lardone J. The part‐time Milwaukee brace treatment of juvenile idiopathic scoliosis. Long‐term follow‐up. Clinical Orthopaedics & Related Research 1982;167:145‐51. [PubMed] [Google Scholar]
Karol 2001 {published data only}
- Karol LA. Effectiveness of bracing in male patients with idiopathic scoliosis. Spine 2001;26(18):2001‐5. [DOI] [PubMed] [Google Scholar]
Katz 1997 {published data only}
- Katz DE, Richards BS, Browne RH, Herring JA. A comparison between the Boston brace and the Charleston bending brace in adolescent idiopathic scoliosis. Spine 1997;22(12):1302‐12. [DOI] [PubMed] [Google Scholar]
Keiser 1976 {published data only}
- Keiser RP, Shufflebarger HL. The Milwaukee brace in idiopathic scoliosis: evaluation of 123 completed cases. Clinical Orthopaedics & Related Research 1976;118:19‐24. [PubMed] [Google Scholar]
Kohashi 1996 {published data only}
- Kohashi Y, Oga M, Sugioka Y. A new method using top views of the spine to predict the progression of curves in idiopathic scoliosis during growth. Spine 1996;21(2):212‐7. [DOI] [PubMed] [Google Scholar]
Korovessis 2000 {published data only}
- Korovessis P, Kyrkos C, Piperos G, Soucacos PN. Effects of thoracolumbosacral orthosis on spinal deformities, trunk asymmetry, and frontal lower rib cage in adolescent idiopathic scoliosis. Spine 2000;25(16):2064‐71. [DOI] [PubMed] [Google Scholar]
Kotwicki 2002 {published data only}
- Kotwicki T, Pietrzak S, Szulc A. Three‐dimensional action of Chêneau brace on thoracolumbar scoliosis. Studies in Health Technology & Informatics 2002;88:226‐9. [PubMed] [Google Scholar]
Kumano 1992 {published data only}
- Kumano K, Maruyama T, Kojima T, Kondoh Y, Shimode M. Results of wearing Milwaukee brace at night for adolescent idiopathic scoliosis. Journal of the Western Pacific Orthopaedic Association 1992;29(2):53‐7. [Google Scholar]
Little 2000 {published data only}
- Little DG, Song KM, Katz D, Herring JA. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. Journal of Bone & Joint Surgery 2000;82(5):685‐93. [DOI] [PubMed] [Google Scholar]
Lonstein 1994 {published data only}
- Lonstein JE, Winter RB. The Milwaukee brace for the treatment of adolescent idiopathic scoliosis. A review of one thousand and twenty patients. Journal of Bone & Joint Surgery 1994;76(8):1207‐21. [DOI] [PubMed] [Google Scholar]
Lou 2004 {published data only}
- Lou E, Raso JV, Hill DL, Mahood JK, Moreau MJ. Correlation between quantity and quality of orthosis wear and treatment outcomes in adolescent idiopathic scoliosis. Prosthetics and Orthotics International 2004;28(1):49‐54. [DOI] [PubMed] [Google Scholar]
Lou 2005 {published data only}
- Lou E, Hill DL, Raso JV, Moreau MJ, Mahood JK. Smart orthosis for the treatment of adolescent idiopathic scoliosis. Medical & Biological Engineering & Computing 2005;43(6):746‐50. [DOI] [PubMed] [Google Scholar]
Mellencamp 1977 {published data only}
- Mellencamp DD, Blount WP, Anderson AJ. Milwaukee brace treatment of idiopathic scoliosis: late results. Clinical Orthopaedics & Related Research 1977;126:47‐57. [PubMed] [Google Scholar]
Miller 1984 {published data only}
- Miller JA, Nachemson AL, Schultz AB. Effectiveness of braces in mild idiopathic scoliosis. Spine 1984;9(6):632‐5. [DOI] [PubMed] [Google Scholar]
Minami 1982 {published data only}
- Minami S. Results of brace treatment in idiopathic scoliosis ‐ evaluation of the patients treated for over 2 years or those who completed the treatment. Journal of the Japanese Orthopaedic Association 1982;56(6):471‐85. [PubMed] [Google Scholar]
Miyasaki 1980 {published data only}
- Miyasaki RA. Immediate influence of the thoracic flexion exercise on vertebral position in Milwaukee brace wearers. Physical Therapy 1980;68(8):1005‐9. [DOI] [PubMed] [Google Scholar]
Moe 1970 {published data only}
- Moe JH, Kettleson DN. Idiopathic scoliosis. Analysis of curve patterns and the preliminary results of Milwaukee‐brace treatment in one hundred sixty‐nine patients. Journal of Bone & Joint Surgery 1970;52(8):1509‐33. [PubMed] [Google Scholar]
Mollon 1984 {published data only}
- Mollon G, Ollier M. Orthopedic treatment from Lyon: ortheses and kinesitherapy. Cahiers de Kinesitherapie 1984;106:45‐58. [Google Scholar]
Montgomery 1989 {published data only}
- Montgomery F, Willner S. Prognosis of brace‐treated scoliosis. Comparison of the Boston and Milwaukee methods in 244 girls. Acta Orthopaedica Scandinavica 1989;60(4):383‐5. [DOI] [PubMed] [Google Scholar]
Montgomery 1990 {published data only}
- Montgomery F, Willner S, Appelgren G. Long‐term follow‐up of patients with adolescent idiopathic scoliosis treated conservatively: an analysis of the clinical value of progression. Journal of Pediatric Orthopedics 1990;10(1):48‐52. [PubMed] [Google Scholar]
Mouilleseaux 1984 {published data only}
- Mouilleseaux B, Pallandre B, Picault C. A gauze corset from Saint‐Etienne for the early treatment of lumbar scoliosis. Cahiers de Kinesitherapie 1984;106:31‐8. [Google Scholar]
Mounier 1984 {published data only}
- Mounier C. The Milwaukee brace. Cahiers de Kinesitherapie 1984;105:65‐74. [Google Scholar]
Negrini 2007 {published data only}
- Negrini S, Marchini G. Efficacy of the symmetric, patient‐oriented, rigid, three‐dimensional, active (SPoRT) concept of bracing for scoliosis: a prospective study of the Sforzesco versus Lyon brace. Europa Medicophysica 2007;43(2):171‐81. [PubMed] [Google Scholar]
Noonan 1996 {published data only}
- Noonan KJ, Weinstein SL, Jacobson WC, Dolan LA. Use of the Milwaukee brace for progressive idiopathic scoliosis. Journal of Bone & Joint Surgery 1996;78(4):557‐67. [DOI] [PubMed] [Google Scholar]
O'Donnell 1988 {published data only}
- O'Donnell CS, Bunnell WP, Betz RR, Bowen JR, Tipping CR. Electrical stimulation in the treatment of idiopathic scoliosis. Clinical Orthopaedics & Related Research 1988;229:107‐13. [PubMed] [Google Scholar]
O'Neill 2005 {published data only}
- O'Neill PJ, Karol LA, Shindle MK, Elerson EE, BrintzenhofeSzoc KM, Katz DE, et al. Decreased orthotic effectiveness in overweight patients with adolescent idiopathic scoliosis. Journal of Bone and Joint Surgery 2005;87(5):1069‐74. [DOI] [PubMed] [Google Scholar]
Park 1977 {published data only}
- Park J, Houtkin S, Grossman J, Levine DB. A modified brace (Prenyl) for scoliosis. Clinical Orthopaedics & Related Research 1977;126:67‐73. [PubMed] [Google Scholar]
Peltonen 1988 {published data only}
- Peltonen J, Poussa M, Ylikoski M. Three‐year results of bracing in scoliosis. Acta Orthopaedica Scandinavica 1988;59(5):487‐90. [DOI] [PubMed] [Google Scholar]
Peterson 1995 {published data only}
- Peterson LE, Nachemson AL. Prediction of progression of the curve in girls who have adolescent idiopathic scoliosis of moderate severity. Logistic regression analysis based on data from The Brace Study of the Scoliosis Research Society. Journal of Bone and Joint Surgery 1995;77(6):823‐7. [DOI] [PubMed] [Google Scholar]
Pham 2007 {published data only}
- Pham VM, Herbaux B, Schill A, Thevenon A. Evaluation of the Cheneau brace in adolescent idiopathic scoliosis. Annales de Readaptation et de Medecine Physique 2007;50(3):125‐33. [DOI] [PubMed] [Google Scholar]
Piazza 1990 {published data only}
- Piazza MR, Bassett GS. Curve progression after treatment with the Wilmington brace for idiopathic scoliosis. Journal of Pediatric Orthopedics 1990;10(1):39‐43. [PubMed] [Google Scholar]
Price 1990 {published data only}
- Price CT, Scott DS, Reed FE Jr, Riddick MF. Nighttime bracing for adolescent idiopathic scoliosis with the Charleston bending brace. Preliminary report. Spine 1990;15(12):1294‐9. [DOI] [PubMed] [Google Scholar]
Price 1997 {published data only}
- Price CT, Scott DS, Reed FR Jr, Sproul JT, Riddick MF. Nighttime bracing for adolescent idiopathic scoliosis with the Charleston Bending Brace: long‐term follow‐up. Journal of Pediatric Orthopedics 1997;17(6):703‐7. [PubMed] [Google Scholar]
Rahman 2005 {published data only}
- Rahman T, Bowen JR, Takemitsu M, Scott C. The association between brace compliance and outcome for patients with idiopathic scoliosis. Journal of Pediatric Orthopaedics 2005;25(4):420‐2. [DOI] [PubMed] [Google Scholar]
Rigo 2003 {published data only}
- Rigo M, Reiter C, Weiss HR. Effect of conservative management on the prevalence of surgery in patients with adolescent idiopathic scoliosis. Pediatric Rehabilitation 2003;6(3‐4):209‐14. [DOI] [PubMed] [Google Scholar]
Roach 1998 {published data only}
- Roach CJ, Andrish JT. Preliminary results of part‐time bracing for the management of idiopathic scoliosis. Journal of Prosthetics and Orthotics 1998;10(3):71‐6. [Google Scholar]
Robinson 1996 {published data only}
- Robinson CM, McMaster MJ. Juvenile idiopathic scoliosis. Curve patterns and prognosis in one hundred and nine patients. Journal of Bone & Joint Surgery 1996;78(8):1140‐8. [DOI] [PubMed] [Google Scholar]
Rosso 1998 {published data only}
- Rosso V, Trucchi F, Collo GL, Marmotti AG. Early treatment of a juvenile idiopathic scoliosis. Charleston Bending orthosis and Olympe orthosis: results after 4 years in 117 cases. Minerva Ortopedica e Traumatologica 1998;49(9):317‐23. [Google Scholar]
Rowe 1997 {published data only}
- Rowe DE, Bernstein SM, Riddick MF, Adler F, Emans JB, Gardner‐Bonneau D. A meta‐analysis of the efficacy of non‐operative treatments for idiopathic scoliosis. Journal of Bone & Joint Surgery 1997;75(9):664‐74. [DOI] [PubMed] [Google Scholar]
Schmitt 1987 {published data only}
- Schmitt O, Jacob S. Development of idiopathic scoliosis in electrostimulation treatment with an implantable system. Zeitschrift fur Orthopadie und Ihre Grenzgebiete 1987;125(5):518‐25. [DOI] [PubMed] [Google Scholar]
Schraudebach 1974 {published data only}
- Schraudebach T, Rossler H, Dennert R. Experience with the Milwaukee brace in an out‐patient department for scoliosis. Zeitschrift fur Orthopadie und Ihre Grenzgebiete 1974;112(6):1265‐74. [PubMed] [Google Scholar]
Scoloveno 1990 {published data only}
- Scoloveno MA, Yarcheski A, Mahon NE. Scoliosis treatment effects on selected variables among adolescents. Western Journal of Nursing Research 1990;12(5):601‐15. [DOI] [PubMed] [Google Scholar]
Shirado 1995 {published data only}
- Shirado O, Ito T, Kaneda K, Strax TE. Kinesiologic analysis of dynamic side‐shift in patients with idiopathic scoliosis. Archives of Physical Medicine and Rehabilitation 1995;76(7):621‐6. [DOI] [PubMed] [Google Scholar]
Skaggs 1996 {published data only}
- Skaggs DL. Effectiveness of treatment with a brace in girl who have adolescent idiopathic scoliosis. A prospective, controlled study based on data from the brace study of the scoliosis research society. Journal of Bone & Joint Surgery 1996;78(1):151. [PubMed] [Google Scholar]
Spoonamore 2004 {published data only}
- Spoonamore MJ, Dolan LA, Weinstein SL. Use of the Rosenberger brace in the treatment of progressive adolescent idiopathic scoliosis. Spine 2004;29(13):1458‐64. [DOI] [PubMed] [Google Scholar]
Tonseth 2005 {published data only}
- Tonseth KA, Wever DJ. Brace treatment of idiopathic scoliosis. Tidsskrift for Den Norske Laegeforening 2005;125(2):170‐2. [PubMed] [Google Scholar]
Trivedi 2001 {published data only}
- Trivedi JM, Thomson JD. Results of Charleston bracing in skeletally immature patients with idiopathic scoliosis. Journal of Pediatric Orthopedics 2001;21(3):277‐80. [PubMed] [Google Scholar]
Upadhyay 1995 {published data only}
- Upadhyay SS, Nelson IW, Ho EK, Hsu LC, Leong JC. New prognostic factors to predict the final outcome of brace treatment in adolescent idiopathic scoliosis. Spine 1995;20(5):537‐45. [DOI] [PubMed] [Google Scholar]
Van Rhijn 2002 {published data only}
- Rhijn LW, Plasmans CMT, Veraart BEEM. Changes in curve pattern after brace treatment for idiopathic scoliosis. Acta Orthopaedica Scandinavica 2002;73(3):277‐81. [DOI] [PubMed] [Google Scholar]
Van Rhijn 2003 {published data only}
- Rhijn LW, Veraart BE, Plasmans CM. Application of a lumbar brace for thoracic and double thoracic lumbar scoliosis: a comparative study. Journal of Pediatric Orthopaedics 2003;12(3):178‐82. [DOI] [PubMed] [Google Scholar]
Veldhuizen 2002 {published data only}
- Veldhuizen AG, Cheung J, Bulthuis GJ, Nijenbanning G. A new orthotic device in the non‐operative treatment of idiopathic scoliosis. Medical Engineering and Physics 2002;24(3):209‐18. [DOI] [PubMed] [Google Scholar]
Vijvermans 2004 {published data only}
- Vijvermans V, Fabry G, Nijs J. Factors determining the final outcome of treatment of idiopathic scoliosis with the Boston brace: a longitudinal study. Journal of Pediatric Orthopaedics 2004;13(3):143‐9. [DOI] [PubMed] [Google Scholar]
Watanabe 2005 {published data only}
- Watanabe K, Hasegawa K, Hirano T, Uchiyama S, Endo N. Use of the scoliosis research society outcomes instrument to evaluate patient outcome in untreated idiopathic scoliosis patients in Japan: Part I: comparison with non‐scoliosis group: preliminary/limited review in a Japanese population. Spine 2005;30(10):1197‐201. [DOI] [PubMed] [Google Scholar]
Weigert 2006 {published data only}
- Weigert KP, Nygaard LM, Christensen FB, Hansen ES, Bunger C. Outcome in adolescent idiopathic scoliosis after brace treatment and surgery assessed by means of the Scoliosis Research Society Instrument 24. European Spine Journal 2006;15(7):1108‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2003 {published data only}
- Weiss HR, Weiss G, Schaar HJ. Incidence of surgery in conservatively treated patients with scoliosis. Pediatric Rehabilitation 2003;6(2):111‐8. [DOI] [PubMed] [Google Scholar]
Weiss 2005 {published data only}
- Weiss H, Weiss GM. Brace treatment during pubertal growth spurt in girls with idiopathic scoliosis (IS): a prospective trial comparing two different concepts. Pediatric Rehabilitation 2005;8(3):199‐206. [DOI] [PubMed] [Google Scholar]
Weiss 2006 {published data only}
- Weiss H, Klein R. Improving excellence in scoliosis rehabilitation: a controlled study of matched pairs. Pediatric Rehabilitation 2006;9(3):190‐200. [DOI] [PubMed] [Google Scholar]
Wessberg 2011 {published data only}
- Wessberg, P, Rune H, Anders N. Night. Time providence bracing compared to fulltime Boston bracing in adolescent idiopathic Scoliosis. A prospective randomized study. Spine: Affiliated Society Meeting Abstracts. 2011.
Wever 2002 {published data only}
- Wever DJ, Tonseth KA, Veldhuizen AG. Curve progression and spinal growth in brace treated idiopathic scoliosis. Studies in Health Technology & Informatics 2002;91:387‐92. [PubMed] [Google Scholar]
Wiley 2000 {published data only}
- Wiley JW, Thomson JD, Mitchell TM, Smith BG, Banta JV. Effectiveness of the Boston brace in treatment of large curves in adolescent idiopathic scoliosis. Spine 2000;25(18):2326‐32. [DOI] [PubMed] [Google Scholar]
Willers 1993 {published data only}
- Willers U, Normelli H, Aaro S, Svensson O, Hedlund R. Long‐term results of Boston brace treatment on vertebral rotation in idiopathic scoliosis. Spine 1993;18(4):432‐5. [PubMed] [Google Scholar]
Yamauchi 1986 {published data only}
- Yamauchi Y, Asaka Y, Chen WS, Tsuji T, Yamaguchi T, Tsuruoka H. Follow‐up results of brace treatment of adolescent idiopathic scoliosis. Journal of the Japanese Orthopaedic Association 1986;60(11):1079‐85. [PubMed] [Google Scholar]
Ylikoski 1989 {published data only}
- Ylikoski M, Peltonen J, Poussa M. Biological factors and predictability of bracing in adolescent idiopathic scoliosis. Journal of Pediatric Orthopaedics 1989;9(6):680‐3. [DOI] [PubMed] [Google Scholar]
Yrjonen 2006 {published data only}
- Yrjonen T, Ylikoski M, Schlenzka D, Kinnunen R, Poussa M. Effectiveness of the Providence nighttime bracing in adolescent idiopathic scoliosis: a comparative study of 36 female patients. European Spine Journal 2006;15(7):1139‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Guo 2014 {published data only}
- Guo J, Lam TP, Wong MS, Ng BK, Lee KM, Liu KL, et al. A prospective randomized controlled study on the treatment outcome of SpineCor brace versus rigid brace for adolescent idiopathic scoliosis with follow‐up according to the SRS standardized criteria. European Spine Journal 2014;23(12):2650‐7. [DOI] [PubMed] [Google Scholar]
Wiemann 2014 {published data only}
- Wiemann JM, Shah SA, Price CT. Nighttime bracing versus observation for early adolescent idiopathic scoliosis. Journal of Pediatric Orthopedics 2014;34(6):603‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Altaf 2013
- Altaf F, Gibson A, Dannawi Z, Noordeen H. Adolescent idiopathic scoliosis. BMJ 2013;346:f2508. [DOI] [PubMed] [Google Scholar]
Asher 2003a
- Asher M, Min Lai S, Burton D, Manna B. Discrimination validity of the Scoliosis Research Society‐22 patient questionnaire: relationship to idiopathic scoliosis curve pattern and curve size. Spine 2003;28:74‐8. [DOI] [PubMed] [Google Scholar]
Asher 2003b
- Asher M, Min Lai S, Burton D, Manna B. The reliability and concurrent validity of the Scoliosis Research Society‐22 patient questionnaire for idiopathic scoliosis. Spine 2003;28:63‐9. [DOI] [PubMed] [Google Scholar]
Boutron 2005
- Boutron I, Moher D, Tugwell P, Giraudeau B, Poiraudeau S, Nizard R, et al. A checklist to evaluate a report of a non pharmacological trial (CLEAR NPT) was developed using consensus. Journal of Clinical Epidemiology 2005;58(12):1233‐40. [DOI] [PubMed] [Google Scholar]
Bunge 2008
- Bunge EM, Koning HJ. Bracing patients with idiopathic scoliosis: design of the Dutch randomized controlled treatment trial. BMC Musculoskeletal Disorders 2008;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Climent 1999
- Climent JM, Sanchez J. Impact of the type of brace on the quality of life of adolescents with spine deformities. Spine 1999;24(18):1903‐8. [DOI] [PubMed] [Google Scholar]
Coillard 2002
- Coillard C, Leroux MA, Badeaux J, Rivard CH. SPINECOR: a new therapeutic approach for idiopathic scoliosis. Studies in Health Technology & Informatics 2002;88:215‐7. [PubMed] [Google Scholar]
Danielsson 2003a
- Danielsson AJ, Nachemson AL. Back pain and function 22 years after brace treatment for adolescent idiopathic scoliosis: a case‐control study ‐ Part I. Spine 2003;28(18):2078‐85. [DOI] [PubMed] [Google Scholar]
Danielsson 2003b
- Danielsson AJ, Nachemson AL. Back pain and function 23 years after fusion for adolescent idiopathic scoliosis: a case‐control study ‐ Part II. Spine 2003;28(18):E373‐83. [DOI] [PubMed] [Google Scholar]
Dickson 1999b
- Dickson RA, Weinstein SL. Bracing (and screening) ‐ yes or no?. Journal of Bone & Joint Surgery 1999;81(2):193‐8. [DOI] [PubMed] [Google Scholar]
Dolan 2007a
- Dolan LA, Weinstein SL. Surgical rates after observation and bracing for adolescent idiopathic scoliosis: an evidence‐based review. Spine 2007;32(19 Suppl):S91‐S100. [DOI] [PubMed] [Google Scholar]
Dolan 2007b
- Dolan LA, Donnelly MJ, Spratt KF, Weinstein SL. Professional opinion concerning the effectiveness of bracing relative to observation in adolescent idiopathic scoliosis. Journal of Pediatric Orthopaedics 2007;27(3):270‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donzelli 2012
- Donzelli S, Zaina F, Negrini S. In defense of adolescents: they really do use braces for the hours prescribed, if good help is provided. Results from a prospective everyday clinic cohort using thermobrace. Scoliosis 2012 May 31;7 (1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freidel 2002a
- Freidel K, Petermann F, Reichel D, Steiner A, Warschburger P, Weiss HR. Quality of life in women with idiopathic scoliosis. Spine 2002;27(4):E87‐91. [DOI] [PubMed] [Google Scholar]
Freidel 2002b
- Freidel K, Reichel D, Steiner A, Warschburger P, Petermann F, Weiss HR. Idiopathic scoliosis and quality of life. Studies in Health Technology & Informatics 2002;88:24‐9. [PubMed] [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder M, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
Fusco 2011
- Fusco C, Zaina F, Atanasio S, Romano M, Negrini A, Negrini S. Physical exercises in the treatment of adolescent idiopathic scoliosis: an updated systematic review. Physiotherapy Theory and Practice 2011;27(1):80‐114. [DOI] [PubMed] [Google Scholar]
Goldberg 1993
- Goldberg CJ, Dowling FE, Hall JE, Emans JB. A statistical comparison between natural history of idiopathic scoliosis and brace treatment in skeletally immature adolescent girls. Spine 1993;18(7):902‐8. [DOI] [PubMed] [Google Scholar]
Grivas 2006
- Grivas TB, Vasiliadis E, Mouzakis V, Mihas C, Koufopoulos G. Association between adolescent idiopathic scoliosis prevalence and age at menarche in different geographic latitudes. Scoliosis 2006;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grivas 2008
- Grivas TB, Vasiliadis ES, Rodopoulos G, Bardakos N. The role of the intervertebral disc in correction of scoliotic curves. A theoretical model of idiopathic scoliosis pathogenesis. Studies in Health Technology & Informatics 2008;140:33‐6. [PubMed] [Google Scholar]
Grivas 2012
- Grivas TB. Spine: maintaining mobility of the spine. Steps toward more effective scoliosis brace treatment to prevent the need for fusion. www.boneandjoint.org.uk/content/maintaining‐mobility‐spine%E2%80%A6 (accessed 29 May 2015).
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hresko 2013
- Hresko MT. Clinical practice. Idiopathic scoliosis in adolescents. New England Journal of Medicine 2013;368(9):834‐41. [DOI] [PubMed] [Google Scholar]
Katz 2001
- Katz DE, Durrani AA. Factors that influence outcome in bracing large curves in patients with adolescent idiopathic scoliosis. Spine 2001;26(21):2354‐61. [DOI] [PubMed] [Google Scholar]
Katz 2010
- Katz DE, Herring JA, Browne RH, Kelly DM, Birch JG. Brace wear control of curve progression in adolescent idiopathic scoliosis. Journal of Bone and Joint Surgery American Version 2010;92(6):1343‐52. [DOI] [PubMed] [Google Scholar]
Landauer 2003
- Landauer F, Wimmer C, Behensky H. Estimating the final outcome of brace treatment for idiopathic thoracic scoliosis at 6‐month follow‐up. Pediatric Rehabilitation 2003;6(3‐4):201‐7. [DOI] [PubMed] [Google Scholar]
Lenssinck 2005
- Lenssinck ML, Frijlink AC, Berger MY, Bierman‐Zeinstra SM, Verkerk K, Verhagen AP. Effect of bracing and other conservative interventions in the treatment of idiopathic scoliosis in adolescents: a systematic review of clinical trials. Physical Therapy 2005;85(12):1329‐39. [PubMed] [Google Scholar]
Lonstein 2006
- Lonstein JE. Scoliosis: surgical versus non‐surgical treatment. Clinical Orthopaedics & Related Research 2006;443:248‐59. [DOI] [PubMed] [Google Scholar]
Lupparelli 2002
- Lupparelli S, Pola E, Pitta L, Mazza O, Santis V, Aulisa L. Biomechanical factors affecting progression of structural scoliotic curves of the spine. Studies in Health Technology & Informatics 2002;91:81‐5. [PubMed] [Google Scholar]
MacLean 1989
- MacLean WE Jr, Green NE, Pierre CB, Ray DC. Stress and coping with scoliosis: psychological effects on adolescents and their families. Journal of Pediatric Orthopaedics 1989;9(3):257‐61. [PubMed] [Google Scholar]
Maruyama 2003
- Maruyama T, Kitagawa T, Takeshita K, Mochizuki K, Nakamura K. Conservative treatment for adolescent idiopathic scoliosis: can it reduce the incidence of surgical treatment?. Pediatric Rehabilitation 2003;6(3‐4):215‐9. [DOI] [PubMed] [Google Scholar]
Mayo 1994
- Mayo NE, Goldberg MS, Poitras B, Scott S, Hanley J. The Ste. Justine Adolescent Idiopathic Scoliosis Cohort Study. Part III: Back pain. Spine 1994;19(14):1573‐81. [DOI] [PubMed] [Google Scholar]
Negrini 2003
- Negrini S, Antonini G, Carabalona R, Minozzi S. Physical exercises as a treatment for adolescent idiopathic scoliosis. A systematic review. Pediatric Rehabilitation 2003;6(3‐4):227‐35. [DOI] [PubMed] [Google Scholar]
Negrini 2005
- Negrini S, Aulisa L, Ferraro C, Fraschini P, Masiero S, Simonazzi P, et al. Italian guidelines on rehabilitation treatment of adolescents with scoliosis or other spinal deformities. Europa Medicophysica 2005;41(2):183‐201. [PubMed] [Google Scholar]
Negrini 2006a
- Negrini S, Grivas TB, Kotwicki T, Maruyama T, Rigo M, Weiss HR, et al. Why do we treat adolescent idiopathic scoliosis? What we want to obtain and to avoid for our patients. SOSORT 2005 Consensus paper. Scoliosis 2006;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Negrini 2006b
- Negrini S, Carabalona R. Social acceptability of treatments for adolescent idiopathic scoliosis: a cross‐sectional study. Scoliosis 2006;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Negrini 2006c
- Negrini S, Marchini G. Efficacy of the Symmetric, Patient‐oriented, Rigid, Three‐dimensional, active (SPoRT) concept of bracing for scoliosis: a prospective study of the Sforzesco versus Lyon brace. Europa Medicophysica 2006;43(2):171‐81. [PubMed] [Google Scholar]
Negrini 2008a
- Negrini S, Fusco C, Minozzi S, Atanasio S, Zaina F, Romano M. Exercises reduce the progression rate of adolescent idiopathic scoliosis: results of a comprehensive systematic review of the literature. Disability Rehabilitation 2008;30(10):772‐85. [DOI] [PubMed] [Google Scholar]
Negrini 2008b
- Negrini S, Atanasio S, Zaina F, Romano M, Parzini S, Negrini A. End‐growth results of bracing and exercises for adolescent idiopathic scoliosis. Prospective worst‐case analysis. Studies in Health Technology & Informatics 2008;135:395‐408. [PubMed] [Google Scholar]
Negrini 2009a
- Negrini S, Atanasio S, Fusco C, Zaina F. Effectiveness of complete conservative treatment for adolescent idiopathic scoliosis (bracing and exercises) based on SOSORT management criteria: results according to the SRS criteria for bracing studies ‐ 2009 SOSORT Award Winner. Scoliosis 2009;4(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Negrini 2009b
- Negrini S, Grivas TB, Kotwicki T, Rigo M, Zaina F, the international Society on Scoliosis Orthopaedic and Rehabilitation Treatment (SOSORT). Guidelines on "Standards of management of idiopathic scoliosis with corrective braces in everyday clinics and in clinical research": SOSORT Consensus 2008. Scoliosis 2009;4(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Negrini 2010b
- Negrini S, Minozzi S, Bettany‐Saltikov J, Zaina F, Chockalingam N, Grivas TB, et al. Braces for idiopathic scoliosis in adolescents. Spine 2010;35(13):1285‐93. [DOI] [PubMed] [Google Scholar]
Negrini 2012
- Negrini S, Aulisa AG, Aulisa L, Circo AB, Mauroy JC, Durmala J, et al. 2011 SOSORT guidelines: Orthopaedic and Rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis 2012;7(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Negrini 2014
- Negrini S. Evidence in adolescent idiopathic scoliosis treatment. European Journal of Physical and Rehabilitation Medicine 2014;50(1):100. [PubMed] [Google Scholar]
Noonan 1997
- Noonan KJ, Dolan LA, Jacobson WC, Weinstein SL. Long‐term psychosocial characteristics of patients treated for idiopathic scoliosis. Journal of Pediatric Orthopaedics 1997;17(6):712‐7. [PubMed] [Google Scholar]
Odermatt 2003
- Odermatt D, Mathieu PA, Beausejour M, Labelle H, Aubin CE. Electromyography of scoliotic patients treated with a brace. Journal of Orthopaedic Research 2003;21(5):931‐6. [DOI] [PubMed] [Google Scholar]
Parent 2005
- Parent S, Newton PO, Wenger DR. Adolescent idiopathic scoliosis: etiology, anatomy, natural history, and bracing. Instructional Course Lectures 2005;54:529‐36. [PubMed] [Google Scholar]
Pehrsson 1992
- Pehrsson K, Larsson S, Oden A, Nachemson A. Long‐term follow‐up of patients with untreated scoliosis. A study of mortality, causes of death, and symptoms. Spine 1992;17(9):1091‐6. [DOI] [PubMed] [Google Scholar]
Pehrsson 2001
- Pehrsson K, Danielsson A, Nachemson A. Pulmonary function in adolescent idiopathic scoliosis: a 25 year follow up after surgery or start of brace treatment. Thorax 2001;56(5):388‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reichel 2003
- Reichel D, Schanz J. Developmental psychological aspects of scoliosis treatment. Pediatric Rehabilitation 2003;6(3‐4):221‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Richards 2005
- Richards BS, Bernstein RM, D'Amato CR, Thompson GH. Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS Committee on Bracing and Nonoperative Management. Spine 2005;30(18):2068‐75. [DOI] [PubMed] [Google Scholar]
Rigo 2006
- Rigo M, Negrini S, Weiss HR, Grivas TB, Maruyama T, Kotwicki T, et al. SOSORT consensus paper on brace action: TLSO biomechanics of correction (investigating the rationale for force vector selection). Scoliosis 2006;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romano 2008
- Romano M, Negrini S. Manual therapy as a conservative treatment for adolescent idiopathic scoliosis: a systematic review. Scoliosis 2008;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romano 2012
- Romano M, Minozzi S, Bettany‐Saltikov J, Zaina F, Chockalingam N, Kotwicki T, et al. Exercises for adolescent idiopathic scoliosis. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD007837.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romano 2013
- Romano M, Minozzi S, Zaina F, Saltikov JB, Chockalingam N, Kotwicki T, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine 2013;38(14):E883‐93. [DOI] [PubMed] [Google Scholar]
Shekelle 1994
- Shekelle PG, Andersson G, Bombardier C, Cherkin D, Deyo R, Keller R. A brief introduction to the critical reading of the clinical literature. Spine 1994;19:2028S‐31S. [DOI] [PubMed] [Google Scholar]
Smania 2008
- Smania N, Picelli A, Romano M, Negrini S. Neurophysiological basis of rehabilitation of adolescent idiopathic scoliosis. Disability Rehabilitation 2008;30(10):763‐71. [DOI] [PubMed] [Google Scholar]
Smith 2003
- Smith GC, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ 2003;327(7429):1459‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
SRS 2006
- Edgar M, Scoliosis Research Society. Brace wear compliance. www.srs.org/professionals/bracing_manuals/section3.pdf (accessed 30 June 2007).
Stokes 2006
- Stokes IA, Burwell RG, Dangerfield PH. Biomechanical spinal growth modulation and progressive adolescent scoliosis ‐ a test of the 'vicious cycle' pathogenetic hypothesis: summary of an electronic focus group debate of the IBSE. Scoliosis 2006;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ugwonali 2004
- Ugwonali OF, Lomas G, Choe JC, Hyman JE, Lee FY, Vitale MG, et al. Effect of bracing on the quality of life of adolescents with idiopathic scoliosis. Spine Journal 2004;4(3):254‐60. [DOI] [PubMed] [Google Scholar]
van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board Cochrane Back Review Group. Updated method guidelines for systemic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Varni 2001
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care 2001;39:800‐12. [DOI] [PubMed] [Google Scholar]
Varni 2003
- Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambulatory Pediatrics 2003;3:329‐41. [DOI] [PubMed] [Google Scholar]
Vasiliadis 2006
- Vasiliadis E, Grivas TB, Savvidou O, Triantafyllopoulos G. The influence of brace on quality of life of adolescents with idiopathic scoliosis. Studies in Health Technology & Informatics 2006;123:352‐6. [PubMed] [Google Scholar]
Vasiliadis 2008
- Vasiliadis ES, Grivas TB. Quality of life after conservative treatment of adolescent idiopathic scoliosis. Studies in Health Technology and Informatics 2008;135:409‐13. [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item Short‐Form Health Survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30:473‐83. [PubMed] [Google Scholar]
Weinstein 2003
- Weinstein SL, Dolan LA, Spratt KF, Peterson KK, Spoonamore MJ, Ponseti IV. Health and function of patients with untreated idiopathic scoliosis: a 50‐year natural history study. JAMA 2003;289(5):559‐67. [DOI] [PubMed] [Google Scholar]
Wells 2008
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 29 May 2015).
Wiklund 1991
- Wiklund I, Karlberg J. Evaluation of quality of life in clinical trials. Selecting quality‐of‐life measures. Controlled Clinical Trials 1991;12(20):4S‐16S. [DOI] [PubMed] [Google Scholar]
Zaina 2009
- Zaina F, Negrini S, Atanasio S, Fusco C, Romano M, Negrini A. Specific exercises performed in the period of brace weaning can avoid loss of correction in Adolescent Idiopathic Scoliosis (AIS) patients: winner of SOSORT's 2008 Award for Best Clinical Paper. Scoliosis 2009;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Negrini 2010a
- Negrini S, Minozzi S, Bettany‐Saltikov J, Zaina F, Chockalingam N, Grivas TB, et al. Braces for idiopathic scoliosis in adolescents. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006850.pub2] [DOI] [PubMed] [Google Scholar]


