Abstract
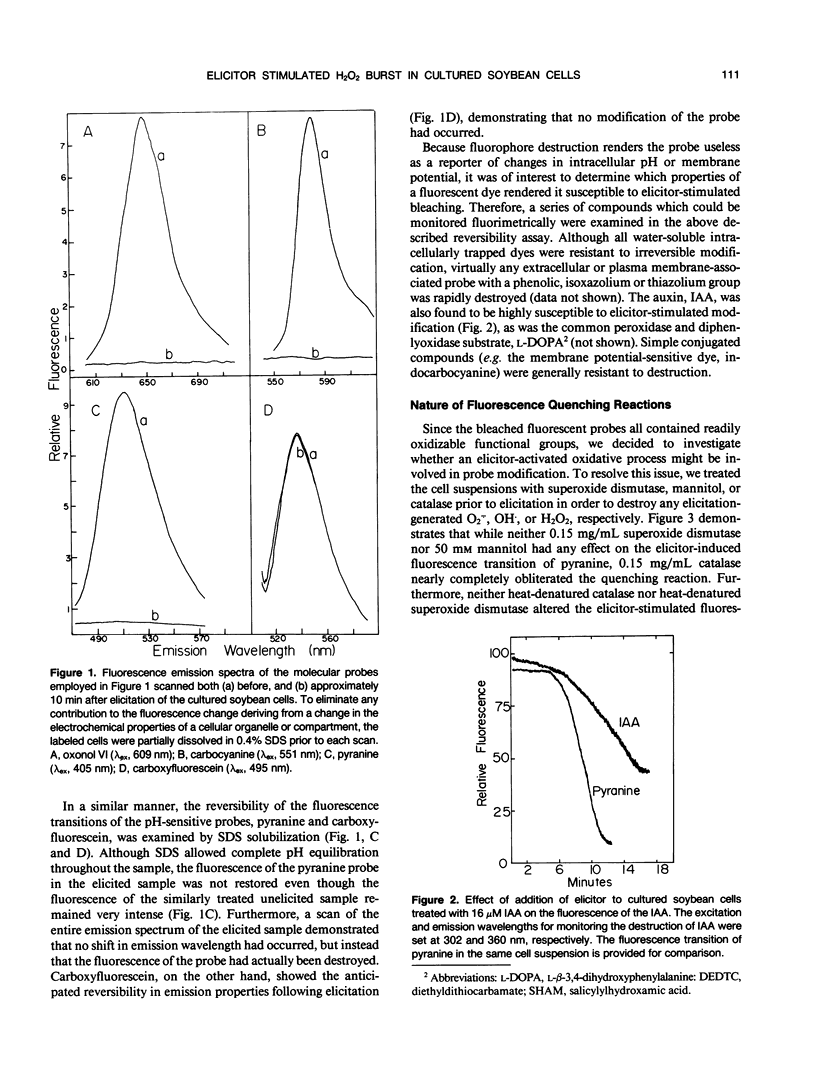
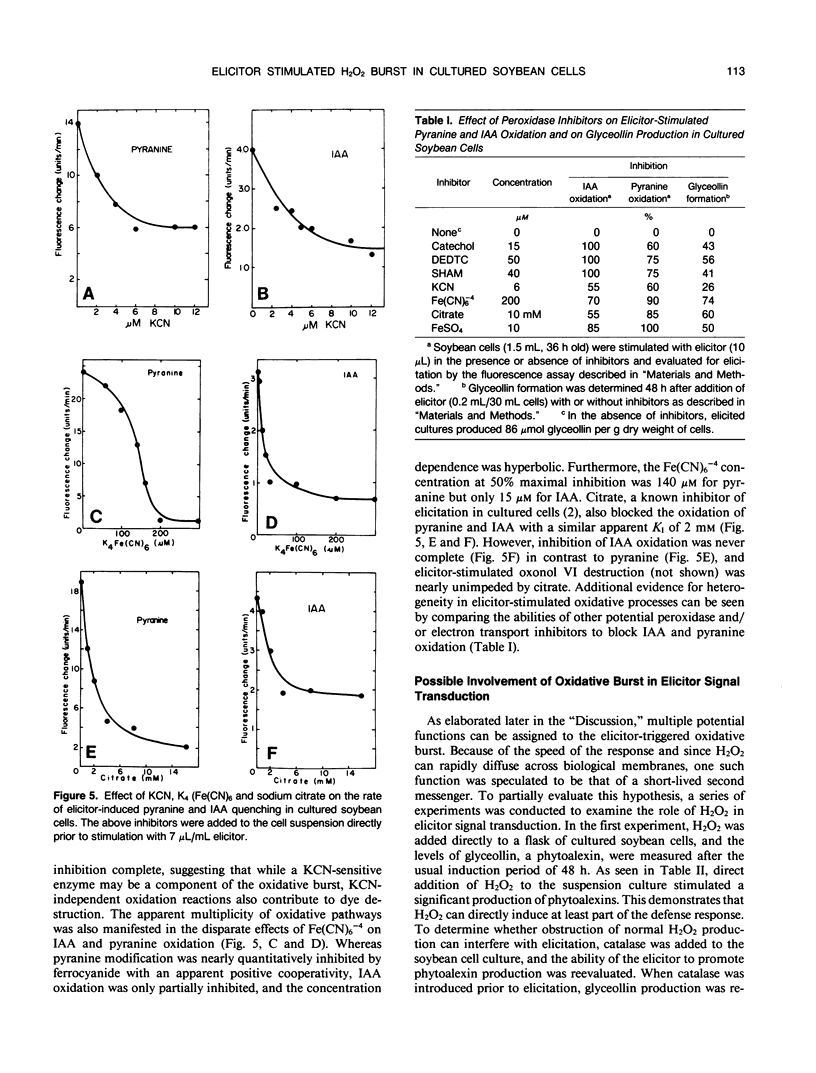
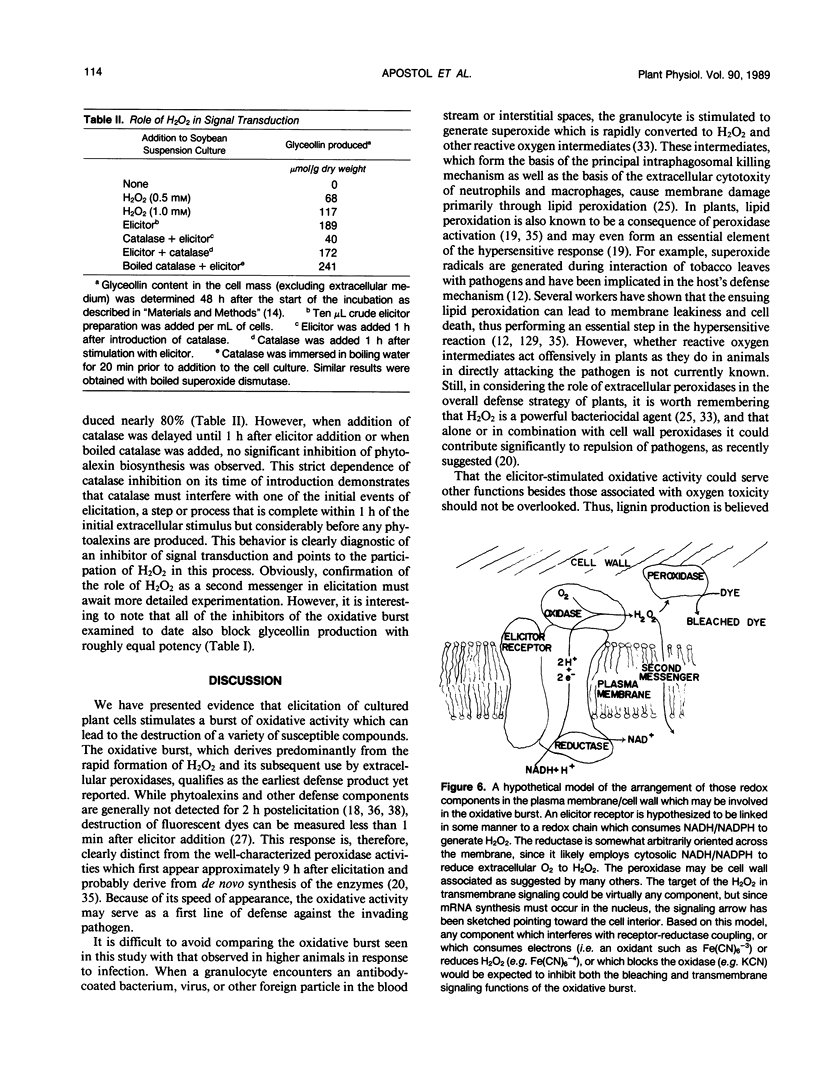
Stimulation of cultured plant cells with elicitors of the defense response leads to the rapid destruction of a variety of water-soluble compounds including indoleacetic acid and certain fluorescent dyes. This destructive activity, which is often vigorously manifested within 5 minutes of elicitor addition, is shown to derive from the rapid production of H2O2 and its use by extracellular peroxidases. Because of its speed of appearance, this oxidative burst may qualify as the first induced line of defense against invading pathogens. Since H2O2 has been implicated as a second messenger of hormone-stimulated metabolic changes in some animal cells, its possible role in transduction of the defense signal in plants was also examined. Not only did exogenous H2O2 alone stimulate phytoalexin production in the plant cell suspension, but inhibition of elicitor-stimulated phytoalexin production was observed upon addition of catalase and other inhibitors of the oxidative burst. Furthermore, for inhibition to occur, the presence of catalase was required during elicitor addition, since if introduction of the enzyme was delayed until 1 hour after addition of the elicitor, no inhibition resulted. These results suggest that H2O2 also plays an important role in inducing subsequent defense responses such as phytoalexin production.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J. G., Storey B. T. Lipid peroxidation and the reactions of superoxide and hydrogen peroxide in mouse spermatozoa. Biol Reprod. 1984 May;30(4):833–841. doi: 10.1095/biolreprod30.4.833. [DOI] [PubMed] [Google Scholar]
- Apostol I., Low P. S., Heinstein P., Stipanovic R. D., Altman D. W. Inhibition of elicitor-induced phytoalexin formation in cotton and soybean cells by citrate. Plant Physiol. 1987 Aug;84(4):1276–1280. doi: 10.1104/pp.84.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman A. O., Barr R., Crane F. L., Morré D. J. Auxin-Stimulated NADH Oxidase Purified from Plasma Membrane of Soybean. Plant Physiol. 1988 Apr;86(4):1264–1269. doi: 10.1104/pp.86.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttger M., Hilgendorf F. Hormone action on transmembrane electron and h transport. Plant Physiol. 1988 Apr;86(4):1038–1043. doi: 10.1104/pp.86.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement N. R., Gould J. M. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. Biochemistry. 1981 Mar 17;20(6):1534–1538. doi: 10.1021/bi00509a019. [DOI] [PubMed] [Google Scholar]
- Crane F. L., Sun I. L., Clark M. G., Grebing C., Löw H. Transplasma-membrane redox systems in growth and development. Biochim Biophys Acta. 1985 Aug 1;811(3):233–264. doi: 10.1016/0304-4173(85)90013-8. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Lawrence J. C., Jr, Lynn W. S. Evidence for the involvement of sulfhydryl oxidation in the regulation of fat cell hexose transport by insulin. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4173–4177. doi: 10.1073/pnas.71.10.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J., Schmidt W. E., Loyal R. Phytoalexin synthesis in soybean cells: elicitor induction of phenylalanine ammonia-lyase and chalcone synthase mRNAs and correlation with phytoalexin accumulation. Arch Biochem Biophys. 1984 Jul;232(1):240–248. doi: 10.1016/0003-9861(84)90540-x. [DOI] [PubMed] [Google Scholar]
- Espelie K. E., Franceschi V. R., Kolattukudy P. E. Immunocytochemical localization and time course of appearance of an anionic peroxidase associated with suberization in wound-healing potato tuber tissue. Plant Physiol. 1986 Jun;81(2):487–492. doi: 10.1104/pp.81.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. H., Meyer H. E., Burke R. E., Feung C. S., Mumma R. O. Metabolism of Indole-3-Acetic Acid: II. Oxindole Pathway in Parthenocissus tricuspidata Crown-Gall Tissue Cultures. Plant Physiol. 1976 Jul;58(1):77–81. doi: 10.1104/pp.58.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshio O., Akanuma Y., Kasuga M. Hydrogen peroxide stimulates tyrosine phosphorylation of the insulin receptor and its tyrosine kinase activity in intact cells. Biochem J. 1988 Feb 15;250(1):95–101. doi: 10.1042/bj2500095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuć J., Rush J. S. Phytoalexins. Arch Biochem Biophys. 1985 Feb 1;236(2):455–472. doi: 10.1016/0003-9861(85)90648-4. [DOI] [PubMed] [Google Scholar]
- Köhle H., Jeblick W., Poten F., Blaschek W., Kauss H. Chitosan-elicited callose synthesis in soybean cells as a ca-dependent process. Plant Physiol. 1985 Mar;77(3):544–551. doi: 10.1104/pp.77.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J. Immunology. A common form of killing. Nature. 1986 Jun 5;321(6070):560–560. doi: 10.1038/321560a0. [DOI] [PubMed] [Google Scholar]
- Low P. S., Heinstein P. F. Elicitor stimulation of the defense response in cultured plant cells monitored by fluorescent dyes. Arch Biochem Biophys. 1986 Sep;249(2):472–479. doi: 10.1016/0003-9861(86)90024-x. [DOI] [PubMed] [Google Scholar]
- May J. M., de Haën C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem. 1979 Apr 10;254(7):2214–2220. [PubMed] [Google Scholar]
- May J. M., de Haën C. The insulin-like effect of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. J Biol Chem. 1979 Sep 25;254(18):9017–9021. [PubMed] [Google Scholar]
- Nothnagel E. A., McNeil M., Albersheim P., Dell A. Host-Pathogen Interactions : XXII. A Galacturonic Acid Oligosaccharide from Plant Cell Walls Elicits Phytoalexins. Plant Physiol. 1983 Apr;71(4):916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Komoriya K., Azuma A., Kurozumi S., Hashimoto Y. Xanthine oxidase-induced histamine release from isolated rat peritoneal mast cells: involvement of hydrogen peroxide. Biochem Pharmacol. 1979;28(2):333–334. doi: 10.1016/0006-2952(79)90524-0. [DOI] [PubMed] [Google Scholar]
- Ramasarma T. Generation of H2O in biomembranes. Biochim Biophys Acta. 1982 Aug 11;694(1):69–93. doi: 10.1016/0304-4157(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Rapid switching of plant gene expression induced by fungal elicitor. Science. 1985 Mar 8;227(4691):1240–1243. doi: 10.1126/science.227.4691.1240. [DOI] [PubMed] [Google Scholar]
- Rogers K. R., Albert F., Anderson A. J. Lipid peroxidation is a consequence of elicitor activity. Plant Physiol. 1988 Feb;86(2):547–553. doi: 10.1104/pp.86.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinshi H., Mohnen D., Meins F. Regulation of a plant pathogenesis-related enzyme: Inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):89–93. doi: 10.1073/pnas.84.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter A. M., Bell J. N., Cramer C. L., Bailey J. A., Varner J. E., Lamb C. J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner A. S. Dye indicators of membrane potential. Annu Rev Biophys Bioeng. 1979;8:47–68. doi: 10.1146/annurev.bb.08.060179.000403. [DOI] [PubMed] [Google Scholar]


