Abstract
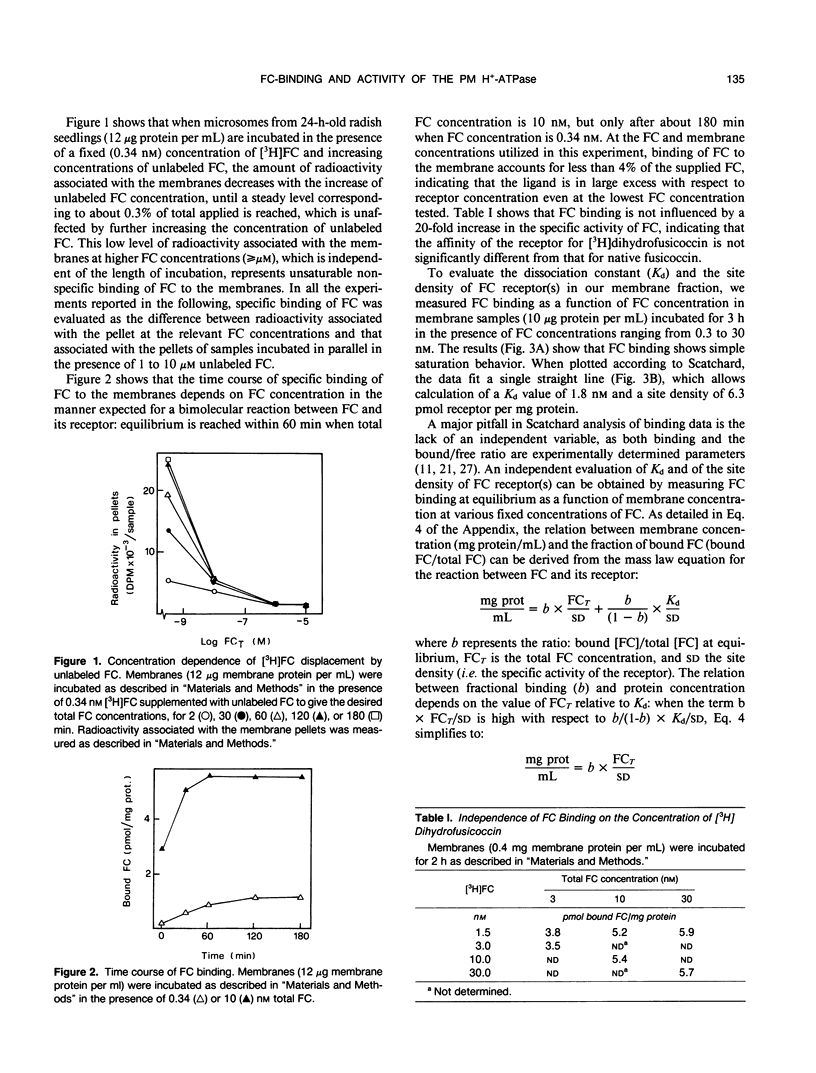
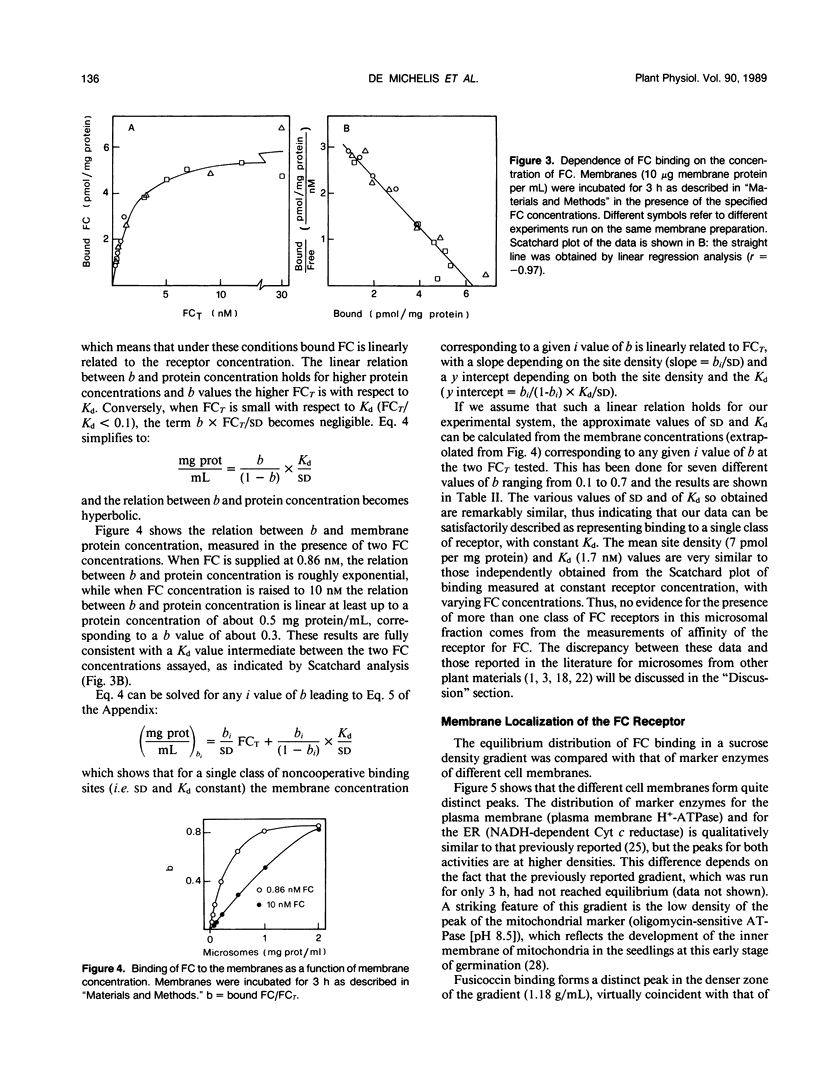
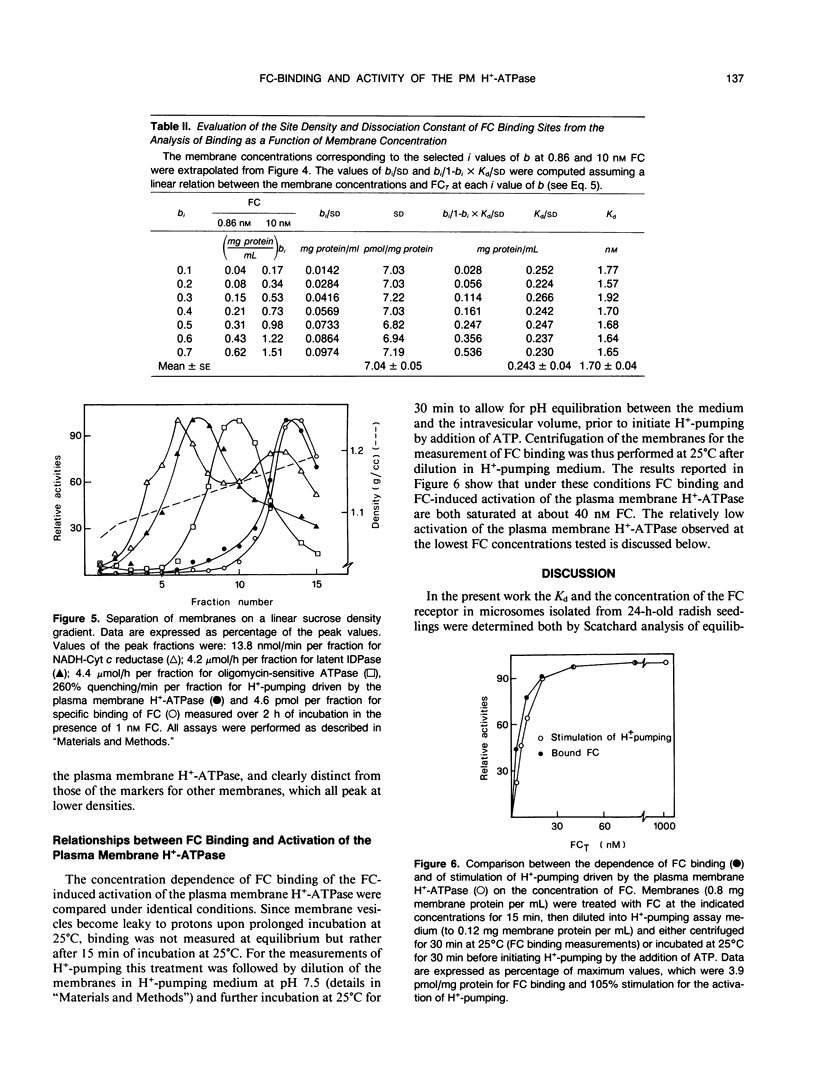
The characteristics of fusicoccin binding were investigated in microsomes from 24-h-old radish (Raphanus sativus L.) seedlings. The time course of fusicoccin binding depended on fusicoccin concentration: equilibrium was reached much faster at 10 nanomolar fusicoccin than at 0.3 nanomolar fusicoccin. Scatchard analysis of equilibrium binding as a function of fusicoccin concentration indicated a single class of receptor sites with a Kd of 1.8 nanomolar and a site density of 6.3 picomoles per milligram protein. Similar values (Kd 1.7 nanomolar and site density 7 picomoles per milligram protein) were obtained from the analysis of the dependence of equilibrium binding on membrane concentration at fixed fusicoccin concentrations. Fusicoccin binding comigrated with the plasma membrane H+-ATPase in an equilibrium sucrose density gradient: both activities formed a sharp peak (1.18 grams per milliliter) clearly distinct from that of markers of other membranes which all peaked at lower densities. The saturation profiles of fusicoccin binding and of fusicoccin-induced activation of the plasma membrane H+-ATPase, measured under identical conditions, were similar, supporting the view that fusicoccin-induced activation of the plasma membrane H+-ATPase is mediated by fusicoccin binding to its plasma membrane receptor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chanson A., McNaughton E., Taiz L. Evidence for a KCl-Stimulated, Mg-ATPase on the Golgi of Corn Coleoptiles. Plant Physiol. 1984 Oct;76(2):498–507. doi: 10.1104/pp.76.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno F., De Michelis M. I., Pugliarello M. C., Marrè E. H-pumping driven by the plasma membrane ATPase in membrane vesicles from radish: stimulation by fusicoccin. Plant Physiol. 1986 Sep;82(1):121–125. doi: 10.1104/pp.82.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., De Michelis M. I. The Ca-Transport ATPase of Plant Plasma Membrane Catalyzes a nH/Ca Exchange. Plant Physiol. 1987 Apr;83(4):994–1000. doi: 10.1104/pp.83.4.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C. Fusicoccin stimulates the H+-ATPase of plasmalemma in isolated membrane vesicles from radish. Biochem Biophys Res Commun. 1985 Nov 27;133(1):280–285. doi: 10.1016/0006-291x(85)91872-8. [DOI] [PubMed] [Google Scholar]
- Stout R. G., Cleland R. E. Partial characterization of fusicoccin binding to receptor sites on oat root membranes. Plant Physiol. 1980 Sep;66(3):353–359. doi: 10.1104/pp.66.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland G. A., Molinoff P. B. Quantitative analysis of drug-receptor interactions: I. Determination of kinetic and equilibrium properties. Life Sci. 1981 Jul 27;29(4):313–330. doi: 10.1016/0024-3205(81)90324-6. [DOI] [PubMed] [Google Scholar]
- Wilson S. B., Bonner W. D. Studies of electron transport in dry and imbibed peanut embryos. Plant Physiol. 1971 Sep;48(3):340–344. doi: 10.1104/pp.48.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]


