Abstract
In order to determine the specificities of PCR-based assays used for detecting Cryptosporidium parvum DNA, eight pairs of previously described PCR primers targeting six distinct regions of the Cryptosporidium genome were evaluated for the detection of C. parvum, the agent of human cryptosporidiosis, and C. muris, C. baileyi, and C. meleagridis, three Cryptosporidium species that infect birds or mammals but are not considered to be human pathogens. The four Cryptosporidium species were divided into two groups: C. parvum and C. meleagridis, which gave the same-sized fragments with all the reactions, and C. muris and C. baileyi, which gave positive results with primer pairs targeting the 18S rRNA gene only. In addition to being genetically similar at each of the eight loci analyzed by DNA amplification, C. parvum and C. meleagridis couldn’t be differentiated even after restriction enzyme digestion of the PCR products obtained from three of the target genes. This study indicates that caution should be exercised in the interpretation of data from water sample analysis performed by these methods, since a positive result does not necessarily reflect a contamination by the human pathogen C. parvum.
Organisms of the genus Cryptosporidium are widespread coccidian protozoans that develop in epithelial cells lining the digestive and respiratory tracts of vertebrates. On the basis of host specificity, pathogenesis, and oocyst morphology, eight Cryptosporidium species are regarded as valid (9): Cryptosporidium muris and C. parvum in mammals (4, 27), C. wrairi in guinea pigs (9), C. felis in domestic cats (9), C. meleagridis and C. baileyi in birds (5, 25), C. nasorum in fish, and C. serpentis in reptiles (9). According to this classification, C. parvum is the agent of clinical cryptosporidiosis in humans and livestock (9). Despite a unique report of C. baileyi infection in an immunocompromised patient (6), C. parvum is the only Cryptosporidium species regarded as a threat to human health.
Human cryptosporidiosis is a worldwide emerging zoonotic disease. Whereas immunocompetent individuals experience short-term gastroenteritis that resolves spontaneously, malnourished children and immunocompromised individuals may suffer from chronic life-threatening diarrhea. Transmission occurs by the fecal-oral route. C. parvum oocysts are shed into the environment by infected mammals who contaminate surface waters. The resistance of these oocysts to standard water disinfectants, as well as the low infective dose of viable C. parvum oocysts (8), accounts for the risk of waterborne transmission of human cryptosporidiosis and for the serious outbreaks that have been reported (12).
Waterborne cryptosporidiosis thus represents a global public health problem, and reliable detection methods are needed in order to control the presence of the parasite in source and finished waters. PCR amplification of Cryptosporidium DNA is a potentially powerful approach in achieving this aim, and several groups have cloned and sequenced Cryptosporidium genes as well as proposed PCR-based methods for identifying C. parvum DNA. However, environmental waters are likely to be contaminated with Cryptosporidium oocysts from diverse vertebrate reservoirs. Therefore, a major requirement regarding the characterization of these techniques should be an accurate evaluation of their specificity with Cryptosporidium oocysts of species other than C. parvum, in order to ultimately develop a technique capable of unambiguously identifying C. parvum oocysts.
In the original studies, PCR-based methods used for the identification and typing of Cryptosporidium isolates were evaluated with C. parvum (3, 15, 18, 28), with C. parvum and C. muris (14), or with DNA from C. parvum, C. muris, and C. baileyi (1, 13, 24), and none of these studies included the bird species C. meleagridis. The aim of the present study was to thoroughly assess the specificities of the eight PCR assays cited above for C. parvum, C. muris, C. baileyi, and C. meleagridis.
MATERIALS AND METHODS
Cryptosporidium isolates.
C. parvum isolate B-97-11 was provided by G. Harly; it was obtained from the diarrheic feces of a naturally infected newborn calf. Oocysts of C. muris, C. meleagridis, and C. baileyi isolate O.96.2 were provided by M. Naciri. The C. muris isolate was obtained from a naturally infected 10-year-old cow with diarrhea. Purified oocysts were ovoid and measured about 7.5 by 5.5 μm. The C. meleagridis isolate was obtained from mucosal scrapings of the cecal pouches of a common quail necropsied during an outbreak of diarrhea and was maintained in chickens by oral inoculation and recovery of the cecal contents. Purified oocysts were spherical and measured 4.5 μm in diameter. C. baileyi isolate O.96.2 originated from the bursa of Fabricius of a newborn duck and was maintained in ducks or chickens by oral inoculation and recovery of the contents of the bursa of Fabricius and the cloaca. Purified oocysts were ovoid and measured 6.9 by 5.5 μm (19). The second C. baileyi isolate utilized in this study, isolate B1, was provided by I. Varga. The oocysts were originally purified from the feces of chickens during an outbreak of avian cryptosporidiosis (7), and the isolate was maintained by serial passage in chickens. Purified oocysts were ovoid and measured 6.2 by 4.2 μm.
Preparation of oocyst lysates as PCR templates.
C. parvum, C. muris, C. meleagridis, and C. baileyi isolate O.96.2 were extracted from fecal material as previously described (2). Purified oocysts were resuspended in 10 mM Tris (pH 8.3)–50 mM KCl at 4°C for DNA extraction or stored in 2.5% potassium dichromate at 4°C until analysis. C. baileyi oocysts from isolate B1 were purified as described elsewhere (5); oocysts in 2.5% potassium dichromate were washed four times by successive pelleting (10,000 × g for 10 min at 4°C) and resuspension in distilled water and were finally suspended in 10 mM Tris (pH 8.3)–50 mM KCl. For DNA extraction, purified oocysts were suspended at a density of 250 oocysts/μl in 100-μl aliquots of 10 mM Tris (pH 8.3)–50 mM KCl containing 0.5% (wt/vol) Tween 20. After freeze-thawing (15 cycles), samples were heated for 15 min at 100°C and then centrifuged for 2 min at 16,000 × g to remove particulate matter. Supernatants were recovered and stored at −20°C until used for PCR amplification (3, 10).
PCR primers.
The sequences of the primers and the sizes of the expected PCR fragments, with reference to their first description, the last names of the primary authors, and the identification of the target genes when characterized, are given in Table 1.
TABLE 1.
Target genes and primers for detection of Cryptosporidium DNA
| Target | Primary investigator (reference) | Primer pair | Fragment size (bp) |
|---|---|---|---|
| Undefined | Laxer (15) | 5′-CCGAGTTTGATCCAAAAAGTTACGAA | 452 |
| 5′-TAGCTCCTCATATGCCTTATTGAGTA | |||
| CpR1 | Laberge (14) | 5′-GCCCACCTGGATATACACTTTC | 358 |
| 5′-TCCCCCTCTCTAGTACCAACAGGA | |||
| CpR1 | Wagner-Wiening (28) | 5′-AGTGTCCTCCAGGTACAAACCTGGTA | 898 |
| 5′-GCACAGCTGGGACAGAATCAGCTTT | |||
| Undefined | Morgan (18) | 5′-GGTACTGGATAGATAGTGGA | 680 |
| 5′-TCGCACGCCCGGATTCTGTA | |||
| 18S rRNA gene | Johnson (13) | 5′-AAGCTCGTAGTTGGATTTCTG | 435 |
| 5′-TAAGGTGCTGAAGGAGTAAGG | |||
| 18S rRNA gene | Awad-El-Kariem (1) | 5′-AGTGCTTAAAGCAGGCAACTG | 556 |
| 5′-CGTTAACGGAATTAACCAGAC | |||
| Hsp70 gene | Rochelle (24) | 5′-AAATGGTGAGCAATCCTCTG | 361 |
| 5′-CTTGCTGCTCTTACCAGTAC | |||
| Undefined | Bonnin (3) | 5′-TTCATTCTATCATGTC | 1500 |
| 5′-ATGGTTATATTTGGG |
PCR amplification and gel analysis of PCR products.
One-microliter volumes of the oocyst lysates were used as amplification templates in 50-μl reaction mixtures containing 75 mM Tris (pH 9); 20 mM (NH4)2SO4; 0.01% (wt/vol) Tween 20; 0.2 mM each dGTP, dATP, dCTP, and dTTP; 2 to 4 mM MgCl2 (Table 2); 50 pM each primer; and 1 to 2 U (Table 2) of GoldStar Taq DNA polymerase (Eurogentec). Reaction mixtures were overlaid with 50 μl of sterile mineral oil and were subjected to denaturation, thermal cycling (Minicycler; MJ Research), and then a final elongation at 72°C. The conditions of denaturation, annealing, and elongation varied depending on the primers (Table 2). PCR products were analyzed on horizontal agarose gels in TAE buffer (40 mM Tris acetate, 2 mM Na2EDTA · 2H2O). Each amplification run included a negative control (PCR water) and a positive control (DNA from C. parvum). All negative samples were reamplified in duplicate by adding DNA from 250 C. parvum oocysts to the reaction mixtures, to ensure that negative results were not due to the copurification of PCR inhibitors. For restriction fragment analysis of the PCR products, 12-μl aliquots of the amplified DNA were treated with restriction enzymes (Table 2) under conditions recommended by the suppliers, prior to electrophoresis.
TABLE 2.
PCRs and thermal-cycling parameters for amplification of Cryptosporidium DNA
| PCR method (reference) | MgCl2 concn (mM) | Amt of Taq (U) | Cycle conditions
|
No. of cycles | Restriction site(s) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Initial denaturation | Denaturation | Annealing | Extension | Final extension | |||||
| Laxer (15) | 2 | 1 | 95°C, 10 min | 94°C, 1 min | 56°C, 90 s | 72°C, 90 s | 72°C, 7 min | 45 | |
| Laberge (14) | 2 | 1 | 95°C, 10 min | 94°C, 1 min | 56°C, 90 s | 72°C, 90 s | 72°C, 7 min | 45 | |
| Wagner-Wiening (28) | 2 | 2 | 94°C, 5 min | 94°C, 1 min | 50°C, 1 min | 72°C, 1 min | 72°C, 10 min | 35 | BamHI |
| Morgan (18) | 2 | 1 | 95°C, 10 min | 94°C, 1 min | 58°C, 90 s | 72°C, 90 s | 72°C, 10 min | 45 | |
| Johnson (13) | 4 | 1 | 94°C, 5 min | 94°C, 30 s | 55°C, 30 s | 72°C, 1 min | 72°C, 10 min | 40 | |
| Awad-El-Kariem (1) | 2 | 2 | 94°C, 5 min | 94°C, 90 s | 47°C, 90 s | 72°C, 3 min | 72°C, 10 min | 45 | MaeI |
| Rochelle (24) | 2 | 1 | 94°C, 2 min | 94°C, 30 s | 55°C, 30 s | 72°C, 90 s | 72°C, 5 min | 40 | |
| Bonnin (3) | 2.5 | 1.5 | 94°C, 20 min | 94°C, 90 s | 45°C, 90 s | 72°C, 90 s | 72°C, 10 min | 45 | HinfI, RsaI |
RESULTS
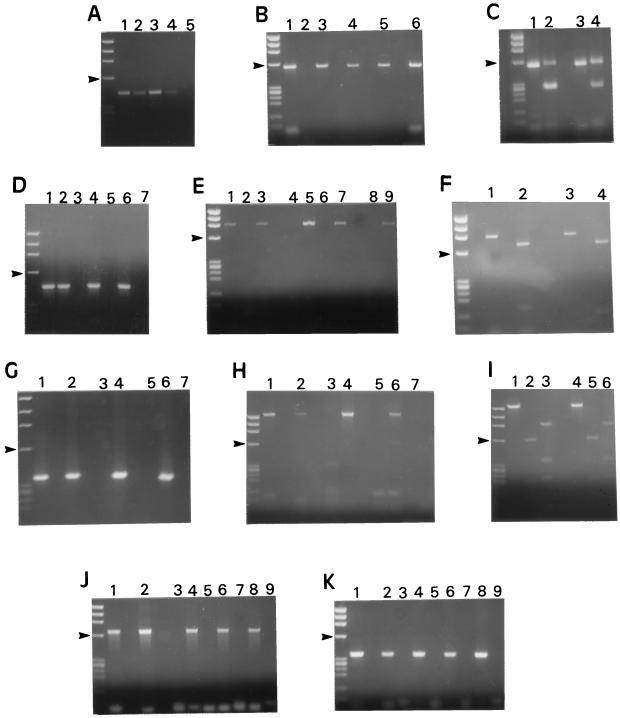
The expected PCR fragments were found to be produced upon analysis of C. parvum DNA with all the techniques evaluated (Fig. 1A, B, D, E, G, H, J, and K). C. baileyi isolates O.96.2 and B1 gave identical results when their DNA was subjected to PCR amplification with the eight techniques utilized (data not shown). No amplification of DNA was detected with the negative-control samples (Fig. 1A, B, D, E, G, H, J, and K). The expected products were obtained by seeding negative samples with C. parvum DNA (Fig. 1D, E, G, H, J, and K), suggesting that the negative results were not due to the copurification of PCR inhibitors.
FIG. 1.
Ethidium bromide-stained agarose gels of PCR products amplified from C. parvum, C. muris, C. baileyi, and C. meleagridis DNA. Size markers are HaeIII-digested ΦX174; arrowheads in all panels point to the 603-bp fragment. (A) Amplification of a 435-bp product of the 18S rRNA gene (method of Johnson [13]). Lanes: 1, C. parvum; 2, C. meleagridis; 3, C. muris; 4, C. baileyi isolate B1; 5, negative control. (B) Amplification of a 556-bp product of the 18S rRNA gene (Awad-El-Kariem [1]). Lanes: 1, C. parvum; 2, negative control; 3, C. meleagridis; 4, C. baileyi isolate B1; 5, C. baileyi isolate O.96.2; 6, C. muris. (C) Electrophoresis of PCR products obtained in the experiment shown in panel B, prior to and after MaeI treatment. As expected (1), incomplete digestion occurred. Lanes: 1, C. parvum; 2, same sample as in lane 1 but after restriction with MaeI; 3, C. meleagridis; 4, same sample as in lane 3 but after restriction with MaeI. (D) Amplification of a 452-bp product of an undefined gene (Laxer [15]). Lanes: 1, C. parvum; 2, C. meleagridis; 3 and 4, C. muris; 5 and 6, C. baileyi isolate B1; 7, negative control. In lanes 4 and 6, C. parvum DNA was added to the reaction mixtures prior to amplification. (E) Amplification of a 898-bp fragment from the CpR1 gene (Wagner-Wiening [28]). Lanes: 1, C. parvum; 2, negative control; 3, C. meleagridis; 4 and 5, C. baileyi isolate B1; 6 and 7, C. baileyi isolate O.96.2; 8 and 9, C. muris. For lanes 5, 7, and 9, C. parvum DNA was added to the reaction mixtures prior to amplification. (F) Electrophoresis of PCR products obtained in the experiment represented in panel E prior to and after treatment with BamHI. Lanes: 1, C. parvum; 2, same sample as in lane 1 but after restriction with BamHI; 3, C. meleagridis; 4, same sample as in lane 3 but after restriction with BamHI. (G) Amplification of a 358-bp fragment of the CpR1 gene (Laberge [14]). Lanes: 1, C. parvum; 2, C. meleagridis; 3 and 4, C. muris; 5 and 6, C. baileyi isolate B1; 7, negative control. For lanes 4 and 6, C. parvum DNA was added to the reaction mixtures prior to amplification. (H) Amplification of a 1,500-bp fragment from an undefined DNA region (Bonnin [3]). Lanes: 1, C. parvum; 2, C. meleagridis; 3 and 4, C. muris; 5 and 6, C. baileyi isolate B1; 7, negative control. For lanes 4 and 6, C. parvum DNA was added to the reaction mixtures prior to amplification. (I) Electrophoresis of PCR products obtained from the 1,500-bp fragment shown in panel H prior to and after treatment with HinfI and RsaI. Lanes: 1, C. parvum; 2 and 3, same product as in lane 1 but after digestion with HinfI and RsaI, respectively; 4, C. meleagridis; 5 and 6, same product as in lane 4 after digestion with HinfI and RsaI, respectively. (J) Amplification of a 680-bp fragment from an undefined gene (Morgan [18]). Lanes: 1, C. parvum; 2, C. meleagridis; 3 and 4, C. muris; 5 and 6, C. baileyi isolate B1; 7 and 8, C. baileyi isolate O.96.2; 9, negative control. For lanes 4, 6, and 8, C. parvum DNA was added to the reaction mixtures prior to amplification. (K) Amplification of a 361-bp fragment of the Hsp70 gene (Rochelle [24]). Lanes: 1, C. parvum; 2, C. meleagridis; 3 and 4, C. muris; 5 and 6, C. baileyi isolate B1; 7 and 8, C. baileyi isolate O.96.2; 9, negative control. For lanes 4, 6, and 8, C. parvum DNA was added to the reaction mixtures prior to amplification.
This multilocus analysis showed that isolates of the four Cryptosporidium species analyzed were divided into two clearly distinct groups according to the allelic combinations they displayed (Fig. 1; Table 3): in the first group, corresponding to C. parvum and C. meleagridis, PCRs gave the same-sized fragments with all the primer pairs evaluated (Fig. 1A, B, D, E, G, H, J, and K), while in the second group, which included C. muris and C. baileyi, only the primer pairs targeting the 18S rRNA gene gave positive results (Fig. 1A and B).
TABLE 3.
Specificity of PCRs for detection of C. parvum, C. muris, C. baileyi, and C. meleagridis
| PCR method (reference) | Resulta for:
|
|||
|---|---|---|---|---|
| C. parvum | C. muris | C. meleagridis | C. baileyi | |
| Laxer (15) | + | − | + | − |
| Laberge (14) | + | − | + | − |
| Wagner-Wiening (28) | + | − | + | − |
| Morgan (18) | + | − | + | − |
| Johnson (13) | + | + | + | + |
| Awad-El-Kariem (1) | + | + | + | + |
| Rochelle (24) | + | − | + | − |
| Bonnin (3) | + | − | + | − |
+, positive PCR; −, negative PCR.
Species in each of the two groups couldn’t be differentiated from each other on the basis of DNA amplification at any of the loci examined. In order to identify genetic differences between C. parvum and C. meleagridis, PCR fragments from 5C12 (Fig. 1I), the 18S rRNA gene (Fig. 1C), and CpR1 (Fig. 1F) were then subjected to restriction enzyme digestion, respectively, with RsaI and HinfI, MaeI, and BamHI. No restriction fragment length polymorphisms (RFLPs) were detected in the PCR products (Fig. 1C, F, and I).
DISCUSSION
In order to determine the specificities of PCR-based diagnosis assays used for detecting C. parvum DNA, we conducted a thorough assessment of eight PCR techniques with isolates of four Cryptosporidium species infecting birds and mammals.
The specificities of the primer pairs targeting the 18S rRNA gene (1, 13) and the Hsp70 gene (24) were determined with C. parvum, C. muris, and C. baileyi in the studies on which the original descriptions of the primers were based, and the results of the present study are similar to those previously reported, confirming that the 18S rRNA regions, but not the 361-bp sequence of the Hsp70 gene, are amplified from C. muris and C. baileyi. Our data also confirm a study by Rochelle et al. (23) showing that the primer pair originally described by Laxer et al. (15) produced no PCR fragment with C. muris or C. baileyi DNA. Similarly, and in agreement with its first description (14), the 358-bp fragment of the C. parvum CpR1 gene was not amplified from C. muris. These results, based on isolates from France and Hungary, strengthen previous reports based on studies done with isolates essentially obtained in North America or the United Kingdom. The other three primer pairs evaluated herein, which target a larger fragment of the CpR1 gene of C. parvum (28) and two undefined sequences of the C. parvum genome (3, 18), were tested with C. parvum DNA only prior to this study. Our data show that the corresponding regions are amplified from C. parvum DNA, but not from C. muris or C. baileyi.
In none of the previous studies was C. meleagridis DNA included to assess PCRs. The isolate used in this study originated from the cecal pouches of a common quail with diarrhea and was maintained by oral inoculation of chickens and recovery of the cecal contents. Thus, host specificity, together with the site of infection and the small size of the oocysts, supports the identification of this isolate as C. meleagridis (5, 17, 25). The hypothesis that PCR fragments obtained with C. meleagridis resulted from nonspecific amplification of DNA from cells or microorganisms copurified with C. meleagridis oocysts is unlikely since all the PCR fragments had the predicted sizes. Amplification of the expected PCR fragments from C. meleagridis DNA with the eight PCRs evaluated is an important finding with respect to the monitoring of water contamination, since a positive PCR result with the primer pairs evaluated in the present paper would not necessarily imply the presence of C. parvum oocysts. We therefore sought for RFLP of the PCR products to differentiate between C. parvum and C. meleagridis. Two of the DNA sequences utilized in the present work contain restriction sites that were previously shown to be polymorphic: MaeI digestion of the 18S rRNA gene distinguished between C. parvum and a group of C. muris-C. baileyi isolates (1), and 5C12 contained RsaI and HinfI sites that distinguished two subpopulations among C. parvum isolates (3). Digestion with the appropriate enzymes showed that the C. meleagridis amplicons had an internal organization identical to that of C. parvum at these loci, ruling out the possibility of discriminating between C. parvum and C. meleagridis based on these RFLPs. Similarly, the BamHI site in the C. parvum CpR1 PCR fragment (28) was present in the corresponding C. meleagridis amplicon. Sequencing of PCR products from both species, or cloning of further regions of the Cryptosporidium genome, may thus be necessary to identify genetic differences between C. meleagridis and C. parvum and to develop appropriate typing assays.
Organization of the four Cryptosporidium species analyzed herein in two groups with respect to results of PCR analysis of eight loci located in six distinct genes is a striking result of the present work. Little is known about the comparative structures of Cryptosporidium species at the molecular level. 125I labeling of outer oocyst wall proteins of C. parvum, C. muris, and C. baileyi revealed common as well as species-specific molecules (26). In a study based on Western blotting, C. parvum and C. baileyi were easily differentiated, while C. muris produced weak bands that were difficult to interpret (20). Isoenzyme analysis showed that C. parvum, C. muris, and C. baileyi had distinct phosphoglucomutase electrophoresis patterns (22). Similarily, double digestion of a large fragment of the 18S rRNA gene produced distinct profiles for C. parvum, C. muris, and C. baileyi (16). Reports based either on the analysis of an undefined DNA sequence of the Cryptosporidium genome (29) or on antigenic reactivity of anti-Cryptosporidium antibodies (21) suggested that C. parvum and C. baileyi are more closely related to one another than to C. muris. On the other hand, PCR-RFLP analysis of the 556-bp region of the 18S rRNA gene showed that C. muris and C. baileyi have a common pattern that differs from the pattern displayed by C. parvum (1). Moreover, in an enzyme immunoassay, C. parvum and C. meleagridis oocysts gave positive reactions while C. muris and C. baileyi gave negative reactions (11), a profile of antigenic reactivity identical to the profiles for the two groups described herein which were separated on the basis of genomic-DNA organization. Whether these molecular profiles have any biological significance, especially with respect to species phylogeny, host adaptation, or differences in virulence will require further investigations.
Differentiation between C. baileyi and C. muris would not be as critical for the quality control of water supplies as a mix-up involving C. parvum, since neither of the first two species is considered a human pathogen. Anyway, two readily available PCR-based techniques may potentially differentiate between C. muris and C. baileyi provided the procedures are optimized for diagnosis samples: a double digestion of cloned PCR products from the 18S rRNA gene produced patterns specific for C. muris and C. baileyi (16), and an undefined DNA region described by Webster et al. was amplified from C. parvum and C. baileyi but not from C. muris (29). However, Rochelle et al. reported low PCR efficiency with the latter sequence (23).
Results of the present study, together with the observation that C. parvum and C. meleagridis share epitopes that are cross-reactive in an enzyme immunoassay (11), indicate that these two species are closely related at the molecular level and emphasize the complexity of the molecular mechanisms involved in host-parasite adaptation. Studies of additional C. meleagridis isolates are needed in order to determine the extent of this biochemical homology and characterize markers that differentiate between C. parvum and C. meleagridis. Furthermore, the specificity of PCR methods available should also be examined with isolates of C. serpentis and C. nasorum, as well as isolates of C. wrairi and C. felis. Insofar as cryptosporidiosis increasingly appears as an environmental threat, our understanding of the epidemiology of cryptosporidiosis and our ability to define appropriate measures to prevent transmission will rely on accurate characterization of the techniques used for testing environmental samples.
ACKNOWLEDGMENTS
This work was supported by grant 95 024 from Agence Nationale de Recherche sur le SIDA (Paris, France) and grant DAF 965112H4 from Conseil Régional de Bourgogne (Dijon, France).
We thank Nicole Gobet for critical reading of the manuscript.
REFERENCES
- 1.Awad-El-Kariem F M, Warhurst D C, McDonald V. Detection and species identification of Cryptosporidium oocysts using a system based on PCR and endonuclease restriction. Parasitology. 1994;109:19–22. doi: 10.1017/s0031182000077714. [DOI] [PubMed] [Google Scholar]
- 2.Bonnin A, Dubremetz J F, Camerlynck P. Characterization of microneme antigens of Cryptosporidium parvum (Protozoa, Apicomplexa) Infect Immun. 1991;59:1703–1708. doi: 10.1128/iai.59.5.1703-1708.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnin A, Fourmaux M N, Dubremetz J F, Nelson R G, Gobet P, Harly G, Buisson M, Puygauthier-Toubas D, Gabriel-Pospisil F, Naciri M, Camerlynck P. Genotyping human and bovine isolates of Cryptosporidium parvum by polymerase chain reaction-restriction fragment length polymorphism analysis of a repetitive DNA sequence. FEMS Microbiol Lett. 1996;137:207–211. doi: 10.1111/j.1574-6968.1996.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 4.Current W L, Reese N C. A comparison of endogenous development of three isolates of Cryptosporidium in suckling mice. J Protozool. 1986;33:98–108. doi: 10.1111/j.1550-7408.1986.tb05567.x. [DOI] [PubMed] [Google Scholar]
- 5.Current W L, Upton S J, Haynes T B. The life cycle of Cryptosporidium baileyi n. sp. (Apicomplexa, Cryptosporidiidae) infecting chickens. J Protozool. 1986;33:289–296. doi: 10.1111/j.1550-7408.1986.tb05608.x. [DOI] [PubMed] [Google Scholar]
- 6.Ditrich O, Palkovic L, Sterba J, Prokopic J, Loudova J, Giboda M. The first finding of Cryptosporidium baileyi in man. Parasitol Res. 1991;77:44–47. doi: 10.1007/BF00934383. [DOI] [PubMed] [Google Scholar]
- 7.Dobos-Kovács M, Varga I, Békési L, Drén C N, Németh I, Farkas T. Concurrent cryptosporidiosis and chicken anaemia virus infection in broiler chickens. Avian Pathol. 1994;23:365–368. doi: 10.1080/03079459408419005. [DOI] [PubMed] [Google Scholar]
- 8.DuPont H L, Chappell C L, Sterling C R, Okhuysen P C, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 9.Fayer R, Speer C A, Dubey J P. The general biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis—1997. Boca Raton, Fla: CRC Press; 1997. pp. 1–41. [Google Scholar]
- 10.Gobet P, Buisson J C, Vagner O, Naciri M, Grappin M, Comparot S, Harly G, Aubert D, Varga I, Camerlynck P, Bonnin A. Detection of Cryptosporidium parvum DNA in formed human feces by a sensitive PCR-based assay including uracil-N-glycosylase inactivation. J Clin Microbiol. 1997;35:254–256. doi: 10.1128/jcm.35.1.254-256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graczyk T K, Cranfield M R, Fayer R. Evaluation of commercial enzyme immunoassay (EIA) and immunofluorescent antibody (IFA) test kits for detection of Cryptosporidium oocysts of species other than Cryptosporidium parvum. Am J Trop Med Hyg. 1996;54:274–279. doi: 10.4269/ajtmh.1996.54.274. [DOI] [PubMed] [Google Scholar]
- 12.Graczyk T K, Fayer R, Cranfield M R. Zoonotic transmission of Cryptosporidium parvum: implications for water-borne cryptosporidiosis. Parasitol Today. 1997;13:348–351. doi: 10.1016/s0169-4758(97)01076-4. [DOI] [PubMed] [Google Scholar]
- 13.Johnson D W, Pieniazek N J, Griffin D W, Misener I, Rose J B. Development of a PCR protocol for sensitive detection of Cryptosporidium oocysts in water samples. Appl Environ Microbiol. 1995;61:3849–3855. doi: 10.1128/aem.61.11.3849-3855.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laberge I, Ibrahim A, Barta J R, Griffiths M W. Detection of Cryptosporidium parvum in raw milk by PCR and oligonucleotide probe hybridization. Appl Environ Microbiol. 1996;62:3259–3264. doi: 10.1128/aem.62.9.3259-3264.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laxer M A, Timblin B K, Patel R J. DNA sequences for the specific detection of Cryptosporidium parvum by the polymerase chain reaction. Am J Trop Med Hyg. 1991;45:688–694. doi: 10.4269/ajtmh.1991.45.688. [DOI] [PubMed] [Google Scholar]
- 16.Leng X, Mosier D A, Oberst R D. Differentiation of Cryptosporidium parvum, C. muris, and C. baileyi by PCR-RFLP analysis of the 18S rRNA gene. Vet Parasitol. 1996;62:1–7. doi: 10.1016/0304-4017(95)00863-2. [DOI] [PubMed] [Google Scholar]
- 17.Lindsay D S, Blagburn B L. Cryptosporidiosis in birds. In: Dubey J P, Speer C A, Fayer R, editors. Cryptosporidiosis of man and animals—1990. Boca Raton, Fla: CRC Press; 1990. pp. 133–148. [Google Scholar]
- 18.Morgan U M, O’Brien P A, Thompson R C A. The development of diagnostic PCR primers for Cryptosporidium using RAPD-PCR. Mol Biochem Parasitol. 1996;77:103–108. doi: 10.1016/0166-6851(96)02577-7. [DOI] [PubMed] [Google Scholar]
- 19.Naciri M, Mancassola R, Répérant J M, Yvoré P. Analysis of humoral immune response in chickens after inoculation with Cryptosporidium baileyi or Cryptosporidium parvum. Avian Dis. 1994;38:832–838. [PubMed] [Google Scholar]
- 20.Nichols G L, MacLauchlin J, Samuel D. A technique for typing Cryptosporidium isolates. J Protozool. 1991;38:237S–240S. [PubMed] [Google Scholar]
- 21.Nina J M S, MacDonald V, Dyson D A, Catchpole J, Uni S, Iseki M, Chiodini P L, MacAdam K P W J. Analysis of oocyst wall and sporozoite antigens from three Cryptosporidium species. Infect Immun. 1992;60:1509–1513. doi: 10.1128/iai.60.4.1509-1513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogunkolade B W, Robinson H A, MacDonald V, Webster K, Evans D A. Isoenzyme variation within the genus Cryptosporidium. Parasitol Res. 1993;79:385–388. doi: 10.1007/BF00931827. [DOI] [PubMed] [Google Scholar]
- 23.Rochelle P A, De Leon R, Stewart M H, Wolfe R L. Comparison of primers and optimization of PCR conditions for detection of Cryptosporidium parvum and Giardia lamblia in water. Appl Environ Microbiol. 1997;63:106–114. doi: 10.1128/aem.63.1.106-114.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rochelle P A, Ferguson D M, Handojo T J, De Leon R, Stewart M H, Wolfe R L. An assay combining cell culture with reverse transcriptase PCR to detect and determine the infectivity of waterborne Cryptosporidium parvum. Appl Environ Microbiol. 1997;63:2029–2037. doi: 10.1128/aem.63.5.2029-2037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slavin D. Cryptosporidium meleagridis (sp. nov.) J Comp Pathol. 1955;65:262–266. doi: 10.1016/s0368-1742(55)80025-2. [DOI] [PubMed] [Google Scholar]
- 26.Tilley M, Upton S J, Blagburn B L, Anderson B C. Identification of outer oocyst wall proteins of three Cryptosporidium (Apicomplexa: Cryptosporidiidae) species by using 125I surface labeling. Infect Immun. 1990;58:252–253. doi: 10.1128/iai.58.1.252-253.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upton S J, Current W L. The species of Cryptosporidium (Apicomplexa: Cryptosporidiidae) infecting mammals. J Parasitol. 1985;71:625–629. [PubMed] [Google Scholar]
- 28.Wagner-Wiening C, Kimmig P. Detection of viable Cryptosporidium parvum oocysts by PCR. Appl Environ Microbiol. 1995;61:4514–4516. doi: 10.1128/aem.61.12.4514-4516.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webster K A, Pow J D E, Giles M, Catchpole J, Woodward M J. Detection of Cryptosporidium parvum using a specific polymerase chain reaction. Vet Parasitol. 1993;50:35–44. doi: 10.1016/0304-4017(93)90005-8. [DOI] [PubMed] [Google Scholar]