Abstract
Cognitive deficits are associated with schizophrenia and show a progressive worsening, often being unresponsive to treatment. New antipsychotic molecules acting as antagonist at the serotoninergic 5-hydroxytryptamine receptor 7 (e.g. lurasidone) or partial agonists at dopamine D3 receptor (e.g. cariprazine) could have an impact on cognition in this patient group. The aim of the systematic review is to explore the efficacy of lurasidone and cariprazine in improving cognition in both animal models and human studies. The following terms: (lurasidone AND cognit*) OR (cariprazine AND cognit*) were searched in Web of Science from inception to December 2021. We included all studies that assessed changes in cognitive function after treatment with cariprazine or lurasidone. Of 201 selected articles, 36 were included. Twenty-four articles used animal models (rats, mice and marmosets), five evaluating the effects of cariprazine and 19 the effects of lurasidone. Twelve articles were clinical studies (cariprazine n = 2; lurasidone n = 10). In both animal and human studies lurasidone showed a greater efficacy on cognitive performance compared to placebo, quetiapine, ziprasidone or treatment-as-usual. Cariprazine was superior to other antipsychotics in improving cognitive functions in both animal and human studies. The cognitive effect of lurasidone could be explained by its potent antagonism at the 5-HT7 receptors combined with partial agonism at 5-HT1A receptors. The pro-cognitive effect of cariprazine is probably explained by its very high affinity for D3 receptors. Head-to-head studies comparing lurasidone and cariprazine are needed to establish the “first-choice” treatment for cognitive dysfunction associated with schizophrenia.
Keywords: Antipsychotics, lurasidone, cariprazine, 5HT7, D3, cognition, psychosis, affective disorder
1. INTRODUCTION
Schizophrenia is a severe psychiatric disorder, affecting more than 20 million people in the world. Schizophrenic patients show higher rates of morbidity and mortality (WHO, 2019) [1] and are subjected to the elevated social stigma which results in significant impairment in quality of life. Cognitive deficits are often associated with schizophrenia, even before its first onset, and show a progressive worsening after the diagnosis, thus representing a core feature of the disease [2, 3]. The biological mechanisms underlying cognitive impairments in schizophrenia seem to involve the cortico-cerebellar-thalamic-cortical pathways, including developmental abnormalities in both maturation and neurogenesis of neurons and synapses [4, 5]: specifically, grey matter alterations in the anterior cingulate are present even before the onset of the disease [6]. Despite the burden associated with cognitive impairment, the development of new medications for schizophrenia over the past decades provided only limited improvement in cognition, giving rise to the need for new pharmacological strategies [7, 8]. Recently, the introduction of antipsychotic molecules acting as an antagonist at the serotoninergic 5-hydroxytryptamine receptor 7 (e.g. lurasidone) or partial agonists at dopamine D3 receptor (e.g. cariprazine) could have an impact on cognitive outcomes in patients with psychotic disorders [9-11]. However, results from studies focusing on cognition after stimulation or blockade of these receptors are still controversial [12-15]. A potential explanation of the role of D3 receptor partial agonists or antagonists on cognition [16] may be the modulation of somatodendritic D3 receptors in the ventral tegmental area, leading to an increase in dopamine release in the prefrontal cortex [17]. On the other hand, antagonism of theserotonergic 5 HT-7 seems to reverse memory deficits through the regulation of hippocampal adenylyl cyclase activity [18].
1.1. Lurasidone
Lurasidone (Figs. 1 and 2) is a potent antagonist of 5-HT7 receptors and a partial agonist of 5-HT1A receptors. Both characteristics are predictive of the pro-cognitive and antidepressant activity of the drug [19, 20]. Lurasidone is also a high-affinity antagonist of 5-HT2A and D2 receptors, and its use is associated with a low risk for extrapyramidal symptoms (EPS). The low affinity of lurasidone for M1 muscarinic, H1 histamine, 5-HT2C and alpha1 adrenergic receptors suggests a low liability to cause peripheral and central anticholinergic adverse effects, sedation, weight gain and hypotension. Lurasidone is also an antagonist of α2c receptors, but the relevance of this mechanism in the overall pharmacological effect of the drug is unknown [19]. Dosing instructions recommend taking lurasidone once daily in the evening, to further reduce the incidence of EPS and sedation. A meal with a caloric value of at least 350 kcal, regardless of the fat content, is recommended to optimize lurasidone absorption. Lurasidone is rapidly absorbed from the gastrointestinal tract and reaches peak plasma concentrations within 1-3 hours. A steady state is reached after 7 days of administration with linear kinetics. Lurasidone has a very high plasma protein binding (99.8%), and is mainly metabolized in the liver by CYP3A4 through hydroxylation processes of the norbornene ring, with the formation of three active (ID-14283, ID-14326 and ID-14614) and two inactive (ID-20219 and ID-20220) metabolites present in percentage less than 10%. Lurasidone crosses the placental barrier and is excreted in the urine and feces. Dose adjustment is expected in patients with impaired renal clearance and severe hepatic insufficiency. Commonly adverse events are somnolence, akathisia, nausea, parkinsonism, and insomnia [21]. Lurasidone treatment is associated with a low risk of hyperprolactinemia, corrected QT (QTc) interval prolongation, weight gain and metabolic disturbances [22, 23]. Compared to olanzapine and quetiapine [24-26], lurasidone is associated with significantly less weight gain but higher rates of akathisia, anxiety and EPS. Lurasidone has a similar safety and tolerability profile to ziprasidone, except for a lower risk of somnolence and QTc prolongation [27].
Fig. (1).
Structure of lurasidone.
Fig. (2).
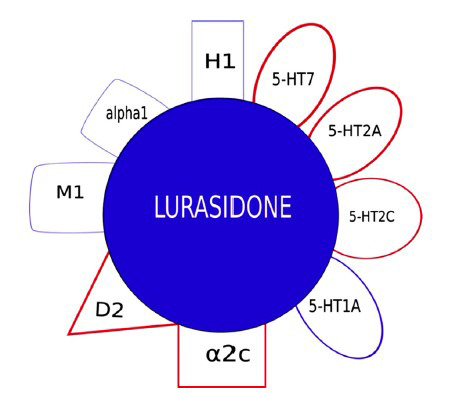
Pharmacological profile of lurasidone.
1.2. Cariprazine
Cariprazine (Figs. 3 and 4) shows a 10-fold higher affinity for human D3 versus human D2 receptors (binding affinity: pKi = 10.07 and 9.31, respectively), behaving as a partial agonist of both receptors. Cariprazine is also a partial agonist of 5-HT1A receptors (pKi = 8.59), a high affinity antagonist of 5-HT2B receptors (pKi = 9.24), and a low affinity antagonist of 5-HT2A (pKi = 7.73), H1 (pKi = 7.63), and 5-HT2C (pKi = 6.87) receptors. By acting as a partial agonist at D3 and 5-HT1A receptors, cariprazine improves negative symptoms, anhedonia and cognitive deficits associated with schizophrenia. Cariprazine has low affinity for adrenergic, and cholinergic receptors. The most common adverse events (≥ 10%) are akathisia, insomnia, weight gain and headache. The risk for cardiovascular adverse effects, sedation, hyperprolactinemia, and metabolic adverse effects is low [28, 29].
Fig. (3).
Structure of cariprazine.
Fig. (4).
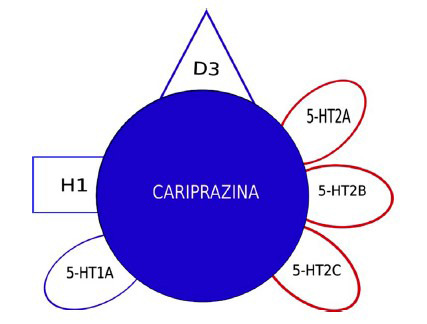
Pharmacological profile of cariprazine.
The time to reach peak plasma concentrations of cariprazine (Tmax) is approximately 3-8 hours. Cariprazine and its major active metabolites are strongly bound (92 to 97%) to plasma proteins. Cariprazine has two active metabolites, one of which has a half-life of 1-3 weeks. The functional total half-life is 7 days. Cariprazine is extensively metabolised by CYP3A4 and to a lesser extent by CYP2D6 to desmethyl cariprazine (DCAR) and didesmethyl cariprazine (DDCAR).
Cariprazine shows a consistent efficacy in improving the negative symptoms of schizophrenia. In a 26-week head-to-head study of cariprazine (3, 4.5, or 6 mg/d) vs. risperidone (3, 4, or 6 mg/d), cariprazine has shown greater efficacy in reducing blunted affect, emotional withdrawal, poor rapport, passive/apathetic social withdrawal, difficulty in abstract thinking, as well as total negative symptoms derived from the Positive and Negative Syndrome Scale (PANSS) of schizophrenia [30].
In another study [31], cariprazine was compared to risperidone in patients affected by schizophrenia with persistent and predominantly negative symptoms. Cariprazine had a greater efficacy on the PANSS negative factor score at week 26. This clinical trial also revealed a significant effect of cariprazine in the following items: self-care, socially useful activities as well as personal and social relationships.
1.3. Aim
Considering the potential influence of lurasidone and cariprazine on cognitive symptoms of schizophrenia, the aim of the present systematic review is to explore the efficacy of these two molecules in improving cognition in both animal models and human subjects.
2. MATERIALS AND METHODS
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [32]. We used the modified Cochrane Collaboration tool to assess the risk of bias for randomized controlled trials.
2.1. Search
The following search terms: (lurasidone AND cognit*) OR (cariprazine AND cognit*) were entered into the Web of Science research engine (all databases) from inception to December 2021. After duplicate removal, articles that were not relevant were excluded. Full-text version of the remaining articles was then obtained and screened according to the pre-specified eligibility criteria described below. Hand-searches of reference lists of potentially relevant articles, clinical studies, and reviews were conducted. The entire search process was conducted independently by three researchers, and disagreements at the final stage were resolved by consensus.
2.2. Inclusion/Exclusion Criteria
Inclusion criteria were: studies in English language that assessed changes in cognitive function in response to treatment with cariprazine or lurasidone. Studies were excluded if they did not report group comparisons or measurements of cognitive functions. Reviews, meta-analyses, conference proceedings/abstracts, book chapters, and unpublished theses were also excluded.
3. RESULTS
Of the 201 selected articles, 36 were included (Fig. 5). Twenty-four were research articles using animal models (rats, mice and marmosets), five evaluating the effects of cariprazine and 19 effects of lurasidone. Twelve articles reported the results of clinical studies using cariprazine (n = 2) or lurasidone (n = 10).
Fig. (5).
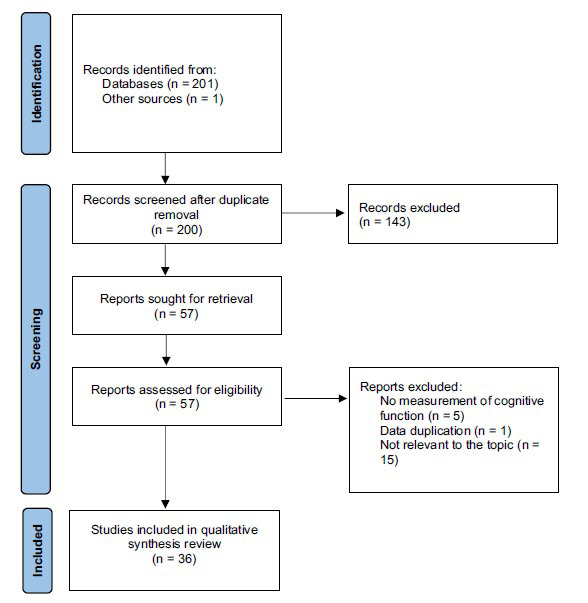
PRISMA flow-chart of the selected studies.
3.1. Animal Studies
General characteristics of the selected studies are presented in Table 1.
Table 1.
General characteristics of animal studies.
|
Author/
Year |
Country | Study Design | Trial Duration | Population | N | Induced Deficits Intervention | Intervention with Lurasidone or Cariprazine |
Other Interventions (Placebo
or Other Antipsychotics) |
Assessment | Cognition Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Barnes et al. (2018) [33] |
USA, Hungary | RCT | 9 days | Male Wistar rats | 96 | PCP 2 mg/kg s.c. | Cariprazine 0.03, 0.1, or 0.3 mg/kg p.o. | 2% Tween80 p.o.; Aripiprazole 1, 3, or 10 mg/kg p.o. | 5-CSRTT | Cariprazine demonstrated potential in attenuating PCP-induced deficits in the 5-CSRTT performance. |
| Calabrese et al. (2020) [39] |
Italy, Poland | RCT | 7 weeks | Male Wistar rats | // | CMS (chronic mild stress) | Lurasidone 3 mg/kg p.o. | 1% hydroxyethylcellulose p.o. | NOR | Seven weeks of CMS induced anhedonia and cognitive impairment, which were normalised by lurasidone. |
| Enomoto et al. (2008) [56] |
Japan | RCT | 5 days | Male Wistar rats | // | MK-801 0.15 and 0.2 mg/kg i.p. | Lurasidone 1 and 3 mg/kg p.o. | Risperidone 0.3 and 1 mg/kg p.o.; Clozapine 3 and 10 mg/kg p.o.; Aripiprazole 0.3 and 1 mg/kg p.o.; Haloperidol 0.3 and 1 mg/kg p.o. | MWM and RAM | Lurasidone potently reversed MK-801-induced learning impairment in the MWM test and reference memory impairment in the RAM test. |
| Gyertyan et al. (2011) [37] |
Hungary | RCT | // | Male Wistar rats | // | PCP 2 mg/kg s.c., MK-801 0.1 mg/kg i.p. | Cariprazine 0.01-2 mg/kg p.o. | Aripiprazole 2-80 mg/kg p.o.; olanzapine 0.3-3 mg/kg p.o.; risperidone 0.05-0.5 mg/kg p.o. | Water-labyrinth learning performance | Cariprazine 0.02-0.08 mg/kg significantly improved the learning performance of scopolamine-treated rats in a water-labyrinth learning paradigm. Risperidone, olanzapine, and aripiprazole were less active against phencyclidine and more cataleptogenic than cariprazine, and had no significant effect in the learning task. |
| Horiguchi et al. (2012) [42] |
USA, Japan | RCT | 22 days | Female Long-Evans rats | 103 | PCP 2 mg/kg bid, i.p. | Lurasidone 0.1 mg/kg or 1 mg/kg i.p. | Tandospirone 5 mg/kg i.p.; pimavanserin 3 mg/kg i.p.; haloperidol 1 mg/kg i.p.; WAY100635 0.6 mg/kg, saline | NOR | Lurasidone (1 mg/kg) but not 0.1 mg/kg, which is effective to acutely reverse the deficit due to subchronic PCP, or tandospirone, but not pimavanserin or haloperidol, significantly prevented the PCP-induced NOR deficit on day 15. The ability of lurasidone co-treatment to prevent the PCP-induced NOR deficit was enduring and still present at day 22. The preventive effect of lurasidone was blocked by WAY100635, a selective 5-HT1A antagonists, further evidence for the importance of 5-HT1A receptor stimulation in the NOR deficit produced by subchronic PCP. |
| Horiguchi et al. (2011) [40] |
USA, Japan | RCT | > 1 month | Female Long-Evans rats | 34 | PCP 2 mg/kg bid, i.p. | Lurasidone 0.01 mg/kg or 0.03 mg/kg, 0.1 mg/kg, 0.5 mg/kg i.p. | Clozapine 0.1-0.3 mg/kg, LY379268 1-3 mg/kg, LY341495 1 mg/kg, haloperidol 0.1 mg/kg, pimavanserin 3 mg/kg, saline |
NOR | mGlu2/3 agonism is relevant to the ability of clozapine and lurasidone to ameliorate the effect of subchronic PCP treatment on NOR, a putative model for the cognitive dysfunction of schizophrenia. |
| Horiguchi et al. (2011) [41] |
USA, Japan | RCT | > 1 month | Female Long-Evans rats | 129 | PCP 2 mg/kg bid i.p | Lurasidone 0.03 and 0.1 mg/kg i.p. | Pimavanserin 3 mg/kg i.p., haloperidol 0.03 mg/kg i.p.; sulpiride 20 and 30 mg/kg i.p.; amisulpride 1 mg/kg i.p.; AS19 5 and 10 mg/kg i.p.; LY379268 1 mg/kg i.p; LY341495 1 mg/kg i.p.; SB269970 0.1, 0.3, and 1 mg/kg i.p.; saline | NOR | Lurasidone (0.1 mg/kg) significantly attenuated the PCP-induced deficit. Lurasidone (0.03 mg/kg) plus 0.1 mg/kg SB269970, but not 0.03 mg/kg lurasidone alone, significantly reversed the PCP-induced NOR deficit. |
| Horiguchi et al. (2012b) [43] |
USA | RCT | > 1 month | Female Long-Evans rats | 86 | PCP 2 mg/kg bid i.p. | Lurasidone 0.1 mg/kg i.p. | Tandospirone 0.2 and 0.6 mg/kg i.p.; F15599 0.16 mg/kg i.p.; WAY100635 0.6 mg/kg i.p.; pimavanserin 3 mg/kg i.p.; buspirone 1 mg/kg i.p.; haloperidol 0.1 mg/kg i.p. | NOR | Lurasidone (0.1 mg/kg) and tandospirone (0.6 mg/kg) ameliorated the subchronic PCP-induced-NOR deficit. The combination of sub-effective doses of tandospirone (0.2 mg/kg) and lurasidone (0.03 mg/kg) also reversed the PCP-induced NOR-deficit. |
| Horiguchi et al. (2016) [44] |
USA, Japan | RCT | 5 weeks | Female Long-Evans rats | 102 | PCP 2 mg/kg bid i.p. | Lurasidone 0.1 and 1 mg/kg i.p. | Tandospirone 0.6 and 5 mg/kg i.p.; WAY100635 0.6 mg/kg i.p.; saline |
NOR | Subchronic lurasidone (1, but not 0.1 mg/kg) significantly reversed the PCP-induced NOR deficit at 24 h after the last injection. The effect of lurasidone persisted for one more week. |
| Horisawa et al. (2013) [53] |
Japan | RCT | // | Male Wistar rats | // | MK-801 0.075 mg/kg, s.c. | Lurasidone 3 mg/kg, p.o. | AS19 1, 3 and 10 mg/kg, s.c. | PA | The 5-HT7 receptor antagonistic activity of lurasidone plays an important role in its effectiveness against MK-801-induced deficits and may contribute to its pharmacological actions in patients with schizophrenia. |
| Huang et al. (2018) [45] |
USA, Republic of Korea | RCT | > 2 weeks | Male wild-type C57BL/6J mice and 5-HT7RKO (constitutive KO) mice |
// | PCP 10 mg/kg i.p., 5HT7-KO | Lurasidone i.p. | AS 19 10 mg/kg i.p.; WAY-100635 0.6 mg/kg i.p.; SB-269970 3 mg/kg i.p., saline |
NOR | Acute lurasidone treatment reversed the cognitive deficit in NOR in subchronic PCP-treated mice. |
| Ishiyama et al. (2007) [54] |
Japan | RCT | // | Male Wistar rats | // | MK-801 0.05 mg/kg, s.c. | Lurasidone 1-30 mg/kg p.o | Quetiapine 1-30 mg/kg p.o.; Haloperidol 0.3 and 1 mg/kg p.o.; Risperidone 0.3-3 mg/kg; Olanzapine 0.3-10 mg/kg; Aripiprazole 0,3-10 mg/kg; Clozapine 0.3-30 mg/kg p.o. |
PA | Lurasidone is superior to other antipsychotics in improving the MK-801-induced memory impairment. |
| Kolaczkowski et al. (2014) [55] | Poland, France | RCT | // | Male Wistar rats | 7-8 per group | MK-801 0.3 mg/kg i.p. | Lurasidone 0.3 to >100 i.p | Aripiprazole 10-100 mg/kg i.p., Olanzapine 0.3-10 mg/kg i.p., risperidone 0.1-10 mg/kg i.p., lurasidone 1-100 mg/kg i.p., asenapine 0.03-10 mg/kg i.p., clozapine 1-100 mg/kg i.p., chlorpromazine 1-30 mg/kg i.p., haloperidol 0.01-0.3 mg/kg s.c., imipramine 3-10 mg/kg |
PA | Lurasidone dose-dependently and significantly antagonized hyperlocomotion, reduced immobility time in the forced swimming test, reduced the step-through latency in the passive avoidance test, inhibited spontaneous locomotion and did not elicit catalepsy. |
| Miyauchi et al. (2016) [46] |
USA, Japan | RCT | >1 month | Female Long-Evans rats | 204 | PCP 2mg/kg bid i.p. | Lurasidone 0.03-0.3 mg/kg i.p. | A-83580 0.3 mg/kg i.p., DHbetaE 3 mg/kg i.p., PNU282987 1 mg/kg i.p., MLA 1 mg/kg i.p., MEC 1 mg/kg i.p. |
NOR | The increase of hippocampal ACh efflux by lurasidone may contribute to its efficacy to restore NOR in the scPCP model. |
| Miyauchi et al. (2017) [47] |
USA, Japan | RCT | > 1 month | Female Long-Evans rats | 223 | PCP 2mg/kg bid i.p. | Lurasidone 0.03-0.1 mg/kg | L-745,870 1 and 3 mg/kg i.p.; PD168077 0.5, 1.5 and 3 mg/kg i.p.; clozapine 0.1 and 0.3mg/kg i.p.; saline | NOR | A SED (subeffective dose) of the D4 agonist, PD168077 (0.5 mg/kg), potentiated the ability of a SED of the atypical APD, lurasidone (0.03 mg/kg), to reverse the sub-chronic PCP-induced NOR deficit, while only partially potentiating a SED of clozapine (0.1 mg/kg). Lastly, D4 antagonism with L-745,870 (1 mg/kg), at a dose that does not disrupt NOR in saline-treated animals, blocked the ability of clozapine (0.3 mg/kg), but not lurasidone (0.1 mg/kg), to reverse the sub-chronic PCP-induced NOR deficit. |
| Miyauchi et al. (2017) [48] |
USA, Japan | RCT | > 1 month | Female Long-Evans rats | 228 | PCP 2mg/kg bid i.p. | Lurasidone 0.03-0.1 mg/kg | Scopolamine 0.03 and 0.1 mg/kg i.p.; VU0255035 3 and 10 mg/kg i.p.; AC260584 1 and 3 mg/kg i.p.; NDMC 0.3 and 3 mg/kg i.p.; clozapine 0.1 and 0.3 mg/kg i.p.; saline | NOR | mAChR antagonism, specifically of the M1 receptor, preferentially disrupts the ability of clozapine and NDMC to fully rescue the subchronic PCP-induced NOR deficit compared to lurasidone; co-administration of the M1 agonist, AC260584, potentiates the effects of NDMC (0.3 mg/kg), clozapine (0.1 mg/kg), and lurasidone (0.03 mg/kg) to reverse the subchronic PCP-induced deficit in NOR. |
| Rajagopal et al. (2013) [49] |
Japan | RCT | // | Male and female common marmosets | 105 | // | Lurasidone 0.3, 1, 3, 10 mg/kg into the stomach | Haloperidol 0-1 mg/kg, olanzapine 0-10 mg/kg, risperidone 0-1 mg/kg, quetiapine 0-30 mg/kg, clozapine 0-10 mg/kg (all drugs into the stomach) | ORD | Lurasidone, unlike conventional antipsychotics, improves cognition associated with executive function. |
| Murai et al. (2014) [52] | Japan | RCT | // | Male and female common marmosets | 23 | // | Lurasidone 0-10 mg/kg into the stomach | Clozapine 0-10 mg/kg, L-745970 0-10 mg/kg, Ro10-5824 0-3 mg/kg (all drugs into the stomach) | ORD | The lack of affinity for the dopamine D4 receptor by lurasidone could contribute to its cognitive-enhancing effect. |
| Neill et al. (2016) [34] | UK, Hungary, USA | RCT | > 2 weeks | Female Lister Hooded rats | 240 | PCP 2 mg/kg i.p. | Cariprazine 0.05, 0.1, or 0.25 mg/kg, p.o. | Risperidone 0.16 mg/kg i.p. | NOR, ORL and SI | Cariprazine was effective to overcome PCP-induced deficits in cognition and social behavior. |
| Percelay et al. (2020) [57] |
France | RCT | 5 weeks | Male C57BL/6J mice | 20 | // | Lurasidone 1 mg/kg i.p., 8.3 mg/kg p.o. | Saline | Behavioural experiment: Open field, spontaneous alternation, sociability and social recognition, NOR, PPI | An impact of chronic lurasidone administration in C57BL/6 mice behaviour wasn't observed. |
| Rajagopal et al. (2016) [50] |
USA | RCT | > 2 weeks | Male C57BL/6J mice | 96 | PCP 10 mg/kg i.p. | Lurasidone 1 or 3 mg/kg; i.p | Tandospirone 0.1, 1, or 5 mg/kg i.p.; WAY100635 0.6 mg/kg i.p.; SB269970 0.5, 1, or 4 mg/kg i.p.; AS 19 10 mg/kg i.p. | ORL | Lurasidone reversed ORL deficit in the scPCP-treated mice. |
| Rajagopal et al. (2018) [49] |
USA | RCT | >1 week | Male C57BL/6J mice | // | PCP 10 mg/kg i.p. | Lurasidone 0.1 mg/kg i.p. | PregS 3-30 mg/kg i.p., Tandospirone 0.03-1 mg/kg i.p., saline, 0.05% methylcellulose and 0.2% Tween80 | NOR, ORL, SI | Acute lurasidone administration rescued scPCP-induced cognitive deficits in male C57BL/6 J mice. |
| Watson et al. (2016) [35] |
UK, Hungary, USA | RCT | > 5 weeks | Male Lister hooded rats | 12-16 | PCP 10 mg/kg i.p. | Cariprazine 0.03-0.3mg/kg i.p. | aripiprazole 0.3-3mg/kg i.p. | NOR | Cariprazine is at least as effective as aripiprazole and in some paradigms, it showed additional beneficial features. |
| Zimnisky et al. (2013) [36] |
USA, Hungary | RCT | // | C57BL/6J mice | // | PCP 1 mg/kg i.p., D3-receptor KO | Cariprazine 0.005 to 0.15 mg/kg i.p. | // | EPM, SI, Recognition Memory, WM, ASST | Cariprazine pretreatment significantly attenuated the emergence of cognitive deficits in PCP-treated wild-type mice, but not in PCP-treated D3-receptor knockout mice. |
Abbreviations: ASST (Attention-Set-Shifting Task), CAR (Conditioned avoidance response), CIAS (Cognitive Impairment Associated with Schizophrenia), EPM (Elevated plus maze), FST (Forced swim test), MWM (Morris water maze), NOR (Novel Object Recognition), ORD (Object Retrieval Detour), ORL (Operant Reversal learning), PA (Passive avoidance), PCP (Phencyclidine), PPI (Prepulse inhibition), RCT (Randomized Clinical Trial), RAM (Radial-arm maze), SI (Social interaction), WM (Working memory), 5-CSRTT (Five-choice serial-reaction time task).
3.1.1. Cariprazine
Five of the 25 identified studies evaluated the cognitive effects of cariprazine [33-37]. They all used the phencyclidine (PCP) model of schizophrenia, in which PCP treatment causes behavioural effects similar to negative symptoms in schizophrenia, as well as cognitive deficits involving long-term memory and executive memory. Barnes et al. 2018 [33] investigated the effect of systemic administration of cariprazine or aripiprazole in reversing memory deficits in a PCP model using the five-choice serial reaction time task (5-CSRTT). The 5-CSRTT is a behavioural test in which the animal must identify which of the five apertures has been briefly illuminated, via a nose poke, in order to receive a sugar reward. They found that all tested cariprazine doses (0.03, 0.1, or 0.3 mg/kg) significantly diminished the PCP-induced increases in incorrect, premature, and timeout responses. However, the two higher doses of cariprazine also reduced the number of completed trials, percent accuracy, and the number of correct responses, suggesting that these doses resulted in a non-specific suppression of responses. Aripiprazole treatment also attenuated PCP-induced deficits. Neill et al. 2016 [34] evaluated the effects of cariprazine in a PCP model using three different tasks: the Novel Object Recognition (NOR), the Operant Reversal Learning (ORL) and the Social Interaction (SI) tasks. The NOR is a commonly used behavioral assay for the investigation of various aspects of learning and memory in mice. During training, the mouse is allowed to explore 2 identical objects. On the test day, one of the training objects is replaced with a novel object. Because mice have an innate preference for novelty, if the mouse recognizes a familiar object, it will spend most of its time with the novel object (Lueptow et al. 2017) [38]. In the ORL test, rats are trained to press either a left or a right lever (only one is active) for food delivery, according to the presence or absence of a visual cue (LED light stimulus above lever). The social interaction test was performed in a square plexiglas open-field box. Two weight-matched rats (one treated test rat and one conspecific), unfamiliar with each other, were placed in the box together with an unfamiliar object (e.g., an unopened drink can). Behaviors (sniffing, avoidance and object exploration) are recorded. Neill and co-authors demonstrated that PCP-induced deficits in cognition and social behavior were significantly improved by cariprazine in a dose-dependent manner in the ORL test while showing efficacy at lower doses in the NOR and SI tests. Watson et al. (2016) [35] compared the effects of cariprazine and aripiprazole in the same PCP rat model to determine their ability to reverse behavioral changes using the NOR and the SI experimental conditions. Results suggest that cariprazine was as effective as aripiprazole in reversing deficits. Zimnisky et al. (2013) [36] tested the efficacy of a cariprazine pre-treatment in reducing the severity of cognitive deficits caused by PCP in wild-type or D3-receptor knockout mice. Cariprazine pre-treatment significantly attenuated the emergence of social recognition, spatial working memory, and attention deficits in PCP-treated wild-type mice, but not in PCP-treated D3-receptor knockout mice. This demonstrated the key role of D3-receptor in mediating the pro-cognitive effect of cariprazine. Gyertyan et al. (2011) [37] studied the effect of cariprazine in rats treated with scopolamine, a muscarinic receptor antagonist affecting learning and memory. Compared to aripiprazole, olanzapine and risperidone, cariprazine was the only antipsychotic agent capable of improving scopolamine-induced learning deficits.
3.1.2. Lurasidone
Nineteen of the 24 identified studies evaluated the effects of lurasidone on cognition. Nine studies assessed the effects of lurasidone using the NOR test [39-48]. Calabrese et al., (2020) [39] examined the effect of lurasidone treatment in rats exposed to chronic mild stress using the NOR test (see above for a description). Chronic mild stress produced an impairment of NOR performance by 30% which was completely reversed by lurasidone treatment. Interestingly, lurasidone did not influence cognitive performance in control rats. Using the same test, Horiguchi et al. (2011) [40] studied lurasidone, amisulpride, and sulpirid in a PCP model in rats. Lurasidone and amisulpride significantly attenuated the cognitive deficit induced by PCP and the effect of lurasidone was prevented by co-administration of a 5HT7 receptor agonist. This indicates that the pro-cognitive effect of lurasidone was mediated by 5-HT7 receptor blockade. In the same year, Horiguchi et al. (2011) [41] used the same model to evaluate the effect of a metabotropic glutamate2/3 (mGlu2/3) receptor agonist combined with either clozapine or lurasidone on cognition. Lurasidone combined with a mGlu2/3 receptor agonist could reverse the deficit in the NOR test, although it was inactive on its own. The same research group (Horiguchi et al., 2012) [42] further examined the pro-cognitive effect of lurasidone in an attempt to clarify its mechanism(s) of action. If co-administered with PCP, lurasidone was able to prevent PCP-induced NOR deficit only at high doses. Of note, the effect of lurasidone was blocked by the co-administration of a 5-HT1A receptor antagonist. The same effect was further confirmed in a subsequent experiment [43] in which the effect of lurasidone was similar to a postsynaptic 5-HT1A agonist, F15599, in reversing PCP-induced deficit. Later, Horiguchi et al., (2016) [44] demonstrated the efficacy of high doses of lurasidone in reversing PCP-induced NOR deficit for a longer period (14 days after the last lurasidone dose), as compared to other 5HT1A agonists (e.g., tandospirone). A similar experiment was conducted by Huang et al., (2018) [45], showing the relevance of 5HT1A receptor activation (to increase the efflux of dopamine and acetylcholine in the cortex) and 5HT7 receptor blockade (involved in the modulation of glutamate efflux in the median prefrontal cortex). Another mechanism of action of lurasidone was studied by Miyauchi et al., (2016) [46] in the same animal model. Specifically, they observed that nicotinic acetylcholine (ACh) receptor (nAChR) agonists (both α4β2* and α7nAChR agonists) could improve PCP-induced NOR deficit and potentiate the effect of sub-effective doses of lurasidone. More recently, the same group [47] observed that a combination of suboptimal doses of a D4 receptor agonist and lurasidone could improve cognition, suggesting that D4 receptors are also involved in the effect of lurasidone. Miyauchi, (2017b) [48] examined the contribution of M1 muscarinic receptors to the overall effect of atypical antipsychotics on cognitive fuction. Drugs with high affinity for M1 receptors, such as clozapine, reduced PCP-induced NOR deficit compared to lurasidone because of the M1 receptor antagonism. Another study [49] used the NOR, ORL, and SI tests to examine whether pregnenolone sulphate (PregS) alone or in combination with lurasidone could rescue persistent deficits in episodic memory, executive functioning, and social behaviour caused by subchronic treatment with PCP. A high dose of PregS significantly rescued subchronic PCP-induced NOR and SI deficits. The same effect was obtained by administering lower doses of PregS and lurasidone. Rajagopal et al. (2016) [50] used only the ORL test to examine the ability of 5-HT1A partial agonism and 5HT7 antagonism to improve cognition in PCP-treated mice. PCP significantly diminished the percent correct responding and increased the total incorrect trials and total incorrect responses in the reversal phase performance of the ORL test; pre-treatment with lurasidone reversed the ORL deficit in PCP-treated mice. The effect of lurasidone was reversed by a selective 5-HT1A antagonist. Two studies assessed the cognitive effects of lurasidone using Object Retrieval with Detours (ORD) [51, 52]. Marmosets were trained to reach the reward (a piece of cake) set in a clear acrylic box open only at one side. The box was placed just outside the animal cage. Each test session consisted of 9 easy trials (reward placed within direct reach) and 8 difficult trials (reward placed deep within the box, and reaching it requires a detour around the box). The order of presentation was fixed and reaching the reward without touching any wall of the box within 30 s was considered “correct”. Marmosets were trained in one test session per day, 2 or 3 days a week. After the 14th training session, animals were treated with antipsychotics and their performance in the ORD task was assessed. Murai et al. (2013) [51] showed that lurasidone did not affect the success rates in the easy trial of the task even at the highest dose while haloperidol, olanzapine, risperidone decreased the success rate; clozapine and quetiapine did not affect the success rate, but they caused adverse effects, such as drowsiness and emesis. Lurasidone increased the success rates also in the difficult trial, whereas haloperidol, olanzapine, quetiapine, risperidone, and clozapine decreased the success rate. Murai et al., (2014) [52] examined the role of D4 receptors in the pro-cognitive effect of lurasidone on executive functions using selective D4 receptor agonists and antagonists. They observed that activation of D4 receptors may improve executive function, whereas D4 and D2 receptor blockade may have the opposite effect. Three studies used the passive avoidance (PA) test in rats treated with the NMDA receptor channel blocker, MK-801 [53-55]. The PA apparatus consisted of a lighted compartment and a dark compartment with a grid floor. These two compartments were separated by a sliding door. In the training session, the animals were placed in the lighted compartment and allowed to explore for 10 sec. The sliding door was then opened, and the step-through latency for animals to enter the dark compartment was measured. As soon as the animals entered the dark compartment, the door was closed: 3 sec later, an inescapable foot-shock was delivered through the grid floor. All animals examined entered the dark compartment within 300 sec as cut-off latency in the training session and received a foot-shock. The test session was performed 24 hours after the training session using the same paradigm, but without the foot-shock, and the step-through latency for animals to enter the dark compartment was measured. Horisawa et al. (2013) [53] showed that 5-HT7 receptor antagonism is involved in the pro-cognitive effects of lurasidone in MK-801-treated rats. Ishiyama et al. (2007) [54] showed that both pre-training and post-training administration of lurasidone significantly and dose-dependently reversed the impairment of the PA response. This may suggest that lurasidone worked, at least in part, by restoring the memory consolidation process disrupted by MK-801. Similar results were obtained by Kolaczkowski et al. (2014) [55]. Enomoto et al. (2008) [56] observed the reversal effect of lurasidone on MK-801-induced impairment of learning and memory using the Morris water maze (MWM) and radial-arm maze (RAM) tests in rats. Lastly, Percelay et al. 2020 [57] analyzed the tolerability of high doses of lurasidone in mice.
3.2. Humans
We identified 12 studies, performed in 7 countries, which met the inclusion criteria and for which the required data were available. Study characteristics are shown in Table 2.
Table 2.
General characteristics of human studies.
|
Author/
Year |
Country | Study Design | Trial Duration | Population | N Tot | N |
N
Ctrl |
Diagnosis | Diagnostic Criteria | Intervention | Intervention Ctrl | Assessment |
Cognition
Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fleischhacker et al. (2019) [30] | Austria, Italy, USA, Hungary | Double blind RCT | 26 weeks | Male and female patients (18-65 years old) | 454 | 227 | 227 | Schizophrenia | DSM-IV-TR | Cariprazine 3, 4.5, or 6 mg/d | Risperidone 3, 4, or 6 mg/d | PANSS/PANSS derived factors | Changes from baseline was significantly different for cariprazine versus risperidone on PANSS-based factors evaluating cognition; (P < .05). |
| Harvey et al. (2011) [64] |
USA, Japan | Double blind RCT | 3 weeks | Male and female outpatients (18-70 years old) | 301 | 150 | 151 | Schizophrenia or Schizoaffective disorder | DSM-IV | Lurasidone 120 mg/d | Ziprasidone 80 mg BID | MCCB and SCoRS | Lurasidone patients demonstrated significant within group-improvement from baseline on the MCCB composite score (p = 0.026) and on the SCoRS (p < 0.001). |
| Harvey et al. (2015) [60] |
USA, Hong Kong, Japan | Double-blind RCT | 32 weeks | // | 488 | 246 | 116; 120 | Schizophrenia | DSM-IV-TR | Lurasidone 40-160 mg/d | Quetiapine XR 200-800 mg/d; Placebo | UPSA-B | All doses of lurasidone were superior to all doses of quetiapine for cognitive performance. |
| Harvey et al. (2017) [67] |
USA | Double-blind RCT | 6 months | // | 292 | 151 | 85; 56 | Acute exacerbation of Schizophrenia | DSM-IV-TR | Lurasidone 40-160 mg/d | Quetiapine XR 200-800 mg/d; Placebo | PANSS, UPSA-B and QWB-SA | At Week 32, improvement in PANSS total score was significantly greater in the lurasidone group compared to the quetiapine XR group. Treatment-related improvement in “insight and judgment” (PANSS-item G12 score) from acute phase baseline to Week 32 was significantly associated with improvement in neurocognitive performance. |
| Kantrowitz et al. (2016) [65] | USA | RCT | 6 months | Male and female patients (18-55 years old) | 120 | 120 | // | Schizophrenia | DSM-IV-TR | Lurasidone 40-160 mg/d | // | MCCB and UPSA-B | Moderate effect size improvements were seen across group for cognitive and symptom outcomes, although no statistically significant between-group differences were seen at study completion. |
| Karpouzian-Rogers et al. (2020) [71] | USA | RCT | 24 weeks | Male and female patients (18-60 years old) | 43 | 21 | 22 | Treatment-resistant schizophrenia | DSM-IV | Lurasidone 80 mg/d | Lurasidone 240 mg/d | EM (eye movement) measures | Stabilization on low dose lurasidone was associated with improved executive control of attention reflected by reduced antisaccade errors. High dose lurasidone resulted in prolonged speed of reflexive and executive shifts of attention and reduced spatial working memory relative to low dose. |
| Meltzer et al. (2020) [69] |
USA | Double blind RCT | 24 weeks | Male and female outpatients | 67 | 34 | 33 | Treatment-resistant schizophrenia | DSM-IV | Lurasidone 80 mg/d | Lurasidone 240 mg/d | PANSS, CGI, PSP, WCST, WISC-R Mazes, Letter Fluency, Category Fluency: Animal Naming, the Brown-Peterson Auditory Consonant Trigrams Test, DSST, Rey Auditory Verbal Learning Test. | Significant non-dose-related improvement in the Positive and Negative Syndrome Scale—Total and subscales and in 2 of 7 cognitive domains, speed of processing and executive function, were noted. |
| Miller et al. (2020) [61] |
USA, Hong Kong | Double blind RCT | 6 weeks | Male and female patients (18-75 years old) | 488 | 125; 121 | 120;122 | Schizophrenia | DSM-IV-TR | Lurasidone 80 and 160 mg/d | Quetiapine XR 600 mg/day; placebo | CRP and CogState | Baseline CRP level combined with measures of metabolic risk significantly moderated the improvement in cognitive performance associated with lurasidone 160 mg/day (vs. placebo) treatment. |
| Nakamura et al. (2009) [68] | Japan | Double blind case-control study | 6 weeks | Male and female patients (18-64 years old) | 180 | 90 | 90 | Acute exacerbation of Schizophrenia | DSM-IV | Lurasidone 80 mg/d | Placebo | BPRSd, PANSS, CGI-S, MADRS | Treatment with lurasidone was associated with significant improvement compared to placebo on the BPRSd, as well as on all secondary efficacy measures, including the PANSS total score, PANSS positive, negativeand general psychopathology subscales. |
| Raison et al. (2020) [62] | USA, Hong Kong | Double blind case-control study | 6 weeks | Male and female patients (10-17 years old) | 343 | 173 | 170 | Patients 10-17 years of age with a DSM-5 diagnosis of bipolar I depression | DSM-5 | Lurasidone 20-80 mg/d | Placebo | CDRS-R, computerized Brief Cogstate Battery | Young patients with bipolar depression with normal weight and higher levels of pre-treatment CRP may show a greater placebo-adjusted improvement in depressive symptoms and cognitive performance when treated with lurasidone. |
| Vieta et al. (2021) [58] |
Spain, USA | Randomized, double-blind, placebo-controlled, parallel-group, fixed-dose study | 8 weeks | Male and female patients (18-65 years old) | 393 | 135; 126 |
132 | Bipolar I disorder without psychotic features | DSM-IV-TR | Cariprazine 1,5 or 3 mg/d | Placebo | FAST | The least squares mean difference (LSMD) in FAST total score change from baseline to week 8 was statically significant in favor of cariprazine 1.5 mg/d versus placebo (p = .0051); the LSMD versus placebo was statistically significant in favor of cariprazine 1.5 mg/d for Autonomy, Occupational Functioning, Cognitive Functioning, and Leisure Time. |
| Yatham et al. (2017) [73] |
Canada | Randomized open-label pilot study | 6 weeks | Male and female patients (19-65 years old) | 34 | 17 | 17 | Euthymic bipolar I disorder | DSM-IV-TR | Lurasidone 20-80 mg/d | TAU | YMRS, MADRS, CGI-BP, CFQ, BFIS, SDS, QoL.BD, ISBD-BANC, CVLT-II and BVMT-R | The magnitude of benefit with lurasidone adjunctive therapy in improving global cognition (effect size 0·46) was greater compared with the improvement observed in the TAU group (0·04). |
Abbreviations: PANSS (Positive and Negative Symtomps Scale), MCCB (Matrics Consensus Cognitive Battery), SCoRS (Schizophrenia cognition rating scale), UPSA-B (The University of California San Diego (UCSD) Performance-based Skills Assessment-Brief version), QWB-SA (Quality of well-being self-administered), CGI (Clinical global impression), PSP (Personal and Social Performance Scale), WCST (Wisconsin card sorting test), WISC-R (Wechsler Intelligence scale for children), DSST (Digit symbol substitution test), CRP (C-Reactive Protein), BPRS (Brief psychiatric rating scale), MADRS (Montgomery Asberg Depression rating scale), CDRS (Clinical dementia rating scale), YMRS (Young mania rating scale), CFQ (Cognitive failure questionnaire), BFIS (Barkley functional impairment scale), SDS (Sheehan Disability Scale), QoL.BD (Quality of Life in Bipolar Disorder), ISBD-BANC (International Society for Bipolar Disorders-Battery for Assessment of Neurocognition), CVLT-II (California Verbal Learning Test–II), BVMT-R (Brief Visuospatial Memory Test—Revised version), TAU (treatment as usual).
3.2.1. Cariprazine
Fleischhacker et al. 2019 [30] evaluated the effect of cariprazine on cognitive function in schizophrenia, assessing changes in PANSS-derived cognitive factor scores. Cariprazine was superior to risperidone in reducing PANSS-based factors evaluating disorganized thoughts, prosocial function, and cognition even at week 26.
Vieta et al. 2021 [58] examined the effects of cariprazine on functional and cognitive outcomes in patients with bipolar I disorder, evaluating mean changes from baseline to week 8 in the Functional Assessment Short Test (FAST), a validated 24-item clinician-rated scale that was designed to measure the areas of functional difficulty associated with bipolar disorder [59]. Cariprazine was superior to placebo in reducing the FAST total score at week 8 at the dose of 1.5 mg/die. On the FAST subscales, cariprazine (1.5 mg/d) was better than placebo in terms of Autonomy, Occupational Functioning, Leisure Time, and Cognitive Functioning.
3.2.2. Lurasidone
Three studies [60-62] assessed the effect of lurasidone using the CogState Computerized Cognitive Battery, a well-established assessment battery for cognitive impairment in schizophrenia [63]. Harvey et al. (2015) [60] performed a 6-month double blind trial of lurasidone compared to quetiapine. They observed schizophrenic patients who received either 120 or 160 mg/die of lurasidone showed significantly greater improvement in the overall cognitive performance compared to quetiapine extended release (XR) at a variable dose of 200-800 mg/die at week 32. Mean changes in neurocognitive composite z-score from baseline were significant for all lurasidone doses at both weeks 19 and 32. Functional capacity scores improved in all treatment groups. However, Miller et al. (2020) [61] did not observe a significant difference in cognitive performance in patients with schizophrenia randomized to lurasidone (80 or 160 mg/die) or quetiapine XR (600 mg/die) at 6 weeks from baseline. Lurasidone (160 mg/die) was superior to placebo in improving cognition and this effect was mediated by inflammation and metabolic risk measures (c-reactive protein levels, and triglyceride/HDL ratio) observed at baseline. In line with these findings, Raison et al. 2020 [62] performed a study in which patients (10 to 17 years of age) with bipolar I disorder were randomized to 6 weeks of double-blind treatment with once-daily, flexibly dosed lurasidone (20-80 mg) or placebo. They found that higher baseline levels of high-sensitivity c-reactive protein were associated with improvement in cognitive function in the lurasidone group (vs. placebo); this was observed however only in patents with normal BMI and not in overweight/ obese patients.
Two studies [64, 65] used the MATRICS Consensus Cognitive Battery (MCCB), a battery recommended by FDA to assess cognitive impairment in schizophrenia [66].
In Harvey et al. (2011) [64] adult outpatients who had schizophrenia or schizoaffective disorder were randomized to 21 days of double-blind treatment with lurasidone (120 mg once daily) or ziprasidone (80 mg twice daily). Lurasidone-treated patients showed a significant improvement from baseline on the MCCB composite score, while ziprasidone-treated patients did not show any improvement.
Kantrowitz et al. (2016) [65] conducted a multicenter, rater-blinded, randomized, controlled study of auditory-focused cognitive remediation used as an augmentation to lurasidone (40-160 mg/die). At study completion, there was a significant improvement in the MCCB composite score both in the auditory processing cognitive remediation and in the comparison group. It had to be noted that both groups were on open-label treatment with lurasidone, and this could have been the cause of the results.
Other three studies [67-69]; used the changes in PANSS-cognitive factors (as defined by Lindenmayer et al. 1994 [70]) as the outcome measures for cognition.
Harvey et al. (2017) [67] evaluated clinically unstable patients with schizophrenia, who were randomized to once-daily, fixed-dose treatment with lurasidone (80 or 160 mg), quetiapine XR (600 mg) or placebo, followed by a long-term, double-blind, flexible-dose continuation. Flexibly dosed lurasidone (40 to 160 mg/die) was found to be associated with significantly greater improvement in insight compared to flexibly dosed quetiapine XR (200 to 800 mg/d) over long-term treatment in patients with schizophrenia.
Nakamura et al. (2009) [68] performed a study in which patients were randomly assigned to 6 weeks of double-blind treatment with a fixed dose of lurasidone (80 mg) or placebo. They found that, at day 42, treatment with lurasidone was associated with significant improvement on the Brief Psychiatric Rating Scale as well as on the PANSS total score and the PANSS positive and general psychopathology subscales, including cognitive items. Significant improvement was seen as early as day 3.
Meltzer et al. (2020) [69] compared two doses of lurasidone (80 vs. 240 mg/die) in time to improve psychopathology and cognition during a 6-month trial in treatment-resistant schizophrenia patients. They found a significant non-dose-related improvement in the PANSS—total and subscales, and in 2 of 7 cognitive domains, i.e. speed of processing and executive function. In the same study, other types of cognitive evaluation focusing on executive functions, memory and attention showed a significant improvement from baseline only in the lurasidone 80 mg/die group.
Karpouzian-Rogers, Stocks et al.’s (2020) [71] study evaluated the effects of high versus low doses of lurasidone on Eye Movement performance in treatment-resistant schizophrenia. Eye movement measures are well-assessed paradigms used to examine the neural systems involved in cognitive and sensorimotor processes. In particular, the anti-saccade task is a reliable and sensitive measure of the processes involved in resolving the conflict between volitional and reflexive behavioral responses (Hutton et al. 2006) [72]. Patients completed the eye movement testing at baseline, on an existing medication regimen, after 6 weeks of low dose (80 mg) of lurasidone, after 12 weeks following randomization to low (80 mg) or high (240 mg) dose of lurasidone, and after 24 weeks of treatment. Six weeks of lurasidone treatment resulted in increased pro-saccade latency and reduced anti-saccade errors, with no change in memory-guided saccade accuracy. After randomization, saccade latencies increased only the high dose group, with no change in antisaccade errors in both groups. Memory-guided saccade error increased in the high-dose group and remained stable in the low-dose group, pointing to higher executive control in this group.
Lastly, Yatham et al. (2017) [73] aimed to examine the efficacy of lurasidone adjunctive therapy compared to treatment as usual in improving cognition in bipolar disorder type I, by administering the International Society for Bipolar Disorders Battery for Assessment of Neurocognition (ISBD-BANC). Lurasidone adjunctive therapy improved global cognition score. The effect of lurasidone on cognition score was of moderate to large magnitude.
4. DISCUSSION
Our systematic review shows the efficacy of lurasidone in improving cognition both in animal and human studies. According to animal models, the effect on cognition could be explained by its potent antagonism at the 5-HT7 receptors combined with partial agonism at 5-HT1A receptors [74]. In fact, several animal studies [41, 42, 49, 50, 53, 55, 56] have shown that the pro-cognitive effect of lurasidone was mediated by either 5-HT7 receptor antagonism and/or 5-HT1A receptor partial agonism. Functionally, the 5-HT7 receptor is associated with several physiological and pathological responses, including serotonin-induced phase shifting of the circadian rhythm, control of memory as well as locomotor and exploratory activity [75, 76]. Of note, two additional studies [46, 47] suggested that the action of lurasidone required the activation of D4 receptors. The D4 receptors are located presynaptically in glutamatergic terminals (thus potentially being involved in long-term potentiation and memory) while the postsynaptic localization is in the dendrites of the GABAergic efferent neurons [77]. The complex mechanism of action of lurasidone may be held responsible for its procognitive effect compared to other antipsychotic molecules which are also strong 5-HT7 antagonists as amisulpride. Of note, considering head-to-head confrontation in animal models, lurasidone was superior to haloperidol, olanzapine, risperidone, aripiprazole, clozapine and quetiapine in improving cognitive functions. We found more studies investigating the effect of lurasidone on cognition than the effect of cariprazine (29 vs. 7). In human studies, lurasidone showed a greater efficacy on cognitive performance compared to placebo [62, 68]; quetiapine [67, 60]; ziprasidone [64], or treatment as usual [72]. Only one study did not report a significant difference with quetiapine [61]. The positive effect on cognition was not consistently dose-dependent [69, 71]. However, the evidence pointing to a significant pro-cognitive effect of lurasidone in humans is still not very strong: in fact, one potential caveat of the included studies is that lurasidone was confronted with molecules (such as quetiapine) that have a higher sedation effect, which could, at least in part, biased the results. Comparisons with less sedating molecules such as aripiprazole are therefore needed.
According to animal models, the pro-cognitive effect of cariprazine is probably explained by its very high affinity for D3 receptors. The involvement of D3 receptors in the action of cariprazine was demonstrated using D3 receptor knockout mice, which were unresponsive to cariprazine. Cariprazine was superior to other antipsychotics in improving cognitive functions in animal studies [33, 34, 37]. In humans, there are little data to draw reliable conclusions: in fact, cariprazine was superior to placebo in one study and risperidone in the other; the same caveat observed for lurasidone is also valid for cariprazine studies, as risperidone has a higher sedation potential compared to cariprazine and this effect could have impact cognitive performance. One potential limitation of our study was the search in only one public database: this may have reduced the original number of articles included, but it is improbable that it may have affected the number of included studies that were thoroughly hand-searched for additional references.
CONCLUSION
In conclusion, our data may support the use of lurasidone which appears effective on the psychopathological front as other “older” antipsychotics, but seems to have a more promising profile on cognition. Therefore, its use could be more frequent in younger patients or first-episode, in order to maintain the cognitive capacity of the individual. There is insufficient evidence for cariprazine, even if its use may seem potentially relevant. Head-to-head studies comparing lurasidone and cariprazine are awaited to establish which of the two drugs may be considered firstly in the treatment of cognitive dysfunction associated with schizophrenia.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- EPS
Extrapyramidal Symptoms
- FAST
Functional Assessment Short Test
- NOR
Novel Object Recognition
- ORL
Operant Reversal Learning
- PA
Passive Avoidance
- PANSS
Positive and Negative Syndrome Scale
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SI
Social Interaction
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Institute of health Metrics and Evaluation (IHME) Global Health Data Exchange (GHDx). Available from: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/27a7644e8ad28e739382d31e77589dd7 (Accessed on: 25 September 2021)
- 2.Zanelli J., Mollon J., Sandin S., Morgan C., Dazzan P., Pilecka I., Reis Marques T., David A.S., Morgan K., Fearon P., Doody G.A., Jones P.B., Murray R.M., Reichenberg A. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am. J. Psychiatry. 2019;176(10):811–819. doi: 10.1176/appi.ajp.2019.18091088. [DOI] [PubMed] [Google Scholar]
- 3.Jonas K., Lian W., Callahan J., Ruggero C.J., Clouston S., Reichenberg A., Carlson G.A., Bromet E.J., Kotov R. The course of general cognitive ability in individuals with psychotic disorders. JAMA Psychiatry. 2022;79(7):659–666. doi: 10.1001/jamapsychiatry.2022.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripathi A., Kar S.K., Shukla R. Cognitive deficits in schizophrenia: Understanding the biological correlates and remediation strategies. Clin. Psychopharmacol. Neurosci. 2018;16(1):7–17. doi: 10.9758/cpn.2018.16.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35(3):509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fusar-Poli P., Smieskova R., Serafini G., Politi P., Borgwardt S. Neuroanatomical markers of genetic liability to psychosis and first episode psychosis: A voxelwise meta-analytical comparison. World J. Biol. Psychiatry. 2014;15(3):219–228. doi: 10.3109/15622975.2011.630408. [DOI] [PubMed] [Google Scholar]
- 7.Bowie C.R., Harvey P.D. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2006;2(4):531–536. doi: 10.2147/nedt.2006.2.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y.S., Marder S.R., Green M.F. Repurposing drugs for cognition in schizophrenia. Clin. Pharmacol. Ther. 2017;101(2):191–193. doi: 10.1002/cpt.529. [DOI] [PubMed] [Google Scholar]
- 9.Okubo R., Hasegawa T., Fukuyama K., Shiroyama T., Okada M. Current limitations and candidate potential of 5-HT7 receptor antagonism in psychiatric pharmacotherapy. Front. Psychiatry. 2021;12:623684. doi: 10.3389/fpsyt.2021.623684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahl S.M. Mechanism of action of cariprazine. CNS Spectr. 2016;21(2):123–127. doi: 10.1017/S1092852916000043. [DOI] [PubMed] [Google Scholar]
- 11.Choi Y.K., Adham N., Kiss B., Gyertyán I., Tarazi F.I. Long-term effects of cariprazine exposure on dopamine receptor subtypes. CNS Spectr. 2014;19(3):268–277. doi: 10.1017/S1092852913000680. [DOI] [PubMed] [Google Scholar]
- 12.Meltzer H.Y., Rajagopal L., Huang M., Oyamada Y., Kwon S., Horiguchi M. Translating the N-methyl-d-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int. J. Neuropsychopharmacol. 2013;16(10):2181–2194. doi: 10.1017/S1461145713000928. [DOI] [PubMed] [Google Scholar]
- 13.Goff D.C., Hill M., Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol. Biochem. Behav. 2011;99(2):245–253. doi: 10.1016/j.pbb.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasbarri A., Pompili A. Serotonergic 5-HT7 receptors and cognition. Rev. Neurosci. 2014;25(3):311–323. doi: 10.1515/revneuro-2013-0066. [DOI] [PubMed] [Google Scholar]
- 15.Sokoloff P., Le Foll B. The dopamine D3 receptor, a quarter century later. Eur. J. Neurosci. 2017;45(1):2–19. doi: 10.1111/ejn.13390. [DOI] [PubMed] [Google Scholar]
- 16.Maramai S., Gemma S., Brogi S., Campiani G., Butini S., Stark H., Brindisi M. Dopamine D3 receptor antagonists as potential therapeutics for the treatment of neurological diseases. Front. Neurosci. 2016;10:451. doi: 10.3389/fnins.2016.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl S.M. Drugs for psychosis and mood: Unique actions at D3, D2, and D1 dopamine receptor subtypes. CNS Spectr. 2017;22(5):375–384. doi: 10.1017/S1092852917000608. [DOI] [PubMed] [Google Scholar]
- 18.Meneses A. 5-HT7 receptor stimulation and blockade: A therapeutic paradox about memory formation and amnesia. Front. Behav. Neurosci. 2014;8:207. doi: 10.3389/fnbeh.2014.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishibashi T., Horisawa T., Tokuda K., Ishiyama T., Ogasa M., Tagashira R., Matsumoto K., Nishikawa H., Ueda Y., Toma S., Oki H., Tanno N., Saji I., Ito A., Ohno Y., Nakamura M. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J. Pharmacol. Exp. Ther. 2010;334(1):171–181. doi: 10.1124/jpet.110.167346. [DOI] [PubMed] [Google Scholar]
- 20.Brennan J.A., Graf R., Grauer S.M., Navarra R.L., Pulicicchio C.M., Hughes Z.A., Lin Q., Wantuch C., Rosenzweig-Lipson S., Pruthi F., Lai M., Smith D., Goutier W., van de Neut M., Robichaud A.J., Rotella D., Feenstra R.W., Kruse C., Broqua P., Beyer C.E., McCreary A.C., Pausch M.H., Marquis K.L. WS-50030 [7-{4-[3-(1H-inden-3-yl)propyl]piperazin-1-yl}-1,3-benzoxazol-2 (3H)-one]: A novel dopamine D2 receptor partial agonist/serotonin reuptake inhibitor with preclinical antipsychotic-like and antidepressant-like activity. J. Pharmacol. Exp. Ther. 2010;332(1):190–201. doi: 10.1124/jpet.109.157388. [DOI] [PubMed] [Google Scholar]
- 21.Sanford M. Lurasidone. Lurasidone: in the treatment of schizophrenia. CNS Drugs. 2013;27(1):67–80. doi: 10.1007/s40263-012-0026-x. [DOI] [PubMed] [Google Scholar]
- 22.Citrome L. Lurasidone for schizophrenia: A review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int. J. Clin. Pract. 2011;65(2):189–210. doi: 10.1111/j.1742-1241.2010.02587.x. [DOI] [PubMed] [Google Scholar]
- 23.Tocco M., Newcomer J.W., Mao Y., Pikalov A., Loebel A. Lurasidone and risk for metabolic syndrome: Results from short- and long-term clinical studies in patients with schizophrenia. CNS Spectr. 2020;14:1–11. doi: 10.1017/S1092852920001698. [DOI] [PubMed] [Google Scholar]
- 24.Loebel A., Cucchiaro J., Xu J., Sarma K., Pikalov A., Kane J.M. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: A 12-month, double-blind, noninferiority study. Schizophr. Res. 2013;147(1):95–102. doi: 10.1016/j.schres.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Loebel A.D., Siu C.O., Cucchiaro J.B., Pikalov A.A., Harvey P.D. Daytime sleepiness associated with lurasidone and quetiapine XR: Results from a randomized double-blind, placebo-controlled trial in patients with schizophrenia. CNS Spectr. 2014;19(2):197–205. doi: 10.1017/S1092852913000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasrallah H.A., Silva R., Phillips D., Cucchiaro J., Hsu J., Xu J., Loebel A. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: A 6-week, randomized, placebo-controlled study. J. Psychiatr. Res. 2013;47(5):670–677. doi: 10.1016/j.jpsychires.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Potkin S.G., Ogasa M., Cucchiaro J., Loebel A. Double-blind comparison of the safety and efficacy of lurasidone and ziprasidone in clinically stable outpatients with schizophrenia or schizoaffective disorder. Schizophr. Res. 2011;132(2-3):101–107. doi: 10.1016/j.schres.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Durgam S., Starace A., Li D., Migliore R., Ruth A., Németh G., Laszlovszky I. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: A phase II, randomized clinical trial. Schizophr. Res. 2014;152(2-3):450–457. doi: 10.1016/j.schres.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 29.Kane J.M., Zukin S., Wang Y., Lu K., Ruth A., Nagy K., Laszlovszky I., Durgam S. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia. J. Clin. Psychopharmacol. 2015;35(4):367–373. doi: 10.1097/JCP.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 30.Fleischhacker W., Galderisi S., Laszlovszky I., Szatmári B., Barabássy Á., Acsai K., Szalai E., Harsányi J., Earley W., Patel M., Németh G. The efficacy of cariprazine in negative symptoms of schizophrenia: Post hoc analyses of PANSS individual items and PANSS-derived factors. Eur. Psychiatry. 2019;58:1–9. doi: 10.1016/j.eurpsy.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Misiak B., Bieńkowski P., Samochowiec J. Cariprazine – a novel antipsychotic drug and its place in the treatment of schizophrenia. Psychiatr. Pol. 2018;52(6):971–981. doi: 10.12740/PP/OnlineFirst/80710. [DOI] [PubMed] [Google Scholar]
- 32.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339(1):b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnes S.A., Young J.W., Markou A., Adham N., Gyertyán I., Kiss B. Correction to: The effects of cariprazine and aripiprazole on PCP-induced deficits on attention assessed in the 5-choice serial reaction time task. Psychopharmacology. 2018;235(5):1621. doi: 10.1007/s00213-018-4884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neill J.C., Grayson B., Kiss B., Gyertyán I., Ferguson P., Adham N. Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur. Neuropsychopharmacol. 2016;26(1):3–14. doi: 10.1016/j.euroneuro.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Watson D.J.G., King M.V., Gyertyán I., Kiss B., Adham N., Fone K.C.F. The dopamine D 3 -preferring D 2/D 3 dopamine receptor partial agonist, cariprazine, reverses behavioural changes in a rat neurodevelopmental model for schizophrenia. Eur. Neuropsychopharmacol. 2016;26(2):208–224. doi: 10.1016/j.euroneuro.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Zimnisky R., Chang G., Gyertyán I., Kiss B., Adham N., Schmauss C. Cariprazine, a dopamine D3-receptor-preferring partial agonist, blocks phencyclidine-induced impairments of working memory, attention set-shifting, and recognition memory in the mouse. Psychopharmacology (Berl.) 2013;226(1):91–100. doi: 10.1007/s00213-012-2896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gyertyán I., Kiss B., Sághy K., Laszy J., Szabó G., Szabados T., Gémesi L.I., Pásztor G., Zájer-Balázs M., Kapás M., Csongor É.Á., Domány G., Tihanyi K., Szombathelyi Z. Cariprazine (RGH-188), a potent D3/D2 dopamine receptor partial agonist, binds to dopamine D3 receptors in vivo and shows antipsychotic-like and procognitive effects in rodents. Neurochem. Int. 2011;59(6):925–935. doi: 10.1016/j.neuint.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Lueptow L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017;30(126):55718. doi: 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calabrese F., Brivio P., Sbrini G., Gruca P., Lason M., Litwa E., Niemczyk M., Papp M., Riva M.A. Effect of lurasidone treatment on chronic mild stress-induced behavioural deficits in male rats: The potential role for glucocorticoid receptor signalling. J. Psychopharmacol. 2020;34(4):420–428. doi: 10.1177/0269881119895547. [DOI] [PubMed] [Google Scholar]
- 40.Horiguchi M., Huang M., Meltzer H.Y. The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J. Pharmacol. Exp. Ther. 2011;338(2):605–614. doi: 10.1124/jpet.111.180638. [DOI] [PubMed] [Google Scholar]
- 41.Horiguchi M., Huang M., Meltzer H.Y. Interaction of mGlu2/3 agonism with clozapine and lurasidone to restore novel object recognition in subchronic phencyclidine-treated rats. Psychopharmacology (Berl.) 2011;217(1):13–24. doi: 10.1007/s00213-011-2251-2. [DOI] [PubMed] [Google Scholar]
- 42.Horiguchi M., Hannaway K.E., Adelekun A.E., Jayathilake K., Meltzer H.Y. Prevention of the phencyclidine-induced impairment in novel object recognition in female rats by co-administration of lurasidone or tandospirone, a 5-HT(1A) partial agonist. Neuropsychopharmacology. 2012;37(10):2175–2183. doi: 10.1038/npp.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horiguchi M., Meltzer H.Y. The role of 5-HT1A receptors in phencyclidine (PCP)-induced novel object recognition (NOR) deficit in rats. Psychopharmacology (Berl.) 2012;221(2):205–215. doi: 10.1007/s00213-011-2561-4. [DOI] [PubMed] [Google Scholar]
- 44.Horiguchi M., Miyauchi M., Neugebauer N.M., Oyamada Y., Meltzer H.Y. Prolonged reversal of the phencyclidine-induced impairment in novel object recognition by a serotonin (5-HT)1A-dependent mechanism. Behav. Brain Res. 2016;301:132–141. doi: 10.1016/j.bbr.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 45.Huang M., Kwon S., Rajagopal L., He W., Meltzer H.Y. 5-HT1A parital agonism and 5-HT7 antagonism restore episodic memory in subchronic phencyclidine-treated mice: Role of brain glutamate, dopamine, acetylcholine and GABA. Psychopharmacology. 2018;235(10):2795–2808. doi: 10.1007/s00213-018-4972-y. [DOI] [PubMed] [Google Scholar]
- 46.Miyauchi M., Neugebauer N.M., Oyamada Y., Meltzer H.Y. Nicotinic receptors and lurasidone-mediated reversal of phencyclidine-induced deficit in novel object recognition. Behav. Brain Res. 2016;301:204–212. doi: 10.1016/j.bbr.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 47.Miyauchi M., Neugebauer N.M., Meltzer H.Y. Dopamine D4 receptor stimulation contributes to novel object recognition: Relevance to cognitive impairment in schizophrenia. J. Psychopharmacol. 2017;31(4):442–452. doi: 10.1177/0269881117693746. [DOI] [PubMed] [Google Scholar]
- 48.Miyauchi M., Neugebauer N.M., Sato T., Ardehali H., Meltzer H.Y. Muscarinic receptor signaling contributes to atypical antipsychotic drug reversal of the phencyclidine-induced deficit in novel object recognition in rats. J. Psychopharmacol. 2017;31(12):1588–1604. doi: 10.1177/0269881117731278. [DOI] [PubMed] [Google Scholar]
- 49.Rajagopal L., Soni D., Meltzer H.Y. Neurosteroid pregnenolone sulfate, alone, and as augmentation of lurasidone or tandospirone, rescues phencyclidine-induced deficits in cognitive function and social interaction. Behav. Brain Res. 2018;350(350):31–43. doi: 10.1016/j.bbr.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Rajagopal L., Massey B.W., Michael E., Meltzer H.Y. Serotonin (5-HT)1A receptor agonism and 5-HT7 receptor antagonism ameliorate the subchronic phencyclidine-induced deficit in executive functioning in mice. Psychopharmacology (Berl.) 2016;233(4):649–660. doi: 10.1007/s00213-015-4137-1. [DOI] [PubMed] [Google Scholar]
- 51.Murai T., Nakako T., Ikejiri M., Ishiyama T., Taiji M., Ikeda K. Effects of lurasidone on executive function in common marmosets. Behav. Brain Res. 2013;246:125–131. doi: 10.1016/j.bbr.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Murai T., Nakako T., Ikeda K., Ikejiri M., Ishiyama T., Taiji M. Lack of dopamine D4 receptor affinity contributes to the procognitive effect of lurasidone. Behav. Brain Res. 2014;261:26–30. doi: 10.1016/j.bbr.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 53.Horisawa T., Nishikawa H., Toma S., Ikeda A., Horiguchi M., Ono M., Ishiyama T., Taiji M. The role of 5-HT7 receptor antagonism in the amelioration of MK-801-induced learning and memory deficits by the novel atypical antipsychotic drug lurasidone. Behav. Brain Res. 2013;244:66–69. doi: 10.1016/j.bbr.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 54.Ishiyama T., Tokuda K., Ishibashi T., Ito A., Toma S., Ohno Y. Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test. Eur. J. Pharmacol. 2007;572(2-3):160–170. doi: 10.1016/j.ejphar.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 55.Kołaczkowski M., Mierzejewski P., Bieńkowski P., Wesołowska A., Newman-Tancredi A. ADN-1184 a monoaminergic ligand with 5-HT(6/7) receptor antagonist activity: Pharmacological profile and potential therapeutic utility. Br. J. Pharmacol. 2014;171(4):973–984. doi: 10.1111/bph.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enomoto T., Ishibashi T., Tokuda K., Ishiyama T., Toma S., Ito A. Lurasidone reverses MK-801-induced impairment of learning and memory in the Morris water maze and radial-arm maze tests in rats. Behav. Brain Res. 2008;186(2):197–207. doi: 10.1016/j.bbr.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Percelay S., Since M., Lagadu S., Freret T., Bouet V., Boulouard M. Antipsychotic lurasidone: Behavioural and pharmacokinetic data in C57BL/6 mice. Pharmacol. Biochem. Behav. 2020;194:172933. doi: 10.1016/j.pbb.2020.172933. [DOI] [PubMed] [Google Scholar]
- 58.Vieta E., Calabrese J.R., Whelan J., Tohen M., Earley W.R. The efficacy of cariprazine on function in patients with bipolar depression: A post hoc analysis of a randomized controlled trial. Curr. Med. Res. Opin. 2021;37(9):1635–1643. doi: 10.1080/03007995.2021.1932446. [DOI] [PubMed] [Google Scholar]
- 59.Rosa A.R., Sánchez-Moreno J., Martínez-Aran A., Salamero M., Torrent C., Reinares M., Comes M., Colom F., Van Riel W., Ayuso-Mateos J., Kapczinski F., Vieta E. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin. Pract. Epidemiol. Ment. Health. 2007;3(1):5. doi: 10.1186/1745-0179-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harvey P.D., Siu C.O., Ogasa M., Loebel A. Effect of lurasidone dose on cognition in patients with schizophrenia: Post-hoc analysis of a long-term, double-blind continuation study. Schizophr. Res. 2015;166(1-3):334–338. doi: 10.1016/j.schres.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Miller B.J., Pikalov A., Siu C.O., Tocco M., Tsai J., Harvey P.D., Newcomer J.W., Loebel A. Association of C-reactive protein and metabolic risk with cognitive effects of lurasidone in patients with schizophrenia. Compr. Psychiatry. 2020;102:152195. doi: 10.1016/j.comppsych.2020.152195. [DOI] [PubMed] [Google Scholar]
- 62.Raison C.L., Siu C., Pikalov A., Tocco M., Loebel A. C-reactive protein and response to lurasidone treatment in children and adolescents with bipolar I depression: Results from a placebo-controlled trial. Brain Behav. Immun. 2020;84:269–274. doi: 10.1016/j.bbi.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Pietrzak R.H., Olver J., Norman T., Piskulic D., Maruff P., Snyder P.J. A comparison of the CogState schizophrenia battery and the measurement and treatment research to improve cognition in schizophrenia (MATRICS) battery in assessing cognitive impairment in chronic schizophrenia. J. Clin. Exp. Neuropsychol. 2009;31(7):848–859. doi: 10.1080/13803390802592458. [DOI] [PubMed] [Google Scholar]
- 64.Harvey P.D., Ogasa M., Cucchiaro J., Loebel A., Keefe R.S.E. Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs. ziprasidone. Schizophr. Res. 2011;127(1-3):188–194. doi: 10.1016/j.schres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Kantrowitz J.T., Sharif Z., Medalia A., Keefe R.S.E., Harvey P., Bruder G., Barch D.M., Choo T., Lee S., Lieberman J.A. A multicenter, rater-blinded, randomized controlled study of auditory processing-focused cognitive remediation combined with open-label lurasidone in patients with schizophrenia and schizoaffective disorder. J. Clin. Psychiatry. 2016;77(6):799–806. doi: 10.4088/JCP.15m09998. [DOI] [PubMed] [Google Scholar]
- 66.Nuechterlein K.H., Green M.F., Kern R.S., Baade L.E., Barch D.M., Cohen J.D., Essock S., Fenton W.S., Frese F.J., III, Gold J.M., Goldberg T., Heaton R.K., Keefe R.S.E., Kraemer H., Mesholam-Gately R., Seidman L.J., Stover E., Weinberger D.R., Young A.S., Zalcman S., Marder S.R. The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. Am. J. Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 67.Harvey P.D., Siu C.O., Loebel A.D. Insight and treatment outcomes in schizophrenia: Post-hoc analysis of a long-term, double-blind study comparing lurasidone and quetiapine XR. Innov. Clin. Neurosci. 2017;14(11-12):23–29. [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura M., Ogasa M., Guarino J., Phillips D., Severs J., Cucchiaro J., Loebel A. Lurasidone in the treatment of acute schizophrenia: A double-blind, placebo-controlled trial. J. Clin. Psychiatry. 2009;70(6):829–836. doi: 10.4088/JCP.08m04905. [DOI] [PubMed] [Google Scholar]
- 69.Meltzer H.Y., Share D.B., Jayathilake K., Salomon R.M., Lee M.A. Lurasidone improves psychopathology and cognition in treatment-resistant schizophrenia. J. Clin. Psychopharmacol. 2020;40(3):240–249. doi: 10.1097/JCP.0000000000001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindenmayer J.P., Bernstein-Hyman R., Grochowski S. A new five factor model of schizophrenia. Psychiatr. Q. 1994;65(4):299–322. doi: 10.1007/BF02354306. [DOI] [PubMed] [Google Scholar]
- 71.Karpouzian-Rogers T., Stocks J., Meltzer H.Y., Reilly J.L. The effect of high vs. low dose lurasidone on eye movement biomarkers of prefrontal abilities in treatment-resistant schizophrenia. Schizophr. Res. 2020;215:314–321. doi: 10.1016/j.schres.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Hutton S.B., Ettinger U. The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology. 2006;43(3):302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 73.Yatham L.N., Mackala S., Basivireddy J., Ahn S., Walji N., Hu C., Lam R.W., Torres I.J. Lurasidone versus treatment as usual for cognitive impairment in euthymic patients with bipolar I disorder: A randomised, open-label, pilot study. Lancet Psychiatry. 2017;4(3):208–217. doi: 10.1016/S2215-0366(17)30046-9. [DOI] [PubMed] [Google Scholar]
- 74.Maroteaux L., Béchade C., Roumier A. Dimers of serotonin receptors: Impact on ligand affinity and signaling. Biochimie. 2019;161:23–33. doi: 10.1016/j.biochi.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 75.Ciranna L., Catania M.V. 5-HT7 receptors as modulators of neuronal excitability, synaptic transmission and plasticity: Physiological role and possible implications in autism spectrum disorders. Front. Cell. Neurosci. 2014;8:250. doi: 10.3389/fncel.2014.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guseva D., Wirth A., Ponimaskin E. Cellular mechanisms of the 5-HT 7 receptor-mediated signaling. Front. Behav. Neurosci. 2014;8:306. doi: 10.3389/fnbeh.2014.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woods A.S. The dopamine D4 receptor, the ultimate disordered protein. J. Recept. Signal Transduct. Res. 2010;30(5):331–336. doi: 10.3109/10799893.2010.513842. [DOI] [PMC free article] [PubMed] [Google Scholar]


